Abstract
A series of 3-deoxy-3-N-arylated-β-d-galactoside and -guloside derivatives have been synthesized by cesium fluoride/trimetylsilylaryl triflate-mediated benzyne generation and N-arylation of 3-deoxy-3-amino-β-d-galactosides and -gulosides, respectively. Evaluation as ligands to galectin-1, 2, 3, 4N (N-terminal domain), 4C (C-terminal domain), 7, 8N, 8C, 9C, and 9N revealed that the galactosides selectively bound galectin-9C, whereas the gulosides selectively bound galectin-9N. Hence, the N-aryl group induces galectin-9 selectivity and the ligand 3C-configuration acts as an epimeric selectivity switch between the two domains of galectin-9. Furthermore, MD simulations revealed that galacto derivatives in galectin-9C and gulo derivatives in galectin-9N find stable poses with specific interactions, which proposes a possible explanation to the gal/gulo 9C/9N selectivity.
Keywords: Galactose, gulose, N-phenyl, epimers, galectin-9, selectivity
Galectins are defined as proteins with an affinity for β-d-galactopyranoside-containing glycoconjugates and a significant similarity in the carbohydrate recognition domain (CRD). Galectins can be found from plants to invertebrate and encompass 15 mammalian proteins1 and have been discovered to be involved in various important biological activities, such as cell signaling, cell trafficking, inflammation, apoptosis, lung fibrosis, and cancer progression.2−5 Different galectins exhibit different expression patterns, localization, and ligand binding specificities, which influences biological functions. Hence, to investigate the biological function of individual galectins, discovery of selective inhibitors as keys tools is critical, but also a daunting challenge as galectins have more than 80% sequence similarities in the CRD. In this context, the use of the natural ligands is limited by their typically low galectin-selectivities, as well as high polarity and sensitivity to hydrolysis and metabolism. Synthetic inhibitors may via specific interactions of synthetic noncarbohydrate structural moieties selectively bind a particular galectin and at the same time possess more favorable ADME-PK properties.
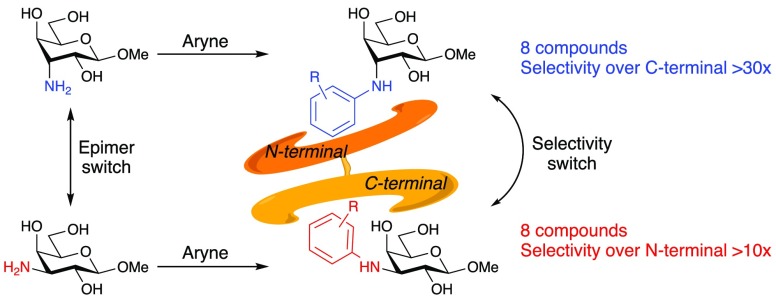
The carbohydrate binding site of the galectins can be compartmentalized into five defined subsites A–E.1 Out of these five subsites, subsite C is the most conserved and involves H-bonds between 4-OH and 6-OH and CH-π stacking with a conserved tryptophan side chain.1 Hence, viable strategies toward galectin inhibitors have often been based on keeping a galactoside core structure and modifying the anomeric and C3 positions toward compounds with improved affinities and/or selectivities. Herein, we show that 3-deoxy-3-N-aryl-β-d-galactopyranosides are highly selective inhibitors of galectin-9C (C-terminal domain) and that inverting the stereochemistry of the 3C atom to carry an axial N-aryl moiety in 3-deoxy-3-N-aryl-β-d-gulopyranosides (C3 epimer of galactose) leads to high selectivity for galectin-9N (N-terminal domain). Hence, the pyranoside 3C stereochemistry acts as an epimeric selectivity switch between the galectin-9 C- and N-terminal domains.
Results and Discussion
Synthesis of Methyl 3-N-Aryl-3-deoxy-β-d-gulopyranosides
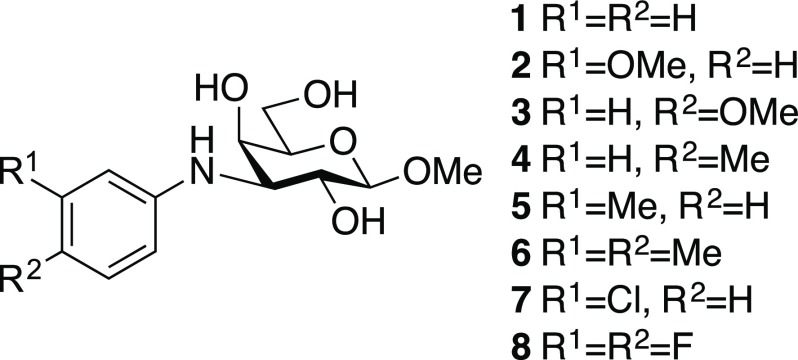
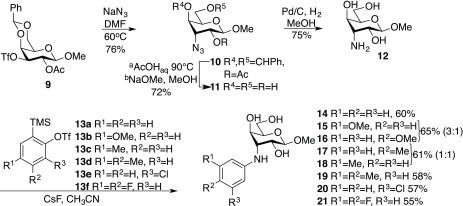
The 3-deoxy-3-N-aryl-β-d-galactoside derivatives 1–8 have been reported earlier (Chart 1).6 The synthetic route toward the 3-N-aryl β-d-gulosides 14–21 started with treatment of the galacto triflate 9(7) with NaN3 in dry DMF at 60 °C to afford the 3-azido guloside 10 (Scheme 1). Acetal hydrolysis of 10, followed by Zemplén de-O-acetylation,8 gave 11. Reduction of the azide 11 via hydrogenation over Pd/C gave the corresponding amine 12. Finally, 12 was treated with 2 equiv of cesium fluoride and a panel of trimetylsilylaryl triflates 13a–f (0.98 equiv) to obtain the N-aryl derivatives 14–21. The reaction time of the N-arylation reactions varied with the substituents of the aryl triflates. It is also noteworthy that compounds 15 and 16 (1:3) and compounds 17 and 18 (1:1) were obtained as regioisomers (separable by preparative HPLC), while compound 20 was formed as a single regioisomer.6
Chart 1. Structures of Methyl 3-N-Aryl-β-d-galactosides 1–8.6.
Scheme 1. Synthesis of Methyl 3-N-Aryl-β-d-gulosides 14–21.
Galectin Affinities of Methyl 3-N-Aryl-3-deoxy-β-d-galacto- and Gulopyranosides
The N-arylated galacto (1–8) and gulo (14–21) derivatives were evaluated as inhibitors of galectin1, 3, 7, 8N, 8C, 9N, and 9C in a competitive fluorescence anisotropy assay as earlier reported in detail9,10 (Table 1). The methyl 3-deoxy-3-N-aryl-β-d-galactopyranoside inhibitors (1–8) showed moderate to good selectivity toward galectin-9C over the other galectins investigated, including a selectivity over galectin-9N. The best affinity for galectin-9C was 130 μM with the 3,4-dimethylphenyl derivative 6, while having an affinity of 1100 μM for galectin-9N. Absence of either one of the methyl groups in 6 (i.e., 4 or 5) resulted in almost 6-fold affinity loss, as well as loss of selectivity for galectin-9C over 9N. Interestingly, the 3-methoxy-substituted 2 bound with an almost 3-fold higher affinity (Kd 140 μM) than the corresponding 4-methoxyphenyl derivative 3 and with complete selectivity for galectin-9C over 9N. In contrast, the 4-methoxyphenyl derivative 3 displayed virtually no selectivity. The 3-chlorophenyl derivative 7 and 3,4-difluorophenyl derivative 8 bound with affinities of 160 μ M and 220 μM, respectively, and with almost an order of magnitude selectivity for galectin-9C over 9N. In contrast, the methyl 3-deoxy-3-N-aryl-gulopyranosides revealed a reversed selectivity with affinities in the mid-μM range toward galectin 9N with comparatively moderate influence by the aryl substitution patterns and no binding to galectin-9C at up to 2 mM concentration (Table 1). Interestingly, the 3,4-difluorophenyl 21 showed weak affinity for galectin-1. The published affinities of methyl β-glycosides of d-galactose13 and d-gulose14 for galectins were included as references to the evaluations. The affinities of galectin-9N and 9C for methyl β-d-galactoside are significantly lower than for 1–8, which shows that the N-aryl moieties of 1–8 interact favorably with both galectin-9N and 9C, but more so with galectin-9C. The methyl β-d-guloside bound only very weakly to galectin-1, 2, and -4C, clearly showing that the N-aryl moieties of 14–21 make favorable and selective interactions with the binding site of galectin-9N.
Table 1. Kd-Values (μM)a of 1–8 and 14–21 against Human Galectin-1, 2, 3, 4N, 4C, 7, 8N, 8C, 9N, and 9C as Measured by a Fluorescence Anisotropy Assayb.
| Galectins |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | 1 | 2 | 3 | 4N | 4C | 7 | 8Nc | 8Cd | 9N | 9C |
| 1 | 1200 ± 40 | NB | NBe | NB | NB | NB | NB | NB | NB | 678 ± 30 |
| 2 | 1300 ± 10 | 1400 ± 438 | NB | 3100 ± 166 | 2400 ± 54 | NB | NB | NB | NB | 140 ± 16 |
| 3 | 1200 ± 22 | NB | NB | 1200 ± 126 | 1300 ± 96 | 1500 ± 100 | NB | NB | 600 ± 26 | 440 ± 22 |
| 4 | 310 ± 6 | NDf | NB | 2300 ± 433 | NB | NB | NB | NB | NB | 770 ± 25 |
| 5 | 850 ± 1 | 5200 ± 774 | NB | NB | 1000 ± 179 | NB | NB | NB | 1700 ± 20 | 670 ± 23 |
| 6 | 750 ± 4 | ND | NB | ND | ND | NB | NB | NB | 1100 ± 30 | 130 ± 9 |
| 7 | 1400 ± 0 | 3500 ± 483 | NB | 1800 ± 195 | 1400 ± 107 | 2000 ± 20 | NB | NB | 1500 ± 300 | 160 ± 6 |
| 8 | 1500 ± 21 | 3200 ± 720 | NB | NB | 1800 ± 160 | NB | NB | NB | NB | 220 ± 7 |
| 14 | NB | NB | NB | NB | NB | NB | NB | NB | 1200 ± 70 | NB |
| 15 | NB | NB | NB | NB | NB | NB | NB | NB | 530 ± 49 | NB |
| 16 | NB | NB | NB | NB | NB | NB | NB | NB | 580 ± 61 | NB |
| 17 | NB | NB | NB | NB | NB | NB | NB | NB | 590 ± 40 | NB |
| 18 | NB | ND | NB | ND | ND | NB | NB | NB | 980 ± 55 | NB |
| 19 | NB | ND | NB | ND | ND | NB | NB | NB | 470 ± 19 | NB |
| 20 | NB | NB | NB | NB | NB | NB | NB | NB | 670 ± 26 | NB |
| 21 | 1800 | NB | NB | NB | NB | NB | NB | NB | 1100 ± 150 | NB |
| Me β-d-galactoside | >1000011 | 1300014 | 410011 | 660014 | 1000014 | 480011 | 630013 | >300008 | 330014 | 860012 |
| Me β-d-guloside13 | 10000 | 10000 | NB | ND | 11000 | ND | NB | NB | NB | NB |
The data are averages and standard deviations of 4–8 single–double point measurements.
Methyl β-d-galacto- and gulopyranosides are included as reference compounds.
N-terminal domain.
C-terminal domain.
Not binding at the highest concentration tested: 2 mM.
Not determined.
Synthesis of Phenyl 3-N-Aryl-3-deoxy-α-d-thio-galacto- and gulopyranosides
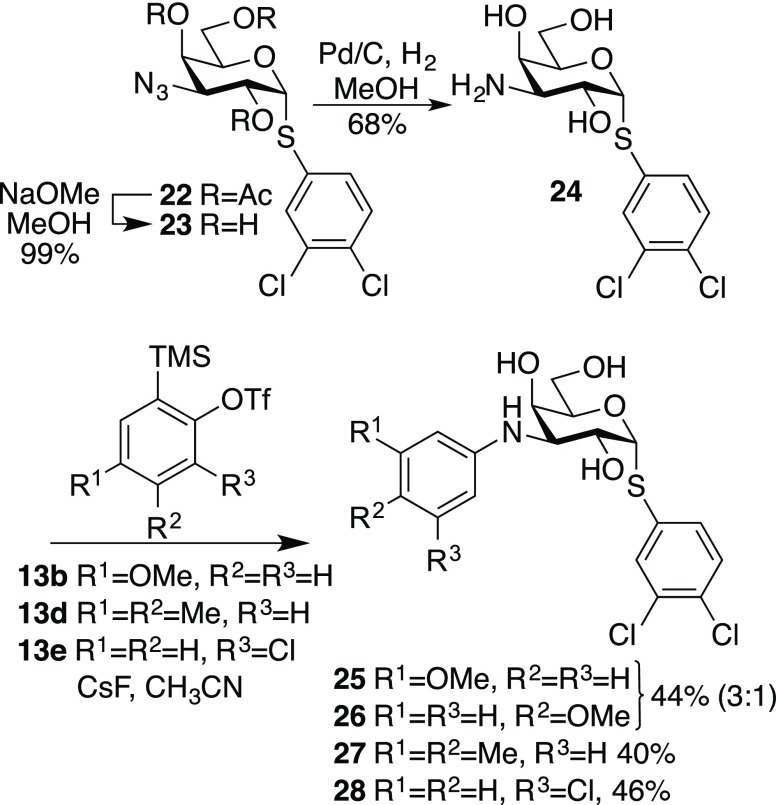
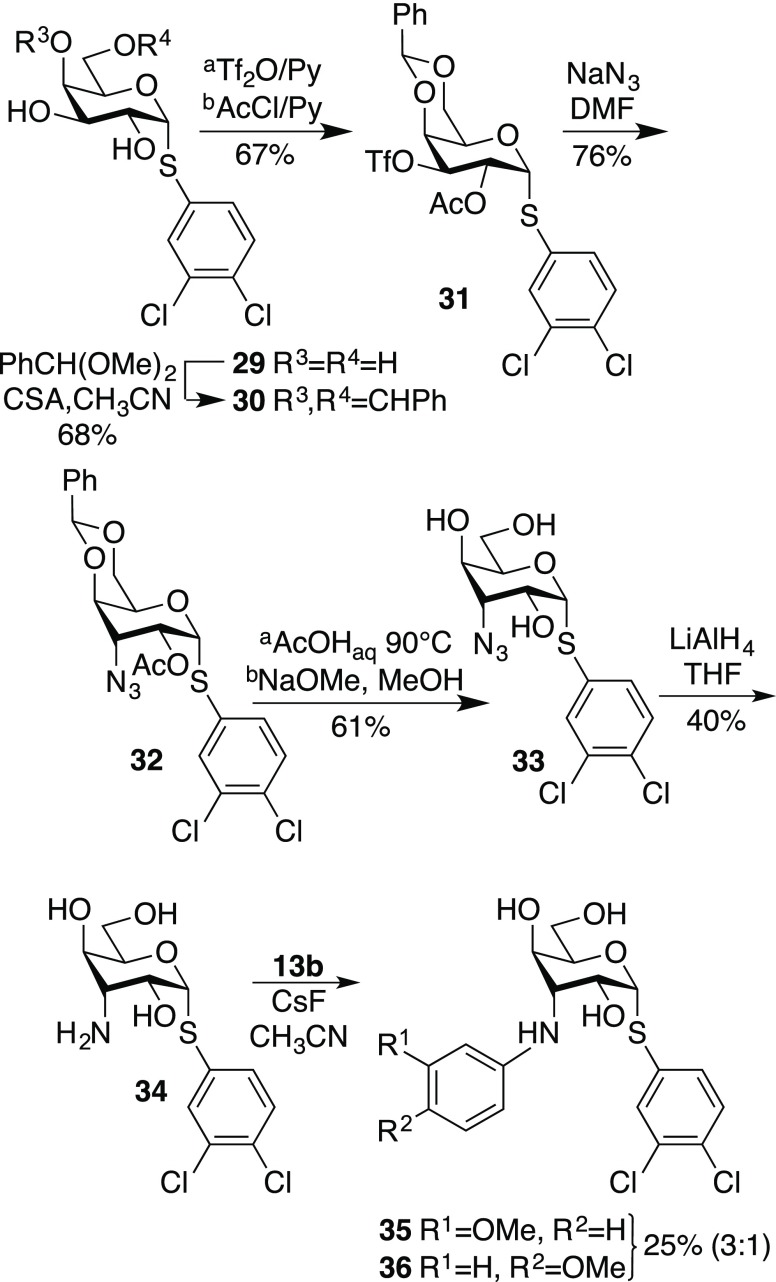
Large affinity enhancements for galectin-3 by halo-substituted α-phenyl thiogalactosides were recently reported,15 while combining the N-aryl moieties of the galacto- and gulo derivatives 1–8 and 14–21 with an α-3,4-dichlorophenyl aglycon was hypothesized to be a viable strategy toward improved affinities toward galectin-9N and 9C. De-O-acetylation of the known 3,4-dichlorophenyl 2,4,6-tri-O-acetyl-3-azido-3-deoxy-α-thiogalactoside 22(15) to give 23, followed by azide reduction, gave the amine 24. N-Arylation of 24 with aryl triflates and cesium fluoride afforded the α-galacto derivatives 25–28 (Scheme 2). In order to synthesize the corresponding gulo derivative, the known 3,4-dichlorophenyl thiogalactoside 29(14) was converted to the benzylidene derivative 30. One-pot selective 3-O-triflation and 2-O-acetylation of 30 gave 31. The 3-azido-3-deoxy-guloside 32 was obtained via substitution of 31 with sodium azide. Debenzylidenation, de-O-acetylation, and azide reduction with LiAlH4 gave the amine 34, which was N-arylated to give a 3:1 mixture of the α-thio-gulosides 35 and 36, which were separated by preparative HPLC (Scheme 3). The yield at the arylation step was disappointingly low, which is likely be due to a steric effect by the thiophenyl aglycon moiety.
Scheme 2. Synthesis of 3-N-Aryl Galactosides 24–27.
Scheme 3. Synthesis of 3-N-Aryl Gulosides 34–35.
Galectin Affinities of Phenyl 3-N-Aryl-3-deoxy-α-d-thio-galacto- and gulopyranosides
The 3,4-dichlorophenyl α-thiogalactosides 25–28 bound with high affinity toward galectin-9C with the best affinity of 2.8 μM for m-OMe derivative 26 (Table 2). However, the selectivity was to some extent compromised; compound 28 showed only 2–3-fold selectivity for galectin-9C over 9N and exhibited binding to other galectins as well. On the other hand, the corresponding gulopyranoside 35 was completely selective for galectin-9 and about 5-fold selective for galectin-9N over 9C. Its other regioisomer 36 displayed somewhat weaker binding but improved selectivity for galectin-9N.
Table 2. Kd-Values (μM)a of 25–28 and 35–36 against Human Galectin-1, 2, 3, 4N, 4C, 7, 8N, 8C, 9N, and 9C as Measured by a Fluorescence Polarization Assay.
| Galectin |
|||||||
|---|---|---|---|---|---|---|---|
| Compound | 1 | 3 | 7 | 8N | 8C | 9N | 9C |
| 25 | 41 ± 1.3 | 79 ± 3 | 36 ± 7.4 | 330 ± 25 | 98 ± 4 | 14 ± 0.6 | 15 ± 0.9 |
| 26 | 120 ± 7 | 79 ± 9 | 13 ± 2.1 | NBb | 240 ± 25 | 6.1 ± 0.1 | 2.8 ± 0.3 |
| 27 | 44 ± 6 | 170 ± 6 | 94 ± 20 | 490 ± 16 | 140 ± 6 | 12 ± 1.3 | 5.8 ± 0.3 |
| 28 | 46 ± 4 | 81 ± 9 | 17 ± 1.8 | 440 ± 15 | 96 ± 6 | 14 ± 0.5 | 4.6 ± 0.4 |
| 35 | NB | NB | NB | NB | NB | 40 ± 0.9 | 210 ± 5.6 |
| 36 | NB | NB | NDc | ND | ND | 260 ± 12 | NB |
The data are average and standard deviation of 4–8 single–double point measurements.
Not binding at the highest concentration tested: 2 mM.
Not determined.
Computational Modeling
In order to understand the difference in binding of the galacto/gulo epimers to galectin-9N and 9C, the galacto compound 6 and its corresponding gulo epimer 18 were subjected to 120 ns molecular dynamics simulations in complexes galectin-9C and 9N, respectively. The galactoside 6 had a stable pose throughout the simulation with galectin-9C and displaced all water molecules from subsite B (Figure 1A). Furthermore, the N-aryl ring of 6 made a T-stacking with His-223, which was stable throughout the simulation. On the other hand, corresponding gulo epimer 18 did not find a stable pose throughout the time scale of the simulation and failed to make any specific interactions with the galectin-9C (Figure 1B). In contrast, the simulations of the guloside 18 in complex with galectin-9N resulted in stable poses with potentially productive interactions (Figure 1D). The N-aryl ring of 18 T-stacked with Trp-82 and shielded a side of Arg-77 from contact with presumably poorly solvated water molecules.16 The galactoside 6 did not find as stable poses or interactions with galectin-9N (Figure 1C) as 18 did. Furthermore, the N-aryl ring of 6 was rotated out of conjugation with the nitrogen in the simulations with galectin-9N.
Figure 1.
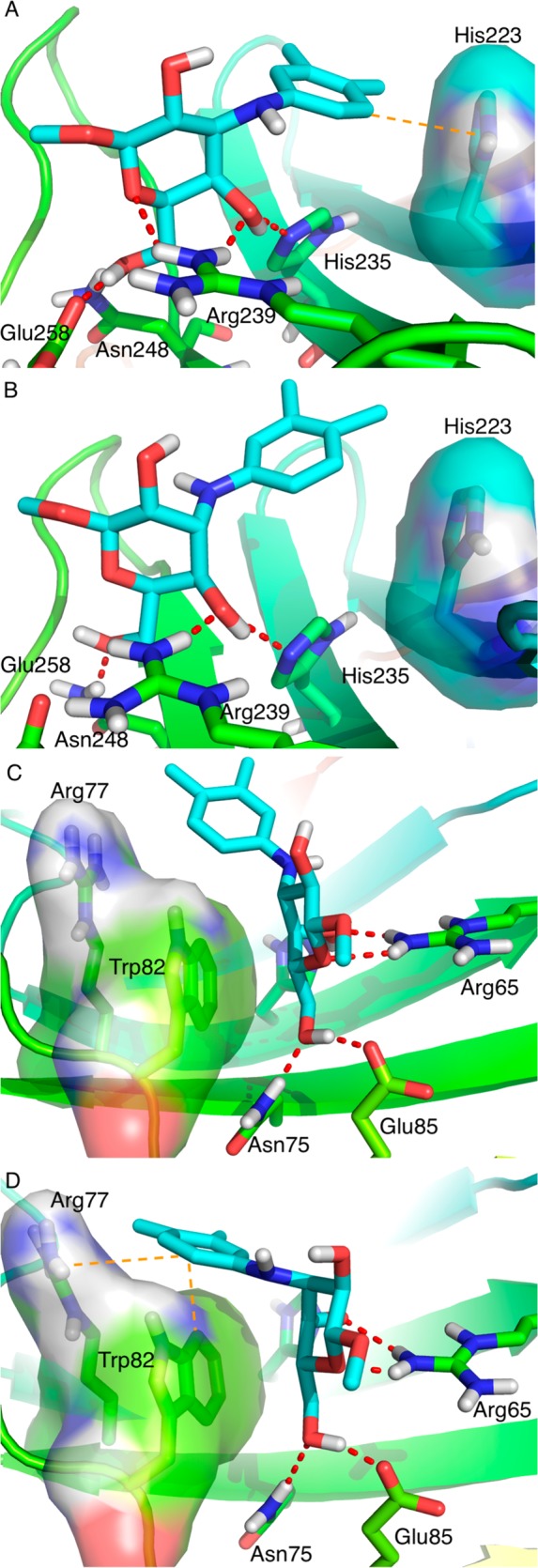
Representative molecular dynamics simulation snapshots of (A) compound 6 with galectin-9C (aryl-His233 stacking is indicated with a dashed orange line), (B) compound 18 with galectin-9C, (C) compound 6 with galectin-9N, and (D) compound 18 with galectin-9N (aryl-Arg77 and Trp82 interactions are indicated with dashed orange lines).
Conclusions
In conclusion, we discovered that galacto/gulo epimers N-arylated at 3C displayed different binding selectivities toward the two different domains of galectin-9; the galactose derivatives were selective for galectin-9C over galectin-9N whereas the N-arylated gulo derivatives (the 3-epimers of galactose) proved to be highly selective for galectin-9N. In quest of enhancing the inhibitor affinities, an α-thiophenyl aglycon fragment was installed on the 3C N-phenyl-galactoside and -guloside structures, which lead to low micromolar affinities for galectin-9C by the galacto derivatives albeit with a compromised selectivity. The corresponding phenyl thio-α-gulo derivatives showed significantly improved affinity toward galectin-9C and retained high selectivity over other galectins. Molecular dynamic simulations suggested that 3C N-phenyl-galactosides engaged in specific interactions with the galectin-9C binding site, which the corresponding gulo derivatives failed to do. In contrast, the molecular dynamic simulations with galectin-9N suggested that the better affinity and selectivity of the gulo derivatives may be explained by selective interactions of the gulo derivatives’ N-phenyl moieties with the binding site of galectin-9N. Altogether, these results demonstrate that high subtype and intradomain galectin-selectivity can be achieved by means of comparatively minor structural modifications of epimeric pyranoside scaffolds. Access to domain-selective galectin-9 inhibitors is valuable as a pharmacological tool to elucidate the molecular basis for the biological roles of galectin-9. Inhibition of galectin-9N and 9C by galactose-derived small molecules has been reported in numerous publications to be very weak and thus only as reference data to support high selectivity toward other galectin, except in one case involving amidine-derivatized galactosides.12 The amidine-derivatized galactosides in this work displayed mid-μM affinities and good selectivity toward galectin-9N with the best galactoside having a Kd value of 42 μM. The most potent galectin-9 monosaccharide-derived inhibitors are α-thiogalactosides carrying substituted phenyltriazoles a C3.15 Albeit having Kd values down to 2.4 and 2.7 μM for galectin-9C and 9N, respectively, these compounds displayed much higher affinities for several other galectins, thus having no selectivity at all for any of galectin-9N or 9C. Larger thiodigalactoside-derivatives have been reported to have sub-μM affinity for galectin-9N and 9C, but again affinities for other galectins were much higher with these derivatives.18 Hence, the N-aryl derivatives presented herein compare favorably with known galectin-9 inhibitors not only in terms of allowing epimer-controlled selectivity switching but also in terms of affinities and selectivities for both galectin-9C and 9N. Hence, the compounds constitute promising lead structures for the development of galectin-9-targeting immune-regulating novel drugs given e.g. the role of galectin-9 in stimulating induced Treg cells via binding to CD44 and subsequent amplification of TGF-β signaling, Foxp3 expression, and Smad phosphorylation.19
Acknowledgments
This work was supported by the Swedish Research Council (Grants No. 621-2012-2978), the Royal Physiographic Society, Lund, a project grant awarded by the Knut and Alice Wallenberg Foundation (KAW 2013.0022), and by Galecto Biotech AB, Lund, Sweden.
Glossary
Abbreviations
- CRD
carbohydrate recognition domain
- ADME
absorption–distribution–metabolism–excretion
- PK
pharmokinetic
- N
N-terminal domain
- C
C-terminal domain
- DMF
N,N-dimethylformamide
- TMS
trimethylsilyl
- Tf
triflic
- CSA
camphor sulfonic acid
- THF
tetrahydrofuran
- HPLC
high performance liquid chromatography
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.9b00396.
Experimental details, physical properties for new compounds, and copies of 1H and 13C NMR spectra (PDF)
Author Contributions
§ M.M. and K.B.P. contributed equally.
The authors declare the following competing financial interest(s): H.L. and U.J.N. are shareholders in Galecto Biotech AB, Lund, Sweden.
Supplementary Material
References
- Johannes L.; Jacob R.; Leffler H. Galectins at a glance. J. Cell Sci. 2018, 131, 1–9. 10.1242/jcs.208884. [DOI] [PubMed] [Google Scholar]
- Rabinovich G. A.; Croci D. O. Regulatory circuits mediated by lectin-glycan interactions in autoimmunity and cancer. Immunity 2012, 36, 322–335. 10.1016/j.immuni.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Rabinovich G. A.; Toscano M. A. Turning ’sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 2009, 9, 338–352. 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- Costa A. F.; Gomes A. M.; Kozlowski E. O.; Stelling M. P.; Pavão M. S. G. Extracellular galectin-3 in tumor progression and metastasis. Front. Oncol. 2014, 4, 1–9. 10.3389/fonc.2014.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput V. K.; Mandal S.; Collins P.; Blanchard H.; Leffler H.; Mackinnon A.; Sethi T.; Schambye H.; Mukhopadhyay B.; Nilsson U. J. Synthesis of thiodigalactoside coumarin hybrids: Discovery of selective nanomolar antagonists evidencing the rate-limiting role of galectin-3 in bleomycin-induced pulmonary fibrosis. J. Med. Chem. 2016, 59, 8141–8147. 10.1021/acs.jmedchem.6b00957. [DOI] [PubMed] [Google Scholar]
- Pal K. B.; Mahanti M.; Nilsson U. J. Arynes in transition- metal-free monoarylation of unprotected carbohydrate amines. Org. Lett. 2018, 20, 616–619. 10.1021/acs.orglett.7b03741. [DOI] [PubMed] [Google Scholar]
- Öberg C. T.; Noresson A.-L.; Delaine T.; Larumbe A.; Johan Tejler J.; von Wachenfeldt H.; Nilsson U. J. Synthesis of 3-azido-3-deoxy-β-d-galactopyranosides. Carbohydr. Res. 2009, 344, 1282–1284. 10.1016/j.carres.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Zemplén G. Abbau der reduzierenden Biosen I. Direkte Konstitutionsermitt-lung der cellobiose. Ber. Dtsch. Chem. Ges. B 1926, 59, 1254. 10.1002/cber.19260590626. [DOI] [Google Scholar]
- Sörme P.; Kahl-Knutson B.; Wellmar U.; Nilsson U. J.; Leffler H. Fluorescence polarization to study galectin−ligand interactions. Methods Enzymol. 2003, 362, 504–512. 10.1016/S0076-6879(03)01033-4. [DOI] [PubMed] [Google Scholar]
- Sörme P.; Kahl-Knutson B.; Huflejt M. U.; Nilsson U. J.; Leffler H. Fluorescence polarization as an analytical tool to evaluate galectin–ligand interactions. Anal. Biochem. 2004, 334, 36–47. 10.1016/j.ab.2004.06.042. [DOI] [PubMed] [Google Scholar]
- Cumpstey I.; Carlsson S.; Leffler H.; Nilsson U. J. Synthesis of a phenyl thio-α-d-galactopyranoside library from 1,5-difluoro-2,4-dinitrobenzene: discovery of efficient and selective monosaccharide inhibitors of galectin-7. Org. Biomol. Chem. 2005, 3, 1922–1932. 10.1039/b502354h. [DOI] [PubMed] [Google Scholar]
- Mandal S.; Rajput V. K.; Sundin A. P.; Leffler H.; Mukhopadhyay B.; Nilsson U. J. Galactose-amidine derivatives as selective antagonists of galectin-9. Can. J. Chem. 2016, 94, 936–939. 10.1139/cjc-2015-0598. [DOI] [Google Scholar]
- Pal K. B.; Mahanti M.; Leffler H.; Nilsson U. J. A galactoside-binding protein tricked into binding unnatural pyranose derivatives: 3-deoxy-3-methylene gulosides selectively inhibit galectin-1. Int. J. Mol. Sci. 2019, 20, 3786–3807. 10.3390/ijms20153786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal K. B.; Mahanti M.; Huang X.; Persson S.; Sundin A. P.; Zetterberg F. R.; Oredsson S.; Leffler H.; Nilsson U. J. Quinoline-galactose hybrids bind selectively with high affinity to a galectin-8 N-terminal domain. Org. Biomol. Chem. 2018, 16, 6295–6305. 10.1039/C8OB01354C. [DOI] [PubMed] [Google Scholar]
- Zetterberg F. R.; Peterson K.; Johnsson R.; Brimert T.; Håkansson M.; Leffler H.; Nilsson U. J. Monosaccharide derivatives with low nM lectin affinity and high selectivity based on combined fluorine-amide, phenyl-arginine, sulfur-π, and halogen bond interactions. ChemMedChem 2018, 13, 133–137. 10.1002/cmdc.201700744. [DOI] [PubMed] [Google Scholar]
- Houriez C.; Meot-Ner M.; Masella M. Solvation of guanidium in pure aqueous environments: A theoretical study from an ab ´initió-based polarizable force field. J. Phys. Chem. B 2017, 121, 11219–11228. 10.1021/acs.jpcb.7b07874. [DOI] [PubMed] [Google Scholar]
- Delaine T.; Collins P.; Mackinnon A.; Sharma G.; Stegmayr J.; Rajput V. K.; Mandal S.; Cumpstey I.; Larumbe A.; Salameh B. A.; Kahl-Knutsson B.; Van Hattum H.; Van Scherpenzeel M.; Pieters R. J.; Sethi T.; Schambye H.; Oredsson S.; Leffler H.; Blanchard H.; Nilsson U. J. Galectin-3-binding glycomimetics that strongly reduce bleomycin-induced lung fibrosis and modulate intracellular glycan recognition. ChemBioChem 2016, 17, 1759–1770. 10.1002/cbic.201600285. [DOI] [PubMed] [Google Scholar]
- Wu C.; Thalhamer T.; Franca R. F.; Xiao S.; Wang C.; Hotta C.; Zhu C.; Hirashima M.; Anderson A. C.; Kuchroo V. K. Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity 2014, 41, 270–282. 10.1016/j.immuni.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.