Abstract
Objective
Systematic reviews and meta-analyses have revealed the associations between H. pylori infection and various health outcomes. We aimed to evaluate the strength and breadth of evidence on the associations.
Design
Umbrella review of systematic reviews and meta-analyses.
Setting
No settings.
Participants
No patients involved.
Data sources
Embase, PubMed, Web of Science, Cochrane Library Databases, CNKI, VIP database and Wangfang database from inception to February 1, 2019.
Outcomes measures
Diverse diseases (such as cancer and ischaemic heart disease).
Results
Sixty articles reporting 88 unique outcomes met the eligible criteria. 74 unique outcomes had nominal significance (p<0.05). Of the outcomes with significance, 61 had harmful associations and 13 had beneficial associations. Furthermore, 73% (64) of the outcomes exhibited significant heterogeneity . Of the these meta-analyses, 32 had moderate to high heterogeneity (I2=50%–75%) and 24 had high heterogeneity (I2>75%). Moreover, 20% exhibited publication bias (p<0.1). In addition, 97% of the methodological qualities were rated ‘critically low’. 36% of the evidence qualities of outcomes were rated ‘low’, 56% of the evidence qualities were rated ‘very low’ and 8% of the evidence qualities were rated ‘moderate’. H. pylori infection may be associated with an increased risk of five diseases and a decreased risk of irritable bowel syndrome.
Conclusion
Although 60 meta-analyses explored 88 unique outcomes, moderate quality evidence only existed for six outcomes with statistical significance. H. pylori infection may be associated with a decreased risk of irritable bowel syndrome and an increased risk of hypertriglyceridemia, chronic cholecystitis and cholelithiasis, gestational diabetes mellitus, gastric cancer and systemic sclerosis.
Trial registration
CRD42019124680.
Keywords: umbrella review, helicobacter pylori, human health
Strengths and limitations of this study.
This umbrella review is the first synthesis of systematic reviews and meta-analyses to consider the associations between H. pylori infection and various health outcomes.
These results provide recommendations about the relationships between H. pylori infection and various health outcomes.
The associations observed in the meta-analyses included in this umbrella review may reflect the uncertainty of most diseases related to H. pylori infection.
Only evidence derived from systematic reviews and meta-analyses was included in our umbrella review. Evidence from original observational studies and/or randomised controlled trials that were not included in the meta-analyses was beyond our scope of discussion. This condition might result in conclusion bias of association between H. pylori infection and human health.
Introduction
H. pylori is a Gram-negative bacterium that affects human health worldwide, and its prevalence ranges from 50.8% to 84%.1–4 Earlier studies demonstrated that H. pylori infection contributes to the development of several digestive diseases (e.g. gastric cancer,5 6 peptic ulcer disease (PUD)7 and dyspepsia).8 These conclusions were supported by recent studies.9–12 Over the last 20 years, the associations between H. pylori infection and a sequence of non-digestive disorders have been investigated extensively. Multiple studies and meta-analyses have revealed that H. pylori infection is harmful to human health by increasing the risk of diverse diseases, including cancers, cardiovascular and cerebrovascular diseases, respiratory disorders, endocrine diseases and neurocognitive disorders. Meta-analyses have further reported that H. pylori infection increases the risk of acquiring hepatocellular carcinoma (HCC) by more than 16-fold,13 cholangiocarcinoma by approximately 9-fold14 and myocardial infarction (MI) nearly 2-fold.15 Subsequently, with further research on H. pylori infection, it may be beneficial to health in some conditions by decreasing the risk of diseases (e.g. asthma,16 inflammatory bowel disease17 and oesophageal cancer).18 Therefore, the causal role of H. pylori infection in these diseases has been widely queried.
The observed associations between H. pylori infection and health outcomes can be causal, indicating that H. pylori infection elicits adverse effects on human health. However, the publication bias, scheme design defects or inconsistencies of studies can lead to a decrease in the strength and validity of evidence. Furthermore, confounding factors, such as age, sex, smoking or drinking status, can affect causality. The lack of adequate controls for confounders may cause reverse causality. Therefore, evidence from meta-analyses may also have uncertainty. If causal, the association of H. pylori infection and public health should be reconsidered, and the role of H. pylori infection in human health must be reanalysed. Once strong associations between H. pylori infection and diseases are confirmed, findings provide an important guidance both for conducting disease diagnosis and treatment. Therefore, the associations of H. pylori infection and health outcomes must be further evaluated.
To provide an overview of the length, validity and credibility of the evidence on the associations between H. pylori infection and human health outcomes, we systematically and comprehensively re-evaluated these pieces of evidence to make them concise for decision-makers and guideline developers. We conducted an umbrella review to estimate the findings and content of meta-analyses that investigated these associations and to estimate the evidence of potential bias and consistency of findings.
Methods
Literature search
Computerised searches on Embase, PubMed, Web of Science, Cochrane Database of Systematic Reviews, CNKI, VIP database and Wangfang database were independently and comprehensively performed by two researchers (Guangwen Chen and Mingbing Chen) to identify the systematic reviews and meta-analyses of epidemiological studies investigating the associations between H. pylori infection and diverse health outcomes. Studies published from inception to February 1, 2019 were collected using a comprehensive search strategy, and the language was limited to English and Chinese. Medical subject heading (MeSH) terms and free-text words were used: meta-analysis, meta analysis, meta-analyses, meta analyses, systematic review, Helicobacter pylori, Campylobacter pylori, Pylorus spirillum and H. pylori. The search strategies are described in online supplementary appendix 1. References from eligible systematic reviews were also manually reviewed. All identified publications were managed with EndNote X7. Two reviewers (Qingzeng Song and Jieru Xie) independently screened the titles, abstracts and full texts for eligible articles based on the inclusion and exclusion criteria. Any discrepancy was resolved by discussion, and all discrepancies that could not be resolved through a discussion were arbitrated by Sheng Xie.
bmjopen-2019-031951supp001.pdf (57.8KB, pdf)
Eligibility criteria and exclusion criteria
Only systematic reviews and meta-analyses of epidemiological studies investigating the associations between H. pylori and multiple diseases were included in this umbrella review. The included systematic reviews and meta-analyses should present the data of pooled summary effects (i.e. relative risks (RRs), odds ratios (ORs), mean difference (MD), standard mean difference (SMD) and their 95% confidence intervals (CIs)), number of included studies, number of cases and participants, publication bias and heterogeneity. Table data (2×2) should be presented if pooled summary effects were unavailable. The population included was not limited to age, sex, ethnicity or country of origin. Articles were not limited to clinical setting, study region or research institution. When more than one meta-analyses were performed for the same review question, the concordance of the main conclusions was checked. If conclusions were inconsistent, the meta-analysis with the largest sample size and the latest date of publication was selected. The meta-analyses of interventional trials and diagnostic trials were unavailable for our research question. Conference abstracts on review questions were also excluded.
Patient and public involvement
Our study is a review of literature, so no patient was involved.
Data extraction
Data from each eligible systematic review and meta-analysis were independently extracted by two investigators (Liqun Li and Jinjing Tan). All of the results were carefully checked by a third investigator (Xiaoyan Huang). Any discrepancy was resolved by discussion, and all discrepancies were arbitrated by a fourth reviewer (Sheng Xie). The name of the first author, the year of publication, outcomes examined, the number of included studies, the total numbers of participants and cases, study design, study region and detection method of H. pylori were extracted by using a predesigned data extraction form. For each eligible systematic review and meta-analysis, the reported relative summary risk estimates (RRs, ORs, SMD or MD) and their 95% CIs were extracted. The p values of the overall pooled effects, Egger’s test and Cochran Q test were extracted. The results of I2 were also extracted. However, if the eligible systematic reviews or meta-analyses did not assess the quality of the included studies, assessing the quality was beyond our task in this umbrella review. If systematic reviews or meta-analyses examined more than one health outcome of interest, each outcome was recorded separately. If the included meta-analyses did not present the results of pooled meta-analysis (RRs, ORs, SMD or MD), I2, Egger’s test or publication bias, the 2×2 table data from studies included in those meta-analyses were extracted for reanalysis.
Assessment of methodological quality
The methodological quality of the included studies was independently assessed by two investigators (Liqun Li and Jianfeng Li) using AMSTAR 2 (A Measurement Tool to Assess systematic Reviews),19 and the results were checked by a third investigator (Xiaoyan Huang) . Inconsistencies were resolved through a discussion or consultation with a fourth reviewer (Sheng Xie). AMSTAR 2 is a reliable, valid and critical assessment tool developed from AMSTAR in 2017.19–21 It contains 16 checklists (7 critical checklists and 9 non-critical checklists) for assessing systematic reviews and meta-analyses, including randomised controlled trial (RCT) studies, observational studies on exposures or both. The rating criteria of AMSTAR 2 were as follows: zero or one non-critical weakness was defined as high quality; more than one non-critical weakness was defined as moderate quality; one critical flaw with or without non-critical weaknesses was defined as low quality; and more than one critical flaw with or without non-critical weaknesses was defined as critically low quality.
Assessment of the quality of evidence
In this umbrella review, we used the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) system to evaluate the quality of evidence for each outcome.22 23 The GRADE system includes five factors for downgrading and three factors for upgrading the quality of evidence. The baseline quality of evidence of health outcomes depends on the design of the primary studies. The summary estimate result of the random-effect model was used if potential heterogeneity was observed. Otherwise, the result of the fixed-effect model was used. When a serious or very serious defect could occur because of downgrading factors, the evidence quality was downgraded by one or two levels, respectively. If the effect was large (RR/OR either >2.0 or <0.5) or very large (RR/OR either>5.0 or <0.2), the evidence quality was upgraded by one level or two levels, respectively. If there was evidence that the influence of all plausible confouding would reduce a demonstrated effect or suggest a apurious effect when results show no effect, the evidence quality was upgraded by one level.The rating criteria of GRADE22 23 were as follows: the primary evidence quality of an observational study was considered ‘low’; the evidence quality was downgraded to ‘very low quality’ by downgrading one level, upgraded to ‘moderate quality’ by increasing one level and upgraded to ‘high quality’ by increasing two levels. The GRADE system approach classifies the evidence quality of outcomes from eligible articles as high, moderate, low and very low.22 23 GRADE classification was independently performed by two investigators (Liqun Li and Jinjing Tan), and the results were checked by a third researcher (Xiaoyan Huang). Any discrepancy was resolved via a discussion, and all discrepancies that could not be resolved through a discussion were arbitrated by Sheng Xie.
Data analysis
If the included meta-analyses did not present results of pooled meta-analysis, they were reanalysed. For example, a study was reanalysed if it did not present the results of pooled meta-analysis (RRs, ORs, SMD or MD), Egger’s test, publication bias or I2. The heterogeneity between different studies was assessed using the I2 metric of inconsistency and the p value of χ2 based on the Cochran Q test. If heterogeneity was observed, a random-effect model was used to calculate the relative summary risk estimates. Otherwise, a fixed-effect model was used.24 25 Publication bias was estimated by using Egger’s test.26 The overall effects of pooled meta-analysis, heterogeneity was considered significant at p value <0.1. Publication bias was cnsidered significant at p<0.1. Statistical analyses were conducted using Stata V.15.
Results
Description of the meta-analyses
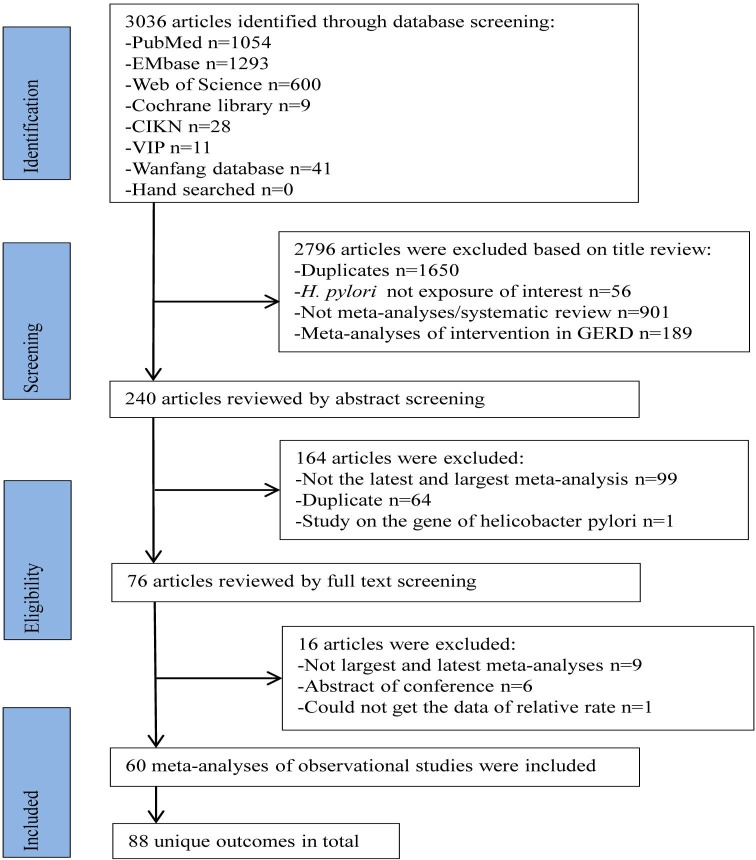
Overall, 3036 articles that met our search criteria were first identified from the seven databases. Sixty articles9–18 27–76 of observational studies were finally selected, covering 88 unique outcomes (figure 1). Fifty-four meta-analyses10–17 28–34 38–40 42–44 47–50 54–60 64–70 72–75 were reanalysed because they did not present all of the results of estimates (i.e. OR, RR), Egger’s test, I2 or publication bias. These 60 eligible non-overlapping meta-analyses are summarised in table 1. A total of 1239 individual study estimates were included in the included meta-analyses. Various measurement methods, including serology, histology, rapid urease test and 18 other detection methods, were used to determine H. pylori positivity. A range of 2–79 study estimates were pooled per meta-analysis, and the median of the study estimate was 10. Among the 1239 individual studies, 274 (22%) were cross-sectional studies, 748 (60%) were case–control studies, 124 (10%) were cohort studies and 93 (8%) were mentioned as observational studies. Furthermore, 1 meta-analysis62 did not present the number of participants, and 12 meta-analyses27 35 36 41 45 49 51 53 54 61–63 72 did not present the number of cases. Among the meta-analyses that indicated the number of cases or participants, the median number of cases was 1032 (28–96 753) and the median number of participants was 3826 (222–377 976). A total of 76 meta-analyses included more than 1000 participants, 34 meta-analyses included more than 1000 cases and 11 meta-analyses included less than 300 cases. The 60 included articles were published from 2003 to 2019, 77% were published between 2014 and 2019, and the number of publication increased yearly before 2016 (figure 2). Various health outcomes associated with H. pylori infection included cancer outcomes (n=12), cardiovascular and cerebrovascular diseases (n=8), respiratory disorders (n=3), endocrine disease (n=10), urological disease (n=2), digestive disorders (n=18), neurocognitive disorders (n=5), pregnancy-related disorders (n=9), ophthalmic diseases (n=2), thyroid disease (n=3), haematological disorders (n=3) and other outcomes (n=13) (figure 3). A total of 23 articles conducted subgroup meta-analysis based on different study region (table 2). H. pylori infection was most harmful to Asians, followed by Europeans.
Figure 1.
Flowchart of study selection process for umbrella review.
Table 1.
Description of 60 meta-analyses of H. pylori infection and prevalence or incidence of diseases included in umbrella review
| Included meta-analyses | Outcomes¶ | HP detection method |
Number of studies | Number of participants | Number of cases | Type of metric | Relative risk (95% CI) | P value* | P value† | I2 (%) | P value‡ | Whether exist publication bias |
| Cancer outcomes | ||||||||||||
| Xuan et al 13 | Hepatocellular carcinoma | HP DNA | 9 CCS; 1 CSS | 522 | 129 | OR | 16.52 (6.63 to 41.12) | 0.00 | 0.07 | 44 | 0 | Yes |
| Mounika 44 |
Lung cancer | ELISA | 5 CCS;1 PNCCS; 1 PCS |
17 951 | 16 244 | OR | 2.29 (1.34 to 3.91) | 0.032 | <0.01 | 83.9 | NA | No§ |
| Xie et al 18 | ESCC in Eastern populations | S; H; R; His +; HpSe + | 16 OS | 7665 | 1961 | OR | 0.66 (0.43 to 0.89) | NA | <0.01 | 74.5 | 0.42 | No |
| Xie et al 18 | EAC in the overall population | S; H; U; His +; HpSe + | 15 OS | 6035 | 1330 | OR | 0.59 (0.51 to 0.68) | NA | 0.13 | 29.9 | 0.37 | No |
| Wang et al 58 | Colorectal adenomatous polyp | S; H; several | 12 CS | 2678 | 1783 | OR | 1.89 (1.59 to 2.25) | 0 | 0.10 | 35.9 | 0.61 | No |
| Xiao et al 14 | Cholangiocarcinoma | PCR; ELISA; WB | 10 CCS | 489 | 220 | OR | 8.88 (3.67 to 21.49) | 0 | 0.02 | 56 | 0.01 | Yes |
| Dong and Hao75 | Colorectal cancer | IgG; UBT; CagA | 21 CCS; 2 CSS | 182 561 | 24 295 | OR | 1.42 (1.38 to 1.46) | 0 | <0.01 | 71 | 0.74 | No |
| Zhou et al 74 | Laryngeal carcinoma | ELISA; H; PCR | 11 CCS | 1030 | 418 | OR | 2.87 (1.7 to 4.84) | 0 | 0.00 | 67.1 | 0.62 | No |
| Liu28 | Colon neoplasia | IgG; IgA; UBT; H; CaA | 24 CCS; 7 CSS; 2 NCCS |
25 897 | 12 145 | OR | 1.63 (1.39 to 1.90) | 0 | <0.01 | 80 | 0.14 | No |
| Li et al 12 | Gastric cancer | WB; Chip; ELISA; neutralisation assay; EIA |
10 CCS | 1094 | 664 | OR | 2.78 (1.98 to 3.89) | 0 | 0.23 | 22.8 | 0.1 | No |
| Ma et al 42 | Oesophagogastric junction adenocarcinoma | NA | 9 CCS; 4 PCS | 5547 | 2893 | OR | 0.95 (0.06 to 1.36) | 0.769 | 0.00 | 78 | 0.61 | No |
| Liu et al 41 | Pancreatic cancer | ELISA | 6 NCCS; 2 PCS | 44 193 | NA | OR | 1.09 (0.81 to 1.47) | 0.58 | <0.01 | 76 | 0.59 | No |
| Cardiovascular and cerebrovascular diseases | ||||||||||||
| Pasceri et al 47 | Ischaemic heart disease | CagA | 3 PCS | 2140 | 966 | OR | 1.26 (1.05 to 1.51) | <0.00001 | 0.01 | 53 | NA | No§ |
| Pasceri et al 47 | Cerebral ischaemia | CagA | 4 RCCS | 1103 | 446 | OR | 2.43 (1.89 to 3.13) | <0.00001 | 0.43 | 0 | NA | No§ |
| Wang et al 55 | Diabetic IHD | S; H | 5 CS | 1805 | 469 | RR | 1.12 (0.95 to 1.32) | 0.172 | 0.14 | 42.30 | 0.21 | No |
| Liu et al 15 | Myocardial infarction | NA | 19 CSS; 7 PCS | 21 960 | 11 156 | OR | 1.73 (1.37 to 2.17) | 0 | 0.00 | 87.9 | 0.71 | No |
| Chen et al 48 | Coronary heart disease | NA | 12 CSS; 5 PCS; 1 NA |
17 514 | 9165 | OR | 1.64 (1.22 to 2.23) | 0.001 | <0.01 | 90 | 0.58 | No |
| Saburi et al 43 | Atherosclerosis | PCR | 4 CCS | 222 | 102 | OR | 5.98 (0.69 to 51.99) | 0.105 | 0.03 | 67.6 | 0.04 | Yes |
| Yan et al 65 | Arrhythmia | IgG; 13C-UBT; UBT | 7 CCS | 2014 | 1032 | OR | 1.80 (1.08 to 2.99) | 0.024 | <0.001 | 80 | 0.28 | No |
| Dong et al 76 | Carotid intima thickness | NA | 9 CCS | 1370 | 694 | SMD | 0.80 (0.69 to 0.92) | <0.01 | 0.00 | 89.7 | NA | No§ |
| Respiratory disorders | ||||||||||||
| Wang et al 57 | COPD | S; 14C-UBT | 9 CCS | 9465 | 3192 | OR | 2.25 (1.73 to 2.92) | 0 | 0.00 | 75.5 | 0.27 | No |
| Wang et al 57 | Chronic bronchitis | S; 14C-UBT | 5 CCS | 5674 | 1824 | OR | 1.57 (1.33 to 1.86) | 0 | 0.04 | 58.2 | 0.51 | No |
| Chen et al 16 | Asthma | SA; ELISA; IgG; 13C-UBT |
8 CCS; 16 CSS | 53 947 | 5648 | OR | 0.83 (0.74 to 0.94) | 0.002 | 0.00 | 53.4 | 0.67 | No |
| Endocrine disease | ||||||||||||
| Upala et al 54 | Metabolic syndrome | S; R; H; SA; UBT; biopsy |
5 CSS; 1 CS | 19 771 | NA | OR | 1.34 (1.17 to 1.53) | NA | <0.01 | 39 | 0.92 | No |
| Upala et al 54 | Fasting blood glucose | S; R; H; SA | 11 CSS; 3 CS | 7905 | NA | MD | 2.37 (0.98 to 3.77) | NA | 0.04 | 55 | 0.92 | No |
| Upala et al 54 | HDL-C | UBT; biopsy | 9 CSS; 3 CS | 7701 | NA | MD | -2.43 (-3.75 to -1.12) | NA | <0.01 | 92 | 0.92 | No |
| Upala et al 54 | Triglyceride level | S; R; H; SA | 8 CSS; 3 CS | 7596 | NA | MD | 8.12 (3.05 to 13.2) | NA | 0.04 | 71 | 0.92 | No |
| Upala et al 54 | Systolic blood pressure | UBT; biopsy; culture | 5 CSS; 1 CS | 7172 | NA | MD | 2.88 (0.20 to 5.57) | NA | 0.01 | 89 | 0.92 | No |
| Upala et al 54 | Body mass index | S; R; H; SA | 8 CSS; 2 CS | 10 707 | NA | MD | 0.30 (0.01 to 0.58) | NA | <0.01 | 57 | 0.92 | No |
| Upala et al 54 | HOMA-IR | UBT; biopsy | 7 CSS; 3 CS | 7935 | NA | MD | 0.38 (0.03 to 0.73) | NA | 0.03 | 85 | 0.92 | No |
| Li et al 39 | DM |
13C-UBT; R; 14C-UBT; SA; biopsy; culture; H |
8 CSS; 68 CCS; 3 PCS |
57 397 | 28 542 | OR | 1.69 (1.47 to 1.95) | 0 | <0.00001 | 86 | 0 | Yes |
| Li et al 39 | T2 DM |
13C-UBT; R; 14C-UBT; SA; biopsy; culture; H |
8 CSS; 57 CCS; 2 PCS |
41 684 | 21 286 | OR | 2.05 (1.67 to 2.52) | 0 | <0.00001 | 89 | 0 | Yes |
| Li et al 39 | T1 DM | 13C-UBT; R; 14C-UBT; biopsy; H | 1 PCS; 11 CCS | 3175 | 969 | OR | 1.23 (0.77 to 1.96) | 0.499 | <0.00001 | 82 | 0.46 | No |
| Urological disease | ||||||||||||
| Wang et al 56 | Diabetic nephropathy | 13C-UBT; ELISA; H | 6 CCS | 636 | 211 | OR | 1.6 (1.1 to 2.33) | 0.018 | 0.44 | 0 | 0.98 | No |
| Wijarnpreecha et al 62 | ESRD in adult | A; H; R; UBT; SA; culture |
33 CSS | NA | NA | RR | 0.71 (0.55 to 0.94) | NA | <0.00001 | 79 | NA | No§ |
| Digestive disorders | ||||||||||||
| Erőss et al 32 | Barrett’s oesophagus | S; H; UBT; PCR; R; SA | 70 CCS | 91 656 | 12 134 | OR | 0.68 (0.58 to 0.79) | 0 | 0.00 | 84 | <0.001 | Yes |
| Li et al 12 | Gastric ulcer | WB; Chip; ELISA; neutralisation assay; EIA |
8 CCS | 517 | 260 | OR | 1.64 (1.02 to 2.62) | 0.042 | 0.26 | 20.8 | 0.96 | No |
| Li et al 12 | Duodenal ulcer | WB; Chip; ELISA; neutralisation assay; EIA |
17 CCS | 2359 | 1333 | OR | 2.06 (1.50 to 2.84) | 0 | 0.01 | 51.3 | 0.63 | No |
| Cremonini et al 30 | GERD in population with HP-negative status | R; S; biopsy; H; UBT; Gram stain; culture; H&E; Giemsa stain |
14 CCS | 2010 | 1683 | OR | 1.34 (1.15 to 1.55) | 0 | <0.001 | NA | NA | No§ |
| Weck and Brenner11 | Chronic atrophic gastritis | NA | 34 OS | 7726 | 5048 | OR | 6.37 (4.01 to 10.11) | 0 | 0.00 | 91.2 | 0.01 | Yes |
| Zhou et al 73 | Biliary lithiasis | ELISA; PCR; culture | 13 CCS | 1333 | 432 | OR | 2.59 (1.21 to 5.55) | 0.014 | <0.0001 | 69.5 | 0.18 | No |
| Shiota et al 10 | Peptic ulcer disease | PCR | 42 CCS | 4601 | 2524 | OR | 1.25 (1.09 to 1.44) | 0.002 | 0.39 | 4.6 | 0.78 | No |
| Jiang et al 37 | Ammonia levels in cirrhotic patients | 14C-UBT; R; H; culture; IgG | 6 OS | 396 | 632 | SMD | 0.34 (0.21 to 0.47) | NA | 0.12 | 42.1 | 0.11 | No |
| Ford et al 9 | Dyspepsia | NA | 13 CSS | 25 305 | 9010 | OR | 1.18 (1.04 to 1.33) | NA | <0.001 | 63 | 0.3 | No |
| Feng et al 33 | Alcoholic cirrhosis in all population | R; UBT; H; ELISA | 8 CCS | 14 226 | 10053 | OR | 0.82 (0.35 to 1.91) | 0.648 | 0.00 | 84.5 | 0.67 | No |
| Feng et al 33 | Alcoholic cirrhosis in European | R; H; ELISA | 3 CCS | 1171 | 516 | OR | 2.14 (1.19 to 3.86) | 0.011 | 0.31 | 15.5 | 0.74 | No |
| Wu et al 17 | Inflammatory bowel disease | IgG; UBT; H; culture | 10 OS | 3116 | 1202 | RR | 0.48 (0.43 to 0.54) | 0 | 0.25 | 21 | 0.2 | No |
| Wang et al 60 | Chronic hepatitis C | PCR; S | 12 CCS | 3826 | 2185 | OR | 2.93 (2.30 to 3.75) | 0 | 0.05 | 45 | 0.31 | No |
| Wang et al 59 | Chronic hepatitis B | S | 15 CCS | 5129 | 2845 | OR | 3.17 (2.38 to 4.22) | 0 | 0.00 | 77.9 | 0.02 | Yes |
| Wijarnpreecha et al 63 | NAFLD | EIA; IgG; 14C-UBT; H; S; SA | 5 CSS; 1 CCS | 38 594 | NA | OR | 1.21 (1.07 to 1.37) | 0.002 | 0.08 | 49 | NA | No§ |
| Cen et al 27 | Chronic cholecystitis and cholelithiasis | H; PCR; culture | 18 CCS | 1544 | NA | OR | 3.02 (1.90 to 4.82) | NA | 0.21 | 20.1 | 0.43 | No |
| Shah et al 49 | Eosinophilic oesophagitis | Biopsy; R; H; IgG; ELISA; EIA; H&E; SA; 13C-UBT | 5 CCS; 3 CS or CCS |
371 274 | 26 442 | OR | 0.63 (0.51 to 0.78) | 0.00 | 0.02 | 57.9 | 0.77 | No |
| Shah et al 49 | Oesophageal eosinophilia | Biopsy; R; H; IgG; ELISA; EIA; H&E; SA; 13C-UBT | 5 CCS; 6 CS or CCS |
377 976 | 28 007 | OR | 0.64 (0.52 to 0.78) | 0.00 | 0.00 | 69.4 | 0.7 | No |
| Neurocognitive disorders | ||||||||||||
| Wang et al 55 | Diabetic neuropathy | S; H | 5 CS | 1607 | 520 | RR | 1.20 (1.03 to 1.40) | 0.018 | 0.29 | 19.1 | 0.99 | No |
| Wang et al 61 | Ischaemic stroke | IgG; CagA; C-UBT | 13 CCS | 4041 | NA | OR | 1.60 (1.21 to 2.11) | NA | 0.00 | 65.2 | 0.01 | Yes |
| Yu et al 70 | Stroke | S | 6 CS; 4 CCS | 166 041 | 1769 | OR | 0.96 (0.78 to 1.14) | NA | 0.03 | 48 | 0.68 | No |
| Shindler-Itskovitch et al 51 | Dementia | Biopsy; IgG; IgA; R; H; CagA | 1 CS; 6 CCS | 86 606 | NA | OR | 1.71 (1.17 to 2.49) | 0.01 | <0.001 | 76.1 | 0.33 | No |
| Shen et al 50 | Parkinson’s disease | ELISA; PCR; 13C-UBT; H; prescriptions for HP eradication drug | 6 CCS; 2 CSS | 28 201 | 1101 | OR | 1.59 (1.37 to 1.85) | 0 | 0.55 | 0 | 0.02 | Yes |
| Pregnancy-related disorders | ||||||||||||
| Ng et al 45 | Hyperemesis gravidarum | Biopsy; H; ELISA; IgG; CagA; EIA; 13C-UBT; SA |
33 CCS; 4 CSS; 1 CS |
10 289 | NA | OR | 1.35 (1.16 to 1.54) | <0.01 | 0.06 | 28 | 0.76 | No |
| Zhan et al 72 | Pre-eclampsia | ELISA; CLIA; Heli‐Blot assay; SA; UBT; WB | 3 CS; 12 CCS; 1 CSS |
10 402 | 1077 | OR | 2.51 (1.18 to 3.34) | 0 | 0.00 | 63 | 0.02 | Yes |
| Zhan et al 72 | Fetal growth restriction | Heli‐Blot assay; ELISA; SA | 3 CCS; 2 CS | 6009 | 202 | OR | 2.28 (1.21 to 4.32) | 0.011 | 0.02 | 66 | 0.17 | No |
| Zhan et al 72 | Gestational DM | ELISA; SA; WB; UBT | 2 CCS; 3 CS | 3697 | 270 | OR | 2.03 (1.56 to 2.64) | 0 | 0.81 | 0 | 0.77 | No |
| Zhan et al 72 | Spontaneous abortion | ELISA; SA | 2 CS; 3 CCS; 1 CSS | 5909 | 226 | OR | 1.5 (1.05 to 2.14) | 0.024 | 0.23 | 27 | 0.76 | No |
| Zhan et al 72 | Birth defect | ELISA; CLIA | 1 CS; 2 CCS | 737 | 132 | OR | 1.63 (1.05 to 2.54) | 0.031 | 0.48 | 0 | 0.14 | No |
| Zhan et al 72 | Stillbirth | SA; ELISA | 1 CS; 1 CCS | 3008 | 28 | OR | 2.53 (0.79 to 8.13) | 0.118 | 0.61 | 0 | 0.79 | No |
| Zhan et al 72 | Low birth weight | NA | 7 CS or CCS | 10 121 | NA | OR | 1.35 (0.88 to 2.08) | NA | 0.16 | 72 | NA | Unclear |
| Zhan et al 72 | Premature delivery | NA | 8 CS or CCS | 12 356 | NA | OR | 1.35 (0.86 to 2.12) | NA | 0.18 | 70 | NA | Unclear |
| Ophthalmic diseases | ||||||||||||
| Wang et al 55 | Diabetic retinopathy | S; H | 7 CS | 1815 | 406 | RR | 1.32 (0.97 to 1.80) | 0.058 | 0.04 | 55 | 0.27 | No |
| Zeng et al 71 | Open-angle glaucoma | H; IgG; 13C-UBT | 18 CCS | 1580 | 695 | OR | 2.08 (1.42 to 3.04) | NA | <0.001 | 63.6 | 0.36 | No |
| Thyroid disease | ||||||||||||
| Hou et al 67 | Autoimmune thyroid diseases | ELISA; WB; UBT; SA | 15 CCS | 3046 | 2408 | OR | 2.25 (1.72 to 2.93) | 0 | 0.00 | 61.6 | 0.68 | No |
| Hou et al 67 | Grave’s disease | ELISA; SA; UBT | 5 CCS | 917 | 498 | OR | 2.78 (1.68 to 4.61) | 0 | 0.07 | 53.4 | 1.51 | No |
| Hou et al 67 | Hashimoto’s thyroiditis | ELISA; SA; UBT; NR | 8 CCS | 1594 | 872 | OR | 2.16 (1.44 to 3.23) | 0 | 0.00 | 68.2 | 0.51 | No |
| Haematological disorders | ||||||||||||
| Hudak et al 35 | Iron deficiency anaemia | R; H; 13C-UBT; 14C-UBT; IgG; SA; IgA; gastroscopy |
11 CSS; 3 CCS | 15 905 | NA | OR | 1.72 (1.23 to 2.42) | NA | 0.00 | 61.5 | 0.38 | No |
| Hudak et al 35 | Iron deficiency | 30 CSS | 23 521 | NA | OR | 1.33 (1.15 to 1.54) | NA | 0.01 | 41.1 | 0.49 | No | |
| Hudak et al 35 | Anaemia | 23 CSS | 11 622 | NA | OR | 1.15 (1.00 to 1.32) | NA | 0.01 | NA | 0.81 | No | |
| Other outcomes | ||||||||||||
| Nweneka and Prentice46 | Circulating ghrelin levels | UBT; ELISA; S; H; culture; R; PCR | 7 CS; 11 CSS; 6 CCS |
956 | 1288 | SMD | −0.42 (−0.57 to −0.27) | <0.00001 | 0.00 | 59 | 0.12 | No |
| Xiong et al 64 | Henoch-Schonlein purpura | R; UBT; IgG; H. pylori antigen | 10 CCS | 1309 | 500 | OR | 3.46 (2.68 to 4.47) | 0 | 0.06 | 46 | 0.03 | Yes |
| Su et al 52 | Migraine | 13C-UBT; ELISA; biopsy | 5 CCS or CSS | 903 | 355 | OR | 1.92 (1.05 to 3.51) | 0.033 | 0.00 | 77.4 | 0.08 | yes |
| Li et al 40 | Recurrent aphthous stomatitis | PCR; UBT | 7 CCS | 510 | 154 | OR | 1.85 (1.24 to 2.74) | 0.002 | 0.21 | 28.5 | 0.49 | No |
| Taye et al 53 | Atopy | H; IgG; ELISA; UBT; SA; IgA | 2 CS; 3 CCS; 11 CSS |
10 968 | NA | OR | 0.82 (0.73 to 0.91) | <0.01 | 0.66 | 0 | 0.85 | No |
| Hwang et al 36 | Chronic tonsillitis | R; PCR; culture; CLO | 6 OS | 436 | NA | OR | 1.99 (0.91 to 4.37) | 0.09 | 0.06 | 53.6 | 0.42 | No |
| Gu et al 34 | Chronic urticaria | ELISA; UBT; S; H; IgG | 16 CCS | 2200 | 984 | OR | 1.66 (1.12 to 2.45) | 0.022 | <0.0001 | 66 | 0.01 | Yes |
| Yao et al 66 | Multiple sclerosis | ELISA; WB; CIA IF |
9 CCS | 2806 | 782 | OR | 0.73 (0.56 to 0.96) | 0 | 0.05 | 48 | 0.07 | yes |
| Dou et al 31 | Halitosis | R; H; BUT; culture; PCR; Gram stain; ELISA; SA; endoscopy; CLO |
6 CCS;1 CSS | 2312 | 467 | OR | 4.03 (1.41 to 11.5) | 0.009 | <0.0001 | 89 | 0.05 | Yes |
| Jørgensen et al 38 | Rosacea | NA | 14 OS | 2455 | 1268 | OR | 1.74 (1.03 to 2.93) | 0.039 | 0.00 | 85.6 | 0.09 | yes |
| Chen et al 29 | Sjogren’s syndrome | Biopsy; ELISA; IgG |
9 CCS | 2018 | 1054 | OR | 1.19 (1.01 to 1.41) | 0.033 | 0.86 | 0 | 0.77 | No |
| Yong et al 68 | Psoriasis | IgG; ELISA; UBT; SA | 4 CSS; 3 PCS; 2 CCS |
1546 | 728 | OR | 1.58 (1.02 to 2.46) | 0.041 | 0.00 | 64 | 0.03 | Yes |
| Yong et al 69 | Systemic sclerosis | IgG; ELISA; IgM; 13C-UBT; R | 7 CSS; 1 PCS | 1446 | 749 | OR | 2.11 (1.62 to 2.76) | 0.00 | 0.33 | 13 | 0.84 | No |
*p value of significance level.
†p value of Q test.
‡p value for Egger’s test.
§The publication bias was assessed using funnel plot.
¶ prevalence or incidence unless otherwise specified.
A, antibody; CagA, cytotoxin-associated gene A; CCS, case–control study; CI, confidence intervals; CLIA, chemiluminescent immunoassay; CLO, Campylobacter-like organism test; COPD, chronic obstructive pulmonary disease; CS, cohort study; CSS, cross-sectional study; DM, diabetes mellitus; EAC, oesophageal adenocarcinoma; EIA, enzyme immunoassay; ESCC, oesophageal squamous cell carcinoma; ESRD, end-stage renal disease; GERD, gastro-oesophageal reflux disease; H, histology; HDL-C, high-density lipoprotein cholesterol; H&E, Hematoxylin and eosin stain; His +, positive histological examination of tissue samples; HOMA-IR, homeostatic model assessment of insulin resistance; HP, H. pylori; HpSe +, seropositivity for antibodies to whole cell; IF, immunofluorescence; IHD, ischaemic heart disease; MD, mean difference; NA, not applicable; NAFLD, non-alcoholic fatty liver disease; NCCS, nested case-control study; NR, not reported; OS, observational study; PCR, polymerase chain reaction; PCS, prospective cohort study; PNCS, prospective nested cohort study; R, rapid urease test; RCCS, retrospective case–control study; RCSS, retrospective cross-sectional study; RR, relative rate; S, serology; SA, stool antigen; several, several methods; SMD, standard mean difference; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; U, rapid urease test; UBT, urea breath test; WB, western blot.
Figure 2.
Number of publications per annum.
Figure 3.
Map of 88 . H. pylori–related outcomes: percentage of outcomes per outcome category for all studies.
Table 2.
Association between H. pylori infection and diverse diseases based on study region
| Study region | Associations between H. pylori infection and outcomes | No association with | |
| Increase risk of | Decrease risk of | ||
| Europe | Cholangiocarcinoma,14 colorectal, cancer,75
diabetes mellitus,39 diabetic nephropathy,56 alcoholic cirrhosis,33 Parkinson’s disease50 |
Barrett's oesophagus32 | Arrhythmia,65 asthma,16 biliary lithiasis,73 migraine,52 recurrent aphthous stomatitis,40 chronic urticaria34 |
| America | Colorectal cancer,75 biliary lithiasis,73 chronic urticaria34 | Barrett’s oesophagus,32 asthma,16 asthma16 | Arrhythmia,65 diabetes mellitus,39 alcoholic cirrhosis,33 recurrent aphthous stomatitis40 |
| East (Asia, China) |
Cholangiocarcinoma,14 colorectal cancer,75 colon neoplasia,28 arrhythmia,65 diabetes mellitus,39 diabetic nephropathy,56 biliary lithiasis,73 ammonia levels in cirrhotic patients,37 chronic cholecystitis and cholelithiasis,27 Parkinson’s disease,50 open-angle glaucoma,71 Henoch-Schonlein purpura,64 migraine,52 chronic urticaria34 | Oesophageal squamous cell carcinoma,13 Barrett’s oesophagus,32 asthma16 | Myocardial infarction,15 COPD,57 biliary lithiasis,73 peptic ulcer disease,10 alcoholic cirrhosis,33 recurrent aphthous stomatitis,40 multiple sclerosis66 |
| West | Colon neoplasia,28 myocardial infarction,15 COPD,57 peptic ulcer disease,10 open-angle glaucoma71 | Oesophageal adenocarcinoma,13 asthma,16 multiple sclerosis66 | Oesophageal squamous cell carcinoma,13 ammonia levels in cirrhotic patients37 |
| Africa | Arrhythmia65 | Barrett’s oesophagus,32 diabetes mellitus,39 peptic ulcer disease10 | |
| Australia | Barrett’s oesophagus32 | ||
| Oceania | Biliary lithiasis73 | ||
COPD, chronic obstructive pulmonary disease.
Summary effect size
Table 1 shows the summary effects of the included meta-analysis. Of the 88 outcomes, 74 (84%) had nominal significance (p<0.05). Of these outcomes, 61 (82%) were harmful associations enumerated as follows: 8 (67%) meta-analyses in cancer outcomes, 6 (75%) in cardiovascular and cerebrovascular diseases, 2 (67%) in respiratory disorders, 6 (60%) in endocrine diseases, 1 (50%) in urological diseases, 12 (67%) in digestive disorders, 4 (80%) in neurocognitive disorders, 6 (67%) in pregnancy-related disorders, 1 (50%) in ophthalmic diseases, 3 (100%) in thyroid diseases, 3 (100%) in haematological disorders and 9 (69%) in other outcomes. These associations had significant pooled estimates (p<0.05). Thus, H. pylori infection was associated with an increased risk of disease and harmful to human health (table 3). By contrast, 13 (15%) evidence from meta-analyses were beneficial associations enumerated as follows: 1 (33%) meta-analyses in respiratory disorders, 2 (15%) in cancer outcomes, 1 (10%) endocrine disease, 1 (50%) urological disease, 5 (28%) digestive disorders and 3 (23%) in other outcomes. These associations had significant pooled estimates (p<0.05), indicating that H. pylori infection was related to a decreased risk of some diseases. These findings could be beneficial to human health in some situations (table 3).
Table 3.
Results of evidence quality for all outcomes classified by GRADE
| Level of evidence | Outcomes | ||||
| Increased risk of | Increase | Decreased risk of | Reduce | No association with | |
| High | – | – | – | ||
| Moderate | Chronic cholecystitis and cholelithiasis,27 gestational diabetes mellitus,72 gastric cancer12 and systemic sclerosis69 | Triglyceride level54 | Inflammatory bowel disease17 | – | Stillbirth72 |
| Low | Hepatocellular carcinoma,13 biliary lithiasis,73 peptic ulcer disease,10 chronic hepatitis C,60 non-alcoholic fatty liver disease,63 diabetic neuropathy,55 Parkinson’s disease,50 hyperemesis gravidarum,45 fetal growth restriction,72 spontaneous abortion,72 birth defect,72 open-angle glaucoma,71 autoimmune thyroid diseases,67 Grave’s disease,67 Hashimoto’s thyroiditis,67 Henoch-Schonlein purpura,64 colorectal adenomatous polyp,58 Sjogren’s syndrome,29 duodenal ulcer,12 laryngeal carcinoma,74 chronic obstructive pulmonary disease,57 diabetic nephropathy,56 gastric ulcer,12 alcoholic cirrhosis in Europian,33 cerebral ischaemia47 | Ammonia levels in patients with cirrhosis37 | Oesophageal adenocarcinoma in the overall population,18 eosinophilic oesophagitis,49 oesophageal eosinophilia,49 atopy53 | – | Diabetic ischemic heart disease, 55 fasting blood glucose54 and diabetic ischaemic heart disease56 |
| Very low | Lung cancer,44 cholangiocarcinoma,14 chronic tonsillitis,36 colorectal cancer, colon neoplasia,28 ischaemic heart disease,47 myocardial infarction,15 coronary heart disease,48 arrhythmia,65 chronic bronchitis,57 metabolic syndrome,54 diabetes mellitus,39 type 2 diabetes mellitus,39 chronic atrophic gastritis,11 dyspepsia,9 chronic hepatitis B,59 ischaemic stroke,61 dementia,51 pre-eclampsia,72 iron deficiency anaemia,35 iron deficiency,31 anaemia,26 migraine,52 recurrent aphthous stomatitis,40 chronic urticaria,34 halitosis,31 rosacea38 and psoriasis68 | Carotid intima thickness,76 body mass index54 and homeostatic model assessment of insulin resistance54 | Oesophageal squamous cell carcinoma in Eastern populations,18 Barrett’s oesophagus,32 asthma,16 end-stage renal disease in adult,62 multiple sclerosis66 and gastro-oesophageal reflux disease30 | High-density lipoprotein cholesterol,54 circulating ghrelin levels46 | Oesophagogastric junction adenocarcinoma,42 pancreatic cancer,41 systolic blood prssure,54 atherosclerosis,43 type 1 diabetes mellitus,39 alcoholic cirrhosis in all populations,28 stroke,70 low birth weight,72 premature delivery72 and diabetic retinopathy70 |
Heterogeneity and publication bias of the included studies
All of the included meta-analyses presented the results of heterogeneity between studies (table 1). In particular, 24 (27%) outcomes of meta-analyses showed no heterogeneity between studies (p≥0.1 of Q test), whereas 64 (73%) exhibited significant heterogeneity (p<0.1 of Q test). Moreover, 32 (57%) of 64 meta-analyses showed moderate to high heterogeneity (I2=50%–75%), and 24 (43%) showed high heterogeneity (I2>75%). Among 88 meta-analyses, 68 (77%) demonstrated no statistical evidence on publication bias according to Egger’s, whereas 18 (20%) of the meta-analyses presented publication bias (p<0.1 of Egger’s test). Only 2 (2%) meta-analyses did not report publication bias.
Summary of the methodological quality of the included meta-analyses
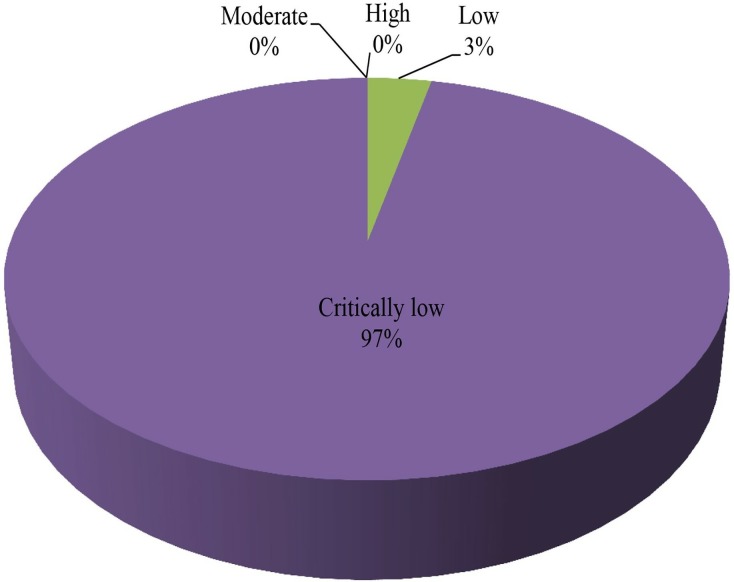
The methodological qualities of the 60 included articles were assessed using AMSTAR 2, and the results are shown in table 4. A total of 52 (87%) meta-analyses did not report a predefined explicit statement or protocol; only 8 (13%) meta-analyses were conducted using a comprehensive literature search strategy, and 24 (40%) meta-analyses did not perform a duplicate selection. Twelve (20%) meta-analyses did not conduct a duplicate data extraction, 53 (88%) meta-analyses provided a list of excluded studies but did not justify the exclusions, 6 (10%) meta-analyses did not provide a list of excluded studies, 50 (83%) meta-analyses partially described the included studies and 22 (37%) meta-analyses did not assess the risk of bias in the included studies. Furthermore, none of the meta-analyses reported the details of funding sources for the included studies, and 28 (47%) meta-analyses did not report potential sources of conflicts of interest. Overall, 85 (97%) methodological qualities of the included meta-analyses were categorised as ‘critically low’, and only 3 (3%) methodological qualities of the included meta-analyses were assessed as low quality (figure 4).
Table 4.
Detail of results for AMSTAR 2 assessing
| Included meta-analyses | AMSTAR 2 checklist | Overall assessment quality | |||||||||||||||
| No. 1 | No. 2 | No 3 | No. 4 | No. 5 | No. 6 | No. 7 | No. 8 | No. 9 | No. 10 | No. 11 | No. 12 | No. 13 | No. 14 | No. 15 | No. 16 | ||
| Xuan et al 13 | Yes | No | Yes | Yes | Yes | Yes | No | Partial yes | No | No | Yes | Yes | Yes | Yes | Yes | No | Critically low |
| Mounika44 | Yes | No | Yes | Partial yes | No | No | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Critically low |
| Xie et al 18 | Yes | No | Yes | No | Yes | Yes | Partial yes | Partial yes | No | No | Yes | Yes | Yes | Yes | Yes | No | Critically low |
| Wang et al 58 | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Critically low |
| Xiao et al 14 | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Dong and Hao75 | Yes | No | Yes | Partial yes | No | No | Partial yes | Partial yes | No | No | No | No | Yes | Yes | Yes | No | Critically low |
| Zhou et al 74 | Yes | No | Yes | Partial yes | No | Yes | Partial yes | Partial yes | No | No | Yes | Yes | Yes | Yes | Yes | No | Critically low |
| Liu28 | Yes | No | Yes | Partial yes | No | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Li et al 12 | Yes | No | Yes | Partial yes | No | Yes | Partial yes | Partial yes | No | No | Yes | No | Yes | Yes | Yes | Yes | Critically low |
| Ma et al 42 | Yes | No | Yes | Partial yes | No | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Critically low |
| Liu et al 41 | Yes | No | Yes | No | Yes | Yes | Partial yes | Partial yes | No | No | Yes | Yes | Yes | Yes | Yes | No | Critically low |
| Erőss et al 32 | Yes | Yes | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Pasceri et al 47 | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | No | No | Yes | Yes | Yes | Yes | Yes | No | Critically low |
| Liu et al 15 | Yes | No | Yes | Partial yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Chen et al 48 | Yes | No | Yes | Partial yes | No | Yes | No | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Saburi et al 43 | Yes | No | Yes | Partial yes | No | No | Partial yes | Partial yes | Yes | No | Yes | No | Yes | No | Yes | Yes | Critically low |
| Yan et al 65 | Yes | No | Yes | Yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Dong et al 76 | Yes | No | No | Yes | No | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Critically low |
| Wang et al 57 | Yes | No | Yes | Partial yes | No | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Chen et al 16 | Yes | No | Yes | No | Yes | Yes | Partial yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Critically low |
| Upala et al 54 | Yes | Yes | Yes | Partial yes | No | Yes | No | Partial yes | No | No | Yes | Yes | Yes | Yes | Yes | No | Critically low |
| Li et al 39 | Yes | No | Yes | Yes | No | No | Partial yes | Partial yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Wang et al 56 | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Wijarnpreecha et al 62 | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | No | No | Yes | No | Critically low |
| Cremonini et al 30 | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Critically low |
| Weck and Brenner11 | Yes | No | No | No | No | No | Partial yes | Yes | No | No | Yes | No | Yes | Yes | Yes | No | Critically low |
| Zhou et al 73 | Yes | No | Yes | Yes | Yes | Yes | Partial yes | Partial yes | No | No | Yes | Yes | Yes | Yes | Yes | No | Critically low |
| Shiota et al 10 | Yes | No | Yes | Partial yes | Yes | No | Partial yes | Partial yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Jiang et al 37 | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Ford et al 9 | Yes | No | Yes | Partial yes | No | Yes | Partial yes | Partial yes | No | No | Yes | Yes | Yes | Yes | Yes | No | Critically low |
| Feng et al 33 | Yes | No | Yes | Partial yes | No | No | Partial yes | Partial yes | No | No | Yes | No | Yes | Yes | Yes | No | Critically low |
| Wu et al 17 | Yes | No | Yes | Partial yes | No | Yes | Partial yes | Partial yes | No | No | Yes | No | Yes | Yes | Yes | No | Critically low |
| Wang et al 60 | Yes | No | Yes | Yes | Yes | Yes | Partial yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Wang et al 59 | Yes | No | Yes | Yes | Yes | No | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Wijarnpreecha et al 63 | Yes | No | Yes | Yes | Yes | Yes | Partial yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Critically low |
| Cen et al 27 | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Shah et al 49 | Yes | No | Yes | Partial yes | No | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Critically low |
| Wang et al 55 | Yes | No | No | Partial yes | Yes | Yes | No | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Wang et al 61 | Yes | No | Yes | Partial yes | No | Yes | Partial yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Yu et al 70 | Yes | No | Yes | Partial yes | No | Yes | Partial yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Shindler-Itskovitch et al 51 | Yes | No | Yes | Partial yes | No | Yes | Partial yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Shen et al 50 | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Ng et al 45 | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | No | No | Yes | No | Yes | Yes | Yes | Yes | Critically low |
| Zhan et al 72 | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Zeng et al 71 | Yes | No | Yes | Partial yes | No | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Critically low |
| Nweneka and Prentice46 | Yes | No | Yes | Partial yes | No | No | Yes | Partial yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Xiong et al 64 | Yes | Yes | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Su et al 52 | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Critically low |
| Li et al 40 | Yes | No | Yes | No | Yes | No | Partial yes | Partial yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Critically low |
| Taye et al 53 | Yes | Yes | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Hwang et al 36 | Yes | No | No | Partial yes | Yes | No | Partial yes | Partial yes | No | No | Yes | No | Yes | Yes | Yes | Yes | Critically low |
| Gu et al 34 | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Yao et al 66 | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Critically low |
| Dou et al 31 | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | No | No | Yes | No | Yes | Yes | No | No | Critically low |
| Jørgensen et al 38 | Yes | No | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Critically low |
| Hou et al 67 | Yes | No | Yes | Partial yes | No | No | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Critically low |
| Hudak et al 35 | Yes | Yes | Yes | Partial yes | No | Yes | Partial yes | Partial yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Critically low |
| Chen et al 29 | Yes | Yes | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | No | No | Yes | Yes | Yes | Yes | Yes | No | Critically low |
| Yong et al 68 | Yes | Yes | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | No | Yes | No | Yes | Yes | Critically low |
| Yong et al 69 | Yes | Yes | Yes | Partial yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | No | Yes | No | Yes | No | Critically low |
AMSTAR 2 checklists: No.1: Did the research questions and inclusion criteria for the review include the components of PICO? No.2: Did the report of the review contain an explicit statement that the review methods were established prior to the conduct of the review and did the report justify any significant deviations from the protocol? No. 3: Did the review authors explain their selection of the study designs for inclusion in the review? No. 4: Did the review authors use a comprehensive literature search strategy? No. 5: Did the review authors perform study selection in duplicate? No. 6: Did the review authors perform data extraction in duplicate? No. 7: Did the review authors provide a list of excluded studies and justify the exclusions? No. 8: Did the review authors describe the included studies in adequate detail? No. 9: Did the review authors use a satisfactory technique for assessing the risk of bias (RoB) in individual studies that were included in the review? No. 10: Did the review authors report on the sources of funding for the studies included in the review? No. 11: If meta-analysis was performed, did the review authors use appropriate methods for statistical combination of results? No. 12: If meta-analysis was performed, did the review authors assess the potential impact of RoB in individual studies on the results of the meta-analysis or other evidence synthesis? No. 13: Did the review authors account for RoB in primary studies when interpreting/discussing the results of the review? No. 14: Did the review authors provide a satisfactory explanation for, and discussion of, any heterogeneity observed in the results of the review? No. 15: If they performed quantitative synthesis, did the review authors carry out an adequate investigation of publication bias (small study bias) and discuss its likely impact on the results of the review? No. 16: Did the review authors report any potential sources of conflict of interest, including any funding they received for conducting the review?
Figure 4.
Map of results of AMSTAR 2: percentage of outcomes per outcome category for 88 meta-analyses.
Evidence classification of the outcomes
The evidence quality of every outcome was assessed using the GRADE system (table 5). None of the evidence quality for any outcome was rated ‘high’. Most of the qualities of evidence were downgraded by the potential risk of bias and serious heterogeneity. A total of 32 (36%) evidence qualities of outcomes were rated ‘low’, 49 (56%) evidence were rated ‘very low’ and only 7 (8%) evidence were rated ‘moderate’ (figure 5). Table 5 shows the results of evidence quality from 88 outcomes.
Table 5.
Details of evidence quality for outcomes classified by GRADE
| Included meta-analyses | Association between H. pylori and* | Downgrade factors | Upgrade factors | GRADE class | |||||
| Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Large effect | Plausible confounding would change the effect | |||
| Cancer outcomes | |||||||||
| Xuan et al 13 | Hepatocellular carcinoma | −1 | 0 | 0 | -1 | −1 | 2 | 1 | Low |
| Mounika44 | Lung cancer | −1 | −2 | 0 | 0 | 0 | 1 | 1 | Very low |
| Xie et al 18 | ESCC in Eastern populations | −1 | −1 | 0 | 0 | 0 | 0 | 1 | Very low |
| Xie et al 18 | EAC in the overall population | −1 | 0 | 0 | 0 | 0 | 0 | 1 | Low |
| Wang et al 58 | Colorectal adenomatous polyp | −1 | 0 | 0 | 0 | 0 | 0 | 1 | Low |
| Xiao et al 14 | Cholangiocarcinoma | −1 | −1 | 0 | −1 | −1 | 2 | 1 | Very low |
| Dong and Hao75 | Colorectal cancer | −1 | −1 | 0 | 0 | 0 | 0 | 1 | Very low |
| Zhou et al 74 | Laryngeal carcinoma | −1 | −1 | 0 | 0 | 0 | 1 | 1 | Low |
| Liu28 | Colon neoplasia | −1 | −2 | 0 | 0 | 0 | 0 | 1 | Very low |
| Li et al 12 | Gastric cancer | −1 | 0 | 0 | 0 | 0 | 1 | 1 | Moderate |
| Ma et al 42 | Oesophagogastric junction adenocarcinoma | −1 | −2 | 0 | 0 | 0 | 0 | 1 | Very low |
| Liu et al 41 | Pancreatic cancer | −1 | −2 | 0 | 0 | 0 | 0 | 1 | Very low |
| Cardiovascular and cerebrovascular diseases | |||||||||
| Pasceri et al 47 | Ischaemic heart disease | −2 | −1 | 0 | 0 | 0 | 0 | 1 | Very low |
| Pasceri et al 47 | Cerebral ischaemia | −2 | 0 | 0 | 0 | 0 | 1 | 1 | Low |
| Wang et al 55 | Diabetic IHD | −1 | 0 | 0 | 0 | 0 | 0 | 1 | Low |
| Liu et al 15 | Myocardial infarction | −1 | −2 | 0 | 0 | 0 | 0 | 1 | Very low |
| Chen et al 48 | Coronary heart disease | −2 | −2 | 0 | 0 | 0 | 0 | 1 | Very low |
| Ramezani-Binabaj et al 43 | Atherosclerosis | −2 | −1 | 0 | −1 | −1 | 0 | 1 | Very low |
| Yan et al 65 | Arrhythmia | −1 | −2 | 0 | 0 | 0 | 0 | 1 | Very low |
| Dong et al 76 | Carotid intima thickness | −1 | −2 | 0 | 0 | 0 | 0 | 1 | Very low |
| Respiratory disorders | |||||||||
| Wang et al 57 | COPD | −1 | −1 | 0 | 0 | 0 | 1 | 1 | Low |
| Wang et al 57 | Chronic bronchitis | −1 | −1 | 0 | 0 | 0 | 0 | 1 | Very low |
| Chen et al 16 | Asthma | −1 | −1 | 0 | 0 | 0 | 0 | 1 | Very low |
| Endocrine disease | |||||||||
| Upala et al 54 | Metabolic syndrome | −1 | −1 | 0 | 0 | 0 | 0 | 1 | Very low |
| Upala et al 54 | Fasting blood glucose | −1 | −1 | 0 | 0 | 0 | 1 | 1 | Low |
| Upala et al 54 | HDL-C | −1 | −2 | 0 | 0 | 0 | 1 | 1 | Very low |
| Upala et al 54 | Triglyceride level | −1 | −1 | 0 | 0 | 0 | 2 | 1 | Moderate |
| Upala et al 54 | Systolic blood pressure | −1 | −2 | 0 | 0 | 0 | 1 | 1 | Very low |
| Upala et al 54 | Body mass index | −1 | −1 | 0 | 0 | 0 | 0 | 1 | Very low |
| Upala et al 54 | HOMA-IR | −1 | −2 | 0 | 0 | 0 | 0 | 1 | Very low |
| Li et al 39 | DM | −1 | −2 | 0 | 0 | −1 | 0 | 1 | Very low |
| Li et al 39 | T2DM | −1 | −2 | 0 | 0 | −1 | 1 | 1 | Very low |
| Li et al 39 | T1DM | −1 | −2 | 0 | 0 | 0 | 0 | 1 | Very low |
| Urological disease | |||||||||
| Wang56 | Diabetic nephropathy | −1 | 0 | 0 | 0 | 0 | 0 | 1 | Low |
| Wijarnpreecha et al 62 | ESRD in adult | −1 | −2 | 0 | 0 | 0 | 0 | 1 | Very low |
| Digestive disorders | |||||||||
| Erőss et al 32 | Barrett’s oesophagus | −1 | −2 | 0 | 0 | −1 | 0 | 1 | Very low |
| Li et al 12 | Gastric ulcer | −1 | 0 | 0 | 0 | 0 | 0 | 1 | Low |
| Li et al 12 | Duodenal ulcer | −1 | −1 | 0 | 0 | 0 | 1 | 1 | Low |
| Cremonini et al 30 | GERD in population with HP-negative status | −1 | −1 | 0 | 0 | 0 | 0 | 1 | Very low |
| Weck and Brenner11 | Chronic atrophic gastritis | −1 | −2 | 0 | 0 | −1 | 2 | 1 | Very low |
| Zhou et al 73 | Biliary lithiasis | −1 | −1 | 0 | 0 | 0 | 1 | 1 | Low |
| Shiota et al 10 | Peptic ulcer disease | −1 | 0 | 0 | 0 | 0 | 0 | 1 | Low |
| Jiang et al 37 | Ammonia levels in cirrhotic patients | −1 | 0 | 0 | 0 | 0 | 0 | 1 | Low |
| Ford et al 9 | Dyspepsia | −1 | −1 | 0 | 0 | 0 | 0 | 1 | Very low |
| Feng et al 33 | Alcoholic cirrhosis in all population | −1 | −2 | 0 | 0 | 0 | 0 | 1 | Very low |
| Feng et al 33 | Alcoholic cirrhosis in European | −1 | 0 | 0 | −1 | 0 | 1 | 1 | Low |
| Wu et al 17 | Inflammatory bowel disease | −1 | 0 | 0 | 0 | 0 | 1 | 1 | Moderate |
| Wang et al 60 | Chronic hepatitis C | −1 | −1 | 0 | 0 | 0 | 1 | 1 | Low |
| Wang et al 59 | Chronic hepatitis B | −1 | −2 | 0 | 0 | −1 | 1 | 1 | Very low |
| Wijarnpreecha et al 63 | NAFLD | −1 | 0 | 0 | 0 | 0 | 0 | 1 | Low |
| Cen et al 27 | Chronic cholecystitis and cholelithiasis | −1 | 0 | 0 | 0 | 0 | 1 | 1 | Moderate |
| Shah et al 49 | Eosinophilic oesophagitis | 0 | −1 | 0 | 0 | 0 | 0 | 1 | Low |
| Shah et al 49 | Oesophageal eosinophilia | 0 | −1 | 0 | 0 | 0 | 0 | 1 | Low |
| Neurocognitive disorders | |||||||||
| Wang et al 55 | Diabetic neuropathy | −1 | 0 | 0 | 0 | 0 | 0 | 1 | Low |
| Wang et al 61 | Ischaemic stroke | −1 | −1 | 0 | 0 | −1 | 0 | 1 | Very low |
| Yu et al 70 | Stroke | −1 | −1 | 0 | 0 | 0 | 0 | 1 | Very low |
| Shindler-Itskovitch et al 51 |
Dementia | −1 | −2 | 0 | 0 | 0 | 0 | 1 | Very low |
| Shen et al 50 | Parkinson’s disease | 0 | 0 | 0 | 0 | −1 | 0 | 1 | Low |
| Pregnancy-related disorders | |||||||||
| Ng et al 45 | Hyperemesis gravidarum | −1 | 0 | 0 | 0 | 0 | 0 | 1 | Low |
| Zhan et al 72 | Pre-eclampsia | −1 | −1 | 0 | 0 | −1 | 1 | 1 | Very low |
| Zhan et al 72 | Fetal growth restriction | −1 | −1 | 0 | 0 | 0 | 1 | 1 | Low |
| Zhan et al 72 | Gestational DM | −1 | 0 | 0 | 0 | 0 | 1 | 1 | Moderate |
| Zhan et al 72 | Spontaneous abortion | −1 | 0 | 0 | 0 | 0 | 0 | 1 | Low |
| Zhan et al 72 | Birth defect | −1 | 0 | 0 | 0 | 0 | 0 | 1 | Low |
| Zhan et al 72 | Stillbirth | −1 | 0 | 0 | 0 | 0 | 1 | 1 | Moderate |
| Zhan et al 72 | Low birth weight | −1 | −1 | 0 | 0 | 0 | 0 | 1 | Very low |
| Zhan et al 72 | Premature delivery | −1 | −1 | 0 | 0 | 0 | 0 | 1 | Very low |
| Ophthalmic diseases | |||||||||
| Wang et al 55 | Diabetic retinopathy | −1 | −1 | 0 | 0 | 0 | 0 | 1 | Very low |
| Zeng et al 71 | Open-angle glaucoma | −1 | −1 | 0 | 0 | 0 | 1 | 1 | Low |
| Thyroid disease | |||||||||
| Hou et al 67 | Autoimmune thyroid diseases | −1 | −1 | 0 | 0 | 0 | 1 | 1 | Low |
| Hou et al 67 | Grave’s disease | −1 | −1 | 0 | 0 | 0 | 1 | 1 | Low |
| Hou et al 67 | Hashimoto’s thyroiditis | −1 | −1 | 0 | 0 | 0 | 1 | 1 | Low |
| Homeopathy disorders | |||||||||
| Hudak et al 35 | Iron deficiency anaemia | −1 | −1 | 0 | 0 | 0 | 0 | 1 | Very low |
| Hudak et al 35 | Iron deficiency | −1 | −1 | 0 | 0 | 0 | 0 | 1 | Very low |
| Hudak et al 35 | Anaemia | −1 | −1 | 0 | 0 | 0 | 0 | 1 | Very low |
| Other outcomes | |||||||||
| Nweneka and Prentice46 | Circulating ghrelin levels | −1 | −1 | 0 | 0 | 0 | 0 | 1 | Very low |
| Xiong et al 64 | Henoch-Schonlein purpura | −1 | 0 | 0 | 0 | −1 | 1 | 1 | Low |
| Su et al 52 | Migraine | −1 | −1 | 0 | 0 | -1 | 0 | 1 | Very low |
| Li et al 40 | Recurrent aphthous stomatitis | −1 | 0 | 0 | −1 | 0 | 0 | 1 | Very low |
| Taye et al 53 | Atopy | −1 | 0 | 0 | 0 | 0 | 0 | 1 | Low |
| Hwang et al 36 | Chronic tonsillitis | −1 | −1 | 0 | 0 | 0 | 0 | 1 | Very low |
| Gu et al 34 | Chronic urticaria | −1 | −1 | 0 | 0 | −1 | 0 | 1 | Very low |
| Yao et al 66 | Multiple sclerosis | −1 | 0 | 0 | 0 | -1 | 0 | 1 | Very low |
| Dou et al 31 | Halitosis | −1 | −2 | 0 | 0 | −1 | 0 | 1 | Very low |
| Jørgensen et al 38 | Rosacea | −1 | −2 | 0 | 0 | 0 | 0 | 1 | Very low |
| Chen et al 29 | Sjogren’s syndrome | −1 | 0 | 0 | 0 | 0 | 0 | 1 | Low |
| Yong et al 68 | Psoriasis | −1 | −1 | 0 | 0 | −1 | 0 | 1 | Very low |
| Yong et al 69 | Systemic sclerosis | −1 | 0 | 0 | 0 | 0 | 1 | 1 | Moderate |
Reference: −1 means downgrade one level; −2 means downgrade two levels; 1 means upgrade one level.
* prevalence or incidence unless otherwise specified.
COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; DM, diabetes mellitus; EAC, oesophgeal adenocarcinoma; ESCC, oesophageal squamous cell carcinoma; ESRD, end-stage renal disease; GERD, gastro-oesophageal reflux disease; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, hemeostatic model assessment of insulin resistance; HP, H. pylori; IHD, ischemic heart disease; NAFLD, non-alcoholic fatty liver disease; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
Figure 5.
Map of results of GRADE: percentage of outcomes per outcome category for 88 evidence.
Harmful outcomes associated with H. pylori infection
Our confidence level in the following was moderate: H. pylori infection is associated with an increased risk of chronic cholecystitis and cholelithiasis,27 gestational diabetes mellitus,72 systemic sclerosis69 and gastric cancer,12 and increased serum triglyceride level.54 Our confidence level in the following was low: H. pylori infection is associated with an increased risk of HCC,13 biliary lithiasis,73 PUD,10 duodenal ulcer,12chronic hepatitis C,60 non-alcoholic fatty liver disease,63 diabetic neuropathy,55 Parkinson’s disease,50 hyperemesis gravidarum,45 fetal growth restriction,72 spontaneous abortion,72 birth defect,72 open-angle glaucoma,71 autoimmune thyroid diseases,67 Grave’s disease,67 Hashimoto’s thyroiditis,67 Henoch-Schonlein purpura,64 diabetic nephropathy,56 gastric ulcer,12 alcoholic cirrhosis Europeans,33 and Sjogren’s syndrome29; ammonia levels decrease in patients with cirrhosis.37 Our confidence level in the following was very low: H. pylori infection is associated with an increased risk of lung cancer,44 cholangiocarcinoma,14 colorectal cancer75, colon neoplasia,28 chronic tonsillitis,36 ischaemic heart disease,47 MI,15 coronary heart disease,48 arrhythmia,65 chronic bronchitis,57 metabolic syndrome,54 diabetes mellitus,39 type 2 diabetes mellitus,39 chronic atrophic gastritis,11 dyspepsia,9 chronic hepatitis B,59 ischaemic stroke,61 dementia,51 pre-eclampsia,72 iron deficiency anaemia,35 iron deficiency,31 anaemia,26 migraine,52 recurrent aphthous stomatitis,40 chronic urticaria,34 halitosis,31 rosacea,38 laryngeal carcinoma,74 cerebral ischaemia,47 chronic obstructive pulmonary disease57 and psoriasis68; an increase in the following parameters is observed: carotid intima thickness,76 body mass index54 and homeostatic model assessment of insulin resistance.54
Beneficial outcomes associated with H. pylori infection
Our confidence level in the following was moderate: H. pylori infection is associated with a decreased risk of irritable bowel syndrome.17 Our confidence level in the following was low: H. pylori infection is associated with a decreased risk of oesophageal adenocarcinoma in the overall population,18 colorectal adenomatous polyp,58 eosinophilic oesophagitis,49 oesophageal eosinophilia49 and atopy53. Our confidence level in the following was very low: H. pylori infection is associated with a decreased risk of oesophageal squamous cell carcinoma in Eastern populations,18 Barrett’s oesophagus,32 asthma,16 end-stage renal disease in adults,62 multiple sclerosis66 and gastro-oesophageal reflux disease30; decreasing high-density lipoprotein cholesterol54 and circulating ghrelin levels are also observed.46
Discussion
Principal findings and possible explanations
This umbrella review summarised the current existing evidence from meta-analyses on the associations between H. pylori infection and diverse health outcomes. In this umbrella review, 60 publications of interest were systematically reviewed. The role of H. pylori infection was explored in relation to a wide range of diseases (74 in total), including cancers, cardiovascular and cerebrovascular diseases, respiratory disorders, endocrine diseases, urological diseases, digestive disorders, neurocognitive disorders, pregnancy-related disorders, ophthalmic diseases, thyroid disease, haematological disorders and other outcomes (figure 3). H . pylori infection is likely more harmful in Asians by increasing the risk of 15 types of diseases (table 2). Through this umbrella review, an uptrend of research on the associations between H. pylori infection and health outcomes was found (figure 2). However, gaps in studies exploring the association between H. pylori infection and the musculoskeletal system diseases were identified as formal meta-analyses were not found. A clear reference exposure time of H. pylori infection could not be obtained because all of the meta-analyses did not present this aspect.
A large proportion (84%) of the health outcomes was associated with H. pylori infection. However, most of them (64%) had serious heterogeneity between studies. The potential heterogeneity might be due to possible confounding factors (e.g. different H. pylori measurement methods, alcohol consumption, smoking, sex, study region, different nationalities and time of follow-up). Substantial heterogeneity affected the results of meta-analyses, indicating that some associations between H. pylori infection and diverse health outcomes might be inflated or false positives. In addition, some of them 20%) had a notable publication bias, revealing that some negative results were not reported. In practice, associations between H. pylori infection and diseases might be found in thousands of individuals. However, only a small proportion of associations were recorded, and an even smaller fraction was finally published. Positive results were probably more easily published than negative results that might not be even published. If researchers strongly believed in the association between H. pylori infection and the risk of developing diseases, their work might be under pressure to comply with the hypothesis during publication. These requirements could cause publication biases in the results. Our result showed that 97% of the meta-analyses had ‘critically low’ methodological quality (figure 4). Evidence was downgraded by serious heterogeneity, potential bias and low method quality. Hence, none of the outcomes had high-quality evidence after evaluation based on the evidence classification criteria. Based on this metric, moderate-quality evidence only existed in six health outcomes, suggesting that H. pylori infection was probably associated with an increased risk of hypertriglyceridemia, chronic cholecystitis and cholelithiasis, gestational diabetes mellitus, gastric cancer and systemic sclerosis and a decreased risk of irritable bowel syndrome. Among these risks, the outcome of triglyceride level exhibited moderate heterogeneity (I2=71%), demonstrating that this association should be cautiously interpreted. This umbrella reivew shows there is no association between H.ylori infection and risk of stillbirth.
Strengths and limitations of the umbrella review
Our umbrella review has several great strengths. An umbrella review systematically searches, collects and assesses the strength and credibility of the evidence derived from various systematic reviews and meta-analyses on any clinical health outcomes related to a particular exposure.77 Studies have also revealed the strengths and significance of umbrella reviews in detail.78–80 Considering that the associations between H. pylori infection and diverse health outcomes have not been systematically and comprehensively assessed, this umbrella review comprehensively evaluated the methodological quality of meta-analyses and assessed the evidence quality of outcomes from the published meta-analyses of observational studies. The quality of the included studies in meta-analyses affects the quality of the meta-analyses. When possible, we reanalysed the summary estimates and explored the heterogeneity and publication bias of the included meta-analyses by using a standardised method. In this umbrella review, seven databases were comprehensively and systematically searched using a standard search strategy to identify eligibility. An uptrend of studies on associations between H. pylori infection and various health outcomes was found, indicating that the associations of H. pylori infection and diseases were widely explored. However, meta-analyses investigated on associations between H. pylori infection and musculoskeletal disorders, and mucosa associated limphoid tissue (MALT) lymphoma were not found in our scope.
We used AMSTAR 2, which is a standard methodological quality assessment approach, to assess the quality of the method used for meta-analyses. Since AMSTAR 2 was developed from AMSTAR in 2017, it has been considered a valid and reliable methodological quality assessment tool.19 81 The lengths of AMSTAR 2 have been described in other studies.21 82 83 This tool helped us identify the highest methodological quality of the meta-analyses of RCTs and also the meta-analyses of observational studies. Therefore, AMSTAR 2 is more practical and applicable than AMSTAR. In this umbrella review, 95% of the methodological qualities of existing meta-analyses studying the associations between H. pylori infection and diverse health outcomes were critically low, suggesting that the results of the meta-analyses might be inconclusive, and further meta-analyses with high methodological quality should be conducted to verify such conclusions.
We also adopted the GRADE system criteria, which are credibility-assessment criteria, to assess the evidence quality of outcomes from meta-analyses. The certainty of evidence is important for the recommendation of guidelines, affecting a patient’s outcomes.84 GRADE re-evaluates the quality of evidence and rates the certainty evidence for clinical decision-makers and guideline developers.22 84 This system is also used worldwide. We downgraded the evidence level because all of the meta-analyses exhibited a potential risk of bias, but we upgraded the evidence level because the result of the included meta-analyses might be affected by various potential confounders, such as age, sex or smoking. Although conducting AMSTAR 2 and GRADE classification was relatively subjective, they were performed by two investigators independently, and the results were checked by another investigator. The inconsistencies were resolved via a discussion, and all discrepancies were arbitrated by another researcher, thereby greatly reducing the subjectivity.
In terms of the study weakness, this umbrella review focused on the existing and published systematic review and meta-analyses and only included publications in Chinese and English. Thus, we might have missed some studies on the associations between H. pylori infection and diverse health outcomes. The potential missing data in other languages might affect the evaluation results. In this umbrella review, only systematic reviews and meta-analyses were included. Evidence from individual observational studies involving undeveloped a meta-analysis was not in the scope of our discussion, such as MALT lymphoma.85 This situation might result in conclusion bias of association between H. pylori infection and human health. In addition, we could not obtain a clear exposure time because most of the meta-analyses did not present the length of time of H. pylori infection. Most of the meta-analyses had heterogeneity, but we did not re-explore the factors causing heterogeneity, such as population characteristics (eg, age, sex and nationality), study design and study region. Common flaws are evident among meta-analyses.86 The evidence quality of meta-analyses depends on the quality of original individual studies included in meta-analyses. However, this umbrella review did not assess the quality of the original individual studies included in the meta-analyses. We extracted the data for calculation from the included meta-analyses but not from the original individual studies, possibly affecting the conclusion of this umbrella review.
Clinical implications and future research
Clinicians have considered whether individuals should be tested for H. pylori or offered eradication therapy for H. pylori infection since multiple unfavourable influences on human health related to H. pylori infection have been found. Different suggestions on addressing H. pylori infection have been provided in different guidelines because of objective factors (eg, local drug resistance, economic level, and medical and health conditions). Several guidelines, including Asian guidelines, recommend screening in every individual, whereas other guidelines recommend no screening.87 The different recommendations regarding H. pylori detection and eradication in different guidelines may cause confusion among clinicians. The significance of our study mostly included the summary of the diseases associated with H. pylori and the clarification of evidence quality to guide clinical practice.
Our umbrella review found that 69% of outcomes were unfavourable influences on human health which should be paid attention to by clinicians, even though most of them were low-quality evidence. In terms of eradicating therapy for H. pylori infection, the beneficial influence (H. pylori infection as a protect factor) on human health might be considered by clinicians. This umbrella review found that a decreased risk of 13 types of conditions (eg, inflammatory bowel disease, laryngeal and oesophageal carcinoma) was also found, even though H. pylori was associated with an increased risk of a large proportion of diseases. H. pylori–eradicating drugs have adverse effects, such as increased resistance of H. pylori.88 Therefore, for an individual who tests positive, whether H. pylori infection is a risk factor or a protective factor should be distinguished before he/she receives eradicating therapy. Before deciding on administering eradicating therapy, clinicians should weigh the advantages and disadvantages of eradicating H. pylori based on an individual’s situation.
Future prospective studies on H. pylori infection and health outcomes should use time-varying exposure (H. pylori infection duration) and confounder information to better model the association between H. pylori infection and health outcomes. Data remain scarce, and a large heterogeneity exists in some associations of H. pylori infection and diseases. Prospective studies should be carried out to better characterise these associations. In the absence of data from RCTs for H. pylori infection and risk of developing diseases, Mendelian randomisation analyses may be useful in determining whether an observed association is likely to be causal. Meta-analyses investigating associations between H. pylori infection and some diseases, such as autoimmune liver disease,89 90 have not been found in our scope. A meta-analysis may be conducted to confirm these conclusions in the future because of the possible inconsistent results in different individual studies.
Conclusion
This umbrella review systematically and comprehensively collected a large amount of existing evidence on the associations between H. pylori infection and diverse health outcomes from published meta-analyses to help clinical decision-makers, guideline developers and investigators evaluate these associations. Although 60 meta-analyses explored 88 unique outcomes, moderate evidence only existed in six outcomes with statistical significance. H. pylori infection may be associated with a decreased risk of irritable bowel syndrome and an increased risk of hypertriglyceridemia, chronic cholecystitis and cholelithiasis, gestational diabetes mellitus, gastric cancer and systemic sclerosis. Further prospective studies and large RCTs with a good assessment of associations between H. pylori infection and health outcomes should be conducted to draw a firm conclusion.
Supplementary Material
Acknowledgments
The authors would like to thank Dr Honghui Li, Shengan Mo and Jiang for their help in this umbrella review.
Footnotes
LL, JT, LL and JL contributed equally.
Funding: This study is supported by National Natural Science Foundation of China (No. 81573914 and No. 81460723).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as online supplementary information.
References
- 1. Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 2017;153:420–9. 10.1053/j.gastro.2017.04.022 [DOI] [PubMed] [Google Scholar]
- 2. Melese A, Genet C, Zeleke B, et al. Helicobacter pylori infections in Ethiopia; prevalence and associated factors: a systematic review and meta-analysis. BMC Gastroenterol 2019;19:8 10.1186/s12876-018-0927-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Venneman K, Huybrechts I, Gunter MJ, et al. The epidemiology of Helicobacter pylori infection in Europe and the impact of lifestyle on its natural evolution toward stomach cancer after infection: a systematic review. Helicobacter 2018;23:e12483 10.1111/hel.12483 [DOI] [PubMed] [Google Scholar]
- 4. Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther 2018;47:868–76. 10.1111/apt.14561 [DOI] [PubMed] [Google Scholar]
- 5. Eslick GD, Lim LL, Byles JE, et al. Association of Helicobacter pylori infection with gastric carcinoma: a meta-analysis. Am J Gastroenterol 1999;94:2373–9. 10.1111/j.1572-0241.1999.01360.x [DOI] [PubMed] [Google Scholar]
- 6. Huang JQ, Sridhar S, Chen Y, et al. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology 1998;114:1169–79. 10.1016/S0016-5085(98)70422-6 [DOI] [PubMed] [Google Scholar]
- 7. Vergara M, Calvet X, Roqué M. Helicobacter pylori is a risk factor for peptic ulcer disease in cirrhotic patients. A meta-analysis. Eur J Gastroenterol Hepatol 2002;14:717–22. 10.1097/00042737-200207000-00002 [DOI] [PubMed] [Google Scholar]
- 8. Jaakkimainen RL, Boyle E, Tudiver F. Is Helicobacter pylori associated with non-ulcer dyspepsia and will eradication improve symptoms? A meta-analysis. BMJ 1999;319:1040–4. 10.1136/bmj.319.7216.1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ford AC, Marwaha A, Sood R, et al. Global prevalence of, and risk factors for, uninvestigated dyspepsia: a meta-analysis. Gut 2015;64:1049–57. 10.1136/gutjnl-2014-307843 [DOI] [PubMed] [Google Scholar]
- 10. Shiota S, Watada M, Matsunari O, et al. Helicobacter pylori iceA, clinical outcomes, and correlation with cagA: a meta-analysis. PLoS One 2012;7:e30354 10.1371/journal.pone.0030354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weck MN, Brenner H. Association of Helicobacter pylori infection with chronic atrophic gastritis: meta-analyses according to type of disease definition. Int J Cancer 2008;123:874–81. 10.1002/ijc.23539 [DOI] [PubMed] [Google Scholar]
- 12. Li Q, Liu J, Gong Y, et al. Serum vacA antibody is associated with risks of peptic ulcer and gastric cancer: a meta-analysis. Microb Pathog 2016;99:220–8. 10.1016/j.micpath.2016.08.030 [DOI] [PubMed] [Google Scholar]
- 13. Xuan S-Y, Xin Y-N, Chen A-J, et al. Association between the presence of H pylori in the liver and hepatocellular carcinoma: a meta-analysis. World J Gastroenterol 2008;14 10.3748/wjg.14.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiao M, Gao Y, Wang Y. Helicobacter species infection may be associated with cholangiocarcinoma: a meta-analysis. Int J Clin Pract 2014;68:262–70. 10.1111/ijcp.12264 [DOI] [PubMed] [Google Scholar]
- 15. Liu J, Wang F, Shi S. Helicobacter pylori infection increase the risk of myocardial infarction: a meta-analysis of 26 studies involving more than 20,000 participants. Helicobacter 2015;20:176–83. 10.1111/hel.12188 [DOI] [PubMed] [Google Scholar]
- 16. Chen C, Xun P, Tsinovoi C, et al. Accumulated evidence on Helicobacter pylori infection and the risk of asthma. Ann Allerg Asthma Im 2017;119:137–45. e132 10.1016/j.anai.2017.05.021 [DOI] [PubMed] [Google Scholar]
- 17. Wu X-W, Ji H-Z, Yang M-F, et al. Helicobacter pylori infection and inflammatory bowel disease in Asians: a meta-analysis. World J Gastroenterol 2015;21:4750–6. 10.3748/wjg.v21.i15.4750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xie F-J, Zhang Y-P, Zheng Q-Q, et al. Helicobacter pylori infection and esophageal cancer risk: an updated meta-analysis. World J Gastroenterol 2013;19:6098–107. 10.3748/wjg.v19.i36.6098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol 2009;62:1013–20. 10.1016/j.jclinepi.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 21. Jung JH, Dahm P. Reaching for the stars—rating the quality of systematic reviews with the Assessment of Multiple Systematic Reviews (AMSTAR) 2. BJU Int 2018;122:717–8. 10.1111/bju.14571 [DOI] [PubMed] [Google Scholar]
- 22. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 23. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401–6. 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 24. DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015;45:139–45. 10.1016/j.cct.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997;127:820–6. 10.7326/0003-4819-127-9-199711010-00008 [DOI] [PubMed] [Google Scholar]
- 26. Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 2000;53:1119–29. 10.1016/s0895-4356(00)00242-0 [DOI] [PubMed] [Google Scholar]
- 27. Cen L, Pan J, Zhou B, et al. Helicobacter pylori infection of the gallbladder and the risk of chronic cholecystitis and cholelithiasis: a systematic review and meta-analysis. Helicobacter 2018;23:e12457 10.1111/hel.12457 [DOI] [PubMed] [Google Scholar]
- 28. Chao Liu PZ. The relationship of Helicobacter pylori infection and the risk of colon neoplasia based on meta-analysis. Int J Clin Exp Med 2016;9:2293–300. [Google Scholar]
- 29. Chen Q, Zhou X, Tan W, et al. Association between Helicobacter pylori infection and Sjögren syndrome: a meta-analysis. Medicine 2018;97:e13528 10.1097/MD.0000000000013528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cremonini F, Di Caro S, Delgado-Aros S, et al. Meta-analysis: the relationship between Helicobacter pylori infection and gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2003;18:279–89. 10.1046/j.1365-2036.2003.01665.x [DOI] [PubMed] [Google Scholar]
- 31. Dou W, Li J, Xu L, et al. Halitosis and Helicobacter pylori infection: a meta-analysis. Medicine 2016;95:e4223 10.1097/MD.0000000000004223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Erőss B, Farkas N, Vincze Áron, et al. Helicobacter pylori infection reduces the risk of Barrett's esophagus: a meta-analysis and systematic review. Helicobacter 2018;23:e12504 10.1111/hel.12504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feng H, Zhou X, Zhang G, et al. Association between cirrhosis and Helicobacter pylori infection. Eur J Gastroenterol Hepatol 2014;26:1–19. 10.1097/MEG.0000000000000220 [DOI] [PubMed] [Google Scholar]
- 34. Gu H, Li L, Gu M, et al. Association between Helicobacter pylori infection and chronic urticaria: a meta-analysis. Gastroenterol Res Pract 2015;2015:486974 10.1155/2015/486974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hudak L, Jaraisy A, Haj S, et al. An updated systematic review and meta-analysis on the association between Helicobacter pylori infection and iron deficiency anemia. Helicobacter 2017;22:e12330 10.1111/hel.12330 [DOI] [PubMed] [Google Scholar]
- 36. Hwang MS, Forman SN, Kanter JA, et al. Tonsillar Helicobacter pylori colonization in chronic tonsillitis: systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg 2015;141:245–9. 10.1001/jamaoto.2014.3296 [DOI] [PubMed] [Google Scholar]
- 37. Jiang H-X, Qin S-Y, Min Z-gang, et al. Association of Helicobacter pylori with elevated blood ammonia levels in cirrhotic patients: a meta-analysis. Yonsei Med J 2013;54:832–8. 10.3349/ymj.2013.54.4.832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jørgensen A-HR, Egeberg A, Gideonsson R, et al. Rosacea is associated with Helicobacter pylori: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol 2017;31:2010–5. 10.1111/jdv.14352 [DOI] [PubMed] [Google Scholar]
- 39. JZ L, JY L, TF W, et al. Helicobacter pylori infection is associated with type 2 diabetes, not type 1 diabetes: an updated meta-analysis. Gastroenterol Res Pract 2017;2017 10.1155/2017/5715403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li L, Gu H, Zhang G. Association between recurrent aphthous stomatitis and Helicobacter pylori infection: a meta-analysis. Clin Oral Investig 2014;18:1553–60. 10.1007/s00784-014-1230-5 [DOI] [PubMed] [Google Scholar]
- 41. Liu H, Chen Y-T, Wang R, et al. Helicobacter pylori infection, atrophic gastritis, and pancreatic cancer risk: a meta-analysis of prospective epidemiologic studies. Medicine 2017;96:e7811 10.1097/MD.0000000000007811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ma S, Ma Q, Li J, SR M, JB L, et al. [Meta-analysis on relationship between Helicobacter pylori infection and esophagogastric junction adenocarcinoma]. Zhonghua Liu Xing Bing Xue Za Zhi 2016;37:418–24. 10.3760/cma.j.issn.0254-6450.2016.03.027 [DOI] [PubMed] [Google Scholar]
- 43. Saburi A, Ramezani-Binabaj M, Jannat AM, et al. The relationship between Helicobacter pylori infection and atherosclerosis: a meta-analysis. Erciyes Med J 2016;38. [Google Scholar]
- 44. Mounika P. Helicobacter pylori infection and risk of lung cancer: a meta-analysis. Lung Cancer Int 2013;2013:1–6. 10.1155/2013/131869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. QX N, Venkatanarayanan N, De Deyn M, et al. A meta-analysis of the association between Helicobacter pylori (H. pylori) infection and hyperemesis gravidarum. Helicobacter 2018;23 10.1111/hel.12455 [DOI] [PubMed] [Google Scholar]
- 46. Nweneka CV, Prentice AM. Helicobacter pylori infection and circulating ghrelin levels—a systematic review. BMC Gastroenterol 2011;11:7 10.1186/1471-230X-11-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pasceri V, Patti G, Cammarota G, et al. Virulent strains of Helicobacter pylori and vascular diseases: a meta-analysis. Am Heart J 2006;151:1215–22. 10.1016/j.ahj.2005.06.041 [DOI] [PubMed] [Google Scholar]
- 48. Chen QS, Huang MH, Chen KH, et al. Meta analysis of the correlation between Helicobacter pylori infection and coronary heart disease. Int J Clin Exp Med 2016;9:1936–1402. [Google Scholar]
- 49. Shah SC, Tepler A, Peek RM, et al. Association between Helicobacter pylori exposure and decreased odds of eosinophilic esophagitis—a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2019;17:2185–98. 10.1016/j.cgh.2019.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shen X, Yang H, Wu Y, et al. Meta-analysis: association of Helicobacter pylori infection with Parkinson's diseases. Helicobacter 2017;22:e12398 10.1111/hel.12398 [DOI] [PubMed] [Google Scholar]
- 51. Shindler-Itskovitch T, Ravona-Springer R, Leibovitz A, et al. A systematic review and meta-analysis of the association between Helicobacter pylori infection and dementia. J Alzheimers Dis 2016;52:1431–42. 10.3233/JAD-160132 [DOI] [PubMed] [Google Scholar]
- 52. Su J, Zhou X-Y, Zhang G-X. Association between Helicobacter pylori infection and migraine: a meta-analysis. World J Gastroenterol 2014;20:14965–72. 10.3748/wjg.v20.i40.14965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Taye B, Enquselassie F, Tsegaye A, et al. Is Helicobacter pylori infection inversely associated with atopy? A systematic review and meta-analysis. Clin Exp Allergy 2015;45:882–90. 10.1111/cea.12404 [DOI] [PubMed] [Google Scholar]
- 54. Upala S, Jaruvongvanich V, Riangwiwat T, et al. Association between Helicobacter pylori infection and metabolic syndrome: a systematic review and meta-analysis. J Dig Dis 2016;17:433–40. 10.1111/1751-2980.12367 [DOI] [PubMed] [Google Scholar]
- 55. Wang F, Fu Y, Lv Z. Association of Helicobacter pylori infection with diabetic complications: a meta-analysis. Endocr Res 2014;39:7–12. 10.3109/07435800.2013.794426 [DOI] [PubMed] [Google Scholar]
- 56. Wang F, Liu J, Lv Z. Association of Helicobacter pylori infection with diabetes mellitus and diabetic nephropathy: a meta-analysis of 39 studies involving more than 20,000 participants. Scand J Infect Dis 2013;45:930–8. 10.3109/00365548.2013.844351 [DOI] [PubMed] [Google Scholar]
- 57. Wang F, Liu J, Zhang Y, et al. Association of Helicobacter pylori infection with chronic obstructive pulmonary disease and chronic bronchitis: a meta-analysis of 16 studies. Infect Dis 2015;47:597–603. 10.3109/00365548.2014.989539 [DOI] [PubMed] [Google Scholar]
- 58. Wang F, Sun MY, Shi SL, et al. Helicobacter pylori infection and normal colorectal mucosa–adenomatous polyp–adenocarcinoma sequence: a meta-analysis of 27 case–control studies. Colorectal Dis 2014;16:246–52. 10.1111/codi.12290 [DOI] [PubMed] [Google Scholar]
- 59. Wang J, Chen R-C, Zheng Y-X, et al. Helicobacter pylori infection may increase the risk of progression of chronic hepatitis B disease among the Chinese population: a meta-analysis. Int J Infect Dis 2016;50:30–7. 10.1016/j.ijid.2016.07.014 [DOI] [PubMed] [Google Scholar]
- 60. Wang J, Li W-T, Zheng Y-X, et al. The association between Helicobacter pylori infection and chronic hepatitis C: a meta-analysis and trial sequential analysis. Gastroenterol Res Pract 2016;2016:8780695 10.1155/2016/8780695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang ZW, Li Y, Huang LY, et al. Helicobacter pylori infection contributes to high risk of ischemic stroke: evidence from a meta-analysis. J Neurol 2012;259:2527–37. 10.1007/s00415-012-6558-7 [DOI] [PubMed] [Google Scholar]
- 62. Wijarnpreecha K, Thongprayoon C, Nissaisorakarn P, et al. Association between Helicobacter pylori and end-stage renal disease: a meta-analysis. World J Gastroenterol 2017;23:1497–506. 10.3748/wjg.v23.i8.1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wijarnpreecha K, Thongprayoon C, Panjawatanan P, et al. Helicobacter pylori and risk of nonalcoholic fatty liver disease: a systematic review and meta-analysis. J Clin Gastroenterol 2018;52:386–91. 10.1097/MCG.0000000000000784 [DOI] [PubMed] [Google Scholar]
- 64. Xiong L-J, Tong Y, Wang Z-L, et al. Is Helicobacter pylori infection associated with Henoch-Schonlein purpura in Chinese children? A meta-analysis. World J Pediatr 2012;8:301–8. 10.1007/s12519-012-0373-1 [DOI] [PubMed] [Google Scholar]
- 65. Yan J, She Q, Zhang Y, et al. The association between arrhythmia and Helicobacter pylori infection: a meta-analysis of case–control studies. Int J Environ Res Public Health 2016;13:1139 10.3390/ijerph13111139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yao G, Wang P, Luo X-D, et al. Meta-analysis of association between Helicobacter pylori infection and multiple sclerosis. Neurosci Lett 2016;620:1–7. 10.1016/j.neulet.2016.03.037 [DOI] [PubMed] [Google Scholar]
- 67. Hou Y, Sun W, Zhang C, et al. Meta-analysis of the correlation between Helicobacter pylori infection and autoimmune thyroid diseases. Oncotarget 2017;8:115691–700. 10.18632/oncotarget.22929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yong WC, Upala S, Sanguankeo A. Association between psoriasis and Helicobacter pylori infection: a systematic review and meta-analysis. Indian J Dermatol 2018;63:193–200. 10.4103/ijd.IJD_531_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yong WC, Upala S, Sanguankeo A, et al. Helicobacter pylori infection in systemic sclerosis: a systematic review and meta-analysis of observational studies. Clin Exp Rheumatol 2018;36 Suppl 113:168–74. [PubMed] [Google Scholar]
- 70. Yu M, Zhang Y, Yang Z, et al. Association between Helicobacter pylori infection and stroke: a meta-analysis of prospective observational studies. J Stroke Cerebrovasc Dis 2014;23:2233–9. 10.1016/j.jstrokecerebrovasdis.2014.04.020 [DOI] [PubMed] [Google Scholar]
- 71. Zeng J, Liu H, Liu X, et al. The relationship between Helicobacter pylori infection and open-angle glaucoma: a meta-analysis. Invest Ophthalmol Vis Sci 2015;56:5238–45. 10.1167/iovs.15-17059 [DOI] [PubMed] [Google Scholar]
- 72. Zhan Y, Si M, Li M, et al. The risk of Helicobacter pylori infection for adverse pregnancy outcomes: a systematic review and meta-analysis. Helicobacter 2019;24:e12562 10.1111/hel.12562 [DOI] [PubMed] [Google Scholar]
- 73. Zhou D, Zhang Y, Gong W, et al. Are Helicobacter pylori and other Helicobacter species infection associated with human biliary lithiasis? A meta-analysis. PLoS One 2011;6:e27390 10.1371/journal.pone.0027390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhou J, Zhang D, Yang Y, et al. Association between Helicobacter pylori infection and carcinoma of the larynx or pharynx. Head Neck 2015;38. [DOI] [PubMed] [Google Scholar]
- 75. Hong-Xia D, Hao L. Correlation between colorectal cancer and Helicobacter pylori infection in different countries: a meta-analysis. Med J Chin PLA 2015;40:236–41. 10.11855/j.issn.0577-7402.2015.03.13 [DOI] [Google Scholar]
- 76. Dong XS, Chen ZL, Fan WX, et al. Relationship between Helicobacter pylori infection and carotid intima thickness by meta-analysis. Chin Cir J 2018;33:366–70. [Google Scholar]
- 77. Aromataris E, Fernandez R, Godfrey CM, et al. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc 2015;13:132–40. 10.1097/XEB.0000000000000055 [DOI] [PubMed] [Google Scholar]
- 78. Veronese N, Demurtas J, Pesolillo G, et al. Magnesium and health outcomes: an umbrella review of systematic reviews and meta-analyses of observational and intervention studies. Eur J Nutr 2019;99 10.1007/s00394-019-01905-w [DOI] [PubMed] [Google Scholar]
- 79. Hong X-Y, Lin J, Gu W-W. Risk factors and therapies in vascular diseases: an umbrella review of updated systematic reviews and meta-analyses. J Cell Physiol 2019;234:8221–32. 10.1002/jcp.27633 [DOI] [PubMed] [Google Scholar]
- 80. Siegfried N, Parry C, NA-Ohoo S. Do alcohol control policies work? An umbrella review and quality assessment of systematic reviews of alcohol control interventions (2006–2017). PLoS One 2019;14:e0214865 10.1371/journal.pone.0214865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gates A, Gates M, Duarte G, et al. Evaluation of the reliability, usability, and applicability of AMSTAR, AMSTAR 2, and ROBIS: protocol for a descriptive analytic study. Syst Rev 2018;7:85 10.1186/s13643-018-0746-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kallmayer MA, Salvermoser M, Knappich C, et al. Quality appraisal of systematic reviews, and meta-analysis of the hospital/surgeon-linked volume–outcome relationship of carotid revascularization procedures. J Cardiovasc Surg 2019;60 10.23736/S0021-9509.19.10943-3 [DOI] [PubMed] [Google Scholar]
- 83. Sanabria A, Kowalski LP, Nixon I, et al. Methodological quality of systematic reviews of intraoperative neuromonitoring in thyroidectomy: a systematic review. JAMA Otolaryngol Head Neck Surg 2019;145 10.1001/jamaoto.2019.0092 [DOI] [PubMed] [Google Scholar]
- 84. Schünemann HJ, Mustafa RA, Brozek J, et al. GRADE guidelines: 22. The GRADE approach for tests and strategies—from test accuracy to patient-important outcomes and recommendations. J Clin Epidemiol 2019;111:69–82. 10.1016/j.jclinepi.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 85. Ishikura N, Usui Y, Ito H, et al. Helicobacter pylori (HP) infection alone, but not HP-induced atrophic gastritis, increases the risk of gastric lymphoma: a case–control study in Japan. Ann Hematol 2019;98:1981–7. 10.1007/s00277-019-03721-y [DOI] [PubMed] [Google Scholar]
- 86. Ioannidis JPA. The mass production of redundant, misleading, and conflicted systematic reviews and meta-analyses. Milbank Q 2016;94:485–514. 10.1111/1468-0009.12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Xie S, Li J, JF L, et al. Quality assessment of guidelines for the management of Helicobacter pylori infection. J Tradit Chin Med 2019;37:263–9. [Google Scholar]
- 88. Almeida N, Romãozinho JM, Donato MM, et al. Helicobacter pylori antimicrobial resistance rates in the central region of Portugal. Clin Microbiol Infect 2014;20:1127–33. 10.1111/1469-0691.12701 [DOI] [PubMed] [Google Scholar]
- 89. Nilsson I, Kornilovs'ka I, Lindgren S, et al. Increased prevalence of seropositivity for non-gastric Helicobacter species in patients with autoimmune liver disease. J Med Microbiol 2003;52:949–53. 10.1099/jmm.0.05344-0 [DOI] [PubMed] [Google Scholar]
- 90. Durazzo M, Pellicano R, Premoli A, et al. Helicobacter pylori seroprevalence in patients with autoimmune hepatitis. Dig Dis Sci 2002;47:380–3. 10.1023/A:1013782408510 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-031951supp001.pdf (57.8KB, pdf)