Abstract
Non-small cell lung cancer (NSCLC) is the most common histological type of lung cancer. Altered expression of centromere protein F (CENPF), a transient kinetochore protein, has been found in a variety of human cancers. However, its clinical significance in NSCLC remains unknown. In the present study the results of quantitative PCR and western blot analyses demonstrated that CENPF and Forkhead box M1 (FOXM1) were significantly higher in NSCLC tissues than in the non-cancerous controls at both transcriptional and translational levels. Immunohistochemical staining results showed 58.7% (44/75) and 64.0% (48/75) of NSCLC tissues displayed high expression of CENPF and FOXM1, respectively. CENPF protein expression showed a positive correlation with tumor size (P=0.0179), vital status (P=0.0008) and FOXM1 expression (P=0.0013) in NSCLC. Poor overall survival was correlated with high levels of CENPF and FOXM1 in NSCLC patients as evaluated by Kaplan-Meier and log rank test. Multivariate analyses showed that CENPF expression was an independent prognostic factor for NSCLC. In conclusion, our study provides evidence of the prognostic function of CENPF in NSCLC.
Keywords: CENPF, FOXM1, prognosis, NSCLC
Introduction
Lung cancer is the second most common cancer and is the leading cause of cancer-related death for men and women worldwide (1–3). Non-small cell lung cancer (NSCLC), accounting for ~85% cases of lung cancer, represents the most common histological type of lung cancer (4). The mortality rate of NSCLC is very high, and the 5-year survival rate is <20% (5), which is due to the lack of reliable tools for early diagnosis or effective therapy. Therefore, investigation is required to identify specific molecules that may contribute to the diagnosis of NSCLC, and serve as prognostic markers.
Centromere protein F (CENPF), a transient kinetochore protein, exhibits a cell-cycle dependent localization, and is completely degraded at the end of cell division (4–6). Evidence has shown that CENPF is overexpressed in head and neck squamous cell carcinomas, hepatocellular carcinoma, breast cancer and prostate cancer, and it may be a prognostic marker for these cancers (7–12). Forkhead box M1 (FOXM1) is a typical proliferation-associated factor and plays an important role in development (13–18). FOXM1 expression is frequently elevated in numerous malignancies and participates actively in the development and progression of various human cancers, including NSCLC (19–21). High expression of FOXM1 is correlated with shorter disease-free survival of NSCLC patients (22,23). The synergistic effect of FOXM1 and CENPF has been found in promoting the growth of prostate cancer and their co-expression predicts poor survival (24–26). However, the clinical significance of CENPF in NSCLC is unknown.
In the present study, the expression of CENPF and FOXM1 in NSCLC was explored by quantitative PCR, western blot analysis and immunohistochemical staining. The relationship between protein expression of CENPF and clinicopathological parameters were investigated to assess the possible prognostic value of CENPF and FOXM1 expression in NSCLC.
Patients and methods
Patients and clinicopathological data
The study was approved by the ethics committee of Shenyang Fifth People Hospital. A total of 75 patients with NSCLC who underwent surgery in Shenyang Fifth People's Hospital between 2009 and 2011 were enrolled after signed informed consent form was received. Tumor tissues and adjacent non-tumorous tissues were obtained from all the patients. Of these samples, 28 pairs of tumor tissues and adjacent non-tumorous tissues were frozen immediately, stored at −80°C and used for quantitative PCR analysis. The samples were formalin-fixed, paraffin-embedded, and cut into 5-µm thick sections.
Quantitative PCR
Total RNA was isolated from collected tissues with TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Then, single-stranded cDNA was generated from 1 µg of total RNA using cDNA synthesis kit (Thermo Fisher Scientific, Inc.). Quantitative PCR was carried out on ABI 7300 system (Applied Biosystem; Thermo Fisher Scientific, Inc.) with SYBR-Green qPCR Master Mixes (Thermo Fisher Scientific, Inc.) as per the manufacturer's instructions. The primers used in the study were: CENPF, forward, 5′-CTCGTTCCATCCCTGTCATC-3′ and reverse primer 5′-TCCTGGTCAGATTCTCCTCC-3′; FOXM1, forward, 5′-GAAACGACCGAATCCAGAG-3′ and reverse primer, 5′-GCAGATCGCCACTAAAGAAC-3′; GAPDH, forward, 5′-AATCCCATCACCATCTTC-3′ and reverse primer 5′-AGGCTGTTGTCATACTTC-3′. CENPF and FOXM1 mRNA expression was calculated using the 2−ΔΔCq method (27).
Western blot analysis
Total protein extracted from collected specimens (0.5 g per sample) was cut into small sections and homogenized in RIPA lysis buffer containing protease and phosphatase inhibitors (Beyotime). After centrifugation at 13,000 × g, at 4°C for 20 min, the supernatant was recovered.
After separation by 6% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), proteins were electroblotted onto nitrocellulose membranes (Millipore) and subjected to western blot analysis, then incubated with primary antibodies, CENPF (rabbit polyclonal, 1:1,000 dilution, ab5), FOXM1 (rabbit polyclonal, 1:1,000dilution, ab226928) (both from Abcam) and GAPDH (rabbit monoclonal, 1:1,000dilution, no. 5174; Cell Signaling Technology), and membranes were incubated with horseradish peroxidase (HRP)-labeled secondary antibody (goat anti rabbit, 1:1,000 dilution, A0208; Beyotime). The immunoreactive signal was detected by the enhanced chemiluminescence kit (Millipore). Quantification of band intensity was analyzed with ImageJ software (http://rsb.info.nih.gov/ij/), and normalized to the intensity of GAPDH.
Immunohistochemical analysis
The sections were deparaffinized in xylene and hydrated in a graded series of ethanol, then antigen retrieved by heat exposure in Tris/EDTA buffer (pH 9.0) for 15 min, and blocked for endogenous peroxidase activity in 3% hydrogen peroxide at room temperature for 10 min. Followed by blocking with 5% normal blocking serum, the sections were reacted with anti-CENPF (1:200 dilution, ab5) (28) or anti-FOXM1 (1:250 dilution, ab207298) (both from Abcam) (29) at 4°C overnight. After probing with HRP-conjugated secondary antibody, immunocomplexes were visualized using 3,3′-diaminobenzidine (DAB) (both from Long Island Biotech Co., Ltd.) and lightly counterstained with hematoxylin. The sections were reviewed and classified by two independent investigators as previously described (27). The percentage of positive stained cancer cells was scored as 0, negative; 1, 1–10%; 2, 11–50% positive; 3, >50% positive. The staining intensity was scored as 0, absent; 1, weak; 2, moderate; 3, strong. The immunoreactive score (IS) was calculated as follows: IS= percentage × staining intensity. The protein was considered to be highly expressed when IS was 3–9, otherwise to be lowly expressed.
Statistical analysis
Statistical analysis was carried out with the Statistical Package for the Social Sciences software (SPSS Inc.). Paired Student's t-test was performed to analyze the difference of mRNA and protein expression between NSCLC tissues and adjacent non-cancerous tissues. Pearsons correlation analysis was used to investigate the relationship between mRNA expression of CENPF and FOXM1 in NSCLC tissues. The Fisher's exact test was used to analyze the relationship between CENPF expression and the clinicopathological features. Kaplan-Meier and log rank test was used to estimate and compare survival. The significance level was set at 0.05.
Results
Association of CENPF and FOXM1 in NSCLC tissues
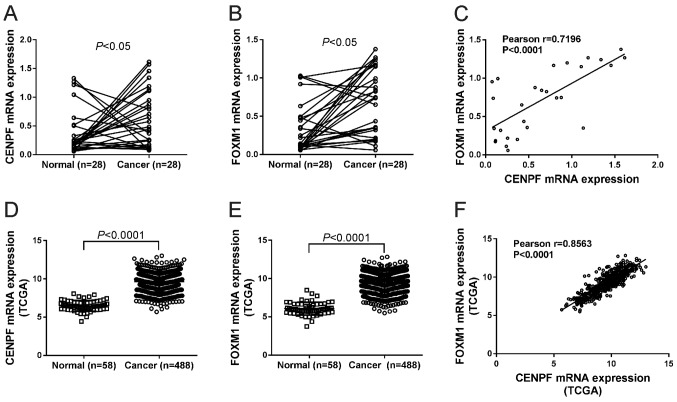
To describe the mRNA expression of CENPF and FOXM1 in NSCLC, we performed quantitative PCR analysis on 28 pairs of NSCLC tissues and adjacent non-cancerous tissues. Fig. 1A and B shows that 67.9% (19 cases) and 78.6% (22 cases) of patients showed high mRNA expression of CENPF and FOXM1, respectively. Paired Student's t-test revealed that mRNA levels of both genes were significantly elevated in NSCLC tissues compared to the non-cancerous tissues (P<0.05). Pearson's r correlation analysis displayed a significant positive association between CENPF and FOXM1 in NSCLC tissues (P<0.0001) (Fig. 1C).
Figure 1.
mRNA expression of CENPF and FOXM1 was elevated in NSCLC tissues. (A and B) mRNA expressions of CENPF (A) and FOXM1 (B) was analyzed by quantitative PCR on 28 pairs of NSCLC tissues and adjacent non-cancerous tissues. (C) Pearsons r correlation analysis between mRNA expression of CENPF and FOXM1 on 28 NSCLC tissues. (D-F) Analysis of CENPF (D) and FOXM1 (E) expression based on mRNA profile data from The Cancer Genome Atlas (TCGA) database. Pearsons r correlation analysis (F) between CENPF and FOXM1 was performed. NSCLC, non-small cell lung cancer; CENPF, centromere protein F; FOXM1, Forkhead box M1.
mRNA profile data of lung cancer tissues and control tissues were downloaded from The Cancer Genome Atlas (TCGA) database. The expression of CENPF (Fig. 1D) and FOXM1 (Fig. 1E) was also significantly higher in lung cancer tissues than in normal control, and CENPF expression was positively correlated with FOXM1 expression in lung cancer tissues (Fig. 1F).
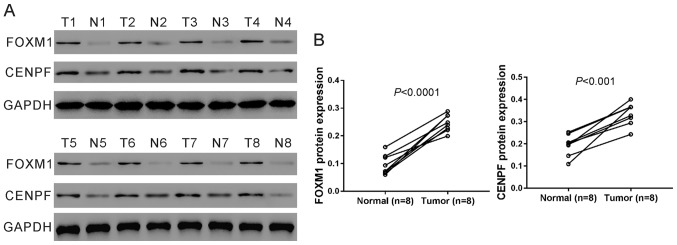
Furthermore, eight pairs of tissue samples were randomly selected from the above 28 pairs of samples and subjected to western blot analysis and the results confirmed the elevated protein levels of CENPF and FOXM1 in NSCLC tissues (Fig. 2A and B).
Figure 2.
Protein expression of CENPF and FOXM1 was up-regulated in NSCLC tissues. Western blot analysis was performed to assess the protein levels of CENPF and FOXM1 on eight pairs of NSCLC (T1-T8) and non-cancerous tissues (N1-N8). GAPDH served as a loading control. Representative images (A) and quantitative analysis (B) of three independent experiments are shown. NSCLC, non-small cell lung cancer; CENPF, centromere protein F; FOXM1, Forkhead box M1.
Elevated expression of CENPF correlated with clinical parameters of NSCLC
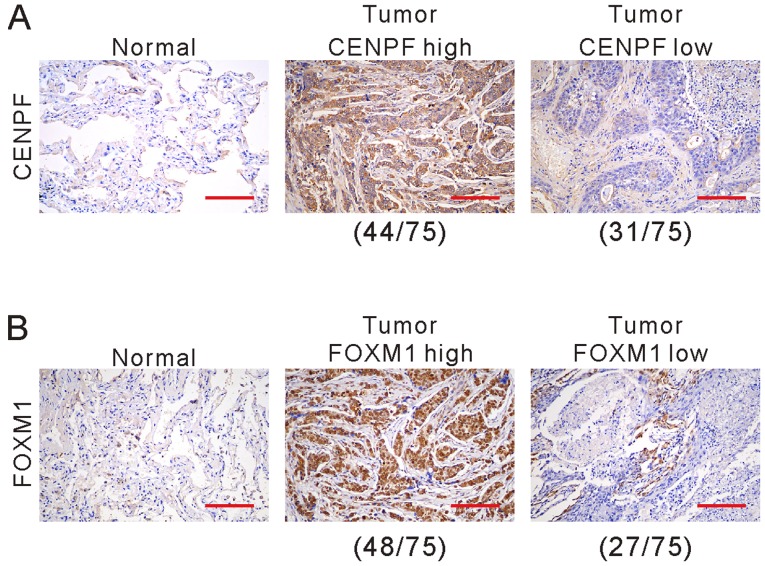
We further detected the protein expression of CENPF and FOXM1 in cancerous specimens and matched non-cancerous specimens from 75 NSCLC patients by immunohistochemistry. The clinical and pathological characteristics of these patients are listed in Table I. CENPF (Fig. 3A) and FOXM1 (Fig. 3B) expression was observed in cytoplasm and nucleus. Of the 75 patients, 58.7% (44 cases) and 41.3% (31 cases) showed high and low expression of CENPF, respectively, while 64.0% (48 cases) and 36.0% (27 cases) showed high and low expression of FOXM1, respectively.
Table I.
Clinicopathological characteristics in NSCLC patients (n=75).
| Characteristic | Cases | % |
|---|---|---|
| Age (years) | ||
| <60 | 37 | 49.3 |
| ≥60 | 38 | 50.7 |
| Sex | ||
| Male | 42 | 56.0 |
| Female | 33 | |
| Smoking status | ||
| Smoker | 25 | 33.3 |
| Non-smoker | 50 | 66.7 |
| Tumor size | ||
| <5 cm | 31 | 41.3 |
| ≥5 cm | 44 | 58.7 |
| TNM stage | ||
| I+II | 30 | 40.0 |
| III | 45 | 60.0 |
| Lymph node metastasis | ||
| Absent | 43 | 57.3 |
| Present | 32 | 42.7 |
| Pathological type | ||
| Adenocarcinoma | 46 | 61.3 |
| Squamous cell carcinoma | 29 | 39.7 |
| Vital status (at follow-up) | ||
| Alive | 24 | 32.0 |
| Dead | 51 | 68.0 |
| CENPF expression | ||
| Low | 31 | 41.3 |
| High | 44 | 58.7 |
| FOXM1 expression | ||
| Low | 27 | 36.0 |
| High | 48 | 64.0 |
NSCLC, non-small cell lung cancer; CENPF, centromere protein F; FOXM1, Forkhead box M1.
Figure 3.
Immunohistochemical staining of CENPF (A) and FOXM1 (B) in NSCLC and non-cancerous tissues. The positive staining for CENPF and FOXM1 is brown in cytoplasm and nucleus. Nucleus is blue in hematoxylin counterstaining. Scale bars, 100 µm. NSCLC, non-small cell lung cancer; CENPF, centromere protein F; FOXM1, Forkhead box M1.
The correlation between CENPF expression and the clinical parameters of NSCLC was analyzed by Fisher's exact test. As shown in Table II, CENPF protein expression was positively correlated with tumor size (P=0.0179), vital status (P=0.0008) and FOXM1 expression (P=0.0013), which suggested clinical significance of CENPF in NSCLC.
Table II.
Correlation of CENPF expression in NSCLC tissues with different clinicopathological features (n=75).
| CENPF | |||
|---|---|---|---|
| Characteristic | Low (n=31) | High (n=44) | P-value |
| Age (years) | 0.6410 | ||
| <60 | 14 | 23 | |
| ≥60 | 17 | 21 | |
| Sex | 0.8163 | ||
| Male | 18 | 24 | |
| Female | 13 | 20 | |
| Smoking status | 0.6210 | ||
| Smoker | 9 | 16 | |
| Non-smoker | 22 | 28 | |
| Tumor size | 0.0179a | ||
| <5 cm | 18 | 13 | |
| ≥5 cm | 13 | 31 | |
| TNM stage | 0.0991 | ||
| I/II | 16 | 14 | |
| III | 15 | 30 | |
| Lymph node metastasis | 0.3474 | ||
| Absent | 20 | 23 | |
| Present | 11 | 21 | |
| Pathological Type | 0.4705 | ||
| Adenocarcinoma | 21 | 25 | |
| Squamous cell carcinoma | 10 | 19 | |
| Vital status (at follow-up) | 0.0008c | ||
| Alive | 17 | 7 | |
| Dead | 14 | 37 | |
| FOXM1 expression | 0.0013b | ||
| Low | 18 | 9 | |
| High | 13 | 35 | |
Clinicopathological features were assessed using the Fisher's exact test.
P<0.05
P<0.01
P<0.0001. NSCLC, non-small cell lung cancer; CENPF, centromere protein F; FOXM1, Forkhead box M1.
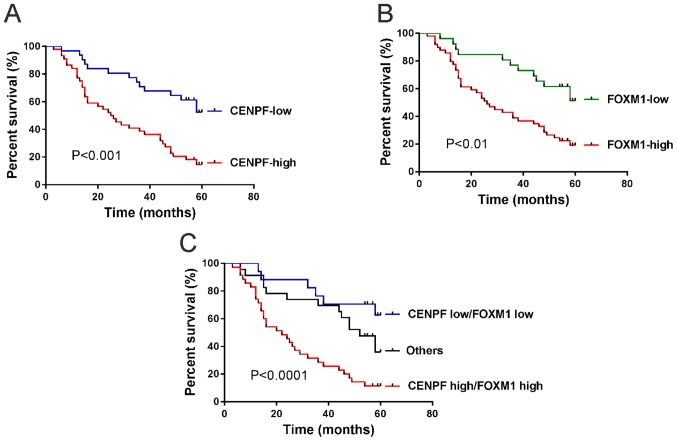
Expression of CENPF is closely related with the poor prognosis of patients with NSCLC. High expression of CENPF (P<0.001; Fig. 4A) and high expression of FOXM1 (P<0.01; Fig. 4B) in NSCLC were correlated with the short survival time of patients by Kaplan-Meier and log-rank test.
Figure 4.
Kaplan-Meier survival plots in NSCLC patients (n=75). The patients were divided into different groups based on (A) CENPF expression, (B) FOXM1 expression, (C) CENPF and FOXM1 coexpression observed by immunohistochemical staining. NSCLC, non-small cell lung cancer; CENPF, centromere protein F; FOXM1, Forkhead box M1.
When both CENPF and FOXM1 were analyzed (Fig. 4C), patients whose tumors exhibited high expression of CENPF and high expression of FOXM1 had the shortest overall survival time, whereas patients with tumors displaying low expression of CENPF and low expression of FOXM1 had the longest overall survival time (P<0.0001).
Finally, a multivariate Cox regression analysis was performed. CENPF (hazard ratio, 2.694; 95% CI, 1.397–5.195; P=0.003) was an independent parameter that was associated with overall survival when compared with tumor size and FOXM1 expression (Table III).
Table III.
Multivariate Cox regression of prognostic parameters for survival in 75 NSCLC patients.
| Multivariate analysis | |||
|---|---|---|---|
| Prognostic parameter | HR | 95% CI | P-value |
| CENPF expression (low vs. high) | 2.694 | 1.397–5.195 | 0.003a |
| Tumor size (<5 vs. ≥5 cm) | 1.045 | 0.574–1.903 | 0.886 |
| FOXM1 expression (low vs. high) | 1.751 | 0.911–3.366 | 0.093 |
P<0.01. HR, hazard ratio; CI, confidence interval; NSCLC, non-small cell lung cancer; CENPF, centromere protein F; FOXM1, Forkhead box M1.
Discussion
Identification of specific biomarkers is important for diagnosis, therapy and prognosis of NSCLC. Previous studies have revealed the potential prognostic values of CENPF in several human cancers except NSCLC (7–12). In the present study, we pinpointed that CENPF expression was elevated in NSCLC tissues at both mRNA and protein levels. Then the protein expression of CENPF in 75 cases of NSCLC and its association with overall survival and clinical characteristics were investigated. The results indicated that there was a significant correlation between CENPF expression and tumor size, vital status, and overall survival. Multivariate Cox regression analysis demonstrated that CENPF expression was an independent prognostic factor of patients with NSCLC. Our data suggest that CENPF expression may serve as a novel prognostic marker for NSCLC although further validation data with larger sample size are required.
FOXM1, a transcription factor, plays a critical role during development (13–18) and carcinogenesis (19–21). Previous studies have demonstrated that FOXM1 is an independent prognostic factor for NSCLC (22,23). FOXM1 and CENPF colocalized in the nucleus of prostate cancer cells, and co-expression of FOXM1 and CENPF is a prognostic indicator for poor survival of prostate cancer (24–26). In the present study, the findings also demonstrated the value of diagnosis and prognosis of FOXM1 in NSCLC. Importantly, we found that CENPF mRNA expression was positively correlated with FOXM1 mRNA expression in NSCLC samples by analyzing TCGA database and our own samples. CENPF protein expression was positively correlated with FOXM1 protein expression in NSCLC specimens as indicated by immunohistochemical staining. In addition, immunohistochemical staining analysis indicated a similar subcellular localization of CENPF and FOXM1 in NSCLC specimens. Patients with high expression of CENPF and FOXM1 had the worst overall survival, whereas patients with low expression of both proteins had the best overall survival. Thus, the present study suggests that CENPF and FOXM1 may co-operate in NSCLC. Aytes et al (24) reported that knockdown of CENPF decreased the binding of FOXM1 to its target genes as revealed by chromatin immunoprecipitation analysis. Similar mechanism may exist in NSCLC cells, which needs to be investigated in the future.
In conclusion, the present study has demonstrated that CENPF expression in NSCLC is correlated with FOXM1 expression and worse clinical outcome. These findings suggest that CENPF may function as a potential prognostic indicator for NSCLC. However, the present findings are based on a small sample size and further study with larger number of patients is needed.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
RL and YL wrote the manuscript. RL, XW and XZ performed PCR and western blot analysis. XZ and HC were responsible for immunohistochemical staining. YM and YL helped with statistical analysis. All the authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Shenyang Fifth People's Hospital. Patients who participated in this research, signed an informed consent and had complete clinical data. Signed informed consents were obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Zheng T, Zhang W. Report of cancer incidence and mortality in China. 2018;4:1–7. doi: 10.3760/cma.j.issn.0253-3766.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Bomont P, Maddox P, Shah JV, Desai AB, Cleveland DW. Unstable microtubule capture at kinetochores depleted of the centromere-associated protein CENP-F. EMBO J. 2005;24:3927–3939. doi: 10.1038/sj.emboj.7600848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varis A, Salmela AL, Kallio MJ. Cenp-F (mitosin) is more than a mitotic marker. Chromosoma. 2006;115:288–295. doi: 10.1007/s00412-005-0046-0. [DOI] [PubMed] [Google Scholar]
- 6.Liao H, Winkfein RJ, Mack G, Rattner JB, Yen TJ. CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis. J Cell Biol. 1995;130:507–518. doi: 10.1083/jcb.130.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark GM, Allred DC, Hilsenbeck SG, Chamness GC, Osborne CK, Jones D, Lee WH. Mitosin (a new proliferation marker) correlates with clinical outcome in node-negative breast cancer. Cancer Res. 1997;57:5505–5508. [PubMed] [Google Scholar]
- 8.de la Guardia C, Casiano CA, Trinidad-Pinedo J, Báez A. CENP-F gene amplification and overexpression in head and neck squamous cell carcinomas. Head Neck. 2001;23:104–112. doi: 10.1002/1097-0347(200102)23:2<104::AID-HED1005>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Kim HE, Kim DG, Lee KJ, Son JG, Song MY, Park YM, Kim JJ, Cho SW, Chi SG, Cheong HS, et al. Frequent amplification of CENPF, GMNN and CDK13 genes in hepatocellular carcinomas. PLoS One. 2012;7:e43223. doi: 10.1371/journal.pone.0043223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Brien SL, Fagan A, Fox EJ, Millikan RC, Culhane AC, Brennan DJ, McCann AH, Hegarty S, Moyna S, Duffy MJ, et al. CENP-F expression is associated with poor prognosis and chromosomal instability in patients with primary breast cancer. Int J Cancer. 2007;120:1434–1443. doi: 10.1002/ijc.22413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shahid M, Lee MY, Piplani H, Andres AM, Zhou B, Yeon A, Kim M, Kim HL, Kim J. Centromere protein F (CENPF), a microtubule binding protein, modulates cancer metabolism by regulating pyruvate kinase M2 phosphorylation signaling. Cell Cycle. 2018;17:2802–2818. doi: 10.1080/15384101.2018.1557496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li P, You S, Nguyen C, Wang Y, Kim J, Sirohi D, Ziembiec A, Luthringer D, Lin SC, Daskivich T, et al. Genes involved in prostate cancer progression determine MRI visibility. Theranostics. 2018;8:1752–1765. doi: 10.7150/thno.23180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang IC, Snyder J, Zhang Y, Lander J, Nakafuku Y, Lin J, Chen G, Kalin TV, Whitsett JA, Kalinichenko VV. Foxm1 mediates cross talk between Kras/mitogen-activated protein kinase and canonical Wnt pathways during development of respiratory epithelium. Mol Cell Biol. 2012;32:3838–3850. doi: 10.1128/MCB.00355-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolte C, Zhang Y, Wang I-C, Kalin TV, Molkentin JD, Kalinichenko VV. Expression of Foxm1 transcription factor in cardiomyocytes is required for myocardial development. PLoS One. 2011;6:e22217. doi: 10.1371/journal.pone.0022217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ustiyan V, Wert SE, Ikegami M, Wang IC, Kalin TV, Whitsett JA, Kalinichenko VV. Foxm1 transcription factor is critical for proliferation and differentiation of Clara cells during development of conducting airways. Dev Biol. 2012;370:198–212. doi: 10.1016/j.ydbio.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim I-M, Ramakrishna S, Gusarova GA, Yoder HM, Costa RH, Kalinichenko VV. The forkhead box m1 transcription factor is essential for embryonic development of pulmonary vasculature. J Biol Chem. 2005;280:22278–22286. doi: 10.1074/jbc.M500936200. [DOI] [PubMed] [Google Scholar]
- 17.Zeng J, Wang L, Li Q, Li W, Björkholm M, Jia J, Xu D. FoxM1 is up-regulated in gastric cancer and its inhibition leads to cellular senescence, partially dependent on p27 kip1. J Pathol. 2009;218:419–427. doi: 10.1002/path.2530. [DOI] [PubMed] [Google Scholar]
- 18.Yang C, Chen H, Tan G, Gao W, Cheng L, Jiang X, Yu L, Tan Y. FOXM1 promotes the epithelial to mesenchymal transition by stimulating the transcription of Slug in human breast cancer. Cancer Lett. 2013;340:104–112. doi: 10.1016/j.canlet.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Ahmad A, Li Y, Banerjee S, Kong D, Sarkar FH. Forkhead box M1 transcription factor: A novel target for cancer therapy. Cancer Treat Rev. 2010;36:151–156. doi: 10.1016/j.ctrv.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu M, Dai B, Kang S-H, Ban K, Huang FJ, Lang FF, Aldape KD, Xie TX, Pelloski CE, Xie K, et al. FoxM1B is overexpressed in human glioblastomas and critically regulates the tumorigenicity of glioma cells. Cancer Res. 2006;66:3593–3602. doi: 10.1158/0008-5472.CAN-05-2912. [DOI] [PubMed] [Google Scholar]
- 21.Kim I-M, Ackerson T, Ramakrishna S, Tretiakova M, Wang IC, Kalin TV, Major ML, Gusarova GA, Yoder HM, Costa RH, et al. The Forkhead Box m1 transcription factor stimulates the proliferation of tumor cells during development of lung cancer. Cancer Res. 2006;66:2153–2161. doi: 10.1158/0008-5472.CAN-05-3003. [DOI] [PubMed] [Google Scholar]
- 22.Xu N, Jia D, Chen W, Wang H, Liu F, Ge H, Zhu X, Song Y, Zhang X, Zhang D, et al. FoxM1 is associated with poor prognosis of non-small cell lung cancer patients through promoting tumor metastasis. PLoS One. 2013;8:e59412. doi: 10.1371/journal.pone.0059412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang DK, Son CH, Lee SK, Choi PJ, Lee KE, Roh MS. Forkhead box M1 expression in pulmonary squamous cell carcinoma: Correlation with clinicopathologic features and its prognostic significance. Hum Pathol. 2009;40:464–470. doi: 10.1016/j.humpath.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Aytes A, Mitrofanova A, Lefebvre C, Alvarez MJ, Castillo-Martin M, Zheng T, Eastham JA, Gopalan A, Pienta KJ, Shen MM, et al. Cross-species regulatory network analysis identifies a synergistic interaction between FOXM1 and CENPF that drives prostate cancer malignancy. Cancer Cell. 2014;25:638–651. doi: 10.1016/j.ccr.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin SC, Kao CY, Lee HJ, Creighton CJ, Ittmann MM, Tsai SJ, Tsai SY, Tsai MJ. Dysregulation of miRNAs-COUP-TFII-FOXM1-CENPF axis contributes to the metastasis of prostate cancer. Nat Commun. 2016;7:11418. doi: 10.1038/ncomms11418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lokody I. Signalling: FOXM1 and CENPF: co-pilots driving prostate cancer. Nat Rev Cancer. 2014;14:450–451. doi: 10.1038/nrc3772. [DOI] [PubMed] [Google Scholar]
- 27.Lee JJ, Maeng CH, Baek SK, Kim GY, Yoo JH, Choi CW, Kim YH, Kwak YT, Kim DH, Lee YK, et al. The immunohistochemical overexpression of ribonucleotide reductase regulatory subunit M1 (RRM1) protein is a predictor of shorter survival to gemcitabine-based chemotherapy in advanced non-small cell lung cancer (NSCLC) Lung Cancer. 2010;70:205–210. doi: 10.1016/j.lungcan.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Mizuno K, Mataki H, Arai T, Okato A, Kamikawaji K, Kumamoto T, Hiraki T, Hatanaka K, Inoue H, Seki N. The microRNA expression signature of small cell lung cancer: Tumor suppressors of miR-27a-5p and miR-34b-3p and their targeted oncogenes. J Hum Genet. 2017;62:671–678. doi: 10.1038/jhg.2017.27. [DOI] [PubMed] [Google Scholar]
- 29.Ma J, Qi G, Xu J, et al. Overexpression of forkhead box m1 and urokinase-type plasminogen activator in gastric cancer is associated with cancer progression and poor prognosis. Oncol Lett. 2017;14:7288–7296. doi: 10.3892/ol.2017.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.