Abstract
Background
Childhood maltreatment may contribute to brain alterations in posttraumatic stress disorder (PTSD). We previously found that PTSD was associated with white matter compromise, or lower fractional anisotropy (FA), in the left inferior longitudinal fasciculus (ILF). In this study, including non‐PTSD controls, we examined whether ILF FA was associated with maltreatment exposures, including those that meet DSM‐IV criterion A (physical abuse, sexual abuse) and those that typically do not (emotional abuse, emotional neglect, physical neglect). We hypothesized that lower FA would be associated with PTSD diagnosis and with both categories of maltreatment.
Methods
Ninety‐three participants (51 female), ages 20–50, were enrolled, including 32 with lifetime DSM‐IV PTSD, 27 trauma‐exposed non‐PTSD controls, and 34 healthy controls. Participants completed structured interviews, the Childhood Trauma Questionnaire (CTQ), and diffusion‐weighted imaging (36 directions). Probabilistic tractography (using FreeSurfer's TRACULA) was used to assess diffusion metrics in the ILF.
Results
Contrary to our hypothesis, there was no significant effect of diagnostic group on FA. In contrast, higher CTQ scores were significantly associated with lower FA in the ILF bilaterally. This association of maltreatment with lower FA remained statistically significant after controlling for diagnostic group, and it was significant for both criterion‐A‐type and noncriterion‐A‐type maltreatment categories.
Conclusions
This work contributes to a growing body of literature indicating that different forms of childhood maltreatment are associated with altered white matter microstructure in the ILF, an association pathway involved in integrating visual information from occipital regions with emotion processing functions of the anterior temporal lobe.
Keywords: childhood maltreatment, diffusion tensor imaging, inferior longitudinal fasciculus, posttraumatic stress disorder, probabilistic tractography
Childhood maltreatment, but not posttraumatic stress disorder (PTSD) diagnostic status, is associated with altered diffusion in occipito‐temporal white matter pathways including lower fractional anisotropy in the inferior longitudinal fasciculus. This work contributes to a growing body of literature indicating that different forms of childhood maltreatment are associated with altered white matter microstructure in the ILF, an association pathway involved in integrating visual information from occipital regions with emotion processing functions of the anterior temporal lobe.
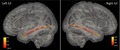
1. INTRODUCTION
Childhood maltreatment is associated with a broad range of negative developmental outcomes, including socioemotional, behavioral, and attachment problems (Cicchetti & Toth, 2005). Maltreated children have higher risk of adverse psychiatric outcomes (Kessler et al., 2010), including posttraumatic stress disorder (PTSD; Widom, 1999). This association likely reflects complex causal relationships. First, childhood maltreatment may lead to PTSD as a direct response to the exposure; prospectively ascertained maltreatment exposure is indeed associated with a markedly increased rate of PTSD (Scott, Smith, & Ellis, 2010). Second, childhood maltreatment may increase risk for the development of PTSD in response to adult trauma (Breslau et al., 2014). From a neurobiological perspective, childhood maltreatment has been associated with physiological processes including chronic inflammation (Coelho, Viola, Walss‐Bass, Brietzke, & Grassi‐Oliveira, 2014) and altered hypothalamic–pituitary–adrenal (HPA) axis reactivity (Heim, Newport, Mletzko, Miller, & Nemeroff, 2008; Suzuki, Poon, Papadopoulos, Kumari, & Cleare, 2014), which may increase the risk for aberrant stress responses and PTSD following subsequent trauma exposure (Carvalho, Coimbra, Ota, Mello, & Belangero, 2017; Eraly et al., 2014). Similarly, childhood maltreatment can disrupt the development of social‐emotional capacities such as attachment (Baer & Martinez, 2006) and social interconnectedness (Hovens, Giltay, van Hemert, & Penninx, 2016), thereby increasing the likelihood of PTSD following subsequent trauma exposure (Lee & Youm, 2011; Platt, Keyes, & Koenen, 2014; Sippel et al., 2017). Collectively, this literature suggests that childhood maltreatment moderates the neural expression of PTSD (Teicher & Samson, 2013).
Previously, we examined 10 white matter (WM) tracts in participants with PTSD versus trauma‐exposed controls, including those connecting canonical fear circuitry regions (e.g., the uncinate fasciculus, UF), and found lower fractional anisotropy (FA) and higher radial diffusivity (RD) in the left inferior longitudinal fasciculus (ILF; Olson et al., 2017). FA and RD, along with mean diffusivity (MD) and axial diffusivity (AD) are diffusion metrics that assess the extent of directionality of water diffusion and reflect brain structural organization. The ILF is a visual limbic pathway that projects between the occipital and anterior temporal lobes, facilitating rapid transfer of visual information to and from temporal regions involved in memory, learning, and emotional processing (Catani, Jones, Donato, & Ffytche, 2003). Choi, Jeong, Polcari, Rohan, and Teicher (2012) also reported lower FA in the left ILF of individuals who witnessed parental domestic violence (WDV) early in life. Our finding was the first report of abnormalities in the ILF in association with PTSD. Because the trauma‐exposed non‐PTSD participants in our sample endorsed minimal exposure to childhood maltreatment, we could not statistically separate effects of childhood maltreatment from effects of PTSD diagnostic status. One of the overarching goals of the current study was to address this limitation in a sample exposed to diverse forms of maltreatment.
One distinction between research on PTSD and research on childhood maltreatment relates to the types of index events under study. PTSD requires exposure to criterion A traumatic event(s) that involve “actual or threatened death or serious injury, or a threat to the physical integrity of self or others,” including “violent personal assault (sexual assault, physical attack).” Physical abuse and sexual abuse typically meet criterion A, while other forms of maltreatment, including emotional abuse, emotional neglect, and physical neglect, typically do not. A growing body of literature suggests that exposure to forms of childhood maltreatment that do not meet criterion A is at least as detrimental as exposure to forms of maltreatment that do meet criterion A. For example, Khan et al. (2015) found that adolescent exposure to verbal abuse, emotional neglect, and nonverbal emotional abuse was more strongly associated with history of major depressive disorder than physical or sexual abuse. Additionally, brain imaging studies have shown that noncriterion‐A‐type maltreatment experiences have significant neurobiological consequences. Specifically, neglect and emotional abuse are associated with amygdala and hippocampus hyperactivity in response to negative emotion (Bogdan, Williamson, & Hariri, 2012; Lee et al., 2015; Maheu et al., 2010), and with amygdala–hippocampus hyperconnectivity in response to psychosocial stress (Fan et al., 2015). Emotional neglect may be uniquely detrimental to social development; in a sample of maltreated adolescents, while controlling for other forms of maltreatment, only emotional neglect independently predicted antisocial features (Ometto et al., 2016).
Directly relevant to the current analyses, the neural correlates of maltreatment and posttraumatic psychopathology overlap substantially. Early life stress is associated with amygdala hyperactivity when viewing negatively valenced facial expressions and with lower hippocampal volume (Dannlowski et al., 2012; Rao et al., 2010; Teicher, Anderson, & Polcari, 2012; Vythilingam et al., 2002), both of which are also robust findings in PTSD patients (Bromis, Calem, Reinders, Williams, & Kempton, 2018; Etkin & Wager, 2007; Hayes, Hayes, & Mikedis, 2012; Smith, 2005). Additionally, both childhood maltreatment and PTSD are associated with alterations in white matter (WM) structural integrity. Tractography studies in PTSD samples versus trauma‐exposed controls have identified significantly lower FA and/or higher mean diffusivity (MD) in fronto‐temporal WM tracts including the UF (Koch et al., 2017), genu of the corpus callosum (Sun et al., 2013), and cingulum (Fani et al., 2016; Reuveni et al., 2016). In the childhood maltreatment literature, many prior reports have focused on reduced FA in the UF, a tract frequently selected for a priori analysis because of its role in facilitating prefrontal regulation of amygdala activity (e.g., Eluvathingal et al., 2006; Hanson, Knodt, Brigidi, & Hariri, 2015; Ho et al., 2017; Souza‐Queiroz et al., 2016). While the UF is often selected as an a priori target, altered diffusion in other tracts also has been identified in the context of diverse types of early adverse experiences, ranging from verbal abuse to early institutionalization, in samples with and without psychopathology. For example, Huang, Gundapuneedi, and Rao (2012) found lower FA in the superior longitudinal fasciculus (SLF), cingulum, inferior fronto‐occipital fasciculus, and splenium of the corpus callosum in adolescents who had experienced physical abuse, sexual abuse, or witnessed domestic violence. In young adults without psychopathology but with prior exposure to chronic parental verbal abuse, Choi, Jeong, Rohan, Polcari, and Teicher (2009) found lower FA in the arcuate fasciculus, cingulum, and fornix. In a sample exposed to early institutional care, Hanson et al. (2013) found widespread alterations in FA that were particularly extensive in posterior cortical regions. Finally, abnormal diffusion in posterior portions of the corpus callosum has been reported in children with maltreatment‐related PTSD (De Bellis et al., 2015; Jackowski et al., 2008). In addition to these cross‐sectional findings, longitudinal studies have examined baseline diffusion as a predictor of the development of psychopathology in maltreated samples, with increased internalizing symptoms developing over time in the context of lower baseline FA in the UF (Hanson et al., 2015), SLF and cingulum (Huang et al., 2012), and corpus callosum and external capsule (Bick, Fox, Zeanah, & Nelson, 2017). Thus, the PTSD and childhood maltreatment tractography literatures both implicate fronto‐temporal white matter tracts, though significant findings extend beyond those fibers to include more posterior cortical regions.
To summarize, the goal of the present study was to extend our previous finding of lower ILF FA in PTSD in a new sample that included comparison subjects with reported exposure to a broad range of childhood maltreatment severity, in order to dissociate possible effects of PTSD versus childhood maltreatment on white matter microstructure. We hypothesized that lower ILF FA would be associated with both PTSD diagnosis and exposure to childhood maltreatment. Additionally, because of the demonstrated significance of emotional neglect, emotional abuse, and physical neglect on neurobiological development, we hypothesized that noncriterion‐A‐type maltreatment exposures would have comparable associations with ILF FA as criterion‐A‐type maltreatment exposures. As a control tract, we examined the corticospinal tract (CST), a pyramidal tract involved in motor control. We were unable to locate any prior reports of altered FA in the CST in individuals with PTSD or histories of childhood maltreatment.
2. MATERIALS AND METHODS
2.1. Participants
Ninety‐three subjects who responded to study advertisements in the Boston metropolitan area participated in this study, including 32 participants with DSM‐IV lifetime PTSD, 27 trauma‐exposed non‐PTSD controls (TENC), and 34 healthy controls (HC). Prior to participating, all subjects received a full explanation of study procedures and provided written informed consent. All subjects were paid for their time ($25/hr, up to $200). Study procedures were authorized by Partners HealthCare's Human Research Committee. For detailed inclusion/exclusion criteria, see Appendix S1.
2.2. Clinical interviews and measures
Participants were evaluated with the Structural Clinical Interview for DSM‐IV Axis I Disorders (SCID‐I/P) (First, Spitzer, Gibbon, & Williams, 1996). PTSD symptom severity was assessed using the Clinician‐Administered PTSD Scale, Current and Lifetime Versions (CAPS‐DX) (Blake et al., 1995). The CAPS was administered to all participants who endorsed at least one DSM‐IV criterion A event on the Life Event Checklist (Gray, Litz, Hsu, & Lombardo, 2004). Clinical interviews were conducted by doctoral‐level clinical psychologists. Lifetime trauma load was assessed using the Life Events Checklist (LEC: Gray et al., 2004); the total score was the sum of experienced and witnessed events).
Childhood Trauma Questionnaire (Bernstein et al., 2003) subscales were summed to generate a total maltreatment exposure variable. The physical and sexual abuse subscales were summed in order to create a combined criterion‐A‐type maltreatment exposure score; and the emotional neglect, physical neglect, and emotional abuse subscales were summed to create a noncriterion‐A‐type maltreatment exposure score.
2.3. MR image acquisition
MRI data were acquired via a 3.0 Tesla Siemens Tim Trio Scanner (Siemens) with the use of a 32‐channel phased‐array design RF head coil operating at 123 MHz. Anatomical whole‐brain images were obtained, including high‐contrast T1‐weighted MPRAGE images. Whole‐brain diffusion tensor images (DTI) were obtained using a 36 direction‐weighting scheme. (For parameters, see Appendix S1.)
2.4. Tractography processing
Automatic probabilistic tractography was performed using TRActs Constrained by UnderLying Anatomy (TRACULA, v 1.22.2.12) tool (Yendiki et al., 2011) within FreeSurfer 5.3.0, using a standard pipeline (see Appendix S1). FA, MD, RD, and axial diffusivity (AD) from each tract (ILF, CST) were extracted for each participant. A modified total motion index was calculated following Yendiki, Koldewyn, Kakunoori, Kanwisher, and Fischl (2014).
2.5. Statistical analyses
Statistical analyses were performed using SPSS Statistics 20 (IBM Corporation). Because of violations of the assumption of homogeneity of variances, group differences in maltreatment exposure were evaluated using Welch's ANOVA with Games‐Howell post hoc tests. Multivariate analyses examined the effect of diagnostic group (HC, TENC, PTSD) and childhood maltreatment exposure on FA, with right and left ILF as dependent variables. Because age and sex can affect FA, these variables were included as nuisance covariates in all analyses. FA was treated as the primary measure of interest; significant findings for FA were followed by analyses of other diffusion metrics (MD, RD, AD) to further characterize changes in the diffusion signal. FA captures the extent to which diffusion occurs preferentially along one direction, AD assesses diffusion along the long axis and is primarily influenced by changes in axonal integrity, RD assesses diffusion perpendicular to the long axis and is primarily influenced by alterations in myelination, and MD captures the overall extent of diffusion restriction (Winklewski et al., 2018).
3. RESULTS
3.1. Childhood maltreatment exposure
Demographics and clinical characteristics of the sample are presented in Table 1. When maltreatment was coded as present or absent based on criteria for at least moderate exposure, 49 participants reported no maltreatment exposure, while 43 participants endorsed at least one category of moderate or severe maltreatment exposure (one participant did not provide complete data). Exposure to maltreatment types was largely overlapping (Figure 1): participants exposed to one maltreatment type tended to be exposed to other maltreatment types as well. Nine participants endorsed exposure only to criterion‐A‐type maltreatment (physical or sexual abuse), and 10 participants endorsed exposure only to noncriterion‐A‐type maltreatment (emotional abuse, emotional neglect, or physical neglect). Independent samples t tests demonstrated no sex differences in the severity of maltreatment exposure, except for sexual abuse, which was more severe in females (M = 8.78, SD = 5.89) than in males (M = 6.32, SD = 3.74), t(85.79) = −2.44, p = .02.
Table 1.
Demographic and clinical characteristics of posttraumatic stress disorder (PTSD), trauma‐exposed non‐PTSD control (TENC), and healthy control (HC) participants (mean ± SD or N (%))
|
PTSD N = 32 |
TENC N = 27 |
HC N = 34 |
Group differences | |
|---|---|---|---|---|
| Sex (female) | 19 (59%) | 15 (56%) | 17 (50%) | X 2(2) = 0.59, p=.74 |
| Age | 34.87 ± 8.11 | 29.99 ± 7.04a | 35.00 ± 8.96 | F(2, 90) = 3.53, p = .03 |
| CAPS current total | 41.59 ± 23.38 | 8.44 ± 10.30 | — | t(44.11) = −7.23, p < .001 |
| Total motion index | 0.49 ± 1.10 | −0.18 ± 1.49 | 0.30 ± 1.65 | F(2, 90) = 1.64, p = .20 |
| CTQ, Emotional abuse | 12.78 ± 6.69 | 9.85 ± 4.29 | 6.71 ± 2.70 | F(2, 50.63) = 14.39, p < .001b |
| CTQ, Physical abuse | 9.66 ± 5.04 | 7.96 ± 3.63 | 5.82 ± 2.30 | F(2, 51.37) = 9.46, p < .001b |
| CTQ, Sexual abuse | 10.25 ± 6.69 | 7.58 ± 4.76 | 5.35 ± 1.61 | F(2, 41.79) = 10.02, p < .001c |
| CTQ, Emotional neglect | 14.66 ± 5.43 | 11.48 ± 4.78 | 8.21 ± 3.59 | F(2, 55.33) = 16.67, p < .001b |
| CTQ, Physical neglect | 9.28 ± 3.74 | 6.81 ± 2.17 | 5.65 ± 1.30 | F(2, 49.34) = 14.85, p < .001d |
| Criterion‐A‐type maltreatment | 19.91 ± 10.42 | 15.46 ± 7.30 | 11.18 ± 3.36 | F(2, 45.13) = 12.65, p < .001b |
| Noncriterion‐A‐type maltreatment | 36.72 ± 14.09 | 28.15 ± 10.15 | 20.56 ± 6.54 | F(2, 51.65) = 19.67, p < .001d |
| Total CTQ score | 56.63 ± 22.11 | 43.11 ± 16.12 | 31.74 ± 9.38 | F(2, 48.83) = 19.55, p < .001d |
| Number of participants taking psychotropic medication | 2 (6%) | 1 (4%) | 0 (0%) | |
| Lifetime trauma load (LEC: experienced plus witnessed events) | 8.41 ± 4.63 | 6.48 ± 2.85 | 2.56 ± 1.94 | F(2, 51.74) = 33.54, p < .001b |
Abbreviations: CAPS, Clinician‐Administered PTSD Scale; CTQ, Childhood Trauma Questionnaire.
TENC < HC, TENC < PTSD.
HC < TENC, HC < PTSD.
HC < PTSD.
HC < TENC < PTSD.
Figure 1.
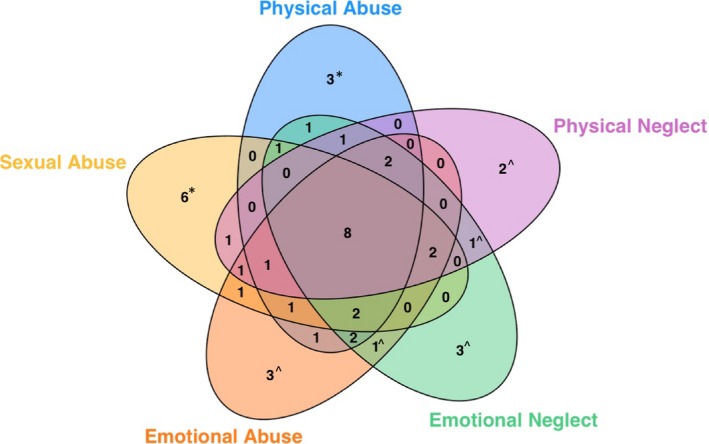
Maltreatment exposures in this sample. Forty‐three of 93 participants endorsed at least moderate maltreatment exposure. As illustrated here, maltreatment exposure categories were largely overlapping. Numbers reflect number of participants reporting exposure to the indicated maltreatment types. Numbers with carets reflect individuals exposed only to noncriterion‐A‐type maltreatment types, while numbers with asterisks reflect individuals exposed to only criterion‐A‐type maltreatment types. All others reported exposure to both maltreatment categories
3.2. ILF FA: Associations with PTSD diagnosis and total childhood maltreatment severity
Contrary to our hypotheses, there was no significant effect of diagnostic group on ILF FA, F(4, 176) = 1.11, p = .36, partial eta squared = 0.03 (Figure 2). In contrast, greater total childhood maltreatment severity was associated with lower ILF FA, F(2, 87) = 3.66, p = .03, partial eta squared = 0.08. This effect was significant bilaterally (left ILF, F(1, 88) = 6.79, p = .01; right ILF, F(1, 88) = 5.35, p = .02). The relationship between childhood maltreatment and lower ILF FA persisted in the combined sample after controlling for diagnostic group, F(2, 85) = 4.12, p = .02, and after controlling for lifetime trauma load, F(2, 86) = 3.233, p = .044, which was not significantly associated with ILF FA, F(2, 86) = 0.140, p = .870. Total childhood maltreatment was also significantly associated with lower ILF FA within the combined TENC and PTSD groups (i.e., after excluding the HC participants), F(2, 53) = 3.55, p = .04 (see also Table 2). There were no significant associations between childhood maltreatment and the other diffusion metrics (MD, RD, AD).
Figure 2.
Tractography of the inferior longitudinal fasciculus in one representative case
Table 2.
Partial correlations between scores on Childhood Trauma Questionnaire (CTQ) subscales and right and left ILF FA, controlling for sex and age. Lower portion of table: in combined three‐group sample (n = 92–93). Upper portion of table: in trauma‐exposed sample (n = 58–59)
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1. CTQ emotional abuse | — | 0.757* | 0.481* | 0.772* | 0.602* | 0.679* | 0.918* | −0.301* | −0.293* |
| 2. CTQ physical abuse | 0.795* | — | 0.578* | 0.662* | 0.479* | 0.862* | 0.733* | −0.352* | −0.213 |
| 3. CTQ sexual abuse | 0.558* | 0.623* | — | 0.419* | 0.325* | 0.912* | 0.470* | −0.240 | −0.145 |
| 4. CTQ emotional neglect | 0.796* | 0.706* | 0.513* | — | 0.683* | 0.594* | 0.931* | −0.275* | −0.169 |
| 5. CTQ physical neglect | 0.672* | 0.571* | 0.446* | 0.723* | — | 0.443* | 0.807* | −0.334* | −0.315* |
| 6. CTQ criterion‐A‐type maltreatment | 0.737* | 0.880* | 0.920* | 0.666* | 0.557* | — | 0.660* | −0.330 * | −0.197 |
| 7. CTQ noncriterion‐A‐type maltreatment | 0.929* | 0.779* | 0.565* | 0.941* | 0.836* | 0.773* | — | −0.333 * | −0.281 * |
| 8. Right ILF FA | −0.242* | −0.261* | −0.201 | −0.164 | −0.257* | −0.250 * | −0.236 * | — | 0.660* |
| 9. Left ILF FA | −0.280* | −0.223* | −0.197 | −0.159 | −0.288* | −0.232 * | −0.257 * | 0.653* | — |
Bold: correlations between broad maltreatment measures and ILF FA.
Abbreviations: FA, fractional anisotropy; ILF, inferior longitudinal fasciculus.
p < .05.
3.3. ILF FA: Relationships with childhood maltreatment types
The association between childhood maltreatment severity and ILF FA was significant for both criterion‐A‐type maltreatment exposures (i.e., physical and sexual abuse), F(2, 87) = 3.15, p = .048, and for noncriterion‐A‐type maltreatment exposures (i.e., emotional abuse, and emotional and physical neglect), F(2, 88) = 3.52, p = .03 (Figure 3). The relationship of noncriterion‐A‐type maltreatment exposures with bilateral ILF FA persisted after controlling for diagnostic group, F(2, 86) = 3.91, p = .02, with a nonsignificant effect of diagnostic status, F(4, 174) = 1.33, p = .26. Similarly, the association of criterion‐A‐type maltreatment exposures with bilateral ILF FA remained after controlling for diagnostic group, F(2, 85) = 3.14, p = .048, with a nonsignificant effect of diagnostic status, F(4, 172) = 1.78, p = .14.
Figure 3.
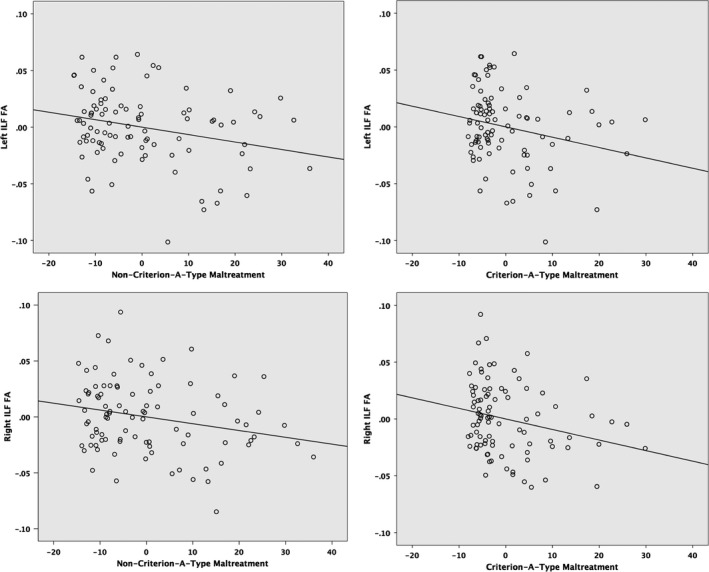
Scatter plots of partial regressions of childhood maltreatment on inferior longitudinal fasciculus (ILF) fractional anisotropy (FA), controlling for age and sex. Left, association between noncriterion‐A‐type maltreatment (physical neglect, emotional neglect, emotional abuse) and left (top) and right (bottom) ILF FA. Right, association between criterion‐A‐type maltreatment (physical and sexual abuse) and left (top) and right (bottom) ILF
3.4. ILF FA: Associations of childhood maltreatment along the trajectory of the ILF pathway
To examine relationships with diffusivity along the trajectory of the ILF pathway, individual results were projected into MNI space along the tract, and p‐values at each point along the normalized tract were computed for the association between total childhood maltreatment exposure and FA (at 53 points along the left ILF, and 52 points along the right ILF; Figure 4). In the left hemisphere, there were extensive regions along the trajectory of the ILF where FA was significantly associated with childhood maltreatment. These ran in parallel with much of the hippocampus, ranging from regions near the anterior hippocampus to regions near the posterior hippocampus and posterior parahippocampal gyrus. Additionally, there was one left hemisphere region near the fusiform gyrus that showed a significant relationship with childhood maltreatment. In the right hemisphere, the findings were somewhat more localized, with significant associations of maltreatment with FA in the right temporal lobe near the mid‐hippocampus, in the posterior temporal lobe near the lingual gyrus, and near the occipital fusiform gyrus.
Figure 4.
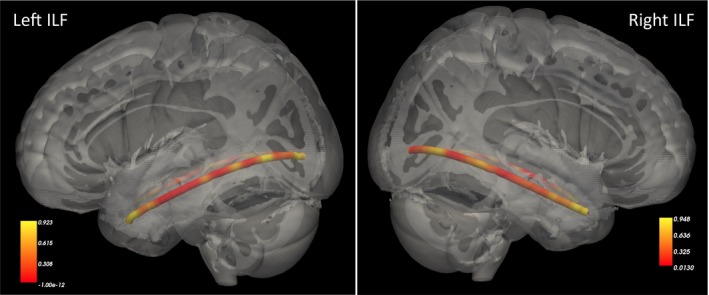
Heat maps illustrating p‐values for the association between total childhood maltreatment (Childhood Trauma Questionnaire total score) and fractional anisotropy along the trajectory of the inferior longitudinal fasciculus
3.5. CST FA: Control tract associations with PTSD diagnosis and total childhood maltreatment severity
Highlighting the specificity of the ILF findings, there was no significant effect of diagnostic group on CST FA, F(4, 176) = 0.69, p = .60, nor a significant effect of total childhood maltreatment on CST FA, F(2, 87) = 0.44, p = .65.
4. DISCUSSION
Greater childhood maltreatment was associated with lower FA in the ILF bilaterally, an effect that persisted after accounting for diagnostic group. In contrast, there were no significant differences in ILF FA between diagnostic groups (PTSD vs. TENC vs. healthy control). Lower ILF FA emerged in association with both criterion‐A‐type childhood maltreatment (physical and sexual abuse) and noncriterion‐A‐type maltreatment (emotional abuse and emotional and physical neglect). There were no significant relationships with other diffusion metrics (MD, RD, AD), and there were no significant associations of childhood maltreatment or PTSD diagnostic status with WM integrity of the corticospinal tract, which was selected as a control tract.
In our previous report (Olson et al., 2017), we found lower ILF FA in adults with PTSD but were unable to determine whether this reflected an association with PTSD diagnosis versus childhood maltreatment because these two variables were statistically overlapping. The present analyses (a) confirm involvement of lower WM integrity in the ILF and (b) clarify that this effect tracks with childhood maltreatment and not PTSD diagnosis. Our findings align with previous research from Choi et al. (2012), who described lower FA in the ILF of young adults who had witnessed domestic violence as children compared with young adults with no maltreatment history. Together, these results confirm that structural integrity of the ILF may be compromised in individuals who experience childhood maltreatment, with effects evident for multiple types of maltreatment. The present study found significant effects on FA but not other diffusion metrics; this pattern can be generated in the context of modest changes in RD and AD that combine to produce significant changes in the extent of directional diffusion, reflecting possible contributions of processes related to both myelination and axonal integrity. Our results contribute to a growing body of work highlighting the importance of considering posterior cortical regions in addition to canonical pathways (e.g., fronto‐temporal pathways) when evaluating effects of maltreatment and trauma exposure (De Bellis et al., 2015; Olson, Kaiser, Pizzagalli, Rauch, & Rosso, 2018).
Although the present study does not allow for differentiation of subcomponents of the ILF, anatomical work has demonstrated that this tract includes three components (fusiform, lingual, and dorsolateral‐occipital), all of which terminate in the anterior or middle temporal lobes (Latini et al., 2017). The ILF directly contacts other association tracts, including the uncinate and arcuate fasciculi (Herbet, Zemmoura, & Duffau, 2018). Like all WM tracts, the developmental trajectory of the ILF is protracted, with peak FA reached after age 25; however, other tracts that have more often been the focus of trauma research show a much later peak FA age (e.g., mid‐40s for the cingulum, over age 35 for the uncinate) (Lebel et al., 2012). It is possible that the earlier maturational process in the ILF makes it particularly vulnerable to insult in childhood or adolescence, since actively maturing regions may be more susceptible to disruption (Rice & Barone, 2000). From a functional perspective, the ILF is involved in a variety of functions including object recognition, facial recognition, and reading (Herbet et al., 2018), as well as semantic memory (Hodgetts et al., 2017). Hanson et al. (2013) found that reduced ILF FA in children exposed to emotional neglect via institutional rearing was correlated with deficits in visual paired associates learning. Importantly, ILF disruption also is associated with deficits in processing facial affect (Genova et al., 2015; Rigon, Voss, Turkstra, Mutlu, & Duff, 2018). The latter may be particularly relevant to children who have experienced maltreatment, who may experience a cascade in which maltreatment results in damage to facial affect processing centers, placing them at risk for further adverse social experiences.
Given the cross‐sectional nature of our data, it is not possible to evaluate the nature of the causal relationships between low ILF FA and maltreatment. There are a number of possibilities. First, stress exposure during childhood may alter ILF development, for example through mechanisms such as alterations in HPA axis responsivity, perhaps mediated by amygdala hyperactivity. Second, ILF FA reductions may occur in the context of neurobiological responses to disrupted attachment. Nonthreat‐based maltreatment including neglect can result in broad disruption to neurodevelopment extending beyond limbic regions (McLaughlin, Sheridan, & Nelson, 2017). Third, in instances where the maltreatment experience is generated by the biological parent, the interpretation of these effects is particularly complex. For example, parents who engage in abnormal attachment‐related behaviors such as emotional neglect may influence the child's neurobiological outcome through the delivery of that maltreatment experience, and also through shared neurobiology (South, Schafer & Ferraro, 2015). Further work with longitudinal imaging throughout childhood, family studies, and studies examining neurocognitive functions supported by the ILF will be necessary in order to disentangle these potential causal influences.
This study has several limitations. First, given the cross‐sectional nature of our data, we are not able to delineate causal relationships. Second, research evaluating effects of PTSD and childhood maltreatment ideally would include a four‐cell design (PTSD with maltreatment, PTSD without maltreatment, maltreatment without PTSD, no PTSD and no maltreatment) to isolate specific associations of diagnosis versus childhood maltreatment on white matter microstructure (De Bellis et al., 2015). Additionally, because we had few participants who had experienced solely criterion‐A‐type maltreatment or solely noncriterion‐A‐type maltreatment, we were unable to directly compare effect sizes across groups. Our cell sizes are also too small for investigation of sex‐specific effects, which may be present and important. Finally, our maltreatment measure did not include information about the timing and duration of exposure during childhood, so we are unable to further investigate those effects. Future work will employ measures (e.g., MACE: Maltreatment and Chronology of Exposure; Teicher & Parigger, 2015) that allow for assessment of these variables.
5. CONCLUSION
To summarize, the present study contributes to a growing body of literature indicating that childhood maltreatment is associated with altered WM microstructure in the ILF, a long association pathway involved in integrating visual information from the posterior cortices with anterior temporal lobe functions including emotion processing. From a clinical perspective, the present work supports the proposition that neglect and emotional abuse can have comparable effects on neurodevelopmental maturation as physical and sexual abuse, highlighting the importance of early detection and intervention with individuals exposed to multiple forms of childhood maltreatment.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare in relation to this work.
Supporting information
Olson EA, Overbey TA, Ostrand CG, Pizzagalli DA, Rauch SL, Rosso IM. Childhood maltreatment experiences are associated with altered diffusion in occipito‐temporal white matter pathways. Brain Behav. 2020;10:e01485 10.1002/brb3.1485
Funding information
This work was supported by the National Institute of Mental Health (5R01MH096987 [IMR]; K23MH112873 [EAO]).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Baer, J. , & Martinez, C. D. (2006). Child maltreatment and insecure attachment: A meta‐analysis. Journal of Reproductive and Infant Psychology, 24(3), 187–197. 10.1080/02646830600821231 [DOI] [Google Scholar]
- Bernstein, D. P. , Stein, J. A. , Newcomb, M. D. , Walker, E. , Pogge, D. , Ahluvalia, T. , … Zule, W. (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect, 27(2), 169–190. 10.1016/S0145-2134(02)00541-0 [DOI] [PubMed] [Google Scholar]
- Bick, J. , Fox, N. , Zeanah, C. , & Nelson, C. A. (2017). Early deprivation, atypical brain development, and internalizing symptoms in late childhood. Neuroscience, 342, 140–153. 10.1016/j.neuroscience.2015.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake, D. D. , Weathers, F. W. , Nagy, L. M. , Kaloupek, D. G. , Gusman, F. D. , Charney, D. S. , & Keane, T. M. (1995). The development of a clinician‐administered PTSD scale. Journal of Traumatic Stress, 8(1), 75–90. 10.1002/jts.2490080106 [DOI] [PubMed] [Google Scholar]
- Bogdan, R. , Williamson, D. E. , & Hariri, A. R. (2012). Mineralocorticoid receptor Iso/Val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. The American Journal of Psychiatry, 169(5), 515–522. 10.1176/appi.ajp.2011.11060855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau, N. , Koenen, K. C. , Luo, Z. , Agnew‐Blais, J. , Swanson, S. , Houts, R. M. , … Moffitt, T. E. (2014). Childhood maltreatment, juvenile disorders and adult post‐traumatic stress disorder: A prospective investigation. Psychological Medicine, 44(9), 1937–1945. 10.1017/S0033291713002651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromis, K. , Calem, M. , Reinders, A. A. T. S. , Williams, S. C. R. , & Kempton, M. J. (2018). Meta‐analysis of 89 structural MRI studies in posttraumatic stress disorder and comparison with major depressive disorder. The American Journal of Psychiatry, 175(10), 989–998. 10.1176/appi.ajp.2018.17111199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho, C. M. , Coimbra, B. M. , Ota, V. K. , Mello, M. F. , & Belangero, S. I. (2017). Single‐nucleotide polymorphisms in genes related to the hypothalamic‐pituitary‐adrenal axis as risk factors for posttraumatic stress disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 174(7), 671–682. 10.1002/ajmg.b.32564 [DOI] [PubMed] [Google Scholar]
- Catani, M. , Jones, D. K. , Donato, R. , & Ffytche, D. H. (2003). Occipito‐temporal connections in the human brain. Brain: A Journal of Neurology, 126(Pt 9), 2093–2107. 10.1093/brain/awg203 [DOI] [PubMed] [Google Scholar]
- Choi, J. , Jeong, B. , Polcari, A. , Rohan, M. L. , & Teicher, M. H. (2012). Reduced fractional anisotropy in the visual limbic pathway of young adults witnessing domestic violence in childhood. NeuroImage, 59(2), 1071–1079. 10.1016/j.neuroimage.2011.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, J. , Jeong, B. , Rohan, M. L. , Polcari, A. M. , & Teicher, M. H. (2009). Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biological Psychiatry, 65(3), 227–234. 10.1016/j.biopsych.2008.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti, D. , & Toth, S. L. (2005). Child maltreatment. Annual Review of Clinical Psychology, 1, 409–438. 10.1146/annurev.clinpsy.1.102803.144029 [DOI] [PubMed] [Google Scholar]
- Coelho, R. , Viola, T. W. , Walss‐Bass, C. , Brietzke, E. , & Grassi‐Oliveira, R. (2014). Childhood maltreatment and inflammatory markers: A systematic review. Acta Psychiatrica Scandinavica, 129(3), 180–192. 10.1111/acps.12217 [DOI] [PubMed] [Google Scholar]
- Dannlowski, U. , Stuhrmann, A. , Beutelmann, V. , Zwanzger, P. , Lenzen, T. , Grotegerd, D. , … Kugel, H. (2012). Limbic scars: Long‐term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry, 71(4), 286–293. 10.1016/j.biopsych.2011.10.021 [DOI] [PubMed] [Google Scholar]
- De Bellis, M. D. , Hooper, S. R. , Chen, S. D. , Provenzale, J. M. , Boyd, B. D. , Glessner, C. E. , … Woolley, D. P. (2015). Posterior structural brain volumes differ in maltreated youth with and without chronic posttraumatic stress disorder. Development and Psychopathology, 27(4 Pt 2), 1555–1576. 10.1017/S0954579415000942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eluvathingal, T. J. , Chugani, H. T. , Behen, M. E. , Juhász, C. , Muzik, O. , Maqbool, M. , …, Makki, M. (2006). Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics, 117(6), 2093–2100. 10.1542/peds.2005-1727 [DOI] [PubMed] [Google Scholar]
- Eraly, S. A. , Nievergelt, C. M. , Maihofer, A. X. , Barkauskas, D. A. , Biswas, N. , & Agorastos, A. , … Marine Resiliency Study Team (2014). Assessment of plasma C‐reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry, 71(4), 423–431. 10.1001/jamapsychiatry.2013.4374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin, A. , & Wager, T. D. (2007). Functional neuroimaging of anxiety: A meta‐analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American Journal of Psychiatry, 164(10), 1476–1488. 10.1176/appi.ajp.2007.07030504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, Y. , Pestke, K. , Feeser, M. , Aust, S. , Pruessner, J. C. , Böker, H. , … Grimm, S. (2015). Amygdala‐hippocampal connectivity changes during acute psychosocial stress: Joint effect of early life stress and oxytocin. Neuropsychopharmacology, 40(12), 2736–2744. 10.1038/npp.2015.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani, N. , King, T. Z. , Shin, J. , Srivastava, A. , Brewster, R. C. , Jovanovic, T. , … Ressler, K. J. (2016). Structural and functional connectivity in posttraumatic stress disorder: Associations with FKBP5. Depression and Anxiety, 33(4), 300–307. 10.1002/da.22483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First, M. B. , Spitzer, R. L. , Gibbon, M. , & Williams, J. B. (1996). Structured clinical interview for the DSM‐IV axis I disorders (SCID PTSD Module). [Google Scholar]
- Genova, H. M. , Rajagopalan, V. , Chiaravalloti, N. , Binder, A. , Deluca, J. , & Lengenfelder, J. (2015). Facial affect recognition linked to damage in specific white matter tracts in traumatic brain injury. Social Neuroscience, 10(1), 27–34. 10.1080/17470919.2014.959618 [DOI] [PubMed] [Google Scholar]
- Gray, M. J. , Litz, B. T. , Hsu, J. L. , & Lombardo, T. W. (2004). Psychometric properties of the life events checklist. Assessment, 11(4), 330–341. 10.1177/1073191104269954 [DOI] [PubMed] [Google Scholar]
- Hanson, J. L. , Adluru, N. , Chung, M. K. , Alexander, A. L. , Davidson, R. J. , & Pollak, S. D. (2013). Early neglect is associated with alterations in white matter integrity and cognitive functioning. Child Development, 84(5), 1566–1578. 10.1111/cdev.12069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, J. L. , Knodt, A. R. , Brigidi, B. D. , & Hariri, A. R. (2015). Lower structural integrity of the uncinate fasciculus is associated with a history of child maltreatment and future psychological vulnerability to stress. Development and Psychopathology, 27(4 Pt 2), 1611–1619. 10.1017/S0954579415000978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, J. P. , Hayes, S. M. , & Mikedis, A. M. (2012). Quantitative meta‐analysis of neural activity in posttraumatic stress disorder. Biology of Mood & Anxiety Disorders, 2(1), 9 10.1186/2045-5380-2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim, C. , Newport, D. J. , Mletzko, T. , Miller, A. H. , & Nemeroff, C. B. (2008). The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology, 33(6), 693–710. 10.1016/j.psyneuen.2008.03.008 [DOI] [PubMed] [Google Scholar]
- Herbet, G. , Zemmoura, I. , & Duffau, H. (2018). Functional anatomy of the inferior longitudinal fasciculus: From historical reports to current hypotheses. Frontiers in Neuroanatomy, 12(September), 1–15. 10.3389/fnana.2018.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, T. C. , King, L. S. , Leong, J. K. , Colich, N. L. , Humphreys, K. L. , Ordaz, S. J. , & Gotlib, I. H. (2017). Effects of sensitivity to life stress on uncinate fasciculus segments in early adolescence. Social Cognitive and Affective Neuroscience, 12(9), 1460–1469. 10.1093/scan/nsx065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgetts, C. J. , Postans, M. , Warne, N. , Varnava, A. , Lawrence, A. D. , & Graham, K. S. (2017). Distinct contributions of the fornix and inferior longitudinal fasciculus to episodic and semantic autobiographical memory. Cortex, 94, 1–14. 10.1016/j.cortex.2017.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovens, J. G. F. M. , Giltay, E. J. , van Hemert, A. M. , & Penninx, B. W. J. H. (2016). Childhood maltreatment and the course of depressive and anxiety disorders: The contribution of personality characteristics. Depression and Anxiety, 33(1), 27–34. 10.1002/da.22429 [DOI] [PubMed] [Google Scholar]
- Huang, H. , Gundapuneedi, T. , & Rao, U. (2012). White matter disruptions in adolescents exposed to childhood maltreatment and vulnerability to psychopathology. Neuropsychopharmacology, 37(12), 2693–2701. 10.1038/npp.2012.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski, A. P. , Douglas‐Palumberi, H. , Jackowski, M. , Win, L. , Schultz, R. T. , Staib, L. W. , … Kaufman, J. (2008). Corpus callosum in maltreated children with posttraumatic stress disorder: A diffusion tensor imaging study. Psychiatry Research, 162(3), 256–261. 10.1016/j.pscychresns.2007.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler, R. C. , McLaughlin, K. A. , Green, J. G. , Gruber, M. J. , Sampson, N. A. , Zaslavsky, A. M. , … Williams, D. R. (2010). Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. The British Journal of Psychiatry, 197(5), 378–385. 10.1192/bjp.bp.110.080499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, A. , McCormack, H. C. , Bolger, E. A. , McGreenery, C. E. , Vitaliano, G. , Polcari, A. , & Teicher, M. H. (2015). Childhood maltreatment, depression, and suicidal ideation: Critical importance of parental and peer emotional abuse during developmental sensitive periods in males and females. Frontiers in Psychiatry, 6, 42 10.3389/fpsyt.2015.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, S. B. J. , van Zuiden, M. , Nawijn, L. , Frijling, J. L. , Veltman, D. J. , & Olff, M. (2017). Decreased uncinate fasciculus tract integrity in male and female patients with PTSD: A diffusion tensor imaging study. Journal of Psychiatry & Neuroscience, 42(5), 331–342. 10.1503/jpn.160129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini, F. , Mårtensson, J. , Larsson, E.‐M. , Fredrikson, M. , Åhs, F. , Hjortberg, M. , … Ryttlefors, M. (2017). Segmentation of the inferior longitudinal fasciculus in the human brain: A white matter dissection and diffusion tensor tractography study. Brain Research, 1675, 102–115. 10.1016/j.brainres.2017.09.005 [DOI] [PubMed] [Google Scholar]
- Lebel, C. , Gee, M. , Camicioli, R. , Wieler, M. , Martin, W. , & Beaulieu, C. (2012). Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage, 60(1), 340–352. 10.1016/j.neuroimage.2011.11.094 [DOI] [PubMed] [Google Scholar]
- Lee, B. , & Youm, Y. (2011). Social network effects on post‐traumatic stress disorder (PTSD) in female North Korean immigrants. Journal of Preventive Medicine and Public Health, 44(5), 191–200. 10.3961/jpmph.2011.44.5.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. W. , Yoo, J. H. , Kim, K. W. , Lee, J.‐S. , Kim, D. , Park, H. W. , … Jeong, B. (2015). Aberrant function of frontoamygdala circuits in adolescents with previous verbal abuse experiences. Neuropsychologia, 79(Pt A), 76–85. 10.1016/j.neuropsychologia.2015.10.029 [DOI] [PubMed] [Google Scholar]
- Maheu, F. S. , Dozier, M. , Guyer, A. E. , Mandell, D. , Peloso, E. , Poeth, K. , … Ernst, M. (2010). A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cognitive, Affective & Behavioral Neuroscience, 10(1), 34–49. 10.3758/CABN.10.1.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, K. A. , Sheridan, M. A. , & Nelson, C. A. (2017). Neglect as a violation of species‐expectant experience: Neurodevelopmental consequences. Biological Psychiatry, 82(7), 462–471. 10.1016/j.biopsych.2017.02.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, E. A. , Cui, J. , Fukunaga, R. , Nickerson, L. D. , Rauch, S. L. , & Rosso, I. M. (2017). Disruption of white matter structural integrity and connectivity in posttraumatic stress disorder: A TBSS and tractography study. Depression and Anxiety, 34(5), 437–445. 10.1002/da.22615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, E. A. , Kaiser, R. H. , Pizzagalli, D. A. , Rauch, S. L. , & Rosso, I. M. (2018). Regional prefrontal resting‐state functional connectivity in posttraumatic stress disorder. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging, 4(4), 390–398. 10.1016/j.bpsc.2018.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ometto, M. , de Oliveira, P. A. , Milioni, A. L. , dos Santos, B. , Scivoletto, S. , Busatto, G. F. , … Cunha, P. J. (2016). Social skills and psychopathic traits in maltreated adolescents. European Child & Adolescent Psychiatry, 25(4), 397–405. 10.1007/s00787-015-0744-y [DOI] [PubMed] [Google Scholar]
- Platt, J. , Keyes, K. M. , & Koenen, K. C. (2014). Size of the social network versus quality of social support: Which is more protective against PTSD? Social Psychiatry and Psychiatric Epidemiology, 49(8), 1279–1286. 10.1007/s00127-013-0798-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, U. , Chen, L.‐A. , Bidesi, A. S. , Shad, M. U. , Thomas, M. A. , & Hammen, C. L. (2010). Hippocampal changes associated with early‐life adversity and vulnerability to depression. Biological Psychiatry, 67(4), 357–364. 10.1016/j.biopsych.2009.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveni, I. , Bonne, O. , Giesser, R. , Shragai, T. , Lazarovits, G. , Isserles, M. , … Levin, N. (2016). Anatomical and functional connectivity in the default mode network of post‐traumatic stress disorder patients after civilian and military‐related trauma. Human Brain Mapping, 37(2), 589–599. 10.1002/hbm.23051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, D. , & Barone, S. (2000). Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environmental Health Perspectives, 108(Suppl), 511–533. 10.1289/ehp.00108s3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigon, A. , Voss, M. W. , Turkstra, L. S. , Mutlu, B. , & Duff, M. C. (2018). White matter correlates of different aspects of facial affect recognition impairment following traumatic brain injury. Social Neuroscience, 14(4), 434–448., 10.1080/17470919.2018.1489302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, K. M. , Smith, D. R. , & Ellis, P. M. (2010). Prospectively ascertained child maltreatment and its association with DSM‐IV mental disorders in young adults. Archives of General Psychiatry, 67(7), 712–719. 10.1001/archgenpsychiatry.2010.71 [DOI] [PubMed] [Google Scholar]
- Sippel, L. M. , Han, S. , Watkins, L. E. , Harpaz‐Rotem, I. , Southwick, S. M. , Krystal, J. H. , … Pietrzak, R. H. (2017). Oxytocin receptor gene polymorphisms, attachment, and PTSD: Results from the National Health and Resilience in Veterans Study. Journal of Psychiatric Research, 94(2), 139–147. 10.1016/j.jpsychires.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, M. E. (2005). Bilateral hippocampal volume reduction in adults with post‐traumatic stress disorder: A meta‐analysis of structural MRI studies. Hippocampus, 15(6), 798–807. 10.1002/hipo.20102 [DOI] [PubMed] [Google Scholar]
- South, S. C. , Schafer, M. H. , & Ferraro, K. F. (2015). Genetic and Environmental Overlap Between Childhood Maltreatment and Adult Physical Health. Twin Research and Human Genetics : The Official Journal of the International Society for Twin Studies, 18(5), 533–544. 10.1017/thg.2015.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Queiroz, J. , Boisgontier, J. , Etain, B. , Poupon, C. , Duclap, D. , D’Albis, M.-A. , …, Houenou, J. (2016). Childhood trauma and the limbic network: a multimodal MRI study in patients with bipolar disorder and controls. Journal of Affective Disorders, 200, 159–164. 10.1016/j.jad.2016.04.038 [DOI] [PubMed] [Google Scholar]
- Sun, Y. , Wang, Z. , Ding, W. , Wan, J. , Zhuang, Z. , Zhang, Y. , … Xu, J. (2013). Alterations in white matter microstructure as vulnerability factors and acquired signs of traffic accident‐induced PTSD. PLoS ONE, 8(12), e83473 10.1371/journal.pone.0083473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, A. , Poon, L. , Papadopoulos, A. S. , Kumari, V. , & Cleare, A. J. (2014). Long term effects of childhood trauma on cortisol stress reactivity in adulthood and relationship to the occurrence of depression. Psychoneuroendocrinology, 50, 289–299. 10.1016/j.psyneuen.2014.09.007 [DOI] [PubMed] [Google Scholar]
- Teicher, M. H. , Anderson, C. M. , & Polcari, A. (2012). Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proceedings of the National Academy of Sciences of the USA, 109(9), E563–E572. 10.1073/pnas.1115396109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher, M. H. , & Parigger, A. (2015). The “Maltreatment and Abuse Chronology of Exposure” (MACE) scale for the retrospective assessment of abuse and neglect during development. PLoS ONE, 10(2), e0117423 10.1371/journal.pone.0117423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher, M. H. , & Samson, J. A. (2013). Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. The American Journal of Psychiatry, 170(10), 1114–1133. 10.1176/appi.ajp.2013.12070957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingam, M. , Heim, C. , Newport, J. , Miller, A. H. , Anderson, E. , Bronen, R. , … Bremner, J. D. (2002). Childhood trauma associated with smaller hippocampal volume in women with major depression. The American Journal of Psychiatry, 159(12), 2072–2080. 10.1176/appi.ajp.159.12.2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom, C. S. (1999). Posttraumatic stress disorder in abused and neglected children grown up. The American Journal of Psychiatry, 156(8), 1223–1229. 10.1176/ajp.156.8.1223 [DOI] [PubMed] [Google Scholar]
- Winklewski, P. J. , Sabisz, A. , Naumczyk, P. , Jodzio, K. , Szurowska, E. , & Szarmach, A. (2018). Understanding the physiopathology behind axial and radial diffusivity changes – What do we know? Frontiers in Neurology, 9, 92 10.3389/fneur.2018.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki, A. , Koldewyn, K. , Kakunoori, S. , Kanwisher, N. , & Fischl, B. (2014). Spurious group differences due to head motion in a diffusion MRI study. NeuroImage, 88, 79–90. 10.1016/j.neuroimage.2013.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki, A. , Panneck, P. , Srinivasan, P. , Stevens, A. , Zöllei, L. , Augustinack, J. , … Fischl, B. (2011). Automated probabilistic reconstruction of white‐matter pathways in health and disease using an atlas of the underlying anatomy. Frontiers in Neuroinformatics, 5, 23 10.3389/fninf.2011.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


