Abstract
Background
Mammalian oocytes initiate meiosis in fetal ovary and are arrested at dictyate stage in prophase I for a long period. It is known that incidence of chromosome segregation errors in oocytes increases with advancing age, but the molecular mechanism underlying this phenomenon has not been clarified.
Methods
Cohesin, a multi‐subunit protein complex, mediates sister chromatid cohesion in both mitosis and meiosis. In this review, molecular basis of meiotic chromosome cohesion and segregation is summarized. Further, the relationship between chromosome segregation errors and cohesin deterioration in aged oocytes is discussed.
Results
Recent studies show that chromosome‐associated cohesin decreases in an age‐dependent manner in mouse oocytes. Furthermore, conditional knockout or activation of cohesin in oocytes indicates that only the cohesin expressed before premeiotic S phase can establish and maintain sister chromatic cohesion and that cohesin does not turnover during the dictyate arrest.
Conclusion
In mice, the accumulating evidence suggests that deterioration of cohesin due to the lack of turnover during dictyate arrest is one of the major causes of chromosome segregation errors in aged oocytes. However, whether the same is true in human remains elusive since even the deterioration of cohesin during dictyate arrest has not been demonstrated in human oocytes.
Keywords: age‐related aneuploidy, chromosome segregation, cohesion, oocyte, sister chromatid cohesion
1. INTRODUCTION
Gametes (ova and spermatozoa) are produced from diploid germ cells (oocytes and spermatocytes) through a special type of cell division called meiosis. Meiosis consists of two successive nuclear divisions without intervening S phase. In the first meiotic division (meiosis I), homologous chromosomes establish the connection with their partners at prophase, forming bivalent chromosomes. Then, at the onset of anaphase I, homologous chromosomes separate from each other by the dissolution of cohesion along chromosome arm region while sister chromatids remain attached at centromere region. In the second meiotic division (meiosis II), sister chromatids separate from each other by the dissolution of the remaining centromeric cohesion.1, 2
Meiosis is sexually dimorphic in mammalian gametogenesis.2, 3, 4 In males, meiosis initiates after puberty and proceeds continuously to the end of meiosis followed by gametogenic differentiation from round spermatids into spermatozoa. In the adult male gonad, meiotic cells called spermatocytes are continuously provided from mitotically dividing stem cells called spermatogonia. In contrast, in females, the number of oocytes reaches a peak at fetal stage and decreases as females get older. Female meiosis starts in the fetal stage (eg, 13.5 days post‐coitum in mice) and proceeds to the extended diplotene stage, also called dictyate stage, at which the first meiotic arrest is imposed (Figure 1). During this arrest, only a limited number of oocytes grow their volume with development of the surrounding follicle. Then, fully grown oocytes resume meiosis and are ovulated at metaphase II stage in each estrous cycle until females go into menopause. The progression from germinal vesicle (GV) stage to metaphase II stage is so‐called oocyte maturation. During oocyte maturation, oocytes conduct germinal vesicle breakdown, segregate homologous chromosomes, and emit the first polar body, and arrest again at metaphase II. During this arrest, oocytes are ovulated and fertilized with spermatozoa. The fertilized oocytes finally complete meiosis by segregating sister chromatids and emitting the second polar body. Hence, in mammalian ovary, majority of oocytes remains arrested at dictyate stage for months, years, or decades depending on species' reproductive lifespans.
Figure 1.
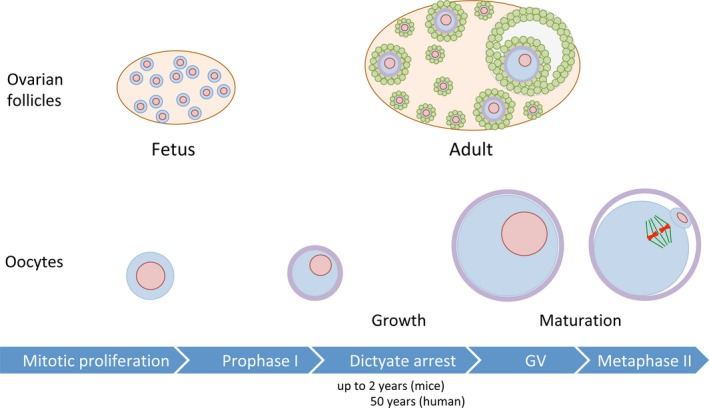
Oogenesis in mammals. Mammalian oocytes initiate meiosis following proliferation by mitotic cell division in fetal ovary. The oocytes conduct synapsis and recombination of homologous chromosomes, progress to diplotene stage at which the first meiotic arrest is imposed. During this arrest, chromatin is decondensed and diffusely distributed unlike male meiosis, and this female meiosis‐specific stage is also called dictyate stage. After birth, some of oocytes start to grow with the development of the surrounding follicles in an estrous cycle dependent manner. Prior to ovulation, oocytes conduct maturation by progressing from GV stage to metaphase II, at which fertilization occurs
The rate of aneuploidy of fertilized eggs in human has been reported to be 10%‐35% from 1990s studies and 30%‐70% in more recent studies, which is much higher than those in other species, for example, 1%‐2% in mice.5, 6 In human, the incidence of some birth defects or congenital disorders increases as the age of pregnant female increases.5 Although chromosome errors can occur in both male and female gametogenesis, paternal age does not contribute to age‐related increase in aneuploidy because cells having chromosome errors are mostly eliminated by cell cycle checkpoint mechanisms regardless of paternal age.7 Thus, the age‐related increase of aneuploidy in fertilized eggs is caused by the increase in oocyte aneuploidy. Since oocytes, unlike spermatogenesis, undergo meiosis at puberty and arrest at meiotic prophase I for long period, it has been thought that deterioration of some key molecules during the prolonged prophase I leads to chromosome segregation errors in aged oocytes. Recent studies suggest that cohesin, a glue protein complex for sister chromatid cohesion, is the most likely candidate for the key molecule.
2. SISTER CHROMATID COHESION MEDIATED BY COHESIN
2.1. Sister chromatid cohesion in somatic cells
In eukaryotes, sister chromatid cohesion must be maintained from S phase to the onset of anaphase for the accurate transmission of genome from mother cells to daughter cells. Cohesin, a multi‐subunit protein complex well conserved among eukaryotes, mediates sister chromatid cohesion.8, 9, 10 The cohesin complex consists of two structural maintenance of chromosome proteins, SMC1α and SMC3, and two non‐SMC proteins, either one of SA1 and SA2, and RAD21 (also called SCC1) (Table 1). Cohesin forms a tripartite ring‐like structure that is composed of V‐shaped SMC1α‐SMC3 heterodimer and RAD21 connecting the heads of the V‐shaped dimer.11 It is believed that cohesin binds sister chromatids by topologically embracing them in its ring‐like structure.12 In the mitotic cell cycle, cohesin is loaded on chromatin through SCC2 (NIPBL)‐SCC4 (MAU2) cohesin loader before DNA replication.13 The function to mediate sister chromatid cohesion is exerted by cohesin which has been recruited at or before DNA replication in mitotic cell cycle.14, 15 Establishment of sister chromatid cohesion by cohesin requires acetyl transferases, ESCO1 and ESCO2, which acetylates lysine residues of SMC3.16, 17 Although cohesin can bind to DNA in an ATPase‐independent manner, topological binding of cohesin to DNA requires ATP hydrolysis by SMC subunits.12 For the entrapment of DNA in cohesin ring, the ring must be opened. The ring opening occurs in two postulated ways: SMC1α‐SMC3 interphase or SMC3‐RAD21 interphase.18 The latter is known to function as “exit gate”. WAPL‐PDS5 open the SMC3‐SCC1 gate and is required for the release of cohesin from chromosomes which promotes sister chromatid resolution prior to anaphase.19 Sororin competes with WAPL for binding to PDS5 and antagonizes WAPL, thereby stabilizing cohesin on chromatin.20, 21 Sororin only binds to cohesin only after SMC3 is acetylated.21
Table 1.
Subunits and accessary proteins of cohesin complex in mammals
| Type | Mitotic (ubiquitous) | Meiosis‐specific | |
|---|---|---|---|
| Cohesin subunits | SMC |
SMC1α SMC3 |
SMC1β |
| Kleisin | RAD21 | REC8 or RAD21L | |
| HEAT repeat | STAG1/SA1 or STAG2/SA2 | STAG3/SA3 | |
| Cohesin regulators | Loading |
SCC2/NIPBL SCC4/MAU2 |
|
| Stabilization and/or removal |
ESCO1, ESCO2 PDS5A, PDS5B SORORIN WAPL |
2.2. Sister chromatid separation at anaphase
In prophase, most of cohesins dissociate from chromosome arms by so‐called prophase pathway which is dependent on WAPL‐PDS5 as well as phosphorylation of SA subunits.22 Then, after nuclear envelope disassembly, condensed chromosomes start to align at spindle equator by capturing spindle microtubules at kinetochores. Sister kinetochores attach to the microtubules extended from the opposite poles of spindle, thereby establishing the bipolar attachment. When all the kinetochores establish the bipolar attachment, spindle assembly checkpoint permits sister chromatids to separate in anaphase by the activation of anaphase‐promoting complex/cyclosome (APC/C).23 The activation of APC/C involves the association of its activator CDC20. APC/CCDC20 ubiquitinates its target proteins, cyclin B and securin, thereby inducing degradation of them by proteasome.24, 25 The degradation, in turn, brings about activation of separase, which cleaves a kleisin subunit RAD21.26 This induces the irreversible opening of cohesin ring, which brings about sister chromatid separation.
3. ESTABLISHMENT OF LINKAGE BETWEEN HOMOLOGOUS CHROMOSOMES AT PROPHASE I
3.1. Synapsis and recombination of homologous chromosomes
For proper segregation of chromosomes in meiosis, homologous chromosomes must be linked with their partners in prophase I. Meiotic recombination (crossover recombination) contributes not only to increasing the genetic diversity in gametes but also to creating the linkage between homologous chromosomes. Recombination initiates by a programed DNA double‐strand break (DSB) with Spo11.27, 28 The DSBs are repaired through several intermediates such as extended D‐loop and double Holliday junction and finally resolved into crossovers and noncrossovers.29 In parallel with the recombination process, homologous chromosomes are juxtaposed and connected with each other by the assembly of the synaptonemal complex (SC).30 At leptotene stage, an axial element (AE) is formed on each chromosome, then the AEs of homologous chromosomes start to be connected by transverse filaments at zygotene stage. Homologous chromosomes are fully connected along the entire length by the assembly of the SC by pachytene stage. The SC assembly is required for proper formation of crossover recombination.31 The connection mediated by SC is only temporal from the assembly of SC at zygotene until the disassembly of the SC at diplotene stage. At later meiotic stages, the connection between homologous chromosomes is maintained by crossover recombination‐mediated linkage. Therefore, crossover formation is tightly regulated to assure accurate chromosome segregation in meiosis I. Firstly, every pair of homologous chromosomes receives at least one crossover, which is called an obligate crossover. Secondly, if multiple crossovers occur, one does not tend to be close to the others, which is so‐called crossover interference. Thirdly, when DSB formation is altered, crossover levels are maintained presumably at the expense of noncrossover.29, 32
3.2. Meiotic cohesins
After the first discovery of Rec8 as a meiotic counterpart of Rad21 in yeast,33, 34 several kinds of meiosis‐specific subunit have been found in various eukaryotes.35 In mammals, four meiosis‐specific subunits, REC8,36, 37 RAD21L,38, 39, 40 SMC1β,41 and STAG3,42 have been found so far and all the meiosis‐specific subunits as well as mitotic cohesin subunits43, 44, 45 are localized on the synaptonemal complex (Table 1).46, 47 Genetic studies using knockout mice have revealed that all the meiosis‐specific cohesin subunits are required for the process of establishing the linkage between homologous chromosomes, such as axial element formation, synapsis, and recombination of homologous chromosome, although different phenotypes have been observed depending on the subunit.48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58 For example, absence of either one of REC8,48, 50 RAD21L,53 or SMC1β,49, 52 causes the partial defects of axial element formation, whereas absence of STAG3 abolishes axial element formation.55, 56 Furthermore, it has been suggested that RAD21L‐containing cohesin may function for the establishment of linkage between homologs whereas REC8‐containing cohesin may function for both homologous chromosome linkage and sister chromatid cohesion.47, 57, 59 The separation of roles by different cohesins in meiosis might be conserved among species.60 Once the crossover recombination between homologous chromosomes has been established, a DNA molecule of one homologous chromosome is interlinked to a DNA molecule of another homologous chromosome. As a result, crossover recombination converts the sister chromatid cohesion distal to chiasma (the position of crossover recombination) into the non‐sister chromatid cohesion between homologous chromosomes (Figure 2). Thus, the linkage between homologous chromosomes is maintained by the function of cohesin to mediate sister chromatid cohesion with the aid of crossover recombination.
Figure 2.
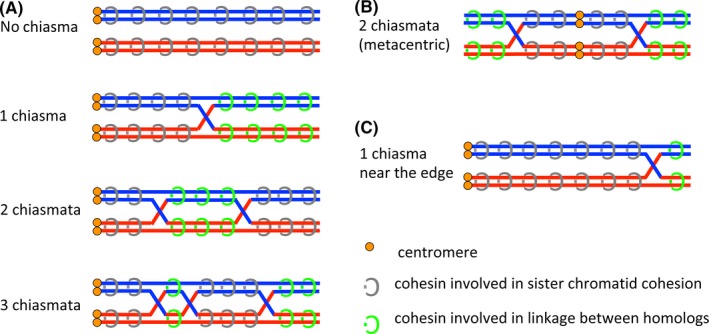
Linkage between homologous chromosomes through chiasma and cohesion. A, Pairs of homologous telocentric chromosomes are described. When no chiasma is present, all cohesins on the chromosomes mediate sister chromatid cohesion. Once a chiasma is formed, cohesins localizing on chromosome arms distal to chiasma (in relation to centromeres) become involved in linkage between homologous chromosomes. When two chiasmata are formed, only the cohesins localizing on chromosome arms between two chiasmata become responsible for the homologous chromosomes linkage. When three chiasmata are formed, the cohesin localizing both between the first and second chiasmata and distal to the third chiasma become responsible for the homologous chromosomes linkage. B, A pair of homologous metacentric chromosomes is described. When two chiasmata are formed on both sides of chromosome arms in relation to centromeres, cohesins localizing distal to chiasmata are responsible for linkage between homologous chromosomes. C, When a chiasma is formed near the edge of telocentric chromosomes, the cohesins involved in linkage between homologous chromosomes are fewer than when a chiasma is formed at the middle of chromosome arms as described in A
4. CHROMOSOME SEPARATION DURING MEIOSIS
In order to segregate homologous chromosomes in meiosis I, sister kinetochores on a univalent chromosome must be attached to the spindle microtubules emanated from the same spindle pole. This is so‐called monopolar attachment requiring centromeric cohesion as well as meiosis‐specific kinetochore protein MEIKIN.35, 61 When all the kinetochores establish the monopolar attachment and bivalent chromosomes align on the spindle equator, APC/C is activated by the binding of CDC20.62 The APC/CCDC20 induces the degradation of cyclin B and securin by ubiquitinating them, which in turn activates separase.63, 64, 65, 66 The activated separase cleaves REC8,67, 68, 69 thereby removing cohesin from chromosome arms while centromeric cohesin is protected by shugoshin, SGO2 in mammals.70, 71 Since the linkage between homologous chromosomes is maintained by sister chromatid cohesion distal to a chiasma, cohesin release from chromosome arm region induces homologous chromosome separation.
5. CHROMOSOME SEGREGATION ERRORS IN MEIOSIS
Chromosome segregation errors in meiosis occur in some different ways, namely nondisjunction, premature separation of sister chromatids, and reverse segregation (Figure 3). Nondisjunction occurs when chromosome segregation fails in meiosis I or meiosis II probably due to the faulty attachment of chromosomes to spindle microtubules such as merotelic attachment instead of amphitelic attachment.72 However, recent studies examining aneuploidy in oocytes by counting the number of chromosomes in the first polar body suggest that errors caused by premature separation of sister chromatids are more common than those caused by nondisjunction in meiosis I.72, 73, 74 Premature sister chromatid cohesion in meiosis I involves untimely dissociation of cohesin from chromosomes or deterioration of cohesin. Reverse segregation occurs when sister chromatids, but not homologous chromosomes, segregate in meiosis I.72 As a result, a correct number of chromosomes are distributed to the secondary oocyte and the first polar body. But, in meiosis II, chromosomes cannot segregate correctly due to the lack of centromeric cohesion, which should be maintained in normal metaphase II oocytes until fertilization. Although it has been considered that differences in cell size, chromosome geometry, spindle assembly, and cell cycle control between oocytes and somatic cells render oocytes so prone to segregation errors,75, 76, 77 I limit the discussion to chromosome cohesion in this review.
Figure 3.
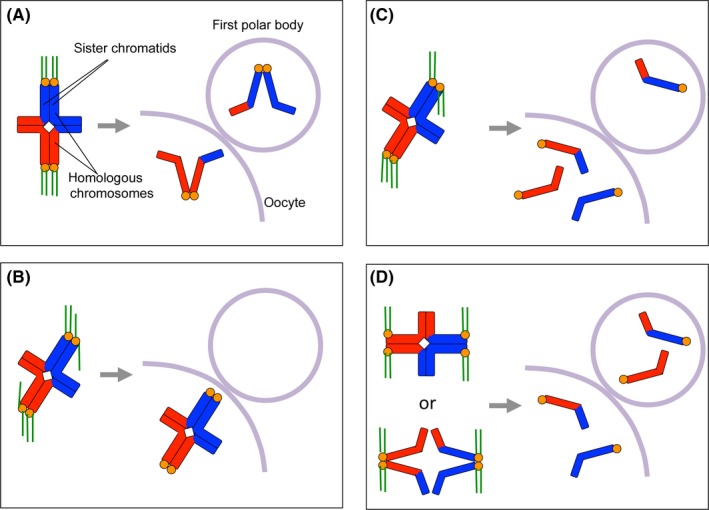
Chromosome segregation and its possible errors in meiosis I. A, In meiosis I, two sister kinetochores on a univalent establish the monopolar attachment to the spindle microtubules so that homologous chromosomes can separate in anaphase I by the dissolution of chromatid arm cohesion. B, When two sister kinetochores on a univalent cannot establish the monopolar attachment, homologs cannot separate in anaphase I, resulting in nondisjunction of homologous chromosomes. C, When two sister kinetochores on a univalent attach to microtubules in a half‐inverted manner instead of monopolar attachment, some of sister chromatids separate precociously in anaphase I. As a result, the secondary oocyte receives an extra number or lesser number of chromatids. D, When centromeric cohesion is precociously lost, sister kinetochores no longer behave as a single unit, resulting in the establishment of bipolar attachment. When chiasma is dissolved by premature loss of arm cohesion, sister kinetochores tend to establish bipolar attachment. In both case, correct number of chromatids might be distributed into the secondary oocyte and the first polar body after anaphase I. However, in meiosis II, chromosome separation does not occur accurately due to the lack of sister chromatid cohesion
6. COHESIN DETERIORATION IN AGED OOCYTES DUE TO THE ABSENCE OF TURNOVER AT PROPHASE I ARREST
6.1. Decrease of chromosome‐associated cohesin in aged oocytes
In mitotically proliferating cells, cohesin that associates with DNA at or prior to S phase can mediate sister chromatid cohesion. If this is applicable to meiosis, only the cohesin expressed in the oocytes in fetal ovary can contribute to meiotic sister chromatid cohesion since mammalian oocytes undergo premeiotic DNA replication at fetal stage. Considering that mammalian oocytes arrest at dictyate stage for a long time up to decades in some species, one can imagine that cohesin might decrease during the arrest. In fact, as has been reported previously,78, 79, 80 REC8 is hardly detected on bivalent chromosomes at metaphase I in aged oocytes from 13‐month‐old mice whereas it is clearly observed in young oocytes from 3‐week‐old mice (Figure 4). Unlike cohesin, another chromosome‐associated SMC protein complex called condensin81 is detectable in both young and aged oocytes (Figure 4). Since substantial amount of condensin is recruited to chromosomes after nuclear envelope disassembly and functions in chromosome condensation and segregation in both mitosis and meiosis,82, 83, 84 prolonged arrest at dictyate stage may not affect the amount of chromosome‐associated condensin in the aged oocytes. It has also been reported that chromosome‐associated REC8 decreases gradually from 3 months old to 9 months old whereas thereafter REC8 level cannot be measured accurately due to the limitation of the detection or quantification ability.79 From the data, half‐life of the chromosome‐associated REC8 seems to be about 2 months during the period. Also, in human, it has been reported that cohesin decreases in aged oocytes, but the amount of reduction is much smaller than that reported in mouse oocytes: More than half amount of cohesin subunits, REC8 and SMC1β, are still retained in the oocytes derived from women aged 40 years and over compared with those aged around 20 years.85 In the human study, the signal intensity might be affected by chromatin‐unbound cohesin in the nucleus, since they estimated the relative cohesin levels by examining the signal intensity of cohesin within nucleus at dictyate stage in contrast to the study in mice using metaphase I‐stage oocytes. Another study examining the cohesin on chromosome spreads from human oocytes has reported that there are no evident differences in the intensity and distribution among oocytes derived from women aged between 18 and 34 years old.86 In summary, it is evident that chromosome‐associated cohesin decreases in oocytes as females get older in mice, but absolute evidence concerning this issue has not been obtained in human.
Figure 4.
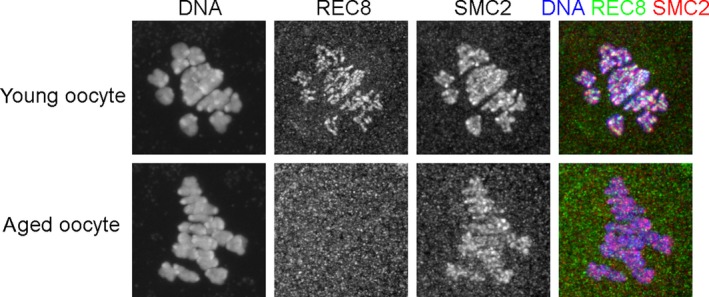
Chromosome‐associated cohesin is decreased in mouse oocytes with advancing age. GV stage oocytes derived from 3‐week‐old or 13‐month‐old mice were cultured for 6 hr in vitro. After culture, the oocytes progressed to metaphase I and were fixed and immunofluorescently labeled with anti‐REC8 antibody and anti‐SMC2 antibody. DNA was counterstained with DAPI. SMC2, one of the subunits of condensin, are detected on chromosomes in both young and aged oocytes. In contrast, REC8 is hardly detected on chromosomes in the aged oocytes although it is clearly detectable in young oocytes
6.2. No cohesin turnover during the dictyate arrest in mouse oocytes
In general, the amount of proteins is determined by rate of synthesis and degradation. Synthesis of cohesin subunits seems to continue in the oocytes after birth because Rec8 mRNA was detected in the ovarian oocytes in adult mice.87 Indeed, immunoblot analysis reveals that the total amount of the proteins is not different between young and aged oocytes.79 So, it is thought that decrease of chromosome‐associated cohesin would be caused by deterioration of cohesin without turnover during the meiotic arrest. To test this hypothesis, conditional knockout or activation strategies have been utilized in mice. A study using REC8TEV/TEV mice, which expresses artificially‐cleavable REC8 by TEV protease, show that injection of TEV protease into metaphase I oocytes induces the premature sister chromatid separation. But, in the oocytes derived from the REC8TEV/TEV mice, the expression of ectopic REC8‐Myc activated by Sox9‐Cre transgene prior to premeiotic DNA replication prevents the TEV protease‐mediated premature sister chromatid separation while the expression of REC8‐Myc activated by Zp3‐Cre transgene during dictyate arrest at the onset of oocyte growth does not rescue the premature sister chromatid separation.88 This suggests that REC8 can establish and maintain sister chromatid cohesion when it is expressed only prior to premeiotic S phase but not when expressed in later meiotic stages. Furthermore, a recent study using drug‐inducible Cre system also supports this notion.89 In addition, inactivation of Smc1β gene shortly after birth at dictyate arrest in oocytes does not affect chiasma positions and sister chromatid cohesion90 whereas conventional knockout of Smc1β does.49 These studies strongly suggest that only the meiotic cohesin expressed before or during premeiotic S phase can establish and maintain sister chromatid cohesion and that there is little or no cohesin turnover during the meiotic arrest at prophase I and thereafter.
6.3. Types of cohesin whose deterioration is potentially involved in age‐related increase of aneuploidy
Supposing that decrease in cohesin causes the age‐related increase of aneuploidy, what types of cohesin relevant to this issue? Each of cohesin subunits except SMC3 has multiple variants including meiosis‐specific one (Table 1). Among three kleisin subunits, RAD21L is expressed mainly in the early stages of prophase I and hardly detected at later meiotic stages.38, 39 Thus, it is thought that RAD21L cannot maintain the sister chromatid cohesion during dictyate arrest. RAD21 does not contribute to sister chromatid cohesion in meiosis since it has been demonstrated that RAD21 cleavage by TEV protease triggers sister chromatid separation in the first embryonic mitosis but not homologous chromosome separation during meiosis.88 Therefore, neither RAD21L nor RAD21 are involved in the age‐related increase of aneuploidy. In contrast, REC8 localizes to the connection sites between chromatids until metaphase II66 and is proved to induce precocious sister chromatid separation in meiosis I when it is cleaved by TEV system.88 Moreover, chromosome‐associated REC8 is indeed decreased in aged oocytes79, 80 (Figure 4). So, among three kleisin subunits, REC8 is the only subunit whose deterioration would be able to cause an increase of chromosome segregation errors in aged oocytes. Among three SA subunits, only STAG3 has been shown to remain at the connection sites of chromatids at least until metaphase I.42 Furthermore, co‐expression of STAG3 with REC8 in somatic cells enables REC8 to enter the nucleus and functionally replace its mitotic counterpart RAD21 and can rescue precocious sister chromatid separation induced by overexpression of hyperactive separase or knockdown of Sgo1 in somatic cells, suggesting that REC8‐STAG3 cohesin can mediate sister chromatid cohesion.91 So, STAG3 is the most likely candidate. Among two SMC1 subunits, SMC1β but not SMC1α localizes on chromosomes after diplotene41 and is associated with REC8 in testis extracts of wild‐type mice.37 However, since SMC1α can substitute for many SMC1β functions,92 SMC1α might mediate sister chromatid cohesion after diplotene if SMC1β is reduced during dictyate arrest. In summary, REC8‐STAG3‐SMC3‐SMC1β (also SMC1α if present after diplotene stage) is likely to be responsible for the maintenance of sister chromatid cohesion at dictyate arrest and thereafter. Thus, its decrease possibly leads to age‐related increase of chromosome segregation errors in oocytes.
6.4. Possible mechanism to reduce chromosome‐associated cohesin in dictyate‐arrested oocytes
How is chromosome‐associated cohesin decreased during meiotic arrest of oocytes? Recent studies have reported the expression of cohesin regulators in meiocytes. NIPBL, a cohesin loading factor, is associated with the AEs of the SC from leptotene to diplotene stage but hardly detected at dictyate stage in mouse oocytes.93, 94 These reports imply that newly synthesized cohesin may be loaded to meiotic chromosomes until diplotene stage but not during dictyate arrest, supporting the hypothesis of no cohesin turnover during dictyate arrest. Human Wapl mRNA and mouse WAPL protein, a removing factor of cohesin, is highly expressed in testis and is localized on AEs the SC in zygotene and pachytene spermatocytes95 and also localized on the SC in pachytene oocytes.96 NIMA‐like kinase‐1 (NEK1) phosphorylates PP1, leading to the dephosphorylation of WAPL, which in turn results in its retention on chromosome cores to promote loss of cohesion at the end of prophase I.97 These studies suggest that WAPL functions in removal of cohesin also in meiosis. Sororin, a stabilizing factor of cohesin, is localized to the central region of the SC interestingly in a synapsis‐dependent manner, but a meiotic cohesin‐independent manner at zygotene and pachytene stages, then accumulate at centromere by late prophase I, and remains there until anaphase II in mouse spermatocytes.98, 99 PDS5B, a cohesin regulator associating alternatively with WAPL or sororin, starts to be detected on the AEs at zygotene stage, culminates at pachytene stage, and is diminished at diplotene stage in mouse spermatocytes.100 Immunoprecipitation analyses reveal that PDS5B is associated with SYCP2 and cohesin subunits such as SMC1β and REC8.100 Furthermore, ectopic expression of meiosis‐specific cohesin subunits reveals that REC8‐STAG3 cohesin physically interacts with PDS5, WAPL, and sororin and that REC8‐STAG3 cohesin is shown to be susceptible to WAPL‐dependent removal and sororin‐mediated protection.91 Overall, WAPL, sororin, and PDS5B are involved in regulation of meiotic cohesin. Thus, untimely activation of WAPL and/or inactivation of sororin might cause the reduction of chromosome‐associated cohesin during dictyate arrest in oocytes I deleted the above sentences according to the suggestion of the reviewer. Since SGO2, a centromeric cohesin protector, is reduced in aged oocytes, the reduction may amplify the cohesin loss during dictyate arrest.80
Little is known about phosphorylation of SA subunits in meiosis except that RAD21L‐associated STAG3 (SA3) is detected as phosphorylated form.38 Whether the phosphorylation of STAG3 associating with REC8 occurs during dictyate arrest should be tested in future studies. Besides above regulations, cohesin can be removed from chromosomes by separase‐dependent cleavage of a kleisin subunit. However, considering the cell cycle stage of meiotic arrest of oocytes, separase‐dependent pathway is not likely unless precocious activation of separase occurs. This is supported by the report that spindle checkpoint function is not impaired in aged oocytes.80
7. IS AGE‐RELATED INCREASE IN CHROMOSOME SEGREGATION ERRORS CAUSED BY COHESIN DETERIORATION?
7.1. Mouse oocytes
It is evident that cohesin deterioration occurs in aged mouse oocytes due to the lack of turnover during dictyate arrest. It is also shown experimentally that total loss of cohesin in metaphase I leads to precocious sister chromatid separation.88 Thus, it seems reasonable to conclude that deterioration of cohesin is one of the major causes for the increase of chromosome segregation errors in aged mouse oocytes. Then, is there any room for argument on this point? In this regard, it must be noted that there is no evidence to show the exact correlation between cohesin decrease and increase of chromosome segregation errors during aging of oocytes. Chromosome‐associated REC8 in mouse oocytes gradually decreases and reaches the bottom level at 9 months old while chromosome segregation errors increase abruptly at 15‐month‐old.79 So, there is a discrepancy in the timing. This discrepancy may be accounted for by the hypothesis that there is a threshold of cohesin level below which sister chromatid cohesion cannot be maintained and that cohesin continues to decrease to the threshold level below the detection limit of the current analysis. But, the hypothesis seems unsound in that a result may be able to be interpreted in two contrary ways. For example, the REC8 staining in an aged oocyte in Figure 4 would be regarded as no or little cohesin when you want to insist the age‐dependent deterioration of cohesin, whereas it would be regarded as the presence of a very small amount of cohesin under detection limit when you want to insist the maintenance of sister chromatid cohesion by the invisible cohesin. Furthermore, there are some issues to consider about threshold; for example, which region of chromosome cohesin dissociates from, and whether functional loss of cohesin occurs. As shown in Figure 2, to maintain the link between homologous chromosomes, the cohesin localizing distal to chiasma should remain functional. So, even if net amount of chromosome‐associated cohesin might remain at a sufficient level in oocytes, the loss of the cohesin functioning for homologous chromosome link (green‐colored cohesin in Figure 2) would evoke chromosome segregation error. We need to know the local threshold level of meiotic cohesin to show a clear correlation between cohesin decrease and increase of chromosome segregation errors during aging of oocytes.
7.2. Human oocytes
As mentioned above, there is no conclusive evidence showing that chromosome‐associated cohesin is decreased in human aged oocytes. Moreover, whether turnover of cohesin occurs or not during dictyate arrest cannot be tested experimentally in human oocytes. Therefore, there are not much data to decide this issue. Comparing human oocytes with mouse oocytes, the timing of increase of chromosome segregation errors is different: The incidence of chromosome segregation errors in oocytes increases over 1 year old in mice while it does over 35 years old in human. What makes the timing so different between these two species is unknown. If cohesin does not turnover during dictyate arrest in human oocytes as seen in mice, meiotic cohesin in human oocytes should be extremely long‐lived proteins. In general, proteins turnover within a few days but a few such as histones, nuclear pore complex proteins, show remarkable stability: The half‐life of histone is ~200 days and that of scaffold proteins of nuclear pore is more than 1 year.101, 102 In addition, the half‐life of histones within cells is estimated to be 117 days in liver and 223 days in brain.101 Thus, half‐life of a protein seems to be dependent on both its property and the surrounding environment. In this context, meiotic cohesin should have its own property of longevity, and human oocytes may provide much better condition for the preservation of long‐life proteins than mouse oocytes if the hypothesis of no cohesin turnover is applied to human oocytes.
8. CONCLUSION
In brief, accumulating evidence in mouse oocytes suggests that deterioration of cohesin is one of the major causes for the age‐related increase in chromosome segregation errors, but we still cannot rule out the possibility that deterioration of molecules other than cohesin or mechanisms might trigger the final switch to chromosome segregation errors in aged oocytes even in mice. CENP‐A, a centromere‐specific histone H3 variant, is shown to persist more than 1 year without detectable turnover in mouse oocytes.103 Therefore, reduction of such long‐lived proteins in combination with cohesin deterioration during oocyte aging might give a deleterious effect on chromosome segregation. In Drosophila oocytes, loading of newly synthesized cohesin by Nipped‐B and establishment of new cohesive linkage by Eco during prophase I after premeiotic S phase is required for maintaining cohesion although the rejuvenation might be needed at earlier stages than the stage at which mammalian oocytes are arrested.104 Further studies examining the cohesin turnover in other species are needed to understand the underlying mechanism of age‐related aneuploidy in human and other mammalian oocytes.
DISCLOSURES
Conflict of interest: Jibak Lee declares that he has no conflict of interest.
Human Rights: This article does not contain any studies with human subjects performed by any of the authors.
Animal studies: This study was approved by the Institutional Animal Care and Use Committee (Permission number: R1‐27‐03‐01) and carried out according to the Kobe University Animal Experimentation Regulations.
Lee J. Is age‐related increase of chromosome segregation errors in mammalian oocytes caused by cohesin deterioration? Reprod Med Biol. 2020;19:32–41. 10.1002/rmb2.12299
Funding information
This work was supported in part by a JSPS KAKENHI grant (18H02353) to JL
REFERENCES
- 1. Miyazaki WY, Orr‐Weaver TL. Sister‐chromatid cohesion in mitosis and meiosis. Annu Rev Genet. 1994;28:167‐187. [DOI] [PubMed] [Google Scholar]
- 2. Bolcun‐Filas E, Handel MA. Meiosis: the chromosomal foundation of reproduction. Biol Reprod. 2018;99:112‐126. [DOI] [PubMed] [Google Scholar]
- 3. Handel MA, Eppig JJ. Sexual dimorphism in the regulation of mammalian meiosis. Curr Top Dev Biol. 1998;37:333‐358. [DOI] [PubMed] [Google Scholar]
- 4. Morelli MA, Cohen PE. Not all germ cells are created equal: Aspects of sexual dimorphism in mammalian meiosis. Reproduction. 2005;130:761‐781. [DOI] [PubMed] [Google Scholar]
- 5. Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genetics. 2001;2:280‐291. [DOI] [PubMed] [Google Scholar]
- 6. Nagaoka S, Hassold TJ, Hunt PA. Human aneuploidy: mechanisms and new insights into an age‐old problem. Nat Rev Genet. 2012;13:493‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vrooman LA, Nagaoka SI, Hassold TJ, Hunt PA. Evidence for paternal age‐related alterations in meiotic chromosome dynamics in the mouse. Genetics. 2014;196:385‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peters JM, Tedeschi A, Schmitz J. The cohesin complex and its roles in chromosome biology. Genes Dev. 2008;22:3089‐3114. [DOI] [PubMed] [Google Scholar]
- 9. Nasmyth K, Haering CH. Cohesin: its roles and mechanisms. Annu Rev Genet. 2009;43:525‐558. [DOI] [PubMed] [Google Scholar]
- 10. Peters JM, Nishiyama T. Sister chromatid cohesion. Cold Spring Harb Perspect Biol. 2012;4:a011130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gruber S, Haering CH, Nasmyth K. Chromosomal cohesin forms a ring. Cell. 2003;112:765‐777. [DOI] [PubMed] [Google Scholar]
- 12. Murayama Y, Uhlmann F. Biochemical reconstitution of topological DNA binding by the cohesin ring. Nature. 2014;505:367‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ciosk R, Shirayama M, Schevchenko A, et al. Cohesin's binding to chromosomes depends on a separate complex consisting of Scc2 and Scc4 proteins. Mol Cell. 2000;5:243‐254. [DOI] [PubMed] [Google Scholar]
- 14. Uhlmann F, Nasmyth K. Cohesion between sister chromatids must be established during DNA replication. Curr Biol. 1998;8:1095‐1101. [DOI] [PubMed] [Google Scholar]
- 15. Rhodes J, Haarhuis J, Grimm JB, Rowland BD, Lavis LD, Nasmyth KA. Cohesin can remain associated with chromosomes during DNA replication. Cell Rep. 2017;20:2749‐2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hou F, Zou H. Two human orthologues of Eco1/Ctf7 acetyltransferases are both required for proper sister‐chromatid cohesion. Mol Biol Cell. 2005;16:3908‐3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rolef Ben‐Shahar T, Heeger S, Lehane C, et al. Eco1‐dependent cohesin acetylation during establishment of sister chromatid cohesion in both human and yeast. Mol Cell. 2008;31:143‐151. [DOI] [PubMed] [Google Scholar]
- 18. Nasmyth K. Cohesin: a catenase with separate entry and exit gates? Nat Cell Biol. 2011;13:1170‐1177. [DOI] [PubMed] [Google Scholar]
- 19. Shintomi K, Hirano T. Releasing cohein from chromosome arms in early mitosis: opposing actions of Wapl‐Pds5 and Sgo1. Genes & Dev. 2009;23:2224‐2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schmitz J, Watrin E, Lenart P, Mechtler K, Peters JM. Sororin is required for stable binding of cohesin to chromatin and for sister chromatid cohesion in interphase. Curr Biol. 2007;17:630‐636. [DOI] [PubMed] [Google Scholar]
- 21. Nishiyama T, Ladurner R, Schmitz J, et al. Sororin mediates sister chromatid cohesion by antagonizing Wapl. Cell. 2010;143:737‐749. [DOI] [PubMed] [Google Scholar]
- 22. Waizenegger IC, Hauf S, Meinke A, Peters JM. Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell. 2000;103:399‐410. [DOI] [PubMed] [Google Scholar]
- 23. Izawa D, Pines J. Mad2 and APC/C compete for the same site on Cdc20 to ensure proper chromosome segregation. J Cell Biol. 2012;199:27‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132‐138. [DOI] [PubMed] [Google Scholar]
- 25. Yu H. Cdc20: A WD40 activator for a cell cycle degradation machine. Mol Cell. 2007;27:3‐16. [DOI] [PubMed] [Google Scholar]
- 26. Uhlmann F, Wernic D, Poupart MA, Koonin EV, Nasmyth K. Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell. 2000;103:375‐386. [DOI] [PubMed] [Google Scholar]
- 27. Keeney S. Mechanism and control of meiotic recombination initiation. Curr Top Dev Biol. 2001;52:1‐53. [DOI] [PubMed] [Google Scholar]
- 28. Kohl KP, Sekelsky J. Meiotic and mitotic recombination in meiosis. Genetics. 2013;194:327‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gray S, Cohen PE. Control of meiotic crossovers: from double‐strand break formation to designation. Annu Rev Genet. 2016;50:175‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Page SL, Hawley RS. The genetics and molecular biology of the synaptonemal complex. Annu Rev Cell Dev Biol. 2004;20:525‐558. [DOI] [PubMed] [Google Scholar]
- 31. de Vries FA, de Boer E, van den Bosch M, et al. Mouse Sycp1 functions in synaptonemal complex assembly, meiotic recombination, and XY body formation. Genes Dev. 2005;19:1376‐1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berchowitz LE, Copenhaver GP. Genetic interference: don't stand so close to me. Curr Genomics. 2010;11:91‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Watanabe Y, Nurse P. Cohesin Rec8 is required for reductional chromosome segregation at meiosis. Nature. 1999;29:461‐464. [DOI] [PubMed] [Google Scholar]
- 34. Klein F, Mahr P, Galova M, et al. A central role for cohesins in sister chromatid cohesion, formation of axial elements, and recombination during yeast meiosis. Cell. 1999;98:91‐103. [DOI] [PubMed] [Google Scholar]
- 35. Ishiguro K. The cohesin complex in mammalian meiosis. Genes Cells. 2019;24:6‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eijpe M, Offenberg H, Jessberger R, Revenkova E, Heyting C. Meiotic cohesin REC8 marks the axial elements of rat synaptonemal complexes before cohesins SMC1beta and SMC3. J Cell Biol. 2003;160:657‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee J, Iwai T, Yokota T, Yamashita M. Temporally and spatially selective loss of Rec8 protein from meiotic chromosomes during mammalian meiosis. J Cell Sci. 2003;116:2781‐2790. [DOI] [PubMed] [Google Scholar]
- 38. Lee J, Hirano T. RAD21L, a novel cohesin subunit implicated in linking homologous chromosomes in mammalian meiosis. J Cell Biol. 2011;192:263‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ishiguro K, Kim J, Fujiyama‐Nakamura S, Kato S, Watanabe Y. A new meiosis‐specific cohesin complex implicated in the cohesin code for homologous pairing. EMBO Rep. 2011;12:267‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gutiérrez‐Caballero C, Herrán Y, Sánchez‐Martín MS, et al. Identification and molecular characterization of the mammalian kleisin RAD21L. Cell Cycle. 2011;10:1477‐1487. [DOI] [PubMed] [Google Scholar]
- 41. Revenkova E, Eijpe M, Heyting C, Gross B, Jessberger R. Novel meiosis‐specific isoform of mammalian SMC1. Mol Cell Biol. 2001;21:6984‐6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Prieto I, Suja JA, Pezzi N, et al. Mammalian STAG3 is a cohesin specific to sister chromatid arms in meiosis I. Nat Cell Biol. 2001;3:761‐766. [DOI] [PubMed] [Google Scholar]
- 43. Eijpe M, Heyting C, Gross B, Jessberger R. Association of mammalian SMC1 and SMC3 proteins with meiotic chromosomes and synaptonemal complexes. J Cell Sci. 2000;113:673‐682. [DOI] [PubMed] [Google Scholar]
- 44. Prieto I, Pezzi N, Buesa J, et al. STAG2 and Rad21 mammalian mitotic cohesins are implicated in meiosis. EMBO Rep. 2002;3:543‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Parra MT, Viera A, Gómez R, et al. Involvement of the cohesin Rad21 and SCP3 in monopolar attachment of sister kinetochores during mouse meiosis I. J Cell Sci. 2004;117:1221‐1234. [DOI] [PubMed] [Google Scholar]
- 46. Suja JA, Barbero JL. Cohesin complexes and sister chromatid cohesion in mammalian meiosis. Genome Dyn. 2009;5:94‐116. [DOI] [PubMed] [Google Scholar]
- 47. Lee J. The regulation and function of cohesin and condensin in mammalian oocytes and spermatocytes. Results Probl Cell Differ. 2017;63:355‐372. [DOI] [PubMed] [Google Scholar]
- 48. Bannister LA, Reinholdt LG, Munroe RJ, Schimenti JC. Positional cloning and characterization of mouse mei8, a disrupted allelle of the meiotic cohesin Rec8. Genesis. 2004;40:184‐194. [DOI] [PubMed] [Google Scholar]
- 49. Revenkova E, Eijpe M, Heyting C, et al. Cohesin SMC1 beta is required for meiotic chromosome dynamics, sister chromatid cohesion and DNA recombination. Nat Cell Biol. 2004;6:555‐562. [DOI] [PubMed] [Google Scholar]
- 50. Xu H, Beasley MD, Warren WD, van der Horst GT, McKay MJ. Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev Cell. 2005;8:949‐961. [DOI] [PubMed] [Google Scholar]
- 51. Hodges CA, Revenkova E, Jessberger R, Hassold TJ, Hunt PA. SMC1beta‐deficient female mice provide evidence that cohesins are a missing link in age‐related nondisjunction. Nat Genet. 2005;37:1351‐1355. [DOI] [PubMed] [Google Scholar]
- 52. Novak I, Wang H, Revenkova E, Jessberger R, Scherthan H, Hoog C. Cohesin Smc1beta determines meiotic chromatin axis loop organization. J Cell Biol. 2008;180:83‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Herrán Y, Gutiérrez‐Caballero C, Sánchez‐Martín M, et al. The cohesin subunit RAD21L functions in meiotic synapsis and exhibits sexual dimorphism in fertility. EMBO J. 2011;30:3091‐3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Llano E, Herran Y, Garcia‐Tunon I, et al. Meiotic cohesin complexes are essential for the formation of the axial element in mice. J Cell Biol. 2012;197:877‐885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Winters T, McNicoll F, Jessberger R. Meiotic cohesin STAG3 is required for chromosome axis formation and sister chromatid cohesion. EMBO J. 2014;33:1256‐1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fukuda T, Fukuda N, Agostinho A, Hernández‐Hernández A, Kouznetsova A, Höög C. STAG3‐mediated stabilization of REC8 cohesin complexes promotes chromosome synapsis during meiosis. EMBO J. 2014;33:1243‐1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ishiguro K, Kim J, Shibuya H, et al. Meiosis‐specific cohesin mediates homolog recognition in mouse spermatocytes. Genes Dev. 2014;28:594‐607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Biswas U, Hempel K, Llano E, Pendas A, Jessberger R. Distinct roles of meiosis‐specific cohesin complexes in mammalian spermatogenesis. PLoS Genet. 2016;12:e1006389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rong M, Miyauchi S, Lee J. Ectopic expression of meiotic cohesin RAD21L promotes adjacency of homologous chromosomes in somatic cells. J Reprod Dev. 2017;63:227‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gyuricza MR, Manheimer KB, Apte V, et al. Dynamic and stable cohesins regulate synaptonemal complex assembly and chromosome segregation. Curr Biol. 2016;26:1688‐1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim J, Ishiguro K‐I, Nambu A, et al. Meikin is a conserved regulator of meiosis‐I‐specific kinetochore function. Nature. 2015;512:466‐471. [DOI] [PubMed] [Google Scholar]
- 62. Jin F, Hamada M, Malureanu L, et al. Cdc20 is critical for meiosis I and fertility of female mice. PLoS Genet. 2010;6:e1001147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Herbert M, Levasseur M, Homer H, Yallop K, Murdoch A, McDougall A. Homologue disjunction in mouse oocytes requires proteolysis of securin and cyclin B1. Nat Cell Biol. 2003;5:1023‐1025. [DOI] [PubMed] [Google Scholar]
- 64. Homer HA, McDougall A, Levasseur M, Yallop K, Murdoch AP, Herbert M. Mad2 prevents aneuploidy and premature proteolysis of cyclin B and securin during meiosis I in mouse coocytes. Genes Dev. 2005;19:202‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Amanai M, Shoji S, Yoshida N, Brahmajosyula M, Perry AC. Injection of mammalian metaphase II oocytes with short interfering RNAs to dissect meiotic and early mitotic events. Biol Reprod. 2006;75:891‐898. [DOI] [PubMed] [Google Scholar]
- 66. Lee J, Okada K, Ogushi S, Miyano T, Miyake M, Yamashita M. Loss of Rec8 from chromosome arm and centromere region is required for homologous chromosome separation and sister chromatid separation, respectively, in mammalian meiosis. Cell Cycle. 2006;5:1448‐1455. [DOI] [PubMed] [Google Scholar]
- 67. Terret ME, Wassmann K, Waizenegger I, Maro B, Peters JM, Verlhac MH. The meiosis I‐to‐meiosis II transition in mouse oocytes requires separase activity. Curr Biol. 2003;13:1797‐1802. [DOI] [PubMed] [Google Scholar]
- 68. Kudo NR, Wassmann K, Anger M, et al. Resolution of chiasmata in oocytes requires separase‐mediated proteolysis. Cell. 2006;126:135‐146. [DOI] [PubMed] [Google Scholar]
- 69. Kudo NR, Anger M, Peters A, et al. Role of cleavage by separase of the Rec8 kleisin subunit of cohesin during mammalian meiosis I. J Cell Sci. 2009;122:2686‐2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lee J, Kitajima TS, Tanno Y, et al. Unified mode of centromeric protection by shugoshin in mammalian oocytes and somatic cells. Nat Cell Biol. 2008;10:42‐52. [DOI] [PubMed] [Google Scholar]
- 71. Llano E, Gómez R, Gutiérrez‐Caballero C, et al. Shugoshin‐2 is essential for the completion of meiosis but not for mitotic cell division in mice. Genes Dev. 2008;22:2400‐2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Webster A, Schuh M. Mechanisms of aneuploidy in human eggs. Trends Cell Biol. 2017;27:55‐68. [DOI] [PubMed] [Google Scholar]
- 73. Pellestor F, Andréo B, Arnal F, Humeau C, Demalle J. Maternal aging and chromosomal abnormalities: new data drawn from in vitro unfertilized human oocytes. Hum Genet. 2003;112:195‐203. [DOI] [PubMed] [Google Scholar]
- 74. Kuliev A, Zlatopolsky Z, Kirillova I, Spivakova J, Janzen JC. Meiosis errors in over 20,000 oocytes studied in the practice of preimplantation aneuploidy testing. Reprod BioMed Online. 2011;22:2‐8. [DOI] [PubMed] [Google Scholar]
- 75. Kitajima T, Ohsugi M, Ellenberg J. Complete kinetochore tracking reveals error‐prone homologous chromosome biorientation in mammalian oocytes. Cell. 2011;146:568‐581. [DOI] [PubMed] [Google Scholar]
- 76. Kyogoku H, Kitajima T. Large cytoplasm is linked to the error‐prone nature of oocytes. Dev Cell. 2017;41:287‐298. [DOI] [PubMed] [Google Scholar]
- 77. Mihajlovic AI, FitzHarris G. Segregating chromosomes in the mammalian oocyte. Curr Biol. 2018;28:R895‐R907. [DOI] [PubMed] [Google Scholar]
- 78. Liu L, Keefe DL. Defective cohesin is associated with age‐dependent misaligned chromosomes in oocytes. Reprod Biomed Online. 2008;16:103‐112. [DOI] [PubMed] [Google Scholar]
- 79. Chiang T, Duncan FE, Schindler K, Schultz RM, Lampson MA. Evidence that weakened centromere cohesion is a leading cause of age‐related aneuploidy in oocytes. Curr Biol. 2010;20:1522‐1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lister LM, Kouznetsova A, Hyslop LA, et al. Age‐related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol. 2010;20:1511‐1521. [DOI] [PubMed] [Google Scholar]
- 81. Hirano T. Condensin‐based chromosome organization from bacteria to vertebrates. Cell. 2016;164:847‐857. [DOI] [PubMed] [Google Scholar]
- 82. Ono T, Losada A, Hirano M, Myers MP, Neuwald AF, Hirano T. Differential contributions of condensin I and condensin II to mitotic chromosome architecture in vertebrate cells. Cell. 2003;115:109‐121. [DOI] [PubMed] [Google Scholar]
- 83. Lee J, Ogushi S, Saitou M, Hirano T. Condensins I and II are essential for construction of bivalent chromosomes in mouse oocytes. Mol Biol Cell. 2011;22:3465‐3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Houlard M, Godwin J, Metson J, Lee J, Hirano T, Nasmyth K. Condensin confers the longitudinal rigidity of chromosomes. Nat Cell Biol. 2015;17:771‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tsutsumi M, Fujiwara R, Nishizawa H, et al. Age‐related decrease of meiotic cohesins in human oocytes. PLoS ONE. 2014;9:e96710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Garcia‐Cruz R, Brieño MA, Roig I, et al. Dynamics of cohesin proteins REC8, STAG3, SMC1 and SMC3 are consistent with a role in sister chromatid cohesion during meiosis in human oocytes. Hum Reprod. 2010;25:2316‐2327. [DOI] [PubMed] [Google Scholar]
- 87. Lee J, Yokota T, Yamashita M. Analyses of mRNA expression patterns of cohesin subunits Rad21 and Rec8 in mice: germ cell‐specific expression of rec8 mRNA in both male and female mice. Zoolog Sci. 2002;19:539‐544. [DOI] [PubMed] [Google Scholar]
- 88. Tachibana‐Konwalski K, Godwin J, van der Weyden L, et al. Rec8‐containing cohesin maintains bivalents without turnover during the growing phase of mouse oocytes. Genes Dev. 2010;24:2505‐2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Burkhardt S, Borsos M, Szydlowska A, et al. Chromosome cohesion established by Rec8‐cohesin in fetal oocytes is maintained without detectable turnover in oocytes arrested for months in mice. Curr Biol. 2016;26:678‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Revenkova E, Herrmann K, Adelfalk C, Jessberger R. Oocyte cohesin expression restricted to predictyate stages provides full fertility and prevents aneuploidy. Curr Biol. 2010;20:1529‐1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wolf PG, Ramos AC, Kenzel J, Neumann B, Stemmann O. Studying meiotic cohesion in somatic cells reveals that Rec8‐containing cohesion requires Stag3 to function and is regulated by Wapl and sororin. J Cell Sci. 2018;131:jcs212100. [DOI] [PubMed] [Google Scholar]
- 92. Biswas U, Stevense M, Jessberger R. SMC1 substitutes for many meiotic functions of SMC1 but cannot protect telomeres from damage. Curr Biol. 2018;28:249‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kuleszewicz K, Fu X, Kudo NR. Cohesin loading factor localizes to chromosome axes during mammalian meiotic prophase. Cell Div. 2013;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Visnes T, Giordano F, Kuznetsova A, et al. Localisation of the SMC loading complex Nipbl/Mau2 during mammalian meiotic prophase I. Chromosoma. 2014;123:239‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kuroda M, Oikawa K, Ohbayashi T, et al. A dioxin sensitive gene, mammalian WAPL, is implicated in spermatogenesis. FEBS Lett. 2005;579:167‐172. [DOI] [PubMed] [Google Scholar]
- 96. Zhang J, Hakansson H, Kuroda M, Yuan L. Wapl localization on the synaptonemal complex, a meiosis‐specific proteinaceous structure that binds homologous chromosomes, in the female mouse. Reprod Domest Anim. 2008;2008(43):124‐126. [DOI] [PubMed] [Google Scholar]
- 97. Brieño‐Enríquez MA, Moak SL, Toledo M, et al. Cohesin removal along the chromosome arms during the first meiotic division depends on a NEK1‐PP1g‐WAPL axis in the mouse. Cell Rep. 2016;17:977‐986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gómez R, Felipe‐Medina N, Ruiz‐Torres M, et al. Sororin loads to the synaptonemal complex central region independently of meiotic cohesin complexes. EMBO Rep. 2016;17:695‐707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jordan PW, Eyster C, Chen J, Pezza RJ, Rankin S. Sororin is enriched at the central region of synapsed meiotic chromosomes. Chromosome Res. 2017;25:115‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fukuda T, Hoog C. The mouse cohesin‐associated protein PDS5B is expressed in testicular cells and is associated with the meiotic chromosome axes. Genes. 2010;1:484‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Commerford SL, Carsten AL, Cronkite EP. Histone turnover within nonproliferating cells. Proc Natl Acad Sci USA. 1982;79:1163‐1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Savas JN, Toyama BH, Xu T, Yates JR III, Hetzer MW. Extremely long‐lived nuclear pore proteins in the rat brain. Science. 2012;335:942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Smoak EM, Stein P, Schultz RM, Lampson MA, Biack BE. Long‐term retention of CENP‐A nucleosomes in mammalian oocytes underpins transgenerational inheritance of centromere identity. Curr Biol. 2016;26:1110‐1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Weng KA, Jeffreys CA, Bickel SE. Rejuvenation of meiotic cohesion in oocytes during prophase I is required for chiasma maintenance and accurate chromosome segregation. PLoS Genet. 2014;10:e1004607. [DOI] [PMC free article] [PubMed] [Google Scholar]


