Abstract
Study Objectives
To test whether the sleep-wake cycle disruption in patients hospitalized with traumatic brain injury (TBI) (1) is also found in patients with traumatic injuries other than TBI (non-TBI) and (2) is associated with a weaker or abnormal circadian clock signal.
Methods
Forty-two non-mechanically ventilated and non-sedated patients hospitalized for moderate-to-severe TBI were compared to 34 non-TBI patients. They wore wrist actigraphs for 9.4 ± 4.2 days, starting 19.3 ± 12.6 days post-injury. Of these, 17 TBI and 14 non-TBI patients had their urine collected every hour for 25 hours, starting 18.3 ± 12.3 days post-injury. We calculated urinary 6-sulfatoxymelatonin concentration to obtain total 24-hour excretion, excretion onset, offset, duration, amplitude, and acrophase. Using Student’s t-tests, we compared groups on actigraphy (daytime activity ratio, nighttime total sleep time, and fragmentation index) and melatonin variables. We investigated associations between melatonin and actigraphy variables using Pearson’s correlations.
Results
TBI patients had poorer daytime activity ratio (TBI: 77.5 ± 9.4%; non-TBI: 84.6 ± 6.9%), shorter nighttime total sleep time (TBI: 353.5 ± 96.6 min; non-TBI: 421.2 ± 72.2 min), and higher fragmentation index (TBI: 72.2 ± 30.0; non-TBI: 53.5 ± 23.6) (all p-values < 0.01). A melatonin rhythm was present in both groups, and no group differences were found on melatonin variables. No associations were found between melatonin and actigraphy variables in TBI patients.
Conclusion
Moderate-to-severe TBI patients have more serious sleep-wake disturbances than non-TBI patients hospitalized in the same environment, suggesting that the brain injury itself alters the sleep-wake cycle. Despite their deregulated 24-hour sleep-wake cycle, TBI patients have a normal circadian clock signal.
Keywords: sleep, circadian rhythms, traumatic brain injury, melatonin, actigraphy, acute care
Statement of Significance.
This is the first study to assess melatonin rhythm in hospitalized patients with a traumatic brain injury (TBI) but who are not mechanically ventilated or continuously sedated, and to use hospitalized trauma patients without TBI to compare sleep-wake and melatonin data, thus controlling for important medical and environmental confounds. Actigraphic results showed more severe sleep-wake disturbances in TBI compared to non-TBI patients, supporting a role of the brain injury itself in the sleep-wake disturbances observed during the hospital stay. However, sleep-wake disturbances do not appear to result from a trauma to the central circadian clock since the 24-hour melatonin rhythm of TBI patients was normal. As a modifiable behavior, sleep remains a promising target to optimize recovery after TBI.
Introduction
A majority of patients with moderate-to-severe traumatic brain injury (TBI) suffer from persistent sleep-wake disturbances [1]. Patients complain of fragmented sleep at night and of difficulties staying awake during the day. These sleep-wake disturbances seriously limit their functional recovery. We previously reported that sleep-wake disturbances, with severe fragmentation of sleep and wake episodes, were already present during the hospital stay, after continuous sedation was discontinued and patients had reached medical stability [2–4]. We also observed that only 50% of these patients regain their ability to maintain continuous periods of daytime wakefulness and nighttime sleep during their hospital stay [2]. Notably, those who returned to a consolidated sleep-wake cycle were also those who showed better recovery from posttraumatic amnesia and lower disability at hospital discharge [2]. A better understanding of the causes underlying sleep-wake disturbances in TBI patients during their hospital stay, including the specific contribution of the brain injury itself, may help develop appropriate interventions to restore sleep-wake consolidation and, potentially, accelerate functional recovery of TBI patients.
Among the possible causes of sleep-wake disturbances observed in hospitalized TBI patients, the most obvious suspects are environmental factors and those associated with pain, medication and frequent nursing care interventions [5]. As these factors are common to all hospitalized patients with severe trauma, comparing TBI patients to patients with severe traumatic injuries but no TBI (non-TBI patients, e.g. orthopedic and spinal cord injuries) may help to identify the specific contribution of brain injury to sleep-wake disturbances.
Another possible cause of sleep-wake disturbances in TBI patients is damage to the complex neural pathways involved in the circadian control of sleep-wake regulation. The main circadian clock, located in the suprachiasmatic nuclei of the hypothalamus, generates rhythms of about 24 hours for all physiological and behavioral functions, including sleep timing. A robust circadian signal is essential to a well-consolidated sleep-wake cycle, with a main sleep episode at night and a long, sustained period of alert wakefulness during the day [6]. Conversely, a weak or disrupted circadian signal results in perturbations similar to those documented in hospitalized TBI patients, including fragmented nighttime sleep and excessive sleepiness and naps during the daytime [2–4].
A recognized marker of circadian output is pineal melatonin production, whose timing is tightly controlled by the main circadian clock [7]. When the circadian clock is correctly synchronized to the external day-night cycle, melatonin production shows elevated levels by night and is almost undetectable by day. The few studies that have investigated the melatonin rhythms of TBI patients hospitalized in the intensive care unit (ICU) reported decreased melatonin production and a disturbed melatonin rhythm [8–10]. However, all of these studies were conducted among mechanically ventilated patients who were under continuous sedation, which not only prevents the observation a natural sleep-wake cycle but could also directly alters circadian function and melatonin production [11]. To date, no study has examined the melatonin rhythm concurrently with the sleep-wake cycle in non-sedated hospitalized TBI patients.
In the present study, we first tested whether TBI patients in the acute phase post-injury, no longer mechanically ventilated or continuously sedated, have more severe sleep-wake cycle disturbances than non-TBI patients with severe traumatic injuries, hospitalized in the same environment. Secondly, we compared the melatonin rhythm of TBI patients to that of non-TBI patients and assessed whether an abnormal circadian rhythm of melatonin production was associated with the sleep-wake disturbances observed among TBI patients during their hospital stay.
Methods
Patients
Forty-two moderate-to-severe TBI patients were recruited from Hôpital du Sacré-Coeur de Montréal, a level-1 trauma center affiliated to the Université de Montréal, between January 2010 and June 2016. TBI was defined as an alteration in brain function or other evidence of brain pathology caused by an external force [12], and TBI severity was assessed upon emergency department admission, prior to intubation, using the Glasgow Coma Scale (GCS) [13]. TBI patients were included if they were hospitalized in the ICU for their TBI.
Thirty-four non-TBI patients having suffered severe traumatic injuries (i.e. orthopedic and/or spinal cord injuries) and requiring extensive medical care and medication were recruited for the control group. They were recruited from the same hospital between January 2012 and January 2016. Severe orthopedic injury was defined as a complex traumatic injury, such as multiple fractures with or without damage to peripheral nerves or to the vascular system, which necessitates intervention by a specialized multidisciplinary team. Although it is difficult to completely rule out mild TBI in patients with orthopedic and spinal cord injury, no patient from the non-TBI group had evidence of brain injury on the CT scan.
Patients were excluded if they were younger than 16 or older than 65 years old; were quadriplegic; had a history of substance abuse; had a diagnosed psychiatric, neurological, or sleep disorder prior to injury; suffered any damage to both eyes or the optic nerve (modifying light perception); or if they had a prior history of TBI. Written informed consent for participation was obtained from patients’ families, and the study was approved by the hospital ethics committee.
Thirty patients of the TBI group were also included as participants in one, two, or three previous publications aiming at answering different questions [2, 4, 14].
Measures
Clinical and demographic measures
Age, sex, GCS score at admission, mechanism of injury, length of ICU stay, and hospital length of stay where recorded in both groups. The Injury Severity Score, a scale that assesses severity of traumatic injuries based on work injury of six body systems (i.e. head/neck, face, chest, abdomen, extremity, external), was also calculated to represent overall injury severity [15]. We also calculated the mean daily dose of sedatives and analgesics (lorazepam, midazolam, propofol, morphine, hydromorphones, and fentanyl) administered during actigraphy recording.
Sleep-wake assessments
Sleep-wake cycle disturbances were assessed using actigraphy. Patients wore a wrist actigraph (Actiwatch-L or Actiwatch-Spectrum, Philips Healthcare, Andover, MA) on a non-paralyzed arm starting in the ICU, and continuing throughout hospitalization on regular neurological/orthopedic wards. To obtain a meaningful assessment of the sleep-wake cycle, actigraphy recordings began when continuous sedation and analgesia had ceased for at least 24 hours, and once patients reached a score ≥3 on the Rancho Los Amigos scale [16], indicative of apparent physical reactivity to internal and external stimuli. At this stage, patients had reached medical stability, defined by the absence of mechanical ventilation, fever, active infections, hemodynamic instability, and elevated intracranial pressure.
Data from the actigraphs were uploaded into dedicated software (Actiware 5.0) and activity counts were derived per 1-minute epoch. For all days of actigraphy recording, activity counts were summed for the daytime (7:00–21:59) and for nighttime (22:00–6:59). Daytime and nighttime were identified according to the light/dark schedule of the hospital. We measured consolidation of the 24-hour sleep-wake cycle using the daytime activity ratio (DAR), as previously described [2, 4]. The DAR is the ratio of total 24-hour activity occurring in the daytime (daytime activity/24-hour activity). Therefore, a higher DAR represents a better consolidation of activity in the daytime and of rest in the nighttime.
In addition to sleep-wake cycle assessments, the Actiware scoring program was applied to nighttime actigraphic data (22:00–6:59) to calculate two parameters estimating nighttime sleep, using an automatic wake detection threshold of 40 activity counts per minute: (1) total sleep time, which is the number of minutes scored as sleep during the nighttime period and (2) nighttime fragmentation index, which is an index of restlessness that quantifies the frequency of switches between immobility and mobility as an estimate of the frequency of sleep interruptions.
Assessment of melatonin rhythm
Melatonin production was estimated using the urinary excretion of 6-sulfatoxymelatonin (aMT6s), melatonin’s main metabolite [7, 17]. Characteristics of the 24-hour melatonin rhythm were evaluated in a subset of 17 TBI and 14 non-TBI patients, before or during their actigraphic assessment. These patients were wearing a urinary catheter, which allowed for hourly urine collection without perturbing the patient’s sleep. This approach also had the advantage of not requiring the collaboration of patients who were still agitated or confused. Collection of all urine also provided the opportunity to estimate total 24-hour melatonin production in addition to hourly concentration of aMT6s [18].
During the 24-hour period of urine collection, the entire contents of the urine drainage bag was collected every hour. After measuring total volume, urine was frozen at −20°C in two 5-mL aliquots. Hourly concentration of aMT6s (in ng/mL) was assessed in duplicate using Bühlmann ELISA kits (ALPCO Diagnostics, Windham, NH). The kit used had a minimum detection limit of 1.5 ng/mL, an intra-assay variation of 7.1% and an inter-assay variation of 11.9%. To obtain total 24-hour excretion (ng), the concentration of aMT6s in each sample was multiplied by the volume and results from all 24 samples were added. The onset and offset of melatonin secretion were estimated by the hour of sampling at which aMT6s concentration exceeded the average of the three preceding (onset) or following (offset) samples by 100% [19, 20]. Duration of melatonin secretion was defined as the interval between estimated onset and offset. A Cosinor analysis [21] was also carried out to quantify amplitude (difference between estimated peak value and estimated mean value, in ng/mL) and acrophase (clock time of the estimated peak value, in h) of the rhythm.
Statistical analyses
Using Student’s t-tests, TBI and non-TBI groups were compared on variables assessed with actigraphy and aMT6s excretion. Chi-square statistics were used to compare nominal data. Bivariate correlation analyses were carried out using Pearson’s correlation coefficient to assess associations between actigraphy and aMT6s variables in TBI patients. Statistical significance was set at p <0.05.
Results
Patient characteristics
Both patient groups were comparable for age and sex (Table 1), and in both groups, the main mechanism of injury was a motor vehicle accident. The TBI group had a longer length of ICU stay and hospital length of stay. Mean daily dose of analgesics during actigraphy recording was higher in the non-TBI group than in the TBI group.
Table 1.
Group characteristics (%; mean ± SD) of the patients with TBI or without TBI (non-TBI)
| Characteristics | TBI (n = 42) | Non-TBI (n = 34) | p-value |
|---|---|---|---|
| Men/women (%) | 69.0% | 67.6% | 0.90 |
| Age | 31.1 ± 14.0 | 33.9 ± 15.2 | 0.41 |
| GCS at admission | 7.8 ± 3.4 | 14.7 ± 1.2 | <0.001 |
| Injury Severity Score | 30.4 ± 10.6 | 19.8 ± 8.9 | <0.001 |
| Length of ICU stay (days) | 21.4 ± 13.7 | 4.9 ± 4.2 | <0.001 |
| Hospital length of stay (days) | 41.5 ± 24.8 | 25.1 ± 12.9 | <0.001 |
| Start of actigraphy (days post-injury) | 20.6 ± 13.0 | 11.8 ± 8.0 | <0.001 |
| Length of actigraphy recording (days) | 10.2 ± 4.6 | 8.5 ± 3.6 | 0.07 |
| Mean daily dose of sedatives administered during actigraphy (mg) | 0.3 ± 1.2 | 0.3 ±1.4 | 0.98 |
| Mean daily dose of analgesics administered during actigraphy (mg) | 4.9 ± 18.9 | 19.8 ± 18.0 | <0.01 |
Sleep-wake cycle and nighttime sleep quality assessed with actigraphy
On average, patients wore the actigraph for 9.5 ± 4.2 days with no significant difference between groups (Table 1). Actigraphic recordings started 16.7 ± 11.8 days post-injury; this delay was shorter for the non-TBI than the TBI group (Table 1).
Patients in the TBI group had a lower DAR than those in the non-TBI group (Table 2), reflecting that their sleep and wake periods were more randomly dispersed throughout the day and night. As in previous studies [2–4], we used a threshold ratio of 0.80 to define a consolidated sleep-wake cycle. Over the duration of the actigraphic recording, TBI patients had a higher percentage of days with a DAR <0.80 than non-TBI patients (Table 2).
Table 2.
Sleep-wake cycle and nighttime sleep characteristics (mean ± SD) in patients with TBI or without TBI (non-TBI)
| Actigraphy variables | TBI (n = 42) | Non-TBI (n = 34) | p-value |
|---|---|---|---|
| Mean DAR (%) | 0.78 ± 0.09 | 0.85 ± 0.07 | <0.001 |
| Days with DAR <0.80 (% of recording) | 48.8 ± 33.0 | 20.5 ± 28.3 | <0.001 |
| Total sleep timea (min) | 353 ± 97 | 421 ± 73 | 0.002 |
| Mean fragmentation indexa | 72.2 ± 30.0 | 53.5 ± 23.6 | 0.006 |
aNighttime.
Regarding estimated nighttime (22:00–6:59) total sleep time, patients in the TBI group slept on average 68 minutes less than non-TBI patients (Table 2). Sleep of TBI patients also showed a higher fragmentation index than non-TBI patients (Table 2), suggesting a more restless sleep.
Melatonin rhythm estimated with urinary aMT6s excretion
Urine collection took place 18.3 ± 12.3 days post-injury (TBI: 23.3 ± 14.1 days post-injury; non-TBI: 12.5 ± 6.4 days post-injury). In 11 TBI and 5 non-TBI patients, actigraphy recording could not be conducted at the same time as urine collection but had to be postponed until their Rancho Los Amigos scale of cognitive functioning score (i.e. level of movement and physical reactivity to internal and external stimuli) reached ≥3 to provide valid recordings of their sleep-wake cycle [16]. On average, melatonin assessment occurred 1.6 ± 6.7 days before the start of actigraphy recording in TBI patients, and 1.4 ± 6.7 days after the start of actigraphic recording in non-TBI patients. In all patients, the melatonin rhythm was always assessed either before or during actigraphic recording.
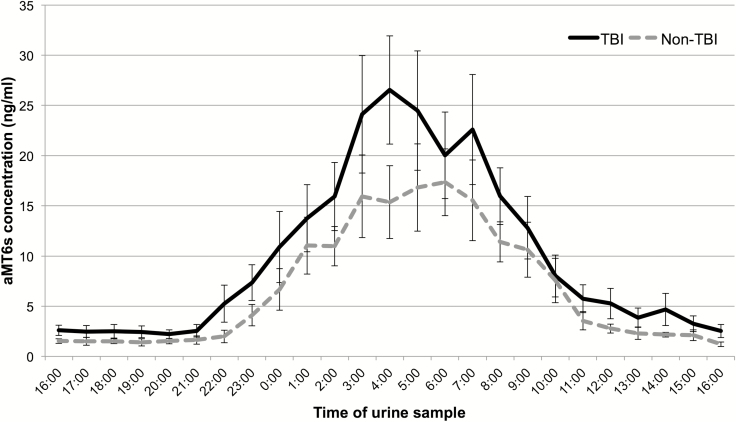
Hourly concentration of aMT6s excretion indicated the presence of a significant 24-hour rhythm of melatonin production that was very similar in the two groups (Figure 1). As shown in Table 3, there was no significant difference on any descriptive variables, including timing of secretion (onset, offset and acrophase), total excretion, and estimated amplitude. Although actigraphic variables were significantly different between the two subgroups (Table 3), TBI patients had a circadian melatonin rhythm comparable to that of non-TBI patients (see Figure 2 for representative patients). Moreover, there was no significant correlation between actigraphic and melatonin variables, either for the entire group of patients (p-values > 0.09) or for the subgroup of TBI patients (p-values > 0.05). These associations were still nonsignificant when correlations were restricted to the first 72 hours following urine collection (this analysis was possible in 23 patients: 10 TBI and 13 non-TBI).
Figure 1.
Average 24-hour melatonin profiles of the TBI and non-TBI groups. Average 24-hour profiles of urinary concentration of aMT6s for the TBI (solid line) and non-TBI (dashed line) groups. Data points represent the hourly concentrations of aMT6s (ng/mL), accumulated in the hour prior to the urine collection time reported on the x-axis. Error bars represent SE.
Table 3.
Characteristics (mean ± SD) of sleep-wake variables, aMT6s excretion, and circadian estimates in the subgroups of patients with TBI or without TBI (non-TBI)
| TBI (n = 17) | Non-TBI (n = 14) | p-value | |
|---|---|---|---|
| Actigraphy variables | |||
| Mean DAR | 0.76 ± 0.09 | 0.86 ± 0.05 | <0.001 |
| Days with DAR <0.80 (% of recording) | 55.2 ± 28.9 | 20.4 ± 28.6 | <0.01 |
| Total sleep timea (min) | 346 ± 101 | 445 ± 54 | <0.01 |
| Mean fragmentation indexa | 80.5 ± 34.0 | 54.7 ± 22.5 | 0.021 |
| aMT6s excretion | |||
| Total 24-hour excretion (ng) | 14 371 ± 9934 | 12 845 ± 5652 | 0.62 |
| Onset (hour:minute) | 23:25 ± 2:22 | 23:38 ± 1:54 | 0.77 |
| Offset (hour:minute) | 08:07 ± 2:51 | 07:38 ± 1:44 | 0.59 |
| Duration of secretion (hour) | 8.7 ± 2.8 | 8.0 ± 2.08 | 0.44 |
| Circadian amplitude (ng/mL) | 12.0 ± 7.5 | 8.1 ± 4.7 | 0.10 |
| Circadian acrophase (hour:minute) | 05:08 ± 2:14 | 05:18 ± 1:35 | 0.81 |
aNighttime.
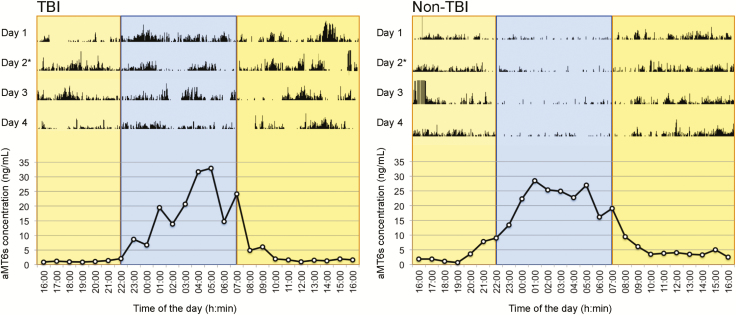
Figure 2.
Example of actograms and 24-hour melatonin profiles of TBI and non-TBI patients. Examples of typical actigraphic findings of a TBI (left) and a non-TBI patient (right), with 24-hour profiles of urinary excretion of aMT6s, corresponding to day 2 of each actogram. For actograms (upper panels), total activity counts for each minute of recording are illustrated by vertical dark lines, on a scale of 0–1000 activity counts. Daytime hours (07:00–22:00 hours) are shown in yellow and nighttime hours (22:00–07:00 hours) in blue. For 24-hour profiles of aMT6s excretion (lower panels), data points represent hourly concentrations (ng/mL).
Discussion
We found more sleep-wake cycle disruptions in TBI patients than in non-TBI patients hospitalized in a similar environment, as shown by weaker sleep-wake cycle consolidation, as well as decreased sleep time and more restless sleep during the night. Despite having a more disturbed sleep-wake cycle, TBI patients had a circadian melatonin rhythm comparable to that of non-TBI patients, in both timing and amplitude. Total melatonin production estimated with 24-hour aMT6s excretion was also similar in the two groups. Moreover, characteristics of the melatonin circadian rhythm were not associated with the sleep-wake disturbances observed in TBI patients during their hospital stay. Our results bring new elements that support a role of the brain injury itself in the sleep-wake cycle disturbances observed in TBI patients during the hospital stay, and suggest that these disturbances are not caused by an alteration in the circadian output signal.
Our study is the first to use a hospitalized trauma population of non-TBI patients as a control group to compare sleep-wake and melatonin data, thus partially controlling for environmental and medical factors influencing sleep and circadian rhythms. Additionally, both patient groups were bedridden at the time of the study, which made actigraphic data comparable between the two groups. Results from non-TBI patients clearly show that it is possible to attain consolidated sleep-wake cycle during the hospital stay, in spite of pain, medication and frequent nursing care, and after a severe traumatic injury. It is therefore unlikely that the severe sleep-wake disturbances observed in TBI patients result essentially from the hospital environment and conditions of nursing care, but rather point to the brain injury itself as the main source of these disturbances.
Contrary to our results, previous studies systematically found a disruption in melatonin secretion in acute TBI [8–10]. This discrepancy between our results and previous studies may be due to the fact that patients in previous studies were tested under continuous sedation and analgesia, and were mechanically ventilated. Continuous sedation and mechanical ventilation are major confounds in the study of sleep and circadian rhythms [11, 22]. Our study is the first to measure the sleep-wake cycle and melatonin rhythm in the waking stage after TBI, being close to the injury itself and still in the acute phase, but enabling the assessment of a more naturally occurring sleep-wake cycle and melatonin rhythm than in previous studies. Moreover, quantifying urinary concentration of aMT6s on an hourly basis provided us with the ability to assess the phase of the melatonin rhythm, which previous acute studies were unable to do. Because our subgroup of patients were all wearing a urinary catheter, such frequent urine collection was possible without causing any sleep disruption, and collection of all urine allowed for the measurement of total 24-hour melatonin production.
Despite that nearly all TBI patients showed an absence of sleep-wake cycle consolidation in the first days of testing, the majority of them were within the normal range of 24-hour aMT6s excretion for healthy adults [23], and the timing of melatonin production reflected a normal nocturnal rhythm, identical to the rhythm observed in non-TBI patients [24]. As melatonin measures took place either slightly before or during actigraphy recording, they suggest that the circadian output signal was normal despite concomitant or subsequent sleep-wake cycle disturbances. Furthermore, the absence of correlation between actigraphy and melatonin variables shows that the circadian timing or amplitude was not related either to sleep-wake cycle consolidation or to estimated nighttime sleep quality. It is not possible to exclude the possibility that injury to the pathways linking the main circadian clock to brain areas involved in sleep-wake regulation contribute to sleep-wake disturbances, but our findings show that sleep-wake disturbances among non-sedated TBI patients are not caused by an impairment of the circadian signal itself.
Our results contradict the hypotheses that the hospital environment and an altered circadian signal explain severe sleep-wake disturbances in TBI patients. However, even if transmission of the circadian signal is intact, it may be that the pathways responsible for subsequently maintaining sleep and wake are affected by the brain injury. The hypothalamus has a crucial role in sleep-wake regulation, but it is also a vulnerable brain region in the context of a TBI [25]. Disruption of hypothalamic function can result in deregulation of wake-promoting neurotransmitters, including orexin and histamine, two wake-promoting neurotransmitters. Another hypothesis suggests that a disconnection in the ascending reticular activating system (ARAS), a brain pathway that is key in regulating wakefulness, could also cause disruptions of wakefulness. As most ARAS regions, namely the pons, the hypothalamus and the thalamus, are vulnerable to TBI [25, 26], disconnection in the ARAS could have a direct impact on TBI patients’ ability to remain awake for extensive periods of time, therefore leading to frequent short bouts of sleep.
Dissociating the effects of injury, hospital environment, and medication on sleep and circadian rhythms
Dissociating the TBI from concomitant injuries, the hospital environment and the treatment intensity remains an important challenge in understanding the direct role of the brain injury in acute sleep-wake disturbances. Although our TBI and non-TBI groups were well matched in terms of age, sex, and absence of pre-existing neurological, psychiatric, or sleep disorders, the non-TBI group had lower Injury Severity Score, shorter ICU stay, and was hospitalized for a shorter period of time. These group differences were expected given the nature of the injuries of each patient group. It took a longer time for TBI patients than non-TBI patients to attain medical stability and a state when meaningful actigraphic assessments of the sleep-wake cycle could be performed. As a result, the interval between injury and data collection was longer in TBI than in non-TBI patients, yet TBI patients showed more severe sleep-wake disturbances. It is possible that the melatonin rhythm is altered in the first days after the TBI and then, returns to normal when medical stability is reached. However, since TBI patients are sedated in the first days post-injury, it is difficult to dissociate the effect of sedation from that of the brain injury itself.
Several studies indicate that key zeitgebers (e.g. light, feeding, pharmacological manipulations, social interactions) are profoundly disturbed in the hospital setting, particularly in the ICU [5, 11, 27, 28]. These rhythm abnormalities can influence both the sleep-wake cycle and circadian function. Given that the TBI group had a significantly longer stay in the ICU than the non-TBI group, this may have contributed to their severely disturbed sleep-wake cycle. However, the fact that melatonin rhythm was normal despite sleep-wake cycle disturbances in the TBI group suggests that this disturbance was not of circadian origin, though it may have resulted in part from a prolonged exposure to the critical care environment. Unfortunately, it remains a challenge to dissociate the effect of the brain injury from that of a prolonged ICU stay.
The non-TBI group also had a higher mean daily dose of analgesics during the actigraphy recording period, which may be due to the importance of their physical (non-brain) injuries. It may also be due to a greater ability to communicate pain than TBI patients. Proper relief from pain may be necessary to ensure sleep continuity in severely injured patients [27–30].
Limitations of the study
A limitation of the study was that the intensity and frequency of nursing care was not directly measured and could have fragmented sleep periods. Therefore, we cannot exclude that the higher fragmentation index observed in the TBI group compared to the non-TBI group could be due to frequent nurse or medical intervention.
Actigraphy has limitations to evaluate sleep quantity and quality. While high activity levels are expected to be incompatible with sleep, the correspondence between rest and sleep is more a matter of interpretation. Differences in estimates of sleep quantity and quality between TBI and non-TBI patients certainly reflect less restful sleep during nighttime in TBI patients, even if a precise evaluation of sleep quality and sleep architecture cannot be done. However, it is not possible to distinguish sleep from rest episodes when low levels of activity are recorded in bedridden patients. Because of this limitation, we have decided to not present total sleep time per 24 hour in our two groups of patients. Despite these limitations, actigraphy remains the method of choice to assess the sleep-wake cycle over many consecutive days. It is well tolerated in severely injured patients and proved to be a very useful and inexpensive tool to assess sleep-wake quality in hospitalized patients.
In 16 participants, it was not possible to record actigraphy and measure melatonin production at the same time. We were constrained to carry out urine collections when patients still had a urinary catheter, but at this point, many patients did not exhibit sufficient movements to render actigraphy recording possible or valid. Therefore, actigraphy had to be postponed for a few days in these patients. However, melatonin data were always collected either before or during actigraphy recording. Our conclusion is that a normal melatonin rhythm observed before or during actigraphic assessment excludes the possibility that sleep-wake disturbances were caused by an abnormal circadian output, as it is unlikely that a melatonin circadian rhythm disappears as the patient recovers.
Conclusion
Using actigraphy, this study showed that non-mechanically ventilated and non-sedated patients hospitalized for a moderate-to-severe TBI have poorer sleep-wake cycle consolidation, shorter nighttime total sleep time, and more sleep fragmentation than non-TBI patients hospitalized in the same environment. These results point to the role of the brain injury itself in altering sleep and wake regulation. Despite severe sleep-wake disturbances, TBI patients had a robust melatonin rhythm, and there was no association between characteristics of the melatonin rhythm and the observed sleep-wake disturbances during the hospital stay. Although many factors could influence the sleep-wake cycle of hospitalized TBI survivors, our study suggests that neural mechanisms other than the circadian clock may be responsible for post-TBI sleep-wake disturbances. Impacts of presenting such a poor sleep in the acute and post-acute stages of TBI on neuronal, cognitive, and functional recovery are still emerging, but suggest that sleep disturbances and poorer brain recovery are associated [4]. Since sleep is a modifiable behavior, it could be a promising target to optimize recovery after TBI.
Funding
This study was supported by the Canadian Institutes of Health Research (grant no. 115172) and by the Fonds de Recherche du Québec - Santé (grant no. 24742).
Acknowledgments
The authors thank Marie-Josée Quinn for dosing melatonin samples. They also wish to thank the patients and their families for their collaboration, as well as the staff of the Intensive Care Unit, Neurological Ward, and Orthopedic Ward for their help in monitoring patients during actigraphy recordings. Address where work was conducted: Hôpital du Sacré-Coeur de Montréal, Centre intégré universitaire de santé et de services sociaux du Nord-de-l’Île-de-Montréal, 5400 boul. Gouin Ouest, local E-0330, Montréal, Québec H4J 1C5, Canada.
Conflict of interest statement. None declared.
References
- 1. Duclos C, et al. Sleep and wake disturbances following traumatic brain injury. Pathol Biol (Paris). 2014;62(5):252–261. [DOI] [PubMed] [Google Scholar]
- 2. Duclos C, et al. Rest-activity cycle disturbances in the acute phase of moderate to severe traumatic brain injury. Neurorehabil Neural Repair. 2014;28(5):472–482. [DOI] [PubMed] [Google Scholar]
- 3. Duclos C, et al. Evolution of severe sleep-wake cycle disturbances following traumatic brain injury: a case study in both acute and subacute phases post-injury. BMC Neurol. 2016;16(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Duclos C, et al. Parallel recovery of consciousness and sleep in acute traumatic brain injury. Neurology. 2017;88(3):268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pisani MA, et al. Sleep in the intensive care unit. Am J Respir Crit Care Med. 2015;191(7):731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dijk DJ, et al. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166(1):63–68. [DOI] [PubMed] [Google Scholar]
- 7. Arendt J. Melatonin: characteristics, concerns, and prospects. J Biol Rhythms. 2005;20(4):291–303. [DOI] [PubMed] [Google Scholar]
- 8. Paparrigopoulos T, et al. Melatonin secretion after head injury: a pilot study. Brain Inj. 2006;20(8):873–878. [DOI] [PubMed] [Google Scholar]
- 9. Paul T, et al. Disturbance of circadian rhythms in analgosedated intensive care unit patients with and without craniocerebral injury. Chronobiol Int. 2007;24(1):45–61. [DOI] [PubMed] [Google Scholar]
- 10. Seifman MA, et al. Measurement of serum melatonin in intensive care unit patients: changes in traumatic brain injury, trauma, and medical conditions. Front Neurol. 2014;5:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Korompeli A, et al. Circadian disruption of ICU patients: a review of pathways, expression, and interventions. J Crit Care. 2017;38:269–277. [DOI] [PubMed] [Google Scholar]
- 12. Menon DK, et al. ; Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health. Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil. 2010;91(11):1637–1640.21044706 [Google Scholar]
- 13. Teasdale G, et al. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. [DOI] [PubMed] [Google Scholar]
- 14. Wiseman-Hakes C, et al. Sleep in the acute phase of severe traumatic brain injury: a snapshot of polysomnography. Neurorehabil Neural Repair. 2016;30(8):713–721. [DOI] [PubMed] [Google Scholar]
- 15. Baker SP, et al. The Injury Severity Score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–196. [PubMed] [Google Scholar]
- 16. Hagen C, et al. Rancho Los Amigos Levels of Cognitive Functioning Scale. Downey, CA: Professional Staff Association of Rancho Los Amigos National Rehabilitation Center; 1972. [Google Scholar]
- 17. Nowak R, et al. The correlation between serum and salivary melatonin concentrations and urinary 6-hydroxymelatonin sulphate excretion rates: two non-invasive techniques for monitoring human circadian rhythmicity. Clin Endocrinol (Oxf). 1987;27(4):445–452. [DOI] [PubMed] [Google Scholar]
- 18. Johnston JD, et al. 60 years of neuroendocrinology: regulation of mammalian neuroendocrine physiology and rhythms by melatonin. J Endocrinol. 2015;226(2):T187–T198. [DOI] [PubMed] [Google Scholar]
- 19. Lushington K, et al. Urinary 6-sulfatoxymelatonin cycle-to-cycle variability. Chronobiol Int. 1996;13(6):411–421. [DOI] [PubMed] [Google Scholar]
- 20. Benhaberou-Brun D, et al. Association between melatonin secretion and daytime sleep complaints in night nurses. Sleep. 1999;22(7):877–885. [DOI] [PubMed] [Google Scholar]
- 21. Cornelissen G. Cosinor-based rhythmometry. Theor Biol Med Model. 2014;11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gehlbach BK, et al. Temporal disorganization of circadian rhythmicity and sleep-wake regulation in mechanically ventilated patients receiving continuous intravenous sedation. Sleep. 2012;35(8):1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mahlberg R, et al. Normative data on the daily profile of urinary 6-sulfatoxymelatonin in healthy subjects between the ages of 20 and 84. Psychoneuroendocrinology. 2006;31(5):634–641. [DOI] [PubMed] [Google Scholar]
- 24. Tzischinsky O, et al. The association between the nocturnal sleep gate and nocturnal onset of urinary 6-sulfatoxymelatonin. J Biol Rhythms. 1993;8(3):199–209. [DOI] [PubMed] [Google Scholar]
- 25. Crompton MR. Hypothalamic lesions following closed head injury. Brain. 1971;94(1):165–172. [DOI] [PubMed] [Google Scholar]
- 26. Edlow BL, et al. Disconnection of the ascending arousal system in traumatic coma. J Neuropathol Exp Neurol. 2013;72(6):505–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oldham MA, et al. Circadian rhythm disruption in the critically Ill: an opportunity for improving outcomes. Crit Care Med. 2016;44(1):207–217. [DOI] [PubMed] [Google Scholar]
- 28. Telias I, et al. Sleep and circadian rhythm in critical illness. Crit Care. 2019;23(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Novaes MA, et al. Stressors in ICU: perception of the patient, relatives and health care team. Intensive Care Med. 1999;25(12):1421–1426. [DOI] [PubMed] [Google Scholar]
- 30. Little A, et al. A patient survey of sleep quality in the intensive care unit. Minerva Anestesiol. 2012;78(4):406–414. [PubMed] [Google Scholar]