Abstract
Objectives
The aim of this study was to investigate the immunity against rubella using the serological status of rubella-specific IgG antibodies (antirubella IgG) in Korean women of childbearing age (15–49 years).
Design
Retrospective cross-sectional study.
Setting
Population-based cross-sectional study in South Korea.
Participants
Between January 2010 and December 2017, test results from Korean women aged 15–49 years who had visited an obstetric private clinic (nationwide institutions) and had requested rubella-specific IgG antibody tests from Green Cross Laboratories were obtained from the laboratory information system.
Results
Between 2010 and 2017, antirubella IgG test results from 328 426 Korean women aged 15–49 years who had visited private obstetric clinics (1438 institutions nationwide) were retrospectively analysed by tested year, age, cohort and geographic regions. Over the 8-year study period, the rate of unimmunised women ranged from 7.8% to 9.7%. Multivariable-adjusted logistic regression models showed that the odds of being immune to rubella (positive and equivocal results of antirubella IgG test) were lower in 2017 compared with 2010, in women in their 40s, in a pre-catch-up cohort and in women living in Incheon, Busan, South Gyeongsang, North and South Jeolla and Jeju provinces (p<0.0001).
Conclusions
In consideration of the factors associated with prevalence of women unimmunised to rubella, future public health efforts should be focused on catch-up activities. The results of this study could be used to strengthen disease control and prevent rubella, including a nationwide immunisation programme.
Keywords: rubella, seroprevalence, immunization, vaccination
Strengths and limitations of this study.
The main strength of the study lies in its sample size, due to the fact that it is a nationwide study with one of the broadest samples to date in South Korea.
The study provided a recent information of the seroprevalence of antirubella IgG that have not been available at this scale before.
The huge sample size of this study allowed for precise information of the age-related seroprevalence of antirubella IgG and this study provides valuable information for establishing a catch-up vaccination programme in South Korea.
One limitation of this study was the lack of detailed clinical information; however, seroprevalence studies are an essential tool to monitor the efficacy of vaccination programmes, to understand population immunity and to identify populations at higher risk of infection.
Introduction
Rubella disease is caused by rubella virus (belonging to the family Togaviridae and the only member of the genus Rubivirus).1 Although most cases of infection lead to a mild, self-limiting measles-like disease, the real threat arises when rubella virus infects the fetus, particularly during the first trimester when infection can lead to miscarriage or congenital rubella syndrome.1 Worldwide, over 100 000 babies are born with congenital rubella syndrome every year, and the WHO recommends that all countries that have not yet introduced a rubella vaccine should consider doing so using existing, well-established measles immunisation programmes.2 The WHO Strategic Advisory Group of Experts on Immunization (SAGE) recommends an increased focus on improving national immunisation systems in general to better control rubella.2 Under the Global Vaccine Action Plan 2011–2020, rubella is targeted for elimination in five WHO Regions by 2020.3 4 As has been reported in Europe, suboptimal coverage levels in childhood (<95%) can lead to a prolonged inter-epidemic period and to a paradoxical shift of disease incidence towards older age groups, including women of childbearing age, with a consequent increase of congenital rubella syndrome.5 Serosurveys may represent an effective instrument to measure infection-induced and vaccine-induced immunity in a specific population, and serosurveys can effectively support strategies aimed at eliminating the disease.5
The incidence of rubella infection in South Korea was 107 cases in 2000 that decreased to 7 cases in 2017, corresponding to incidence rates below 0.1 per 100 000 persons according to the Infectious Diseases Surveillance Yearbook, 2017.6 Although the exact number of cases for congenital rubella syndrome was not available for the surveillance book, 17 cases in 2010 of congenital rubella syndrome were reported, which using the Korean Classification of Disease code P350 for congenital rubella syndrome on the Healthcare Bigdata Hub by the Health Insurance Review and Assessment Service (HIRA).7 According to the reported measles and rubella cases and incidence rates by WHO member states, 0–3947 confirmed rubella cases corresponding to incidence rates of 0–11.54 per 1 000 000 total population were reported in 2018 in the western pacific region.8
In Korea, a rubella vaccination programme using the measles, mumps in rubella (MMR) vaccine has been included in the national immunisation programme since 1985 for disease control and prevention.9 A second MMR vaccine dose was introduced in 1997, and a catch-up measles-rubella (MR) vaccine for school-aged children was introduced in 2001.9 In 2002, a two-dose MMR keep-up programme through the verification of vaccination history was introduced at elementary schools (6–7 years).9 A new vaccination policy was formed by the 2012 Military Healthcare Service, and since then, MMR vaccines have been routinely administered to all new recruits early in basic training.10 The national guidelines in Korea regarding ascertainment of rubella immunity are based on laboratory evidence for rubella antibodies and the Korea Centers for Disease Control and Prevention recommends that women of childbearing age whose antirubella specific IgG is negative should receive 1 dose of the MMR vaccine although they did have histories of rubella vaccination (total numbers of vaccination in one individual should be ≤3).11
Although there have been several studies on rubella in Korea, most of the studies have only been focused on surveillance of newly identified cases, seroprevalences of rubella IgG in children or had been conducted in the early 1990s.9 10 12–16 Although a recent meta-analysis assessing global seroprevalence of rubella among pregnant and childbearing age women, no data from Korean populations were included in the study.5 In a recent 16-year review of seroprevalence studies on rubella, only one Korean study on children and adolescents was included.3 To our knowledge, no recent data have been collected on rubella immunisation status with rubella-specific IgG antibodies in Korean women of childbearing age in a large study population, which could provide basic knowledge on nationwide immunisation strategies. Green Cross Laboratories is one of the largest referral clinical laboratories throughout South Korea that has its own bio-logistics and provides clinical specimen analysis services including rubella-specific IgG antibody tests to nationwide clinics and hospitals. According to the provider data on the National Health Insurance Statistical Yearbook 2017 published by HIRA in South Korea, 1319 private obstetric clinics and 1433 hospitals with or without obstetric clinics are providing health services.17 Among a total of 91 545 healthcare providing institutions (public and private), 4.1% (3746 institutions) were public or national provider institutions.17 According to the review records of delivery by provider type in the same book, 89.9% (523/582) of delivery institutions nationwide were private obstetric clinics and hospitals.17 Among the 358 285 deliveries carried out in 2017, 93.5% (335 119) were delivered in private obstetric clinics and hospitals.17
Therefore, in this study, we aimed to investigate the immunity against rubella and to share baseline data for future immunisation policies in South Korea. The aim of this study was to investigate the epidemiology of rubella immunisation status using serological assays for rubella-specific IgG antibodies in Korean women of childbearing age. In addition, we assessed rubella immunisation status according to year and age group.
Materials and Methods
Participants’ involvement and data collection
No patients were involved in the development of the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients were asked for advice regarding the interpretation or writing of results. There are no plans to disseminate the study results to the relevant patient community.
Study populations
Between January 2010 and December 2017, test results from Korean women aged 15–49 years who had visited an obstetric private clinics and hospitals (nationwide institutions) and had requested rubella-specific IgG antibody tests from Green Cross Laboratories were obtained from the laboratory information system. Missing data for age, sex and geographic regions were excluded. Test results from women whose tests were duplicated were excluded. All data were anonymised before being transferred to analysis for age-specific, year-specific, birth cohort and geographical region-specific antirubella IgG seroprevalences. This study was conducted according to guidelines in the Declaration of Helsinki.
Data collection
Annual incidence of rubella infection in South Korea was obtained from reported cases in the Infectious Diseases Surveillance Yearbook, 2017 by the Korea Centers for Disease Control and Prevention.6 Data for the incidence of congenital rubella syndrome was obtained from the Healthcare Bigdata Hub by HIRA using Korean Classification of Disease code P350 in South Korea.7
Analytical procedures
All serum samples were tested for antirubella IgG using a chemiluminescent microparticle immunoassay (Architect i2000SR, Abbott Diagnostics, Abbott Park, Illinois, USA) according to the manufacturer's instructions. For the rubella IgG assay, the presence of ≥10 IU/mL was defined as ‘positive’. Antibody levels of 0.0–4.9 IU/mL were defined as ‘negative’, and antibody levels between 5.0 and 9.9 IU/mL were defined as ‘equivocal’. During the 8-year study period, the laboratory protocol was maintained without any changes and all tests requested for antirubella specific IgG were analysed automatically and tested once without retest.
Definition
Positive rubella-specific IgG results are indicative of past exposure to rubella virus or being vaccinated.18 Women who had ‘negative’ results were defined as ‘unimmunised’. Women were classified as ‘immune’ if their antirubella IgG was positive or showed equivocal results.18 Birth cohorts were defined based on the vaccination programme: pre-catch-up, 1976–1984; catch-up, 1985–1993 and keep-up, ≥1994.9 The pre-catch-up (1976–1984) cohort was women who had presumptively limited MMR vaccination coverage with only one dose provided by the public programme. The catch-up (1985–1993) cohort was woman who had limited MMR vaccination coverage but were given the MR vaccine during the 2001 catch-up campaign.9 The keep-up (≥1994) cohort was women who were candidates for the keep-up programme.9
Statistical analysis
Categorical variables are presented as frequencies and percentages. The χ² test was used to compare categorical variables. The Cochran-Armitage test for trend was performed to evaluate the seroprevalence of antirubella IgG by year and cohort. Multivariable-adjusted logistic regression models were used to estimate the OR of being immune to rubella based on the results of the antirubella IgG seroprevalence test for the tested years, age, birth cohort and geographic region in South Korea. Variables with univariate p values less than 0.05 were included as adjusted variables for the multivariable analysis. Statistical analysis was executed using MedCalc Statistical Software V.18.5 (MedCalc Software bvba, Ostend, Belgium). P values were considered significant at the 0.05 level.
Results
General characteristics of the study population
Between January 2010 and December 2017, antirubella IgG test results from 328 426 Korean women age 15–49 years who had visited obstetric private clinics (from 1438 institutions nationwide) and had requested rubella-specific IgG antibody tests from Green Cross Laboratories were obtained from the laboratory information system and included in the study. The numbers for antirubella IgG results for the study subjects by each year and age group are summarised in table 1.
Table 1.
Test results for antirubella IgG by each tested-year and age for 328 465 Korean women tested for rubella IgG antibodies
| Test year | 15–20 years | 21–30 years | 31–40 years | 41–49 years | ||||||||||||
| N | E | P | Total | N | E | P | Total | N | E | P | Total | N | E | P | Total | |
| 2010 | 8 | 48 | 312 | 368 | 1332 | 2499 | 13 628 | 17 459 | 1640 | 1601 | 16 691 | 19 932 | 87 | 102 | 623 | 812 |
| 2.2% | 13.0% | 84.8% | 9.4% | 7.6% | 14.3% | 78.1% | 14.1% | 8.2% | 8.0% | 83.7% | 10.4% | 10.7% | 12.6% | 76.7% | 8.6% | |
| 2011 | 25 | 64 | 451 | 540 | 1717 | 3024 | 13 376 | 18 117 | 2167 | 2600 | 17 668 | 22 436 | 120 | 103 | 687 | 910 |
| 4.6% | 11.9% | 83.5% | 13.8% | 9.5% | 16.7% | 73.8% | 14.6% | 9.7% | 11.6% | 78.8% | 11.8% | 13.2% | 11.3% | 75.5% | 9.6% | |
| 2012 | 30 | 105 | 439 | 574 | 1381 | 2899 | 13 388 | 17 668 | 2321 | 3438 | 19 407 | 25 166 | 225 | 137 | 1125 | 1487 |
| 5.2% | 18.3% | 76.5% | 14.7% | 7.8% | 16.4% | 75.8% | 14.2% | 9.2% | 13.7% | 77.1% | 13.2% | 15.1% | 9.2% | 75.7% | 15.8% | |
| 2013 | 23 | 113 | 379 | 515 | 1195 | 2491 | 11 989 | 15 675 | 2477 | 3867 | 18 106 | 24 450 | 135 | 106 | 875 | 1116 |
| 4.5% | 21.9% | 73.6% | 13.2% | 7.6% | 15.9% | 76.5% | 12.6% | 10.1% | 15.8% | 74.1% | 12.8% | 12.1% | 9.5% | 78.4% | 11.8% | |
| 2014 | 35 | 100 | 405 | 540 | 778 | 2032 | 11 793 | 14 603 | 2142 | 3662 | 17 906 | 23 710 | 111 | 108 | 919 | 1138 |
| 6.5% | 18.5% | 75.0% | 13.8% | 5.3% | 13.9% | 80.8% | 11.8% | 9.0% | 15.4% | 75.5% | 12.4% | 9.8% | 9.5% | 80.8% | 12.1% | |
| 2015 | 29 | 84 | 398 | 511 | 674 | 2032 | 11 596 | 14 302 | 2407 | 4361 | 18 467 | 25 235 | 137 | 91 | 997 | 1225 |
| 5.7% | 16.4% | 77.9% | 13.1% | 4.7% | 14.2% | 81.1% | 11.5% | 9.5% | 17.3% | 73.2% | 13.2% | 11.2% | 7.4% | 81.4% | 13.0% | |
| 2016 | 39 | 79 | 389 | 507 | 651 | 1887 | 11 152 | 13 690 | 2573 | 4532 | 18 304 | 25 409 | 142 | 105 | 1029 | 1276 |
| 7.7% | 15.6% | 76.7% | 13.0% | 4.8% | 13.8% | 81.5% | 11.0% | 10.1% | 17.8% | 72.0% | 13.3% | 11.1% | 8.2% | 80.6% | 13.5% | |
| 2017 | 39 | 78 | 228 | 345 | 779 | 1985 | 9922 | 12 686 | 2689 | 4709 | 17 151 | 24 549 | 162 | 118 | 1196 | 1476 |
| 11.3% | 22.6% | 66.1% | 8.8% | 6.1% | 15.6% | 78.2% | 10.2% | 11.0% | 19.2% | 69.9% | 12.9% | 11.0% | 8.0% | 81.0% | 15.6% | |
| Total | 228 | 671 | 3001 | 3900 | 8507 | 18 849 | 96 844 | 124 200 | 18 416 | 28 770 | 143 700 | 190 886 | 1119 | 870 | 7451 | 9440 |
| 5.8% | 17.2% | 76.9% | 6.8% | 15.2% | 78.0% | 9.6% | 15.1% | 75.3% | 11.9% | 9.2% | 78.9% | |||||
E, equivocal; N, negative; P, positive.
Rubella immunity in Korean women of childbearing age
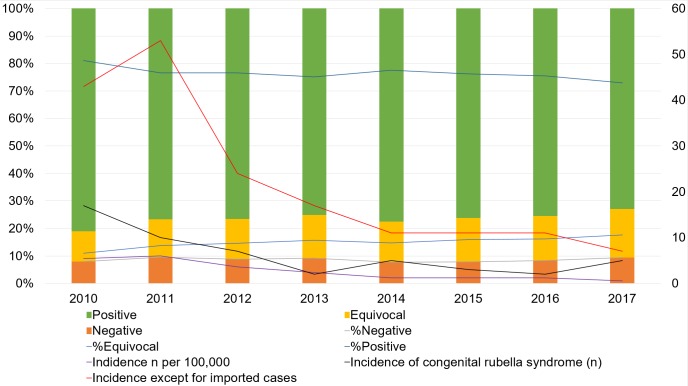
The overall proportion of IgG-negative women who were defined as ‘unimmunised’ was 8.6%, and the overall proportion of IgG-equivocal women was 15.0% and IgG-positive women was 76.4%. Rubella-specific IgG antibody test results with an annual incidence of rubella infection and congenital rubella syndrome from surveillance data by year are summarised in figure 1. There were significant differences in the rate of unimmunised women during the 8-year study period (p<0.05), although there was no significant trend (p>0.05). There was a decrease in the rate of women who had positive rubella-specific IgG antibody results (from 81.0% in 2010 to 73.0% in 2017, p<0.05) and an increase in the rate of women who had ‘equivocal’ results from 2010 to 2017 (11.0% in 2010 to 17.6% in 2017, p<0.05, figure 1). There were significant differences in the rate of unimmunised women among different age groups, cohorts and geographic regions (p<0.05). For example, less than 1000 women had been tested for antirubella IgG in the Gangwon province and Ulsan.
Figure 1.
Rubella-specific IgG antibody test results with annual incidence of rubella infection and congenital rubella syndrome from surveillance data by year (2010–2017). Percentage of rubella specific IgG results in this study (left axis) and numbers of cases for incidence of rubella from surveillance data (right axis) are plotted against years tested.
Multivariable–adjusted logistic regression models showed that the odds of being immune to rubella (positive and equivocal results of antirubella IgG tests) were decreased in 2017 compared with 2010 (OR 0.63, 95% CI 0.60 to 0.67, p<0.0001) and women in their 40s (OR 0.85, 95% CI 0.79 to 0.90, p<0.0001, table 2). Among different cohorts, catch-up (being born in 1985–1993) and keep-up (born ≥1994) cohorts had higher ORs for being immune to rubella compared with pre-catch-up cohorts (born in 1976–1984, p<0.0001). Among different geographic regions, women living in Incheon, Busan, South Gyeongsang, North and South Jeolla and Jeju provinces had lower ORs and women living in Sejong city and Daejeon had higher ORs for being immune to rubella in comparison with women living in Seoul (p<0.0001).
Table 2.
Association between seroprevalence of antirubella IgG (being immune to rubella)* and population characteristics
| Total | Immune | Univariable logistic regression | Multivariable logistic regression | ||||||
| n | n | % | OR | 95% CI | P value | OR | 95% CI | P value | |
| Tested year | |||||||||
| 2010 | 38 571 | 35 504 | 92.0 | ||||||
| 2011 | 42 002 | 37 973 | 90.4 | 0.81 | 0.78 to 0.86 | <0.0001 | 0.79 | 0.75 to 0.83 | <0.0001 |
| 2012 | 44 895 | 40 938 | 91.2 | 0.89 | 0.85 to 0.94 | <0.0001 | 0.85 | 0.81 to 0.89 | <0.0001 |
| 2013 | 41 756 | 37 926 | 90.8 | 0.86 | 0.81 to 0.90 | <0.0001 | 0.78 | 0.74 to 0.82 | <0.0001 |
| 2014 | 39 991 | 36 925 | 92.3 | 1.04 | 0.99 to 1.10 | 0.1368 | 0.91 | 0.86 to 0.96 | 0.0003 |
| 2015 | 41 273 | 38 026 | 92.1 | 1.01 | 0.96 to 1.07 | 0.6586 | 0.84 | 0.80 to 0.89 | <0.0001 |
| 2016 | 40 882 | 37 477 | 91.7 | 0.95 | 0.90 to 1.00 | 0.0520 | 0.75 | 0.72 to 0.79 | <0.0001 |
| 2017 | 39 056 | 35 387 | 90.6 | 0.83 | 0.79 to 0.88 | <0.0001 | 0.63 | 0.60 to 0.67 | <0.0001 |
| Age of women | |||||||||
| 15–20 years | 3900 | 3672 | 94.2 | ||||||
| 21–30 years | 124 200 | 115 693 | 93.2 | 0.84 | 0.74 to 0.97 | <0.0001 | |||
| 31–40 years | 190 886 | 172 470 | 90.4 | 0.58 | 0.51 to 0.67 | <0.0001 | |||
| 41–49 years | 9440 | 8321 | 88.1 | 0.46 | 0.40 to 0.54 | <0.0001 | 0.85 | 0.79 to 0.90 | <0.0001 |
| Cohort | |||||||||
| Pre-catch-up (1976–1984) | 228 176 | 205 536 | 90.1 | ||||||
| Catch-up (1985–1993) | 94 056 | 88 887 | 94.5 | 1.89 | 1.84 to 1.95 | <0.0001 | 1.99 | 1.92 to 2.05 | <0.0001 |
| Keep-up (≥1994) | 6194 | 5733 | 92.6 | 1.37 | 1.24 to 1.51 | <0.0001 | 1.50 | 1.36 to 1.65 | <0.0001 |
| Geographic locations | |||||||||
| Seoul | 65 380 | 59 821 | 91.5 | ||||||
| Gyeonggi Province | 131 157 | 120 183 | 91.6 | 1.02 | 0.98 to 1.05 | 0.3078 | |||
| Incheon | 9611 | 8747 | 91.0 | 0.94 | 0.87 to 1.01 | 0.1111 | 0.93 | 0.86 to 1.00 | 0.0382 |
| Gangwon Province | 703 | 654 | 93.0 | 1.24 | 0.93 to 1.66 | 0.1478 | |||
| Sejong City | 3859 | 3623 | 93.9 | 1.43 | 1.25 to 1.63 | <0.0001 | 1.20 | 1.05 to 1.37 | 0.0076 |
| Daejeon | 12 496 | 11 553 | 92.5 | 1.14 | 1.06 to 1.22 | 0.0004 | 1.07 | 1.00 to 1.15 | 0.0484 |
| North Chungcheong Province | 11 186 | 10 306 | 92.1 | 1.09 | 1.01 to 1.17 | 0.0252 | |||
| South Chungcheong Province | 8390 | 7710 | 91.9 | 1.05 | 0.97 to 1.14 | 0.2178 | |||
| Daegu | 14 781 | 13 473 | 91.2 | 0.96 | 0.90 to 1.02 | 0.1739 | |||
| Ulsan | 660 | 625 | 94.7 | 1.66 | 1.18 to 2.34 | 0.0037 | |||
| North Gyeongsang Province | 2075 | 1891 | 91.1 | 0.96 | 0.82 to 1.11 | 0.5577 | |||
| South Gyeongsang Province | 4426 | 3994 | 90.2 | 0.86 | 0.78 to 0.95 | 0.0039 | 0.85 | 0.77 to 0.95 | 0.0023 |
| Busan | 12 574 | 11 376 | 90.5 | 0.88 | 0.83 to 0.94 | 0.0002 | 0.86 | 0.81 to 0.91 | <0.0001 |
| Gwangju | 2035 | 1845 | 90.7 | 0.90 | 0.78 to 1.05 | 0.1848 | |||
| North Jeolla Province | 11 911 | 10 890 | 91.4 | 0.99 | 0.92 to 1.06 | 0.8031 | 0.93 | 0.87 to 0.99 | 0.0213 |
| South Jeolla Province | 13 621 | 12 233 | 89.8 | 0.82 | 0.77 to 0.87 | <0.0001 | 0.79 | 0.75 to 0.84 | <0.0001 |
| Jeju Province | 23 561 | 21 232 | 90.1 | 0.85 | 0.81 to 0.89 | <0.0001 | 0.83 | 0.79 to 0.87 | <0.0001 |
*Positive and equivocal results of antirubella specific IgG test results were defined as ‘immune’ in this study.18
Discussion
In this study, we investigated the seroprevalence of rubella in Korean women of childbearing age within the past 8 years. The strength of this study was the large study population over a long study period (8 years) and the novelty of the study population (Korean women of childbearing age were assessed for the first time in Korea). Because previous studies focused on the different measurement methods and immunisation status, this suggested that equivocal results might be due to being immune to rubella infection;18 19 thus, the authors focused on and analysed factors associated with those whose antirubella IgG results were negative.
Understanding the spread of infectious diseases and designing optimal control strategies is a major goal of public health.20 21 In the present study, the seronegativity prevalence was 8.6% in Korean women of childbearing age. A recent 16-year review of seroprevalence studies on rubella assessing 97 articles between January 1998 and June 2014 had reported that seroprevalence ranged from 53.0% to 99.3% for rubella studies.3 A recent meta-analysis of rubella among pregnant and childbearing age women had reported that approximately 88% of the studies conducted on pregnant women had reported a seronegativity rate >5%, and the pooled rubella seronegativity prevalence was 9.3%.5 The study had reported that global seronegativity prevalence was of concern, considering that WHO set the rubella susceptibility threshold at 5% for women of childbearing age. Previous studies that had been included in the meta-analysis had used more than 1000 subjects and had been published within the past 10 years are summarised in table 3.
Table 3.
Previous studies on rubella seronegativity in women that included more than 1000 subjects and were published within the past 10 years, grouped by WHO region
| WHO region | Publication year | N | Country | Seronegativity (%) | Population | Reference | Measurement method |
| AFR | 2009 | 7430 | South Africa | 6.2 | WCBA | Schoub et al 26 | Bio-Rad Platelia Rubella IgG ELISA |
| AMR | 2009 | 8939 | Brazil | 28.4 | Pregnant | Inagaki et al 27 | Q-Preven IgG-DBS kit |
| AMR | 2011 | 9610 | Brazil | 11.6 | Pregnant | Artimos de Oliveira et al 28 | Beckman Coulter Access RUBELLA IgG ChLIA or bioMérieux VIDAS RUB IgG II ELFA |
| AMR | 2016 | 54 717 | Brazil | 4.5 | Pregnant | Avila Moura et al 29 | Q-Preven IgG-DBS kit |
| AMR | 2009 | 5783 | Canada | 7.0 | Pregnant | McElroy et al 30 | Hemagglutination inhibition test |
| AMR | 2013 | 459 963 | Canada | 4.4 | WCBA | Lim et al 31 | Abbott AxSYM Rubella IgG MEIA |
| AMR | 2015 | 157 763 | Canada | 15.9 | Pregnant | Lai et al 32 | Abbott ARCHITECT Rubella IgG CMIA |
| EMR | 2014 | 4062 | Kuwait | 6.8 | Pregnant | Madi et al 33 | Abbott ARCHITECT Rubella IgG CMIA |
| EMR | 2013 | 2284 | Morocco | 9.8 | Pregnant | Belefquih et al 34 | Siemens Enzygnost Anti-Rubella-Virus IgG EIA |
| EMR | 2014 | 10 276 | Saudi Arabia | 8.7 | Pregnant | Alsibiani et al 35 | Dade Behring ELISA BP III |
| EUR | 2012 | 424 876 | England | 2.6 | Pregnant | Byrne et al 36 | Microgen Mercia Rubella G EIA |
| EUR | 2013 | 1090 | Germany | 1.6 | Pregnant | Enders et al 37 | Hemagglutination inhibition test |
| EUR | 2013 | 74 810 | Ireland | 6.2 | Pregnant | O’Dwyer et al 38 | Method not described |
| EUR | 2012 | 2385 | Italy | 8.0 | Pregnant | De Paschale et al 39 | DiaSorin ETI-RUBEK-G PLUS EIA |
| EUR | 2015 | 22 681 | Spain | 5.9 | Pregnant | Vilajeliu et al 40 | Siemens ADVIA Centaur Rubella G ChLIA |
| EUR | 2010 | 41 637 | Sweden | 4.2 | Pregnant | Kakoulidou et al 41 | Abbott AxSYM Rubella IgG MEIA |
| EUR | 2009 | 1972 | Turkey | 3.9 | Pregnant | Tamer et al 42 | Abbott AxSYM Rubella IgG MEIA |
| EUR | 2012 | 5959 | Turkey | 1.9 | Pregnant | Uysal et al 43 | bioMérieux VIDAS RUB IgG II ELFA |
| EUR | 2011 | 11 987 | UK | 4.4 | Pregnant | Matthews et al 44 | DiaSorin ETI-RUBEK-G EIA |
| EUR | 2016 | 19 046 | UK | 6.3 | Pregnant | Ogundele et al 45 | Roche E602 MODULAR analyzer |
| SEAR | 2011 | 2224 | Nepal | 9.2 | WCBA | Upreti et al 46 | Enzygnost Anti-Rubella-Virus IgG EIA |
| SEAR | 2014 | 1988 | Vietnam | 28.9 | Pregnant | Miyakawa et al 47 | bioMérieux Mini VIDAS EIA |
| WPR | 2008 | 1020 | Australia | 2.7 | WCBA | Nardone et al 48 | Siemens Enzygnost Anti-Rubella-Virus IgG EIA |
| WPR | 2008 | 2741 | Japan | 6.7 | Pregnant | Okuda et al 49 | Hemagglutination inhibition test |
| WPR | 2013 | 13 924 | Japan | 2.7 | Pregnant | Hanaoka et al 50 | Hemagglutination inhibition test |
| WPR | 2014 | 20 363 | Japan | 4.7 | Pregnant | Yamada et al 51 | Hemagglutination inhibition test |
| WPR | 2017 | 782 293 | China | 33.8 | WCBA | Liu et al 52 | Method not described |
| WPR | 2011 | 43 640 | Taiwan | 10.9 | Pregnant | Lin et al 53 | Abbott AxSYM Rubella IgG MEIA and Beckman Coulter Access RUBELLA IgG ChLIA |
| WPR | 2012 | 14 090 | Taiwan | 6.5 | Pregnant | Lin et al 54 | Abbott AxSYM Rubella IgG MEIA |
| WPR | 2019 | 327 637 | Republic of Korea | 8.7 | WCBA | This study | Abbott ARCHITECT Rubella IgG CMIA |
AFR, Africa region; AMR, American region; EMR, Eastern Mediterranean Region; EUR, European region; SEAR, South-East Asian region; WCBA, women of childbearing age; WPR, Western Pacific region.
The seroprevalence of rubella in Korean populations was assessed previously in infants, children and adolescents.12–16 One study on 5393 students from eight elementary schools in the Gyeonggi province, Korea in 1993, 1996 and 1996 had reported that the age-adjusted rubella susceptibility rate was 22.9%.14 Another study performed during the same study period had reported that rubella antibody loss rates were 14.3%–15.8% in Korean children.12 In a 2005 population-based survey in Nonsan, Korea, age-appropriate immunisation among urban-rural children aged 24–35 months had reported that the age-appropriate MMR immunisation rate was 61.1%–97.4%.16 A recent study conducted between September 2009 and December 2010 assessing seroprevalence of rubella in 295 infants and 80 of their mothers had reported that seropositive rates were 22.4% in infants and 98.8% in mothers (79/80).13 In that study, because none of the infants had a history of MMR vaccination, natural infection or contact with an infected person, it was assumed that specific antibodies were passed from their mothers to their infants.13 Moreover, among the 80 mothers, 55 (68.8%) had experienced either immunisation or past rubella infection.13
The historical immunisation coverage in preschool children right before admission to elementary school, which was evaluated based on a telephone survey, reported 99.5% in 2001 and 97.3% of school-aged children (catch-up cohort) were vaccinated with the MR vaccine.22 According to the Infectious Disease Surveillance Yearbook 2017, published by the Korean Ministry of Health and Welfare and the Korean Centers for Disease Control and Prevention, the incidence rate of rubella from 2001 to 2017 decreased (from 0.17 per 100 000 population in 2001 to 0.01 per 100 000 population in 2017).6 In this study, ORs for being immune to rubella infection were higher in the catch-up (born 1985–1993) and keep-up (born ≥1994) cohorts than in pre-catch-up cohorts (born 1976–1984) which suggests that catch-up and keep-up immunisation was effective.22 The vaccine coverage rate was maintained at >95% from 2010 to 2017 in South Korea (ranges 97.0% in 2012 to 99.8% in 2010).22 No rubella outbreak had been reported in South Korea over 8 years (2010–2017) according to the Infectious Disease Surveillance Yearbook. Among the different age groups, older women were more likely to have negative IgG results and no protection from rubella infection. Women in their 30s had the lowest rate of IgG+ results in this study. According to recent data from Korean Statistical Information (KOSIS), the average maternal age at delivery for Korean women was 32.4 years in 2016. Because of this, public health efforts should be focused on catch-up activities. The results of this study could be used as basic knowledge to support strengthening disease control and prevention of rubella, including a nationwide immunisation programme.
In South Korea, national guidelines in force to control and prevention measles and rubella include national immunisation programme and active disease surveillance system.2 4 22 MMR vaccination has been covered by national health insurance that provides free of charge immunisation to all children aged ≤12 years, and clinical laboratory screening for rubella immunisation status using antirubella-specific IgG tests in pregnant women has been covered by the national health insurance free of charge for women visiting obstetrics clinics.17 Susceptible woman of childbearing age is indeed a priority, and public health efforts should be focused on catch-up activities in order to reduce the rate of susceptible young adults, especially for all women of childbearing age.23 Gynaecologists and general practitioners should be encouraged to propose rubella screening for women of childbearing age before they become pregnant to identify those women who lack rubella antibodies, whether acquired as the result of vaccination or a natural infection.23 Finally, active surveillance from laboratories that perform rubella immunity testing should be planned; laboratories should notify the Public Health Authority about every woman of childbearing age with a negative test, and the Public Health Authority should engage these women to promote immunisation against rubella.23 Serological surveillance is an important tool for the evaluation of vaccination programmes and avoids the limitations of passive disease reporting systems; this is one of the entry points for congenital rubella syndrome surveillance, where gaps limit the ability to monitor progress towards its elimination.23
In this study, women living in Sejong city were the most protected from rubella infection. In early 2007, the South Korean government had created a special administrative district from parts of the South Chungcheong and North Chungcheong provinces, near Daejeon, to relocate nine ministries and four national agencies from Seoul. Various government programmes for encouraging more births, such as incentives, in different regions may have affected the results.4 In this study, less than 1000 women had been tested for antirubella IgG in the Gangwon province and Ulsan. This may affect the per cent seropositivity of antirubella IgG in the present study. Future studies are needed to define the effect of regional differences of government strategies on rubella seroprevalences.
One limitation of this study was the lack of clinical information, such as vaccination history or contact history with rubella-infected individuals. The results of this study were prone to ascertainment bias because the study population was based on mostly private obstetric clinics; thus, results might be different from those obtained from individuals using national or public healthcare providing institutions, although the use of a population-based study minimised selection bias.24 Because the exact proportions of pregnant women in Korea who used public health facilities to test for antirubella IgG, and their sociodemographics as well as rubella vaccine coverage among the population seeking healthcare from private and public sectors and the proportion of pregnant women as well as the general population seeking care from the private sector across provinces were not available, future studies to evaluate those factors associated with rubella control and prevention are needed. However, we do not yet understand what surrogate markers, other than antibodies, show longer-term cell-mediated immunity and protection from disease.1 Seroprevalence studies are an essential tool to monitor the efficacy of vaccination programmes, to understand population immunity and to identify populations at higher risk of infection.25 This study is a cross-sectional study and merely descriptive analyses were adopted in this study. The results of this study were prone to ascertainment bias. The present study did not include men, women with older ages or foreigners living in South Korea. Therefore, the findings are not generalisable to these groups. A systems-level approach to understanding the development and maintenance of acute and long-term immunity to rubella and a rubella-containing vaccine is needed.1
Conclusion
In conclusion, this study investigated immunisation status of rubella among Korean women of childbearing age. Considering the immunisation status by age group and the increased prevalence of women with equivocal results, future public health efforts should be focused on catch-up activities. The results of this study could be used as foundational knowledge for strengthening disease control and prevention of rubella, including a nationwide immunisation programme.
Supplementary Material
Footnotes
Contributors: All authors contributed to manuscript preparation. RC and SGL: conception, design, statistical analyses and interpretation of the data. RC, YoO, SHK and SGL: data acquisition. RC: article drafting. RC, SGL and EHL: critical article revision for important intellectual content. SGL and EHL: obtaining funding. RC, YoO, YeO and SHK: administrative and technical support. RC, YoO and SHK: collection and assembly of data. All authors read and approved the final manuscript.
Funding: This work was supported by Abbott Diagnostics Korea.
Disclaimer: The sponsor had no involvement in the study design, data interpretation, or writing of the manuscript.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: All procedures involving human subjects were approved by the Institutional Review Board of Green Cross Laboratories (GCL 2017-1010-02).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request.
References
- 1. Lambert N, Strebel P, Orenstein W, et al. Rubella. Lancet 2015;385:2297–307. 10.1016/S0140-6736(14)60539-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization WHO fact sheet for rubella 2017. Available: https://www.who.int/en/news-room/fact-sheets/detail/rubella [Accessed Jan 2019].
- 3. Dimech W, Mulders MN. A 16-year review of seroprevalence studies on measles and rubella. Vaccine 2016;34:4110–8. 10.1016/j.vaccine.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization Global measles and rubella strategic plan 2012, 2012. [Google Scholar]
- 5. Pandolfi E, Gesualdo F, Rizzo C, et al. Global seroprevalence of rubella among pregnant and childbearing age women: a meta-analysis. Eur J Public Health 2017;27:530–7. 10.1093/eurpub/ckw259 [DOI] [PubMed] [Google Scholar]
- 6. Ministry of Health and Welfare and Korea Centers for Disease Control and Prevention Infectious diseases surveillance Yearbook, 2017, 2018. [Google Scholar]
- 7. Healthcare Bigdata Hub Health insurance review and assessment service. Available: https://opendata.hira.or.kr/home.do
- 8. World Health Organization Measles and rubella surveillance data. Available: https://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/active/measles_monthlydata/en/
- 9. Choe YJ, Eom H-E, Cho S-I. Trend of measles, mumps, and rubella incidence following the measles-rubella catch up vaccination in the Republic of Korea, 2001. J Med Virol 2017;89:1528–31. 10.1002/jmv.24808 [DOI] [PubMed] [Google Scholar]
- 10. Heo JY, Choe K-W, Yoon C-G, et al. Vaccination policy in Korean armed forces: current status and future challenge. J Korean Med Sci 2015;30:353–9. 10.3346/jkms.2015.30.4.353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Korea centers for disease control and prevention Chapter XVI. Rubella. Available: https://nip.cdc.go.kr/irgd/reference.do
- 12. Ki M, Kim MH, Choi BY, et al. Rubella antibody loss rates in Korean children. Epidemiol Infect 2002;129:557–64. 10.1017/S0950268802007811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cho HK, Lee H, Kim HW, et al. Seroprevalences of specific IgG antibodies to measles, mumps, and rubella in Korean infants. J Korean Med Sci 2016;31:1957–62. 10.3346/jkms.2016.31.12.1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ki MR, Choi BY, Kim M-H, et al. Rubella seroprevalence in Korean children. J Korean Med Sci 2003;18:331–6. 10.3346/jkms.2003.18.3.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee H, Kim HW, Cho HK, et al. Reappraisal of MMR vaccines currently used in Korea. Pediatr Int 2011;53:374–80. 10.1111/j.1442-200X.2010.03244.x [DOI] [PubMed] [Google Scholar]
- 16. Kim E-Y, Lee M-S. Related factors of age-appropriate immunization among urban-rural children aged 24-35 months in a 2005 population-based survey in Nonsan, Korea. Yonsei Med J 2011;52:104–12. 10.3349/ymj.2011.52.1.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Health Insurance Review & Assessment Service National health insurance statistical Yearbook 2017, 2018. [Google Scholar]
- 18. Bouthry E, Furione M, Huzly D, et al. Assessing Immunity to Rubella Virus: a Plea for Standardization of IgG (Immuno)assays. J Clin Microbiol 2016;54:1720–5. 10.1128/JCM.00383-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization Manual for the laboratory-based surveillance of measles, rubella, and congenital rubella syndrome, 2019. Available: https://www.who.int/immunization/monitoring_surveillance/burden/laboratory/manual_section91/en/
- 20. Salathé M, Jones JH. Dynamics and control of diseases in networks with community structure. PLoS Comput Biol 2010;6:e1000736 10.1371/journal.pcbi.1000736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu Z, Zu Z, Zheng T, et al. Comparative analysis of the effectiveness of three immunization strategies in controlling disease outbreaks in realistic social networks. PLoS One 2014;9:e95911 10.1371/journal.pone.0095911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Korea centers for disease control and prevention 2017 disease control and prevention white paper, 2018. [Google Scholar]
- 23. Gallone MS, Gallone MF, Larocca AMV, et al. Lack of immunity against rubella among Italian young adults. BMC Infect Dis 2017;17:199 10.1186/s12879-017-2724-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sedgwick P. Bias in observational study designs: cross sectional studies. BMJ 2015;350:h1286 10.1136/bmj.h1286 [DOI] [PubMed] [Google Scholar]
- 25. Dimech W, Mulders MN. A review of testing used in seroprevalence studies on measles and rubella. Vaccine 2016;34:4119–22. 10.1016/j.vaccine.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 26. Schoub BD, Harris BN, McAnerney J, et al. Rubella in South Africa: an impending Greek tragedy? S Afr Med J 2009;99. [Google Scholar]
- 27. Inagaki ADdeM, Oliveira LARde, Oliveira MFBde, et al. [Seroprevalence of antibodies for toxoplasmosis, rubella, cytomegalovirus, syphilis and HIV among pregnant women in Sergipe]. Rev Soc Bras Med Trop 2009;42:532–6. 10.1590/s0037-86822009000500010 [DOI] [PubMed] [Google Scholar]
- 28. Artimos de Oliveira S, Bastos Camacho LA, Uzeda Barreto MC, et al. Serologic status of women in an urban population in Brazil before and after rubella immunization campaign using routine screening data. J Infect Dis 2011;204 Suppl 2:S664–8. 10.1093/infdis/jir356 [DOI] [PubMed] [Google Scholar]
- 29. Avila Moura A, Mello MJGde, Correia JB. Serological statuses of pregnant women in an urban Brazilian population before and after the 2008 rubella immunization campaign. Vaccine 2016;34:445–50. 10.1016/j.vaccine.2015.12.019 [DOI] [PubMed] [Google Scholar]
- 30. McElroy R, Laskin M, Jiang D, et al. Rates of rubella immunity among immigrant and non-immigrant pregnant women. J Obstet Gynaecol Can 2009;31:409–13. 10.1016/S1701-2163(16)34171-8 [DOI] [PubMed] [Google Scholar]
- 31. Lim GH, Harris T, Desai S, et al. Rubella immunity among prenatal women in Ontario, 2006-2010. BMC Infect Dis 2013;13:362 10.1186/1471-2334-13-362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lai FY, Dover DC, Lee B, et al. Determining rubella immunity in pregnant Alberta women 2009-2012. Vaccine 2015;33:635–41. 10.1016/j.vaccine.2014.12.022 [DOI] [PubMed] [Google Scholar]
- 33. Madi N, Al-Tawalah H, Abdul Khalik D, et al. A relatively high number of pregnant women in Kuwait remain susceptible to rubella: a need for an alternative vaccination policy. Med Princ Pract 2014;23:145–8. 10.1159/000356892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Belefquih B, Kasouati J, Doblali T, et al. Rubella seroprevalence in pregnant women at the military teaching Hospital, Rabat, Morocco. Int J Gynaecol Obstet 2013;120:191–2. 10.1016/j.ijgo.2012.08.026 [DOI] [PubMed] [Google Scholar]
- 35. Alsibiani SA. Rubella immunity among pregnant women in Jeddah, Western region of Saudi Arabia. Obstet Gynecol Int 2014;2014:659838 10.1155/2014/659838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Byrne L, Brant L, Reynolds C, et al. Seroprevalence of low rubella IgG antibody levels among antenatal women in England tested by NHS blood and transplant: 2004-2009. is rubella susceptibility increasing? Vaccine 2012;30:161–7. 10.1016/j.vaccine.2011.11.045 [DOI] [PubMed] [Google Scholar]
- 37. Enders M, Bartelt U, Knotek F, et al. Performance of the Elecsys rubella IgG assay in the diagnostic laboratory setting for assessment of immune status. Clin Vaccine Immunol 2013;20:420–6. 10.1128/CVI.00688-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O'Dwyer V, Bonham S, Mulligan A, et al. Antenatal rubella immunity in Ireland. Ir Med J 2013;106:232–5. [PubMed] [Google Scholar]
- 39. De Paschale M, Manco MT, Paganini A, et al. Rubella antibody screening during pregnancy in an urban area of northern Italy. Infect Dis Rep 2012;4:e17 10.4081/idr.2012.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vilajeliu A, García-Basteiro AL, Valencia S, et al. Rubella susceptibility in pregnant women and results of a postpartum immunization strategy in Catalonia, Spain. Vaccine 2015;33:1767–72. 10.1016/j.vaccine.2015.02.043 [DOI] [PubMed] [Google Scholar]
- 41. Kakoulidou M, Forsgren M, Lewensohn-Fuchs I, et al. Serum levels of rubella-specific antibodies in Swedish women following three decades of vaccination programmes. Vaccine 2010;28:1002–7. 10.1016/j.vaccine.2009.10.130 [DOI] [PubMed] [Google Scholar]
- 42. Tamer GS, Dundar D, Caliskan E. Seroprevalence of Toxoplasma gondii, rubella and cytomegalovirus among pregnant women in Western region of turkey. Clin Invest Med 2009;32:43–7. 10.25011/cim.v32i1.5086 [DOI] [PubMed] [Google Scholar]
- 43. Uysal A, Taner CE, Cüce M, et al. Cytomegalovirus and rubella seroprevalence in pregnant women in Izmir/Turkey: follow-up and results of pregnancy outcome. Arch Gynecol Obstet 2012;286:605–8. 10.1007/s00404-012-2353-z [DOI] [PubMed] [Google Scholar]
- 44. Matthews LA, Lawrance LM, Gray D, et al. An audit of rubella IgG antibody status in antenatal women in a NHS trust over 5 years (2005-2009). Epidemiol Infect 2011;139:1720–6. 10.1017/S0950268810002748 [DOI] [PubMed] [Google Scholar]
- 45. Ogundele M, Ghebrehewet S, Chawla A. Some factors affecting rubella seronegative prevalence among pregnant women in a North West England region between April 2011 and March 2013. J Public Health (Oxf) 2016;38:243–9. 10.1093/pubmed/fdv033 [DOI] [PubMed] [Google Scholar]
- 46. Upreti SR, Thapa K, Pradhan YV, et al. Developing rubella vaccination policy in Nepal--results from rubella surveillance and seroprevalence and congenital rubella syndrome studies. J Infect Dis 2011;204(Suppl 1):S433–8. 10.1093/infdis/jir078 [DOI] [PubMed] [Google Scholar]
- 47. Miyakawa M, Yoshino H, Yoshida LM, et al. Seroprevalence of rubella in the cord blood of pregnant women and congenital rubella incidence in Nha Trang, Vietnam. Vaccine 2014;32:1192–8. 10.1016/j.vaccine.2013.08.076 [DOI] [PubMed] [Google Scholar]
- 48. Nardone A, Tischer A, Andrews N, et al. Comparison of rubella seroepidemiology in 17 countries: progress towards international disease control targets. Bull World Health Organ 2008;86:118–25. 10.2471/BLT.07.042010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Okuda M, Yamanaka M, Takahashi T, et al. Positive rates for rubella antibody in pregnant women and benefit of post-partum vaccination in a Japanese perinatal center. J Obstet Gynaecol Res 2008;34:168–73. 10.1111/j.1447-0756.2007.00689.x [DOI] [PubMed] [Google Scholar]
- 50. Hanaoka M, Hisano M, Watanabe N, et al. Changes in the prevalence of the measles, rubella, varicella-zoster, and mumps virus antibody titers in Japanese pregnant women. Vaccine 2013;31:2343–7. 10.1016/j.vaccine.2013.03.012 [DOI] [PubMed] [Google Scholar]
- 51. Yamada T, Mochizuki J, Hanaoka M, et al. Effects of campaign for postpartum vaccination on seronegative rate against rubella among Japanese women. BMC Infect Dis 2014;14:152 10.1186/1471-2334-14-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu F, Zhang S, Liu J, et al. Sociodemographic and economic characteristics of susceptibility to rubella among women preparing for pregnancy in rural China. Int J Infect Dis 2017;62:112–8. 10.1016/j.ijid.2017.07.013 [DOI] [PubMed] [Google Scholar]
- 53. Lin C-C, Yang C-Y, Shih Y-L, et al. Rubella seroepidemiology and estimations of the catch-up immunisation rate and persistence of antibody titers in pregnant women in Taiwan. BJOG 2011;118:706–12. 10.1111/j.1471-0528.2011.02903.x [DOI] [PubMed] [Google Scholar]
- 54. Lin C-C, Yang C-Y, Shih Y-L, et al. Persistence and titer changes of rubella virus antibodies in primiparous women who had been vaccinated with strain RA 27/3 in junior high school. Clin Vaccine Immunol 2012;19:1–4. 10.1128/CVI.05334-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.