Abstract
Background and aim
The primary aim of our study was to evaluate percutaneous endoscopic gastrostomy (PEG) tube placement depending on body weight and body mass index in patients undergoing radiotherapy (RT) for head and neck cancer (HNC). A secondary aim was to evaluate the course of weight change following PEG placement.
Methods
We retrospectively reviewed the medical records of 186 patients with HNC undergoing radiotherapy (RT) or chemoradiotherapy (CRT) at our institution between January 2010 and August 2017. Initial weight and nutritional intake were analyzed prior to RT initiation and then followed throughout treatment until completion. Based on these data, the indication of PEG placement was determined. Medical records were also reviewed to analyze PEG-related acute toxicities.
Results
A total of 186 patients met inclusion criteria. Patients were most commonly male (n=123, 66.1%) with squamous cell carcinoma (n=164, 88.2%). Patients who had dysphagia prior to treatment initiation as well as patients with a BMI <18.5 kg/m2 needed PEG placement earlier during the treatment course. Low-grade toxicities related to PEG insertion were observed in 10.7% patients, with peristomal pain and redness adjacent to the PEG tube insertion site being most common. High-grade toxicities, such as peritonitis and organ injury, were found in 4.9% of patients.
Conclusion
Underweight patients and those with preexisting dysphagia should be closely screened during RT for weight loss and decreased oral intake. For weight loss greater than 4.5% during the treatment of HNC, early PEG-tube placement should be considered. Further prospective studies are needed to confirm these findings, and delineate a scoring system for timing of PEG use (prophylactic vs reactive) as well as assess the quality of life in patients with HNC who receive PEG placement.
Keywords: head and neck cancer, radiotherapy, PEG placement, body mass index, toxicity
Introduction
Head and neck cancer (HNC) is the sixth most common malignancy worldwide.1,2 HNC is most commonly of squamous differentiation and includes a variety of tumors located within the oral and nasal cavities, pharynx, lips, tongue, larynx and salivary glands.3,4 Radiotherapy (RT) or chemoradiotherapy (CRT), administered as primary or adjuvant treatment, has become an important treatment strategy over the past several decades for patients with locally advanced disease or those who are unable to undergo surgical resection.4–6 A major problem seen among patients with HNC is malnutrition, the prevalence of which is estimated to be around 35–60%. Over the past few years, several interventions have been developed to improve the nutritional status of this cohort and to decrease weight loss in patients undergoing RT; these include dietary counseling, nutritional supplements, and the use of a percutaneous endoscopic gastrostomy (PEG) tube.7 The tumor location itself can often cause nutritional complications before treatment begins, with 5–52% of patients reporting dysphagia before undergoing CRT or RT.8 In addition, the side effects of RT and CRT, including esophagitis, mucositis, xerostomia, dysphagia, odynophagia, nausea, and fatigue, can contribute to poor nutritional intake in this cohort.9 PEG tube placement through a minimally invasive technique is an accepted strategy for enteral feeding,4,10–13 as demonstrated by several previous studies which showed that PEG tube placement is effective in preventing weight loss and providing sufficient nutritional intake to prevent malnutrition. This is a commonly employed technique because there is no longer a delay in treatment initiation due to the minimally invasive technique.12,13 Nevertheless, there remain different opinions regarding the timing of PEG tube use: prophylactic vs reactive placement has been discussed in a controversial manner.15,18 Furthermore, not all patients with cancer benefit from a prophylactic feeding tube. In fact, a number of studies have shown excellent results without PEG placement.15,18–20 Additionally, a lower quality of life and a higher rate of complications was observed among patients who completed RT and received a prophylactic PEG tube placement.14–17 On the other hand, several studies have indicated that PEG tube placement is associated with a decrease in treatment-related weight loss.15,18–20
At our center, physicians decide before treatment whether PEG tube placement based on the presence of a number of symptoms such as dysphagia and anorexia, which could negatively affect the treatment or the patient’s ability to maintain adequate nutritional intake. The primary objective of this retrospective study was to evaluate the period of time between RT initiation and PEG tube placement. The secondary endpoints were to analyze body mass index (BMI) and body weight (kilogram (kg)) of patients over the period of RT in an attempt to better understand any potential impact or influence on time of PEG insertion, even the impact of the planning target volume (PTV) on time when PEG tube was necessary and evaluate the PEG-insertion-related complications. Gaining additional insight could help improve clinical decision-making regarding PEG tube placement, underscoring the rationale for our study.
Patients and Methods
Study Design
A retrospective analysis was conducted using the clinical, operative and hospital records of patients who received gastric PEG tube placement either before or during administration of RT in the Department of Radiation Oncology at the University Hospital of Heidelberg between January 2010 and August 2017. Ethical approval was obtained prior to study initiation and procedures were carried out according to national and institutional ethical standards.
Study Population
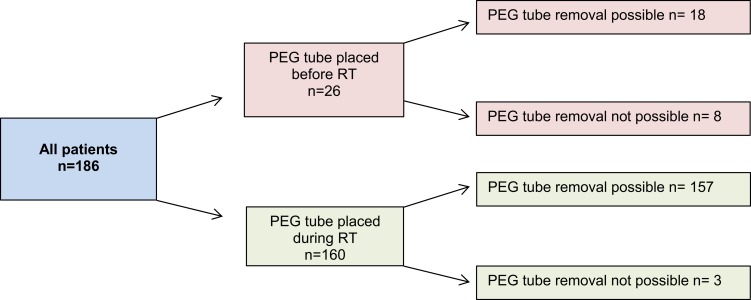
Each patient with HNC cancer who underwent PEG tube placement either before or during RT at our department was included in this study. Patients receiving either concurrent chemotherapy or immunotherapy with RT were also included. Patients who did not require PEG tube placement were excluded. The total study population consisted of 186 patients (Figure 1). All patients were given the opportunity to have a nutritional consultation at the NCT (Nationales Zentrum für Tumorerkrankungen) Heidelberg, but these data are not characterized in the present work given a lack of documentation. Each patient was referred through social services to a nursing service at the end of treatment, which provided home care instructions regarding utilization of the PEG tube.
Figure 1.
Schematic representation of patient subgroups in the present study.
Abbreviations: RT, radiotherapy; PEG, percutaneous endoscopic gastrostomy.
We evaluated patients’ demographic characteristics, tumor location in head and neck, tumor stage (TNM), reason for PEG tube placement and complications related to PEG tube placement.
Regarding patient’s nutritional status, the BMI [weight (kg)/height squared (m2)] and body weight in kilogram (kg) were analyzed at three different times: a) at the start of RT, b) at the day of PEG tube placement and c) at the end of therapy. For statistical analysis we categorized all patients into the following three groups based on their initial BMI score (kg/m2):
group 1:<18.5 kg/m2
group 2: 18.5–24.9 kg/m2
group 3: ≥25.0 kg/m2
Patient Characteristics
A total of 186 patients met inclusion criteria, with 123 patients (66.1%) being male and 63 patients (33.9%) female. Histologically, 164 patients (88.2%) had squamous cell carcinoma, 2 patients (1.1%) had adenocarcinoma, 6 patients (3.2%) had adenoid cystic carcinoma and 14 patients (3.2%) had other histologies. In terms of staging, 68.8% had locally advanced disease (T3-4) and 76.9% were node-positive (N+). Four patients (2.2%) presented with distant metastases. Detailed patient and tumor characteristics are shown in Table 1.
Table 1.
Patient Characteristics
| Characteristics | All Patients | |
|---|---|---|
| n | % | |
| No. of patients | 186 | 100 |
| Age at PEG placement | ||
| Median (years) | 67 | |
| Range (years) | 21–88 | |
| Sex | ||
| Male | 123 | 66.1 |
| Female | 63 | 33.9 |
| Histology | ||
| SCC | 164 | 88.2 |
| Adenocarcinoma | 2 | 1.1 |
| Adenoid cystic carcinoma (ACC) | 6 | 3.2 |
| Others | 14 | 7.5 |
| Primary tumor locations | ||
| Oral cavity | 71 | 38.2 |
| Hypopharynx | 30 | 16.1 |
| Glottis region | 20 | 10.8 |
| Oropharynx | 15 | 8.1 |
| Larynx | 12 | 6.5 |
| Paranasal sinus | 4 | 2.2 |
| Nasopharynx | 4 | 2.2 |
| Thyroid gland | 2 | 1.1 |
| Lung | 1 | 0.54 |
| Carcinoma of unknown primary (CUP) | 6 | 3.2 |
| TNM stage | ||
| T1 | 15 | 8.1 |
| T2 | 37 | 19.9 |
| T3 | 55 | 29.6 |
| T4 | 73 | 39.2 |
| Tx | 6 | 3.2 |
| N0 | 43 | 23.1 |
| N+ | 143 | 76.9 |
| M0 | 153 | 82.3 |
| M1 | 4 | 2.2 |
| Mx | 29 | 15.5 |
Nutritional Status
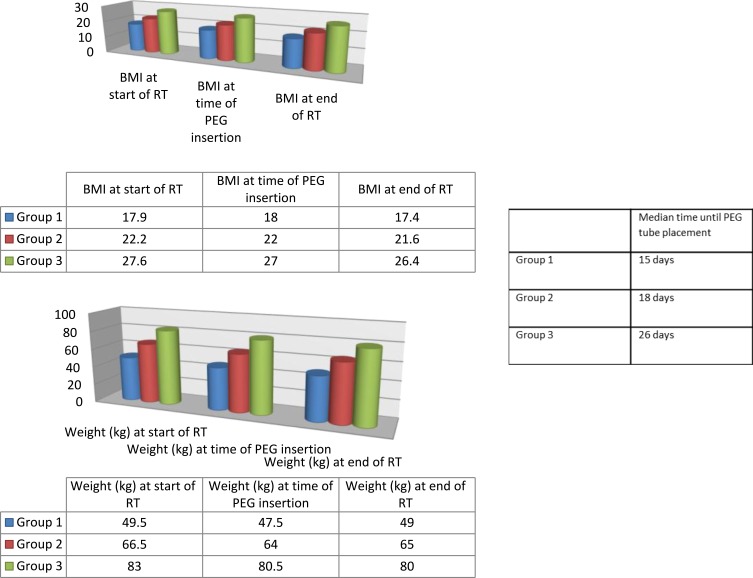
The development of body weight and BMI for patients who received PEG insertion during RT is shown in Figure 2. Median time to PEG insertion in group 1 was 15 days, group 2 18 days and in group 3, 26 days. Table 3 shows changes in body weight and BMI in patients who received PEG insertion before RT. Weight loss is expressed as a percentage decrease from baseline and is displayed in association with the median number of days until PEG was placed during RT. Table 2 illustrates weight loss from time of RT initiation to PEG tube placment for the entire cohort. Figure 2 illustrates weight loss according to the three BMI groups as well as the elapsed days until PEG tube placement.
Figure 2.
BMI and body weight development according to group among the 160 patients who received PEG placement prior to RT or at the end of RT. BMI and weight development in the three groups: before RT (Group 1 BMI/weight range 12.4–18.4/33kg–63kg; Group 2 BMI/weight range 12–18/33kg-63kg; Group 3 BMI/weight range 12.6–20.1/34kg–64kg), at time of PEG insertion (Group 1 BMI/weight range 18.5–25.0/48kg–85kg; Group 2 BMI/weight range 18–25/45kg–84kg; Group 3 BMI/weight range 16.4–25.6/47kg–81kg) and after completion of RT (Group 1 BMI/weight range 25–36.8/64kg-112kg; Group 2 BMI/weight range 25–37/58kg–;105kg; Group 3 BMI/weight range 20.9–34.0/59kg–110kg). Right table shows Synopsis of the three groups for median time of PEG insertion after treatment start.
Table 3.
BMI and Body Weight (Ranges) Development by Group Among the 26 Patients Who Received PEG Placement During RT
| Weight Initial (kg) | BMI Initial | Weight (kg) End of Treatment | BMI End of Treatment | |
|---|---|---|---|---|
| Group 1 | 57 (43–63) | 18.1 (13.8–18.4) | 54 (47–64) | 17.9 (15.2–20.1) |
| Group 2 | 67 (53–77) | 21.8 (19.8–24.9) | 67 (51–76) | 21.8 (19.4–24.2) |
| Group 3 | 83.5 (67–106) | 27.8 (25.6–35.0) | 83.5 (67–99) | 27.6 (27.8–32.7) |
Table 2.
Weight Loss from Time of RT Initiation to PEG Tube Placement for the Entire Cohort
| Weight Loss (%) | n (%) |
|---|---|
| ≤0% | 47 (25.3) |
| ≤5% | 69 (37.1) |
| >5–10% | 42 (22.6) |
| >10% | 14 (7.5) |
Data Collection and Treatment Toxicity
Patient records were reviewed to collect nutritional status information prior to radiation treatment, during treatment, and after treatment completion. Beyond general demographic characteristics, body weight, BMI, oral intake and patient-reported dysphagia and treatment-related toxicities were specifically examined.
Acute treatment-related toxicity was evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) criteria (version 4.03, US Department of Health and Human Services, Washington, DC, USA). Symptoms of grades 1 and 2 according to CTCAE were referred to as low-grade and grades 3 and 4 as high-grade toxicities. All patients were seen by a doctor once a week to detect side effects. Adverse events which led to PEG-insertion (RT related complications) like mucositis, dysphagia and esophageal stenosis were monitored. Following PEG-tube placement, patients were required to recover in the hospital. Therefore, the medical record was reviewed to analyze PEG-related toxicity, including skin toxicity (redness, dryness, pus), pain, infection, or anemia.
Statistical Analysis
Statistical analysis was conducted using IBM SPSS software version 24. The results are presented as mean and percentage. PEG-free interval (PFI) for the whole cohort, for patients with lower BMI (<18.5 kg/m2), preexisting dysphagia and higher treated RT doses (total doses ≥66Gy) was calculated by Kaplan-Meier estimates. The observed time was defined as the time from treatment initiation to PEG-insertion.
Univariate analysis was conducted using logistic regression analysis. As this is a retrospective analysis, p-values are of a descriptive nature. A descriptive p-value ≤0.05 was considered statistically significant. Variables that were tested for significance were selected for a multivariate logistic regression model. Variables that were not significant in univariate analysis, but were deemed to have clinical significance based on literature were also included in the multivariate model (BMI groups and body weight). Subgroups were compared using the log rank test. Odds ratios were accompanied with 95% confidence intervals. For comparison between groups, the chi-squared and Student’s t-tests were performed for categorical and continuous variables, respectively.
Ethics
This study was performed following institutional guidelines and the Declaration of Helsinki of 1975 in its most recent version. Ethical approval for the study was given from the local ethics committee at University Hospital Heidelberg. As the data were analyzed retrospectively and anonymously and treatment of patients was not affected by this study, no written informed consent from each individual patient was necessary according to institutional standards and the local ethics committee decision.
Results
RT Planning Characteristics
For planning purposes, a computed tomography (CT) with a maximum slice thickness of 3mm was performed for each patient. Contrast-enhanced images were obtained when not clinically contra-indicated. Individual head fixation including neck and shoulders was achieved by the use of a thermoplastic mask fitted for each patient. Target volume definition was defined according to ICRU guidelines. Total dose was prescribed with regard to tumor location, staging and treatment intent, whether curative or palliative. RT was administered to 149 patients (80.1%) as primary treatment and to 37 patients (19.9%) in adjuvantly following surgical resection. In total, 74.7% (n=139) of patients received concomitant systemic therapy: 55.9% (n=104) received chemotherapy and 18.8% (n=35) received immunotherapy. Patients were treated with either three-dimensional conformal RT (3D-CRT) (n=3, 1.6%), intensity-modulated RT (IMRT) (n=162, 87.1%, VMAT 53.1%, TomoTherapy® 34%), combined therapy with IMRT and carbon-ions (C12) (n=17, 9.1%) or protons alone (n=3, 1.6%). Radiation was delivered in two phases: a primary phase encompassing all sites of disease and respective clinical margins and a boost phase encompassing gross disease with a small clinical margin. The median primary phase dose was 56Gy (range, 23–72Gy), administered using a median of 1.8Gy/fraction (range, 1.8–3.0Gy/fraction). The boost was given either simultaneously or after completing the primary phase, with a median dose of 2.2Gy/fraction (range, 1.0–3.3Gy/fraction) to a median total dose of 70Gy (range, 48–82Gy). Treatment was most commonly delivered in five fractions per week for photon treatment and 5–6 fractions per week for particle therapy. A total of 183 patients (98.4%) completed RT as planned; 3 patients aborted treatment because of a fulminant gastrointestinal infection unrelated to treatment. The median PTV during the primary course was 771mL (range, 51–1843mL), and 158mL (range, 1.0–3.3mL) during the boost-phase. Detailed treatment characteristics are shown in Table 4.
Table 4.
Treatment Characteristics
| Characteristics | All Patients | |
|---|---|---|
| n | % | |
| No. of patients | 186 | 00 |
| RT setting | ||
| Definitive | 149 | 80.1 |
| Adjuvant | 37 | 19.9 |
| RT-technique | ||
| IMRT | 163 | 87.6 |
| 3D-CRT | 3 | 1.6 |
| IMRT + Carbon-Ions | 17 | 9.1 |
| Protons | 3 | 1.6 |
| Concomitant therapy | ||
| Chemotherapy | 98 | 52.7 |
| Immunotherapy | 32 | 47.3 |
| Neck irradiation | ||
| Ipsilateral neck | 57 | 30.6 |
| Bilateral neck | 102 | 54.8 |
| Radiotherapy dose (Gy) | ||
| Main course | ||
| median cumulative dose (range) | 56.0 (48.0–72) | |
| median dose per fraction (range) | 1.8 (1.8–3.0) | |
| Boost | ||
| median cumulative dose (range) | 70.0 (48–92) | |
| median dose per fraction (range) | 2.1 (2.1–3.0) | |
| PTV (mL) | ||
| Main course | ||
| Median | 771 | |
| Range | 51–1843 | |
| Q1–Q3 | 595–1007 | |
| Boost | ||
| Median | 158 | |
| Range | 26–1359 | |
| Q1–Q3 | 108–265 | |
Clinical Factors Affecting Timing of PEG-Placement
The median age at the time of PEG tube placement was 67 years (range, 21–88years). The indication for the 26 patients who received PEG insertion before RT treatment was pronounced dysphagia and resulting weight loss. The median age at the time of PEG tube placement was 67 years (range, 21–88years). The indication for the 26 patients who received PEG insertion before RT treatment was pronounced dysphagia and resulting weight loss. Forty-seven patients (25.3%) had problems with progressive dysphagia during RT in which pain prevented adequate oral intake, necessitating PEG tube placement. After PEG insertion eight patients of these 47 had a stable weight (± 0kg) at the end of RT; the remaining patients lost between one to six kilograms until the end of RT: −1kg in 10 patients, −2kg in 7 patients, −3kg in 3 patients, −1kg in 1 patient, −5kg in 3 patients, −6kg in 3 patients. The other eleven patients retained their body weight (within 1 to 5 kg) after PEG insertion until treatment completion.
From the cohort as a whole, 69 patients (37.1%) gained between 1% and <5% in body weight, 42 patients (22.6%) gained between 5% and 10% in body weight, and 14 patients (7.5%) lost >10% of their initial body weight. In comparing the cohort based on PEG placement either before or during treatment, there was no significant difference in median loss of body weight among the two groups.
The median time to PEG-placement in patients with preexisting dysphagia was 6 days, whereas time to PEG-placement was 23 days among patients without preexisting dysphagia.
Univariable and Multivariable Analyses
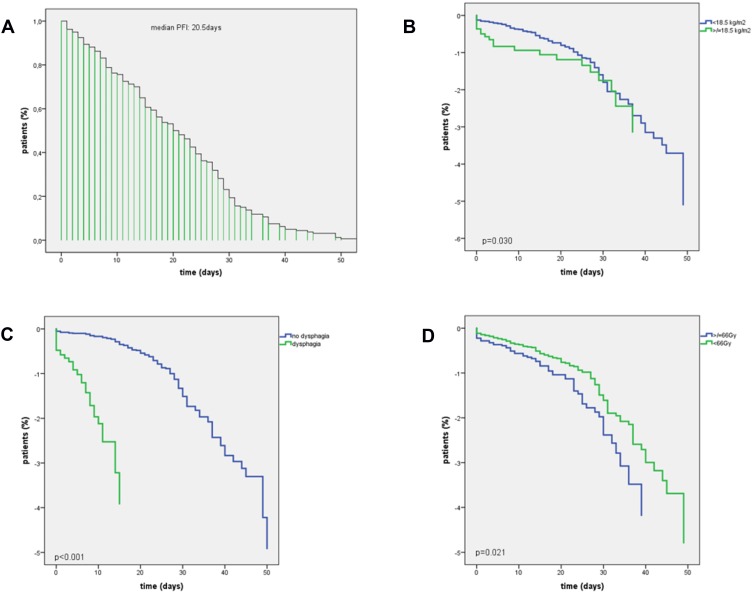
Univariable and multivariable Cox regression models were used to examine the effect of radiotherapy on earlier PEG-placement time for all BMI groups. Kaplan Meier estimates for the cohort showed a median PEG-free-interval (PFI) from the start of RT to PEG insertion of 20.5 days. Severely underweight patients (<18.5 kg/m2) were found to have a significantly earlier time to PEG insertion than normal or overweight patients (p=0.030). Similarly, patients with dysphagia before treatment (p<0.001) as well as those requiring RT doses >66Gy (p=0.021) were found to have significantly earlier PEG insertion. Results of univariable and multivariable cox regression models are summarized in Table 5 and Figure 3.
Table 5.
Univariable and Multivariable Cox Regression Models for Earlier PEG Insertion Time
| Parameter | p value | CI 95% |
|---|---|---|
| Age <60 years vs ≥60 years | 0.578 | 0.797–1.502 |
| Gender male vs female | 0.591 | 0.673–1.253 |
| BMI group 1 vs group 2/3 | 0.030 | 1.098–6.211 |
| T stage T1/2 vs T3/4 | 0.110 | 0.567–1.060 |
| N stage N0 vs N+ | 0.461 | 0.624–1.239 |
| Dysphagia symptoms at diagnosis CTCAE grade ≥3 | <0.001 | 4.515–9.858 |
| Concomitant therapy | 0.273 | 0.863–1.685 |
| Cumulative RT-dose <60Gy vs ≥60Gy | 0.021 | 0.514–0.948 |
| PTV size <800mL vs ≥800mL | 0.480 | 0.830–1.484 |
Figure 3.
Kaplan-Meier estimates for PEG-free interval (PFI). (A) median time for all patients to PEG placement was 20.5 days (B) significantly earlier time of PEG insertion in patients with BMI <18.5kg/m2 (p=0.030, CI 1.098–6.211); (C) significantly earlier time of PEG insertion in patients with dysphagia CTCAE ≥grade 3 at baseline (p<0.001, CI 4.515–9.858); (D) significantly earlier time of PEG insertion in patients with requiring cumulative RT-doses ≥66Gy (p=0.021, CI 0.514–0.984).
Abbreviations: PEG, percutaneous endoscopic gastrostomy; PFI, PEG-free interval.
RT-Related Toxicity
Forty-seven patients (25.3%) reported dysphagia before treatment initiation: grade 1 and 2 (low-grade) in 36 patients (76.6%) and grade 3 and 4 (high-grade) in 11 patients (5.9%).
The most common acute RT-related complication that led to PEG insertion was mucositis (83 patients (44.4%): low grade in 21 patients (11.2%) and high grade in 62 patients (33.2%)). RT-related toxicity including dysphagia and esophagitis was detected in 100 patients (53.5%), low grade in 46 patients (24.6%) and high grade in 54 patients (28.9%). During RT, three patients (1.6%) developed esophageal stenosis.
PEG-Related Toxicity
A total of 160 patients (69%) underwent endoscopic PEG tube placement, while the remaining 26 patients (14%) underwent surgical intervention for placement. Acute toxicities associated with minor complications after PEG tube placement were detected in 20 patients (10.7%) and included tube dislodgement, tube clogging, wound infection, and peri-stomal wound leakage. Eight patients (4.3%) developed high-grade complications with laparotomy, including peritonitis in five patients (2.7%) and organ perforation (intestinal) in three patients (1.6%).
In follow-up, the median duration of the PEG tube remaining in-situ was 8.7 months (range, 3.2–13.7 months). As a result of pharyngeal stenosis in follow-up examinations, PEG tube removal was not possible in 11 patients (5.9%). Of these, 8 patients had preexisting dysphagia, while 3 patients developed dysphagia during RT (Figure 1).
Discussion
RT or CRT for head-and-neck cancers can cause severe mucositis, intolerable pain, swallowing dysfunction, and dysphagia, often resulting in significant malnutrition. Consequence of these complications include weight loss due to reduced food intake. The European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines have identified that malnutrition during the treatment of cancers decreases the quality and activity of daily life, increases side effects, and decreases response to treatment interventions.28,33 PEG tubes have been used to provide nutritional support and decrease the incidence of malnutrition during RT.12,21–26,28
This retrospective study demonstrated that patients with an initial low BMI (<18.5kg/m2) and patients with preexisting tumor-associated dysphagia were significantly more likely to receive earlier PEG placement. In total, 25.3% of patients developed dysphagia before the start of RT and underwent earlier PEG tube placement (after 6.0 days of RT), when compared to patients not reporting this symptom. RT-related toxicities including dysphagia and esophagitis were detected in 100 patients (53.5%). Worldwide, some centers avoid PEG tube placement unless patients show signs of weight loss or swallowing disability, while other centers prophylactically place them before treatment initiation. There are no internationally accepted standard methods for assessing the nutritional status of oncologic patients to assess the value of a prophylactic PEG tube use.12,21–26,30
In 1999, Morrison and Hark published a grading system for weight loss within the first week, describing weight loss of 1–2% as significant and >2% weight loss as a severe side effect of therapy. A weight loss of >5% from baseline in the first month after the start of therapy could lead to severe consequences.27 Weight loss is a nutritional indicator that reflects reduced intake or nutritional discrepancy. Indeed, the indication among the 26 patients who received PEG insertion prior to treatment initiation was pronounced dysphagia resulting in weight loss. In the present study, the results of our analysis on timing of PEG tube placement showed that obese patients had a median weight loss of 2.5% and received a PEG tube within 26 days. Those patients who were underweight with BMIs <18.5kg/m2 had a median weight loss of 2.0%, followed by PEG tube placement after a median of 15 days. Based on the published data by Morrison and Hark,27 patients in our study collectively underwent PEG tube placement when there was a significant weight loss (1-2%). These results imply that patients with HNC are generally identified as a group that may require assistance through caloric supplementation via PEG placement. The literature lacks studies correlating target volume size (PTV) with the timing of PEG tube placement. The idea that a larger PTV leads to earlier PEG tube placement was not confirmed in our analysis (p=0.480). Similar to our data, Takahasi et al showed that PEG tube placement constituted a practicable nutritional intervention with quite a few benefits, such as effective reduction of treatment-related weight loss with stable weight at the end of RT: median weight at PEG tube placement was 67.4 vs 67.3 kg at the end of RT.34 These data underscore the importance of monitoring the nutritional status of HNC patients, indicating that timing is important as well.
Several previous studies in the literature have described that PEG tube insertion is a well-tolerated procedure with minimal morbidity and mortality, allowing a good nutritional support in patients who are unable to safely tolerate oral intake.29–31 The literature reports that about 13–40% of patients receiving PEG tube placement experience minor complications such as maceration due to leakage of gastric contents around the tube and peristomal pain.30,31 Serious complications requiring further intervention have been reported in 0.4–4.4% of the procedures and include peristomal leakage with peritonitis, necrotizing fasciitis of the anterior abdominal wall, gastric bleeding, injury to internal organs, tumor seeding at the PEG site, and death.29–31 These results are comparable with our findings, with low-grade toxicity found in 10.7% of patients, due to peristomal pain and erythema adjacent to the PEG tube insertion site, and high-grade toxicity observed in 4.9% of patients due to peritonitis and organ injury.
Despite the improvement in maintaining nutritional intake, the literature also reports several negative aspects of PEG tube placement when performed as a prophylactic measure, such as more severe and long-standing dysphagia caused by muscle disuse and atrophy as well as PEG-associated complications.32 These findings are similar to those presented in the present study, wherein we observed that PEG tube removal was not possible in 11 patients because of severe dysphagia caused by pharyngeal fibrosis/stenosis. Of these, 8 patients had preexisting dysphagia and needed PEG insertion before the start of RT, while 3 patients developed dysphagia during RT and needed the PEG insertion later. Therefore, the early identification of patients who could benefit from PEG tube insertion is a critical aspect that should be routinely considered.
Limitations of this study include its retrospective nature and the lack of subjective data to measure patient quality of life. Selection bias could also be at play, because receiving a PEG tube was based on the clinical decision making and subject to the patient’s consent; compliant and better informed patients would be more prone to receiving PEG tube placement, which could have affected the results of this study. Therefore, a prospective follow-up study is needed to confirm the findings presented herein. Additionally, prospective studies could be used to develop a prognostic scoring system for patients with HNC based on the results obtained in the present analysis.
Conclusion
Patients with preexisting dysphagia or who are initially underweight should be closely screened during RT for weight loss and oral intake. For a weight loss of more than 4.5% during the HNC treatment, early PEG-placement should be considered in an attempt to maintain adequate nutrition to improve treatment tolerance and reducing side effects. Further prospective studies could be used to delineate a scoring system for prophylactic vs reactive PEG placement and assess the quality of life in patients with HNC receiving PEG placement.
Funding Statement
The study was financed by the Department of Radiation Oncology of the University Hospital Heidelberg. There was no external funding source. We acknowledge financial support by Deutsche Forschungsgemeinschaft within the funding programme Open Access Publishing, by the Baden-Württemberg Ministry of Science, Research and the Arts and by Ruprecht-Karls-Universität Heidelberg.
Abbreviations
HNC, head and neck cancer; SCC, squamous cell carcinoma; CIRT, carbon ion radiotherapy; RT, radiotherapy; PEG, percutaneous endoscopic gastrostomy; CRT, chemoradiotherapy; BMI, body mass index; TNM, Tumor Node Metastasis; UICC, International Union against Cancer; WHO, World Health Organization; CTCAE, Common Terminology Criteria for Adverse Events; PTV, planning target volume; CT, chemotherapy; IT, immunotherapy.
Ethical Considerations
The final protocol was approved by the ethics committee of the University of Heidelberg, Heidelberg, Germany (S-421/2015).
Disclosure
Dr Sebastian Adeberg report personal fees from Merck Serono GmbH, other from Novocure, personal fees from Accuray International Sarl, outside the submitted work. Prof. Dr. Jürgen Debus report grants from Merck Serono, grants from Accuray, grants, non-financial support from Siemens Healthineers, non-financial support from Research Labs, outside the submitted work. The authors report no other conflict of interest in this work.
References
- 1.Patterson JM, Brady GC, Roe JW. Research into the prevention and rehabilitation of dysphagia in head and neck cancer: a UK perspective. Curr Opin Otolaryngol Head Neck Surg. 2016;24(3):208–214. doi: 10.1097/MOO.0000000000000260 [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 3.Devlin J, Sherman E. Combined modality treatment of squamous cell cancer of the head and neck. Clin Adv Hematol Oncol. 2005;3(5):373–382. [PubMed] [Google Scholar]
- 4.Moleiro J, Faias S, Fidalgo C, et al. Usefulness of prophylactic percutaneous gastrostomy placement in patients with head and neck cancer treated with chemoradiotherapy. Dysphagia. 2016;31(1):84–89. doi: 10.1007/s00455-015-9661-y [DOI] [PubMed] [Google Scholar]
- 5.Chan AT, Gregoire V, Lefebvre J-L, et al. Nasopharyngeal cancer: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl Supplement 5):v187–v189. doi: 10.1093/annonc/mdq186 [DOI] [PubMed] [Google Scholar]
- 6.Chan AT, Gregoire V, Lefebvre J-L, et al. Nasopharyngeal cancer: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii83–vii85. doi: 10.1093/annonc/mds266 [DOI] [PubMed] [Google Scholar]
- 7.Takenaka Y, Yamamoto M, Nakahara S, et al. Factors associated with malnutrition in patients with head and neck cancer. Acta Otolaryngol. 2014;134(10):1079–1085. doi: 10.3109/00016489.2014.906750 [DOI] [PubMed] [Google Scholar]
- 8.Platteaux N, Dirix P, Dejaeger E, et al. Dysphagia in head and neck cancer patients treated with chemoradiotherapy. Dysphagia. 2010;25(2):139–152. doi: 10.1007/s00455-009-9247-7 [DOI] [PubMed] [Google Scholar]
- 9.Marcy P-Y, Magné N, Bensadoun R-J, et al. Systematic percutaneous fluoroscopic gastrostomy for concomitant radiochemotherapy of advanced head and neck cancer: optimization of therapy. Support Care Cancer. 2000;8(5):410–413. doi: 10.1007/s005200050010 [DOI] [PubMed] [Google Scholar]
- 10.Bossola M. Nutritional interventions in head and neck cancer patients undergoing chemoradiotherapy: a narrative review. Nutrients. 2015;7(1):265–276. doi: 10.3390/nu7010265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silander E, Nyman J, Bove M, et al. Impact of prophylactic percutaneous endoscopic gastrostomy on malnutrition and quality of life in patients with head and neck cancer: a randomized study. Head Neck. 2012;34(1):1–9. doi: 10.1002/hed.v34.1 [DOI] [PubMed] [Google Scholar]
- 12.Raykher A, Correa L, Russo L, et al. The role of pretreatment percutaneous endoscopic gastrostomy in facilitating therapy of head and neck cancer and optimizing the body mass index of the obese patient. JPEN J Parenter Enteral Nutr. 2009;33(4):404–410. doi: 10.1177/0148607108327525 [DOI] [PubMed] [Google Scholar]
- 13.Bradley PT, Brown T, Paleri V. Gastrostomy in head and neck cancer: current literature, controversies and research. Curr Opin Otolaryngol Head Neck Surg. 2015;23(2):162–170. doi: 10.1097/MOO.0000000000000135 [DOI] [PubMed] [Google Scholar]
- 14.Baschnagel A, Yadav S, Marina O, et al. Toxicities and costs of placing prophylactic and reactive percutaneous gastrostomy tubes in patients with locally advanced head and neck cancers treated with chemoradiotherapy. Head Neck. 2013;36(8):1155–1161. doi: 10.1002/pro.v36.8 [DOI] [PubMed] [Google Scholar]
- 15.Madhoun MF, Blankenship MM, Blankenship DM, et al. Prophylactic PEG placement in head and neck cancer: how many feeding tubes are unused (and unnecessary)? World J Gastroenterol. 2011;17(8):1004–1008. doi: 10.3748/wjg.v17.i8.1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams GF, Teo MT, Sen M, Dyker KE, Coyle C, Prestwich RJ. Enteral feeding outcomes after chemoradiotherapy for oropharynx cancer: a role for a prophylactic gastrostomy? Oral Oncol. 2003;48:434–440. doi: 10.1016/j.oraloncology.2011.11.022 [DOI] [PubMed] [Google Scholar]
- 17.Clavel S, Fortin B, Després P, et al. Enteral feeding during chemoradiotherapy for advanced head‐and-neck cancer: a single‐institution experience using a reactive approach. Int J Radiat Oncol Biol Phys. 2011;79:763–769. doi: 10.1016/j.ijrobp.2009.12.032 [DOI] [PubMed] [Google Scholar]
- 18.Kramer S, Newcomb M, Hessler J, et al. Prophylactic versus reactive PEG tube placement in head and neck cancer. Otolaryngol Head Neck Surg. 2014;150(3):407–412. doi: 10.1177/0194599813517081 [DOI] [PubMed] [Google Scholar]
- 19.Hutcheson KA, Barringer DA, Rosenthal DI, et al. Swallowing outcomes after radiotherapy for laryngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2008;134(2):178–183. doi: 10.1001/archoto.2007.33 [DOI] [PubMed] [Google Scholar]
- 20.Raynor EM, Williams MF, Martindale RG, et al. Timing of percutaneous endoscopic gastrostomy tube placement in head and neck cancer patients. Otolaryngol Head Neck Surg. 1999;120(4):479–482. doi: 10.1053/hn.1999.v120.a91408 [DOI] [PubMed] [Google Scholar]
- 21.Rustom IK, Jebreel A, Tayyab M, et al. Percutaneous endoscopic, radiological and surgical gastrostomy tubes: a comparison study in head and neck cancer patients. J Laryngol Otol. 2006;120(6):463–466. doi: 10.1017/S0022215106000661 [DOI] [PubMed] [Google Scholar]
- 22.Raykher A, Russo L, Schattner M, et al. Enteral nutrition support of head and neck cancer patients. Nutr Clin Pract. 2007;22(1):68–73. doi: 10.1177/011542650702200168 [DOI] [PubMed] [Google Scholar]
- 23.Hunter AMB. Nutrition management of patients with neoplastic disease of the head and neck treated with radiation therapy. Nutr Clin Pract. 1996;11(4):157–169. doi: 10.1177/0115426596011004157 [DOI] [PubMed] [Google Scholar]
- 24.Murayama KM. Enteral feeding tube placement in head and neck cancer patients: special considerations. Nutr Clin Pract. 1997;12(1 Suppl):S34–S37. doi: 10.1177/088453369701200112 [DOI] [PubMed] [Google Scholar]
- 25.Lee JH, Machtay M, Unger LD, et al. Prophylactic gastrostomy tubes in patients undergoing intensive irradiation for cancer of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124(8):871–875. doi: 10.1001/archotol.124.8.871 [DOI] [PubMed] [Google Scholar]
- 26.Romesser PB, Romanyshyn JC, Schupak KD, et al. Percutaneous endoscopic gastrostomy in oropharyngeal cancer patients treated with intensity-modulated radiotherapy with concurrent chemotherapy. Cancer. 2012;118(24):6072–6078. doi: 10.1002/cncr.27633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hark MA. Grading of unintentional weight loss. Concise Manual Hematol Oncol. 1999. [Google Scholar]
- 28.Langmore S, Krisciunas GP, Miloro KV, et al. Does PEG use cause dysphagia in head and neck cancer patients? Dysphagia. 2012;27(2):251–259. doi: 10.1007/s00455-011-9360-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larson DE, Burton DD, Schroeder KW, et al. Percutaneous endoscopic gastrostomy. Indications, success, complications, and mortality in 314 consecutive patients. Gastroenterology. 1987;93(1):48–52. doi: 10.1016/0016-5085(87)90312-X [DOI] [PubMed] [Google Scholar]
- 30.Grant MD, Rudberg MA, Brody JA. Gastrostomy placement and mortality among hospitalized Medicare beneficiaries. JAMA. 1998;279(24):1973–1976. doi: 10.1001/jama.279.24.1973 [DOI] [PubMed] [Google Scholar]
- 31.Chen AM, Li B-Q, Lau DH, et al. Evaluating the role of prophylactic gastrostomy tube placement prior to definitive chemoradiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2010;78(4):1026–1032. doi: 10.1016/j.ijrobp.2009.09.036 [DOI] [PubMed] [Google Scholar]
- 32.Siddiqi AM, Hamilton RD, Minocha A. Malignant seeding of percutaneous endoscopic gastrostomy tract in patient with head and neck cancer. Am J Med Sci. 2008;336(3):291–292. doi: 10.1097/MAJ.0b013e31815b60ba [DOI] [PubMed] [Google Scholar]
- 33.Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11–48. doi: 10.1016/j.clnu.2016.07.015 [DOI] [PubMed] [Google Scholar]
- 34.Takahashi M, Kosaka N, Wakui E, et al. Role of intensive nutrition support and prophylactic percutaneous endoscopic gastrostomy during concomitant chemoradiotherapy for oropharyngeal cancer. Int J Clin Oncol. 2018;23:1023. doi: 10.1007/s10147-018-1328-x [DOI] [PubMed] [Google Scholar]