Important Compound Classes
Title
Modulators of Proteolysis and Associated Methods of Use
Patent Application Number
WO 2019/195609 A2
Publication Date
October 10, 2019
Priority Application
US 62/652,672
Priority Date
April 04, 2018
Inventors
Crew, A. P.; Hornberger, K. R.; Wang, J.; Dong, H.; Berlin, M.; Crews, C. M.
Assignee Company
Arvinas Operations, Inc.; 5 Science Park, New Haven, Connecticut 06488. Yale University; 2 Whitney Avenue, New Haven, Connecticut 06510.
Disease Area
Cancers
Biological Target
Kirsten Ras Sarcoma Protein (KRAS)
Summary
Ras proteins belong to the small GTPase family that are involved in transmitting growth, survival, and proliferation signals within cells. They cycle between a GTP-bound state and a GDP-bound inactive state, and the transition between these states is regulated by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs). The primary structural changes occur in the switch domains, switch 1 (SW1) and switch 2 (SW2), which constitute a major portion of the guanine nucleotide binding pocket. In the GTP-bound active state, Ras interacts with effector proteins and activates a number of downstream cellular signal transduction pathways, including the PI3K-AKT-mTOR, RAF-MEKERK, and RalGDS pathways.
In humans, there are three (KRAS, HRAS, and NRAS) common RAS genes, and their mutation frequencies and distribution are not uniform among these three family members. The most frequently mutated gene (KRAS) is altered in 86% of RAS-altered cancers, and RAS proteins are associated with about 16% of all human cancers. The RAS GTP binding sites of G12, G13, and Q61 are three hot spot point mutations, and G12D mutations are predominantly found in pancreatic, colon, and rectal carcinomas. Activation of RAS by receptor tyrosine kinases such as epidermal growth factor receptors and guanine nucleotide exchange factors (GEFs) is recruited to KRAS and initiates exchange of GDP for GTP. The active KRAS interacts with effectors such as Raf in the MAPK pathway and PI3K in the AKT pathway, which drives cell growth and proliferation. Upon GTP hydrolysis during the RAS cycle, signaling is turned off. However, oncogenic mutations that impair its GAP-mediated or intrinsic GTPase activity render KRAS constitutively active and cause uncontrolled cell growth, metabolic reprogramming, and sustained proliferation, which may lead to cancer. Consequently, mutant KRAS is a highly sought-after anticancer drug target. In spite of the decades of research efforts, drugging KRAS and RAS proteins in general have remained a formidable drug target. This is due to the lack of a deep, hydrophobic pocket on the surface of KRas, which is suitable for potent and selective small molecule binding. Second, KRas exerts its biological effects via protein–protein interactions, which are often difficult to disrupt with small molecules, due to their large surface areas. Thus, competitive inhibition becomes impractical and efforts to avoid off-target activities become challenging. An alternate strategy involves the indirect RAS inhibition by targeting its interaction partner proteins or membrane localization, which direct the allosteric KRAS inhibitors, and this has been a major focus by many research groups.
Recent development has shown that E3 ubiquitin ligases confer substrate specificity for ubiquitination and may provide exciting therapeutic potential. The discovery of Nutlins, the first small molecule E3 ligase mouse double minute 2 homologue (MDM2) inhibitors, helped in the discovery of additional compounds that target MDM2. The von Hippel–Lindau (VHL) tumor suppressor, an E3 ligase with promising therapeutic potential, has Hypoxia Inducible Factor 1α (HIF-1α) as the primary substrate. Furthermore, cereblon (CRBN) forms an E3 ubiquitin ligase complex with damaged DNA binding protein 1 (DDB1), Cullin-4A (CUL4A), and regulator of cullins 1 (ROC1), which ubiquitinates a number of other proteins.
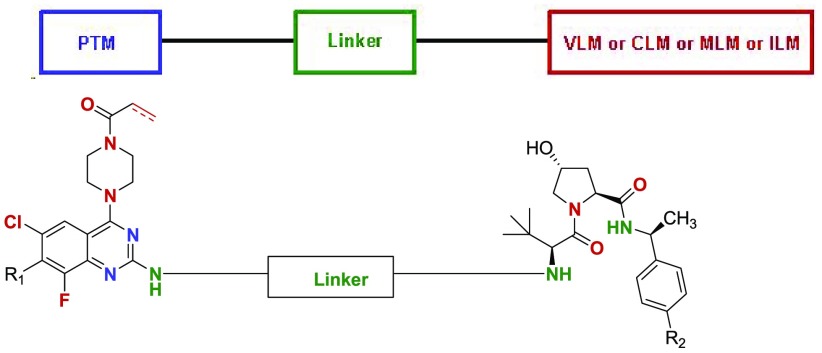
Compounds in this Patent Highlight are bifunctional or proteolysis targeting chimeric (PROTAC) compounds, which are modulators of targeted ubiquitination of a variety of polypeptides and other proteins, which are then degraded or inhibited. The compounds displayed broad range of pharmacological activities from virtually any protein class or family and using an effective amount of the compounds for the treatment or amelioration of a disease condition such as cancer, including colon cancer, pancreatic cancer, colorectal cancer, nonsmall cell lung cancer, cervical cancer, bladder cancer, liver cancer, and breast cancer. The PROTAC compound comprises a ligand for an E3 ubiquitin ligase or “ULM” group such as VHL or CRBN; L is a linker and a moiety that binds the target protein (“PTM” group such as KRAS), which is placed in close proximity to the ubiquitin ligase to effect degradation of that protein.
Definitions
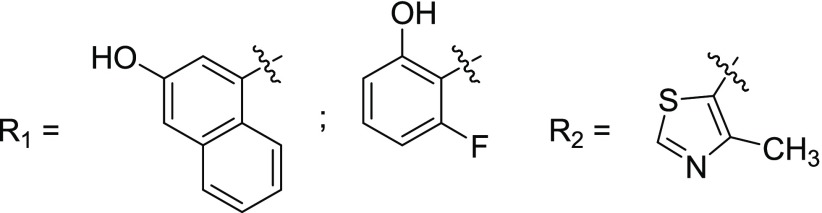
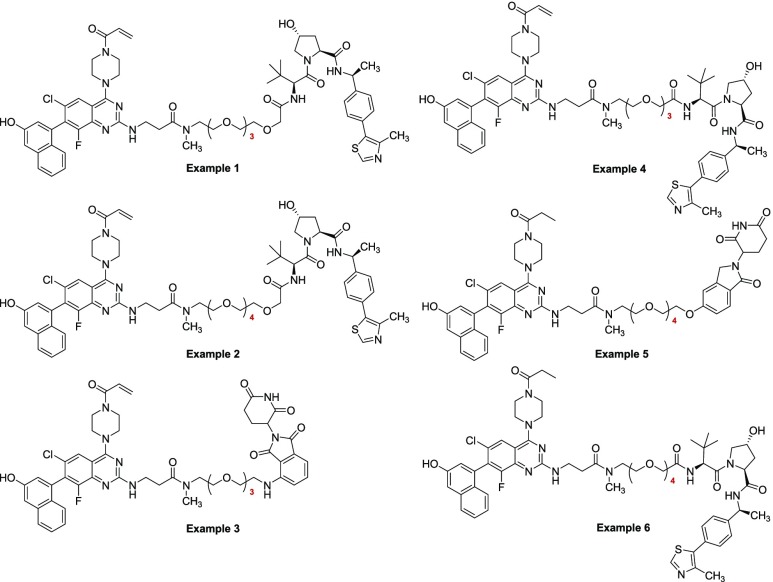
Key Structures
Biological Assay
Western Blotting. KRAS was detected by immunoblotting using LSBio antibody.
Biological Data
The Table below represents PROTAC compounds
that demonstrated KRAS protein degradation. Values indicate maximal
observed degradation (Dmax) at the given
concentrations. Percent degradation: C < 25; 25 ≤ B <
50; A ≥ 50.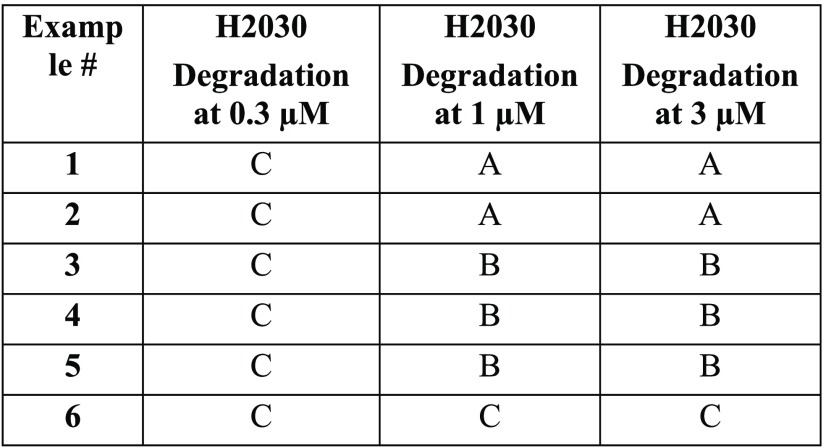
Recent Review Articles
-
1.
Munoz-Maldonado C.; Zimmer Y.; Medova M.; Munoz-Maldonado C.; Zimmer Y.; Medova M.. Front. Oncol 2019, 91088.
-
2.
Galvano A.; Incorvaia L.; Badalamenti G.; Rizzo S.; Guarini A.; Cusenza S.; Castellana L.; Barraco N.; Calo V.. et al. Cancers 2019, 11, 1189.
-
3.
Dvorak K.; Higgins A.; Palting J.; Cohen M.; Brunhoeber P.. Patho. Oncol. Res. 2019, 25, 349.
-
4.
Sideris M.; Emin E. I.; Hanrahan J.; Abdullah Z.; Hollingworth T.; Odejinmi F.; Vimplis S.; Willmott F.. et al. Anticancer Res. 2019, 39, 533.
-
5.
Li S.; Counter C. M.; Balmain A.. Nat. Rev. Cancer 2018, 18, 767.
-
6.
Ostrem J. M.; Shokat K. M.. Nat. Rev. Drug Discovery 2016, 15, 771.
The author declares no competing financial interest.