Abstract
Recordings from infants who died suddenly and unexpectedly demonstrate the occurrence of recurring apneas, ineffective gasping, and finally, failure to restore eupnea and arouse prior to death. Immunohistochemical and autoradiographic data demonstrate a constellation of serotonergic defects in the caudal raphe nuclei in infants who died of Sudden Infant Death Syndrome (SIDS). The purpose of this review is to synthesize what is known about adaptive responses of the infant to severely hypoxic conditions, which unleash a flood of neuromodulators that inhibit cardiorespiratory function, thermogenesis, and arousal and the emerging role of serotonin, which combats this cardiorespiratory inhibition to foster autoresuscitation, eupnea, and arousal to ensure survival following an hypoxic episode. The laryngeal and carotid body chemoreflexes are potent in newborns and infants, and both reflexes can induce apnea and bradycardia, which may be adaptive initially, but must be terminated if an infant is to survive. Serotonin has a unique ability to touch on each of the processes that may be required to recover from hypoxic reflex apnea: gasping, the restoration of heart rate and blood pressure, termination of apneas and, eventually, stimulation of eupnea and arousal are all modulated by serotonin. Recurrent apneic events, bradycardia, ineffective gasping and a failure to terminate apneas and restore eupnea are observed in animals harboring defects in the caudal serotonergic system models – all of these phenotypes are reminiscent of and compatible with the cardiorespiratory recordings made in infants who subsequently died of SIDS. The caudal serotonergic system provides an organized, multi-pronged defense against reflex cardiorespiratory inhibition and the hypoxia that accompanies prolonged apnea, bradycardia and hypotension, and any deficiency of caudal serotonergic function will increase the propensity for sudden unexplained infant death.
Keywords: Sudden Infant Death Syndrome, arousal, 5-HT, caudal raphe, autoresuscitation, apnea, adenosine, gamma-amino butyric acid (GABA), autonomic neuroscience
1 Introduction
Sudden Infant Death Syndrome (SIDS) and asphyxial deaths represent the single largest causes of deaths in infancy (age 1–12 months ) (2016). Occasionally, whether by design or by accident, the events leading up to the deaths of infants attributed to SIDS have been recorded with a variety of accompanying physiological data. The data among a diverse set of studies and recording methods are consistent: before death, these infants experience a series of apneas and associated bradycardias from which they initially recover, albeit sometimes only partially, until they succumb to final a period of severe apnea and bradycardia when gasping fails to restore heart rate and eupnea (Meny et al., 1994; Poets et al., 1999; Poets et al., 1993; Sridhar et al., 2003) (Fig. 1). Therefore, any hypothesis about the pathogenesis of SIDS must explain the two key features of these recordings before death: the origin of the apneas and the failure of recovery from these apneas. We believe that the designation Sudden Unexpected Infant Death (SUID), which includes SIDS, asphyxial deaths, and other poorly defined causes of death in infants less than one year of age, better captures the population of infants at risk for prolonged apneas, failed gasping, and a failure to restore eupnea and arouse that may lead to the death of an infant (Carlin and Moon, 2017; Hunt et al., 2015). Therefore, we have been pursuing studies to understand how the risk factors for SUID (and the risk factors for SIDS and asphyxial deaths within SUID are remarkably similar) either promote reflex apneas, regardless of sleep position, or inhibit or suppress effective gasping, restoration of eupnea and arousal after apneas (Leiter and Böhm, 2007).
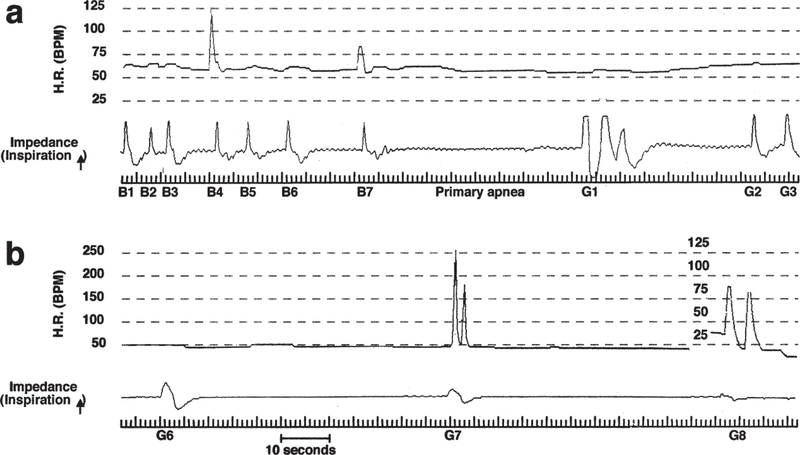
Figure 1.
Recordings of heart rate (H.R.) by ECG and respiratory activity by thoracic impedance in an infant classified as a SIDS case. (a) Breaths (B) 1 through 7 show a slowing of respiratory rate (i.e. progressively longer apnea) in a background of severe bradycardia (Normal HR = ~150 bpm). As hypoxia becomes more severe respiratory activity ceases altogether (primary hypoxic apnea). Three gasps (G1–3) then emerge, the respiratory component of “autoresuscitation”. (b) Terminal gasps (6 through 8) in the same record are shown – note that gasping has not succeeded in elevating H.R. or re-establishing eupnea; i.e. failed autoresuscitation. Reproduced from Sridhar et al., 2003.
Reflex apnea: The gateway to SUID
The main focus of this review is the multifaceted role of 5-HT in the processes that prevent or shorten prolonged apneas and promote gasping, restoration of eupnea, and arousal. However, the control of apnea initiation and duration intersects with the serotonergic system in the brainstem, and so a discussion of the putative origin of apneas in the context of SUID is a necessary prologue to further discussion of the important and protean actions of 5-HT in babies responding to hypoxic reflex apnea during sleep.
Fetal Responses to hypoxia
Apnea is elicited by hypoxia in young animals when a ventilatory response to hypoxia cannot be sustained (Mortola, 2004). The physiological adjustments to conserve oxygen as part of the response to hypoxia are best understood as a spectrum of adaptations appropriate to each particular environment and developmental state as animals pass from fetal life to adulthood.
The apneic response to hypoxia originates in utero where a ventilatory response to hypoxia provides no benefit; the fetus is completely dependent on oxygen delivery from uterine blood flow and its own conservative reflex responses to hypoxia. When neonatal animals are first exposed to hypoxia, minute ventilation may increase, a response that originates from the carotid body (Blanco et al., 1984) (Bureau et al., 1985). The large surface area to body mass ratio of small or young animals makes the maintenance of body temperature energetically costly, and a hyperventilatory response to hypoxia sufficient to maintain oxygen delivery to support a constant body temperature is prohibitively expensive energetically (Mortola, 1996). Thus, for many neonatal mammals, including humans (Cross and Oppe, 1952; Cross and Warner, 1951), a hyperventilatory response to hypoxia cannot be maintained, and ventilation declines as an oxygen conserving strategy (Bissonnette, 2000). Moreover, thermogenesis is blunted in the neonate and body temperature falls (i.e. hypoxic hypometabolism (Mortola, 1999, 2004)). Oxygen consumption in infants is correlated with the availability of oxygen - the neonate behaves like a ‘regulated oxygen conformer,’ and part of the response to hypoxia in neonates is inhibition of metabolism (Asakura et al., 1990; Mortola, 2004). Breathing is also inhibited, sometimes to the point of apnea. If primary hypoxic apnea develops, bradycardia, redistribution of blood flow, reduced thermoregulation, and depressed cerebral oxygen consumption ensue to provide a comprehensive and integrated mechanism of oxygen conservation (all of which recapitulate the fetal response to hypoxia). The persistence of a hypometabolic response, while initially adaptive, may compromise the re-establishment of normal cardiovascular function and body temperature control between apneic events. The repetitive, incomplete recoveries of heart rate and breathing evident in physiological records from babies who died of SIDS may reflect incomplete recovery of central cardiorespiratory and thermoregulatory control between apneic events (Meny et al., 1994; Poets et al., 1999; Poets et al., 1993; Sridhar et al., 2003).
Since the work of J.D. Wood in the 1960s, it has also been realized that a flood of GABA (Wood et al., 1968), possibly involving reduced GABA reuptake (Hagberg et al., 1985), contributes directly to inhibitory neuromodulation in the hypoxic mammalian brain. Increased GABAergic drive specifically within respiratory circuits contributes to hypoxic respiratory depression (Melton et al., 1990). Adenosine is also released in abundance during severe hypoxia and, acting through excitatory A2A receptors, may activate GABAergic neurons (Abu-Shaweesh, 2007; Mayer et al., 2006; Wilson et al., 2004; Zaidi et al., 2006). Adenosine, acting through adenosine A1 receptors, inhibits excitatory neurotransmitter systems (Cunha, 2001) and may permit prolonged apneas by decreasing CO2 sensitivity (James et al., 2018). Thus, adenosine and GABA inhibit neuronal activity and cause profound suppression of cerebral metabolic activity. We believe that the reduction in cerebral metabolism, like the centrally-mediated inhibition of respiration and thermogenesis (Bissonnette, 2000; Mortola, 2004), is a controlled and regulated process that enhances survival and is neuroprotective under severe hypoxic conditions (Hochachka, 1986). Hypoxia suppresses active sleep (AS) in the fetus (a REM-like state with a relatively high cerebral metabolic rate), and the suppression of cerebral metabolism in the fetus, neonates and infants generates a state analogous to NREM sleep in adults (though infants lack the cortical maturity to manifest the EEG characteristics of NREM sleep). The analogy is apt, however, in that NREM sleep is the sleep state associated with the lowest metabolic rate, and hypothermic responses to hypoxic stress are likely entered through NREM sleep in adult animals as well (Berger, 1975; Berger and Phillips, 1993; Heller, 1988). Moreover, adenosine is a sleep-promoting substance that fosters the appearance of slow-wave sleep (Radulovacki, 1985; Strecker et al., 2000). NREM and REM sleep are associated with active suppression of sensory afferent information; NREM REM sleep isolate the brain from outside stimuli and disruptions. In the same way, the structured, hypometabolic brain state caused by hypoxia during prolonged apneas actively isolates the infants from external stimuli. This may be one reason infants in the midst of prolonged hypoxic apneas fail to respond to resuscitation efforts, even when resuscitation is started promptly after the apneic and bradycardic events are first observed (Meny et al., 1994; Poets et al., 1999).
How do apneic responses leading to hypoxia begin?
Many investigators have recognized that the dive reflex, primary hypoxic apnea, and the laryngeal chemoreflex (LCR) have features in common with the events recorded in infants who died of SIDS. All three reflexes may be associated with apnea and bradycardia, redistribution of blood flow to vital organs, and often suppression of consciousness depending on the severity and duration of hypoxia accompanying the apnea (Li et al., 2018). These reflex apneas are also much more potent in young, immature animals and small mammals. Each of these reflexes has been thought to contribute to SIDS (Downing and Lee, 1975; Guntheroth and Kawabori, 1975; Kovar et al., 1979; Lanier et al., 1983; Page and Jeffery, 1998; Perkett and Vaughan, 1982; Thach, 1997; Wolf, 1966). The dive reflex is elicited by circumstances that seem far removed from SIDS, but primary hypoxic apnea and the LCR seem like strong candidates to initiate a process that may end in sudden death if apnea terminating and eupnea restoring processes are ineffective (Li et al., 2018).
The LCR is a protective reflex elicited by water, low chloride concentration solutions, or acid in the larynx (Boggs and Bartlett, 1982; St. Hilaire et al., 2005) and consists of a complex set of behaviors, apnea (sometimes profound), swallowing, coughing, sometimes bradycardia and redistribution of blood flow to vital organs. Many investigators have proposed that the respiratory inhibition and bradycardia associated with the LCR may result in the death of infants if they are not reversed before severe hypoxemia ensues (Downing and Lee, 1975; Page and Jeffery, 2000; Thach, 2001). Apneas can only exist if eupnea is inhibited, and this digital, reflex - on or off – switch between apnea and eupnea seems to originate in the NTS (where afferent fibers from the carotid body and larynx terminate) and requires an amplification process and likely reciprocal inhibitory mechanisms so that the animal can switch rapidly between apnea and eupnea. The rapid switching is achieved within the NTS by presynaptic modulation of glutamate release by the A- and C-fiber afferents mediating reflex apneas (Doyle et al., 2002; Fawley et al., 2014; Hermes et al., 2016; Jin et al., 2004). If the LCR is a gateway to SUID, then the physiological effects of many risk factors for SUID, such as thermal stress, maternal nicotine, fetal tobacco smoke exposure and inflammation, ought to increase the sensitivity or severity of the LCR in animals; which has been confirmed in multiple studies (Xia et al., 2011, 2016; Xia et al., 2009; Xia et al., 2010). Presynaptic transient receptor potential vanilloid 1 (TRPV1) receptors, which are abundant on C-fibers in the NTS and also participate in central sensitization of pain, provide a mechanism through which many risk factors for SUID may modulate presynaptic signaling in the NTS (Xia et al., 2011, 2016). Thus, risk factors for SUID may sensitize the LCR (Li et al., 2018), analogous to central sensitization of pain fibers, and produce reflex allodynia in infants (potentiation of the LCR by stimuli that would otherwise be innocuous) and increase the likelihood of severe apneic events leading to sudden infant death.
Parallels exist between the cellular mechanisms underlying the LCR and those underlying peripheral chemoreflex response to hypoxia. Stimulation of the LCR and the hypoxic chemoreflex may cause apnea and bradycardia, both reflexes originate from cranial nerves, and the initially processing of both reflexes occurs in the caudal NTS. In addition to sensitizing the LCR, TRPV1 activation also increases the sensitivity of the carotid body to hypoxia (Roy et al., 2018). Central apneas are more frequent in infants at risk for SIDS (Kahn et al., 1988; Kahn et al., 1992; Kato et al., 2001), and increased carotid body sensitivity increases the loop gain of the respiratory control system and therefore decreases respiratory stability (Alvaro et al., 2012; Boros and Reynolds, 1976; Khoo et al., 1991; Nakayama et al., 2003; Smith et al., 2007; Zhao et al., 2011). Thus, risk factors for SIDS that sensitize the LCR may also sensitize carotid body function and increase respiratory instability, especially during sleep when other influences that stabilize breathing are absent (Horner, 2017; Phillipson, 1978), thereby exposing infants to a greater risk of prolonged apneas and the subsequent severe hypoxia during sleep.
Apnea during sleep: Serotonin rides in wearing a white hat
The recordings from infants who died of SIDS reveal a sequence of events in which apnea was followed by ineffective gasping, failed restoration of eupnea and failed arousal (Meny et al., 1994; Poets et al., 1999; Poets et al., 1993; Sridhar et al., 2003). Most infants experience repetitive apneas during neonatal life that diminish in frequency as infants mature (Kahn et al., 1992; Kato et al., 2001). Termination of severe apneas relies on autoresuscitation (Guntheroth and Kawabori, 1975), and autoresuscitation from apneas involves gasping, termination of apnea, restoration of eupnea, and often, arousal from sleep. It is our hypothesis that in this stereotypical train of events, each step is dependent on the preceding event; the process of successful autoresuscitation starts with gasping and reversal of bradycardia. Oxygenation must improve to allow the next processes to emerge: apnea cessation and restoration of eupnea, after which arousal from sleep may occur. Serotonin contributes to each process in a way that promotes eupnea and arousal. Figure 2 shows a schematic of the processes and sequencing of recovery from hypoxic reflex apnea and the role of serotonin in each process.
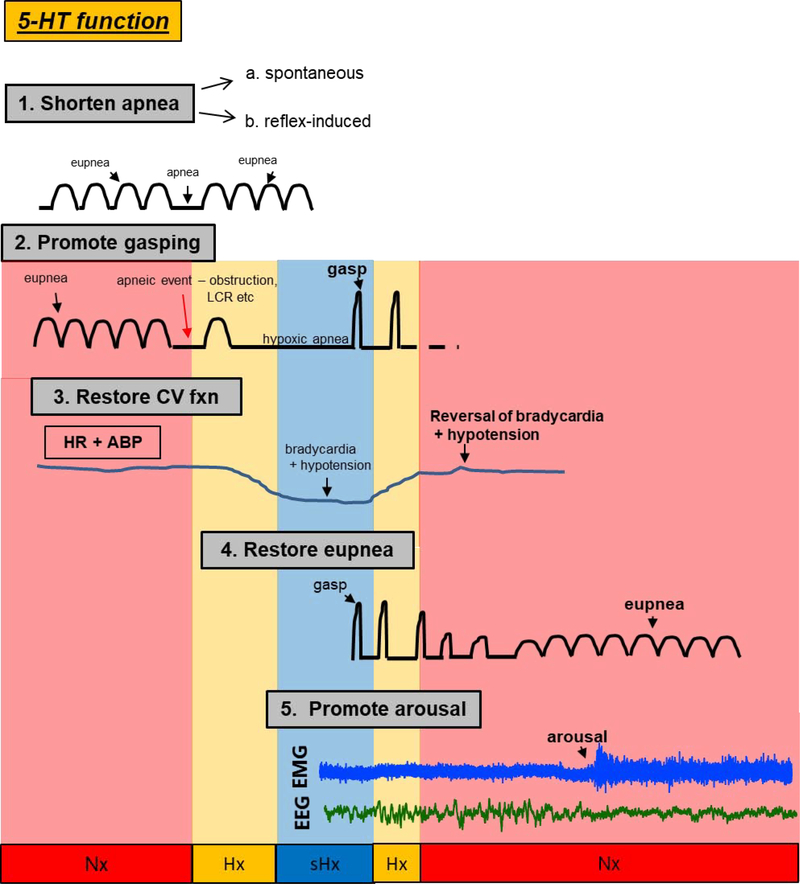
Figure 2.
The critical role of central serotonin (5-HT) on cardiorespiratory homeostasis and arousal following spontaneous, reflex- or hypoxia-induced apnea. Shown are specific functions of 5-HT on: 1. the prevention or mitigation of spontaneous (see Erickson et al.,2007, Hodges et al., 2009, Ptak et al., 2009, Cummings et al., 2010, Kaplan et al. 2016, Young et al., 2017) or reflex-induced apneas (i.e. those initiated by the laryngeal chemoreflex (LCR) (see Donnelly et al., 2016, 2017); 2. the promotion of gasping during severe hypoxia (see Pena and Ramirez, 2002, Tribe et al., 2006, Erickson and Sposato, 2009, Cummings et al., 2011, Chen et al., 2013, Dosumu-Johnson et al., 2018), 3. the restoration of cardiovascular function during the gasping phase (see Cummings et al., 2011, Chen et al., 2013, Yang and Cummings, 2013, Dosumu-Johnson et al., 2018), 4. the eventual restoration of eupneic breathing (see St. John and Leiter, 2007, Erickson and Sposato, 2009, Cummings et al., 2011, Chen et al. 2013, Dosumu-Johnson et al., 2018), and finally, 5. arousal (see Buchanan and Richerson, 2010, Buchanan et al., 2015, Darnall et al., 2011, Darnall et al. 2016, Kaur et al., 2013, Kaur and Saper, 2019). Gasp: high-amplitude, low frequency respiratory motor output in response to severe hypoxia; eupnea: normal breathing; apnea: no breathing; CV: cardiovascular; HR: heart rate; ABP: arterial blood pressure; EMG: electromyograph; EEG: electroencephalograph. Colored regions represent normoxic (Nx, red), hypoxic (Hx, orange), and severely hypoxic (sHx, blue) conditions.
Multiple abnormalities in caudal serotonergic neurons have been described in infants who died of SIDS, and the core neuropathological lesions of SIDS are found among the serotonergic neurons in the paragigantocellularis lateralis, gigantocellularis, intermediate reticular zone, caudal raphe, and arcuate nucleus (Kinney and Haynes, 2019). These neurons send projections rostrally to a variety of respiratory-related and arousal-related nuclei, and the 5-HT derived from these nuclei has important effects on each element of the autoresuscitation process whereby apneas are terminated and regular breathing is restored. Moreover, each of the elements of autoresuscitation originates in a different part of the brainstem and seems to receive serotonergic inputs from the caudal raphe that act through different sets of 5-HT receptors (Fig. 3).
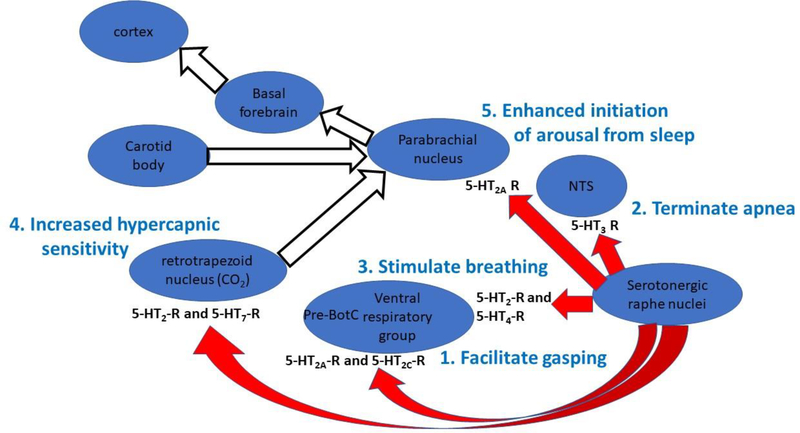
Figure 3.
The schematic representation of brainstem nuclei portrays the important role of serotonin originating from the caudal raphe interacting with different nuclei within the brainstem to organize the sequential processes that are essential to restore normal breathing following reflex, hypoxic apneas and bradycardia. Within each target nucleus, a different set of serotonin receptors seems to mediate the action of serotonin, but ultimately, the serotonergic neurons within the caudal raphe drive these processes. Moreover, the processes of recovery from hypoxic, reflex apnea seem to proceed from the caudal brainstem rostrally to the cortex through the brainstem nuclei and following the numbered sequence shown above. Autoresuscitation appears first (if the hypoxic apnea is sufficiently severe), termination of apnea follows, eupnea is restored next, and finally arousal is facilitated by enhanced hypercapnic sensitivity and amplification of arousing inputs to the parabrachial nucleus. Failure or deficiency of any one of these processes may increase the likelihood that a sleeping infant does not recovery successfully from hypoxic, reflex apnea.
Gasping requires disinhibition and serotonergic facilitation
Active inhibition originating from the pons and possibly higher brain centers inhibit respiratory circuits responsible for the generation of gasping (e.g. pre-BotC), thereby preventing gasping behavior during eupnea (Lumsden, 1923; St John et al., 1984; St John, 1985; St John and Knuth, 1981). However, there is likely a decrease in this inhibition during severe hypoxia, which permits gasping to emerge. Glycinergic mechanisms are perhaps paramount inhibiting gasping; in situ experiments utilizing phrenic nerve recordings have shown that, when coupled with an increase in extracellular K+ (a situation that is known to occur naturally under anoxic conditions) and glycinergic blockade (Blank and Kirshner, 1977), the eupneic pattern of breathing switches to a gasping pattern (St. John et al., 2002). In addition to the removal of this glycinergic braking, there is an increase in both glutamatergic and serotonergic drive within the local Pre-BotC microcircuitry during hypoxia that expedites or facilitates gasping (but is perhaps is not necessary for its initiation) (Solomon, 2004). Astrocytes are an additional key source of excitatory drive during hypoxia that counteracts GABA/glycinergic inhibition – this includes the release of gasotransmitters such as H2S as well as ATP that binds P2Y receptors within the inspiratory pre-BotC network (da Silva et al., 2017; Rajani et al., 2018).
Serotonergic facilitation of the gasp
In reduced preparations, serotonergic neurons provide excitatory drive to the Pre-BotC to facilitate gasping. For example, 5-HT, acting on 5-HT2A receptors, differentially affected pacemaker neurons; cadmium-insensitive pacemaker neurons – i.e. those that rely on persistent sodium currents necessary for gasping but not eupnea – require endogenous 5-HT2A receptor activation for bursting (Pena and Ramirez, 2002; St. John and Leiter, 2008; Tryba et al., 2006). Pharmacological activation of PKC blocks the effect of 5-HT2A receptor antagonism on fictive respiratory activity, suggesting that PKC activation is a key step in the signaling transduction pathways leading from 5-HT2A receptors to the downstream effector molecules (Pena and Ramirez, 2002). In addition to 5-HT, Substance P is also released from serotonergic neurons in the raphe obscurus, modulating background cation leak currents to increase the excitability of pre-BotC neurons (Pena and Ramirez, 2004; Ptak et al., 2009). These effects are mediated by 5-HT2A, 5-HT2C and NK-1 receptors. One can reasonably conclude from the above findings that during prolonged or severe hypoxia, there is a shift away from inhibition of the gasping centers (via glycinergic disinhibition) that, when combined with 5-HT2A and 5-HT2C activation and increased extracellular K+, releases the constraint on the Pre-BotC and allows gasping to emerge.
The findings from slice preparations have largely been recapitulated in whole animals, but with an important caveat: the role of 5-HT is highly dependent on stage of postnatal development. For example, studies on Pet-1−/− mice lacking about two-thirds of the usual number of serotonergic neurons demonstrated that 5-HT has little role in the genesis of gasping once the animals are beyond about 2 weeks of age (Chen et al., 2013; Cummings et al., 2011; Erikson and Sposato, 2009; St. John et al., 2009). Serotonin appears to be most efficacious terminating hypoxic apnea and initiating gasping in ~P7–10 mice; Pet-1−/− mice have profoundly delayed gasping only at this age (Chen et al., 2013; Cummings et al., 2011). This is of particular relevance to SIDS because this age is arguably comparable to human infancy (Clancy et al., 2001). The neurophysiological basis for the relatively narrow developmental window in which 5-HT facilitates gasping has not been elucidated. It is not a function of “immature” respiratory neuronal networks; in the first few days of life (postnatal day 4–5), 5-HT-deficient Pet-1−/− mice are completely normal in terms of gasping and autoresuscitation (Cummings et al., 2011). Two subsequent studies addressed whether 5-HT exerts a physiological or developmental role within gasping centers of the brainstem. Like Pet-1−/− mice, rat pups in which 5-HT neurons were chemically lesioned postnatally a few days before testing have delayed gasping and decreased survival during severely hypoxic conditions. This suggests a physiological, rather than developmental, role for serotonin as a facilitator of gasping. Perhaps more convincing evidence that serotonin exerts a physiological role comes from mice in which 5-HT neurons were acutely “silenced” using Designer Receptor Exclusively Activated by Designer Drug (DREADD) in mice. During activation of DREADD receptors, the mice demonstrated delayed and less effective gasping (Dosumu-Johnson et al., 2018). These findings strongly support an acute facilitation of the gasping by 5-HT in neonatal animals, likely through 5-HT2A and 5-HT2C receptors (Pena and Ramirez, 2002).
Effective autoresuscitation depends on gasping, but there is a cardiovascular component as well. During each gasp, there is a dramatic increase in heart rate that increases cardiac output and, coupled with the dramatic increase in pulmonary ventilation, helps reverse systemic hypoxia. The primary effect of carotid body stimulation is bradycardia when there are no respiratory efforts; when vagally-mediated information associated with successful respiratory efforts is present, hypoxia is associated with a tachycardia (Angell-James and Daly, 1969, 1975; Daly et al., 1979). Therefore, the tachycardia seen with each gasp is part of a coordinated reversal of the inhibitory, apneic-bradycardic response to hypoxia to an excitatory cardiorespiratory response more appropriate for air breathing. Successful autoresuscitation cannot occur if gasping and tachycardia do not improve systemic oxygenation (Guntheroth and Kawabori, 1975). In this respect, it is interesting that in 5-HT deficient mice and rats, whether they were Pet1−/− or rat pups treated with 5–7 DHT, a reduction in 5-HT was associated with both reduced effectiveness of gasping and a less effective reversal of the bradycardic response to hypoxia. Moreover, compared to rats replete with 5-HT, rat pups lacking 5-HT experience a more profound decrease in arterial blood pressure that compromises survival during severely hypoxic conditions. Along with reduced cardiac output, reduced blood pressure during apnea and bradycardia undoubtedly leads to brain hypoperfusion and more profound tissue hypoxia that cannot be mitigated by gasping alone (Yang and Cummings, 2013). While the mechanisms for the support of blood pressure by 5-HT have not been revealed, it may be that 5-HT facilitates the carotid body-mediated sympatho-excitation that occurs during apnea and/or hypoxia, possibly at the level of the NTS. It is clear from these animal studies that 5-HT contributes to each component of the coordinated cardiorespiratory response to severe hypoxia; i.e. conversion of apnea to gasping and the recovery of normal heart rate and blood pressure (Fig. 2).
Serotonergic inhibition of apnea
Apnea and eupnea are mutually exclusive. Activation of the LCR and primary hypoxic apnea hold the respiratory pattern generator in a post-inspiratory apneusis, which precludes progress through the usual three phases of eupnea: inspiration, post-inspiration, and expiration. Therefore, a necessary step in the restoration of eupnea is either the waning of the apnogenic influence arising from within the NTS, or active inhibition of that apnogenic process, so that the post-inspiratory apneusis may be terminated and eupnea may be resumed. Termination of apnea during the LCR was facilitated by activation of 5-HT3 receptor activation in the NTS (Donnelly et al., 2016). Serotonin 3 receptors are expressed on C-fibers within the superior laryngeal nerve, and they are densely expressed within the NTS where the axons of these fibers terminate in the area postrema (AP) and NTS (Barnes et al., 2009; Pratt and Bowery, 1989; Waeber et al., 1989). We speculated that the 5-HT3 receptors were on presynaptic terminals of GABAergic neurons, which when activated, inhibit (as yet electrophysiologically unidentified) apnogenic neurons in the NTS, which are presumably glutamatergic (Czyzyk-Krzeska and Lawson, 1991; Kubin and Davies, 1995; Remmers et al., 1986). The 5-HT that interacts with the 5-HT3 receptors in the NTS originates from the caudal raphe (mainly the raphe obscurus) (Donnelly et al., 2017). Serotonin originating from the raphe obscurus also blunts the bradycardic response to carotid body stimulation by activating 5-HT3 receptors in the commissural nucleus of the NTS (Weissheimer and Machado, 2007). Thus, both apnea termination and the termination of the bradycardic response to hypoxic apnea are associated with activation of 5-HT3 receptors in the NTS, and inhibition of apnea and bradycardia set the stage for a sustained, excitatory cardiorespiratory response to hypoxia and restoration of eupnea. A deficiency of either 5-HT3 receptors in the NTS or a deficiency of activation and release of 5-HT from caudal raphe neurons could be associated with prolonged and difficult to reverse apneas and bradycardia, and SUID may be associated with a hyposerotonergic state associated with or derived from numerous neuropathological abnormalities in the caudal raphe of babies who died of SIDS (Kinney and Haynes, 2019). Moreover, intermittent hypoxia administered to pregnant rat dams resulted in sensitization of the LCR, reduced ability of 5-HT to shorten the LCR (which as noted above depends on 5-HT3 receptors), and a marked reduction in 5-HT3 receptor binding in the rat pups at the same postnatal age when the LCR was prolonged (cite paper in this journal).
Thus, prenatal hypoxia appears to alter brain development in a way that sensitizes apnogenic reflexes, like the LCR, and reduces the capacity of 5-HT, which originates at least in part from the caudal raphe, to shorten and terminate reflex apnea. There are no similar studies of regulation of apnea duration following primary hypoxic apnea, but given the convergence of apnea control processes within the NTS, an effect of 5-HT3 receptor activation on the duration of primary hypoxic apnea seems plausible. In summary, serotonergic mechanisms exist within the caudal raphe and NTS that may terminate reflex apneas and clear the way for restoration of eupnea. Should any of these processes be deficient, as we believe they are in SUID, apneas will be more difficult to terminate, and eupnea and arousal, the essential process to protect infants against prolonged hypoxic apneas, will be more difficult to initiate and maintain (Fig. 2).
Serotonergic support of eupnea
In general, 5-HT has a stabilizing effect on respiration (Lalley, 1994; Richter et al., 2003), although activation of the 5-HT1A receptor can inhibit elements of the respiratory controller (Lalley et al., 1997; Lalley et al., 1994). This stabilizing effect is most clearly seen in animals in which 5-HT is deficient. In Lmx1bf/f/p mice, which lack serotoninergic neurons, younger animals had repeated, long spontaneous apneas. When these animals were treated with 5-HT or 5-HT agonists, the respiratory pattern was regularized and the frequency of breaths increased. This serotonergic effect was achieved through 5-HT2A and/or neurokinin 1 (NK-1) receptors, presumably in the ventral medullary regions controlling the respiratory pattern (Hodges et al., 2009). The stabilizing effect of 5-HT on neonatal breathing has also been demonstrated in neonatal Pet-1−/− mice (Cummings et al., 2010) and tryptophan hydroxylase 2-deficient rat pups (Kaplan et al., 2016; Young et al., 2017). The latter specifically lack 5-HT, retaining other co-released neuromodulators like substance P and thyrotropin releasing factor that have excitatory effects on breathing. In reduced preparations, 5-HT, arising from the caudal raphe, also acts to increase respiratory frequency and stabilize breathing by stimulation 5-HT2A/2C, 5-HT4, substance P and/or NK-1 receptors in the respiratory network (Ptak et al., 2009).
Other aspects of serotonergic activity coordinate the regular transition between inspiration and expiration, and through these processes reduce the occurrence or duration of apnea. For example, 5-HT signaling through 5-HT1A receptors inhibits the Kolliker Fuse (KF) nucleus, a region in the dorsolateral pons that facilitates the transition from inspiration to expiration (Dutschmann and Dick, 2012). Disinhibition of the KF after blockade of inhibitory 5-HT1A receptors increased the frequency of apneas (Dhingra et al., 2016). In addition to stimulating eupnea, 5-HT facilitates regular cycling between inspiration and expiration, which is essential for the restoration of eupnea following hypoxic apnea.
It is beyond the scope of this review, but caudally projecting serotonergic neurons provide important inputs to thermogenesis (Morrison, 2016), and it may be that the serotonergic inputs to thermogenesis are important in re-establishing respiratory stability and thermal homeostasis following the hypometabolic, oxygen conforming state that occurs during hypoxic, reflex apneas in neonates and infants (Mortola, 1999, 2004). Moreover, lack of serotonin in Lmx1bf/f/p mice was associated both with apneas, respiratory instability, and reduced thermogenic capacity (Hodges et al., 2009). Carotid bodies also contribute to the regulation of thermogenesis during hypoxia, albeit mostly during the recovery phase once oxygen becomes available after the apnea has been terminated (Hemelrijk et al., 2019; Mortola, 2004). This suggests that the most immature mammals, including human infants - those that perhaps have the lowest carotid body sensitivity to hypoxia (Bissonnette, 2000), experience a more prolonged blunting of thermogenesis that persists even after cessation of the hypoxic stimulus, perhaps in keeping with the adaptive nature of the oxygen-conserving response. It is interesting that the recordings of infants who subsequently died of SIDS often demonstrate incomplete recovery of eupnea or full arousal from sleep. Hence, it appears that failure to restore thermal homeostasis, possibly as a result of deficient serotonergic function, may contribute to the lack of respiratory stability following repeated hypoxic apneic events.
Sudden infant death invariably occurs during sleep, but there is no clear indication whether SUID occurs preferentially in NREM sleep or REM sleep (analogous to AS in infants). Respiratory activity is more variable during REM or active sleep (Horner, 2017), and it seems likely that apneic and bradycardic events leading to sudden death occur predominantly in REM or AS, which is also the sleep state associated with the least serotonergic activity. Young and colleagues asked whether the apneas displayed by neonatal serotonin-deficient rodents showed any dependency on state; i.e. were they more frequent in quiet sleep (analogous to NREM in adults) or active sleep? Compared to wild-type littermates, TPH2−/− pups displayed increased spontaneous apneas, specifically during prolonged periods of AS (Young et al., 2017). The increased apnea of TPH2−/− rat pups was ameliorated by central administration of atropine, suggesting that elevated cholinergic drive to respiratory centers - probably originating from the pontine tegmentum, a key “REM driver” – contributed to apneic phenotype (Davis et al., 2019). Even in normal infants and neonates, AS may be considered a “risky” state, given the destabilized respiratory, heart rate and arterial blood pressure that characterize this state (Horner, 2012; Horner, 2017). A loss of 5-HT may put an infant at greater risk for apnea and the initiation of events that may lead to SUID specifically in AS. In addition to more apnea (Kahn et al., 1988; Kahn et al., 1992; Kato et al., 2001), there is evidence that infants who subsequently died of SIDS cases had more AS than quiet sleep (Schechtman et al., 1992). Serotonergic dysfunction may have a role in both of increasing the amount of AS and decreasing respiratory stability during that stage of sleep in infants at risk for SIDS/SUID.
Serotonergic support of arousal
Arousal from sleep is associate with set of physiological responses, usually in a stereotypical sequence, including an increase in heart rate, blood pressure and muscle tone (reversing the relatively low heart rate and blood pressure and laxity of muscle tone associated with sleep), a sustained respiratory effort (though there may be a brief pause in breathing), and activation of the EEG. In human infants, arousal begins with an augmented breath (sometimes followed by a brief apnea) or a startle, increased heart rate and a change in the EEG activity reflecting the arousal (usually low voltage faster activity) (McNamara et al., 2002; McNamara et al., 1998). This stereotypical sequence has been described in piglets and rat pups and may occur spontaneously or may be elicited by exposure to hypoxia and/or hypercapnia (BuSha et al., 2001; Darnall et al., 2010; Dauger et al., 2001). Based on the timing and pattern of the events within the arousal response, the arousals in infants seem to begin with autonomic changes originating from the brainstem (respiratory and heart rate changes) and then ascend, partially or completely, to the cortex. Hence, arousals originate in the brainstem (BuSha et al., 2001; McNamara et al., 2002; McNamara et al., 1998).
SIDS is associated with serotonergic defects in the caudal raphe (Kinney and Haynes, 2019), and arousal responses appear to be inadequate in babies who died of SIDS (Harper and Bandler, 1998; Hunt, 1981; Kahn et al., 2002; McCulloch et al., 1982). Numerous studies have linked deficient serotonergic activity in the caudal raphe to failed or deficit arousal responses. Toxigenic lesion studies using 5,7-DHT, a toxin that specifically kills serotoninergic neurons, injected into the medullary raphe in P2 rat pups resulted in an 80% reduction in medullary 5-HT neurons. When these animals were studied at P5, P15, and P25, pups injected with 5,7-DHT had longer arousal latencies and a reduced respiratory frequency response to hypoxia at all three ages tested compared to control rat pups (Darnall et al., 2016). Similar results were obtained in Pet-1−/− knock-out mice, which have dramatically reduced numbers of serotonergic neurons. Pups between the ages P6-P10 were exposed to four episodes of hypoxia during sleep.
After the onset of hypoxia, the latencies to arousal in Pet-1−/− pups were significantly longer than in wild-type control animals. The arousal latency tends to habituate (get longer) after repeated exposure to hypoxia, and arousal habituation was also greater in Pet-1−/− knock-out mice compared to wild-type control animals (Darnall et al., 2011). Moreover, TPH2−/− pups have delayed arousal responses to increasing CO2 in both QS and AS, while arousal responses to hypoxia were unaffected (Young et al., 2017). Hypercapnia appears to be a more potent arousing stimulus than hypoxia (Kaur et al., 2013), as discussed below, even though both stimuli are present during hypoxic reflex apnea.
Serotonergic facilitation of arousal: central and peripheral mechanisms
As noted above, arousals seem to begin caudally and project rostrally. The cortical component of arousals are generated by an ascending arousal system that originates in the rostral pons and includes the parabrachial nucleus, which projects to the basal forebrain and then to the cortex (Fuller et al., 2011). The parabrachial nucleus integrates arousing stimuli related to hypercapnia (Kaur et al., 2013), hypoxia (Darnall, 2013), and vagally-mediated mechanoreceptor information from the airways (Kaur and Saper, 2019). Serotonergic neurons in the caudal raphe send projections to the parabrachial nucleus (Bang et al., 2012; Miller et al., 2011), where 5-HT may enhance arousals to hypercapnic stimuli by acting through 5-HT2A receptors (Buchanan and Richerson, 2010; Buchanan et al., 2015). The 5-HT mediating these arousal enhancing effects may originate in part from the raphe magnus (Darnall et al., 2016), but possibly other caudal raphe nuclei. In addition, hypoxia-sensitive neurons in the rostral ventrolateral medulla may also contribute to arousal response, though whether this depends on serotonergic mechanisms is less clear (Guyenet and Abbott, 2013). Serotonin may amplify the arousing effects of carotid body and vagal stimuli at the level of the parabrachial nucleus, but the role of amplification of hypercapnic inputs to arousal at the level of the parabrachial nucleus has been more fully elaborated (Kaur and Saper, 2019).
The foregoing survey of studies makes it clear that 5-HT originating from the caudal raphe participates in multiple processes that are important in reversing hypoxic apnea and bradycardia, but this leaves unanswered the question, what stimulates serotonergic neurons as the apnea progresses so that they can overcome the inhibition that is central to the hypometabolic apneic response to hypoxia? The simplest answer is that the duration of the apnea leads to progressive hypercapnia and hypoxia. Carotid body chemoreceptors detect hypoxia and to a lesser extent, hypercapnia and increase ventilation when activated. Central chemoreceptors detect hypercapnia, and there are oxygen sensitive neurons in the ventral medulla that may participate in the restoration of eupnea following apneas in infants (Bamford and Carroll, 1999; Carroll and Fitzgerald, 1993; Gauda et al., 2009; Guyenet and Abbott, 2013; Kholwadwala and Donnelly, 1992). Activity from the carotid bodies also contributes to behavioral arousal in response to hypoxia, and denervation of the carotid bodies reduced arousal in response to hypoxia and airway obstruction (Fewell et al., 1989; Fewell et al., 1990). The involvement of serotonergic neurons in carotid body-mediated arousal is supported by the observation that stimulation of the carotid sinus nerves induces FOS-like protein (a marker of neuronal activation) in regions of the medullary raphe (Erickson and Millhorn, 1991). Hypercapnia appears to be a potent activator of arousal through the parabrachial nucleus, which is enhanced by serotonergic inputs (Kaur and Saper, 2019). In a similar way, the hypercapnic response generated within the retrotrapezoid nucleus (RTN) may also be amplified by serotonergic inputs. There are also projections from midline serotonergic neurons to the RTN (Brust et al., 2014; Rosin et al., 2006), and serotonergic projections from the medullary raphe to the RTN amplify the neuronal and ventilatory responses to hypercapnia originating within the RTN (Dias et al., 2008; Mulkey et al., 2007; Wu et al., 2019). The amplification of CO2 sensitivity within the RTN depends on 5-HT2 and 5-HT7 receptors acting on two different ph-sensitive channels mediating CO2 sensitivity of RTN neurons: HCN channels are modulated by 5-HT2 receptors (Hawkins et al., 2015) and KCNQ channels were modulated by 5-HT7 receptors (Mulkey et al., 2007). Any amplification of CO2 sensitivity within the RTN will further enhance the arousing potential of hypercapnia, which may be further amplified by serotonergic inputs to the parabrachial nucleus discussed above. Thus, the projections from the RTN to arousal-promoting neurons in the parabrachial nucleus (Rosin et al., 2006) and carotid body afferents to the parabrachial nucleus likely concurrently stimulate ventilation and promote arousal as a dual-pronged approach to overcome the inhibition of breathing associated with hypoxic reflex apnea.
Serotonin and the risk of SUID
Serotonergic defects are common in babies who died of SIDS (Duncan et al., 2010; Paterson et al., 2006) and also in babies who died of under circumstances that were consistent with asphyxia (Randall et al., 2013). We think it is likely that even though there has been diagnostic drift (Hunt et al., 2015), deaths of infants that are labeled as asphyxial, may still reflect disorders of the serotonergic system. First, the risk factors for SUID, which reflect SIDS and asphyxial deaths, encompass many of the factors that were previously associated with SIDS and may contribute to the serotonergic defects identified in infants who died in circumstances consistent with asphyxia (Randall et al., 2013). It is our belief that deficiencies of gasping, termination of apneas due to hypoxia, restoration of eupnea and arousal are likely also deficient in infants whose deaths are labeled asphyxia. Hence, difficulty terminating apnea, restoring eupnea and arousing from sleep likely contribute to deaths in infants that were attributed to both to asphyxia and to SIDS. Based on the hypotheses presented above, the severity of serotonergic defects, whether the cause of death is listed as SIDS or as asphyxia, will dictate the extent to which infants can overcome hypoxic apneic events. In addition to the benefit of reoxygenation associated with gasping, serotonin is the primary neurotransmitter organizing the recovery from reflex apneas and hypoxia.
Summary
Surviving episodic hypoxia is essential, and neonates and infants are in a difficult transitional stage in which they do not have sufficient body mass or energy reserves to mount an effective and sustained euthermic response to hypoxia. Therefore, an oxygen conserving and hypothermic response to that permits oxygen consumption to decline during hypoxia is essential to prolong survival, but there must be a mechanism of reevaluation of this strategy, a way to reassess, and a way to reverse the hypometabolic, apneic and bradycardic response to hypoxia. The intrinsic hypoxic sensitivity of the gasping mechanism provides this opportunity to reevaluate the hypometabolic strategy and begins the process whereby infants may re-establish eupnea. If gasping restores oxygenation, the apnea may be terminated, the cardiorespiratory response may be converted from an inhibitory pattern to an excitatory pattern and the respiratory rate and heart rate may increase, and the infant may arouse as the final step in complete restoration of a vigorous waking response to hypoxia. In our analysis, hypoxia, adenosine and GABA are part of a centrally controlled and structured pattern of responses to survive hypoxia, and oxygen and 5-HT are arrayed against these inhibitory influences to reduce the impact of adenosine (reoxygenation) and provide excitatory inputs to the key events necessary to restore normal breathing. There are surely more excitatory neurotransmitters brought into play during the recovery from apnea, but 5-HT is a major factor. Infants who died of SIDS have an array of defects that mainly involve the caudal raphe (Kinney and Haynes, 2019); it is our hypothesis that these defects compromise the ability of infants at risk for SIDS to mount an effect and timely defense against the potent evolutionary adaptations fostering apneic and hypometabolic responses to hypoxia. In summary, we believe that infants at risk for SIDS (and SUID) have both an increased propensity for apneas derived from sensitization of the LCR and/or carotid body activity, which increases the strength of the apneic, hypometabolic response to hypoxia, and defects in serotonergic function that limit the effectiveness of those behaviors that are meant to reverse hypoxia. This sequence of events, an increased frequency and/or severity of apneic events and bradycardia and a reduced capacity to generate effective gasping, terminate apneas, restore eupnea and arouse, is compatible with the cardiorespiratory recordings made in infants who subsequently died of SIDS (Kelly et al., 1986).
Highlights.
Apnea and bradycardia can be an appropriate response to hypoxia in infants when a ventilatory response cannot be maintained
Autoresuscitation, inhibition of apnea, restoration of eupnea and arousal are essential to terminate apnea and bradycardia in infants
Infants at risk for SIDS may have heightened sensitivity of the reflex mechanisms precipitating apneas and bradycardias
The caudal serotonergic system supports all the processes necessary to terminate apnea and bradycardia: serotonin facilitates gasping and autoresuscitation, shortens or terminates reflex apneas, stimulates eupnea and drives arousal from the caudal brainstem rostrally to the cortex
Each of these processes is associated with activation of a different set of serotonergic receptors in specific regions of the brainstem targeted by serotonergic neurons in the caudal raphe.
Acknowledgments
Funding sources: This work was funded by grant 36379 from the NICHD.
1 Abbreviations go here
- AS
Active sleep
- GABA
gamma-amino butyric acid
- LCR
laryngeal chemoreflex
- NREM
non-rapid eye movement
- NTS
nucleus of the solitary tract
- REM
rapid eye movement
- SIDS
Sudden Infant Death Syndrome
- SUID
Sudden Unexpected Infant Death
- TRPV
transient receptor potential vanilloid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 2016. US Centers for Disease Control and Prevention, Infant deaths: linked birth/infant death records., http://wonder.cdc.gov/lbd.html.
- Abu-Shaweesh JM, 2007. Activation of central adenosine A2A receptors enhances superior laryngeal nerve stimulation-induced apnea in piglets via a GABAergic pathway. J. Appl. Physiol 103, 1205–1211. [DOI] [PubMed] [Google Scholar]
- Alvaro RE, Khalil M, Qurashi M, Al-Saif S, Al-Matary A, Chiu A, Minski J, Manfreda J, Kwiatkowski K, Cates D, Rigatto H, 2012. CO(2) inhalation as a treatment for apnea of prematurity: a randomized double-blind controlled trial. J. Pediatr 160, 252–257 e251. [DOI] [PubMed] [Google Scholar]
- Angell-James JE, Daly M.d.B., 1969. Cardiovascular responses in apnoeic asphyxia: role of arterial chemoreceptors and the modification of their effects by a pulmonary vagal inflation reflex. J. Physiol. (London) 201, 87–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angell-James JE, Daly M.d.B., 1975. Interactions between cardiac reflexes from the lungs and those elicited from carotid bodies and larynx in the dog. J. Physiol. (London) 254, 55P–56P. [PubMed] [Google Scholar]
- Asakura H, Ball KT, Power GG, 1990. Interdependence of arterial PO2 and O2 consumption in the fetal sheep. Journal of developmental physiology 13, 205–213. [PubMed] [Google Scholar]
- Bamford OS, Carroll JL, 1999. Dynamic ventilatory responses in rats: normal development and effects of prenatal nicotine exposure. Respir. Physiol 117, 29–40. [DOI] [PubMed] [Google Scholar]
- Bang SJ, Jensen P, Dymecki SM, Commons KG, 2012. Projections and interconnections of genetically defined serotonin neurons in mice. The European journal of neuroscience 35, 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Hales TG, Lummis SC, Peters JA, 2009. The 5-HT3 receptor--the relationship between structure and function. Neuropharmacology 56, 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger RJ, 1975. Bioenergetic functions of sleep and activity rhythms and their possible relevance to aging. Fed. Proc 34, 97–102. [PubMed] [Google Scholar]
- Berger RJ, Phillips NH, 1993. Sleep and energy conservation. NIPS 8, 276–281. [Google Scholar]
- Bissonnette JM, 2000. Mechanisms regulating hypoxic respiratory depression during fetal and postnatal life. American journal of physiology. Regulatory, integrative and comparative physiology 278, R1391–1400. [DOI] [PubMed] [Google Scholar]
- Blanco CE, Dawes GS, Hanson MA, McCooke HB, 1984. The response to hypoxia of arterial chemoreceptors in fetal sheep and new-born lambs. The Journal of physiology 351, 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank WF Jr., Kirshner HS, 1977. The kinetics of extracellular potassium changes during hypoxia and anoxia in the cat cerebral cortex. Brain research 123, 113–124. [DOI] [PubMed] [Google Scholar]
- Boggs DF, Bartlett D Jr., 1982. Chemical specificity of a laryngeal apneic reflex in puppies. J. Appl. Physiol 53, 455–462. [DOI] [PubMed] [Google Scholar]
- Boros SJ, Reynolds JW, 1976. Prolonged apnea of prematurity: Treatment with continuous airway distending pressure delivered by nasopharyngeal tube. Clin. Pediatr 15, 123–134. [DOI] [PubMed] [Google Scholar]
- Brust RD, Corcoran AE, Richerson GB, Nattie E, Dymecki SM, 2014. Functional and developmental identification of a molecular subtype of brain serotonergic neuron specialized to regulate breathing dynamics. Cell reports 9, 2152–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, Richerson GB, 2010. Central serotonin neurons are required for arousal to CO2. Proc. Natl. Acad. Sci. U S A 107, 16354–16359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, Smith HR, MacAskill A, Richerson GB, 2015. 5-HT2A receptor activation is necessary for CO2-induced arousal. J. Neurophysiol 114, 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau MA, Lamarche J, Foulon P, Dalle D, 1985. The ventilatory response to hypoxia in the newborn lamb after carotid body denervation. Respiration physiology 60, 109–119. [DOI] [PubMed] [Google Scholar]
- BuSha BF, Leiter JC, Curran A, Li A, Nattie EE, Darnall RA, 2001. Spontaneous arousals during quiet sleep in piglets: A visual and wavelet-based analysis. Sleep 24, 499–513. [DOI] [PubMed] [Google Scholar]
- Carlin RF, Moon RY, 2017. Risk Factors, Protective Factors, and Current Recommendations to Reduce Sudden Infant Death Syndrome: A Review. JAMA Pediatr 171, 175–180. [DOI] [PubMed] [Google Scholar]
- Carroll JL, Fitzgerald RS, 1993. Carotid chemoreceptor responses to hypoxia and hypercapnia in developing kittens. Adv. Exp. Med. Biol 337, 387–391. [DOI] [PubMed] [Google Scholar]
- Chen J, Magnusson J, Karsenty G, Cummings KJ, 2013. Time- and agedependent effects of serotonin on gasping and autoresuscitation in neonatal mice. J. Appl. Physiol 114, 1668–1676. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finaly BL, 2001. Translating developmental time across mammalian species. Neurosci. 105, 7–17. [DOI] [PubMed] [Google Scholar]
- Cross KW, Oppe TE, 1952. The effect of inhalation of high and low concentrations of oxygen on the respiration of the premature infant. The Journal of physiology 117, 38–55. [PMC free article] [PubMed] [Google Scholar]
- Cross KW, Warner P, 1951. The effect of inhalation of high and low oxygen concentrations on the respiration of the newborn infant. The Journal of physiology 114, 283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KJ, Commons KG, Hewitt JC, Daubenspeck JA, Li A, Kinney HC, Nattie EE, 2011. Failed heart rate recovery at a critical age in 5-HT-deficient mice exposed to episodic anoxia: implications for SIDS. J. Appl. Physiol 111, 825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KJ, Li A, Deneris ES, Nattie EE, 2010. Bradycardia in serotonin-deficient Pet-1−/− mice: influence of respiratory dysfunction and hyperthermia over the first 2 postnatal weeks. American journal of physiology. Regulatory, integrative and comparative physiology 298, R1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA, 2001. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem. Int 38, 107–125. [DOI] [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Lawson EE, 1991. Synaptic events in ventral respiratory neurones during apnoea induced by laryngeal nerve stimulation in neonatal piglet. J. Physiol 436, 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva GSF, Sabino JPJ, Rajani V, Alvares TS, Pagliardini S, Branco LGS, Funk GD, 2017. Excitatory Modulation of the preBotzinger Complex Inspiratory Rhythm Generating Network by Endogenous Hydrogen Sulfide. Frontiers in physiology 8, 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly MD, Angell-James JE, Elsner R, 1979. Role of carotid-body chemoreceptors and their reflex interactions in bradycardia and cardiac arrest. Lancet 1, 764–767. [DOI] [PubMed] [Google Scholar]
- Darnall RA, 2013. The carotid body and arousal in the fetus and neonate. Respir. Physiol. Neurobiol 185, 132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall RA, McWilliams S, Schneider RW, Tobia CM, 2010. Reversible blunting of arousal from sleep in response to intermittent hypoxia in the developing rat. J Appl Physiol (1985) 109, 1686–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall RA, Schneider RW, Tobia CM, Commons KG, 2016. Eliminating medullary 5-HT neurons delays arousal and decreases the respiratory response to repeated episodes of hypoxia in neonatal rat pups. J Appl Physiol 120, 514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall RA, Schneider RW, Tobia CM, Webster CA, Zemel BM, Cummings KJ, Kinney HC, Nattie EE, 2011. PET1 knockout mouse pups have impaired arousal in response to intermittent hypoxia: Implications for the Sudden Infant Death Syndrome (SIDS). Society for Neuroscience 286.12/SS8. [Google Scholar]
- Dauger S, Aizenfisz S, Renolleau S, Durand E, Vardon G, Gaultier C, Gallego J, 2001. Arousal response to hypoxia in newborn mice. Respiration physiology 128, 235–240. [DOI] [PubMed] [Google Scholar]
- Davis MR, Magnusson JL, Cummings KJ, 2019. Increased central cholinergic drive contributes to the apneas of serotonin-deficient rat pups during active sleep. J Appl Physiol (1985) 126, 1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra RR, Dutschmann M, Dick TE, 2016. Blockade of dorsolateral pontine 5HT1A receptors destabilizes the respiratory rhythm in C57BL6/J wild-type mice. Respiratory physiology & neurobiology 226, 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie EE, 2008. Focal CO2 dialysis in raphe obscurus does not stimulate ventilation but enhances the response to focal CO2 dialysis in the retrotrapezoid nucleus. J. Appl. Physiol 105, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly WT, Bartlett D Jr., Leiter JC, 2016. Serotonin in the solitary tract nucleus shortens the laryngeal chemoreflex in anesthetized neonatal rats. Exp. Physiol (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly WT, Bartlett D Jr., Leiter JC, 2017. Activation of serotonergic neurons in the medullary caudal raphe shortens the laryngeal chemoreflex in anaesthetized neonatal rats. Exp. Physiol 102, 1007–1018. [DOI] [PubMed] [Google Scholar]
- Dosumu-Johnson RT, Cocoran AE, Chang Y, Nattie E, Dymecki SM, 2018. Acute perturbation of Pet1-neuron activity in neonatal mice impairs cardiorespiratory homeostatic recovery. eLife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing SE, Lee JC, 1975. Laryngeal chemosensitivity: A possible mechanism of sudden infant death. Pediatrics 55, 640–649. [PubMed] [Google Scholar]
- Doyle MW, Bailey TW, Jin Y-H, Andresen MC, 2002. Vanilloid receptors presynaptically modulate cranial visceral afferent synaptic transmission in nucleus tractus solitarius. J. Neurosci 22, 8222–8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg F, Kinney HC, 2010. Brainstem serotonergic deficiency in sudden infant death syndrome. Jama 303, 430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Dick TE, 2012. Pontine mechanisms of respiratory control. Comprehensive Physiology 2, 2443–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE, 1991. Fos-like protein is induced in neurons of the medulla oblongata after stimulation of the carotid sinus nerve in awake and anesthetized rats. Brain research 567, 11–24. [DOI] [PubMed] [Google Scholar]
- Erikson JT, Sposato BC, 2009. Autoresuscitation responses to hypoxia-induced apnea are delayed in newborn 5-HT-deficient Pet-1 homozygous mice. J. Appl. Physiol (in press). [DOI] [PubMed] [Google Scholar]
- Fawley JA, Hofmann ME, Andresen MC, 2014. Cannabinoid 1 and transient receptor potential vanilloid 1 receptors discretely modulate evoked glutamate separately from spontaneous glutamate transmission. J. Neurosci 34, 8324–8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewell JE, Kondo CS, Dascalu V, Filyk SC, 1989. Influence of carotid denervation on the arousal and cardiopulmonary response to rapidly developing hypoxemia in lambs. Pediatr. Res 25, 473–477. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Taylor BJ, Kondo CS, Dascalu V, Filyk SC, 1990. Influence of carotid denervation on the arousal and cardiopulmonary responses to upper airway obstruction in lambs. Pediatric research 28, 374–378. [DOI] [PubMed] [Google Scholar]
- Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J, 2011. Reassessment of the structural basis of the ascending arousal system. The Journal of comparative neurology 519, 933–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauda EB, Carroll JL, Donnelly DF, 2009. Developmental maturation of chemosensitivity to hypoxia of peripheral arterial chemoreceptors--invited article. Advances in experimental medicine and biology 648, 243–255. [DOI] [PubMed] [Google Scholar]
- Guntheroth WG, Kawabori I, 1975. Hypoxic apnea and gasping. J. Clin. Invest 56, 1371–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Abbott SB, 2013. Chemoreception and asphyxia-induced arousal. Respiratory physiology & neurobiology 188, 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg H, Lehmann A, Sandberg M, Nystrom B, Jacobson I, Hamberger A, 1985. Ischemia-induced shift of inhibitory and excitatory amino acids from intra- to extracellular compartments. J Cereb Blood Flow Metab 5, 413–419. [DOI] [PubMed] [Google Scholar]
- Harper RM, Bandler R, 1998. Finding the failure mechanism in Sudden Infant Death Syndrome. Nature medicine 4, 157–158. [DOI] [PubMed] [Google Scholar]
- Hawkins VE, Hawryluk JM, Takakura AC, Tzingounis AV, Moreira TS, Mulkey DK, 2015. HCN channels contribute to serotonergic modulation of ventral surface chemosensitive neurons and respiratory activity. Journal of neurophysiology 113, 1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller HG, 1988. Sleep and hypometabolism. Can. J. Zool 66, 61–89. [Google Scholar]
- Hemelrijk SD, van Gulik TM, Heger M, 2019. Carotid chemoreceptor denervation does not impair hypoxia-induced thermal downregulation but vitiates recovery from a hypothermic and hypometabolic state in mice. Scientific reports 9, 5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes SM, Andresen MC, Aicher SA, 2016. Localization of TRPV1 and P2X3 in unmyelinated and myelinated vagal afferents in the rat. Journal of chemical neuroanatomy 72, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW, 1986. Defense strategies against hypoxia and hypothermia. Science 231, 234–241. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB, 2009. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J. Neurosci 29, 10341–10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner RL, 2012. Neural control of the upper airway: integrative physiological mechanisms and relevance for sleep disordered breathing. Comprehensive Physiology 2, 479–535. [DOI] [PubMed] [Google Scholar]
- Horner R.l., 2017. Respiratory Physiology, in: M.H., K., Roth T, Dement WC (Eds.), Principles and Practice of Sleep Medicine. Elsevier Saunders, St. Louis, pp. 155166.e155. [Google Scholar]
- Hunt CE, 1981. Abnormal hypercarbic and hypoxic sleep arousal responses in near-miss SIDS infants. Pediatr. Res 15, 1462–1464. [DOI] [PubMed] [Google Scholar]
- Hunt CE, Darnall RA, McEntire BL, Hyma BA, 2015. Assigning cause for sudden unexpected infant death. Forensic Sci. Med. Pathol 11, 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SD, Hawkins VE, Falquetto B, Ruskin DN, Masino SA, Moreira TS, Olsen ML, Mulkey DK, 2018. Adenosine Signaling through A1 Receptors Inhibits Chemosensitive Neurons in the Retrotrapezoid Nucleus. eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y-H, Bailey TW, Li B, Schild JH, Andresen MC, 2004. Puringergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J. Neurosci 24, 4709–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn A, Blum D, Rebuffat E, Sottiaux M, Levitt JG, Bochner A, Alexander M, Grosswasser J, Muller MF, 1988. Polysomnographic studies of infants who susbsequently died of Sudden Infant Death Syndrome. Pediatrics 82, 721–727. [PubMed] [Google Scholar]
- Kahn A, Groswasser J, Franco P, Scaillet S, Sawaguchi T, Kelmanson I, Bernanrd D, 2002. Sudden infant deaths: arousal as a survival mechanism. Sleep medicine 3 Suppl 2, S11–14. [DOI] [PubMed] [Google Scholar]
- Kahn A, Groswasser J, Rebuffat E, Scottiaux M, Blum D, Foerster M, Franco P, Bochner A, Alexander M, Bachy A, Richard P, Verghote M, Le Polain D, Wayenberg JL, 1992. Sleep and cardiorespiratory characteristics of infant victims of sudden death: a prospective case-control study. Sleep 15, 287–292. [DOI] [PubMed] [Google Scholar]
- Kaplan K, Echert AE, Massat B, Puissant MM, Palygin O, Geurts AM, Hodges MR, 2016. Chronic central serotonin depletion attenuates ventilation and body temperature in young but not adult Tph2 knockout rats. J Appl Physiol (1985) 120, 1070–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato I, Groswasser J, Franco P, Scaillet S, Kelmanson I, Togari H, Kahn A, 2001. Developmental characteristics of apnea in infants who succumb to sudden infant death syndrome. American journal of respiratory and critical care medicine 164, 1464–1469. [DOI] [PubMed] [Google Scholar]
- Kaur S, Pedersen NP, Yokota S, Hur EE, Fuller PM, Lazarus M, Chamberlin NL, Saper CB, 2013. Glutamatergic signaling from the parabrachial nucleus plays a critical role in hypercapnic arousal. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 7627–7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Saper CB, 2019. Neural Circuitry Underlying Waking Up to Hypercapnia. Front Neurosci 13, 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DH, Golub H, Carley D, Shannon DC, 1986. Pneumograms in infants who subsequently died of sudden infant death syndrome. J. Pediatr 109, 249–254. [DOI] [PubMed] [Google Scholar]
- Kholwadwala D, Donnelly DF, 1992. Maturation of carotid chemoreceptor sensitivity to hypoxia: in vitro studies in the newborn rat. J. Physiol. (Lond) 453, 461–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo MCK, Gottschalk A, Pack AI, 1991. Sleep-induced periodic breathing and apnea: a theoretical study. J. Appl. Phys 70, 2014–2024. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Haynes RL, 2019. The Serotonin Brainstem Hypothesis for the Sudden Infant Death Syndrome. J Neuropathol Exp Neurol 78, 765–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar I, Selstam U, Catterton WZ, Stahlman MT, Sundell HW, 1979. Laryngeal chemoreflex in newborn lambs: Respiratory and swallowing response to salts, acids, and sugars. Pediatr. Res 13, 1144–1149. [DOI] [PubMed] [Google Scholar]
- Kubin L, Davies RO, 1995. Central pathways of pulmonary and airway vagal afferents, in: Dempsey JA, Pack AI (Eds.), Regulation of Breathing. Marcel Dekker, New York, pp. 219–284. [Google Scholar]
- Lalley PM, 1994. The excitability and rhythm of medullary respiratory neurons in the cat are altered by the serotonin receptor agonist 5-methoxy-N,N, dimethyltryptamine. Brain research 648, 87–98. [DOI] [PubMed] [Google Scholar]
- Lalley PM, Benacka R, Bischoff AM, Richter DW, 1997. Nucleus raphe obscurus evokes 5-HT-1A receptor-mediated modulation of respiratory neurons. Brain Res. 747, 156–159. [DOI] [PubMed] [Google Scholar]
- Lalley PM, Bischoff AM, Richter DW, 1994. 5-HT-1A receptor-mediated modulation of medullary expiratory neurones in the cat. The Journal of physiology 476, 117–130. [PMC free article] [PubMed] [Google Scholar]
- Lanier B, Richardson MA, Cummings C, 1983. Effect of hypoxia on laryngeal reflex apnea - implications for sudden infant death. Otolaryngol. Head Neck Surg 91, 597. [DOI] [PubMed] [Google Scholar]
- Leiter JC, Böhm I, 2007. Mechanisms of pathogenesis in the Sudden Infant Death Syndrome (SIDS). Respir. Physiol. Neurobiol 159, 127–138. [DOI] [PubMed] [Google Scholar]
- Li A, Darnall RA, Dymecki S, Leiter JC, 2018. Animal Models: Illuminating the Pathogenesis of Sudden Infant Death Syndrome, in: Byard RW, Duncan J (Eds.), SIDS, Sudden Infant and Early Childhood Death: The Past, the Present and the Future. University of Adelaide Press. [PubMed] [Google Scholar]
- Mayer CA, Haxhiu MA, Martin RJ, Wilson CG, 2006. Adenosine A2A receptors mediate GABAergic inhibition of respiration in rats. J. Appl. Physiol 100, 91–97. [DOI] [PubMed] [Google Scholar]
- McCulloch K, Brouillette RT, Guzzetta AJ, Hunt CE, 1982. Arousal responses in near-miss sudden infant death syndrome and in normal infants. J. Pediatrics 101, 911–917. [DOI] [PubMed] [Google Scholar]
- McNamara F, Lijowska AS, Thach BT, 2002. Spontaneous arousal activity in infants during NREM and REM sleep. J. Physiol. (Lond) 538.1, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara F, Wulbrand H, Thach BT, 1998. Characteristics of the infant arousal response. J. Appl. Physiol 85, 2314–2321. [DOI] [PubMed] [Google Scholar]
- Melton JE, Neubauer JA, Edelman NH, 1990. GABA antagonism reverses hypoxic respiratory depression in the cat. J Appl Physiol (1985) 69, 1296–1301. [DOI] [PubMed] [Google Scholar]
- Meny RG, Carroll JL, Carbone MT, Kelly DH, 1994. Cardiorespiratory recordings from infants dying suddenly and unexpectedly at home. Pediatrics 93, 44–49. [PubMed] [Google Scholar]
- Miller RL, Stein MK, Loewy AD, 2011. Serotonergic inputs to FoxP2 neurons of the pre-locus coeruleus and parabrachial nuclei that project to the ventral tegmental area. Neuroscience 193, 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, 2016. Central neural control of thermoregulation and brown adipose tissue. Auton Neurosci 196, 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortola JP, 1996. Ventilatory responses to hypoxia in mammals, in: Haddad GG, Lister G (Eds.), Tissue Oxygen Deprivation: Developmental, Molecular and Integrated Function. Marcel Dekker, New York, pp. 43–477. [Google Scholar]
- Mortola JP, 1999. How newborn mammals cope with hypoxia. Respir. Physiol 116, 95–103. [DOI] [PubMed] [Google Scholar]
- Mortola JP, 2004. Implications of hypoxic hypometabolism during mammalian ontogenesis. Respiratory physiology & neurobiology 141, 345–356. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West GB, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG, 2007. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J. Neurosci 27, 14218–14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Smith CA, Rodman JR, Skatrud JB, Dempsey JA, 2003. Carotid body denervation eliminates apnea in response to transient hypocapnia. J Appl Physiol (1985) 94, 155–164. [DOI] [PubMed] [Google Scholar]
- Page M, Jeffery HE, 1998. Airway protection in sleeping infants in response to pharyngeal stimulation in the supine position. Pediatr. Res 44, 691–698. [DOI] [PubMed] [Google Scholar]
- Page M, Jeffery HE, 2000. The role of gastro-oesophageal reflux in the aetiology of SIDS. Early Hum. Dev 59, 127–149. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall RA, Chadwick AE, Krous HF, Kinney HC, 2006. Multiple serotonergic brainstem abnormalities in the sudden infant death syndrome. J.A.M.A 296, 2124–2132. [DOI] [PubMed] [Google Scholar]
- Pena F, Ramirez JM, 2002. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. The Journal of neuroscience : the official journal of the Society for Neuroscience 22, 11055–11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Ramirez JM, 2004. Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. The Journal of neuroscience : the official journal of the Society for Neuroscience 24, 7549–7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkett EA, Vaughan RL, 1982. Evidence for a laryngeal chemoreflex in some human preterm infants. Acta Paediatr. Scand 71, 969–972. [DOI] [PubMed] [Google Scholar]
- Phillipson EA, 1978. Control of breathing during sleep. Am. Rev. Respir. Dis 118, 909–939. [DOI] [PubMed] [Google Scholar]
- Poets CF, Meny RG, Chobanian MR, Bonofiglo RE, 1999. Gasping and other cardiorespiratory patterns during sudden infant death. Pediatr. Res 45, 350–354. [DOI] [PubMed] [Google Scholar]
- Poets CF, Samuels MP, Noyes JP, Hewertson J, Hartmann H, Holder A, Southall DP, 1993. Home event recordings of oxygenation, breathing movements, and heart rate and rhythm in infants with recurrent life-threatening events. The Journal of pediatrics 123, 693–701. [DOI] [PubMed] [Google Scholar]
- Pratt GD, Bowery NG, 1989. The 5-HT3 receptor ligand, [3H]BRL 43694, binds to presynaptic sites in the nucleus tractus solitarius of the rat. Neuropharmacology 28, 1367–1376. [DOI] [PubMed] [Google Scholar]
- Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC, 2009. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J. Neurosci 29, 3720–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovacki M, 1985. Role of adenosine in sleep in rats. Rev. Clin. Basic Pharmacol 5, 327–339. [PubMed] [Google Scholar]
- Rajani V, Zhang Y, Jalubula V, Rancic V, SheikhBahaei S, Zwicker JD, Pagliardini S, Dickson CT, Ballanyi K, Kasparov S, Gourine AV, Funk GD, 2018. Release of ATP by pre-Botzinger complex astrocytes contributes to the hypoxic ventilatory response via a Ca(2+) -dependent P2Y1 receptor mechanism. The Journal of physiology 596, 3245–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall BB, Paterson DS, Haas EA, Broadbelt KG, Duncan JR, Mena OJ, Krous HF, Trachtenberg FL, Kinney HC, 2013. Potential asphyxia and brainstem abnormalities in sudden and unexpected death in infants. Pediatrics 132, e1616–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers JE, Richter DW, Ballantyne D, Bainton CR, Klein JP, 1986. Reflex prolongation of stage I of expiration. Pflügers Arch. 407, 190–198. [DOI] [PubMed] [Google Scholar]
- Richter DW, Manzke T, Wilken B, Ponimaskin E, 2003. Serotonin receptors: guardians of stable breathing. Trends Mol Med 9, 542–548. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG, 2006. Afferent and efferent connections of the rat retrotrapezoid nucleus. The Journal of comparative neurology 499, 64–89. [DOI] [PubMed] [Google Scholar]
- Roy A, Farnham MMJ, Derakhshan F, Pilowsky PM, Wilson RJA, 2018. Acute intermittent hypoxia with concurrent hypercapnia evokes P2X and TRPV1 receptor-dependent sensory long-term facilitation in naive carotid bodies. The Journal of physiology 596, 3149–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechtman VL, Harper RM, Wilson AJ, Southall DP, 1992. Sleep state organization in normal infants and victims of the sudden infant death syndrome. Pediatrics 89, 865–870. [PubMed] [Google Scholar]
- Smith CA, Chenuel BJ, Henderson KS, Dempsey JA, 2007. The apneic threshold during non-REM sleep in dogs: sensitivity of carotid body vs. central chemoreceptors. J Appl Physiol (1985) 103, 578–586. [DOI] [PubMed] [Google Scholar]
- Solomon IC, 2004. Ionotropic excitatory amino acid receptors in pre-Botzinger complex play a modulatory role in hypoxia-induced gasping in vivo. J Appl Physiol (1985) 96, 1643–1650. [DOI] [PubMed] [Google Scholar]
- Sridhar R, Thach BT, Kelly DH, Henslee JA, 2003. Characterization of successful and failed autoresuscitation in human infants. Pediatr. Pulmonol 36, 113–122. [DOI] [PubMed] [Google Scholar]
- St. Hilaire M, Nsegbe E, Gagnon-Gervais K, Samson N, Moreau-Bussière F, Fortier P-H, Praud J-P, 2005. Laryngeal chemoreflexes induced by acid, water and saline in non-sedated newborn lambs during quiet sleep. J. Appl. Physiol 102, 1429–1438. [DOI] [PubMed] [Google Scholar]
- St. John WM, Leiter JC, 2008. Maintenance of gasping and restoration of eupnea after hypoxia is impaired following blockers of α−1 adrenergic receptors and serotonin 5HT2 receptors J. Appl. Physiol 104, 665–673. [DOI] [PubMed] [Google Scholar]
- St. John WM, Li A, Leiter JC, 2009. Genesis of gasping is independent of levels of serotonin in the PET-1 knockout mouse. J. Appl. Physiol. (in press) doi: 10.1152/japplphysiol.91461.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John WM, Rybak I, Paton JFR, 2002. Switch from eupnoea to fictive gasping following blockade of glycine transmission and potassium channels. Amer. J. Physiol [DOI] [PubMed] [Google Scholar]
- Strecker RE, Morairty S, Thakkar MM, Porkka-Heiskanen T, Basheer R, Dauphin LJ, Rainnie DG, Portas CM, Greene RW, McCarley RW, 2000. Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behavioural brain research 115, 183–204. [DOI] [PubMed] [Google Scholar]
- Thach BT, 1997. Reflux associated apnea in infants: Evidence for a laryngeal chemoreflex. Am. J. Med 103(5A), 120S–124S. [DOI] [PubMed] [Google Scholar]
- Thach BT, 2001. Maturation and transformation of reflexes that protect the laryngeal airway from liquid aspiration from fetal to adult life. Am. J. Med 111, 69S–77S. [DOI] [PubMed] [Google Scholar]
- Tryba AK, Peña F, Ramirez J-M, 2006. Gasping activity in vitro: A rhythm dependent on 5-HT2A receptors. J. Neurosci 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waeber C, Hoyer D, Palacios JM, 1989. 5-hydroxytryptamine3 receptors in the human brain: autoradiographic visualization using [3H]ICS 205–930. Neuroscience 31, 393–400. [DOI] [PubMed] [Google Scholar]
- Weissheimer KV, Machado BH, 2007. Inhibitory modulation of chemoreflex bradycardia by stimulation of the nucleus raphe obscurus is mediated by 5-HT3 receptors in the NTS of awake rats. Auton. Neurosci 132, 27–36. [DOI] [PubMed] [Google Scholar]
- Wilson CG, Martin RJ, Jaber M, Abu-Shaweesh JM, Jafri A, Haxhiu MA, Zaidi S, 2004. Adenosine A2A receptors interact with GABAergic pathways to modulate respiration in neonatal piglets. Respir. Physiol. Neurobiol 141, 201–211. [DOI] [PubMed] [Google Scholar]
- Wolf S, 1966. Sudden death and the oxygen-conserving reflex. American heart journal 71, 840–841. [DOI] [PubMed] [Google Scholar]
- Wood JD, Watson WJ, Ducker AJ, 1968. The effect of hypoxia on brain gammaaminobutyric acid levels. Journal of neurochemistry 15, 603–608. [DOI] [PubMed] [Google Scholar]
- Wu Y, Proch KL, Teran FA, Lechtenberg RJ, Kothari H, Richerson GB, 2019. Chemosensitivity of Phox2b-expressing retrotrapezoid neurons is mediated in part by input from 5-HT neurons. The Journal of physiology 597, 2741–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Bartlett D Jr., Leiter JC, 2011. TRPV1 channels in the nucleus of the solitary tract mediate thermal prolongation of the LCR in decerebrate piglets. Respir. Physiol. Neurobiol 176, 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Bartlett D Jr., Leiter JC, 2016. Interleukin-1beta and interleukin-6 enhance thermal prolongation fo the LCR in decerebrate piglets. Resp. Physiol. Neurobiol in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Crane-Godreau MA, Leiter JC, Bartlett D Jr., 2009. Gestational cigarette smoke exposure and hyperthermic enhancement of laryngeal chemoreflex in rat pups. Respir. Physiol. Neurobiol 165, 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Leiter JC, Bartlett D Jr., 2010. Gestational nicotine exposure exaggerates hyperthermic enhancement of laryngeal chemoreflex in rat pups. Respir. Physiol. Neurobiol 171, 17–21. [DOI] [PubMed] [Google Scholar]
- Yang HT, Cummings KJ, 2013. Brain stem serotonin protects blood pressure in neonatal rats exposed to episodic anoxia. J. Appl. Physiol 115, 1733–1741. [DOI] [PubMed] [Google Scholar]
- Young JO, Geurts A, Hodges MR, Cummings KJ, 2017. Active sleep unmasks apnea and delayed arousal in infant rat pups lacking central serotonin. J Appl Physiol (1985) 123, 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi SIA, Jafri A, Martin RJ, Haxhui MA, 2006. Adenosine A2A receptors are expressed by GABAergic neurons of medulla oblongata in developing rat. Brain Res. 1071, 42–53. [DOI] [PubMed] [Google Scholar]
- Zhao J, Gonzalez F, Mu D, 2011. Apnea of prematurity: from cause to treatment. Eur. J. Pediatr 170, 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]