Abstract
Alzheimer's disease (AD) is a neurodegenerative disorder characterized by learning and memory impairments. Recent studies have suggested that AD can be induced by multiple factors, such as cholinergic system dysfunction and β-amyloid (Aβ) neurotoxicity. It was reported that 6-bromo-N-propionyltryptamine could treat neurological diseases, including AD. In the present study, 6-bromotryptamine A, a derivative of 6-bromo-N-propionyltryptamine, was synthesized by the condensation of 2-(6-bromo-1H-indol-3-yl)ethan-1-amine and 2-(4-bromophenyl)acetic acid, and was used as a potential anti-AD molecule. Furthermore, scopolamine can induce impairments of learning and memory, and was widely used to establish AD animal models. The results demonstrated that 6-bromotryptamine A significantly prevented scopolamine-induced short-term cognitive impairments, as revealed by various behavioral tests in mice. Furthermore, an acetylcholinesterase (AChE) activity assay revealed that 6-bromotryptamine A directly inhibited AChE activity. Notably, it was observed that 6-bromotryptamine A blocked the formation of Aβ oligomer, as evaluated by the dot blot assay. All these results suggested that 6-bromotryptamine A may be used to prevent impairments in short-term learning and memory ability possibly via the inhibition of AChE and the blockade of Aβ oligomer formation.
Keywords: Alzheimer's disease, 6-bromotryptamine A, β-amyloid, acetylcholinesterase, scopolamine, one-molecule multi-target strategy
Introduction
As a multifaceted neurodegenerative disorder, Alzheimer's disease (AD) greatly impacts the health of the affected elderly population (1). However, the pathophysiology of AD has not been completely elucidated to date. Cholinergic deficiency and β-amyloid (Aβ) deposition are widely accepted as important pathological events in the progress of AD (2). Previous studies have suggested that overactivation of acetylcholinesterase (AChE) may be responsible for the dysfunction of the cholinergic system, leading to cognitive impairments in AD (3,4). In addition, Aβ oligomer-induced neurotoxicity is considered as the main cause of neuronal loss in AD (5). Due to the complexity of the pathology of this disease, a one-molecule multi-target strategy may be useful for identifying novel anti-AD drugs (6).
Scopolamine, a muscarinic cholinergic antagonist, can induce short-term learning and memory impairments in mice (7), while scopolamine-induced amnesia is associated with deficits in cholinergic neurotransmission, which is also observed in patients with AD (8). Therefore, a scopolamine-induced mouse model can be used for the evaluation of cognition-enhancing drugs for treating AD (7,9).
In our previous studies, 6-bromo-N-propionyltryptamine from marine bacterium Pseudoalteromonas rubra QD1-2 was synthesized (10), and it was observed that this compound acts on 5-hydroxytryptamine (5-HT) receptors in the central nervous system and may be used for treating neurological diseases, including AD (11). In the present study, another compound was further synthesized, namely 6-bromotryptamine A, which is a molecule with a similar structure to that of 6-bromo-N-propionyltryptamine (11). Next, the effects of 6-bromotryptamine A on scopolamine-induced short-term impairments in recognition and spatial cognition was evaluated in mice. Furthermore, the study examined whether 6-bromotryptamine A is able to directly inhibit AChE activity and reduce the formation of Aβ oligomer.
Materials and methods
Synthesis of 6-bromotryptamine A
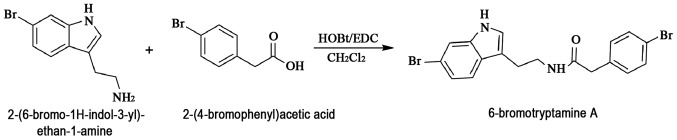
The molecule 6-bromotryptamine A was synthesized through the condensation of 2-(6-bromo-1H-indol-3-yl)ethan-1-amine and 2-(4-bromophenyl)acetic acid, as shown in Fig. 1. For this reaction, hydroxybenzotriazole and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride in dichloromethane solution were used at room temperature for 12 h. The purity of 6-bromotryptamine A was greater than 99%.
Figure 1.
Chemical synthesis of 6-bromotryptamine A.
Animals and drug treatment
Male ICR mice weighing 25–30 g were purchased from Zhejiang Academy of Medical Sciences (Hangzhou, China). The animals were maintained on a 12-h light/dark cycle under controlled temperature (22±2°C) and humidity (50±10%), and given standard diet and water access. The animals were allowed to acclimatize for 3 days before the experiments. All animal experiments followed the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (publication no. 80–23, revised 1996), and were approved by the Animal Ethics and Welfare Committee of Ningbo University (Ningbo, China; approval no. SYXK-2008-0110).
Prior to administration, 6-bromotryptamine A was dissolved in sterile saline containing 0.1% DMSO, 0.5% Tween-20 and 1% ethanol, while scopolamine was dissolved in sterile saline. Intraperitoneal (i.p.) injection of scopolamine at the dose of 1–5 mg/kg has previously been reported for the establishment of an amnesia model and for evaluation of cognition-enhancing drugs (12,13). Therefore, in the present study, mice were intraperitoneally injected with 3 mg/kg scopolamine to establish an AD animal model. Briefly, the mice were randomly distributed into six groups of 8 animals each, and treated accordingly, as follows: Control, 3 mg/kg scopolamine, and 3 mg/kg scopolamine plus low (0.5 mg/kg), medium (1.5 mg/kg) or high (5.0 mg/kg) dose of 6-bromotryptamine A. Administration of 6-bromotryptamine A was conducted by i.p. injection at the same time as scopolamine. All drugs were administered 30 min prior to behavioral tests once a day for 10 successive days. The open-field test was performed on the first day of the experiment, while the novel object recognition (NOR) test was conducted for the following three days. Subsequently, the Morris water maze task was performed on day 5–10. After behavioral tests, the mice were sacrificed for biochemical study. All the animals were given the last injection of drugs 30 min prior to sacrifice.
Open-field test
Open-field test was used to analyze the activities of exploration and locomotion (14). In the present study, open-field test was performed according to the protocols described previously, with certain modifications (15). Briefly, the animals were placed in the left rear quadrant of a 50×50×39 cm open field with white plywood walls and a brown floor divided into four identical squares of equal dimensions (25×25 cm). The mice were placed in turns in the middle of the box and allowed to explore it for 5 min. Stopwatches and hand-operated counters were used to score the number of line crossing and the number of rearings (defined as the number of times the animal stood on its hind legs), which were used as indicators of the locomotor and exploratory activities, respectively. The researcher performing the counting was blinded to the drug status of the animals. To avoid perturbation of the animals due to urine and feces, the apparatus was cleaned with 10% ethanol solution and a dry cloth following each test.
NOR test
The NOR test is typically used to evaluate the object recognition abilities of rodents (16). In the current study, the NOR test was performed according to a previously described protocol, with modifications (17). Briefly, an open-field arena (30×30×30 cm) was built with polyvinyl chloride plastic, plywood and transparent acrylic. The task was composed of three sessions, including the habituation, familiarization and test phases, which were performed over a period of three consecutive days. On the first day of the experiment, the animals were habituated to the experimental arena by allowing them to freely explore the arena for 5 min. On the second day, the animals were allowed to explore two identical objects for 5 min. On the third day, one of the objects was changed to a novel one with a different shape and color, and the animals were allowed to explore for 5 min. The field was cleansed with 10% ethanol solution and dried following occupancy by each mouse.
Exploration was defined as sniffing or touching the objects with the nose and/or forepaws at a distance of <2 cm. Sitting on or turning around the objects closely was not considered as exploratory behavior. The exploratory track was manually recorded using a video camera positioned over the arena by an observer blinded to the testing conditions. The total exploration time was defined as the amount of time spent exploring the two objects. The object recognition ability of mice was expressed as the ratio of the time spent exploring either of the two objects (familiarization phase) or the novel object (test phase) over the total exploration time.
Morris water maze task
The Morris water maze task was used to measure the spatial learning and memory of animals (18), and performed as previously described with minor modification (19). The water maze apparatus consisted of a circular pool with a diameter of 110 cm, which was filled with water at 23±2°C to cover a platform. The platform was always laid in the center of the northeast quadrant, except on the last day of the experiment. Each mouse's swimming was observed by a video camera linked to a computer-based image analyzer. The learning performance was assessed for four consecutive days, beginning on day 5 after the first injection of scopolamine (training trials). Each mouse was trained to find the platform through four trials per day. In each trial, the time required to escape onto the hidden platform was recorded. On day 10, a probe trial was arranged by removing the platform and allowing each mouse to swim for 90 sec in order to find it. The swimming time in each of the four quadrants of the pool was calculated. The tendency of mice to swim in the target area indicated that they had acquired and remembered the spatial task.
Measurement of AChE activity
A colorimetric method, which was adapted to 96-well-plates with a final volume of 200 µl, was used for the detection of AChE activity (20). In brief, following anesthesia by intraperitoneal injection of sodium pentobarbital (50 mg/kg), mice were decapitated and brains were immediately collected to examine the AChE activity. The brain was weighted, and then a 10X volume of lysis buffer was added (containing 1 mM EGTA, 10 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM EDTA and 0.5% Triton X-100). Following vortexing on ice for 15 min, tissue homogenization was achieved. The supernatant was then obtained by centrifugation for 15 min at 956 × g at 4°C. The assay medium included 5% supernatant as mentioned above, 10 mM DTNB, 1 mM acetylthiolcholine iodide and 0.1 M Na2HPO4 (pH 7.5). The assay medium was incubated with 0.1 mM ethopropazine hydrochloride for 5 min to inhibit the butyrylcholinesterase activity. Subsequently, 6-bromotryptamine A was mixed with assay medium and pre-incubated at 37°C for 15 min, and the AChE activity was then determined by measuring the absorbance at 412 nm. The half maximal inhibitory concentration (IC50) of 6-bromotryptamine A on AChE inhibition was automatically calculated using GraphPad Prism (version 5.0; GraphPad Software. Inc).
Molecular docking analysis
Molecular docking analyses were conducted with the SYBYL 2.0 (Tripos Inc.) software and associated programs. The three-dimensional (3D) crystal structure of AChE was obtained from the Protein Data Bank (PDB cod: 1EVE) (21). The three-dimensional structure of 6-bromotryptamine A was constructed using the geometric parameters of SYBYL and then optimized by the Powell method (7). The Surflex-Dock program, which uses an empirically-derived scoring function based on the binding affinities of protein-ligand complexes, was then used to perform docking analysis. As a flexible docking method, Surflex-Dock has been proven to be efficient in analyzing a variety of receptors (22). The active site of AChE was further defined relative to the coordinates of donepezil. During the simulations, the rotatable bonds of the ligands were defined, whereas the receptor was kept rigid.
Preparation of Aβ1–42 oligomer
Soluble Aβ1–42 oligomer was obtained as previously described (23). Briefly, Aβ1–42 (GL Biochem) was added in hexafluoroisopropanol (HFIP) to form the Aβ1–42 monomer, which was further spin-vacuumed in 10% HFIP solution. HFIP was then evaporated to obtain the Aβ1–42 solution. Next, the Aβ1–42 solution, with or without 6-bromotryptamine A, was incubated for 2 days at 25°C under stirring and centrifuged at 14,000 × g for 15 min at 4°C. The supernatant, which contained mainly soluble Aβ1–42 oligomer, was subsequently collected and quantified by a BCA assay (Thermo Fisher Scientific, Inc.).
Dot blot analysis
A nitrocellulose membrane was divided into equal grids, and a 2-µl sample (Aβ1–42 oligomer treated with or without 6-bromotryptamine A as previously described) was dotted onto the membrane and then air-dried. The membrane was blocked in a Tris-buffered saline/Tween-20 (TBST) solution (containing 50 mM Tris, 150 mM NaCl and 0.1% Tween-20) with 10% milk at 25°C overnight. Subsequently, the membrane was incubated with anti-oligomer antibody A11 (1:1,000; cat. no. AHB0052, Thermo Fisher Scientific, Inc.) or anti-Aβ1–17 antibody 6E10 (1:1,000; cat. no. MAB1560, Sigma-Aldrich, Merck KGaA) at 25°C for 1 h with gentle shaking. Following three washes with TBST, the membrane was incubated with secondary antibodies (cat. no. 14708, Cell Signaling Technology) at 25°C for 1 h and then developed with an enhanced chemiluminescence plus kit (24). The optical density of each band was further quantified by using ImageJ (1.50i; National Institutes of Health).
Statistical analysis
The data are expressed as the mean ± standard error of the mean. Statistically significant differences were determined by one-way analysis of variance (ANOVA) followed by Tukey's or Dunnett's test for post hoc multiple comparison, with the exception of mean escape latency, which was analyzed using two-way repeated-measures ANOVA followed by the least significant difference post hoc test. Differences were considered as statistically significant at P<0.05.
Results
6-bromotryptamine A does not affect the locomotor activity of mice in the open-field test
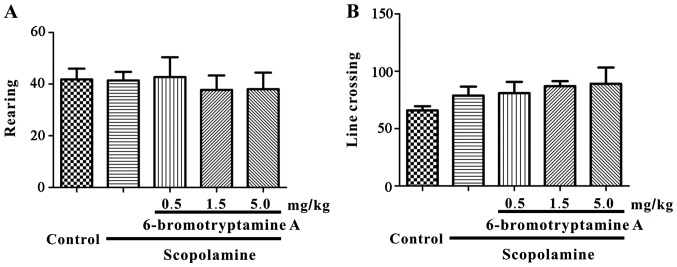
The protocol of the animal study and the tests performed are displayed in detail in Fig. 2. The mice were treated with drugs 30 min prior to the daily testing. In the open-field test, the number of line crossings and rearings were recorded for 5 min. As demonstrated in Fig. 3, none of the experimental group mice exhibited a significant change in the number of line crossings or rearings following treatment [for line crossing, one-way ANOVA, F(4, 35)=0.960, P=0.4418; for rearing, F(4, 35)=0.167, P=0.9539; detailed data are provided in Table SI]. In this study, F-test was used to determine whether group means are equal, and the F-value was calculated as the variability between groups divided by the variability within group. These results suggested that 6-bromotryptamine A treatment did not alter the motor functions of mice.
Figure 2.
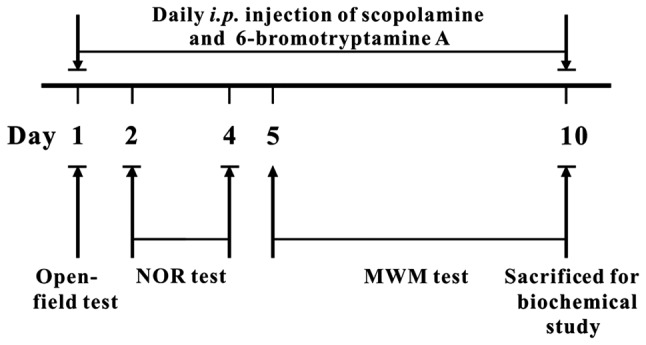
Experimental protocol of animal tests. NOR, novel object recognition; MWM, Morris water maze; i.p., intraperitoneal.
Figure 3.
Locomotor activity was not significantly affected by 6-bromotryptamine A treatment in mice. The numbers of (A) line crossings and (B) rearings are shown. Data are expressed as the mean ± standard error of the mean (n=8).
6-bromotryptamine A prevents scopolamine-induced short-term cognitive impairments
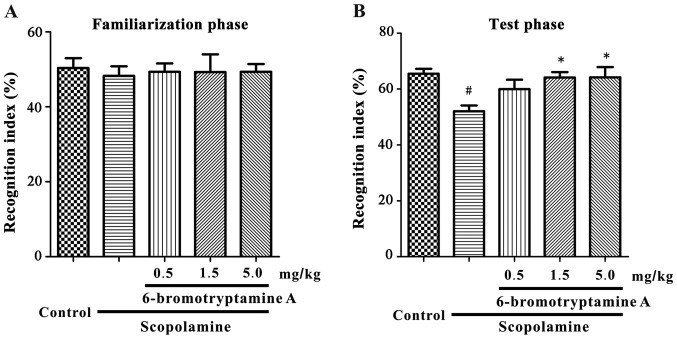
On day 1 of NOR test, mice were accustomed to the experimental arena without any behaviorally-relevant stimulus. Following this habituation phase, the familiarization phase of the test was performed, and the exploration time of two identical objects was recorded. As shown in Fig. 4A, in the familiarization phase, all groups exhibited similar recognition indexes for identical objects [F(4, 35)=0.061, P=0.9927; detailed data are provided in Table SII]. On the following day, the test phase was conducted. As shown in Fig. 4B, the recognition index for the novel object was significantly altered in the treated mice compared with the control mice [one-way ANOVA, F(4, 35)=4.058, P=0.0083; detailed data are provided in Table SII]. The recognition index was significantly reduced in the scopolamine-treated mice compared with that in the control mice (Tukey's test, P<0.05), whereas 6-bromotryptamine A treatment (1.5 and 5.0 mg/kg) significantly prevented the scopolamine-induced decrease in this index (Tukey's test, P<0.05; Fig. 4B).
Figure 4.
Prevention of scopolamine-induced recognition dysfunction by 6-bromotryptamine A was assessed with the novel object recognition test. (A) The recognition index of the two objects during the familiarization phase was similar in all groups. (B) The recognition index during the test phase is demonstrated. 6-bromotryptamine A significantly prevented the scopolamine-induced decrease in the recognition index. Data are presented as the mean ± standard error of the mean (n=8). #P<0.05 vs. control group; *P<0.05 vs. scopolamine-treated group (one-way analysis of variance and Tukey's test).
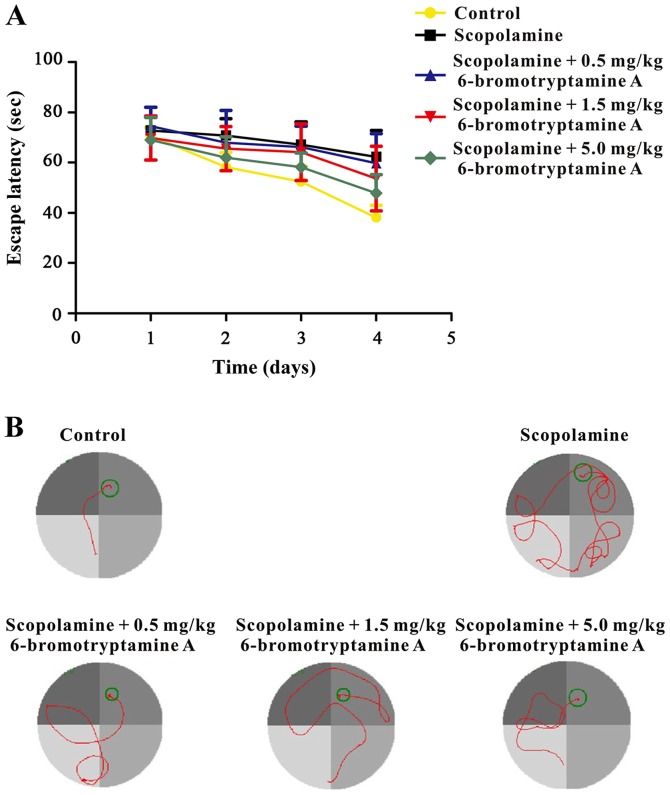
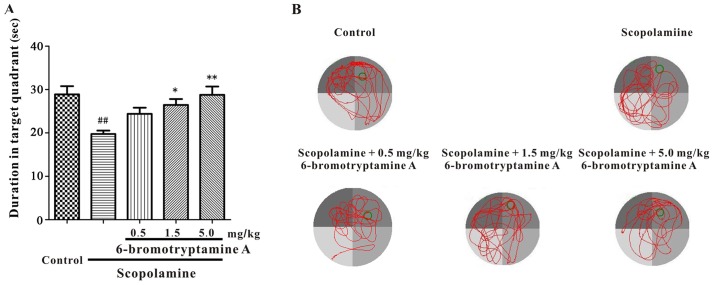
Furthermore, a Morris water maze test was conducted to examine whether 6-bromotryptamine A was able to prevent scopolamine-induced spatial cognitive impairments. The performance of mice in all groups improved throughout the training session, as indicated by the shortened escape latency (Fig. 5; detailed data are provided in Table SIII). However, during the probe trial, the time spent in the target quadrant was significantly different among all groups, as shown in Fig. 6 [one-way ANOVA, F(4, 35)=6.102, P=0.008; detailed data are provided in Table SIII]. Scopolamine treatment markedly reduced the time mice spent in the target quadrant, as compared with the control group. By contrast, treatment with 6-bromotryptamine A at a dose of 1.5 and 5 mg/kg significantly increased the swimming time of mice in the target quadrant compared with that in the scopolamine group (Tukey's test, P<0.05; Fig. 6).
Figure 5.
6-bromotryptamine A prevents scopolamine-induced spatial learning deficits during the training session of the Morris water maze test. (A) Mean latencies to escape from the water onto the hidden platform in training trials. Each mouse was subjected to four trials per day for four consecutive days. Data are expressed as the mean ± standard error of the mean (n=8). (B) Representative swimming-tracking paths of different groups during the training session.
Figure 6.
6-bromotryptamine A prevents scopolamine-induced spatial memory deficits in the probe trial of the Morris water maze test. (A) Swimming time in the target quadrant during the probe trial. (B) Representative swimming-tracking paths of different groups during the probe trial. Data are expressed as the mean ± standard error of the mean (n=8). ##P<0.01 vs. control group; *P<0.05 and **P<0.01 vs. scopolamine-treated group (analysis of variance and Tukey's test).
6-bromotryptamine A directly inhibits AChE activity
AChE inhibitors have been reported to prevent learning and memory impairments caused by scopolamine (25). Therefore, it was speculated that 6-bromotryptamine A may also act on AChE. In the current study, an AChE activity assay was conducted to examine this. It was observed that 6-bromotryptamine A dose-dependently inhibited the activity of AChE in the test-tube assay, and 6-bromotryptamine A at a dose of 50 µM reduced the AChE activity to ~45% of the control group without treatment of 6-bromotryptamine A (Table I). The IC50 of 6-bromotryptamine A on the inhibition of AChE was predicted to be 73.73 µM, as calculated using GraphPad Prism based on the results shown in Table I.
Table I.
Inhibitory effect of 6-bromotryptamine A on AChE activity.
| 6-bromotryptamine A (µM) | Inhibition of AChE (% of control) |
|---|---|
| 0.1 | 3.97±0.94 |
| 0.3 | 5.98±0.81 |
| 1 | 7.19±1.15 |
| 3 | 11.58±1.34 |
| 10 | 29.32±0.31 |
| 25 | 33.42±1.68 |
| 50 | 44.97±0.46 |
AChE, acetylcholinesterase.
Interaction between 6-bromotryptamine A and AChE
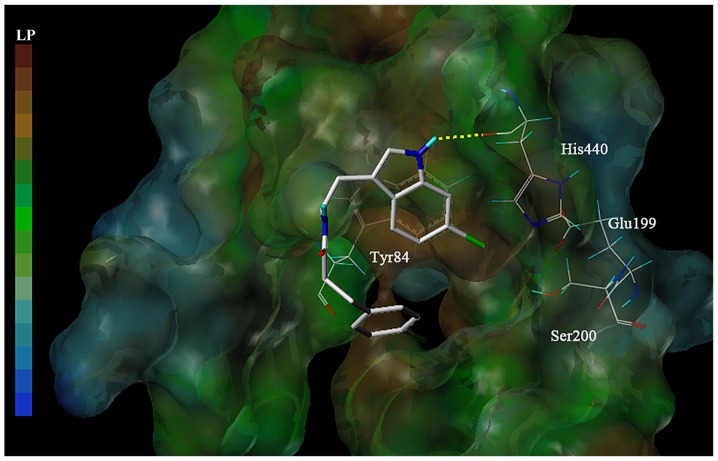
Molecular docking analysis was subsequently performed to investigate the interaction between 6-bromotryptamine A and AChE. The docking model suggested that 6-bromotryptamine A may form a hydrogen bond with His440 residue at the catalytic active site (CAS) within AChE (Fig. 7). Furthermore, the benzene ring of 6-bromotryptamine A may extend to the peripheral anionic site (PAS) of AChE.
Figure 7.
Binding of the low energy conformation model of 6-bromotryptamine A to AChE. 6-bromotryptamine A is depicted as a stick model, showing carbon (white), oxygen (red), nitrogen (dark blue), bromine (green), hydrogen (light blue), hydrogen bond (dotted yellow line). LP, lipophilic potential.
6-bromotryptamine A inhibits Aβ1–42 oligomer formation
A dot blot assay was further performed to evaluate the effects of 6-bromotryptamine A on the formation of Aβ oligomer. In the control group, Aβ1–42 monomer formed Aβ1–42 oligomer after 2 days of incubation under stirring. Notably, co-incubation with 6-bromotryptamine A at doses of 3 and 10 µM significantly reduced the amount of the Aβ1–42 oligomer compared with the control condition (Tukey's test, P<0.05; Fig. 8).
Figure 8.
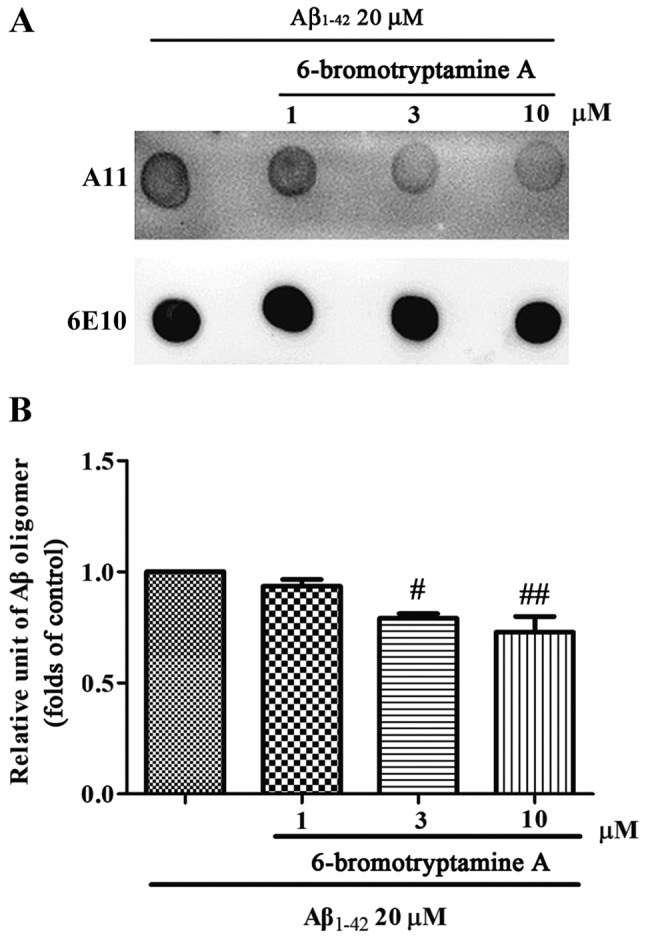
6-bromotryptamine A reduces Aβ1–42 oligomer formation in a dose-dependent manner. The Aβ1–42 monomer was co-incubated with 6-bromotryptamine A at the indicated doses for 2 days. The solution was centrifuged, and the supernatants were then analyzed. (A) Dot blot analysis was performed with A11 (an anti-oligomer antibody) and 6E10 (an anti-Aβ antibody). (B) The bands from three independent experiments were quantified via densitometry and are represented graphically. Data are presented as the mean ± standard error of the mean. #P<0.05 and ##P<0.01, vs. control group (analysis of variance and Tukey's test).
Discussion
In the present study, the data demonstrated that 6-bromotryptamine A treatment prevented scopolamine-induced short-term learning and memory impairments in vivo, suggesting that this compound may serve as an anti-AD agent (26). In addition, it was observed that 6-bromotryptamine A inhibited AChE, possibly by acting on both the CAS and PAS of AChE. However, compared with the clinically used AChE inhibitors, such as donepezil (IC50 on AChE activity, 12.3 nM) and galantamine (IC50 on AChE activity, 410 nM), the AChE inhibition activity of 6-bromotryptamine A was not as potent (27).
Several studies have reported that AChE is involved not only in neurotransmission, but also in the regulation of cell proliferation, differentiation and apoptosis (28), while overexpression of AChE was observed in numerous types of cancer (29,30). Notably, a number of AChE inhibitors are currently used or are under investigation for their potential application in cancer treatment. For instance, donepezil has been reported to induce apoptosis of HL-60 human promyelocytic leukemia cells (31). Galantamine has also been proven to produce anti-proliferation effects against 3T3 cells (28). Similarly, certain approved chemotherapeutics have been demonstrated to inhibit AChE at their efficient anti-cancer concentrations. Irinotecan, a chemotherapeutic used for colon and ovarian cancer therapy, inhibited AChE with an IC50 value of 0.97 µM (32). Furthermore, cyclophosphamide monohydrate, an alkylating agent used for the treatment of lymphoma and leukemia, inhibited AChE activity with an IC50 of 511 µM (33). Therefore, in the present study, it is speculated that 6-bromotrypamine A, a novel AChE inhibitor, may be used not only in the treatment of AD, but also in the treatment of cancer.
The moderate AChE inhibitory activity may not fully explain the cognitive-enhancing effects of 6-bromotryptamine A. Cheng et al (34) have demonstrated that (−)-meptazinol-indole amine hybrids, which have structures similar to 6-bromotryptamine A, are potential anti-AD candidates with dual inhibitory potency against AChE and Aβ aggregation. Their results further indicated that the indole group of chemicals may lead to the deposition of Aβ aggregates. In the present study, it was speculated that 6-bromotryptamine A may also act on Aβ aggregation due to its similar pharmacophore. Indeed, the results of dot blot analysis further suggested that 6-bromotryptamine A was able to directly inhibit Aβ oligomerization, indicating that the interactions between 6-bromotryptamine A and Aβ may decrease the interaction between Aβ molecules and therefore prevent the formation of Aβ aggregates. Previous studies have reported that several Aβ oligomer inhibitors, such as epigallocatechin gallate and brazilin, interact with Aβ by hydrogen bonds and π-π interactions (35,36). The compound 6-bromotryptamine A has a benzene ring and indole group to form π-π interactions, and active atoms to form hydrogen bonds. Thus, it is speculated that 6-bromotryptamine A may also interact with Aβ through hydrogen bonds and π-π interactions, and consequently inhibit the intermolecular interactions among Aβ oligomers.
To date, there are no effective drugs available for AD treatment (37), and the current therapeutic approach of ‘one molecule-one target’ strategy for AD fails due to the complexity of AD (38). Furthermore, the pharmaceutical combination of single-target drugs has certain challenges, such as different degrees of bioavailability and metabolisms of various drugs (39). Therefore, an alternative one-molecule multi-target strategy may be suitable for treating AD. The present study has provided evidence that 6-bromotryptamine A may be such a multi-target anti-AD molecule.
Although the current study demonstrated that 6-bromotryptamine A was able to prevent learning and memory impairments in mice, the possible applications of this compound in humans cannot be clearly determined based on these preliminary results. The main limitation of the present study is the lack of examination of the toxicity, absorption, distribution, metabolism and excretion of 6-bromotryptamine A, which would impact its application in humans. Although i.p. injection of 5.0 mg/kg 6-bromotryptamine A + scopolamine did not significantly alter the motor functions of mice, it cannot be concluded that 6-bromotryptamine A at this concentration is safe for animals. Prior to the use of this compound in clinical trials, the acute and chronic in vivo toxicity should be first evaluated by administering 6-bromotryptamine A at high concentrations. In addition, the pharmacokinetics and bioavailability of 6-bromotryptamine A should be investigated in various animal models.
The quality of the finding reported in present study may also be enhanced by performing in vivo experiments to verify whether 6-bromotryptamine A reduces Aβ oligomer formation. During the AD process, the formation of Aβ oligomer is relatively slow and cannot be observed in the normal neurotoxin-induced AD animal models, such as the model established by i.p. scopolamine injection or intracerebroventricular Aβ injection of rodents. Typically, in vivo formation of Aβ oligomer can only be tested in AD transgenic mice, including APP/PS1 and APP/PS1/tau mice. In such transgenic mice, drugs should be used for a long period (for example, 3–6 months) and the quantity of Aβ oligomer can only be measured in older animals (6-9 month of age). However, such in vivo experiments were not conducted in the present study due to the low supply of 6-bromotryptamine A available.
Besides the cholinergic system dysfunction and Aβ neurotoxicity, numerous other factors contribute to the onset of AD. For instance, tau aggregates in the cortex precede Aβ deposition at the early stage of AD (40). Furthermore, there are several mutations of APP, PSEN1 or PSEN2 in familial AD cases (41). However, the present study mainly evaluated the effects of 6-bromotryptamine A on AChE and Aβ oligomer inhibition, which is a limitation of the study. Further studies are required to investigate whether this compound acts on other AD targets, such as tau, PSEN1 or PSEN2, to exert its anti-AD effects.
In conclusion, the present study revealed that 6-bromotryptamine A, a novel tryptamine derivative, inhibited AChE activity and Aβ oligomer formation, as well as prevented the scopolamine-induced short-term impairments in learning and memory in vivo. These results suggest that 6-bromotryptamine A, a molecule with multiple targets, may be used to treat AD.
Supplementary Material
Acknowledgements
Not applicable.
Glossary
Abbreviations
- AD
Alzheimer's disease
- AChE
acetylcholinesterase
- Aβ
β-amyloid
- CAS
catalytic active site
- NIH
National Institutes of Health
- i.p.
intraperitoneal
- NOR
novel object recognition
- PAS
peripheral anionic site
Funding
This study was supported by the National Natural Science Foundation of China (grant nos. 81673407, 31600402, U1503223, 41776168, 41706167 and 31801165), the Applied Research Project on Nonprofit Technology of Zhejiang Province (grant nos. 2016C37110 and 2015C33155), the Ningbo Natural Science Foundation (grant nos. 2017A610216, 2015A610219 and 2018A610213), the Ningbo Sci & Tech Project for Common Wealth (grant nos. 2017C50042 and 2017C10016), the Ningbo Municipal Innovation Team of Life Science and Health (grant no. 2015C110026), the 111 Project (grant no. D16013), the Li Dak Sum Yip Yio Chin Kenneth Li Marine Biopharmaceutical Development Fund, and the K. C. Wong Magna Fund in Ningbo University.
Availability of data and materials
All data generated or analyzed during this study are included in this published study.
Authors' contributions
WC and SH conceived and designed the experiments. XJ, MW, JS, CH, YB, HP, DZ, ZY, XX, HZ, LD, QW and XW performed the experiments. XJ and MW analyzed the data. MW and WC wrote the manuscript. All authors read and approved the final manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All animal experiments followed the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (publication no. 80-23, revised 1996), and were approved by the Animal Ethics and Welfare Committee of Ningbo University (Ningbo, China; approval no. SYXK-2008-0110).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
References
- 1.Luo W, Wang T, Hong C, Yang YC, Chen Y, Cen J, Xie SQ, Wang CJ. Design, synthesis and evaluation of 4-dimethylamine flavonoid derivatives as potential multifunctional anti-Alzheimer agents. Eur J Med Chem. 2016;122:17–26. doi: 10.1016/j.ejmech.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 2.Bhounsule AS, Bhatt LK, Prabhavalkar KS, Oza M. Cyclin dependent kinase 5: A novel avenue for Alzheimer's disease. Brain Res Bull. 2017;132:28–38. doi: 10.1016/j.brainresbull.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Zhao XJ, Gong DM, Jiang YR, Guo D, Zhu Y, Deng YC. Multipotent AChE and BACE-1 inhibitors for the treatment of Alzheimer's disease: Design, synthesis and bio-analysis of 7-amino-1,4-dihydro-2H-isoquilin-3-one derivates. Eur J Med Chem. 2017;138:738–747. doi: 10.1016/j.ejmech.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Cieslikiewicz-Bouet M, Chao S, Jean L, et al. Toward an innovative treatment of Alzheimer's disease: Synthesis and evaluation of multi-target directed ligands (MTDLs) targeting acetylcholinesterase (AChE) and alpha7 nicotinic acetylchloline receptors (alpha7 nAChRs) J Neurochem. 2017;142:209–210. [Google Scholar]
- 5.Barnett R. Alzheimer's disease. Lancet. 2019;393:1589. doi: 10.1016/S0140-6736(19)30851-7. [DOI] [PubMed] [Google Scholar]
- 6.Saxena M, Dubey R. Target enzyme in Alzheimer's disease: Acetylcholinesterase inhibitors. Curr Top Med Chem. 2019;19:264–275. doi: 10.2174/1568026619666190128125912. [DOI] [PubMed] [Google Scholar]
- 7.Huang L, Lin J, Xiang S, Zhao K, Yu J, Zheng J, Xu D, Mak S, Hu S, Nirasha S, et al. Sunitinib, a clinically used anticancer drug, is a potent AChE inhibitor and attenuates cognitive impairments in mice. Acs Chem Neurosci. 2016;7:1047–1056. doi: 10.1021/acschemneuro.5b00329. [DOI] [PubMed] [Google Scholar]
- 8.Ghumatkar PJ, Patil SP, Jain PD, Tambe RM, Sathaye S. Nootropic, neuroprotective and neurotrophic effects of phloretin in scopolamine induced amnesia in mice. Pharmacol Biochem Behav. 2015;135:182–191. doi: 10.1016/j.pbb.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Sang ZP, Qiang XM, Li Y, Xu R, Cao Z, Song Q, Wang T, Zhang X, Liu H, Tan Z, Deng Y. Design, synthesis and evaluation of scutellarein-O-acetamidoalkylbenzylamines as potential multifunctional agents for the treatment of Alzheimer's disease. Eur J Med Chem. 2017;135:307–323. doi: 10.1016/j.ejmech.2017.04.054. [DOI] [PubMed] [Google Scholar]
- 10.Ding L, He S, Wu W, Jin H, Zhu P, Zhang J, Wang T, Yuan Y, Yan X. Discovery and structure-based optimization of 6-bromotryptamine derivatives as potential 5-HT2A receptor antagonists. Molecules. 2015;20:17675–17683. doi: 10.3390/molecules200917675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He S, Ding L, Yan X. New 6-bromotryptamine derivatives from marine bacterium pseudoalteromonas rubra QD1-2 and the impact of side chain length on their cytotoxicity. Planta Med. 2013;79:845. doi: 10.1055/s-0033-1348634. [DOI] [Google Scholar]
- 12.Chen HX, Xiang SY, Huang L, Lin J, Hu S, Mak SH, Wang C, Wang Q, Cui W, Han Y. Tacrine(10)-hupyridone, a dual-binding acetylcholinesterase inhibitor, potently attenuates scopolamine-induced impairments of cognition in mice. Metab Brain Dis. 2018;33:1131–1139. doi: 10.1007/s11011-018-0221-7. [DOI] [PubMed] [Google Scholar]
- 13.Bae HJ, Sowndhararajan K, Park HB, Kim SY, Kim S, Kim DH, Choi JW, Jang DS, Ryu JH, Park SJ. Danshensu attenuates scopolamine and amyloid-β-induced cognitive impairments through the activation of PKA-CREB signaling in mice. Neurochem Int. 2019;131:104537. doi: 10.1016/j.neuint.2019.104537. [DOI] [PubMed] [Google Scholar]
- 14.Kraeuter AK, Guest PC, Sarnyai Z. The open field test for measuring locomotor activity and anxiety-like behavior. Methods Mol Biol. 2019;1916:99–103. doi: 10.1007/978-1-4939-8994-2_9. [DOI] [PubMed] [Google Scholar]
- 15.Overstreet DH. The open field test for two. J Psychopharmacol. 2007;21:140. doi: 10.1177/0269881107074490. [DOI] [PubMed] [Google Scholar]
- 16.Lueptow LM. J Vis Exp; 2017. Novel object recognition test for the investigation of learning and memory in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L, Huang C, Shentu J, Wang M, Yan S, Zhou F, Zhang Z, Wang C, Han Y, Wang Q, Cui W. Indirubin derivative 7-bromoindirubin-3-oxime (7Bio) attenuates Aβ oligomer-induced cognitive impairments in mice. Front Mol Neurosci. 2017;10:393. doi: 10.3389/fnmol.2017.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/S0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, Wu X, Gu X, Zhou Y, Ye L, Zhang K, Pan H, Wang J, Wei H, Zhu B, et al. Tacrine(10)-hupyridone prevents post-operative cognitive dysfunction via the activation of BDNF pathway and the inhibition of AChE in aged mice. Front Cell Neurosci. 2018;12:396. doi: 10.3389/fncel.2018.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santos WP, da Silva Carvalho AC, dos Santos Estevam C, Santana AE, Marçal RM. In vitro and ex vivo anticholinesterase activities of Erythrina velutina leaf extracts. Pharm Biol. 2012;50:919–924. doi: 10.3109/13880209.2011.649429. [DOI] [PubMed] [Google Scholar]
- 21.Li FJ, Liu Y, Yuan Y, Yang B, Liu ZM, Huang LQ. Molecular interaction studies of acetylcholinesterase with potential acetylcholinesterase inhibitors from the root of Rhodiola crenulata using molecular docking and isothermal titration calorimetry methods. Int J Biol Macromol. 2017;104:527–532. doi: 10.1016/j.ijbiomac.2017.06.066. [DOI] [PubMed] [Google Scholar]
- 22.Jain AN. Surflex-Dock 2.1: Robust performance from ligand energetic modeling, ring flexibility, and knowledge-based search. J Comput Aided Mol Des. 2007;21:281–306. doi: 10.1007/s10822-007-9114-2. [DOI] [PubMed] [Google Scholar]
- 23.Xiang S, Liu F, Lin J, Chen H, Huang C, Chen L, Zhou Y, Ye L, Zhang K, Jin J, et al. Fucoxanthin inhibits β-amyloid assembly and attenuates β-amyloid oligomer-induced cognitive impairments. J Agric Food Chem. 2017;65:4092–4102. doi: 10.1021/acs.jafc.7b00805. [DOI] [PubMed] [Google Scholar]
- 24.Chunhui H, Dilin X, Ke Z, Jieyi S, Sicheng Y, Dapeng W, Qinwen W, Wei C. J Vis Exp; 2018. A11-positive β-amyloid oligomer preparation and assessment using dot blotting analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun K, Bai Y, Zhao R, Guo Z, Su X, Li P, Yang P. Neuroprotective effects of matrine on scopolamine-induced amnesia via inhibition of AChE/BuChE and oxidative stress. Metab Brain Dis. 2019;34:173–181. doi: 10.1007/s11011-018-0335-y. [DOI] [PubMed] [Google Scholar]
- 26.Nakako T, Iwamura Y, Matsumoto A, Matsumoto K, Ikejiri M, Ikeda K. Effects of donepezil on scopolamine-induced cognitive impairment and Alzheimer's disease-like change in quantitative EEG analysis in rhesus monkeys. Eur Neuropsychopharm. 2017;27:S736–S737. doi: 10.1016/S0924-977X(17)31352-4. [DOI] [Google Scholar]
- 27.Li WM, Kan KK, Carlier PR, Pang YP, Han YF. East meets west in the search for Alzheimer's therapeutics-novel dimeric inhibitors from tacrine and huperzine a. Curr Alzheimer Res. 2007;4:386–396. doi: 10.2174/156720507781788918. [DOI] [PubMed] [Google Scholar]
- 28.Lazarevic-Pasti T, Leskovac A, Momic T, Petrovic S, Vasic V. Modulators of acetylcholinesterase activity: From Alzheimer's disease to anti-cancer drugs. Curr Med Chem. 2017;24:3283–3309. doi: 10.2174/0929867324666170705123509. [DOI] [PubMed] [Google Scholar]
- 29.Xi HJ, Wu RP, Liu JJ, Zhang LJ, Li ZS. Role of acetylcholinesterase in lung cancer. Thorac Cancer. 2015;6:390–398. doi: 10.1111/1759-7714.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz-Espejo F, Cabezas-Herrera J, Illana J, Campoy FJ, Muñoz-Delgado E, Vidal CJ. Breast cancer metastasis alters acetylcholinesterase activity and the composition of enzyme forms in axillary lymph nodes. Breast Cancer Res Tr. 2003;80:105–114. doi: 10.1023/A:1024461108704. [DOI] [PubMed] [Google Scholar]
- 31.Ki YS, Park EY, Lee HW, Oh MS, Cho YW, Kwon YK, Moon JH, Lee KT. Donepezil, a potent acetylcholinesterase inhibitor, induces caspase-dependent apoptosis in human promyelocytic leukemia HL-60 cells. Biol Pharm Bull. 2010;33:1054–1059. doi: 10.1248/bpb.33.1054. [DOI] [PubMed] [Google Scholar]
- 32.Hyatt JL, Tsurkan L, Morton CL, Yoon KJ, Harel M, Brumshtein B, Silman I, Sussman JL, Wadkins RM, Potter PM. Inhibition of acetylcholinesterase by the anticancer prodrug CPT-11. Chem-Biol Interact. 2005;157-158:247–252. doi: 10.1016/j.cbi.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 33.al-Jafari AA, Duhaiman AS, Kamal MA. Inhibition of human acetylcholinesterase by cyclophosphamide. Toxicology. 1995;96:1–6. doi: 10.1016/0300-483X(94)02848-O. [DOI] [PubMed] [Google Scholar]
- 34.Cheng S, Zheng W, Gong P, Zhou Q, Xie Q, Yu L, Zhang P, Chen L, Li J, Chen J, et al. (−)-Meptazinol-melatonin hybrids as novel dual inhibitors of cholinesterases and amyloid-β aggregation with high antioxidant potency for Alzheimer's therapy. Bioorg Med Chem. 2015;23:3110–3118. doi: 10.1016/j.bmc.2015.04.084. [DOI] [PubMed] [Google Scholar]
- 35.Liu FF, Dong XY, He LZ, Middelberg APJ, Sun Y. Molecular insight into conformational transition of amyloid β-peptide 42 inhibited by (−)-epigallocatechin-3-gallate probed by molecular simulations. J Phys Chem B. 2011;115:11879–11887. doi: 10.1021/jp202640b. [DOI] [PubMed] [Google Scholar]
- 36.Du WJ, Guo JJ, Gao MT, Hu SQ, Dong XY, Han YF, Liu FF, Jiang S, Sun Y. Brazilin inhibits amyloid β-protein fibrillogenesis, remodels amyloid fibrils and reduces amyloid cytotoxicity. Sci Rep. 2015;5:7992. doi: 10.1038/srep07992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aykac A, Ozbeyli D, Uncu M, Ertaş B, Kılınc O, Şen A, Orun O, Sener G. Evaluation of the protective effect of Myrtus communis in scopolamine-induced Alzheimer model through cholinergic receptors. Gene. 2019;689:194–201. doi: 10.1016/j.gene.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Bird TD. Genetic aspects of Alzheimer disease. Genet Med. 2008;10:231–239. doi: 10.1097/GIM.0b013e31816b64dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang T, Liu XH, Guan J, Ge S, Wu MB, Lin JP, Yang LR. Advancement of multi-target drug discoveries and promising applications in the field of Alzheimer's disease. Eur J Med Chem. 2019;169:200–223. doi: 10.1016/j.ejmech.2019.02.076. [DOI] [PubMed] [Google Scholar]
- 40.Jagust W. Imaging the evolution and pathophysiology of Alzheimer disease. Nat Rev Neurosci. 2018;19:687–700. doi: 10.1038/s41583-018-0067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM. Alzheimer's disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published study.