Abstract
Background
People with urothelial carcinoma of the bladder are at risk for recurrence and progression following transurethral resection of a bladder tumour (TURBT). Mitomycin C (MMC) and Bacillus Calmette‐Guérin (BCG) are commonly used, competing forms of intravesical therapy for intermediate‐ or high‐risk non‐muscle invasive (Ta and T1) urothelial bladder cancer but their relative merits are somewhat uncertain.
Objectives
To assess the effects of BCG intravesical therapy compared to MMC intravesical therapy for treating intermediate‐ and high‐risk Ta and T1 bladder cancer in adults.
Search methods
We performed a systematic literature search in multiple databases (CENTRAL, MEDLINE, Embase, Web of Science, Scopus, LILACS), as well as in two clinical trial registries. We searched reference lists of relevant publications and abstract proceedings. We applied no language restrictions. The latest search was conducted in September 2019.
Selection criteria
We included randomised controlled trials (RCTs) that compared intravesical BCG with intravesical MMC therapy for non‐muscle invasive urothelial bladder cancer.
Data collection and analysis
Two review authors independently screened the literature, extracted data, assessed risk of bias and rated the quality of evidence according to GRADE per outcome. In the meta‐analyses, we used the random‐effects model.
Main results
We identified 12 RCTs comparing BCG versus MMC in participants with intermediate‐ and high‐risk non‐muscle invasive bladder tumours (published from 1995 to 2013). In total, 2932 participants were randomised.
Time to death from any cause: BCG may make little or no difference on time to death from any cause compared to MMC (hazard ratio (HR) 0.97, 95% confidence interval (CI) 0.79 to 1.20; participants = 1132, studies = 5; 567 participants in the BCG arm and 565 in the MMC arm; low‐certainty evidence). This corresponds to 6 fewer deaths (40 fewer to 36 more) per 1000 participants treated with BCG at five years. We downgraded the certainty of the evidence two levels due to study limitations and imprecision.
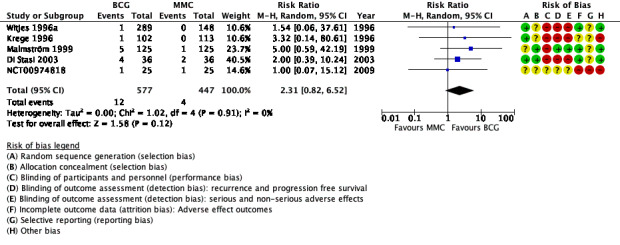
Serious adverse effects: 12/577 participants treated with BCG experienced serious non‐fatal adverse effects compared to 4/447 participants in the MMC group. The pooled risk ratio (RR) is 2.31 (95% CI 0.82 to 6.52; participants = 1024, studies = 5; low‐certainty evidence). Therefore, BCG may increase the risk for serious adverse effects compared to MMC. This corresponds to nine more serious adverse effects (one fewer to 37 more) with BCG. We downgraded the certainty of the evidence two levels due to study limitations and imprecision.
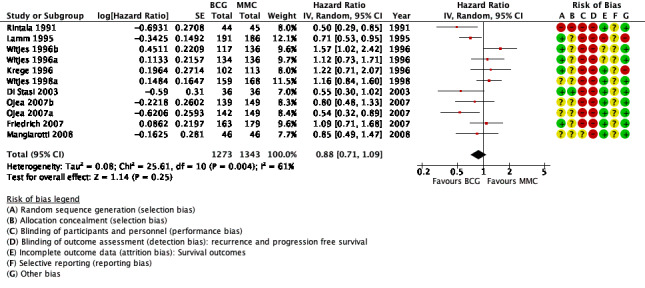
Time to recurrence: BCG may reduce the time to recurrence compared to MMC (HR 0.88, 95% CI 0.71 to 1.09; participants = 2616, studies = 11, 1273 participants in the BCG arm and 1343 in the MMC arm; low‐certainty evidence). This corresponds to 41 fewer recurrences (104 fewer to 29 more) with BCG at five years. We downgraded the certainty of the evidence two levels due to study limitations, imprecision and inconsistency.
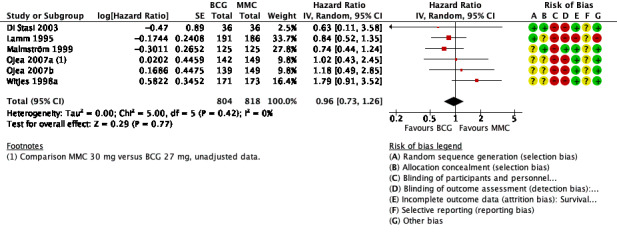
Time to progression: BCG may make little or no difference on time to progression compared to MMC (HR 0.96, 95% CI 0.73 to 1.26; participants = 1622, studies = 6; 804 participants in the BCG arm and 818 in the MMC arm; low‐certainty evidence). This corresponds to four fewer progressions (29 fewer to 27 more) with BCG at five years. We downgraded the certainty of the evidence two levels due to study limitations and imprecision.
Quality of life: we found very limited data for this outcomes and were unable to estimate an effect size.
Authors' conclusions
Based on our findings, BCG may reduce the risk of recurrence over time although the Confidence Intervals include the possibility of no difference. It may have no effect on either the risk of progression or risk of death from any cause over time. BCG may cause more serious adverse events although the Confidence Intervals once again include the possibility of no difference. We were unable to determine the impact on quality of life. The certainty of the evidence was consistently low, due to concerns that include possible selection bias, performance bias, given the lack of blinding in these studies, and imprecision.
Plain language summary
Bacillus Calmette‐Guérin or mitomycin C for treatment of non‐muscle‐invasive bladder cancer
Review question
In people with cancer of the inner lining of the bladder, how do two different medicines, that are called Bacillus Calmette‐Guérin (BCG) and mitomycin (MMC), that are put into the bladder, after the tumour is taken out, compare?
Background
Tumours of the superficial layers of the bladder, so‐called non‐muscle‐invasive bladder cancer, are treated by putting small instruments into the bladder and shaving them out. This works well but these tumours often come back. When they do come back they can be more aggressive and advanced than before. Different types of medicines put into the bladder afterwards can make that happen less often, with BCG and MMC being those used most often. We are not sure how the two treatments compare when it comes to wanted and unwanted effects.
Study characteristics
The content of this review is current to September 2019. We included only studies where chance determined what treatment people in the study would get.
Key results
We found 12 studies including 2932 people who matched our question.
We found that BCG may lead to similar risk of dying from any cause over time (low‐quality evidence), but may increase the risk of serious unwanted effects (low‐quality evidence), although it is possible that it does not make a difference.
BCG may reduce the risk that the tumour comes back over time (low‐quality evidence), although it is possible that it does not make a difference.
BCG may have little or no effect on the risk that the tumour gets worse over time (low‐quality evidence).
We found no data on quality of life.
Quality of the evidence
The quality of the evidence was consistently rated as low, meaning that our confidence is limited, and future research may change these findings.
Summary of findings
Summary of findings for the main comparison. Bacillus Calmette‐Guérin (BCG) compared to mitomycin C (MMC) for Ta and T1 bladder cancer.
| BCG compared to MMC for Ta and T1 bladder cancer | |||||
|
Participants: Adults (≥18 years) with intermediate and high‐risk
non‐muscle invasive urothelial bladder cancer Setting: hospital Intervention: BCG Comparison: MMC | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with MMC | Risk difference with BCG | ||||
|
Time to death from any cause (absolute effect size estimates based on
event rate at 5 years). Follow‐up: range 3.5–20 years |
1132 (5 RCTs) | ⊕⊕⊝⊝ Lowa,b | HR 0.97 (0.79 to 1.20) | Study population | |
| 210 per 1000c | 6 fewer per 1000 (40 fewer to 36 more) | ||||
|
Serious adverse effects Follow‐up: range 1.6–10 years |
1024 (5 RCTs) | ⊕⊕⊝⊝ Lowa,b | RR 2.31 (0.82 to 6.52) | Study population | |
| 7 per 1000 | 9 more per 1000 (1 fewer to 37 more) | ||||
|
Time to recurrence (absolute effect size estimates based on event rate
at 5 years) Follow‐up: range 3–20 years |
2616 (11 RCTs) | ⊕⊝⊝⊝ Lowa,b,d | HR 0.88 (0.71 to 1.09) | Study population | |
| 450 per 1000e | 41 fewer per 1000 (104 fewer to 29 more) | ||||
|
Time to progression (absolute effect size estimates based on event rates
at 5 years) Follow‐up: range 1.6–20 years |
1622 (6 RCTs) | ⊕⊕⊝⊝ Lowa,b | HR 0.96 (0.73 to 1.26) | Study population | |
| 112 per 1000c | 4 fewer per 1000 (29 fewer to 27 more) | ||||
|
Quality of life (measured using EORTC QLQ‐BLS24 at baseline and after each installation weekly for 6 weeks) |
110 (1 RCT) | Not estimablef | Not estimable | There was no evidence of a difference between BCG and MMC groups, except for abdominal bloating and flatulence, which was worse in the BCG group.f | |
| *The risk in the intervention group (and its 95% confidence interval) is
based on the assumed risk in the comparison group and the relative effect
of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level for study limitations: concerns with performance or detection bias (or both), as well as with regard to allocation concealment and selective outcome reporting.
bDowngraded one level for imprecision: 95% CI was consistent with the possibility for important benefit and large harm.
cThe assumed risk was based on five‐year mortality rate from Gardmark 2007.
dDowngraded one level for inconsistency: variation in point estimates or substantial heterogeneity among studies (or both).
eThe assumed risk is based on five‐year mortality rate based on Ojea 2007b
fMore detailed results on quality of life were not available (conference abstract only)
Background
Description of the condition
Urinary bladder cancer affects men and women worldwide, though it is more common in the Western world. Bladder cancer is the fourth most common cancer diagnosed in men in the USA and Europe. It is placed at seventh and eighth position in cancer‐related mortality in the USA (Siegel 2018) and Europe, respectively (Ferlay 2013). The tumour appears three to four times more frequently in men than in women (Fajkovic 2011). One in 26 men will develop bladder cancer in their lifetime (Siegel 2018). Overall five‐year survival rates in Europe are around 68% (De Angelis 2014), but it has been noted that women present with more advanced disease and have a worse prognosis (Shariat 2010). Age, tobacco smoking and exposure to cancerous substances have been reported as potential risk factors (Burger 2013).
Approximately 75% of newly diagnosed cases are non‐muscle invasive bladder cancers, where the tumour affects only the mucous membrane or submucosal layer (also called non‐muscle invasive urothelial carcinoma of the bladder) (Babjuk 2018). About 25% of people diagnosed with bladder cancer have muscle invasive disease and will have poor prognosis even after receiving treatment. The prevalence of bladder cancer is high, as the tumour recurs frequently even after initial treatment and it requires long‐term clinical monitoring. Therefore, this type of cancer is very bothersome to those affected, causes substantial morbidity and affects quality of life (Griffiths 2013).
In economic terms, bladder cancer has the highest lifetime treatment cost per patient. Compared to all other cancers, the per‐patient expenditures range from USD 89,287 to USD 202,203 per patient from diagnosis to death (Sievert 2009), because of high medical expenditures on diagnosis, treatment and continued surveillance using invasive techniques (Svatek 2014). The disease is very costly for the healthcare system and for society, because of work loss and loss of productivity.
Description of the intervention
Although transurethral resection of the bladder (TURB) can eradicate Ta and T1 bladder tumours, intravesical therapy is recommended in most people with intermediate‐ or high‐risk non‐muscle invasive bladder tumours (Ta, T1 and Cis) due to the high chance of tumour recurrence (about 80%) or progression to muscle invasive disease (about 45%) (Babjuk 2018; van Rhijn 2009). Therapy includes either immunotherapy with Bacillus Calmette‐Guérin (BCG) or chemotherapy with cytotoxics, the most commonly used being mitomycin C (MMC) (Ragonese 2016). Other intravesical cytotoxics include gemcitabine, epirubicin and doxorubicin. Intravesical therapies are used to prevent cancer recurrence after primary treatment, and have shown efficacy during recent years of regular utilisation (Abern 2013; Perlis 2013; Sylvester 2004). After the instillation of intravesical agents into the bladder, the solution should be retained for 1.5 to 2 hours. The patient is encouraged to move positions every 30 to 45 minutes to allow the intravesical solution to contact all parts of the bladder wall. After this time, the patient voids to remove the solution.
BCG is provided as a freeze‐dried powder and is diluted with saline before it is instilled into the bladder. Different strains of BCG are available. The original BCG strain was developed at the Pasteur Institute from an attenuated strain of Mycobacterium bovis. Subcultures were made and sent to other parts of the world: Tice and TheraCys substrains are available in the USA, while the Tokyo 172 and the Danish substrains are available outside the USA. There is some evidence that different strains might differ in their clinical efficacy, but this evidence is still limited (Rentsch 2014; Sengiku 2013). Contraindications to BCG therapy are gross haematuria, traumatic catheterisation, recent bladder tumour resection (less than two weeks after TURB), urinary incontinence, symptomatic urinary tract infection and immunosuppression. A BCG sepsis might occur, which presents as an acute tuberculosis‐like illness. Signs and symptoms of a life‐threatening septicaemia are high‐grade fevers, hepatotoxicity, respiratory distress, chills, haemodynamic instability and mental status changes. Local adverse effects might include symptoms of cystitis, haematuria, symptomatic granulomatous prostatitis and epididymo‐orchitis.
MMC powder is diluted with saline and is administered through a catheter directly into the bladder. The recommended dosage depends on patient and tumour characteristics, such as age and prior cytostatic therapy. Although bladder cancer occurs mostly in older people, there are only limited data available about the use of MMC in people aged over 65 years. MMC was isolated from Streptomyces caespitosus or Streptomyces lavendulae in the 1950s. Trade names are Amétycine, Mitem, Urocin and Mito‐medac, as well as other diverse generic products. Contraindications to MMC use are: reduced bone marrow function; bleeding predisposition; damage to liver, lung or kidney; general bad health; and hypersensitivity against MMC; as well as haematuria, perforation of bladder, and urinary tract infection. It is systemically absorbed to a very limited degree when administered intravesically, and systemic adverse effects are rare. Common adverse effects might include cystitis, dysuria, nocturia, pollakisuria, haematuria, local bladder wall reactions and allergic reactions of the skin. The administration of MMC with local microwave‐induced hyperthermia to enhance the effectiveness of therapy is still experimental, with limited evidence but promising results (Lammers 2011; Slater 2014). Also, the use of an electrical current to improve the delivery of intravesical agents (electromotive drug administration) has been a matter of research. Recent evidence suggests a delay in time to recurrence in selected people with non‐muscle invasive bladder cancer, while the effect about its impact on serious adverse effects is still uncertain (Jung 2017). Other heating devices are currently tested in clinical trials.
The type of intravesical therapy which is chosen for the individual patient depends on the patient's risk group (Babjuk 2018). While for low‐risk tumours (primary, solitary, Ta G1, less than 3 cm, no carcinoma in situ (Cis)) an immediate single instillation of chemotherapy is sufficient, intermediate‐risk tumours (between the category of low and high risk) will need additional instillations of either chemotherapy (i.e. MMC) or immunotherapy (i.e. BCG) for one year (reference current European Association of Urology (EAU) guideline). For high‐risk tumours (T1 or G3 or Cis or multiple, recurrent, greater than 3 cm Ta G1‐2, or a combination of these) BCG instillations for one to three years may be more effective in preventing tumour recurrence than TURB alone or TURB and chemotherapy, but people experience significantly more adverse effects (Malmström 2009a; Shang 2011, current EAU guideline). There are still contradictory results concerning the beneficial effect of BCG over MMC on tumour progression (Böhle 2004; Malmström 2009a; Shelley 2010; Sylvester 2004).
How the intervention might work
The mechanism of action of BCG therapy is not clearly understood. The therapeutic effect might be the result of an immune response against BCG surface antigens that cross‐react with bladder tumour antigens. The BCG organisms enter macrophages, where they induce the same type of histological and immunological reaction as found in people with tuberculosis. BCG therapy also has been shown to have a predilection for entering bladder cancer cells, where the proteins are broken down and fragments are combined with histocompatibility antigens and displayed on the cell surface. This induces a cytokine and direct cell‐to‐cell cytotoxicity response, which targets these cells for destruction. The overall response to BCG is limited if the patient is immunosuppressed. BCG induction therapy (primary treatment) is usually given in six‐week schedules. Many different maintenance schedules (following therapy) are used, ranging from a total of 10 instillations given at 18‐week intervals to 27 instillations given over a three‐year period (Lamm 2000: Packiam 2017).
MMC is a mutagenic substance and is used as a chemotherapeutic agent. The mechanism of effect is based preliminarily on alkylation of DNA with corresponding inhibition of DNA synthesis. The degree of damage correlates with the clinical effect and is less in resistant cells than in sensitive cells. The biological half‐life time is short at about 40 to 50 minutes. A single and immediate instillation of chemotherapy is effective and reduces the recurrence rate by 12% to 13% compared to TURB alone (Abern 2013; Perlis 2013; Sylvester 2004). The agent acts by destroying free intravesical tumour cells resulting from the TURB and by an ablative effect on residual tumour cells at the resection site (Soloway 1980). Immediate instillation is necessary, as remaining free tumour cells in the bladder are implanted and covered by extracellular matrix within a few hours (Pode 1986). The prognostic factors of the patient indicate the further need for adjuvant intravesical instillations (chemotherapy or immunotherapy). There is still controversy about which patient groups might benefit the most from an immediate chemotherapy instillation (Abern 2013).
Why it is important to do this review
Although several systematic reviews and meta‐analyses have been conducted on this topic (Böhle 2003; Shelley 2010), the debate on whether MMC or BCG is more effective with less toxicity is still ongoing. It furthermore remains unclear what the optimal treatment dose and schedule might be, as well as the question of which people benefit most from one or the other agent.
One systematic review by Shelley and colleagues identified over 80 randomised controlled trials (RCTs) and 11 meta‐analyses that studied the effectiveness of different intravesical therapies in non‐muscle invasive bladder cancer (Shelley 2010). Although in their general conclusion intravesical administration of BCG was judged to be superior to chemotherapy in terms of complete response and disease‐free survival, there was no conclusive evidence to show the superiority of one agent over the other in terms of overall survival.
In the direct comparison of BCG versus MMC, BCG seemed to be superior to MMC in terms of preventing tumour recurrence in people with high‐risk bladder cancer and reducing the risk of tumour progression in intermediate‐ and high‐risk tumours, but it appeared to be more toxic (Shang 2011; Shelley 2010). There was no significant difference in disease progression and overall survival in this patient population. In intermediate‐risk groups, MMC and BCG might be equally effective in preventing cancer recurrence (Shelley 2010).
The differences in findings among primary studies are the result of the clinical complexity of the disease: dosage, frequency and duration might vary considerably, also the time between TURB and intravesical therapy might differ, as well as patient characteristics, length of follow‐up and study power. All these factors complicate and limit the value of the conclusions that can be drawn. The optimal schedule for BCG immunotherapy, in terms of number of inductions, and frequency and duration of maintenance, remains unknown.
The first Cochrane Review dealing with this topic was published in 2003 (Shelley 2003). This Cochrane Review serves to update the previous review, includes the new findings from the results of recent RCTs and addresses new subgroup analyses that incorporate new developments and clinical practice in this field. The methodology was adapted to the new standards of reporting and conducting Cochrane Reviews. Therefore, this systematic review provides the best available evidence that exists to date and includes independent 'Risk of bias' assessment and certainty rating according to the GRADE methodology.
Objectives
To assess the effects of Bacillus Calmette‐Guérin (BCG) intravesical therapy compared to mitomycin C (MMC) intravesical therapy for treating adults with intermediate‐ and high‐risk non‐muscle invasive bladder cancer.
Methods
Criteria for considering studies for this review
Types of studies
All randomised clinical trials (RCTs), parallel‐grouped or quasi‐randomised trials that compared intravesical BCG with intravesical MMC therapy for non‐muscle invasive urothelial bladder cancer were considered for inclusion. Studies were not excluded on the basis of publication status or language of publication. Studies that included other intravesical agents, but had treatment groups allowing a comparison of BCG and MMC were also considered for inclusion, if the results were reported separately. Studies comparing BCG to placebo/no intervention or MMC versus placebo/no intervention were excluded. We identified no cross‐over trials.
Types of participants
This review considered studies reporting on adults (aged 18 years or greater) with intermediate‐ and high‐risk non‐muscle invasive urothelial bladder cancer (Sobin 2009). We also considered studies including participants with Cis of the bladder. If studies also included participants with muscle invasive bladder cancer, only data of the subset of participants with non‐muscle invasive bladder cancer were considered, if these studies presented data stratified for people with intermediate‐ and high‐risk non‐muscle invasive bladder cancer.
Eligible people were those who were at intermediate or high risk of tumour recurrence or progression, or both. If studies also included participants with low risk for tumour recurrence and/or progression, we again assessed data of the subset of participants with intermediate or high risk (or both) if these data were reported separately.
The risk for recurrence and progression was defined using the EAU guidelines (Babjuk 2018), which refer to the European Organisation for Research and Treatment of Cancer (EORTC) risk tables (Sylvester 2006):
low risk is defined as: primary, solitary, Ta G1 (papillary urothelial neoplasm of low malignant potential, low grade), less than 3 cm, no Cis;
intermediate‐risk tumours are defined as: all tumours between the categories of low and high risk;
high risk refers to any of the following four requirements: T1 tumours; high grade G3 (high grade) tumour; Cis; multiple, recurrent and large (greater than 3 cm) Ta G1G2/low‐grade tumours (all these conditions must be presented).
Following the latest clinical guideline (Babjuk 2018), we also included people at highest risk for recurrence/progression that was defined as T1 G3 tumours associated with concurrent bladder Cis or recurrent T1 G3 (or both), T1 G3 with Cis in prostatic urethra, atypical histology of urothelial carcinoma or lymphovascular invasion.
Types of interventions
Single agent intravesical therapy with BCG or MMC for the prevention or treatment of intermediate‐ and high‐risk non‐muscle invasive urothelial bladder cancer after TURB was eligible for inclusion. BCG of any schedule or strain was considered appropriate for inclusion, as well as any dose or schedule of MMC.
Types of outcome measures
We did not use the measurement of the outcomes assessed in this review as an eligibility criterion for study inclusion.
Primary outcomes
Time to death from any cause (defined as the time from the date of randomisation to the date of death).
Serious adverse effects (adverse effects were considered serious when they required hospitalisation, were life‐threatening or were reported as serious by the authors of the original publication).
Secondary outcomes
Time to recurrence (defined as the date from randomisation to the date of diagnosis of recurrence or death).
Time to progression (defined as the date from randomisation to the date of diagnosis of progression, in stage or grade or death).
Adverse effects (such as dysuria, painful urination, haematuria, cystitis, nocturia, pollakisuria or allergic reactions).
Quality of life (measured with validated instruments).
Main outcomes for 'Summary of findings' table
The 'Summary of findings' table included the following outcomes.
Time to death from any cause.
Serious adverse effects.
Time to recurrence.
Time to progression.
Quality of life.
Findings and quality of the available evidence were reported according to the GRADE methodology (Schünemann 2011). For the time‐to‐event outcomes, we used published evidence to estimate the baseline risk (see Table 1).
Search methods for identification of studies
We performed a comprehensive literature search with no restrictions on the language of publication or publication status.
Electronic searches
We applied no date or language restrictions.
We searched the following databases: Cochrane Central Register of Controlled Trials (CENTRAL; included in the Cochrane Library; 2018, Issue 11) latest issue (Appendix 1), MEDLINE and MEDLINE in Process via Ovid from 1946 to 13 November 2017 (Appendix 2), Embase via Ovid from 1974 to 13 November 2017 (Appendix 3), Scopus from 1966 to 16 November 2017 (Appendix 4), Web of Science (Thomson Reuters Web of Knowledge) from 1900 to 16 November 2017 (Appendix 5), and LILACS from 1982 to 16 November 2017 (Appendix 6).
The electronic search were complemented by a search of the World Health Organization International Clinical Trials Registry Platform Search Portal (WHO ICTRP Search Portal; www.who.int/ictrp/en/, no restricted time period) (Appendix 7) and ClinicalTrials.gov (clinicaltrials.gov/, no restricted time period) (Appendix 8) to identify further completed or ongoing trials.
We updated the searches for all relevant databases shortly before publication of the review (23th September 2019) and screened the results for further potentially eligible studies. We documented and reported the search process in detail.
Searching other resources
We manually screened the reference lists of included articles to identify potentially relevant citations. We searched the American Society of Clinical Oncology (ASCO) database for grey literature (2011 to 2018; meetinglibrary.asco.org/). We contacted authors to request missing information.
Data collection and analysis
In this review, we followed the methodological recommendations given by Cochrane (Higgins 2011a).
Selection of studies
Two review authors (SS and RD or DD) independently reviewed titles and abstracts of identified references according to the predefined inclusion criteria. Two review authors (SS and RD or DD) independently assessed the full texts of all potentially relevant studies. We resolved disagreements by discussion or, if necessary, with the help of a third review author (JJM or FK). We recorded the reasons for study exclusion in the Characteristics of excluded studies table. We identified duplicate publication of studies by checking potentially relevant references for author names, locations and settings, details of interventions, numbers of participants, baseline data, study date and duration of the study. We used EndNote software to manage the references (endnote.com/).
Data extraction and management
Two review authors (SS and DD) independently extracted relevant data on study characteristics, participant population and study setting, follow‐up time, tumour characteristics and relevant comorbidities, intervention characteristics on agent and administration, study methodology, study results and author conclusion using a data extraction form. A third review author (KJ) checked the extracted outcome data relevant to this review as needed for calculation of summary statistics and measures of variance. The data extraction form was based on the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), and was pilot tested before routine use. The review authors resolved any potential disagreement by consensus or through discussion with a third review author (JJM or FK). In addition, when necessary, we contacted the original investigators. We collected and used the most detailed numerical data in order to facilitate similar analyses of included studies. We displayed the information in the Characteristics of included studies table.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary study, we maximised yield of information by mapping all publications to unique studies and collating all available data. We used the most complete dataset aggregated across all known publications. In case of doubt, we gave priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes
Assessment of risk of bias in included studies
For the 'Risk of bias' assessment, we used the Cochrane 'Risk of bias' tool for RCTs (Higgins 2011b). Two review authors (SS and DD or LMK) independently assessed all included studies for potential risk of bias. We resolved discrepancies through discussion or by contacting a third review author (JJM or FK). We assessed the following domains.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other sources of bias.
We judged risk of bias domains as 'low risk', 'high risk' or 'unclear risk' and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We presented a 'Risk of bias' summary figure to illustrate these findings.
We further summarised the risk of bias across domains for each outcome in each included study, as well as across studies and domains for each outcome.
For performance bias (blinding of participants and personnel), we considered all outcomes similarly susceptible to performance bias and assessed them in one group.
For detection bias (blinding of outcome assessment), we grouped outcomes as susceptible to detection bias (subjective) or not susceptible to detection bias (objective). Objective outcomes: time to death from any cause. Subjective outcomes: serious adverse effects, time to recurrence, time to progression, adverse effects and quality of life.
We assessed attrition bias (incomplete outcome data) on a per‐outcome basis and created groups of outcomes based on similar reporting characteristics. Time‐to‐event outcomes: time to death from any cause, time to recurrence, time to progression; adverse effects outcomes: serious adverse effects, adverse effects; quality‐of‐life outcomes.
Measures of treatment effect
We extracted hazard ratios (HRs) with 95% confidence intervals (CIs) for time to event outcomes (time to recurrence, time to progression and time to death from any cause). Adjusted HRs based on multivariate analysis were preferred to univariate HRs. An indirect estimation method was used to calculate HRs and their variances if they were not reported (Parmar 1998; Tierney 2007; Williamson 2002). We expressed results of dichotomous outcomes (e.g. serious adverse effects, adverse effects) as risk ratios (RRs) with 95% CIs, results of continuous outcomes (e.g. quality of life) as mean difference (MD) with corresponding 95% CI, unless different studies use different measures to assess the same outcome, in which case we expressed data as standardised mean differences (SMDs) with 95% CIs.
Unit of analysis issues
The unit of analysis was the individual participant. In the event we identified trials with more than two intervention groups for inclusion in the review, we handled these in accordance with guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Dealing with missing data
We contacted the corresponding author of the original publication to request any missing data. We did not impute missing data and considered only the available data in the analyses. We did not conduct best‐case and worst‐case scenarios.
Assessment of heterogeneity
We examined statistical heterogeneity using the I² statistic. The thresholds for interpretation of the I² statistic are in accordance with the definitions presented in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011):
0% to 40% might not be important;
30% to 60% may represent moderate heterogeneity;
60% to 90% may represent substantial heterogeneity;
75% to 100% considerable heterogeneity.
Subgroup analyses was done for the examination of clinical heterogeneity. For details, see Subgroup analysis and investigation of heterogeneity.
Assessment of reporting biases
To account for possible publication bias, we conducted a combination of electronic and manual searches of multiple databases without language restrictions. In case of sufficient data, we created funnel plots to assess the likelihood of publication bias. Several explanations can be offered for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials) and publication bias. Therefore, we interpreted results with caution (Sterne 2011).
Data synthesis
We performed data synthesis using Review Manager 5 software provided by Cochrane (Review Manager 2014).
In the meta‐analyses, we used the random‐effects model that assumes that the treatment effect among studies varies and, therefore, incorporates the heterogeneity among studies in the synthesis of primary study results. We combined the estimated log HRs using the generic inverse‐variance method, the result of which is presented as pooled HR with 95% CI on a logarithmic scale. HRs were given for BCG compared to MMC, therefore, an HR less than 1 indicates a benefit of BCG. We calculated summary statistics with respect to the RR and its 95% CI using the Mantel‐Haenszel method (Lane 2013).
Three‐arm trials comparing two BCG arms with one MMC arm but without clinical relevant difference in the BCG treatment approaches were included in the meta‐analysis with both treatment arms of BCG versus MMC (Ojea 2007a; Ojea 2007b; Witjes 1996a; Witjes 1996b). The standard error of the HRs were adjusted according to Woods 2010 in order to avoid a unit‐of‐analysis error (i.e. using the participants of the MMC group twice). In Friedrich 2007, we included one MMC arm (six weeks) in the primary meta‐analysis to give attention to the comparable duration of medication in the BCG and the MMC arm. The second MMC arm (three years) was used for a sensitivity analysis.
For adverse effect outcomes, we did not pool study data to give an overall result on adverse effects, as in all studies (except Ojea 2007b) adverse effects were not reported on a per‐patient basis, but as the number of the different adverse effects that had occurred. We chose to present cystitis as a patient‐relevant outcome in Table 1.
Subgroup analysis and investigation of heterogeneity
We explored the following potential sources of clinical heterogeneity using the following subgroup analyses:
different doses of BCG installations;
different doses of MMC installations;
different strains of BCG;
different BCG maintenance therapies (posthoc subgroup analyses).
We used the fixed‐effect models for the subgroup analyses due to the limited number of available studies (Bender 2018).
Sensitivity analysis
We aimed at examining the methodological quality according to risk of bias, by conducting separate meta‐analyses for low risk of bias studies, excluding studies judged as high or unclear (or both) risk of bias. As there were no studies with low risk of bias, this analysis was not performed.
Instead we tested the robustness of results using sensitivity analysis. The fixed‐effect model was used to explore visually if results of the meta‐analysis varied substantially when using a model that does assume homogeneity of effects among studies and gives greater weight to larger studies. Furthermore, the second MMC arm in Friedrich 2007 (three years) was used instead of the six weeks MMC arm for a sensitivity analysis.
'Summary of findings' table
We presented the overall certainty of the evidence for each outcome according to the GRADE approach, which takes into account five criteria related to internal validity (risk of bias, inconsistency, imprecision, publication bias), and external validity, such as directness of results (Guyatt 2008). For each comparison, two review authors (SS and JJM) independently rated the certainty of evidence for each outcome as 'high', 'moderate', 'low' or 'very low' using GRADEpro GDT. We resolved any discrepancies by consensus, or, if needed, by arbitration by a third review author (PD). For each comparison, we presented a summary of the evidence for the main outcomes in Table 1, which provides key information about the best estimate of the magnitude of the effect in relative terms and absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome; and the rating of the overall confidence in effect estimates for each outcome (Guyatt 2011; Schünemann 2011).
Results
Description of studies
Results of the search
The literature search identified 1125 records, of which 12 studies fulfilled our inclusion criteria (based on 29 publications). Eleven were included in the meta‐analyses. The one study that was not included in the meta‐analysis was only available as a conference proceeding (Michielsen 2013), which did not provide sufficient data for inclusion in the analysis. Figure 1 shows the flow chart for the selection of studies. For one study, there was only the trial registry entry available (NCT00974818). This study has been terminated early due to accrual problems. For this review, we used the results available from the clinical trial website for analyses (clinicaltrials.gov/ct2/show/NCT00974818). We identified no relevant ongoing trials.
1.
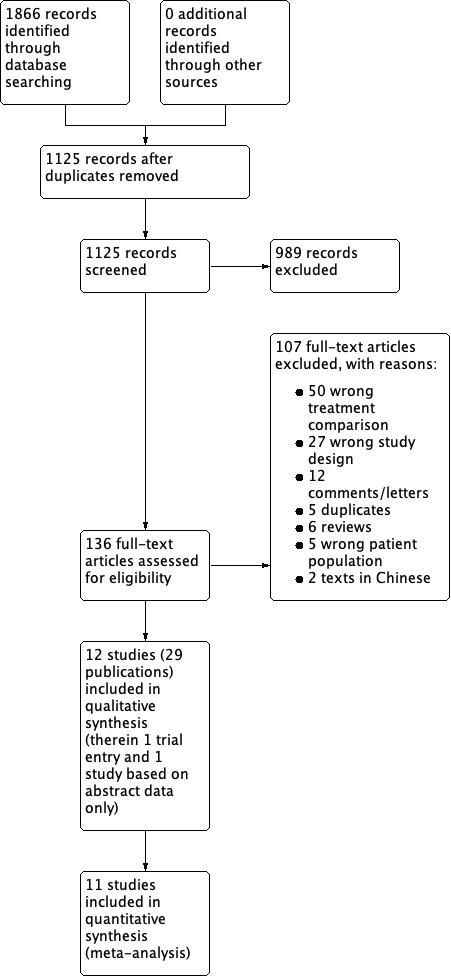
Study flow diagram.
Included studies
The 12 included studies are: Di Stasi 2003; Friedrich 2007; Krege 1996; Lamm 1995; Malmström 1999; Mangiarotti 2008; Michielsen 2013; NCT00974818; Ojea 2007b and Ojea 2007a; Rintala 1991; Witjes 1998a; and Witjes 1996a and Witjes 1996b. In total, the studies randomised 3080 participants. Table 7 gives a detailed description of interventions of included studies, and the Characteristics of included studies table and Table 8 give a detailed description of included studies.
1. Description of interventions.
| Study | Intervention (route, frequency, total dose/day) | Comparator (route, frequency, total dose/day) |
| Michielsen 2013 | I1: BCG group (full dose) for 6 weeks; each group had a specific maintenance programme. | C1: MMC group (40 mg in 50 mL saline) weekly for 6 weeks; each group had a specific maintenance programme. |
| NCT00974818 | I1: MMC 40 mg, dissolved in 20 mL sterile water. | C1: BCG 81 mg, dissolved in 53 mL of diluent and saline. |
| Mangiarotti 2008 | I1: therapy started 1 month after TUR. BCG Tice, weekly instillations for 6 weeks, thereafter once a month for 1 year. | C1: therapy started 1 month after TUR. MMC 40 mg in 50 mL saline for once a week for 8 weeks, thereafter for once a month for 1 year. |
| Friedrich 2007 | I1: 6 weekly instillations of BCG RIVM 2 × 108 cfu (BCG 6 week). Therapy started 4 weeks after TUR. | C1: 6 weekly instillations of MMC 20 mg (MMC 6 week). Therapy started 4 weeks after TUR. |
| C2: 6 weekly instillations of MMC 20 mg followed by monthly instillations of MMC 20 mg for 3 years (MMC 3 year). Therapy started 4 weeks after TUR. | ||
| Ojea 2007b; Ojea 2007a | I1: low‐dose BCG 27 mg. Connaught strain. Instillations started 14–21 days after TUR. The instillations were repeated once a week for 6 weeks followed by another 6 instillations given once every 2 weeks for 12 weeks. | C1: MMC 30 mg, instillations started 14–21 days after TUR. The instillations were repeated once a week for 6 weeks followed by another 6 instillations given once every 2 weeks for 12 weeks. |
| I2: very low‐dose BCG 13.5 mg. Connaught strain. Instillations started 14–21 days after TUR. The instillations were repeated once a week for 6 weeks followed by another 6 instillations given once every 2 weeks for 12 weeks. | ||
| Di Stasi 2003 | I1: Pasteur BCG instillations with 81 mg wet weight (mean 10.2, SEM 9.0 ×
108 cfu). Lyophilised BCG was suspended in 50 mL
bacteriostatic‐free 0.9% saline solution. Suspension was instilled and
retained for 120 minutes. Treatment started 3 weeks after TUR. Participants who had a complete response to the initial 6 weekly treatments underwent a further 10 monthly instillations. If cancer persisted at 3 months, a second 6‐week course was given. If disease persisted at 6 months, there was a cross‐over to a 6‐week second‐line course of BCG for participants in the 2 MMC groups and electromotive MMC for participants in the BCG group. |
C1: participants were placed on fluid restriction and oral sodium bicarbonate before intravesical MMC treatments. Under ultrasound control, the bladder was thoroughly drained by repositioning the catheter or participant, or both. MMC 40 mg with 960 mg excipient NaCl dissolved in 100 mL water was instilled and retained in the bladder for 60 minutes. Treatment started 3 weeks after TUR. |
| C2: participants were placed on fluid restriction and oral sodium bicarbonate before intravesical MMC treatments. Under ultrasound control the bladder was thoroughly drained by repositioning the catheter or participant. Electromotive instillations of MMC 40 mg with 960 mg excipient NaCl dissolved in 100 mL water, retained for 30 minutes with 20 mA pulsed electric current (600 mA minute). Treatment started 3 weeks after TUR. | ||
| Malmström 1999 | I1: BCG (Danish strain 1331) 120 mg containing 1 × l09 cfu, dissolved in 50 mL saline. Therapy was begun 1–3 weeks after TUR or biopsies, and was given weekly for 6 weeks, then monthly for up to 1 year and every 3 months during year 2. | C1: MMC 40 mg dissolved in 50 mL phosphate buffer (pH 7.4). Therapy was begun 1–3 weeks after TUR or biopsies, and was given weekly for 6 weeks, then monthly for up to 1 year and every 3 months during year 2. |
| Witjes 1998a | I1: Intravesical therapy was started 7–15 days after resection. BCG‐RIVM (5 × 108 bacilli in 50 mL saline) was given weekly for 6 consecutive weeks. In case of a recurrence at 3 months, a complete resection was performed, where after in BCG‐treated participants a second course was given. | C1: intravesical therapy was started 7–15 days after resection. MMC 30 mg in 50 mL saline was given weekly for 4 consecutive weeks and thereafter monthly for 5 months. In case of a recurrence at 3 months, a complete resection was performed, and instillations were continued. |
| Krege 1996 | I1: 6 weeks after TUR, BCG 120 mg Connaught strain in 50 mL sodium chloride was instilled intravesically for 1 hour. At the same time, BCG 0.5 mg was applied subcutaneously by multiple punctures in the forearm. Therapy was continued once weekly for 6 weeks and once a month for 4 months. | C1: 6 weeks after TUR, MMC 20 mg in 50 mL sodium chloride was instilled via a catheter and kept in the bladder for 2 hours. Instillations were performed every 2 weeks during year 1 and once a month during year 2. |
| Witjes 1996a; Witjes 1996b | I1: Treatment start 7–20 days after TUR. BCG‐RIVM 5 × 108 bacilli in 50 mL saline was administered once a week for 6 weeks. If disease recurred within 6 months in the BCG treatment group, a second course of 6 weekly instillations was administered after complete tumour resection. | C1: treatment start 7–20 days after TUR. MMC 30 mg in 50 mL saline instilled once a week for 1 month (weeks 1–4) and thereafter once a month for 6 months. If a recurrence was detected in the MMC group, complete resection was carried out and the MMC treatment continued monthly for another 3 months. |
| I2: Treatment start 7–20 days after TUR. BCG‐Tice 5 × 108 bacilli in 50 mL saline was administered once a week for 6 weeks. If disease recurred within 6 months in the BCG treatment group, a second course of 6 weekly instillations was administered after complete tumour resection. | ||
| Lamm 1995 | I1: lyophilised Tice BCG 50 mg 5 × 108 cfu diluted in 50 mL of sterile, preservative‐free saline. The 50 mL suspension was instilled into the bladder by gravity flow. Participants were instructed to lie on their abdomen for 15 minutes and on their left, right and back for 15 minutes each and to retain the suspension, if possible, for 2 hours. Treatments were repeated weekly for 6 weeks and at 8 and 12 weeks, then monthly to 1 year. Treatment was initiated no sooner than 1 week and no later than 2 weeks after TUR. | C1: MMC 20 mg in 20 mL of sterile water. Treatments were repeated weekly for 6 weeks and at 8 and 12 weeks, then monthly to 1 year. Treatment was initiated no sooner than 1 week and no later than 2 weeks after TUR. |
| Rintala 1991 | I1: Intravesical BCG 75 mg in 50 mL distilled water for 2 hours 6 × 108 cfu Pasteur Strain F. Instillations started 2 weeks after TUR. Weekly repetition during the first month, then once a month for 2 years. | C1: MMC 20–40 mg (AUC method) for 2 hours. Instillations started 2 weeks after TUR. Weekly repetition during the first month, then once a month for 2 years. |
aThe term 'clinical practice setting' refers to the specification of the intervention/comparator as used in the course of a standard medical treatment (such as dose, dose escalation, dosing scheme, provision for contraindications and other important features).
AUC: area under the curve; BCG: Bacillus Calmette Guérin; C: comparator; cfu: colony‐forming units; I: intervention; MMC: mitomycin C; NaCl: sodium chloride, TUR: transurethral resection.
2. Baseline characteristics.
| Study | Intervention(s) and comparator(s) | Duration of intervention (duration of follow‐up) | Description of participants | Trial period | Country | Setting |
| Michielsen 2013 | I1: BCG full dose | Weekly for 6 weeks, each group with specific maintenance programme. | Intermediate‐risk non‐muscle invasive urothelial carcinoma of the bladder | — | Belgium | Hospital |
| C1: MMC 40 mg | ||||||
| Mangiarotti 2008 | I1: BCG Tice | BCG weekly for 6 weeks, then 1 × month for 1 year. MMC 1 × week for 8 weeks, then 1 × month for 1 year (follow‐up 42–45 months). |
Intermediate‐risk non‐muscle invasive urothelial carcinoma of the bladder, Ta‐T1 G1‐2 | — | Italy | Hospital |
| C1: MMC 40 mg | ||||||
| Friedrich 2007 | I1: BCG RIVM 2 × 108 cfu | All 3 treatments for 6 weeks; long‐term MMC continued for 3 years | Intermediate‐risk pTa G1 tumours or pTa G2 up to pT1 tumours (G1‐3) | 1995–2002 | Germany | Hospital |
| C1: MMC 20 mg | ||||||
| C2: MMC 20 mg long‐term | ||||||
| Ojea 2007b; Ojea 2007a | I1: BCG Connaught strain low‐dose 27 mg | Once a week for 6 weeks, followed by another 6 instillations every 2 weeks for 12 weeks. | Intermediate‐risk Ta G2 and T1 G1‐2 without Cis | 1995–1998 | Spain | Hospital, multicentre |
| I2: BCG Connaught strain very low‐dose 13.5 mg | ||||||
| C1: MMC 30 mg | ||||||
| Di Stasi 2003 | I1: BCG Pasteur 81 mg | Weekly for 6 weeks, a further 6 weeks for non‐responders and a follow‐up 10 monthly treatments. | Multifocal Cis and most had concurrent pT1 | 1994–2001 | Italy | Hospital, multicentre |
| C1: MMC 40 mg | ||||||
| C2: MMC 40 mg electromotive | ||||||
| Malmström 1999 | I1: BCG 120 mg Danish strain | Weekly for 6 weeks, then monthly for 1 year and then every 3 months for 3 years. | Ta G1‐3 or T1 G1‐2 | 1987–1992 | Sweden‐Norway | Hospital, multicentre |
| C1: MMC 40 mg | ||||||
| Witjes 1998a | I1: BCG RIVM | MMC: weekly for 4 weeks, then monthly for 5 months. BCG: weekly for 6 weeks. |
pTa and pT1 including Cis | 1985–1986 | Europe | Hospital, multicentre |
| C1: MMC 30 mg | ||||||
| Krege 1996 | I: TUR | BCG: weekly for 6 weeks, then monthly for 4 months. MMC: every 2 weeks for 12 months, then once a months for 2 years. |
pTa/1 G1‐3 | 1985–1992 | Germany | Hospital, multicentre |
| C1: BCG 120 mg Connaught strain | ||||||
| C2: MMC 20 mg | ||||||
| Witjes 1996a; Witjes 1996b | I1: BCG RIVM 5 × 108 bacilli | BCG: weekly for 6 weeks, a further 6 weeks for non‐responders. MMC: once a week for 1 month, then once a month for 6 months, for non‐responders monthly another 3 months. |
Ta or T1 including Cis | 1987–1990 | — | Hospital, multicentre |
| I2: BCG Tice 5 × 108 bacilli | ||||||
| C1: MMC 30 mg | ||||||
| Lamm 1995 | I1: BCG Tice 50 mg (5 × 108 cfu) | Weekly for 6 weeks and at 8 and 12 weeks, then monthly to 1 year. | Ta or T1 at increased risk | — | — | Hospital, multicentre |
| C1: MMC 20 mg | ||||||
| Rintala 1991 | I1: BCG Pasteur strain 75 mg | Weekly for 1 month, then once per months for 2 years. | Cis G1‐3, Ta‐T1 G1‐3 | 1984–1987 | — | Hospital, multicentre |
| C1: MMC 20–40 mg |
BCG: Bacillus Calmette‐Guérin; Cis: carcinoma in situ; cfu: colony‐forming units; MMC: mitomycin C; NaCl: sodium chloride, TUR: transurethral resection.
Study design and settings
Most of the studies were multicentre prospective RCTs, except Mangiarotti 2008, which was a single‐centre study. Di Stasi 2003; Friedrich 2007; Krege 1996; Ojea 2007b; Ojea 2007a; Witjes 1996a; Witjes 1996b were three arm studies. The studies of Ojea and Witjes are introduced twice in the reference section, as we have used the arms separately in the analyses. All trials were conducted in the hospital setting and most were conducted in Europe. Studies were published from 1991 to 2013.
Participants
A total of 2932 participants were randomised to either BCG or MMC. Follow‐up ranged from 20 month to 20 years. Rintala 1991 reported the longest follow‐up. Trials included men and women with histologically confirmed pTa/T1 grades 1 to 3 of intermediate‐ or high‐risk non‐muscle invasive transitional cell carcinoma of the bladder. Participants had undergone a prior transurethral resection without prior adjuvant therapy. Major exclusion criteria were: prior cancer, muscle invasive disease, concurrent treatment with chemotherapy or radiotherapy and pregnancy.
Interventions and comparators
BCG dosages ranged from 120 mg (Krege 1996; Malmström 1999) to 13.5 mg (very low dose, Ojea 2007a). Studies used different BCG strains (Tice, RIVM, Connaught and Pasteur). Most studies administered BCG weekly for six weeks, followed by different maintenance schemes. Rintala 1991 started BCG therapy with weekly instillations for four weeks. MMC dosages were 20 mg (Friedrich 2007; Krege 1996; Lamm 1995), 30 mg (Ojea 2007b; Witjes 1996a; Witjes 1996b; Witjes 1998a), or 40 mg (Di Stasi 2003; Malmström 1999; Mangiarotti 2008; Michielsen 2013). Rintala 1991 administered MMC 20 mg to 40 mg. Instillations were mostly given weekly for six weeks. Mangiarotti 2008 used a weekly schedule of eight weeks, Witjes 1996a; Witjes 1996b; Witjes 1998a; and Rintala 1991 used weekly for four weeks and Krege 1996 used every two weeks instillations for 12 months.
Outcomes
Most data were available for time to recurrence (11 studies, 2616 participants), followed by adverse effects. Five studies reported time to death from any cause (Di Stasi 2003; Lamm 1995; Malmström 1999; Rintala 1991; Witjes 1998a; 1132 participants). Six studies provided information on time to progression (Di Stasi 2003; Lamm 1995; Malmström 1999; Ojea 2007a; Ojea 2007b; Witjes 1998a; 1622 participants). Reporting of adverse effects was inhomogeneous. Studies reported on 18 different adverse effects. Only one study aimed at evaluating quality of life in these participant groups (Michielsen 2013). Information was only available in abstract form (conference proceeding) and hence gave no further insights.
Funding sources and conflicts
Three studies had at least one coauthor with a financial relationship with a company or the study was at least partly financed by a company (Di Stasi 2003; Friedrich 2007; Malmström 1999). Four studies provided no information on funding (Mangiarotti 2008; Michielsen 2013; Ojea 2007b; Witjes 1996a).
Excluded studies
A list of 95 excluded studies is in the Characteristics of excluded studies table.
Risk of bias in included studies
The Characteristics of included studies table, Figure 2, and Figure 3 show the detailed risk of bias evaluation. In summary, unclear or incomplete reporting in primary studies seriously hindered definitive risk of bias assessment.
2.
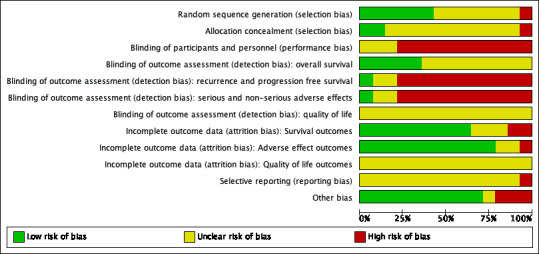
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
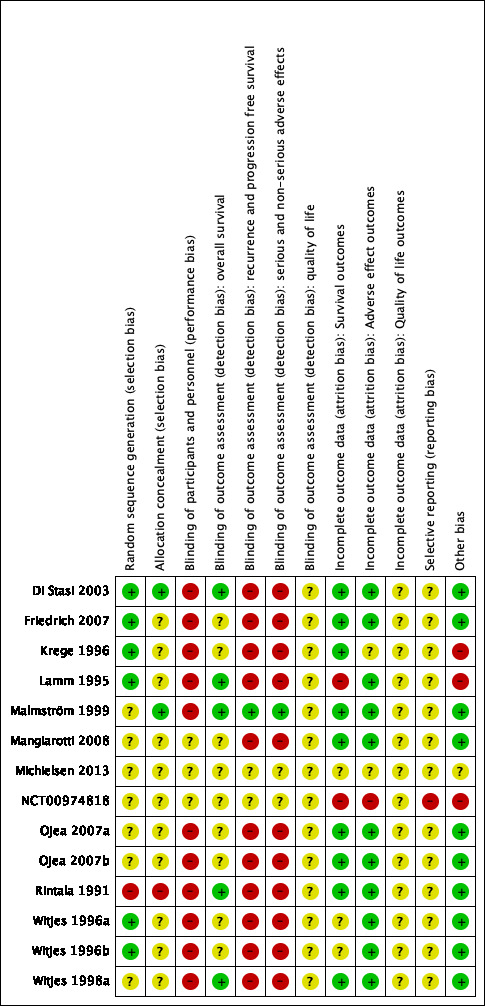
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Malmström 1999; Mangiarotti 2008; Michielsen 2013; NCT00974818; Ojea 2007b; Ojea 2007a; and Witjes 1998a had unclear random sequence generation. One study randomised participants to treatment arms, but based allocation on date of birth and so was judged at high risk of bias (Rintala 1991). The remaining studies had low risk of random sequence generation (Di Stasi 2003; Friedrich 2007; Krege 1996; Lamm 1995; Witjes 1996a; Witjes 1996b).
Allocation concealment
Most studies did not report allocation concealment and, therefore, this domain was at unclear risk of bias (Friedrich 2007; Krege 1996; Lamm 1995; Mangiarotti 2008; Ojea 2007a; Witjes 1996a; Witjes 1996b; Witjes 1998a). Only Di Stasi 2003 and Malmström 1999 reported the method for allocation concealment, which was adequate and at low risk. Rintala 1991 was at high risk as participant selection was based on date of birth, which might have influenced the concealment of the allocation.
Blinding
Blinding of participants and personnel
None of the studies reported that blinding was done. Given that blinding is a well‐known mechanism to reduce bias in trials, we assumed that if blinding was not reported, it was not done. Therefore, we judged this domain at high risk of bias for most outcomes. For the clinical trial entry (NCT00974818) and the study that was only available as conference proceeding (Michielsen 2013), we rated this domain as unclear.
Blinding of outcome assessment
We judged that a lack of blinding had no effect on assessment of objective outcomes, such as survival or death. For the studies that evaluated time to death from any cause, this domain was rated at low risk of bias, although blinding was not performed.
For outcomes based on a more subjective assessment (time to recurrence and time to progression, adverse effects and serious adverse effects), we judged this domain at high risk of bias.
Only one study assessed quality of life (Michielsen 2013), Unfortunately, the conference proceeding did not provide sufficient information on trial methodology and conduct. Therefore, all studies were at unclear risk of bias for quality of life.
Incomplete outcome data
Most studies clearly reported participant flow and there was no indication of important attrition bias.
Time‐to‐event outcomes
In the study of Lamm 1995 there was a concern regarding the time to death from any cause outcomes as only 85% (BCG) and 84% (MMC) of participants were included in the analyses. In NCT00974818, there was no analysis for time to death from any cause due to a lack of accrual. Also, the number of participants throughout the website entry was not congruent. Thus, we rated it at high risk of bias.
Adverse effect outcomes
The only concern was in the NCT00974818 study where the number of participants throughout the website entry was not congruent, which might indicate a possible bias. In the Krege 1996 study, there was no precise information on the number of patients included in this analysis. In the conference proceeding of Michielsen 2013 there is insufficient information to rate the bias due to attrition.
Quality of life outcomes
One study assessed quality of life but the conference proceeding gave no detailed results and was at high risk of bias (Michielsen 2013). Therefore, all studies were at unclear risk of bias.
Selective reporting
Most studies had no study protocol available. Therefore, we judged this domain as unclear in all but one study (NCT00974818). In NCT00974818, there was no information why data on the primary outcome (relapse rate) were not reported but data on the secondary outcomes (adverse effects) were. Therefore, we rated this domain at high risk of bias.
Other potential sources of bias
We identified no other sources of bias.
Effects of interventions
See: Table 1
The effects of the intervention are presented in Table 1 for the main outcomes. All other effects are presented in Figure 4; Figure 5; Figure 6; Figure 7; Figure 8; and Figure 9. None of the included studies calculated the sample size with respect to time to death from any cause to achieve a certain power.
4.
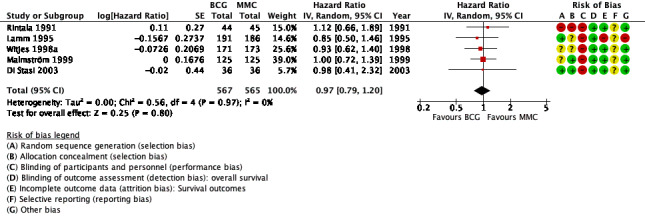
Forest plot of comparison: 1 Bacillus Calmette‐Guérin (BCG) versus mitomycin C (MMC), outcome: 1.1 Time to death from any cause.
5.
Forest plot of comparison: 1 Bacillus Calmette‐Guérin (BCG) versus mitomycin C (MMC), outcome: 1.2 Serious adverse effects.
6.
Forest plot of comparison: 1 Bacillus Calmette‐Guérin (BCG) versus mitomycin C (MMC), outcome: 1.3 Time to recurrence.
7.
Funnel plot of comparison: 1 Bacillus Calmette‐Guérin (BCG) versus mitomycin C (MMC), outcome: 1.3 Time to recurrence.
8.
Forest plot of comparison: 1 Bacillus Calmette‐Guérin (BCG) versus mitomycin C (MMC), outcome: 1.4 Time to progression.
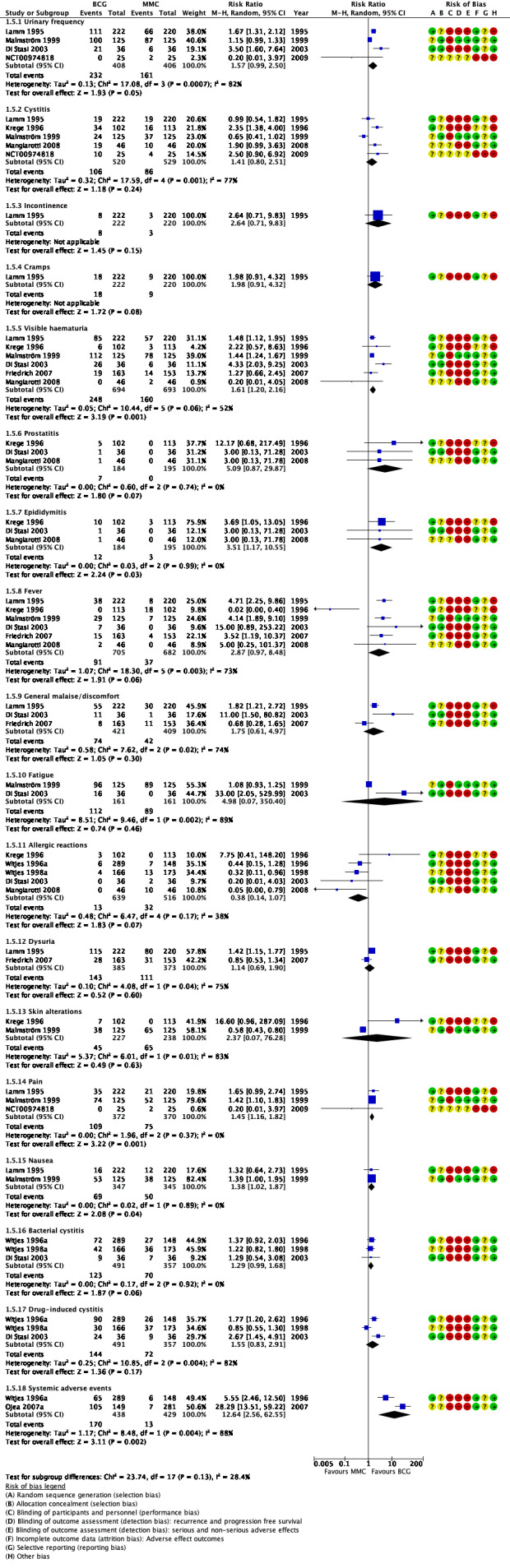
9.
Forest plot of comparison: 1 Bacillus Calmette‐Guérin (BCG) versus mitomycin C (MMC), outcome: 1.5 Adverse effects.
1 Bacillus Calmette‐Guérin versus mitomycin C
1.1 Primary outcomes
1.1.1 Time to death from any cause
BCG may have little or no effect on time to death from any cause in adults with intermediate‐ and high‐risk non‐muscle invasive bladder cancer (HR 0.97, 95% CI 0.79 to 1.20; studies = 5, participants = 1132; 567 participants in the BCG arm and 565 in the MMC arm; I² = 0%; Analysis 1.1; Figure 4). This corresponds to six fewer deaths (40 fewer to 36 more) per 1000 participants with BCG at five years.
1.1. Analysis.
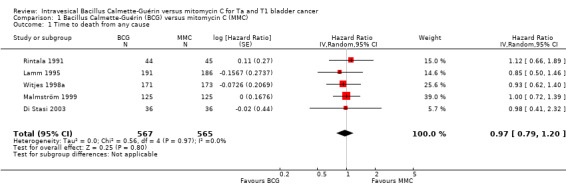
Comparison 1 Bacillus Calmette‐Guérin (BCG) versus mitomycin C (MMC), Outcome 1 Time to death from any cause.
Certainty of the evidence was low because of study limitations (performance bias and allocation concealment) and imprecision (the CIs were wide with a possibility for either important benefit or harm). The results are based on study data with different lengths of follow‐up (3.5 to 20 years).
1.1.2 Serious adverse effects
Twelve of 577 participants on BCG had serious non‐fatal adverse effects compared to four of 447 participants in the MMC group. BCG may increase the risk of experiencing a serious adverse event. The pooled RR was 2.31 (95% CI 0.82 to 6.52; studies = 5, participants = 1024; I² = 0%; Analysis 1.2; Figure 5); although BCG may increase the risk for serious adverse effects compared to MMC, the 95% CI includes the possibility of no difference. This corresponds to nine more serious adverse effects (1 fewer to 37 more) with BCG. Certainty of the evidence was low because of study limitations (performance bias and allocation concealment) and the CIs were wide and were consistent with both no effect and clinically relevant harm). Length of follow‐up among the studies ranged from 1.6 to 10 years.
1.2. Analysis.
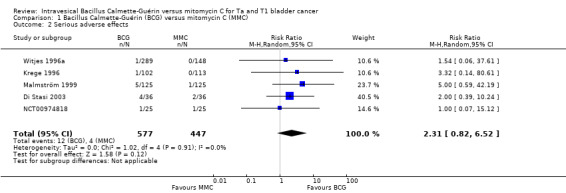
Comparison 1 Bacillus Calmette‐Guérin (BCG) versus mitomycin C (MMC), Outcome 2 Serious adverse effects.
1.2 Secondary outcomes
1.2.1 Time to recurrence
Pooled data demonstrated a 12% hazard reduction over time for BCG (HR 0.88, 95% CI 0.71 to 1.09; studies = 11, participants = 2616; 1273 participants in the BCG arm and 1343 in the MMC arm; I² = 61%; Analysis 1.3; Figure 6). This corresponds to 41 fewer recurrences (104 fewer to 29 more) with BCG at five years. These data are based on a follow‐up from 3 to 20 years.
1.3. Analysis.
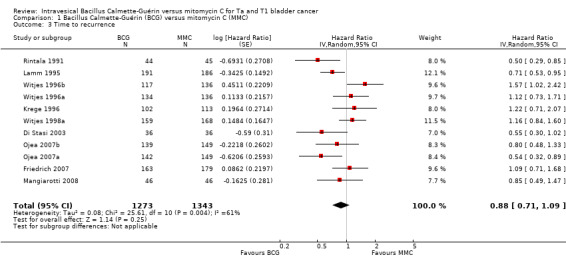
Comparison 1 Bacillus Calmette‐Guérin (BCG) versus mitomycin C (MMC), Outcome 3 Time to recurrence.
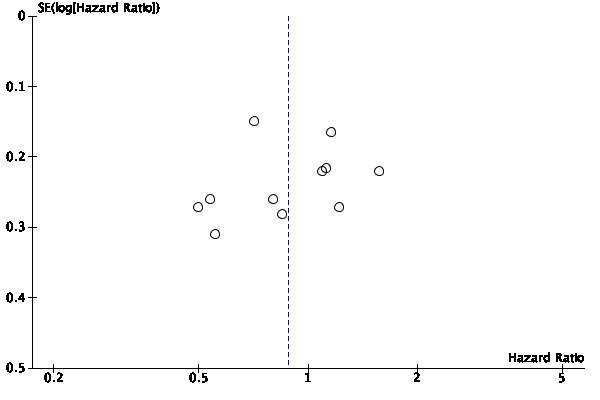
Certainty of the evidence was low because of study limitations (performance bias and allocation concealment), the CIs were imprecise (possibility for either important benefit or large harm), and the results of the point estimates of primary studies varied substantially and showed inconsistency. In aggregate, we downgraded twice. The funnel plot showed no asymmetry (Figure 7). Hence, we did not downgrade for publication bias.
1.2.2 Time to progression
BCG may have little to no effect on time to progression in adults with intermediate‐ and high‐risk non‐muscle invasive bladder cancer (HR 0.96, 95% CI 0.73 to 1.26; studies = 6, participants = 1622; 804 participants in the BCG arm and 818 in the MMC arm; I² = 0%; Analysis 1.4; Figure 8). This corresponds to four fewer progressions (29 fewer to 27 more) with BCG at five years. Certainty of the evidence was low because of study limitations (performance bias and allocation concealment) and the CIs were imprecise (possibility for both important benefit or large harm). Length of follow‐up ranged from 1.6 to 20 years.
1.4. Analysis.
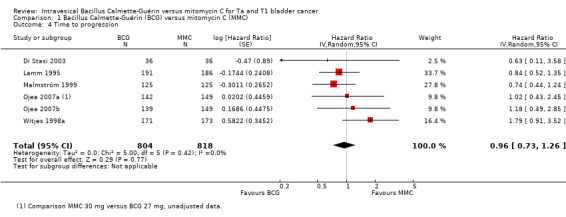
Comparison 1 Bacillus Calmette‐Guérin (BCG) versus mitomycin C (MMC), Outcome 4 Time to progression.
1.2.3 Adverse effects
Reporting of adverse effects was heterogeneous in the included studies. The studies reported 18 different adverse effects. Adverse events were as follows (Analysis 1.5; Figure 9):
1.5. Analysis.
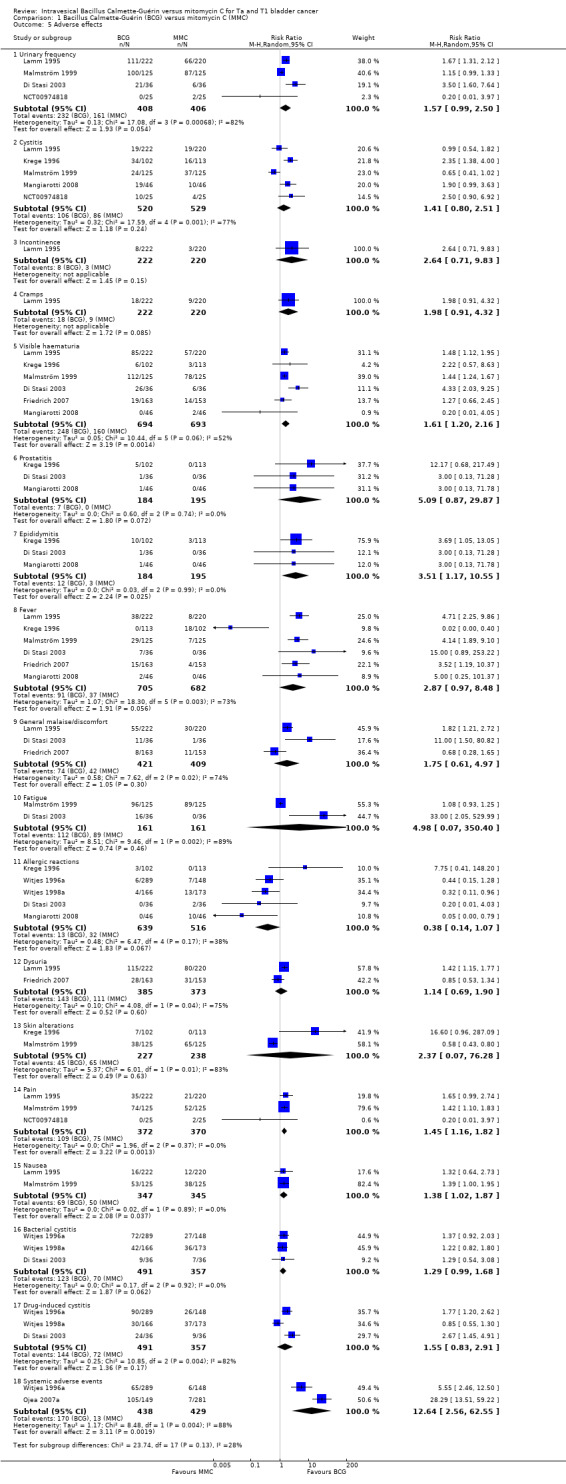
Comparison 1 Bacillus Calmette‐Guérin (BCG) versus mitomycin C (MMC), Outcome 5 Adverse effects.
urinary frequency: RR 1.57, 95% CI 0.99 to 2.50; studies = 4, participants = 814; I² = 82%;
cystitis: RR 1.41, 95% CI 0.80 to 2.51; studies = 5, participants = 1049; I² = 77%;
incontinence: RR 2.64, 95% CI 0.71 to 9.83; studies = 1, participants = 442; I² = 0%;
cramps: RR 1.98, 95% CI 0.91 to 4.32; studies = 1, participants = 442; I² = 0%;
visible haematuria: RR 1.61, 95% CI 1.20 to 2.16; studies = 6, participants = 1387; I² = 52%;
prostatitis: RR 5.09, 95% CI 0.87 to 29.87; studies = 3, participants = 379; I² = 0%;
epididymitis: RR 3.51, 95% CI 1.17 to 10.55; studies = 3, participants = 379; I² = 0%;
fever: RR 2.87, 95% CI 0.97 to 8.48; studies = 6, participants = 1387; I² = 73%;
general malaise/discomfort: RR 1.75, 95% CI 0.61 to 4.97; studies = 3, participants = 830; I² = 74%;
fatigue: RR 4.98, 95% CI 0.07 to 350.40; studies = 2, participants = 322; I² = 0%;
allergic reactions: RR 0.38, 95% CI 0.14 to 1.07; studies = 5, participants = 1155; I² = 38%);
dysuria: RR 1.14, 95% CI 0.69 to 1.90; studies = 2, participants = 758; I² = 75%);
skin alterations: RR 2.37, 95% CI 0.07 to 76.28; studies = 2, participants = 465; I² = 83%);
pain: RR 1.45, 95% CI 1.16 to 1.82; studies = 3, participants = 742; I² = 0%);
nausea: RR 1.38, 95% CI 1.02 to 1.87; studies = 2, participants = 692; I² = 0%);
bacterial cystitis: RR 1.29, 95% CI 0.99 to 1.68; studies = 3, participants = 848; I² = 0%);
drug‐induced cystitis: RR 1.55, 95% CI 0.83 to 2.91; studies = 3, participants = 848; I² = 82%);
systemic adverse effects: RR 12.64, 95% CI 2.56 to 62.55; studies = 2, participants = 867; I² = 88%).
1.2.4 Quality of life
One study evaluated quality of life (Michielsen 2013). Information was only available as a conference proceeding. The study used the EORTC‐BLS‐24 instrument. There were no statistical differences when comparing groups, except for abdominal bloating and flatulence, which was worse in the BCG group.
More detailed results on quality of life were not available.
2 Subgroup analyses
Below we present the results of the subgroup analyses. All other initially planned subgroup analyses could not be conducted due to a lack of data.
2.1 Different doses of Bacillus Calmette‐Guérin installations (subgroup analyses)
In Analysis 2.1, we tested the effect of different doses of BCG on serious adverse effects. Compared to MMC, BCG 120 mg (RR 4.46, 95% CI 0.76 to 26.16; studies = 2, participants = 465; I² = 0%) showed higher serious adverse effects than BCG administered in lower doses (less than 120 mg: RR 1.64, 95% CI 0.46 to 5.86; studies = 3, participants = 559; I² = 0%). The difference of the subgroup test showed no statistical difference (P = 0.37, I² = 0%). This was the only subgroup analysis possible in this context. Results are shown graphically in Figure 10.
2.1. Analysis.
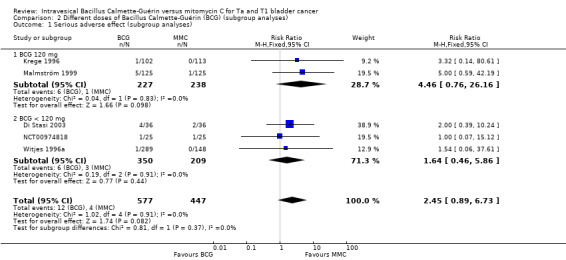
Comparison 2 Different doses of Bacillus Calmette‐Guérin (BCG) (subgroup analyses), Outcome 1 Serious adverse effect (subgroup analyses).
10.
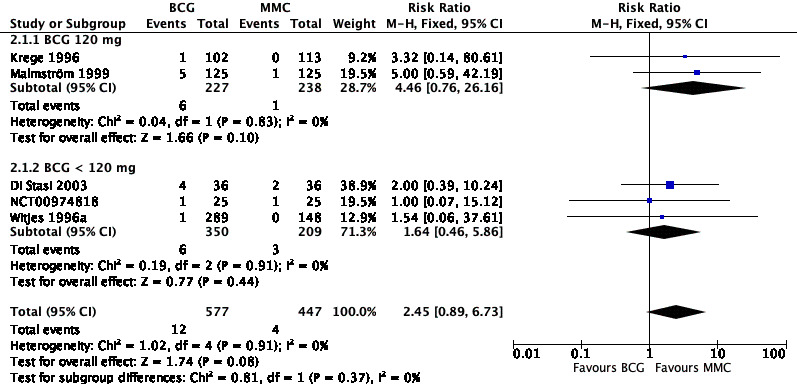
Forest plot of comparison: 2 Different doses of Bacillus Calmette‐Guérin (BCG) (subgroup analyses), outcome: 2.1 Serious adverse effect (subgroup analyses).
2.2 Different doses of mitomycin C installations (subgroup analyses)
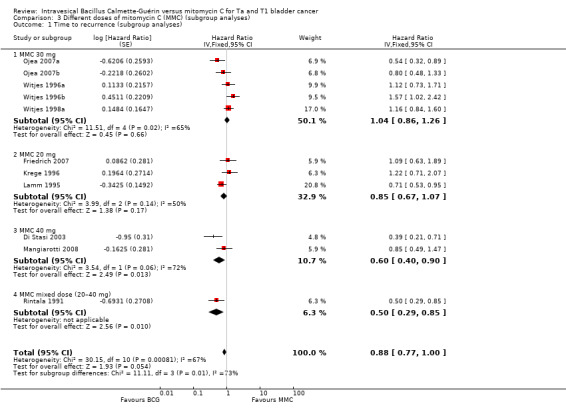
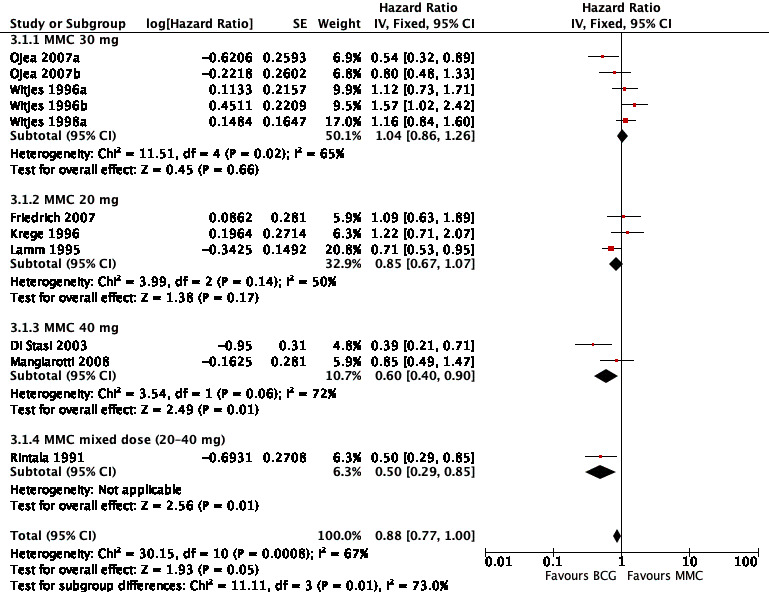
In Analysis 3.1, we tested the effect of different doses of MMC on time to recurrence (see Figure 11). Compared to BCG, MMC 30 mg (HR 1.04, 95% CI 0.86 to 1.26; studies = 5, participants = 0; I² = 65%) showed little or no effect compared to MMC 20 mg (HR 0.85, 95% CI 0.67 to 1.07; studies = 3, participants = 0; I² = 50%). MMC 40 mg had a longer time to recurrences (HR 0.60, 95% CI 0.40 to 0.90; studies = 2, participants = 0; I² = 72%), but data were based on two studies with high heterogeneity. The difference of the subgroup test showed statistical difference (P = 0.01, I² = 73%). This was the only outcome we could address.
3.1. Analysis.
Comparison 3 Different doses of mitomycin C (MMC) (subgroup analyses), Outcome 1 Time to recurrence (subgroup analyses).
11.
Forest plot of comparison: 3 Different doses of mitomycin C (MMC) (subgroup analyses), outcome: 3.1 Time to recurrence (subgroup analyses).
2.3 Different strains of Bacillus Calmette‐Guérin (subgroup analyses)
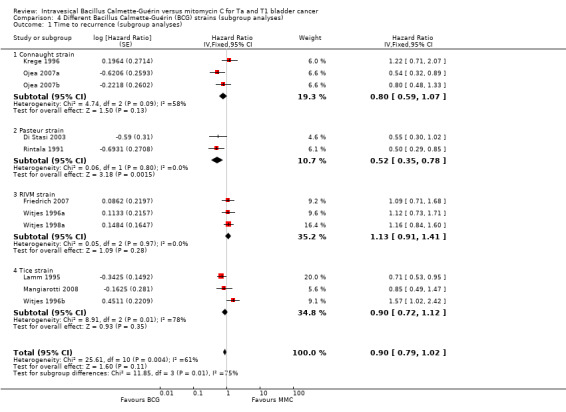
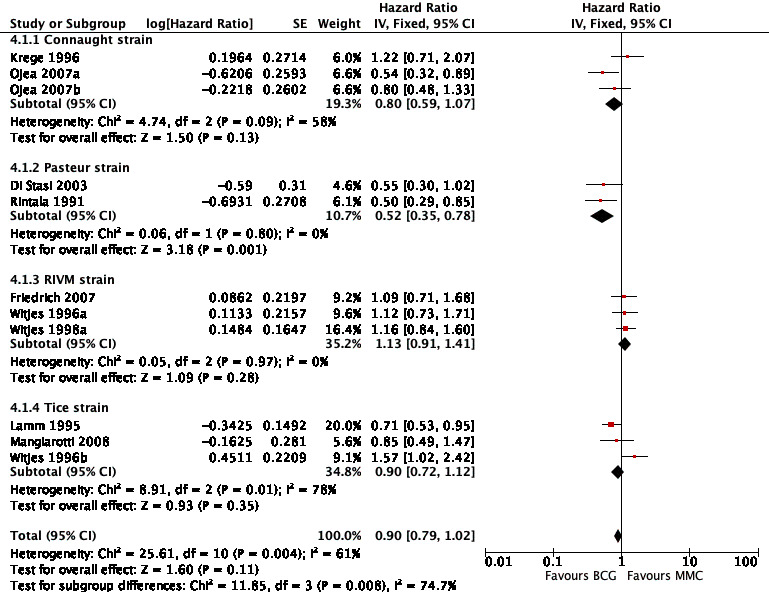
In Analysis 4.1, we tested the effect of different BCG strains compared to MMC on time to recurrence. Findings suggested that there might be relevant differences among BCG strains regarding time to recurrence. Especially the Pasteur strain, but also the Connaught and Tice strains showed some effects on recurrence. The RIVM strain might be less effective. Results are presented in Figure 12.
4.1. Analysis.
Comparison 4 Different Bacillus Calmette‐Guérin (BCG) strains (subgroup analyses), Outcome 1 Time to recurrence (subgroup analyses).
12.
Forest plot of comparison: 4 Different Bacillus Calmette‐Guérin (BCG) strains (subgroup analyses), outcome: 4.1 Time to recurrence (subgroup analyses).
Connaught strain: HR 0.80, 95% CI 0.59 to 1.07; studies = 3; I² = 58%.
Pasteur strain: HR 0.52, 95% CI 0.35 to 0.78; studies = 2; I² = 0%.
RIVM strain: HR 1.13, 95% CI 0.91 to 1.41; studies = 3; I² = 0%.
Tice strain: HR 0.90, 95% CI 0.72 to 1.12; studies = 3; I² = 78%.
The test for subgroup differences was statistically significant (P = 0.008, I² = 74.7%).
2.4 Different Bacillus Calmette‐Guérin maintenance therapies (subgroup analyses)
In Analysis 5.1; Analysis 5.2; Analysis 5.3; and Analysis 5.4, we tested the effect of different BCG maintenance therapies between each other. We compared induction regimens (six weeks or greater) versus maintenance regimens (greater than one year).
5.1. Analysis.
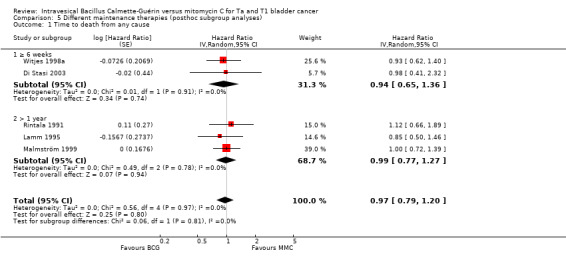
Comparison 5 Different maintenance therapies (posthoc subgroup analyses), Outcome 1 Time to death from any cause.
5.2. Analysis.
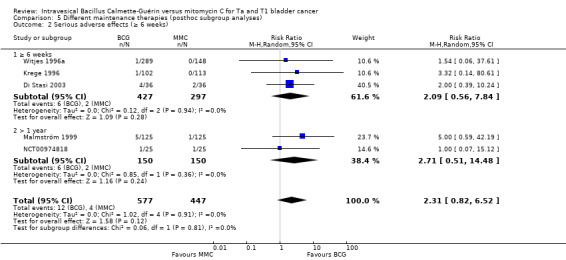
Comparison 5 Different maintenance therapies (posthoc subgroup analyses), Outcome 2 Serious adverse effects (≥ 6 weeks).
5.3. Analysis.
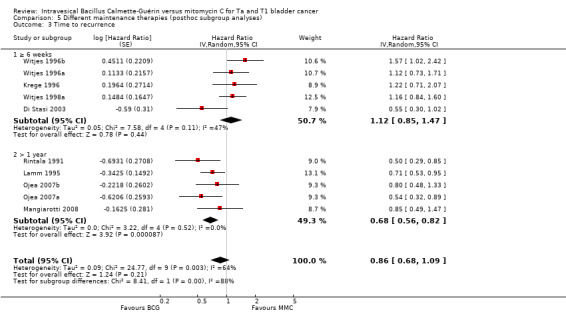
Comparison 5 Different maintenance therapies (posthoc subgroup analyses), Outcome 3 Time to recurrence.
5.4. Analysis.
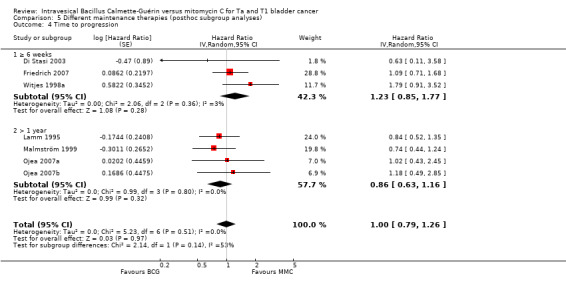
Comparison 5 Different maintenance therapies (posthoc subgroup analyses), Outcome 4 Time to progression.
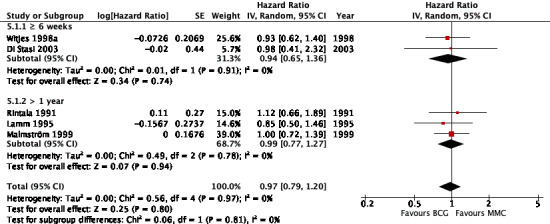
2.4.1 Time to death from any cause
Figure 13 shows the results of the time to death from any cause analysis. Results were as follows: six weeks or greater: HR 0.94, 95% CI 0.65 to 1.36; studies = 2, participants = 416; greater than one year group: HR 0.99, 95% CI 0.77 to 1.27; studies = 3, participants = 339 (Analysis 5.1). The test for subgroup effect was not significant (P = 0.81).
13.
Forest plot of comparison: 5 Different maintenance therapies (posthoc subgroup analyses), outcome: 5.1 Time to death from any cause.
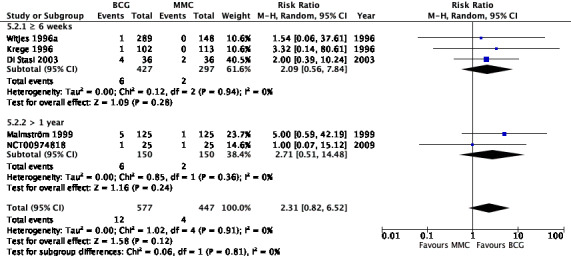
2.4.2 Serious adverse effects
Results for the subgroup analyses for serious adverse effects were as follows: BCG induction therapy six weeks or greater: RR 2.09, 95% CI 0.56 to 7.84; studies = 3, participants = 724; I² = 0%); BCG maintenance therapy greater than one year: RR 2.71, 95% CI 0.51 to 14.48; studies = 2, participants = 300; I² = 0%) (Analysis 5.2; Figure 14). The test for subgroup effect was not significant (P = 0.81).
14.
Forest plot of comparison: 5 Different maintenance therapies (posthoc subgroup analyses), outcome: 5.2 Serious adverse effects (greater than six weeks).
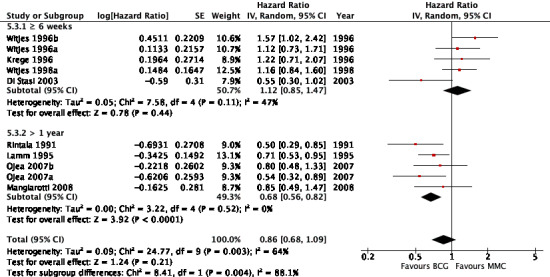
2.4.3 Time to recurrence
Eight studies reported data on time to recurrence for this subgroup analysis. Results were as follows: six weeks or greater group (HR 1.12, 95% CI 0.85 to 1.47; participants = 1137; studies = 4; Analysis 5.3; Figure 15). BCG maintenance therapy greater than one year (HR 0.68, 95% CI 0.56 to 0.82; studies = 4, participants = 89). The test for subgroup effect was significant (P = 0.004), but showed high heterogeneity (I² = 88%).
15.
Forest plot of comparison: 5 Different maintenance therapies (posthoc subgroup analyses), outcome: 5.3 Time to recurrence.
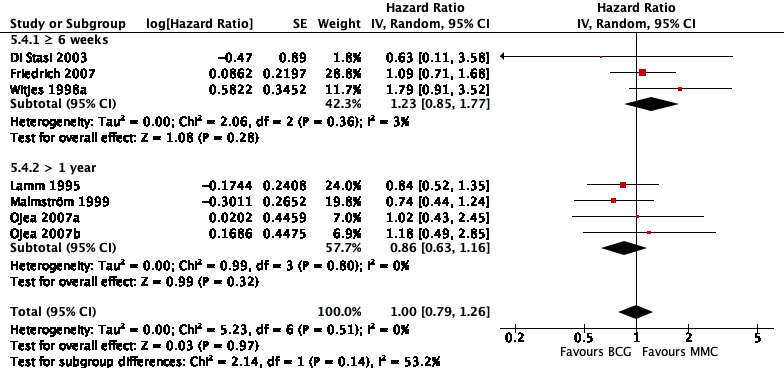
2.4.4 Time to progression
Six studies reported data on time to progression for this subgroup analysis. Results were as follows: six weeks or greater group (HR 1.23, 95% CI 0.85 to 1.77; participants = 416; studies = 3); BCG maintenance therapy greater than one year (HR 0.86, 95% CI 0.63 to 1.16; studies = 3, participants = 250). The test for subgroup effect was not significant (P = 0.14; Analysis 5.4; Figure 16).
16.
Forest plot of comparison: 5 Different maintenance therapies (posthoc subgroup analyses), outcome: 5.4 Time to progression.
3 Sensitivity analyses
The use of the fixed‐effect model compared to the random‐effect model showed no relevant differences (data not shown). Friedrich 2007 only reported summary data for time to recurrence. In a sensitivity analysis using the BCG six weeks arm versus the MMC three years arm for Friedrich 2007 (instead of MMC six weeks arm; adjusted HR 2.87, 95% CI 1.67 to 4.90) resulted in an overall HR of 0.95 (95% CI 0.71 to 1.26; I² = 76%; random‐effect model) and thus a smaller treatment difference for recurrence‐free survival.
Discussion
Summary of main results
This latest update of a prior Cochrane Review (Shelley 2003) on the question of BCG versus MMC for people with intermediate‐ or high‐grade non‐muscle invasive bladder tumours based on 12 RCTs provides evidence of low certainty for all outcomes except quality of life to inform clinical and health policy decision‐making.
Data suggested that BCG probably reduces the risk of recurrence over time (450 recurrences per 1000 participants treated with MMC and 41 fewer recurrences with BCG), but may result in more serious adverse effects (7 serious adverse effects per 1000 participants treated with MMC and 9 more serious adverse effects with BCG). BCG may have little or no effect on time to death from any cause or time to progression. Studies reported several adverse effects with BCG and MMC treatment. We found no available RCT evidence for quality of life.
Overall completeness and applicability of evidence
This review was based on 12 RCTs of people with intermediate‐ and high‐risk non‐muscle invasive bladder tumours. Results were based on a systematic literature search including several databases. Two review authors assessed studies for inclusion and evaluated the certainty of the evidence. The characteristics of participants and treatments are likely to reflect daily clinical practice. Thus, included studies provide direct evidence to the review question.
The first Cochrane Review on this topic was published in 2003 (Shelley 2003), and included seven trials based on 1901 participants. This review update includes further five trials and was based on 3080 participants. It now reflects also the current Cochrane methodology, which includes the certainty of the evidence assessment according to the GRADE approach.
We identified substantial heterogeneity in our analyses (I² = 66% for the analyses of time to recurrence and I² = 77% for cystitis). This may be due to differences in study design (e.g. in length of follow‐up, BCG strains used, treatment dosage and schedule) as well as due to different baseline risks for recurrence and progression of included participants.
In this review, we used the EAU risk categories, which differ from the risk categories set up by the American Urological Association (AUA). Applying the AUA risk categories would likely impact the results of this review.
We were unable to assess treatment effects between intermediate‐ and high‐risk groups, which may differ.
Quality of the evidence
The judgement of low certainty of the evidence for all outcomes with available data means that further research is very likely to have an important impact on the confidence in the estimates of effects and is likely to change the estimates.
Of the 12 identified studies, six were planned and conducted in the 1990s and do not meet 2019s methodological quality standards. Only one trial was conducted after 2010 but results of this trial have not been published yet. One trial (recruitment 2009 to 2012) was closed prior to finalisation due to a lack of accrual. Blinding of participants did not take place in any of the 12 trials. General concerns, which led to downgrading, were study limitations (performance bias and allocation concealment), wide CIs resulting in imprecision (possibility for either important benefit or large harm) and study heterogeneity.
The availability of low‐certainty evidence for non‐muscle‐invasive bladder cancer only is not surprising. One meta‐analysis revealed that the evidence on transurethral resection versus transurethral resection plus chemotherapy (MMC and other) was also low to very low (Perlis 2013). Although this is not the study question addressed in the review here, it highlights similar methodological issues.
Potential biases in the review process
We conducted an extensive systematic literature search without language or publication date restrictions as well as a search in clinical trial registries for unpublished, planned or ongoing studies. Therefore, we have probably identified all relevant information on this topic. However, there is always a possibility that relevant publications may not have been identified.
This review follows standard Cochrane methodology including the latest MECIR standards. No funding was received for this review and the authors state that they have no financial conflicts of interests.
Agreements and disagreements with other studies or reviews
The Agency for Healthcare Research and Quality conducted a systematic review with quality evaluation of included evidence (AHRQ 2016). The authors identified no difference between BCG and MMC therapy for cancer recurrence (RR 0.95, 95% CI 0.81 to 1.11; 10 trials). This is in contrast to our results that included two additional studies (Michielsen 2013; Rintala 1991). Our findings suggested an effect (HR 0.88, 95% CI 0.71 to 1.09) although the CIs did cross the line of no effect. Based on a subgroup analysis, the AHRQ review further indicated a decreased risk for cancer recurrence using BCG versus MMC (RR 0.79, 95% CI 0.71 to 0.87; 5 trials). It found no difference between BCG and MMC for all‐cause mortality, bladder cancer‐specific mortality or progression (all‐cause mortality: RR 0.94, 95% CI 0.83 to 1.0, 7 trials; bladder cancer‐specific mortality: RR 0.77, 95% CI 0.54 to 1.10, 5 trials; progression: RR 0.88, 95% CI 0.66 to 1.17, 7 trials). However, BCG also increased the risk of local adverse events and fever when compared with MMC (AHRQ 2016).
One individual participant data meta‐analysis based on 9/12 RCTs included in this review concluded that only when BCG was used in the form of maintenance therapy was it superior to MMC with regard to prevention of recurrences (Malmström 2009a). There was no meaningful difference between BCG and MMC unless treatment was stratified by the receipt of maintenance therapy. Also, there was no difference concerning overall survival, cancer‐specific survival, and progression. The effect on recurrence for the BCG maintenance therapy group remained statistically significant independently of prior chemotherapy treatment (Malmström 2009a). Three per cent of included participants belonged to the low‐risk group, 74% to the intermediate‐risk group and 23% to the high‐risk group (median follow‐up of 4.4 years, maximum 17.7 years). This meta‐analysis further concluded that the optimal strain, dosage and duration of BCG maintenance therapy remains unknown (Malmström 2009b).
One systematic review with network meta‐analyses (including 65 trials of 12,246 participants) not limited to MMC as a comparator concluded that no definitive conclusion could be drawn regarding superiority of a given BCG strain and recurrence reduction (Boehm 2017). Available clinical trials lack important methodological safeguards against bias; therefore, higher‐quality head‐to‐head comparisons are needed to address this question (Boehm 2017; Miyazaki 2018). Our subgroup results suggested a relevant positive effect among BCG strains, especially for the Pasteur strain, but also for the Connaught and Tice strains on time to recurrence. The RIVM strain may be less effective. However, these subgroup analyses are based on few studies and few participants and should be interpreted with caution.
Differences in the results of existing systematic reviews might be due to differences in included participants of primary trials. The non‐muscle invasive bladder cancer participant group is highly heterogeneous, and may include BCG‐refractory, BCG‐relapsing, BCG‐intolerant and BCG‐unresponsive participants (Packiam 2017). A mixture of these participants in trials can cause difficulty in interpreting the results, especially because some failure types such as BCG‐relapsing participants have superior outcomes in comparison with others (Packiam 2017). Also, different dosing of BCG and MMC regimens (dosage and schedules) may result in heterogeneity of data, making it difficult to draw definite conclusions. Results should also be interpreted with caution due to the methodological limitations of primary studies as reflected in the low certainty of evidence rating.
Further administration modes have been developed and tested. Intravesical substances can be delivered via electromotive drug administration (EMDA). One small RCT demonstrated the efficacy of MMC using EMDA sequentially combined with BCG in people with high‐risk tumours (Di Stasi 2006a). One Cochrane Review concluded that the use of EMDA to administer intravesical MMC may result in a delay in time to recurrence in selected participant populations, but that there is no information on serious adverse effects yet (Jung 2017). Hyperthermic intravesical chemotherapy administration can also be used for MMC delivery. This procedure increases the temperature of instilled MMC. This RCT compared one year of BCG with one year of MMC and microwave‐induced hyperthermia in people with intermediate‐ and high‐risk bladder cancer and found reduced time to recurrence at 24 months in the MMC group (Arends 2016). However, these newer techniques of application of MMC are not included in this review, which addresses the standard mode of administration.
There is also the option of sequential BCG and MMC administration, but there is still controversy about the effectiveness of this approach (AHRQ 2016; Kaasinen 2016; Solsona 2016). One phase III trial of high‐risk participants is currently ongoing (NCT02948543). Furthermore, BCG and MMC efficacy and toxicity may depend on manufacturing components, which might influence participant outcomes.
Patient care might not always follow scientific evidence but is dependent on practical issues, such as supply shortages. Due to the current worldwide need of BCG and manufacturing problems in the past, there has been a delay in BCG supply for some countries (Abufaraj 2018; Cernuschi 2018). This issue has to be kept in mind when prospectively planning patient care. Therefore, BCG usage must be further studied to predict patients who respond most to BCG therapy, and to determine the optimal schedule and amount of BCG delivery per patient.
Authors' conclusions
Implications for practice.
Treatment decisions and patient counselling for intermediate‐ and high‐risk bladder cancer on choice of Bacillus Calmette‐Guérin (BCG) or mitomycin C (MMC) is based on evidence of low certainty. BCG may improve time to recurrence but may not impact time to death from any cause or time to progression. Serious adverse events may be increased as might minor adverse events. There is no meaningful data concerning patient‐reported quality of life.
Implications for research.
High‐quality randomised controlled trials in people with intermediate‐ and high‐risk bladder cancer with adequate randomisation and blinding are warranted. They should address quality of life, adverse effects and time to progression to provide more reliable results for this patient population.
Notes
None.
Acknowledgements
We wish to thank the Cochrane Urology Group for their support and patience during this review process.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Urinary Bladder Neoplasms] explode all trees
#2 bladder*:ti,ab,kw near/3 (cancer* or carcinoma* or neoplas* or tumo?r* or malignan*):ti,ab,kw
#3 #1 or #2
#4 MeSH descriptor: [BCG Vaccine] explode all trees
#5 (bacillus calmette guerin or BCG or calmette guerin):ti,ab,kw
#6 calmette*:ti,ab,kw near/3 vaccine*:ti,ab,kw
#7 #4 or #5 or #6
#8 MeSH descriptor: [Mitomycin] explode all trees
#9 (mitomycin or mitocinc or mitocin c or ametycine or mutamycin or mitocin‐c or nsc26980 or nsc‐26980 or nsc 26980 or mitomycin‐c or 50SG953SK6):ti,ab,kw
#10 #8 or #9
#11 #3 and #7 and #10
Appendix 2. MEDLINE (Ovid) search strategy
| 1 | exp urinary bladder neoplasms/ |
| 2 | (bladder* adj3 (cancer* or carcinoma* or neoplas* or tumo?r* or malignan*)).tw. |
| 3 | 1 or 2 |
| 4 | BCG vaccine/ |
| 5 | (bacillus calmette guerin or BCG or calmette guerin).mp. |
| 6 | (calmette* adj3 vaccine*).mp. |
| 7 | or/4‐6 |
| 8 | Mitomycin/ |
| 9 | 50SG953SK6.rn. |
| 10 | (mitomycin or mitocinc or mitocin c or ametycine or mutamycin or mitocin‐c or nsc26980 or nsc‐26980 or nsc 26980 or mitomycin‐c).mp. |
| 11 | or/8‐10 |
| 12 | 3 and 7 and 11 |
| 13 | randomized controlled trial.pt. |
| 14 | controlled clinical trial.pt. |
| 15 | randomized.ab. |
| 16 | placebo.ab. |
| 17 | drug therapy.fs. |
| 18 | randomly.ab. |
| 19 | trial.ab. |
| 20 | groups.ab. |
| 21 | or/13‐20 |
| 22 | exp animals/ not humans.sh. |
| 23 | 21 not 22 |
| 24 | 12 and 23 |
Appendix 3. Embase (Ovid) search strategy
| 1 | exp bladder tumor/ |
| 2 | (bladder* adj3 (cancer* or carcinoma* or neoplas* or tumo?r* or malignan*)).tw. |
| 3 | 1 or 2 |
| 4 | exp BCG vaccine/ |
| 5 | (bacillus calmette guerin or BCG or calmette guerin).mp. |
| 6 | (calmette* adj3 vaccine*).mp. |
| 7 | or/4‐6 |
| 8 | exp mitomycin C/ |
| 9 | mitomycin.rn. |
| 10 | (mitomycin or mitocinc or mitocin c or ametycine or mutamycin or mitocin‐c or nsc26980 or nsc‐26980 or nsc 26980 or mitomycin‐c or 50SG953SK6).mp. |
| 11 | or/8‐10 |
| 12 | 3 and 7 and 11 |
| 13 | Crossover Procedure/ |
| 14 | double‐blind procedure/ |
| 15 | randomized controlled trial/ |
| 16 | single‐blind procedure/ |
| 17 | (random$ or factorial$ or crossover$ or cross over$ or placebo$ or assign$ or allocat$ or volunteer$).mp. |
| 18 | ((doubl$ or singl$) adj blind$).mp. |
| 19 | or/13‐18 |
| 20 | 12 and 19 |
Appendix 4. Scopus search strategy
((TITLE‐ABS‐KEY(bladder* W/3 (cancer* OR carcinoma* OR tumor* OR tumour* OR neoplas*))) AND (TITLE‐ABS‐KEY(“bacillus calmette Guerin” OR bcg OR calmette)) AND (TITLE‐ABS‐KEY(mitomycin OR mitocinc OR mitocin c OR ametycine OR mutamycin OR mitocin‐c OR nsc26980 OR nsc‐26980 OR nsc 26980 OR mitomycin‐c OR 50sg953sk6))) AND (TITLE‐ABS‐KEY("clinical trial*" OR "research design" OR "comparative stud*" OR "evaluation stud*" OR "controlled trial*" OR "follow‐up stud*" OR "prospective stud*" OR random* OR placebo* OR "single blind*" OR "double blind*"))
Appendix 5. Web of Science search strategy
| # 1 | TS=(bladder* NEAR/3 (cancer* or carcinoma* or tumor* or tumour* or
neoplas*)) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=1900‐2015 |
| # 2 | TS=(BCG vaccine) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=1900‐2015 |
| # 3 | TS=(bacillus calmette guerin or BCG or calmette guerin) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=1900‐2015 |
| # 4 | TS=(calmette* NEAR/3 vaccine*). Indexes=SCI‐EXPANDED, CPCI‐S Timespan=1900‐2015 |
| # 5 | #4 OR #3 OR #2 Indexes=SCI‐EXPANDED, CPCI‐S Timespan=1900‐2015 |
| # 6 | TS=(mitomycin or mitocinc or mitocin c or ametycine or mutamycin or
mitocin‐c or nsc26980 or nsc‐26980 or nsc 26980 or mitomycin‐c or
50SG953SK6) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=1900‐2015 |
| # 7 | #6 AND #5 AND #1 Indexes=SCI‐EXPANDED, CPCI‐S Timespan=1900‐2015 |
| # 8 | TS= clinical trial* OR TS=research design OR TS=comparative stud* OR
TS=evaluation stud* OR TS=controlled trial* OR TS=follow‐up stud* OR
TS=prospective stud* OR TS=random* OR TS=placebo* OR TS=(single blind*) OR
TS=(double blind*) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=1900‐2015 |
| # 9 | #8 AND #7 Indexes=SCI‐EXPANDED, CPCI‐S Timespan=1900‐2015 |
Appendix 6. LILACS search strategy
(bladder* OR vejiga OR bexiga OR vesical) AND (bcg OR bacillus calmette guerin OR calmette) AND (mitomycin OR mitocinc OR mitocin c OR ametycine OR mutamycin OR mitocin‐c ) AND ((PT randomized controlled trial OR PT controlled clinical trial OR PT multicenter study OR MH randomized controlled trials as topic OR MH controlled clinical trials as topic OR MH multicenter study as topic OR MH random allocation OR MH double‐blind method OR MH single‐blind method) OR ((ensaio$ OR ensayo$ OR trial$) AND (azar OR acaso OR placebo OR control$ OR aleat$ OR random$ OR enmascarado$ OR simpleciego OR ((simple$ OR single OR duplo$ OR doble$ OR double$) AND (cego OR ciego OR blind OR mask))) AND clinic$)) AND NOT (MH animals OR MH rabbits OR MH rats OR MH primates OR MH dogs OR MH cats OR MH swine OR PT in vitro)
Appendix 7. WHO ICTRP search strategy
Bladder* AND BCG AND mitomycin
Bladder* AND “Bacillus Calmette Guerin” AND mitomycin
Appendix 8. ClinicalTrials.gov search strategy
Bladder* AND BCG AND mitomycin
Bladder* AND “Bacillus Calmette Guerin” AND mitomycin
Data and analyses
Comparison 1. Bacillus Calmette‐Guérin (BCG) versus mitomycin C (MMC).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to death from any cause | 5 | 1132 | Hazard Ratio (Random, 95% CI) | 0.97 [0.79, 1.20] |
| 2 Serious adverse effects | 5 | 1024 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [0.82, 6.52] |
| 3 Time to recurrence | 11 | 2616 | Hazard Ratio (Random, 95% CI) | 0.88 [0.71, 1.09] |
| 4 Time to progression | 6 | 1622 | Hazard Ratio (Random, 95% CI) | 0.96 [0.73, 1.26] |
| 5 Adverse effects | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Urinary frequency | 4 | 814 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.99, 2.50] |
| 5.2 Cystitis | 5 | 1049 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.80, 2.51] |
| 5.3 Incontinence | 1 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 2.64 [0.71, 9.83] |
| 5.4 Cramps | 1 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [0.91, 4.32] |
| 5.5 Visible haematuria | 6 | 1387 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [1.20, 2.16] |
| 5.6 Prostatitis | 3 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 5.09 [0.87, 29.87] |
| 5.7 Epididymitis | 3 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 3.51 [1.17, 10.55] |
| 5.8 Fever | 6 | 1387 | Risk Ratio (M‐H, Random, 95% CI) | 2.87 [0.97, 8.48] |
| 5.9 General malaise/discomfort | 3 | 830 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.61, 4.97] |
| 5.10 Fatigue | 2 | 322 | Risk Ratio (M‐H, Random, 95% CI) | 4.98 [0.07, 350.40] |
| 5.11 Allergic reactions | 5 | 1155 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.14, 1.07] |
| 5.12 Dysuria | 2 | 758 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.69, 1.90] |
| 5.13 Skin alterations | 2 | 465 | Risk Ratio (M‐H, Random, 95% CI) | 2.37 [0.07, 76.28] |
| 5.14 Pain | 3 | 742 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.16, 1.82] |
| 5.15 Nausea | 2 | 692 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.02, 1.87] |
| 5.16 Bacterial cystitis | 3 | 848 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.99, 1.68] |
| 5.17 Drug‐induced cystitis | 3 | 848 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.83, 2.91] |
| 5.18 Systemic adverse events | 2 | 867 | Risk Ratio (M‐H, Random, 95% CI) | 12.64 [2.56, 62.55] |
Comparison 2. Different doses of Bacillus Calmette‐Guérin (BCG) (subgroup analyses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serious adverse effect (subgroup analyses) | 5 | 1024 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [0.89, 6.73] |
| 1.1 BCG 120 mg | 2 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.46 [0.76, 26.16] |
| 1.2 BCG < 120 mg | 3 | 559 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.46, 5.86] |
Comparison 3. Different doses of mitomycin C (MMC) (subgroup analyses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to recurrence (subgroup analyses) | 11 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.77, 1.00] | |
| 1.1 MMC 30 mg | 5 | Hazard Ratio (Fixed, 95% CI) | 1.04 [0.86, 1.26] | |
| 1.2 MMC 20 mg | 3 | Hazard Ratio (Fixed, 95% CI) | 0.85 [0.67, 1.07] | |
| 1.3 MMC 40 mg | 2 | Hazard Ratio (Fixed, 95% CI) | 0.60 [0.40, 0.90] | |
| 1.4 MMC mixed dose (20–40 mg) | 1 | Hazard Ratio (Fixed, 95% CI) | 0.50 [0.29, 0.85] |
Comparison 4. Different Bacillus Calmette‐Guérin (BCG) strains (subgroup analyses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to recurrence (subgroup analyses) | 11 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.79, 1.02] | |
| 1.1 Connaught strain | 3 | Hazard Ratio (Fixed, 95% CI) | 0.80 [0.59, 1.07] | |
| 1.2 Pasteur strain | 2 | Hazard Ratio (Fixed, 95% CI) | 0.52 [0.35, 0.78] | |
| 1.3 RIVM strain | 3 | Hazard Ratio (Fixed, 95% CI) | 1.13 [0.91, 1.41] | |
| 1.4 Tice strain | 3 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.72, 1.12] |
Comparison 5. Different maintenance therapies (posthoc subgroup analyses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to death from any cause | 5 | Hazard Ratio (Random, 95% CI) | 0.97 [0.79, 1.20] | |
| 1.1 ≥ 6 weeks | 2 | Hazard Ratio (Random, 95% CI) | 0.94 [0.65, 1.36] | |
| 1.2 > 1 year | 3 | Hazard Ratio (Random, 95% CI) | 0.99 [0.77, 1.27] | |
| 2 Serious adverse effects (≥ 6 weeks) | 5 | 1024 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [0.82, 6.52] |
| 2.1 ≥ 6 weeks | 3 | 724 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [0.56, 7.84] |
| 2.2 > 1 year | 2 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 2.71 [0.51, 14.48] |
| 3 Time to recurrence | 10 | Hazard Ratio (Random, 95% CI) | 0.86 [0.68, 1.09] | |
| 3.1 ≥ 6 weeks | 5 | Hazard Ratio (Random, 95% CI) | 1.12 [0.85, 1.47] | |
| 3.2 > 1 year | 5 | Hazard Ratio (Random, 95% CI) | 0.68 [0.56, 0.82] | |
| 4 Time to progression | 7 | Hazard Ratio (Random, 95% CI) | 1.00 [0.79, 1.26] | |
| 4.1 ≥ 6 weeks | 3 | Hazard Ratio (Random, 95% CI) | 1.23 [0.85, 1.77] | |
| 4.2 > 1 year | 4 | Hazard Ratio (Random, 95% CI) | 0.86 [0.63, 1.16] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Di Stasi 2003.
| Methods |
Study design: multicentre, prospective, randomised clinical
trial Number of study centres: unclear Study dates: June 1994 to March 2001, follow‐up 42–45 months Participants randomly assigned: 108 (36 in each group) |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group A: MMC 40 mg with 960 mg excipient saline dissolved in 100 mL
water instilled and retained in the bladder for 60 minutes Group B: 81 mg wet weight (mean 10.2, SEM 9.0 × 108 cfu) intravesical Pasteur BCG. Lyophilised BCG was suspended in 50 mL bacteriostatic‐free saline 0.9% solution. Instillations retained for 120 minutes. Group C: MMC 40 mg with 960 mg excipient saline dissolved in 100 mL water instilled and retained in the bladder for 30 minutes with 20 mA pulsed electric current (600 mA minute) Procedure:
|
|
| Outcomes | Time to first recurrence, time to progression, time to death from any cause, adverse effects | |
| Funding sources | Supported by grants Progetti di Ricerca di Ateneo ex 60% 1999–2000 and 2000–2001 from Tor Vergata University of Rome. Electromotive equipment provided by Physion Srl, Medolla, Italy. | |
| Declarations of interest | No interest, except 1 coauthor, who reported financial interest with the company. | |
| Notes | 53 participants underwent cross‐over: 25 with electromotive MMC and 15 with MMC switched to a 6‐week BCG course; 13 with BCG failure switched to electromotive MMC. Here we only considered the MMC data with passive administration, not the electromotive MMC data. 1 of the study authors declared financial interest with the company providing the electromotive equipment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Randomization and data collection were performed using a central
computer." Comment: we assumed a low risk for the domain. |
| Allocation concealment (selection bias) | Low risk | Quote "Randomization and data collection were performed using a central
computer. Patients were allocated to 1 of 3 treatment arms by blocked
randomisation across 8 (2x2x2) strata resulting from 3 factors, namely Tis
[Cis] only vs Tis with concurrent T1 papillary tumours, grades III vs II
concurrent T1 papillary tumours and multifocal vs unifocal concurrent T1
papillary tumours." Comment: we assumed a low risk for the domain. |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Comment: no information on blinding. We assumed that there was no blinding and that the outcomes might have been influenced by differences in performance due to a lack of blinding. |
| Blinding of outcome assessment (detection bias) overall survival | Low risk | Comment: no information on blinding. We assumed that there was no blinding but that the absence of blinding had not affected this objective outcome. |
| Blinding of outcome assessment (detection bias) recurrence and progression free survival | High risk | Comment: no information on blinding. We assumed that there was no blinding. We assumed that the absence of blinding might have had an effect on the detection and measurement of subjective outcomes. |
| Blinding of outcome assessment (detection bias) serious and non‐serious adverse effects | High risk | Comment: no information on blinding. We assumed that there was no blinding. We assumed that the absence of blinding might have had an effect on the detection and measurement of subjective outcomes. |
| Blinding of outcome assessment (detection bias) quality of life | Unclear risk | Outcome not reported. |
| Incomplete outcome data (attrition bias) Survival outcomes | Low risk | Comment: all randomised participants (72/72) were considered in the analyses. Participant flow was clearly reported. |
| Incomplete outcome data (attrition bias) Adverse effect outcomes | Low risk | Comment: all randomised participants (72/72) were considered in the analyses. Participant flow is clearly reported. |
| Incomplete outcome data (attrition bias) Quality of life outcomes | Unclear risk | Outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol available. |
| Other bias | Low risk | Comment: we assumed that there was no risk for other bias. |
Friedrich 2007.
| Methods |
Study design: multicentre, prospective, randomised open‐label
clinical trial Number of study centres: unclear Study dates: 1995–2002, follow‐up 2.9 years Participants randomly assigned: 495 |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group A: 6 weekly instillations of MMC 20 mg (MMC 6 week) Group B: 6 weekly instillations of BCG RIVM (BCG 6 week) Group C: 6 weekly instillations of MMC 20 mg followed by monthly instillations of MMC 20 mg for 3 years (MMC 3 years) Procedure:
|
|
| Outcomes | Recurrence‐free survival, adverse effects | |
| Funding sources | Quote: "The work was supported in part by Fa. Medac GmbH, Wedel, Germany. Dr Pichlmeier is an employee of Medac GmbH." | |
| Declarations of interest | Quote: "None of the authors will benefit financially from the publication of the manuscript." | |
| Notes | Quote: "None of the authors will benefit financially from the publication
of the manuscript. The work was supported in part by Fa. Medac GmbH, Wedel,
Germany. Dr Pichlmeier is an employee of Medac GmbH." Our meta‐analyses included groups A and B. Group C was considered in the sensitivity analyses. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed by use of a stratified permuted block
randomisation scheme, balanced for treatment groups. Stratification was
performed by hospital or private urologists." Comment: therefore, we assumed this item to be of low risk for bias. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Comment: no information on blinding. We assumed that there was no blinding and that the outcomes might have been influenced by differences in performance due to a lack of blinding. |
| Blinding of outcome assessment (detection bias) overall survival | Unclear risk | Outcome not reported. |
| Blinding of outcome assessment (detection bias) recurrence and progression free survival | High risk | Comment: no information on blinding. We assumed that there was no blinding. We assumed that the absence of blinding might have had an effect on the detection and measurement of subjective outcomes. |
| Blinding of outcome assessment (detection bias) serious and non‐serious adverse effects | High risk | Comment: no information on blinding. We assumed that there was no blinding. We assumed that the absence of blinding might have had an effect on the detection and measurement of subjective outcomes. |
| Blinding of outcome assessment (detection bias) quality of life | Unclear risk | Outcome not reported. |
| Incomplete outcome data (attrition bias) Survival outcomes | Low risk | Comment: all participants were considered in the analysis (Group A 179/179, Group B 163/163, Group C 153/153). |
| Incomplete outcome data (attrition bias) Adverse effect outcomes | Low risk | Comment: all participants were considered in the analyses (Group A 179/179, Group B 163/163, Group C 153/153). |
| Incomplete outcome data (attrition bias) Quality of life outcomes | Unclear risk | Outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol available. |
| Other bias | Low risk | Comment: we assumed that there was no risk for other bias. |
Krege 1996.
| Methods |
Study design: multicentre, prospective, randomised clinical
trial Number of study centres: 14 Study dates: August 1985 to September 1992, follow‐up 20.2 months Participants randomly assigned: 327 |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group A: 112 participants randomised to TUR alone Group B: 113 participants randomised to TUR followed by intravesical MMC 20 mg in 50 mL saline Group C: 102 participants randomised to TUR followed by intravesical BCG 120 mg Connaught strain in 50 mL saline, plus concomitant subcutaneous BCG 0.5 mg. Procedure:
|
|
| Outcomes | Time to recurrence, progression rate and adverse effects | |
| Funding sources | Supported by a grant from the Ministry of Science and Technology, Germany. | |
| Declarations of interest | No information reported. | |
| Notes | Sample size calculations demanded the admission of 402 participants into the study. However, despite an extended recruitment phase to September 1992, only 337 participants were enrolled. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomised by permuted block method after stratification with respect to primary or recurrent tumours, as well as the canters involved to ensure balanced group sizes within strata after every 6 participants. We assumed that sequence generation was done adequately. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Comment: no information on blinding. We assumed that there was no blinding and that the outcomes might have been influenced by differences in performance due to a lack of blinding. |
| Blinding of outcome assessment (detection bias) overall survival | Unclear risk | Outcome not reported. |
| Blinding of outcome assessment (detection bias) recurrence and progression free survival | High risk | Comment: no information on blinding. We assumed that there was no blinding. We assumed that the absence of blinding might have had an effect on the detection and measurement of subjective outcomes. |
| Blinding of outcome assessment (detection bias) serious and non‐serious adverse effects | High risk | Comment: no information on blinding. We assumed that there was no blinding. We assumed that the absence of blinding might have had an effect on the detection and measurement of subjective outcomes. |
| Blinding of outcome assessment (detection bias) quality of life | Unclear risk | Outcome not reported. |
| Incomplete outcome data (attrition bias) Survival outcomes | Low risk | Comment: nearly all participants were included in statistical analysis (Group B 112/113, Group C 102/102). |
| Incomplete outcome data (attrition bias) Adverse effect outcomes | Unclear risk | Comment: no precise information on participants included in analyses. |
| Incomplete outcome data (attrition bias) Quality of life outcomes | Unclear risk | Outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol available. |
| Other bias | High risk | Quote: "(...) despite an extended recruitment phase (...), only 337
patients were found." Comment: sample size calculations showed a need for 134 participants per treatment arm. Thus, the reduced number of study participants might have had led to reduced power to detect any effects. Study was supported by a grant from the Ministry of Science and Technology, Germany. Conflicts of interests are not reported. We assumed there was no other potential risk of bias. |
Lamm 1995.
| Methods |
Study design: multicentre, prospective, randomised clinical
trial Number of study centres: 65 institutions Study dates: not reported Participants randomly assigned: 447 |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group A: lyophilised Tice BCG 50 mg (5 × 108 cfu) diluted
in 50 mL of sterile, preservative‐free saline Group B: MMC 20 mg in 20 mL of sterile water Procedure:
|
|
| Outcomes | Recurrence‐free survival, worsening‐free survival (progression to higher‐stage disease), overall survival, adverse effects | |
| Funding sources | investigation was supported in part by the following PH.5 Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA46113, CA22433, CA13612, CA42777, C.446441, CA46282, CA13238, 'X45560, CA20319, C‐427057, CA16385, 'X28862. CA35192, CA35431, CA12213, 'X22411, CA35090, CA32734, CA35178, C‐435281, CA14028, CA35261, CA35117, CA45450, CA52420, CA37981, CA04919, CA36020. CA38926, CA32102, CA49957, CA21076. | |
| Declarations of interest | No information on declaration of interests reported. | |
| Notes | Trial of the Southwest Oncology Group. Early Trial Closure: quote: "The trial opened for accrual in December of 1988. The first planned interim analysis was performed in May 1992. It provided strong evidence of BCG arm superiority over the MMC arm with respect to prolonging the time to first recurrence in patients without Cis. Based primarily on the strength of this evidence the trial was closed by its data monitoring committee prior to the completion of planned accrual. The intent‐to treat analysis presented in this article preserves the between arm comparability implemented through randomisation. However, because the trial was closed early with an indication of BCG superiority it is possible that patients randomised to MMC were switched to BCG treatment. If a large number of patients randomised to the MMC arm were switched to BCG treatment then the intent‐to‐treat analysis will underestimate the relative magnitude of the BCG effect size (as compared to MMC)." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomisation according to dynamic balancing algorithm. Balancing factor was the absence of Cis. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this trial forms part of the Southwest Oncology Group Study. Nevertheless, there was no information on allocation concealment in the text. |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Comment: no information on blinding. We assumed that there was no blinding and that the outcomes might have been influenced by differences in performance due to a lack of blinding. |
| Blinding of outcome assessment (detection bias) overall survival | Low risk | Comment: no information on blinding. We assumed that there was no blinding but that the absence of blinding did not affect this objective outcome. |
| Blinding of outcome assessment (detection bias) recurrence and progression free survival | High risk | Comment: no information on blinding. We assumed that there was no blinding. We assumed that the absence of blinding might have had an effect on the detection and measurement of subjective outcomes. |
| Blinding of outcome assessment (detection bias) serious and non‐serious adverse effects | High risk | Comment: no information on blinding. We assumed that there was no blinding. We assumed that the absence of blinding might have had an effect on the detection and measurement of subjective outcomes. |
| Blinding of outcome assessment (detection bias) quality of life | Unclear risk | Outcome not reported. |
| Incomplete outcome data (attrition bias) Survival outcomes | High risk | Quote: "All subsequent analyses are based on eligible patients." Comment: participants included in the survival analyses: Group A 191/225 (85%), Group B 186/222 (84%). |
| Incomplete outcome data (attrition bias) Adverse effect outcomes | Low risk | Comment: nearly all participants were included in statistical analysis (Group A 222/225, Group B 220/222). |
| Incomplete outcome data (attrition bias) Quality of life outcomes | Unclear risk | Outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol was available. |
| Other bias | High risk | Quote: "Early Closure: The trial opened for accrual in December of 1988.
The first planned interim analysis was performed in May 1992. It provided
strong evidence of BCG arm superiority over the MMC arm with respect to
prolonging the time to first recurrence in patients without Cis. Based
primarily on the strength of this evidence the trial was closed by its data
monitoring committee prior to the completion of planned accrual. The
intent‐to‐treat analysis presented in this article preserves the between arm
comparability implemented through randomisation. However, because the trial
was closed early with an indication of BCG superiority it is possible that
patients randomised to MMC were switched to BCG treatment. If a large number
of patients randomised to the MMC arm were switched to BCG treatment then
the intent‐to‐treat analysis will underestimate the relative magnitude of
the BCG effect size (as compared to MMC)." Quote: "There were 43 patients for which pre‐randomisation pathologic stage from review is unavailable and 39 patients for whom pathologic grade from review is unavailable, primarily because a box of specimens was lost." Comment: this might have affected the results. |
Malmström 1999.
| Methods |
Study design: multicentre, prospective, randomised clinical
trial Number of study centres: 12 Study dates: 1987–1992, follow‐up of 10 years Participants randomly assigned: 261 |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group A: MMC 40 mg dissolved in 50 mL phosphate buffer (pH 7.4) Group B: BCG (Danish strain 1331) 120 mg containing 1 × 109 cfu, dissolved in 50 mL saline Procedure:
|
|
| Outcomes | Recurrence‐free survival, progression‐free survival, overall survival | |
| Funding sources | No information on funding in the first study publication reported. The later publications referred to governmental funding sources. | |
| Declarations of interest | No information on declaration of interests in the first study publication. In the publication of 1999, 1 author reported "financial interest and/or other relationship with Statens Serum Institute;" in the publication of 2007, the authors declared no conflicts of interests. | |
| Notes | Supported by Grant 2323‐Bg5‐09XBB from the Swedish Cancer Society. First author declared financial interest or other relationship with Statens Serum Institute, or both. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information |
| Allocation concealment (selection bias) | Low risk | Comment: randomisation via centralised procedure. |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Comment: no information on blinding. We assumed that there was no blinding and that the outcomes might have been influenced by differences in performance due to a lack of blinding. |
| Blinding of outcome assessment (detection bias) overall survival | Low risk | Comment: no information on blinding. We assumes that there was no blinding but that the absence of blinding did not affect this objective outcome. |
| Blinding of outcome assessment (detection bias) recurrence and progression free survival | Low risk | Quote: "Immunostaining evaluation was performed blindly, without knowledge
of clinical history, by 2 observers (K. W. and C. B.) in collaboration over
a conference microscope." (for the 5‐year outcome paper). Comment: we assumed there was low risk for this item. |
| Blinding of outcome assessment (detection bias) serious and non‐serious adverse effects | Low risk | Quote: "Immunostaining evaluation was performed blindly, without knowledge
of clinical history, by 2 observers (K. W. and C. B.) in collaboration over
a conference microscope." (for the 5‐year outcome paper). Comment: we assumed there was low risk for this item. |
| Blinding of outcome assessment (detection bias) quality of life | Unclear risk | Outcome not reported. |
| Incomplete outcome data (attrition bias) Survival outcomes | Low risk | Comment: 125/130 participants in the MMC group and 125/131 (95%) in the BCG group were included in the analyses. |
| Incomplete outcome data (attrition bias) Adverse effect outcomes | Low risk | Comment: 125/130 participants in the MMC group and 125/131 (95%) in the BCG group were included in the analyses. |
| Incomplete outcome data (attrition bias) Quality of life outcomes | Unclear risk | Outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol available. |
| Other bias | Low risk | Comment: we assumed that there was no risk for other bias. |
Mangiarotti 2008.
| Methods |
Study design: prospective, randomised clinical trial Number of study centres: 1 Study dates: recruitment period not reported, follow‐up 12–108 months Participants randomly assigned: 92 |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group A: MMC 40 mg in 50 mL saline Group B: BCG Tice Procedure:
|
|
| Outcomes | Recurrence rate, recurrence‐free survival, adverse effects | |
| Funding sources | Not reported. | |
| Declarations of interest | No information on interests reported. | |
| Notes | Sample size estimation required 97 participants to allow a 5% dropout and 92 remaining participants (46 in each group). The article reported on the 92 participants and on the 46 per group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information |
| Blinding of participants and personnel (performance bias) all outcomes | Unclear risk | Comment: no information |
| Blinding of outcome assessment (detection bias) overall survival | Unclear risk | Outcome not reported. |
| Blinding of outcome assessment (detection bias) recurrence and progression free survival | High risk | Comment: no information on blinding. We assumed that there was no blinding. We assumed that the absence of blinding might have had an effect on the detection and measurement of subjective outcomes. |
| Blinding of outcome assessment (detection bias) serious and non‐serious adverse effects | High risk | Comment: no information on blinding. We assumed that there was no blinding. We assumed that the absence of blinding might have had an effect on the detection and measurement of subjective outcomes. |
| Blinding of outcome assessment (detection bias) quality of life | Unclear risk | Outcome not reported. |
| Incomplete outcome data (attrition bias) Survival outcomes | Low risk | Comment: all participants entered the analysis (46/46 in each group). |
| Incomplete outcome data (attrition bias) Adverse effect outcomes | Low risk | Comment: all participants entered the analysis (46/46 in each group). |
| Incomplete outcome data (attrition bias) Quality of life outcomes | Unclear risk | Outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol available. |
| Other bias | Low risk | Comment: we assumed that there was no risk for other bias. |
Michielsen 2013.
| Methods |
Study design: prospective, randomised controlled clinical trial Number of study centres: 1 probably Study dates: not reported Participants randomly assigned: unclear |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group A: MMC 40 mg in 50 mL 0.9% saline Group B: BCG full dose Procedure:
|
|
| Outcomes | Disease‐specific quality of life, measured with EORTC QLQ BLS24 | |
| Funding sources | No information reported. | |
| Declarations of interest | No information reported. | |
| Notes | Congress abstract available only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information reported to allow a judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information reported to allow a judgement. |
| Blinding of participants and personnel (performance bias) all outcomes | Unclear risk | Comment: no information reported to allow a judgement. |
| Blinding of outcome assessment (detection bias) overall survival | Unclear risk | Comment: no information reported to allow a judgement. |
| Blinding of outcome assessment (detection bias) recurrence and progression free survival | Unclear risk | Comment: no information reported to allow a judgement. |
| Blinding of outcome assessment (detection bias) serious and non‐serious adverse effects | Unclear risk | Comment: no information reported to allow a judgement. |
| Blinding of outcome assessment (detection bias) quality of life | Unclear risk | Comment: no information reported to allow a judgement. |
| Incomplete outcome data (attrition bias) Survival outcomes | Unclear risk | Comment: no information reported to allow a judgement. |
| Incomplete outcome data (attrition bias) Adverse effect outcomes | Unclear risk | Comment: no information reported to allow a judgement. |
| Incomplete outcome data (attrition bias) Quality of life outcomes | Unclear risk | Comment: no information reported to allow a judgement. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol available. |
| Other bias | Unclear risk | Comment: no information reported to allow a judgement. |
NCT00974818.
| Methods |
Study design: prospective, randomised clinical trial Number of study centres: 3 Study dates: September 2009 to March 2012 Participants randomly assigned: 50 |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group A: MMC 40 mg, dissolved in 20 mL sterile water Group B: BCG 81 mg, dissolved in 53 mL of diluent and saline Procedure:
|
|
| Outcomes | Response to treatment (relapse rate), serious adverse effects, adverse effects | |
| Funding sources | Sponsor was Memorial Sloan Kettering Cancer Center. | |
| Declarations of interest | No information reported. | |
| Notes | Study has been terminated due to lack of accrual. Only the trial entry is
available: NCT00974818. Official
name: mitomycin C versus Bacillus Calmette‐Guérin in the intravesical
treatment of non‐muscle‐invasive bladder cancer participants: a randomized
phase III non‐inferiority trial. Quote: "Due to a lack of patients accrued to the protocol the protocol was closed and the analysis of the 2 year relapse rates could not be compared." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information reported to allow a judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information reported to allow a judgement. |
| Blinding of participants and personnel (performance bias) all outcomes | Unclear risk | Comment: no information reported to allow a judgement. |
| Blinding of outcome assessment (detection bias) overall survival | Unclear risk | Outcome not assessed. |
| Blinding of outcome assessment (detection bias) recurrence and progression free survival | Unclear risk | Comment: no information reported to allow a judgement. |
| Blinding of outcome assessment (detection bias) serious and non‐serious adverse effects | Unclear risk | Comment: no information reported to allow a judgement. |
| Blinding of outcome assessment (detection bias) quality of life | Unclear risk | Outcome not assessed. |
| Incomplete outcome data (attrition bias) Survival outcomes | High risk | Comment: analysis was not done due to a lack of participants. Unclear why this analysis could not be done. |
| Incomplete outcome data (attrition bias) Adverse effect outcomes | High risk | Comment: we judged this item at high risk, as the reported numbers on the webpage were not congruent. But analyses here included the 50 participants (25 per arm). |
| Incomplete outcome data (attrition bias) Quality of life outcomes | Unclear risk | Outcome not assessed. |
| Selective reporting (reporting bias) | High risk | Comment: no information why data on the primary outcome (relapse rate) was not reported but there were data on the secondary outcomes (adverse effects). |
| Other bias | High risk | Comment: study was affected by a lack of reporting. |
Ojea 2007a.
| Methods |
Study design: multicentre, prospective, randomised clinical
trial Number of study centres: unclear Study dates: March 1995 to May 1998, follow‐up 52.6 months Participants randomly assigned: 430 |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group A: low‐dose BCG 27 mg Connaught strain Group B: very low‐dose BCG 13.5 mg Connaught strain Group C: MMC 30 mg Procedure:
|
|
| Outcomes | Disease‐free interval, time to progression, overall survival, adverse effects | |
| Funding sources | No information | |
| Declarations of interest | The authors reported that they had nothing to disclose. | |
| Notes | CUETO study 95011 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Comment: no information on blinding. We assumed that there was no blinding and that the outcomes might have been influenced by differences in performance due to a lack of blinding. |
| Blinding of outcome assessment (detection bias) overall survival | Unclear risk | Outcome not reported. |
| Blinding of outcome assessment (detection bias) recurrence and progression free survival | High risk | Comment: no information on blinding. We assumed that there was no blinding. We assumed that the absence of blinding might have had an effect on the detection and measurement of subjective outcomes. |
| Blinding of outcome assessment (detection bias) serious and non‐serious adverse effects | High risk | Comment: no information on blinding. We assumed that there was no blinding. We assumed that the absence of blinding might have had an effect on the detection and measurement of subjective outcomes. |
| Blinding of outcome assessment (detection bias) quality of life | Unclear risk | Outcome not reported. |
| Incomplete outcome data (attrition bias) Survival outcomes | Low risk | Comment: all participants entered the analysis (Group A 142/142, Group B 139/139, Group C 149/149). |
| Incomplete outcome data (attrition bias) Adverse effect outcomes | Low risk | Comment: all participants entered the analysis (Group A 142/142, Group B 139/139, Group C 149/149). |
| Incomplete outcome data (attrition bias) Quality of life outcomes | Unclear risk | Outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol available. |
| Other bias | Low risk | Comment: we assumed that there was no risk for other bias. |
Ojea 2007b.
| Methods |
Study design: multicentre, prospective, randomised clinical
trial Number of study centres: unclear Study dates: March 1995 to May 1998, follow‐up 52.6 months Participants randomly assigned: 430 |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group A: low‐dose BCG 27 mg Connaught strain Group B: very low‐dose BCG 13.5 mg Connaught strain Group C: MMC 30 mg Procedure:
|
|
| Outcomes | Disease‐free interval, time to progression, overall survival, adverse effects | |
| Funding sources | No information | |
| Declarations of interest | The authors reported that they had nothing to disclose. | |
| Notes | CUETO study 95011 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Comment: no information on blinding. We assumed that there was no blinding and that the outcomes might have been influenced by differences in performance due to a lack of blinding. |
| Blinding of outcome assessment (detection bias) overall survival | Unclear risk | Outcome not reported. |
| Blinding of outcome assessment (detection bias) recurrence and progression free survival | High risk | Comment: no information on blinding. We assumed that there was no blinding. We assumed that the absence of blinding might have had an effect on the detection and measurement of subjective outcomes. |
| Blinding of outcome assessment (detection bias) serious and non‐serious adverse effects | High risk | Comment: no information on blinding. We assumed that there was no blinding. We assumed that the absence of blinding might have had an effect on the detection and measurement of subjective outcomes. |
| Blinding of outcome assessment (detection bias) quality of life | Unclear risk | Outcome not reported. |
| Incomplete outcome data (attrition bias) Survival outcomes | Low risk | Comment: all participants entered the analysis (Group A 142/142, Group B 139/139, Group C 149/149). |
| Incomplete outcome data (attrition bias) Adverse effect outcomes | Low risk | Comment: all participants entered the analysis (Group A 142/142, Group B 139/139, Group C 149/149). |
| Incomplete outcome data (attrition bias) Quality of life outcomes | Unclear risk | Outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol available. |
| Other bias | Low risk | Comment: we assumed that there was no risk for other bias. |
Rintala 1991.
| Methods |
Study design: multicentre, prospective, randomised clinical
trial Number of study centres: unclear Study dates: 1984–1987, for a subgroup of participants there is a follow‐up of 20 years Participants randomly assigned: 89 |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group A: BCG Pasteur Strain F, 75 mg Group B: MMC 20–40 mg (AUC method) Procedure:
|
|
| Outcomes | Recurrence rate, recurrence index, overall mortality, progression, disease‐specific mortality | |
| Funding sources | Finnish Cancer Foundation, Academy of Finland Paolo Foundation and Research and Science Foundation of Farmos | |
| Declarations of interest | No information reported. | |
| Notes | FinnBladder I study group. Jarvinen reported 20‐year follow‐up data based on a subgroup of participants with TaT1 disease and without Cis (91/109 participants). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: method of randomisation was based on date of birth. |
| Allocation concealment (selection bias) | High risk | Comment: method of randomisation was based on date of birth. |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Comment: no information on blinding. We assumed that there was no blinding and that the outcomes might have been influenced by differences in performance due to a lack of blinding. |
| Blinding of outcome assessment (detection bias) overall survival | Low risk | Comment: no information on blinding. We assumed that there was no blinding but that the absence of blinding has not affected this objective outcome. |
| Blinding of outcome assessment (detection bias) recurrence and progression free survival | High risk | Comment: no information on blinding. We assumed that there was no blinding. We assumed that the absence of blinding might have had an effect on the detection and measurement of subjective outcomes. |
| Blinding of outcome assessment (detection bias) serious and non‐serious adverse effects | High risk | Comment: no information on blinding. We assumed that there was no blinding. We assumed that the absence of blinding might have had an effect on the detection and measurement of subjective outcomes. |
| Blinding of outcome assessment (detection bias) quality of life | Unclear risk | Outcome not reported. |
| Incomplete outcome data (attrition bias) Survival outcomes | Low risk | Comment: all participants were considered in the analyses (Group A 44/44, Group B 45/45). |
| Incomplete outcome data (attrition bias) Adverse effect outcomes | Low risk | Comment: all participants were considered in the analyses (Group A 44/44, Group B 45/45). |
| Incomplete outcome data (attrition bias) Quality of life outcomes | Unclear risk | Outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol available. |
| Other bias | Low risk | Comment: we assumed that there was no risk for other bias. |
Witjes 1996a.
| Methods |
Study design: multicentre, prospective, randomised clinical
trial Number of study centres: 27 Study dates: 1987–1990, follow‐up 36 months (2–81 months) Participants randomly assigned: 437 |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group A: MMC 30 mg in 50 mL saline Group B: BCG‐RIVM 5 × 108 bacilli in 50 mL saline Group C: BCG‐Tice 5 × 108 bacilli in 50 mL saline Procedure:
|
|
| Outcomes | Recurrence‐free survival, progression‐free survival, adverse effects | |
| Funding sources | No information reported. | |
| Declarations of interest | No information reported. | |
| Notes | Dutch South East Cooperative Trial. 1 pathologist determined stage and grade. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: restricted block‐wise (block size 6 equals 3 treatments times 2 participants per treatment) randomisation was used. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Comment: no information on blinding. We assumed that there was no blinding and that the outcomes might have been influenced by differences in performance due to a lack of blinding. |
| Blinding of outcome assessment (detection bias) overall survival | Unclear risk | Outcome not reported. |
| Blinding of outcome assessment (detection bias) recurrence and progression free survival | High risk | Comment: no information on blinding. We assumed that there was no blinding. We assumed that the absence of blinding might have had an effect on the detection and measurement of subjective outcomes. |
| Blinding of outcome assessment (detection bias) serious and non‐serious adverse effects | High risk | Comment: no information on blinding. We assumed that there was no blinding. We assumed that the absence of blinding might have had an effect on the detection and measurement of subjective outcomes. |
| Blinding of outcome assessment (detection bias) quality of life | Unclear risk | Outcome not reported. |
| Incomplete outcome data (attrition bias) Survival outcomes | Unclear risk | Outcome not reported. |
| Incomplete outcome data (attrition bias) Adverse effect outcomes | Low risk | Comment: all participants were considered in the analyses (Group A 136/136, Group B 134/134, Group C 140/140). |
| Incomplete outcome data (attrition bias) Quality of life outcomes | Unclear risk | Outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol available. |
| Other bias | Low risk | Comment: we assumed that there was no risk for other bias. |
Witjes 1996b.
| Methods |
Study design: multicentre, prospective, randomised clinical
trial Number of study centres: 27 Study dates: 1987–1990, follow‐up 36 months (2–81 months) Participants randomly assigned: 437 |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group A: MMC 30 mg in 50 mL saline Group B: BCG‐RIVM 5 × 108 bacilli in 50 mL saline Group C: BCG‐Tice 5 × 108 bacilli in 50 mL saline Procedure:
|
|
| Outcomes | Recurrence‐free survival, progression‐free survival, adverse effects | |
| Funding sources | No information reported. | |
| Declarations of interest | No information reported. | |
| Notes | Dutch South East Cooperative Trial. 1 pathologist determined stage and grade. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: restricted block‐wise (block size 6 equals 3 treatments times 2 participants per treatment) randomisation was used. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Comment: no information on blinding. We assumed that there was no blinding and that the outcomes might have been influenced by differences in performance due to a lack of blinding. |
| Blinding of outcome assessment (detection bias) overall survival | Unclear risk | Outcome not reported. |
| Blinding of outcome assessment (detection bias) recurrence and progression free survival | High risk | Comment: no information on blinding. We assumed that there was no blinding. We assumed that the absence of blinding might have had an effect on the detection and measurement of subjective outcomes. |
| Blinding of outcome assessment (detection bias) serious and non‐serious adverse effects | High risk | Comment: no information on blinding. We assumed that there was no blinding. We assumed that the absence of blinding might have had an effect on the detection and measurement of subjective outcomes. |
| Blinding of outcome assessment (detection bias) quality of life | Unclear risk | Outcome not reported. |
| Incomplete outcome data (attrition bias) Survival outcomes | Unclear risk | Outcome not reported. |
| Incomplete outcome data (attrition bias) Adverse effect outcomes | Low risk | Comment: all participants were considered in the analyses (Group A 136/136, Group B 134/134, Group C 140/140). |
| Incomplete outcome data (attrition bias) Quality of life outcomes | Unclear risk | Outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol available. |
| Other bias | Low risk | Comment: we assumed that there was no risk for other bias. |
Witjes 1998a.
| Methods |
Study design: prospective, randomised clinical trial Number of study centres: 24 Study dates: January 1985 to October 1986, median follow‐up 7.2 years Participants randomly assigned: 344 |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group A: MMC 30 mg in 50 mL saline Group B: BCG‐RIVM 5 × 108 bacilli in 50 mL saline Procedure:
|
|
| Outcomes | Time to first recurrence, time to progression, adverse effects | |
| Funding sources | This work was supported by grants 5U10 CA11488‐26 and 5U10 CA11488‐27 from the National Cancer Institute, Bethesda, MD. | |
| Declarations of interest | No information reported. | |
| Notes | Joint effort of the European Organisation for Research and Treatment of Cancer (EORTC) Genito‐Urinary Tract Cancer Collaborative Group and the Dutch South East Cooperative Urological Group (protocol 30845). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Comment: no information on blinding. We assumed that there was no blinding and that the outcomes might have been influenced by differences in performance due to a lack of blinding. |
| Blinding of outcome assessment (detection bias) overall survival | Low risk | Comment: no information on blinding. We assumed that there was no blinding but that the absence of blinding has not affected this objective outcome. |
| Blinding of outcome assessment (detection bias) recurrence and progression free survival | High risk | Comment: no information on blinding. We assumed that there was no blinding. We assumed that the absence of blinding might have had an effect on the detection and measurement of subjective outcomes. |
| Blinding of outcome assessment (detection bias) serious and non‐serious adverse effects | High risk | Comment: no information on blinding. We assumed that there was no blinding. We assumed that the absence of blinding might have had an effect on the detection and measurement of subjective outcomes. |
| Blinding of outcome assessment (detection bias) quality of life | Unclear risk | Outcome not reported. |
| Incomplete outcome data (attrition bias) Survival outcomes | Low risk | Comment: participants considered in the analyses were: BCG 159/171 (93%), MMC 168/173 (97%). |
| Incomplete outcome data (attrition bias) Adverse effect outcomes | Low risk | Comment: participants considered in the analyses were: BCG 166/171 (97%), MMC 173/173. |
| Incomplete outcome data (attrition bias) Quality of life outcomes | Unclear risk | Outcome not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol available. |
| Other bias | Low risk | Comment: we assumed that there was no risk for other bias. |
AUC: area under the curve; BCG: Bacillus Calmette‐Guérin; cfu: colony‐forming units; Cis: carcinoma in situ; EORTC QLQ BLS24: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire – 24‐question Superficial Bladder Cancer; MMC: mitomycin C; SEM: standard error of the mean; TUR: transurethral resection.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12613000513718 | Wrong treatment comparison. |
| Allona 1988 | Wrong treatment comparison. |
| Altay 2000 | Wrong treatment comparison. |
| Arends 2014 | Wrong treatment comparison. |
| Ayres 2010 | Wrong study design. |
| Badalato 2011 | Wrong study design. |
| Bassi 2001 | Wrong study design. |
| Bismarck 2004 | Comment/letter. |
| Boccafoschi 1991 | Wrong study design. |
| Bochner 2006 | Comment/letter. |
| Bohle 2008 | Comment/letter. |
| Braasch 2008 | Wrong study design. |
| Brausi 1998 | Wrong study design. |
| Chen 2012 | Wrong study design. |
| Chen 2019 | Wrong study design. |
| Cho 2012 | Wrong study design. |
| Crawford 1995 | Comment/letter. |
| Dalbagni 2009 | Comment/letter. |
| de Jong 1989 | Wrong study design. |
| deVere 2000 | Wrong study design. |
| Di Stasi 2004a | Wrong treatment comparison. |
| Di Stasi 2004b | Wrong treatment comparison. |
| Di Stasi 2006b | Wrong treatment comparison. |
| Di Stasi 2012a | Wrong treatment comparison. |
| Di Stasi 2012b | Wrong treatment comparison. |
| Di Stasi 2013 | Wrong treatment comparison. |
| Di Stasi 2015 | Wrong treatment comparison. |
| El Kader 2010 | Wrong treatment comparison. |
| EUCTR2008‐005428‐99‐GB | Wrong treatment comparison. |
| EUCTR2011‐000607‐41‐BE | Wrong treatment comparison. |
| FinnBladder 4 | Wrong treatment comparison. |
| FinnBladder II | Wrong treatment comparison. |
| Gao 2002 | Wrong study design. |
| Gazzaniga 2009 | Wrong study design. |
| Gelabert‐Mas 1993 | Wrong treatment comparison. |
| Gelabert‐Mas 1997 | Wrong treatment comparison. |
| Gianneo 1997 | Wrong study design. |
| Grossman 2006 | Comment/letter. |
| Guerrero‐Ramos 2019 | Wrong treatment comparison. |
| Gulpinar 2012 | Wrong treatment comparison. |
| Han 2015 | Wrong study design. |
| Hausladen 2003 | Wrong study design. |
| Hayne 2011 | Wrong treatment comparison. |
| Huang 2010 | Wrong treatment comparison. |
| Iavarone 1996 | Wrong study design. |
| ISRCTN85785327 | Duplicate. |
| Jarvinen 2012 | Wrong treatment comparison. |
| Jarvinen 2013 | Comment/letter. |
| Jarvinen 2014 | Wrong treatment comparison. |
| Jarvinen 2015 | Wrong treatment comparison. |
| Jauhiainen 1993 | Wrong study design. |
| Kaasinen 2000 | Wrong treatment comparison. |
| Kaasinen 2002 | Wrong treatment comparison. |
| Kaasinen 2003 | Wrong treatment comparison. |
| Kaasinen 2014 | Wrong treatment comparison. |
| Kelly 2015 | Wrong treatment comparison. |
| Kirkali 2010 | Wrong treatment comparison. |
| Kurth 2000 | Wrong study design. |
| Lamm 1991 | Wrong study design. |
| Leblanc 1999 | Wrong study design. |
| Liberati 2012 | Wrong treatment comparison. |
| Lundholm 1999 | Duplicate. |
| Malmström 2009b | Comment/letter. |
| Matsumoto 2010 | Wrong study design. |
| Matsumoto 2012 | Wrong study design. |
| Mondal 2016 | No distinction of low‐ and mid‐/high‐risk participants. |
| Morales 1999 | Wrong treatment comparison. |
| Murillo 2019 | Wrong study design. |
| NCT00023842 | Wrong treatment comparison. |
| NCT00384891 | Wrong treatment comparison. |
| NCT01094964 | Duplicate. |
| NCT01442519 | Wrong treatment comparison. |
| Nishimura 1996 | Wrong study design. |
| Nohales 1996 | Wrong study design. |
| Nouhaud 2017 | Wrong treatment comparison. |
| Ooi 2011 | Comment/letter. |
| Peyromaure 2004 | Wrong study design. |
| Raviv 2005 | Wrong study design. |
| Saxena 2006 | Wrong study design. |
| Sekine 2001 | Wrong treatment comparison. |
| Shelley 2015b | Wrong study design. |
| Smits 1998 | Wrong study design. |
| Soloway 1990 | Wrong study design. |
| Stasi 2004 | Wrong treatment comparison. |
| Steinberg 2017 | Wrong treatment comparison. |
| Study 30993 | Wrong treatment comparison. |
| Sylvester 2009 | Wrong study design. |
| Tong 2003 | Duplicate. |
| van der Meijden 1989 | Duplicate. |
| van Gils‐Gielen 1995 | Wrong study design. |
| Wang 1992 | Wrong participant population. |
| Wang 2011 | No distinction of low‐ and mid‐/high‐risk participants. |
| Witjes 1998b | Wrong treatment comparison. |
| Witjes 1999a | Wrong treatment comparison. |
| Witjes 1999b | Comment/letter. |
| Yabusaki 1991 | Wrong treatment comparison. |
| Yang 1999 | Wrong study design. |
| Yari 2010 | Wrong treatment comparison. |
Differences between protocol and review
This review is based on a published protocol (Schmidt 2015).
Adverse effects: we omitted adverse effects from the list of main outcomes for the 'Summary of findings' table, as adverse effects were reported very heterogeneously and were not clearly defined among included studies.
Secondary outcomes: We have added "quality of life" to the list of secondary outcomes.
Sensitivity analyses: we planned to examine the methodological quality according to risk of bias, by conducting separate meta‐analyses for low risk of bias studies, excluding studies judged as high or unclear (or both) risk of bias. As there was no study rated as low risk of bias, we could not perform these sensitivity analyses.
Subgroup: we planned to explore clinical heterogeneity by testing high risk versus intermediate risk of tumour recurrence. These analyses were not conducted as included studies did not report information for these subgroups separately. We also aimed at exploring effects of different schedules of BCG installations versus different schedules of MMC (less than one year versus more than one year) at the protocol stage. Due to the heterogeneity and paucity of data, these subgroup analyses were not conducted.
Subgroup (posthoc): we added the comparison of different BCG maintenance therapy strategies (BCG administration greater than six weeks and BCG administration greater than one year) as posthoc subgroup analyses as we found this to be of clinical importance.
We have excluded two studies in Chinese, as we were unable to translate them.
Contributions of authors
SS: project co‐ordination of this update, protocol writing, data extraction, quality assessment, analyses and draft manuscript.
FK: critical feedback, review protocol and manuscript.
BC: search strategy, and protocol and manuscript review.
DD: manuscript review, data extraction and quality assessment.
LMK: protocol and manuscript review, data extraction and quality assessment.
RD: protocol and manuscript review, study selection and quality assessment.
SK: statistical advice, statistical analyses and manuscript review.
KJ: statistical advice, statistical analyses, and protocol and manuscript review.
PD: critical feedback, protocol and manuscript review.
JJM: methodological guidance, critical feedback, protocol and manuscript review.
All authors approved the final version of this review.
Sources of support
Internal sources
-
Deutsche Gesellschaft für Urologie e.V., Germany.
The primary author received internal support from her institution in the form of her regular salary.
External sources
No sources of support supplied
Declarations of interest
SS: none.
FK: none.
BC: none.
DD: none.
LMK: none.
RD: received lecture fees by Roche and travel grants by Biogen.
SK: employed by the Institute of Medical Biometry and Informatics, University Heidelberg, which receives support by the German Society for Urology (Deutsche Gesellschaft für Urologie, DGU) for conducting urology‐related systematic reviews.
KJ: employed by the Institute of Medical Biometry and Informatics, University Heidelberg, which receives support by the German Society for Urology (Deutsche Gesellschaft für Urologie, DGU) for conducting urology‐related systematic reviews.
PD: none.
JJM: none.
New
References
References to studies included in this review
Di Stasi 2003 {published data only}
- Stasi SM, Giannantoni A, Stephen RL, Capelli G, Navarra P, Massoud R, et al. Intravesical electromotive mitomycin C versus passive transport mitomycin C for high risk superficial bladder cancer: a prospective randomized study. Journal of Urology 2003;170(3):777‐82. [DOI] [PubMed] [Google Scholar]
Friedrich 2007 {published data only}
- Friedrich MG, Pichlmeier U, Schwaibold H, Conrad S, Huland H. Long‐term intravesical adjuvant chemotherapy further reduces recurrence rate compared with short‐term intravesical chemotherapy and short‐term therapy with Bacillus Calmette‐Guérin (BCG) in patients with non‐muscle‐invasive bladder carcinoma. European Urology 2007;52(4):1123‐29. [DOI] [PubMed] [Google Scholar]
- Isbarn H, Budäus L, Pichlmeier U, Conrad S, Huland H, Friedrich MG. Comparison of the effectiveness between long‐term instillation of mitomycin C and short‐term prophylaxis with MMC or bacille Calmette‐Guérin. Study of patients with non‐muscle‐invasive urothelial cancer of the urinary bladder. Urologe 2008;47(5):608‐15. [DOI] [PubMed] [Google Scholar]
Krege 1996 {published data only}
- Krege S, Giani G, Meyer R, Otto T, Rübben H, participating clinics. A randomized multicenter trial of adjuvant therapy in superficial bladder cancer: transurethral resection only versus transurethral resection plus mitomycin C versus transurethral resection plus Bacillus Calmette‐Guerin. Journal of Urology 1996;156(3):962‐6. [DOI] [PubMed] [Google Scholar]
Lamm 1995 {published data only}
- Lamm DL, Blumenstein BA, Crawford DE, Crissman JD, Lowe BA, Smith JA Jr, et al. Randomized intergroup comparison of Bacillus Calmette‐Guerin immunotherapy and mitomycin C chemotherapy prophylaxis in superficial transitional cell carcinoma of the bladder a southwest oncology group study. Urologic Oncology 1995;1(3):119‐26. [DOI] [PubMed] [Google Scholar]
Malmström 1999 {published data only}
- Gardmark T, Jahnson S, Wahlquist R, Wijkström H, Malmström PU. Analysis of progression and survival after 10 years of a randomized prospective study comparing mitomycin‐C and Bacillus Calmette‐Guérin in patients with high‐risk bladder cancer. BJU International 2007;99(4):817‐20. [DOI] [PubMed] [Google Scholar]
- Gardmark T, Malmström PU, Wiklund P, Jahnson S, Wijkstrom H, Wahlquist R. Long term follow up of a randomised study of BCG versus mitomycin‐C in high risk superficial bladder cancer. Journal of Urology 2004;171(4):73. [Google Scholar]
- Lundholm C, Malmström PU, Norlen BJ. Comparative study of mitomycin‐C and BCG in treatment of superficial bladder cancer. Scandinavian Journal of Urology and Nephrology 1994;164 Suppl:34‐5. [Google Scholar]
- Lundholm C, Norlen BJ, Ekman P, Jahnson S, Lagerkvist M, Lindeborg T, et al. A randomized prospective study comparing long‐term intravesical instillations of mitomycin C and Bacillus Calmette‐Guerin in patients with superficial bladder carcinoma. Journal of Urology 1996;156(2):372‐6. [DOI] [PubMed] [Google Scholar]
- Malmström P, Lundholm C, Ekman P, Jahnson S, Lagerkvist M, Lindeborg T, et al. A five‐year follow‐up of a randomized prospective study comparing long‐term intravesical instillations of mitomycin‐C and Bacille Calmette‐Guerin in patients with superficial bladder carcinoma. Scandinavian Journal of Urology and Nephrology 1998;Suppl 197:9‐10. [DOI] [PubMed] [Google Scholar]
- Malmström PU, Wijkström H, Lundholm C, Wester K, Busch C, Norlén BJ, Swedish‐Norwegian Bladder Cancer Study Group. 5‐year followup of a randomized prospective study comparing mitomycin C and Bacillus Calmette‐Guerin in patients with superficial bladder carcinoma. Journal of Urology 1999;161(4):1124‐7. [PubMed] [Google Scholar]
Mangiarotti 2008 {published data only}
- Mangiarotti B, Trinchieri A, Nero A, Montanari E. A randomized prospective study of intravesical prophylaxis in non‐muscle invasive bladder cancer at intermediate risk of recurrence: mitomycin chemotherapy vs BCG immunotherapy. Archivio Italiano di Urologia, Andrologia 2008;80(4):167‐71. [PubMed] [Google Scholar]
Michielsen 2013 {published data only}
- Michielsen D, Coomans D. Intravesical chemotherapy or immunotherapy for intermediate‐risk non‐muscle invasive bladder cancer: does the patient mention a different quality of life?. Urology 2013;82:S130‐1. [Google Scholar]
NCT00974818 {published data only}
- NCT00974818. A randomized prospective study of intravesical prophylaxis in non‐muscle invasive bladder cancer at intermediate risk of recurrence: mitomycin chemotherapy vs BCG immunotherapy. clinicaltrials.gov/ct2/show/NCT00974818 (first received 10 September 2009; study terminated due to lack of accrual). [PubMed]
Ojea 2007a {published data only}
- Nogueira March JL, Solsona E, Unda M, Ojea A, Rodrigez‐Molina A, Fernandez J. A multicenter and randomised prospective study comparing three intravesical therapies, two with Bacillus Calmette‐Guerin and one with mitomycin C chemotherapy in medium and low risk superficial bladders tumours. European Urology 2001;Suppl 5:Abstract 119. [Google Scholar]
- Ojea A, Nogueira JL, Solsona E, Flores N, Gómez JM, Molina JR, et al. CUETO Group. A multicentre, randomised prospective trial comparing three intravesical adjuvant therapies for intermediate‐risk superficial bladder cancer: low‐dose Bacillus Calmette‐Guerin (27 mg) versus very low‐dose Bacillus Calmette‐Guerin (13.5 mg) versus mitomycin C. European Urology 2007;52(5):1396‐406. [DOI] [PubMed] [Google Scholar]
Ojea 2007b {published data only}
- Nogueira March JL, Solsona E, Unda M, Ojea A, Rodrigez‐Molina A, Fernandez J. A multicenter and randomised prospective study comparing three intravesical therapies, two with Bacillus Calmette‐Guerin and one with mitomycin C chemotherapy in medium and low risk superficial bladders tumours. European Urology 2001;Suppl 5:Abstract 119. [Google Scholar]
- Ojea A, Nogueira JL, Solsona E, Flores N, Gómez JM, Molina JR, et al. CUETO Group. A multicentre, randomised prospective trial comparing three intravesical adjuvant therapies for intermediate‐risk superficial bladder cancer: low‐dose Bacillus Calmette‐Guerin (27 mg) versus very low‐dose Bacillus Calmette‐Guerin (13.5 mg) versus mitomycin C. European Urology 2007;52(5):1396‐406. [DOI] [PubMed] [Google Scholar]
Rintala 1991 {published data only}
- Järvinen R, Kaasinen E, Sankila A, Rintala E, FinnBladder Group. Long‐term efficacy of maintenance Bacillus Calmette‐Guérin versus maintenance mitomycin C instillation therapy in frequently recurrent TaT1 tumours without carcinoma in situ: a subgroup analysis of the prospective, randomised FinnBladder I study with a 20‐year follow‐up. European Urology 2009;56(2):260‐5. [DOI] [PubMed] [Google Scholar]
- Rintala E, Jauhiainen K, Alfthan O, Hansson E, Juusela H, Kanerva K, et al. Intravesical chemotherapy (mitomycin C) versus immunotherapy (Bacillus Calmette‐Guérin) in superficial bladder cancer. European Urology 1991;20(1):19‐25. [DOI] [PubMed] [Google Scholar]
- Rintala E, Jauhiainen, K, Alfthan O. Mitomycin‐C and BCG in intravesical chemotherapy and immunotherapy of superficial bladder cancer. FinnBladder Research Group. Progress in Clinical and Biological Research 1989;310:271‐4. [PubMed] [Google Scholar]
Witjes 1996a {published data only}
- Mulders PF, Meyden AP, Doesburg WH, Oosterhof GO, Debruyne FM. Prognostic factors in pTa‐pT1 superficial bladder tumours treated with intravesical instillations. The Dutch South‐Eastern Urological Collaborative Group. British Journal of Urology 1994;73(4):403‐8. [DOI] [PubMed] [Google Scholar]
- Vegt PD, Witjes JA, Witjes WP, Doesburg WH, Debruyne FM, Meijden AP. A randomized study of intravesical mitomycin C, Bacillus Calmette‐Guerin Tice and Bacillus Calmette‐Guerin RIVM treatment in pTa‐pT1 papillary carcinoma and carcinoma in situ of the bladder. Journal of Urology 1995;153(3 Pt 2):929‐33. [PubMed] [Google Scholar]
- Witjes JA, Meijden AP, Witjes WP, Doesburg W, Schaafsma HE, Debruyne FM. A randomised prospective study comparing intravesical instillations of mitomycin‐C, BCG‐Tice, and BCG‐RIVM in pTa‐pT1 tumours and primary carcinoma in situ of the urinary bladder. Dutch South‐East Cooperative Urological Group. European Journal of Cancer 1993;29A(12):1672‐6. [DOI] [PubMed] [Google Scholar]
- Witjes WP, Witjes JA, Oosterhof GO, Debruyne MJ, Dutch South East Cooperative Urological Group. Update on the Dutch Cooperative Trial: mitomycin versus Bacillus Calmette‐Guérin‐Tice versus Bacillus Calmette‐Guérin RIVM in the treatment of patients with pTA‐pT1 papillary carcinoma and carcinoma in situ of the urinary bladder. Seminars in Urologic Oncology 1996;14(S1):10‐6. [PubMed] [Google Scholar]
- Witjes WP, Meijden PM, Roos EP, Witjes JA, Steerenberg PA, Doesburg W, et al. BCG‐RIVM versus BCG‐Tice versus mitomycin‐C in superficial bladder cancer. Rationale, design, and interim analysis of the trial of the South‐East Cooperative Urological Group, The Netherlands. Progress in Clinical and Biological Research 1992;378:59‐67. [PubMed] [Google Scholar]
Witjes 1996b {published data only}
- Mulders PF, Meyden AP, Doesburg WH, Oosterhof GO, Debruyne FM. Prognostic factors in pTa‐pT1 superficial bladder tumours treated with intravesical instillations. The Dutch South‐Eastern Urological Collaborative Group. British Journal of Urology 1994;73(4):403‐8. [DOI] [PubMed] [Google Scholar]
- Vegt PD, Witjes JA, Witjes WP, Doesburg WH, Debruyne FM, Meijden AP. A randomized study of intravesical mitomycin C, Bacillus Calmette‐Guerin Tice and Bacillus Calmette‐Guerin RIVM treatment in pTa‐pT1 papillary carcinoma and carcinoma in situ of the bladder. Journal of Urology 1995;153(3 Pt 2):929‐33. [PubMed] [Google Scholar]
- Witjes JA, Meijden AP, Witjes WP, Doesburg W, Schaafsma HE, Debruyne FM. A randomised prospective study comparing intravesical instillations of mitomycin‐C, BCG‐Tice, and BCG‐RIVM in pTa‐pT1 tumours and primary carcinoma in situ of the urinary bladder. Dutch South‐East Cooperative Urological Group. European Journal of Cancer 1993;29A(12):1672‐6. [DOI] [PubMed] [Google Scholar]
- Witjes WP, Witjes JA, Oosterhof GO, Debruyne MJ, Dutch South East Cooperative Urological Group. Update on the Dutch Cooperative Trial: mitomycin versus Bacillus Calmette‐Guérin‐Tice versus Bacillus Calmette‐Guérin RIVM in the treatment of patients with pTA‐pT1 papillary carcinoma and carcinoma in situ of the urinary bladder. Seminars in Urologic Oncology 1996;14(S1):10‐6. [PubMed] [Google Scholar]
- Witjes WP, Meijden PM, Roos EP, Witjes JA, Steerenberg PA, Doesburg W, et al. BCG‐RIVM versus BCG‐Tice versus mitomycin‐C in superficial bladder cancer. Rationale, design, and interim analysis of the trial of the South‐East Cooperative Urological Group, The Netherlands. Progress in Clinical and Biological Research 1992;378:59‐67. [PubMed] [Google Scholar]
Witjes 1998a {published data only}
- DeBruyne FM, Meijden AP, Franssen MP. BCG‐(RIVM) versus mitomycin intravesical therapy in patients with superficial bladder cancer. EORTC GU Group and the Dutch South‐East Collaborative Group. Progress in Clinical and Biological Research 1989;303:435‐46. [PubMed] [Google Scholar]
- DeBruyne FM, Meijden AP, Geboers AD, Franssen MP, Leeuwen MJ, Steerenberg PA, et al. BCG (RIVM) versus mitomycin intravesical therapy in superficial bladder cancer. First results of randomized prospective trial. Urology 1988;31(3 Suppl):20‐5. [PubMed] [Google Scholar]
- Debruyne FM, Meijden AP, Schreinemachers LM, Geboers AD, Franssen MP, Leeuwen MJ, et al. BCG‐RIVM intravesical immunoprophylaxis for superficial bladder cancer. Progress in Clinical and Biological Research 1988;269:511‐24. [PubMed] [Google Scholar]
- Witjes JA, Meijden AP, Collette L, Sylvester R, Debruyne FM, Aubel A, et al. Long‐term follow‐up of an EORTC randomized prospective trial comparing intravesical bacille Calmette‐Guérin‐RIVM and mitomycin C in superficial bladder cancer. EORTC GU Group and the Dutch South East Cooperative Urological Group. European Organisation for Research and Treatment of Cancer Genito‐Urinary Tract Cancer Collaborative Group. Urology 1998;52(3):403‐10. [DOI] [PubMed] [Google Scholar]
- Meijden PM, Debruyne FM, Steerenberg PA, Jong WH, Doesburg W. BCG‐RIVM versus BCG‐Tice versus mitomycin‐C in superficial bladder cancer. Rationale and design of the trial of the Southeast Co‐operative Urological Group, The Netherlands. Progress in Clinical and Biological Research 1989;310:285‐98. [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12613000513718 {published data only}
- ACTRN12613000513718. Adding mitomycin C to Bacillus of Calmette‐Guerin (BCG) as adjuvant intravesical therapy for high‐risk, non‐muscle‐invasive bladder cancer: a randomised phase 3 trial. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364165 (first received 8 May 2013).
Allona 1988 {published data only}
- Allona Moncada A, García Vaquero S, Zuloaga Gómez A, Martínez Torres JL, López‐Pardo R, Molina J, et al. Preliminary results of a multicenter study with mitomycin C in superficial bladder tumors (Ta, T1). Actas Urologicas Espanolas 1988;12(5):424‐9. [PubMed] [Google Scholar]
Altay 2000 {published data only}
- Altay B, Girgin C, Kefi A, Cikili N. The best management of superficial bladder tumours: comparing TUR alone versus TUR combined with intravesical chemotherapy modalities?. International Urology and Nephrology 2000;32(1):53‐8. [DOI] [PubMed] [Google Scholar]
Arends 2014 {published data only}
- Arends T, vander Heijden A, Witjes A. Results of a randomized controlled trial comparing intravesical combined chemohyperthermia with mitomycin‐C versus BCG for adjuvant treatment of patients with intermediate and high risk non‐muscle invasive bladder cancer. Urology 2014;1:S45‐6. [DOI] [PubMed] [Google Scholar]
- Arends TJ, Nativ O, Maffezzini M, Cobelli O, Heijden AG, Witjes JA. Results of the first randomized controlled trial comparing intravesical radiofrequency induced chemohyperthermia with mitomycin‐C versus BCG for adjuvant treatment of patients with intermediate‐ and high‐risk non‐muscle invasive bladder cancer. European Urology, Supplements 2015;14(2):944e. [DOI] [PubMed] [Google Scholar]
- Arends TJ, Nativ O, Maffezzini M, Cobelli O, Canepa G, Verweij F, et al. Results of a randomised controlled trial comparing intravesical chemohyperthermia with mitomycin C versus Bacillus Calmette‐Guerin for adjuvant treatment of patients with intermediate‐ and high‐risk non‐muscle‐invasive bladder cancer. European Urology 2016;69(6):1046‐52. [DOI] [PubMed] [Google Scholar]
Ayres 2010 {published data only}
- Ayres BE, Crew JP. Is immediate postoperative intravesical chemotherapy beneficial in non‐muscle‐invasive bladder cancer?. British Journal of Urology 2010;105 Suppl:14‐7. [DOI] [PubMed] [Google Scholar]
Badalato 2011 {published data only}
- Badalato GM, Hruby G, Razmjoo M, McKiernan JM. Maximizing intravesical therapy options: is there an advantage to the administration of perioperative mitomycin C prior to an induction course of BCG?. Canadian Journal of Urology 2011;18(5):5890‐5. [PubMed] [Google Scholar]
Bassi 2001 {published data only}
- Bassi P, Iafrate M, Spinadin R, Carando R, Iannello R, Repele M, et al. Superficial bladder neoplasia unresponsive to endocavitary treatment: when should the treatment approach be changed?. Archivio Italiano di Urologia 2001;73(4):181‐6. [PubMed] [Google Scholar]
Bismarck 2004 {published data only}
- Bismarck E, Schmitz‐Dräger BJ. Adjuvant and neoadjuvant therapy of urinary bladder carcinoma. Onkologe 2004;10(Suppl 1):S14‐5. [Google Scholar]
Boccafoschi 1991 {published data only}
- Boccafoschi C, Geraci E, Annoscia S, Montefiore F, Lozzi C, Leva G. Local immunoprophylaxis with BCG versus local chemoprophylaxis with doxorubicin or mitomycin C in the prevention of recurrence of superficial bladder cancer: comparative study. Acta Urologica Italica 1991;5(6 Suppl 1):149‐52. [Google Scholar]
Bochner 2006 {published data only}
- Bochner BH. Intravesical Bacillus Calmette‐Guerin combined with electromotive mitomycin for high‐risk superficial bladder cancer. Nature Clinical Practice Oncology 2006;3(9):474‐5. [DOI] [PubMed] [Google Scholar]
Bohle 2008 {published data only}
- Bohle A. A multicentre, randomised prospective trial comparing three intravesical adjuvant therapies for intermediate‐risk superficial bladder cancer: low‐dose Bacillus Calmette‐Guerin (27 mg) versus very low‐dose Bacillus Calmette‐Guerin (13.5 mg) versus mitomycin C. International Braz J Urol 2008;34(1):117‐8. [DOI] [PubMed] [Google Scholar]
Braasch 2008 {published data only}
- Braasch MR, Bohle A, O'Donnell MA. Risk‐adapted use of intravesical immunotherapy. BJU International 2008;102(9B):1254‐64. [DOI] [PubMed] [Google Scholar]
Brausi 1998 {published data only}
- Brausi M, Hurle R, Lembo A, Palladini PD. Immunotherapy with BCG in high risk recurrent bladder cancers previously treated with multiple cycles of chemotherapy: long‐term results. Acta Urologica Italica 1998;12(2):131‐2. [Google Scholar]
Chen 2012 {published data only}
- Chen C‐H, Yang H‐J, Shun C‐T, Huang C‐Y, Huang K‐H, Yu H‐J, et al. A cocktail regimen of intravesical mitomycin‐C, doxorubicin, and cisplatin (MDP) for non‐muscle‐invasive bladder cancer. Urologic Oncology 2012;30(4):421‐7. [DOI] [PubMed] [Google Scholar]
Chen 2019 {published data only}
- Chen HR, Kao CC, Tsao CW, Tang SH, En M, Cha TL, Sun GH, Wu ST, Yu DS. Comparison of Different Treatment Schedules of Mitomycin C Intravesical Instillation in High‐Risk Superficial Bladder Cancer Patients. Aktuelle Urologie 2019;50(3):292‐297. [DOI] [PubMed] [Google Scholar]
Cho 2012 {published data only}
- Cho IC, Kim EK, Joung JY, Seo HK, Chung J, Park WS, et al. Adjuvant intravesical instillation for primary T1G3 bladder cancer: BCG versus MMC in Korea. Anticancer Research 2012;32(4):1493‐8. [PubMed] [Google Scholar]
Crawford 1995 {published data only}
- Crawford ED. Randomized study of intravesical mitomycin‐C, Bacillus‐Calmette‐Guerin, Tice and Bacillus‐Calmette‐Guerin, RIVM treatment in pTa‐pT1 papillary carcinoma and carcinoma in‐situ of bladder – comment. Journal of Urology 1995;153(3):933. [PubMed] [Google Scholar]
Dalbagni 2009 {published data only}
- Dalbagni G. Is intravesical Bacillus Calmette‐Guerin better than mitomycin for intermediate‐risk bladder cancer?. European Urology 2009;56(2):257‐8; discussion 258. [DOI] [PubMed] [Google Scholar]
de Jong 1989 {published data only}
- Jong Z, Pontonnier F, Plante P, Mansat A, Centa F. The course of 203 superficial urothelial malignant tumors of the bladder during conservative treatment. Annales d'Urologie 1989;23(4):269‐74. [PubMed] [Google Scholar]
deVere 2000 {published data only}
- deVere White RW, Deitch AD, Daneshmand S, Blumenstein B, Lowe BA, Sagalowsky AI, et al. The prognostic significance of S‐phase analysis in stage Ta/T1 bladder cancer. A Southwest Oncology Group Study. European Urology 2000;37(5):595‐600. [DOI] [PubMed] [Google Scholar]
Di Stasi 2004a {published data only}
- Stasi SM, Giannantoni A, Stephen RL, Capelli G, Giurioli A, Zampa G, et al. Sequential Bacillus Calmette Guerin and electromotive mitomycin‐C versus Bacillus Calmette Guerin alone for high‐risk superficial bladder cancer: a prospective controlled study. Journal of Clinical Oncology 2004;22(14):391S. [Google Scholar]
Di Stasi 2004b {published data only}
- Stasi SM, Giannantoni A, Stephen RL, Virgill G, Giurioli A, Storti L, et al. Sequential intravesical Bacillus Calmette Guerin and electromotive mitomycin‐C for high risk superficial bladder cancer: a prospective controlled study. Journal of Urology 2004;171(4):74. [Google Scholar]
Di Stasi 2006b {published data only}
- Stasi SM, Giannantoni A, Giurioli A, Valenti M, Zampa G, Storti L, et al. Sequential BCG and electromotive mitomycin versus BCG alone for high‐risk superficial bladder cancer: a randomised controlled trial. Lancet Oncology 2006;7(1):43‐51. [DOI] [PubMed] [Google Scholar]
- Stasi SM, Giannantoni A, Giurioli A, Vespasiani G, Zampa G, Storti L, et al. Long‐term follow‐up of a randomised trial comparing sequential Bacillus Calmette‐Guerin and electromotive mitomycin‐C with Bacillus Calmette Guerin alone in high‐risk superficial bladder cancer. European Urology Supplements 2006;5(2):191. [Google Scholar]
- Stasi SM, Giannantoni A, Giurioli A, Vespasiani G, Zampa G, Storti L, et al. Sequential Bacillus Calmette‐Guerin and electromotive mitomycin‐C versus Bacillus Calmette‐Guerin alone for high risk superficial bladder cancer: long‐term follow‐up results of a prospective controlled study. Journal of Urology 2006;175(4):268. [Google Scholar]
Di Stasi 2012a {published data only}
- Stasi SM, Verri C, Liberati E, Masedu F, Topazio L, Valenti M. Intravesical sequential Bacillus Calmette‐Guerin and electromotive mitomycin versus Bacillus Calmette‐Guerin alone for stage PT1 urothelial bladder cancer. Journal of Urology 2012;1:e674. [Google Scholar]
Di Stasi 2012b {published data only}
- Stasi SM, Verri C, Liberati E, Zampa G, Masedu F, Valenti M. Intravesical sequential BCG and electromotive mitomycin versus BCG alone in high risk non‐muscle invasive bladder cancer. Journal of Clinical Oncology 2012;30(Suppl 1):15. [Google Scholar]
Di Stasi 2013 {published data only}
- Stasi SM, Verri C, Liberati E, Masedu F, Valenti M. Intravesical sequential BCG and electromotive mitomycin versus BCG alone for stage pT1 urothelial bladder cancer. European Urology 2013;12(1):e698‐9. [Google Scholar]
Di Stasi 2015 {published data only}
- Stasi SM, Riedl C, Giannantoni A, Verri C, Celestino F, Carlo F, et al. Is intravesical BCG alone still the only truly effective intravesical therapy for non‐muscle invasive bladder cancer?. Journal of Urology 2015;1:e381. [Google Scholar]
El Kader 2010 {published data only}
- Kader OA. Immediate mitomycin C instillation followed by usual BCG course versus usual BCG alone for superficial transitional cell carcinoma of the bladder (4 years experience). Journal of Urology 2010;1:e567‐8. [Google Scholar]
EUCTR2008‐005428‐99‐GB {published data only}
- EUCTR2008‐005428‐99‐GB. A randomised controlled phase III trial comparing hyperthermia plus mitomycin to a second course of Bacillus Calmette‐Guerin or standard therapy in patients with recurrence of non‐muscle invasive bladder cancer following induction or maintenance Bacillus Calmette‐Guerin therapy. Hyperthermia plus mitomycin (HYMN). www.clinicaltrialsregister.eu/ctr‐search/trial/2008‐005428‐99/GB (first registered 26 July 2010).
EUCTR2011‐000607‐41‐BE {published data only}
- EUCTR2011‐000607‐41‐BE. A phase I/II multicentric Belgian prospective novel sequential chemo‐immunotherapy regimen for adjuvant treatment in non‐muscle invasive bladder cancer. Novel chemo‐immunotherapy for non‐muscle invasive bladder cancer. apps.who.int/trialsearch/Trial3.aspx?trialid=EUCTR2011‐000607‐41‐BE (first received 11 February 2011).
FinnBladder 4 {published data only}
- Svatek RS. Long‐term outcomes of the FinnBladder‐4 study. European Urology 2015;68(4):618‐9. [DOI] [PubMed] [Google Scholar]
FinnBladder II {published data only}
- Rintala E. Results of a randomized phase III trial of sequential, intravesical therapy with mitomycin C and Bacillus Calmette‐Guerin versus mitomycin C alone in patients with superficial bladder cancer – comment. Journal of Urology 1998;160(5):1671‐2. [PubMed] [Google Scholar]
- Rintala E, Jauhiainen K, Kaasinen E, Nurmi M, Alfthan O. Alternating mitomycin C and Bacillus Calmette‐Guerin instillation prophylaxis for recurrent papillary (stages Ta to T1) superficial bladder cancer. FinnBladder Group. Journal of Urology 1996;156(1):56‐9; discussion 59. [PubMed] [Google Scholar]
- Rintala E, Jauhiainen K, Rajala P, Ruutu M, Kaasinen E, Alfthan O. Alternating mitomycin C and Bacillus Calmette‐Guerin instillation therapy for carcinoma in situ of the bladder. The FinnBladder Group. Journal of Urology 1995;154(6):2050‐3. [PubMed] [Google Scholar]
Gao 2002 {published data only}
- Gao CZ, Zhang JJ, Lu Yl, Zhao HY. Instillation of BCG vaccine and mitomycin for the prevention of bladder neoplasms recurrence. China Journal of Cancer Prevention and Treatment 2002;6:644‐5. [Google Scholar]
Gazzaniga 2009 {published data only}
- Gazzaniga P, Gradilone A, Berardinis E, Sciarra A, Cristini C, Naso G, et al. A chemosensitivity test to individualize intravesical treatment for non‐muscle‐invasive bladder cancer. BJU International 2009;104(2):184‐8. [DOI] [PubMed] [Google Scholar]
Gelabert‐Mas 1993 {published data only}
- Gelabert‐Mas A, Arango Toro O, Bielsa Gali O, Llado Carbonell C. A prospective and randomized study of the complete response, index of recurrences and progression in superficial bladder carcinoma treated with mitomycin C alone versus mitomycin C and BCG alternatively. Archivos Espanoles de Urologia 1993;46(5):379‐82. [PubMed] [Google Scholar]
Gelabert‐Mas 1997 {published data only}
- Gelabert‐Mas A. Six years of follow up. A prospective study on intravesical chemoprophylaxis with mitomycin‐C and BCG alternating. Complete response rate and much lower tumor progression rate. British Journal of Urology 1997;80(Suppl 2):34. [Google Scholar]
Gianneo 1997 {published data only}
- Gianneo E, Conti G. BCG vs mitomycin in patients affected by monocentric T1G2 bladder cancer: randomized study in diploid and aneuploid TCC. British Journal of Urology 1997;80(Suppl 2):40. [Google Scholar]
Grossman 2006 {published data only}
- Grossman HB. Sequential BCG and electromotive mitomycin versus BCG alone for high‐risk superficial bladder cancer: a randomised controlled trial. Urologic Oncology: Seminars and Original Investigations 2006;24(3):271‐2. [DOI] [PubMed] [Google Scholar]
Guerrero‐Ramos 2019 {published data only}
- Gonzalez Padilla DA, Gonzalez Diaz A, Miranda‐Utrera N, Rosa Kehrmann F, Villacampa‐Auba F, Guerrero‐Ramos F. HIVEC HR: Chemohyperthermia with mitomycin C vs BCG for high‐risk non‐muscle invasive bladder cancer. Preliminary results from a randomized controlled trial. European Urology. 2019; Vol. 18 (1):e768‐e770.
- Guerrero‐Ramos F, Gonzalez‐Padilla DA, Gonzalez‐Diaz A, Duarte‐Ojeda JM, Miranda‐Utrera N, Villacampa‐Auba F, Rosa‐Kehrmann F. BCG VS CHEMOHYPERTHERMIA WITH MITOMYCIN C FOR HIGH‐RISK NON‐MUSCLE INVASIVE BLADDER CARCINOMA: PRELIMINARY RESULTS OF HIVEC‐HR RANDOMIZED CLINICAL TRIAL. Journal of Urology. 2019; Vol. 201(4):E620‐E620.
Gulpinar 2012 {published data only}
- Gulpinar O, Halilioglu AH, Gokce MI, Gogus C, Baltaci S. The value of perioperative mitomycin C instillation in improving subsequent Bacillus Calmette‐Guerin instillation efficacy in intermediate and high‐risk patients with non‐muscle invasive bladder cancer: a prospective randomized study. International Braz J Urol 2012;38(4):474‐9. [DOI] [PubMed] [Google Scholar]
Han 2015 {published data only}
- Han KS, You D, Jeong IG, Kwon T, Hong B, Hong JH, et al. Is intravesical Bacillus Calmette‐Guérin therapy superior to chemotherapy for intermediate‐risk non‐muscle‐invasive bladder cancer? An ongoing debate. Journal of Korean Medical Science 2015;30(3):252‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hausladen 2003 {published data only}
- Hausladen DA, Wheeler MA, Altieri DC, Colberg JW, Weiss RM. Effect of intravesical treatment of transitional cell carcinoma with Bacillus Calmette‐Guerin and mitomycin C on urinary survival levels and outcome. Journal of Urology 2003;170(1):230‐4. [DOI] [PubMed] [Google Scholar]
Hayne 2011 {published data only}
- Hayne D, Stockler M, Ives A, Houghton B, Braganza P, Chalasani V, et al. Sequential BCG and mitomycin intravesical therapy versus BCG alone for high‐risk non‐muscle invasive bladder cancer (NMIBC): pilot study and proposal for a 2‐stage randomized phase III trial. BJU International 2011;107:25‐6. [Google Scholar]
Huang 2010 {published data only}
- Huang J, Wang GZ, Xu CI. Clinical trial of effect and adverse reaction of domestic therapeutic BCG for prevention of recurrence of superficial bladder cancer. Chinese Journal of Biologicals 2010;23(4):419‐21. [Google Scholar]
Iavarone 1996 {published data only}
- Iavarone C, Minocchi L, Arecchi S, Nicolucci D, Porcelli C, D'Orazi V, et al. A comparative study of BCG and mitomycin C in superficial carcinomas of the bladder. Giornale di Chirurgia 1996;17(5):289‐91. [PubMed] [Google Scholar]
ISRCTN85785327 {published data only}
- ISRCTN85785327. HYMN: a trial comparing hyperthermia and mitomycin chemotherapy with a second BCG treatment, or other standard treatment, for bladder cancer that has come back. www.isrctn.com/ISRCTN85785327 (first received 28 May 2009).
Jarvinen 2012 {published data only}
- Jarvinen R, Kaasinen E, Rintala E, the FinnBladder Group. Long‐term results of maintenance treatment of mitomycin C or alternating mitomycin C and Bacillus Calmette‐Guerin instillation therapy of patients with carcinoma in situ of the bladder: a subgroup analysis of the prospective FinnBladder 2 study with a 17‐year follow‐up. Scandinavian Journal of Urology and Nephrology 2012;46(6):411‐7. [DOI] [PubMed] [Google Scholar]
Jarvinen 2013 {published data only}
- Jarvinen R, Kaasinen E, Rintala E, Group T F. Re: long‐term results of maintenance treatment of mitomycin C or alternating mitomycin C and Bacillus Calmette‐Guérin instillation therapy of patients with carcinoma in situ of the bladder: a subgroup analysis of the prospective FinnBladder 2 study with a 17‐year follow‐up. Journal of Urology 2013;190(6):2019. [DOI] [PubMed] [Google Scholar]
Jarvinen 2014 {published data only}
- Jarvinen R, Kaasinen E, Rintala E, Liukkonen T, Puolakka VM, Kallio J, et al. 15‐year outcome of patients with frequently recurrent non‐muscle‐invasive bladder carcinoma (NMIBC) treated with 5 weekly mitomycin C (MMC) instillations followed by monthly Bacillus‐Calmette Guerin (BCG) or alternating BCG and interferon‐alpha2b (IFN) instillations. European Urology 2014;13(1):e1107. [Google Scholar]
Jarvinen 2015 {published data only}
- Jarvinen R, Marttila T, Kaasinen E, Rintala E, Aaltomaa S, Kallio J, et al. Long‐term outcome of patients with frequently recurrent non‐muscle‐invasive bladder carcinoma treated with one perioperative plus four weekly instillations of mitomycin C followed by monthly Bacillus Calmette‐Guérin (BCG) or alternating BCG and interferon‐α2b instillations: prospective randomised FinnBladder‐4 study. European Urology 2015;68(4):611‐7. [DOI] [PubMed] [Google Scholar]
Jauhiainen 1993 {published data only}
- Jauhiainen K, Rintala E. Superficial urinary bladder cancer. Results from the FinnBladder studies and a review on instillation treatments. Annales Chirurgiae et Gynaecologiae 1993;206:31‐8. [PubMed] [Google Scholar]
Kaasinen 2000 {published data only}
- Kaasinen E, Rintala E, Pere AK, Kallio J, Puolakka VM, Liukkonen T, et al. Weekly mitomycin C followed by monthly Bacillus Calmette‐Guerin or alternating monthly interferon‐alpha2B and Bacillus Calmette‐Guerin for prophylaxis of recurrent papillary superficial bladder carcinoma. Journal of Urology 2000;164(1):47‐52. [PubMed] [Google Scholar]
Kaasinen 2002 {published data only}
- Kaasinen E, Rintala E, Hellstrom P, Viitanen J, Juusela H, Rajala P, et al. Factors explaining recurrence in patients undergoing chemoimmunotherapy regimens for frequently recurring superficial bladder carcinoma. European Urology 2002;42(2):167‐74. [DOI] [PubMed] [Google Scholar]
Kaasinen 2003 {published data only}
- Kaasinen E, Wijkstrom H, Malmström PU, Hellsten S, Duchek M, Mestad O, et al. Alternating mitomycin C and BCG instillations versus BCG alone in treatment of carcinoma in situ of the urinary bladder: a Nordic study. European Urology 2003;43(6):637‐45. [DOI] [PubMed] [Google Scholar]
Kaasinen 2014 {published data only}
- Kaasinen E, Wijkstrom H, Malmström PU, Rintala E, Jahnsson S. 17 year follow‐up of the Nordic CIS study: long‐term results of 1 year BCG monotherapy versus alternating therapy with mitomycin C and BCG in patients with carcinoma in situ of the urinary bladder. European Urology 2014;13(1):e1006. [DOI] [PubMed] [Google Scholar]
Kelly 2015 {published data only}
- Kelly J, Buckley L, Devall AJ, Loubiere LS, Barnwell JM, Mostafid H, et al. HYMN: a randomised controlled phase III trial comparing hyperthermia plus mitomycin to a second course of BCG or institutional standard in patients with recurrence of non‐muscle invasive bladder cancer (NMIBC) following induction or maintenance BCG therapy. BJU International 2015;115:12. [Google Scholar]
Kirkali 2010 {published data only}
- Kirkali Z, Oosterlinck W, Sylvester R, Silva FC, Busch C, Algaba F, et al. Sequential chemo‐immunotherapy with mitomycin C (MMC) and Bacillus Calmette‐Guerin (BCG) versus BCG alone in patients with carcinoma in situ (CIS) of the urinary bladder. Results of EORTC GU group randomized phase II study 30993. Journal of Urology 2010;1:e568. [Google Scholar]
Kurth 2000 {published data only}
- Kurth KH, Bouffioux C, Sylvester R, Meijden AP, Oosterlinck W, Brausi M. Treatment of superficial bladder tumors: achievements and needs. The EORTC Genitourinary Group. European Urology 2000;37 Suppl 3:1‐9. [DOI] [PubMed] [Google Scholar]
Lamm 1991 {published data only}
- Lamm DL. Comparison of BCG with other intravesical agents. Urology 1991;37(5 Suppl):30‐2. [DOI] [PubMed] [Google Scholar]
Leblanc 1999 {published data only}
- Leblanc B, Duclos AJ, Bénard F, Côté J, Valiquette L, Paquin JM, et al. Long‐term followup of initial Ta grade 1 transitional cell carcinoma of the bladder. Journal of Urology 1999;162(6):1946‐50. [DOI] [PubMed] [Google Scholar]
Liberati 2012 {published data only}
- Liberati E, Verri C, Topazio L, Valenti M, Stasi SM. Intravesical sequential BCG and electromotive mitomycin‐C versus BCG alone for stage PT1 urothelial bladder cancer. Anticancer Research 2012;32(5):1861‐2. [Google Scholar]
Lundholm 1999 {published data only}
- Lundholm C, Wester K, Busch C, Norlen BJ, Ekman P, Karlberg L, et al. 5‐Year followup of a randomized prospective study comparing mitomycin C and Bacillus Calmette‐Guerin in patients with superficial bladder carcinoma. Journal of Urology 1999;161(4):1124‐7. [PubMed] [Google Scholar]
Malmström 2009b {published data only}
- Malmström PU, Sylvester RJ. Rebuttal from authors re: Guido Dalbagni. Is intravesical Bacillus Calmette‐Guérin better than mitomycin for intermediate‐risk bladder cancer?. European Urology 2009;56(2):258‐9. [DOI] [PubMed] [Google Scholar]
Matsumoto 2010 {published data only}
- Matsumoto K, Kikuchi E, Horiguchi Y, Tanaka N, Miyajima A, Nakagawa K, et al. Late recurrence and progression in non‐muscle‐invasive bladder cancers after 5‐year tumor‐free periods. Urology 2010;75(6):1385‐90. [DOI] [PubMed] [Google Scholar]
Matsumoto 2012 {published data only}
- Matsumoto K, Kikuchi E, Shirakawa H, Hayakawa N, Tanaka N, Ninomiya A, et al. Risk of subsequent tumour recurrence and stage progression in bacille Calmette‐Guerin relapsing non‐muscle‐invasive bladder cancer. BJU International 2012;110(11 Pt B):E508‐13. [DOI] [PubMed] [Google Scholar]
Mondal 2016 {published data only}
- Mondal HP, Yirang K, Mukhopadhyay C, Adhikary SS, Dutta B, Bhoj SS. Prospective randomized study between intravesical BCG and mitomycin‐C for non‐muscle‐invasive urothelial carcinoma of urinary‐bladder post TURBT. Bangladesh Journal of Medical Science 2016;15(1):74‐77. [Google Scholar]
Morales 1999 {published data only}
- Morales A. Results of a randomized phase III trial of sequential intravesical therapy with mitomycin C and Bacillus Calmette‐Guerin versus mitomycin C alone in patients with superficial bladder cancer. Journal of Urology 1999;161(5):1582‐3. [DOI] [PubMed] [Google Scholar]
Murillo 2019 {published data only}
- Murillo I, Barja S. Treatment of High‐risk Non‐muscle Invasive Bladder Cancer With IMUNO BGC Moreau RJ as Prophylaxis. https://clinicaltrials.gov/ct2/show/NCT03982797 2019.
NCT00023842 {published data only}
- NCT00023842. BCG with or without mitomycin in treating patients with bladder cancer. clinicaltrials.gov/ct2/show/NCT00023842 (first received 27 January 2003).
NCT00384891 {published data only}
- NCT00384891. Hyperthermia treatment in conjunction with mitomycin C versus BCG for superficial bladder cancer. clinicaltrials.gov/ct2/show/NCT00384891 (first received 6 October 2006).
NCT01094964 {published data only}
- NCT01094964. Hyperthermia and mitomycin C, Bacillus Calmette‐Guerin, or standard therapy as second‐line therapy in treating patients with recurrent bladder cancer. clinicaltrials.gov/ct2/show/NCT01094964 (first received 29 March 2010).
NCT01442519 {published data only}
- NCT01442519. Sequential Bacillus Calmette‐Guérin (BCG) and electromotive mitomycin‐C versus Bacillus Calmette‐Guérin (BCG) alone for high risk superficial bladder cancer. clinicaltrials.gov/ct2/show/NCT01442519 (first received 28 September 2011).
Nishimura 1996 {published data only}
- Nishimura K, Higashino M, Hara T, Oka T, Sugao H, Osafune M. Clinical study of intravesical instillation therapy for superficial bladder cancer. Journal of Urology 1996;58(4):362‐6. [Google Scholar]
Nohales 1996 {published data only}
- Nohales TG, Cortadellas ÁR, Arango TO, Bielsa GO, Gelabert MA. Resultados de un estudio prospective de quimioprofilaxis con mitomycina‐C y BCG alternadas: respuesta completa, Índice de recidivas y de progresión. Archivos Espanoles de Urologia 1996;49(7):689‐92. [PubMed] [Google Scholar]
Nouhaud 2017 {published data only}
- Nouhaud FX, Rigaud J, Saint F, Colombel M, Irani J, Soulie M, et al. Final results of the phase III URO‐BCG 4 multicenter study: efficacy and tolerance of one‐third dose BCG maintenance in nonmuscle invasive bladder cancer. Anticancer Drugs 2017;28(3):335‐40. [DOI] [PubMed] [Google Scholar]
Ooi 2011 {published data only}
- Ooi Wei L, Stockler M, Hayne D. Re: Willem Oosterlinck, Ziya Kirkali, Richard Sylvester, et al. Sequential intravesical chemoimmunotherapy with mitomycin C and Bacillus Calmette‐Guerin and with Bacillus Calmette‐Guerin alone in patients with carcinoma in situ of the urinary bladder: results of an EORTC Genito‐urinary Group Randomized Phase 2 Trial (30993). Eur Urol 2011;59:438‐46. European Urology 2011;60(1):e1; author reply e2‐3. [DOI] [PubMed] [Google Scholar]
Peyromaure 2004 {published data only}
- Peyromaure M, Zerbib M. T1G3 transitional cell carcinoma of the bladder: recurrence, progression and survival. BJU International 2004;93(1):60‐3. [DOI] [PubMed] [Google Scholar]
Raviv 2005 {published data only}
- Raviv G, Pinthus JH, Shefi S, Mor Y, Kaufman‐Francis K, Levron J, et al. Effects of intravesical chemotherapy and immunotherapy on semen analysis. Urology 2005;65(4):765‐7. [DOI] [PubMed] [Google Scholar]
Saxena 2006 {published data only}
- Saxena S, Agrawal U, Agarwal A, Murthy NS, Mohanty NK. Adjuvant intravesical therapy based on an in vitro cytotoxicity assay in the management of superficial transitional cell cancer of the urinary bladder. BJU International 2006;98(5):1012‐7. [DOI] [PubMed] [Google Scholar]
Sekine 2001 {published data only}
- Sekine H, Ohya K, Kojima S, Igarashi K, Fukui I. Equivalent efficacy of mitomycin C plus doxorubicin instillation to Bacillus Calmette‐Guerin therapy for carcinoma in situ of the bladder. International Journal of Urology 2001;8(9):483‐6. [DOI] [PubMed] [Google Scholar]
Shelley 2015b {published data only}
- Shelley M, Court JB, Kynaston HG, Wilt TJ, Coles B, Mason M. WITHDRAWN: Intravesical Bacillus Calmette‐Guerin versus mitomycin C for Ta and T I bladder cancer. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD003231.pub2] [DOI] [PubMed] [Google Scholar]
Smits 1998 {published data only}
- Smits G, Schaafsma E, Kiemeney L, Caris C, Debruyne F, Witjes JA. Microstaging of pT1 transitional cell carcinoma of the bladder: identification of subgroups with distinct risks of progression. Urology 1998;52(6):1009‐13; discussion 1013. [DOI] [PubMed] [Google Scholar]
Soloway 1990 {published data only}
- Soloway MS. Follow up of patients receiving treatment for superficial bladder cancer with mitomycin C and BCG. Progress in Clinical and Biological Research 1990;350:71‐9. [PubMed] [Google Scholar]
Stasi 2004 {published data only}
- Stasi SM, Giannantoni A, Stephen RL, Capelli G, Giurioli A, Zampa G, et al. Sequential Bacillus Calmette Guérin and electromotive mitomycin‐C versus Bacillus Calmette Guérin alone for high‐risk superficial bladder cancer: a prospective controlled study. Annual Meeting Proceedings of the American Society of Clinical Oncology. 2004:390.
Steinberg 2017 {published data only}
- Steinberg RL, Brooks NA, Thomas LJ, Mott SL, O'Donnell MA. Bacillus Calmette‐Guerin strain may not effect recurrence‐free survival when used intravesically with interferon‐alpha2b for non‐muscle‐invasive bladder cancer. Urologic Oncology 2017;35(5):201‐7. [DOI] [PubMed] [Google Scholar]
Study 30993 {published data only}
- Oosterlinck W, Kirkali Z, Sylvester R, Silva FC, Busch C, Algaba F, et al. Sequential intravesical chemoimmunotherapy with mitomycin C and Bacillus Calmette‐Guerin and with Bacillus Calmette‐Guerin alone in patients with carcinoma in situ of the urinary bladder: results of an EORTC genito‐urinary group randomized phase 2 trial (30993). European Urology 2011;59(3):438‐46. [DOI] [PubMed] [Google Scholar]
- Oosterlinck W, Kirkali Z, Sylvester RJ, Calais Da Silva F, Busch C, Algaba F, et al. Sequential chemo‐immunotherapy with mitomycin C (MMC) and Bacillus Calmette‐Guerin (BCG) versus BCG alone in patients with carcinoma in situ (CIS) of the urinary bladder results of EORTC GU group randomized phase II study 30993. European Urology 2010;9(2):91. [DOI] [PubMed] [Google Scholar]
Sylvester 2009 {published data only}
- Sylvester RJ. Bacillus Calmette‐Guerin versus mitomycin C for the treatment of intermediate‐risk non‐muscle‐invasive bladder cancer: the debate continues. European Urology 2009;56(2):266‐8; discussion 268. [DOI] [PubMed] [Google Scholar]
Tong 2003 {published data only}
- Tong M, Yu LZ, Ding Y, Liu LB, Pan BN, Na YQ. Prevention of postoperative recurrence of human bladder carcinoma by intravesical instillation of immunotoxin, a clinical study. Zhonghua Yi Xue za Zhi 2003;83(3):201‐3. [PubMed] [Google Scholar]
van der Meijden 1989 {published data only}
- Meijden PM, Debruyne FM, Steerenberg PA, Jong WH, Doesburg W. BCG‐RIVM versus BCG‐Tice versus mitomycin‐C in superficial bladder cancer. Rationale and design of the trial of the Southeast Co‐operative Urological Group, The Netherlands. Progress in Clinical and Biological Research 1989;310:285‐98. [PubMed] [Google Scholar]
van Gils‐Gielen 1995 {published data only}
- Gils‐Gielen RJ, Witjes WP, Caris CT, Debruyne FM, Witjes JA, Oosterhof GO. Risk factors in carcinoma in situ of the urinary bladder. Dutch South East Cooperative Urological Group. Urology 1995;45(4):581‐6. [DOI] [PubMed] [Google Scholar]
Wang 1992 {published data only}
- Wang SH. Intravesical BCG, mitomycin‐C and thiotepa in the prevention of bladder tumor recurrence after operation. Chinese Journal of Surgery 1992;30(7):410‐2. [PubMed] [Google Scholar]
Wang 2011 {published data only}
- Wang F, Qin W, Zhang G, Zhang Y, Liu H, Yang L, et al. Prospective clinical studies at the efficacy of Brucea javanica oil, mitomycin and BCG for preventing postoperative relapse of superficial bladder cancer through perfusion. Chinese‐German Journal of Clinical Oncology 2011;10(4):P228‐31. [Google Scholar]
- Wang FL, Qin WJ, Wang H, Dong QC, Liu HL, Zhang YT, et al. Efficacy of Brucea javanica oil, mitomycin and BCG for prophylaxis of recurrent superficial bladder cancer. Chinese Journal of Cancer Prevention and Treatment 2010;17(8):622‐4. [Google Scholar]
Witjes 1998b {published data only}
- Witjes JA, Caris CT, Mungan NA, Debruyne FM, Witjes WP. Results of a randomized phase III trial of sequential intravesical therapy with mitomycin C and Bacillus Calmette‐Guerin versus mitomycin C alone in patients with superficial bladder cancer. Journal of Urology 1998;160(5):1668‐71; discussion 1671‐2. [PubMed] [Google Scholar]
Witjes 1999a {published data only}
- Witjes JA, Caris CT, Mungan NA, Debruyne FM, Witjes WP. Results of a randomized phase III trial of sequential intravesical therapy with mitomycin C and Bacillus Calmette‐Guerin versus mitomycin C alone in patients with superficial bladder cancer. Journal of Urology 1999;160(5):1668‐72. [PubMed] [Google Scholar]
Witjes 1999b {published data only}
- Witjes JA, Caris CT, Mungan NA, Debruyne FM, Witjes WP, Morales A. Re: results of a randomized phase III trial of sequential intravesical therapy with mitomycin C and Bacillus Calmette‐Guerin versus mitomycin C alone in patients with superficial bladder cancer (multiple letters). Journal of Urology 1999;161(5):1582‐3. [Google Scholar]
Yabusaki 1991 {published data only}
- Yabusaki N, Komatsu H, Tago K, Yamada Y, Ueno A. Intravesical instillation of Bacillus Calmette‐Guerin for superficial bladder carcinoma: study on significance of additional maintenance instillations of Bacillus Calmette‐Guerin. Nippon Hinyokika Gakkai Zasshi 1991;82(2):290‐6. [DOI] [PubMed] [Google Scholar]
Yang 1999 {published data only}
- Yang D, Li S, Wang H, Li X, Liu S, Han W, et al. Prevention of postoperative recurrence of bladder cancer: a clinical study. Zhonghua Wai Ke za Zhi [Chinese Journal of Surgery] 1999;37(8):464‐5. [PubMed] [Google Scholar]
Yari 2010 {published data only}
- Yari H, Fallahnezhad M, Haji KB, Tavasoli SS. Comparison of full‐dose intravesical BCG versus half dose BCG and mitomycin‐C in treatment of patients with superficial bladder cancer. European Urology 2010;9(6):595‐6. [Google Scholar]
Additional references
Abern 2013
- Abern MR, Owusu RA, Anderson MR, Rampersaud EN, Inman BA. Perioperative intravesical chemotherapy in non‐muscle‐invasive bladder cancer: a systematic review and meta‐analysis. Journal of the National Comprehensive Cancer Network : JNCCN 2013;11(4):477‐84. [DOI] [PubMed] [Google Scholar]
Abufaraj 2018
- Abufaraj M, Mostafid H, Shariat SF, Babjuk M. What to do during Bacillus Calmette‐Guérin shortage? Valid strategies based on evidence. Current Opinion in Urology 2018;28(6):570‐6. [DOI] [PubMed] [Google Scholar]
AHRQ 2016
- Chou R, Buckley D, Fu R, Gore JL, Gustafson K, Griffin J, et al. Emerging Approaches to Diagnosis and Treatment of Non‐Muscle‐Invasive Bladder Cancer. AHRQ Publication No. 15(16)‐EHC017‐EF. Rockville (MD): Agency for Healthcare Research and Quality, 2016. [PubMed] [Google Scholar]
Arends 2016
- Arends TJ, Nativ O, Maffezzini M, Cobelli O, Canepa G, Verweij F, et al. Results of a randomised controlled trial comparing intravesical chemohyperthermia with mitomycin C versus Bacillus Calmette‐Guérin for adjuvant treatment of patients with intermediate‐ and high‐risk non‐muscle‐invasive bladder cancer. European Urology 2016;69(6):1046‐52. [DOI] [PubMed] [Google Scholar]
Babjuk 2018
- Babjuk M, Burger M, Compérat E, Gontero P, Mostafid AH, Palou J, et al. EAU Guidelines on Non‐Muscle‐Invasive Bladder Cancer (TaT1 and CIS). Arnhem (The Netherlands): European Association of Urology, 2018. [Google Scholar]
Bender 2018
- Bender R, Friede T, Koch A, Kuss O, Schlattmann P, Schwarzer G, et al. Methods for evidence synthesis in the case of very few studies. Research Synthesis Methods 2018;9(3):382‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boehm 2017
- Boehm BE, Cornell JE, Wang H, Mukherjee N, Oppenheimer JS, Svatek RS. Efficacy of Bacillus Calmette‐Guérin strains for treatment of nonmuscle invasive bladder cancer: a systematic review and network meta‐analysis. Journal of Urology 2017;198(3):503‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burger 2013
- Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. European Urology 2013;63(2):234‐41. [DOI] [PubMed] [Google Scholar]
Böhle 2003
- Böhle A, Jocham D, Bock PR. Intravesical Bacillus Calmette‐Guerin versus mitomycin C for superficial bladder cancer: a formal meta‐analysis of comparative studies on recurrence and toxicity. Journal of Urology 2003;169(1):90‐5. [DOI] [PubMed] [Google Scholar]
Böhle 2004
- Böhle A, Bock PR. Intravesical bacille Calmette‐Guérin versus mitomycin C in superficial bladder cancer: formal meta‐analysis of comparative studies on tumor progression. Urology 2004;63(4):682‐6. [DOI] [PubMed] [Google Scholar]
Cernuschi 2018
- Cernuschi T, Malvolti S, Nickels E, Friede M. Bacillus Calmette‐Guérin (BCG) vaccine: a global assessment of demand and supply balance. Vaccine 2018;36(4):498‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Angelis 2014
- Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, et al. Cancer survival in Europe 1999‐2007 by country and age: results of EUROCARE‐5 – a population‐based study. Lancet Oncology 2014;15(1):23‐34. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Di Stasi 2006a
- Stasi SM, Giannantoni A, Giurioli A, Valenti M, Zampa G, Storti L, et al. Sequential BCG and electromotive mitomycin versus BCG alone for high‐risk superficial bladder cancer: a randomised controlled trial. Lancet Oncology 2006;7(1):43‐51. [DOI] [PubMed] [Google Scholar]
Fajkovic 2011
- Fajkovic H, Halpern JA, Cha EK, Bahadori A, Chromecki TF, Karakiewicz PI, et al. Impact of gender on bladder cancer incidence, staging, and prognosis. World Journal of Urology 2011;29(4):457‐63. [DOI] [PubMed] [Google Scholar]
Ferlay 2013
- Ferlay J, Steliarova‐Foucher E, Lortet‐Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. European Journal of Cancer 2013;49(6):1374‐403. [DOI] [PubMed] [Google Scholar]
Gardmark 2007
- Gardmark T, Jahnson S, Wahlquist R, Wijkstrom H, Malmström PU. Analysis of progression and survival after 10 years of a randomized prospective study comparing mitomycin‐C and Bacillus Calmette‐Guerin in patients with high‐risk bladder cancer. BJU International 2007;99(4):817‐20. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed Mai 2019.
Griffiths 2013
- Griffiths TR, Action on Bladder Cancer. Current perspectives in bladder cancer management. International Journal of Clinical Practice 2013;67(5):435‐48. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Schünemann HJ, et al. GRADE: what is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, BrozekJ, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jung 2017
- Jung JH, Gudeloglu A, Kiziloz H, Kuntz GM, Miller A, Konety BR, et al. Intravesical electromotive drug administration for non‐muscle invasive bladder cancer. Cochrane Database of Systematic Reviews 2017, Issue 9. [DOI: 10.1002/14651858.CD011864.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaasinen 2016
- Kaasinen E, Wijkström H, Rintala E, Mestad O, Jahnson S, Malmström PU. Seventeen‐year follow‐up of the prospective randomized Nordic CIS study: BCG monotherapy versus alternating therapy with mitomycin C and BCG in patients with carcinoma in situ of the urinary bladder. Scandinavian Journal of Urology 2016;50(5):360‐8. [DOI] [PubMed] [Google Scholar]
Lamm 2000
- Lamm DL, Blumenstein BA, Crissman JD, Montie JE, Gottesman JE, Lowe BA, et al. Maintenance Bacillus Calmette‐Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group study. Journal of Urology 2000;163(4):1124‐9. [PubMed] [Google Scholar]
Lammers 2011
- Lammers RJ, Witjes JA, Inman BA, Leibovitch I, Laufer M, Nativ O, et al. The role of a combined regimen with intravesical chemotherapy and hyperthermia in the management of non‐muscle‐invasive bladder cancer: a systematic review. European Urology 2011;60(1):81‐93. [DOI] [PubMed] [Google Scholar]
Lane 2013
- Lane PW. Meta‐analysis of incidence of rare events. Statistical Methods in Medical Research 2013;22(2):117‐32. [DOI] [PubMed] [Google Scholar]
Malmström 2009a
- Malmström PU, Sylvester RJ, Crawford DE, Friedrich M, Krege S, Rintala E, et al. An individual patient data meta‐analysis of the long‐term outcome of randomised studies comparing intravesical mitomycin C versus Bacillus Calmette‐Guérin for non‐muscle‐invasive bladder cancer. European Urology 2009;56(2):247‐56. [DOI] [PubMed] [Google Scholar]
Miyazaki 2018
- Miyazaki J, Onozawa M, Takaoka E, Yano I. Bacillus Calmette‐Guérin strain differences as the basis for immunotherapies against bladder cancer. International Journal of Urology 2018;25(5):405‐13. [DOI] [PubMed] [Google Scholar]
NCT02948543
- NCT02948543. Adding mitomycin C to Bacillus of Calmette‐Guerin (BCG) as adjuvant intravesical therapy for high‐risk, non‐muscle‐invasive bladder cancer (BCG+MMC). clinicaltrials.gov/ct2/show/NCT02948543 (first received 28 October 2016).
Packiam 2017
- Packiam VT, Johnson SC, Steinberg GD. Non‐muscle‐invasive bladder cancer: intravesical treatments beyond bacille Calmette‐Guérin. Cancer 2017;123(3):390‐400. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Perlis 2013
- Perlis N, Zlotta AR, Beyene J, Finelli A, Fleshner NE, Kulkarni GS. Immediate post‐transurethral resection of bladder tumor intravesical chemotherapy prevents non‐muscle‐invasive bladder cancer recurrences: an updated meta‐analysis on 2548 patients and quality‐of‐evidence review. European Urology 2013;64(3):421‐30. [DOI] [PubMed] [Google Scholar]
Pode 1986
- Pode D, Alon Y, Horowitz AT, Vlodavsky I, Biran S. The mechanism of human bladder tumor implantation in an in vitro model. Journal of Urology 1986;136(2):482‐6. [DOI] [PubMed] [Google Scholar]
Ragonese 2016
- Ragonese M, Racioppi M, Bassi PF, Gianfrancesco L, Lenci N, Filianoti A, et al. Mitomycin C: new strategies to improve efficacy of a well‐known therapy. Urologia 2016;83(Suppl 2):24‐8. [DOI] [PubMed] [Google Scholar]
Rentsch 2014
- Rentsch CA, Birkhäuser FD, Biot C, Gsponer JR, Bisiaux A, Wetterauer C, et al. Bacillus Calmette‐Guérin strain differences have an impact on clinical outcome in bladder cancer immunotherapy. European Urology 2014;66(4):677‐88. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schmidt 2015
- Schmidt S, Kunath F, Coles B, Draeger D, Krabbe L, Dersch R, Jensen K, Dahm P, Meerpohl JJ. Intravesical bacillus Calmette‐Guérin versus mitomycin C for Ta and T1 bladder cancer. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD011935] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sengiku 2013
- Sengiku A, Ito M, Miyazaki Y, Sawazaki H, Takahashi T, Ogura K. A prospective comparative study of intravesical Bacillus Calmette‐Guérin therapy with the Tokyo or Connaught strain for nonmuscle invasive bladder cancer. Journal of Urology 2013;190(1):50‐4. [DOI] [PubMed] [Google Scholar]
Shang 2011
- Shang PF, Kwong J, Wang ZP, Tian J, Jiang L, Yang K, et al. Intravesical Bacillus Calmette‐Guérin versus epirubicin for Ta and T1 bladder cancer. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD006885.pub2] [DOI] [PubMed] [Google Scholar]
Shariat 2010
- Shariat SF, Sfakianos JP, Droller MJ, Karakiewicz PI, Meryn S, Bochner BH. The effect of age and gender on bladder cancer: a critical review of the literature. BJU International 2010;105(3):300‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shelley 2010
- Shelley MD, Mason MD, Kynaston H. Intravesical therapy for superficial bladder cancer: a systematic review of randomised trials and meta‐analyses. Cancer Treatment Reviews 2010;36(3):195‐205. [DOI] [PubMed] [Google Scholar]
Siegel 2018
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a Cancer Journal for Clinicians 2018;68(1):7‐30. [DOI] [PubMed] [Google Scholar]
Sievert 2009
- Sievert KD, Amend B, Nagele U, Schilling D, Bedke J, Horstmann M, et al. Economic aspects of bladder cancer: what are the benefits and costs?. World Journal of Urology 2009;27(3):295‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Slater 2014
- Slater SE, Patel P, Viney R, Foster M, Porfiri E, James ND, et al. The effects and effectiveness of electromotive drug administration and chemohyperthermia for treating non‐muscle invasive bladder cancer. Annals of the Royal College of Surgeons of England 2014;96(6):415‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sobin 2009
- Sobin LH, Gospodariwicz M, Wittekind C. TNM Classification of Malignant Tumors. 7th Edition. Geneva (Switzerland): UICC International Union Against Cancer, 2009. [Google Scholar]
Soloway 1980
- Soloway MS, Masters S. Urothelial susceptibility to tumor cell implantation: influence of cauterization. Cancer 1980;46(5):1158‐63. [DOI] [PubMed] [Google Scholar]
Solsona 2016
- Solsona E, Madero R, Chantada V, Fernandez JM, Zabala JA, Portillo JA, et al. Members of Club Urológico Español de Tratamiento Oncológico. Sequential combination of mitomycin C plus Bacillus Calmette‐Guérin (BCG) is more effective but more toxic than BCG alone in patients with non‐muscle‐invasive bladder cancer in intermediate‐ and high‐risk patients: final outcome of CUETO 93009, a randomized prospective trial. European Urology 2016;67(3):508‐16. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Svatek 2014
- Svatek RS, Hollenbeck BK, Holmäng S, Lee R, Kim SP, Stenzl A, et al. The economics of bladder cancer: costs and considerations of caring for this disease. European Urology 2014;66(2):253‐62. [DOI] [PubMed] [Google Scholar]
Sylvester 2004
- Sylvester RJ, Oosterlinck W, Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta‐analysis of published results of randomized clinical trials. Journal of Urology 2004;171(6 Pt 1):2186‐90. [DOI] [PubMed] [Google Scholar]
Sylvester 2006
- Sylvester RJ, Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. European Urology 2006;49(3):466‐77. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Rhijn 2009
- Rhijn BW, Burger M, Lotan Y, Solsona E, Stief CG, Sylvester RJ, et al. Recurrence and progression of disease in non–muscle‐invasive bladder cancer: from epidemiology to treatment strategy. European Urology 2009;56:430‐42. [DOI] [PubMed] [Google Scholar]
Williamson 2002
- Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta‐analysis with time‐to‐event outcomes. Statistics in Medicine 2002;1(22):3337‐51. [DOI] [PubMed] [Google Scholar]
Woods 2010
- Woods BS, Hawkins N, Scott DA. Network meta‐analysis on the log‐hazard scale, combining count and hazard ratio statistics accounting for multi‐arm trials: a tutorial. BMC Medical Research Methodology 2010;10:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Shelley 2003
- Shelley MD, Court JB, Kynaston H, Wilt TJ, Coles B, Mason M. Intravesical Bacillus Calmette‐Guerin versus mitomycin C for Ta and T1 bladder cancer [updated in 2015 and then withdrawn]. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD003231] [DOI] [PubMed] [Google Scholar]
Shelley 2015a
- Schmidt S, Kunath F, Coles B, Draeger DL, Krabbe LM, Dersch R, et al. Intravesical bacillus Calmette‐Guérin versus mitomycin C for Ta and T1 bladder cancer. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD011935] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shelley 2015c
- Shelley M, Court JB, Kynaston HG, Wilt TJ, Coles B, Mason M. WITHDRAWN: Intravesical Bacillus Calmette‐Guerin versus mitomycin C for Ta and T I bladder cancer. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD003231.pub2] [DOI] [PubMed] [Google Scholar]


