Abstract
Study Objectives
To determine the associations of sleep-disordered breathing (SDB) with subsequent healthcare costs and utilization including inpatient and post-acute care facility stays among community-dwelling older men.
Methods
Participants were 1,316 men (mean age 76.1 [SD = 5.7] years) in the Outcomes of Sleep Disorders in Older Men (MrOS sleep) study (from December 2003 to March 2005), who were enrolled in a Medicare Fee-For-Service plan. Primary SDB measures including apnea hypopnea index (AHI) and oxygen desaturation index (ODI) were collected using in-home level 2 polysomnography. Incident healthcare costs and utilization were determined from claims data in the subsequent 3-year period post-MrOS sleep visit.
Results
Five hundred and twenty-nine (40.2%) men had at least one hospitalization in the 3-year period. Compared with those without sleep apnea (AHI < 5/hour), men with moderate to severe sleep apnea (AHI ≥ 15/hour) had a higher odds of all-cause hospitalization (odds ratio [OR] adjusted for age and site 1.43, 95% confidence interval [CI]: 1.07–1.90). This association was slightly attenuated after further adjustment for traditional prognostic factors including education, body mass index, comorbid medical conditions, and health status (OR = 1.36; 95% CI: 1.01–1.83). Similar associations were observed for ODI. However, measures of SDB were not related to subsequent healthcare costs (total or outpatient) or odds of post-acute skilled nursing facility stay.
Conclusions
Older men with SDB have an increased risk of hospitalization, not entirely explained by the greater prevalence of comorbid conditions, but not higher subsequent total healthcare costs. These findings indicate a need to evaluate the impact of SDB treatment on subsequent healthcare utilization.
Keywords: sleep-disordered breathing, sleep apnea, Medicare, hospitalization, healthcare costs and utilizations, older men
Statement of Significance.
Previous studies have examined associations between sleep apnea and healthcare costs and utilization among middle-aged and younger populations. However, these studies were limited by case–control and cross-sectional designs, lack of adjustment for potential confounders, and under-representation of older adults who experience high rates of healthcare utilization. We utilized a prospective cohort design and linked our cohort study participants with their Medicare claim data to investigate whether objective, polysomnography-measured sleep-disordered breathing (SDB) is associated with higher healthcare costs and utilization among older community-dwelling men. Moderate to more severe SDB was associated with an increased risk of hospitalization, but not higher total healthcare costs. The presence of SDB may help identify individuals at high risk for hospitalization. Future research should evaluate the effect of treatment of SDB on subsequent healthcare utilization.
Introduction
Sleep-disordered breathing (SDB) is a common disorder characterized by repeated pauses or reductions in breathing during sleep with a prevalence of 25% in older community-dwelling men [1, 2]. SDB is associated with prevalent and incident cardiovascular disease (CVD) including hypertension, coronary heart disease, cardiac conduction abnormalities, heart failure, and stroke [3–10]. SDB is also associated with perioperative complications, motor vehicle accidents, cognitive impairment, and cognitive decline [11–13]. Given that SDB is associated with adverse health outcomes, especially CVD events, SDB may be associated with higher healthcare costs and utilization across a variety of healthcare settings. If SDB is associated with higher subsequent healthcare costs and utilization, future intervention studies would be warranted to determine whether treatment of SDB lowers these measures of healthcare burden.
A number of studies primarily in younger or middle-aged populations have evaluated the association of SDB and healthcare utilization [14–23]. However, previous studies were limited by use of cross-sectional or case–control study designs [16–19, 22] and inadequate control of potential confounders including body mass index (BMI) [14, 23]. One study used the modified Chronic Disease Score (CDS) as a proxy measure for healthcare utilization [23] and another study relied on administrative claims for the diagnosis of the obstructive sleep apnea (OSA) [21]. Only three studies focused on older men and results were not consistent between studies [14, 16, 21].
Our aim was to examine the association of objective measures of SDB with subsequent total healthcare costs and utilization in community-dwelling older men. To address this question, we used a unique longitudinal data set comprising 1,316 men participating in the Outcomes of Sleep Disorders in Older Men (MrOS Sleep) prospective cohort study linked with their Medicare claim data.
Methods
Study population and linkage to Medicare claim data
We studied participants enrolled in MrOS study, a prospective cohort study of 5,994 community-dwelling older men, aged ≥65 years. Men were recruited between March 2000 and April 2002 from six US cities: Birmingham, AL; Minneapolis, MN; Palo Alto, CA; Monongahela Valley near Pittsburgh, PA; Portland, OR; and San Diego, CA. Details of the MrOS study design and recruitment have been described elsewhere [24, 25]. Linkage of MrOS cohort data to Medicare Claims Files was completed by submitting participant social security and/or Medicare numbers to the Centers for Medicare and Medicaid Services (CMS). Linkage to Medicare enrollment data was successful for 5,876 men (98%) as of January 1, 1999.
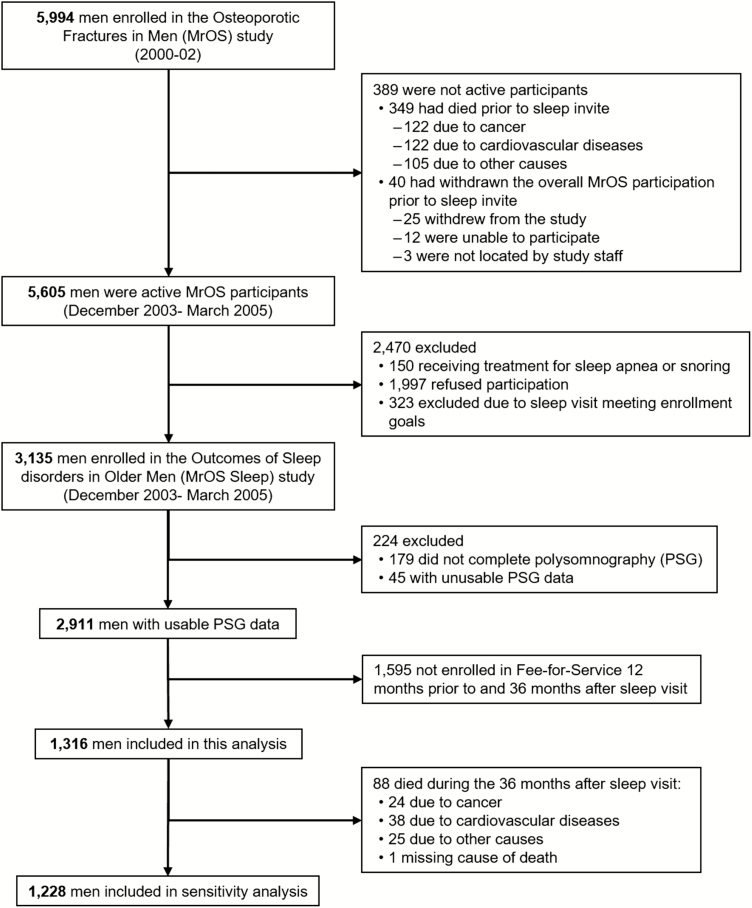
Recruitment for Outcomes of Sleep Disorders in Older Men (MrOS Sleep) study occurred from December 2003 to March 2005 among the pool of 5,605 active participants (Figure 1). Among these men, 150 were not eligible for the MrOS Sleep study because they were receiving treatment for sleep apnea or snoring, 1,997 were invited but refused to participate, and 323 were not asked to participate because recruitment goals had already been met. Thus, a total of 3,135 (57%) men agreed to participate in the Sleep study (exceeding the recruitment goal of 3,000 men).
Figure 1.
Analysis cohort.
Of the 3,135 men who participated in the sleep visit, 2,911 men had usable overnight polysomnography (PSG) recording. Of these 2,911 men, 1,316 (45.2%) men who were enrolled continuously in a Medicare Fee-For-Service (FFS) program (Parts A and B [and not Part C, Medicare Advantage]) during the 12 months prior to and 36 months after the sleep visit (or until death within this period) were included in the analytical cohort for this study (Figure 1).
SDB exposures
In-home sleep studies were completed using in-home, level 2 PSG (Safiro, Compumedics, Inc., Melbourne, Australia). The PSG recordings were obtained within 1 month of the clinic visit (mean 6.9 ± 15.8 days from visit), with 78% of recordings gathered within 1 week of the clinic visit. The recording montage was as follows: C3/A2 and C4/A1 electroencephalograms, bilateral electrooculograms, and a bipolar submental electromyogram to determine sleep stage; thoracic and abdominal respiratory inductance plethysmography to determine respiratory effort; airflow (by nasal-oral thermocouple and nasal pressure cannula); finger pulse oximetry (SpO2) for measuring oxygen saturation; lead I EKG; body position (mercury switch sensor); and bilateral tibialis leg movements (piezoelectric sensors). Centrally trained and certified staff performed home visits to set up the unit, verify the values of the impedances for each channel, confirm calibration of position sensors, and note any problems encountered during set-up, similar to the protocol used in the Sleep Heart Health Study [26]. Staff returned the next morning to collect the equipment and download the data to the Central Sleep Reading Center (Cleveland, OH) to be scored by certified research polysomnologists blinded to all other data. PSG data quality was excellent, with a failure rate of less than 4% and more than 70% of studies graded as being of excellent or outstanding quality.
Apneas were defined as a complete or almost complete cessation of airflow for more than 10 seconds. Hypopneas were defined as a >30% reduction in amplitude of either respiratory effort or airflow for more than 10 seconds associated with an oxygen desaturation of ≥4% [27].
The primary measures of SDB in this study were the apnea hypopnea index (AHI) and oxygen desaturation index (ODI). AHI was defined as the average number of apneas and hypopneas per hour of sleep. Severity of sleep apnea was defined as normal if AHI was <5/hour, mild if AHI was 5 to <15/hour, and moderate to severe if AHI was ≥15/hour [28]. ODI was defined as the mean number of oxygen desaturation events (≥4% decrease in SpO2) per hour of sleep. Severity of ODI was considered normal if ODI ≤ 5/hour, mild if ODI was between 5 and ≤10/hour, moderate if ODI was between 10 and ≤15/hour, and severe if ODI >15/hour [29]. Secondary SDB measures included the percent of sleep time with SpO2 < 90%, and OSA calculated as the sum of obstructive apneas (excluding central apneas) plus hypopneas associated with a ≥4% desaturation. Severity of nocturnal hypoxemia (% of total sleep time with SaO2 < 90% [%TST<90]) was considered normal if %TST<90 was less than 1%, mild if %TST<90 was 1.0% to less than 3.5%, and at least moderate if %TST<90 was 3.5% or greater. OSA severity was categorized using the same cut points as for AHI.
Outcome measures
The primary outcome was total healthcare costs (an aggregate measure of overall healthcare burden) for the 36 months after the MrOS sleep visit. Secondary outcomes included all-cause and CVD-related hospitalizations. Annualized total healthcare costs were calculated as the sum of standardized inpatient hospital costs, Part A paid skilled nursing facility (SNF) costs, inpatient rehabilitation facility (IRF) costs, outpatient costs, and home healthcare costs. Inpatient hospital stays and stays in post-acute care facilities (SNF or IRF) were identified using the Medical Provider Analysis and Review (MedPAR) file. Standardized costs for hospital stays, SNF stays, and IRF stays were calculated using previously validated and published methods [30–32]. Costs for Part A paid SNF stays, for IRF stays, home healthcare utilization, and outpatient utilization were calculated using allowable charges for these services in the MedPAR, Home Healthcare, Carrier, and Outpatient Medicare claim files. All costs were adjusted for healthcare cost inflation to US 2017 dollars [32].
Secondary outcomes including all-cause hospitalizations, CVD-related hospitalizations, and SNF stays were identified from claims data. A CVD-related hospitalization was defined as a hospitalization with a primary or secondary discharge diagnosis of coronary heart disease (ICD-9 codes 414.xx), congestive heart failure (ICD-9 codes 398.91, 428.0), myocardial infarction (ICD-9 codes 410.xx, 412, 429.7x), or cerebrovascular accident (stroke) (ICD-9 codes 433.xx, 434.xx).
Other measurements
Participants completed a questionnaire on demographics, history of selected medical conditions, self-reported health status, smoking status (never, former, current) at the time of the sleep visit. Participants were asked about physician diagnosis of diabetes, coronary heart disease (including angina, myocardial infarction, angioplasty, or coronary artery bypass), stroke, congestive heart failure, or chronic obstructive pulmonary disease (COPD). CVD was defined as having a self-reported history of coronary heart disease, stroke, or congestive heart failure. Hypertension was defined using self-reported hypertension, use of antihypertensive medications, having systolic blood pressure ≥ 140 mm Hg, or having diastolic blood pressure ≥ 90 mm Hg [33]. Depressive symptoms were assessed using the Geriatric Depression Scale (GDS); a participant with GDS score ≥ 6 was considered to have depression [34]. Participants who attended an in-clinic visit also had measurements of blood pressure, body weight, and height collected. BMI was calculated as weight in kilograms divided by the square of height in meters. Participants were also asked to bring all medication containers used within the preceding 30 days to the clinic visit. Drugs were identified and recorded by clinic staff, and the information was stored in an electronic drugs inventory database. All medications recorded by the clinics were entered into an electronic medication inventory (San Francisco Coordinating Center, San Francisco, CA). Each medication was matched to its Ingredient(s) based on the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA) [35].
Statistical analysis
Characteristics of the 1,316 men were compared across categories of SDB measures using chi-square or Fisher’s exact test for categorical variables, ANOVA for continuous variables with normal distributions and nonparametric Kruskal–Wallis tests for variables with skewed distributions.
The associations of SDB measures with annualized total healthcare costs and outpatient costs were estimated using generalized linear models (GLMs). GLMs with log link and gamma distribution were used to account for the highly right-skewed distributions of total healthcare costs and outpatient costs and to ensure that the models were well specified based on the results of the Modified Park [36] and Pregibon link [37] tests. Logistic regression models were used to estimate the association of SDB measures with odds of one or more hospitalizations and odds of one or more SNF stays during the 3-year follow-up period.
Base models were adjusted for age and clinical site. Multivariable models were further adjusted for traditional prognostic variables including education, health status, diabetes, hypertension, CVD, COPD, and body mass index at the sleep visit.
Analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC) or Stata, version 14 (StataCorp LLC, College Station, TX).
Results
Study population
Among the 1,316 men in the analytical cohort, mean (SD) age was 76.5 years (5.7); 92.2% were non-Hispanic white; and 12% reported their health status as fair/poor/very poor. There were 529 men (40.2%) who had no evidence of sleep apnea as indicated by AHI < 5/hour, 444 (33.7%) with mild sleep apnea (AHI 5 to <15/hour), and 343 (26.1%) with moderate to severe sleep apnea (AHI ≥ 15/hour). SDB as indicated by ODI was also common in this cohort of older men, with 21.0% of the study participants with ODI between 5 and ≤10/hour (mild), 12.8% with ODI between 10 and ≤15/hour (moderate), and 32.1% with ODI greater than 15/hour (severe). Men with greater severity of sleep apnea as manifested by higher AHI or ODI were older, more likely to report poorer health status and fewer years of education and have diabetes, hypertension, congestive heart failure and higher BMI (Table 1 and Supplementary Table S1).
Table 1.
Characteristics of 1,316 participants by category of apnea hypopnea index at baseline
| AHI | ||||
|---|---|---|---|---|
| Normal (<5.0/hour) | Mild (5.0 to <15/hour) | At least moderate (≥15/hour) | ||
| Baseline Characteristic | (N = 529) | (N = 444) | (N = 343) | P* |
| Age, years, mean (SD) | 76.2 (5.7) | 76.2 (5.7) | 77.3 (5.6) | .01 |
| Caucasian, n (%) | 489 (92.4) | 411 (92.6) | 313 (91.3) | .76 |
| Education, n (%) | .02 | |||
| Less than high school | 17 (3.2) | 20 (4.5) | 14 (4.1) | |
| High school | 56 (10.6) | 58 (13.1) | 63 (18.4) | |
| Beyond high school | 456 (86.2) | 366 (82.4) | 266 (77.6) | |
| Fair, poor, or very poor health status, n (%) | 53 (10.0) | 60 (13.5) | 45 (13.1) | .19 |
| Smoking status, n (%) | .23 | |||
| Never | 216 (40.8) | 181 (40.9) | 147 (42.9) | |
| Past | 302 (57.1) | 254 (57.3) | 195 (56.9) | |
| Current | 11 (2.1) | 8 (1.8) | 1 (0.3) | |
| Diabetes, n (%) | 54(10.2) | 61 (13.8) | 53 (15.5) | .06 |
| Hypertension, n (%) | 344 (65.0) | 316 (71.3) | 262 (76.6) | .001 |
| Coronary heart disease†, n (%) | 161 (30.4) | 124 (27.9) | 114 (33.2) | .27 |
| Stroke, n (%) | 23 (4.3) | 15 (3.4) | 11 (3.2) | .62 |
| Congestive heart failure, n (%) | 18 (3.4) | 30 (6.8) | 25 (7.3) | .02 |
| CVD**, n (%) | 178 (33.6) | 144 (32.4) | 130 (37.9) | .25 |
| COPD or emphysema, n (%) | 29 (5.5) | 24 (5.4) | 11 (3.2) | .25 |
| Body mass index, kg/m2, mean (SD) | 26.2 (3.3) | 27.3 (3.6) | 28.2 (4.1) | <.001 |
| Depression (GDS score ≥ 6), n (%) | 31 (5.9) | 17 (3.8) | 23 (6.7) | .17 |
| Hospitalized in the year prior to sleep visit, n (%) | 67 (12.7) | 65 (14.6) | 43 (12.5) | .59 |
| Outcomes‡ | ||||
| Hospitalized, n (%) | 188 (35.5) | 176 (39.6) | 159 (46.4) | .006 |
| CVD-related hospitalization, n (%) | 96 (18.1) | 87 (19.6) | 89 (25.9) | .02 |
| SNF stay, n (%) | 34 (6.4) | 24 (5.4) | 19 (5.5) | .76 |
| Dead, n (%) | 32 (6.0) | 29 (6.5) | 27 (7.9) | .55 |
| Annualized total healthcare costs | .06 | |||
| Mean (SD) | $7,441 (9,904) | $6,945 (8,644) | $8,305 (10,079) | |
| Median (IQR) | $3,672 (1,536–9,128) | $3,746 (1,641–8,799) | $4,674 (1,947–10,227) | |
| Annualized outpatient costs | .16 | |||
| Mean (SD) | $3,955 (5,192) | $3,641 (3,962) | $4,181 (5,193) | |
| Median (IQR) | $2,772 (1,344–4,950) | $2,708 (1,287–4,632) | $2,980 (1,601–5,064) | |
AHI = apnea hypopnea index; N = number of men in each category; n (%) = number (proportion); CVD = cardiovascular disease; COPD = chronic obstructive pulmonary disease; GDS = Geriatric Depression Scale; SNF = skilled nursing facility; IQR = interquartile range.
*ANOVA (or nonparametric equivalent, i.e. Kruskal–Wallis test) for continuous variables, and chi-square test (or Fisher’s exact test) for categorical variables.
†Coronary heart disease was defined by a self-reported history of a physician diagnosis of angina, myocardial infarction, angioplasty, or coronary artery bypass.
**CVD was defined as having either one of these three conditions: coronary heart disease, stroke, or congestive heart failure.
‡During the 3-year follow-up post-MrOS Sleep visit.
Characteristics (including the distributions of SDB measures) of the 1,316 men in the analytical cohort were similar to those of the 1,595 MrOS men attending the sleep visit who were excluded from analyses because they were not enrolled in an FFS plan (Supplementary Table S2). Although differences in race, educational level, hypertension, and depression were statistically significant, these differences were small in magnitude.
Associations of measures of SDB with total healthcare and outpatient costs
The annualized unadjusted mean (SD) total healthcare and outpatient costs (2017 US dollars) during the 36-month follow-up period were $7,499 (SD 9,552) and $3,908 (SD 4,813), respectively. Mean annualized total healthcare costs during the 3 years following the sleep visit were $7,441 (SD 9,904) for men without SDB, $6,945 (SD 8,644) for men with mild SDB, and $8,305 (SD 10,079) for men with moderate or more severe SDB (p-value for difference in mean total healthcare costs across categories of SDB = 0.06, Table 1). After consideration of age and study enrollment site, there was no evidence of an association between severity of SDB as manifested by higher AHI or ODI and total healthcare costs (Table 2). For example, the cost ratios (CR) of mean total healthcare and outpatient costs were slightly higher among men with moderate to severe sleep apnea (AHI ≥ 15/hour) compared with those without sleep apnea (AHI < 5/hour), but the associations were not significant as shown by the respective CR and the 95% confidence intervals (CI): 1.10 (0.92–1.31) and 1.01 (0.76–1.36). Further consideration of other potential confounders in multivariable models did not alter these results. Among secondary measures of SDB, neither OSA nor %TST<90 was associated with total healthcare costs. When considering outpatient costs, greater %TST<90 was associated with lower outpatient costs in full multivariable models.
Table 2.
Associations of measures of sleep-disordered breathing with mean total and outpatient healthcare costs
| Cost ratio (95% CI) | ||||
|---|---|---|---|---|
| Total healthcare costs | Outpatient care costs | |||
| SDB measure | Age and site adjusted | Multivariable adjusted* | Age and site adjusted | Multivariable adjusted* |
| AHI† | ||||
| <5.0 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 5.0 to <15.0 | 0.93 (0.79–1.10) | 0.91 (0.78–1.08) | 0.87 (0.66–1.14) | 0.86 (0.66–1.13) |
| ≥15.0 | 1.10 (0.92–1.31) | 1.04 (0.86–1.24) | 1.01 (0.76–1.36) | 0.93 (0.69–1.25) |
| ODI† | ||||
| <5.0 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 5.0 to ≤10 | 0.95 (0.78–1.15) | 0.95 (0.78–1.16) | 0.94 (0.68–1.29) | 0.93 (0.68–1.27) |
| 10 to ≤15 | 0.87 (0.69–1.10) | 0.86 (0.68–1.09) | 0.74 (0.51–1.07) | 0.72 (0.50–1.04) |
| >15.0 | 1.07 (0.90–1.28) | 1.01 (0.84–1.21) | 0.93 (0.70–1.23) | 0.84 (0.63–1.13) |
| OSA† | ||||
| <5.0 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 5.0 to <15.0 | 0.96 (0.81–1.14) | 0.94 (0.80–1.11) | 0.87 (0.67–1.15) | 0.86 (0.66–1.13) |
| ≥15.0 | 1.10 (0.92–1.31) | 1.06 (0.89–1.27) | 1.01 (0.76–1.35) | 0.95 (0.71–1.27) |
| %TST<90 | ||||
| <1.0 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 1.0 to <3.5 | 1.02 (0.86–1.22) | 0.97 (0.81–1.15) | 0.88 (0.66–1.17) | 0.80 (0.61–1.06) |
| ≥3.5 | 0.98 (0.82–1.17) | 0.95 (0.78–1.14) | 0.78 (0.59–1.04) | 0.68 (0.51–0.90) |
CI = confidence interval; SDB = sleep-disordered breathing; AHI = apnea hypopnea index; ODI = oxygen desaturation index; OSA = obstructive apneas plus hypopneas with a 4% desaturation; %TST<90 = percent of total sleep time with oxygen saturation <90%.
*Adjusted for age, site, education, health status, comorbid medical conditions (including diabetes, hypertension, CVD, and COPD), and body mass index at the sleep visit.
†Unit is per hour.
Association of measures of SDB with incident all-cause hospitalization, CVD-related hospitalization, and SNF stay
There were 523 (39.7%) men who were hospitalized on at least one occasion during the 3 years following their PSG study including 272 (20.7%) with at least one CVD-related hospitalization (Table 1). A total of 77 men (5.9%) had at least 1 SNF stay and 88 (6.7%) men died during the 3-year-follow-up period.
Men with greater SDB as manifested by high levels of AHI, ODI, or OSA were more likely to experience at least one hospitalization including a CVD-related hospitalization during the subsequent 36 months. In particular, men with moderate to severe sleep apnea (AHI ≥ 15/hour) had a 1.4-fold higher odds of subsequent hospitalization (odds ratio [OR] = 1.43, 95% CI: 1.07–1.90) compared with those without sleep apnea (AHI < 5/hour) in models adjusted for age and study enrollment site (Table 3). Results were similar in models substituting ODI or OSA for AHI. The association of moderate to severe SDB with hospitalization was only slightly attenuated in multivariate models further adjusted for additional potential confounders (OR for AHI ≥ 15/hour vs. <5/hour = 1.36, 95% CI: 1.01–1.83). The associations of moderate to severe SDB as manifested by higher AHI, ODI, or OSA with odds of subsequent hospitalization due to CVD appeared to be similar in magnitude to associations of these measures with all-cause hospitalization, though the latter associations did not reach significance (Supplementary Table S3). Associations of mild SDB as manifested by intermediate values of AHI, ODI, or OSA the subsequent odds of hospitalization and CVD-related hospitalization were weaker in magnitude and not statistically significant. Among men hospitalized, there was no difference in mean length of hospital stays (LOS) according to severity of sleep apnea; mean LOS was 8.8 days for men without sleep apnea, 6.4 days for men with mild sleep apnea, and 8.1 days for those with moderate to severe sleep apnea. Greater nocturnal hypoxemia as manifested by higher %TST<90 was not related to odds of hospitalization. In addition, there was no association between any of the measures of SDB and odds of an SNF stay in either the minimally adjusted or fully adjusted models.
Table 3.
Associations of measures of sleep-disordered breathing with odds of hospitalization and skilled nursing facility stays
| SDB measure | Odds ratio (95% CI) | |||
|---|---|---|---|---|
| Inpatient hospital stays | SNF stays | |||
| Age and site adjusted | Multivariable adjusted* | Age and site adjusted | Multivariable adjusted* | |
| AHI† | ||||
| <5.0 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 5.0 to <15.0 | 1.15 (0.88–1.50) | 1.13 (0.86–1.49) | 0.85 (0.49–1.48) | 0.72 (0.41–1.28) |
| ≥15.0 | 1.43 (1.07–1.90) | 1.36 (1.01–1.83) | 0.80 (0.44–1.46) | 0.60 (0.32–1.13) |
| ODI† | ||||
| <5.0 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 5.0 to ≤10 | 0.96 (0.70–1.33) | 0.96 (0.69–1.33) | 0.45 (0.20–1.00) | 0.44 (0.19–0.98) |
| 10 to ≤15 | 1.32 (0.92–1.92) | 1.33 (0.91–1.93) | 0.78 (0.36–1.72) | 0.67 (0.30–1.49) |
| >15.0 | 1.38 (1.04–1.83) | 1.33 (0.99–1.78) | 1.02 (0.59–1.76) | 0.75 (0.42–1.34) |
| OSA† | ||||
| <5.0 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 5.0 to <15.0 | 1.09 (0.83–1.44) | 1.07 (0.81–1.41) | 0.79 (0.45–1.39) | 0.67 (0.37–1.20) |
| ≥15.0 | 1.41 (1.06–1.88) | 1.37 (1.02–1.84) | 0.80 (0.45–1.42) | 0.61 (0.33–1.13) |
| %TST<90 | ||||
| <1.0 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 1.0 to <3.5 | 1.04 (0.79–1.38) | 1.00 (0.75–1.34) | 0.97 (0.54–1.74) | 0.82 (0.45–1.51) |
| ≥3.5 | 1.22 (0.93–1.62) | 1.20 (0.89–1.61) | 1.11 (0.62–1.97) | 0.82 (0.44–1.53) |
CI = confidence interval; SNF = skilled nursing facility; SDB = sleep-disordered breathing; AHI = apnea hypopnea index; ODI = oxygen desaturation index; OSA = obstructive apneas plus hypopneas with a 4% desaturation; %TST<90 = percent of total sleep time with oxygen saturation <90%.
*Adjusted for age, site, education, health status, comorbid medical conditions (including diabetes, hypertension, CVD, and COPD), and body mass index at the sleep visit.
†Unit is per hour.
Additional analyses
Further sensitivity analyses restricting the study sample to the 1,228 men, who survived 36 months after the sleep visit did not alter the results (data not shown). In addition, analyses excluding the 72 (5.5%) men who initiated treatment for sleep apnea during the 3-year follow-up period post-sleep visit yielded similar results (data not shown).
Discussion
In this longitudinal study of older community-dwelling men, we found that SDB as manifested by higher AHI, ODI, or OSA was similarly associated with a higher risk of hospitalization even after consideration of multiple traditional prognostic indicators. However, measures of SDB were not related to healthcare costs or risk of post-acute SNF stays. The presence of SDB may help identify individuals at increased risk for hospitalization. Future research should evaluate the effect of treatment of SDB on subsequent healthcare utilization.
Our findings suggest a 1.4-fold increase in the risk of hospitalization among older men with moderate to severe SDB, an association that is only explained in part by the greater burden of cardiovascular and other medical conditions among men with SDB. SDB is associated with chronic cardiac, pulmonary, metabolic, and liver disease, which has been attributed to chronic effects of sleep fragmentation, increased work of breathing, and hypoxemia. Although we adjusted for prevalent health problems, those with moderate to severe SDB may have had more severe underlying organ dysfunction or less reserve, resulting in greater vulnerability to decompensation requiring inpatient hospitalization. SDB also has been associated with immune dysregulation and accelerated biological aging [38–40], which may accelerate inflammatory processes and reduce resiliency [41]. Individuals with underlying lung disease and SDB may have more hypoxemia than individuals with either condition and when faced with an exacerbation of lung disease, such individuals may more likely require hospitalization [42]. In addition, patients with SDB may be chronically fatigued and have impaired cognition, which may adversely affect adherence to chronic disease medical regiments.
Our results are in general agreement with other studies that have suggested that SDB is associated with increased risk of hospitalization [17, 18, 21]. A small case–control study (mean age 47.1 years), conducted in 1996 in Manitoba, Canada, comprising 97 obese patients with OSA and 97 controls without OSA, reported that patients in the OSA group had statistically significant higher total number of nights spent in hospitals during a 2-year follow-up period (251 nights for OSA group vs. 90 nights for the control group) [17]. Another Canadian case–control study reported similar findings where OSA patients spent more nights in hospitals than controls (1,118 nights vs. 676 nights) [18]. A large retrospective cohort study in 1,867,876 older veterans (age ≥ 65) done at the Veterans Health Administration (VHA) reported that patients with incident OSA had a 4.5-fold higher odds of hospitalization compared with those without OSA [21]. The magnitude of the association between OSA and hospitalization in these studies is higher than the magnitude of the association between SDB and hospitalization in our study. A plausible explanation for this difference in results may be the difference in the study design (case–control vs. prospective cohort study). In addition, the retrospective cohort study performed at the VHA used the International Classification of Diseases (ICD-9) codes to obtain diagnosis of OSA that may not be as accurate as objectively measured OSA using PSG [21]. Our study strengthens and expands on these earlier findings through our use of objective, quantifiable measures of SDB through PSG, prospective design, and use of community-based sampling (rather than the common practice of clinic-based sampling) of older men not selected on the basis of disease status.
Few previous studies have estimated the associations between SDB and total healthcare costs and utilization in older men and arrived at inconsistent conclusions. Similar to findings from our study, a cross-sectional analysis of a small subgroup of 198 Taiwanese men at least 70 years of age, reported that there was no difference in the total healthcare costs between those with and without sleep apnea [14]. In contrast, utilizing a case–control study in an Israeli population, Tarasiuk et al. found that OSA patients had 1.8-fold higher healthcare costs compared to controls without OSA in a small total sample of 185 older (age ≥65 years) patients [16]. Another cross-sectional study analyzed 6,440 participants (mean age 63.6 years) from the Sleep Heart Health Study (SHHS) and reported that severe SDB problems (AHI > 29.9) and greater nocturnal hypoxemia were associated with higher healthcare utilization, as indirectly measured using the modified CDS [23]. A small case–control study performed in middle-aged adults by Kapur et al. found that medical costs among patients with undiagnosed sleep apnea were almost double the medical costs for controls without sleep apnea, matched for age and gender, but this study did not consider other potential confounders of the association [15]. Potential reasons for these discrepant results could be due to other studies selection of either younger patients (SHHS) or sicker elderly patients compared with the healthier community-dwelling men in MrOS. For example, among the elderly group of 158 participants, Tarasiuk et al. reported 42% of them with poor health status, and among the OSA group, comorbidities such as CVD and hyperlipidemia were highly prevalent [16]. Compared with previously published studies, the advantages of our study include a prospective cohort design, consideration of several potential confounders and use of well-established Medicare claim data to estimate, with validated methods, standardized healthcare costs representative for the older male US Medicare population.
The increased risk of hospitalization for those with moderate to severe sleep apnea compared with those without sleep apnea did not translate to higher subsequent total healthcare costs, outpatient costs, or SNF utilization. Results were generally consistent regardless of which SDB measure was used. This casts doubt on the hypothesis that the costs of SDB treatment can be partially offset by saving health care costs associated with untreated SDB. Results for sensitivity analyses restricting to those who survived at least 36 months following the sleep visit or excluding those who initiated treatment for SDB during follow-up were similar to the finding of the primary analyses. Surprisingly, we found that men with moderate to severe nocturnal hypoxemia had lower outpatient costs compared with men without hypoxemia in the full multivariable model. We do not have a plausible explanation for this finding that may be a spurious result due to random chance alone or numerous comparisons performed. Hence, although treatment of sleep apnea may yield health benefits, our data suggest that lower healthcare costs will likely not be among those benefits.
This study has several strengths, including the use of a prospective cohort study with comprehensively assessed participant characteristics, objective measures of SDB using PSG, enrollment of participants not on the basis of sleep apnea status, published and validated methodology to compute standardized healthcare costs and ascertain healthcare utilization from administrative data, linkage of cohort participants to their Medicare claim data, and inclusion of several possible confounding and mediating factors. However, this study has limitations. First, the cohort included healthy community-dwelling older men, with few non-Caucasian participants. Thus, the results might not be generalizable to women, others from different racial or ethnic groups, older men in poorer health, or those residing in other institutions such as nursing homes. Future studies are needed to confirm our findings and to further investigate associations of SDB and other outcomes, such as long-term nursing home placement. Second, data on total healthcare and outpatient costs, hospital and SNF stays were only available for those men enrolled in Medicare FFS, but not for those enrolled in Medicare Advantage. This limitation is mitigated by the fact that characteristics of men enrolled in Medicare FFS, including proportions with SDB, were similar to those of men enrolled in Medicare Advantage. Third, although our data do not preclude a weak association between moderate to severe sleep apnea and higher total health care cost, the association was not significant perhaps because we did not have a sufficient sample size in the moderate to severe sleep apnea group to detect a small effect. Fourth, we do not have the sample size to establish whether shifts of costs from outpatient or SNF settings to acute care hospitals account for our findings of higher odds of hospitalization, yet no clear increase in total health care costs among those with OSA. Fifth, although we adjusted for education in our analysis, we did not account for other socioeconomic status variables such as income and occupation, which might have confounded the association between sleep apnea and healthcare costs and utilization. Finally, even though we utilized a longitudinal cohort study and controlled for potential confounders and mediators, causality of the relationship between SDB and risk of subsequent hospitalizations cannot be strongly inferred due to the potential for residual confounding.
In conclusion, our results suggest that SDB is associated with higher risk of hospitalization (but not with total healthcare costs) in community-dwelling older men. This association is not entirely explained by a greater number of cardiovascular or medical conditions among those men with SDB. Future studies are needed to evaluate the association between SDB and healthcare costs and utilization among other patient populations and to evaluate the effect of treatment of SDB on these measures of healthcare burden.
Funding
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128.
The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. Dr. Redline was supported in part by 5R35 HL135818. The funding agencies had no direct role in the conduct of the study; the collection, management, analyses, and interpretation of the data; or preparation or approval of the manuscript.
Supplementary Material
Acknowledgments
This manuscript is the result of work supported with resources and use of facilities of the Minneapolis VA Health Care System. The contents do not represent the views of the US Department of Veterans Affairs or the United States Government.
Conflict of interest statement: None declared.
References
- 1. Mehra R, et al. ; Osteoporotic Fractures in Men Study Prevalence and correlates of sleep-disordered breathing in older men: Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2007;55(9):1356–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Young T, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. [DOI] [PubMed] [Google Scholar]
- 3. Cintra FD, et al. Sleep apnea and nocturnal cardiac arrhythmia: a populational study. Arq Bras Cardiol. 2014;103(5):368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gottlieb DJ, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guilleminault C, et al. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol. 1983;52(5):490–494. [DOI] [PubMed] [Google Scholar]
- 6. Hla KM, et al. Coronary heart disease incidence in sleep disordered breathing: the Wisconsin Sleep Cohort Study. Sleep. 2015;38(5):677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kwon Y, et al. ; Osteoporotic Fractures in Men (MrOS) Study Group Sleep-disordered breathing and daytime cardiac conduction abnormalities on 12-lead electrocardiogram in community-dwelling older men. Sleep Breath. 2016;20(4):1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peppard PE, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. [DOI] [PubMed] [Google Scholar]
- 9. Redline S, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182(2):269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shahar E, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163(1):19–25. [DOI] [PubMed] [Google Scholar]
- 11. Blackwell T, et al. ; Osteoporotic Fractures in Men Study Group Associations between sleep-disordered breathing, nocturnal hypoxemia, and subsequent cognitive decline in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2015;63(3):453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yaffe K, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Young T, et al. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. [DOI] [PubMed] [Google Scholar]
- 14. Kao LT, et al. Healthcare service utilization by patients with obstructive sleep apnea: a population-based study. PLoS One. 2015;10(9):e0137459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kapur V, et al. The medical cost of undiagnosed sleep apnea. Sleep. 1999;22(6):749–755. [DOI] [PubMed] [Google Scholar]
- 16. Tarasiuk A, et al. The effect of obstructive sleep apnea on morbidity and health care utilization of middle-aged and older adults. J Am Geriatr Soc. 2008;56(2):247–254. [DOI] [PubMed] [Google Scholar]
- 17. Kryger MH, et al. Utilization of health care services in patients with severe obstructive sleep apnea. Sleep. 1996;19(9 Suppl):S111–S116. [DOI] [PubMed] [Google Scholar]
- 18. Ronald J, et al. Health care utilization in the 10 years prior to diagnosis in obstructive sleep apnea syndrome patients. Sleep. 1999;22(2):225–229. [DOI] [PubMed] [Google Scholar]
- 19. Tarasiuk A, et al. Determinants affecting health-care utilization in obstructive sleep apnea syndrome patients. Chest. 2005;128(3):1310–1314. [DOI] [PubMed] [Google Scholar]
- 20. Albarrak M, et al. Utilization of healthcare resources in obstructive sleep apnea syndrome: a 5-year follow-up study in men using CPAP. Sleep. 2005;28(10):1306–1311. [DOI] [PubMed] [Google Scholar]
- 21. Diaz K, et al. Obstructive sleep apnea is associated with higher healthcare utilization in elderly patients. Ann Thorac Med. 2014;9(2):92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Greenberg-Dotan S, et al. Gender differences in morbidity and health care utilization among adult obstructive sleep apnea patients. Sleep. 2007;30(9):1173–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kapur VK, et al. ; Sleep Heart Health Research Group The relationship between chronically disrupted sleep and healthcare use. Sleep. 2002;25(3):289–296. [PubMed] [Google Scholar]
- 24. Blank JB, et al. Overview of recruitment for the Osteoporotic Fractures in Men Study (MrOS). Contemp Clin Trials. 2005;26(5):557–568. [DOI] [PubMed] [Google Scholar]
- 25. Orwoll E, et al. Design and baseline characteristics of the Osteoporotic Fractures in Men (MrOS) Study – a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–585. [DOI] [PubMed] [Google Scholar]
- 26. Redline S, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21(7): 759–767. [PubMed] [Google Scholar]
- 27. Quan SF, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20(12):1077–1085. [PubMed] [Google Scholar]
- 28. Iber C, Ancoli-Israel S, Chesson A, Quan SF.. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 29. Martin JL, et al. Nighttime oxygen desaturation and symptoms of sleep-disordered breathing in long-stay nursing home residents. J Gerontol A Biol Sci Med Sci. 2005;60(1): 104–108. [DOI] [PubMed] [Google Scholar]
- 30. Schousboe JT, et al. Estimation of standardized hospital costs from Medicare claims that reflect resource requirements for care: impact for cohort studies linked to Medicare claims. Health Serv Res. 2014;49(3):929–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schousboe JT, et al. Pre-fracture individual characteristics associated with high total health care costs after hip fracture. Osteoporos Int. 2017;28(3):889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schousboe JT, et al. Estimating true resource costs of outpatient care for Medicare beneficiaries: standardized costs versus Medicare payments and charges. Health Serv Res. 2016;51(1):205–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fung MM, et al. ; Osteoporotic Fractures in Men Research Group Decreased slow wave sleep increases risk of developing hypertension in elderly men. Hypertension. 2011;58(4):596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sheikh JI, Yesavage JA. Geriatric depression scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. [Google Scholar]
- 35. Pahor M, et al. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10(4):405–411. [DOI] [PubMed] [Google Scholar]
- 36. Manning WG, et al. Estimating log models: to transform or not to transform? J Health Econ. 2001;20(4):461–494. [DOI] [PubMed] [Google Scholar]
- 37. Pregibon D. Goodness of link tests for generalized linear models. Appl Stat. 1980;29:154. [Google Scholar]
- 38. Riestra P, et al. Obstructive sleep apnea risk and leukocyte telomere length in African Americans from the MH-GRID study. Sleep Breath. 2017;21(3):751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jackowska M, et al. Short sleep duration is associated with shorter telomere length in healthy men: findings from the Whitehall II cohort study. PLoS One. 2012;7(10):e47292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cribbet MR, et al. Cellular aging and restorative processes: subjective sleep quality and duration moderate the association between age and telomere length in a sample of middle-aged and older adults. Sleep. 2014;37(1):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Geovanini GR, et al. Elevations in neutrophils with obstructive sleep apnea: the Multi-Ethnic Study of Atherosclerosis (MESA). Int J Cardiol. 2018;257:318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sanders MH, et al. ; Sleep Heart Health Study. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med. 2003;167(1):7–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.