Abstract
Acetylcholinesterase cysteine-targeted insecticides against malaria vector Anopheles gambia and other mosquitos have already been introduced. We have applied the olefin metathesis for the preparation of cysteine-targeted insecticides in high yields. The prepared compounds with either a succinimide or maleimide moiety were evaluated on Anopheles gambiae and human acetylcholinesterase with relatively high irreversible inhibition of both enzymes but poor selectivity. The concept of cysteine binding was not proved by several methods, and poor stability was observed of the chosen most potent/selective compounds in a water/buffer environment. Thus, our findings do not support the proposed concept of cysteine-targeted selective insecticides for the prepared series of succinimide or maleimide compounds.
Keywords: Acetylcholinesterase, inhibitor, insecticide, Anopheles gambiae, human, selectivity
According to the World Malaria Report 2017, there were 216 million cases of malaria with 445,000 deaths in 2016. Almost 2.7 billion people were reported at risk of malaria. Based on these alarming data, malaria remains a huge threat.1 Chemical insecticides are one of the major tools used for insect elimination and/or prevention of the spread of vector-borne diseases.2,3 However, traditional chemical insecticides are often associated with significant toxicity to mammals and beneficial insects such as honey bees or bumble bees.4,5
The principal mechanism of insecticide action is the formation of a covalent bond to acetylcholinesterase (AChE, EC 3.1.1.7), a vital enzyme that is responsible for rapid hydrolysis of the neurotransmitter acetylcholine (ACh).6−8 The standard organophosphate and carbamate insecticides covalently block Ser360 (Anopheles gambiae acetylcholinesterase, AgAChE) and thus disable the process of ACh hydrolysis in invertebrates as well as in vertebrates. In recent years, several compounds were introduced having very good and selective inhibition potency against AgAChE over human AChE.9−11 Cysteine-targeted insecticides aimed at the Cys447 residue (full-length numbering; in Torpedo californica the numbering is Cys286) in the peripheral anionic site (PAS) of some insect species have been proposed to overcome insecticide resistance.12 The uniqueness of this approach depends on the fact that the Cys residue is missing in the mammalian enzyme. Moreover, the Cys residue is sterically hindered in some insects such as Drosophila melanogaster and it is accessible to standard insecticides only with difficulty.12,13 Thus, it is believed that Cys-targeted insecticides might possess significant selectivity favoring Anopheles AChE over mammalian AChE.3,11,14
In this paper we report the optimization and total synthesis of some of the previously reported Cys-targeted insecticides. Insecticides targeted to free cysteine were first proposed by computational studies in 2012, and some of them have already been investigated.12,15 Herein, we describe a novel and more straightforward synthetic strategy for Cys-targeted insecticides starting from commercially available reagents. In other recent work, a similar synthetic approach was applied for development of several novel insecticides. The maleimide-containing compounds were prepared as Cys-targeted molecules that should directly bind to Cys447, whereas succinimide-containing compounds were developed for comparison purposes. All of them were then evaluated in vitro for their potency to block the action of recombinant Anopheles gambiae acetylcholinesterase (AgAChE1), human acetylcholinesterase (HssAChE), and human butyrylcholinesterase (HssBChE).
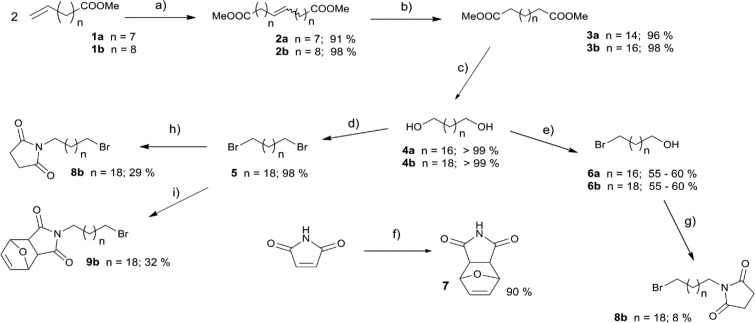
For the chemical synthesis, the long polymethylene chains were required as the basic scaffolds for the outlined compounds. The methyl esters with terminal double bond (1a and 1b) were chosen as the starting material. Olefin metathesis was applied (instead of using Grignard reactions) with Grubbs reaction yielding dimeric intermediates 2a and 2b in nearly quantitative yields (Scheme 1). There was no need to use a stereoselective reaction, because hydrogenation of the double bond took place in the next step. For this reason, a cheaper first generation Grubbs catalyst was preferred and used to obtain esters 3a and 3b instead of using one of the second generation.16 Subsequent reduction of the esters provided alcohols 4a and 4b in quantitative yields. The ester reduction can be managed by two synthetic routes. Besides lithium aluminum hydride, diisobutylaluminum hydride (DIBAL-H) was efficiently applied to obtain α,ω-dihydroxy compounds 4a and 4b (Scheme 1).
Scheme 1. Application of Olefin Metathesis to Obtain Diversely Substituted Long Chain Alkanes.
Reagents and conditions: (a) Grubbs 1st gen. (0.02 equiv), reduced pressure up to 2 mbar, RT up to 50 °C; (b) Pd(OH)2 on C (20%; 0.2 equiv), H2, MeOH/EA (2:1), RT; (c) LiAlH4 2 M solution in THF (2.5 equiv), THF, reflux; (d) NBS (3.0 equiv), PPh3 (3.0 equiv), THF, RT; (e) HBr 48% solution in H2O (3.0 equiv), toluene, reflux; (f) furan (3.0 equiv), dioxane, 90 °C; (g) DIAD (1.3 equiv), PPh3 (1.3 equiv), THF, 0 °C to RT; (h) succinimide (1.5 equiv), K2CO3 (1.5 equiv), DMF, 60°C; (i) 7 (1.5 equiv), K2CO3, (1.5 equiv), DMF, 60 °C.
A challenging step was the attempt at selective protection of one hydroxyl group to successively and selectively proceed toward the formation of alkylating agent. Initial attempts for selective protection were not successful and are described in the Supporting Information (SI section 2.4). A convenient approach consisted in the use of HBr as a selective reagent with 4a and 4b resulting in monobromide intermediates 6a and 6b.17 HBr was also employed for the synthesis in previous work; however, the authors did not fully describe how they obtained monobromide intermediates.15
Further reactions were analogous to those published.15 Diels–Alder reaction of maleimide with furan provided imide 7.18 Imide 7 and succinimide were then coupled with brominated alcohols 6a and 6b. The intermediates 8a/b and 9a/b were obtained in two steps via coupling reaction and subsequent bromination with N-bromosuccinimide (NBS) in high yields (>70%) (Scheme 2). The original procedure was simplified by skipping isolation of the alcohol intermediates (17a, 17b, 18a, and 18b; described in SI) by direct bromination to get 9a and 9b. Succinimide was used for analogous N-alkylation to obtain 8a and 8b. In this way better yields were achieved and further reduction of maleimide into a succinimide scaffold, as was described in the original work, was avoided.15
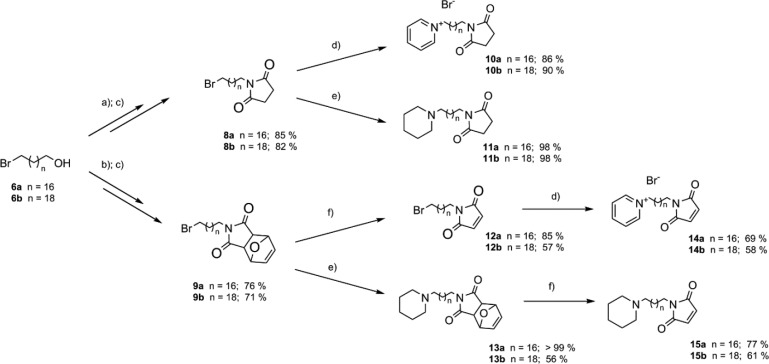
Scheme 2. Synthetic Approach for Cys-Targeted Insecticides.
Reagents and conditions: (a) succinimide (1.5 equiv), K2CO3 (1.5 equiv), DMF, 60°C; (b) 7 (1.5 equiv), K2CO3, (1.5 equiv), DMF, 60°C; (c) NBS (1.5 equiv); PPh3 (1.5 equiv), THF, RT; (d) microwave irradiation (MW), dynamic curve with 100 W and 300 PSI max cap, pyridine (2.0 equiv), MeCN, 90 °C; (e) MW, dynamic curve with 100 W and 300 PSI max cap, piperidine (2.0 equiv), K2CO3 (3.0 equiv), MeCN, 90 °C; (f) vacuum app. 1 mbar, 130 °C.
The final compounds containing the succinimide moiety (10a/b and 11a/b) were obtained using microwave irradiation. The N-alkylation of 8a/b achieved almost quantitative yields in the case of the piperidine analogues (11a and 11b) and around 90% for the pyridine analogues (10a and 10b; Scheme 2). The final preparation of compounds 14a/b and 15a/b containing a maleimide residue required a slightly different approach. In the case of the pyridinium compounds 14a and 14b, retro Diels–Alder reaction was used prior to the N-alkylation. For the piperidine analogues 15a and 15b, the N-alkylation of 9a/b had to precede the retro Diels–Alder reaction to achieve the desired products. Both the N-alkylation and the retro Diels–Alder reactions were carried out with good yields over 58% (Scheme 2). These final steps were slightly distinct from those reported in the original work, by using different conditions and by implementation of microwave (MW) irradiation.15 Note that compounds 14a/b correspond to previously published compounds under code names PM18 and PM20, and compounds 10b, 15a, and 11a are analogous to PMS20, PY18, and PYS18, respectively (only the salts are different).15 The rest of the compounds 10a, 11b, and 15b are reported herein for the first time.
The crucial mechanism of action should be based on the formation of a covalent bond between the maleimide moiety and Cys447 in AgAChE1, resulting in irreversible inhibition. A detailed in vitro evaluation was made by Dou et al. in 2013.15 For in vitro purposes, recombinant AgAChE1 was prepared and purified in our laboratories (see SI Figure S1). The kinetic parameters of the enzyme were evaluated (see SI Figure S2). The KM constant of AgAChE1 was found to be 54.63 μM for acetylthiocholine iodide (ATCh) as a substrate, which is in very good correlation with the previously published data.19,20 To verify the enzyme and assay results, the standard inhibitors including paraoxon and carbamates (bendiocarb, carbofuran) were evaluated.
The inhibitory ability of the prepared insecticides toward HssAChE and AgAChE1 was determined using the spectrophotometric method described by Ellman et al. (Table 1).21HssBChE was used as a common off-target for AChE inhibitors. As shown in the Supporting Information, the HssBChE inhibition levels observed for the prepared compounds were over 60 μM (see SI Table S1). Maleimides 14a, 14b, and 15a were found to be the most potent inhibitors of AgAChE1 and several-fold more potent than corresponding succinimides. For HssAChE, maleimides 14a, 14b, and 15b are similar in potency to the related succinimides 10a, 10b, and 11b. However, maleimide 15a was found to be several-fold more potent than its corresponding succinimide 11a for HssAChE. As HssAChE does not contain a free cysteine residue which could react with maleimide 15a, the inhibitory ability of this compound was found unexpected and cannot be rationalized by interaction with Cys447.
Table 1. Inhibitory Activity toward AgAChE1 and HssAChE.
| IC50 ± SEM (μM)a |
||||
|---|---|---|---|---|
| Compound | AgAChE1 | HssAChE | Selectivity indexb | |
| 10a | --- | 3.60 ± 0.095 | 3.38 ± 0.143 | 0.94 |
| 10b | PMS20 | 2.06 ± 0.040 | 3.39 ± 0.338 | 1.65 |
| 11a | PYS18 | 9.97 ± 0.213 | 21.48 ± 0.843 | 2.15 |
| 11b | --- | 25.87 ± 1.704 | 29.30 ± 2.536 | 1.13 |
| 14a | PM18 | 0.465 ± 0.020 | 3.26 ± 0.194 | 7.00 |
| 14b | PM20 | 0.810 ± 0.060 | 2.03 ± 0.138 | 2.51 |
| 15a | PY18 | 0.545 ± 0.028 | 3.52 ± 0.523 | 6.45 |
| 15b | --- | 2.65 ± 0.344 | 39.50 ± 4.598 | 14.89 |
| paraoxon | 0.0072 ± 0.0004 | 0.0084 ± 0.0004 | 1.16 | |
| bendiocarb | 0.0024 ± 0.0003 | 0.0309 ± 0.0008 | 12.70 | |
| carbofuran | 0.0062 ± 0.0008 | 0.0221 ± 0.0012 | 3.59 | |
IC50 values measured by modified Ellman’s assay 15 min after introduction of inhibitor.
Selectivity for AgAChE1 is determined as the ratio IC50 (HssAChE)/IC50 (AgAChE1).
The selectivity for AgAChE1 was rather poor, and most compounds were found to be relatively potent inhibitors of HssAChE. Only in the case of 15b was the selectivity toward the insect enzyme found to be over 10-fold. However, the prepared compounds possess only poor inhibitory activity toward AgAChE1 compared to paraoxon or bendiocarb.
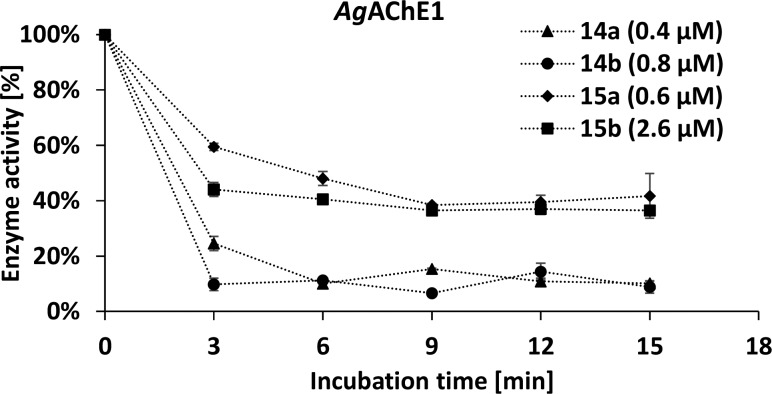
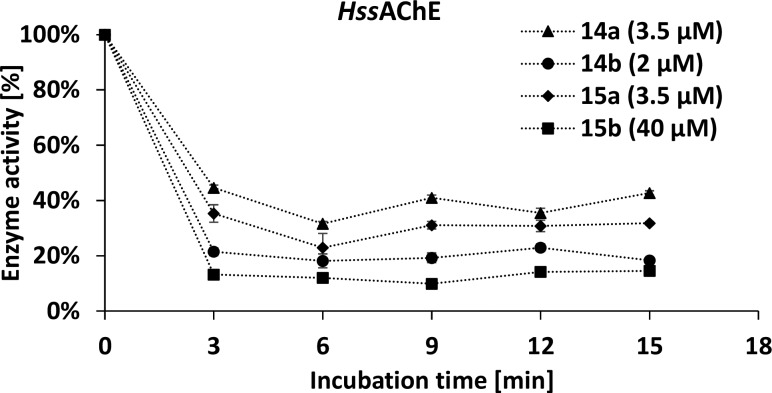
Further, time-dependent inhibition was used to determine differences between both enzymes. A rapid decrease in enzyme activity was observed within 3 min, and the activity remained almost steady for 15 min for both HssAChE and AgAChE1 (Figure 1 and 2). Surprisingly, increased potency of maleimides vs succinimides for AgAChE1 is not necessarily evidence of covalent binding, since the same phenomenon is seen for HssAChE. Additionally, the rate of enzyme–inhibitor complex formation could be explained by noncovalent interaction between pyridine/piperidine parts of the tested compounds with both enzymes. Such interactions are in general very rapid, and there is probably no covalent bond formation involved in this short time period.22,23 The previously published time-dependency15 did not prove covalent binding of the compounds but depicted only a slower or faster rate of binding to the enzyme.
Figure 1.
Time-dependent inhibition of maleimide derivatives on AgAChE1.
Figure 2.
Time-dependent inhibition of maleimide derivatives on HssAChE.
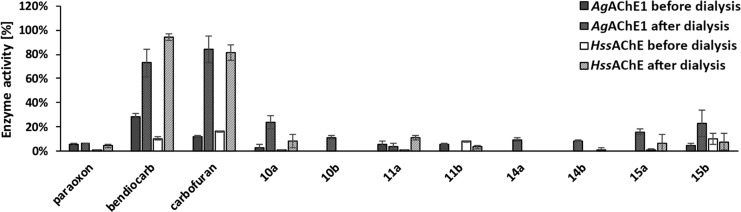
Thus, the mechanism of interaction is truly crucial for this series of compounds. We have tested the reversibility of the reaction using the removal of the selected insecticides by dialysis (Figure 3). Apparently, none of the tested compounds showed reversible action of inhibition for either AgAChE1 or HssAChE. Based on the previous work,15 maleimide-harboring compounds were presumed to act as irreversible inhibitors and succinimide compounds were prepared only as the controls to prove insect-specific cysteine involvement in covalent bond formation. The presented results are showing different findings, with all succinimide- or maleimide-containing compounds found to be irreversible-acting inhibitors when incubated 30 min with enzymes and further dialyzed overnight (Figure 3). The irreversible inhibition was shown toward HssAChE as well, although in this enzyme the Cys is substituted by Phe (Figure 4). It should also be noted that maleimide is able to form a covalent bond with other amino acid residues (except free cysteine) as was formerly reported.24 However, the most surprising fact is that all succinimides (10a/b, 11a/b) were found to be irreversible inhibitors of both enzymes with recovery less than 20%. Such findings could be plausibly explained by, e.g., precipitation or denaturation of the enzymes by the detergent-like succinimides.
Figure 3.
Reversibility of inhibition on AgAChE1 and HssAChE using the dialysis method.
Figure 4.
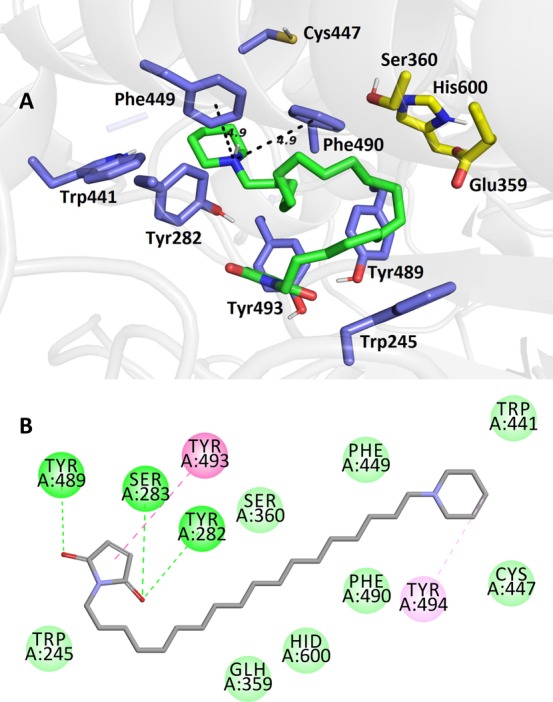
Superimposition of compound 15a in the AgAChE1 active site (PDB ID: 6ARX, G119S mutation). The close-up view for ligand is presented as three-dimensional (4A; 15a is presented in green, important amino acid residues in blue, and catalytic triad residues in yellow) and two-dimensional (4B) diagrams.
Additionally, a molecular docking study was conducted. Compound 15a was selected from all the tested compounds for its valuable and higher inhibition activity on AgAChE1 and moderate effect on HssAChE/BChE. Two protein structures were used (PDB IDs: 6ARX in Figure 4 and SI Figure S3; 5YDH only in SI Figure S3) to reveal and better understand the binding interactions between ligand 15a and the AgAChE1 active site.25 The top docking-scored pose of 15a was found different in each of the used proteins. For 6ARX with G119S mutation (Figure 4), ligand 15a resulted in binding of the maleimide moiety close to the catalytic triad and interaction with tyrosine residues (Tyr489 and Tyr493), with the piperidine ring attached at the rim of the gorge (Phe449, Phe 490, and Tyr494). Differently in the 5YDH protein (SI Figure S3), the maleimide moiety was found interacting with the rim of the AgAChE1 gorge (Cys447, Phe449, Tyr494), with the piperidine scaffold attached close to the active site (Trp245, Tyr291, Glu359). The literature was checked to elucidate these contradictory results for the involvement of tyrosine residues in maleimide binding, but there were no literature data supporting the hypothesis of covalent bond formation between a hydroxyl from either tyrosine or serine residues, and thus different binding of ligand 15a in 6ARX (Figure 4). Apart from sulfhydryl cysteine residues, only primary amines can be expected to possibly conjugate with maleimide at pH values higher than 7.5; however, for 15a this is not the case.26
The contradictory finding within in vitro evaluation and molecular docking raised the question of binding of the presented maleimide/succinimide molecules to AgAChE1 or HssAChE. The enzymes were treated with 100 molar excess of iodoacetamide (IAA) to determine the affinity of the presented inhibitors toward Cys447. IAA is a commonly used blocking agent of free thiol groups.27 Although IAA is not strictly thiol selective (as well as maleimide), the reaction should result in AgAChE1 enzyme that is resistant to irreversible inhibition by maleimide-based inhibitors. However, both enzymes treated with IAA were found irreversibly inhibited by selected compounds 11a (succinimide scaffold) and 15b (maleimide scaffold; Figure 5). Apparently, these results indicate that irreversible inhibition is most probably not mediated by the free cysteine residue Cys447 in AgAChE1 as was formerly reported.15 The maleimide or succinimide moiety can also interact with other amino acid residues, e.g. with histidine from the catalytic triad of AgAChE1 or HssAChE, and thus may irreversibly inhibit both enzymes this way.
Figure 5.
Reversibility of inhibition on AgAChE1 and HssAChE using dialysis method after iodoacetamide (IAA) pretreatment.
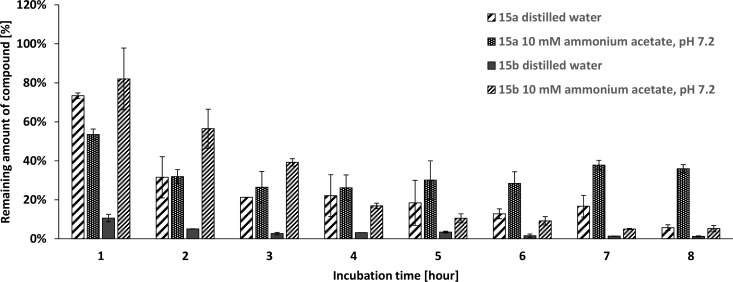
From the point of practical use for insecticidal purposes, stability of the most potent and most selective compounds for AgAChE1 (15a and 15b) was inspected by incubation at 40 °C (Figure 6). Fast degradation of both compounds was observed in a water or buffer environment, with the water degradation found to be apparently more rapid. In particular, compound 15b (the most selective for AgAChE1) was almost completely degraded within 1 h in water. Both compounds were more stable in ammonium acetate buffer, but a degradation ratio of over 50% was observed in 3 h.
Figure 6.
Stability determination of 15a and 15b over time in distilled water or buffer.
In summary, eight compounds with a succinimide or maleimide moiety were prepared and evaluated as cysteine-targeted insecticides. Some of the compounds were shown as potent AgAChE1 inhibitors, but they were found to be potent inhibitors of HssAChE with a limited selectivity ratio as well. The time-dependent inhibition of the compounds was very fast, resulting in inhibition of both enzymes within minutes. The presented compounds acted in an irreversible manner to both AgAchE1 and HssAChE. Molecular docking on two distinct AgAChE1 proteins indicated contradictory results showing the possibility of different interactions than the proposed Cys-targeted binding. This phenomenon was further confirmed by cysteine blocking treatment of both enzymes, after which they were still found irreversibly inhibited by the tested compounds, suggesting a different mode of irreverible binding. The potential use of the tested compounds might be limited by the relatively low stability depicted within the degradation assay. Thus, our findings do not support the proposed concept of cysteine-targeted selective insecticides. We presume that this class of molecules will not be effective as insecticides due to the aforementioned findings.
Acknowledgments
The work was supported by Ministry of Health of the Czech Republic (no. NV16-34390A), University of Hradec Kralove (no. SV2115-2018, no. VT2019-2021 and postdoctoral job positions at UHK), and University of Defence (Faculty of Military Health Sciences, Long-term development plan and SV/FVZ2019/01). The authors are grateful to Ian McColl MD PhD for assistance with the manuscript.
Glossary
Abbreviations
- ACh
acetylcholine
- AChE
acetylcholinesterase
- ATCh
acetylthiocholine iodide
- AgAChE1
Anopheles gambiae acetylcholinesterase
- DIBAL-H
diisobutylaluminum hydride
- HssAChE
human acetylcholinesterase
- HssBChE
human butyrylcholinesterase
- IAA
iodoacetamide
- IC50
median inhibitory concentration
- MW
microwave irradiation
- NBS
N-bromosuccinimide
- PAS
peripheral active site
- TBAF
tetrabutylammonium fluoride
- TBDPSCl
tert-butyl(chloro)diphenylsilane.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.9b00477.
General synthetic methods; general procedure and spectral data; HPLC analysis of final compounds; recombinant AgAChE1 production and characterization; enzymatic assays; molecular modeling studies; time-dependent stability measurement (PDF)
Author Contributions
⊥ L.G., R.A., and M.S. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- World Malaria Report 2016; World Health Organization: Geneva; 2016. Licence: CC BY-NC-SA 3.0 IGO.
- Fox C. M.; Kim K.-S.; Cregan P. B.; Hill C. B.; Hartman G. L.; Diers B. W. Inheritance of Soybean Aphid Resistance in 21 Soybean Plant Introductions. Theor. Appl. Genet. 2014, 127 (1), 43–50. 10.1007/s00122-013-2199-1. [DOI] [PubMed] [Google Scholar]
- Pang Y. P. Insect Acetylcholinesterase as a Target for Effective and Environmentally Safe Insecticides. Adv. Insect Physiol. 2014, 46, 435–494. 10.1016/B978-0-12-417010-0.00006-9. [DOI] [Google Scholar]
- Johnson R. M. Honey Bee Toxicology. Annu. Rev. Entomol. 2015, 60, 415–434. 10.1146/annurev-ento-011613-162005. [DOI] [PubMed] [Google Scholar]
- Casida J. E.; Durkin K. A. Neuroactive Insecticides: Targets, Selectivity, Resistance, and Secondary Effects. Annu. Rev. Entomol. 2013, 58, 99–117. 10.1146/annurev-ento-120811-153645. [DOI] [PubMed] [Google Scholar]
- Gorecki L.; Korabecny J.; Musilek K.; Malinak D.; Nepovimova E.; Dolezal R.; Jun D.; Soukup O.; Kuca K. SAR Study to Find Optimal Cholinesterase Reactivator against Organophosphorous Nerve Agents and Pesticides. Arch. Toxicol. 2016, 90 (12), 2831–2859. 10.1007/s00204-016-1827-3. [DOI] [PubMed] [Google Scholar]
- Colović M. B.; Krstić D. Z.; Lazarević-Pašti T. D.; Bondžić A. M.; Vasić V. M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11 (3), 315–335. 10.2174/1570159X11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engdahl C.; Knutsson S.; Ekström F.; Linusson A. Discovery of Selective Inhibitors Targeting Acetylcholinesterase 1 from Disease-Transmitting Mosquitoes. J. Med. Chem. 2016, 59 (20), 9409–9421. 10.1021/acs.jmedchem.6b00967. [DOI] [PubMed] [Google Scholar]
- Wong D. M.; Li J.; Chen Q.-H.; Han Q.; Mutunga J. M.; Wysinski A.; Anderson T. D.; Ding H.; Carpenetti T. L.; Verma A.; et al. Select Small Core Structure Carbamates Exhibit High Contact Toxicity to “Carbamate-Resistant” Strain Malaria Mosquitoes, Anopheles Gambiae (Akron). PLoS One 2012, 7 (10), e46712 10.1371/journal.pone.0046712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartsel J. A.; Wong D. M.; Mutunga J. M.; Ma M.; Anderson T. D.; Wysinski A.; Islam R.; Wong E. A.; Paulson S. L.; Li J.; et al. Re-Engineering Aryl Methylcarbamates to Confer High Selectivity for Inhibition of Anopheles Gambiae versus Human Acetylcholinesterase. Bioorg. Med. Chem. Lett. 2012, 22 (14), 4593–4598. 10.1016/j.bmcl.2012.05.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier P. R.; Bloomquist J. R.; Totrov M.; Li J. Discovery of Species-Selective and Resistance-Breaking Anticholinesterase Insecticides for the Malaria Mosquito. Curr. Med. Chem. 2017, 24 (27), 2946–2958. 10.2174/0929867324666170206130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y.-P.; Brimijoin S.; Ragsdale D. W.; Zhu K. Y.; Suranyi R. Novel and Viable Acetylcholinesterase Target Site for Developing Effective and Environmentally Safe Insecticides. Curr. Drug Targets 2012, 13 (4), 471–482. 10.2174/138945012799499703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel M.; Kryger G.; Rosenberry T. L.; Mallender W. D.; Lewis T.; Fletcher R. J.; Guss J. M.; Silman I.; Sussman J. L. Three-Dimensional Structures of Drosophila Melanogaster Acetylcholinesterase and of Its Complexes with Two Potent Inhibitors. Protein Sci. 2000, 9 (6), 1063–1072. 10.1110/ps.9.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M.; Hrabcova V.; Jun D.; Kuca K.; Musilek K. Vector Control and Insecticidal Resistance in the African Malaria Mosquito Anopheles Gambiae. Chem. Res. Toxicol. 2018, 31 (7), 534–547. 10.1021/acs.chemrestox.7b00285. [DOI] [PubMed] [Google Scholar]
- Dou D.; Park J. G.; Rana S.; Madden B. J.; Jiang H.; Pang Y.-P. Novel Selective and Irreversible Mosquito Acetylcholinesterase Inhibitors for Controlling Malaria and Other Mosquito-Borne Diseases. Sci. Rep. 2013, 3, 1068. 10.1038/srep01068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzaskowski J.; Quinzler D.; Bährle C.; Mecking S. Aliphatic Long-Chain C20 Polyesters from Olefin Metathesis. Macromol. Rapid Commun. 2011, 32 (17), 1352–1356. 10.1002/marc.201100319. [DOI] [PubMed] [Google Scholar]
- Chang J.; Zhang S.-J.; Jiang Y.-W.; Xu L.; Yu J.-M.; Zhou W.-J.; Sun X. Design, Synthesis, and Antibacterial Activity of Demethylvancomycin Analogues against Drug-Resistant Bacteria. ChemMedChem 2013, 8 (6), 976–984. 10.1002/cmdc.201300011. [DOI] [PubMed] [Google Scholar]
- Fujita D.; Suzuki K.; Sato S.; Yagi-Utsumi M.; Kurimoto E.; Yamaguchi Y.; Kato K.; Fujita M. Synthesis of a Bridging Ligand with a Non-Denatured Protein Pendant: Toward Protein Encapsulation in a Coordination Cage. Chem. Lett. 2012, 41 (3), 313–315. 10.1246/cl.2012.313. [DOI] [Google Scholar]
- Jiang H.; Liu S.; Zhao P.; Pope C. Recombinant Expression and Biochemical Characterization of the Catalytic Domain of Acetylcholinesterase-1 from the African Malaria Mosquito, Anopheles Gambiae. Insect Biochem. Mol. Biol. 2009, 39 (9), 646–653. 10.1016/j.ibmb.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engdahl C.; Knutsson S.; Fredriksson S.-Å.; Linusson A.; Bucht G.; Ekström F. Acetylcholinesterases from the Disease Vectors Aedes Aegypti and Anopheles Gambiae: Functional Characterization and Comparisons with Vertebrate Orthologues. PLoS One 2015, 10 (10), e0138598 10.1371/journal.pone.0138598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman G. L.; Courtney K. D.; Andres V.; Feather-Stone R. M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Ramsay R. R.; Tipton K. F. Assessment of Enzyme Inhibition: A Review with Examples from the Development of Monoamine Oxidase and Cholinesterase Inhibitory Drugs. Molecules 2017, 22 (7), 1192. 10.3390/molecules22071192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandolfi F.; De Vita D.; Bortolami M.; Coluccia A.; Di Santo R.; Costi R.; Andrisano V.; Alabiso F.; Bergamini C.; Fato R.; et al. New Pyridine Derivatives as Inhibitors of Acetylcholinesterase and Amyloid Aggregation. Eur. J. Med. Chem. 2017, 141, 197–210. 10.1016/j.ejmech.2017.09.022. [DOI] [PubMed] [Google Scholar]
- Trujillo-Ferrara J.; Vázquez I.; Espinosa J.; Santillan R.; Farfán N.; Höpfl H. Reversible and Irreversible Inhibitory Activity of Succinic and Maleic Acid Derivatives on Acetylcholinesterase. Eur. J. Pharm. Sci. 2003, 18 (5), 313–322. 10.1016/S0928-0987(03)00023-X. [DOI] [PubMed] [Google Scholar]
- Cheung J.; Mahmood A.; Kalathur R.; Liu L.; Carlier P. R. Structure of the G119S Mutant Acetylcholinesterase of the Malaria Vector Anopheles Gambiae Reveals Basis of Insecticide Resistance. Structure 2018, 26 (1), 130–136.e2. 10.1016/j.str.2017.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda J. S.; Lorsch J. R. Labeling of a Protein with Fluorophores Using Maleimide Derivitization. Methods Enzymol. 2014, 536, 79–86. 10.1016/B978-0-12-420070-8.00007-6. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Zhang Y.; Zhang H.; Harrington P. B.; Chen H. Fast and Selective Modification of Thiol Proteins/Peptides by N-(Phenylseleno)phthalimide. J. Am. Soc. Mass Spectrom. 2012, 23 (3), 520–529. 10.1007/s13361-011-0317-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.