Abstract
Systemic vasculitis is diverse group of autoimmune disorders which are characterized by inflammation of blood vessel walls with deep aching and burning pain. Their underlying etiology and pathophysiology still remain poorly understood. Extracellular vesicles (EVs), including exosomes, microvesicles (MVs), and apoptotic bodies, are membrane vesicular structures that are released either during cell activation, or when cells undergo programmed cell death, including apoptosis, necroptosis, and pyroptosis. Although EVs were thought as cell dusts, but now they have been found to be potently active since they harbor bioactive molecules, such as proteins, lipids, nucleic acids, or multi-molecular complexes. EVs can serve as novel mediators for cell-to-cell communications by delivery bioactive molecules from their parental cells to the recipient cells. Earlier studies mainly focused on MVs budding from membrane surface. Recent studies demonstrated that EVs may also carry molecules from cytoplasm or even from nucleus of their parental cells, and these EVs may carry autoantigens and are important in vasculitis. EVs may play important roles in vasculitis through their potential pathogenic involvements in inflammation, autoimmune responses, procoagulation, endothelial dysfunction/damage, angiogenesis, and intimal hyperplasia. EVs have also been used as specific biomarkers for diagnostic use or disease severity monitoring. In this review, we have focused on the aspects of EV biology most relevant to the pathogenesis of vasculitis, discussed their perspective insights, and summarized the exist literature on EV relevant studies in vasculitis, therefore provides an integration of current knowledge regarding the novel role of EVs in systemic vasculitis.
Keywords: extracellular vesicles, systemic vasculitis, inflammation, autoimmunity
1. Introduction
Systemic vasculitis is a multisystem autoimmune disorder characterized by inflammation of blood vessel walls, including weakening, thickening, narrowing or scarring, in any type, size and location of blood vessels, leading to aneurysm, stenosis, occlusion, thrombosis and superficial phlebitis. Systemic vasculitis is a heterogeneous group of diseases, including takayasu arteritis (TA), giant cell arteritis (GCA), polyarteritis nodosa (PAN), Kawasaki disease (KD), antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (AAV), Behçet’s disease (BD), etc[1, 2]. Though the progress has been continuously made in the past few decades, many aspects of the systemic vasculitis, including the etiology and pathogenic mechanisms, potential diagnostic biomarkers and therapeutic targets still remain poorly understood.
Genetic susceptibility, environmental factors, as well as abnormal innate and acquired immunity play important roles in pathogenesis of systemic vasculitis [3, 4]. A meta-analysis study reported that 33 genetic variants were identified to be associated with AAV [5]. Different AAV serotypes have different genetic variants [6]. Human leukocyte antigen (HLA) *52:01 and non-HLA genes, the IL-12B region, were associated with TA susceptibility [7]. However, GCA had a significant association with HLA-DR4, which is different from TA [7]. In addition, environmental toxins, pharmacological therapies, and infections can act as triggers of vasculitis and contribute to the disease onset [8]. Furthermore, abnormal activation of innate immune cells, such as neutrophils, monocytes and dendritic cells (DC), can release pro-inflammatory cytokines and activate adaptive immunity excessively [9]. A predominance of the T-help (Th)1 and Th17 cells and reduced number or functional impairment of T regulatory (Treg) cells promote the development of systemic vasculitis [10–12].
When cells die, they can trigger inflammatory responses in the body. The dead cells can always be found in the site of inflammation [13, 14]. Extracellular vesicles (EVs) are membrane vesicles which can be released by a variety of cell types during cell activation or programmed cell death [15–17]. EVs have been shown to mediate intercellular communications, and are involved in various physiological and pathological processes [17–19], including inflammation, autoimmune responses, endothelial dysfunction/damage, procoagulation, angiogenesis and intimal hyperplasia, the pathological conditions are known to be involved in vasculitis. In this review, we will summarize the latest literature on recent advances in our understanding of the biological characteristics and pathogenic functions of EVs, and their roles of EVs in pathogenesis of systemic vasculitis.
2. Classification and characteristics of extracellular vesicles
EVs are composed of a phospholipid bilayer and cytoplasmic components, including proteins, lipids, DNA, mRNA, microRNAs [17], or multi-molecular complexes [20] derived from their parental cells. EVs can be released from different cell types, including normal cells (platelets, erythrocytes, endothelial cells, monoctyes, lymphocytes, etc) and malignant cells during cell activation, or undergo programmed cell death [15–17]. EVs can be found in blood, urine, synovial fluid, and other body fluids, many diseased organ/tissues, and even feces [21, 22]. EVs are in low concentrations under normal physiological conditions, while the levels of EVs may be increased in various pathologic conditions or diseases, such as cancer, inflammatory, autoimmune, cardio-metabolic diseases [18–23]. EVs are always heterogeneous and can be classified into three types, namely exosomes, microvesicles (MVs), and apoptotic bodies, according to their size and different biogenesis [19] (Figure 1).
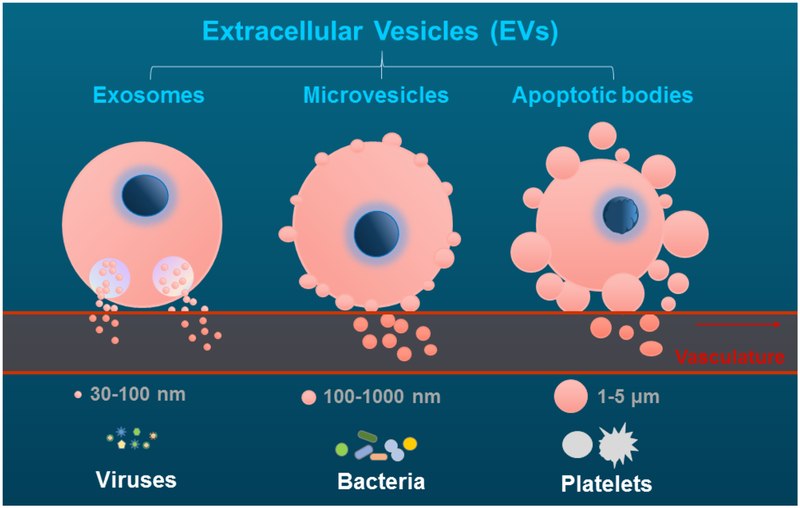
Figure 1. The formation and release of extracellular vesicles.
The schematic figure indicates the release of exosomes, microvesicles and apoptotic bodies, from their parental cells, as well as their corresponding comparisons in size to viruses, bacteria, and platelets.
Exosomes are the smallest membrane vesicles, ranging 30 nm to 100 nm in diameter. Exosomes were first described by the Johnstone group in the 1980s, and they found that transferrin receptor was shed from multivesicular elements during reticulocyte maturation [24, 25]. Exosomes are derived from the inward budding of endosomal compartments, then resulting in the formation of multivesicular bodies and released to the extracellular milieu by exocytosis [26]. The formation of exosome can be regulated by Endosomal Sorting Complex Required for Transport (ESCRT) machinery [27, 28] or ESCRT-independent machinery, such as sphingolipid ceramide [29] and the tetraspanin CD63 [30]. Composition of exosomes is variable owing to their cellular origin. Exosomes are rich in lipids, such as cholesterol and sphingolipids, but phosphatidylserine (PS) is usually not present on exosomes as they are not budding from plasma membrane surface [31]. However, some studies report different results which may be due to their different cell origin [32, 33]. In addition, MHC molecules, adhesion molecules, tetraspanins (CD63, CD9, CD81, CD82), transferrin receptors have been identified on the surface of exosomes [26]. Exosomes may also contain nucleic acids, including messenger RNA, microRNA and DNA, originated from their parental cells [33].
MVs, also called microparticles, are small heterogeneous membranous vesicles which are approximately 100–1,000 nm in diameter. MVs were first described by Peter Wolf in 1967 as “platelet dusts” which were originated from platelets [34]. MVs are generated by budding directly from cell plasma membrane [17]. Numerous signal transduction pathways participate in the production of MVs from activated cells, including activation-induced protein tyrosine dephosphorylation, protein phosphorylation, and calmodulin activation [35]. Procoagulation is one of the most studied functions of MVs due to their transport of tissue factor (TF), a transmembrane molecule that is responsible for the initiation of the extrinsic coagulation cascade and thrombus formation [36]. Our previous publications have reported the release of prothrombotic TF-positive MVs from human monocytes when they are exposed to high levels of cholesterol [37] or tobacco smoke extract [38]. The majority of MVs expose PS on their surface, which is also involved in blood coagulation by providing phospholipid surface [39]. Since MVs have specific cell surface glycoproteins from their parental cells, these cell surface markers can be used for identification of their cellular origin. For example, endothelial cells-derived MVs carry CD105 (Endoglin), CD31, CD34, CD51, CD146 or CD62 (E-Selectin), lymphocyte carry CD45, erythrocyte carry glycophorin, while platelet-derived MVs are characterized by glycoprotein Ibα polypeptide (GPIbα/CD42b) , P-selectin and CD61 (GPIIIb, integrin β3) [17, 40, 41].
In addition to the classically discussed apoptotic MVs [17], several recent studies have also shown MVs can also be released from necroptotic [16, 42], and pyroptotic [15, 43, 44] cells, two novel types of programmed cell death, which are known to be related to inflammation and autoimmune diseases, including vasculitis [45]. It has been shown that pyroptotic cells can release cytokine-containing MVs [15], i.e. IL-1β containing pyroptotic MVs [43, 44]. Studies have demonstrated that both ANCA vasculitis [46] and vasculitis syndrome – KD [47] are related to IL-1β. However, there is no direct investigation yet to determine if pyroptotic or necroptotic MVs are involved in the pathogenesis of vasculitis. The majority of necroptotic MVs are approximately 0.2–0.8 μm in size [16, 42], similar to apoptotic MVs [17]. Necroptotic cells also expose PS on their plasma membrane surface in which externalization of PS is regulated by phosphorylated mixed lineage kinase-like (MLKL) when it translocates to cell membrane [42]. MLKL can also regulate endosomal trafficking and promote the release of EVs [16]. In addition, necroptotic cells lead to the release of danger-associated molecular patterns (DAMPs) from different intracellular compartments [13].
In contrast to the above mentioned several types of cell death, NETosis is a unique form of neutrophil cell death in which nuclear DNA is decondensed and released to form neutrophil extracellular traps (NETs) [48, 49] from ruptured nuclear envelope [50] and broken plasma membranes. As we discussed in the above, EVs can serve as an extracellular platform for intercellular transferring of bioactive molecules. Similarly, neutrophil NETs can also serve as a novel type of extracellular platform for transferring of nuclear DNA and histone from nucleus, as well as the NET-associated MPO or PR3 from cytoplasm of neutrophils, and therefore play important roles in pathogenesis of vasculitis [51].
DAMPs compose of a group of heterogenous molecules, such as DNA/RNA, high mobility group protein B1 (HMGB1), histones, heat shock proteins, biglycan, decorin and fibronectin [52, 53]. These DAMP molecules, when released to the extracellular space, can be carried by both EVs and NETs. When neutrophils undergo NETosis, nuclear DNA and histones in the decondensed chromatin are released from ruptured nuclear and plasma membranes forming ‘net-like’ extracellular trap structure. Therefore, as the major components of the NETs, DNA and histones are associated with NETs and play an important role in autoimmune diseases [51]. Our recent publication indicated that HMGB1 can be externalized and released with MVs [54]. On the other hand, HMGB1 could exaggerate NET formation by interaction with TLR2, TLR4 and the receptor for advanced glycation end products (RAGE) reported by Ma and colleagues [55]. DAMPs can trigger autoimmune response and promote inflammatory response by acting as auto-adjuvant and auto-antigen and activating pattern recognition receptors (PRRs), including toll-like receptors (TLRs), purinergic receptors, and inflammasomes [56, 57]. DAMPs can also drive tissue regeneration and fibrosis by promoting TGF-β receptor signaling [58].
Apoptotic bodies are dead corpse with approximately 1000–5000 nm in diameter, and they are released in the late stage apoptotic cells [59]. Apoptosis is a programmed cell death, and occurs in various physiological and pathological processes, such as cell turnover in physiological condition, embryonic, and immune system development [60]. Rho kinase plays a crucial role in regulation of the formation of membrane blebs and re-localization of fragmented DNA into blebs and apoptotic bodies [61]. Apoptotic bodies are recognized and cleared by phagocytes under physiological condition [62]. PS is located almost exclusively in the inner leaflet of the double layer cell membrane in resting conditions, while flip to the outer leaflet membrane, therefore exposed on the membrane surface during the formation of apoptotic cells [63, 64]. PS serves as a “eat me” signal and is critical to drive recognition and phagocytosis. However, the phagocytic capability can be impaired in pathologic conditions, i.e. in patients with autoimmune diseases [65].
3. Detection of extracellular vesicles
Source identification of EVs is important for clinical studies. However, there are no standardized methods for the purification and detection of EVs until now [17]. Flow cytometry is the most widely used to detect cell-derived EVs according to their phenotype and size [17]. However, it is well known that conventional flow cytometry detect particles based on light scattering signal, and it is unable to detect EVs smaller than 200nm because these particles cannot be distinguished from background noise [22, 66], however fluorescent-labeled antibody against specific cell surface marker would be helpful. Along with the advance of biotechnology, a high-resolution flow cytometer which detects vesicles based on fluorescence intensity is introduced. High-resolution flow cytometer bright fluorescent labeling of cell-derived vesicles and can detect EVs with a diameter of 100nm [67, 68]. Other methods are also available for detecting EVs, including nanoparticle tracking analysis, electron microscopy, confocal fluorescent microscopy, fluorescence-based antibody array system, enzyme-linked immunosorbent assay (ELISA), western blotting, immune electrophoresis, have been used by our [17, 37, 38, 54] and other groups [69–71].
In addition, the process of EVs analysis can be greatly affected by many factors, including sampling process, the type of collection tube, transport conditions, phlebotomy conditions, centrifugation steps, and freezing conditions, which need to be carefully conducted [72]. Lacroix, et al. reported that three major pre-analytical parameters were the delay before the first centrifugation, agitation of the tubes during transportation and the centrifugation protocol. A double centrifugation of whole blood at 2500 – g for 15 min at room temperature can generate less artificial MVs [73]. Exosomes are generally isolated by sequential centrifugation [74].
4. Cell-Derived extracellular vesicles that are related to vasculitis
EVs derived from different cell types carry various different molecules and display different characteristics and functions. Here, we discuss several subsets of EVs with different cellular origin, i.e. platelets, endothelial cells, monocyte/ macrophages, neutrophils, and lymphocytes, which might be involved in vasculitis.
4.1. Platelet-derived extracellular vesicles
Platelets are the first responders in endothelial disruption and accumulates at the sites of vascular injury [75]. Platelet counts closely correlate with disease activity and may serve as a marker of vasculitis [76]. Platelet-derived EVs are released from activated platelets and involve in various pathophysiological processes, including thrombosis formation, inflammatory responses and intercellular signal transduction [77, 78]. Platelet-derived MVs may play more important roles in thrombogenesis than platelet-derived exosomes as MVs have more PS exposure on their surface [79]. Increased levels of platelet-derived MVs have been reported in patients with acute-phase KD, while anti-platelet therapy can decrease the MVs levels [80, 81]. Furthermore, platelet-derived MVs can be used as biomarkers for evaluating platelet activation dynamics [80] and the effect of anti-platelet therapy in KD [81]. Daniel et al. have reported the elevated levels of platelet-derived MVs, neutrophil-derived MVs, and aggregates of neutrophil/platelet MVs in acute vasculitis, therefore these MVs can be considered as non-specific markers of neutrophil activation in acute vasculitis [82]. Moreover, platelet-derived MVs can enhance leukocyte-leukocyte interactions through P-selectin, leading to the activation and aggregation of inflammatory cells [83, 84]. Platelet-derived MVs also employ effects on vascular inflammation by transferring pro-inflammatory mediators to endothelial cells, and promoting secretion of pro-inflammatory cytokines and chemokines [85]. Interestingly, aspirin has been reported to decrease the expression of HMGB1 and a-granule chemokines CXCL4 and CXCL7 on platelet-derived exosomes [86]. So platelet-derived EVs can serve as biomarkers of platelet and neutrophil activation, and play key roles in the pathogenesis of vasculitis by promoting thrombogenesis and inflammatory responses.
4.2. Endothelial cell-derived extracellular vesicles
Many studies have reported to use endothelial cell-derived EVs as markers of endothelial activation/dysfunction or vascular injury [40, 87, 88]. Increased levels of endothelial cell-derived EVs has been found in patients with systemic vasculitis, and these EVs can serve as biological markers for monitoring disease activity in systemic vasculitis [40, 87–90]. Circulating endothelial cell-derived MVs can stimulate vascular endothelium by impairing acetylcholine-induced vaso-relaxation and nitric oxide (NO) production in a concentration-dependent fashion [91]. In addition, Taguchi K et al. reported that endothelial cell-derived MVs could decrease the expression of endothelial NO synthase protein through activation of ERK1/2 [92]. Furthermore, endothelial cell-derived EVs also have prothrombotic and proinflammatory effects [93, 94], and endothelial cell-derived EVs have significant procoagulant activity that can be partly blocked by an anti-TF antibody [95]. Therefore, endothelial cell-derived EVs can lead to endothelial dysfunction though various mechanisms, which are important in pathology of vasculitis.
4.3. Monocyte/macrophage-derived extracellular vesicles
Monocyte/macrophge-derived EVs are critical players in procoagulation, endothelial activation/damage, inflammation and, vascular complications [96, 97]. Our published works reported that monocyte-derived EVs carry both TF and PS on their surface, therefore demonstrate procoagulant activities [98, 99]. Aharon and colleagues confirmed our findings that monocyte-derived EVs harbor TF [96], and also enhance endothelial expression of TF, and decrease the levels of anticoagulant tissue factor pathway inhibitor and thrombomodulin [96]. Our study also found that cholesterol-induced monocyte MVs containing biologically active danger signals could stimulate endothelial activation and leukocyte recruitment to vasculature [56]. Nair et al reported that EVs released from LPS-stimulated macrophages can be coated with histones [100], which are recently shown to induce endothelial dysfunction or damage [101, 102]. On the other hand, our published work demonstrated that MVs from monocyte/macrophages can carry proteolytic MMP14 which can degrade collagen, therefore contribute to the weakness/rupture of vascular wall [37]. Furthermore, monocyte-derived MVs may carry IL-1β and inflammasome that can amplify inflammatory responses through activation of ERK1/2 and NF-κB pathways [103]. Since pyroptotic monocytes can release IL-1β and MVs [15], one can propose that there might be IL-1β containing pyroptotic MVs. However, there is very little published work about pyroptotic MVs. In addition, exosomes released from monocyte-derived DCs can promote adaptive immune responses by enhancing the survival and activation of naive CD4+ T cells [104, 105]. Our study indicates that HMGB1 can be released with EVs into extracellular milieu from activated monocytes [54], and HMGB1-positive EVs may be important in the development of inflammatory and autoimmune diseases [54]. So monocyte-derived EVs may participate pathogenesis and development of vasculitis due to their roles in coagulation, inflammation and immune responses.
4.4. Neutrophil-derived extracellular vesicles
Neutrophils and their cytoplasmic proteinase 3 (PR3) or myeloperoxidase (MPO) are important in AAV [106–108]. Both PR3 [106] and MPO [107, 108] can be carried by EVs, and these EV-associated PR3 and MPO may serve as autoantigens to trigger production of the corresponding anti-PR3 and anti-MPO autoantibodies, therefore contributing to the development of AAV. Furthermore, PR3-ANCA and MPO-ANCA can induce neutrophil activation and stimulate the release of NETs, which also contain auto-antigens, including PR3 and MPO [51, 109, 110]. These process propagates the release of autoantigens, therefore, further enhance ANCA-associated vasculitis. On the other hand, Pitanga and colleagues reported that neutrophil-derived MVs can lead to endothelial damage in MPO-dependent manner [107]. Similar to neutrophil NETosis, neutrophil EVs may also carry nuclear molecules, including DNA/histone, from their parental cells, or even other cells [111]. EV-associated histones may also cause endothelial damage [101, 102]. Circulating levels of TF-positive neutrophil EVs are increased in patients with active AAV, suggesting the potential association between these pro-thrombotic and pro-inflammatory EVs with active AAV [112]. Furthermore, neutrophil-derived MVs express active and functional integrin αMβ2 that can also activate resting platelets and promote pro-thrombotic effects through AKT dependent pathway [113]. Neutrophil-derived MVs can promote the release of IL-6 and IL-8 and expression of intercellular adhesion molecule-1 adhesion molecule in endothelial cells, contributing to inflammatory response in AAV [114]. However, neutrophil-derived EVs may also enhance the release of anti-inflammatory factors, suggesting their role in regulation of the balance of pro-inflammatory and anti-inflammatory responses [115, 116]. In addition, Our study found that neutrophil MVs carry enzymatically active a disintegrin and metalloprotease-10 (ADAM10) and ADAM17 that could contribute to the damage/weakness of aortic wall development of human abdominal aortic aneurysm [38]. Therefore, neutrophil-derived EVs may contribute to the pathogenesis of systemic vasculitis through various mechanisms.
4.5. T-cell and B-cell derived extracellular vesicles
Lymphocytes are also important in vasculitis. The levels of T-cell, but not B-cell, derived EVs are increased in KD patients compared to healthy controls, while intravenous immunoglobulin administration results in the reduced levels of both T-cell and B-cell derived MVs [117]. Shefler et al reported that EVs derived from activated T cells contain functional miR-4443 and these EVs can activate mast cells to release proinflammatory cytokine IL-8 through down-regulation of the tyrosine phosphatase protein tyrosine phosphatase receptor type J [118]. In contrast, CD4+ T cell derived exosomes may have immunosuppressive effects for inhibition of CD8+ cytotoxic T-lymphocyte responses [119]. Exosomes derived from Treg cells can suppress Th1 cell proliferation and cytokine production by transferring microRNA Let-7d to Th1 cells [120]. Furthermore, B-cell derived EVs can carry multiple short noncoding RNA molecules that may be involved in a variety of processes in immunity and inflammation [121]. In addition, B cell-derived exosomes express functional integrins that can mediate cell-cell adherence during inflammation [122]. So T-cell and B-cell derived EVs may be involved in pathophysiology of systemic vasculitis through promoting inflammatory and autoimmune responses.
5. Potential pathologic effects of Extracellular Vesicles on systemic vasculitis
EVs can mediate cell-cell communication by transferring bioactive molecules from their parental cells to target cells through different mechanisms. Ligands or other cell surface molecules released with EVs can bind to receptors of target cells, and then trigger intracellular signaling, and result in proinflammatory responses or cytokine release. Other cellular components, namely proteins, lipids, DNA, mRNA and microRNA, multimolecular complexes, can also be transferred to target cells and promote inflammatory responses [123, 124]. EVs may be involved in pathogenesis of systemic vasculitis through their role in inflammation, autoimmunity, pro-coagulation, endothelial damage/dysfunction, angiogenesis, and intimal hyperplasia [17, 18, 125–127].
5.1. Inflammation
EVs may exert either pro-inflammatory or anti-inflammatory properties. As summarized in figure 2, EVs can transport cytokines, chemokines or other pro-inflammatory molecules, therefore emerged as a novel mechanism for spread and maintenance of inflammation. It has been reported that EVs derived from activated monocytes/macrophages contain IL-1β and inflammasome, therefore promote inflammation responses [20, 103, 128, 129]. On the other hand, EVs can stimulate the synthesis and secretion of proinflammatory mediators from immune cells through cell-cell interactions [130]. EVs have been shown to enhance the synthesis of IL-1β, TNFα, IL-6, IL-8, monocyte chemoattractant protein 1 (MCP-1), and MCP-2 [131–133]. Our recent studies also showed that MVs could induce the release of TNF-α from cultured peripheral blood mononuclear cells [134, 135]. In addition, Esser J et al. have reported that macrophages and DCs derived exosomes containing functional enzymes can induce the biosynthesis of leukotrienes which are potent proinflammatory lipid mediators [136]. A recent work reported that PS-exposed necroptotic cells can be phagocytosed and leaded to the secretion of pro-inflammatory cytokines IL-6 and TNFα [42]. Necroptotic bodies released from necroptotic cells contain PS that may serve as specific “find me” and “eat me” signals and modulate the inflammatory response [42] , while these results remain to be confirmed in future [42]. In contrast, neutrophil-derived EVs can enhance the release of potent anti-inflammatory cytokine transforming growth factor β1 (TGFβ1) in activated or resting macrophages, and inhibit the secretion of pro-inflammatory cytokine, IL-8 and TNFα, in activated macrophages, thus suppress the inflammatory process in the early stage of inflammation [115, 116]. Furthermore, neutrophil NETs can trigger plasmacytoid DCs and other immune cells for production of proinflammatory cytokines, thus involve in pathogenesis of systemic vasculitis [51, 137–140].
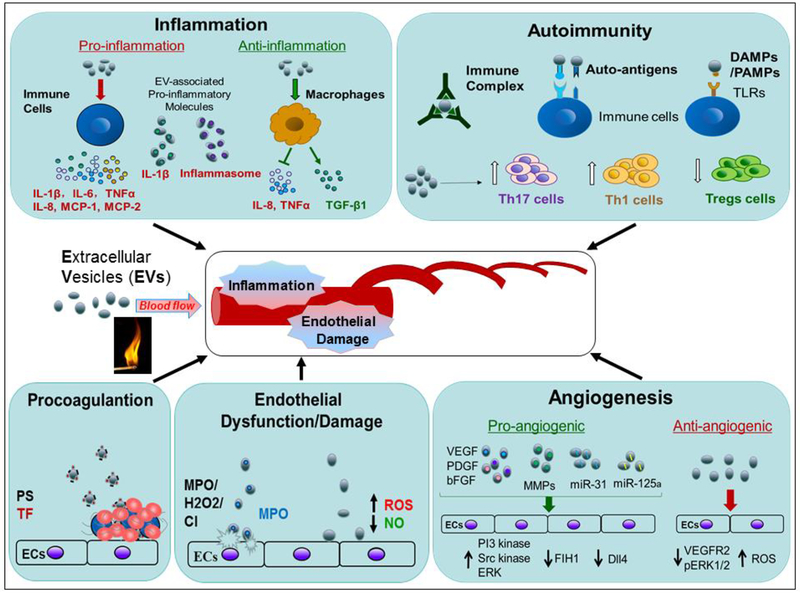
Figure 2.
Roles of extracellular vesicles in systemic vasculitis The schematic figures indicate the potential pathogenic effects of EVs on systemic vasculitis through their roles in inflammation, autoimmunity, procoagulation, endothelial dysfunction/damage, and angiogenesis. EVs can enhance inflammation through their associated proinflammatory cytokines/molecules, i.e. IL-1β and inflammasome, and promotion the release of proinflammtory cytokines, i.e. IL-1β, IL-6, TNFα, IL-8, MCP-1 and MCP-2. In contrast, EVs can also exert anti-inflammatory effects by stimulation of release of anti-inflammatory molecules, i.e. TGFβ1 or inhibition of the secretion of pro-inflammatory IL-8 and TNFα from macrophages. For the roles in autoimmunity, EVs can activate immune cells by EV-associated immune complexes, auto-antigens, and DAMPs/PAMPs. The EVs can serve as antigenic surface for formation of functional ICs. EVs can also enhance differentiation of Th1/17 cells, and inhibit the differentiation of Tregs. Increased procoagulant activity is important in pathogenesis of vasculitis, and EVs are highly procoagulant due to their associated PS and TF. As the important pathologic changes in vasculitis, endothelial dysfunction/damage can also be induced by EVs through increasing ROS production, reducing NO production and bioavailability, as well as the EVs-associated bioactive MPO or nuclear histone. In addition, EVs can not only exert pro-angiogenic function owing to their expression of VEGF, bFGF, PDGF, MMPs, MiR-125a and miRNA-31, but also suppress angiogenesis through augmentation of ROS generation and down-regulation of the protein levels of VEGFR2 and pERK1/2.
5.2. Autoimmunity
In the field of autoimmune diseases, increased levels of circulating EVs have been described in systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), Sjögren’s syndrome, systemic sclerosis, and systemic vasculitis [17, 125] [141, 142]. Our recent publication systematically reviewed the role of MVs in autoimmune diseases [17]. EVs have been shown to affect immune responses through various mechanisms. EVs can activate both innate and adaptive immune cells as EVs have been found to express various immune response molecules, including auto-antigens and peptide–MHC complexes [143]. It has been reported that both PR3 and MPO can be associated with EVs, and these PR3-positive EVs [106] and MPO-positive EVs [107, 108] may serve as auto-antigens and contribute to autoimmune responses in AAV. Similarly, neutrophil NET-associated extracellular PR3 and MPO can also serve as autoantigens and play an important role in autoimmunity in vasculitis [51]. In addition, EVs may also participate in the formation of immune complexes (ICs) which can stimulate inflammatory responses and development of autoimmune diseases. Cloutier et al. found that ICs containing EVs existed in synovial fluid of RA patients [144]. The EVs could serve as antigenic surface for formation of functional ICs by recognition of citrullinated autoantigens [144]. Similarly, EVs exhibit nucleosomal molecules in an antigenic form and serve as a source of ICs in SLE [145]. The levels of IgG, IgM, and C1q are increased in circulating EVs in SLE patients, and IgG-EVs are associated with autoantibodies, such as anti-dsDNA, anti-ENA and anti-histone antibodies [145, 146]. Furthermore, EV-associated ICs may interrupt the recognition and clearance of these EVs by phagocytes [147]. Our unpublished works suggested EVs may carry CD47 and affect cell phagocytosis through CD47-signal regulatory protein α (SIRPα)–mediated inhibition [148]. Attenuated phagocytosis may result in the accumulation of proinflammatory EVs, debris and dead cells, and contribute to the propagation of autoimmune responses. EVs derived from infected cells and stressed or injured tissues carry pathogen-associated molecular patterns (PAMPs) and DAMPs, and these EVs can activate PRRs in recipient cells, and then induce inflammatory and autoimmune responses [149–151]. In addition, EVs can increase the expression of T-bet mRNA and protein, and then significantly enhance the differentiation of Th1 cells [152]. Circulating EVs can also inhibit the differentiation of Foxp3+ Tregs and induce the expression of interferon-γ by CD4+ lymphocytes as well as the differentiation of Th17 pathogenic cells [153]. Similar to EVs, NETs can also promote CD4+ T cells and CD19+ B cells proliferation, B cells maturation, and autoreactive B cell activation, thus contribute to autoimmune responses in vasculitis [51, 154].
5.3. Procoagulation
Elevated levels of circulating EVs have been reported in thrombotic disorders, particularly in cardiovascular and autoimmune diseases [17, 155]. EVs are associated with thrombotic diseases due to their carriage of procoagulant PS and TF. Platelets are the major source of PS+-EVs in blood, and EVs derived from monocytes, lymphocytes, or endothelial cells also present PS on their surface [155, 156]. The plasma membrane of normal cells has an asymmetrical distribution of lipids between the inner and outer plasma membranes in which PS is localized in the inner plasma membrane. PS externalization can lead to the procoagulant activity of EVs due to an electrostatic interaction between positively charged carboxyglutamic acid in the clotting proteins and negatively charged PS on the membrane [157]. TF, released with monocytic EVs [98, 99], triggers extrinsic blood coagulation cascade, so the presence of TF on EVs dramatically increases their procoagulant activity in the studies from our [98, 99] and others [158]. Furthermore, the structural shape of the NET network, NET-associated DNA, histones, and TF, also provide procoagulant activities of neutrophil NETs, therefore contribute to vasculitis [51, 159–163]. It is reported that platelet-derived EVs have higher procoagulant activity than activated platelets [164]. However, Berckmans et al. found that EVs originated from platelets, erythrocytes, granulocytes and endothelial cells in healthy individuals had negative correlation with the concentrations of thrombin-antithrombin complex in plasma, indicating EVs also had an anticoagulant function [165].
5.4. Endothelial dysfunction/damage
Endothelial dysfunction characterized by reduced vasodilation and increased pro-inflammatory and pro-thrombic properties is an important manifestation in systemic vasculitis [166, 167]. Plasma levels of EVs are elevated when vascular endothelium are activated or damaged, thus EVs can serve as potential markers of endothelial dysfunction [92, 168]. EVs may be directly implicated in endothelial dysfunction by impairing endothelium-dependent vasorelaxation and reducing the production and bioavailability of NO [91]. In addition, EVs can bind to and activate endothelium through increasing the production of reactive oxygen species (ROS) [114]. MVs derived from active neutrophils expressing active MPO can activate myeloperoxidase-hydrogen peroxide-chloride pathway, leading to endothelial cell injury in vasculitis [107]. Furthermore, macrophage-derived EVs may carry nuclear histones from their parental cells[100]. These EV-associated histones have been recently shown to induce endothelial dysfunction or damages [101, 102], thus result in the onset of vasulitis. Since neutrophil NETs also contain histones, that may result in the induction of endothelial damage [159, 169]. In addition, NETs may also induce endothelial dysfunction through activation of MMP2 [169], thus contribute to vasculitis. However, further studies are still needed regarding the roles of EVs or NETs in the pathogenesis of vasculitis.
5.5. Angiogenesis
Angiogenesis, defined as the formation of new blood vessels from pre-existing vessels, plays a vital role in the pathogenesis of sysmetic vasculitis [170–172]. Increased circulating markers of angiogenesis, such as matrix metalloproteinases (MMPs) and vascular endothelial growth factor (VEGF), have been demonstrated in vasculitis, and MMP-2/9 are strongly associated with aneurysm formation [170–173]. It has been reported that EVs can exert pro-angiogenic and anti-angiogenic effects via a variety of mechanisms [174]. Endothelial- or macrophage-derived EVs contain pro-angiogenic mediators MMP-2, MMP-9 [175], and MT1-MMP [37], which can cause extracellular matrix remodeling that leads to endothelium invasion and revascularization [175]. Platelet-derived EVs can trigger an angiogenic response due to the expression of VEGF, basic fibroblast growth factor (bFGF) and platelet derived growth factor (PDGF), which can activate PI3-kinase, Src kinase and ERK [176]. MiR-125a and miRNA-31, while suppress antiangiogenic genes- factor inhibiting HIF-1 (FIH1) and delta-like 4 (Dll4), also can be transferred to vascular endothelial cells via EVs [177, 178]. In contrast, lymphocyte-derived EVs can suppress angiogenesis by augmenting ROS generation and down-regulating the VEGF signaling pathway [179]. Furthermore, Aldabbous et al have reported the role of neutrophil NETs in angiogenesis and provide a functional link between NETs and inflammatory angiogenesis [180]. Lavoie also reported that NETs may contribute to angiopoietin-mediated pro-angiogenic activities [181]. Therefore, NETs may involve in vasculitis through promotion of angiogenesis.
5.6. Intimal hyperplasia
Intimal hyperplasia is the thickening of the tunica intima of a blood vessel as a complication of a reconstruction procedure, which eventually results in narrowing and obstruction of the vasculature in vasculitis [182]. In lesions of vasculitis, activated DCs recruit T cells and macrophages, and dysregulated vascular smooth muscle cells (VSMCs) migrate towards the lumen, eventually leading to luminal stenosis or occlusion [182, 183]. HMGB1 has been reported to drive inflammation and intimal hyperplasia after arterial injury [184]. Our recent publication indicated that HMGB1 can be externalized and release with MVs from macrophages [54]. In contrast, Liu R et al. has reported that EVs derived from adipose mesenchymal stem cells (ADMSC-EVs) can inhibit intimal hyperplasia by interfering VSMCs proliferation and migration, as well as macrophage migration and inflammatory cytokine expression [127]. Therefore, EVs may be involved in vasculitis through their effects on intimal hyperplasia in different aspect.
6. Extracellular vesicles in systemic vasculitis
Over the past decade, increasing numbers of publications have demonstrated that EVs may play important roles in the pathogenesis of systemic vasculitis as we summarized in Table 1 [40, 80–82, 87–90, 112, 114, 117, 162, 185–201]. Brogan, et al. first reported that the plasma levels of MVs in children with active vasculitis were significantly higher than those in children with inactive vasculitis group and healthy controls, including platelet MVs expressing CD42a and endothelial MVs expressing CD105 or E-selectin. Endothelial MVs levels were closely associated with Birmingham Vasculitis Activity Score (BVAS) and the acute-phase reactant levels, suggesting that these MVs may be useful biomakers for diagnosing and monitoring disease activity in children with systemic vasculitis [185]. It is generally known that endothelial activation or injury contribute to the development of vasculitis [166, 167]. MVs isolated from GPA patients have been shown to activate vascular endothelial cells and platelets in vitro [186]. The numbers of CD144+ endothelial MVs, circulating endothelial cells, VEGF, and endothelial progenitor cells are increased in children with active vasculitis as compared to their healthy controls, and declined after effective treatments [90]. The levels of endothelial cell-derived MVs are increased in KD patients [87, 88, 187–189], particularly in patients with coronary artery aneurysms [189], and these MVs are closely related to decreased values of flow-mediated dilation [88]. Dursun et al. reported that circulating endothelial MV levels are significantly higher in children with Henoch-Schonlein Purpura (HSP) in both active and remission periods as compared to healthy controls, suggesting that circulating endothelial MVs may serve as biomarkers for subclinical inflammation in HSP [190]. Similarly, endothelial cell-derived MVs are also increased in adult patients with active AAV as compared to their healthy controls and the inactive AAV patients, and the levels of these MVs are correlated with the disease activity [40]. Therefore circulating endothelial cell-derived MVs may be a sensitive biomarker for identification of endothelial dysfunction and inflammation in patients with vasculitis.
Table 1.
Summary of the Published Studies about EVs in Vasculitis
| EV origin | Cell origin and markers | Associated Specific component | Samples | Techniques | Diseased/affected vessels | Presumed Pathogenenic involvements | References |
|---|---|---|---|---|---|---|---|
| MVs | Endothelial cells CD144 | miR-145–5p, miR-320a | Platelet-poor plasma | Flow cytometry | KD/ medium vessels | Upregulation of proinfammatory cytokine | 187 |
| MVs | Leukocytes CD45, neutrophils CD66 | B1-receptors | Plasma, renal biopsies | Flow cytometry, electron microscopy | AAV, HSP/ small vessels | Kinin-associated inflammation | 192 |
| MVs | Endothelial cells CD105/CD144 | B1-receptors | Platelet-free plasma | Flow cytometry | MPA, GPA, HSP/ small vessels | Kinin-associated inflammation | 193 |
| MVs | Platelets CD42a/CD62P | ND | Plasma | Flow cytometry | BD/ vessels of any sizes | Inflammation | 201 |
| MVs | Endothelial cells CD144/CD146 | ND | Platelet-free plasma | Flow cytometry | HSP/ small vessels | Inflammation | 190 |
| Exosomes | ND | miR-328,miR-575,miR-134,miR-671–5p | Serum | Microarray | KD/ medium vessels | Inflammation | 195 |
| MVs | Neutrophils CD66b platelets CD41a | ND | Platelet-poor plasma | Flow cytometry | AAV/ small vessels | Inflammation and procoagulation | 87 |
| MVs | Neutrophils annexin V/CD66b | Tissue factor | Serum | Flow cytometry | AAV/ small vessels | Thrombosis and inflammation | 112 |
| Exosomes | CD9/CD81/flotillin | 38 differentially expressed proteins | Platelet-poor plasma | Electron microscopy, Western blot, 2D-electrophoresis/MALDI-TOF mass spectrometry | KD/ medium vessels | Inflammation and procoagulation | 197 |
| Exosomes | CD9/flotillin | 69 differential proteins | Serum | Electron microscopy, Western blot, 2D-electrophoresis/MALDI-TOF mass spectrometry | KD/ medium vessels | Inflammation, procoagulation and autoimmunity | 196 |
| MVs | Annexin V neutrophils CD66b/CD11b/MPO/PR3/CD18 | CD18/CD11b/PR3/MPO/PS | Supernatant of cultured neutrophils, platelet-poor plasma | Flow cytometry | AAV/ small vessels | Inflammation, procoagulation and endothelium activation | 114 |
| MVs | Annexin V | Tissue factor | Supernatant of cultured neutrophils | Flow cytometry | AAV/ small vessels | Procoagulation | 162 |
| MVs | Annexin V | ND | Platelet-free plasma | ELISA | BD/ vessels of any sizes | Procoagulation | 198 |
| MVs | Annexin V | Tissue factor | Platelet-poor plasma | Flow cytometry | BD/ vessels of any sizes | Procoagulation | 199 |
| MVs | Annexin V, Platelets CD41 endothelial cells CD62E neutrophils CD 11b | Tissue factor/PS | Platelet-poor plasma | Flow cytometry | MPA, GPA, PAN, KD, BD,UCV / vessels of any sizes | Procoagulation | 191 |
| MVs | Platelets CD62 | ND | Whole blood | Flow cytometry | BD/ vessels of any sizes | Procoagulation | 200 |
| MVs | Platelets CD42b/CD42a | ND | Platelet-poor plasma | ELISA | KD/ medium vessels | Evaluation of platelet activation | 80 |
| MVs | Annexin V | ND | Platelet-poor plasma | ELISA | KD/medium vessels | Evaluation of platelet activation | 81 |
| MVs | Annexin V endothelial cells CD 105/ CD 144, platelets CD41, leukocytes CD 18, neutrophils CD 16b, monocytes CD 14 | ND | Platelet-poor plasma | Flow cytometry | GPA/ small vessels | Platelets activation and endothelial activation | 186 |
| MVs | Annexin V/CD62E/CD31 | ND | The supernatant of cultured HUVECs | Flow cytometry, ELISA, electron microscopy | KD/ medium vessels | Endothelial damage | 87 |
| MVs | Annexin V, endothelial cells CD105/CD62E, platelets CD42a/ CD62P, leukocytes CD45/ CD11b | ND | Platelet-poor plasma | Flow cytometry | AAV/ small vessels | Endothelial damage | 41 |
| MVs | Endothelial cells CD144+/CD42b−、 CD62E、CD 105 | ND | Platelet-poor plasma | Flow cytometry | KD/ medium vessels | Endothelial dysfunction | 88 |
| MVs | Annexin V, endothelial cells vWF/CD105/CD62e, leukocytes CD45/ CD11b, platelets CD42a/CD62P | ND | Platelet-poor plasma | Flow cytometry | CSS/ small vessels | Endothelial damage | 89 |
| MVs | Annexin V, endothelial cells CD 144/CD62E | ND | Platelet-poor plasma | Flow cytometry | MPA, GPA, PAN, KD, TA,BD, UCV/vessels of any sizes | Endothelial damage | 90 |
| MVs | Platelets CD42, erythrocytes CD235, neutrophils CD66b, monocles CD 14, T cells CD3, B cells CD 19, endothelial cells CD 144 | ND | Platelet-poor plasma | Flow cytometry | KD/ medium vessels | Endothelial damage | 117 |
| MVs | Annexin V, platelets CD42a/CD62P endothelial cells CD62E CD105/ICAM-1/VCAM-1 | ND | Platelet-poor plasma | Flow cytometry | MPA,GPA, PAN,KD/ small and medium vessels | Endothelial activation | 185 |
| MVs | Endothelial cells CD31/CD146 | ND | Platelet-poor plasma | Flow cytometry | KD/ medium vessels | Endothelial damage | 188 |
| MVs | Endothelial cells CD 105/CD62E/ICAM-1/VCAM-1/CD144/CD31 | ND | Platelet-poor plasma | Flow cytometry | KD/ medium vessels | Endothelial damage | 189 |
| Exosomes | CD9/CD81/TSG101 | miR-1246,miR-4436b-5p,miR-197–3p,miR-671–5p | Serum | Microarray, real-time quantitative PCR, western blot | KD/ medium vessels | Diagnostic biomarkers | 194 |
The current investigations showed a potential role of neutrophil and platelet-derived MVs in the pathogenesis of vasculitis [80–82, 114]. Danie et al. reported that the levels of plasma neutrophil MVs, platelet MVs and neutrophil/platelet mixed MVs were strikingly higher in patients with acute vasculitis as compared to those in healthy controls [82]. It demonstrates that neutrophil and platelet-derived MVs are non-specific markers of neutrophil activation in acute phase of vasculitis. Polyclonal PR3-ANCA and MPO-ANCA from AAV patients and chimeric PR3-ANCA can induce the release of neutrophil MVs from cytokine-primed neutrophils in vitro [114]. The plasma levels of neutrophil MVs in children with active AAV are higher than those in healthy controls [114]. These neutrophil MVs can bind to endothelial cells in a CD18-dependent manner, resulting in the release of pro-inflammatory cytokines IL-6 and IL-8, increased expression of intercellular adhesion molecule-1 and production of reactive oxygen species [114]. PR3 and MPO can be carried by EVs, and these EVs can promote endothelial cell damage and inflammation reactions in AAV [106–108]. The levels of platelet-derived MVs are significantly higher in acute-phase KD patients as compared to those in healthy controls [80]. Platelet-derived MVs could be used as a biomarker for evaluation of platelet activation dynamics [80], and the therapeutic responses to anti-platelet therapy in KD [81]. Furthermore, ANCA-positive IgG can promote C5aprimed neutrophils to release TF-expressing MVs or neutrophil extracellular traps which may promote hypercoagulability in AAV [162]. Compared to children with inactive vasculitis and healthy controls, higher peak thrombin has been shown in children with active vasculitis, especially in the active vasculitis with thrombosis [191], and peak thrombin is associated with the levels of MVs. Therefore, MVs-mediated thrombin generation can be used as a biomarker to assess clinical thrombotic events in children with vasculitis [191].
The kinin system, also called the contact system, contributes to inflammatory responses and the development of vasculitis [202, 203]. Activation of the kinin system has been reported in children and adults with vasculitis [202, 203]. Kahn and colleagues recently reported that the leukocyte-derived MVs were associated with kinin B1-receptors [192], which played an important role in neutrophil chemotaxis and chronic inflammation [204]. Functional B1-receptors can be transferred to target organ cells via MVs and then promote kinin-associated inflammation [192]. Interestingly, plasma levels of the kinin B1-receptor-positive MVs are increased in patients with vasculitis as compared to the healthy controls [192]. C1-inhibitor is the major inhibitor of the kinin system that can inhibit the release of B1-receptor-positive MVs during vascular inflammation [193]. Thus, blockage of the B1-receptor may be potential new therapeutic strategy for vasculitis.
The numbers of total MVs are significantly increased in the acute phase of KD [117]. These MVs are derived from endothelial cells, platelet and T cells, and these MVs are decreased after intravenous immunoglobulin treatment [117]. Four serum exosomal microRNAs, namely miR-1246, miR-4436b-5p, miR-197–3p and miR-671–5p, have been identified as diagnostic biomarkers for KD [194]. Zhang et al. also explored serum exosomal microRNAs associated with KD [195]. It demonstrated that serum exosomal miR-328, miR-575, miR-134 and miR-671–5p could affect the expression of inflammatory genes, and these microRNAs might be used as potential biomarkers for the diagnosis of KD and the prediction of therapeutic outcomes of intravenous immunoglobulin therapy [195]. In addition, sixty nine differential proteins have been found in serum exosomes from KD patients compared to healthy controls, and some protein levels changed after intravenous immunoglobulin therapy, i.e. complement C3, apolipoprotein A-IV, and insulin-like growth factor-binding protein complex acid labile subunit. So these proteins may be used as potential biomarkers for monitoring intravenous immunoglobulin therapy in KD [196]. Zhang et al. reported that the proteome profile of serum exosomes was associated with coronary artery dilatation which was caused by KD [197]. They found 13 up-regulated proteins and 25 down-regulated proteins in patients with coronary artery dilatation caused by KD as compared to healthy controls, and most of the differentially expressed proteins are involved in inflammation response and coagulation cascades [197]. Kümpers et al. reported that endothelial, leukocytic and platelet-derived MVs markedly increased on initial stage in a patient with Churg-Strauss vasculitis-induced cardiomyopathy, while these MVs decreased during disease remission [89]. Therefore, the levels of these MVs subtypes are closely associated with the disease activity [89]. The endothelial MVs might be an important biomarker for diagnose and monitoring of the suspected vasculitic cardiac involvement in Churg-Strauss syndrome (CSS) [89]. The above studies suggested that EVs may serve as a novel biomarker for clinical application in diagnosis and monitoring of the disease activity and therapy.
In constrast, plasma levels of procoagulant MVs were increased in BD patients, but they were not related to gender or any clinical manifestations, including thrombotic events [198]. There was no significant difference between the levels of MVs after the previous or current treatments [198], indicating that MVs levels cannot be used for therapeutic monitoring. Khan et al. reported that BD patients had significantly higher plasma levels of total MVs and TF-positive MVs as compared to healthy controls, especially in BD patients with a history of thrombosis. A low balance between tissue factor pathway inhibitor (TFPI)-positive MVs and TF-positive MVs may predispose to thrombosis. So the balance between TF-positive and TFPI-positive MVs may be used to assess the risk of thrombosis in BD [199]. But there was no difference in the numbers of platelet-derived MVs between BD patients and healthy controls [200]. Another study showed that the levels of CD62P+ platelet MVs were significantly increased in patients in 20–50-year of age, but not in the older BD patients >50 years old. These results might suggest that activity of this disease was decreasing with age [201].
7. Conclusion
EVs release from various cell types, and composition of MVs is different owing to their different cell origin and biological status. EVs can act as important mediators of intercellular communication through a variety of approaches, such as cell-to-cell direct contact and secretion of soluble mediators and effectors. Increasing evidences suggest that EVs play important roles in inflammation, autoimmune responses, pro-coagulation, endothelial dysfunction/damage, angiogenesis and intimal hyperplasia, the crucial pathological processes in vasculitis. As another extracellular platform, NETs may also involve in vasculitis by affecting the above mentioned pathologic processes. EVs may serve as biomarkers for diagnosing and monitoring disease activity and therapeutic efficacy in patients with systemic vasculitis. However, whether EVs can be the therapeutic targets in systemic vasculitis are not fully understood.
Further studies are necessary to standardize the methodology for measurement of clinical samples, explore the potential novel therapeutic strategy to target EVs, and search the signalling pathways activated by EVs in vasculitis. The long-time follow-up studies are also needed to evaluate the possible roles of EVs as biomarkers to define any clinical manifestations and predict prognostic outcomes in vasculitis.
Take-home messages:
Extracellular vesicles, including exosomes, microvesicles and apoptotic bodies, are membrane vesicles released from various cell types during cell activation or programmed cell death.
Extracellular vesicles can act as mediators for cell-to-cell communications by transferring membrane surface molecules, cytoplasmic or nuclear components from their parental cells to target cells via different mechanisms.
Extracellular vesicles are involved in pathophysiology of systemic vasculitis through their effects on inflammation, autoimmunity, pro-coagulation, endothelial dysfunction/damage, angiogenesis, and intimal hyperplasia.
Abbreviations:
- ANCA
antineutrophil cytoplasmic autoantibody
- AAV
antineutrophil cytoplasmic autoantibody-associated vasculitis
- ADMSC
adipose mesenchymal stem cells
- ADAM
a disintegrin and metalloprotease-10
- BD
Behçet’s disease
- BVAS
Birmingham Vasculitis Activity Score
- bFGF
basic fibroblast growth factor
- CRP
C reactive protein
- CAD
coronary artery dilatation
- CSS
Churg-Strauss syndrome
- DC
dendritic cells
- DAMPs
danger-associated molecular patterns
- Dll4
delta-like lagand 4
- EVs
extracellular vesicles
- ESR
erythrocyte sedimentation rate
- ELISA
enzyme-linked immunosorbent assay
- ESCRT
Endosomal Sorting Complex Required for Transport
- FIH1
factor inhibiting HIF-1
- GCA
giant cell arteritis
- GPA
granulomatosis with polyangiitis
- HLA
human leukocyte antigen
- HMGB1
high mobility group protein B1
- HSP
Henoch–Schönlein purpura
- ICs
immune complexe
- ICAM-1
intercellular adhesion molecule 1
- KD
Kawasaki disease
- MVs
microvesicles
- MPO
myeloperoxidase
- MMPs
matrix metalloproteinases
- MLKL
mixed lineage kinase-like
- MCP
chemoattractant protein
- MPA
microscopic polyangiitis
- MALDI-TOF
matrix-assisted laser desorption/ionization-time-of-flight
- ND
not detectable
- NO
nitric oxide
- ND
not detectable
- NETs
neutrophil extracellular traps
- PAN
polyarteritis nodosa
- PRRs
pattern recognition receptors
- PAMPs
pathogen-associated molecular patterns
- PS
phosphatidylserine
- PR3
proteinase 3
- PDGF
platelet derived growth factor
- PAN
polyarteritis nodosa
- RA
rheumatoid arthritis
- ROS
reactive oxygen species
- RAGE
receptor for advanced glycation end products
- SLE
systemic lupus erythematosus
- TA
takayasu arteritis
- TF
tissue factor
- TFPI
tissue factor pathway inhibitor
- TLRs
toll-like receptors
- TGF-β
transforming growth factor β
- Treg
T regulatory
- Th
T-help
- UCV
unclassified vasculitis
- VEGF
vascular endothelial growth factor
- VSMCs
vascular smooth muscle cells
- 2D
two dimentional
Footnotes
Disclosure
The authors declare that there is no conflict of interests regarding the publication of this review.
Reference
- [1].Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med 2003;349:160–9. 10.1056/NEJMra022694. [DOI] [PubMed] [Google Scholar]
- [2].Seo P, Stone JH. Small-vessel and medium-vessel vasculitis. Arthritis Rheum 2007;57:1552–9. 10.1002/art.23105. [DOI] [PubMed] [Google Scholar]
- [3].Elefante E, Monti S, Bond M, Lepri G, Quartuccio L, Talarico R, et al. One year in review 2017: systemic vasculitis. Clin Exp Rheumatol 2017;35 Suppl 103:5–26. [PubMed] [Google Scholar]
- [4].Jennette JC, Falk RJ. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat Rev Rheumatol 2014;10:463–73. 10.1038/nrrheum.2014.103. [DOI] [PubMed] [Google Scholar]
- [5].Rahmattulla C, Mooyaart AL, van Hooven D, Schoones JW, Bruijn JA, Dekkers OM, et al. Genetic variants in ANCA-associated vasculitis: a meta-analysis. Ann Rheum Dis 2016;75:1687–92. 10.1136/annrheumdis-2015-207601. [DOI] [PubMed] [Google Scholar]
- [6].Lyons PA, Rayner TF, Trivedi S, Holle JU, Watts RA, Jayne DR, et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med 2012;367:214–23. 10.1056/NEJMoa1108735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Terao C. Revisited HLA and non-HLA genetics of Takayasu arteritis--where are we? J Hum Genet 2016;61:27–32. 10.1038/jhg.2015.87. [DOI] [PubMed] [Google Scholar]
- [8].Hoffman GS, Calabrese LH. Vasculitis: determinants of disease patterns. Nat Rev Rheumatol 2014;10:454–62. 10.1038/nrrheum.2014.89. [DOI] [PubMed] [Google Scholar]
- [9].Misra DP, Agarwal V. Innate immune cells in the pathogenesis of primary systemic vasculitis. Rheumatol Int 2016;36:169–82. 10.1007/s00296-015-3367-1. [DOI] [PubMed] [Google Scholar]
- [10].Abdulahad WH, Lamprecht P, Kallenberg CG. T-helper cells as new players in ANCA-associated vasculitides. Arthritis Res Ther 2011;13:236 10.1186/ar3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mendoza-Pinto C, Garcia-Carrasco M, Jimenez-Hernandez M, Jimenez Hernandez C, Riebeling-Navarro C, Nava Zavala A, et al. Etiopathogenesis of Behcet’s disease. Autoimmun Rev 2010;9:241–5. 10.1016/j.autrev.2009.10.005. [DOI] [PubMed] [Google Scholar]
- [12].Samson M, Bonnotte B. [Pathogenesis of large vessel vasculitis]. Rev Med Interne 2016;37:264–73. 10.1016/j.revmed.2015.10.350. [DOI] [PubMed] [Google Scholar]
- [13].Kolb JP, Oguin TH, 3rd, Oberst A, Martinez J. Programmed Cell Death and Inflammation: Winter Is Coming. Trends Immunol 2017;38:705–18. 10.1016/j.it.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wallach D, Kang TB, Dillon CP, Green DR. Programmed necrosis in inflammation: Toward identification of the effector molecules. Science 2016;352:aaf2154. 10.1126/science.aaf2154. [DOI] [PubMed] [Google Scholar]
- [15].Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 2009;7:99–109. 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yoon S, Kovalenko A, Bogdanov K, Wallach D. MLKL, the Protein that Mediates Necroptosis, Also Regulates Endosomal Trafficking and Extracellular Vesicle Generation. Immunity 2017;47:51–65 e7. 10.1016/j.immuni.2017.06.001. [DOI] [PubMed] [Google Scholar]
- [17].Liu ML, Williams KJ, Werth VP. Microvesicles in Autoimmune Diseases. Adv Clin Chem 2016;77:125–75. 10.1016/bs.acc.2016.06.005. [DOI] [PubMed] [Google Scholar]
- [18].Buzas EI, Gyorgy B, Nagy G, Falus A, Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol 2014;10:356–64. 10.1038/nrrheum.2014.19. [DOI] [PubMed] [Google Scholar]
- [19].Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 2013;200:373–83. 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zuo P, Lin X, Li X, Zhang Y. Macrophage-derived extracellular vesicles transfer inflammasome components to endothelial cells and induces endothelial injury. The FASEB Journal 2017;31:825.13-.13. 10.1096/fasebj.31.1_supplement.825.13. [DOI] [Google Scholar]
- [21].Park KS, Lee J, Lee C, Park HT, Kim JW, Kim OY, et al. Sepsis-Like Systemic Inflammation Induced by Nano-Sized Extracellular Vesicles From Feces. Front Microbiol 2018;9:1735 10.3389/fmicb.2018.01735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev 2012;64:676–705. 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- [23].Chen Y, Li G, Liu ML. Microvesicles as Emerging Biomarkers and Therapeutic Targets in Cardiometabolic Diseases. Genomics Proteomics Bioinformatics 2018;16:50–62. 10.1016/j.gpb.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol 1985;101:942–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 1983;33:967–78. [DOI] [PubMed] [Google Scholar]
- [26].Lin J, Li J, Huang B, Liu J, Chen X, Chen XM, et al. Exosomes: novel biomarkers for clinical diagnosis. ScientificWorldJournal 2015;2015:657086 10.1155/2015/657086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci 2013;126:5553–65. 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- [28].Juan T, Furthauer M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin Cell Dev Biol 2018;74:66–77. 10.1016/j.semcdb.2017.08.022. [DOI] [PubMed] [Google Scholar]
- [29].Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008;319:1244–7. 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- [30].van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, et al. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell 2011;21:708–21. 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002;2:569–79. 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- [32].Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 2004;104:3257–66. 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- [33].Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 2014;30:255–89. 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- [34].Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol 1967;13:269–88. [DOI] [PubMed] [Google Scholar]
- [35].Morel O, Jesel L, Freyssinet JM, Toti F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler Thromb Vasc Biol 2011;31:15–26. 10.1161/ATVBAHA.109.200956. [DOI] [PubMed] [Google Scholar]
- [36].Giesen PL, Rauch U, Bohrmann B, Kling D, Roque M, Fallon JT, et al. Blood-borne tissue factor: another view of thrombosis. Proc Natl Acad Sci U S A 1999;96:2311–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Li CJ, Liu Y, Chen Y, Yu D, Williams KJ, Liu ML. Novel proteolytic microvesicles released from human macrophages after exposure to tobacco smoke. Am J Pathol 2013;182:1552–62. 10.1016/j.ajpath.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Folkesson M, Li C, Frebelius S, Swedenborg J, Wagsater D, Williams KJ, et al. Proteolytically active ADAM10 and ADAM17 carried on membrane microvesicles in human abdominal aortic aneurysms. Thromb Haemost 2015;114:1165–74. 10.1160/TH14-10-0899. [DOI] [PubMed] [Google Scholar]
- [39].Distler JH, Huber LC, Gay S, Distler O, Pisetsky DS. Microparticles as mediators of cellular cross-talk in inflammatory disease. Autoimmunity 2006;39:683–90. 10.1080/08916930601061538. [DOI] [PubMed] [Google Scholar]
- [40].Erdbruegger U, Grossheim M, Hertel B, Wyss K, Kirsch T, Woywodt A, et al. Diagnostic role of endothelial microparticles in vasculitis. Rheumatology (Oxford) 2008;47:1820–5. 10.1093/rheumatology/ken373. [DOI] [PubMed] [Google Scholar]
- [41].Horstman LL, Jy W, Jimenez JJ, Bidot C, Ahn YS. New horizons in the analysis of circulating cell-derived microparticles. Keio J Med 2004;53:210–30. [DOI] [PubMed] [Google Scholar]
- [42].Zargarian S, Shlomovitz I, Erlich Z, Hourizadeh A, Ofir-Birin Y, Croker BA, et al. Phosphatidylserine externalization, “necroptotic bodies” release, and phagocytosis during necroptosis. PLoS Biol 2017;15:e2002711 10.1371/journal.pbio.2002711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Brough D, Rothwell NJ. Caspase-1-dependent processing of pro-interleukin-1beta is cytosolic and precedes cell death. J Cell Sci 2007;120:772–81. 10.1242/jcs.03377. [DOI] [PubMed] [Google Scholar]
- [44].Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ 2007;14:1590–604. 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Schreiber A, Rousselle A, Becker JU, von Massenhausen A, Linkermann A, Kettritz R. Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proc Natl Acad Sci U S A 2017;114:E9618–E25. 10.1073/pnas.1708247114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Borgmann S, Endisch G, Hacker UT, Song BS, Fricke H. Proinflammatory genotype of interleukin-1 and interleukin-1 receptor antagonist is associated with ESRD in proteinase 3-ANCA vasculitis patients. Am J Kidney Dis 2003;41:933–42. [DOI] [PubMed] [Google Scholar]
- [47].Alphonse MP, Duong TT, Shumitzu C, Hoang TL, McCrindle BW, Franco A, et al. Inositol-Triphosphate 3-Kinase C Mediates Inflammasome Activation and Treatment Response in Kawasaki Disease. J Immunol 2016;197:3481–9. 10.4049/jimmunol.1600388. [DOI] [PubMed] [Google Scholar]
- [48].Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science 2004;303:1532–5. 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- [49].Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med 2017;23:279–87. 10.1038/nm.4294. [DOI] [PubMed] [Google Scholar]
- [50].Liu M, Li Y, Sharma M, Werth VP. Nuclear translocation of PKCα is important in neutrophil NETosis and UVB induced-skin inflammation. J Invest Dermatol. 2018; 139 Suppl1, S195. [Google Scholar]
- [51].Lee KH, Kronbichler A, Park DD, Park Y, Moon H, Kim H, et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: A comprehensive review. Autoimmun Rev 2017;16:1160–73. 10.1016/j.autrev.2017.09.012. [DOI] [PubMed] [Google Scholar]
- [52].Monneret G, Venet F, Cour M, Argaud L. Danger associated molecular patterns in injury: a double-edged sword? J Thorac Dis 2016;8:1060–1. 10.21037/jtd.2016.04.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol 1994;12:991–1045. 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- [54].Chen Y, Li G, Liu Y, Werth VP, Williams KJ, Liu ML. Translocation of Endogenous Danger Signal HMGB1 From Nucleus to Membrane Microvesicles in Macrophages. J Cell Physiol 2016;231:2319–26. 10.1002/jcp.25352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ma YH, Ma TT, Wang C, Wang H, Chang DY, Chen M, et al. High-mobility group box 1 potentiates antineutrophil cytoplasmic antibody-inducing neutrophil extracellular traps formation. Arthritis Res Ther 2016;18:2 10.1186/s13075-015-0903-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Liu ML, Scalia R, Mehta JL, Williams KJ. Cholesterol-induced membrane microvesicles as novel carriers of damage-associated molecular patterns: mechanisms of formation, action, and detoxification. Arterioscler Thromb Vasc Biol 2012;32:2113–21. 10.1161/ATVBAHA.112.255471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Cavassani KA, Ishii M, Wen H, Schaller MA, Lincoln PM, Lukacs NW, et al. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med 2008;205:2609–21. 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Anders HJ, Schaefer L. Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J Am Soc Nephrol 2014;25:1387–400. 10.1681/ASN.2014010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci 2011;68:2667–88. 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol 2007;35:495–516. 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol 2001;3:339–45. 10.1038/35070009. [DOI] [PubMed] [Google Scholar]
- [62].Erwig LP, Henson PM. Clearance of apoptotic cells by phagocytes. Cell Death Differ 2008;15:243–50. 10.1038/sj.cdd.4402184. [DOI] [PubMed] [Google Scholar]
- [63].Lauber K, Blumenthal SG, Waibel M, Wesselborg S. Clearance of apoptotic cells: getting rid of the corpses. Mol Cell 2004;14:277–87. [DOI] [PubMed] [Google Scholar]
- [64].Li MO, Sarkisian MR, Mehal WZ, Rakic P, Flavell RA. Phosphatidylserine receptor is required for clearance of apoptotic cells. Science 2003;302:1560–3. 10.1126/science.1087621. [DOI] [PubMed] [Google Scholar]
- [65].Kimani SG, Geng K, Kasikara C, Kumar S, Sriram G, Wu Y, et al. Contribution of Defective PS Recognition and Efferocytosis to Chronic Inflammation and Autoimmunity. Front Immunol 2014;5:566 10.3389/fimmu.2014.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Jy W, Horstman LL, Jimenez JJ, Ahn YS, Biro E, Nieuwland R, et al. Measuring circulating cell-derived microparticles. J Thromb Haemost 2004;2:1842–51. 10.1111/j.1538-7836.2004.00936.x. [DOI] [PubMed] [Google Scholar]
- [67].van der Vlist EJ, Nolte-’t Hoen EN, Stoorvogel W, Arkesteijn GJ, Wauben MH. Fluorescent labeling of nano-sized vesicles released by cells and subsequent quantitative and qualitative analysis by high-resolution flow cytometry. Nat Protoc 2012;7:1311–26. 10.1038/nprot.2012.065. [DOI] [PubMed] [Google Scholar]
- [68].Chandler WL, Yeung W, Tait JF. A new microparticle size calibration standard for use in measuring smaller microparticles using a new flow cytometer. J Thromb Haemost 2011;9:1216–24. 10.1111/j.1538-7836.2011.04283.x. [DOI] [PubMed] [Google Scholar]
- [69].Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJ, Hole P, et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine 2011;7:780–8. 10.1016/j.nano.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].van der Pol E, Coumans FA, Grootemaat AE, Gardiner C, Sargent IL, Harrison P, et al. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J Thromb Haemost 2014;12:1182–92. 10.1111/jth.12602. [DOI] [PubMed] [Google Scholar]
- [71].Maas SL, de Vrij J, van der Vlist EJ, Geragousian B, van Bloois L, Mastrobattista E, et al. Possibilities and limitations of current technologies for quantification of biological extracellular vesicles and synthetic mimics. J Control Release 2015;200:87–96. 10.1016/j.jconrel.2014.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2013;2 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lacroix R, Judicone C, Poncelet P, Robert S, Arnaud L, Sampol J, et al. Impact of pre-analytical parameters on the measurement of circulating microparticles: towards standardization of protocol. J Thromb Haemost 2012;10:437–46. 10.1111/j.1538-7836.2011.04610.x. [DOI] [PubMed] [Google Scholar]
- [74].Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 2006;Chapter 3:Unit 3 22. 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- [75].Stalker TJ, Welsh JD, Brass LF. Shaping the platelet response to vascular injury. Curr Opin Hematol 2014;21:410–7. 10.1097/MOH.0000000000000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Willeke P, Kumpers P, Schluter B, Limani A, Becker H, Schotte H. Platelet counts as a biomarker in ANCA-associated vasculitis. Scand J Rheumatol 2015;44:302–8. 10.3109/03009742.2015.1006247. [DOI] [PubMed] [Google Scholar]
- [77].Tao SC, Guo SC, Zhang CQ. Platelet-derived Extracellular Vesicles: An Emerging Therapeutic Approach. Int J Biol Sci 2017;13:828–34. 10.7150/ijbs.19776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Aatonen MT, Ohman T, Nyman TA, Laitinen S, Gronholm M, Siljander PR. Isolation and characterization of platelet-derived extracellular vesicles. J Extracell Vesicles 2014;3 10.3402/jev.v3.24692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 1999;94:3791–9. [PubMed] [Google Scholar]
- [80].Yahata T, Suzuki C, Yoshioka A, Hamaoka A, Ikeda K. Platelet activation dynamics evaluated using platelet-derived microparticles in Kawasaki disease. Circ J 2014;78:188–93. [DOI] [PubMed] [Google Scholar]
- [81].Kim HJ, Choi EH, Lim YJ, Kil HR. The Usefulness of Platelet-derived Microparticle as Biomarker of Antiplatelet Therapy in Kawasaki Disease. J Korean Med Sci 2017;32:1147–53. 10.3346/jkms.2017.32.7.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Daniel L, Fakhouri F, Joly D, Mouthon L, Nusbaum P, Grunfeld JP, et al. Increase of circulating neutrophil and platelet microparticles during acute vasculitis and hemodialysis. Kidney Int 2006;69:1416–23. 10.1038/sj.ki.5000306. [DOI] [PubMed] [Google Scholar]
- [83].Forlow SB, McEver RP, Nollert MU. Leukocyte-leukocyte interactions mediated by platelet microparticles under flow. Blood 2000;95:1317–23. [PubMed] [Google Scholar]
- [84].Baj-Krzyworzeka M, Majka M, Pratico D, Ratajczak J, Vilaire G, Kijowski J, et al. Platelet-derived microparticles stimulate proliferation, survival, adhesion, and chemotaxis of hematopoietic cells. Exp Hematol 2002;30:450–9. [DOI] [PubMed] [Google Scholar]
- [85].Vajen T, Mause SF, Koenen RR. Microvesicles from platelets: novel drivers of vascular inflammation. Thromb Haemost 2015;114:228–36. 10.1160/TH14-11-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Goetzl EJ, Goetzl L, Karliner JS, Tang N, Pulliam L. Human plasma platelet-derived exosomes: effects of aspirin. FASEB J 2016;30:2058–63. 10.1096/fj.201500150R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Tian J, Lv HT, An XJ, Ling N, Xu F. Endothelial microparticles induce vascular endothelial cell injury in children with Kawasaki disease. Eur Rev Med Pharmacol Sci 2016;20:1814–8. [PubMed] [Google Scholar]
- [88].Ding YY, Ren Y, Feng X, Xu QQ, Sun L, Zhang JM, et al. Correlation between brachial artery flow-mediated dilation and endothelial microparticle levels for identifying endothelial dysfunction in children with Kawasaki disease. Pediatr Res 2014;75:453–8. 10.1038/pr.2013.240. [DOI] [PubMed] [Google Scholar]
- [89].Kumpers P, Erdbrugger U, Grossheim M, Meyer GP, Hiss M, Gwinner W, et al. Endothelial microparticles as a diagnostic aid in Churg-Strauss vasculitis-induced cardiomyopathy. Clin Exp Rheumatol 2008;26:S86–9. [PubMed] [Google Scholar]
- [90].Clarke LA, Hong Y, Eleftheriou D, Shah V, Arrigoni F, Klein NJ, et al. Endothelial injury and repair in systemic vasculitis of the young. Arthritis Rheum 2010;62:1770–80. 10.1002/art.27418. [DOI] [PubMed] [Google Scholar]
- [91].Brodsky SV, Zhang F, Nasjletti A, Goligorsky MS. Endothelium-derived microparticles impair endothelial function in vitro. Am J Physiol Heart Circ Physiol 2004;286:H1910–5. 10.1152/ajpheart.01172.2003. [DOI] [PubMed] [Google Scholar]
- [92].Taguchi K, Hida M, Narimatsu H, Matsumoto T, Kobayashi T. Glucose and angiotensin II-derived endothelial extracellular vesicles regulate endothelial dysfunction via ERK1/2 activation. Pflugers Arch 2017;469:293–302. 10.1007/s00424-016-1926-2. [DOI] [PubMed] [Google Scholar]
- [93].Vitkova V, Zivny J, Janota J. Endothelial cell-derived microvesicles: potential mediators and biomarkers of pathologic processes. Biomark Med 2018;12:161–75. 10.2217/bmm-2017-0182. [DOI] [PubMed] [Google Scholar]
- [94].Liu Y, Zhang R, Qu H, Wu J, Li L, Tang Y. Endothelial microparticles activate endothelial cells to facilitate the inflammatory response. Mol Med Rep 2017;15:1291–6. 10.3892/mmr.2017.6113. [DOI] [PubMed] [Google Scholar]
- [95].Holnthoner W, Bonstingl C, Hromada C, Muehleder S, Zipperle J, Stojkovic S, et al. Endothelial Cell-derived Extracellular Vesicles Size-dependently Exert Procoagulant Activity Detected by Thromboelastometry. Sci Rep 2017;7:3707 10.1038/s41598-017-03159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Aharon A, Tamari T, Brenner B. Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb Haemost 2008;100:878–85. [DOI] [PubMed] [Google Scholar]
- [97].Kanazawa S, Nomura S, Kuwana M, Muramatsu M, Yamaguchi K, Fukuhara S. Monocyte-derived microparticles may be a sign of vascular complication in patients with lung cancer. Lung Cancer 2003;39:145–9. [DOI] [PubMed] [Google Scholar]
- [98].Li M, Yu D, Williams KJ, Liu ML. Tobacco smoke induces the generation of procoagulant microvesicles from human monocytes/macrophages. Arterioscler Thromb Vasc Biol 2010;30:1818–24. 10.1161/ATVBAHA.110.209577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Liu ML, Reilly MP, Casasanto P, McKenzie SE, Williams KJ. Cholesterol enrichment of human monocyte/macrophages induces surface exposure of phosphatidylserine and the release of biologically-active tissue factor-positive microvesicles. Arterioscler Thromb Vasc Biol 2007;27:430–5. 10.1161/01.ATV.0000254674.47693.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Nair RR, Mazza D, Brambilla F, Gorzanelli A, Agresti A, Bianchi ME. LPS-Challenged Macrophages Release Microvesicles Coated With Histones. Front Immunol 2018;9:1463 10.3389/fimmu.2018.01463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Kumar SV, Kulkarni OP, Mulay SR, Darisipudi MN, Romoli S, Thomasova D, et al. Neutrophil Extracellular Trap-Related Extracellular Histones Cause Vascular Necrosis in Severe GN. J Am Soc Nephrol 2015;26:2399–413. 10.1681/ASN.2014070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Perez-Cremades D, Bueno-Beti C, Garcia-Gimenez JL, Ibanez-Cabellos JS, Hermenegildo C, Pallardo FV, et al. Extracellular histones disarrange vasoactive mediators release through a COX-NOS interaction in human endothelial cells. J Cell Mol Med 2017;21:1584–92. 10.1111/jcmm.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Wang JG, Williams JC, Davis BK, Jacobson K, Doerschuk CM, Ting JP, et al. Monocytic microparticles activate endothelial cells in an IL-1beta-dependent manner. Blood 2011;118:2366–74. 10.1182/blood-2011-01-330878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Matsumoto K, Morisaki T, Kuroki H, Kubo M, Onishi H, Nakamura K, et al. Exosomes secreted from monocyte-derived dendritic cells support in vitro naive CD4+ T cell survival through NF-(kappa)B activation. Cell Immunol 2004;231:20–9. 10.1016/j.cellimm.2004.11.002. [DOI] [PubMed] [Google Scholar]
- [105].Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol 2002;3:1156–62. 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- [106].Martin KR, Kantari-Mimoun C, Yin M, Pederzoli-Ribeil M, Angelot-Delettre F, Ceroi A, et al. Proteinase 3 Is a Phosphatidylserine-binding Protein That Affects the Production and Function of Microvesicles. J Biol Chem 2016;291:10476–89. 10.1074/jbc.M115.698639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Pitanga TN, de Aragao Franca L, Rocha VC, Meirelles T, Borges VM, Goncalves MS, et al. Neutrophil-derived microparticles induce myeloperoxidase-mediated damage of vascular endothelial cells. BMC Cell Biol 2014;15:21 10.1186/1471-2121-15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Slater TW, Finkielsztein A, Mascarenhas LA, Mehl LC, Butin-Israeli V, Sumagin R. Neutrophil Microparticles Deliver Active Myeloperoxidase to Injured Mucosa To Inhibit Epithelial Wound Healing. J Immunol 2017;198:2886–97. 10.4049/jimmunol.1601810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 2009;15:623–5. 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Takeuchi H, Kawasaki T, Shigematsu K, Kawamura K, Oka N. Neutrophil extracellular traps in neuropathy with anti-neutrophil cytoplasmic autoantibody-associated microscopic polyangiitis. Clin Rheumatol 2017;36:913–7. 10.1007/s10067-017-3546-4. [DOI] [PubMed] [Google Scholar]
- [111].Chennakrishnaiah S, Meehan B, D’Asti E, Montermini L, Lee TH, Karatzas N, et al. Leukocytes as a reservoir of circulating oncogenic DNA and regulatory targets of tumor-derived extracellular vesicles. J Thromb Haemost 2018;16:1800–13. 10.1111/jth.14222. [DOI] [PubMed] [Google Scholar]
- [112].Kambas K, Chrysanthopoulou A, Vassilopoulos D, Apostolidou E, Skendros P, Girod A, et al. Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease. Ann Rheum Dis 2014;73:1854–63. 10.1136/annrheumdis-2013-203430. [DOI] [PubMed] [Google Scholar]
- [113].Pluskota E, Woody NM, Szpak D, Ballantyne CM, Soloviev DA, Simon DI, et al. Expression, activation, and function of integrin alphaMbeta2 (Mac-1) on neutrophil-derived microparticles. Blood 2008;112:2327–35. 10.1182/blood-2007-12-127183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Hong Y, Eleftheriou D, Hussain AA, Price-Kuehne FE, Savage CO, Jayne D, et al. Anti-neutrophil cytoplasmic antibodies stimulate release of neutrophil microparticles. J Am Soc Nephrol 2012;23:49–62. 10.1681/ASN.2011030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Gao J, Wang S, Wang Z. High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVs) for anti-inflammation therapy. Biomaterials 2017;135:62–73. 10.1016/j.biomaterials.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Gasser O, Schifferli JA. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood 2004;104:2543–8. 10.1182/blood-2004-01-0361 [DOI] [PubMed] [Google Scholar]
- [117].Guiducci S, Ricci L, Romano E, Ceccarelli C, Distler JH, Miniati I, et al. Microparticles and Kawasaki disease: a marker of vascular damage? Clin Exp Rheumatol 2011;29:S121–5. [PubMed] [Google Scholar]
- [118].Shefler I, Salamon P, Levi-Schaffer F, Mor A, Hershko AY, Mekori YA. MicroRNA-4443 regulates mast cell activation by T cell-derived microvesicles. J Allergy Clin Immunol 2018;141:2132–41 e4. 10.1016/j.jaci.2017.06.045. [DOI] [PubMed] [Google Scholar]
- [119].Zhang H, Xie Y, Li W, Chibbar R, Xiong S, Xiang J. CD4(+) T cell-released exosomes inhibit CD8(+) cytotoxic T-lymphocyte responses and antitumor immunity. Cell Mol Immunol 2011;8:23–30. 10.1038/cmi.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T, et al. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity 2014;41:89–103. 10.1016/j.immuni.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Almanza G, Zanetti M. High-efficiency Generation of Multiple Short Noncoding RNA in B-cells and B-cell-derived Extracellular Vesicles. Mol Ther Nucleic Acids 2015;4:e271 10.1038/mtna.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Clayton A, Turkes A, Dewitt S, Steadman R, Mason MD, Hallett MB. Adhesion and signaling by B cell-derived exosomes: the role of integrins. FASEB J 2004;18:977–9. 10.1096/fj.03-1094fje. [DOI] [PubMed] [Google Scholar]
- [123].Loyer X, Vion AC, Tedgui A, Boulanger CM. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ Res 2014;114:345–53. 10.1161/CIRCRESAHA.113.300858. [DOI] [PubMed] [Google Scholar]
- [124].Lawson C, Kovacs D, Finding E, Ulfelder E, Luis-Fuentes V. Extracellular Vesicles: Evolutionarily Conserved Mediators of Intercellular Communication. Yale J Biol Med 2017;90:481–91. [PMC free article] [PubMed] [Google Scholar]
- [125].Turpin D, Truchetet ME, Faustin B, Augusto JF, Contin-Bordes C, Brisson A, et al. Role of extracellular vesicles in autoimmune diseases. Autoimmun Rev 2016;15:174–83. 10.1016/j.autrev.2015.11.004. [DOI] [PubMed] [Google Scholar]
- [126].Ardoin SP, Shanahan JC, Pisetsky DS. The role of microparticles in inflammation and thrombosis. Scand J Immunol 2007;66:159–65. 10.1111/j.1365-3083.2007.01984.x. [DOI] [PubMed] [Google Scholar]
- [127].Liu R, Shen H, Ma J, Sun L, Wei M. Extracellular Vesicles Derived from Adipose Mesenchymal Stem Cells Regulate the Phenotype of Smooth Muscle Cells to Limit Intimal Hyperplasia. Cardiovasc Drugs Ther 2016;30:111–8. 10.1007/s10557-015-6630-5. [DOI] [PubMed] [Google Scholar]
- [128].MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity 2001;15:825–35. [DOI] [PubMed] [Google Scholar]
- [129].Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol 2007;179:1913–25. [DOI] [PubMed] [Google Scholar]
- [130].Hezel MEV, Nieuwland R, Bruggen RV, Juffermans NP. The Ability of Extracellular Vesicles to Induce a Pro-Inflammatory Host Response. Int J Mol Sci 2017;18 10.3390/ijms18061285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Lee JY, Park JK, Lee EY, Lee EB, Song YW. Circulating exosomes from patients with systemic lupus erythematosus induce an proinflammatory immune response. Arthritis Res Ther 2016;18:264 10.1186/s13075-016-1159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Distler JH, Jungel A, Huber LC, Seemayer CA, Reich CF 3rd, Gay RE, et al. The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc Natl Acad Sci U S A 2005;102:2892–7. 10.1073/pnas.0409781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Straat M, Boing AN, Tuip-De Boer A, Nieuwland R, Juffermans NP. Extracellular Vesicles from Red Blood Cell Products Induce a Strong Pro-Inflammatory Host Response, Dependent on Both Numbers and Storage Duration. Transfus Med Hemother 2016;43:302–5. 10.1159/000442681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Liu M, Xu H, Bashir M, Sharma M, Williams K, Anyanwu C, et al. Proinflammatory microvesicles in patients with cutaneous lupus erythematosus. Journal of Investigative Dermatology 2014;134. [Google Scholar]
- [135].Liu M-L, Bashir M, Sharma M, Xu H, Williams KJ, Anyanwu C. Novel proinflammatory microvesicles that carry LL-37 in patients with cutaneous lupus 2013;65:1860. [Google Scholar]
- [136].Esser J, Gehrmann U, D’Alexandri FL, Hidalgo-Estevez AM, Wheelock CE, Scheynius A, et al. Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. J Allergy Clin Immunol 2010;126:1032–40, 40 e1–4. 10.1016/j.jaci.2010.06.039. [DOI] [PubMed] [Google Scholar]
- [137].Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007;449:564–9. 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- [138].Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med 2011;3:73ra19. 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Kahlenberg JM, Carmona-Rivera C, Smith CK, Kaplan MJ. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J Immunol 2013;190:1217–26. 10.4049/jimmunol.1202388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015;349:316–20. 10.1126/science.aaa8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Katsiougiannis S. Extracellular Vesicles: Evolving Contributors in Autoimmunity. For Immunopathol Dis Therap 2015;6:163–70. 10.1615/ForumImmunDisTher.2016016491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Sellam J, Proulle V, Jungel A, Ittah M, Miceli Richard C, Gottenberg JE, et al. Increased levels of circulating microparticles in primary Sjogren’s syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res Ther 2009;11:R156 10.1186/ar2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Kapsogeorgou EK, Abu-Helu RF, Moutsopoulos HM, Manoussakis MN. Salivary gland epithelial cell exosomes: A source of autoantigenic ribonucleoproteins. Arthritis Rheum 2005;52:1517–21. 10.1002/art.21005. [DOI] [PubMed] [Google Scholar]
- [144].Cloutier N, Tan S, Boudreau LH, Cramb C, Subbaiah R, Lahey L, et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle-associated immune complexes. EMBO Mol Med 2013;5:235–49. 10.1002/emmm.201201846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Ullal AJ, Reich CF 3rd, Clowse M, Criscione-Schreiber LG, Tochacek M, Monestier M, et al. Microparticles as antigenic targets of antibodies to DNA and nucleosomes in systemic lupus erythematosus. J Autoimmun 2011;36:173–80. 10.1016/j.jaut.2011.02.001. [DOI] [PubMed] [Google Scholar]
- [146].Nielsen CT, Ostergaard O, Stener L, Iversen LV, Truedsson L, Gullstrand B, et al. Increased IgG on cell-derived plasma microparticles in systemic lupus erythematosus is associated with autoantibodies and complement activation. Arthritis Rheum 2012;64:1227–36. 10.1002/art.34381. [DOI] [PubMed] [Google Scholar]
- [147].Mobarrez F, Gunnarsson I, Svenungsson E. Altered beta2 -glycoprotein I expression on microparticles in the presence of antiphospholipid antibodies. J Thromb Haemost 2017;15:1799–806. 10.1111/jth.13765. [DOI] [PubMed] [Google Scholar]
- [148].Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: structure, function, and therapeutic target. Annu Rev Immunol 2014;32:25–50. 10.1146/annurev-immunol-032713-120142. [DOI] [PubMed] [Google Scholar]
- [149].Maugeri N, Rovere-Querini P, Baldini M, Baldissera E, Sabbadini MG, Bianchi ME, et al. Oxidative stress elicits platelet/leukocyte inflammatory interactions via HMGB1: a candidate for microvessel injury in sytemic sclerosis. Antioxid Redox Signal 2014;20:1060–74. 10.1089/ars.2013.5298. [DOI] [PubMed] [Google Scholar]
- [150].Kouwaki T, Okamoto M, Tsukamoto H, Fukushima Y, Oshiumi H. Extracellular Vesicles Deliver Host and Virus RNA and Regulate Innate Immune Response. Int J Mol Sci 2017;18 10.3390/ijms18030666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 2007;110:3234–44. 10.1182/blood-2007-03-079152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Lu Y, Li L, Yan H, Su Q, Huang J, Fu C. Endothelial microparticles exert differential effects on functions of Th1 in patients with acute coronary syndrome. Int J Cardiol 2013;168:5396–404. 10.1016/j.ijcard.2013.08.050. [DOI] [PubMed] [Google Scholar]
- [153].Rodriguez-Munoz A, Martinez-Hernandez R, Ramos-Levi AM, Serrano-Somavilla A, Gonzalez-Amaro R, Sanchez-Madrid F, et al. Circulating Microvesicles Regulate Treg and Th17 Differentiation in Human Autoimmune Thyroid Disorders. J Clin Endocrinol Metab 2015;100:E1531–9. 10.1210/jc.2015-3146. [DOI] [PubMed] [Google Scholar]
- [154].Lange C, Csernok E, Moosig F, Holle JU. Immune stimulatory effects of neutrophil extracellular traps in granulomatosis with polyangiitis. Clin Exp Rheumatol 2017;35 Suppl 103:33–9. [PubMed] [Google Scholar]
- [155].Niccolai E, Emmi G, Squatrito D, Silvestri E, Emmi L, Amedei A, et al. Microparticles: Bridging the Gap between Autoimmunity and Thrombosis. Semin Thromb Hemost 2015;41:413–22. 10.1055/s-0035-1549850. [DOI] [PubMed] [Google Scholar]
- [156].Tripisciano C, Weiss R, Eichhorn T, Spittler A, Heuser T, Fischer MB, et al. Different Potential of Extracellular Vesicles to Support Thrombin Generation: Contributions of Phosphatidylserine, Tissue Factor, and Cellular Origin. Sci Rep 2017;7:6522 10.1038/s41598-017-03262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Morel O, Toti F, Hugel B, Bakouboula B, Camoin-Jau L, Dignat-George F, et al. Procoagulant microparticles: disrupting the vascular homeostasis equation? Arterioscler Thromb Vasc Biol 2006;26:2594–604. 10.1161/01.ATV.0000246775.14471.26. [DOI] [PubMed] [Google Scholar]
- [158].Egorina EM, Sovershaev MA, Olsen JO, Osterud B. Granulocytes do not express but acquire monocyte-derived tissue factor in whole blood: evidence for a direct transfer. Blood 2008;111:1208–16. 10.1182/blood-2007-08-107698. [DOI] [PubMed] [Google Scholar]
- [159].Qi H, Yang S, Zhang L. Neutrophil Extracellular Traps and Endothelial Dysfunction in Atherosclerosis and Thrombosis. Front Immunol 2017;8:928 10.3389/fimmu.2017.00928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood 2011;118:1952–61. 10.1182/blood-2011-03-343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].McDonald B, Davis RP, Kim SJ, Tse M, Esmon CT, Kolaczkowska E, et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood 2017;129:1357–67. 10.1182/blood-2016-09-741298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Huang YM, Wang H, Wang C, Chen M, Zhao MH. Promotion of hypercoagulability in antineutrophil cytoplasmic antibody-associated vasculitis by C5a-induced tissue factor-expressing microparticles and neutrophil extracellular traps. Arthritis Rheumatol 2015;67:2780–90. 10.1002/art.39239. [DOI] [PubMed] [Google Scholar]
- [163].Nakazawa D, Tomaru U, Yamamoto C, Jodo S, Ishizu A. Abundant neutrophil extracellular traps in thrombus of patient with microscopic polyangiitis. Front Immunol 2012;3:333 10.3389/fimmu.2012.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Sinauridze EI, Kireev DA, Popenko NY, Pichugin AV, Panteleev MA, Krymskaya OV, et al. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost 2007;97:425–34. [PubMed] [Google Scholar]
- [165].Berckmans RJ, Nieuwland R, Boing AN, Romijn FP, Hack CE, Sturk A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost 2001;85:639–46. [PubMed] [Google Scholar]
- [166].Bacon PA. Endothelial cell dysfunction in systemic vasculitis: new developments and therapeutic prospects. Curr Opin Rheumatol 2005;17:49–55. [DOI] [PubMed] [Google Scholar]
- [167].Salmela A, Ekstrand A, Joutsi-Korhonen L, Raisanen-Sokolowski A, Lassila R. Activation of endothelium, coagulation and fibrinolysis is enhanced and associates with renal anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrol Dial Transplant 2015;30 Suppl 1:i53–9. 10.1093/ndt/gfu379. [DOI] [PubMed] [Google Scholar]
- [168].Liu ML, Williams KJ. Microvesicles: potential markers and mediators of endothelial dysfunction. Curr Opin Endocrinol Diabetes Obes 2012;19:121–7. 10.1097/MED.0b013e32835057e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Carmona-Rivera C, Zhao W, Yalavarthi S, Kaplan MJ. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis 2015;74:1417–24. 10.1136/annrheumdis-2013-204837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Monach PA, Tomasson G, Specks U, Stone JH, Cuthbertson D, Krischer J, et al. Circulating markers of vascular injury and angiogenesis in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2011;63:3988–97. 10.1002/art.30615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].O’Neill L, Rooney P, Molloy D, Connolly M, McCormick J, McCarthy G, et al. Regulation of Inflammation and Angiogenesis in Giant Cell Arteritis by Acute-Phase Serum Amyloid A. Arthritis Rheumatol 2015;67:2447–56. 10.1002/art.39217. [DOI] [PubMed] [Google Scholar]
- [172].Keskin D, Keskin G, Inal A, Ozisik L. Serum angiostatin levels in patients with Behcet’s disease: does angiogenesis play a role in the pathogenesis of Behcet’s disease? Acta Clin Belg 2014;69:246–50. 10.1179/2295333714Y.0000000030. [DOI] [PubMed] [Google Scholar]
- [173].Pay S, Abbasov T, Erdem H, Musabak U, Simsek I, Pekel A, et al. Serum MMP-2 and MMP-9 in patients with Behcet’s disease: do their higher levels correlate to vasculo-Behcet’s disease associated with aneurysm formation? Clin Exp Rheumatol 2007;25:S70–5. [PubMed] [Google Scholar]
- [174].Todorova D, Simoncini S, Lacroix R, Sabatier F, Dignat-George F. Extracellular Vesicles in Angiogenesis. Circ Res 2017;120:1658–73. 10.1161/CIRCRESAHA.117.309681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Taraboletti G, D’Ascenzo S, Borsotti P, Giavazzi R, Pavan A, Dolo V. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol 2002;160:673–80. 10.1016/S0002-9440(10)648870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Brill A, Dashevsky O, Rivo J, Gozal Y, Varon D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res 2005;67:30–8. 10.1016/j.cardiores.2005.04.007. [DOI] [PubMed] [Google Scholar]
- [177].Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci 2016;129:2182–9. 10.1242/jcs.170373. [DOI] [PubMed] [Google Scholar]
- [178].Kang T, Jones TM, Naddell C, Bacanamwo M, Calvert JW, Thompson WE, et al. Adipose-Derived Stem Cells Induce Angiogenesis via Microvesicle Transport of miRNA-31. Stem Cells Transl Med 2016;5:440–50. 10.5966/sctm.2015-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Yang C, Mwaikambo BR, Zhu T, Gagnon C, Lafleur J, Seshadri S, et al. Lymphocytic microparticles inhibit angiogenesis by stimulating oxidative stress and negatively regulating VEGF-induced pathways. Am J Physiol Regul Integr Comp Physiol 2008;294:R467–76. 10.1152/ajpregu.00432.2007. [DOI] [PubMed] [Google Scholar]
- [180].Aldabbous L, Abdul-Salam V, McKinnon T, Duluc L, Pepke-Zaba J, Southwood M, et al. Neutrophil Extracellular Traps Promote Angiogenesis: Evidence From Vascular Pathology in Pulmonary Hypertension. Arterioscler Thromb Vasc Biol 2016;36:2078–87. 10.1161/ATVBAHA.116.307634. [DOI] [PubMed] [Google Scholar]
- [181].Lavoie SS, Dumas E, Vulesevic B, Neagoe PE, White M, Sirois MG. Synthesis of Human Neutrophil Extracellular Traps Contributes to Angiopoietin-Mediated In Vitro Proinflammatory and Proangiogenic Activities. J Immunol 2018;200:3801–13. 10.4049/jimmunol.1701203. [DOI] [PubMed] [Google Scholar]
- [182].Weyand CM, Goronzy JJ. Immune mechanisms in medium and large-vessel vasculitis. Nat Rev Rheumatol 2013;9:731–40. 10.1038/nrrheum.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Hid Cadena R, Abdulahad WH, Hospers GAP, Wind TT, Boots AMH, Heeringa P, et al. Checks and Balances in Autoimmune Vasculitis. Front Immunol 2018;9:315 10.3389/fimmu.2018.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Cai J, Yuan H, Wang Q, Yang H, Al-Abed Y, Hua Z, et al. HMGB1-Driven Inflammation and Intimal Hyperplasia After Arterial Injury Involves Cell-Specific Actions Mediated by TLR4. Arterioscler Thromb Vasc Biol 2015;35:2579–93. 10.1161/ATVBAHA.115.305789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Brogan PA, Shah V, Brachet C, Harnden A, Mant D, Klein N, et al. Endothelial and platelet microparticles in vasculitis of the young. Arthritis Rheum 2004;50:927–36. 10.1002/art.20199. [DOI] [PubMed] [Google Scholar]
- [186].Hajj-Ali RA, Major J, Langford C, Hoffman GS, Clark T, Zhang L, et al. The interface of inflammation and subclinical atherosclerosis in granulomatosis with polyangiitis (Wegener’s): a preliminary study. Transl Res 2015;166:366–74. 10.1016/j.trsl.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Nakaoka H, Hirono K, Yamamoto S, Takasaki I, Takahashi K, Kinoshita K, et al. MicroRNA-145–5p and microRNA-320a encapsulated in endothelial microparticles contribute to the progression of vasculitis in acute Kawasaki Disease. Sci Rep 2018;8:1016 10.1038/s41598-018-19310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Tan Z, Yuan Y, Chen S, Chen Y, Chen TX. Plasma Endothelial Microparticles, TNF-a and IL-6 in Kawasaki Disease. Indian Pediatr 2013;50:501–3. [DOI] [PubMed] [Google Scholar]
- [189].Shah V, Christov G, Mukasa T, Brogan KS, Wade A, Eleftheriou D, et al. Cardiovascular status after Kawasaki disease in the UK. Heart 2015;101:1646–55. 10.1136/heartjnl-2015-307734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Dursun I, Dusunsel R, Poyrazoglu HM, Gunduz Z, Patiroglu T, Ulger H, et al. Circulating endothelial microparticles in children with Henoch-Schonlein purpura; preliminary results. Rheumatol Int 2011;31:1595–600. 10.1007/s00296-010-1528-9. [DOI] [PubMed] [Google Scholar]
- [191].Eleftheriou D, Hong Y, Klein NJ, Brogan PA. Thromboembolic disease in systemic vasculitis is associated with enhanced microparticle-mediated thrombin generation. J Thromb Haemost 2011;9:1864–7. 10.1111/j.1538-7836.2011.04434.x. [DOI] [PubMed] [Google Scholar]
- [192].Kahn R, Mossberg M, Stahl AL, Johansson K, Lopatko Lindman I, Heijl C, et al. Microvesicle transfer of kinin B1-receptors is a novel inflammatory mechanism in vasculitis. Kidney Int 2017;91:96–105. 10.1016/j.kint.2016.09.023. [DOI] [PubMed] [Google Scholar]
- [193].Mossberg M, Stahl AL, Kahn R, Kristoffersson AC, Tati R, Heijl C, et al. C1-Inhibitor Decreases the Release of Vasculitis-Like Chemotactic Endothelial Microvesicles. J Am Soc Nephrol 2017;28:2472–81. 10.1681/ASN.2016060637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Jia HL, Liu CW, Zhang L, Xu WJ, Gao XJ, Bai J, et al. Sets of serum exosomal microRNAs as candidate diagnostic biomarkers for Kawasaki disease. Sci Rep 2017;7:44706 10.1038/srep44706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Zhang X, Xin G, Sun D. Serum exosomal miR-328, miR-575, miR-134 and miR-671–5p as potential biomarkers for the diagnosis of Kawasaki disease and the prediction of therapeutic outcomes of intravenous immunoglobulin therapy. Exp Ther Med 2018;16:2420–32. 10.3892/etm.2018.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Zhang L, Song QF, Jin JJ, Huang P, Wang ZP, Xie XF, et al. Differential protein analysis of serum exosomes post-intravenous immunoglobulin therapy in patients with Kawasaki disease. Cardiol Young 2017;27:1786–96. 10.1017/S1047951117001433. [DOI] [PubMed] [Google Scholar]
- [197].Zhang L, Wang W, Bai J, Xu YF, Li LQ, Hua L, et al. Proteomic analysis associated with coronary artery dilatation caused by Kawasaki disease using serum exosomes. Rev Port Cardiol 2016;35:265–73. 10.1016/j.repc.2015.11.016. [DOI] [PubMed] [Google Scholar]
- [198].Mejia JC, Ortiz T, Tassies D, Solanich X, Vidaller A, Cervera R, et al. Procoagulant microparticles are increased in patients with Behcet’s disease but do not define a specific subset of clinical manifestations. Clin Rheumatol 2016;35:695–9. 10.1007/s10067-015-2903-4. [DOI] [PubMed] [Google Scholar]
- [199].Khan E, Ambrose NL, Ahnstrom J, Kiprianos AP, Stanford MR, Eleftheriou D, et al. A low balance between microparticles expressing tissue factor pathway inhibitor and tissue factor is associated with thrombosis in Behcet’s Syndrome. Sci Rep 2016;6:38104 10.1038/srep38104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Martinez M, Ricart JM, Ruiz-Aja S, Rus A, Todoli J, Calvo J, et al. Platelet activation and red blood cell phosphatidylserine exposure evaluated by flow cytometry in patients with Behcet’s disease: are they related to thrombotic events? Pathophysiol Haemost Thromb 2007;36:18–22. 10.1159/000112635. [DOI] [PubMed] [Google Scholar]
- [201].Macey M, Hagi-Pavli E, Stewart J, Wallace GR, Stanford M, Shirlaw P, et al. Age, gender and disease-related platelet and neutrophil activation ex vivo in whole blood samples from patients with Behcet’s disease. Rheumatology (Oxford) 2011;50:1849–59. 10.1093/rheumatology/ker177. [DOI] [PubMed] [Google Scholar]
- [202].Kahn R, Herwald H, Muller-Esterl W, Schmitt R, Sjogren AC, Truedsson L, et al. Contact-system activation in children with vasculitis. Lancet 2002;360:535–41. 10.1016/S0140-6736(02)09743-X. [DOI] [PubMed] [Google Scholar]
- [203].Kahn R, Hellmark T, Leeb-Lundberg LM, Akbari N, Todiras M, Olofsson T, et al. Neutrophil-derived proteinase 3 induces kallikrein-independent release of a novel vasoactive kinin. J Immunol 2009;182:7906–15. 10.4049/jimmunol.0803624. [DOI] [PubMed] [Google Scholar]
- [204].Duchene J, Lecomte F, Ahmed S, Cayla C, Pesquero J, Bader M, et al. A novel inflammatory pathway involved in leukocyte recruitment: role for the kinin B1 receptor and the chemokine CXCL5. J Immunol 2007;179:4849–56. [DOI] [PMC free article] [PubMed] [Google Scholar]