Abstract
The microsporidian parasite Nosema ceranae is a highly prevalent, global honey bee pathogen. Apis mellifera is considered to be a relatively recent host for this microsporidia, which raises questions as to how it affects its host’s physiology, behavior and longevity, both at the individual and colony level. As such, honey bees were inoculated with fresh purified spores of this pathogen, both individually (Group A) or collectively (Group B) and they were studied from 0 to 15 days post-emergence (p.e.) to evaluate the effect of bee age and the method of inoculation at 7 days post-infection. The level of infection was analyzed individually by qPCR by measuring the relative amount of the N. ceranae polar tubule protein 3 (PTP3) gene. The results show that the bee’s age and the method of infection directly influence parasite load, and thus, early disease development. Significant differences were found regarding bee age at the time of infection, whereby the youngest bees (new-born and 1 day p.e.) developed the highest parasite load, with this load decreasing dramatically in bees infected at 2 days p.e. before increasing again in bees infected at 3–4 days p.e. The parasite load in bees infected when older than 4 days p.e. diminished as they aged. When the age cohort data was pooled and grouped according to the method of infection, a significantly higher mean concentration and lower variation in N. ceranae infection was evident in Group A, indicating greater variation in experimental infection when spores were administered collectively to bees through their food. In summary, these data indicate that both biological and experimental factors should be taken into consideration when comparing data published in the literature.
Keywords: Apis mellifera, Nosema ceranae, host-parasite interactions, age of infection, epidemiology, method of infection, parasite load
1. Introduction
Beekeeping is a primary sector practice principally aimed at producing nutritive and healthy products for human consumption [1,2], yet it also contributes significantly to the preservation of global biodiversity through the pollinating service that bees provide [2,3]. One of the major threats to A. mellifera honey bees are transmissible diseases [4,5]. In this respect, the microsporidian parasite Nosema ceranae is a prevalent global parasite of bees [6,7,8,9,10,11] and it is associated with colony weakness and loss, especially in the temperate Mediterranean areas [6,12,13].
This microsporidia is a unicellular obligate parasite that infects the bee’s digestive tract [14]. Infection occurs after ingestion of mature spores in food or water, mainly transmitted by trophallaxis [15] or via the fecal-oral route (fecal waste cleaning) in infected nests [16,17]. Once infection occurs, the parasite proliferates inside the ventricular cells, causing morphological and physiological changes [17,18,19,20], as well as epithelial degeneration [18,19,20]. By exploiting the host cell’s machinery to obtain resources, N. ceranae infection affects vital functions and modifies the host’s immune response, ultimately causing cell lysis [21] and digestive disorders, provoking changes in vital functions and modifying the host’s immune response, and significantly compromising the survival of the infected bee [22,23,24,25,26,27,28,29]. This pathogen also affects aspects of honey bee neurobiology, such as olfaction, learning and memory [30], and the orientation and homing skills of workers [31], accelerating bee aging and their related tasks [32,33,34].
Many factors may influence the pathogeny (course and development) of N. ceranae infection such as the genetic diversity of honey bees [25,35,36]; beekeeping practices [37,38,39], and climatic and environmental differences [40,41,42,43]. Other factors that could influence the development of the infection are the age of the bees, the means of transmission and the source of the spores. Moreover, for assays performed in a laboratory, the results of the assay may be affected by the specific conditions used, including the duration of the experiment, the temperature, method of infection, humidity, source and type of food, type of cages, number of bees in the cages, wax access, queen hormone, etc. [11,44]. Currently, there is very limited information about the influence of bee age on N. ceranae infection. Studies developed under field conditions have demonstrated that older worker bees are the most strongly infected [45,46,47]. By contrast, younger queens were found to be more susceptible than older ones after inoculation with N. ceranae spores in the laboratory [48]. However, there is little data regarding the susceptibility of worker bees to infection by this microsporidium. Likewise, it remains unclear how bee age influences the pathophysiology of infection [49], as the age and caste affect the replicative activity of intestinal stem cells in A. mellifera [50].
Consequently, we set out to study if the age of worker bees at the moment of exposure to N. ceranae spores has any influence on the development of the infection. Similarly, as most studies in apiculture science and insect pathology are performed in laboratory cages under controlled conditions [51] in order to overcome the difficulties in controlling certain variables in open field studies [5,11,34,51,52,53], we evaluated how the method of bee inoculation with N. ceranae spores influences the success of infection.
2. Materials and Methods
2.1. Source of Bees and Rearing Conditions
The experiments presented here were carried out in August–September 2017. To minimize any potential colony-level effects on the results [51], frames of capped brood were obtained from 5 healthy, Nosema-free (confirmed by PCR; [54,55]) colonies of Apis mellifera iberiensis located in an experimental apiary 20 km from the Centro de Investigación Apícola y Agroambiental (CIAPA) in Marchamalo (Castilla-La Mancha, Marchamalo, Spain). The frames were kept in an incubator at 34 °C (±1 °C) to provide a supply of newly emerged Nosema-free honey bees over 15 days. All new-born worker bees were removed daily from the brood combs, they were carefully confined to steel mesh cages [56] in groups of 20 and they were kept in a different incubator (33 °C ± 1 °C) until infection. The age of the bees (days post-emergence: p.e.) was recorded and all the bees in a cage were of the same age (age cohort). The bees were fed ad libitum with a freshly prepared sucrose solution (50% w/w in dH2O) supplemented with 2% Promotor L® (Calier Lab., Les Franqueses del Vallès, Spain), a commercial mixture of amino acids and vitamins. Honey and pollen were not used to feed the bees to avoid any possible contamination [57] with infective Nosema spp. spores and to avoid problems of standardization. Dead bees were removed daily from the cages and in this way, cages with bee cohorts from 0 to 15 days p.e. were available for Nosema-spore inoculation at almost all times.
2.2. N. ceranae Spores
Fresh spores were isolated two days before their use from around 200 heavily infected A. m. iberiensis bees collected from three naturally infected colonies at CIAPA (see the detailed procedures in Higes et al. [58]). Briefly, groups of 30–40 bees were macerated in dH2O (PCR quality) for 120 s at low speed (Stomacher 80-Microbiomaster®) in a filter bag (Seward, AK, USA, BA6040). The macerate was centrifuged for 6 min at 800 g and the pellet was purified by gradient separation [59] on 95% isotonic Percoll® (in dH2O) at a ratio of 1:9 (spores-pellet:Percoll), centrifuging at 11,000 g for 40 min. Subsequently, the clean spores were washed three times and recovered by centrifugation at 800 g for 6 min, the supernatant was decanted between washes and the sediment was resuspended uniformly in 1 mL sterile ddH2O. The species of Nosema that the spores corresponded to was confirmed by PCR, as described previously [54,55], and the mean N. ceranae spore concentration was obtained by counting the purified spores four times under a light microscope using a Neubauer® haemocytometer as described in [60]. The final spore concentration was established as 57,000 spores µL−1 and the stock spore solution was then immediately divided into aliquots (50 µL, spore inoculum), and the vials were stored at room temperature in darkness until they were used two days later.
2.3. Infection Experiments: Group A and Group B
Two assays were carried out in parallel. In the first of these, the bees were infected individually (Group A) and in the second, the bees were infected collectively (Group B). Each age cohort (n = 80, 20 bees per cage) from days 0–15 p.e. was assigned to 4 cages and they were distributed in a way that 3 cages were used for the individual infections (Group A) with the fourth cage designated for the collective infection (Group B). In addition, cages with bees of different ages were established (n = 300 bees) for use as a control groups; Group CA and Group CB for Group A and Group B, respectively. These control groups were used to ensure the absence of any initial infection.
Both assays were designed so that infections were only performed on two different days. This strategy allowed the bees of different ages and of the two infection methods to be inoculated on the same day, such that no bias was introduced by the aliquot or through the spore conservation. The bees in the control group were the last ones to be manipulated after inoculating the study groups at both infection times.
All infections took place after bees were starved for 2 h and anesthetized by a 90 sec exposure to CO2 for easy manipulation. Regardless of the method of infection, all the bees were inoculated with 114,000 N. ceranae spores to promote rapid multiplication such that it could be detected in the breeding conditions employed [25,33,40,47,48,52,61,62,63,64].
2.3.1. Group A: Individual Infection of Bees at Different Ages
Bees from the Group A (3 cages of 20 bees from each age cohort) were held individually by the wings and when they started to wake up, they were administered a 2 µL drop of the spore solution (57,000 spores µL−1) through a micropipette [65]. The spore solution was vortexed after every third bee to ensure the suspension remained uniform.
In this assay, bees aged 0 to 15 days p.e. were inoculated (except for those on day 3 that were not available). A control group of bees was included at 0, 1, 4, 5, 8, 11, 13 and 14 days p.e. (1 cage of 20 bees per age), and they were individually fed 2 µL of spore-free water. Bees that did not consume the entire water droplet were discarded and a cardboard barrier was used to physically separate the cages of the different cohorts, avoiding contact between the bees of different ages in the same incubator (Memmert® Mod. IPP500). Infected and uninfected groups of bees were kept in separate incubators under the same conditions (darkness, 33 ± 1 °C and 80% relative humidity). The age cohorts and number of bees used in the study are shown in Table 1.
Table 1.
Total number of bees in each cohort and infection type.
| Experimental Design | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age Cohort | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| Group A | 90 | 60 | 60 | - | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | - |
| Group CA | 20 | 20 | - | - | 20 | 20 | - | - | 20 | - | - | 20 | - | 20 | 20 | - | - |
| Group B | - | 20 | 20 | 20 | - | - | 20 | 20 | 20 | 20 | 20 | 20 | - | 20 | 20 | 20 | 20 |
| Group CB | - | 20 | - | - | - | - | - | 20 | 20 | - | 20 | 20 | - | - | 20 | - | - |
Group A, individually infected bees (n = 20 bees × 3 cages), Group B, collectively infected bees (n = 20 bees × 1 cage) and uninfected control Groups: CA (individually fed with spore-free water) and CB (collectively fed with the plain sucrose solution). Hyphen (-) indicates bees not available. The bees that died were removed from the study.
2.3.2. Group B: Collective Infection of Groups of Bees at Different Ages
In parallel to the aforementioned assay, a cage of each age group of bees (from 0 to 15 days p.e.) was used to inoculate bees collectively (Group B). To this end, a syrup solution was prepared containing purified spores (using the same final inoculum as above) at an equivalent dose of 5700 µL−1 spores in 400 µL of fresh syrup per 20 bee cage, assuming that each bee consumes 20 µL of food per day and that all bees consume the same amount. On the day of inoculation (spore administration), the feeders of all the Group B cages were replaced for 24 h with feeders containing the sucrose solution with the spores. Complete consumption of the food administered was verified after 24 h, such that the minimum age of the bees at the time of infection was considered as 1 day p.e. and thus, a cohort of 0 days was not available for the assay of Group B (Table 1). The food was then replaced with spore-free syrup (sucrose solution), which was administered ad libitum thereafter. In addition, control bees of 1, 7, 8, 10, 11 and 14 days p.e. that were not inoculated with spores (1 cage of 20 bees of each age received 400 µL of fresh syrup without spores) were also included to determine the absence of initial infection. Note that in these studies, no bees aged 4, 5 and 12 days p.e. were available. The cages with infected bees were kept in an incubator distinct to that used for Group A and again, physically separated to avoid cross-infection. The cages with uninfected bees were kept in a different incubator next to the cages with uninfected bees of Group A.
2.4. Molecular Detection of N. ceranae Infection (DNA Extraction and qPCR Analysis)
On day 7 post inoculation (p.i.), the number of surviving bees was recorded and they were frozen at −20 °C. When available, 10 bees were taken from each Group A (30 bees per cohort) and Group B (10 bees per cohort) cage, and each bee’s abdomen was carefully separated from the thorax and was placed individually into one well of a 96-well plate. Each of these wells contained four 2 mm glass beads (Sigma®) and 180 µL of sterile H2OmiliQ® to homogenize the tissue by stirring at 30 Hz for 6 min (TyssueLyser II, Qiagen®). DNA was extracted from the resulting macerate and 50 μL was transferred on ice to a new multi-well plate (Eppendorf®, Hamburg, Germany) containing 50 μL of a Tris-HCl lysis solution (10 mM Tris-HCl [pH 8.0], 1 mM EDTA) and 15 μL of Proteinase K (Qiagen®, No. 1019499). The plates were incubated for 20 min at 95 °C in a Mastercycler ep Gradient S (Eppendorf®), with a well left with no sample between the bees from different cohorts and used as a DNA-extraction control (with H2OmiliQ® and all the reagents: EC). Nosema ceranae was assessed by quantitative real time-PCR (qPCR) to detect DNA for the polar tubule protein 3 (PTP3) gene [55] in a Roche LightCycler® 480 thermocycler. The qPCR was performed in 384 plates and in a final reaction volume of 10 μL, using the LightCycler® 480 Probes Master Mix (Roche) according to the manufacturer’s instructions: primers at 500 nM and universal probes at 10 nM (UPL, Roche Molecular Systems, Basel, Switzerland). Each amplification cycle was analyzed with the LightCycler® 480 software v1.5.1 (Roche Diagnostics GmbH, Basel, Switzerland) and the crossing point (Cp) was recorded in all samples. Two replicates for each sample were included in the same kinetic qPCR run (intra-assay variation). The Cp value was calculated for each qPCR reaction from the standard curve using the second maximum derivative method.
The parasitic load was quantified in all samples relative to the specific synthetic oligonucleotides (gBlocks®, IDT DNA Technologies, Coralville, Iowa, USA) for the N. ceranae PTP3 gene fragment (the sequence of the PTP3 gBlock® Gene Fragment is shown in Table 2), as designed using the gBlock® Gene Fragment ordering tool. The non-variable areas of the gene were selected based on the sequence data available in GenBank and our in-house database [66]. The standard curve for quantification was prepared following the manufacturer’s protocol at an initial concentration of 10 ng/μL (in TE) and using serial dilutions up to 1 × 10−14 ng/µL.
Table 2.
PTP3 gBlock® Gene Fragment.
| Method | Organism | PTP3 gBlock® Gene Fragment | Source |
|---|---|---|---|
| qPCR | N. ceranae | 5′_TGAAGCTAAAAAAGAAGAACAACTTGACCAAATAGCTAAAAAGAATGCAGAGACAGAGAAACAACACAGAGAGGTACTTCTCAAAGAACATCAAGATGCTGATGTTATGGCTACAGAAGAAAGACTTGCTAAAAATAATAGAgccaggaaGATTAGTGAGGCAGGAATTAAAGCAGCGCAATCTGTATTGAAAACTGGAGGAACAATAGAAGAAGCAAGAGCAGCTAAGGCGGCAGCTGAAAAAGCTATATTGCAAGAAATTGAGAGTAGAGAAGCGCAAA_3′ | gi|557790804|gb|KC520145.1| |
A 281 nucleotide fragment of the N. ceranae genome including the NC-PTP3 primer sequences (underlined in bold), and the sequence of the UPL#72 probe in bold and lowercase.
To detect possible contamination in each qPCR reaction, the ECs and non-template controls (NTC) were analyzed in parallel in the same reaction plate. In addition, a positive control with DNA from N. ceranae extracted from naturally infected bees was included in each reaction to detect possible amplification failures.
2.5. Statistical Analysis: N. ceranae Infection According to Bee Age and the Mode of Infection
The average infection of N. ceranae was calculated in all the available bee cohorts and for each study group (Table 1) based on the average concentration value of the N. ceranae-PTP3 gene at 7 days p.i. For each age cohort tested, 30 bee abdomens from Group A and 10 bee abdomens from Group B were analyzed individually.
To evaluate the possible effect of age and the method of infection on the extent of infection for each group of bees tested, generalized linear models (GLMs) were used. These GLMs allow variables to be modeled that do not meet the requirements for standard linear models (i.e., normality and homoscedasticity: Levene’s test), thus permitting the modeling of variables that follow distributions other than a normal distribution. In this study, the distribution that best fit the response variable-average DNA concentrations of N. ceranae was considered to be the gamma distribution (GLMz log link function).
To determine whether the “cage” (random factor) significantly affected the extent of infection within each age cohort (fixed factor) in Group A (3 cages per age cohort) a mixed GLM (GLMMz) was used. The infection levels were then compared in three different ways: (a) to evaluate the effect of the age cohort, the method of infection and their interaction in the study (Group A and Group B) by GLMz; (b) between different age cohorts within the same assay (Group A or Group B) using a Kruskal-Wallis and post-hoc Mann-Whitney-U test; and (c) between both assays (Group A vs. Group B) also using a Mann-Whitney-U test to compare the pooled data (n = 423 and n = 108 for Group A and B, respectively). p values <0.05 were considered significant and all statistical analyses were carried out using the IBM SPSS Statistics V24 software by the Statistics Unit of the Scientific Computing Area at the SGAI-CSIC (Madrid, Spain).
3. Results
All the bees used in the study were negative for Nosema spp. infection at birth, as were all the bees in the control groups (Group CA: n = 46; Group CB: n = 17) throughout. Likewise, no Nosema spp. were detected in the controls at the DNA extraction or PCR steps (EC and NTC), indicating that there was no cross-contamination during the molecular analysis.
The sensitivity of the qPCR was 1 × 10−11 ng/µL of N. ceranae-PTP3 DNA and the number of bees analyzed individually from each infection group (A and B) and from each age group is shown in Table 3.
Table 3.
Number of bees and the percentage of bees infected by N. ceranae at 7 days post-inoculation (p.i.).
| Infected Bees (7 Days p.i.) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age Cohort | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
| Group A | n | 30 | 30 | 24 | - | 30 | 30 | 30 | 30 | 21 | 30 | 30 | 30 | 30 | 30 | 30 | 19 * | - |
| qPCR+ | 30 | 29 | 24 | - | 30 | 30 | 30 | 30 | 21 | 30 | 30 | 30 | 30 | 30 | 30 | 19 | - | |
| % | 100 | 97 | 100 | - | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | - | |
| Group B | n | - | 10 | 10 | 10 | - | - | 10 | 10 | 10 | 10 | 10 | 10 | - | 10 | 10 | 10 | 10 |
| qPCR+ | - | 10 | 9 | 10 | - | - | 10 | 10 | 9 | 10 | 9 | 8 | - | 5 | 8 | 1 | 10 | |
| % | - | 100 | 90 | 100 | - | - | 100 | 100 | 90 | 100 | 90 | 80 | - | 50 | 80 | 10 | 100 | |
Group A, bees infected individually with N. ceranae spores; (*) n < 30 bees due to the high accumulated mortality of the bees at 7 days p.i. Group B, bees infected collectively with the same spores. All control bees analyzed (Group CA and Group CB) were negative for N. ceranae infection and they are not shown in the table. n: number of samples analyzed. qPCR +: indicates number of positive samples.
Inoculation was more successful in the bees infected individually (n = 424; 99.76%) than when they were infected collectively (n = 130; 90%). In fact, virtually every bee exposed individually to the spores was infected (Group A), irrespective of their age, whereas there was considerably more variation in the rates of infection found in Group B, with the lowest levels of infection detected in bees exposed to the spores at 13 days p.e. and especially at 15 days p.e., when only one bee was infected 7 days p.i. (Table 3). By contrast, all bees were infected when they were inoculated at 16 days p.e.
3.1. Level of Infection in Bees of Different Ages (Group A)
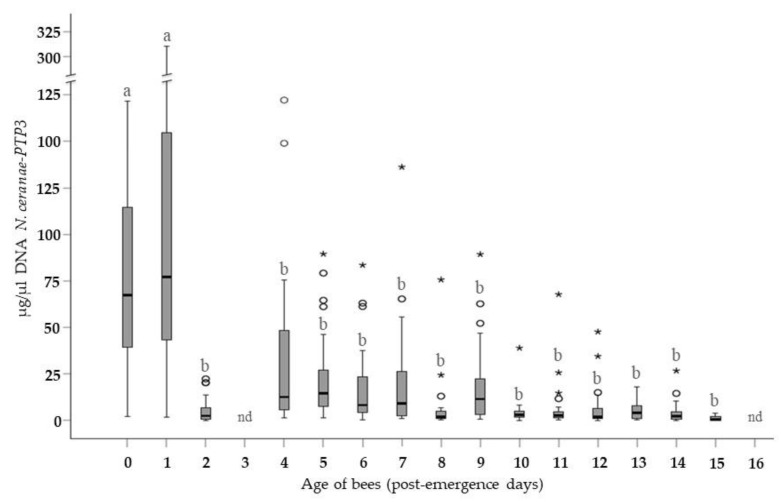
The average level of infection of Group A bees was analyzed, and as can be seen (Figure 1), the age of the bees at the time of exposure to the spores significantly influenced the degree of infection evident at 7 days p.i. (GLMz, Wald Chi-Square test; p < 0.001). The largest amount of N. ceranae-PTP3 DNA amplified was obtained from bees infected at ages 0–24 h p.e. Infection was a significantly milder in bees exposed to the spores at 48 h p.e. (Mann-Whitney test, p ≤ 0.05), leading to an approximately 20-fold reduction in the parasite load than in the younger bees. The level of infection again increased in bees exposed at 4 days p.e., although this increase was not associated with a significant rise in the parasitic load relative to the bees exposed to the spores on day 2 p.e. However, the levels were lower at all the remaining ages. The parasitic load remained low in the cohorts exposed between 4 and 9 days p.e., with the lowest levels detected in infected bees exposed to the spores at more advanced ages, from 10 to 15 days p.e. Thus, there was an indirect trend towards lower levels of infection as the age of the bees increased, which was up to an order of magnitude lower than that of the immediately younger bees. The average concentration of the pathogen (µg/µL) was statistically different between the bees at 0 and 1 days p.e. when compared to the rest of the cohort.
Figure 1.
Average concentration of the N. ceranae-PTP3 DNA (µg/µL) in Group A bees 7 days p.i. The letters (a, b) indicate statistical significance p < 0.05 (Mann-Whitney-U test). *: extreme cases (any value > Q1 − 1.5 * IQR or > Q3 + 1.5 * IQR; Q: quartile; IQR: interquartile range). o: very unlikely cases (any value > Q1 – 3 * IQR or > Q3 + 3 * IQR; Q: quartile; IQR: interquartile range). Extreme cases and very unlikely cases were calculated separately within each age. nd: no data.
The mean standard deviation observed in the cohorts studied was relatively high due to the large variation in the degree of parasitization between each individual bee within the same age group. The effect of the “cage” on the response was apparently not significant (GLMMz, Wald Chi-Squared test: p = 0.582) and thus, the average DNA concentration of N. ceranae was not modified when the 30 values registered from bees of the same age were grouped.
3.2. Level of Infection According to Age and Method of Infection (Group A vs. Group B)
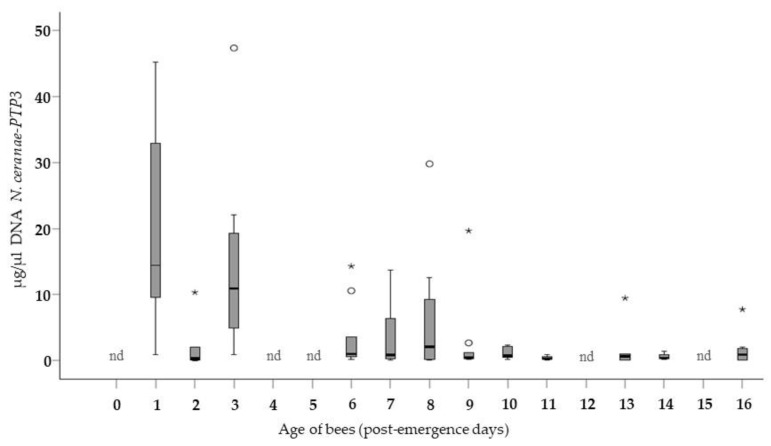
When the average concentration of the N. ceranae-PTP3 gene DNA was assessed in all the collectively inoculated cohorts (Figure 2), the data confirmed the trend observed in the bees infected individually (Figure 1). Thus, the evolution of the infection in the Group B bees was very similar to that in Group A, and the interaction effect (age * method) was not significant (GLMz, Wald Chi-Squared; p = 0.75). Accordingly, when the youngest bees were infected (1 day p.e.) they had more N. ceranae DNA than those bees that were exposed to the spores later in life, at 2 days p.e. and older. Bees exposed to the parasite at 2 days p.e. developed microsporidium infection on average ≈8 times less than that of the bees exposed to the spores on day 1 p.e. The average parasitization of the bees infected at 3 days p.e. was greater than in those infected on the previous day, yet not higher than that registered in bees exposed to the parasite on day 1 p.e. Moreover, this level was maintained in the infected cohorts until 10 days p.e. The lowest average levels of infection was detected in the more mature bees, those exposed to the parasite from 11 to 16 days p.e. In bees of these ages, the values were an order of magnitude lower than in the immediately younger bees, confirming that less N. ceranae DNA was detected as the age at infection increased. In this group of collectively infected bees, the distribution of the average concentration was not statistically different among the bees inoculated at the different ages.
Figure 2.
Average concentration of the N. ceranae-PTP3 DNA (µg/µL) 7 days after the collective infection of the bees in Group B: *: extreme cases (any value > Q1 − 1.5 * IQR or > Q3 + 1.5 * IQR; Q: quartile; IQR: interquartile range). o: very unlikely cases (any value > Q1 − 3 * IQR or > Q3 + 3 * IQR; Q: quartile; IQR: interquartile range). Extreme cases and very unlikely cases were calculated separately within each age. nd: no data. As indicated, bees were exposed collectively to the N. ceranae spores on day 0 p.e. and analyzed 24 h later (after consuming the contaminated food).
The levels of infection in Group A (Figure 1) were generally higher than those in Group B (Figure 2) when bees exposed to the spores at the same age were compared. When the values were grouped according to the method of infection (Group A vs. Group B), a significantly higher average concentration of microsporidia (Mann-Whitney-U Test: p = 0.004) was observed in the individually infected bees relative to those infected collectively. The largest amount of N. ceranae DNA in the entire study (7 days p.i.) was observed in the bees infected individually at the age of 1 day p.e.
4. Discussion
The objective of this study was to assess how the age at the time of inoculation, and the method used for this influences the parasite infection that develops. The N. ceranae parasitic load was quantified without analyzing its effect on the viability of the bees. Accordingly, it was evident that both these factors, the age of the bee and the method of inoculation, affect the development of N. ceranae infection.
In this study, a dose of spores was administered that was expected to ensure 100% infection of the bees [25,44,61,64,67,68] using spores from different naturally infected colonies to favor the natural heterogeneity of the regional population of N. ceranae [24,69,70]. The study design involved assessing infection at 7 days p.i. to prevent the proliferation of spores to equal levels between all groups over longer periods [71], and to assess the infection at a specific time point before many of the bees had died due to the infection [18,72]. Thus, we avoided the prolonged confinement that increases both the levels of Nosema spp. infection [73] and bee mortality [18,72].
The results obtained here demonstrate that the success of infection under controlled conditions and/or the ability of N. ceranae to multiply depend significantly on the age of the bees at the time of infection, as well as on the method of spore administration. The youngest adult bees were especially susceptible to infection (0–1 day p.e.) and the parasitic load at 7 days p.i. was significantly higher following exposure to spores at that age. A lower parasitic N. ceranae load was detected in bees infected at 2 days p.e., a trend that was observed in both study groups (individually and collectively infected). The differences in the level of infection of the bees of different ages cannot be attributed to the origin of the spores or bees, since all were fed with the same N. ceranae spore mixture and all came from the same breeding frames.
In agreement with these results, Chaimanee et al. [48] showed that queen bees infected collectively 1 day p.e. were more susceptible to infection by N. ceranae and they developed significantly higher levels of infection than queen bees infected at 6 and 12 days p.e. Conversely, Huang et al. [25] reported that newly emerged bees were the least susceptible to N. ceranae whereas bees infected at 5 days p.e. were more susceptible to these parasites, and older honey bees infected by N. ceranae developed more intense infections (spore number at 12 days p.i.) and survived better than younger bees [74].
It is known that bees undergo particularly important natural modifications in the composition and succession of their intestinal microbiota over the initial days of their adult development. Indeed, new-born bees begin to acquire their intestinal microbiota within a matter of hours and they are fully colonized by the sixth day p.e. [75,76,77,78]. The bacterial community that colonizes the bee gut is resilient to changes in the nutritional, hive, and social environment [77]. However, it has been suggested that active microbiota differ in species richness and total abundance across the ontogenetic stage of honey bee and hive location [78], as has also been proposed for many other animal species. Consequently, the success of N. ceranae infection may be related to the microbiota becoming established in the intestine of the bees and its competition with the parasite for the host’s resources, as well as to the physiological events inherent to bee development [49,50]. These modifications could affect the biological cycle of microsporidia [79] and perhaps the intensity of infection [80]. In fact, the promotion of diseases in honey bees, and therefore the health of the colony, may be associated with changes in the composition and abundance of the intestinal microbiota of individual bees [79,81,82,83,84]. This phenomenon might explain why the greatest differences in infection were seen here among the youngest cohorts of 0 and 1 days p.e. However, the relative abundance of some members of the microbiota is associated with the environment in which they develop [85], and the succession of communities is strongly associated with the breed and age of the bees [86]. In our study, the bees were kept in an incubator and not in the colony, which most likely influences the establishment of normal microbiota. Moreover, the strict dependence on host energy for the development of microsporidia [87,88] and the greater availability of essential amino acids in young bees [89] or even the absence of a well-established peritrophic membrane could also favor the multiplication of the parasite in younger bees [87,88,90].
Other elements that are likely to influence the pathogenic events mediated by N. ceranae infection include the host’s immune response, which varies with age through a phenomenon generally referred to as immunosenescence (reviewed in [90]). This senescence often weakens the immune response, either cellular (capsulation, nodulation or melanization) or humoral (antimicrobial peptide synthesis and oxidative response), frequently enhancing the intensity of infection in older insects [91]. Indeed, older honey bees develop a more intense N. ceranae infection than younger bees, producing less prophenoloxidase, which is also negatively correlated with the intensity of parasite infection [74]. However, it is not yet clear what effect the innate humoral response of honey bees has on the extent of N. ceranae infection, particularly how the microsporidia are affected by antimicrobial peptides and chemical signaling cascades. This may be because the parasite passes directly through the intestinal lumen to the midgut epithelial layer where the infection develops, and the effects of immune peptides and chemical cascades are less likely to reach the parasite at this location. In fact, one host cell mechanism used to combat intracellular parasites is the apoptosis of infected cells, which is dampened following infection by N. ceranae and other microsporidia [92,93,94].
It is commonly accepted that the older forager bees in hives have the highest frequency and most intense infection [45,46,95,96], which is thought to provoke an acceleration of foraging behavior in bees infected by N. ceranae [32,33,34,58,97]. However, given the greater susceptibility of younger worker bees evident in this study, it is possible that various strategies may be employed in the super-organism to reduce contamination by Nosema spp. in the nucleus of the hive where they develop. Social insects are known to develop social immunity, which is expressed through a variety of sanitary behaviors and the use of antimicrobials to reduce the risk of infection and the pathogen load of exposed individuals [98]. Some interactions among colony members also contribute to limiting pathogen spread at the colony-level and to decrease the infection risk of valuable individuals, providing “organizational immunity” [98]. In fact, bee colonies are highly compartmentalized structures, and the connectivity and spatial overlap is considerable among same-age worker bees, yet weak among different-age workers [99]. In the case of N. ceranae, infection increased the frequency of antennal contact [100], probably because infected bees solicit more food as they are more responsive to sucrose and less inclined to share this food with other bees, a response that might influence the transmission of infection [101]. On the other hand, the spatial distribution of various age classes established in the bee colony reflects a degree of immunity in young individuals [102]. Foragers (the most intensely infected population in a colony) are at the periphery of the social network, while the colony core is formed by young bees and the queen [99]. As such, the latter are protected from potentially harmful external agents [98], such as N. ceranae spores. As they age, worker bees shift from tasks in the interior to peripheral tasks, like food processing and nest maintenance, and at the end of their lives they perform outside-nest tasks [98]. However, N. ceranae infection produces behavioral and physiological changes in bees [100,103], inducing infected bees to become early foragers and thereby delaying the onset of foraging in their healthy nest-mates [103]. This has been proposed to as defense mechanism in infected colonies, decreasing the spatial overlap of healthy bees with infected workers and delaying the drain on the nursing force induced by Nosema infection [98]. This whole battery of strategies has been proposed to prevent the infection of young queen bees [48], young worker bees [104] and immature bees [68,105], which have a lower risk of developing the disease even when the prevalence of infection in adult bees is relatively high [55].
In addition to the biological factors associated with the different life stages of the bees, N. ceranae infection itself can affect different aspects of the physiology (metabolism and immune response), morphology and behavior of honey bees [21,34,92,105,106,107], thereby influencing the viability and intensity of the infection. Some evidence suggests that parasite manipulation of intestinal homeostasis and the inhibition of intestinal epithelial renewal [92,108] are correlated with an increased susceptibility of bees to N. ceranae infection due to their diminished capacity to repair the intestinal damage [108]. Ventricular epithelial cells in young bees 1–2 days p.e. are still undergoing morphogenetic development [109], which may be associated with the ability to accumulate infected apoptotic cells in the midgut and rectum when they are experimentally infected [94].
Although the collective assay (Group B) was carried out on just one cage while three cages were used for the individual assay (Group A), a similar trend was observed in both assays. The prevalence of infection and the average levels of infection were significantly higher in individually infected bees than in those infected collectively, which may explain some of the conflicting results found in the literature. In this sense, important differences in food intake have been described between bees caged together within the first 48 h [110]. These observations suggest bees are exposed to distinct numbers of N. ceranae spores during collective infection. Indeed, in some studies only 23.3% [111] or 78% [32] of caged bees become infected 12–15 days after collective administration of spores in food.
Trophallaxis favors the transmission of spores among bees and it has been shown that while only three infected bees were found in five samples analyzed in the inoculated cages at 5 days p.i., all bees sampled at 10 and 15 days p.i. were infected [112]. However, infected bees may be less willing to share food with their cage mates [101] or conversely, their trophallactic behavior may be enhanced [32]. In addition, the number of infected bees in a cage and their parasite load are factors that influence the exchange of food between infected and uninfected bees [113].
The levels of N. ceranae infection in both groups (Group A and Group B) showed similar trends, with significantly higher values in bees exposed to spores in the first 48 h p.e. and lower values as the bees aged. Therefore, considering all the variables that could modulate the frequency and intensity of infection, and the exchange of contaminated food between caged bees, we consider that the age of the bee at the time of infection influences the degree of infection and the development of nosemosis, as suggested for N. apis infection [104]. In addition, the number of uninfected bees following collective exposure to the spores was higher than that of individual infections, particularly when old bees (11–15 days p.e.) were infected. These phenomena could affect the interpretation of the results of experimental studies, and thus, both these variables must be considered in laboratory studies. Likewise, in addition to cage experiments, more field tests should be carried out as differences have been found in the morphology, physiology, behavior and gene expression of honey bees (workers and queens) reared in the laboratory when compared to those naturally infected by microsporidia [81,114,115,116,117].
5. Conclusions
The bees infected in the first 48 h p.e. develop significantly high levels of N. ceranae infection and as their age increases, there is a trend towards milder infection independent of the method used to administer the spores. Hence, younger bees appear to be more susceptible to infection and/or the microsporidia is more capable of multiplying better in these bees. Moreover, our results demonstrate that the effect of age on N. ceranae infection is similar in bees exposed individual or collectively. Collective inoculation was less successful and the bees, when infected, had lower parasitic charge. Thus, the results presented here demonstrate that bee age and the method of inoculation ultimately determine the extent of infection, which is important to take into account when comparing experimental studies carried out in the laboratory. Future research aimed at elucidating the physiological differences between bees of different ages might help explain their different susceptibility to N. ceranae infection, potential paving the way to design strategies to combat this pathogen.
Acknowledgments
The authors wish to thank V. Albendea, T. Corrales, M. Gajero, C. Uceta, J. Almagro and J. García of the Honey Bee Pathology Laboratory for their technical support.
Author Contributions
Conceptualization and Methodology: R.M.-H. and A.U.-M.; Formal Analysis and Investigation: R.M.-H.; M.H. and A.U.-M.; Statistical Analysis: L.B.; A.U.-M. and M.H.; Data collection and Curation: A.U.-M.; Preparation of the First Draft: A.U.-M. and R.M.-H.; Manuscript Review & Editing: A.U.-M.; R.M.-H.; M.H. and A.M.; Visualization, Supervision, Project Administration: R.M.-H.; M.H.; A.U.-M. and A.M.; Funding Acquisition: R.M.-H.
Funding
This research was funded by The National Institute for Agricultural and Food Research and Technology (INIA) and FEDER funds (RTA2015-00013-C03-01 and RTA2012-00076-C02-01).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Alvarez-Suarez J.M. In: Bee Products-Chemical and Biological Properties. Facultad de Ciencias de la Salud, Universidad de Las Americas, editor. Springer International Publishing AG; Quito, Ecuador: 2017. [Google Scholar]
- 2.European Commission . EU Pollinators Initiative: Roadmap. European Commission; Brussels, Belgium: 2017. pp. 1–3. [Google Scholar]
- 3.IPBES . In: Summary for Policymakers of the Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on Pollinators, Pollination and Food Production. Potts S.G., Imperatriz-Fonseca V.L., Ngo H.T., Biesmeijer J.C., Breeze T.D., Dicks L.V., Garibaldi L.A., Hill R., Settele J., Vanbergen A.J., et al., editors. Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Bonn, Germany: 2016. 36p. [Google Scholar]
- 4.Grozinger C.M., Flenniken M.L. Bee Viruses: Ecology, Pathogenicity, and Impacts. Annu. Rev. Entomol. 2019;64:205–226. doi: 10.1146/annurev-ento-011118-111942. [DOI] [PubMed] [Google Scholar]
- 5.Goblirsch M. Nosema ceranae disease of the honey bee (Apis mellifera) Apidologie. 2018;49:131–150. doi: 10.1007/s13592-017-0535-1. [DOI] [Google Scholar]
- 6.Klee J., Besana A.M., Genersch E., Gisder S., Nanetti A., Tam D.Q., Chinh T.X., Puerta F., Ruz J.M., Kryger P., et al. Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. J. Invertebr. Pathol. 2007;96:1–10. doi: 10.1016/j.jip.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y., Evans J.D., Smith I.B., Pettis J.S. Nosema ceranae is a long-present and wide-spread microsporidian infection of the European honey bee (Apis mellifera) in the United States. J. Invertebr. Pathol. 2008;97:186–188. doi: 10.1016/j.jip.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Tokarev Y.S., Zinatullina Z.Y., Ignatieva A.N., Zhigileva O.N., Malysh J.M., Sokolova Y.Y. Detection of two Microsporidia pathogens of the European honey bee Apis mellifera (Insecta: Apidae) in Western Siberia. Acta Parasitol. 2018;63:728–732. doi: 10.1515/ap-2018-0086. [DOI] [PubMed] [Google Scholar]
- 9.Ostroverkhova N.V., Kucher A.N., Golubeva E.P., Rosseykina S.A., Konusova O.L. Study Of Nosema spp. In The Tomsk Region, Siberia: Co-Infection Is Widespread In Honeybee Colonies. Far East. Entomol. 2019;378:12–22. doi: 10.25221/fee.378.3. [DOI] [Google Scholar]
- 10.Gisder S., Schüler V., Horchler L.L., Groth D., Genersch E. Long-Term Temporal Trends of Nosema spp. Infection Prevalence in Northeast Germany: Continuous Spread of Nosema ceranae, an Emerging Pathogen of Honey Bees (Apis mellifera), but No General Replacement of Nosema apis. Front. Cell. Infect. Microbiol. 2017;7:1–14. doi: 10.3389/fcimb.2017.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martín-Hernández R., Bartolomé C., Chejanovsky N., Le Conte Y., Dalmon A., Dussaubat C., García-Palencia P., Meana A., Pinto M.A., Soroker V., et al. Nosema ceranae in Apis mellifera: A 12 years post-detection perspective. Environ. Microbiol. 2018;20:1302–1329. doi: 10.1111/1462-2920.14103. [DOI] [PubMed] [Google Scholar]
- 12.Botías C., Martín-Hernández R., Garrido-Bailón E., González-Porto A., Martínez-Salvador A., De La Rúa P., Meana A., Higes M. The growing prevalence of Nosema ceranae in honey bees in Spain, an emerging problem for the last decade. Res. Vet. Sci. 2012;93:150–155. doi: 10.1016/j.rvsc.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Cepero A., Martín-Hernández R., Bartolomé C., Gómez-Moracho T., Barrios L., Bernal J., Teresa Martín M., Meana A., Higes M. Passive laboratory surveillance in Spain: Pathogens as risk factors for honey bee colony collapse. J. Apic. Res. 2016;54:525–531. doi: 10.1080/00218839.2016.1162978. [DOI] [Google Scholar]
- 14.Huang W.F., Solter L.F. Comparative development and tissue tropism of Nosema apis and Nosema ceranae. J. Invertebr. Pathol. 2013;113:35–41. doi: 10.1016/j.jip.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Smith M.L. The honey bee parasite Nosema ceranae: Transmissible via food exchange? PLoS ONE. 2012;7:e43319. doi: 10.1371/journal.pone.0043319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webster T. Nosema apis spore transmission among honey bees. Am. Bee J. 1993;133:869–870. [Google Scholar]
- 17.Higes M., Martin-Hernandez R., Garcia-Palencia P., Martin P., Meana A.M. Horizontal transmission of Nosema ceranae (Microsporidia) from worker honeybees to queens (Apis mellifera) Environ. Microbiol. Rep. 2009;1:495–498. doi: 10.1111/j.1758-2229.2009.00052.x. [DOI] [PubMed] [Google Scholar]
- 18.Higes M., García-Palencia P., Martín-Hernández R., Meana A. Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia) J. Invertebr. Pathol. 2007;94:211–217. doi: 10.1016/j.jip.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 19.García-Palencia P., Martín-Hernández R., González-Porto A.-V., Marin P., Meana A., Higes M. Natural infection by Nosema ceranae causes similar lesions as in experimentally infected caged-worker honey bees (Apis mellifera) J. Apic. Res. 2010;49:278–283. doi: 10.3896/IBRA.1.49.3.08. [DOI] [Google Scholar]
- 20.Higes M., García-Palencia P., Urbieta A., Nanetti A., Martín-Hernández R. Nosema apis and Nosema ceranae Tissue Tropism in Worker Honey Bees (Apis mellifera) Vet. Pathol. 2019 doi: 10.1177/0300985819864302. [DOI] [PubMed] [Google Scholar]
- 21.Dussaubat C., Brunet J.L., Higes M., Colbourne J.K., Lopez J., Choi J.H., Martín-Hernández R., Botías C., Cousin M., McDonnell C., et al. Gut pathology and responses to the microsporidium Nosema ceranae in the honey bee Apis mellifera. PLoS ONE. 2012;7:e37017. doi: 10.1371/journal.pone.0037017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alaux C., Brunet J.L., Dussaubat C., Mondet F., Tchamitchan S., Cousin M., Brillard J., Baldy A., Belzunces L.P., Le Conte Y. Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera) Environ. Microbiol. 2010;12:774–782. doi: 10.1111/j.1462-2920.2009.02123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aufauvre J., Misme-Aucouturier B., Viguès B., Texier C., Delbac F., Blot N. Transcriptome Analyses of the Honeybee Response to Nosema ceranae and Insecticides. PLoS ONE. 2014;9:e91686. doi: 10.1371/journal.pone.0091686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doublet V., Natsopoulou M.E., Zschiesche L., Paxton R.J. Within-host competition among the honey bees pathogens Nosema ceranae and Deformed wing virus is asymmetric and to the disadvantage of the virus. J. Invertebr. Pathol. 2015;124:31–34. doi: 10.1016/j.jip.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Huang W.F., Solter L., Aronstein K., Huang Z. Infectivity and virulence of Nosema ceranae and Nosema apis in commercially available North American honey bees. J. Invertebr. Pathol. 2015;124:107–113. doi: 10.1016/j.jip.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz R.S., Evans J.D. Single and mixed-species trypanosome and microsporidia infections elicit distinct, ephemeral cellular and humoral immune responses in honey bees. Dev. Comp. Immunol. 2013;40:300–310. doi: 10.1016/j.dci.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Mayack C., Naug D. Parasitic infection leads to decline in hemolymph sugar levels in honeybee foragers. J. Insect Physiol. 2010;56:1572–1575. doi: 10.1016/j.jinsphys.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Williams G.R., Shutler D., Burgher-MacLellan K.L., Rogers R.E.L. Infra-population and -community dynamics of the parasites Nosema apis and Nosema ceranae, and consequences for honey bee (Apis mellifera) hosts. PLoS ONE. 2014;9:5–10. doi: 10.1371/journal.pone.0099465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vidau C., Panek J., Texier C., Biron D.G., Belzunces L.P., Le Gall M., Broussard C., Delbac F., El Alaoui H. Differential proteomic analysis of midguts from Nosema ceranae-infected honeybees reveals manipulation of key host functions. J. Invertebr. Pathol. 2014;121:89–96. doi: 10.1016/j.jip.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Gage S.L., Kramer C., Calle S., Carroll M., Heien M., DeGrandi-Hoffman G. Nosema ceranae parasitism impacts olfactory learning and memory and neurochemistry in honey bees (Apis mellifera) J. Exp. Biol. 2018;221:jeb161489. doi: 10.1242/jeb.161489. [DOI] [PubMed] [Google Scholar]
- 31.Wolf S., McMahon D.P., Lim K.S., Pull C.D., Clark S.J., Paxton R.J., Osborne J.L. So near and yet so far: Harmonic radar reveals reduced homing ability of Nosema infected honeybees. PLoS ONE. 2014;9:e103989. doi: 10.1371/journal.pone.0103989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lecocq A., Jensen A.B., Kryger P., Nieh J.C. Parasite infection accelerates age polyethism in young honey bees. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep22042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleites-Ayil F.A., Quezada-Euán J.J.G., Medina-Medina L.A. Onset of foraging and lifespan of Africanized honey bees (Apis mellifera) infected with different levels of Nosema ceranae spores in Neotropical Mexico. Apidologie. 2018;49:781–788. doi: 10.1007/s13592-018-0602-2. [DOI] [Google Scholar]
- 34.Goblirsch M., Huang Z.Y., Spivak M. Physiological and Behavioral Changes in Honey Bees (Apis mellifera) Induced by Nosema ceranae Infection. PLoS ONE. 2013;8:e58165. doi: 10.1371/journal.pone.0058165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourgeois A.L., Rinderer T.E., Sylvester H.A., Holloway B., Oldroyd B.P. Patterns of Apis mellifera infestation by Nosema ceranae support the parasite hypothesis for the evolution of extreme polyandry in eusocial insects. Apidologie. 2012;43:539–548. doi: 10.1007/s13592-012-0121-5. [DOI] [Google Scholar]
- 36.Fontbonne R., Garnery L., Vidau C., Aufauvre J., Texier C., Tchamitchian S., Alaoui H.E., Brunet J.L., Delbac F., Biron D.G. Comparative susceptibility of three Western honeybee taxa to the microsporidian parasite Nosema ceranae. Infect. Genet. Evol. 2013;17:188–194. doi: 10.1016/j.meegid.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 37.Giersch T., Berg T., Galea F., Hornitzky M. Nosema ceranae infects honey bees ( Apis mellifera ) and contaminates honey in Australia. Apidologie. 2009;40:117–123. doi: 10.1051/apido/2008065. [DOI] [Google Scholar]
- 38.van der Zee R., Pisa L., Andonov S., Brodschneider R., Charrière J.-D., Chlebo R., Coffey M.F., Crailsheim K., Dahle B., Gajda A., et al. Managed honey bee colony losses in Canada, China, Europe, Israel and Turkey, for the winters of 2008–9 and 2009–10. J. Apic. Res. 2012;51:100–114. doi: 10.3896/IBRA.1.51.1.12. [DOI] [Google Scholar]
- 39.Malone L.A., Gatehouse H.S. Effects of Nosema apis Infection on Honey Bee (Apis mellifera) Digestive Proteolytic Enzyme Activity. J. Invertebr. Pathol. 1998;71:169–174. doi: 10.1006/jipa.1997.4715. [DOI] [Google Scholar]
- 40.Martín-Hernández R., Meana A., García-Palencia P., Marín P., Botías C., Garrido-Bailón E., Barrios L., Higes M. Effect of temperature on the biotic potential of honeybee microsporidia. Appl. Environ. Microbiol. 2009;75:2554–2557. doi: 10.1128/AEM.02908-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martín-Hernández R., Meana A., Prieto L., Salvador A.M., Garrido-Bailón E., Higes M. Outcome of colonization of Apis mellifera by Nosema ceranae. Appl. Environ. Microbiol. 2007;73:6331–6338. doi: 10.1128/AEM.00270-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fenoy S., Rueda C., Higes M., Martín-Hernández R., Del Aguila C. High-level resistance of Nosema ceranae, a parasite of the honeybee, to temperature and desiccation. Appl. Environ. Microbiol. 2009;75:6886–6889. doi: 10.1128/AEM.01025-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tapaszti Z., Forgách P., Kővágó C., Békési L., Bakonyi T., Rusvai M. First detection and dominance of Nosema ceranae in Hungarian honeybee colonies. Acta Vet. Hung. 2009;57:383–388. doi: 10.1556/AVet.57.2009.3.4. [DOI] [PubMed] [Google Scholar]
- 44.Fries I., Chauzat M.-P., Chen Y.-P., Doublet V., Genersch E., Gisder S., Higes M., McMahon D.P., Martín-Hernández R., Natsopoulou M., et al. Standard methods for Nosema research. J. Apic. Res. 2013;52:1–28. doi: 10.3896/IBRA.1.52.1.14. [DOI] [Google Scholar]
- 45.Meana A., Martín-Hernández R., Higes M. The reliability of spore counts to diagnose Nosema ceranae infections in honey bees. J. Apic. Res. 2010;49:212–214. doi: 10.3896/IBRA.1.49.2.12. [DOI] [Google Scholar]
- 46.Smart M.D., Sheppard W.S. Nosema ceranae in age cohorts of the western honey bee (Apis mellifera) J. Invertebr. Pathol. 2012;109:148–151. doi: 10.1016/j.jip.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 47.Jack C.J., Lucas H.M., Webster T.C., Sagili R.R. Colony level prevalence and intensity of Nosema ceranae in honey bees (Apis mellifera L.) PLoS ONE. 2016;11:e0163522. doi: 10.1371/journal.pone.0163522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chaimanee V., Chantawannakul P., Chen Y., Evans J.D., Pettis J.S. Effects of host age on susceptibility to infection and immune gene expression in honey bee queens (Apis mellifera) inoculated with Nosema ceranae. Apidologie. 2014;45:451–463. doi: 10.1007/s13592-013-0258-x. [DOI] [Google Scholar]
- 49.VanEngelsdorp D., Traynor K.S., Andree M., Lichtenberg E.M., Chen Y., Saegerman C., Cox-Foster D.L. Colony Collapse Disorder (CCD) and bee age impact honey bee pathophysiology. PLoS ONE. 2017;12:e0179535. doi: 10.1371/journal.pone.0179535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ward K.N., Coleman J.L., Clinnin K., Fahrbach S., Rueppell O. Age, caste, and behavior determine the replicative activity of intestinal stem cells in honeybees (Apis mellifera L.) Exp. Gerontol. 2008;43:530–537. doi: 10.1016/j.exger.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 51.Williams G.R., Alaux C., Costa C., Csáki T., Doublet V., Eisenhardt D., Fries I., Kuhn R., Mcmahon D.P., Medrzycki P., et al. Standard methods for maintaining adult Apis mellifera in cages under in vitro laboratory conditions. J. Apic. Res. 2013;52:1–36. doi: 10.3896/IBRA.1.52.1.04. [DOI] [Google Scholar]
- 52.Porrini M.P., Sarlo E.G., Medici S.K., Garrido P.M., Porrini D.P., Damiani N., Eguaras M.J. Nosema ceranae development in Apis mellifera: Influence of diet and infective inoculum. J. Apic. Res. 2011;50:35–41. doi: 10.3896/IBRA.1.50.1.04. [DOI] [Google Scholar]
- 53.Di Pasquale G., Salignon M., Le Conte Y., Belzunces L.P., Decourtye A., Kretzschmar A., Suchail S., Brunet J.-L.L., Alaux C. Influence of Pollen Nutrition on Honey Bee Health: Do Pollen Quality and Diversity Matter? PLoS ONE. 2013;8:e72016. doi: 10.1371/journal.pone.0072016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martín-Hernández R., Botías C., Bailón E.G., Martínez-Salvador A., Prieto L., Meana A., Higes M. Microsporidia infecting Apis mellifera: Coexistence or competition. Is Nosema ceranae replacing Nosema apis? Environ. Microbiol. 2012;14:2127–2138. doi: 10.1111/j.1462-2920.2011.02645.x. [DOI] [PubMed] [Google Scholar]
- 55.Urbieta-Magro A., Higes M., Meana A., Gómez-Moracho T., Rodríguez-García C., Barrios L., Martín-Hernández R. The levels of natural Nosema spp. infection in Apis mellifera iberiensis brood stages. Int. J. Parasitol. 2019;49:657–667. doi: 10.1016/j.ijpara.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 56.Martín-Hernández R., Botías C., Barrios L., Martínez-Salvador A., Meana A., Mayack C., Higes M. Comparison of the energetic stress associated with experimental Nosema ceranae and Nosema apis infection of honeybees (Apis mellifera) Parasitol. Res. 2011;109:605–612. doi: 10.1007/s00436-011-2292-9. [DOI] [PubMed] [Google Scholar]
- 57.Higes M., Martín-Hernández R., Garrido-Bailón E., García-Palencia P., Meana A. Detection of infective Nosema ceranae (Microsporidia) spores in corbicular pollen of forager honeybees. J. Invertebr. Pathol. 2008;97:76–78. doi: 10.1016/j.jip.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 58.Higes M., Martín-Hernández R., Garrido-Bailón E., Botías C., García-Palencia P., Meana A. Regurgitated pellets of Merops apiaster as fomites of infective Nosema ceranae (Microsporidia) spores. Environ. Microbiol. 2008;10:1374–1379. doi: 10.1111/j.1462-2920.2007.01548.x. [DOI] [PubMed] [Google Scholar]
- 59.Taupin V., Méténier G., Vivarès C.P., Prensier G. An improved procedure for Percoll gradient separation of sporogonial stages in Encephalitozoon cuniculi (Microsporidia) Parasitol. Res. 2006;99:708–714. doi: 10.1007/s00436-006-0231-y. [DOI] [PubMed] [Google Scholar]
- 60.Cantwell G.E. Standard methods for counting nosema spores. Am. Bee J. 1970;110:222–223. [Google Scholar]
- 61.Forsgren E., Fries I. Comparative virulence of Nosema ceranae and Nosema apis in individual European honey bees. Vet. Parasitol. 2010;170:212–217. doi: 10.1016/j.vetpar.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 62.Milbrath M.O., van Tran T., Huang W.F., Solter L.F., Tarpy D.R., Lawrence F., Huang Z.Y. Comparative virulence and competition between Nosema apis and Nosema ceranae in honey bees (Apis mellifera) J. Invertebr. Pathol. 2015;125:9–15. doi: 10.1016/j.jip.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 63.Jack C.J., Uppala S.S., Lucas H.M., Sagili R.R. Effects of pollen dilution on infection of Nosema ceranae in honey bees. J. Insect Physiol. 2016;87:12–19. doi: 10.1016/j.jinsphys.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 64.McGowan J., De la Mora A., Goodwin P.H., Habash M., Hamiduzzaman M.M., Kelly P.G., Guzman-Novoa E. Viability and infectivity of fresh and cryopreserved Nosema ceranae spores. J. Microbiol. Methods. 2016;131:16–22. doi: 10.1016/j.mimet.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 65.Malone L.A., Giacon H.A., Newton M.R. Comparison of the responses of some New Zealand and Australian honey bees (Apis mellifera L) to Nosema apis Z. Apidologie. 1995;26:495–502. doi: 10.1051/apido:19950606. [DOI] [Google Scholar]
- 66.Gómez Moracho T. Análisis de Los Patrones de Diversidad Genética de Nosema Ceranae, un Patógeno Emergente de Apis Mellifera. Universidade de Santiago de Compostela; Galicia, Spain: 2015. [Google Scholar]
- 67.Suwannapong G., Yemor T., Boonpakdee C., Benbow M.E. Nosema ceranae, a new parasite in Thai honeybees. J. Invertebr. Pathol. 2011;106:236–241. doi: 10.1016/j.jip.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 68.Eiri D.M., Suwannapong G., Endler M., Nieh J.C. Nosema ceranae Can Infect Honey Bee Larvae and Reduces Subsequent Adult Longevity. PLoS ONE. 2015;10:e0126330. doi: 10.1371/journal.pone.0126330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sagastume S., Martín-Hernández R., Higes M., Henriques-Gil N. Genotype diversity in the honey bee parasite Nosema ceranae: Multi-strain isolates, cryptic sex or both? BMC Evol. Biol. 2016;16:216. doi: 10.1186/s12862-016-0797-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Natsopoulou M.E., McMahon D.P., Doublet V., Bryden J., Paxton R.J. Interspecific competition in honeybee intracellular gut parasites is asymmetric and favours the spread of an emerging infectious disease. Proc. R. Soc. B Biol. Sci. 2014;282:20141896. doi: 10.1098/rspb.2014.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fries I. Infectivity and Multiplication of Nosema apis Z. in the Ventriculus of the Honey Bee. Apidologie. 1988;19:319–328. doi: 10.1051/apido:19880310. [DOI] [Google Scholar]
- 72.Pettis J.S., Vanengelsdorp D., Johnson J., Dively G. Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften. 2012;99:153–158. doi: 10.1007/s00114-011-0881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stear M.J. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Cambridge University Press; Cambridge, UK: 2018. OIE Nosemosis of Honey Bess; pp. 744–749. [Google Scholar]
- 74.Roberts K.E., Hughes W.O.H. Immunosenescence and resistance to parasite infection in the honey bee, Apis mellifera. J. Invertebr. Pathol. 2014;121:1–6. doi: 10.1016/j.jip.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 75.Powell J.E., Martinson V.G., Urban-Mead K., Moran N.A. Routes of acquisition of the gut microbiota of the honey bee Apis mellifera. Appl. Environ. Microbiol. 2014;80:7378–7387. doi: 10.1128/AEM.01861-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Engel P., Bartlett K.D., Moran N.A. The bacterium Frischella perrara causes scab formation in the gut of its honeybee host. MBio. 2015;6:e00193-15. doi: 10.1128/mBio.00193-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anderson K.E., Rodrigues P.A.P., Mott B.M., Maes P., Corby-Harris V. Ecological Succession in the Honey Bee Gut: Shift in Lactobacillus Strain Dominance During Early Adult Development. Microb. Ecol. 2016 doi: 10.1007/s00248-015-0716-2. [DOI] [PubMed] [Google Scholar]
- 78.Hroncova Z., Havlik J., Killer J., Doskocil I., Tyl J., Kamler M., Titera D., Hakl J., Mrazek J., Bunesova V., et al. Variation in Honey Bee Gut Microbial Diversity Affected by Ontogenetic Stage, Age and Geographic Location. PLoS ONE. 2015;10:e0118707. doi: 10.1371/journal.pone.0118707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maes P.W., Rodrigues P.A.P., Oliver R., Mott B.M., Anderson K.E. Diet-related gut bacterial dysbiosis correlates with impaired development, increased mortality and Nosema disease in the honeybee (Apis mellifera) Mol. Ecol. 2016;25:5439–5450. doi: 10.1111/mec.13862. [DOI] [PubMed] [Google Scholar]
- 80.Rubanov A., Russell K.A., Rothman J.A., Nieh J.C., McFrederick Q.S. Intensity of Nosema ceranae infection is associated with specific honey bee gut bacteria and weakly associated with gut microbiome structure. Sci. Rep. 2019;9:3820. doi: 10.1038/s41598-019-40347-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ptaszyńska A.A., Paleolog J., Borsuk G. NosemaceranaeInfection Promotes Proliferation of Yeasts in Honey Bee Intestines. PLoS ONE. 2016;11:e0164477. doi: 10.1371/journal.pone.0164477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anderson K.E., Ricigliano V.A. Honey bee gut dysbiosis: A novel context of disease ecology. Curr. Opin. Insect Sci. 2017;22:125–132. doi: 10.1016/j.cois.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 83.Regan T., Barnett M.W., Laetsch D.R., Bush S.J., Wragg D., Budge G.E., Highet F., Dainat B., de Miranda J.R., Watson M., et al. Characterisation of the British honey bee metagenome. Nat. Commun. 2018;9:4995. doi: 10.1038/s41467-018-07426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li J.H., Evans J.D., Li W.F., Zhao Y.Z., DeGrandi-Hoffman G., Huang S.K., Li Z.G., Hamilton M., Chen Y.P. New evidence showing that the destruction of gut bacteria by antibiotic treatment could increase the honey bee’s vulnerability to Nosema infection. PLoS ONE. 2017;12:e0187505. doi: 10.1371/journal.pone.0187505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jones J.C., Fruciano C., Hildebrand F., Al Toufalilia H., Balfour N.J., Bork P., Engel P., Ratnieks F.L., Hughes W.O. Gut microbiota composition is associated with environmental landscape in honey bees. Ecol. Evol. 2018;8:441. doi: 10.1002/ece3.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Anderson K.E., Ricigliano V.A., Mott B.M., Copeland D.C., Floyd A.S., Maes P. The queen’s gut refines with age: Longevity phenotypes in a social insect model. Microbiome. 2018;6:108. doi: 10.1186/s40168-018-0489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burri L., Williams B.A.P., Bursac D., Lithgow T., Keeling P.J. Microsporidian mitosomes retain elements of the general mitochondrial targeting system. Proc. Natl. Acad. Sci. USA. 2006;103:15916–15920. doi: 10.1073/pnas.0604109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cornman R.S., Chen Y.P., Schatz M.C., Street C., Zhao Y., Desany B., Egholm M., Hutchison S., Pettis J.S., Lipkin W.I., et al. Genomic analyses of the microsporidian Nosema ceranae, an emergent pathogen of honey bees. PLoS Pathog. 2009;5:e1000466. doi: 10.1371/journal.ppat.1000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Crailsheim K., Leonhard B. Amino acids in honeybee worker haemolymph. Amino Acids. 1997;13:141–153. doi: 10.1007/BF01373212. [DOI] [Google Scholar]
- 90.Weidner E., Findley A.M., Dolgikh V., Sokolova J. Microsporidian Biochemistry and Physiology. In: Wittne M., Weiss L.M., editors. The Microsporidia and Microsporidiosis. ASM Press; Washington, DC, USA: 1999. pp. 172–195. [Google Scholar]
- 91.Hillyer J.F. Insect immunology and hematopoiesis. Dev. Comp. Immunol. 2016;58:102–118. doi: 10.1016/j.dci.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martín-Hernández R., Higes M., Sagastume S., Juarranz Á., Dias-Almeida J., Budge G.E., Meana A., Boonham N. Microsporidia infection impacts the host cell’s cycle and reduces host cell apoptosis. PLoS ONE. 2017;12:e0170183. doi: 10.1371/journal.pone.0170183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kurze C., Le Conte Y., Kryger P., Lewkowski O., Müller T., Moritz R.F.A. Infection dynamics of Nosema ceranae in honey bee midgut and host cell apoptosis. J. Invertebr. Pathol. 2018;154:1–4. doi: 10.1016/j.jip.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 94.Kurze C., Le Conte Y., Dussaubat C., Erler S., Kryger P., Lewkowski O., Müller T., Widder M., Moritz R.F.A. Nosema Tolerant Honeybees (Apis mellifera) Escape Parasitic Manipulation of Apoptosis. PLoS ONE. 2015;10:e0140174. doi: 10.1371/journal.pone.0140174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Higes M., Martín-Hernández R., Botías C., Bailón E.G., González-Porto A.V., Barrios L., Del Nozal M.J., Bernal J.L., Jiménez J.J., Palencia P.G., et al. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ. Microbiol. 2008;10:2659–2669. doi: 10.1111/j.1462-2920.2008.01687.x. [DOI] [PubMed] [Google Scholar]
- 96.Li W., Evans J.D., Li J., Su S., Hamilton M., Chen Y. Spore load and immune response of honey bees naturally infected by Nosema ceranae. Parasitol. Res. 2017;116:3265–3274. doi: 10.1007/s00436-017-5630-8. [DOI] [PubMed] [Google Scholar]
- 97.Holt H., Villar G., Grozinger C.M. Molecular, physiological and behavioral responses of honey bee (Apis mellifera) drones to infection with microsporidian parasites. J. Invertebr. Pathol. 2018;155:14–24. doi: 10.1016/j.jip.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 98.Stroeymeyt N., Casillas-Pérez B., Cremer S. Organisational immunity in social insects. Curr. Opin. Insect Sci. 2014;5:1–15. doi: 10.1016/j.cois.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 99.Baracchi D., Cini A. A Socio-Spatial Combined Approach Confirms a Highly Compartmentalised Structure in Honeybees. Ethology. 2014;120:1167–1176. doi: 10.1111/eth.12290. [DOI] [Google Scholar]
- 100.Natsopoulou M.E., McMahon D.P., Paxton R.J. Parasites modulate within-colony activity and accelerate the temporal polyethism schedule of a social insect, the honey bee. Behav. Ecol. Sociobiol. 2016;70:1019–1031. doi: 10.1007/s00265-015-2019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Naug D., Gibbs A. Behavioral changes mediated by hunger in honeybees infected with Nosema ceranae. Apidologie. 2009;40:595–599. doi: 10.1051/apido/2009039. [DOI] [Google Scholar]
- 102.Naug D. Structure of the social network and its influence on transmission dynamics in a honeybee colony. Behav. Ecol. Sociobiol. 2008;62:1719–1725. doi: 10.1007/s00265-008-0600-x. [DOI] [Google Scholar]
- 103.Dussaubat C., Maisonnasse A., Crauser D., Beslay D., Costagliola G., Soubeyrand S., Kretzchmar A., Le Conte Y. Flight behavior and pheromone changes associated to Nosema ceranae infection of honey bee workers (Apis mellifera) in field conditions. J. Invertebr. Pathol. 2013;113:42–51. doi: 10.1016/j.jip.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 104.Woyciechowski M., Moroń D. Life expectancy and onset of foraging in the honeybee (Apis mellifera) Insectes Soc. 2009;56:193–201. doi: 10.1007/s00040-009-0012-6. [DOI] [Google Scholar]
- 105.Benvau L.R., Nieh J.C. Larval honey bees infected with Nosema ceranae have increased vitellogenin titers as young adults. Sci. Rep. 2017;7:1–8. doi: 10.1038/s41598-017-14702-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aliferis K.A., Copley T., Jabaji S. Gas chromatography-mass spectrometry metabolite profiling of worker honey bee (Apis mellifera L.) hemolymph for the study of Nosema ceranae infection. J. Insect Physiol. 2012;58:1349–1359. doi: 10.1016/j.jinsphys.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 107.Holt H.L., Aronstein K.A., Grozinger C.M. Chronic parasitization by Nosema microsporidia causes global expression changes in core nutritional, metabolic and behavioral pathways in honey bee workers (Apis mellifera) BMC Genom. 2013;14:799. doi: 10.1186/1471-2164-14-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Panek J., Paris L., Roriz D., Mone A., Dubuffet A., Delbac F., Diogon M., EI Alaoui H. Impact of the microsporidian Nosema ceranae on the gut epithelium renewal of the honeybee, Apis mellifera. J. Invertebr. Pathol. 2018;159:121–128. doi: 10.1016/j.jip.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 109.Pipan N., Rakovec V. Cell Death in the Midgut Epithelium of the Worker Honey Bee (Apis mellifera carnica) During Metamorphosis. Zoomorphologie. 1980;94:217–224. doi: 10.1007/BF01081936. [DOI] [Google Scholar]
- 110.Brodschneider R., Libor A., Kupelwieser V., Crailsheim K. Food consumption and food exchange of caged honey bees using a radioactive labelled sugar solution. PLoS ONE. 2017;12:e0174684. doi: 10.1371/journal.pone.0174684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pettis J.S., Lichtenberg E.M., Andree M., Stitzinger J., Rose R., VanEngelsdorp D. Crop Pollination Exposes Honey Bees to Pesticides Which Alters Their Susceptibility to the Gut Pathogen Nosema ceranae. PLoS ONE. 2013;8:e70182. doi: 10.1371/journal.pone.0070182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Badaoui B., Fougeroux A., Petit F., Anselmo A., Gorni C., Cucurachi M., Cersini A., Granato A., Cardeti G., Formato G., et al. RNA-sequence analysis of gene expression from honeybees (Apis mellifera) infected with Nosema ceranae. PLoS ONE. 2017;12:e0173438. doi: 10.1371/journal.pone.0173438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Biganski S., Kurze C., Müller M.Y., Moritz R.F.A. Social response of healthy honeybees towards Nosema ceranae-infected workers: Care or kill? Apidologie. 2018;49:325. doi: 10.1007/s13592-017-0557-8. [DOI] [Google Scholar]
- 114.Blacquière T., Smagghe G., Van Gestel C.A.M., Mommaerts V. Neonicotinoids in bees: A review on concentrations, side-effects and risk assessment. Ecotoxicology. 2012;21:973–992. doi: 10.1007/s10646-012-0863-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.De Smet L., Hatjina F., Ioannidis P., Hamamtzoglou A., Schoonvaere K., Francis F., Meeus I., Smagghe G., De Graaf D.C. Stress indicator gene expression profiles, colony dynamics and tissue development of honey bees exposed to sub-lethal doses of imidacloprid in laboratory and field experiments. PLoS ONE. 2017;12:e0171529. doi: 10.1371/journal.pone.0171529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jones J.C., Fruciano C., Marchant J., Hildebrand F., Forslund S., Bork P., Engel P., Hughes W.O.H. The gut microbiome is associated with behavioural task in honey bees. Insectes Soc. 2018;65:419–429. doi: 10.1007/s00040-018-0624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.De Souza D.A., Kaftanoglu O., De Jong D., Page R.E., Jr., Amdam G.V., Wang Y. Differences in the morphology, physiology and gene expression of honey bee queens and workers reared in vitro versus in situ. Biol. Open. 2018;7:bio036616. doi: 10.1242/bio.036616. [DOI] [PMC free article] [PubMed] [Google Scholar]