Abstract
Background
Chronic suppurative otitis media (CSOM), sometimes referred to as chronic otitis media (COM), is a chronic inflammation and often polymicrobial infection (involving more than one micro‐organism) of the middle ear and mastoid cavity, characterised by ear discharge (otorrhoea) through a perforated tympanic membrane. The predominant symptoms of CSOM are ear discharge and hearing loss. Topical antibiotics, the most common treatment for CSOM, act to kill or inhibit the growth of micro‐organisms that may be responsible for the infection. Antibiotics can be used alone or in addition to other treatments for CSOM, such as antiseptics or ear cleaning (aural toileting).
Objectives
To assess the effects of topical antibiotics (without steroids) for people with CSOM.
Search methods
The Cochrane ENT Information Specialist searched the Cochrane ENT Register; Central Register of Controlled Trials (CENTRAL via the Cochrane Register of Studies); Ovid MEDLINE; Ovid Embase; CINAHL; Web of Science; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 1 April 2019.
Selection criteria
We included randomised controlled trials (RCTs) with at least a one‐week follow‐up involving participants (adults and children) who had chronic ear discharge of unknown cause or CSOM, where the ear discharge had continued for more than two weeks.
The interventions were any single, or combination of, topical antibiotic agent(s) of any class, applied directly into the ear canal as ear drops, powders or irrigations, or as part of an aural toileting procedure.
The two main comparisons were topical antibiotic compared to a) placebo or no intervention and b) another topical antibiotic (e.g. topical antibiotic A versus topical antibiotic B).
Within each comparison we separated studies where both groups of participants had received topical antibiotic a) alone or with aural toileting and b) on top of background treatment (such as systemic antibiotics).
Data collection and analysis
We used the standard Cochrane methodological procedures. We used GRADE to assess the certainty of the evidence for each outcome.
Our primary outcomes were: resolution of ear discharge or 'dry ear' (whether otoscopically confirmed or not), measured at between one week and up to two weeks, two weeks to up to four weeks and after four weeks; health‐related quality of life using a validated instrument; ear pain (otalgia) or discomfort or local irritation. Secondary outcomes included hearing, serious complications and ototoxicity measured in several ways.
Main results
We included 17 studies with a total of 2198 participants. Twelve studies reported the sample size in terms of participants (not ears); these had a total of 1797 participants. The remaining five studies reported both the number of participants and ears, representing 401 participants, or 510 ears.
A: Topical antibiotics versus placebo or no treatment (with aural toilet in both arms and no other background treatment)
One small study compared a topical antibiotic (ciprofloxacin) with placebo (saline). All participants received aural toilet. Although ciprofloxacin was better than saline in terms of resolution of discharge at one to two weeks: 84% versus 12% (risk ratio (RR) 6.74, 95% confidence interval (CI) 1.82 to 24.99; 35 participants, very low‐certainty evidence), the very low certainty of the evidence means that it is very uncertain whether or not one intervention is better or worse than the other. The study authors reported that "no medical side‐effects and worsening of audiological measurements related to this topical medication were detected" (very low‐certainty evidence).
B: Topical antibiotics versus placebo or no treatment (with use of oral antibiotics in both arms)
Four studies compared topical ciprofloxacin to no treatment (three studies; 190 participants) or topical ceftizoxime to no treatment (one study; 248 participants). In each study all participants received the same antibiotic systemically (oral ciprofloxacin, injected ceftizoxime). In at least one study all participants received aural toilet. Useable data were only available from the first three studies; ciprofloxacin was better than no treatment, resolution of discharge occurring in 88.2% versus 60% at one to two weeks (RR 1.47, 95% CI 1.20 to 1.80; 2 studies, 150 participants; low‐certainty evidence). None of the studies reported ear pain or discomfort/local irritation.
C: Comparisons of different topical antibiotics
The certainty of evidence for all outcomes in these comparisons is very low.
Quinolones versus aminoglycosides
Seven studies compared an aminoglycoside (gentamicin, neomycin or tobramycin) with ciprofloxacin (734 participants) or ofloxacin (214 participants). Whilst resolution of discharge at one to two weeks was higher in the quinolones group the very low certainty of the evidence means that it is very uncertain whether or not one intervention is better or worse than the other (RR 1.95, 95% CI 0.88 to 4.29; 6 studies, 694 participants). One study measured ear pain and reported no difference between the groups.
Quinolones versus aminoglycosides/polymyxin B combination ±gramicidin
We identified three studies but data on our primary outcome were only available in one study. Comparing ciprofloxacin to a neomycin/polymyxin B/gramicidin combination, for an unknown treatment duration (likely four weeks), ciprofloxacin was better (RR 1.12, 95% CI 1.03 to 1.22, 186 participants). A "few" patients experienced local irritation upon the first instillation of topical treatment (numbers/groups not stated).
Others
Other studies examined topical gentamicin versus a trimethoprim/sulphacetamide/polymixin B combination (91 participants) and rifampicin versus chloramphenicol (160 participants). Limited data were available and the findings were very uncertain.
Authors' conclusions
We are uncertain about the effectiveness of topical antibiotics in improving resolution of ear discharge in patients with CSOM because of the limited amount of low‐quality evidence available. However, amongst this uncertainty there is some evidence to suggest that the use of topical antibiotics may be effective when compared to placebo, or when used in addition to a systemic antibiotic. There is also uncertainty about the relative effectiveness of different types of antibiotics; it is not possible to determine with any certainty whether or not quinolones are better or worse than aminoglycosides. These two groups of compounds have different adverse effect profiles, but there is insufficient evidence from the included studies to make any comment about these. In general, adverse effects were poorly reported.
Plain language summary
Topical antibiotics for people with chronic suppurative otitis media
What is the aim of this review?
The aim of this Cochrane Review was to find out if topical antibiotics are effective in treating chronic suppurative otitis media and whether one type of topical antibiotic treatment is more effective than any other. We collected and analysed all relevant studies to answer this question.
Key messages
There is a lot of uncertainty as to whether or not topical antibiotics improve the resolution of ear discharge in patients with chronic suppurative otitis media (CSOM). However, among this uncertainty there is some evidence to suggest that the use of topical antibiotics may be effective when compared to placebo, or when used in addition to a systemic antibiotic (oral or injected). There is also lots of uncertainty about which type of topical antibiotic is the most effective. Overall, the certainty of the evidence was very low.
What was studied in the review?
Chronic suppurative otitis media, sometimes referred to as chronic otitis media (COM), is a long‐term (chronic) swelling and infection of the middle ear, with ear discharge (otorrhoea) through a perforated tympanic membrane (eardrum). The main symptoms of CSOM are ear discharge and hearing loss. Topical antibiotics (administered into the ear canal as ear drops, ointments, sprays or creams) are the most commonly used treatment for CSOM. Topical antibiotics kill or stop the growth of the micro‐organisms that may be responsible for the infection. Topical antibiotics can be used on their own or added to other treatments for CSOM, such as antiseptics or ear cleaning (aural toileting) or systemic antibiotics (antibiotics taken either by mouth or by an injection into a muscle or vein). It was important in this review to examine whether there were any adverse effects from using topical antibiotics as they can cause irritation of the skin within the outer ear, which may cause discomfort, pain or itching. This review also examined whether different types of antibiotics were more effective at treating CSOM than others, as some antibiotics (such as aminoglycosides) may have the potential to be toxic to the inner ear (ototoxicity), with potential to cause irreparable hearing loss (sensorineural), dizziness or ringing in the ear (tinnitus).
What are the main results of the review?
We found 17 studies examining at least 2126 participants, but it was difficult to determine precisely how many participants were included as a number of studies did not clearly report the number. A number of different types of antibiotics and combinations of antibiotics were used.
Comparison of topical antibiotics to placebo or no treatment
One study compared topical antibiotics to a saline (salt water) ear wash. The topical antibiotics appeared to be more effective than the saline ear wash when assessed one to two weeks after treatment, but this study was too small to provide any certainty of the findings (very low‐certainty evidence).
Comparison of topical antibiotics in addition to systemic (oral or injected) antibiotics
Four studies compared treatment with topical antibiotic (ciprofloxacin) drops in addition to a systemic (oral or injected) antibiotic. Treatment marginally favoured the combined topical and oral antibiotics compared to oral antibiotics only for resolution of discharge at one to two weeks and two to four weeks. These studies were too small to provide any certainty of the findings (low‐certainty evidence).
Comparisons of different topical antibiotics
There were 12 studies that examined the effectiveness of different types of antibiotics. The certainty of the evidence for all outcomes in these comparisons is very low. Two studies did not report the number of included participants, or reported only the number of ears treated, so the total number of participants could not be calculated. Due to the low certainty of evidence it is not known which type of topical antibiotic is the most effective.
How up to date is this review?
The evidence is up to date to April 2019.
Summary of findings
Summary of findings for the main comparison. Topical antibiotics versus placebo/no treatment for chronic suppurative otitis media.
| Topical antibiotics (ciprofloxacin) versus placebo/no treatment for chronic suppurative otitis media | |||||||
|
Patient or population: patients with mucopurulent otorrhoea Settings: specialist hospital in Thailand Intervention: ciprofloxacin ear drops Comparison: saline | |||||||
| Outcomes | Relative effect (95% CI) | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | Comments | ||
| Without topical antibiotic | With topical antibiotic | Difference | |||||
| Resolution of ear discharge ‐ measured at 1 to 2 weeks Follow‐up: 7 days |
RR 6.74 (1.82 to 24.99) | 35 (1 RCT) | Study population | ⊕⊝⊝⊝ very low1 | Topical antibiotics may increase the number of patients with resolution of ear discharge at 7 days compared with placebo, but we are very uncertain about the results. | ||
| 12.5% | 84.2% (22.8 to 100.0) |
71.8% more (10.3 to 299.9) |
|||||
| Resolution of ear discharge ‐ measured after 4 weeks | No study measured this outcome. | ||||||
| Health‐related quality of life | No study measured this outcome. | ||||||
| Ear pain (otalgia) or discomfort or local irritation Follow‐up: 7 days |
— | 35 (1 RCT) | Authors reported "no medical side‐effects and worsening of audiological measurements related to this topical medication were detected". | ⊕⊝⊝⊝ very low2 | — | ||
| Hearing Follow‐up: 7 days |
— | 35 (1 RCT) | Authors reported "no ... worsening of audiological measurements related to this topical medication were detected." | ⊕⊝⊝⊝ very low3 | — | ||
| Serious complications | No studies reported that any participant died or had any intracranial or extracranial complications. | ||||||
| Suspected ototoxicity Follow‐up: 7 days |
— | 35 (1 RCT) | Authors report "no suspected ototoxicity" but it is unclear how this was measured. | ⊕⊝⊝⊝ very low2 | — | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||||
1Downgraded to very low certainty: downgraded by one level due to study limitations (risk of bias) because there were concerns about incomplete data (50 people entered the study but results are only reported for 35). Downgraded by two levels due to imprecision as there was one very small study (35 participants) with wide confidence intervals.
2Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) because there were concerns about incomplete data (50 people entered the study but results are only reported for 35) and it is unclear how this outcome was measured as the paper just reports "no medical side effects". Downgraded by one level due to imprecision as numeric results were not provided and it was only one very small study (35 participants).
3Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) because of concerns about incomplete data (50 people entered the study but results are only reported for 35) and the methods used for measuring hearing were not provided in the paper. Downgraded by one level due to imprecision as numeric results were not reported and it was only one very small study (35 participants).
Summary of findings 2. Topical antibiotics on top of systemic antibiotics for chronic suppurative otitis media.
| Topical antibiotics (ciprofloxacin) on top of systemic antibiotics (ciprofloxacin) for chronic suppurative otitis media | |||||||
|
Patient or population: CSOM, recurrence of CSOM or suppuration following mastoidectomy or tympanoplasty Settings: secondary care clinics in Spain and Italy Intervention: ciprofloxacin (topical) plus ciprofloxacin (systemic) Comparison: ciprofloxacin (systemic) | |||||||
| Outcomes | Relative effect (95% CI) | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | Comments | ||
| Without topical antibiotics | With topical antibiotics | Difference | |||||
| Resolution of ear discharge‐ measured at 1 to 2 weeks Follow‐up: 7 to 10 days |
RR 1.47 (1.20 to 1.80) |
150 (2 RCTs) | Study population | ⊕⊕⊝⊝ low1 | Topical antibiotics in addition to systemic antibiotics may increase the number of patients with resolution of ear discharge at 7 to 10 days compared with systemic antibiotics alone. The NNTB is 4 (95% CI 3 to 9). | ||
| 60.0% | 88.2% (72.0 to 100) |
28.2% more (12 more to 48 more) |
|||||
| Resolution of ear discharge ‐ measured after 4 weeks | No studies reported this outcome. | ||||||
| Health‐related quality of life | No studies reported this outcome. | ||||||
| Ear pain (otalgia) or discomfort or local irritation | No studies reported this outcome. | ||||||
| Hearing | No studies reported results for this outcome. | ||||||
| Serious complications | No studies reported that any participant died or had any intracranial or extracranial complications. | ||||||
| Suspected ototoxicity Follow‐up: 7 to 10 days | — | 190 (3 RCTs) | Three studies reported that they did not suspect ototoxicity in any participants, but it is unclear how this was measured (de Miguel 1999; Esposito 1990; Ramos 2003). | ⊕⊝⊝⊝ very low2 | — | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB: number needed to treat to benefit; RCT: randomised controlled trial; RR: risk ratio | |||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||||
1Downgraded to low certainty: downgraded by one level due to study limitations (risk of bias) as both studies had unclear randomisation and allocation concealment and were unblinded. Downgraded by one level due to imprecision as there were only two small studies (150 participants) with the confidence interval crossing the line of minimally important benefit.
2Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) as all three studies had unclear randomisation, allocation concealment and were unblinded studies. It was also unclear how the outcome was reported. Downgraded by one level due to imprecision as numeric results were not reported and there were only three small studies (190 participants).
Summary of findings 3. Quinolones versus aminoglycosides for chronic suppurative otitis media.
| Quinolones versus aminoglycosides for chronic suppurative otitis media | |||||||
|
Patient or population: CSOM Settings: secondary care settings in Israel, Turkey, Jordan, Spain and Pakistan Intervention: ciprofloxacin versus tobramycin (2 studies); ciprofloxacin versus gentamycin (3 studies); ofloxacin versus gentamycin (2 studies) Comparison: other antibiotic | |||||||
| Outcomes | Relative effect (95% CI) | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | Comments | ||
| Aminoglycosides | Quinolones | Difference | |||||
| Resolution of ear discharge ‐ measured at 1 to 2 weeks Follow‐up: range 8 days to 2 weeks |
RR 1.95 (0.88 to 4.29) | 694 (6 RCTs) | 33.7%1 | 65.7 (29.7% to 100%) |
32.0% more (4.0% lower to 110.9% higher) | ⊕⊝⊝⊝ very low2 | We used a random‐effects model due to high heterogeneity. Resolution of ear discharge at 1 to 2 weeks was higher in the quinolones group but the very low certainty of the evidence means that it is very uncertain whether or not one intervention is better or worse than the other. |
| Resolution of ear discharge ‐ Measured after 4 weeks | None of the studies measured this outcome. | ||||||
| Health‐related quality of life | None of the studies measured this outcome. | ||||||
| Ear pain (otalgia) or discomfort or local irritation Follow‐up: 30 days |
— | 308 (1 RCT) | One study measured ear pain on a 3‐point scale (Lorente 1995). Results were presented as a mean score. Both groups had a mean score of 0 at 30 days. There was no difference between the groups. | ⊕⊝⊝⊝ very low3 | — | ||
| Hearing Follow‐up: 10 days |
— | 132 (4 RCTs) |
One study presents the hearing levels per group but does not present the data in a way that can be analysed (Tutkun 1995). One study stated in the methods that hearing was measured but only mentioned that neither group showed significant differences (Nawasreh 2001). | ⊕⊝⊝⊝ very low4 | — | ||
| Serious complications Follow‐up: 10 to 30 days |
None of the studies reported that any participant died or had any intracranial or extracranial complications. | ||||||
| Suspected ototoxicity Follow‐up: 10 to 30 days |
— | 352 (2 RCTs) |
One study (Lorente 1995) assessed for ototoxicity and did not find any cases. One study (Tutkun 1995) reported assessment of ototoxicity in the methods but did not provide results. None of the studies reported assessment nor any cases of suspected ototoxicity. | ⊕⊝⊝⊝ very low5 | — | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CSOM: chronic suppurative otitis media; RCT: randomised controlled trial; RR: risk ratio | |||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||||
1Average event rates in the control group were calculated without the Lorente 1995 study, as this seemed to show a very high rate of resolution (94%) compared to the other studies (range between 28% and 55%).
2Downgraded to very low certainty. Downgraded due to study limitations (risk of bias) as six of seven studies were unblinded and in general the methods were poor. Downgraded due to imprecision as the point estimate shows that more people with quinolones had resolution of discharge compared with aminoglycosides BUT there is a large confidence interval, which includes 'no effect' and a very large effect (four times as many people had resolution with quinolones compared to aminoglycosides). Downgraded due to inconsistency as there was high heterogeneity (I2 = 97%) within the results.
3Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) as all elements of the risk of bias assessment were unclear. Downgraded by one level due to imprecision as the results come from one relatively small study (308 patients).
4Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) as the studies were assessed as either high risk or unclear risk for all elements of the risk of bias assessment. Downgraded by one level due to imprecision as numeric results were not presented and the results came from two small studies (132 patients).
5Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) as many were unblinded and in general the studies had methodological issues and/or were badly reported. In addition, it is not clear how the outcome was measured. Downgraded by one level due to imprecision as numeric results were not reported and there were only two studies (352 participants) that identified ototoxicity as an outcome.
Background
This is one of a suite of Cochrane Reviews evaluating the comparative effectiveness of non‐surgical interventions for chronic suppurative otitis media (CSOM) using topical antibiotics, topical antibiotics with corticosteroids, systemic antibiotics, topical antiseptics and aural toileting (ear cleaning) methods (Table 4).
1. Table of Cochrane Reviews.
| Topical antibiotics with steroids | Topical antibiotics | Systemic antibiotics | Topical antiseptics | Aural toileting (ear cleaning) | |
| Topical antibiotics with steroids | Review CSOM‐4 | ||||
| Topical antibiotics | Review CSOM‐4 | Review CSOM‐1 | |||
| Systemic antibiotics | Review CSOM‐4 | Review CSOM‐3 | Review CSOM‐2 | ||
| Topical antiseptics | Review CSOM‐4 | Review CSOM‐6 | Review CSOM‐6 | Review CSOM‐5 | |
| Aural toileting | Review CSOM‐4 | Not reviewed | Not reviewed | Not reviewed | Review CSOM‐7 |
| Placebo (or no intervention) | Review CSOM‐4 | Review CSOM‐1 | Review CSOM‐2 | Review CSOM‐5 | Review CSOM‐7 |
CSOM‐1: Topical antibiotics for chronic suppurative otitis media (Brennan‐Jones 2018b).
CSOM‐2: Systemic antibiotics for chronic suppurative otitis media (Chong 2018a).
CSOM‐3: Topical versus systemic antibiotics for chronic suppurative otitis media (Chong 2018b).
CSOM‐4: Topical antibiotics with steroids for chronic suppurative otitis media (Brennan‐Jones 2018a).
CSOM‐5: Topical antiseptics for chronic suppurative otitis media (Head 2018a).
CSOM‐6: Antibiotics versus topical antiseptics for chronic suppurative otitis media (Head 2018b).
CSOM‐7: Aural toilet (ear cleaning) for chronic suppurative otitis media (Bhutta 2018).
This review compares the effectiveness of topical antibiotics (without corticosteroids) against placebo/no treatment, or another topical antibiotic for CSOM.
Description of the condition
Chronic suppurative otitis media (CSOM), which is also often referred to as chronic otitis media (COM), is a chronic inflammation and infection of the middle ear and mastoid cavity, characterised by ear discharge (otorrhoea) through a perforated tympanic membrane.
The predominant symptoms of CSOM are ear discharge and hearing loss. Ear discharge can be persistent or intermittent, and many sufferers find it socially embarrassing as the discharge is often visible and odorous (Orji 2013). Some patients also experience discomfort or earache. Most patients with CSOM experience temporary or permanent hearing loss with average hearing levels typically between 10 and 40 decibels (Jensen 2013). The hearing loss can be disabling, and it can have an impact on speech and language skills, employment prospects, and on children's psychosocial and cognitive development, including academic performance (Elemraid 2010; Olatoke 2008; WHO 2004). Consequently, quality of life can be affected. CSOM can also progress to serious complications in rare cases (and more often when cholesteatoma is present): both extracranial complications (such as mastoid abscess, postauricular fistula and facial palsy) and intracranial complications (such as otitic meningitis, lateral sinus thrombosis and cerebellar abscess) have been reported (Dubey 2007; Yorgancılar 2013).
CSOM is estimated to have a global incidence of 31 million episodes per year, or 4.8 new episodes per 1000 people (all ages), with 22% of cases affecting children under five years of age (Monasta 2012; Schilder 2016). The prevalence of CSOM varies widely between countries, but it disproportionately affects people at socio‐economic disadvantage. It is rare in high‐income countries, but common in many low‐ and middle‐income countries (Mahadevan 2012; Monasta 2012; Schilder 2016; WHO 2004).
Definition of disease
There is no universally accepted definition of CSOM. Some define CSOM in patients with a duration of otorrhoea of more than two weeks but others may consider this an insufficient duration, preferring a minimum duration of six weeks or more than three months (Verhoeff 2006). Some include diseases of the tympanic membrane within the definition of CSOM, such as tympanic perforation without a history of recent ear discharge, or the disease cholesteatoma (a growth of the squamous epithelium of the tympanic membrane).
In accordance with a consensus statement, here we use CSOM only to refer to tympanic membrane perforation, with intermittent or continuous ear discharge (Gates 2002). We have used a duration of otorrhoea of two weeks as an inclusion criterion, in accordance with the definition used by the World Health Organization, but we have used subgroup analyses to explore whether this is a factor that affects observed treatment effectiveness (WHO 2004).
Many people affected by CSOM do not have good access to modern primary health care, let alone specialised ear and hearing care, and in such settings health workers may be unable to view the tympanic membrane to definitively diagnose CSOM. It can also be difficult to view the tympanic membrane when the ear discharge is profuse. Therefore we have also included, as a subset for analysis, studies where participants have had chronic ear discharge for at least two weeks, but where the diagnosis is unknown.
At‐risk populations
Some populations are considered to be at high risk of CSOM. There is a high prevalence of disease among Indigenous people such as the Aboriginal and Torres Strait Islander Australian, Native American and Inuit populations. This is likely due to an interplay of factors, including socio‐economic deprivation and possibly differences resulting from population genetics (Bhutta 2016). Those with primary or secondary immunodeficiency are also susceptible to CSOM. Children with craniofacial malformation (including cleft palate) or chromosomal mutations such as Down syndrome are prone to chronic non‐suppurative otitis media ('glue ear'), and by extrapolation may also be at greater risk of suppurative otitis media. The reasons for this association with craniofacial malformation are not well understood, but may include altered function of the Eustachian tube, coexistent immunodeficiency, or both. These populations may be less responsive to treatment and more likely to develop CSOM, recurrence or complications.
Children who have a grommet (ventilation tube) in the tympanic membrane to treat glue ear or recurrent acute otitis media may be more prone to develop CSOM; however, their pathway to CSOM may differ and therefore they may respond differently to treatment. Children with grommets who have chronic ear discharge meeting the CSOM criteria are therefore considered to be a separate high‐risk subgroup (van der Veen 2006).
Treatment
Treatments for CSOM may include topical antibiotics (administered into the ear) with or without steroids, systemic antibiotics (given either by mouth or by injection), topical antiseptics and ear cleaning (aural toileting), all of which can be used on their own or in various combinations. Whereas primary healthcare workers or patients themselves can deliver some treatments (for example, some aural toileting and antiseptic washouts), in most countries antibiotic therapy requires prescription by a doctor. Surgical interventions to repair the tympanic membrane are an option in cases where complications arise or in patients who have not responded to other treatments; however, there is a range of practice in terms of the type of surgical intervention that should be considered and the timing of the intervention. In addition, access to or availability of surgical interventions is setting‐dependent. This series of Cochrane Reviews therefore focuses on non‐surgical interventions. In addition, most clinicians consider cholesteatoma to be a variant of CSOM, but acknowledge that it will not respond to non‐surgical treatment (or will only respond temporarily) (Bhutta 2011). Therefore, studies in which more than half of the participants were identified as having cholesteatoma are not included in these reviews.
Description of the intervention
Antibiotics are the most commonly used treatment for CSOM. They can be administered topically (as drops, ointments, sprays or creams to the affected area) or systemically (either by mouth or by injection into a vein (intravenous) or muscles (intramuscular)). Topical antibiotics are often used in preference to systemic antibiotics as there may be immediate adverse effects of systemic antibiotics such as gastrointestinal upset. A broader concern is the association of the overuse of systemic antibiotics with increasing bacterial resistance among community‐ and hospital‐acquired pathogens (Costelloe 2010; ECDC 2011; Laxminarayan 2013).
Topical application has the advantage of potentially delivering high concentrations of antibiotic to the affected area, whereas systemic antibiotics are absorbed and distributed throughout the body. However, the penetration of topical antibiotics into the middle ear may be compromised if the perforation in the tympanic membrane is small or there is copious mucopurulent discharge in the ear canal that cannot be cleaned. It may also be difficult to achieve compliance with topical dosing in both children and adults. In these cases, systemic antibiotics may have an advantage.
How the intervention might work
CSOM is a chronic and often polymicrobial (involving more than one micro‐organism) infection of the middle ear. Broad‐spectrum antibiotics such as second‐generation quinolones and aminoglycosides, which are often active against the most frequently cultured micro‐organisms (Pseudomonas aeruginosa andStaphylococcus aureus) are therefore commonly used (Mittal 2015) (Table 5). It is possible that antibiotics for CSOM that target Pseudomonas aeruginosa may have an advantage over antibiotics that do not. Dose and duration of treatment are also important factors but are less likely to affect relative effectiveness if given within the therapeutic range. Generally, treatment for at least five days is necessary and a duration of one to two weeks is sufficient to resolve uncomplicated infections. However, in some cases it may take more than to two weeks for the ear to become dry and therefore longer follow‐up (more than four weeks) may be needed to monitor for recurrence of discharge. Some antibiotics (such as aminoglycosides) may have the potential to be toxic to the inner ear (ototoxicity), which might be experienced as sensorineural hearing loss, dizziness or tinnitus, but this is less likely to be a risk when applied topically in patients with CSOM (Phillips 2007). Local discomfort, ear pain or itching may occur through the action of putting ear drops into the ear or because the topical antibiotics or their excipients cause chemical or allergic irritation of the skin of the outer ear.
2. Examples of antibiotics classes and agents with anti‐Pseudomonas activity.
| Class of antibiotics | Examples | Route of administration |
| Quinolones | Ciprofloxacin, ofloxacin, levofloxacin | Oral, intravenous, topical |
| Aminoglycosides | Gentamicin, tobramycin | Topical or parenteral |
| Neomycin/framycetin | Only topical | |
| Cephalosporins | Ceftazidime | Parenteral |
| Penicillins | Ticarcillin plus clavulanic acid | Parenteral |
| Monobactams | Aztreonam | Parenteral |
Why it is important to do this review
Topical antibiotics (without steroids) are widely recommended as a first‐line treatment for CSOM. Opinions about the safety of topical antibiotics for treatment of CSOM, particularly the use of aminoglycosides, differ between professional groups and amongst ENT specialists across different countries. There remain concerns about local toxicity to the inner ear (ototoxicity), particularly with the use of topical aminoglycosides (Phillips 2007; Youngs 2016). Quinolone antibiotics are considered by many to have the best overall risk‐benefit profile, but the evidence base contains only a few small trials with high risk of bias. They are also not licensed for the treatment of CSOM (Youngs 2016). In addition, the cost of treatment, especially with quinolones, may be an issue in some settings. Evidence‐based knowledge of their effectiveness and the relative effectiveness of different topical antibiotics could help to optimise their use.
Objectives
To assess the effects of topical antibiotics (without steroids) for people with chronic suppurative otitis media (CSOM).
Methods
Criteria for considering studies for this review
Types of studies
We included studies with the following design characteristics:
Randomised controlled trials (including cluster‐randomised trials where the unit of randomisation is the setting or operator) and quasi‐randomised trials.
Patients were followed up for at least one week.
We excluded studies with the following design characteristics:
Cross‐over trials, because CSOM is not expected to be a stable chronic condition. Unless data from the first phase were available, we excluded such studies.
Types of participants
We included studies with patients (adults and children) who had:
chronic ear discharge of unknown cause; or
chronic suppurative otitis media.
We defined patients with chronic ear discharge as patients with at least two weeks of ear discharge, where the cause of the discharge was unknown.
We defined patients with chronic suppurative otitis media (CSOM) as patients with:
chronic or persistent ear discharge for at least two weeks; and
a perforated tympanic membrane.
We did not exclude any populations based on age, risk factors (cleft palate, Down syndrome), ethnicity (e.g. Australian Aboriginal or Torres Strait Islanders) or the presence of ventilation tubes (grommets). Where available, we recorded these factors in the patient characteristics section during data extraction from the studies. If any of the included studies recruited these patients as a majority (80% or more), we analysed them in a subgroup analysis (see Subgroup analysis and investigation of heterogeneity).
We excluded studies where the majority (more than 50%) of participants:
had an alternative diagnosis to CSOM (e.g. otitis externa);
had underlying cholesteatoma;
had ear surgery within the last six weeks.
We did not include studies designed to evaluate interventions in the immediate peri‐surgical period, which were focused on assessing the impact of the intervention on the surgical procedure or outcomes.
Types of interventions
Intervention
We included all (topical) antibiotics applied directly into the ear canal. The most common formulations are ear drops but we also included other formulations such as sprays.
We excluded studies that conducted swabs and tests for antimicrobial sensitivity and then based the choice of antibiotics for each participant on the results of the laboratory test.
Duration
At least five days of treatment with antibiotics was required, except for antibiotics where a shorter duration has been proven to be equivalent.
Dose
There was no limitation on the dose, concentration, volume or frequency of application.
Comparisons
The following were the comparators:
Placebo, no intervention (topical antibiotic versus placebo; topical antibiotic versus no intervention).
Another topical antibiotic (topical antibiotic A versus topical antibiotic B).
We analysed these as three main scenarios depending on which common therapy was applied in the background:
Topical antibiotics as a single treatment (main therapy): this included studies where all participants in both treatment groups either received no other treatment or only received aural toileting. This also included situations where antiseptics were applied only once (e.g. as part of microsuction at the start of treatment).
Topical antibiotics as an add‐on therapy to antiseptics: this included studies where all participants in both treatment groups also used a daily antiseptic, with or without aural toileting.
Topical antibiotics as an add‐on therapy to systemic or another topical antibiotic: this included studies where all participants in both treatment groups also received a systemic or topical antibiotic, which was a different type to the antibiotic under investigation, with or without aural toileting or antiseptics.
If either one or both intervention and comparison arms also received a topical steroid, we considered the data in an accompanying review in this series ('Topical antibiotics with steroids for chronic suppurative otitis media') (Brennan‐Jones 2018a).
Many comparison pairs were possible in this review. The main comparisons of interest that we summarised and presented in the 'Summary of findings' table are:
topical antibiotics as a single treatment (main therapy) versus placebo or no intervention;
topical antibiotics as a single treatment (main therapy) versus placebo or no intervention, on top of systemic antibiotics; and
topical quinolone versus topical aminoglycoside (both as single treatments).
Types of outcome measures
We analysed the following outcomes in the review, but we did not use them as a basis for including or excluding studies.
We extracted and reported data from the longest available follow‐up for all outcomes.
Primary outcomes
-
Resolution of ear discharge or 'dry ear' (whether otoscopically confirmed or not), measured at:
between one week and up to two weeks;
two weeks to up to four weeks; and
after four weeks.
Health‐related quality of life using a validated instrument for CSOM (e.g. Chronic Otitis Media Questionnaire (COMQ)‐12 (Phillips 2014a; Phillips 2014b; van Dinther 2015), Chronic Otitis Media Outcome Test (COMOT)‐15 (Baumann 2011), Chronic Ear Survey (CES) (Nadol 2000).
Ear pain (otalgia) or discomfort or local irritation.
Secondary outcomes
Hearing, measured as the pure‐tone average of air conduction thresholds across four frequencies tested (500 Hz, 1000 Hz, 2000 Hz and 4000 Hz) of the affected ear. If this was not available, we reported the pure‐tone average of the thresholds measured.
Serious complications, including intracranial complications (such as otitic meningitis, lateral sinus thrombosis and cerebellar abscess) and extracranial complications (such as mastoid abscess, postauricular fistula and facial palsy) and death.
-
Ototoxicity; this was measured as 'suspected ototoxicity' as reported by the studies where available, and as the number of people with the following symptoms that may be suggestive of ototoxicity:
sensorineural hearing loss;
balance problems/dizziness/vertigo;
tinnitus.
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 1 April 2019.
Electronic searches
The Information Specialist searched:
the Cochrane ENT Register (searched via the Cochrane Register of Studies to 1 April 2019);
the Cochrane Central Register of Controlled Trials (CENTRAL) (searched via the Cochrane Register of Studies Web to 1 April 2019);
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to 1 April 2019);
Ovid EMBASE (1974 to 1 April 2019);
EBSCO CINAHL (1982 to 1 April 2019);
LILACS (Latin American and Caribbean Health Science Information database), lilacs.bvsalud.org (search to 1 April 2019);
Web of Knowledge, Web of Science (1945 to 1 April 2019);
ClinicalTrials.gov, www.clinicaltrials.gov (search via the Cochrane Register of Studies to 1 April 2019);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (search to 1 April 2019).
We also searched:
IndMed (search to 22 March 2018);
African Index Medicus (search to 22 March 2018).
The search strategies for major databases are detailed in Appendix 1. The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. The search strategies were designed to identify all relevant studies for a suite of reviews on various interventions for chronic suppurative otitis media (Bhutta 2018; Brennan‐Jones 2018a; Brennan‐Jones 2018b; Chong 2018a; Chong 2018b; Head 2018a; Head 2018b). Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011)
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched Ovid MEDLINE to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Information Specialist also ran non‐systematic searches of Google Scholar to retrieve grey literature and other sources of potential trials.
We did not perform a separate search for adverse effects. We considered the adverse effects described in the included studies only.
We contacted original authors for clarification and further data if trial reports were unclear and we arranged translations of papers where necessary.
Data collection and analysis
Selection of studies
At least two review authors (KH/LYC) independently screened all titles and abstracts of the references obtained from the database searches to identify potentially relevant studies. At least two review authors (KH/LYC) evaluated the full text of each potentially relevant study to determine whether it met the inclusion and exclusion criteria for this review.
We resolved any differences by discussion and consensus, with the involvement of a third author for clinical and methodological input where necessary.
Data extraction and management
At least two review authors (KH/LYC/CBJ/MB) independently extracted data from each study using a standardised data collection form (see Appendix 2). Whenever a study had more than one publication, we retrieved all publications to ensure complete extraction of data. Where there were discrepancies in the data extracted by different review authors, we checked these against the original reports and resolved any differences by discussion and consensus, with the involvement of a third author or a methodologist where appropriate. We contacted the original study authors for clarification or for missing data whenever possible. If differences were found between publications of a study, we contacted the original authors for clarification. We used data from the main paper(s) if no further information was found.
We included key characteristics of the included studies, such as study design, setting (including location), year of study, sample size, age and sex of participants, and how outcomes were defined or collected in the studies. In addition, we also collected baseline information on prognostic factors or effect modifiers (see Appendix 2). For this review, this included the following information whenever available:
duration of ear discharge at entry to the study;
diagnosis of CSOM (as opposed to patients with chronic ear discharge but without a diagnosis of CSOM);
number of participants who may have been at higher risk of CSOM, including those with cleft palate or Down syndrome;
ethnicity of participants including the number who were from Indigenous populations;
number of participants who had previously had ventilation tubes (grommets) inserted (and, where known, the number who had tubes still in place);
number of participants who had previous ear surgery;
number of participants who had previous treatments for CSOM (non‐responders, recurrent versus new cases).
We recorded concurrent treatments alongside the details of the interventions used. See the 'Data extraction form' in Appendix 2 for more details.
For the outcomes of interest to the review, we extracted the findings of the studies on an available case analysis basis, i.e. we included data from all participants available at the time points based on the treatment randomised whenever possible, irrespective of compliance or whether participants had received the treatment as planned.
In addition to extracting pre‐specified information about study characteristics and aspects of methodology relevant to risk of bias, we extracted the following summary statistics for each trial and each outcome:
For continuous data: the mean values, standard deviations and number of patients for each treatment group. Where endpoint data were not available, we extracted the values for change from baseline. We analysed data from disease‐specific quality of life scales such as COMQ‐12, COMOT‐15 and CES as continuous data.
For binary data: the number of participants who experienced an event and the number of patients assessed at the time point.
For ordinal scale data: if the data appeared to be approximately normally distributed or if the analysis that the investigators performed suggested parametric tests were appropriate, then we treated the outcome measures as continuous data. Alternatively, if data were available, we converted it into binary data.
Time‐to‐event outcomes: we did not expect any outcomes to be measured as time‐to‐event data. However, if outcomes such as resolution of ear discharge were measured in this way, we reported the hazard ratios.
For resolution of ear discharge, we extracted the longest available data within the time frame of interest, defined as from one week up to (and including) two weeks (7 days to 14 days), from two weeks up to (and including) four weeks (15 to 28 days), and after four weeks (28 days or one month).
For other outcomes, we reported the results from the longest available follow‐up period.
Extracting data for pain/discomfort and adverse effects
For these outcomes, there were variations in how studies had reported the outcomes. For example, some studies reported both 'pain' and 'discomfort' separately whereas others did not. Prior to the commencement of data extraction, we agreed and specified a data extraction algorithm for how data should be extracted.
We extracted data for serious complications as a composite outcome. If a study reported more than one complication and we could not distinguish whether these occurred in one or more participants, we extracted the data with the highest incidence to prevent double counting.
Extracting data from figures
Where values for primary or secondary outcomes were shown as figures within the paper, we attempted to contact the study authors to try to obtain the raw values. When the raw values were not provided, we extracted information from the graphs using an online data extraction tool, using the best quality version of the relevant figures available.
Assessment of risk of bias in included studies
At least two review authors (KH/LYC/CBJ/MB) independently assessed the risk of bias of each included study. We followed the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), using the Cochrane 'Risk of bias' tool. With this tool we assessed the risk of bias as 'low', 'high' or 'unclear' for each of the following six domains:
sequence generation;
allocation concealment;
blinding of participants, personnel and outcome assessment;
incomplete outcome data;
selective reporting;
other sources of bias.
Measures of treatment effect
We summarised the effects of dichotomous outcomes (e.g. proportion of patients with complete resolution of ear discharge) as risk ratios (RR) with confidence intervals (CIs). For the key outcomes that are presented in the 'Summary of findings' table, we expressed the results as absolute numbers based on the pooled results and compared to the assumed risk. We also calculated the number needed to treat to benefit (NNTB) using the pooled results. The assumed baseline risk was typically either (a) the median of the risks of the control groups in the included studies, this being used to represent a 'medium‐risk population' or, alternatively, (b) the average risk of the control groups in the included studies, which is used as the 'study population' (Handbook 2011). If a large number of studies were available, and where appropriate, we also attempted to present additional data based on the assumed baseline risk in (c) a low‐risk population and (d) a high‐risk population.
For continuous outcomes, we expressed treatment effects as a mean difference (MD) with standard deviation (SD). If different scales were used to measure the same outcome, we used the standardised mean difference (SMD) and provided a clinical interpretation of the SMD values.
Unit of analysis issues
Cross‐over studies
This review did not use data from phase II of cross‐over studies.
The ear as the unit of randomisation: within‐patient randomisation in patients with bilateral ear disease
For data from studies where 'within‐patient' randomisation was used (i.e. studies where both ears (right versus left) were randomised), we adjusted the analyses for the paired nature of the data (Elbourne 2002; Stedman 2011), as outlined in section 16.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
The ear as the unit of randomisation: non‐paired randomisation in patients with bilateral ear disease
Some patients with bilateral disease may have received the same treatment in both ears, whereas others received a different treatment in each ear. We did not exclude these studies, but we only reported the data if specific pairwise adjustments were completed or if sufficient data were obtained to be able to make the adjustments.
The patient as the unit of randomisation
Some studies randomised by patient and those with bilateral CSOM received the same intervention for both ears. In some studies the results may be reported as a separate outcome for each ear (the total number of ears is used as the denominator in the analysis). The correlation of response between the left ear and right ear when given the same treatment was expected to be very high, and if both ears were counted in the analysis this was effectively a form of double counting, which may be especially problematic in smaller studies if the number of people with bilateral CSOM was unequal. We did not exclude these studies, but we only reported the results if the paper presented the data in such a way that we could include the data from each participant only once (one data point per participant) or if we had enough information to reliably estimate the effective sample size or inflated standard errors as presented in chapter 16.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). If this was not possible, we attempted to contact the authors for more information. If there was no response from the authors, then we did not include data from these studies in the analysis.
If we found cluster‐randomised trials by setting or operator, we analysed these according to the methods in section 16.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
Dealing with missing data
We attempted to contact the study authors via email whenever the outcome of interest was not reported but the methods of the study suggested that the outcome had been measured. We did the same if not all of the data required for the meta‐analysis were reported, unless the missing data were standard deviations. If standard deviation data were not available, we approximated these using the standard estimation methods from P values, standard errors or 95% CIs if these were reported, as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). Where it was impossible to estimate these, we contacted the study authors.
Apart from imputations for missing standard deviations, we did not conduct any other imputations. We extracted and analysed data for all outcomes using the available case analysis method.
Assessment of heterogeneity
We assessed clinical heterogeneity (which may be present even in the absence of statistical heterogeneity) by examining the included studies for potential differences in the types of participants recruited, interventions or controls used, and the outcomes measured. We did not pool studies where the clinical heterogeneity made it unreasonable to do so.
We assessed statistical heterogeneity by visually inspecting the forest plots and by considering the Chi² test (with a significance level set at P value < 0.10) and the I² statistic, which calculated the percentage of variability that is due to heterogeneity rather than chance, with I² values over 50% suggesting substantial heterogeneity (Handbook 2011).
Assessment of reporting biases
We assessed reporting bias as within‐study outcome reporting bias and between‐study publication bias.
Outcome reporting bias (within‐study reporting bias)
We assessed within‐study reporting bias by comparing the outcomes reported in the published report against the study protocol, whenever this could be obtained. If the protocol was not available, we compared the outcomes reported to those listed in the methods section. If results were mentioned but not reported adequately in a way that allowed analysis (e.g. the report only mentioned whether the results were statistically significant or not), bias in a meta‐analysis was likely to occur. We tried to find further information from the study authors, but if no further information could be obtained, we noted this as being a high risk of bias. Where there was insufficient information to judge the risk of bias, we noted this as an unclear risk of bias (Handbook 2011).
Publication bias (between‐study reporting bias)
We intended to create funnel plots if sufficient trials (more than 10) were available for an outcome. If we observed asymmetry of the funnel plot, we would have conducted a more formal investigation using the methods proposed by Egger 1997.
Data synthesis
We conducted all meta‐analyses using Review Manager 5.3 (RevMan 2014). For dichotomous data, we analysed treatment differences as a risk ratio (RR) calculated using the Mantel‐Haenszel methods. We analysed time‐to‐event data using the generic inverse variance method.
For continuous outcomes, if all the data was from the same scale, we pooled the mean values obtained at follow‐up with change outcomes and reported this as a MD. However, if the SMD had to be used as an effect measurement, we did not pool change and endpoint data.
When statistical heterogeneity is low, random‐effects versus fixed‐effect methods yield trivial differences in treatment effects. However, when statistical heterogeneity is high, the random‐effects method provides a more conservative estimate of the difference.
Subgroup analysis and investigation of heterogeneity
We subgrouped studies where most participants (80% or more) met the criteria stated below in order to determine whether the effect of the intervention was different compared to other patients. Due to the risks of reporting and publication bias with unplanned subgroup analyses of trials, we only analysed subgroups reported in studies if these were prespecified and stratified at randomisation.
We planned to conduct subgroup analyses regardless of whether statistical heterogeneity was observed for studies that included patients identified as high risk (i.e. thought to be less responsive to treatment and more likely to develop CSOM, recurrence or complications) and patients with ventilation tubes (grommets). 'High risk' patients include Indigenous populations (e.g. Australian Aboriginal and Torres Strait Islanders, Native Americans and Inuit populations of Alaska, Canada and Greenland), people with craniofacial malformation (e.g. cleft palate), Down syndrome and people with known immunodeficiency.
We planned to present the main analyses of this review in the form of forest plots based on this main subgroup analysis.
For the high‐risk group, this applied to the outcomes resolution of ear discharge (dry ear), quality of life, pain/discomfort, development of complications and hearing loss.
For patients with ventilation tubes, this applied to the outcome resolution of ear discharge (dry ear) for the time point of four weeks or more because this group was perceived to be at lower risk of treatment failure and recurrence than other patient groups. If statistical heterogeneity was observed, we also conducted subgroup analysis for the effect modifiers below. If there were statistically significant subgroup effects, we presented these subgroup analysis results as forest plots.
For this review, effect modifiers included:
Diagnosis of CSOM: it was likely that some studies would include patients with chronic ear discharge but who had not had a diagnosis of CSOM. Therefore, we subgrouped studies where most patients (80% or more) met the criteria for CSOM diagnosis in order to determine whether the effect of the intervention was different compared to patients where the precise diagnosis was unknown and inclusion into the study was based purely on chronic ear discharge symptoms.
Duration of ear discharge: there is uncertainty about whether the duration of ear discharge prior to treatment has an impact on the effectiveness of treatment and whether more established disease (i.e. discharge for more than six weeks) is more refractory to treatment compared with discharge of a shorter duration (i.e. less than six weeks).
Patient age: patients who were younger than two years old versus patients up to six years old versus adults. Patients under two years are widely considered to be more difficult to treat.
We presented the results as subgroups regardless of the presence of statistical heterogeneity based on the following two factors:
Class of antibiotics: We grouped by pharmacological class, e.g. quinolones, aminoglycosides, penicillins etc. The rationale for this was that different classes may have had different effectiveness and side effect profiles.
Spectrum of activity against Pseudomonas aeruginosa (groups with known activity against Pseudomonas aeruginosa versus groups without activity against Pseudomonas aeruginosa). This is the most commonly found bacteria in patients with CSOM and its presence is associated with tissue damage.
When other antibiotics were also used as a common treatment in both the intervention and comparison group, we investigated the class and antipseudomonal activity when statistical heterogeneity was present and could not be explained by the other subgroup analyses.
No other subgroups based on the pharmacological properties of antibiotics were planned, but we considered the method and frequency of aural toileting if there was remaining unexplained heterogeneity despite conducting the other subgroup analyses.
Sensitivity analysis
We planned to carry out sensitivity analyses to determine whether the findings were robust to the decisions made in the course of identifying, screening and analysing the trials. We planned to conduct sensitivity analysis for the following factors, whenever possible:
Impact of model chosen: fixed‐effect versus random‐effects model.
Risk of bias of included studies: excluding studies with high risk of bias (we defined these as studies that have a high risk of allocation concealment bias and a high risk of attrition bias (overall loss to follow‐up of 20%, differential follow‐up observed)).
Where there was statistical heterogeneity, studies that only recruited patients who had previously not responded to one of the treatments under investigation in the RCT. Studies that specifically recruited patients who did not respond to a treatment could potentially have reduced the relative effectiveness of an agent.
If any of these investigations found a difference in the size of the effect or heterogeneity, we mentioned this in the 'Effects of interventions' section and/or presented the findings in a table.
GRADE and 'Summary of findings' table
Using the GRADE approach, at least two review authors (KH/LYC) independently rated the overall certainty of evidence using the GDT tool (http://www.guidelinedevelopment.org/) for the main comparison pairs listed in the Types of interventions section. The certainty of evidence reflects the extent to which we were confident that an estimate of effect was correct and we applied this in the interpretation of results. There were four possible ratings: 'high', 'moderate', 'low' and 'very low' (Handbook 2011). A rating of 'high' certainty evidence implies that we were confident in our estimate of effect and that further research was very unlikely to change our confidence in the estimate of effect. A rating of 'very low' certainty implies that any estimate of effect obtained was very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high certainty. However, several factors could lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading was determined by the seriousness of these factors:
study limitations (risk of bias);
inconsistency;
indirectness of evidence;
imprecision;
publication bias.
The 'Summary of findings' table presents the following outcomes:
-
resolution of ear discharge or 'dry ear':
at between one week and up to two weeks;
after four weeks;
health‐related quality of life;
ear pain (otalgia) or discomfort or local irritation;
hearing;
serious complications;
suspected ototoxicity.
Results
Description of studies
Results of the search
The searches retrieved a total of 7256 references and we identified five additional references from other sources. This was reduced to 3147 after removal of duplicates. We screened the titles and abstracts and subsequently removed 2935 references. We assessed 212 full‐text references for eligibility of which we discarded 187; we excluded 150 (including unpublished studies) of these references (122 studies) with reasons recorded in the review (see Excluded studies).
We included 23 references (17 studies). There are two references awaiting classification (Abdul 2005; Abes 1998). See Characteristics of studies awaiting classification. We did not identify any ongoing studies.
A flow chart of study retrieval and selection is provided in Figure 1.
1.
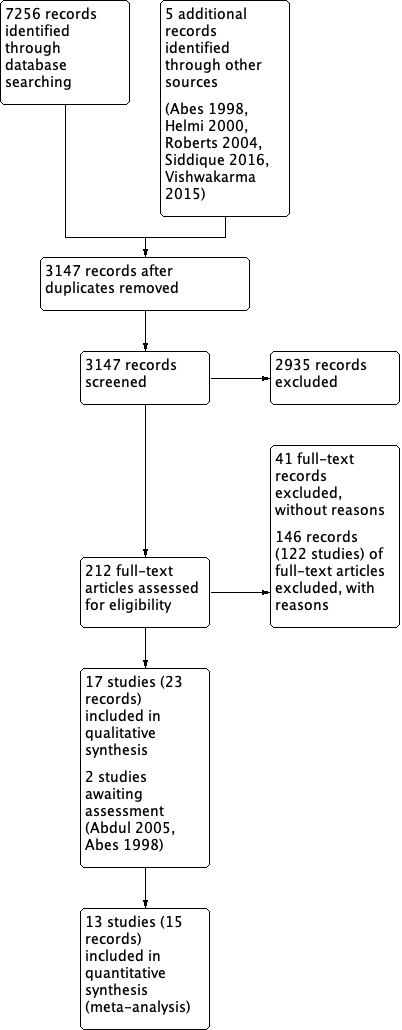
Study flow diagram
Included studies
Seventeen studies were included (Asmatullah 2014; de Miguel 1999; Esposito 1990; Fradis 1997; Gyde 1978; Jamalullah 2016; Kasemsuwan 1997; Kaygusuz 2002; Liu 2003; Lorente 1995; Mira 1993; Nawasreh 2001; Ramos 2003; Siddique 2016; Tutkun 1995; van Hasselt 1997; van Hasselt 1998a). Table 6 provides a summary of the included studies.
3. Summary of study characteristics.
|
Ref ID (no. participants) |
Setting | Population | Intervention 1 | Intervention 2 | Treatment duration | Follow‐up | Background Treatment | Notes |
| Topical antibiotics versus placebo/no treatment (no background or aural toileting) | ||||||||
|
Kasemsuwan 1997 (n = 50) |
Specialist hospital, Thailand | Mucopurulent otorrhoea with perforated tympanic membrane (CSOM) | Ciprofloxacin 250 mg/mL, 5 drops per 8 hours | Saline, 5 drops per 8 hours | 1 week | 1 week | Aural toilet on day 1, 4 and 7 | Randomised by person |
| Topical antibiotic versus placebo/no treatment (systemic antibiotic as background treatment) | ||||||||
|
de Miguel 1999 (n = 50) |
General hospital, Spain | Simple chronic otitis media (36%), osteitic chronic otitis media (25.6%), cholesteatomas chronic otitis media (13.6%), post surgery cases (24.8%) | Topical ciprofloxacin 0.2%, 3 drops per 8 hours and oral ciprofloxacin, 500 mg per 12 hours | No treatment | 7 days | 15 days | Aural toileting before beginning treatment, analgesics and antipyretics. Oral ciprofloxacin, 500 mg per 12 hours | Part of 5‐arm trial Randomised by person |
|
Esposito 1990 (n = 40) |
University clinic, Italy | Mild or moderate CSOM in acute stage | Ciprofloxacin 250 µg/mL, 3 drops per 12 hours | No treatment | 5 to 10 days | 2 weeks | Oral ciprofloxacin, 250mg per 12 hours | Part of 3‐arm trial Randomised by person |
|
Mira 1993 (n = 50) |
University clinic, Italy | Recurrence of CSOM or suppuration following mastoidectomy or tympanoplasty | Ceftizoxime 500 µg/mL, 2 x 2 mL washes per 12 hours | Saline, 2 x 2 mL washes per 12 hours | 1 week | 3 weeks | Systemic ceftizoxime by intramuscular route every 12 hours Aural toilet at first visit |
Randomised by person |
|
Ramos 2003 (n = 100) |
ENT departments, Spain | Simple chronic otitis media (42.7%), chronic otitis media with osteolysis (19%), chronic cholesteatoma (14%), chronic otorrhoea in operated ears 24.3%) | Ciprofloxacin 0.2%, 0.5 mL per 8 hours | No treatment | 1 week | 10 days | Oral ciprofloxacin, 500 mg per 12 hours | Part of a 6‐arm trial Randomised by person |
| Quinolones versus aminoglycosides | ||||||||
|
Asmatullah 2014 (n = 134) |
ENT department, Pakistan | Active tubotympanic type CSOM | Ofloxacin 0.3%, 12 drops per day | Gentamycin 0.3%, 12 drops per day | 10 days | 2 weeks | None mentioned | Randomised by person |
|
Fradis 1997 (n = 40) |
Outpatient clinic, Israel | Chronic otitis media | Ciprofloxacin (no conc), 15 drops per day | Tobramycin (no conc), 15 drops per day | 3 weeks | 3 weeks | None mentioned | Part of 3‐arm trial Randomised by ear |
|
Kaygusuz 2002 (n = 40) |
University ENT clinic, Turkey | CSOM | Ciprofloxacin 0.3%, 6 drops per day | Tobramycin 0.3%, 6 drops per day | 3 weeks | 3 weeks | Daily aspiration | Translated from Turkish Part of 4‐arm trial Randomised by person. |
|
Nawasreh 2001 (n = 88) |
Unclear setting, Jordan | CSOM and intermittent mucopurulent heavy discharge for more than 1 year | Ciprofloxacin 200 µg/mL (0.02%), 15 drops per day | Gentamicin 5 mg/mL, 15 drops per day | 10 days | 2 weeks | None mentioned | Randomised by person |
|
Lorente 1995 (n = 308) |
Hospital ENT clinics, Spain | CSOM (purulent discharge > 3 months and perforated membrane) | Ciprofloxacin 0.3%, 15 drops per day | Gentamycin 0.3%, 15 drops per day | 8 days | 30 days | Unclear | Translated from Spanish Assume this is same as Sabater paper Randomised by person |
|
Tutkun 1995 (n = 44) |
University hospital, Turkey | CSOM and purulent discharge for more than 1 year | Ciprofloxacin 200 µg/mL (0.02%), 15 drops per day | Gentamicin 5 mg/mL, 15 drops per day | 10 days | 10 days | None mentioned | Randomised by person |
|
Jamalullah 2016 (n = 80) |
Otolaryngology department, Pakistan | CSOM (tubotympanic type) | Ofloxacin 0.6%, 12 drops per day | Gentamycin 0.3%, 12 drops per day | 2 weeks | 2 weeks | One aural toilet at start | Randomised by person |
| Quinolones versus others | ||||||||
|
Siddique 2016 (n = 200) |
Specialist hospital, Pakistan | Tubotympanic type of CSOM | Ciprofloxacin (no conc), 3 drops per 12 hours | Neomycin/polymixin/gramicidin‐D (no conc), 2 drops per 12 hours | Unclear (probably 4 weeks) | 4 weeks | No information | Randomised by person |
|
van Hasselt 1997 (n = 50) |
Rural setting, Malawi | Children with CSOM | Ofloxacin 0.3%, 3 drops per 8 hours | Neomycin 0.5%/polymixin B 0.1%, 3 drops per 8 hours | 2 weeks | 2 weeks | Aural toilet at start and weekly | Part of a 3‐arm trial Only presented as an internal report Unclear unit of randomisation, results reported by ear |
|
van Hasselt 1998a (n = unclear) |
Rural setting, Malawi | "Mainly children" with CSOM | Ofloxacin 0.3%, 6 drops per 12 hours | Neomycin/polymixin B (no conc), 6 drops per 12 hours | 2 weeks | 8 weeks | Aural toilet at start and weekly | Only a presentation given at a conference available Unclear unit of randomisation, results presented by ear Part of 4‐arm trial ‐ once weekly arms have not been included. |
| Aminoglycosides versus trimethoprim, sulphacetamide and polymixin B (TSP) | ||||||||
|
Gyde 1978 (n = 91) |
Outpatient clinic, Canada | Otitis externa (21%), CSOM (51%), subacute otitis (16%), postoperative infection (21%) | Trimethoprim, sulphacetamide and polymyxin B, 16 drops per day | Gentamicin 0.3%, 16 drops per day | Mean: 16 days | 12 months | Not reported | Translated from French Randomised by person but reported by ear Semi cross‐over trial |
| Rifampicin versus chloramphenicol | ||||||||
|
Liu 2003 (n = 160) |
Outpatient department, China | CSOM | Rifampicin 0.1%, 9 drops per day | Chloramphenicol 0.25%, 9 drops per day | 2 weeks | 2 weeks | 3% hydrogen peroxide ear wash daily | Translated from Chinese Randomised by person |
CSOM: chronic suppurative otitis media
Study design
Ten studies were two‐arm trials (Asmatullah 2014; Gyde 1978; Jamalullah 2016; Kasemsuwan 1997; Liu 2003; Lorente 1995; Mira 1993; Nawasreh 2001; Siddique 2016; Tutkun 1995) and three studies were three‐arm trials (Esposito 1990; Fradis 1997; van Hasselt 1997). Two studies were part of a five‐arm trial (de Miguel 1999; Ramos 2003), where only the arms that compared topical antibiotics plus systemic antibiotics with systemic antibiotics alone were used in this review. Two studies were part of a four‐arm trial (Kaygusuz 2002; van Hasselt 1998a), but only two arms are presented in this review. Details of the other study arms for each study can be found in the Characteristics of included studies table.
All studies provided an indication that they were 'randomised controlled trials' and were parallel‐group studies, apart from Gyde 1978, which was a cross‐over RCT.
Sample size
The total number of participants was 2198. Twelve studies reported the sample size in terms of participants (not ears); these had a total of 1797 participants (Asmatullah 2014; de Miguel 1999; Esposito 1990; Jamalullah 2016; Kasemsuwan 1997; Liu 2003; Lorente 1995; Mira 1993; Nawasreh 2001; Ramos 2003; Siddique 2016; Tutkun 1995). The remaining five studies reported both the number of patients and ears, representing 401 participants, or 510 ears (Fradis 1997; Gyde 1978; Kaygusuz 2002; van Hasselt 1997; van Hasselt 1998a).
Unit of randomisation
The individual (rather than the ear) was randomised to treatment group in 14 studies (Asmatullah 2014; de Miguel 1999; Esposito 1990; Gyde 1978; Jamalullah 2016; Kasemsuwan 1997; Kaygusuz 2002; Liu 2003; Lorente 1995; Mira 1993; Nawasreh 2001; Ramos 2003; Siddique 2016; Tutkun 1995). (See Table 7). Of these 14 studies, only one reported the number of patients with bilateral disease (15 patients (8%) had bilateral disease), but as the denominator was by person, it is assumed that no double counting occurred (Siddique 2016). Although Gyde 1978 was randomised by person, the results were reported by ear. The study Gyde 1978 stated that if there was a treatment failure 'ears' were transferred to the alternative treatment group, thus effectively breaking randomisation. These results have not been included in the analysis.
4. Resolution of ear discharge outcome.
| Reference | Unit of randomisation | Reported | Definition | Otoscopically confirmed? | Time points | Notes |
| Asmatullah 2014 | Person | Person | "no discharge" | Yes | 1 to 2 weeks (10 days) | — |
| de Miguel 1999 | Person | Person | "global index of clinic microbiological cure" | Yes | 1 to 2 weeks (7 days) | — |
| Esposito 1990 | Person | Person | "cured" (no definition but assumed to be no discharge) | Unclear, paper states "clinically examined" | 1 week to 2 weeks (6 to 11 days) and 2 to 4 weeks (19 to 24 days) | 1 to 2 weeks examined but not reported |
| Fradis 1997 | Ear | Ear | "clinical success as defined as cessation of otorrhea and eradication of the microorganisms in the post treatment culture" | Unclear | 2 to 4 weeks (21 days) | Unclear how many patients had bilateral ear disease in each group |
| Gyde 1978 | Person | Ear | "dry ear" and a negative culture at 3 weeks or a real improvement in at 3 weeks and the cessation of discharge at 6 weeks | Unclear | 2 to 4 weeks (3 weeks) and after 4 weeks (6 weeks) | Semi cross‐over trial. It does not appear that any consideration of the correlation of results between ears has been taken into account. If there was a treatment failure 'ears' were transferred to the alternative groups. These results have not been included in the analysis. If the ear was not dry on review at 6 months, treatment for 3 weeks with the alternative treatment was completed with review after 6 months |
| Jamalullah 2016 | Person | Person | "absence" of aural discharge | Yes | 2 to 4 weeks (2 weeks) | — |
| Kasemsuwan 1997 | Person | Person | "cure" | Unclear | 1 to 2 weeks (7 days) | — |
| Kaygusuz 2002 | Person | Ear | Assessed using 3‐point scale (2 points = no drainage) | Yes | 2 to 4 weeks (day 14 and 21) | Unclear method of allocation, unsure if random selection of study ear |
| Liu 2003 | Person | Person | "Cured: otorrhea disappeared, mucosal hyperaemia of the tympanic membrane and tympanic cavity disappeared. Significantly effective: no complaints of otorrhea, no visible purulence in the ear canal and tympanic cavity, and nonvisible or slight hyperaemia of the tympanic membrane and the tympanic canal" |
Unclear | 1 to 2 weeks (2 weeks) | — |
| Lorente 1995 | Person | Person | "Complete resolution of ear discharge" | Yes | 1 to 2 weeks (8 days) and after 4 weeks (30 days) | — |
| Mira 1993 | Person | Person | Not reported in a way that could be used in the review | N/A | N/A | Paper plotted the time course of otorrhoea (quantity) on a scale of 0 to 3 at 3, 7 and 21 days |
| Nawasreh 2001 | Person | Person | "cessation of otorrhea" | Yes | 1 to 2 weeks (10 days) | — |
| Ramos 2003 | Person | Person | "cured" according to "indices de curacion" | Unclear | 1 to 2 weeks (10 days) | — |
| Siddique 2016 | Person | Person | "absence of discharge from middle ear cavity and no inflammation/congestion in middle ear mucosa and tympanic membrane" | Unclear | 2 to 4 weeks (4 weeks) | 15 patients (8%) had bilateral disease but how these cases were handled is not stated. The denominator in the trials is the person so it is assumed that no double counting occurred. |
| Tutkun 1995 | Person | Person | "cessation of otorrhea" | Yes | 1 to 2 weeks (10 days) | — |
| van Hasselt 1997 | Unclear, most likely person | Ear | "dry ear" | Unclear | 1 to 2 weeks (1 week) and after 4 weeks (> 2 weeks) | Counting bilateral ears separately. All ears reported separately. Data come from an unpublished report. In the analysis 3/11 (27.27%), 10/30 (33%) and 11/28 (39%) of patients had bilateral disease in the ofloxacin, neomycin and antiseptic acid groups respectively. |
| van Hasselt 1998a | Unclear | Ear | "inactive ear" ‐ completely dry middle ear | Unclear | 1 to 2 weeks (1 week), 2 to 4 weeks (2 weeks) and after 4 weeks (8 weeks) | Counting bilateral ears separately |
N/A: not applicable
In the remaining one study randomisation occurred by ear and it appears that the analysis occurred without allowing for correlation/adjustment for the response between paired ears (Fradis 1997). The unit of randomisation for two other studies is unclear: it is most likely by person but the results are reported by ear (van Hasselt 1997; van Hasselt 1998a). In the two studies by van Hasselt, bilateral ears were counted separately, but the number of patients per group was not reported. Data from van Hasselt 1997 come from an unpublished report, with the analysis indicating that 3/11 (27.27%), 10/30 (33%) and 11/28 (39%) of patients had bilateral disease in the ofloxacin, neomycin and antiseptic acid groups respectively. The results of these studies were not included in the analysis due to the risk of double counting.
Location
The studies were conducted in 10 countries including: Israel, Thailand, China, Canada, Jordan, Turkey, Italy, Malawi, Pakistan and Spain (see Table 6).
Setting of trial
With regard to clinical setting, six studies were in outpatient departments of hospitals/medical centres (Fradis 1997; Gyde 1978; Jamalullah 2016; Liu 2003; Mira 1993; Ramos 2003); two studies were based in secondary care from the ENT departments of hospitals (Asmatullah 2014; Lorente 1995); two were in a specialist hospital (Kasemsuwan 1997; Siddique 2016); and one study was in the general hospital (de Miguel 1999). Three studies were undertaken in university clinics/hospitals (Esposito 1990; Kaygusuz 2002; Tutkun 1995), while two studies were community studies taking place in rural settings (van Hasselt 1997; van Hasselt 1998a). The setting of the study was unclear in one trial (Nawasreh 2001).
The years in which the studies were conducted were often not well reported. There was one study from the 1970s (Gyde 1978), and one study in which it is unclear but was most likely to have been conducted in the 1980s (Esposito 1990). Nine studies were conducted in the 1990s (de Miguel 1999; Fradis 1997; Kasemsuwan 1997; Lorente 1995; Mira 1993; Nawasreh 2001; Tutkun 1995; van Hasselt 1997; van Hasselt 1998a), three were published in the 2000s (Kaygusuz 2002; Liu 2003; Ramos 2003), and three were published post 2010 (Asmatullah 2014; Jamalullah 2016; Siddique 2016). See Table 6 for further details.
Population
Age and sex
Two studies did not provide any patient characteristics, with the age only referred to as "mainly children" (van Hasselt 1997; van Hasselt 1998a).
Fifteen studies provided information on the age of participants, with the mean age ranging from 25.8 to 44.4 years old (Asmatullah 2014; de Miguel 1999; Esposito 1990; Fradis 1997; Gyde 1978; Jamalullah 2016; Kasemsuwan 1997; Kaygusuz 2002; Liu 2003; Lorente 1995; Mira 1993; Nawasreh 2001; Ramos 2003; Siddique 2016; Tutkun 1995). The de Miguel 1999 study reported a mean age of 39.6 years, however 17/25 participants were children.
All 15 studies that provided participant characteristics reported that they included both males and females. Of the 2010 participants reported, 860 (43%) were female, with the percentage of females in studies ranging from 18% to 63%.
High‐risk populations
None of the studies reported the inclusion of any of the 'high‐risk' populations as defined by our inclusion criteria (cleft palate, Down syndrome, Indigenous groups, immunocompromised patients). Two studies specifically stated "no diabetes or other comorbidities" (Esposito 1990) or "0% immunocompromised" (Siddique 2016), while the remaining 15 did not report any high‐risk populations (Asmatullah 2014; de Miguel 1999; Fradis 1997; Gyde 1978; Jamalullah 2016; Kasemsuwan 1997; Kaygusuz 2002; Liu 2003; Lorente 1995; Mira 1993; Nawasreh 2001; Ramos 2003; Tutkun 1995; van Hasselt 1997; van Hasselt 1998a). However, the two van Hasselt studies were conducted in rural community areas in Malawi, with the van Hasselt 1997 study noting that hygiene was 'poor'.
Diagnosis
Eight studies provided the diagnosis method for confirmation of tympanic membrane perforation or presence of mucopurulent discharge via otoscopy or microscopic examination (de Miguel 1999; Fradis 1997; Jamalullah 2016; Kasemsuwan 1997; Kaygusuz 2002; Ramos 2003; Tutkun 1995; van Hasselt 1997), while an additional study confirmed perforation of the tympanic membrane but did not provide a method (Lorente 1995). The diagnostic method was not reported in eight studies (Asmatullah 2014; Esposito 1990; Gyde 1978; Liu 2003; Mira 1993; Nawasreh 2001; Siddique 2016; van Hasselt 1998a).
Duration of ear discharge
Nine studies reported the duration of symptoms/mucopurulent discharge for diagnosis, with one study including patients with ear discharge for more than six weeks or sporadically with three or more episodes in a year (Ramos 2003), one study with discharge for more than two months (van Hasselt 1998a), four studies with ear discharge for more than three months (Jamalullah 2016; Kasemsuwan 1997; Kaygusuz 2002; Lorente 1995), and a further three studies longer than one year (Fradis 1997; Nawasreh 2001; Tutkun 1995). Eight studies did not have inclusion criteria or provide details of the average duration of ear discharge at the start of the study (Asmatullah 2014; de Miguel 1999; Esposito 1990; Gyde 1978; Liu 2003; Mira 1993; Siddique 2016; van Hasselt 1997).
Other important effect modifiers
Nine studies did not report on any important effect modifiers (Asmatullah 2014; Kasemsuwan 1997; Liu 2003; Lorente 1995; Nawasreh 2001; Siddique 2016; Tutkun 1995; van Hasselt 1997; van Hasselt 1998a). One study reported the previous use of grommets (Ramos 2003; n = 12; 4%). Five studies provided alternative diagnoses (de Miguel 1999 (n = 17; 13.6%); Gyde 1978 (n = 24; 21%); Mira 1993 (n = 52; 21%); Ramos 2003 (n = 42; 14%)). Five studies reported the number having previous surgery (de Miguel 1999 (n = 31; 24.8%); Fradis 1997 (n = 8; 15.7%); Kaygusuz 2002 (none); Mira 1993 (n = 52; 21%); Ramos 2003 (n = 73; 24.3%)). Four studies reported the number having previous antibiotic treatment for CSOM (de Miguel 1999 (n = 79; 63.2%); Esposito 1990 (n = 38; 63%); Fradis 1997 (n = 46; 90.2%); Ramos 2003 (n = 197; 65.6%)).
Intervention
Intervention details
Details of the interventions, background treatments and treatment durations for each of the included studies are summarised in Table 6.
Topical antibiotics
Fourteen studies used topical quinolones, with four of these using ofloxacin (Asmatullah 2014; Jamalullah 2016; van Hasselt 1997; van Hasselt 1998a) and the remaining 10 using ciprofloxacin (de Miguel 1999; Esposito 1990; Fradis 1997; Kasemsuwan 1997; Kaygusuz 2002; Lorente 1995; Nawasreh 2001; Ramos 2003; Siddique 2016; Tutkun 1995).
Nine studies used aminoglycosides, with six using gentamycin or gentamicin (Asmatullah 2014; Gyde 1978; Jamalullah 2016; Lorente 1995; Nawasreh 2001; Tutkun 1995), two using tobramycin (Fradis 1997; Kaygusuz 2002), and one using neomycin (Siddique 2016).
Two studies used neomycin/polymixin B (van Hasselt 1997; van Hasselt 1998a). The remaining four studies used other topical antibiotics, including rifampicin (Liu 2003), chloramphenicol (Liu 2003), ceftizoxime (Mira 1993) and trimethoprim, sulphacetamide and polymixin B (TSP) combination (Gyde 1978).
Background treatment
Nine studies used aural toileting at different frequencies: two at the start of trial (Jamalullah 2016; Mira 1993), four before each treatment (de Miguel 1999; Gyde 1978; Kaygusuz 2002; Liu 2003), two at the start of trial and at one and two weeks (van Hasselt 1997; van Hasselt 1998a), and one at days one, four and seven (Kasemsuwan 1997).
Three studies used systemic antibiotics as a background treatment, with three via the oral route (de Miguel 1999; Esposito 1990; Ramos 2003) and one via the intramuscular route (Mira 1993). One study also had a background treatment of analgesics and antipyretics (de Miguel 1999).
A further six studies did not mention the use of any background treatments (Esposito 1990; Fradis 1997; Lorente 1995; Nawasreh 2001; Siddique 2016; Tutkun 1995).
Duration of intervention
Nine studies treated for less than two weeks (Asmatullah 2014; de Miguel 1999; Esposito 1990; Kasemsuwan 1997; Lorente 1995; Mira 1993; Nawasreh 2001; Ramos 2003; Tutkun 1995). Seven studies treated for between two to four weeks (Fradis 1997; Gyde 1978; Jamalullah 2016; Kaygusuz 2002; Liu 2003; van Hasselt 1997; van Hasselt 1998a). One study treated for four weeks (Siddique 2016).
Comparison
One study analysed topical antibiotic versus placebo:
Kasemsuwan 1997 ‐ ciprofloxacin versus placebo (saline).
Sixteen studies compared different topical antibiotics (topical antibiotic A versus topical antibiotic B).
-
Four studies analysed topical antibiotic versus no treatment/placebo (with systemic antibiotics as background):
de Miguel 1999 ‐ ciprofloxacin versus no treatment;
Esposito 1990 ‐ ciprofloxacin versus no treatment;
Mira 1993 ‐ ceftizoxime versus placebo (saline);
Ramos 2003 ‐ ciprofloxacin versus no treatment.
-
Seven studies analysed quinolones versus aminoglycosides:
Asmatullah 2014 ‐ ofloxacin versus gentamycin;
Fradis 1997 ‐ ciprofloxacin versus tobramycin;
Jamalullah 2016 ‐ ofloxacin versus gentamycin;
Kaygusuz 2002 ‐ ciprofloxacin versus tobramycin;
Lorente 1995 ‐ ciprofloxacin versus gentamycin;
Nawasreh 2001 ‐ ciprofloxacin versus gentamicin;
Tutkun 1995 ‐ ciprofloxacin versus gentamicin.
-
Three studies analysed quinolones versus aminoglycosides/polymixin B combination ± gramicidin:
Siddique 2016 ‐ ciprofloxacin versus neomycin/polymixin/gramicidin‐D;
van Hasselt 1997 ‐ ofloxacin versus neomycin/polymixin B;
van Hasselt 1998a ‐ ofloxacin versus neomycin/polymixin B.
-
Two studies analysed other antibiotics:
Outcome
Resolution of ear discharge
The definitions, methods and timing of assessment differed between studies, and these are summarised in Table 7.
Health‐related quality of life using a validated instrument
No studies reported health‐related quality of life.
Ear pain (otalgia) or discomfort or local irritation
Four studies measured adverse effects associated with treatment: one described measuring local sensitivity (Gyde 1978), one specified symptoms further, examining ear pain, itching and stinging (measured on a four‐point scale: 0 = none, 3 = severe) (Lorente 1995), whilst the other two broadly mentioned that this was measured in relation to the topical medication (Kasemsuwan 1997; Siddique 2016).
Hearing
Six studies indicated that they measured hearing pre‐ and post‐treatment, however none of these provided details regarding the methods used including whether air or bone conduction methods were used or the frequencies of testing (Fradis 1997; Gyde 1978; Kasemsuwan 1997; Nawasreh 2001; Tutkun 1995; van Hasselt 1997). All results were reported narratively.
Serious complications (including intracranial complications, extracranial complications and death)
Although serious complications, including intracranial complications (such as otitic meningitis, lateral sinus thrombosis and cerebellar abscess) and extracranial complications (such as mastoid abscess, postauricular fistula and facial palsy) and death were not specifically listed in the methods as outcomes that would be recorded, three papers report that no side effects occurred in any participants, which would include the defined serious complications (Gyde 1978; Siddique 2016; Tutkun 1995).
Suspected ototoxicity
One study stated that they tested audiometric and vestibular function to measure suspected ototoxicity outcomes (Esposito 1990). Another study assessed suspected ototoxicity with an audiogram, although a specific definition was not stated, and reported that 0/125 suffered from ototoxicity associated with treatment (Ramos 2003). Three additional studies listed suspected ototoxicity as a potential outcome, but did not describe how it would be assessed (de Miguel 1999; Kasemsuwan 1997; Siddique 2016).
Excluded studies
We excluded 146 papers (150 papers including unpublished studies) (122 studies) after reviewing the full text. Further details for the reasons for exclusion can be found in the Characteristics of excluded studies table. These are the main reasons for the exclusion:
We excluded 40 studies (59 references, 63 including unpublished studies)) because the comparisons were not appropriate for this review, but were relevant to another review in this suite of reviews (Boesorire 2000; Browning 1988; Couzos 2003; Crowther 1991; Eason 1986; Esposito 1992; Fliss 1990; Gendeh 2001; Ghosh 2012; Gupta 2015; Helmi 2000; I‐HEAR‐BETA (in progress study); Indudharan 2005; Jaya 2003; Kiris 1998; Lazo Saenz 1999; Leach 2008; Legent 1994; Loock 2012; Macfadyen 2005; Minja 2006; Miro 2000; Nwokoye 2015; Onali 2018; Panchasara 2015; Papastavros 1989; Picozzi 1983; Picozzi 1984; Povedano 1995; Renukananda 2014; Rotimi 1990; Sanchez Gonzales 2001; Smith 1996; Somekh 2000; Subramaniam 2001; Tong 1996; van der Veen 2007; van Hasselt 1998b; Vishwakarma 2015; Yuen 1994).
We excluded 38 studies (39 references) on the basis of their study design (Baba 1986; Baba 2008; Bluestone 2001; Brook 1979; Brook 1980; Deguchi 1985; Deguchi 1986; Deitmer 2002; Dohar 2002; Esposito 2000; Gehanno 1997; Harris 2016; Hwang 2015; Jahn 1984; Jang 2004; Kadar 2003; Kashiwamura 2004; Kenna 1986; Kothari 1969; Kovacic 1999; Kurilin 1976; Lancaster 1999; Lancaster 2003; Lang 1992; Lautala 1983; Manolidis 2004; Merifield 1993; Morgon 1976; Otwombe 2003; Poliakova 1991; Singhal 1992; Sultan 2017; Sumitsawan 1995; Supiyaphun 1995; Tachibana 1986; Thomsen 1976; Wintermeyer 1997; Wright 2009).
We excluded 22 studies (24 references) due to the population characteristics included in their study (Abbott 2016; Alper 2000; Baba 1982b; Baba 1983; Baba 1983b; Baba 1987; Berman 1990; Block 2000; Bross Soriano 1996; Clayton 1990; Garcia‐Rodriguez 1993; Granath 2007; Gyde 1981; Gyde 1982; Mendelman 1992; Mesure 1973; Principi 1995; Quick 1973; Quick 1975; Saez‐Llorens 2005; Stenstrom 1991; van Dongen 2014).
We excluded 17 studies (18 references) because the interventions were outside of the protocol (Blekher 1967; Browning 1983; Connolly 1997; Dellamonica 1995; Fraysse 1988; ISRCTN12149720; ISRCTN84220089; ISRCTN86106121; Jiang 2016; Khanna 2000; Li 2004; Mora 2012; NCT02592096; NCT02817347; Shkil' 1964; Wilde 1995; Xu 1999).
Five studies (six references) had multiple reasons for exclusion (Baba 1980; Fombeur 1994; Hemlin 1997; Khon 2012; Lorentzen 1978).
Risk of bias in included studies
See Figure 2 for a 'Risk of bias' graph (our judgements about each risk of bias item presented as percentages across all included studies) and Figure 3 for a 'Risk of bias' summary (our judgements about each risk of bias item for each included study).
2.
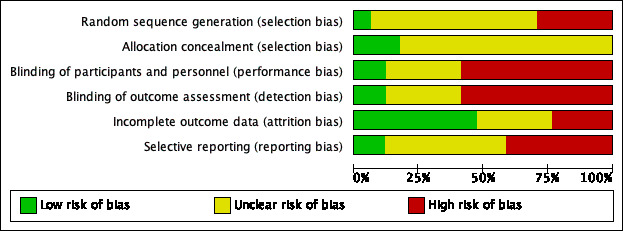
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
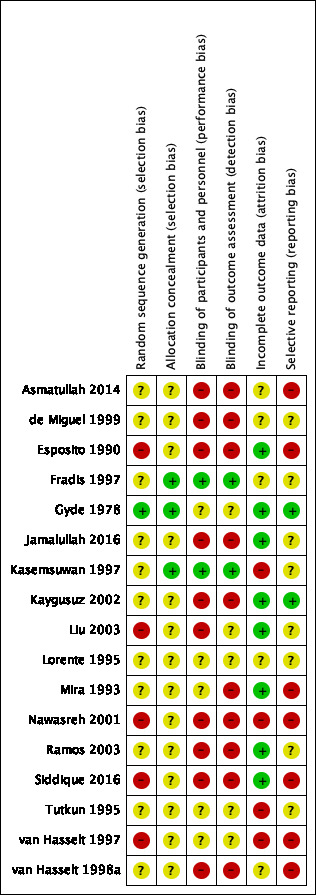
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
We assessed five studies to be at high risk of selection bias with regards to randomisation (Esposito 1990; Liu 2003; Nawasreh 2001; Siddique 2016; van Hasselt 1997). For Liu 2003, it is not clear whether or not the patients were randomised. Similarly for Siddique 2016 it was unclear what the actual process for the 'non‐probability' sampling was and how to ensure that the allocation was random. Esposito 1990 did not clearly specify the randomisation method and while it was noted that 38/60 patients were previously unsuccessfully treated with at least five days of antibiotics, it was unclear how this was distributed across groups. The abstract for Nawasreh 2001 mentions that randomisation occurred but there was no mention of randomisation or methods for randomisation in the full paper, which only described patients as being "divided" between the two groups. It is unclear if the groups were evenly distributed as there was 40 in one group and 48 in the other, with baseline characteristics between the group not provided. The original report for van Hasselt 1997 indicates this was a "pilot trial", with no reference to blinding or randomisation. However, this was mentioned as a "randomised" trial in the introduction of a 2002 paper by the author. If randomisation was done, there was also no clear ratio for randomisation, with 46, 38 and 12 in the three treatment groups and the cheapest intervention having the most participants.
We assessed one study as low risk (Gyde 1978) and the remaining 11 studies as 'unclear risk' as they did not provide enough information (Asmatullah 2014; de Miguel 1999; Fradis 1997; Jamalullah 2016; Kasemsuwan 1997; Kaygusuz 2002; Lorente 1995; Mira 1993; Ramos 2003; Tutkun 1995; van Hasselt 1998a).
Allocation concealment
We assessed three studies to be at low risk of allocation concealment bias (Fradis 1997; Gyde 1978; Kasemsuwan 1997).
We assessed the remaining 14 studies as at unclear risk as they did not provide enough information with regards to allocation concealment (Asmatullah 2014; de Miguel 1999; Esposito 1990; Jamalullah 2016; Kaygusuz 2002; Liu 2003; Lorente 1995; Mira 1993; Nawasreh 2001; Ramos 2003; Siddique 2016; Tutkun 1995; van Hasselt 1997; van Hasselt 1998a).
Blinding
Performance bias
We assessed 11 studies as high risk for performance bias. Two were at high risk due to a lack of blinding for patients and healthcare practitioners even when it was possible to do (Asmatullah 2014; Jamalullah 2016). Seven were at high risk due to blinding being impossible because of the differences in treatment regimens/administration that mean it is likely to be known to which group they were allocated (de Miguel 1999; Esposito 1990; Liu 2003; Nawasreh 2001; Ramos 2003; Siddique 2016; van Hasselt 1998a). One study was at high risk due to the absence of a clear statement on blinding (Kaygusuz 2002), and one study did not provide details of who was blinded during the "single‐blinded" study (Mira 1993). In this case we assumed that it was the patients who were blinded to treatment, but as the main outcomes were physician‐reported, blinding the patients would not have prevented performance bias. We assessed two studies as low risk because the used sufficient blinding methods (Fradis 1997; Kasemsuwan 1997), while four were at unclear risk due to lack of information (Gyde 1978; Lorente 1995; Tutkun 1995; van Hasselt 1997).
Detection bias
We assessed nine studies to be at high risk of bias. Four of these were due to the subjective nature of the judgement of outcomes (Asmatullah 2014; de Miguel 1999; Esposito 1990; Ramos 2003), whereas three did not mention any attempts to blind assessors even though it would have been feasible (Jamalullah 2016; Nawasreh 2001; Siddique 2016). One study stated that it was double‐blind, although the treatment regimens were not the same and no placebo was used (Kaygusuz 2002). One study did not provide a clear statement on blinding (van Hasselt 1998a). We assessed two studies to be low risk because blinding appeared to be adequate to make detection unlikely (Fradis 1997; Kasemsuwan 1997). We assessed six studies as at unclear risk because there was no mention of blinding or further clarification was necessary but omitted (Gyde 1978; Liu 2003; Lorente 1995; Mira 1993; Tutkun 1995; van Hasselt 1997).
Incomplete outcome data
We assessed four studies to be at high risk of attrition bias (Kasemsuwan 1997; Nawasreh 2001; Tutkun 1995; van Hasselt 1997). Two of these were due to issues regarding participation, with high dropout rates ‐ over 25% in the short time periods within the trials (Kasemsuwan 1997; van Hasselt 1997). It was also noted that Nawasreh 2001 and van Hasselt 1997 had an imbalance of participants between the allocation groups, which could have led to bias. We deemed one study to be at high risk due to a lack of clarity in the statement regarding exclusion of patients ‐ whether this was part of the recruitment criteria or after randomisation (Tutkun 1995).
We assessed eight studies to be low risk, as all or more than 90% of participants appearing in the trial are accounted for in results (Esposito 1990; Gyde 1978; Jamalullah 2016; Kaygusuz 2002; Liu 2003; Mira 1993; Ramos 2003; Siddique 2016). We assessed a further five studies as having unclear risk due to lack of a statement or reasoning (Asmatullah 2014; de Miguel 1999; Fradis 1997; Lorente 1995; van Hasselt 1998a).
Selective reporting
None of the 17 studies had protocols identified through searches of clinical trials registries.
We assessed seven studies to be at high risk of selective reporting bias: three of these studies were due to the methods section not being well presented (Asmatullah 2014; Mira 1993; Nawasreh 2001), three were due to discrepancies in time point outcome reporting (Esposito 1990; Siddique 2016; van Hasselt 1997), and one was due to the study not being published, making it difficult to evaluate the methods fully (van Hasselt 1998a).
We assessed two studies as low risk due to adequate outcome reporting between the methods and results, however no protocols were found (Gyde 1978; Kaygusuz 2002). We assessed the remaining eight studies as having unclear risk of selective reporting (de Miguel 1999; Fradis 1997; Jamalullah 2016; Kasemsuwan 1997; Liu 2003; Lorente 1995; Ramos 2003; Tutkun 1995).
Other potential sources of bias
Funding
Esposito 1990 stated that "the ciprofloxacin tablets and powder used in this study were kindly provided by Bayer Italia Spa, Milan, Italy." Although it was not stated, we assumed one study to have funding from the Christian Blind Mission, as other studies by the same authors were funded through this avenue (van Hasselt 1997). One study specifically stated that "no funding was received from any agency or institution" (Siddique 2016), while the remaining 14 studies provided no information (Asmatullah 2014; de Miguel 1999; Fradis 1997; Gyde 1978; Jamalullah 2016; Kasemsuwan 1997; Kaygusuz 2002; Liu 2003; Lorente 1995; Mira 1993; Nawasreh 2001; Ramos 2003; Tutkun 1995; van Hasselt 1998a).
Declarations of interest
Siddique 2016 stated that "[the] abstract and results of this study were accepted and presented in an oral presentation at the International conference on Medical Education, organised by Association for Excellence in Medical Education (AEME) and held on 07th‐09th March 2014 at University of Health Sciences (UHS) Lahore, Pakistan." The remaining studies did not provide any information about conflicts of interest (Asmatullah 2014; de Miguel 1999; Esposito 1990; Fradis 1997; Gyde 1978; Jamalullah 2016; Kasemsuwan 1997; Kaygusuz 2002; Liu 2003; Lorente 1995; Mira 1993; Nawasreh 2001; Ramos 2003; Tutkun 1995; van Hasselt 1997; van Hasselt 1998a).
Effects of interventions
See: Table 1; Table 2; Table 3
Comparison 1: Topical antibiotics versus placebo or no treatment
One study was included in this comparison: Kasemsuwan 1997 (50 participants), which compared topical ciprofloxacin to saline. See also Table 1.
Primary outcomes
Resolution of ear discharge or 'dry ear'
Between one week and up to two weeks
Kasemsuwan 1997 identified that topical antibiotics appeared to be more effective than saline at one to two weeks follow‐up (risk ratio (RR) 6.74, 95% confidence interval (CI) 1.82 to 24.99; 35 participants; very low‐certainty evidence; Analysis 1.1).
1.1. Analysis.
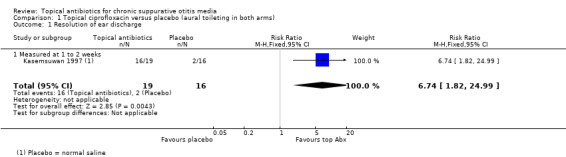
Comparison 1 Topical ciprofloxacin versus placebo (aural toileting in both arms), Outcome 1 Resolution of ear discharge.
Two weeks to up to four weeks and after four weeks
The study did not report the results per person for this outcome at between two to four weeks or after four weeks.
Health‐related quality of life using a validated instrument
This outcome was not reported.
Ear pain (otalgia) or discomfort or local irritation
Kasemsuwan 1997 reports that "no medical side‐effects and worsening of audiological measurements related to this topical medication were detected" (very low‐certainty evidence).
Secondary outcomes
Hearing
Kasemsuwan 1997 reported "no worsening of audiological outcomes", but specific information relating to hearing levels was not presented (very low‐certainty evidence).
Serious complications (including intracranial complications, extracranial complications and death)
The study did not report that any participant died or had any intracranial or extracranial complications.
Suspected ototoxicity
Kasemsuwan 1997 reported "no suspected ototoxicity" but it is unclear how this was measured.
Subgroup analysis
With only one study included in the quantitative analysis, subgroup analysis was not possible (very low‐certainty evidence).
Comparison 2: Topical antibiotics versus placebo or no treatment (with systemic antibiotics as background treatment)
Four studies (190 participants) were included in this comparison, three of which compared treatment with topical ciprofloxacin drops and systemic ciprofloxacin to systemic ciprofloxacin only (de Miguel 1999; Esposito 1990; Ramos 2003). The remaining study, Mira 1993 (248 participants), compared topical ceftizoxime to placebo ear drops (saline) for seven days with all participants in both treatment arms received intravenous ceftizoxime. None of the primary or secondary outcomes were reported. Two of the studies had reported exactly the same percentages of "cure" across all but one of the intervention arms of the studies (de Miguel 1999; Ramos 2003). We had concerns that these could have been the same set of patients, but the authors clarified that these were entirely different patients (and studies). See Table 2 for the main comparison.
Primary outcomes
Resolution of ear discharge or 'dry ear'
Between one week and up to two weeks
Ramos 2003 and de Miguel 1999 found that topical and systemic antibiotics resulted in more dry ears compared to the systemic antibiotics only group (RR 1.47, 95% CI 1.20 to 1.80; 150 participants; 2 studies; I2 = 0%; low‐certainty evidence; Analysis 2.1).
2.1. Analysis.
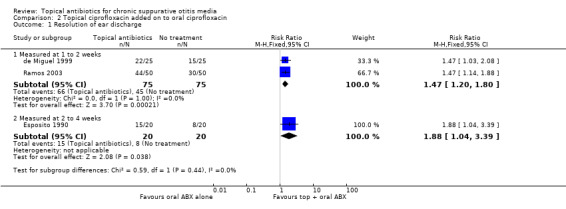
Comparison 2 Topical ciprofloxacin added on to oral ciprofloxacin, Outcome 1 Resolution of ear discharge.
Two weeks to up to four weeks
Esposito 1990 found that topical and systemic antibiotics resulted in more dry ears compared to the systemic antibiotics only group (RR 1.88, 95% CI 1.04 to 3.39; 40 participants; Analysis 2.1).
After four weeks
No studies measured the results at this time point.
Health‐related quality of life using a validated instrument
None of the studies measured this outcome.
Ear pain (otalgia) or discomfort or local irritation
None of the studies measured this outcome although one study reported that "no side effect was recorded in any patient..." (Esposito 1990).
Secondary outcomes
Hearing
Although three studies reported hearing as an outcome in their methods section (de Miguel 1999; Esposito 1990; Ramos 2003), none of the studies reported results for this outcome.
Serious complications (including intracranial complications, extracranial complications and death)
No studies reported that any participant died or had any intracranial or extracranial complications.
Suspected ototoxicity
Three studies reported that they did not suspect ototoxicity in any participants, but it is unclear how this was measured (de Miguel 1999; Esposito 1990; Ramos 2003) (very low‐certainty evidence).
de Miguel 1999 reported that the data did not show cochleovestibular dysfunction during treatment or further follow‐up. They also stated that all post‐treatment audiometries showed a lack of antimicrobial ototoxicity, both for oral and topical routes. However, there was no definition of 'ototoxicity' provided and it may be that they only measured air conduction audiological thresholds rather than bone conduction thresholds as well.
Esposito 1990 stated that "no side effect was recorded in any patient and no worsening of the audiometric function related to the local therapy was observed". Audiometric measurement and vestibular tests were performed before and 24 hours after the end of the therapy in patients receiving topical treatment only.
Ramos 2003 reported a lack of symptoms suggesting vestibular problems, but did not provide details on how this was measured or defined.
Subgroup analysis
No subgroup analysis was completed as there were no differences in any of the identified subgroups.
High‐risk populations ‐ none of the studies reported high‐risk populations as defined in our methods.
Patients with ventilation tubes ‐ none of the studies reported the inclusion of patients with ventilation tubes.
Diagnosis of CSOM ‐ most of the studies included mixed populations of CSOM along with ear discharge from other causes.
Duration of ear discharge ‐ only one study reported the duration of discharge.
Patient age ‐ none of the studies included participants younger than two years of age. Although two studies included children from five years old stratification by age does not appear to have taken place (de Miguel 1999; Ramos 2003).
Comparison 3: Quinolones versus aminoglycosides
Seven studies (734 participants) were included in this comparison (Asmatullah 2014; Fradis 1997; Jamalullah 2016; Kaygusuz 2002; Lorente 1995; Nawasreh 2001; Tutkun 1995). For details of the interventions and comparisons see Table 6. See also Table 3.
Primary outcome
Resolution of ear discharge or 'dry ear'
Between one week and up to two weeks
Six studies (694 participants) reported this outcome and found that more participants given topical quinolones had resolution of ear discharge at between one to two weeks compared with topical aminoglycosides (RR 1.95, 95% CI 0.88 to 4.29; 694 participants; 6 studies; I2 = 97%; very low‐certainty evidence; Analysis 3.1). However, we noted that the heterogeneity was very high. This was driven by the largest study (Lorente 1995), which had an extremely high resolution rate in both arms: 95% and 94% in the quinolone and aminoglycoside arms respectively compared with an average of 75% and 34% in the quinolone and aminoglycoside arms in the remaining studies. After an investigation of the study details, it was not possible to identify why the results were so different, and so we kept the study in the analysis and used a random‐effects model.
3.1. Analysis.
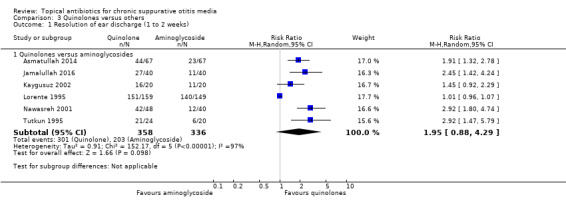
Comparison 3 Quinolones versus others, Outcome 1 Resolution of ear discharge (1 to 2 weeks).
Two weeks to up to four weeks
Kaygusuz 2002 found no difference between topical and quinolones compared to aminoglycosides (RR 1.88, 95% CI 1.04 to 3.39; 40 participants; Analysis 3.2).
3.2. Analysis.
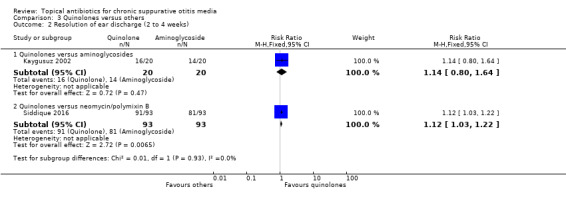
Comparison 3 Quinolones versus others, Outcome 2 Resolution of ear discharge (2 to 4 weeks).
After four weeks
No studies reported the results per person for this outcome.
Health‐related quality of life using a validated instrument
None of the studies measured this outcome.
Ear pain (otalgia) or discomfort or local irritation
Lorente 1995 (308 participants) measured ear pain on a three‐point scale. Results were presented as a mean score. No differences between the two groups were identified (very low‐certainty evidence).
Secondary outcomes
Hearing
Tutkun 1995 (44 participants) presented mean air and bone conduction hearing levels at 500 Hz, 1000 Hz, 2000 Hz and 4000 Hz pre‐ and post‐treatment by treatment group. No standard deviations were presented although the authors assert that "the difference between these two groups was not statistically significant (p>0.01)". Nawasreh 2001 (88 participants) stated that hearing was measured and that neither group showed significant differences. This evidence is of very low certainty.
Serious complications (including intracranial complications, extracranial complications and death)
No studies reported that any participant died or had any intracranial or extracranial complications.
Suspected ototoxicity
Two studies reported that they had assessed patients for suspected ototoxicity (Lorente 1995; Tutkun 1995). Whilst Lorente 1995 did not present any results, Tutkun 1995 indicated that "There were no side effects, and audiometric evaluation yielded no evidence of ototoxicity as reflected by the pure tone threshold and speech discrimination scores in either group" (very low‐certainty evidence).
Subgroup analysis
With the exception of type of antibiotic, no subgroup analysis could be completed:
High‐risk populations ‐ none of the studies reported the inclusion of high‐risk populations as defined in our methods.
Patients with ventilation tubes ‐ none of the studies reported the inclusion of patients with ventilation tubes.
Diagnosis of CSOM ‐ all of the studies reported patients with 'CSOM'.
Duration of ear discharge ‐ where given the duration of ear discharge at the start of the study was between three months and two years. There were no studies using a two‐week or six‐week criteria.
Patient age ‐ where given, the youngest patients included were nine years of age and no studies stratified participants by age.
Comparison 4: Quinolones versus aminoglycosides/polymixin B ± gramicidin
Three studies were included in this comparison (van Hasselt 1997 (50 participants), van Hasselt 1998a (unclear number of participants) and Siddique 2016 (200 participants)). van Hasselt 1997 and van Hasselt 1998a both compared topical ofloxacin to topical neomycin/polymyxin B, however the unit of randomisation was unclear in both studies and therefore only Siddique 2016, which compared topical ciprofloxacin to topical neomycin/polymixin B and gramicidin, could be included in the analysis.
Primary outcomes
Resolution of ear discharge or 'dry ear'
Between one week and up to two weeks
No studies reported this outcome.
Two weeks to up to four weeks
Siddique 2016 found that treatment marginally favoured the topical quinolones compared to neomycin/polymyxin B and gramicidin for resolution of discharge (RR 1.12, 95% CI 1.03 to 1.22) at two to four weeks (Analysis 3.2).
After four weeks
No studies reported the results per person for this outcome.
Health‐related quality of life using a validated instrument
None of the studies measured this outcome.
Ear pain (otalgia) or discomfort or local irritation
A "few" patients experienced local irritation upon the first instillation of topical treatment. No information was given regarding the number or treatment arm of the participants.
Secondary outcomes
Hearing
None of the studies measured this outcome.
Serious complications (including intracranial complications, extracranial complications and death)
No studies reported that any participant died or had any intracranial or extracranial complications.
Suspected ototoxicity
None of the studies measured this outcome.
Subgroup analysis
With only one study included in the quantitative analysis, subgroup analysis was not possible.
Comparison 5: Aminoglycosides versus trimethoprim, sulphacetamide and polymyxin B (TSP)
One study was included in this comparison: Gyde 1978 (91 participants) compared a topical aminoglycoside to topical trimethoprim, sulphacetamide and polymyxin B. Participants were randomised by ear and there was no adjustment for the paired nature of this data. Whilst resolution of discharge was assessed at two to four weeks there was no extractable efficacy data.
Primary outcomes
Resolution of ear discharge or 'dry ear'
Between one week and up to two weeks
The study did not report this outcome.
Two weeks to up to four weeks
There were no extractable efficacy data.
After four weeks
The study did not report the results per person for this outcome.
Health‐related quality of life using a validated instrument
The study did not measure this outcome.
Ear pain (otalgia) or discomfort or local irritation
The study reported no signs of local sensitivity or fungal proliferation.
Secondary outcomes
Hearing
The study did not measure this outcome.
Serious complications (including intracranial complications, extracranial complications and death)
No side effects were reported.
Suspected ototoxicity
The study reported no signs of ototoxicity. It is unclear how this outcome was measured.
Subgroup analysis
With only one study included in the quantitative analysis, subgroup analysis was not possible.
Comparison 6: Rifampicin versus chloramphenicol
One study was included in this comparison: Liu 2003 (160 participants) compared topical rifampicin to topical chloramphenicol. While resolution of discharge was assessed at one to two weeks there were no extractable efficacy data.
Primary outcomes
Resolution of ear discharge or 'dry ear'
Between one week and up to two weeks
Liu 2003 found that rifampicin resulted in more dry ears compared to chloramphenicol (RR 1.78, 95% CI 1.35 to 2.34) (Analysis 4.1).
4.1. Analysis.
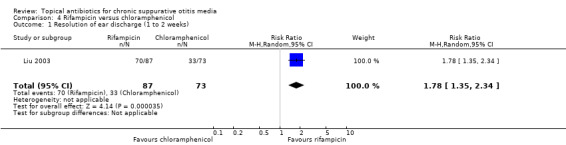
Comparison 4 Rifampicin versus chloramphenicol, Outcome 1 Resolution of ear discharge (1 to 2 weeks).
Two weeks to up to four weeks
The study did not measure this outcome.
After four weeks
The study did not measure this outcome.
Health‐related quality of life using a validated instrument
The study did not measure this outcome.
Ear pain (otalgia) or discomfort or local irritation
The study reported no signs of local sensitivity or fungal proliferation.
Secondary outcomes
Hearing
The study did not measure this outcome.
Serious complications (including intracranial complications, extracranial complications and death)
The study did not report that any participant died or had any intracranial or extracranial complications.
Suspected ototoxicity
The study reported no signs of ototoxicity. It is unclear how this outcome was measured.
Subgroup analysis
With only one study included in the quantitative analysis, subgroup analysis was not possible.
Discussion
Summary of main results
We found 17 studies reporting on six different comparisons (Asmatullah 2014; de Miguel 1999; Esposito 1990; Fradis 1997; Gyde 1978; Jamalullah 2016; Kasemsuwan 1997; Kaygusuz 2002; Liu 2003; Lorente 1995; Mira 1993; Nawasreh 2001; Ramos 2003; Siddique 2016; Tutkun 1995; van Hasselt 1997; van Hasselt 1998a). Due to the choice of outcome measures used in these studies and the incomplete reporting of results, for many of the comparisons we were not able to find a substantial amount of evidence. Of the 17 studies, one examined the effectiveness of topical antibiotics compared to no treatment (Comparison 1), four examined topical antibiotics compared to no treatment with systemic antibiotics as a background treatment (Comparison 2), and 12 studies directly compared different topical antibiotics (Comparisons 3 to 6).
The following is a summary of the key findings for the comparisons:
Comparison 1: Topical antibiotics versus placebo or no treatment (without background treatment)
We included one study examining topical antibiotics versus no treatment, with no background treatment (Kasemsuwan 1997; 50 participants). This study compared ciprofloxacin drops to saline with resolution of discharge measured at one to two weeks. It was unclear if the resolution of discharge was otoscopically confirmed. Topical antibiotics appeared to be more effective than saline (risk ratio (RR) 6.74, 95% confidence interval (CI) 1.82 to 24.99). However, this study was too small to provide any certainty of the findings (GRADE assessment: very low‐certainty evidence). No adverse events, suspected ototoxicity or worsening of audiological measurements were reported and the other outcomes were either not measured or not reported. See Table 1 for the comparison.
Comparison 2: Topical antibiotics versus placebo or no treatment (with background treatment)
We included two studies examining topical antibiotics versus no treatment, with background treatment (de Miguel 1999; Esposito 1990; 90 participants). Both compared treatment with topical ciprofloxacin drops and systemic ciprofloxacin to systemic ciprofloxacin only. Only one study reported that the resolution of discharge was otoscopically confirmed (de Miguel 1999). Resolution of discharge was measured at one to two weeks and two to four weeks. Treatment marginally favoured the topical and systemic antibiotics group compared to the systemic antibiotics only group for resolution of discharge (RR 1.47, 95% CI 1.20 to 1.80 at one to two weeks and RR 1.88. 95% CI 1.04 to 3.39 at two to four weeks). These studies were too small to provide any certainty of the findings (GRADE assessment: low‐certainty evidence). The other outcomes were either not measured or poorly reported. See Table 2 for the comparison.
Comparison 3: Aminoglycoside versus quinolones
We found seven studies (Asmatullah 2014; Fradis 1997; Jamalullah 2016; Kaygusuz 2002; Lorente 1995; Nawasreh 2001; Tutkun 1995; 734 participants). Five studies used the quinolone ciprofloxacin (Fradis 1997; Kaygusuz 2002; Lorente 1995; Nawasreh 2001; Tutkun 1995). The studies used varying dosages of six (Kaygusuz 2002) or 15 (Fradis 1997; Lorente 1995; Nawasreh 2001; Tutkun 1995) drops per day, and variable concentrations including 0.3% (Kaygusuz 2002; Lorente 1995) and 0.6%, 200 µg/mL (Nawasreh 2001; Tutkun 1995), or did not report the concentration (Fradis 1997). Two studies used the quinolone ofloxacin (Asmatullah 2014; Jamalullah 2016). Both studies had the same dosage of 12 drops per day, whilst Jamalullah 2016 used a 0.6% concentration and Asmatullah 2014 used a 0.3% concentration. Five studies reported that the resolution of discharge was otoscopically confirmed (Asmatullah 2014; Jamalullah 2016; Kaygusuz 2002; Lorente 1995; Tutkun 1995).
We found that resolution of discharge at one to two weeks was almost twice as likely in the quinolones group, although this was not statistically significant (GRADE assessment: very low‐certainty evidence). See Table 3 for the comparison.
Tutkun 1995 (44 participants) presented mean air and bone conduction hearing levels at 500 Hz, 1000 Hz, 2000 Hz and 4000 Hz pre‐ and post‐treatment by treatment group. No standard deviations were presented although the authors assert that "the difference between these two groups was not statistically significant (p>0.01)". Nawasreh 2001 (88 participants) stated that hearing was measured and that neither group showed significant differences. Lorente 1995 (308 participants) measured ear pain on a three‐point scale. Results were presented as a mean score. There was no difference in hearing levels between the groups.
Comparison 4: Quinolones versus aminoglycosides/polymixin B ± gramicidin
We found three studies for this comparison (van Hasselt 1997 (50 participants); van Hasselt 1998a (unknown number of participants); Siddique 2016 (186 participants). Both van Hasselt 1997 and van Hasselt 1998a used ofloxacin at 0.3% with dosages of three drops per eight hours and six drops per 12 hours respectively and compared this to a combination of neomycin and polymixin B of varying or unclear concentration and dosages. None of the studies reported that the resolution of discharge was otoscopically confirmed. Treatment duration was two weeks for both studies. Siddique 2016 compared ciprofloxacin of unknown concentration with a dosage of three drops per 12 hours with neomycin and polymixin B with gramicidin D of unknown concentration with a dosage of two drops per 12 hours. Treatment duration was unclear but likely lasted four weeks.
Only Siddique 2016 reported resolution of discharge and this was at four weeks post‐treatment. More patients experienced resolution of discharge with quinolones at four weeks compared with neomycin and polymixin B with gramicidin D (RR 1.12, 95% CI 1.03 to 1.22).
Comparison 5: Aminoglycosides versus trimethoprim sulphacetamide and polymyxin B
We included only one study examining topical aminoglycosides (gentamicin) versus a combination of trimethoprim sulphacetamide and polymyxin B (Gyde 1978; 91 participants, 100 ears). The study was translated from French and 51% of participants had CSOM (other diagnoses were otitis externa (21%), 'subacute otitis' (16%) and postoperative discharge (12%)). It was not reported whether the resolution of discharge was otoscopically confirmed. Participants were randomised by ear, with no adjustment for the paired nature of the data. Whilst resolution of discharge was assessed at two to four weeks there were no extractable efficacy data.
The study authors reported no signs of ototoxicity, excessive fungal proliferation or any local sensitivity to the ear drops. Health‐related quality of life, hearing level and serious complications were not reported.
Comparison 6: Rifampicin versus chloramphenicol
We found one study examining this comparison (Liu 2003; 160 participants), which was translated from Chinese. This study compared topical rifampicin (0.1%, nine drops per day, treatment duration of two weeks) with topical chloramphenicol (0.25%, nine drops per day, treatment duration of two weeks) with a background treatment of 3% hydrogen peroxide ear wash daily in both arms. It was not reported whether the resolution of discharge was otoscopically confirmed. Topical rifampicin appeared to be more effective than chloramphenicol for resolving ear discharge one to two weeks after completion of treatment (RR 1.78, 95% CI 1.35 to 2.34). However, this study was too small to provide any certainty of the findings (GRADE assessment: very low‐certainty evidence). No adverse events, suspected ototoxicity or worsening of audiological measurements were reported and the other outcomes were either not measured or not reported.
Overall completeness and applicability of evidence
The doses used in the studies were in keeping with manufacturers' recommendations and are applicable to the population being studied. The population of patients with CSOM are likely to receive treatment in both primary and secondary care settings.
Whilst the inclusion criteria was ear discharge for more than two weeks, reflecting the World Health Organization (WHO) guidelines for CSOM diagnosis, the majority of studies included patients who had ear discharge for more than six weeks before intervention, which is in keeping with a number of local treatment protocols and the practice of many tertiary‐based otolaryngologists. The length of follow‐up in most studies was between one to four weeks, meaning that there was limited evidence regarding the long‐term effectiveness of topical antibiotics for the resolution of discharge for people with CSOM.
No studies examined children under two years of age. As the peak prevalence of otitis media is in children under two years of age this leaves us with no information on this important patient group. Similarly, no studies included participants classed as 'high‐risk' in our protocol, including Indigenous populations and immunocompromised patients. Patients in these high‐risk groups can be a challenge for clinicians to treat effectively and evidence to support best‐practice interventions for these people is needed. The effectiveness of topical antibiotics is likely to be influenced by the sensitivity of the antibiotic to the micro‐organisms present. We were unable to carry out a subgroup analysis of the spectrum of antibiotic activity as the data were either not in the included studies or heterogeneity was not observed, which leaves us with no information on this aspect of antibiotic treatment.
Disease‐specific health‐related quality of life, which is both specific to the disease and important to patients, was not used in the included studies as an outcome measure. There is therefore no information at all on whether the different types of antibiotics used have an impact on patients' quality of life.
Quality of the evidence
The certainty of the evidence for all outcomes in these comparisons was very low (GRADE assessment), due to the small number of participants available for analysis (resulting in large confidence intervals) and limitations in the methods of study conduct and reporting. Accuracy of the diagnosis was also a potential issue throughout the studies included in this review. Of the 17 included studies, only six described the use of otoscopic confirmation of resolution of discharge. This may have impacted on the accuracy of the diagnostic outcome and therefore the response to treatment.
Potential biases in the review process
In most cases the studies did not report enough information for us to further analyse the results. We have had to take readings from graphs using a digital graph reader and impute standard deviations based on the P values reported. They were often only reported as 'P value < 0.05' or 'P value < 0.01' in comparisons where the studies found statistical significance. Our imputations are based on these values (using P value = 0.01 or P value = 0.05) and we are therefore conservative in our estimation of the standard deviations. However, this lack of information about non‐significant results could have prevented us from drawing more conclusive results about the lack of difference between groups.
Agreements and disagreements with other studies or reviews
This review is part of a series of reviews on CSOM (Bhutta 2018; Brennan‐Jones 2018a; Brennan‐Jones 2018b; Chong 2018a; Chong 2018b; Head 2018a; Head 2018b). A companion review looks at the effectiveness of topical antibiotics with steroids for the treatment of CSOM (Brennan‐Jones 2018a).
There are few previous reviews or guidelines for CSOM. The WHO in 2004 suggested that first‐line treatment of CSOM should comprise aural toilet and topical antibiotic drops, with second‐line treatment comprising an alternative topical antibiotic (guided by results of microbiological culture) or parenteral antibiotics (WHO 2004). The Australian government recommendations from 2010 for the treatment of Aboriginal and Torres Strait islanders gave similar recommendations, with first‐line treatment comprising aural toilet (or antiseptic washout) followed by topical antibiotics, and second‐line treatment with parenteral antibiotics (Morris 2010). An expert panel of the American Academy of Otolaryngologists in 2000 came to a similar conclusion (Hannley 2000).
These reviews supersede a pair of previous Cochrane Reviews examining topical antibiotics for CSOM (Macfadyen 2005a; Macfadyen 2005b).
Although we planned subgroup analyses for different participant characteristics (age, high‐risk, ventilation tubes), treatment duration and spectrum of antibiotic activity these were not carried out either because the data were not available or heterogeneity was not observed.
Authors' conclusions
Implications for practice.
We are uncertain about the effectiveness of topical antibiotics (without steroids) in improving resolution of ear discharge in patients with chronic suppurative otitis media (CSOM) because of the limited amount of low‐quality evidence available. However, amongst this uncertainty there is some evidence to suggest that the use of topical antibiotics (without steroids) may be effective when compared to placebo, or when used in addition to a systemic antibiotic. There is also uncertainty about the relative effectiveness of different types of antibiotics; it is not possible to determine with any certainty whether or not quinolones are better or worse than aminoglycosides. These two groups of compounds have different adverse effect profiles, but there is insufficient evidence from the included studies to make any comment about these. In general, adverse effects were poorly reported.
Implications for research.
The results of this review, current to April 2019, show that there is very low‐certainty evidence that, for people with CSOM, treatment with topical antibiotics (without steroids) may be beneficial in improving the short‐term resolution of ear discharge when compared to placebo, or when used in addition to a systemic antibiotic. The low certainty of the evidence for CSOM treatments in this review is common throughout this suite of seven reviews of CSOM treatments.
There is insufficient evidence to address the implications of topical antibiotics (without steroids) for high‐risk groups such as immunocompromised patients or Indigenous populations. Potential adverse effects and hearing outcomes were poorly reported and the impact of background treatment with aural toileting and/or systemic antibiotics is also unclear.
Prior to commencing these reviews, we conducted a scoping review that identified three key questions that clinicians, researchers and consumers would like to see answered:
Are topical antibiotics effective when added to other interventions (e.g. aural toileting, systematic antibiotics)?
Which topical antibiotic is more effective (when compared to each other)?
Which type of topical antibiotic is more effective when added to other interventions?
Due to the low certainty of the available evidence these questions cannot yet be addressed with any certainty. There is clearly room for more trials examining the impact of topical antibiotics for people with CSOM, including trials that assess the class of antibiotic and the dosing/duration. Whilst the largest number of studies compared the use of topical quinolones to topical aminoglycosides, the certainty of the evidence is still very low (GRADE) for this comparison.
Long‐term effects (effectiveness and harms) are also important. In addition to clinical trials, health services should establish prospective databases for patients with CSOM to record (long‐term) outcomes for resolution of discharge, adverse effects and hearing outcomes for people receiving treatment.
Suggestions for future trials
This review is one of a suite of reviews of treatments for CSOM, each of which features its own research recommendations. Across all reviews, key features of future research are as follows:
Design and methods
Where the intent is to assess the effectiveness of interventions, randomised controlled trials should be conducted. These trials (including those testing non‐systemic interventions), should randomise, analyse and report results by person (not ears).
In patients with bilateral CSOM, for outcomes that can be reported by ear, such as resolution of ear discharge or recurrence, only one finding should be analysed and reported per person. We suggest that a single ear be included in the trial (the decision on which ear is to be included and analysed must be made a priori, and the method or criteria for the decision must explicitly specified in the trial protocol and report). Since there are limited data on whether people with bilateral CSOM respond to treatment in the same way as people with unilateral CSOM, and whether both ears respond in the same way to treatment, reporting these factors would be useful.
Trials need to use appropriate methods for randomisation and allocation concealment to avoid selection bias, and they should be adequately powered.
Attempts should be made by the investigators to blind participants, healthcare professionals and study personnel to the treatment allocation. This could be through the use of a placebo and ensuring that the treatment regimens are the same between treatment arms. A double placebo design should be used where dosage form and/or regimen are different. Where it is not possible to blind participants and/or clinicians to the treatment received, efforts to blind the outcome assessment and analysis personnel should be made.
Population
Diagnosis of CSOM should be according to the World Health Organization (WHO) criteria, be otoscopically confirmed and include an assessment of hearing level.
Potentially important patient characteristics (such as existence of ear grommets) should be recorded and presented in the report.
If patients from 'high‐risk' groups are included, these characteristics should be accounted for and explored in the design of the study.
Interventions
All interventions (adjunctive therapies and/or allowed treatment) should be the same apart from the treatments being evaluated.
Clear reporting of the therapies used, including dose, frequency and duration, and clear descriptions of any adjunctive therapies used across the treatment groups (including aural toileting), should be provided.
Outcomes
There is currently no core outcome set for CSOM, or a widely agreed set of priority outcomes and definitions for CSOM trials. The development of core outcome sets for CSOM, using established methods (Kirkham 2017), would be beneficial for future trials. This would help to ensure that trials are consistent, high‐quality and examine appropriate outcomes. The standardisation of outcomes allows for analysis and comparison of data across trials (and treatments) using network meta‐analysis or individual participant data meta‐analysis.
The assessment of adverse effects should be defined in the protocol and these should be systematically sought during trials using explicit methods.
All outcomes (including hearing) should be measured and reported using valid and predefined methods.
A validated quality of life instrument should be used whenever possible.
Studies should follow up patients for at least six months and preferably over one year to identify the rate of recurrence of ear discharge, using a pre‐agreed definition of recurrence.
Trials should be registered in a regional or international clinical trials registry and, when published, adhere to reporting guidelines such as CONSORT (CONSORT 2010). Where publication in a peer‐reviewed journal is not possible, results should be included in the clinical trial report.
Acknowledgements
This project was funded by the NHMRC Centre of Research Excellence for Ear and Hearing Health of Aboriginal and Torres Strait Islander Children (NHMRC CRE_ICHEAR) and the Western Australian Department of Health through a Future Health Merit Award. The contents of the publications arising from this work are solely the responsibility of the authors and do not reflect the views of NHMRC or the Department of Health.
We are grateful to Professor Amanda Leach for peer reviewing the protocol for this review, to Mr Iain Swan for peer reviewing the protocol and review, and to consumer referee Joan Blakely for her helpful comments at all stages. We would also like to thank Dr Adrian James, as Acting Co‐ordinating Editor for Cochrane ENT, for his insightful comments and advice, and the other members of the Cochrane ENT editorial board for their input and encouragement.
We would like to sincerely thank Jenny Bellorini and Samantha Cox from the Cochrane ENT team for their invaluable help, which has enabled the completion of this suite of reviews, and Jessica Daw for assisting with preparation and collation of the final reviews.
We would also like to thank the following clinicians, scientists and consumers who provided comments on the initial scoping review and prioritisation exercise for this suite of reviews into CSOM: Amanda Leach, Chris Perry, Courtney McMahen, De Wet Swanepoel, Deborah Lehmann, Eka Dian Safitri, Francis Lannigan, Harvey Coates, Has Gunasekera, Ian Williamson, Jenny Reath, Kathy Brooker, Kathy Currie, Kelvin Kong, Matthew Brown, Pavanee Intakorn, Penny Abbot, Samantha Harkus, Sharon Weeks, Shelly Chadha, Stephen O'Leary, Victoria Stroud and Yupitri Pitoyo.
We are indebted to Therese Dalsbø, Artur Gevorgyan, Nathan Gonik, Anna Kashchuk, Esther Martin, Stefano Morettini, Jussi Mustonen, Irina Telegina, Yu‐Tian Xiao, Ibrahim Ethem Yayali, Francine Choi, Chiara Arienti, Maria Paula Garcia, Karen Sagomonyants and Elizabeth Weeda for translating and identifying primary studies for inclusion or exclusion for this suite of reviews.
We are also indebted to Erika Ota from Cochrane Japan for organising a group of MSc students, Shunka Cho, Kiriko Sasayama, Asuka Ohashi, Noyuri Yamaji and Mika Kato, to help with translating and identifying primary studies for inclusion or exclusion for this suite of reviews.
We thank Carolyn McFadyen for her help and support in providing documents from the previous Cochrane Reviews.
We thank Dr Nathan Tu for his contributions and clinical guidance at the protocol stage of this review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies
| CENTRAL (the Cochrane Register of Studies) | MEDLINE (Ovid) | Embase (Ovid) |
| 1 MESH DESCRIPTOR Otitis Media EXPLODE ALL AND CENTRAL:TARGET 2 ("otitis media" or OME):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 3 MESH DESCRIPTOR Tympanic Membrane Perforation EXPLODE ALL AND CENTRAL:TARGET 4 MESH DESCRIPTOR Tympanic Membrane EXPLODE ALL AND CENTRAL:TARGET 5 ("ear drum*" or eardrum* or tympanic):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 6 #4 OR #5 AND CENTRAL:TARGET 7 (perforat* or hole or ruptur*):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 8 #6 AND #7 AND CENTRAL:TARGET0 9 #1 OR #2 OR #3 OR #8 AND CENTRAL:TARGET 10 MESH DESCRIPTOR Suppuration EXPLODE ALL AND CENTRAL:TARGET 11 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or discomfort or earach* or mucopurulen*):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 12 (pain):AB,TI,TO AND CENTRAL:TARGET 13 #10 or #11 or #12 AND CENTRAL:TARGET 14 MESH DESCRIPTOR Chronic Disease EXPLODE ALL AND CENTRAL:TARGET 15 MESH DESCRIPTOR Recurrence EXPLODE ALL AND CENTRAL:TARGET 16 (chronic* or persist* or recurr* or repeat*):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 17 #14 OR #15 OR #16 AND CENTRAL:TARGET 18 #9 AND #17 AND #13 AND CENTRAL:TARGET 19 ((chronic* or persist* or recurr* or repeat*) NEAR (ear or ears or aural) NEAR (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or mucopurulen* or pain* or discomfort or disease*)):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 20 ((earach* near (chronic or persist* or recurr* or repeat*))):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 21 MESH DESCRIPTOR Otitis Media, Suppurative EXPLODE ALL AND CENTRAL:TARGET 22 (CSOM):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 23 #20 OR #21 OR #22 OR #18 OR #19 AND CENTRAL:TARGET | 1 exp Otitis Media/ 2 ("otitis media" or OME).ab,ti. 3 exp Tympanic Membrane Perforation/ 4 exp Tympanic Membrane/ 5 ("ear drum*" or eardrum* or tympanic).ab,ti. 6 4 or 5 7 (perforat* or hole or ruptur*).ab,ti. 8 6 and 7 9 1 or 2 or 3 or 4 or 8 10 exp Suppuration/ n 11 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or moist or wet or mucopurulen* or discomfort or pain* or earach*).ab,ti. 12 10 or 11 13 exp Chronic Disease/ 14 exp Recurrence/ 15 (chronic* or persist* or recurr* or repeat*).ab,ti. 16 13 or 14 or 15 17 9 and 12 and 16 18 ((chronic or persist*) adj3 (ear or ears or aural) adj3 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or mucopurulen* or pain* or discomfort)).ab,ti. 19 CSOM.ab,ti. 20 exp Otitis Media, Suppurative/ 21 (earach* adj6 (chronic or persist* or recurr* or repeat*)).ab,ti. 22 17 or 18 or 19 or 20 or 21 |
1 exp otitis media/ 2 ("otitis media" or OME).ab,ti. 3 exp eardrum perforation/ 4 exp eardrum/ 5 ("ear drum*" or eardrum* or tympanic).ab,ti. 6 4 or 5 7 (perforat* or hole or ruptur*).ab,ti. 8 6 and 7 9 1 or 2 or 3 or 8 10 exp suppuration/ 11 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or moist or wet or mucopurulen* or discomfort or pain* or earach*).ab,ti. 12 10 or 11 13 exp chronic disease/ 14 exp recurrent disease/ 15 (chronic* or persist* or recurr* or repeat*).ab,ti. 16 13 or 14 or 15 17 9 and 12 and 16 18 exp suppurative otitis media/ 19 CSOM.ab,ti. 20 ((chronic or persist*) adj3 (ear or ears or aural) adj3 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or mucopurulen* or pain* or discomfort or disease*)).ab,ti. 21 (earach* adj3 (chronic or persist* or recurr* or repeat*)).ab,ti. 22 17 or 18 or 19 or 20 or 21 |
| Web of Science (Web of Knowledge) | CINAHL (EBSCO) | Cochrane ENT Register (the Cochrane Register of Studies) |
| #1 TOPIC: ("otitis media" or OME) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years #2 TOPIC: (("ear drum*" or eardrum* or tympanic) AND (perforat* or hole or ruptur*)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years #3 #2 OR #1 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years #4 TOPIC: ((suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or moist or wet or mucopurulen* or discomfort or pain* or earach*) AND (chronic* or persist* or recurr* or repeat*)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years #5 #4 AND #3 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years #6 TOPIC: (((chronic or persist*) NEAR/3 (ear or ears or aural) NEAR/3 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or mucopurulen* or pain* or discomfort))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years #7 TOPIC: ((earach* NEAR/3 (chronic or persist* or recurr* or repeat*))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years #8 #7 OR #6 OR #5 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
S21 S17 OR S18 OR S19 OR S20 S20 TX ((chronic or persist*) N3 (ear or ears or aural) N3 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or mucopurulen* or pain* or discomfort)) S19 TX (earach* N3 (chronic or persist* or recurr* or repeat*)) S18 TX csom S17 S9 AND S12 AND S16 S16 S13 OR S14 OR S15 S15 TX chronic* or persist* or recurr* or repeat* S14 (MH "Recurrence") S13 (MH "Chronic Disease") S12 S10 OR S11 S11 TX suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or moist or wet or mucopurulen* or discomfort or pain* or earach*) S10 (MH "Suppuration+") S9 S1 OR S2 OR S3 OR S8 S8 S6 AND S7 S7 TX perforat* or hole or ruptur* S6 S4 OR S5 S5 TX "ear drum*" or eardrum* or tympanic S4 (MH "Tympanic Membrane") S3 (MH "Tympanic Membrane Perforation") S2 TX "otitis media" or OME S1 (MH "Otitis Media+") |
1 ("otitis media" or OME):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 2 (("ear drum*" or eardrum* or tympanic)):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 3 (perforat* or hole or ruptur*):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 4 #2 AND #3 AND INREGISTER 5 #4 OR #1 AND INREGISTER 6 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or discomfort or earach* or mucopurulen*):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 7 (pain):AB,TI,TO AND INREGISTER 8 #6 OR #7 AND INREGISTER 9 (chronic* or persist* or recurr* or repeat*):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 10 #5 AND #8 AND #9 AND INREGISTER 11 (csom):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 12 (((chronic* or persist* or recurr* or repeat*) and (ear or ears or aural) and (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or mucopurulen* or pain* or discomfort or disease*))):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 13 ((earach* and (chronic or persist* or recurr* or repeat*))):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 14 #10 OR #11 OR #12 OR #13 AND INREGISTER |
| ClinicalTrials.gov | ICTRP (WHO Portal) | Other |
|
Search 1 (clinicaltrials.gov): (chronic OR persistent OR recurrence OR recurrent) AND (suppuration OR pus OR discharge OR otorrhea or active OR mucopurulent) AND Condition: "Otitis Media" OR OME AND Study type: interventional Search 2 (clinicaltrials.gov): (chronic OR persistent OR recurrence OR recurrent) AND (earache OR "ear ache" OR "ear pain" OR "ear discharge" OR "wet ear" OR "moist ear" OR "weeping ear") AND Study type: interventional Search 3 (clinicaltrials.gov): ("ear drum" OR eardrum OR "tympanic membrane") AND (hole OR perforation OR rupture) AND Study type: interventional Search 4 (the Cochrane Register of Studies): 1 ("otitis media" or OME):AB,EH,KW,KY,MC,MH,TI,TO AND INSEGMENT 2 (("ear drum*" or eardrum* or tympanic)):AB,EH,KW,KY,MC,MH,TI,TO AND INSEGMENT 3 (perforat* or hole or ruptur*):AB,EH,KW,KY,MC,MH,TI,TO AND INSEGMENT 4 #2 AND #3 AND INSEGMENT 5 #4 OR #1 AND INSEGMENT 6 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or discomfort or earach* or Mucopurulen*):AB,EH,KW,KY,MC,MH,TI,TO AND INSEGMENT 7 (pain):AB,TI,TO AND INSEGMENT 8 #6 OR #7 AND INSEGMENT 9 (chronic* or persist* or recurr* or repeat*):AB,EH,KW,KY,MC,MH,TI,TO AND INSEGMENT 10 #5 AND #8 AND #9 AND INSEGMENT 11 (csom):AB,EH,KW,KY,MC,MH,TI,TO AND INSEGMENT 12 (((chronic* or persist* or recurr* or repeat*) and (ear or ears or aural) and (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or Mucopurulen* or pain* or discomfort or disease*))):AB,EH,KW,KY,MC,MH,TI,TO AND INSEGMENT 13 ((earach* and (chronic or persist* or recurr* or repeat*))):AB,EH,KW,KY,MC,MH,TI,TO AND INSEGMENT 14 #10 OR #11 OR #12 OR #13 AND INSEGMENT 15 (nct*):AU AND INSEGMENT 16 #14 AND #15 |
otitis media AND chronic OR ear discharge OR earache OR wet ear OR weeping ear OR moist ear OR CSOM OR OME AND chronic OR tympanic membrane AND perforation OR eardrum AND hole OR eardrum AND perforation |
LILACS TW:"otitis media" OR "TW:"ear discharge" OR TW:earache OR ((TW:eardrum OR TW:tympanic) AND (TW:perforation OR hole)) OR ((TW:wet OR moist OR weeping) AND TW:ear) AND: Filter: Controlled Clinical Trial IndMed Chronic Suppurative Otitis Media OR Chronic Otitis Media OR CSOM African Index Medicus “chronic suppurative otitis media" OR "chronic otitis media“ OR CSOM |
Appendix 2. Data extraction form
| REF ID: | Study title: |
| Date of extraction: | Extracted by: |
| Name and email address of correspondence authors: |
| General comments/notes (internal for discussion): |
FLOW CHART OF TRIAL:
|
Intervention (name the intervention) |
Comparison (name the intervention) |
|
| No. of people screened | ||
| No. of participants randomised ‐ all | ||
| No. randomised to each group | ||
| No. receiving treatment as allocated | ||
| No. not receiving treatment as allocated ‐ Reason 1 ‐ Reason 2 |
||
| No. that dropped out1 (no follow‐up data for any outcome available) |
||
| No. excluded from analysis2 (for all outcomes) ‐ Reason 1 ‐ Reason 2 |
||
1This includes patients who withdrew and provided no data, or did not turn up for follow‐up. 2This should be the people who were excluded from all analyses (e.g. because the data could not be interpreted or the outcome was not recorded for some reason). This is the number of people who dropped out, plus the people who were excluded by the authors for some reason (e.g. non‐compliant).
INFORMATION TO GO INTO THE 'CHARACTERISTICS OF INCLUDED STUDIES' TABLE:
| Methods | X arm, double‐/single‐/non‐blinded, [multicentre] parallel‐group/cross‐over/cluster RCT, with x duration of treatment and x duration of follow‐up |
| Participants |
Location: [country, rural?, no. of sites etc.] Setting of recruitment and treatment: [specialist hospital? general practice? school? state YEAR] Sample size:
Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria: |
| Interventions |
Intervention (n = x): drug name, method of administration, dose per day/frequency of administration, duration of treatment For aural toileting: who does it, methods or tools used, frequency, duration Comparator group (n = y): Concurrent treatment: Use of additional interventions (common to both treatment arms): |
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes
|
| Funding sources | "No information provided"/"None declared"/State source of funding |
| Declarations of interest | "No information provided"/"None declared"/State conflict |
| Notes |
Clinical trial registry no: (if available) Unit of randomisation: person/ears/other (e.g. cluster‐randomised by hospital/school) [In the case of randomisation by person]: Methods for including patients with bilateral disease, for example:
|
RISK OF BIAS TABLE:
(See table 8.5d in the Cochrane Handbook for Systematic Reviews of Interventions: http://handbook.cochrane.org/).
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High/low/unclear risk | Quote: "…" Comment: |
| Allocation concealment (selection bias) | High/low/unclear risk | Quote: "…" Comment: |
| Blinding of participants and personnel (performance bias) | High/low/unclear risk | Quote: "…" Comment: |
| Blinding of outcome assessment (detection bias) | High/low/unclear risk | Quote: "…" Comment: |
| Incomplete outcome data (attrition bias) | High/low/unclear risk | Quote: "…" Comment: |
| Selective reporting (reporting bias) | High/low/unclear risk | Quote: "…" Comment: |
FINDINGS OF STUDY
CONTINUOUS OUTCOMES
| Results (continuous data table) | |||||||
| Outcome | Intervention (name the intervention) |
Comparison (name the intervention) |
Other summary statistics/Notes | ||||
| Mean | SD | N | Mean | SD | N | Mean difference (95% CI), P values etc. | |
|
Disease‐specific health‐related quality of life (COMQ‐12, COMOT‐15, CES)1 Time point: (state) |
|||||||
|
Hearing: [Measurement method: include frequencies and report results separately if they are presented in the paper] Time point: [xx] |
|||||||
| Comments: [If there is no information apart from (vague) narration, quote here] [If information is in the form of graphs, used this software to read it: http://arohatgi.info/WebPlotDigitizer/app/, and save a copy of your charts in a folder] | |||||||
1State the measurement method: this will be instrument name/range for patient‐reported outcomes.
DICHOTOMOUS OUTCOMES
| Results (dichotomous data table) | ||||||
| Outcome | Applicable review/ Intervention1 |
Group A ‐ intervention arm | Group B – control | Other summary statistics/Notes | ||
| No. of people with events | No. of people analysed | No. of people with events | No. of people analysed | P values, RR (95% CI), OR (95% CI) | ||
|
Resolution of ear discharge or 'dry ear' at 1 to 2 weeks [Measurement method or definition used: not/unclear if/otoscopically confirmed]1 Time point: [State actual time point] |
||||||
|
Resolution of ear discharge or 'dry ear' at 2 to 4 weeks [Measurement method or definition used: not/unclear if/otoscopically confirmed] Time point: [xx] |
||||||
|
Resolution of ear discharge or 'dry ear' after 4 weeks [Measurement method or definition used: not/unclear if/otoscopically confirmed] Time point: [xx] |
||||||
|
Ear pain/discomfort/local irritation
[Measurement method or definition used e.g. patient‐reported] Time point: [xx] |
||||||
|
Suspected ototoxicity [Measurement method or definition used] Time point: [xx] |
||||||
|
Sensorineural hearing loss [Measurement method or definition used] Time point: [xx] |
||||||
|
Tinnitus [Measurement method or definition used] Time point: [xx] |
||||||
|
Dizziness/vertigo/balance [Measurement method or definition used] Time point: [xx] |
||||||
|
Serious complications:
[State whether the paper had prespecified looking for this event, how it was diagnosed] Time point: state length of follow‐up of the trial |
Note down the page number/table where info was found for ease of checking | |||||
|
Otitic meningitis [How was this diagnosed?] |
||||||
|
Lateral sinus thrombosis [How was this diagnosed?] |
||||||
|
Cerebellar abscess [How was this diagnosed?] |
||||||
|
Mastoid abscess/mastoiditis [How was this diagnosed?] |
||||||
|
Postauricular fistula [How was this diagnosed?] |
||||||
|
Facial palsy [How was this diagnosed?] |
||||||
|
Other complications [How was this diagnosed?] |
||||||
|
Death [How was this diagnosed?] |
||||||
|
Multiple serious complications [How was this diagnosed?] |
||||||
| Comment/additional notes: If any calculations are needed to arrive at the data above, note this down here. | ||||||
1State briefly how this was measured in the study, especially whether there was deviation from what was expected in the protocol.
For adverse events, note down how these were collected, e.g. whether the adverse event was one of the prespecified events that the study planned to collect, when it was collected and how/who measured it (e.g. as reported by patients, during examination and whether any scoring system was used).
Data and analyses
Comparison 1. Topical ciprofloxacin versus placebo (aural toileting in both arms).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Resolution of ear discharge | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.74 [1.82, 24.99] |
| 1.1 Measured at 1 to 2 weeks | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.74 [1.82, 24.99] |
Comparison 2. Topical ciprofloxacin added on to oral ciprofloxacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Resolution of ear discharge | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Measured at 1 to 2 weeks | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.20, 1.80] |
| 1.2 Measured at 2 to 4 weeks | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.04, 3.39] |
Comparison 3. Quinolones versus others.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Resolution of ear discharge (1 to 2 weeks) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Quinolones versus aminoglycosides | 6 | 694 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.88, 4.29] |
| 2 Resolution of ear discharge (2 to 4 weeks) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Quinolones versus aminoglycosides | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.80, 1.64] |
| 2.2 Quinolones versus neomycin/polymixin B | 1 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [1.03, 1.22] |
Comparison 4. Rifampicin versus chloramphenicol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Resolution of ear discharge (1 to 2 weeks) | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [1.35, 2.34] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Asmatullah 2014.
| Methods | Two‐arm, non‐blinded, parallel‐group RCT, with 10 days duration of treatment and 2‐week duration of follow‐up | |
| Participants |
Location: Pakistan, 1 site Setting of recruitment and treatment: ENT Department, Hayatabad Medical Complex, Peshawar, January to July 2012 Sample size: 134
Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention (n = 67): gentamycin 0.3% ear drops, 4 drops 3 times daily, treatment continued for 10 days Comparator group (n = 67): ofloxacin 0.3% ear drops, 4 drops 3 times daily, treatment continued for 10 days Concurrent treatment: none listed. No aural toileting methods mentioned. |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes |
Unit of randomisation: person Methods for including patients with bilateral disease: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All patients were allocated into two groups by randomization done by lottery method." Comment: not enough information to know whether this method was sufficient to generate a sufficiently randomised sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: no methods regarding allocation concealment were reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: there is no mention of blinding for any of the outcomes despite the ability to blind the trial (same treatment regimen) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: there is no mention of blinding the treatment outcome. There may have been bias when judging the marginal cases such as the boundary between 'mild' and 'no' discharge. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the description of patient allocation is not clear within the paper. Although the paper does not appear to have lost patients to follow‐up, there is no clear statement of this. It is unclear whether only patients included in the outcome were reported. |
| Selective reporting (reporting bias) | High risk | Comment: no trial protocol was mentioned in the paper or found on the clinicaltrials.gov website. The levels of discharge were not well defined in the methods section. In addition, there was no defined measuring or indeed mention of adverse events anywhere within the paper. |
de Miguel 1999.
| Methods | Five‐arm, non‐blinded, parallel‐group RCT, with 7‐day duration of treatment and 15‐day duration of follow‐up | |
| Participants |
Location: Canary Islands, Spain Setting of recruitment and treatment: general hospital, published in 1999 Sample size: 125
Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group A (n = 25): oral ciprofloxacin, 500 mg/12 hours for 7 days Group B (n = 25): topical ciprofloxacin 0.2%, 3 eardrops/8 hours for 7 days Group C (n = 25): topical ciprofloxacin 0.5%, 3 eardrops/8 hours for 7 days Group D (n = 25): topical ciprofloxacin 0.2%, 3 eardrops/8 hours for 7 days PLUS oral ciprofloxacin, 500 mg/12 hours for 7 days simultaneously Group E (n = 25): topical polymixin B + neomycin + hydrocortisone, 3 eardrops/8 hours for 7 days Concurrent treatment: all patients had aspiration and cleaning of ear secretions before beginning treatment. Analgesics and antipyretics. |
|
| Outcomes |
Outcomes of interest in the review: Primary outcome:
Secondary outcomes:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes |
Unit of randomisation: person Methods for including patients with bilateral disease: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "125 patients were analysed for two years attending to health system with chronic otorrhea as the mean symptom." Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomized to five therapeutic groups." Comment: insufficient information about allocation concealment method provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no information provided about blinding method or use of placebo. The treatment arms involved different dosage forms (oral versus ear drops) – blinding of these interventions is impossible without the use of a placebo |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no information provided regarding to who assessed the outcomes. For subjective outcomes (otoscopy examinations, hearing test or adverse events) is probable that the knowledge of treatment group influenced the results |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no dropouts or missing data reported; no statements about missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to permit judgement of 'low‐risk' or 'high‐risk'. Protocol for trial not available. |
Esposito 1990.
| Methods | Three‐arm, non‐blinded, single‐centre, parallel‐group RCT, with 5 to 10 days of treatment and 24 hours and 14 days follow‐up after end of treatment | |
| Participants |
Location: Naples, Italy Setting of recruitment and treatment: Clinic of Infectious Diseases and Otolaryngology, University of Naples Sample size: 60
Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Topical plus systemic ciprofloxacin (n = 20): 3 drops topical ciprofloxacin 250 µg/mL in saline solution locally twice a day PLUS oral ciprofloxacin 250 mg twice a day Topical ciprofloxacin (n = 20): 3 drops topical ciprofloxacin 250 µg/mL in saline solution locally twice a day Oral ciprofloxacin (n = 20): oral ciprofloxacin 250 mg twice a day All interventions given for at least 5 days. Those not cured at 5 days carried on up to 10 days. Concurrent treatment: no other treatment or use of aural toileting was mentioned |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
|
|
| Funding sources | "The ciprofloxacin tablets and powder used in this study were kindly provided by Bayer Italia Spa, Milan, Italy." | |
| Declarations of interest | No information provided | |
| Notes | Topical ciprofloxacin was prepared from ciprofloxacin powder in sterile saline and tested for stability and activity for 10 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Ciprofloxacin was randomly administered according to the following schedules." Comment: randomisation method not clearly specified. 38/60 patients were previously unsuccessfully treated with at least 5 days of antibiotics – unclear how this was distributed across groups. 12/20 in the oral ciprofloxacin only group had pseudomonas versus 8/20 in other groups. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Group A (20 patients), 250mg orally twice a day; group B (20 patients), 3 drops containing 250ug/mL of ciprofloxacin in saline solution locally twice a day; and group C (20 patients), both the previous treatments twice a day." Comment: most likely that participants were not blinded as the routes of administration (oral versus topical) were different among groups and it was not mentioned that placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Patients were clinically examined before, during (every 2 to 3 days) and after the therapy." Comment: not specified who assessed the outcomes and the assessment method was not specifically standardised. "Cure", "improvement" and "failure" seemed to be more of a subjective judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropouts were reported. All of the patients randomised are presented in the results of the study. |
| Selective reporting (reporting bias) | High risk | Comment: there is no protocol for the trial on clinicaltrials.gov or in the EU register of clinical trials. Some of the results mentioned in the methods section are not fully presented in the results section. "Cure" or resolution of discharge is only reported at one time point, most likely 14 days after end of treatment. The other time point, 24 hours after end of treatment (i.e. 6 to 11 days), was not reported. |
Fradis 1997.
| Methods | Three‐arm, double‐blind, parallel‐group RCT, with 3 weeks duration of treatment and follow‐up | |
| Participants |
Location: Israel, 1 site Setting of recruitment and treatment: otolaryngology outpatient clinic of Bnai Zion Medical Centre, January 1994 to December 1995 Sample size:
Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group A (n = 20 ears): ciprofloxacin (no concentration given) ear drops, 5 drops, 3 times daily for a period of 3 weeks Group B (n = 20 ears): tobramycin (no concentration given) ear drops, 5 drops, 3 times daily for a period of 3 weeks Group C (n = 20 ears): Burow's solution (1% aluminium acetate solution) ear drops, 5 drops 3 times daily for a period of 3 weeks Concurrent treatment: no information about concurrent treatment All other medications were discontinued 2 weeks prior to beginning participation in the study |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | This is a 3‐arm trial comparing topical ciprofloxacin, topical tobramycin and Burow's solution (aluminium acetate – topical antiseptic) Unit of randomisation: ears Methods for including patients with bilateral disease: not stated. No adjustments made. Unclear how many patients had bilateral ear disease in each group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly divided into 3 groups of 20 ears each…" Comment: insufficient information about the sequence generation is available to determine whether this is a 'high' or 'low' risk |
| Allocation concealment (selection bias) | Low risk | Quote: "… All patients … received similar appearing bottles of ear drops in a randomized manner. Neither the patients nor the treating physician knew what type of ear drops was given to each patient." Comment: it does not appear that the treating physician could determine the allocation to treatment group |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All numbered bottles were retained in the hospital pharmacy and during the study only the head of the pharmacy department knew what each bottle contained. The code of bottle contents was broken only at the end of the study to summarize the results of the investigation." Comment: participants and trial personnel were sufficiently blinded to treatment group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All numbered bottles were retained in the hospital pharmacy and during the study only the head of the pharmacy department knew what each bottle contained. The code of bottle contents was broken only at the end of the study to summarize the results of the investigation." Comment: those assessing outcomes were blinded to treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Only 54 of 60 ears were available for re‐examination after 3 weeks of treatment. Six patients (1 from group 1, 2 from group 2, and 3 from group 3) who entered the study were unavailable for follow‐up." Comment: loss to follow‐up was 10% (6/60) in total. No reasons for loss to follow‐up are provided. |
| Selective reporting (reporting bias) | Unclear risk | Quote: "All patients underwent an audiological examination at the end of the treatment period." Comment: the methods section states that audiological examination was completed at the end of treatment but this is not presented in the results section. No protocol for the trial was identified. |
Gyde 1978.
| Methods | Two‐arm, double‐blind, cross‐over RCT, with unclear duration of treatment (up to 3 weeks) and 12 months duration of follow‐up | |
| Participants |
Location: Canada, unclear number of sites Setting of recruitment and treatment: unclear. Outpatient department, published in 1978. Sample size: 91 people (100 ears)
Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention (n = 50 ears): trimethoprim (1 mg/mL), sulphacetamide (5 mg/mL) and polymyxin B (10,000 units/mL) (Burroughs Wellcome Ltd), ear drops, 8 drops/12 hours. Duration of treatment: up to 3 weeks (average treatment time for those with 'success' was 16.4 days). Comparator group (n = 50 ears): gentamycin (Garamycin), 0.3% (3mg/mL), ear drops, 8 drops/12 hours. Duration of treatment: up to 3 weeks (average treatment time for those with 'success' was 22.8 days). Concurrent treatment: aural toileting: dry mopping and suction cleaning before each treatment. No details on method. No further details on any other treatment. |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes |
Unit of randomisation: person Methods for reporting outcomes of patients with bilateral disease: it does not appear that any consideration of the correlation of results between ears has been taken into account. If there was a treatment failure 'ears' were transferred to the alternative groups. These results have not been included in the analysis. If an ear was not dry on review at 6 months, treatment for 3 weeks with the alternative treatment was completed with review after 6 months. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: (translated) "We used the method minimisation technique of Taves to allocate the patients to the treatment groups using the type of infection as the principal criterium." Comment: references are given to support the method of participant selection |
| Allocation concealment (selection bias) | Low risk | Comment: if the Taves method of minimisation was used correctly, it is unlikely that the allocating physician could have predicted the treatment group to which the patient would have been allocated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blinded study" Comment: no further information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blinded study" Comment: no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: it appears that all of the participants who started the trial were accounted for in the results |
| Selective reporting (reporting bias) | Low risk | Comment: no protocol was identified on the WHO clinical trials registry |
Jamalullah 2016.
| Methods | Two‐arm, non‐blinded, parallel‐group, quasi‐randomised controlled trial, with 2‐week duration of treatment and follow‐up | |
| Participants |
Location: Pakistan, 1 site Setting of recruitment and treatment: Department of Otolaryngology, Pakistan Institute of Medical Sciences, Islamabad, September 2009 to March 2010 Sample size: 80
Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention (n = 40): ofloxacin 0.6%, ear drops, 4 drops 3 times daily. Duration of treatment = 2 weeks. Comparator group (n = 40): gentamycin 0.3%, ear drops, 4 drops 3 times daily. Duration of treatment = 2 weeks. Concurrent treatment: aural toileting: "aural toilets or all the patients were done before initiating any therapy." No other information about additional treatments was given. |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes |
Unit of randomisation: person Methods for including patients with bilateral disease: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This randomized controlled trial ..." Comment: no methods of sequence generation were described |
| Allocation concealment (selection bias) | Unclear risk | Comment: methods of allocation concealment were not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding of participants was attempted despite blinding being a possibility as both of the treatments were topical ear drops and the regimens were the same |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: the paper does not mention any attempts to blind the outcome assessors, despite this being feasible because the outcomes were the presence or absence of ear discharge |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: it appears that all of the patients who started the trial were included in the analysis. There was no mention of any participants being lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol for the paper was found on the WHO clinical trial database (ICTRP). The paper does not mention a protocol. The outcomes listed in the methods section are reported in the results section although there is a greater emphasis on subgroups relating to the severity of initial otorrhoea and type of mucosa (polypoidal or erythematous) in the results. It is unclear if these were pre‐determined subgroups or whether the results were analysed in this way post‐hoc. |
Kasemsuwan 1997.
| Methods | Two‐arm, double‐blind, parallel‐group RCT, with 7 days duration of treatment and follow‐up | |
| Participants |
Location: Thailand, 1 site Setting of recruitment and treatment: Ramathibodi hospital (specialist hospital), Bangkok, October to December 1993 Sample size: 50
Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention (n = 19): ciprofloxacin in saline solution (250 mg/mL), ear drops, 5 drops, 3 times daily. Duration of treatment = 7 days. Comparator group (n = 16): saline solution, ear drops. 5 drops, 3 times daily. Duration of treatment = 7 days Concurrent treatment: on day 1, 4 and 7 ear cleaning was performed. No other information regarding the concurrent treatment was provided. |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes |
Unit of randomisation: person Methods for including patients with bilateral disease: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…randomly allocated treatment…" Comment: no information was provided about how the sequence was generated |
| Allocation concealment (selection bias) | Low risk | Quote: "…They were randomly allocated treatment with either ciprofloxacin in saline solution or solution without antibiotics in a double blind method. The medications were prepared, and given codes which were known only in the pharmaceutical laboratory." Comment: it is implied that the treatment allocation was completed by physicians without knowledge of the randomisation schedule |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: it is implied that the medications were coded, rather than labelled and so the treatment group was not known by the participants or the personnel in the trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: it is implied that the trial was blinded and the personnel assessing the outcome (ear discharge) were unaware of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: the paper states that 15/50 (30%) participants did not complete the trial due to "lack of attendance". The paper does not provide an analysis of which group they were allocated to or why they did not attend. Given that the trial was for 7 days, this seems to be a very high dropout rate. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol for the trial was identified through clinicaltrials.gov or the Thai registry of clinical trials. Outcomes that were detailed in the methods are presented in the full paper although the results of the audiological assessments are not presented explicitly, just as a single statement stating no difference. |
Kaygusuz 2002.
| Methods | Four‐arm, non‐blinded, single‐centre, parallel‐group RCT, with 3 weeks duration of treatment and follow‐up | |
| Participants |
Location: Turkey, 1 site Setting of recruitment and treatment: university ENT clinic; no dates (published in 2002) Sample size: 80 patients (103 ears)
Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Topical ciprofloxacin (n = 20): 0.3% ciprofloxacin hydrochloride eardrops, 2 drops, 3 times in a day, treatment duration = 21 days Topical tobramycin (n = 20): 0.3% tobramycin eardrops, 2 drops, 3 times in a day, treatment duration = 21 days Topical ciprofloxacin + dexamethasone (n = 20): 0.3% ciprofloxacin hydrochloride PLUS 0.1% dexamethasone combination eardrops, 2 drops, 3 times in a day, treatment duration = 21 days Topical tobramycin + dexamethasone (n = 20): 0.3% tobramycin PLUS 0.1% dexamethasone combination ear drops, 2 drops, 3 times in a day, treatment duration = 21 days Concurrent treatment: daily aspiration during exam for 3 weeks |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | Part of a 4‐arm trial with the following treatment groups:
Only the first 2 arms are presented in this review Unit of randomisation: person Methods for including patients with bilateral disease: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no clear statement regarding "randomized". No description about randomisation method. |
| Allocation concealment (selection bias) | Unclear risk | Comment: there is no information about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: there is no clear statement regarding whether the study was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: there is no clear statement regarding whether the study was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no participants were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: there was no study protocol mentioned within the paper and no protocol was found on clinicaltrials.gov All of the outcomes mentioned in the methods section are reported in the results |
Liu 2003.
| Methods | Two‐arm, blinding not described, parallel‐group RCT, with 2‐week duration of treatment and follow‐up | |
| Participants |
Location: China, 1 site Setting of recruitment and treatment: outpatient department, department of Otolaryngology, Wenzhou Second People's Hospital, published in 2003 Sample size: 160
Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group A (n = 87): rifampicin (0.1%) eye drops, through the ear canal, 3 drops, 3 times a day. Duration of treatment: 2 weeks. Group B (n = 73): chloramphenicol (0.25%) eye drops, through the ear canal, 3 drops, 3 times a day. Duration of treatment: 2 weeks. Concurrent treatment: aural toileting, "wash the ear canal with 3% H2O2 solution and dry the ear" before administration of ear drops |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes: Complete resolution of ear discharge measured at 2 weeks: Quote: "Criteria for assessment of outcomes:
The outcome was presented as the number of patients whose treatment was considered curative, significantly effective, or non‐effective, respectively Secondary outcomes:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes |
Unit of randomisation: person Methods for including patients with bilateral disease: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "We randomly selected 160 patients who were diagnosed with chronic suppurative otitis media in outpatient department (male 92, female 58, age 16 to 65, mean 30). These patients were divided into rifampicin group (group A, 87 patients) and chloramphenicol group (group B, 73 patients). The two group showed no significant difference in terms of gender, age, and course of disease." Comment: it is not clear whether or not the patients were randomised |
| Allocation concealment (selection bias) | Unclear risk | Quote: "We randomly selected 160 patients who were diagnosed with chronic suppurative otitis media in outpatient department (male 92, female 58, age 16 to 65, mean 30). These patients were divided into rifampicin group (group A, 87 patients) and chloramphenicol group (group B, 73 patients). The two group showed no significant difference in terms of gender, age, and course of disease." Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not mentioned. Given the different colour of the 2 different eye drops, blinding would be difficult to achieve. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition not reported. It seems that no participant was lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol for this trial is not available |
Lorente 1995.
| Methods | Two‐arm, double‐blinded, multicentre, parallel‐group RCT, with 8 days duration of treatment and 30 days duration of follow‐up | |
| Participants |
Location: Spain, 4 centres Setting of recruitment and treatment: hospital ENT clinics, Barcelona, no dates (published in 1995) Sample size: 308
Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention (n = 159): ciprofloxacin 0.3% (3 mg/mL), ear drops, 5 drops, 3 times a day, duration of treatment = 8 days Comparator group (n = 149): gentamicin 0.3% (3 mg/mL), ear drops, 5 drops, 3 times a day, duration of treatment = 8 days Concurrent treatment: not reported |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes |
Unit of randomisation: person Methods for including patients with bilateral disease: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: the study indicates that it was "randomised" but does not provide any further details |
| Allocation concealment (selection bias) | Unclear risk | Comment: the study does not provide any details about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: the study indicates that it is "double‐blind" but does not provide any details on how blinding of participants and healthcare professionals was ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: the study indicates that it is "double‐blind" but does not indicate if this includes those people who assessed the outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the paper does not mention that any participants were lost to follow‐up. The paper presents the number of people available for analysis at the end of the trial but does not specifically state how many were randomised to treatment. In a trial of 300 participants for 30 days it would be unusual to have no participants withdrawing from treatment. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol could be identified on clinicaltrials.gov or in the European clinical trials registry. Although the outcomes as presented in the methods section are presented in the results section, the level of reporting is not always clear. The hearing results just show that there was no significant difference between the groups. |
Mira 1993.
| Methods | Two‐arm, single‐blinded, parallel‐group RCT, with 7 days duration of treatment and 21 days follow‐up | |
| Participants |
Location: Italy, 1 site Setting of recruitment and treatment: Department of Otolaryngology, published in 1993 Sample size: 248
Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention (n = 128): ceftizoxime 2 g dissolved in 4 mL of saline twice per day for 7 days. Method of administration: 2 mL of solution was instilled into the auditory canal while the patient was supine and keeping their head turned to one side. The patient remained in this position for 3 to 5 minutes, after which they released the excess liquid by turning their head to the opposite side. The remaining part of the supplied solution was then also instilled and the canal was padded with an ear gauze. Morning treatment: ear pad was removed after 2 hours Evening treatment: ear pad kept in situ overnight and removed the next morning Comparator group (n = 120): saline solution – same method as per intervention arm above Concurrent treatment: at the first visit a specialist aspirated local secretions All patients received systemic antibiotics 2 g daily of ceftizoxime by intramuscular route (1 vial every 12 hours) for 7 days |
|
| Outcomes | Ear discharge was reported but not in a way that we could use in this review | |
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes |
Unit of randomisation: person Methods for including patients with bilateral disease: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was a single‐blind randomised investigation." Comment: there is no information about the method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: there is no information regarding allocation concealment. It is not clear whether the healthcare professionals could have influenced the allocation to treatment group |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The study was a single‐blind randomised investigation." Comment: although the study indicates that the study was "single‐blinded" it does not provide details of who was blinded to treatment. It is assumed that it was the participants who were blinded to treatment. The saline placebo was used at the same volume and with the same method of application as the antibiotic ear drops. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The study was a single‐blind randomised investigation." Comment: it is assumed that the single blinding was blinding of the participants. In this case the outcome assessors would have known the treatment to which the participants were allocated when assessing outcomes. This could have led to bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: it appears that only 1 participant in the topical antibiotics group did not complete the trial (1/128; 0.7%) |
| Selective reporting (reporting bias) | High risk | Comment: a protocol was not available through clinicaltrials.gov or the European Clinical Trials Registry. The outcomes as reported in the methods section are not all well presented in the results section. The methods sections reports some information about the scales used to measure symptom scores but it is not clear which symptoms were measured. Similarly the methods section gives definitions of the outcomes 'recovered', 'improved' and 'unchanged or worsened' but these are not presented in the results. The results are given descriptively without any information about precision. |
Nawasreh 2001.
| Methods | Two‐arm, non‐blinded, parallel‐group RCT, with 10‐day duration of treatment and follow‐up | |
| Participants |
Location: Jordan, 1 site Setting of recruitment and treatment: unclear setting, January to August 1999 Sample size: 88 at the end of the trial
Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention (n = 48): ciprofloxacin in distilled water (200 µg/mL), ear drops, 5 drops, 3 times per day. Treatment duration = 10 days Comparator group (n = 40): gentamicin sulphate (5 mg/mL) ear drops, 5 drops, 3 times per day. Treatment duration = 10 days Concurrent treatment: none listed. No mention of aural toileting. |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes |
Unit of randomisation: person Methods for including patients with bilateral disease: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "…[patients] were randomly placed…." Comment: the abstract mentions that randomisation occurred but there is no mention of randomisation or methods for randomisation in the full paper. The full paper describes the patients as "divided" between the 2 groups. It is unclear if the groups were evenly distributed as there were 40 in one group and 48 in the other but baseline characteristics between the groups were not provided. |
| Allocation concealment (selection bias) | Unclear risk | Comment: there is no information within the paper about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it does not appear that personnel or participants were blinded to treatment group, although both groups had the same treatment regimen so this would have been possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: it does not appear that any efforts were made to keep the outcome assessors blind to the treatment group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Patients who failed to use the topical solution regularly or who took other medication during the study period were excluded." Comment: there is no information about how many people were in these categories or into which treatment group they had been allocated to show whether there was a difference between the groups that could have led to bias in the results. There is an imbalance in participants between the groups. There were nearly 20% fewer participants in the gentamicin group. |
| Selective reporting (reporting bias) | High risk | Comment: no trial protocol could be identified in clinicaltrials.gov. Some of the results that were identified in the methods section were not well presented in the paper (e.g. hearing, where there is only a statement that gives the basic significant difference between the start and end of treatment in each group, rather than a between treatment group comparison). Only 10‐day treatment results are presented, not 1‐, 5‐ and 7‐day treatment results. |
Ramos 2003.
| Methods | Five‐arm, open‐label, parallel‐group RCT, with 7‐day duration of treatment and 10 days of follow‐up. Follow‐up to 3 days after finishing the treatment | |
| Participants |
Location: Spain Setting of recruitment and treatment: 3 ENT departments of 3 tertiary hospitals Sample size: 300 patients
Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group A (n = 50): oral ciprofloxacin 500 mg 12‐hourly PLUS topical ciprofloxacin 0.2% 0.5 mL 8‐hourly for 7 days Group B (n = 50): topical ciprofloxacin 0.3% PLUS fluocinolone 0.5 mL 8‐hourly for 7 days Group C (n = 50): topical ciprofloxacin 0.5%, 0.5 mL 8‐hourly for 7 days Group D (n = 50): topical ciprofloxacin 0.2%, 0.5 mL 8‐hourly for 7 days Group E (n = 50): topical polymyxin 10,000 IU, neomycin 0.0035 g, hydrocortisone 0.00025 g, 8‐hourly for 7 days Group F (n = 50): oral ciprofloxacin 500 mg twice 12‐hourly for 7 days Concurrent treatment: not reported |
|
| Outcomes |
Outcomes of interest in the review: Primary outcome:
Secondary outcomes
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes |
Unit of randomisation: person Methods for including patients with bilateral disease: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly allocated into 6 groups." Comment: insufficient information about the sequence generation. In addition, the study stated that children were not randomised to oral ciprofloxacin; it is unclear how this was done. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomly allocated into 6 groups." Comment: insufficient information about allocation concealment. There is no information about how they maintained allocation concealment but did not randomise children to ciprofloxacin. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no information provided about blinding method or use of placebo. The treatment arms involved different dosage forms (oral versus ear drops) – blinding of these interventions is impossible without the use of placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no information provided regarding who assessed the outcomes. For subjective outcomes (otoscopy examinations) the knowledge of treatment group may influence the results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 2 patients on oral treatment were reported as withdrawn due to gastrointestinal adverse events. It is unclear in which group this was and whether these patients were counted in the percentages reported. The percentage of withdrawals is small. |
| Selective reporting (reporting bias) | Unclear risk | Comment: an audiogram was performed at baseline and the end of treatment, but not reported |
Siddique 2016.
| Methods | Two‐arm, unblinded, parallel‐group RCT, with unclear duration of treatment (probably 4 weeks) and 4‐week follow‐up | |
| Participants |
Location: Pakistan, 1 site Setting of recruitment and treatment: specialist/tertiary military hospital, Peshawar, January to December 2013 Sample size: 200
Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention (n = 93): 'standard dosage' ciprofloxacin (Cipotec ear drops), 3 drops/12 hours. Unclear treatment duration (probably 4 weeks). Comparator group (n = 93): 'standard dosage' neomycin (Neosporin ear drops), 2 drops/12 hours. Unclear treatment duration (probably 4 weeks). Concurrent treatment: there is no information about the use of aural toileting or any additional treatments |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
|
|
| Funding sources | "No funding was received from any agency or institution" | |
| Declarations of interest | "Abstract and results of this study were accepted and presented in an oral presentation at the International conference on Medical Education, organised by Association for Excellence in Medical Education (AEME) and held on 07th‐09th March 2014 at University of Health Sciences (UHS) Lahore, Pakistan" No other conflicts were mentioned |
|
| Notes |
Unit of randomisation: person Methods for including patients with bilateral disease: 15 patients (8%) had bilateral disease but how these cases were handled is not stated. The denominator in the trials is the person so it is assumed that no double counting occurred |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quotes: "Randomized clinical trial" and "patients… assigned to one or the other group based on consecutive non‐probability sampling." Comment: it is unclear what the actual process for the 'non‐probability' sampling was and how it ensured that the allocation was random |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients… assigned to one or the other group based on consecutive non‐probability sampling." Comment: it is not clear how much impact the investigators had in terms of allocating people to treatment groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it does not appear that participants or personnel were blinded to treatment group. The treatment groups had different treatment regimens. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: it does not appear that the outcome assessors were blinded to treatment group although this would have been feasible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the 200 patients included in the study 14 were lost to follow up." Comment: the rate of loss to follow‐up is low (7%) but there is no explanation of why patients were lost to follow‐up and to which groups they had been allocated. Due to the small numbers this is unlikely to have influenced the results. |
| Selective reporting (reporting bias) | High risk | Comment: no trial protocol was identified on clinicaltrials.gov or the WHO clinical trials registry site. Comment: whilst the methods state that the amount of ear discharge at both 2 weeks and 4 weeks was measured, only the amount of ear discharge at 4 weeks is reported. |
Tutkun 1995.
| Methods | Two‐arm, non‐blinded, single‐centre, parallel‐group RCT, with 10 days duration of treatment and follow‐up | |
| Participants |
Location: Turkey, 1 site Setting of recruitment and treatment: tertiary medical centre, university hospital, Istanbul; November 1993 to June 1994 Sample size: 44
Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention (n = 24): ciprofloxacin hydrochloride 200 µg/mL (0.02%), ear drop, 5 drops (equivalent volume unknown), 3 times daily, duration of 10 days (solution prepared by dissolving ciprofloxacin HCL in distilled water) Comparator group (n = 21): gentamicin sulfate 5 mg/mL, ear drops, 5 drops, 3 times daily, duration of 10 days Concurrent treatment: not reported All patients were free of any medications for at least 10 days before the treatment and were advised not to take any other medications during the course of the study |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes |
Unit of randomisation: person Methods for including patients with bilateral disease: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly divided into two groups." Comment: specific method not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: no method reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no blinding or placebo mentioned. No other information on whether there was other concurrent treatment such as aural toileting, except that "taking any other medication… were excluded". The treatment regimens were the same for both groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no blinding or placebo mentioned. The treatment regimens were the same for both groups. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Patients who did not use the topical solutions regularly and those who had taken any other medication during the study period were also excluded…" Comment: it is unclear whether this statement referred to recruitment criteria or exclusion of patients after randomisation. There is no information regarding how many patients this related to, nor whether the patients who were excluded were different to those who completed. The study reported the results of 24 patients in the ciprofloxacin group and 20 patients in the gentamicin group It is unclear if there were dropouts from the intervention arm. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no trial protocol was identified on clinicaltrials.gov or the European Clinical Trials registry. All of the outcomes listed in the methods section of the report are also presented in the results section. Standard deviations are not provided for continuous outcomes. Insufficient information to judge. |
van Hasselt 1997.
| Methods | Three‐arm, non‐blinded, parallel‐group RCT, with 2‐week duration of treatment and 8‐week duration of follow‐up | |
| Participants |
Location: Malawi, rural (Nkota Kota District) Setting of recruitment and treatment: Nkota Kota District. Community setting. Conducted between 4 and 23 August 1997 Sample size: 96
Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention A (n = 12): ofloxacin 0.3% (Exocin) ear drops, 3 drops/8 hours. Duration of treatment = 2 weeks. Intervention B (n = 38): neomycin 0.5%/polymixin B 0.1%, ear drops, 3 drops/8 hours. Duration of treatment = 2 weeks. Comparator group (n = 46): acetic acid 2% in spirit 25% and glycerine 30%, ear drops, 3 drops/8 hours. Duration of treatment = 2 weeks. In all groups the participants were asked to keep the affected ear uppermost for 10 minutes after instillation. Concurrent treatment: for aural toileting: suction cleaning in all groups at the start of the trial and at the review appointments at 1 and 2 weeks after the start of the trial |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
|
|
| Funding sources | It is assumed the funding was from the Christian Blind Mission International | |
| Declarations of interest | No information provided | |
| Notes |
Unit of randomisation: unclear if randomised by patient or by ear. Most likely by person. Methods for reporting outcomes of patients with bilateral disease: counting bilateral ears separately. All ears reported separately. Data come from an unpublished report. In the analysis 3/11 (27.27%), 10/30 (33%) and 11/28 (39%) of patients had bilateral disease in the ofloxacin, neomycin and antiseptic acid groups respectively. The costs of treatment were DM 10.00 for ofloxacin ear drops, DM 0.60 for neomycin/polymyxin B ear drops and DM 0.25 for acetic acid/spirit drops |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: original report that this is a "pilot trial" with no reference to blinding or randomisation. This was mentioned as a "randomised" trial in a 2002 paper by the author. If randomisation was done, it is unclear whether the unit of randomisation was the child or the ears (most likely per person). There was no clear ratio of randomisation, with 46 in the acetic acid group, 38 in the neomycin/polymyxin group and 12 in the ofloxacin group, and the cheapest intervention had most participants. |
| Allocation concealment (selection bias) | Unclear risk | Comment: there is no mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: there is no mention of blinding. The same treatment regimen was used for each treatment group but the treatments would have been difficult to blind due to the differences in smell between the drops. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: as above, there is no mention of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: high overall dropout rate (27/96 = 28%). Unequally distributed between the treatment groups: 18 (39%), 8 (21%) and 1 (8%) did not complete the trial in the acetic acid/spirit, neomycin/polymyxin B and ofloxacin groups respectively |
| Selective reporting (reporting bias) | High risk | Quote: "The children of the present trial will be reviewed after 8 weeks. The results will be presented at the next PAFOS Conference (Pan‐African Federation of Otorhinolaryngological Societies) in Nairobi, 7‐10 June 1998" Comment: no information on the planned outcomes. The report suggested that the patients were followed up to 8 weeks, but the results of outcome could not be found. There is no protocol available on the WHO clinical trial registry. |
van Hasselt 1998a.
| Methods | Four‐arm, double‐blind, parallel‐group RCT, with 2‐week duration of treatment and 8‐week duration of follow‐up | |
| Participants |
Location: Malawi, rural, unclear number of sites Setting of recruitment and treatment: community‐based, study presented in 1998 Sample size: 107 children (151 ears)
Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention 1: neomycin/polymyxin‐B, ear drops, no information on concentration, 6 drops/7 days. Duration of treatment = 2 weeks. Intervention 2: neomycin/polymyxin‐B, ear drops, no information on concentration, 6 drops/12 hours. Duration of treatment = 2 weeks. Intervention 3: ofloxacin 0.3%, ear drops, 6 drops/7 days. Duration of treatment = 2 weeks. Intervention 4: ofloxacin 0.3%, ear drops, 6 drops/12 hours. Duration of treatment = 2 weeks. Concurrent treatment: weekly suction cleaning in all groups (no information about methods used). No other information. |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes |
Unit of randomisation: unclear Methods for reporting outcomes of patients with bilateral disease: counting bilateral ears separately |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomised" Comment: no information about sequence generation. A lack of baseline characteristics makes it difficult to determine whether there was bias due to the randomisation sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Double‐blind" Comment: authors state that the trial was double‐blind but the treatment regimens were not the same (once weekly versus twice daily) and there was no mention of placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Double‐blind" Comment: authors state that the trial was double‐blind but the treatment regimens were not the same (once weekly versus twice daily) and there was no mention of placebo used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the number of "defaulting ears" was 15% at 8 weeks, however it is not possible to determine whether the people that "defaulted" were evenly balanced across the different treatment groups. No reasons are provided for dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: the study was not published and was only reported as a conference presentation. It is not possible to evaluate the methods fully due to lack of information presented. No protocol was available on the WHO clinical trials registry. |
CSOM: chronic suppurative otitis media; F: female; HPMC: hydroxypropyl methyl‐cellulose; M: male; RCT: randomised controlled trial; WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbott 2016 | POPULATION: not CSOM (acute otitis media without perforation) |
| Alper 2000 | POPULATION: animal study (monkeys) |
| Baba 1980 | INTERVENTION: comparison of antibiotics within same class and spectrum of activity (cefroxadine versus cephalexin); cefroxadine is a withdrawn drug DURATION: only 6 days of follow‐up |
| Baba 1982b | POPULATION: acute suppurative otitis media, including acute otitis media |
| Baba 1983 | POPULATION: acute suppurative otitis media |
| Baba 1983b | POPULATION: acute suppurative otitis media |
| Baba 1986 | STUDY DESIGN: not a RCT (all patients received same treatment, aztreonam) |
| Baba 1987 | POPULATION: acute suppurative otitis media |
| Baba 2008 | STUDY DESIGN: not a RCT (all patients received the same intervention) |
| Berman 1990 | POPULATION: middle ear effusion, not CSOM |
| Blekher 1967 | INTERVENTION: not interventions of interest |
| Block 2000 | POPULATION: not CSOM (acute otitis media without perforation of tympanic membrane) |
| Bluestone 2001 | STUDY DESIGN: not a RCT (systematic review) |
| Boesorire 2000 | COMPARISON: steroids added onto topical antibiotics (see CSOM‐4) |
| Brook 1979 | STUDY DESIGN: not a RCT ‐ (alternative treatment) aminoglycosides only added when Gram‐negative organisms present in large numbers |
| Brook 1980 | STUDY DESIGN: not a RCT (all patients received the same intervention, additional intervention only added based on bacteriological findings) |
| Bross Soriano 1996 | POPULATION: AOM; patients with CSOM were excluded |
| Browning 1983 | INTERVENTION: standard antibiotics were not given, the choice was dependent on cultures |
| Browning 1988 | COMPARISON: variety of topical antibiotics + steroids (see CSOM‐4) |
| Clayton 1990 | POPULATION: less than 20% had otorrhoea with "central perforation"; others were patients with otitis externa and mastoid cavity problems |
| Connolly 1997 | INTERVENTION: compared method of administration, i.e. delivery system (spray versus drops) of neomycin‐dexamethasone |
| Couzos 2003 | COMPARISON: steroids added onto topical antibiotics (see CSOM‐4) |
| Crowther 1991 | COMPARISON: topical antibiotic + variety of steroids (see CSOM‐4) |
| Deguchi 1985 | STUDY DESIGN: not a RCT |
| Deguchi 1986 | STUDY DESIGN: not a RCT |
| Deitmer 2002 | STUDY DESIGN: not a RCT |
| Dellamonica 1995 | INTERVENTION: within‐class comparison (cephalosporin) |
| Dohar 2002 | STUDY DESIGN: not a RCT (short review) |
| Eason 1986 | COMPARISON: systemic antibiotics versus none (see CSOM‐2), antiseptics versus none (see CSOM‐5), aural toilet versus none (see CSOM‐7) |
| Esposito 1992 | COMPARISON: topical versus systemic antibiotics (see CSOM‐3) |
| Esposito 2000 | STUDY DESIGN: not a RCT (all patients had the same intervention ‐ ceftazidime) |
| Fliss 1990 | COMPARISON: variety of systemic antibiotics (see CSOM‐2) |
| Fombeur 1994 | STUDY DESIGN: not a RCT (no mention of randomisation); INTERVENTION: high‐dose versus low‐dose ciprofloxacin |
| Fraysse 1988 | INTERVENTION: fenspiride (a bronchodilator/anti‐inflammatory agent) is not an intervention under investigation |
| Garcia‐Rodriguez 1993 | POPULATION: mixture of patients, less than half had CSOM. Patients were not stratified by diagnosis |
| Gehanno 1997 | STUDY DESIGN: not a RCT (all patients had the same intervention) |
| Gendeh 2001 | COMPARISON: steroids added onto topical antibiotics (see CSOM‐4) |
| Ghosh 2012 | COMPARISON: variety of systemic antibiotics (see CSOM‐2) |
| Granath 2007 | POPULATION: not CSOM (patients with recurrent AOM with discharge through tympanostomy tube) |
| Gupta 2015 | COMPARISON: antibiotic versus antiseptic (see CSOM‐6) |
| Gyde 1981 | POPULATION: less than 50% (27/68) had CSOM |
| Gyde 1982 | POPULATION: less than 50% had CSOM |
| Harris 2016 | STUDY DESIGN: not a RCT (systematic review) |
| Helmi 2000 | COMPARISON: steroids added onto topical antibiotics (see CSOM‐4) |
| Hemlin 1997 | POPULATION: unilateral or bilateral secretory otitis media (COME); INTERVENTION: systemic corticosteroids |
| Hwang 2015 | STUDY DESIGN: not a RCT (case control study) |
| I‐HEAR‐BETA | COMPARISON: systemic antibiotic versus none (see CSOM‐2), topical antiseptic versus none (see CSOM‐5), topical antiseptic versus topical antibiotic (see CSOM‐6) |
| Indudharan 2005 | COMPARISON: steroids added onto topical antibiotics (see CSOM‐4) |
| ISRCTN12149720 | INTERVENTION: antimicrobial peptide OP145 |
| ISRCTN84220089 | INTERVENTION: antimicrobial peptide OP145 |
| ISRCTN86106121 | INTERVENTION: not an intervention of interest to the review (oral zinc sulphate) |
| Jahn 1984 | STUDY DESIGN: not a RCT |
| Jang 2004 | STUDY DESIGN: not a RCT (mentioned use of a "control group", no mention of randomisation) |
| Jaya 2003 | COMPARISON: topical antibiotic versus topical antiseptic (see CSOM‐6) |
| Jiang 2016 | INTERVENTION: comparison of 2 agents of the same class of antibiotics (erythromycin versus azithromycin) used in addition to a traditional Chinese medicine product |
| Kadar 2003 | STUDY DESIGN: not a RCT |
| Kashiwamura 2004 | STUDY DESIGN: cohort (no comparison group); POPULATION: less than 50% with CSOM |
| Kenna 1986 | STUDY DESIGN: not a RCT ‐ cohort study (no comparison group) |
| Khanna 2000 | INTERVENTION: culture sensitivity‐based prescribing |
| Khon 2012 | POPULATION: not CSOM ‐ either diffuse otitis externa or acute otitis externa; STUDY DESIGN: no evidence of randomisation |
| Kiris 1998 | COMAPRISON: daily aural toilet versus singular aural toilet (see CSOM‐7) |
| Kothari 1969 | STUDY DESIGN: not a RCT (no comparison) |
| Kovacic 1999 | STUDY DESIGN: not a RCT (compared ofloxacin in patients who had previous ear surgery versus no previous ear surgery) |
| Kurilin 1976 | STUDY DESIGN: not a RCT (no mention of randomised controlled study design or control group included for comparison) |
| Lancaster 1999 | STUDY DESIGN: not a RCT (cross‐sectional survey) |
| Lancaster 2003 | STUDY DESIGN: not a RCT (compared compliance) |
| Lang 1992 | STUDY DESIGN: not a RCT (case series) |
| Lautala 1983 | STUDY DESIGN: not a RCT (case series) |
| Lazo Saenz 1999 | COMPARISON: steroids added onto topical antibiotics (see CSOM‐4) |
| Leach 2008 | COMPARISON: steroids added onto topical antibiotics (see CSOM‐4) |
| Legent 1994 | COMPARSION: variety of systemic antibiotics (see CSOM‐2) |
| Li 2004 | INTERVENTION: not an intervention of interest to the review (self‐prepared Chinese herbal medicine ear drops) |
| Loock 2012 | COMPARISON: variety of topical antiseptics (see CSOM‐5), topical antibiotic versus topical antiseptic (see CSOM‐6) |
| Lorentzen 1978 | POPULATION: AOM with intact or spontaneously erupted tympanic membrane; INTERVENTION: surgery |
| Macfadyen 2005 | COMPARISON: topical antibiotic versus topical antiseptic (see CSOM‐6) |
| Manolidis 2004 | STUDY DESIGN: not a RCT (review) |
| Mendelman 1992 | POPULATION: acute suppurative otitis media (symptoms of 7 days or less) |
| Merifield 1993 | STUDY DESIGN: not a RCT (case series) |
| Mesure 1973 | POPULATION: in clinical trial part of study (part 2) only 1 case of chronic otitis media |
| Minja 2006 | COMPARISON: systemic antibiotic versus none (see CSOM‐2) and topical antiseptic versus none (see CSOM‐5) |
| Miro 2000 | COMPARISON: steroids added onto topical antibiotics (see CSOM‐4) |
| Mora 2012 | INTERVENTION: polyvalent bacterial lysate (antigens) |
| Morgon 1976 | STUDY DESIGN: single‐arm study |
| NCT02592096 | INTERVENTION: phase I dose finding trial ‐ compared different concentrations of pazufloxacin |
| NCT02817347 | INTERVENTION: phase II trial ‐ compared different concentrations of piperacillin against tazobactam plus dexamethasone |
| Nwokoye 2015 | COMPARISON: variety of systemic antibiotics (see CSOM‐2) |
| Onali 2018 | COMPARISON: systemic antibiotic versus none (see CSOM‐2) |
| Otwombe 2003 | STUDY DESIGN: systematic review, not a RCT |
| Panchasara 2015 | COMPARISON: steroids added onto topical antibiotics (see CSOM‐4) |
| Papastavros 1989 | COMPARISON: topical antiseptic versus none (see CSOM‐5) |
| Picozzi 1983 | COMPARISON: topical antibiotic + steroid versus none (see CSOM‐4) |
| Picozzi 1984 | COMPARISON: systemic metronidazole versus placebo in people who already had gentamicin plus hydrocortisone ear drops (see CSOM‐2) |
| Poliakova 1991 | STUDY DESIGN: not a RCT |
| Povedano 1995 | COMPARISON: systemic versus topical antibiotics (see CSOM‐3) |
| Principi 1995 | POPULATION: acute and recurrent otitis media |
| Quick 1973 | POPULATION: not CSOM (included acute tonsillitis, acute pharyngitis, acute sinusitis, acute otitis media, chronic sinusitis and peritonsillar) |
| Quick 1975 | POPULATION: not CSOM (only 6/145 patients had otitis media) |
| Renukananda 2014 | COMPARISON: systemic antibiotics added onto topical antibiotics (see CSOM‐2) |
| Rotimi 1990 | COMPARISON: variety of systemic antibiotics (see CSOM‐2) |
| Saez‐Llorens 2005 | POPULATION: AOM |
| Sanchez Gonzales 2001 | COMPARISON: variety of systemic antibiotics (see CSOM‐2) |
| Shkil' 1964 | INTERVENTION: no comparison of interest (antiseptic arms used a number of different agents ‐ unclear which) |
| Singhal 1992 | STUDY DESIGN: not a RCT (no comparison group) |
| Smith 1996 | COMPARISON: aural toilet versus no treatment (see CSOM‐7) |
| Somekh 2000 | COMPARISON: variety of systemic antibiotics (see CSOM‐2) |
| Stenstrom 1991 | POPULATION: acute otitis media, not CSOM |
| Subramaniam 2001 | COMPARISON: steroids added onto topical antibiotics (see CSOM‐4) |
| Sultan 2017 | STUDY DESIGN: not a RCT ‐ single intervention (oral levofloxacin) studied |
| Sumitsawan 1995 | STUDY DESIGN: not a RCT ‐ single intervention (ofloxacin drops) studied |
| Supiyaphun 1995 | STUDY DESIGN: not a RCT (cohort ‐ all patients received same treatment) |
| Tachibana 1986 | STUDY DESIGN: not a RCT (all patients received same treatment) |
| Thomsen 1976 | STUDY DESIGN: not a RCT; POPULATION: acute suppurative otitis media |
| Tong 1996 | COMPARISON: steroids added onto topical antibiotics (see CSOM‐4) |
| van der Veen 2007 | COMPARISON: systemic antibiotics versus none (see CSOM‐2) |
| van Dongen 2014 | POPULATION: 1) inclusion of minimum 2 weeks (review defined exclusion of 6 weeks perioperatively); 2) max duration of otorrhoea was 1 week |
| van Hasselt 1998b | COMPARISON: povidone iodine added in hydroxypropyl methylcellulose ear drops (see CSOM‐5) |
| Vishwakarma 2015 | COMPARISON: topical antiseptic versus topical antibiotic (see CSOM‐6) |
| Wilde 1995 | INTERVENTION: different ways of administering the same ear drops (ribbon gauze versus drops) |
| Wintermeyer 1997 | STUDY DESIGN: not a RCT (cohort) |
| Wright 2009 | STUDY DESIGN: not a RCT (review) |
| Xu 1999 | INTERVENTION: not a comparison of interest ‐ antibiotics versus Chinese medicine |
| Yuen 1994 | COMPARISON: systemic versus topical antibiotics (see CSOM‐3) |
AOM: acute otitis media; CSOM: chronic suppurative otitis media; RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Abdul 2005.
| Methods | Unclear, "comparative study" |
| Participants | Active chronic suppurative otitis media |
| Interventions | Local ciprofloxacin versus aluminium acetate 3.5% versus no treatment |
| Outcomes | Unclear |
| Notes | Unable to locate paper. It is not clear if there was a control arm from the title of the paper. |
Abes 1998.
| Methods | Randomised controlled clinical trial; single‐blinded |
| Participants | Patients 9 to 54 years old with CSOM (3 weeks) |
| Interventions | Experimental intervention: ofloxacin 0.3% otic solution, 5 drops twice daily for 2 to 4 weeks Control intervention: polymixin otic solution, 3 to 5 drops to the affected ear 3 times daily for 2 to 4 weeks |
| Outcomes | Cure rate; resolution of ear discharge; bacterial eradication rate; adverse drug event |
| Notes | Concealment of allocation is not clear Data from Abes 2003 |
CSOM: chronic suppurative otitis media
Contributions of authors
Christopher G Brennan‐Jones: clinical guidance at all stages of the review; reviewed the analyses; wrote, reviewed and edited the text of the review.
Lee Yee Chong: scoped the review, designed and wrote the protocol. Screened the search results and selected studies, carried out data extraction, 'Risk of bias' assessment and statistical analyses, reviewed and edited the text of the review.
Karen Head: scoped the review, designed and wrote the protocol. Screened the search results and selected studies, carried out data extraction, 'Risk of bias' assessment and statistical analyses, wrote the text of the review.
Martin J Burton: clinical guidance at all stages of the review; reviewed the analyses and reviewed and edited the text of the review. Wrote the abstract for the review.
Anne GM Schilder: clinical guidance at all stages of the review; reviewed the analyses and reviewed and edited the text of the review.
Mahmood F Bhutta: helped to scope, design and write the protocol; reviewed the analyses of results and provided clinical guidance at all stages of the review. Reviewed and edited the text of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
Infrastructure funding for Cochrane ENT
NHMRC Centre of Research Excellence in Ear and Hearing Health of Aboriginal and Torres Strait Islander Children, Australia.
-
Department of Health, Western Australia, Australia.
Infrastructure funding
-
NHMRC Fellowship for CG Brennan‐Jones #1142897, Australia.
Personnel support
-
WA Department of Health, Australia.
Future Health Merit Award
Declarations of interest
Christopher G Brennan‐Jones: Dr Brennan‐Jones's research team is primarily funded by the Australian NHMRC and the WA Department of Health. He sits on the national Technical Advisory Group responsible for developing treatment guidelines for otitis media in Australia.
Karen Head: none known.
Lee Yee Chong: none known.
Martin J Burton: Professor Martin Burton is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review.
Anne GM Schilder: Professor Anne Schilder is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review. Her evidENT team at UCL is supported in part by the National Institute of Health Research (NIHR) University College London Hospitals Biomedical Research Centre. The research is funded by the NIHR and EU Horizon2020. She is the national chair of the NIHR Clinical Research Network ENT Specialty. She is the Surgical Specialty Lead for ENT for the Royal College of Surgeons of England's Clinical Trials Initiative. In her role as director of the NIHR UCLH BRC Deafness and Hearing Problems Theme, she acts as an advisor on clinical trial design and delivery to a range of biotech companies, most currently Novus Therapeutics.
Mahmood F Bhutta: Dr Mahmood Bhutta has received an honorarium from Novus Therapeutics for advice on an experimental treatment for otitis media (not related to any treatment in this review).
New
References
References to studies included in this review
Asmatullah 2014 {published data only}
- Asmatullah, Khan Q, Nawaz G, Ullah G, Iqbal J, Khan M, et al. Comparison of efficacy of topical ofloxacin and gentamycin in tubotympanic type of chronic suppurative otitis media. Medical Forum Monthly 2014;25(12):68‐71. [CENTRAL: CN‐01084518; CRS: 1815087; EMBASE: 2015070065] [Google Scholar]
de Miguel 1999 {published data only (unpublished sought but not used)}
- Miguel Martinez I, Vasallo Morillas JR, Ramos Macias A. Antimicrobial therapy in chronic suppurative otitis media [Terapeutica antimicrobiana en otitis media cronica supurada]. Acta Otorrinolaringologica Espanola 1999;50(1):15‐9. [CENTRAL: CN‐00161091; CRS: 1091988; PUBMED: 10091344] [PubMed] [Google Scholar]
Esposito 1990 {published data only}
- Esposito S, D'Errico G, Montanaro C. Topical and oral treatment of chronic otitis media with ciprofloxacin. A preliminary study. Archives of Otolaryngology‐Head & Neck Surgery 1990;116(5):557‐9. [CENTRAL: CN‐00067110; CRS: 1002139; PUBMED: 2328112] [DOI] [PubMed] [Google Scholar]
Fradis 1997 {published data only}
- Fradis M, Brodsky A, Ben‐David J, Srugo I, Larboni J, Podoshin L. Chronic otitis media treated topically with ciprofloxacin or tobramycin. Archives of Otolaryngology‐‐Head & Neck Surgery 1997;123(10):1057‐60. [CENTRAL: CN‐00144425; CRS: 1078973; PUBMED: 9339980] [DOI] [PubMed] [Google Scholar]
- Podoshin L, Brodzki A, Fradis M, Ben‐David J, Larboni J, Srugo I. Local treatment of purulent chronic otitis media with ciprofloxacin. Harefuah 1998;134(1):32‐6, 78. [CENTRAL: CN‐00682735; CRS: 1487878; PUBMED: 9517277] [PubMed] [Google Scholar]
Gyde 1978 {published data only}
- Gyde MC, Randall RF. Double‐blind comparative study of trimethroprim‐sulphacetamide‐polymyxin B and gentamicin in the treatment of otorrhoea [Etude comparative a double insu de la trimethorprime‐sulfacetamide=polymyxine B et de la gentamicine dans le traitement de l'otorrhee]. Annales d'Oto‐laryngologie et de Chirurgie Cervico Faciale 1978;95(1‐2):43‐55. [CENTRAL: CN‐00018235; CRS: 953322; PUBMED: 207210] [PubMed] [Google Scholar]
Jamalullah 2016 {published data only}
- Jamalullah M, Babat M, Choudhury IM. Comparison of efficacy of topical gentamycin 0.3% with topical ciprofloxacin 0.6% in patients with active tubotympanic type of chronic suppurative otitis media. Isra Medical Journal 2016;8(1):14‐8. [CENTRAL: CN‐01601161; CRS: 8519228] [Google Scholar]
Kasemsuwan 1997 {published data only}
- Kasemsuwan L, Clongsuesuek P. A double blind, prospective trial of topical ciprofloxacin versus normal saline solution in the treatment of otorrhoea. Clinical Otolaryngology and Allied Sciences 1997;22(1):44‐6. [CENTRAL: CN‐00138220; CRS: 1072793; PUBMED: 9088679] [DOI] [PubMed] [Google Scholar]
Kaygusuz 2002 {published data only}
- Kaygusuz I, Karlidag T, Gok U, Yalcin S, Keles E, Demirbag E, et al. Efficacy of topical ciprofloxacin and tobramycin in combination with dexamethasone in the treatment of chronic suppurative otitis media [Kronik supuratif otitis media tedavisinde topikal siprofloksasin ve tobramisinin deksametazon ile kullanimi]. Kulak Burun Bogaz Ihtisas Dergisi: KBB [Journal of Ear, Nose, and Throat] 2002;9(2):106‐11. [CENTRAL: CN‐00397704; CRS: 1259444; PUBMED: 12122630] [PubMed] [Google Scholar]
Liu 2003 {published data only}
- Liu J. The curative effect of rifampicin solution in the treatment of chronic suppurative otitis media. Journal of Preclinical Medicine College of Shangdong University 2003;17(1):8‐9. [CENTRAL: CN‐00475923; CRS: 1324675] [Google Scholar]
Lorente 1995 {published data only}
- Lorente J, Sabater F, Maristany M, Jimenez R, Menem J, Vinas J, et al. Multicenter study comparing the efficacy and tolerance of topical ciprofloxacin (0.3%) versus topical gentamicin (0.3%) in the treatment of simple, non‐cholesteatomatous chronic otitis media in the suppurative phase [Estudio multicentrico comparativo de la eficacia y tolerancia de ciprofloxacino topico (0.3%) versus gentamicina topica (0.3%) en el tratamiento de la otitis media cronica simple no colesteatomatosa en fase supurativa]. Anales Otorrinolaringologicos Ibero‐americanos 1995;22(5):521‐33. [CENTRAL: CN‐00120014; CRS: 1054615; PUBMED: 7485860] [PubMed] [Google Scholar]
- Sabater F, Maristany M, Mensa J, Villar E, Traserra J. Prospective double‐blind randomized study of the efficacy and tolerance of topical ciprofloxacin vs topical gentamicin in the treatment of simple chronic otitis media and diffuse external otitis [Estudio prospectivo doble‐ciego randomizado de la eficacia y tolerancia de ciprofloxacino topico versus gentamicina topica en el tratamiento de la otitis media cronica supurada simple y de la otitis externa difusa]. Acta Otorrinolaringologica Espanola 1996;47(3):217‐20. [CENTRAL: CN‐00129550; CRS: 1064142; PUBMED: 8924287] [PubMed] [Google Scholar]
Mira 1993 {published data only}
- Mira E, Benazzo M, Mira E, Benazzo M. Ceftizoxime as local therapy in the treatment of recurrences of chronic suppurative otitis media. Journal of Drug Development Supplement 1993;6(Suppl 2):39‐44. [CENTRAL: CN‐00362597; CRS: 1231417] [Google Scholar]
- Mira E, Benazzo M, Mira E, Benazzo M. Clinical evaluation of ceftizoxime (EposerinR) as local therapy in the treatment of recurrences of chronic suppurative otitis media [Uso topico delle cefalosporine nel trattamento delle otiti medie purulente: valutazione della ceftizoxima (eposerin R)]. Rivista Italiana di Otorinolaringologia Audiologia e Foniatria 1992;12(4):219‐25. [CENTRAL: CN‐00624623; CRS: 1442776] [Google Scholar]
Nawasreh 2001 {published data only}
- Nawasreh O, Fraihat A. Topical ciprofloxacin versus topical gentamicin for chronic otitis media. La Revue de Sante de la Mediterranee Orientale/Al‐Majallah Al‐sihhiyah Li‐sharq Al‐mutawassit [Eastern Mediterranean Health Journal] 2001;7(1‐2):26‐30. [CENTRAL: CN‐00413339; CRS: 1273001; PUBMED: 12596948] [PubMed] [Google Scholar]
Ramos 2003 {published data only}
- Ramos A, Ayudarte F, Miguel I, Cuyas JM, Cenjor C. Use of topical ciprofloxacin in chronic suppurating otitis media [Utilizacion del ciprofloxacino topico en la otitis media cronica supurada]. Acta Otorrinolaringologica Espanola 2003;54(7):485‐90. [CENTRAL: CN‐00614808; CRS: 1435687; PUBMED: 14671920] [DOI] [PubMed] [Google Scholar]
Siddique 2016 {published data only}
- Siddique W, Hakeem A, Ashfaq K, Khan M, Gul AA. Comparison between the efficacy of topical ciprofloxacin with neomycin in the management of chronic suppurative otitis media. Pakistan Armed Forces Medical Journal 2016;66(2):235‐9. [CENTRAL: CN‐01601188; CRS: 8527048] [Google Scholar]
Tutkun 1995 {published data only}
- Ozagar A, Koc A, Ciprut A, Tutkun A, Akdas F, Sehitoglu MA. Effects of topical otic preparations on hearing in chronic otitis media. Otolaryngology ‐ Head & Neck Surgery 1997;117(4):405‐8. [CENTRAL: CN‐00144419; CRS: 1078967; PUBMED: 9339804] [DOI] [PubMed] [Google Scholar]
- Tutkun A, Ozagar A, Koc A, Batman C, Uneri C, Sehitoglu MA. Treatment of chronic ear disease. Topical ciprofloxacin vs topical gentamicin. Archives of Otolaryngology‐Head & Neck Surgery 1995;121(12):1414‐6. [CENTRAL: CN‐00121174; CRS: 1055775; PUBMED: 7488373] [DOI] [PubMed] [Google Scholar]
van Hasselt 1997 {published data only}
- Hasselt P, Hasselt P. Pilot trial of treatment of chronic suppurative otitis media (CSOM) with several types of ear drops in Nkota Kota District, Malawi. Internal Report of the Christian Blind Mission International. Bensheim (DE), 1997. [CENTRAL: CN‐00519675; CRS: 1362364]
- Hasselt P, Kregten E. Treatment of chronic suppurative otitis media with ofloxacin in hydroxypropyl methylcellulose ear drops: a clinical/bacteriological study in a rural area of Malawi. International Journal of Pediatric Otorhinolaryngology 2002;63(1):49‐56. [CENTRAL: CN‐00519676; CRS: 1362365] [DOI] [PubMed] [Google Scholar]
van Hasselt 1998a {published data only}
- Hasselt P. A controlled trial of the treatment of CSOM in rural Malawi. Conference of the Pan‐African Federation of Otorhinolaryngological Societies (PAFOS); Nairobi. 1998. [CENTRAL: CN‐00519673; CRS: 1362362]
- Hasselt P, Kregten E. Treatment of chronic suppurative otitis media with ofloxacin in hydroxypropyl methylcellulose ear drops: a clinical/bacteriological study in a rural area of Malawi. International Journal of Pediatric Otorhinolaryngology 2002;63(1):49‐56. [CENTRAL: CN‐00519676; CRS: 1362365] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abbott 2016 {published data only}
- Abbott P, Gunasekera H, Leach AJ, Askew D, Walsh R, Kong K, et al. A multi‐centre open‐label randomised noninferiority trial comparing watchful waiting to antibiotic treatment for acute otitis media without perforation in low‐risk urban Aboriginal and Torres Strait Islander children (the WATCH trial): study protocol for a randomised controlled trial. Trials 2016;17(1):119. [CENTRAL: CN‐01297248; CRS: 5185798; EMBASE: 613896996] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alper 2000 {published data only}
- Alper CM, Dohar JE, Gulhan M, Ozunlu A, Bagger‐Sjobak D, Hebda PA, et al. Treatment of chronic suppurative otitis media with topical tobramycin and dexamethasone. Archives of Otolaryngology‐‐Head & Neck Surgery 2000;126(2):165‐73. [CRS: 1231391] [DOI] [PubMed] [Google Scholar]
Baba 1980 {published data only}
- Baba S, Hondo J, Wada K. Evaluation of cefroxadine (CGP‐9000) in acute tonsillitis, acute suppurative otitis media and acute exacerbation of chronic otitis media. A comparative double blind study with cephalexin. Japanese Journal of Chemotherapy 1980;28(Suppl 3):590‐618. [CENTRAL: CN‐00333804; CRS: 1209073; EMBASE: 1981031803] [Google Scholar]
Baba 1982b {published data only}
- Baba S, Ito H, Kinoshita H. A well controlled comparative study on cefmetazole and cefazolin in the treatment of suppurative otitis media. Japanese Journal of Antibiotics 1982;35(6):1523‐52. [CRS: 7773666] [PubMed] [Google Scholar]
Baba 1983 {published data only}
- Baba S, Murai K, Kinoshita H. Evaluation of BRL25000 (clavulanic acid‐amoxicillin) in acute purulent otitis media and acute exacerbation of chronic purulent otitis media. A comparative double blind study with amoxicillin. Chemotherapy 1983;31(Suppl 2):97‐112. [CENTRAL: CN‐00174613; CRS: 1102889; EMBASE: 1983209351] [Google Scholar]
Baba 1983b {published data only}
- Baba S, Murai K, Kinoshita H. The double‐blind trial of cefroxadine and cephalexin in the treatment of acute suppurative otitis media and acute exacerbation of chronic suppurative otitis media. Japanese Journal of Antibiotics 1983;36(9):2595‐634. [CENTRAL: CN‐00334158; CRS: 1209415; EMBASE: 1984002024] [PubMed] [Google Scholar]
Baba 1986 {published data only}
- Baba S, Kinoshita H, Mori Y, Suzuki K, Furuuchi C, Baba K, et al. Efficacy evaluation of aztreonam for suppurative otitis media. Japanese Journal of Antibiotics 1986;39(1):159‐76. [CRS: 7773668] [PubMed] [Google Scholar]
Baba 1987 {published data only}
- Baba S, Kinoshita H, Mori Y, Suzuki K, Shimada J, Kawamura S, et al. A parallel comparative double blind study of cefixime with cefaclor in the treatment of acute suppurative otitis media in children. Japanese Journal of Antibiotics 1987;40(1):1‐24. [CENTRAL: CN‐00048250; CRS: 983303; PUBMED: 3295321] [PubMed] [Google Scholar]
Baba 2008 {published data only}
- Baba S, Suzuki K, Yamanaka N, Yamashita H, Kurono Y, Hori S. Efficacy and safety of sitafloxacin in patients with otorhinolaryngological infections and its tissue distribution in the otorhinolaryngological field. Japanese Journal of Chemotherapy 2008;56(Suppl 1):110‐20. [CRS: 7773673] [Google Scholar]
Berman 1990 {published data only}
- Berman S, Grose K, Nuss R, Huber‐Navin C, Roark R, Gabbard SA, et al. Management of chronic middle ear effusion with prednisone combined with trimethoprim‐sulfamethoxazole. Pediatric Infectious Disease Journal 1990;9(8):533‐8. [CENTRAL: CN‐00071176; CRS: 1006118; PUBMED: 2235167] [DOI] [PubMed] [Google Scholar]
Blekher 1967 {published data only}
- Blekher LB. Methods of treatment of chronic suppurative otitis [K metodike lecheniia khronicheskikh gnoinykh otitov]. Zhurnal Ushnykh, Nosovykh I Gorlovykh Boleznei [Journal of Otology, Rhinology, and Laryngologie [sic]] 1967;27(1):87‐90. [CENTRAL: CN‐00404420; CRS: 1265353] [PubMed] [Google Scholar]
Block 2000 {published data only}
- Block SL, McCarty JM, Hedrick JA, Nemeth MA, Keyserling CH, Tack KJ. Comparative safety and efficacy of cefdinir vs amoxicillin/clavulanate for treatment of suppurative acute otitis media in children. Pediatric Infectious Disease Journal 2000;19(12 Suppl):S159‐65. [CENTRAL: CN‐00327731; CRS: 1203110; EMBASE: 2001001390; PUBMED: 11144398] [DOI] [PubMed] [Google Scholar]
Bluestone 2001 {published data only}
- Bluestone CD. Efficacy of ofloxacin and other ototopical preparations for chronic suppurative otitis media in children. Pediatric Infectious Disease Journal 2001;20(1):111‐5. [CRS: 7211634] [DOI] [PubMed] [Google Scholar]
Boesorire 2000 {published data only}
- Boesoirie T. A comparative study between ofloxacin ear drop and neomycin‐polymixin b‐hydrocortisone ear drops on the chronic suppurative otitis media. 9th ASEAN ORL Head and Neck Congress 31 March‐1 April, 2001. Singapore, 2001.
- Boesoirie T. A comparative study between ofloxacin ear drops and neomycin‐polymixin b‐hydrocortisone ear drops on the chronic suppurative otitis media. Department of ENT Head and Neck Surgery, University of Padjajaran, Bandung, Indonesia Unpublished, 2000.
Brook 1979 {published data only}
- Brook I. Bacteriology and treatment of chronic otitis media. Laryngoscope 1979;89(7 Pt 1):1129‐34. [CRS: 6794900; PUBMED: 449555] [PubMed] [Google Scholar]
Brook 1980 {published data only}
- Brook I. Clindamycin in treatment of chronic recurrent suppurative otitis media in children. Journal of Laryngology and Otology 1980;94(6):607‐15. [CRS: 7773768] [DOI] [PubMed] [Google Scholar]
Bross Soriano 1996 {published data only}
- Bross Soriano D, Arrieta Gomez JR, Corvera Behar G, Ledon Perez LE. Comparison of the efficacy and safety of ceftibuten and cefaclor in the treatment of acute otitis medica and of the exacerbation of chronica otitis media in children [Comparacion de la eficacia y seguridad del ceftibuten y el cefaclor en el tratamiento de la otitis media aguda y la otitis media cronica agudizada en ninos]. Anales de Otorrinolaringologia Mexicana: Anales de la Sociedad Mexicana de Otorrinolaringologia 1996;41(1):18‐23. [CENTRAL: CN‐01050653; CRS: 1781238] [Google Scholar]
Browning 1983 {published data only}
- Browning GG, Picozzi GL, Calder IT, Sweeney G. Controlled trial of medical treatment of active chronic otitis media. British Medical Journal (Clinical Research Ed.) 1983;287(6398):1024. [CENTRAL: CN‐00032195; CRS: 967258; PUBMED: 6412934] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picozzi CL, Calder I, Browning GG, Sweeney G. Controlled trial of medical treatment of active chronic otitis media. Clinical Otolaryngology and Allied Sciences 1982;7:137‐8. [CENTRAL: CN‐00262047; CRS: 1161168] [Google Scholar]
Browning 1988 {published data only}
- Browning GG, Gatehouse S, Calder IT. Medical management of active chronic otitis media: a controlled study. Journal of Laryngology and Otology 1988;102(6):491‐5. [CENTRAL: CN‐00054939; CRS: 989984; PUBMED: 3294318] [DOI] [PubMed] [Google Scholar]
Clayton 1990 {published data only}
- Clayton MI, Osborne JE, Rutherford D, Rivron RP. A double‐blind, randomized, prospective trial of a topical antiseptic versus a topical antibiotic in the treatment of otorrhoea. Clinical Otolaryngology and Allied Sciences 1990;15(1):7‐10. [CENTRAL: CN‐00066816; CRS: 1001848; PUBMED: 2323085] [DOI] [PubMed] [Google Scholar]
Connolly 1997 {published data only}
- Connolly AA, Picozzi GL, Browning GG. Randomized trial of neomycin/dexamethasone spray vs drop preparation for the treatment of active chronic mucosal otitis media. Clinical Otolaryngology and Allied Sciences 1997;22(6):529‐31. [CENTRAL: CN‐00147553; CRS: 1082075; PUBMED: 9466064] [DOI] [PubMed] [Google Scholar]
Couzos 2003 {published data only}
- Couzos S. The Naccho Ear Trial ‐ a community‐based RCT to improve chronic suppurative otitis media affecting Aboriginal children. 2005 International Meeting of Australian Society of Paediatric Oto‐rhino‐laryngology; 2005 Jul 11‐13; Denarau Island (Fiji). Denarau Island (Fiji), 2005. [CENTRAL: CN‐00614782; CRS: 1435671]
- Couzos S, Lea T, Mueller R, Coates H. A community‐based, multi‐centre, double‐blind randomized controlled trial comparing the effectiveness of topical ciprofloxacin and Sofradex as treatment for chronic suppurative otitis media (CSOM) in Aboriginal children. 8th International Symposium on Recent Advances in Otitis Media; 2003 Jun 3‐7; Ft. Lauderdale (FL). 2003. [CENTRAL: CN‐00449257; CRS: 1302426]
- Couzos S, Lea T, Mueller R, Murray R, Culbong M. Effectiveness of ototopical antibiotics for chronic suppurative otitis media in Aboriginal children: a community‐based, multicentre, double‐blind randomised controlled trial. Medical Journal of Australia 2003;179(4):185‐90. [CENTRAL: CN‐00439955; CRS: 1294300; EMBASE: 37038200; PUBMED: 12914507] [DOI] [PubMed] [Google Scholar]
- Couzos S, Lea T, Murray R, Culbong M. 'We are not just participants‐we are in charge': the NACCHO ear trial and the process for Aboriginal community‐controlled health research. Ethnicity & Health 2005;10(2):91‐111. [CENTRAL: CN‐00512515; CRS: 1355899; EMBASE: 2005‐04153‐001; PUBMED: 15804658] [DOI] [PubMed] [Google Scholar]
- Dugdale AE. Management of chronic suppurative otitis media. Medical Journal of Australia 2004;180(2):91. [CRS: 7773922] [DOI] [PubMed] [Google Scholar]
Crowther 1991 {published data only}
- Crowther JA, Simpson D. Medical treatment of chronic otitis media: steroid or antibiotic with steroid ear‐drops?. Clinical Otolaryngology and Allied Sciences 1991;16(2):142‐4. [CENTRAL: CN‐00076715; CRS: 1011630; PUBMED: 2070529] [DOI] [PubMed] [Google Scholar]
Deguchi 1985 {published data only}
- Deguchi K, Fukayama S, Nishimura Y, Nishike A, Oda S, Sato S, et al. Study of clinical bacteriological efficacy of cefmenoxime ototopical solution. Japanese Journal of Antibiotics 1985;38(7):1739‐49. [CENTRAL: CN‐00357486; CRS: 1227057; EMBASE: 1986002477] [PubMed] [Google Scholar]
Deguchi 1986 {published data only}
- Deguchi K, Yokota N, Tanaka S, Fukayama S, Nishimura Y, Yoshihara H, et al. A clinical bacteriological efficacy study on a fosfomycin otic solution. Japanese Journal of Antibiotics 1986;39(9):2344‐54. [CENTRAL: CN‐00045975; CRS: 981029; PUBMED: 3099028] [PubMed] [Google Scholar]
Deitmer 2002 {published data only}
- Deitmer T. Topical and systemic treatment for chronic suppurative otitis media. Ear, Nose, & Throat Journal 2002;81(8 Suppl 1):16‐7. [CRS: 7773900] [PubMed] [Google Scholar]
Dellamonica 1995 {published data only}
- Dellamonica P, Choutet P, Lejeune JM, Lucht F, Morgon A, Pessey JJ, et al. Efficacy and safety of cefotiam hexetil in the treatment of chronic otitis media. A comparative double blind randomized study versus cefuroxime axetil [Efficacite et tolerance du cefotiam hexetil dans le traitement des otites moyennes chroniques. etude randomisee, en double aveugle, comparaison au cefuroxime axetil]. Medecine et Maladies Infectieuses 1995;25(5):733‐9. [CENTRAL: CN‐00169813; CRS: 1100109; EMBASE: 1995207381] [Google Scholar]
Dohar 2002 {published data only}
- Dohar JE. Topical quinolones in the treatment of chronic suppurative otitis media and recurrent otorrhea. Ear, Nose, & Throat Journal 2002;81(8 Suppl 1):20. [CRS: 5716094] [PubMed] [Google Scholar]
Eason 1986 {published data only}
- Eason RJ, Harding E, Nicholson R, Nicholson D, Pada J, Gathercole J. Chronic suppurative otitis media in the Solomon Islands: a prospective, microbiological, audiometric and therapeutic survey. New Zealand Medical Journal 1986;99:812‐5. [CENTRAL: CN‐00045614; CRS: 980668; PUBMED: 3466089] [PubMed] [Google Scholar]
Esposito 1992 {published data only}
- Esposito S, Noviello S, D'Errico G, Montanaro C. Topical ciprofloxacin vs intramuscular gentamicin for chronic otitis media. Archives of Otolaryngology‐Head & Neck Surgery 1992;118(8):842‐4. [CENTRAL: CN‐00086057; CRS: 1020830; PUBMED: 1642836] [DOI] [PubMed] [Google Scholar]
Esposito 2000 {published data only}
- Esposito S, Noviello S, Ianniello F, D'Errico G. Ceftazidime for outpatient parenteral antibiotic therapy (OPAT) of chronic suppurative otitis media due to Pseudomonas aeruginosa. Journal of Chemotherapy (Florence, Italy) 2000;12(1):88‐93. [CRS: 7773949] [DOI] [PubMed] [Google Scholar]
Fliss 1990 {published data only}
- Fliss DM, Dagan R, Houri Z, Leiberman A. Medical management of chronic suppurative otitis media without cholesteatoma in children. Journal of Pediatrics 1990;116(6):991‐6. [CENTRAL: CN‐00067997; CRS: 1003023; PUBMED: 2189979] [DOI] [PubMed] [Google Scholar]
- Leiberman A, Fliss DM, Dagan R. Medical treatment of chronic suppurative otitis media without cholesteatoma in children‐a two‐year follow‐up. International Journal of Pediatric Otorhinolaryngology 1992;24(1):25‐33. [CRS: 7072188; PUBMED: 1399301] [DOI] [PubMed] [Google Scholar]
Fombeur 1994 {published data only}
- Fombeur JP, Barrault S, Koubbi G, Laurier JN, Ebbo D, Lecomte F, et al. Study of the efficacy and safety of ciprofloxacin in the treatment of chronic otitis. Chemotherapy 1994;40 Suppl 1:29‐34. [CENTRAL: CN‐00108584; CRS: 1043206; PUBMED: 7805428] [DOI] [PubMed] [Google Scholar]
Fraysse 1988 {published data only}
- Fraysse B, Calvet H, Faure P, Schutz D. Value of fenspiride (Pneumorel (R) 80 mg) in the preoperative treatment of chronic open tympanum otitis. Double‐blind placebo‐controlled study. Rhinology 1988;26(Suppl 4):31‐41. [CENTRAL: CN‐00187914; CRS: 1114954; EMBASE: 1989095354] [PubMed] [Google Scholar]
Garcia‐Rodriguez 1993 {published data only}
- Garcia Rodriguez JA, Garcia Sanchez JE, Garcia Garcia MI, Garcia Sanchez E, Munoz Bellido JL, Ramos Macias A. Efficacy of topical ciprofloxacin in the treatment of ear infections in adults. Journal of Antimicrobial Chemotherapy 1993;31(3):452‐3. [CENTRAL: CN‐00092937; CRS: 1027589; PUBMED: 8486586] [DOI] [PubMed] [Google Scholar]
Gehanno 1997 {published data only}
- Gehanno P. Multicenter study of the efficacy and safety of oral ciprofloxacin in the treatment of chronic suppurative otitis media in adults. The French Study Group. Otolaryngology ‐ Head and Neck Surgery 1997;117(1):83‐90. [CENTRAL: CN‐00141764; CRS: 1076322; PUBMED: 9230329] [DOI] [PubMed] [Google Scholar]
Gendeh 2001 {published data only}
- Gendeh S. A comparative study of ofloxacin otic drops vs framycetin sulfate‐dexamethasone‐gramicidin otic drops in the medical treatment of otitis externa and chronic suppurative otitis media. 9th ASEAN ORL Head and Neck Congress, 31 March‐1 April, 2001. Singapore, 2001.
- Gendeh S. A comparative study of ofloxacin otic drops vs. framycetin sulfate‐dexamethasone‐gramicidin otic drops in the medical treatment of otitis externa and chronic suppurative otitis media. Department of ORL, Hospital University Kabangsaan Malaysia, Kuala Lumpur, Malaysia Unpublished, 2000.
Ghosh 2012 {published data only}
- CTRI/2011/10/002079. Comparison of effectiveness of ciprofloxacin and cefpodoxime in patients with acute attack of chronic middle ear infection [Comparison of efficacy and safety of ciprofloxacin and cefpodoxime in acute exacerbation of chronic suppurative otitis media: a randomized open labeled study]. Http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=3489 (first received 19 October 2011). [CENTRAL: CN‐01013255; CRS: 1743872]
- Ghosh A, Jana U, Khaowas A, Das S, Mandal A, Das N. Comparison of the effectiveness and safety of cefpodoxime and ciprofloxacin in acute exacerbation of chronic suppurative otitis media: a randomized, open‐labeled, phase IV clinical trial. Journal of Pharmacology & Pharmacotherapeutics 2012;3(4):320‐4. [CENTRAL: CN‐00905716; CRS: 1676041; EMBASE: 2013360430; PUBMED: 23326103] [DOI] [PMC free article] [PubMed] [Google Scholar]
Granath 2007 {published data only}
- Granath A, Lindberg K, Rynnel‐Dagoo B. Tube associated otorrhea in children with recurrent otitis media: results of a prospective randomized treatment study. 9th International Symposium on Recent Advances in Otitis Media; 2007 Jun 3‐7; St. Pete Beach (FL). St. Pete Beach (FL), 2007. [CENTRAL: CN‐00614760; CRS: 1435660]
Gupta 2015 {published data only}
- Gupta C, Agrawal A, Gargav ND. Role of acetic acid irrigation in medical management of chronic suppurative otitis media: a comparative study. Indian Journal of Otolaryngology‐Head & Neck Surgery 2015;67(3):314‐8. [CENTRAL: CN‐01098823; CRS: 1829333; PUBMED: 26405670] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gyde 1981 {published data only}
- Gyde MC. A double‐blind comparative study of trimethoprim‐polymyxin B versus trimethoprim‐sulfacetamide‐polymyxin B otic solutions in the treatment of otorrhea. Journal of Laryngology and Otology 1981;95(3):251‐9. [CENTRAL: CN‐00024381; CRS: 959452; PUBMED: 6257815] [DOI] [PubMed] [Google Scholar]
- Gyde MC. Double‐blind comparative trial of trimethoprim‐polymyxin B and trimethoprim‐sulphacetamide‐polymyxin B ear drops in the treatment of otorrhoea [Etude comparative a double insu sur les solutions otiques de trimethoprime ‐ polymyxine B et de trimethoprime ‐ sulfacetamide ‐ polymyxine B dans le traitement de l'otorrhee]. Annales d'Oto‐laryngologie et de Chirurgie Cervico Faciale 1981;98(1‐2):37‐40. [CENTRAL: CN‐00026056; CRS: 961126; EMBASE: 1982037351; PUBMED: 6269476] [PubMed] [Google Scholar]
Gyde 1982 {published data only}
- Gyde MC, Norris D, Kavalec EC. The weeping ear: clinical re‐evaluation of treatment. Journal of International Medical Research 1982;10(5):333‐40. [CENTRAL: CN‐00029419; CRS: 964485; PUBMED: 6754505] [DOI] [PubMed] [Google Scholar]
Harris 2016 {published data only}
- Harris AS, Elhassan HA, Flook EP. Why are ototopical aminoglycosides still first‐line therapy for chronic suppurative otitis media? A systematic review and discussion of aminoglycosides versus quinolones. Journal of Laryngology and Otology 2016;130(1):2‐7. [CRS: 8519203; PUBMED: 26584651] [DOI] [PubMed] [Google Scholar]
Helmi 2000 {published data only}
- Helmi A, Ratna D, Zainul A, Sosialisman E, Alfian FH, Bambang H. The efficacy and safety of ofloxacin otic solution for active suppurative otitis media. Faculty of Medicine, University of Indonesia, Jakarta, Indonesia Unpublished, 2000.
- Helmi A, et al. The efficacy and safety of ofloxacin otic solution for active suppurative otitis media. 9th ASEAN ORL Head and Neck Congress, 31 Mar‐1 Apr, 2001. Singapore, 2001.
Hemlin 1997 {published data only}
- Hemlin C, Carenfelt C, Papatziamos G. Single dose of betamethasone in combined medical treatment of secretory otitis media. Annals of Otology, Rhinology, and Laryngology 1997;106(5):359‐63. [CENTRAL: CN‐00139646; CRS: 1074207; PUBMED: 9153098] [DOI] [PubMed] [Google Scholar]
Hwang 2015 {published data only}
- Hwang JH, Lee JH, Hwang JH, Chung KM, Lee EJ, Yoon YJ, et al. Comparison of arbekacin and vancomycin in treatment of chronic suppurative otitis media by methicillin resistant Staphylococcus aureus. Journal of Korean Medical Science 2015;30(6):688‐93. [CENTRAL: CN‐01257881; CRS: 4910884; PUBMED: 26028918] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Hwang JH, Moon MK, Kim JS, Lee CS. Comparison of the usefulness of arbekacin and vancomycin in treating chronic suppurative otitis media due to methicillin resistant staphylococcus aureus. International Journal of Antimicrobial Agents 2015;45(52):S60‐1. [CRS: 7774347] [Google Scholar]
I‐HEAR‐BETA {published data only}
- Morris P, Leach AJ. Among Aboriginal children (2 months of age and up to 17 years of age) with chronic suppurative otitis media, is 4 months of povidone‐iodine ear wash and/or oral cotrimoxazole in addition to standard treatment (cleaning and dry mopping with tissue spears plus topical ciprofloxacin) superior to standard treatment alone for resolving ear discharge? A 2x2 factorial randomised controlled trial. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=365450 (first received 5 March 2014). [ACTRN12614000234617]
Indudharan 2005 {published data only}
- Indudharan R, Ashraful Haq J, Mohamad H, Mohamad Z, Mohamad Z. Topical antibiotic versus antibiotic steroid combination in the management of chronic suppurative otitis media. XVI World Congress of Otorhinolaryngology Head and Neck Surgery; 1997 Mar 2‐7; Sydney. 1997:1153‐8. [CENTRAL: CN‐00549107; CRS: 1379203]
- Indudharan R, Valuyeetham KA, Raju SS. Role of glucocorticoids in ototopical antibiotic‐steroid preparations in the treatment of chronic suppurative otitis media. Archives of Medical Research 2005;36(2):154‐8. [CENTRAL: CN‐00512134; CRS: 1355519; EMBASE: 2005181801; PUBMED: 15847949] [DOI] [PubMed] [Google Scholar]
ISRCTN12149720 {published data only}
- ISRCTN12149720. Treatment of chronic suppurative otitis media with the antimicrobial peptide OP‐145 (AMP60.4Ac) in adults. Http://www.isrctn.com/isrctn12149720 (first received 13 October 2015). [CENTRAL: CN‐01601160; CRS: 8519222]
ISRCTN84220089 {published data only}
- ISRCTN84220089. An interventional, randomized, double‐blind, placebo‐controlled, parallel‐assignment, safety/efficacy study for treatment of chronic middle ear infection in adult patients with the antimicrobial peptide OP‐145. Http://isrctn.org/ISRCTN84220089 (first received 18 January 2008). [CENTRAL: CN‐01013171; CRS: 1743788]
ISRCTN86106121 {published data only}
- ISRCTN86106121. Treatment of chronic suppurative otitis media (CSOM) in Kenyan children: a double blind, two‐group comparative randomised placebo controlled trial [Randomised trial of treatment of chronic suppurative otitis media (CSOM) in Kenyan children]. Http://isrctn.org/ISRCTN86106121 (first received 17 December 2008). [CENTRAL: CN‐00766897; CRS: 1564692]
Jahn 1984 {published data only}
- Jahn AF, Abramson M. Medical management of chronic otitis media. Otolaryngologic Clinics of North America 1984;17(4):673‐7. [CRS: 7774187] [PubMed] [Google Scholar]
Jang 2004 {published data only}
- Jang CH, Song CH, Wang PC, Wang PC. Topical vancomycin for chronic suppurative otitis media with methicillin‐resistant Staphylococcus aureus otorrhoea. Journal of Laryngology and Otology 2004;118(8):645‐7. [CENTRAL: CN‐00497367; CRS: 1342862] [DOI] [PubMed] [Google Scholar]
Jaya 2003 {published data only}
- Jaya C, Job A, Mathai E, Antonisamy B. Evaluation of topical povidone‐iodine in chronic suppurative otitis media. Archives of Otolaryngology‐Head & Neck Surgery 2003;129(10):1098‐100. [CENTRAL: CN‐00452670; CRS: 1305097; EMBASE: 37248310; PUBMED: 14568795] [DOI] [PubMed] [Google Scholar]
Jiang 2016 {published data only}
- Jiang S‐L, Li H‐Y, Gong Q‐H. Efficacy and safety compare of two antibiotics combined with Chinese patent medicine for chronic suppurative otitis media in children. International Journal of Clinical and Experimental Medicine 2016;9(2):4089‐94. [CENTRAL: CN‐01199922; CRS: 4437723; EMBASE: 20160256082] [Google Scholar]
Kadar 2003 {published data only}
- Kadar AA, Usman M, Tirmizi S. Topical quinolones versus topical aminoglycosides in the medical management of chronic suppurative otitis media: a comparative trial. Journal of Surgery Pakistan 2003;8(4):6‐9. [CENTRAL: CN‐00597098; CRS: 1421747] [Google Scholar]
Kashiwamura 2004 {published data only}
- Kashiwamura M, Chida E, Matsumura M, Nakamaru Y, Suda N, Terayama Y, et al. The efficacy of Burow's solution as an ear preparation for the treatment of chronic ear infections. Otology & Neurotology 2004;25(1):9‐13. [CRS: 1342915] [DOI] [PubMed] [Google Scholar]
Kenna 1986 {published data only}
- Kenna MA, Bluestone CD, Reilly JS, Lusk RP. Medical management of chronic suppurative otitis media without cholesteatoma in children. Laryngoscope 1986;96(2):146‐51. [CRS: 8519234; PUBMED: 3945144] [DOI] [PubMed] [Google Scholar]
Khanna 2000 {published data only}
- Khanna V, Chander J, Nagarkar NM, Dass A. Clinicomicrobiologic evaluation of active tubotympanic type chronic suppurative otitis media. Journal of Otolaryngology 2000;29(3):148‐53. [CENTRAL: CN‐00331653; CRS: 1207025; PUBMED: 10883827] [PubMed] [Google Scholar]
Khon 2012 {published data only}
- Khon EM, Dzhenzhera GE, Ovchinnikov AI. Local antibacterial therapy for the inflammatory diseases of the external and middle ear. Vestnik Otorinolaringologii 2012, (3):92‐4. [CENTRAL: CN‐00850158; CRS: 1626026; EMBASE: 365865807; PUBMED: 22951697] [PubMed] [Google Scholar]
Kiris 1998 {published data only}
- Kiris M, Berktas M, Egeli E, Kutluhan A. The efficacy of topical ciprofloxacin in the treatment of chronic suppurative otitis media. Ear, Nose, & Throat Journal 1998;77(11):904‐5, 909. [CENTRAL: CN‐00306946; CRS: 1191282; PUBMED: 9846467] [PubMed] [Google Scholar]
Kothari 1969 {published data only}
- Kothari A. Treatment of 'resistant' otorrhea with acetic acid. Laryngoscope 1969;79(3):494‐8. [CRS: 7774298; PUBMED: 5776741] [DOI] [PubMed] [Google Scholar]
Kovacic 1999 {published data only}
- Kovacic M, Dzelalija B. Clinical success of treatment of chronic otitis media using topical and peroral administration of ofloxacin. Lijecnicki Vjesnik 1999;121(6):185‐7. [CRS: 7774299] [PubMed] [Google Scholar]
Kurilin 1976 {published data only}
- Kurilin IA, Mokhort NA, Iurina RV, Garankina LA. Complex conservative method of treatment of chronic suppurative otitis media with the use of mefenamine sodium salt [Kompleksnyi konservativnyi metod lecheniia khronicheskikh gnoinykh srednykh otitov s primeneniem mefenamina natrievoi soli]. Zhurnal Ushnykh, Nosovykh I Gorlovykh Boleznei [Journal of Otology, Rhinology, and Laryngologie [sic]] 1976;2:64‐70. [CRS: 6614468; PUBMED: 1266398] [PubMed] [Google Scholar]
Lancaster 1999 {published data only}
- Lancaster JL, Makura ZG, Porter G, McCormick M. Topical aminoglycosides in the management of active mucosal chronic suppurative otitis media. Journal of Laryngology and Otology 1999;113(1):10‐2. [CRS: 7774332] [DOI] [PubMed] [Google Scholar]
Lancaster 2003 {published data only}
- Lancaster J, Matthews J, Williams RS, Thussey C, Kent SE. Comparison of compliance between topical aural medications. Clinical Otolaryngology and Allied Sciences 2003;28(4):331‐4. [CRS: 3455627] [DOI] [PubMed] [Google Scholar]
Lang 1992 {published data only}
- Lang R, Goshen S, Raas‐Rothschild A, Raz A, Ophir D, Wolach B, et al. Oral ciprofloxacin in the management of chronic suppurative otitis media without cholesteatoma in children: preliminary experience in 21 children. Pediatric Infectious Disease Journal 1992;11(11):925‐9. [CRS: 5037742; PUBMED: 1454433] [DOI] [PubMed] [Google Scholar]
Lautala 1983 {published data only}
- Lautala P, Vare M, Vuorinen O, Kaar M L. Ceftazidime in the treatment of chronic suppurative otitis media in children. Journal of Antimicrobial Chemotherapy 1983;12 Suppl A:365‐7. [CRS: 7774341] [DOI] [PubMed] [Google Scholar]
Lazo Saenz 1999 {published data only}
- Lazo Saenz GJ, Alonzo Rojo SE, Perez Blanco A. Topical treatment in chronic otitis media [Tratamiento atopico en otitis media cronica]. Annales de Otorinolaringologia Mexicana 1999;45(1):17‐9. [CENTRAL: CN‐00477445; CRS: 1325497] [Google Scholar]
Leach 2008 {published data only}
- Leach A, Wood Y, Gadil E, Stubbs E, Morris P. Topical ciprofloxin versus topical framycetin‐gramicidin‐dexamethasone in Australian aboriginal children with recently treated chronic suppurative otitis media: a randomized controlled trial. Pediatric Infectious Disease Journal 2008;27(8):692‐8. [CENTRAL: CN‐00650088; CRS: 1463553; EMBASE: 2009258884; PUBMED: 18664984] [DOI] [PubMed] [Google Scholar]
- Morris P, Leach A, Gadil E, Wood Y. Topical ciprofloxacin versus topical Sofradex in children with persistent chronic suppurative otitis media: a randomized controlled trial. 8th International Symposium on Recent Advances in Otitis Media; 2003 Jun 3‐7; Fort Lauderdale (FL). 2003:289. [CENTRAL: CN‐00449347; CRS: 1302492]
Legent 1994 {published data only}
- Legent F, Bordure P, Beauvillain C, Berche P, Bordure PH. Controlled prospective study of oral ciprofloxacin versus amoxycillin/clavulanic acid in chronic suppurative otitis media in adults. Chemotherapy 1994;40(Suppl 1):16‐23. [CENTRAL: CN‐00108583; CRS: 1043205; EMBASE: 1994301734; PUBMED: 7805426] [DOI] [PubMed] [Google Scholar]
Li 2004 {published data only}
- Li KY, Zhao NJ, Zhu JC. Clinical observation on treatment of chronic suppurative otitis media caused large tympanic membranes perforation by ear‐dropping with combined Chinese and Western drugs. Zhongguo Zhong Xi Yi Jie He Za Zhi [Chinese Journal of Integrated Traditional and Western Medicine] 2004;24(11):989‐91. [CENTRAL: CN‐00504865; CRS: 1349072; PUBMED: 15609596] [PubMed] [Google Scholar]
Loock 2012 {published data only}
- Loock J. Strategies in the medical treatment of active mucosal chronic otitis media suitable for all levels of healthcare: a randomized controlled trial. Clinical Otolaryngology 2012;37(Suppl 1):165‐6. [CENTRAL: CN‐01008068; CRS: 1738685; EMBASE: 71023646] [Google Scholar]
- Loock JW. A randomised controlled trial of active chronic otitis media comparing courses of eardrops versus one‐off topical treatments suitable for primary, secondary and tertiary healthcare settings. Clinical Otolaryngology 2012;37(4):261‐70. [CENTRAL: CN‐00850193; CRS: 1626058; EMBASE: 365535141; PUBMED: 22804826] [DOI] [PubMed] [Google Scholar]
Lorentzen 1978 {published data only}
- Lorentzen P, Haugsten P. Treatment of acute suppurative otitis media. Paracentesis and/or antibiotics [Behandlingen av akutt mellomorebetennelse. Paracentese og/eller antibiotika]. Tidsskrift for Den Norske Laegeforening 1978;98(26):1273‐5. [CENTRAL: CN‐01402427; CRS: 6616425; PUBMED: 754336] [PubMed] [Google Scholar]
- Lorentzen P, Haugsten P, Lorentzen P, Haugsten P. Treatment of acute suppurative otitis media. Journal of Laryngology and Otology 1977;4:331‐40. [CENTRAL: CN‐00452743; CRS: 1305161] [DOI] [PubMed] [Google Scholar]
Macfadyen 2005 {published data only}
- Macfadyen C, Gamble C, Garner P, Macharia I, Mackenzie I, Mugwe P, et al. Topical quinolone vs. antiseptic for treating chronic suppurative otitis media: a randomized controlled trial. Clinical Otolaryngology 2005;30(2):193‐4. [CENTRAL: CN‐00521519; CRS: 1363906; EMBASE: 2005185914; PUBMED: 15839875] [DOI] [PubMed] [Google Scholar]
- Macfadyen C, Gamble C, Garner P, Macharia I, Mackenzie I, Mugwe P, et al. Topical quinolone vs. antiseptic for treating chronic suppurative otitis media: a randomized controlled trial. Tropical Medicine & International Health 2005;10(2):190‐7. [CENTRAL: CN‐00502876; EMBASE: 2005088099; 1347086; 1347086; PUBMED: 15679563] [DOI] [PubMed] [Google Scholar]
Manolidis 2004 {published data only}
- Manolidis S, Friedman R, Hannley M, Roland PS, Matz G, Rybak L, et al. Comparative efficacy of aminoglycoside versus fluoroquinolone topical antibiotic drops. Otolaryngology ‐ Head & Neck Surgery 2004;130(3 Suppl):S83‐8. [CRS: 7774411] [DOI] [PubMed] [Google Scholar]
Mendelman 1992 {published data only}
- Mendelman PM, Beccaro MA, McLinn SE, Todd WM. Cefpodoxime proxetil compared with amoxicillin‐clavulanate for the treatment of otitis media. Journal of Pediatrics 1992;121(3):459‐65. [CENTRAL: CN‐00086772; CRS: 1021542; PUBMED: 1517926] [DOI] [PubMed] [Google Scholar]
Merifield 1993 {published data only}
- Merifield DO, Parker NJ, Nicholson NC. Therapeutic management of chronic suppurative otitis media with otic drops. Otolaryngology ‐ Head & Neck Surgery 1993;109(1):77‐82. [CENTRAL: CN‐00343156; CRS: 1215768; PUBMED: 8393167] [DOI] [PubMed] [Google Scholar]
Mesure 1973 {published data only}
- Mesure R. Double‐blind study of the association of sulfamethoxazole and trimethoprim in otorhinolaryngological infections [Essai en double aveugle de l'association de sulfamethoxazole et de trimethoprime dans les infections oto‐rhino‐laryngologiques]. Acta Oto‐rhino‐laryngologica Belgica 1973;27(1):27‐33. [CENTRAL: CN‐00008422; CRS: 944928; PUBMED: 4697134] [PubMed] [Google Scholar]
Minja 2006 {published data only}
- Minja BM, Moshi NH, Ingvarsson L, Bastos I, Grenner J. Chronic suppurative otitis media in Tanzanian school children and its effects on hearing. East African Medical Journal 2006 Jun;83(6):322‐5. [CENTRAL: CN‐00568108; CRS: 1397424; PUBMED: 16989377] [DOI] [PubMed] [Google Scholar]
Miro 2000 {published data only}
- Miro N. Controlled multicenter study on chronic suppurative otitis media treated with topical applications of ciprofloxacin 0.2% solution in single‐dose containers or combination of polymyxin B, neomycin, and hydrocortisone suspension. Otolaryngology ‐ Head & Neck Surgery 2000;123(5):617‐23. [CENTRAL: CN‐00331261; CRS: 1206633; PUBMED: 11077352] [DOI] [PubMed] [Google Scholar]
Mora 2012 {published data only}
- Mora R, Salzano FA, Mora E, Guastini L. Efficacy of a topical suspension of bacterial antigens for the management of chronic suppurative otitis media. European Archives of Oto‐rhino‐laryngology 2012;269(6):1593‐7. [CENTRAL: CN‐00849461; CRS: 1625459; PUBMED: 22037722] [DOI] [PubMed] [Google Scholar]
Morgon 1976 {published data only}
- Morgon A. Role of antibiotic therapy with clindamycin in the treatment of chronic suppurative otitis media [Place de l'antibiotherapie par la clindamycine dans le traitement des otites moyennes suppurees chroniques]. JFORL. Journal Francais d'Oto‐rhino‐laryngologie; Audiophonologie et Chirurgie Maxillo‐faciale 1976;25(4):353‐4. [CRS: 6791598; PUBMED: 135074] [PubMed] [Google Scholar]
NCT02592096 {published data only}
- NCT02592096. Pazufloxacin mesilate ear drops clinical trial protocol [A single dose phase I clinical study of pazufloxacin mesilate ear drops for the patients with otitis media]. Https://clinicaltrials.gov/show/nct02592096 (first received 30 October 2015). [CENTRAL: CN‐01104441; CRS: 1834950]
NCT02817347 {published data only}
- NCT02817347. A clinical trial of YH1177 in patients with otitis media and otorrhea [A randomized, double‐blind, multicenter, phase2 trial to evaluate the safety and efficacy of YH1177 or YH1177‐D otic solution in patients with otitis media and otorrhea]. Https://clinicaltrials.gov/show/nct02817347 (first received 29 June 2016). [CENTRAL: CN‐01383192; CRS: 6333841]
Nwokoye 2015 {published data only}
- Bakshi SS. Occurrence of otitis media in children and assessment of treatment options. Journal of Laryngology and Otology 2015;129(12):1253. [CRS: 8521489; PUBMED: 26429519] [DOI] [PubMed] [Google Scholar]
- Nwokoye NN, Egwari LO, Olubi OO. Occurrence of otitis media in children and assessment of treatment options. Journal of Laryngology and Otology 2015;129(8):779‐83. [CRS: 8519954; PUBMED: 26072993] [DOI] [PubMed] [Google Scholar]
Onali 2018 {published data only}
- Onali MA, Bareeqa SB, Zia S, Ahmed SI, Owais A, Ahmad AN. Efficacy of empirical therapy with combined ciprofloxacin versus topical drops alone in patients with tubotympanic chronic suppurative otitis media: a randomized double‐blind controlled trial. Clinical Medicine Insights. Ear Nose & Throat 2018;11:1179550617751907. [CENTRAL: CN‐01445979; CRS: 7538061; PUBMED: 29348711] [DOI] [PMC free article] [PubMed] [Google Scholar]
Otwombe 2003 {published data only}
- Otwombe K N, Ogutu B. Intention‐to‐treat analysis in the chronic suppurative otitis media trials. East African Medical Journal 2003;80(6):308‐11. [CRS: 7774599] [DOI] [PubMed] [Google Scholar]
Panchasara 2015 {published data only}
- CTRI/2012/07/002784. Efficacy and safety of ofloxacin and its combination with dexamethasone in chronic suppurative otitis media ‐ a randomized, double blind, parallel group, comparative study. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=4129 (first received 11 July 2012). [CENTRAL: CN‐00867279; CRS: 1641742] [PMC free article] [PubMed]
- Panchasara A, Singh A, Mandavia D, Jha S, Tripathi C. Efficacy and safety of ofloxacin and its combination with dexamethasone in chronic suppurative otitis media. A randomised, double blind, parallel group, comparative study. Acta Otorhinolaryngologica Italica 2015;35(1):39‐44. [CENTRAL: CN‐01098783; CRS: 1829293; EMBASE: 615600505; PUBMED: 26015650] [PMC free article] [PubMed] [Google Scholar]
Papastavros 1989 {published data only}
- Papastavros T, Giamarellou H, Varlejides S. Preoperative therapeutic considerations in chronic suppurative otitis media. Laryngoscope 1989;99(6 Pt 1):655‐9. [CENTRAL: CN‐00060222; CRS: 995265; PUBMED: 2725163] [DOI] [PubMed] [Google Scholar]
Picozzi 1983 {published data only}
- Picozzi G, Browning G, Calder I. Controlled trial of gentamicin and hydrocortisone ear drops with and without systemic metronidazole in the treatment of active chronic otitis media. Clinical Otolaryngology 1983;8:367‐8. [CENTRAL: CN‐00262065; CRS: 1161186] [Google Scholar]
Picozzi 1984 {published data only}
- Picozzi GL, Browning GG, Calder IT. Controlled trial of gentamicin and hydrocortisone ear drops with and without systemic metronidazole in the treatment of active chronic otitis media. Clinical Otolaryngology and Allied Sciences 1984;9:305. [CENTRAL: CN‐00262101; CRS: 1161222] [Google Scholar]
Poliakova 1991 {published data only}
- Poliakova TS, Voznesenskii NL, Mironov AA. "Otinum" in the therapy of middle ear diseases. Vestnik Otorinolaringologii 1991, (2):56‐8. [CRS: 8524870; PUBMED: 2048256] [PubMed] [Google Scholar]
Povedano 1995 {published data only}
- Povedano Rodriguez V, Seco Pinero MJ, Jurado Ramos A, Lopez Villarejo P. Efficacy of topical ciprofloxacin in the treatment of chronic otorrhea [Eficacia del ciprofloxacino topico en el tratamiento de la otorrea cronica]. Acta Otorrinolaringologica Espanola 1995;46(1):15‐8. [CENTRAL: CN‐00113534; CRS: 1048153; PUBMED: 7734157] [PubMed] [Google Scholar]
Principi 1995 {published data only}
- Principi N. Multicentre comparative study of the efficacy and safety of azithromycin compared with amoxicillin/clavulanic acid in the treatment of paediatric patients with otitis media. European Journal of Clinical Microbiology & Infectious Diseases 1995;14(8):669‐76. [CENTRAL: CN‐00120943; CRS: 1055544; EMBASE: 1995280756; PUBMED: 8565983] [DOI] [PubMed] [Google Scholar]
Quick 1973 {published data only}
- Quick CA, Wagner D. Trimethoprim‐sulfamethoxazole in the treatment of infections of the ears, nose, and throat. Journal of Infectious Diseases 1973;128(Suppl):S696‐700. [CENTRAL: CN‐00009316; CRS: 945820; PUBMED: 4202209] [DOI] [PubMed] [Google Scholar]
Quick 1975 {published data only}
- Quick CA. Comparison of penicillin and trimethoprim‐sulfamethoxazole in the treatment of ear, nose and throat infections. Canadian Medical Association Journal 1975;112(13):83‐6. [CENTRAL: CN‐00697942; CRS: 1501105; PUBMED: 805651] [PMC free article] [PubMed] [Google Scholar]
Renukananda 2014 {published data only}
- Renukananda GS, Santosh UP, George NM. Topical vs combination ciprofloxacin in the management of discharging chronic suppurative otitis media. Journal of Clinical and Diagnostic Research 2014;8(6):KC01‐4. [CENTRAL: CN‐00995335; CRS: 1725967; EMBASE: 2014430779; PUBMED: 25121008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rotimi 1990 {published data only}
- Rotimi VO, Olabiyi DA, Banjo TO, Okeowo PA. Randomised comparative efficacy of clindamycin, metronidazole, and lincomycin, plus gentamicin in chronic suppurative otitis media. West African Journal of Medicine 1990;9(2):89‐97. [CENTRAL: CN‐00072366; CRS: 1007294; PUBMED: 2268574] [PubMed] [Google Scholar]
Saez‐Llorens 2005 {published data only}
- Saez‐Llorens X, Rodriguez A, Arguedas A, Hamed KA, Yang J, Pierce P, et al. Randomized, investigator‐blinded, multicenter study of gatifloxacin versus amoxicillin/clavulanate treatment of recurrent and nonresponsive otitis media in children. Pediatric Infectious Disease Journal 2005;24(4):293‐300. [CENTRAL: CN‐00515218; CRS: 1358586; EMBASE: 2005176460; PUBMED: 15818287] [DOI] [PubMed] [Google Scholar]
Sanchez Gonzales 2001 {published data only}
- Sanchez Gonzalez A, Gonzalez Galindo T. An open, comparative study of treatment of chronic middle ear otitis with levofloxacine vs amoxicillin/clavulanate. [Spanish] [Estudio abierto comparativo del tratamiento de otitis media cronica con levofloxacino vs amoxicilina/clavulanato]. Investigacion Medica Internacional 2001;28(1):33‐6. [CENTRAL: CN‐00425055; CRS: 1281854; EMBASE: 2001355708] [Google Scholar]
Shkil' 1964 {published data only}
- Shkil' AM. On the complex treatment of chronic suppurative otitis media [O kompleksnom lechenii khronicheskikh gnoinykh srednikh otitov]. Zhurnal Ushnykh, Nosovykh I Gorlovykh Boleznei [Journal of Otology, Rhinology, and Laryngologie [sic]] 1964;24(6):17‐22. [CRS: 6614470; PUBMED: 5876597] [PubMed] [Google Scholar]
Singhal 1992 {published data only}
- Singhal S, Sharma SC, Singhal KC. Adverse reactions to gentamycin in patients with ear, nose or throat infections. Indian Journal of Physiology and Pharmacology 1992;36(3):189‐98. [CRS: 8527046] [PubMed] [Google Scholar]
Smith 1996 {published data only}
- Mackenzie IJ, Smith AW, Hatcher J, Machria I. Randomized controlled trial of treatment of chronic suppurative otitis media in Kenyan school children [abstract]. Clinical Otolaryngology 1997;22:81. [CENTRAL: CN‐00262473; CRS: 1161574] [Google Scholar]
- Smith AW, Hatcher J, Mackenzie IJ, Thompson S, Bal I, Macharia I, et al. Randomised controlled trial of treatment of chronic suppurative otitis media in Kenyan school children. Lancet 1996;348(9035):1128‐33. [CENTRAL: CN‐00132594; CRS: 1067178; EMBASE: 1996327505; PUBMED: 8888166] [DOI] [PubMed] [Google Scholar]
Somekh 2000 {published data only}
- Somekh E, Cordova Z. Ceftazidime versus aztreonam in the treatment of pseudomonal chronic suppurative otitis media in children. Scandinavian Journal of Infectious Diseases 2000;32(2):197‐9. [CENTRAL: CN‐00296955; CRS: 1183249; PUBMED: 10826908] [DOI] [PubMed] [Google Scholar]
Stenstrom 1991 {published data only}
- Stenstrom C, Lundgren K, Ingvarsson L, Bertilson SO. Amoxycillin/clavulanate versus amoxycillin in recurrent otitis media and therapeutic failure in children. Acta Oto‐laryngologica 1991;111(1):120‐9. [CENTRAL: CN‐00074552; CRS: 1009472; EMBASE: 1991091483; PUBMED: 1901686] [DOI] [PubMed] [Google Scholar]
Subramaniam 2001 {published data only}
- Subramaniam K, Jalaludin M, Krishnan G. Comparative study of ofloxacin otic drops versus neomycin‐polymixin b‐hydrocortisone in the medical management of chronic suppurative otitis media. Department of ORL, University Malaya Medical Center, Kuala Lumpur, Malaysia Unpublished, 2000.
- Subramaniam K, Jaludin M, Krishnan G. Comparative study of ofloxacin otic drops versus neomycin‐polymixin hydrocortisone in the medical management of chronic suppurative otitis media. 9th ASEAN ORL Head and Neck Congress, 31 March‐1 April, 2001. Singapore, 2001.
Sultan 2017 {published data only}
- Sultan SSN, Alsaady MA. Role of levofloxacin for treatment of chronic suppurative otitis media: sample of Iraqi patients. Asian Journal of Pharmaceutical and Clinical Research 2017;10(9):358‐60. [CRS: 7778490; EMBASE: 618371938] [Google Scholar]
Sumitsawan 1995 {published data only}
- Sumitsawan Y, Tharavichitkul P, Prawatmuang W, Ingsuwan B, Sriburi P. Ofloxacin otic solution as treatment of chronic suppurative otitis media and diffuse bacterial otitis externa. Chotmaihet Thangphaet [Journal of the Medical Association of Thailand] 1995;78(9):455‐9. [CRS: 6796558; PUBMED: 7561571] [PubMed] [Google Scholar]
Supiyaphun 1995 {published data only}
- Supiyaphun P, Chochaipanichnon L, Tonsakulrungruang K, Chongtateong A, Samart Y. The treatment of chronic suppurative otitis media and otitis externa with 0.3 per cent ofloxacin otic solution: a clinico‐microbiological study. Chotmaihet Thangphaet [Journal of the Medical Association of Thailand] 1995;78(1):18‐21. [CRS: 1305324] [PubMed] [Google Scholar]
Tachibana 1986 {published data only}
- Tachibana M, Ohshima W, Mizukoshi O, Yanohara K, Nishimura T. Clinical trial of BRL 28500 (clavulanic acid‐ticarcillin) in the treatment of chronic suppurative otitis media. Chemotherapy 1986;34(Suppl 4):1120‐4. [CRS: 7774884] [Google Scholar]
Thomsen 1976 {published data only}
- Thomsen VF, Olsen JS, Sorensen H, Thomsen J. Bacteriology and antibiotics in acute suppurative otitis media. Journal of Otolaryngology 1976;5(4):289‐97. [CRS: 7774909] [PubMed] [Google Scholar]
Tong 1996 {published data only}
- Tong MC, Woo JK, Hasselt CA. A double‐blind comparative study of ofloxacin otic drops versus neomycin‐polymyxin B‐hydrocortisone otic drops in the medical treatment of chronic suppurative otitis media. Journal of Laryngology and Otology 1996;110(4):309‐14. [CENTRAL: CN‐00127151; CRS: 1061746; PUBMED: 8733449] [DOI] [PubMed] [Google Scholar]
van der Veen 2007 {published data only}
- Boonacker CW, Veen EL, Wilt GJ, Schilder AG, Rovers MM. Trimethoprim‐sulfamethoxazole in children with chronic otitis media: a randomized comparison of costs and effects. Otology & Neurotology 2008;29(7):961‐4. [CENTRAL: CN‐00666283; CRS: 1473130; PUBMED: 18758386] [DOI] [PubMed] [Google Scholar]
- Miller JL, Honey BL, Johnson PN, Hagemann TM. Effectiveness of trimethoprim/sulfamethoxazole for children with chronic active otitis media. Pediatrics 2007;120(6):1403. [8527167; PUBMED: 18055693] [DOI] [PubMed] [Google Scholar]
- NCT00189098. Effectiveness of sulfamethoxazole‐trimethoprim in the treatment of chronic otitis media. Https://clinicaltrials.gov/show/nct00189098 (first received 16 September 2005). [CENTRAL: CN‐01039521; CRS: 1770119]
- Verhoeff M, Rovers MM, Sanders EAM, Schilder AGM. The COCO‐study: a randomized clinical trial of the efficacy of trimethoprim‐sulfamethoxazole (co‐trimoxazole) in children with chronic suppurative otitis media. Clinical Otolaryngology and Allied Sciences 2004;29(4):460. [CENTRAL: CN‐00874123; CRS: 1647964] [Google Scholar]
- Veen EL, Rovers MM, Albers FW, Sanders EA, Schilder AG. Effectiveness of trimethoprim/sulfamethoxazole for children with chronic active otitis media: a randomized, placebo‐controlled trial. Pediatrics 2007;119(5):897‐904. [CENTRAL: CN‐00588516; CRS: 1415321; PUBMED: 17473089] [DOI] [PubMed] [Google Scholar]
- Veen EL, Schilder AG, Timmers TK, Rovers MM, Fluit AC, Bonten MJ, et al. Effect of long‐term trimethoprim/sulfamethoxazole treatment on resistance and integron prevalence in the intestinal flora: a randomized, double‐blind, placebo‐controlled trial in children. Journal of Antimicrobial Chemotherapy 2009;63(5):1011‐6. [CENTRAL: CN‐00697186; CRS: 1500349; PUBMED: 19297377] [DOI] [PubMed] [Google Scholar]
van Dongen 2014 {published data only}
- NTR1481. Comparison of ototopical antibiotic‐steroid drops or oral antibiotics versus watchful waiting in children with acute tympanostomy tube otorrhea [Comparison of bacicoline‐B eardrops or oral Augmentin versus watchful waiting in children with acute tympanostomy tube otorrhea]. Http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=1481 (first received 6 October 2008). [CENTRAL: CN‐00974948; CRS: 1705907]
- Dongen TMA, Heijden GJMG, Venekamp RP, Rovers MM, Schilder AGM. A trial of treatment for acute otorrhea in children with tympanostomy tubes. New England Journal of Medicine 2014;370(8):723‐33. [CENTRAL: CN‐00982484; CRS: 1713315; EMBASE: 2014119997; PUBMED: 24552319] [DOI] [PubMed] [Google Scholar]
van Hasselt 1998b {published data only}
- Hasselt P. Management of chronic suppurative otitis media in developing countries. 2nd European Congress on Tropical Medicine; 1998; Liverpool, UK. 1998:57. [CENTRAL: CN‐00519674; CRS: 1362363]
- Hasselt P, Kregten E. Treatment of chronic suppurative otitis media with ofloxacin in hydroxypropyl methylcellulose ear drops: a clinical/bacteriological study in a rural area of Malawi. International Journal of Pediatric Otorhinolaryngology 2002;63(1):49‐56. [CENTRAL: CN‐00519676; CRS: 1362365] [DOI] [PubMed] [Google Scholar]
Vishwakarma 2015 {published data only}
- Vishwakarma K, Khan FA, Nizamuddin S, Signh P, Yadav L. Role of topical acetic acid in comparison to gentamicin for the management of chronic suppurative otitis media. International Archives of Biomedical and Clinical Research 2015;1(1):13‐6. [CENTRAL: CN‐01601165; CRS: 8527169] [Google Scholar]
Wilde 1995 {published data only}
- Wilde AD, England J, Jones AS. An alternative to regular dressings for otitis externa and chronic supperative otitis media?. Journal of Laryngology and Otology 1995;109(2):101‐3. [CENTRAL: CN‐00112706; CRS: 1047325; EMBASE: 1995094313; PUBMED: 7535834] [DOI] [PubMed] [Google Scholar]
Wintermeyer 1997 {published data only}
- Wintermeyer SM, Hart MC, Nahata MC. Efficacy of ototopical ciprofloxacin in pediatric patients with otorrhea. Otolaryngology ‐ Head & Neck Surgery 1997;116(4):450‐3. [CRS: 7775009] [DOI] [PubMed] [Google Scholar]
Wright 2009 {published data only}
- Wright D, Safranek S. Treatment of otitis media with perforated tympanic membrane. American Family Physician 2009;79(8):650‐2. [CRS: 7775012] [PubMed] [Google Scholar]
Xu 1999 {published data only}
- Xu Y, Yu J, Song Q. Clinical and experimental study on treatment of chronic pyogenic tympanitis with shenlian ear‐drops. Zhongguo Zhong Xi Yi Jie He za Zhi [Chinese Journal of Integrated Traditional and Western Medicine] 1999;19(8):477‐80. [CENTRAL: CN‐00376881; CRS: 1242470; PUBMED: 11783228] [PubMed] [Google Scholar]
Yuen 1994 {published data only}
- Yuen PW, Lau SK, Chau PY, Hui Y, Wong SF, Wong S, et al. Ofloxacin eardrop treatment for active chronic suppurative otitis media: prospective randomized study. American Journal of Otology 1994;15(5):670‐3. [CENTRAL: CN‐00122894; CRS: 1057491; EMBASE: 1994288260; PUBMED: 8572070] [PubMed] [Google Scholar]
References to studies awaiting assessment
Abdul 2005 {published data only}
- Abdul ME, Shabana Y, Ghonim M. Comparative study of the efficacy of local ciprofloxacin versus aluminum acetate 3.5% in the management of active chronic suppurative otitis media [CSOM]. New Egyptian Journal of Medicine 2005;32:190‐3. [Google Scholar]
Abes 1998 {published data only}
- Abes J, Jamir J, Gloria‐Cruz MT, Gumban V, Seredrica G. Comparative efficacy and safety of ofloxacin and polymixin otic drops for chronic suppurative otitis media. UPM Journal 1998;4:59–70. [Google Scholar]
Additional references
Baumann 2011
- Baumann I, Gerendas B, Plinkert PK, Praetorius M. General and disease‐specific quality of life in patients with chronic suppurative otitis media‐a prospective study. Health and Quality of Life Outcomes 2011;9:48. [DOI: 10.1186/1477-7525-9-48] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhutta 2011
- Bhutta MF, Williamson IG, Sudhoff HH. Cholesteatoma. BMJ 2011;342:d1088. [DOI: 10.1136/bmj.d1088] [DOI] [PubMed] [Google Scholar]
Bhutta 2016
- Bhutta MF. Evolution and otitis media: a review, and a model to explain high prevalence in indigenous populations. Human Biology 2016;87(2):92‐108. [DOI] [PubMed] [Google Scholar]
Bhutta 2018
- Bhutta MF, Head K, Chong LY, Tu N, Schilder AGM, Burton MJ, et al. Aural toilet (ear cleaning) for chronic suppurative otitis media. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD013057] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brennan‐Jones 2018a
- Brennan‐Jones CG, Chong LY, Head K, Tu N, Burton MJ, Schilder AGM, et al. Topical antibiotics with steroids for chronic suppurative otitis media. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD013054] [DOI] [PubMed] [Google Scholar]
Chong 2018a
- Chong LY, Head K, Richmond P, Snelling T, Schilder AGM, Burton MJ, et al. Systemic antibiotics for chronic suppurative otitis media. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD013052] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chong 2018b
- Chong LY, Head K, Richmond P, Snelling T, Schilder AGM, Burton MJ, et al. Topical versus systemic antibiotics for chronic suppurative otitis media. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD013053] [DOI] [PMC free article] [PubMed] [Google Scholar]
CONSORT 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dubey 2007
- Dubey SP, Larawin V. Complications of chronic suppurative otitis media and their management. Laryngoscope 2007;117(2):264‐7. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Elemraid 2010
- Elemraid MA, Brabin BJ, Fraser WD, Harper G, Faragher B, Atef Z, et al. Characteristics of hearing impairment in Yemeni children with chronic suppurative otitis media: a case‐control study. International Journal of Pediatric Otorhinolaryngology 2010;74(3):283‐6. [DOI] [PubMed] [Google Scholar]
Gates 2002
- Gates GA, Klein JO, Lim DJ, Mogi G, Ogra PL, Pararella MM, et al. Recent advances in otitis media. 1. Definitions, terminology, and classification of otitis media. Annals of Otology, Rhinology & Laryngology. Supplement 2002;188:8‐18. [DOI] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hannley 2000
- Hannley MT, Denneny JC, Holzer SS. Use of ototopical antibiotics in treating 3 common ear diseases. Otolaryngology ‐ Head and Neck Surgery 2000;122(6):934‐40. [DOI] [PubMed] [Google Scholar]
Head 2018a
- Head K, Chong LY, Bhutta MF, Morris PS, Vijayasekaran S, Burton MJ, et al. Topical antiseptics for chronic suppurative otitis media. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD013055.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Head 2018b
- Head K, Chong LY, Bhutta MF, Morris PS, Vijayasekaran S, Burton MJ, et al. Antibiotics versus topical antiseptics for chronic suppurative otitis media. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD013056.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 2013
- Jensen RG, Koch A, Homøe P. The risk of hearing loss in a population with a high prevalence of chronic suppurative otitis media. International Journal of Pediatric Otorhinolaryngology 2013;77(9):1530‐5. [DOI: 10.1016/j.ijporl.2013.06.025] [DOI] [PubMed] [Google Scholar]
Kirkham 2017
- Kirkham JJ, Davis K, Altman DG, Blazeby JM, Clarke M, Tunis S, Williamson PR. Core Outcome Set ‐ STAndards for Development: The COS‐STAD recommendations. PLoS Medicine 2017;14(11):e1002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Macfadyen 2005a
- Macfadyen C, Gamble C, Garner P, Macharia I, Mackenzie I, Mugwe P, et al. Topical quinolone vs. antiseptic for treating chronic suppurative otitis media: a randomized controlled trial. Clinical Otolaryngology 2005;30(2):193‐4. [CENTRAL: CN‐00521519; CRS: 1363906; CRSREP: 1363906; EMBASE: 2005185914; PUBMED: 15839875] [DOI] [PubMed] [Google Scholar]
Macfadyen 2005b
- Macfadyen C, Gamble C, Garner P, Macharia I, Mackenzie I, Mugwe P, et al. Topical quinolone vs. antiseptic for treating chronic suppurative otitis media: a randomized controlled trial. Tropical Medicine & International Health 2005;10(2):190‐7. [CENTRAL: CN‐00502876; CRS: 1347086; CRSREP: 1347086; EMBASE: 2005088099; PUBMED: 15679563] [DOI] [PubMed] [Google Scholar]
Mahadevan 2012
- Mahadevan M, Navarro‐Locsin G, Tan HK, Yamanaka N, Sonsuwan N, Wang PC, et al. A review of the burden of disease due to otitis media in the Asia‐Pacific. International Journal of Pediatric Otorhinolaryngology 2012;76(5):623‐35. [DOI: 10.1016/j.ijporl.2012.02.031] [DOI] [PubMed] [Google Scholar]
Mittal 2015
- Mittal R, Lisi CV, Gerring R, Mittal J, Mathee K, Narasimhan G, et al. Current concepts in the pathogenesis and treatment of chronic suppurative otitis media. Journal of Medical Microbiology 2015;64(10):1103‐16. [DOI: 10.1099/jmm.0.000155] [DOI] [PMC free article] [PubMed] [Google Scholar]
Monasta 2012
- Monasta L, Ronfani L, Marchetti F, Montico M, Vecchi Brumatti L, Bavcar A, et al. Burden of disease caused by otitis media: systematic review and global estimates. PloS One 2012;7(4):e36226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 2010
- Morris P, Leach A, Shah P, Nelson S, Anand A, Allnutt R, et al. Recommendations for Clinical Care Guidelines on the Management of Otitis Media: In Aboriginal and Torres Strait Islander Populations. Canberra: Office for Aboriginal and Torres Strait Islander Health, Australian Government, 2010. [Google Scholar]
Nadol 2000
- Nadol JB Jr, Staecker H, Gliklich RE. Outcomes assessment for chronic otitis media: the Chronic Ear Survey. Laryngoscope 2000;110(3 Pt 3):32‐5. [DOI: 10.1097/00005537-200003002-00009] [DOI] [PubMed] [Google Scholar]
Olatoke 2008
- Olatoke F, Ologe FE, Nwawolo CC, Saka MJ. The prevalence of hearing loss among schoolchildren with chronic suppurative otitis media in Nigeria, and its effect on academic performance. Ear, Nose, & Throat Journal 2008;87(12):E19. [PubMed] [Google Scholar]
Orji 2013
- Orji F. A survey of the burden of management of chronic suppurative otitis media in a developing country. Annals of Medical and Health Sciences Research 2013;4(3):598‐601. [DOI: 10.4103/2141-9248.122126] [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillips 2007
- Phillips JS, Yung MW, Burton MJ, Swan IR. Evidence review and ENT‐UK consensus report for the use of aminoglycoside‐containing ear drops in the presence of an open middle ear. Clinical Otolaryngology 2007;32(5):330‐6. [DOI] [PubMed] [Google Scholar]
Phillips 2014a
- Phillips JS, Haggard M, Yung M. A new health‐related quality of life measure for active chronic otitis media (COMQ‐12): development and initial validation. Otology & Neurotology 2014;35(3):454‐8. [DOI: 10.1097/mao.0000000000000205] [DOI] [PubMed] [Google Scholar]
Phillips 2014b
- Phillips JS, Yung MW. COMQ‐12 scores in adult patients without chronic middle ear disease. Clinical Otolaryngology 2014;39(6):362‐7. [DOI: 10.1111/coa.12306] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schilder 2016
- Schilder AG, Chonmaitree T, Cripps AW, Rosenfeld RM, Casselbrant ML, Haggard MP, et al. Otitis media. Nature Reviews Disease Primers 2016;2:16063. [DOI: 10.1038/nrdp.2016.63] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stedman 2011
- Stedman MR, Curtin F, Elbourne DR, Kesselheim AS, Brookhart MA. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2011;40(6):1732‐4. [DOI: 10.1093/ije/dyp345] [DOI] [PubMed] [Google Scholar]
van der Veen 2006
- Veen EL, Schilder AG, Heerbeek N, Verhoeff M, Zielhuis GA, Rovers MM. Predictors of chronic suppurative otitis media in children. Archives of Otolaryngology‐‐Head & Neck Surgery 2006;132(10):1115‐8. [DOI: 10.1001/archotol.132.10.1115] [DOI] [PubMed] [Google Scholar]
van Dinther 2015
- Dinther J, Droessaert V, Camp S, Vanspauwen R, Maryn Y, Zarowski A, et al. Validity and test‐retest reliability of the Dutch Version of the Chronic Otitis Media Questionnaire 12 (COMQ‐12). Journal of International Advanced Otology 2015;11(3):248‐52. [DOI: 10.5152/iao.2015.1701] [DOI] [PubMed] [Google Scholar]
Verhoeff 2006
- Verhoeff M, Veen EL, Rovers MM, Sanders EA, Schilder AG. Chronic suppurative otitis media: a review. International Journal of Pediatric Otorhinolaryngology 2006;70(1):1‐12. [DOI] [PubMed] [Google Scholar]
WHO 2004
- World Health Organization. Chronic Suppurative Otitis Media (CSOM): Burden of Illness and Management Options. Geneva, Switzerland: World Health Organization, 2004. [Google Scholar]
Yorgancılar 2013
- Yorgancılar E, Yildirim M, Gun R, Bakir S, Tekin R, Gocmez C, et al. Complications of chronic suppurative otitis media: a retrospective review. European Archives of Oto‐rhino‐laryngology 2013;270(1):69‐76. [DOI: 10.1007/s00405-012-1924-8] [DOI] [PubMed] [Google Scholar]
Youngs 2016
- Youngs R, Fisher E, Hussain M, Fishman J. Topical aural antibiotic use in the UK ‐ time for a change of policy?. Journal of Laryngology and Otology 2016;130(1):1. [DOI: 10.1017/S002221511500331X] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Brennan‐Jones 2018b
- Brennan‐Jones CG, Head K, Chong LY, Tu N, Burton MJ, Schilder AGM, et al. Topical antibiotics for chronic suppurative otitis media. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD013051] [DOI] [PMC free article] [PubMed] [Google Scholar]


