Abstract
Bone morphogenetic proteins (BMPs) are members of the transforming growth factor-β (TGF-β) family and may play an important role in the regulation of malignant cells in bladder cancer. The aim of the present study was to investigate BMP expression in non-muscle invasive bladder cancer. Tumor tissue samples from 71 patients treated with transurethral resection and 10 samples of normal bladder tissue were stained using immunohistochemistry for BMP-2, −4, −6 and −7. The levels of BMP were correlated with the number and size of tumors in the bladder, the pathohistological findings as well as with tumor recurrence and progression. The results of the present study demonstrated that BMP-2 and −7 are highly expressed in normal bladder tissue, but significantly downregulated in cancer samples. This reduction correlates with a faster rate of tumor recurrence as well as with an increase in the number of recurrent tumors. There was no evident interrelation between BMP-2 and −7 reduction and changes in tumor grade and stage. In conclusion, BMP-2 and −7 are potential prognostic factors for tumor recurrence and further studies on BMP and bladder cancer are needed to confirm these results.
Keywords: non-muscle invasive bladder cancer, bone morphogenic protein-2, bone morphogenic protein-4, bone morphogenic protein-6, bone morphogenic protein-7, prognostic factor, tumor recurrence
Introduction
Transitional cell carcinoma (TCC) of the bladder is the most common malignancy in the urinary tract. American Cancer Society estimates about 80,470 new cases and about 17,670 deaths from bladder cancer in 2019 in United States, with an almost three times higher incidence in male patients (1). Bladder cancer incidence peaks between ages 50 and 70 years and the leading risk factors for bladder cancer development are tobacco smoking and occupational exposure to various chemical substances (mainly benzene and aryl amines) (2,3).
When diagnosed for the first time, usually by transurethral bladder resection, approximately 75% of tumors are identified as non-muscle invasive bladder cancer (NMIBC) and are confined to the bladder mucosa and submucosa (4). The remaining are muscle invasive bladder cancers which have a much worse prognosis and require more aggressive treatment with very little chance of bladder preservation. But NMIBC is also a serious disease, well known for its recurrence and progression rates, which are not easy to predict. Furthermore, it is estimated that up to 20% of NMIBC will progress to muscle invasive disease (5,6). The most important risk factors for recurrence and progression are number of tumors (single vs. two or more tumors), tumor grade (low vs. high), tumor size (< vs. ≥3 cm), presence of CIS and prior recurrence rate (≤ vs. >1 recurrence/year) (4). It has also been shown that the response to intravesical treatment with bacillus Calmette-Guerin (BCG) is an important prognostic factor for NMIBC (7).
Bone morphogenetic proteins (BMPs), named so because they were first isolated from bone, are members of the transforming growth factor beta family (TGF-β). More than 20 members of BMP have been isolated to date. BMPs have a critical function during normal mammalian development and cellular differentiation (8–10). Recent studies have also shown that they may play an important role in the regulation of malignant cells, including prostate (11) and bladder cancer (12,13). We have investigated BMP-6 and −7 in human clear cell renal carcinoma and we have found a significantly higher BMP-6 mRNA expression in malignant than in healthy tissue. Nevertheless, BMP-6 mRNA and protein expression have not shown a significant correlation with disease presentation, disease progression and patients' characteristics (14). BMP-7 expression was down-regulated in our clear cell renal carcinoma samples (15). Our research has been limited by a relatively small number of samples and a relatively short follow-up period.
BMPs' expression and a possible role in urological cancers are still not clear. Presumably, it is different for distinct members of BMPs and diverse urological cancers as well as for their histology, grade, stage and tissue heterogeneity. Thus, in order to proceed with our research of BMPs in urological cancer, in this study we aimed to examine their (BMP-2, −4, −6, and −7 proteins) expression in NMIBC and their possible role in the progression and recurrence of the disease.
Huge efforts have been invested in finding novel pathways associated with the evolution of different malignancies, which may lead to an improvement in patients' prognosis. It is necessary to identify molecular markers that may predict the outcome or potentially serve as therapeutic targets for NMIBC. Preliminary data have shown that BMPs are important molecular markers for urothelial carcinomas (16–19).
Materials and methods
This study is based on tissue samples from 71 patients with NMIBC treated with transurethral resection (TUR) at our institution from 2007–2010. Written informed consent was obtained in accordance with the local Ethics Code. The study was conducted in accordance with the Helsinki Declaration, and was approved by the Ethics Committee of the University Hospital Center Zagreb. Patient data, as well as data on previous surgery for bladder cancer, including the time to recurrence, were obtained. Number of tumors in the bladder, size of tumors in cm, and pathohistological findings were collected. We also stained 10 samples of macroscopically and histologically healthy bladder tissue obtained during brain death, heart-beating donor organ explantation. The pathological stage was assigned according to the American Joint Committee on Cancer criteria using the 2004TNM staging system.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissues (3–4 µm) were deparaffinized in xylene and then rehydrated through graded alcohol. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide for 10 min. The sections were blocked with 20% normal rabbit serum for 30 min. prior to 1 h of incubation with primary antibody (mouse monoclonal BMP-2, −4, −6 antibodies, (Abcam, UK) and antihuman BMP-7 monoclonal antibody [(R&D Systems, USA)]. The slides were washed twice in Tris-buffered saline and incubated with biotinylated rabbit-antimyocyte antibody (DAKO, Glostrup, Denmark) diluted 1:500 in blocking serum. The detection of antibody reaction was carried out with a standard streptavidin-biotin complex (Dako, Glostrup, Denmark). Negative control sections were processed in an identical manner after omitting the primary antibody: They showed no staining. Immunohistochemical staining for BMPs was evaluated semiquantitatively and the specimens were scored according to the distribution of positive cells. Immunostaining results were graded according to the following protocol: 3+, more than 50% of cells positive; 2+, 10–50% of cells positive; 1+, up to 10% of cells positive; and 0, if cells demonstrated no positive staining. The cellular localization and pattern of immunoreactivity were examined in a blinded fashion independently by two investigators.
The patients were followed-up every 3 months for 2 years, then every 6 months for 2 years and annually thereafter. The routine check-up included office visits, serum electrolytes, urine cytology, cystoscopy and ultrasound. Bladder and upper urinary tracts, using multislice computed tomography were imaged at the discretion of the treating physician. The patients who were followed elsewhere were evaluated by correspondence or a phone interview. Time to recurrence was calculated as the time interval from surgical intervention to the first evidence of recurrence or until the last follow-up if the patient did not have recurrence.
Statistical analysis
Statistical analysis was performed using the STATISTICA software package version 6.1, SN AGA304B211928E61, StatSoft Inc.; USA.
Generalized one-way ANOVA as test in Generalized Linear/Nonlinear Model for Poisson distribution was performed from pairwise/multiple group comparison (Comparison between BMP categories in numerical parameters). Descriptive statistics and t-tests for single samples were also performed. Comparison between BMP categories in qualitative parameters were assessed using cross-tabulation tables and Pearson chi-square test. All statistical analyses were 2-tailed, with P<0.05 considered to indicate a statistically significant difference..
Results
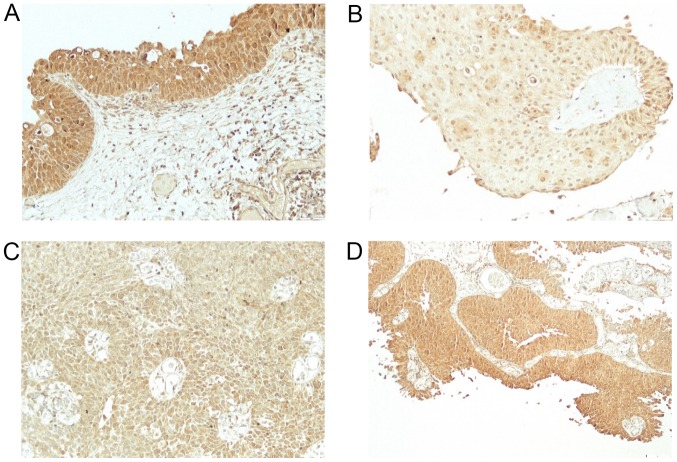
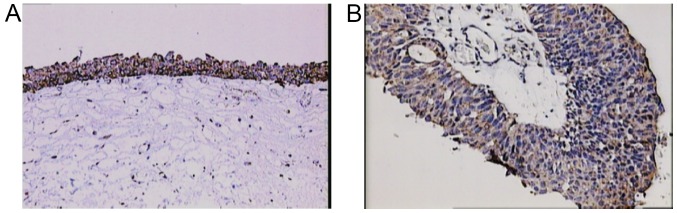
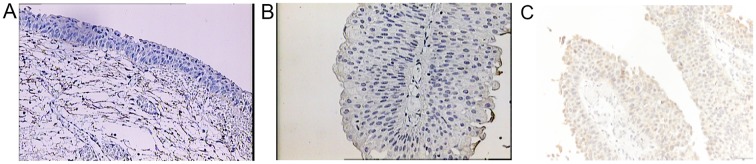
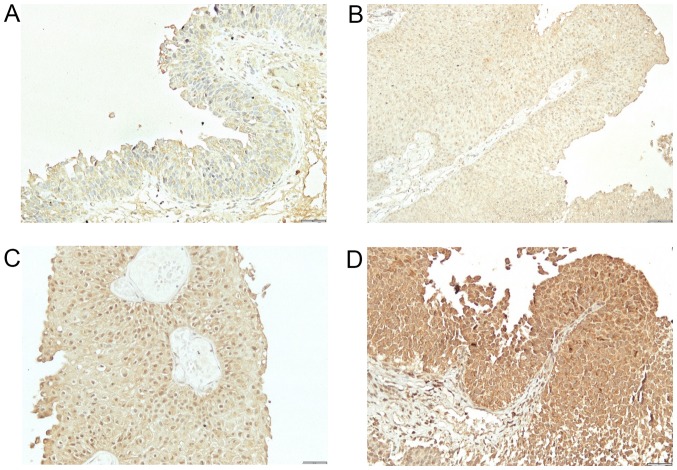
The study included 49 men and 22 women. Mean age at the time of TUR was 65.5 years (from 39–84). Out of 71 patients, 24 (34%) had previous TUR for bladder cancer. Based on pathohistological findings, 38 patients (54%) had low-grade and 33 (46%) high-grade bladder cancer. Twenty-eight patients (39%) had Ta and 43 (61%) T1 stage cancer. The patients were followed-up from 5 to 67 months (mean 33). Thirty-four patients (48%) had at least one recurrence, mean 1.6 (from 1 to 4) (Table I). Out of 34 patients with recurrence, 11 (32%) had a change in the grade status (three progressed to a higher grade and eight showed downgrading to a lower grade). Regarding the stage, five patients progressed from Ta to T1 cancer, and six patients demonstrated a downstaging from T1 to Ta cancer. Expression of BMPs was as follows: BMP-2 in normal bladder tissue from 2 to 3 (2.9) (Fig. 1A) and in cancer tissue from 1–3 (2.5) (Fig. 1B-D), BMP-4 in normal bladder tissue from 1–2 (1.1) (Fig. 2A) and in cancer tissue from 0–3 (0.9) (Fig. 2B), BMP-6 in normal bladder tissue from 0–1 (0.9) (Fig. 3A) and in cancer tissue from 0–2 (0.3) (Fig. 3B), BMP-7 in normal bladder tissue from 2–3 (2.7) (Fig. 4A) and in bladder cancer tissue from 0–3 (0.4) (Fig. 4B-D). Comparison of expression levels in normal tissue and in cancer tissue revealed statistically significant changes of BMP-2 (P<0.01), BMP-6 (P<0.01) and BMP-7 (P<0.01) but not BMP-4 (P=0.32) (Table II). In normal tissue and in tumor samples, the staining of BMP proteins was confined within the cytoplasm, magnification 20× and for BMP-7 in cancer tissue 40×.
Table I.
Patient characteristics and pathohistological results.
| Characteristics | Total n (%) |
|---|---|
| Mean age (range), years | 65.5 (39–84) |
| Previous TUR | |
| Yes | 24 (34) |
| No | 47 (66) |
| Tumor grade | |
| Low | 38 (54) |
| High | 33 (46) |
| Tumor stage | |
| Ta | 28 (39) |
| T1 | 43 (61) |
| No. of tumors, average (range) | 2.3 [1-multiple (≥8)] |
| Size of tumor in cm, average (range) | 4 (1–10) |
| Mean follow-up, months (range) | 33 (5–67) |
| Tumor recurrence | |
| No | 37 (52) |
| Yes | 34 (48) |
| Mean no. of recurring tumors, n (range) | 1.6 (1–4) |
TUR, transurethral resection.
Figure 1.
Staining for BMP2. (A) Immunohistochemical staining for BMP2 demonstrated strong and uniform cytoplasmic positivity in the normal bladder urothelium. (B) Weak cytoplasmic positivity in urothelial carcinoma cells of the bladder. (C) Moderate cytoplasmic positivity in urothelial carcinoma cells of the bladder. (D) Strong cytoplasmic positivity in urothelial carcinoma cells of the bladder. Magnification, ×200. BMP2, bone morphogenetic protein 2.
Figure 2.
Staining for BMP4. (A) Moderate cytoplasmic positivity for BMP4 in normal urothelial cells and (B) weak cytoplasmic positivity in urothelial carcinoma cells. Magnification, ×200. BMP4, bone morphogenetic protein 4.
Figure 3.
Staining for BMP6. (A) Negative immunohistochemical staining for BMP6 in normal urothelial cells. (B) Negative immunohistochemical staining in urothelial carcinoma cells. (C) Weak positive immunohistochemical reaction for BMP6 in the cytoplasm of the urothelial carcinoma cells. Magnification, ×200. BMP6, bone morphogenetic protein 6.
Figure 4.
Staining for BMP7. (A) Weak cytoplasmic positivity in urothelial cells for BMP7. (B) Weak cytoplasmic positivity in urothelial carcinoma cells. (C) Moderate cytoplasmic positivity in urothelial carcinoma cells. (D) Strong cytoplasmic positivity in urothelial carcinoma cells. Magnification, ×200. BMP7, bone morphogenetic protein 7.
Table II.
Levels of BMP-2, −4, −6 and −7 expression in normal and cancer tissues.
| BMP type | Normal tissues | Cancer tissues | P-value |
|---|---|---|---|
| BMP-2 levels | 2–3 (2.9±0.32) | 1–3 (2.5±0.56) | <0.01 |
| BMP-4 levels | 1–2 (1.1±0.32) | 0–3 (0.9±0.95) | 0.32 |
| BMP-6 levels | 0–1 (0.9±0.32) | 0–2 (0.3±0.54) | <0.01 |
| BMP-7 levels | 2–3 (2.7±0.01) | 0–3 (0.4±0.80) | <0.01 |
Data are presented as the average (mean ± standard deviation).
The average number of tumors in the bladder was 2.3 [ranging from 1 to multiple tumors (≥8)], (Table I). By comparing the number of tumors in the bladder and BMP expression we found that only the decrease in BMP-7 was significant and almost statistically significant (P=0.07). For other BMP proteins no significant change was found [BMP-2 (P=0.93), BMP-4 (P=0.99), BMP-6 (P=0.89)]. When we divided the subjects according to the number of tumors in the bladder into those with 1 and those with >1 tumors, the results were similar: BMP-7 (P=0.06), BMP-2 (P=0.55), BMP-4 (P=0.5) and BMP-6 (P=0.86). The average tumor size in the bladder was 4 cm (from 1 to 10), (Table I). The tumor size and BMP expression did not show any correlation [BMP-2 (P=0.49), BMP-4 (P=0.63), BMP-6 (P=0.92), BMP-7 (P=0.95)]. When we divided the subjects according to the tumor size into two groups: Patients with tumors up to 3 cm, and patients with tumors ≥3 cm, no statistically significant change was found either: BMP-2 (P=0.92), BMP-4 (P=0.56), BMP-6 (P=0.17) and BMP-7 (P=0.54). Since 24 patients (34%) had a previous TUR for bladder cancer, we compared BMP proteins expression in this group and in the group of patients without any previous history of bladder cancer. Time from previous TUR ranged from 4 to 234 months (mean 9). For BMP-2 expression, patients who had <50% of cells positive compared to those with ≥50% have a significantly shorter time period from previous surgery for bladder cancer, i.e. 5 vs. 13 months (P<0.01). Loss of BMP-7 positivity was also significantly related with a shorter period of time from previous surgery, i.e. 6 vs. 22 months (P<0.01), (Table III). When comparing patients with previous recurrence ≤1 year, and those with tumor reappearance >1 year, eleven patients had recurrence in a period ≤1 year while in 13 patients more than a year passed between the previous tumor and the tumor from which the samples for this study were obtained. However, the size of the two patient groups was too small for a valid statistical analysis.
Table III.
BMP-2 and −7 levels and time to tumor recurrence in months and number of recurrent tumors.
| Immunostaining score | |||
|---|---|---|---|
| Expression group | 0–2 | 3 | P-value |
| Time from previous TUR, months | |||
| BMP-2 positive | 5 | 13 | <0.01 |
| BMP-7 positive | 6 | 22 | <0.01 |
| First recurrence, months | |||
| BMP-2 positive | 5 | 8 | <0.01 |
| BMP-7 positive | 6 | 9 | <0.01 |
| No. of recurrent tumors | |||
| BMP-2 positive | 0.5 | 1.1 | 0.015 |
| BMP-7 positive | 0.6 | 1.2 | 0.019 |
Where ‘time from previous TUR’ refers to the time period in months between the previous TUR and the TUR when the samples were obtained for immunohistochemistry, and ‘first recurrence’ refers to the time period in months between TUR and when the first recurrent tumor is observed. The following immunostaining grading system was applied: 3+, >50% of positive cells; 2+, 10–50% of positive cells; 1+, up to 10% of positive cells; and 0, if cells demonstrated no positive staining. Statistical analysis was performed via generalized one-way ANOVA with the generalized linear/nonlinear model for Poisson distribution. BMP, bone morphogenetic protein; TUR, transurethral resection.
For patients exhibiting BMP-2 positive tumors with <50% of positive cells, a shorter time to first recurrence and a higher number of tumor recurrence in the follow-up period was observed than in patients with ≥50% of positive cells (5 vs. 8 months, P<0.01; 0.5 vs. 1.1 P=0.015; respectively). In BMP-7 patients without any expression (i.e. patients who lost their BMP-7 high positivity found in normal tissue) there was a significant difference in time elapsed to primary recurrence in comparison to patients with BMP-7 positive samples, i.e. 6 vs. 9 months (P<0.01), as well as in the number of tumor recurrences (0.6 vs. 1.2) in the follow-up period (P=0.019), (Table III). We found no correlation for BMP-4 and BMP-6.
We did not find any statistically significant difference between the levels of BMP expression and tumor presence in lamina (positive vs. negative) (BMP-2 P=0.96, BMP-4 P=0.82, BMP-6 P=0.755, BMP-7 P=0.084). It is however worth mentioning that our results for BMP-7 almost reached statistical significance: This difference may possibly be proven on a larger sample. No statistically significant results were found for tumor grade (low vs. high) and BMP expression (BMP-2, P=0.74, BMP-4, P=0.48, BMP-6 P=0.85, BMP-7 P=0.71). The changes in tumor grade and stage were not significant either, most probably because of the small number of such patients in comparison to patients who showed no change, i.e. 11 vs. 60.
Discussion
Numerous studies have linked members of the BMP family, BMP antagonists, and BMP receptors to cancer (11–24). However, available data on involvement of BMP family members in cancerogenesis are conflicting. Different members of the BMP group induce different effects in various types of cancer. For example a decrease of BMP-3 and an increase of BMP-4 have been related to progression and poor prognosis of breast cancer (25,26). Increased levels of BMP-6 and −7 have been correlated with bone metastases in prostate cancer (27,28) while the loss of BMP and BMP receptors has been associated with a higher tumor grade and pathological stage, increased rate of recurrence and a lower survival rate (29). Since BMPs are members of TGN-β, which has in general inhibitory characteristics on malignant cells, the same effect would be expected in bladder cancer cells. However, expression of BMP-9 in bladder cancer tissue has recently been established. Given that the up regulation of BMP-9 promotes proliferation and migration of bladder cancer cells, it could be used as a novel marker of tumor aggressiveness (13).
We have shown that there is a significant change: A loss of BMP proteins in cancer tissue when compared to the normal tissue. Although this loss is probably an important event in tumor development, it is not clear what causes it. BMP-2 expression has been shown in bladder cancer cell line (T24) (30) as well as in NMIBC, MIBC and metastatic bladder cancer in progressive scale. By contrast, we have found high levels of expression in normal (for BMP-2 and −7) tissue and a decrease of expression levels in some NMIBC, but in more than 50% of our samples the level of BMP-2 expression remained high. Moreover, the patients with a reduced number of BMP-2 positive cells (<50%) also displayed a significantly shorter time from previous TUR if they underwent it. They also had a shorter time to first recurrence as well as a higher number of recurrent tumors, although we did not detect any significant change in tumor stage and grade related to BMP levels. The same results were observed in BMP-7, but not in BMP-4 and −6. Likewise, using real-time polymerase chain reaction, Kuzaka et al (31) have found BMP-2 and BMP-7 downregulation in infiltrating urothelial carcinoma, while BMP-4 was downregulated in non-invasive tumors. Furthermore, they have shown that BMP-2 and BMP-7 correlated with prolonged time to recurrence (31). Regarding BMP-4, we have also shown low, although not significant, downregulation in our group of NMIBC. On the other hand, using a human bladder cancer patient samples, Martínez et al (32) have recently shown, increased expression of BMP-4 in advanced and undifferentiated tumors. Although we can only speculate about the reasons for these observations, different members of the BMP group probably have diverse roles in normal and in tumor tissue. Furthermore, dissimilar results from different studies can be related to choice of methodology and cancer sample variants used for detection of BMP expression.
Also, changes in BMP expression levels in bladder cancer can most likely be related to a significant heterogeneity within the subgroups of TCC (33).
Kim et al (12) have also shown that tissue samples from TCC frequently loss expression of BMP receptor and that overexpression of BMP receptor in BMP resistant cell line leads to a restoration of BMP signaling and a decreased rate of tumor growth. These data as well as high levels of expression (for BMP-2 and −7) found in the normal healthy bladder tissue suggest that BMP may play an important protective role in the urinary bladder. Furthermore, a significant loss of BMP protein in cancer tissue can be related to an increased number of and a shorter time period for tumor recurrence, as we have shown in our study. Our results have to be verified on larger number of samples and with additional molecular medicine methods, such as polymerase chain reaction for detecting BMP messenger ribonucleic acid and Western blot for protein detection in tissue samples. However, we have shown that there is a correlation between the loss of BMP-2 and −7 and tumor recurrence. This finding is important for the treatment and follow-up of patients with NMIBC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
TH conceived and designed the project and protocol, collected the tissue samples, analysed the data and wrote the manuscript. ZK, PK and NBJ conceived and designed the project and protocol, and edited the manuscript. AES conceived and designed the project and protocol. MB analyzed the data and edited the manuscript. MC and DT analyzed the tissue samples and data.
Ethics approval and consent to participate
The present study was revised and approved by the Ethics Committee of the University Hospital Center Zagreb (Zagreb, Croatia). Written informed consent for the use of their tissue was obtained from all participants.
Patient consent for publication
Written informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare that they have no competing interests.
References
- 1.American Cancer Society: Key statistics for bladder cancer. https://www.cancer.org/cancer/bladder-cancer/about/key-statistics.html. [Mar 1;2019 ]; [Google Scholar]
- 2.Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011;306:737–745. doi: 10.1001/jama.2011.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pesch B, Taeger D, Johnen G, Gawrych K, Bonberg N, Schwentner C, Wellhäusser H, Kluckert M, Leng G, Nasterlack M, et al. Screening for bladder cancer with urinary tumor markers in chemical workers with exposure to aromatic amines. Int Arch Occup Environ Health. 2014;87:715–724. doi: 10.1007/s00420-013-0916-3. [DOI] [PubMed] [Google Scholar]
- 4.M Babjuk M, Burger M, Zigeuner R, Shariat S, Van Rhijn B, Compérat E, Sylvester R, Kaasinen E, Böhle A, Palou J, Rouprêt M. Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and CIS) http://www.uroweb.org/gls/pdf/05_TaT1_Bladder_Cancer_LR.pdf. 2013 doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW, Kurth K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–475. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez-Gomez J, Madero R, Solsona E, Unda M, Martinez-Piñeiro L, Gonzalez M, Portillo J, Ojea A, Pertusa C, Rodriguez-Molina J, et al. Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guerin: The CUETO scoring model. J Urol. 2009;182:2195–2203. doi: 10.1016/j.juro.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Isharwal S, Konety B. Non-muscle invasive bladder cancer risk stratification. Indian J Urol. 2015;31:289–296. doi: 10.4103/0970-1591.166445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: Molecular clones and activites. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 9.Sampath TK, Maliakal JC, Hauschka PV, Jones WK, Sasak H, Tucker RF, White KH, Coughlin JE, Tucker MM, Pang RH, et al. Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J Biol Chem. 1992;267:20352–20362. [PubMed] [Google Scholar]
- 10.Hogan BL. Bone morphogenic proteins: Multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- 11.Kim IY, Lee DH, Ahn HJ, Tokunaga H, Song W, Devereaux LM, Jin D, Sampath TK, Morton RA. Expression of bone morphogenetic protein receptors type-IA, -IB and II correlates with tumor grade in human prostate cancer tissues. Cancer Res. 2000;60:2840–2844. [PubMed] [Google Scholar]
- 12.Kim IY, Lee DH, Lee DK, Kim WJ, Kim MM, Morton RA, Lerner SP, Kim SJ. Restoration of bone morphogenic protein receptor type II Expression leads to a decreased rate of tumor growth in bladder transitional cell carcinoma cell lines TSU-Pr1. Cancer Res. 2004;64:7355–7360. doi: 10.1158/0008-5472.CAN-04-0154. [DOI] [PubMed] [Google Scholar]
- 13.Gou L, Liu M, Xia J, Wan Q, Jiang Y, Sun S, Tang M, Zhou L, He T, Zhang Y. BMP9 promotes the proliferation and migration of bladder cancer cells through up-regulating lncRNA UCA1. Int J Mol Sci. 2018;19(pii):E1116. doi: 10.3390/ijms19041116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basic-Jukic N, Radic-Antolic M, Hudolin T, Coric M, Zadro R, Pasini J, Kastelan Z, Kes P. Immunolocalization and mRNA expression of bone morphogenetic protein-6 in human clear cell renal carcinoma. Kidney Blood Press Res. 2009;32:445–450. doi: 10.1159/000266479. [DOI] [PubMed] [Google Scholar]
- 15.Basic-Jukic N, Hudolin T, Radic-Antolic M, Coric M, Zadro R, Kastelan Z, Pasini J, Bandic-Pavlovic D, Kes P. Bone morphogenetic protein-7 expression is down-regulated in human clear cell renal carcinoma. J Nephrol. 2011;24:91–97. doi: 10.5301/JN.2010.2020. [DOI] [PubMed] [Google Scholar]
- 16.Hung TT, Wang H, Kingsley EA, Risbridger GP, Russell PJ. Molecular profiling of bladder cancer: Involvement of the TGF-beta pathway in bladder cancer progression. Cancer Lett. 2008;265:27–38. doi: 10.1016/j.canlet.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 17.Komai Y, Morimoto S, Saito K, Urushibara M, Sakai K, Ikeda S. Possible involvement of bone morphogenetic protein 2 in heterotopic ossification in metastatic lesion from urothelial carcinoma of bladder. Int J Urol. 2006;13:1126–1128. doi: 10.1111/j.1442-2042.2006.01488.x. [DOI] [PubMed] [Google Scholar]
- 18.Zaravinos A, Lambrou GI, Boulalas I, Delakas D, Spandidos DA. Identification of common differentially expressed genes in urinary bladder cancer. PLoS One. 2011;6:e18135. doi: 10.1371/journal.pone.0018135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang ZJ, Liu FX, Yang YS, Yang X, Zhu GX. Expression of bone-morphogenetic protein 2 and tumor necrosis factor α correlates with bone metastases in bladder urothelial carcinoma. Ann Diagn Pathol. 2013;17:51–53. doi: 10.1016/j.anndiagpath.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Alarmo EL, Rauta J, Kauraniemi P, Karhu R, Kuukasjärvi T, Kallioniemi A. Bone morphogenetic protein 7 is widely overexpressed in primary breast cancer. Genes Chromosomes Cancer. 2006;45:411–419. doi: 10.1002/gcc.20307. [DOI] [PubMed] [Google Scholar]
- 21.Haudenschild DR, Palmer SM, Moseley TA, You Z, Reddi AH. Bone morphogenetic protein (BMP)-6 signaling and BMP antagonist noggin in prostate cancer. Cancer Res. 2004;64:8276–8284. doi: 10.1158/0008-5472.CAN-04-2251. [DOI] [PubMed] [Google Scholar]
- 22.Franzen A, Heldin NE. BMP-7-induced cell cycle arrest of anaplastic thyroid carcinoma cells via p21(CIP1) and p27(KIP1) Biochem Biophys Res Commun. 2001;285:773–781. doi: 10.1006/bbrc.2001.5212. [DOI] [PubMed] [Google Scholar]
- 23.Yanagita M. BMP antagonists: Their roles in development and involvement in patophysiology. Cytokin Growth Factor Rev. 2005;16:309–317. doi: 10.1016/j.cytogfr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Deng H, Makizumi R, Ravikumar TS, Dong H, Yang W, Yang WL. Bone morphogenetic protein-4 is overexpressed in colonic adenocarcinomas and promotes migration and invasion of HCT116 cells. Exp Cell Res. 2007;313:1033–1044. doi: 10.1016/j.yexcr.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 25.Davies SR, Watkins G, Douglas-Jones A, Mansel RE, Jiang WG. Bone morphogenetic proteins 1 to 7 in human breast cancer, expression pattern and clinical/prognostic relevance. J Exp Ther Oncol. 2008;7:327–338. [PubMed] [Google Scholar]
- 26.Alarmo EL, Kuukasjaävi T, Karhu R, Kallioniemi A. A comprehensive expression survey of bone morphogenic proteins in breast cancer highlights the importance of BMP4 and BMP7. Breast Cancer Res Treat. 2007;103:239–246. doi: 10.1007/s10549-006-9362-1. [DOI] [PubMed] [Google Scholar]
- 27.Autzen P, Robson CN, Bjartell A, Malcolm AJ, Johnson MI, Neal DE, Hamdy FC. Bone morphogenic protein 6 in skeletal metastases from prostate cancer and other common human malignancies. Br J Cancer. 1998;78:1219–1223. doi: 10.1038/bjc.1998.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masuda H, Fukabori Y, Nakano K, Takezawa Y, Csuzuki T, Yamanaka H. Increased expression of bone morphogenic protein-7 in bone metastatic prostate cancer. Prostate. 2003;54:268–274. doi: 10.1002/pros.10193. [DOI] [PubMed] [Google Scholar]
- 29.Kim IY, Lee DH, Ahn HJ, Ahn HJ, Kim MM, Kim SJ, Morton RA. Loss of expression of bone morphogenic protein receptor type II in human prostate cancer cells. Oncogene. 2004;23:7651–7659. doi: 10.1038/sj.onc.1207924. [DOI] [PubMed] [Google Scholar]
- 30.Hatakeyama S, Gao YH, Nemoto-Ohara Y, Kataoka H, Satoh M. Expression of bone morphogenic proteins of human neoplastic epithelial cells. Biochem Mol Biol Int. 1997;42:497–505. doi: 10.1080/15216549700202901. [DOI] [PubMed] [Google Scholar]
- 31.Kuzaka B, Janiak M, Włodarski KH, Radziszewski P, Włodarski PK. Expression of bone morphogenetic protein-2 and −7 in urinary bladder cancer predicts time to tumor recurrence. Arch Med Sci. 2015;11:378–384. doi: 10.5114/aoms.2014.46796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martínez VG, Rubio C, Martínez-Fernández M, Segovia C, López-Calderón F, Garín MI, Teijeira A, Munera-Maravilla E, Varas A, Sacedón R, et al. BMP4 induces M2 macrophage polarization and favors tumor progression in bladder cancer. Clin Cancer Res. 2017;23:7388–7399. doi: 10.1158/1078-0432.CCR-17-1004. [DOI] [PubMed] [Google Scholar]
- 33.Kim IY, Kim SJ. Role of bone morphogenic proteins in transitional cell carcinoma cells. Cancer Lett. 2006;241:118–123. doi: 10.1016/j.canlet.2005.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the present study are available from the corresponding author on reasonable request.