Abstract
The mandible is a crucial organ in both clinical and biological fields due to the high frequency of congenital anomalies and the significant morphological changes during evolution. Primary cilia play a critical role in many biological processes, including the determination of left/right axis patterning, the regulation of signaling pathways, and the formation of bone and cartilage. Perturbations in the function of primary cilia are known to cause a wide spectrum of human diseases: the ciliopathies. Craniofacial dysmorphologies, including mandibular deformity, are often seen in patients with ciliopathies. Mandibular development is characterized by chondrogenesis and osteogenesis; however, the role of primary cilia in mandibular development is not fully understood. To address this question, we generated mice with mesenchymal deletions of the ciliary protein, Ift88 (Ift88 fl/fl ;Wnt1Cre). Ift88 fl/fl ;Wnt1Cre mice showed ectopic mandibular bone formation, whereas Ift88 mutant mandible was slightly shortened. Meckel's cartilage was modestly expanded in Ift88 fl/fl ;Wnt1Cre mice. The downregulation of Hh signaling was found in most of the mesenchyme of Ift88 mutant mandible. However, mice with a mesenchymal deletion of an essential molecule for Hh signaling activity, Smo (Smo fl/fl ;Wnt1Cre), showed only ectopic mandibular formation, whereas Smo mutant mandible was significantly shortened. Ift88 is thus involved in chondrogenesis and osteogenesis during mandibular development, partially through regulating Sonic hedgehog (Shh) signaling.
Keywords: Hedgehog signaling, Ift88, mandibular bone, Meckel's cartilage
Mandibular bone formation was duplicated in mice with mesenchymal conditional deletion of Ift88.
![]()
Introduction
Approximately one‐third of all congenital defects include craniofacial anomalies. In particular, mandible anomalies, including micrognathia, have been shown to occur at high frequency. The mandible is also known to be a key factor in evolution due to the significant morphological changes that occurred during evolution. Therefore, understanding the molecular mechanisms regulating mandibular development is crucial for both biological and clinical fields.
The mandible shows unique developmental processes (Achilleos & Trainor, 2015; Parada & Chai, 2015; Terrazas et al. 2017). Mandibular development relies on chondrogenesis (Meckel's cartilage) and osteogenesis (mandibular bone). Meckel's cartilage is believed to act as a transient supportive tissue for mandibular bone formation during embryogenesis and disappears at late gestation and in early neonates. Meckel's cartilage initiates in the molar tooth region of the mandibular process and then extends in both directions along the anterior–posterior axis. The mandibular bone is first seen as condensed mesenchymal cells that proliferate and differentiate into osteogenic cells. The ossification of the mandibular bone mainly begins in the mesenchyme buccal and extends until Meckel's cartilage at the molar tooth region, and the developing mandibular bone subsequently surrounds Meckel's cartilage. However, in murine mandible, except in the incisor region, the bulk of the mandibular bone forms in the mesenchyme buccal to Meckel's cartilage, and only a thin portion of bone is formed in the mesenchyme lingual to Meckel's cartilage.
Primary cilia are immotile organelles on the surface of almost all mammalian cells. Cilia play important roles in many biological processes, including determination of left/right axis patterning and regulation of signaling pathways (Bisgrove & Yost, 2006; Zaghloul & Brugmann, 2011). Hedgehog (Hh) signaling is activated within primary cilia. Primary cilium comprises a membrane‐bound cylinder surrounding nine doublet microtubules that extend from a basal body. Cilia are assembled and maintained by an intraflagellar transport (IFT) system, in which multiple protein complexes move bidirectionally along the axoneme by the coordinated action of IFT motor proteins. IFT is a highly conserved system in all ciliated eukaryotic cells, and perturbations in the function of primary cilia are implicated in a wide spectrum of human diseases: the ciliopathies (Bisgrove & Yost, 2006). Mandibular abnormalities are observed in many ciliopathy patients. Primary cilia are reportedly involved in chondrogenesis and osteogenesis (Yuan & Yang, 2016); however, their role in mandibular development is not fully understood (Kolpakova‐Hart et al. 2007; Gray et al. 2009; Brugmann et al. 2010; Zhang et al. 2011, 2011; Adel Al‐Lami et al. 2016; Cela et al. 2018; Kitami et al. 2019).
Ift88 encodes a protein that is required for IFT (Murcia et al. 2000). By analyzing mice with tissue‐specific conditional deletions of Ift88, we discovered that Ift88 regulates mandibular development by controlling chondrogenesis and osteogenesis.
Materials and methods
Production and analysis of transgenic mice
P53 −/− , Ift88 fl/fl, Wnt1Cre, R26SmoM2, and K14Cre mice were produced as described previously (Danielian et al. 1998; Jeong et al. 2004; Narai et al. 2006; Yi et al. 2006; Haycraft et al. 2007). Embryonic day 0 (E0) was taken to be midnight prior to finding a vaginal plug.
In situ hybridization
In situ hybridization was carried out as described previously (Ohazama et al. 2008).
Skeletal preparation
To analyze the skeleton, pups were stained with Alcian Blue for nonmineralized cartilage and Alizarin Red for bone. Briefly, mice were fixed in 100% ethanol and then stained for 5 days in 0.1% Alizarin Red S (in 95% ethanol), 0.3% Alcian Blue (in 70% ethanol), 100% acetic acid, and ethanol, followed by alkaline hydrolysis and glycerol clearing.
3D reconstruction of Meckel's cartilage
The 3D reconstructions of Meckel's cartilage were made from serial sections as described previously (Kawasaki et al. 2014) using the amira software package (Template Graphics Software).
Cell proliferation
For detection of cell proliferation, pregnant females were injected intraperitoneally with BrdU (Roche) labeling reagent (45 mg g−1 body weight) at E12.5. One hour after injection, embryos were fixed in 4% paraformaldehyde (PFA) fixative and embedded in paraffin wax, from which sections were cut. Immunodetection of BrdU was performed using the BrdU labeling and detection kit (Roche) according to the manufacturer's instructions.
Results
Mandible phenotypes in Ift88 fl/fl ;Wnt1Cre mice
Global mutations in Ift88 have been shown to lead to early embryonic lethality (Murcia et al. 2000). Therefore, mice with tissue conditional mutations in Ift88 were generated using the Cre–LoxP system. First, we generated mice with a mesenchymal loss of Ift88 in neural crest‐derived cells using Wnt1Cre (Ift88 fl/fl ;Wnt1Cre). Ift88 fl/fl ;Wnt1Cre mice died at birth and had no tongue (Tian et al. 2017). Skeletal preparation analysis showed that Ift88 mutant mandibles were slightly shorter and thicker than those of wild‐type mice along the anterior–posterior and left/right axis, respectively at E18.5 (Fig. 1A,B,D,E,G,H). In wild‐type mice, developing mandibular bone extends along the anterior–posterior axis and, subsequently, the posterior end of the mandible starts to form three mandibular processes that are classified as secondary cartilage: condylar, coronoid, and angular. In wild‐type mice, cartilage was observed only in the angular and condylar processes at birth (Fig. 1A,D,G,J). In Ift88 fl/fl ;Wnt1Cre mice, extra cartilage formation was observed in the lingual side of the mandible at the posterior end of the mandible (Fig. 1E,K). This extra cartilage was isolated from other endogenous cartilage. Examination of the aboral side of Ift88 mutant mandibles indicated that two mandibular bones were present, and the extra cartilage at the posterior end of the mandible was found to form in the lingual side of the mandibular bone (Fig. 1H, Supporting Information Fig. S1B). These two mandibular bones could not be observed in the anterior mandibles of Ift88 fl/fl ;Wnt1Cre mice (Fig. 1H). The oral side of Ift88 mutant mandibles did not show these two mandibular bones, except at the posterior end of the mandibles (Fig. S1D,F). The anterior mutant mandible was slightly enlarged along the left/right axis (Figs 1E,H and S1F). Examination of the buccal and lingual sides of the mandibles indicated that Ift88 mutant mandibular bone was also enlarged along the oral–aboral axis and that the coronoid process was ablated in Ift88 fl/fl ;Wnt1Cre mice (Fig. 1N,Q). Histological analysis revealed that mandibular bone oral, lingual, and buccal to Meckel's cartilage was enlarged in the anterior mandible of Ift88 fl/fl ;Wnt1Cre mice (Fig. 2B). In the middle and posterior mandibles, enlarged mandibular bone lingual to Meckel's cartilage (lingual mandibular bone) and the mandibular bone buccal to Meckel's cartilage (buccal mandibular bone) were observed in Ift88 fl/fl ;Wnt1Cre mice (Fig. 2E, data not shown). There was a gap between the lingual and buccal mandibular bone at the aboral side of the mandible, but thin bone formation was observed between them at the oral side of the mandible (Fig. 2H). To examine Meckel's cartilage present between the lingual and buccal mandibular bone in Ift88 fl/fl ;Wnt1Cre mice, in addition to the analysis of skeletal preparations using Alcian Blue, we performed a 3D reconstruction of Meckel's cartilage from histological specimens to obtain a more detailed picture of Meckel's cartilage. Both analyses showed that Meckel's cartilage was slightly shortened and thickened in Ift88 fl/fl ;Wnt1Cre mice (Fig. 3B, data not shown). The enlargement of chondrocytes could not be detected in Ift88 fl/fl ;Wnt1Cre mice (data not shown). These mandibular bone phenotypes were observed to be fully penetrant in Ift88 fl/fl ;Wnt1Cre mice. To determine whether Ift88 in epithelium is also involved in mandibular development, we next generated mice with epithelial conditional Ift88 mutations using Keratin(K)14Cre (Ift88 fl/fl ;K14Cre). We found that Ift88 fl/fl ;K14Cre mice exhibited no obvious abnormalities in mandibular development (data not shown).
Figure 1.
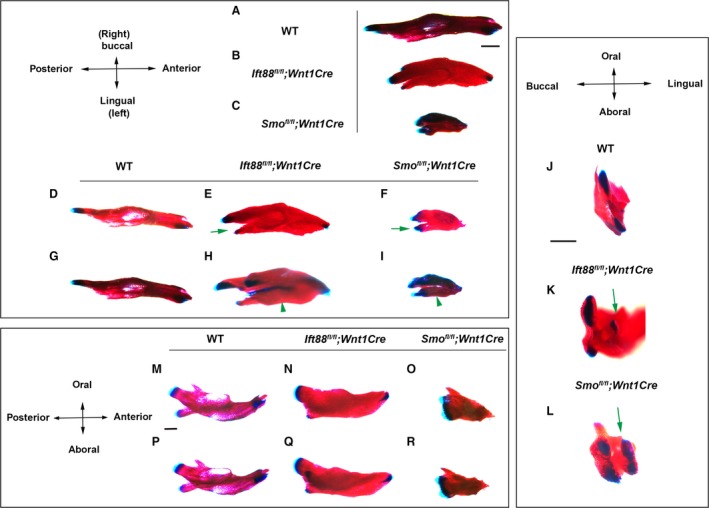
Mandibular bone phenotypes in Ift88 fl/fl ;Wnt1Cre and Smo fl/fl ;Wnt1Cre mice. Oral (A–F), aboral (G–I), buccal (M–O) and lingual (P–R) view of skeletal preparation of wild‐type, Ift88 fl/fl ;Wnt1Cre and Smo fl/fl ;Wnt1Cre mice at E18.5. (J–L) Proximal end of skeletal preparation of wild‐type, Ift88 fl/fl ;Wnt1Cre and Smo fl/fl ;Wnt1Cre mice at E18.5. Green arrows and arrowheads indicate extra cartilage formation and ectopic mandibular bone formation, respectively. Scale bars: 1 mm.
Figure 2.
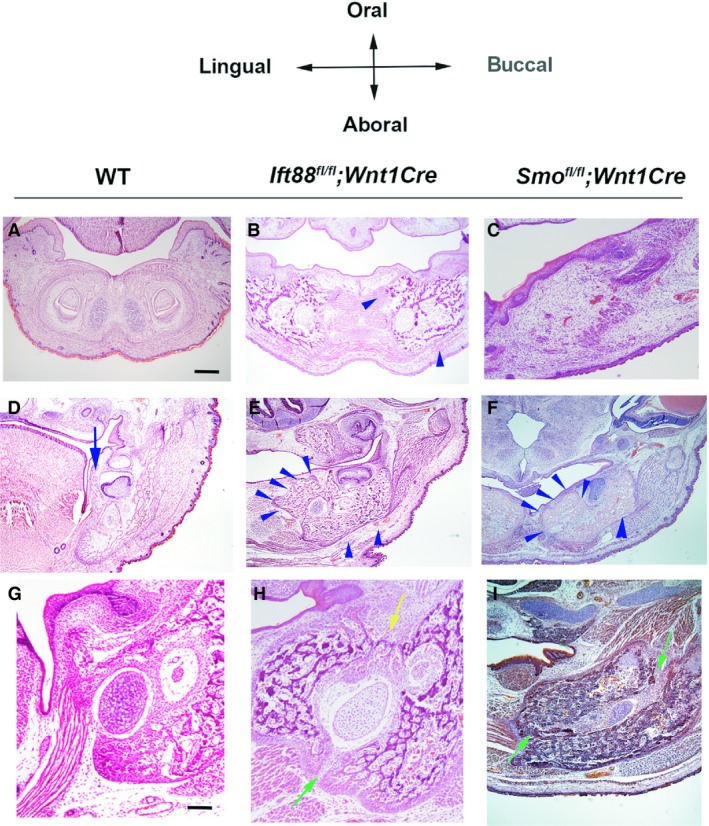
Histological mandibular bone phenotypes in Ift88 fl/fl ;Wnt1Cre and Smo fl/fl ;Wnt1Cre mice. Frontal sections show the developing mandibular bone in wild‐type, Ift88 fl/fl ;Wnt1Cre and Smo fl/fl ;Wnt1Cre mice at E18.5. Blue arrow and arrowheads indicate wild‐type endogenous lingual mandibular bone and mutant excess mandibular bone, respectively. Green arrow indicates the gap between lingual and buccal bone. Yellow arrow indicate the thin bone between lingual and buccal bone. Scale bars: 500 μm (A–F), 250 μm (G–I).
Figure 3.
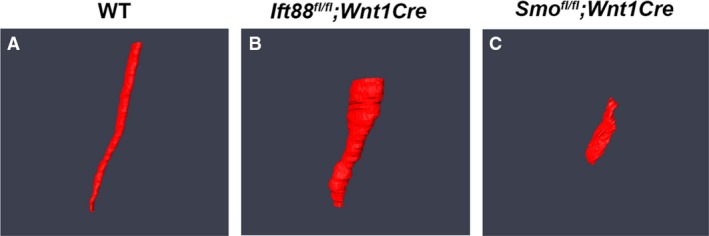
Meckel's cartilage in Ift88 fl/fl ;Wnt1Cre and Smo fl/fl ;Wnt1Cre mice. 3D reconstruction of Meckel's cartilage of wild‐type, Ift88 fl/fl ;Wnt1Cre and Smo fl/fl ;Wnt1Cre mice at E14.5.
Initiation of abnormal mandible formation in Ift88 fl/fl ;Wnt1Cre mice
Ectopic condensed mesenchyme and cell proliferation examined by BrdU were observed in the region lingual to Meckel's cartilage in Ift88 fl/fl ;Wnt1Cre mice (Fig. S2B,D). To further identify the region where abnormal mandibular development initiates in Ift88 fl/fl ;Wnt1Cre mice, we analyzed the osteoblast differentiation marker, Runx2, at early stages of mandibular development. At E12.5, Runx2 was expressed in mesenchyme buccal to Meckel's cartilage in wild‐type mice, which was slightly expanded in Ift88 fl/fl ;Wnt1Cr mice (Fig. 4A,B,D,E). Ift88 fl/fl ;Wnt1Cre mice also showed ectopic Runx2 expression in mesenchyme lingual to Meckel's cartilage, which was observed as an oblique expression domain in Ift88 mutant mandibles from the anterior mandible to the lingual end of the posterior mandible (Fig. 4B,E).
Figure 4.
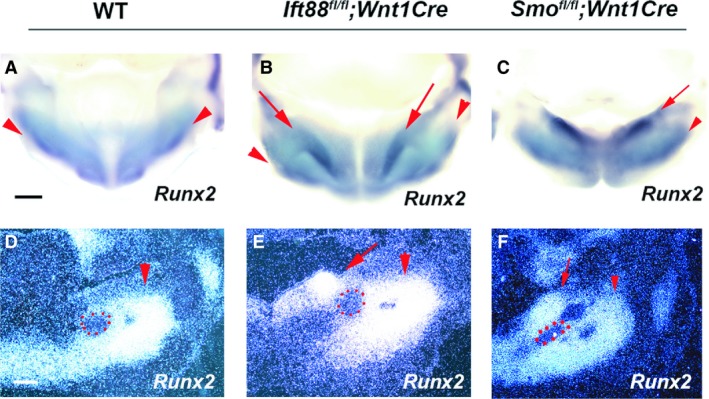
Initiation of mandibular bone formation. (A–C) Oral view of whole mount showing Runx2 expression. (D–F) Frontal sections showing in situ hybridization of Runx2. Arrowheads and arrows indicate endogenous and ectopic mandibular bone region, respectively. Scale bars: 500 μm.
Molecular changes in the developing mandible of Ift88 fl/fl ;Wnt1Cre mice
To identify candidate molecules related to the mandibular phenotypes in Ift88 fl/fl ;Wnt1Cre mice, we performed in situ hybridization and immunohistochemistry analyses in the region where extra bone formation initiated at E11.5 and E12.5. It has been shown that Fgf, Wnt, Tgfβ, and Bmp signaling pathways are involved in craniofacial development, including mandibular development (Brugmann et al. 2007; Mina et al. 2007; Oka et al. 2008; Komatsu et al. 2013). No Ift88 fl/fll ;Wnt1Cre mice exhibited any significant differences in the expression of marker molecules for these pathways in developing mandibles (data not shown). Hh signaling is activated within primary cilia and is involved in craniofacial development (Jeong et al. 2004; Bisgrove & Yost, 2006; Zaghloul & Brugmann, 2011; Kurosaka et al. 2014). Therefore, expression of Ptch1 and Gli1 (direct targets of Hh signaling) was examined in developing mandibular. In wild‐type mandibular mesenchyme, Ptch1 and Gli1 expression was broadly observed at E11.5 (Fig. 5A,B). At E12.5, Ptch1 and Gli1 expression was also found in entire mesenchyme of the anterior mandible, but became restricted in the middle and posterior mandible (Fig. 5C,E,G,I). In the middle and posterior mandibles, both Ptch1 and Gli1 showed expression in the endogenous bone region at E12.5. Ptch1 was also expressed in mesenchyme lingual to Meckel's cartilage, whereas Gli1 expression could not be detected in the region (Fig. 5E,I). Ptch1 and Gli1 expression was significantly downregulated in most of the mesenchyme from the anterior to posterior mandible, including the region corresponding to endogenous and ectopic mandibular bone formation in Ift88 fl/fl ;Wnt1Cre mice (Fig. 5D,F,H,J). Unlike in mesenchyme, both Ptch1 and Gli1 expression was found to be increased in Ift88 mutant mandibular epithelium, indicating that Hh signaling was overactivated in mandibular epithelium of Ift88 fl/fl ;Wnt1Cre mice. The jaw is known to develop via an epithelial–mesenchymal interaction (Billmyre & Klingensmith, 2015; Li et al. 2017). It has been shown that mice with overexpression of Shh in the epithelium (K14‐Shh) show enlarged mandibles, suggesting the possibility that the overactivation of Hh signaling in mandibular epithelium results in extra mandibular bone formation (Cobourne et al. 2009). However, Hh signaling is likely overactivated both in epithelium and mesenchyme in K14‐Shh mice, since overexpression of Shh protein can bind to receptors expressing both in epithelium and mesenchyme. Hh signaling was overactivated only in mandibular epithelium of Ift88 fl/fl ;Wnt1Cre mice. To address this question, we generated mice with overactive Hh signaling only in the epithelium using R26SmoM2, since Hh signaling is overactivated only in epithelium, when R26SmoM2 mice are crossed with K14Cre driver mice (R26SmoM2;K14Cre). R26SmoM2;K14Cre mice exhibited no extra mandibular bone formation, suggesting that the upregulated Hh signaling observed in Ift88 mutant mandibular epithelium is not the cause of extra mandibular bone formation (Supporting Information Fig. S3).
Figure 5.
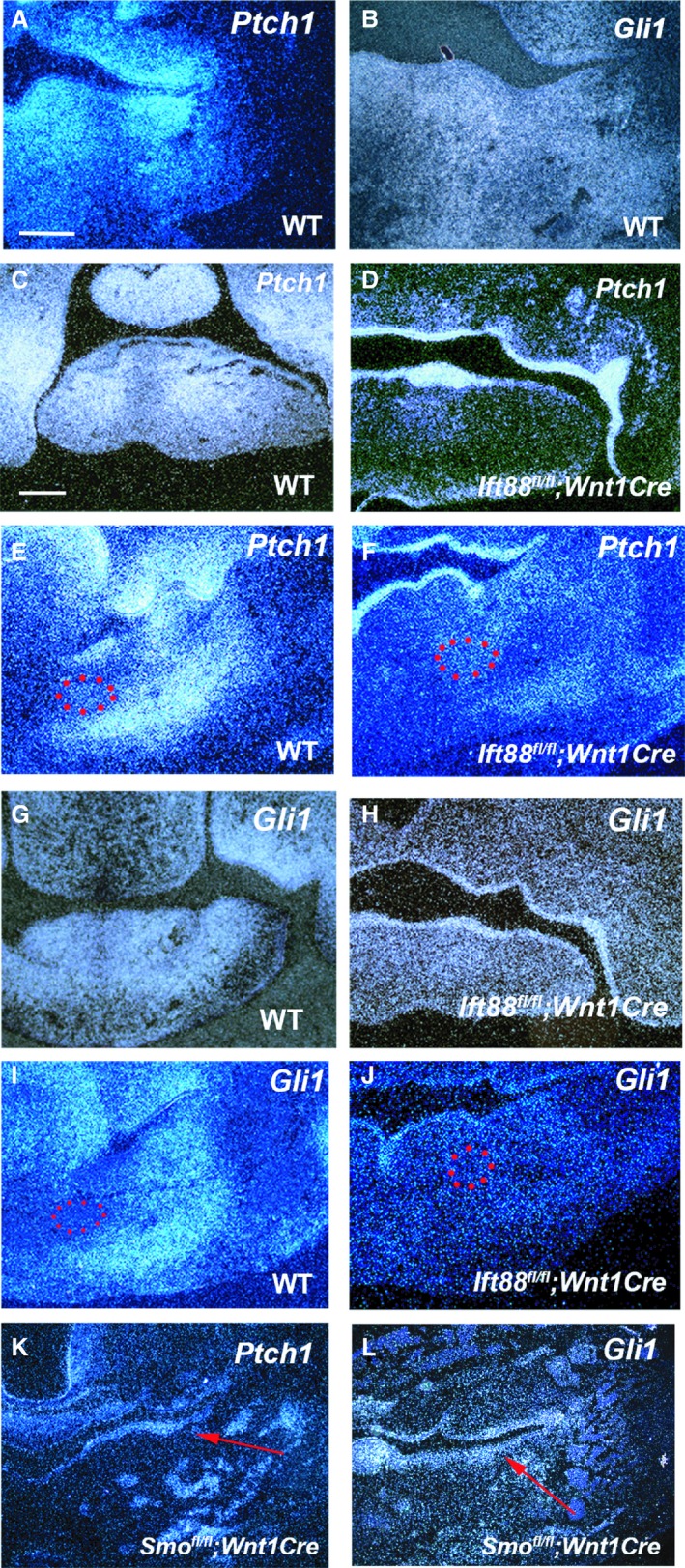
Shh signaling in mandibles in Ift88 fl/fl ;Wnt1Cre and Smo fl/fl ;Wnt1Cre mice. Frontal sections show in situ hybridization of Ptch1 (A,C–F,K) and Gli1 (B,G–J,L) in the anterior (C,D,G,H) and middle (molar) region (A,B,E,F,I–L) of wild‐type (A,B,C,E,G,I), Ift88 fl/fl ;Wnt1Cre (D,F,H,J) and Smo fl/fl ;Wnt1Cre (K, L) mice at E11.5 (A, B) and E12.5 (C–L). Meckel's cartilage is outlined by red dots. Scale bars: 250 μm.
Hh signaling in Ift88 mutant mandibles
It has been shown that mice with a mesenchymal conditional mutation in Smo (an essential molecule in Hh signaling) generated using Wnt1Cre mice (Smo fl/fl ;Wnt1Cre) exhibit abnormal mandibular formation (Jeong et al. 2004; Xu et al. 2019). To investigate whether abnormal mandibular bone formation in Ift88 fl/fll ;Wnt1Cre mice was the result of the downregulation of the Hh signaling pathway in mesenchyme, we compared the mandibular phenotypes in Ift88 fl/fll ;Wnt1Cre and Smo fl/fl ;Wnt1Cre mice. Smo fl/fl ;Wnt1Cre mice have been shown to exhibit significantly smaller lower jaws than wild‐type mice (Fig. 1C; Jeong et al. 2004; Xu et al. 2019). In common with Ift88 fl/fll ;Wnt1Cre mice, Smo fl/fl ;Wnt1Cre mice exhibited extra cartilage at the posterior end of the mandible, which was separated from endogenous cartilage in the angular and condylar processes (Fig. 1F,L). The extra cartilage in the Smo fl/fl ;Wnt1Cre mice was bigger than that in the Ift88 fl/fll ;Wnt1Cre mice. Contrary to Ift88 fl/fll ;Wnt1Cre mice, the coronoid process was also present in Smo fl/fl ;Wnt1Cre mice (Fig. 1O,R). In common with Ift88 fl/fll ;Wnt1Cre mice, the aboral side of the Smo mutant mandibles showed two mandibular bones (Fig. 1I). However, unlike in Ift88 fl/fll ;Wnt1Cre mice, these two mandibular bones were observed along the entire mandible of Smo fl/fl ;Wnt1Cre mice. Through histological analysis, no bone formation was observed in the anterior mandible of Smo fl/fl ;Wnt1Cre mice (Fig. 2C). Like Ift88 fl/fll ;Wnt1Cre mice, enlarged lingual and buccal mandibular bone was observed in the middle and posterior part of Smo mutant mandibles (Fig. 2F, data not shown). In Smo fl/fl ;Wnt1Cre mice, Meckel's cartilage was also present between the lingual and buccal mandibular bone and was significantly shortened and slightly thickened (Fig. 3C; Jeong et al. 2004; Billmyre & Klingensmith, 2015; Xu et al. 2019). There was a gap between the lingual and buccal bone at both the oral and aboral side of mandible in the Smo mutant mandible (Fig. 2I). Runx2 expression was slightly expanded in the region buccal to Meckel's cartilage, whereas ectopic Runx2 expression was observed in mesenchyme lingual to Meckel's cartilage in Smo fl/fl ;Wnt1Cre mice (Fig. 4C,F). Unlike in Ift88 fl/fll ;Wnt1Cre mice, the ectopic Runx2 expression domain was retained at the lingual end of Smo fl/fl ;Wnt1Cre mutant mandibles. Interestingly, upregulation of Ptch1 and Gli1 expression were also observed in the mandibular epithelium of Smo fl/fl ;Wnt1Cre mice (Fig. 5K,L).
The relationship between ectopic bone formation in the palatal shelf and the mandible
We previously reported that ectopic bone is formed in the maxillary processes of Ift88 fl/fl ;Wnt1Cre mice as a result of abnormal apoptosis. The ectopic bone disappears when the apoptosis regulating molecule p53 is deleted from Ift88 fl/fl ;Wnt1Cre mice (Ift88 fl/fl ;Wnt1Cre;p53 −/−; Watanabe et al. 2019). However, in the present study, no abnormal apoptotic activity was detected in Ift88 mutant mandible, and the abnormal mandibular bone showed no changes in Ift88 fl/fl ;Wnt1Cre;p53 −/− mice (Supporting Information Fig. S4, data not shown).
Discussion
In wild‐type mice, only thin bone formation was observed in the region lingual to Meckel's cartilage in the middle and posterior mandible. Runx2 was not obviously expressed in that region at the early stage of mandibular development. In Ift88 fl/fll ;Wnt1Cre mice, Runx2 was ectopically expressed in the region and the subsequent lingual mandibular bone was significantly enlarged. Extra cartilage formation was also observed at the posterior end of the Ift88 mutant mandible along with the angular and condylar processes, which was formed in the enlarged lingual mandibular bone. It has been shown that cartilage in the angular and condylar processes is derived from the periosteum of the ossifying mandible in wild‐type mammals (Shibata et al. 2013), indicating that extra cartilage formation at the posterior end of the Ift88 mutant mandible is derived from the enlarged lingual mandibular bone. The enlarged lingual mandibular bone in Ift88 fl/fl ;Wnt1Cre mice was thus programmed as another mandibular bone. In addition, there was a gap between lingual and buccal mandibular bone formation in the mutant, and Meckel's cartilage was present in the gap. Ift88 fl/fl ;Wnt1Cre mice thus exhibited mirror‐image mandibles between the lingual and buccal sides of the mandible. These indicate the possibility that mandibular bone formation was duplicated in Ift88 fl/fll ;Wnt1Cre mice. However, lingual mandibular bone separated from buccal mandibular bone could not be detected on the oral side or the anterior part of the mandible from Ift88 fl/fll ;Wnt1Cre mice. The duplication of mandibular bone is likely partially to have occurred in Ift88 fl/fl ;Wnt1Cre mice.
The lack of Hh signaling was observed in most of the mesenchyme from the anterior to the posterior region of Ift88 mutant mandible. Smo fl/fl ;Wnt1Cre mice also showed similar mandibular phenotypes, including two mandibular formations and extra cartilage at the posterior end of the mandibles (Xu et al. 2019). Mirror‐image mandibles were also observed in Smo fl/fl ;Wnt1Cre mice. These results indicate that mandibular duplication was caused by downregulated Hh signaling. Shh signaling has recently been shown to regulate the patterning of mandibular development (Xu et al. 2019). Shh signaling is activated within primary cilia, and the primary cilia are known to regulate axis patterning (Bisgrove & Yost, 2006; Bimonte et al. 2011; Zaghloul & Brugmann, 2011). It is conceivable that duplication of mandibular bone in Ift88 fl/fl ;Wnt1Cre mice was caused by abnormal patterning through perturbation of primary cilia function due to Ift88 deletion and subsequent reduced Shh signaling. The primary cilia thus likely regulate the direction of mandibular bone formation through Hh signaling. On the other hand, deficiency of Ift88 in osteoblasts and osteocytes has been shown to result in increased bone formation (Yuan & Yang, 2016). The deletion of another ciliary protein, Kif3a, has also been reported to lead to ectopic bone formation in the craniobase (Koyama et al. 2007). In addition to abnormal patterning, it is also possible that increased bone formation by perturbation of primary cilia function contributes to enlarged lingual mandibular bone formation in Ift88 fl/fl ;Wnt1Cre mice. The primary cilia might play a role in limiting bone formation in mesenchyme lingual to Meckel's cartilage in wild‐type mandible. Extra bone formation is also observed in maxillary processes of Ift88 fl/fl ;Wnt1Cre mice, indicating the possibility that limiting bone formation by the primary cilia takes place in many regions during craniofacial development (Watanabe et al. 2019). However, in Ift88 fl/fl ;Wnt1Cre;p53 −/− mice, abnormal bone formation disappears in maxillary processes, but not in mandibles (Watanabe et al. 2019). Molecular mechanisms controlling bone formation by the primary cilia are thus likely to be different between regions.
Hh signaling was downregulated in most of the mandibular mesenchyme of Ift88 fl/fl ;Wnt1Cre mice. However, the anterior mandibular bone was present and slightly enlarged in Ift88 fl/fl ;Wnt1Cre mice, although the anterior mandibular bone was absent in Smo fl/fl ;Wnt1Cre mice. In addition, the coronoid process was observed in Smo fl/fl ;Wnt1Cre mice but not in Ift88 fl/fll ;Wnt1Cre mice. Thus, the lack of Hh signaling is likely to result in only abnormal lingual mandibular bone formation. Other phenotypes observed in Ift88 fl/fl ;Wnt1Cre mice including the lack of the coronoid process and the presence and enlargement of the anterior mandibular bone were independent of the lack of Hh signaling. Furthermore, the mandible in Smo fl/fl ;Wnt1Cre mice was much smaller than those in Ift88 fl/fl ;Wnt1Cre mice. The phenotypes of Meckel's cartilage were significantly different between Ift88 fl/fl ;Wnt1Cre and Smo fl/fl ;Wnt1Cre mice. Thus, other molecular changes should have occurred in Ift88 fl/fll ;Wnt1Cre mice, probably resulting in different phenotypes in Ift88 fl/fll ;Wnt1Cre mice.
Hh signaling was upregulated in the mandibular epithelium in Ift88 fl/fl ;Wnt1Cre mice, which was also observed in Smo fl/fl ;Wnt1Cre mice. These results suggest that Hh activity in the epithelium is determined by Hh signaling in the mandibular mesenchyme. It is possible that overactivation of Hh signaling in epithelium leads to abnormal mandibular bone formation, since it has been shown that mice with overexpression of Shh in the epithelium (K14‐Shh) exhibit enlarged mandibles (Cobourne et al. 2009). However, we found no extra mandibular bone formation or abnormal Meckel's cartilage in R26SmoM2;K14Cre mice, suggesting that the upregulation of Hh signaling in mandibular epithelium was not the cause of enlarged mandibles in Ift88 fl/fl ;Wnt1Cre mice. Hh signaling in R26SmoM2;K14Cre mice should only be overactivated in the epithelium. However, in K14‐Shh mice, it is highly likely that overactivation of Shh protein (the Hh ligand) from epithelium can bind to receptors expressed in both epithelium and mesenchyme. It is possible that enlarged mandibles are caused by upregulation of Shh signaling in both the epithelium and mandibular mesenchyme in K14‐Shh mice.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Fig. S1. Mandibular bone phenotypes in Ift88 fl/fl ;Wnt1Cre. Aboral (A,B) and oral (C–F) view of skeletal preparation of wild‐type and Ift88 fl/fl ;Wnt1Cre in the posterior (A–D) and anterior (E,F) region at E18.5. Scale bars: 1 mm.
Fig. S2. Initiation of extra mandibular bone formation. (A,B) Frontal sections showing the developing mandible at E13.5. Arrows indicating ectopic condensed mesenchyme. (C,D) Frontal sections showing BrdU‐positive cells in wild‐type and Ift88 fl/fl ;Wnt1Cre mice at E12.5. Arrows indicating region corresponding ectopic bone. Meckel's cartilage was outlined by blue dots. Scale bars: 500 μm.
Fig. S3. Overactivation of Hh signaling in epithelium in mandibular development. Frontal sections showing the developing mandibular bone in wild‐type and R26SmoM2 fl/fl ;K14Cre mice. Scale bars: 500 μm.
Fig. S4. Mandibular bone phenotype in Ift88 fl/fl ;Wnt1Cre;p53 −/−. Frontal sections showing the developing mandibular bone in Ift88 fl/fl ;Wnt1Cre (A) and Ift88 fl/fl ;Wnt1Cre;p53 −/− (B,C) mice at E18.5. Scale bars: 500 μm.
Acknowledgements
This research was funded by the Japan Society for the Promotion of Science (JSPS; 18K09762).
References
- Achilleos A, Trainor PA (2015) Mouse models of rare craniofacial disorders. Curr Top Dev Biol 115, 413–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adel Al‐Lami H, Barrell WB, Liu KJ (2016) Micrognathia in mouse models of ciliopathies. Biochem Soc Trans 44, 1753–1759. [DOI] [PubMed] [Google Scholar]
- Billmyre KK, Klingensmith J (2015) Sonic hedgehog from pharyngeal arch 1 epithelium is necessary for early mandibular arch cell survival and later cartilage condensation differentiation. Dev Dyn 244, 564–576. [DOI] [PubMed] [Google Scholar]
- Bimonte S, De Angelis A, Quagliata L, et al. (2011) Ofd1 is required in limb bud patterning and endochondral bone development. Dev Biol 349, 179–191. [DOI] [PubMed] [Google Scholar]
- Bisgrove BW, Yost HJ (2006) The roles of cilia in developmental disorders and disease. Development 133, 4131–4143. [DOI] [PubMed] [Google Scholar]
- Brugmann SA, Goodnough LH, Gregorieff A, et al. (2007) Wnt signaling mediates regional specification in the vertebrate face. Development 134, 3283–3295. [DOI] [PubMed] [Google Scholar]
- Brugmann SA, Allen NC, James AW, et al. (2010) A primary cilia‐dependent etiology for midline facial disorders. Hum Mol Genet 19, 1577–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cela P, Hampl M, Shylo NA, et al. (2018) Ciliopathy protein Tmem107 plays multiple roles in craniofacial development. J Dent Res 97, 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobourne MT, Xavier GM, Depew M, et al. (2009) Sonic hedgehog signalling inhibits palatogenesis and arrests tooth development in a mouse model of the nevoid basal cell carcinoma syndrome. Dev Biol 331, 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, et al. (1998) Modification of gene activity in mouse embryos in utero by a tamoxifen‐inducible form of Cre recombinase. Curr Biol 8, 1323–1326. [DOI] [PubMed] [Google Scholar]
- Gray RS, Abitua PB, Wlodarczyk BJ, et al. (2009) The planar cell polarity effector Fuz is essential for targeted membrane trafficking, ciliogenesis and mouse embryonic development. Nat Cell Biol 11, 1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft CJ, Zhang Q, Song B, et al. (2007) Intraflagellar transport is essential for endochondral bone formation. Development 134, 307–316. [DOI] [PubMed] [Google Scholar]
- Jeong J, Mao J, Tenzen T, et al. (2004) Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev 18, 937–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki M, Porntaveetus T, Kawasaki K, et al. (2014) R‐spondins/Lgrs expression in tooth development. Dev Dyn 243, 844–851. [DOI] [PubMed] [Google Scholar]
- Kitami M, Yamaguchi H, Ebina M, et al. (2019) IFT20 is required for the maintenance of cartilaginous matrix in condylar cartilage. Biochem Biophys Res Commun 509, 222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolpakova‐Hart E, Jinnin M, Hou B, et al. (2007) Kinesin‐2 controls development and patterning of the vertebrate skeleton by Hedgehog‐ and Gli3‐dependent mechanisms. Dev Biol 309, 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y, Yu PB, Kamiya N, et al. (2013) Augmentation of Smad‐dependent BMP signaling in neural crest cells causes craniosynostosis in mice. J Bone Miner Res 28, 1422–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama E, Young B, Nagayama M, et al. (2007) Conditional Kif3a ablation causes abnormal hedgehog signaling topography, growth plate dysfunction, and excessive bone and cartilage formation during mouse skeletogenesis. Development 134, 2159–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaka H, Iulianella A, Williams T, et al. (2014) Disrupting hedgehog and WNT signaling interactions promotes cleft lip pathogenesis. J Clin Invest 124, 1660–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Fu G, Liu Y, et al. (2017) ISLET1‐dependent β‐Catenin/hedgehog signaling is required for outgrowth of the lower jaw. Mol Cell Biol 31, pii: e00590–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mina M, Havens B, Velonis DA (2007) FGF signaling in mandibular skeletogenesis. Orthod Craniofac Res 10, 59–66. [DOI] [PubMed] [Google Scholar]
- Murcia NS, Richards WG, Yoder BK, et al. (2000) The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left‐right axis determination. Development 127, 2347–2355. [DOI] [PubMed] [Google Scholar]
- Narai S, Kodama Y, Maeda Y, et al. (2006) Trp53 affects the developmental anomaly of clefts of the palate in irradiated mouse embryos but not clefts of the lip with or without the palate. Radiat Res 166, 877–882. [DOI] [PubMed] [Google Scholar]
- Ohazama A, Johnson EB, Ota MS, et al. (2008) Lrp4 modulates extracellular integration of cell signaling pathways in development. PLoS ONE 3, e4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka K, Oka S, Hosokawa R, et al. (2008) TGF‐beta mediated Dlx5 signaling plays a crucial role in osteo‐chondroprogenitor cell lineage determination during mandible development. Dev Biol 321, 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada C, Chai Y (2015) Mandible and tongue development. Curr Top Dev Biol 115, 31–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Sato R, Murakami G, et al. (2013) Origin of mandibular condylar cartilage in mice, rats, and humans: periosteum or separate blastema? J Oral Biosci 55, 208–216. [Google Scholar]
- Terrazas K, Dixon J, Trainor PA, et al. (2017) Rare syndromes of the head and face: mandibulofacial and acrofacial dysostoses. Wiley Interdiscip Rev Dev Biol 6, e263 10.1002/wdev.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Feng J, Li J, et al. (2017) Intraflagellar transport 88 (IFT88) is crucial for craniofacial development in mice and is a candidate gene for human cleft lip and palate. Hum Mol Genet 26, 860–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Kawasaki M, Kawasaki K, et al. (2019) Ift88 limits bone formation in maxillary process through suppressing apoptosis. Arch Oral Biol 27, 101, 43–50. [DOI] [PubMed] [Google Scholar]
- Xu J, Liu H, Lan Y, et al. (2019) Hedgehog signaling patterns the oral‐aboral axis of the mandibular arch. Elife 8, e40315 10.7554/eLife.40315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, O'Carroll D, Pasolli HA, et al. (2006) Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nat Genet 38, 356–362. [DOI] [PubMed] [Google Scholar]
- Yuan X, Yang S (2016) Primary cilia and intraflagellar transport proteins in bone and cartilage. J Dent Res 95, 1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul NA, Brugmann SA (2011) The emerging face of primary cilia. Genesis 49, 231–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wlodarczyk BJ, Niederreither K, et al. (2011) Fuz regulates craniofacial development through tissue specific responses to signaling factors. PLoS ONE 6, e24608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Mandibular bone phenotypes in Ift88 fl/fl ;Wnt1Cre. Aboral (A,B) and oral (C–F) view of skeletal preparation of wild‐type and Ift88 fl/fl ;Wnt1Cre in the posterior (A–D) and anterior (E,F) region at E18.5. Scale bars: 1 mm.
Fig. S2. Initiation of extra mandibular bone formation. (A,B) Frontal sections showing the developing mandible at E13.5. Arrows indicating ectopic condensed mesenchyme. (C,D) Frontal sections showing BrdU‐positive cells in wild‐type and Ift88 fl/fl ;Wnt1Cre mice at E12.5. Arrows indicating region corresponding ectopic bone. Meckel's cartilage was outlined by blue dots. Scale bars: 500 μm.
Fig. S3. Overactivation of Hh signaling in epithelium in mandibular development. Frontal sections showing the developing mandibular bone in wild‐type and R26SmoM2 fl/fl ;K14Cre mice. Scale bars: 500 μm.
Fig. S4. Mandibular bone phenotype in Ift88 fl/fl ;Wnt1Cre;p53 −/−. Frontal sections showing the developing mandibular bone in Ift88 fl/fl ;Wnt1Cre (A) and Ift88 fl/fl ;Wnt1Cre;p53 −/− (B,C) mice at E18.5. Scale bars: 500 μm.


