Abstract
Background
Previous systematic reviews have not shown clear benefit of glucocorticoids for acute viral bronchiolitis, but their use remains considerable. Recent large trials add substantially to current evidence and suggest novel glucocorticoid‐including treatment approaches.
Objectives
To review the efficacy and safety of systemic and inhaled glucocorticoids in children with acute viral bronchiolitis.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2012, Issue 12), MEDLINE (1950 to January week 2, 2013), EMBASE (1980 to January 2013), LILACS (1982 to January 2013), Scopus® (1823 to January 2013) and IRAN MedEx (1998 to November 2009).
Selection criteria
Randomised controlled trials (RCTs) comparing short‐term systemic or inhaled glucocorticoids versus placebo or another intervention in children under 24 months with acute bronchiolitis (first episode with wheezing). Our primary outcomes were: admissions by days 1 and 7 for outpatient studies; and length of stay (LOS) for inpatient studies. Secondary outcomes included clinical severity parameters, healthcare use, pulmonary function, symptoms, quality of life and harms.
Data collection and analysis
Two authors independently extracted data on study and participant characteristics, interventions and outcomes. We assessed risk of bias and graded strength of evidence. We meta‐analysed inpatient and outpatient results separately using random‐effects models. We pre‐specified subgroup analyses, including the combined use of bronchodilators used in a protocol.
Main results
We included 17 trials (2596 participants); three had low overall risk of bias. Baseline severity, glucocorticoid schemes, comparators and outcomes were heterogeneous. Glucocorticoids did not significantly reduce outpatient admissions by days 1 and 7 when compared to placebo (pooled risk ratios (RRs) 0.92; 95% confidence interval (CI) 0.78 to 1.08 and 0.86; 95% CI 0.7 to 1.06, respectively). There was no benefit in LOS for inpatients (mean difference ‐0.18 days; 95% CI ‐0.39 to 0.04). Unadjusted results from a large factorial low risk of bias RCT found combined high‐dose systemic dexamethasone and inhaled epinephrine reduced admissions by day 7 (baseline risk of admission 26%; RR 0.65; 95% CI 0.44 to 0.95; number needed to treat 11; 95% CI 7 to 76), with no differences in short‐term adverse effects. No other comparisons showed relevant differences in primary outcomes.
Authors' conclusions
Current evidence does not support a clinically relevant effect of systemic or inhaled glucocorticoids on admissions or length of hospitalisation. Combined dexamethasone and epinephrine may reduce outpatient admissions, but results are exploratory and safety data limited. Future research should further assess the efficacy, harms and applicability of combined therapy.
Plain language summary
Glucocorticoids for acute viral bronchiolitis in infants and young children under two years of age
Bronchiolitis is the most common acute infection of the airways and lungs during the first years of life. It is caused by viruses, the most common being respiratory syncytial virus. The illness starts similar to a cold, with symptoms such as a runny nose, mild fever and cough. It later leads to fast, troubled and often noisy breathing (for example, wheezing). While the disease is often mild for most healthy babies and young children, it is a major cause of clinical illness and financial health burden worldwide. Hospitalisations have risen in high‐income countries, there is substantial healthcare use and bronchiolitis may be linked with preschool wheezing disorders and the child later developing asthma.
There is variation in how physicians manage bronchiolitis, reflecting the absence of clear scientific evidence for any treatment approach. Anti‐inflammatory drugs like glucocorticoids (for example, prednisolone or dexamethasone) have been used based on apparent similarities between bronchiolitis and asthma. However, no clear benefit of their use has been shown.
Our systematic review found 17 controlled studies involving 2596 affected children that used these drugs for a short duration and assessed short‐term outcomes. When comparing glucocorticoids to placebo, no differences were found for either hospital admissions or length of hospital stay. There was no substantial benefit in other health outcomes. These findings are consistent and likely to be applicable in diverse settings.
Exploratory results from one large high‐quality trial suggest that combined treatment of systemic glucocorticoids (dexamethasone) and bronchodilators (epinephrine) may significantly reduce hospital admissions. There were no relevant short‐term adverse effects that were any different from those seen with an inactive placebo, while long‐term safety was not assessed. Further research is needed to confirm the efficacy, safety and applicability of this promising approach.
Summary of findings
Background
Description of the condition
Acute viral bronchiolitis is the most common acute infection of the lower respiratory tract during the first year of life (Wright 1989). It is diagnosed clinically in infants and young children, based on a history of rhinorrhoea and low‐grade fever that progress to cough and respiratory distress, with findings of tachypnoea, chest retractions and wheeze, crackles, or both, on examination (Bush 2007; Smyth 2006; Taussig 2008). Respiratory syncytial virus (RSV) is responsible for the majority of cases, usually in seasonal epidemics (Smyth 2006; Yusuf 2007). Other viral agents, particularly rhinovirus, human metapneumovirus, bocavirus and adenovirus, may also be involved as single or dual infections (Calvo 2010; Kusel 2006; Mansbach 2008b; Mansbach 2012). Although bronchiolitis is usually a straightforward diagnosis, some variability in its definition exists. This may be due to poor agreement on the identification of early childhood wheezing phenotypes and worldwide differences in disease semantics (Brand 2008; Everard 2009; Mansbach 2008a).
Bronchiolitis is a major cause of clinical morbidity and its financial health burden is substantial. Population‐based studies in developed countries suggest an incidence ratio of approximately 10% within the first year of life, with hospital admissions up to 3% (Koehoorn 2008; Mansbach 2005; Shay 1999; Wright 1989). While mortality is rare, hospitalisations have increased steadily in North America and Europe over the past 10 to 20 years (Langley 2003; Shay 1999; van Woensel 2002), with rising inpatient health care costs (Langley 1997; Paramore 2004; Pelletier 2006). Additionally, a majority of cases with mild illness cared for in the community are responsible for a considerable number of outpatient visits, loss of parental work time and decreased quality of life (Carroll 2008; Mansbach 2007; Robbins 2006). RSV infection, including bronchiolitis, is a major cause of childhood morbidity and mortality at a global level (Nair 2010).
Bronchiolitis involves acute inflammation of the bronchiolar airways initiated by viral infection, regardless of the causative agent. Airway oedema, necrosis and mucous plugging are the hallmark pathological features, and air flow obstruction ensues (Taussig 2008). Factors underlying disease severity are only partially understood, but clinical determinants include lower age, prematurity, chronic lung, heart or neurological disease, immunodeficiency and ethnicity (Damore 2008; Figueras‐Aloy 2008; Meissner 2003; Simoes 2003; Simoes 2008). There is likely a complex interplay between host (i.e. genetic markers), agent (i.e. viral loads, specific agents and co‐infections) and environmental factors (i.e. crowding, tobacco smoke exposure) (Colosia 2012; Collins 2008; DiFranza 2012; Mansbach 2012; Miyairi 2008;Papadopoulos 2002). Basic, translational and clinical research studies are elucidating the association between bronchiolitis, preschool wheezing disorders and later asthma (Martinez 2005; Perez‐Yarza 2007; Singh 2007; Sly 2010).
Description of the intervention
The current treatment for bronchiolitis is controversial. There is substantial variation in its management throughout the world, reflecting the absence of clear evidence for any single treatment approach (Babl 2008; Barben 2003; Brand 2000; Gonzalez 2010; Mansbach 2005; Plint 2004). Many interventions failed to show consistent and relevant effects (Bialy 2011). Recently, both nebulised epinephrine and hypertonic saline have emerged as options for improving relevant outcomes in outpatient and inpatient populations, respectively (Hartling 2011a; Zhang 2011). However, no routine treatment is yet recommended by most evidence‐based clinical practice guidelines worldwide (AAP 2006; Baumer 2007; Turner 2008).
The case of glucocorticoids highlights the uncertainties of research in this field. Trials assessing their use date back to the 1960s, with different potencies, modes of administration, dosages and regimens of these drugs having been recommended (Connolly 1969; Leer 1969). However, results from randomised clinical trials (RCTs) have been heterogeneous, leading to ongoing controversy regarding their use. Differences in participants, care settings and outcomes may account for these conflicting results, and have led to distinct interpretations (Everard 2009; Guilbert 2011; Hall 2007; Weinberger 2003; Weinberger 2007).
How the intervention might work
Glucocorticoid use in bronchiolitis was originally thought to have equivalent benefits to those in acute asthma. Similarities between clinical findings were expected to express equivalent biological and physiological mechanisms attributable to inflammation (Leer 1969). However, evidence suggests there is heterogeneity in inflammatory pathways and mediators activated in different wheezing phenotypes which may underlie bronchiolitis (for example, neutrophil‐ versus eosinophil‐mediated inflammation) (Halfhide 2008). Mechanistic studies have shown that glucocorticoids have limited anti‐inflammatory effects in this condition (Buckingham 2002; Somers 2009) and there is an ongoing debate regarding their efficacy in acute virus‐induced wheezing in preschool children (Bush 2009; Ducharme 2009; Panickar 2009). Further, potential benefits need to be considered in light of possible short‐ and long‐term adverse effects of glucocorticoid use. While the interactive effect of bronchodilators and glucocorticoids has been widely known in asthma, both at a clinical and biological level, its use as a putative treatment option in bronchiolitis has only been explored recently (Plint 2009).
Why it is important to do this review
While guideline implementation has changed prescription patterns, glucocorticoids are still widely used (Barben 2000; Barben 2008; David 2010). The latest version of this review integrated critical results from the two largest multi‐centre studies in this area (Corneli 2007; Plint 2009) and examined the use of combined therapy with bronchodilators or adrenaline. We continue to update the current body of evidence in order to adequately assess the efficacy and safety of glucocorticoids in bronchiolitis.
Objectives
To review the efficacy and safety of systemic and inhaled glucocorticoids in children with acute viral bronchiolitis.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs irrespective of risk of bias, sample size, publication status or language of publication.
Types of participants
Studies should include infants and young children ≤ 24 months of age with acute viral bronchiolitis. Bronchiolitis was defined clinically as a first episode of acute wheezing, respiratory distress and clinical evidence of a viral infection (cough, coryza, fever). Many bronchiolitis trial reports do not specify clinical findings required for participant inclusion (King 2004); we included all studies if other diagnoses (for example, pneumonia) could be excluded. We did not restrict inclusion based on specific findings on examination (for example, crackles) or viral aetiology.
We excluded studies in which any participant had a history of wheezing or respiratory distress (one or more previous episodes), a formal diagnosis of asthma, or if reporting of these items was unclear. We focused on first time wheezing so results could be directly pertinent to infants with 'typical' viral bronchiolitis, as opposed to children with acute recurrent wheezing. We did not exclude trials based on other reported participant characteristics, including gestational age and co‐morbidities.
We included studies of both inpatients and outpatients (ambulatory care and/or emergency department), and excluded trials in the intensive care setting or with intubated and/or ventilated participants.
Types of interventions
The interventions of interest were short‐term systemic or inhaled glucocorticoids administered for the acute care of bronchiolitis. We considered all types of glucocorticoids, dosages, durations and routes of administration. Glucocorticoids could be administered alone or combined with co‐interventions (for example, bronchodilators), used with or without a fixed protocol. We excluded trials assessing the use of longer courses of glucocorticoids started during the acute phase for the prevention of post‐bronchiolitic wheezing.
Comparators included either placebo or another intervention (for example, bronchodilators, other glucocorticoid). Inhaled isotonic saline is frequently used as a placebo control for inhaled drugs. We excluded studies comparing different doses or regimens of the same glucocorticoid.
Types of outcome measures
We selected primary outcomes based a priori on clinical relevance and patient importance; secondary outcomes assessed other relevant health domains (clinical severity, pulmonary function, healthcare use, patient/parent‐reported symptoms and status, and harms). We included studies if they reported numeric data on at least one primary or secondary outcomes assessed within the first month after acute bronchiolitis. We considered different timings of outcome assessment, based on a priori relevance and available data.
Primary outcomes
Rate of admission by days one and seven for outpatient studies.
Length of stay (LOS) for inpatient studies.
Secondary outcomes
Clinical severity scores.
O2 saturation, respiratory rate and heart rate.
Hospital re‐admissions (for inpatient studies) and return healthcare visits (for all studies); LOS (for outpatient studies).
Pulmonary function tests.
Symptoms and quality of life.
Short‐ and long‐term adverse events.
We selected the following time points and intervals for clinical scores, O2 saturation, respiratory and heart rate: 60 and 120 minutes, three to six hours, six to 12 hours, 12 to 24 hours, 24 to 72 hours, and three to 10 days. The time points selected for re‐admissions and return visits were days 1 to 10, and 11 to 30. We also considered data on all other reported outcomes.
Search methods for identification of studies
The previous version of this review used an inclusive search strategy as part of a comprehensive systematic review evaluating the effect of three types of interventions in bronchiolitis (glucocorticoids, epinephrine and other bronchodilators) (Hartling 2011b).
Electronic searches
Previously we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2009, Issue 4), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (1950 to November Week 2, 2009), EMBASE (1980 to Week 47, 2009), LILACS (Latin American and Caribbean Center on Health Sciences Information) (1982 to 25 November 2009), Scopus® (1823 to 25 November 2009) and IRAN MedEx (1998 to 26 November 2009).
We developed search strings by scanning search strategies of relevant systematic reviews and examining index terms of potentially relevant studies. We applied and modified a validated RCT filter according to each database (Glanville 2006). We applied no publication or language restrictions. The search strings for each database can be found in Appendix 1 to Appendix 6.
For this 2013 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2012, Issue 12, part of The Cochrane Library, www.thecochranelibrary.com (accessed 21 January 2013), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (October 2009 to January week 2, 2013), EMBASE (November 2009 to January 2013), LILACS (Latin American and Caribbean Center on Health Sciences Information) (2009 to January 2013) and Scopus (2009 to January 2013) (Appendix 7).
Searching other resources
To identify unpublished studies and studies in progress we searched the following clinical trials registers on 1 August 2012: ClinicalTrials.gov and ICTRP Search Portal – World Health Organization. We searched the following conference proceedings: Pediatric Academic Societies (2003 to 2012), European Respiratory Society (2003 to 2011), American Thoracic Society (2006 to 2012).
We identified additional published, unpublished or ongoing studies by handsearching reference lists and included or excluded studies of relevant reviews. In addition, we contacted topic specialists.
Data collection and analysis
Selection of studies
Five review authors (AP, LB, LH, NH or RF) independently screened the titles, keywords and abstracts (when available) to determine if an article met the inclusion criteria. These review authors independently assessed the full text of all articles classified as 'include' or unclear' using a standardised form. We resolved disagreements by consensus or by an arbitrator (AP, TK, DJ, or RF).
Data extraction and management
We extracted data using a standardised form in paper or electronic format (available from authors). Seven review authors extracted data (LB, LH, AM, HM, RF, OT or JF) and three review authors (LB, AM or RF) independently checked for accuracy and completeness. We resolved discrepancies by consensus or in consultation with a third review author (TK, AP or DJ). A statistician (BV) checked all quantitative data during analysis. Extracted data included study characteristics, funding, inclusion/exclusion criteria, participant characteristics, interventions, outcomes and results.
Assessment of risk of bias in included studies
We used the Cochrane 'Risk of bias' assessment tool, which includes seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other sources of bias (Higgins 2011). We assessed blinding and incomplete outcome data separately for the following groups of outcomes: healthcare use (rate of admission, LOS, hospital re‐admissions and return healthcare visits); clinical parameters (clinical severity scores, O2 saturation, respiratory rate and heart rate); pulmonary function; patient/parent‐reported outcomes (symptoms and quality of life measures) and other outcomes such as adverse events. Where trial protocols or trial registers were unavailable, we assessed selective outcome reporting by comparing outcomes reported in the methods and results sections. We summarised risk of bias for each study across outcomes based on individual domain assessments ('high' if one or more domains were high; 'low' if all domains were low; 'unclear' for all other studies).
Three review authors (LB, LH or RF) independently assessed the risk of bias of the included studies; we resolved discrepancies by consensus. One review author (OT) assessed study reports written in Turkish. We pilot tested the risk of bias tool on a sample of five studies and used the results to adapt decision rules (available from authors).
Grading the body of evidence
We used the Evidence‐Based Practice Centers Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, based on the standard GRADE system (GRADE 2009; Owens 2010), to assess domain‐specific and overall strength of evidence on three relevant outcomes: length of stay or admission rate, clinical severity scores and adverse events. Two review authors (LH, RF) independently graded the body of evidence using adapted decision rules.
We examined the following domains: risk of bias, consistency, directness and precision. Risk of bias was considered as low or medium, as we only included RCTs. There is limited evidence regarding clinically significant and patient‐important between‐group differences in this field. We therefore defined a priori thresholds of clinical relevance based on expert opinion and GRADE guidance for the precision domain: risk ratio reduction > 20% for admissions, reduction in LOS > 0.5 days and clinical scale effect sizes based on GRADE guidance (GRADE 2009). We graded overall strength of evidence 'high', 'moderate' or 'low' based on the likelihood of further research changing our confidence in the estimate of effect (when evidence was unavailable or did not permit estimation of an effect, it was considered insufficient).
All decisions were made explicitly and inter‐rater agreement was calculated (data available from authors). We resolved discrepancies by consensus among two review authors (LH, RF).
Measures of treatment effect
We pooled dichotomous variables using risk ratios (RRs). We derived the number needed to treat to benefit (NNTB) for significant results from primary outcomes. Since the only comparison with significant differences was based on a single trial, the NNTB is shown for that trial's baseline risk.
We analysed measurement scale outcomes as continuous variables. For continuous variables measured on the same scale (for example, respiratory rate), we calculated mean differences (MD) for individual studies and mean differences for the pooled estimates. For those measured on different scales (for example, clinical scores), we calculated MDs for separate studies and standardised MD (SMD) for the pooled estimates. We used changes from baseline for all continuous variables.
Unit of analysis issues
Some of the studies included in this review were multi‐arm or factorial studies in which more than two intervention groups were eligible to contribute several comparisons to a single meta‐analysis. For example, a trial might compare glucocorticoid versus placebo in two arms, and glucocorticoid + bronchodilator versus placebo + bronchodilator in another two arms, with both contributing to the overall glucocorticoid versus placebo comparison. When the comparisons were independent, i.e. with no intervention group in common, we included data from these arms with no transformation and we shown them separately in each forest plot. If needed and feasible, we pooled the active groups to avoid double‐counting of the comparator group when there was more than one active group: for example, two glucocorticoid groups versus placebo. We did not include any treatment groups twice in the same meta‐analysis.
Guidance regarding the analysis of factorial trials mandates caution when results suggest positive interaction/additive effects ('synergism') between study treatments (McAlister 2003; Montgomery 2003). This was the case for a large trial included in this review. We therefore chose to include comparisons separately in meta‐analysis ('within the table analysis'): for example, for the glucocorticoid versus placebo comparison, we included separately glucocorticoid + bronchodilator versus placebo + bronchodilator and glucocorticoid + placebo versus double placebo. We also performed sensitivity analysis pooling all arms ('at the margins analysis').
Dealing with missing data
We extracted information on incomplete outcome data and we classified trials that performed intention‐to‐treat (ITT) analysis as either ITT with all data, ITT with imputation of missing data, ITT with available case analysis, per protocol analysis or treatment‐received analysis (Higgins 2011). We did not impute missing data for drop‐outs. We estimated unreported means from figures or imputed from medians if possible. We computed standard deviations (SDs) from available data (i.e. standard errors, confidence intervals (CI) or P values) when missing. Failing this, we estimated them from ranges and inter‐quartile ranges, or imputed them from a similar study. When standard deviations of change from baseline values were unavailable, we estimated correlation at 0.5 (Follmann 1992; Wiebe 2006). We occasionally encountered clinical score results presented as dichotomous data, for example, using a cut‐off score or time‐to‐event analysis. When methods were feasible and assumptions judged reasonable, we used existing approaches to re‐express odds ratios as standardised mean differences, thus allowing dichotomous and continuous data to be pooled together (Higgins 2011). When data were unavailable for one of the predefined timings of outcome measurement, we used the time point closest or any time point in the range. If there was more than one time point, we chose the one with the largest magnitude of change.
We did not contact trial authors of the individual studies to obtain additional data.
Assessment of heterogeneity
We quantified statistical heterogeneity using the I2 statistic. We used the following intervals for interpreting I2 statistic values: 0% to 30% low heterogeneity; 30% to 50% moderate heterogeneity; 50% to 75% substantial heterogeneity; and 75% to 100% considerable heterogeneity (Higgins 2011).
Assessment of reporting biases
We assessed reporting biases for the main comparisons and primary outcomes by visual interpretation of funnel plots and testing for funnel plot asymmetry (Egger test) (Higgins 2011).
Data synthesis
We meta‐analysed quantitative results within the different comparisons when studies were consistent on clinical grounds and had available outcome data; we imposed no restrictions based on risk of bias. We performed separate meta‐analyses for studies involving inpatients and outpatients.
We combined results using random‐effects models regardless of heterogeneity, due to expected differences in interventions, outcomes and measurement instruments. We calculated fixed‐effect models in a sensitivity analysis. We conducted meta‐analyses of dichotomous outcomes using Mantel‐Haenszel methods. We used inverse variance methods for continuous outcomes and measurement scales, and combined dichotomous and continuous data into a standardised mean difference whenever needed (Higgins 2011). All results are reported with 95% CI. We used Review Manager software for data management and analysis (RevMan 2012).
Subgroup analysis and investigation of heterogeneity
We planned to investigate heterogeneity by conducting subgroup analyses based on pre‐specified study‐ and participant‐level characteristics. The following subgroups were considered:
Protocolised use of bronchodilators (studies with protocolised use versus no/unclear protocolised use).
RSV status (studies with all participants exclusively RSV‐positive versus some RSV‐negative/unspecified RSV status).
Age of participants (studies with all participants exclusively less than 12 months of age versus some participants older than 12 months/unspecified age).
Atopy (studies with all participants exclusively atopic versus some participants not atopic/unspecified atopic status).
Glucocorticoid: type of glucocorticoids; and daily and overall dose (high versus low).
We explored potential positive or negative (i.e. 'synergistic' or 'antagonistic') interactions between glucocorticoids and bronchodilators by distinguishing trials where bronchodilator use was protocolised (i.e. comparing glucocorticoids + bronchodilator versus placebo + bronchodilator) from studies where use was either at the discretion of the physician or not allowed (Gurusamy 2009). The choice of RSV, age and atopy was based on clinical or biological evidence suggesting possible effect modification of glucocorticoid effects by these parameters. We studied drug type and dose to explore distinct glucocorticoid pharmacokinetic and pharmacodynamic properties; dosing was based on prednisolone equivalents.
We planned to perform subgroup analyses only on the review's primary outcomes. We also collected data from studies that analysed these subgroups at a study level. We assessed subgroup differences comparing changes in effect estimate and CI overlap; statistical tests or meta‐regression techniques were not used.
Sensitivity analysis
We decided a priori to perform sensitivity analyses on primary outcome results of trials with overall low risk of bias. We also checked for differences in the direction and magnitude of primary outcome results when using fixed‐effect models, as well as using pooled data from all factorial trial arms ('at the margins analysis').
Results
Description of studies
Results of the search
The initial 2009 comprehensive search of all electronic databases identified 2249 records, of which 344 were potentially relevant. Handsearching had identified four more studies and overall 348 full‐text articles had been assessed for eligibility. Of 91 studies that used glucocorticoids, 17 trials fulfilled inclusion criteria.
The 2013 search identified 280 further records, of which 13 were assessed for eligibility using full text but all were excluded (flowchart in Figure 1).
1.
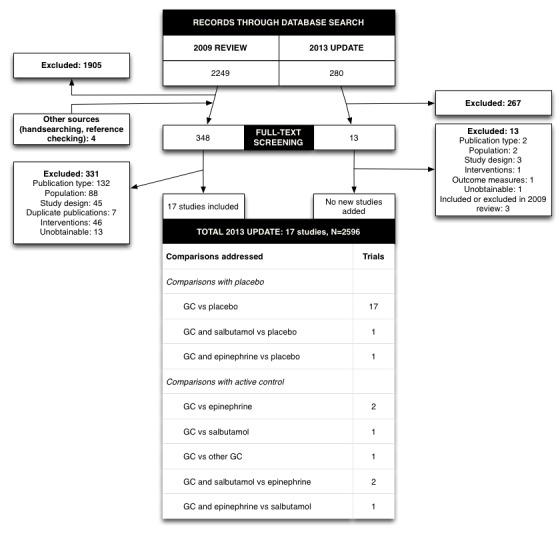
Flow of citations through the search and screening procedures of the 2009 review and this 2012 update, studies included in the review and comparisons addressed (GC: glucocorticoids)
Included studies
We included 17 trials with 2596 randomised participants. We considered different comparisons separately between glucocorticoids, alone or with fixed co‐interventions, and either placebo or active controls. Included trials contributed to one or more comparisons, depending on trial arms (Figure 1).
Design, centres and sample sizes
Fifteen trials were parallel‐designed, 14 of which were double‐armed (Bentur 2005; Berger 1998; Cade 2000; Corneli 2007; De Boeck 1997; Goebel 2000; Gomez 2007; Klassen 1997; Mesquita 2009; Richter 1998; Roosevelt 1996; Schuh 2002; Teeratakulpisarn 2007; Zhang 2003) and one was six‐armed (Barlas 1998). Two trials were factorial two‐by‐two (Kuyucu 2004; Plint 2009).
Eleven trials were single‐centred and five included multiple centres (range: 2 to 20) (Cade 2000; Corneli 2007; Goebel 2000; Plint 2009; Teeratakulpisarn 2007); one trial did not clearly report this item (Bentur 2005). All trials were conducted in a single country, either in North, Central or South America, Europe and the Middle East or Asia.
Sample size calculations were reported in 12 trials (Bentur 2005; Berger 1998; Cade 2000; Corneli 2007; Klassen 1997; Mesquita 2009; Plint 2009; Richter 1998; Roosevelt 1996; Schuh 2002; Teeratakulpisarn 2007; Zhang 2003); the outcome used for sample size calculation was the reported primary outcome in all except one trial (Richter 1998). The overall median number of participants per trial was 72 (range 32 to 800), with two large trials counting 600 and 800 (Corneli 2007; Plint 2009, respectively), and all others fewer than 200.
Funding was reported in nine studies, three of which had pharmaceutical industry support (Cade 2000; Richter 1998; Schuh 2002).
Setting and participants
Outpatients were included in eight trials, with 1824 randomised participants and a median of 85 participants per trial (range: 42 to 800) (Barlas 1998; Berger 1998; Corneli 2007; Goebel 2000; Kuyucu 2004; Mesquita 2009; Plint 2009; Schuh 2002). Outpatient settings mostly included paediatric emergency departments. Nine trials included inpatients only, with 772 participants and a median of 61 participants per trial (range: 32 to 179) (Bentur 2005; Cade 2000; De Boeck 1997; Gomez 2007; Klassen 1997; Richter 1998; Roosevelt 1996; Teeratakulpisarn 2007; Zhang 2003). Few details were reported regarding criteria for hospitalisation and the type of admission unit in which patients received care, except for one inpatient trial report (Teeratakulpisarn 2007).
In most trials bronchiolitis was defined by clinical findings; wheezing was always required. Three trials restricted inclusion to bronchodilator responders (Goebel 2000 ‐ outpatients; Teeratakulpisarn 2007 and Zhang 2003 ‐ inpatients). Seven trials only included participants under the age of 12 months, all of which had a mean or median participant age below six months (Bentur 2005; Cade 2000; Corneli 2007; Plint 2009; Richter 1998; Roosevelt 1996; Zhang 2003).
Bronchiolitis severity thresholds were used for inclusion in eight outpatient (Barlas 1998; Berger 1998; Corneli 2007; Goebel 2000; Kuyucu 2004; Mesquita 2009; Plint 2009; Schuh 2002) and two inpatient trials (Gomez 2007; Klassen 1997). Severity was based on clinical scales or respiratory parameters, and thresholds varied. The Respiratory Distress Assessment Instrument (RDAI) baseline score thresholds varied between two and six (less than four usually considered mild bronchiolitis).
Thirteen trials reported testing for RSV at least in a portion of participants, and three trials only included RSV‐positive patients (Bentur 2005; Cade 2000; De Boeck 1997). Prevalence of RSV in the remaining 10 trials varied from 33% to 89% (Barlas 1998; Berger 1998; Corneli 2007; Goebel 2000; Klassen 1997; Mesquita 2009; Plint 2009; Richter 1998; Roosevelt 1996; Schuh 2002).
Atopic status was reported in nine trials (Barlas 1998; Berger 1998; Cade 2000; Plint 2009; Richter 1998; Roosevelt 1996; Schuh 2002; Teeratakulpisarn 2007; Zhang 2003), while one trial reported a family history of wheezing (Corneli 2007). Definitions for atopy and methods of assessment were rarely provided, and when reported were heterogeneous. No trials excluded participants with a history of atopy.
Children with chronic cardiac, pulmonary or neurological conditions or immunodeficiency were frequently excluded. All or some premature infants were explicitly excluded in seven trials (Cade 2000; Corneli 2007; De Boeck 1997; Goebel 2000; Plint 2009; Schuh 2002; Teeratakulpisarn 2007). Other criteria for exclusion were length of illness and glucocorticoid‐related parameters (previous use, history of adverse events, specific contraindications to their use).
Subgroup analyses within studies were reported in five trials (Bentur 2005; Cade 2000; Corneli 2007; Plint 2009; Teeratakulpisarn 2007), two of which being pre‐specified (Corneli 2007; Plint 2009). Subgroups were based on age, RSV status, family or personal history of atopy and eczema, duration and severity of illness, and exposure to smoke and/or dampness.
Interventions
There was heterogeneity regarding the choice of glucocorticoid, its dosage, route of administration and duration of treatment. Dexamethasone was the most frequently tested drug (11 trials). Nine trials used systemic dexamethasone, either oral (Corneli 2007; Klassen 1997; Mesquita 2009; Plint 2009; Schuh 2002), intramuscular (Kuyucu 2004; Roosevelt 1996; Teeratakulpisarn 2007) or intravenous (De Boeck 1997). Single‐day doses were administered for one to five days. Initial dosing was higher (0.5 to 1 mg/kg), with later doses ranging from 0.15 to 0.6 mg/kg. The highest overall dose was seen in Plint 2009 and Schuh 2002 (1 mg/kg followed by 0.6 mg/kg for five days), and the lowest in Mesquita 2009 (single‐dose 0.5 mg/kg). Two trials used inhaled dexamethasone (0.2 mg to 0.25 mg every four to six hours), at least for one day, or until discharge for inpatients (Bentur 2005; Gomez 2007). Systemic prednisone or prednisolone were tested in four trials, three oral (Berger 1998; Goebel 2000; Zhang 2003) and one intravenous (Barlas 1998). Duration varied between one and five days (1 to 2 mg/kg/day, once or twice daily). Three trials used inhaled budesonide (0.5 mg to 1 mg, once or twice daily) for one to six weeks (Barlas 1998; Cade 2000; Richter 1998).
Details on placebos were reported in nine trials. Inhaled placebos included mist (Barlas 1998) and 0.9% saline (Bentur 2005; Richter 1998). Protocolised standard of care was used as a control arm in Zhang 2003.
Eleven trials used protocolised bronchodilators in both glucocorticoid and placebo arms. The choice of bronchodilator, its dose and frequency varied substantially. Seven trials used salbutamol (Barlas 1998; Berger 1998; Goebel 2000; Gomez 2007; Klassen 1997; Kuyucu 2004; Schuh 2002), four used epinephrine (Bentur 2005; Kuyucu 2004; Mesquita 2009; Plint 2009) and one used salbutamol and ipratropium bromide (De Boeck 1997). Nebulised salbutamol was administered during emergency department stay (first two to four hours), or each four to six hours at home or during hospitalisation (1.5 mg to 2.5 mg, or 0.15 mg/kg). Oral administration was also allowed in Goebel 2000. Nebulised epinephrine was administered every six hours to inpatients, or once or twice in the emergency department for outpatients (1 mg to 3 mg). All other trials used bronchodilators at the discretion of the attending physician, often with guidance on the choice of drug and dosage. Additional use of glucocorticoids was often restricted. Supportive measures, i.e. oxygen and intravenous or nasogastric fluids, were usually reported.
Outcomes
Pre‐defined primary outcomes were specified in 12 trials (Cade 2000; Corneli 2007; Goebel 2000; Klassen 1997; Kuyucu 2004; Mesquita 2009; Plint 2009; Richter 1998; Roosevelt 1996; Schuh 2002; Teeratakulpisarn 2007; Zhang 2003), three of which reported more than one primary outcome (Kuyucu 2004; Richter 1998; Teeratakulpisarn 2007). Only the two largest trials used admission as a primary outcome (Corneli 2007; Plint 2009). Other primary outcomes included clinical scales (Goebel 2000; Klassen 1997; Kuyucu 2004; Mesquita 2009; Richter 1998; Schuh 2002), clinical severity parameters or duration of disease (Kuyucu 2004; Roosevelt 1996; Teeratakulpisarn 2007) and symptoms (Cade 2000; Zhang 2003). Timings of primary outcome assessment were reported in 11 trials, six of which used multiple time points. Sample size calculations were either not reported or based on secondary outcomes in Goebel 2000, Kuyucu 2004 and Richter 1998.
Reported outcomes included healthcare use domains and clinical severity parameters (all trials), pulmonary function (De Boeck 1997), patient/parent‐reported symptoms and status (seven trials: Berger 1998; Cade 2000; Plint 2009; Roosevelt 1996; Schuh 2002; Teeratakulpisarn 2007; Zhang 2003) and other outcomes, including adverse events (10 trials: Bentur 2005; Cade 2000; Corneli 2007; Klassen 1997; Kuyucu 2004; Plint 2009; Richter 1998; Roosevelt 1996; Teeratakulpisarn 2007; Zhang 2003). Not all outcome and time point results were reported.
Admission rates were assessed in all eight outpatient trials, both by day 1 (all trials) and day 7 (three trials; Corneli 2007; Plint 2009; Schuh 2002). Kuyucu 2004 and Goebel 2000 reported admissions by days 5 and 6, respectively, and were pooled with day 7 results. LOS was reported in eight of nine inpatient trials (except Roosevelt 1996) and three outpatient trials (Berger 1998; Corneli 2007; Goebel 2000). Criteria for admission or discharge were rarely reported. Considerable variability was found in control group admission rates (from 0% to 44% by day 1, and 0% to 49% by day 7) and mean LOS (0.8 to 6.6 days) (Table 3). Hospital re‐admissions for inpatients and return healthcare visits up to one month were mentioned in six trials, with variable assessment methods (Berger 1998; Klassen 1997; Plint 2009; Roosevelt 1996; Schuh 2002; Teeratakulpisarn 2007).
1. Placebo group risk of admission/length of stay.
| Study | Placebo group ‐ participants | Placebo group ‐ primary outcomes | |
| OUTPATIENT STUDIES | Risk of admission day 1 (%) | Risk of admission day 7 (%) | |
| Barlas 1998 | 30 | 17% | NR |
| Berger 1998 | 18 | 11% | NR |
| Corneli 2007 | 295 | 41% | 49% |
| Goebel 2000 | 24 | 8% | 21% |
| Kuyucu 2004 | 11 | 0% | 0% |
| Mesquita 2009 | 32 | 22% | NR |
| Plint 2009 | 201 | 18% | 26% |
| Schuh 2002 | 34 | 44% | 47% |
| INPATIENT STUDIES | Length of stay (mean ± SD days) | ||
| Bentur 2005 | 32 | 6.3 ± 8.8 | |
| Cade 2000 | 79 | 2 ± 2.2 | |
| De Boeck 1997 | 15 | 6.6 ± 1.2 | |
| Gomez 2007 | 25 | 0.8 ± 0.2 | |
| Klassen 1997 | 32 | 2 ± 0.7 | |
| Richter 1998 | 19 | 3 ± 1.6 | |
| Teeratakulpisarn 2007 | 85 | 2.8 ± 1.7 | |
| Zhang 2003 | 24 | 5 ± 3.3 | |
NR = not reported SD = standard deviation
Clinical severity scales were assessed in all except one trial (Zhang 2003), often using more than one scale (Corneli 2007; Plint 2009; Richter 1998; Schuh 2002). Measurement instruments were developed specifically for nine trials (Barlas 1998; Bentur 2005; Berger 1998; Cade 2000; De Boeck 1997; Goebel 2000; Richter 1998; Roosevelt 1996; Teeratakulpisarn 2007), mostly based on previous scales by Schuh 1990, Tal 1983 and Westley 1978. The RDAI was used in eight trials (Corneli 2007; Gomez 2007; Klassen 1997; Kuyucu 2004; Mesquita 2009; Plint 2009; Richter 1998; Schuh 2002). Corneli 2007 and Plint 2009 also used the Respiratory Assessment Change Score (RACS), based on RDAI and respiratory rate (both originally reported by Lowell 1987). All scales included items on wheezing and accessory muscle use; other respiratory items (for example, timing or location of wheezing) or disease domains (for example, general status, nutrition) were less frequently used. Oxygen saturation, respiratory and heart rates were reportedly measured in most trials. Heterogeneity in timings of repeated measurements was found; the two most frequently time points assessed were 60 minutes and three to six hours.
Measurement of patient/parent‐reported symptoms was inconsistent. Five trials reported symptoms data (Cade 2000; Plint 2009; Richter 1998; Roosevelt 1996; Teeratakulpisarn 2007). There were differences in the specific symptoms addressed (for example, respiratory, feeding), the measurement instrument used (i.e. questionnaires, diaries) and the time points of assessment. No trial reported the use of generic or disease‐specific quality of life instruments.
Other reported outcomes included temperature measurements (Corneli 2007; Plint 2009; Roosevelt 1996), time to resolution or length of illness (Roosevelt 1996; Zhang 2003), and duration of oxygen therapy or fluids (Bentur 2005; Richter 1998; Roosevelt 1996; Teeratakulpisarn 2007; Zhang 2003). Data on the use of bronchodilator co‐interventions were often reported as an outcome.
Adverse events were mentioned in six trials (Corneli 2007; Goebel 2000; Klassen 1997; Kuyucu 2004; Plint 2009; Teeratakulpisarn 2007). Five of these studies assessed specific gastrointestinal, endocrine or infectious complications. There was heterogeneity and incomplete reporting regarding which adverse events were pre‐specified, their definitions and measurement methods. All adverse effects were short‐term and no study assessed long‐term harms.
Excluded studies
Eighty‐four out of 361 excluded papers involved glucocorticoids. Motives for exclusion from this subset mostly included inappropriate population (for example, trials including participants with a history of previous wheezing, or > 24 months old), type of publication and non‐RCT study design (Characteristics of excluded studies).
Risk of bias in included studies
We assessed overall risk of bias as 'low' in three trials, as 'high' in seven and 'unclear' in seven. The glucocorticoid and epinephrine versus placebo comparison included one low risk of bias trial. All other comparisons included mostly high risk of bias trials (Figure 2).
2.
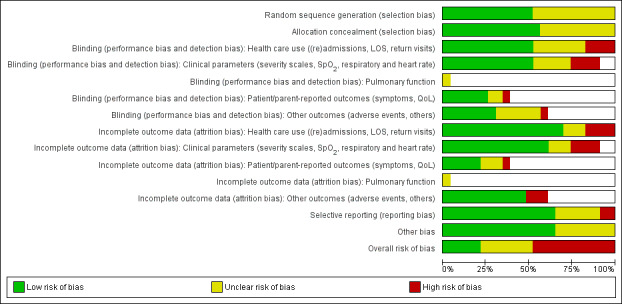
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.*
*For multi‐arm studies (Barlas 1998, Kuyucu 2004 and Plint 2009), we included one overall assessment for all trial comparisons, and two assessments for each separate comparison of glucocorticoids versus placebo (with or without protocolised bronchodilator, or with epinephrine or salbutamol).
We found adequate sequence generation and allocation concealment in 10 and 11 trials, respectively (Figure 3). We considered blinding adequate in 10 out of 17 trials for the review primary outcomes and clinical severity parameters. Incomplete reporting explained most 'unclear' assessments. Incomplete outcome data were adequately addressed in 12 out of 17 studies for the review primary outcomes, and 11 out of 17 for clinical severity outcomes; it was unclear or inadequate when there was imbalanced attrition between groups, mostly in longer follow‐up assessments.
3.
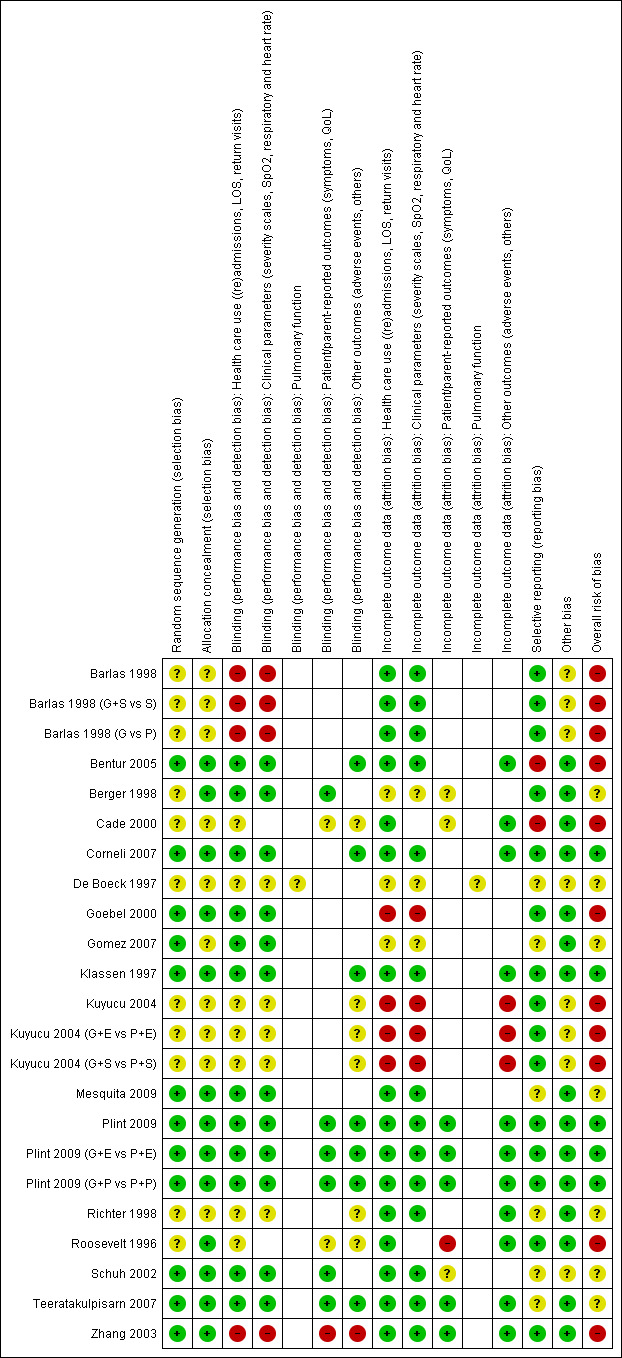
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.*
*For multi‐arm studies (Barlas 1998, Kuyucu 2004 and Plint 2009), we included one overall assessment for all trial comparisons, and two assessments for each separate comparison of glucocorticoids versus placebo (with or without protocolised bronchodilator, or with epinephrine or salbutamol).
We considered nine out of 17 studies free from risk of selective outcome reporting. Assessment of this item was challenging given the large number of outcomes reported, the diversity of measurement time points, and the fact that trial protocols were not available. Using trial registry searches, we identified three trial registers and used that data to complete assessments (Corneli 2007; Plint 2009; Teeratakulpisarn 2007).
Regarding publication bias and small study effects, there was no asymmetry in funnel plots for the primary outcomes in the glucocorticoids versus placebo comparison by visual inspection or statistical testing (Egger test for admissions and length of stay, P = 0.98 and P = 0.77, respectively) (Figure 4; Figure 5).
4.
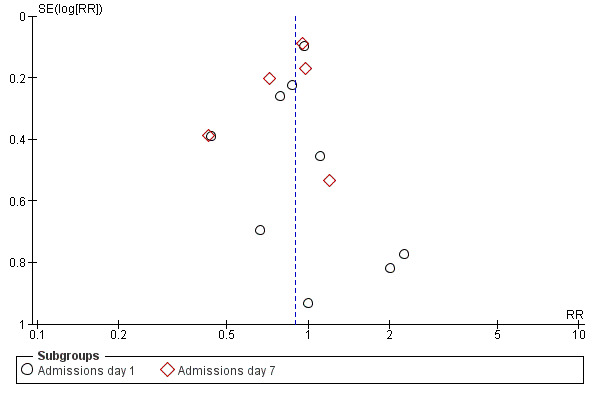
Funnel plot of comparison: 1 Steroid versus placebo, outcome: 1.1 Admissions (days 1 and 7) (outpatients) ‐ review primary outcome.
5.
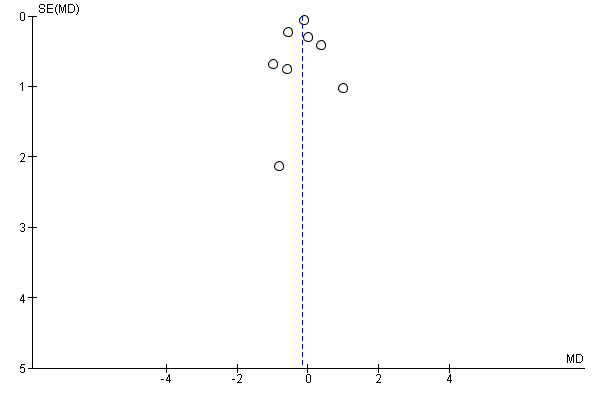
Funnel plot of comparison: 1 Steroid versus placebo, outcome: 1.2 Length of stay (inpatients) ‐ review primary outcome.
Other types of bias assessed as 'unclear' included baseline imbalances, or active arm contamination with other related co‐interventions (Kuyucu 2004 and Schuh 2002, respectively).
Effects of interventions
Summary of findings for the main comparison. Glucocorticoid versus placebo: summary of findings.
| Glucocorticoid versus placebo for acute viral bronchiolitis in infants and young children | |||||
| Patient or population: infants and young children with acute viral bronchiolitis Settings: outpatients and inpatients Intervention: glucocorticoid versus placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Steroid versus placebo | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk1 | Corresponding risk | ||||
| Placebo | Steroid | ||||
|
Admissions (outpatients) Follow‐up: day 1 |
Medium risk population |
RR 0.92 (0.78 to 1.08) |
1762 (8) | high | |
| 162 per 1000 | 149 per 1000 (126 to 175) | ||||
|
Admissions (outpatients) Follow‐up: day 7 |
Medium risk population |
RR 0.86 (0.7 to 1.06) |
1530 (5) | moderate | |
| 250 per 1000 | 215 per 1000 (175 to 265) | ||||
|
Length of stay (inpatients) days |
The mean length of stay ranged across control groups from 0.8 to 6.6 days | The mean length of stay in the intervention groups was 0.18 lower (0.39 lower to 0.04 higher) | 633 (8) | high | |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Assumed risk for admissions was based on the median control group risks across the studies included in the meta‐analysis (medium risk).
Summary of findings 2. Glucocorticoid and epinephrine versus placebo: summary of findings.
| Glucocorticoid and epinephrine versus placebo for acute viral bronchiolitis in infants and young children | ||||||
| Patient or population: infants and young children with acute viral bronchiolitis Settings: outpatients Intervention: glucocorticoid and epinephrine versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Steroid versus placebo | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| Placebo | Steroid | |||||
|
Admissions (outpatients) Follow‐up: day 1 |
179 per 1000 | 115 per 1000 (72 to 186) | RR 0.65 (0.4 to 1.05) | 400 (1) | Low | NNT: not calculated for non‐significant findings |
|
Admissions (outpatients) Follow‐up: day 7 |
264 per 1000 | 169 per 1000 (116 to 251) |
RR 0.65 (0.44 to 0.95) |
400 (1) | Low | NNT: 11 (95% CI 7 to 76) (based on unadjusted analysis results) |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval NNT: number needed to treat RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Assumed risk for admissions was based on the control group risk in the single study included (Plint 2009).
Results are summarised by comparison, setting and type of outcome. GRADE assessments for the two main comparisons ‐ glucocorticoid versus placebo and glucocorticoid and bronchodilator versus placebo are shown in Table 4 and Table 5. All meta‐analyses used random‐effects models; fixed‐effect models did not modify the direction and magnitude of results unless mentioned.
2. GRADE assessments: glucocorticoid versus placebo.
| Population | Outcome | Number of studies | Number of participants | GRADE domains | Strength of evidence | Intervention favoured | |||
| Risk of bias | Consistency | Directness | Precision | ||||||
| GLUCOCORTICOID versus PLACEBO | |||||||||
| Inpatients | Length of stay | 8 | 633 | Medium | Consistent | Direct | Precise | High | No difference |
| Clinical score : 3 to 6 hours | 1 | 26 | Medium | Unknown | Direct | Imprecise | Low | Glucocorticoid | |
| Clinical score : 6 to 12 hours | 3 | 175 | Medium | Consistent | Direct | Imprecise | Moderate | Glucocorticoid | |
| Clinical score : 12 to 24 hours | 3 | 230 | Medium | Consistent | Direct | Imprecise | Moderate | No difference (glucocorticoid favoured) | |
| Clinical score : 24 to 72 hours | 4 | 113 | Medium | Inconsistent | Direct | Imprecise | Low | No difference (glucocorticoid favoured; very close to significant) | |
| Outpatients | Admissions day 1 | 8 | 1762 | Medium | Consistent | Direct | Precise | High | No difference |
| Admissions up to day 7 | 5 | 1530 | Low | Consistent | Direct | Imprecise | Moderate | No difference | |
| Clinical score: 60 minutes | 4 | 1006 | Low | Consistent | Direct | Precise | High | No difference | |
| Clinical score: 120 minutes | 3 | 214 | Medium | Consistent | Direct | Imprecise | Moderate | No difference | |
| Clinical score: 3 to 6 hours | 2 | 808 | Medium | Inconsistent | Direct | Precise | Moderate | No difference | |
| Clinical score: 12 to 24 hours | 1 | 69 | Medium | Unknown | Direct | Imprecise | Low | No difference | |
| Clinical score: 3 to 10 days | 4 | 224 | Medium | Inconsistent | Direct | Imprecise | Low | No difference | |
| Inpatients/outpatients | Adverse events | 5 | 1123 | Low | Consistent | Direct | Precise | Moderate | No difference |
3. GRADE assessments: glucocorticoid and epinephrine versus placebo.
| Population | Outcome | Number of studies | Number of participants | GRADE domains | Strength of evidence | Intervention favoured | |||
| Risk of bias | Consistency | Directness | Precision | ||||||
| GLUCOCORTICOID AND EPINEPHRINE versus PLACEBO | |||||||||
| Outpatients | Admissions day 1 | 1 | 400 | Low | Unknown | Direct | Imprecise | Low | Favours epi + dex but NS |
| Admissions day 7 | 1 | 400 | Low | Unknown | Direct | Imprecise | Low | Epi + dex | |
| Clinical score: 60 minutes | 1 | 400 | Low | Unknown | Direct | Precise | Moderate | Epi + dex | |
| Adverse events | 1 | 400 | Low | Unknown | Direct | Imprecise | Low | No difference | |
dex = dexamethasone epi = epinephrine NS = non‐significant
Glucocorticoid versus placebo
Outpatients
Primary outcomes
All eight outpatient studies reported admissions by day 1, and five also reported admissions by day 7. Complete outcome data were available for 1762 participants by day 1 (out of 1824 randomised) and 1530 participants by day 7 (out of 1612 randomised).
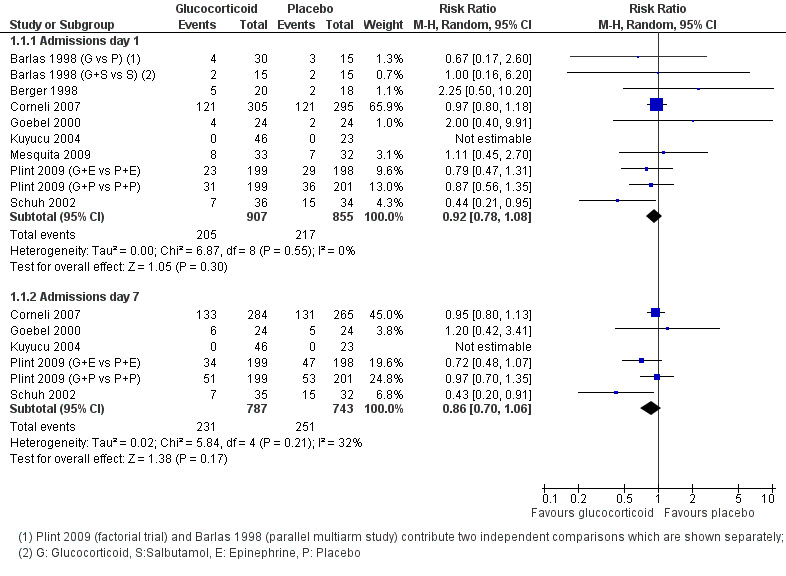
The pooled risk ratios (RRs) for admissions by days 1 and 7 were 0.92 (95% confidence interval (CI) 0.78 to 1.08) and 0.86 (95% CI 0.7 to 1.06), respectively, with no significant differences between groups (Analysis 1.1; Figure 6). Heterogeneity was low for day 1 results and moderate for day 7 (I2 statistic = 0% and 31%, respectively). There was no relevant change in the magnitude or direction of results when using pooled data from both Plint 2009 arms. Sensitivity analyses for both trials with low overall risk of bias showed comparable results (Analysis 1.22). Overall strength of evidence for these findings was high for day 1 results and moderate for day 7, the latter due to some imprecision in the effect estimate (Table 4; Table 1).
1.1. Analysis.
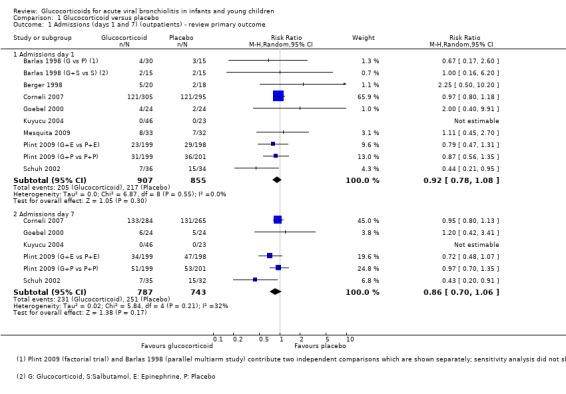
Comparison 1 Glucocorticoid versus placebo, Outcome 1 Admissions (days 1 and 7) (outpatients) ‐ review primary outcome.
6.
Forest plot of comparison: 1 Steroid versus placebo, outcome: 1.1 Admissions (days 1 and 7) (outpatients) ‐ review primary outcome.
1.22. Analysis.
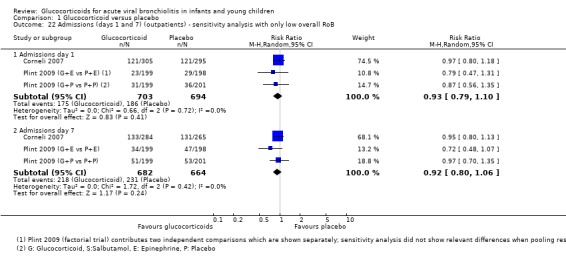
Comparison 1 Glucocorticoid versus placebo, Outcome 22 Admissions (days 1 and 7) (outpatients) ‐ sensitivity analysis with only low overall RoB.
Subgroup analysis of studies using protocolised bronchodilator found lower pooled RRs for admissions by both days 1 and 7, but the CIs between subgroups overlapped (Analysis 1.15; Analysis 1.16). For admissions by day 7, the estimate for RR was 0.68 (95% CI 0.44 to 1.05) for protocolised bronchodilator trials (four trials, 581 participants), and 0.95 (95% CI 0.82 to 1.11) for other trials (two trials, 949 participants). Heterogeneity was low in both subgroups.
1.15. Analysis.
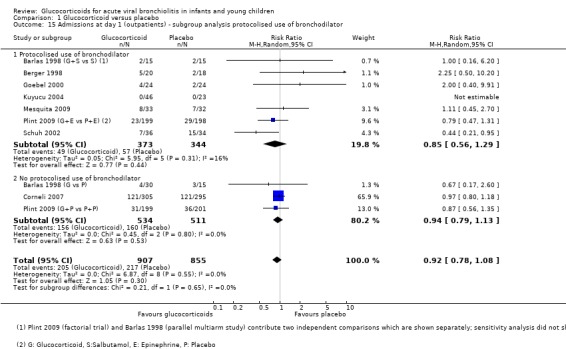
Comparison 1 Glucocorticoid versus placebo, Outcome 15 Admissions at day 1 (outpatients) ‐ subgroup analysis protocolised use of bronchodilator.
1.16. Analysis.
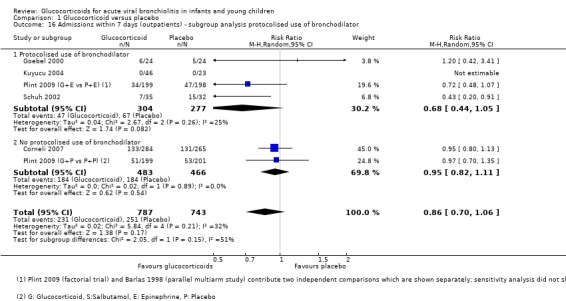
Comparison 1 Glucocorticoid versus placebo, Outcome 16 Admissions within 7 days (outpatients) ‐ subgroup analysis protocolised use of bronchodilator.
The two largest outpatient studies only included participants under 12 months of age, while six smaller studies also included older patients (Analysis 1.17; Analysis 1.18). For admissions by day 7, estimates were 0.92 (95% CI 0.80 to 1.06) and 0.67 (95% CI 0.25 to 1.83), for < 12 months (two trials, 1346 participants) and trials including older participants (three trials, 184 participants), respectively. Trials including older participants had a lower effect estimate, but a large CI overlapped with the other subgroup and there was substantial heterogeneity (I2 statistic = 60%).
1.17. Analysis.
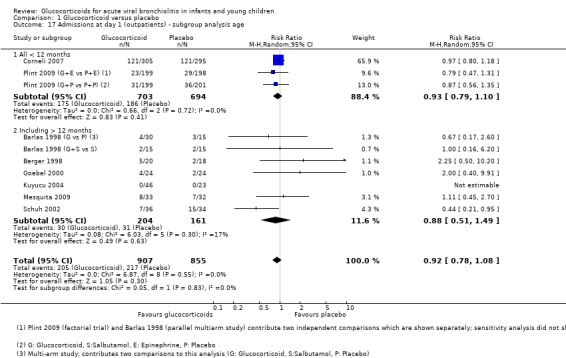
Comparison 1 Glucocorticoid versus placebo, Outcome 17 Admissions at day 1 (outpatients) ‐ subgroup analysis age.
1.18. Analysis.
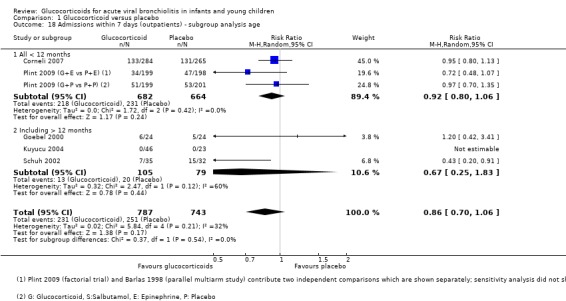
Comparison 1 Glucocorticoid versus placebo, Outcome 18 Admissions within 7 days (outpatients) ‐ subgroup analysis age.
No subgroup analysis according to respiratory syncytial virus (RSV) or atopic status was performed, since no outpatient trial restricted inclusion based on these parameters. Corneli 2007 and Plint 2009 reported pre‐specified subgroup analyses based on atopic status, with no statistically significant differences. Plint 2009 also reported no differences according to RSV status, duration of illness and severity. We chose not to perform analyses based on glucocorticoid type or dose due to heterogeneity in glucocorticoid schemes.
Secondary outcomes
Clinical score data were available for time points/intervals between 60 minutes and 3 to 10 days (Analysis 1.4; Figure 7). Different sets of studies with different scales contributed to each time point, with most data at 60 minutes (four trials, 1006 participants); no trial assessed the period between 24 to 72 hours. There were no significant differences between groups at any time point. Strength of evidence for these findings was high at 60 minutes, with precise and consistent results (standardised mean difference (SMD) ‐0.04; 95% CI ‐0.16 to 0.09; I2 statistic = 0%). Evidence was weaker for later results due to imprecision and substantial heterogeneity.
1.4. Analysis.
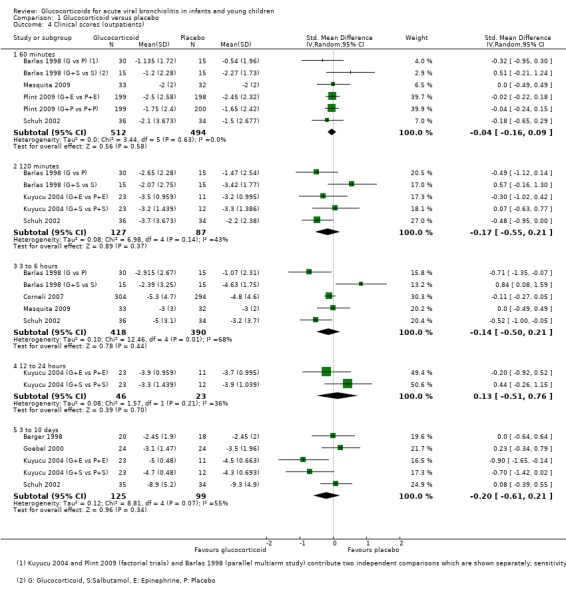
Comparison 1 Glucocorticoid versus placebo, Outcome 4 Clinical scores (outpatients).
7.
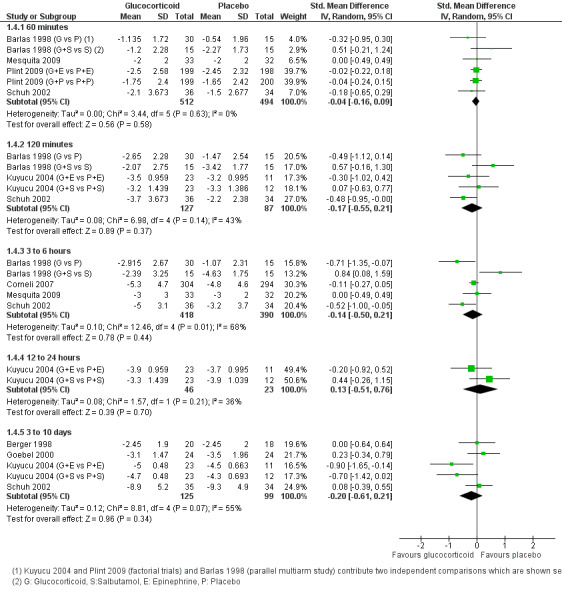
Forest plot of comparison: 1 Steroid versus placebo, outcome: 1.4 Clinical scale scores (outpatients) (change from baseline data).
Six trials reported outcome data on oxygen saturation between 60 minutes and 24 to 72 hours (Analysis 1.6). Data were most frequently reported at 60 minutes (three trials, 936 participants). At three to six hours, results favoured placebo (mean difference (MD) ‐0.43; 95% CI ‐0.84 to ‐0.02; units: %), while for all other time points there were no significant differences between groups.
1.6. Analysis.
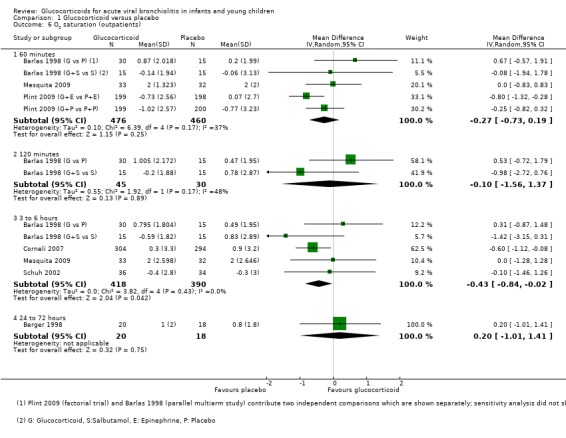
Comparison 1 Glucocorticoid versus placebo, Outcome 6 O2 saturation (outpatients).
Respiratory and heart rate data were both reported in six outpatient trials, between 60 minutes and 3 to 10 days (Analysis 1.8; Analysis 1.10). The most frequently assessed time point for both outcomes was 60 minutes; no trial assessed the period between 24 to 72 hours. There were no significant differences between groups for any of these outcomes.
1.8. Analysis.
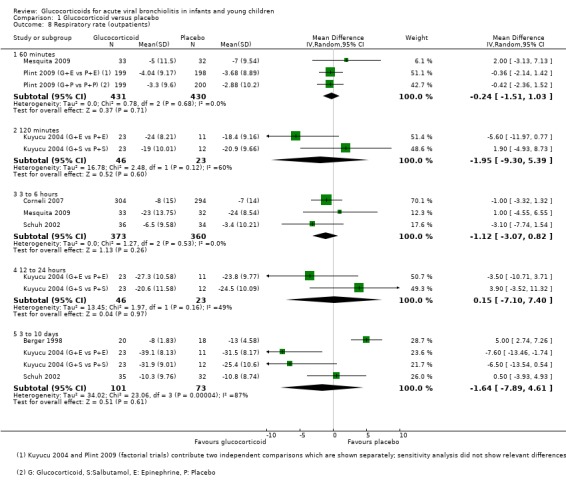
Comparison 1 Glucocorticoid versus placebo, Outcome 8 Respiratory rate (outpatients).
1.10. Analysis.
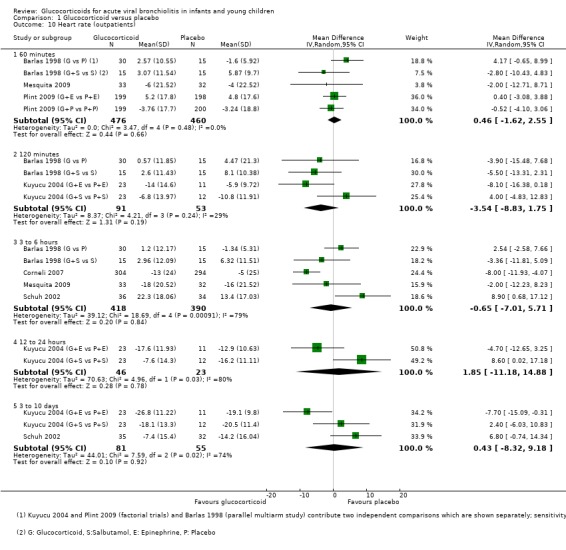
Comparison 1 Glucocorticoid versus placebo, Outcome 10 Heart rate (outpatients).
Regarding other health services outcomes, pooled data from three trials (255 participants) reporting length of stay (LOS) of admitted patients did not show significant differences between groups (Analysis 1.3). Return to healthcare visits for bronchiolitis symptoms were only assessed in two trials (863 participants), both showing considerable event rate for a three to four‐week follow‐up period (26% to 53% in all groups; Table 6). Pooled results did not show significant differences between groups (RR 1.04; 95% CI 0.80 to 1.35) (Analysis 1.14).
1.3. Analysis.
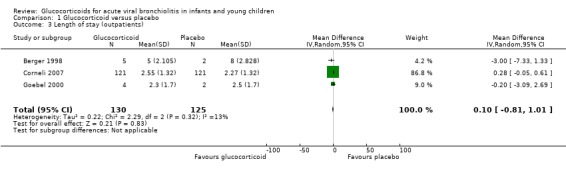
Comparison 1 Glucocorticoid versus placebo, Outcome 3 Length of stay (outpatients).
4. Hospital re‐admissions and return healthcare visits (in‐ and outpatients).
| Study | Population | Duration of follow‐up | Glucocorticoid‐including group | Placebo or comparator group | Notes |
| GLUCOCORTICOID AND EPINEPHRINE versus PLACEBO: HOSPITAL RE‐ADMISSIONS | |||||
| Roosevelt 1996 | Inpatients | Days 1 to 14 | 0 | 0 | (No events in either group) |
| Klassen 1997 | Inpatients | Days 1 to 7 | 4/35 (11%) | 1/32 (3%) | P = 0.36 |
| Teeratakulpisarn 2007 | Inpatients | Days 1 to 30 | 3/89 (3%) | 7/85 (8%) | |
| GLUCOCORTICOID versus PLACEBO: RETURN HEALTHCARE VISITS* | |||||
| Plint 2009 (epinephrine ‐ E; dexamethasone ‐ D; placebo ‐ P) |
Outpatients | Days 1 to 22 | D + E 95/199 (48%) |
P + E 93/198 (47%) |
Return to the health care provider for bronchiolitis symptoms Difference between dexamethasone + placebo versus placebo + placebo, was significant in the unadjusted analysis (P = 0.04) |
| D + P 106/199 (53%) |
P + P 86/201 (43%) |
||||
| Schuh 2002 | Outpatients | Days 7 to 28 | 9/35 (26%) | 14/32 (44%) | Medical visits for continuing symptoms; P = 0.069 |
| Klassen 1997 | Inpatients | Days 1 to 7 | 29/35 (83%) | 24/32 (75%) | P = 0.77 |
| Roosevelt 1996 | Inpatients | Days 1 to 14 | 16/65 (25%) | 5/53 (9%) | P = 0.01; reported on visits made by the physician; 69% were for non‐respiratory difficulties |
| Teeratakulpisarn 2007 | Inpatients | Days 1 to 30 | 17/89 (19%) | 26/85 (31%) | Visit to emergency room or a private clinic because of respiratory symptoms |
| GLUCOCORTICOID versus EPINEPHRINE: RETURN HEALTHCARE VISITS | |||||
| Plint 2009 (dexamethasone + placebo versus epinephrine + placebo) |
Outpatients | Days 1 to 22 | 106/199 (53%) | 93/198 (47%) | ‐ |
| GLUCOCORTICOID AND EPINEPHRINE versus PLACEBO: RETURN HEALTHCARE VISITS | |||||
| Plint 2009 (dexamethasone + epinephrine versus placebo + placebo) |
Outpatients | Days 1 to 22 | 95/198 (48%) | 86/201 (43%) | ‐ |
*Berger 1998: no difference between groups, but did not report quantitative data. Data presented as n/N (%)
1.14. Analysis.
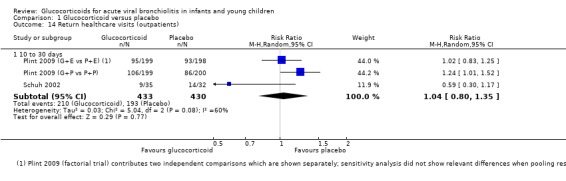
Comparison 1 Glucocorticoid versus placebo, Outcome 14 Return healthcare visits (outpatients).
Plint 2009 reported data on parent‐reported symptoms regarding time to return to normal feeding, sleeping, breathing and no coughing (Table 7). There were no statistically significant differences between glucocorticoid and placebo groups. No outpatient trials assessed or reported pulmonary function or quality of life outcomes.
5. Symptoms and quality of life (in‐ and outpatients)*.
| Study | Population | Duration of follow‐up | Parameter | Glucocorticoid‐including group | Placebo or comparator group | Notes |
| GLUCOCORTICOID versus PLACEBO | ||||||
| Teeratakulpisarn 2007 | Inpatients | Days 1 to 30 | Time from treatment to being symptom free ‐ mean ± SD | 7.0 ± 5.9 | 9.0 ± 6.4 | P = 0.035 |
| Cade 2000 | Inpatients | Days 1 to 28 | Time taken for half of infants to become asymptomatic for 48 hours (95% CI) ‐ time to event analysis | 10 (10 to 13) | 12 (10 to 16) | HR 1.41 (95% CI 0.98 to 2.04), P = 0.07 |
| Days with coughing or wheezing episodes ‐ mean ± SD | 17.0 ± 7.6 days | 17.1 ± 8.5 | Mean difference: 0.91 days (95% CI ‐2.72 to 2.41), P = 0.91 | |||
| Roosevelt 1996# | Inpatients | Day 10 to 14 | No current difficulty breathing ‐ n/N (%) | 45/45 (100) | 37/42 (88) | P = 0.07 |
| Feeding and drinking well ‐ n/N (%) | 45/45 (100) | 40/42 (95) | P = 0.57 | |||
| GLUCOCORTICOID versus PLACEBO, GLUCOCORTICOID versus EPINEPHRINE, GLUCOCORTICOID AND EPINEPHRINE versus PLACEBO | ||||||
| Plint 2009 (epinephrine ‐ E; dexamethasone ‐ D; placebo ‐ P) | Outpatients | Days 1 to 22 | Time to return to normal feeding ‐ median (IQR) | D + E: 0.6 (0.2 to 1.3) D + P: 0.8 (0.3 to 1.9) P + E: 0.5 (0.2 to 1.2) P + P: 0.9 (0.3 to 2.1) | Time to return to normal feeding ‐ mean symptom duration ratio D + E versus P + P: 0.63 (unadjusted 95% CI 0.5 to 0.8)¶ Time to return to quiet breathing ‐ mean symptom duration ratio D + E versus P + P: 0.83 (unadjusted 95% CI 0.69 to 1.00) No other reported comparison was statistically significant in adjusted analysis |
|
| Time to return to normal sleeping ‐ median (IQR) | D + E: 0.7 (0.2 to 1.7) D + P: 0.8 (0.3 to 1.9) P + E: 0.8 (0.3 to 1.9) P + P: 0.8 (0.3 to 1.8) | |||||
| Time to no coughing ‐ median (IQR) | D + E: 12.6 (7.8 to 18.5) D + P: 13.8 (8.5 to 20.2) P + E: 13.2 (8.1 to 19.3) P + P: 13.3 (8.2 to 19.5) | |||||
| Time to quiet breathing ‐ median (IQR) | D + E: 3.1 (1.4 to 6.1) D + P: 3.7 (1.6 to 7.1) P + E: 3.6 (1.5 to 6.9) P + P: 3.7 (1.6 to 7.2) | |||||
*Units in days unless otherwise stated; no study assessed or reported data from generic or disease‐specific quality of life instruments; Richter 1998 also reported number of symptom‐free days for a 6‐week follow‐up period
#Roosevelt 1996 primary outcome was time to resolution (defined as number of 12 h periods needed to achieve: O2 saturation > 95% at room air, accessory muscle score = 0, wheeze = 0 or 1, and normal feeding); only association measures were reported: HR 1.3 (95% CI 0.9 to 1.3), P = 0.22
¶time to symptom relief was analysed by means of parametric survival models with Weibull distributions assumed; 95% CI adjusted for multiple analysis in a factorial trial.
CI = confidence interval HR = hazard ratio IQR = interquartile range SD = standard deviation
Inpatients
Primary outcomes
Eight inpatient trials reported data on LOS (633 participants), with no significant mean difference between glucocorticoid and placebo groups (MD ‐0.18 days; 95% CI ‐0.39 to 0.04; I2 statistic = 16%) (Analysis 1.2; Figure 8). On a sensitivity analysis using fixed‐effect models and including all studies, the mean difference reached statistical significance favouring glucocorticoids, with a similar magnitude (MD ‐0.14 days; 95% CI ‐0.25 to ‐0.03). We graded the strength of evidence as high given its precision, consistency and 'Risk of bias' assessments for all included trials (Table 4; Table 1).
1.2. Analysis.
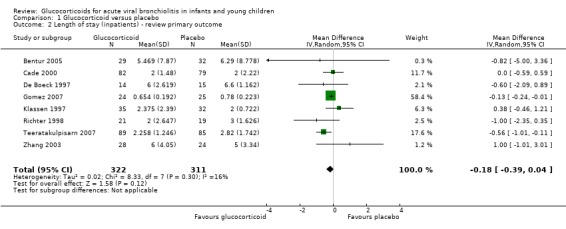
Comparison 1 Glucocorticoid versus placebo, Outcome 2 Length of stay (inpatients) ‐ review primary outcome.
8.
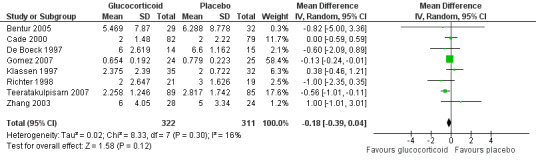
Forest plot of comparison: 1 Steroid versus placebo, outcome: 1.2 Length of stay (inpatients) ‐ review primary outcome.
Subgroup analyses showed a statistically significant reduction in LOS in trials with protocolised bronchodilator (‐0.12 days; 95% CI ‐0.23 to ‐0.00; four trials, 206 participants), although CIs overlapped between subgroups (Analysis 1.19). Heterogeneity was low in the protocolised group results (I2 statistic = 0%) and moderate in the other subgroup (I2 statistic = 38%).
1.19. Analysis.
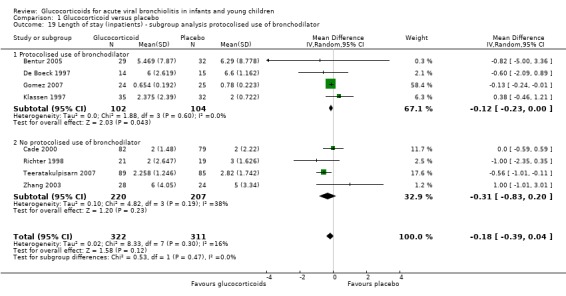
Comparison 1 Glucocorticoid versus placebo, Outcome 19 Length of stay (inpatients) ‐ subgroup analysis protocolised use of bronchodilator.
In subgroup analyses according to age and RSV status, CIs overlapped between subgroups for both parameters (Analysis 1.20 and Analysis 1.21). Heterogeneity was low in both < 12 months and RSV‐only trial results, and moderate in the other subgroups.
1.20. Analysis.
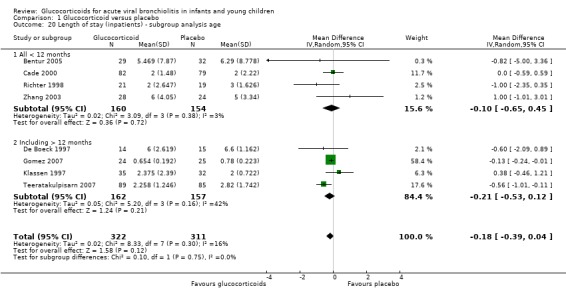
Comparison 1 Glucocorticoid versus placebo, Outcome 20 Length of stay (inpatients) ‐ subgroup analysis age.
1.21. Analysis.
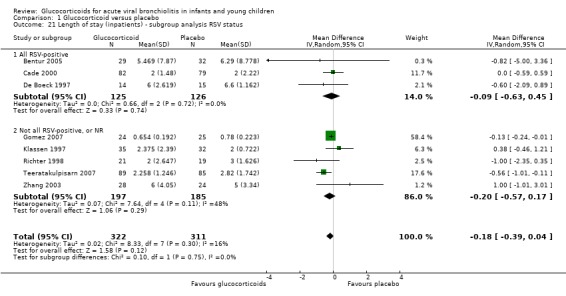
Comparison 1 Glucocorticoid versus placebo, Outcome 21 Length of stay (inpatients) ‐ subgroup analysis RSV status.
We did not perform subgroup analyses based on atopic status and glucocorticoid type and dose for the reasons mentioned previously.
Secondary outcomes
Clinical score data were only available for intervals between three to six hours and 24 to 72 hours (Analysis 1.5; Figure 9). Glucocorticoids were favoured at earlier time points (three to six hours, one trial, 174 participants: SMD ‐1.03 (95% CI ‐1.87 to ‐0.19); and 6 to 12 hours, three trials, 269 participants: SMD ‐0.62 (95% CI ‐1.00 to ‐0.23). There were no statistically significant differences at later time points. We assessed the overall strength of evidence for these findings as low or moderate, due to imprecision and low or unknown consistency, often with considerable heterogeneity.
1.5. Analysis.
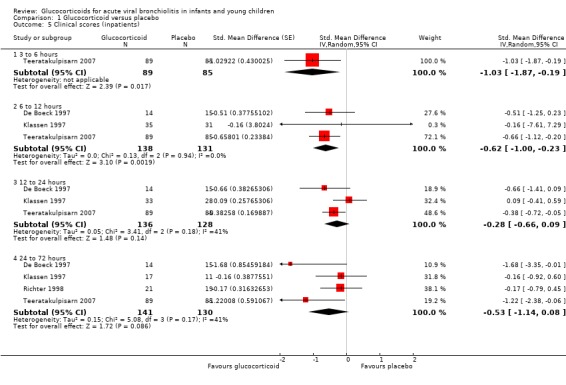
Comparison 1 Glucocorticoid versus placebo, Outcome 5 Clinical scores (inpatients).
9.
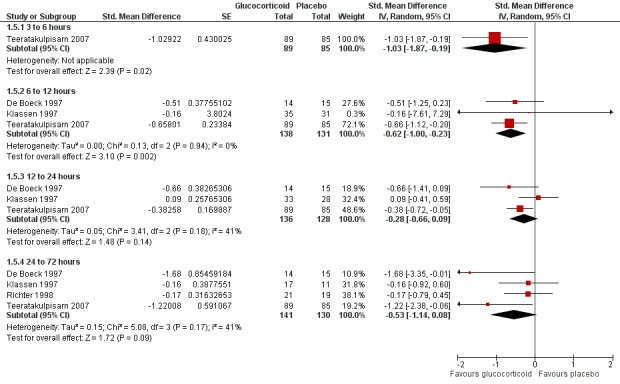
Forest plot of comparison: 1 Glucocorticoid versus placebo, outcome: 1.6 Clinical scores (inpatients) (change from baseline data).
Only two trials reported outcomes of oxygen saturation and respiratory rate at time points between 6 to 12 hours and 24 to 72 hours, one of which also reported heart rate at 12 to 24 hours (Analysis 1.7; Analysis 1.9; Analysis 1.11). There were no significant differences between groups for any outcome or time point.
1.7. Analysis.
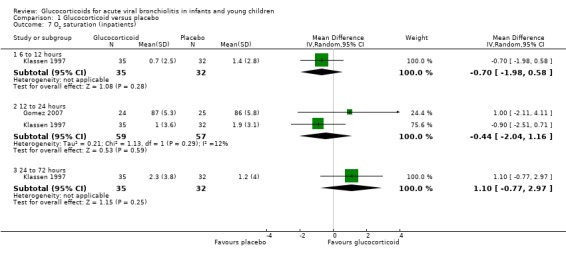
Comparison 1 Glucocorticoid versus placebo, Outcome 7 O2 saturation (inpatients).
1.9. Analysis.
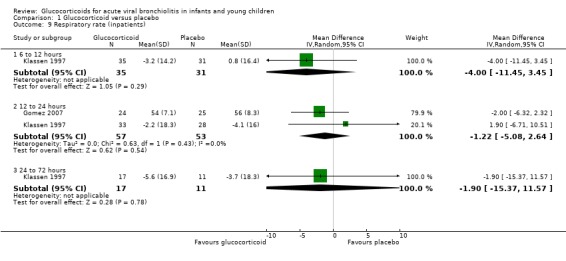
Comparison 1 Glucocorticoid versus placebo, Outcome 9 Respiratory rate (inpatients).
1.11. Analysis.
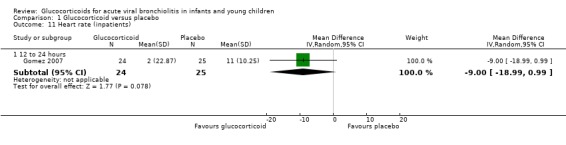
Comparison 1 Glucocorticoid versus placebo, Outcome 11 Heart rate (inpatients).
Both hospital re‐admissions and return healthcare visits were reported by three inpatient studies, with distinct durations of follow‐up; no significant differences were found between groups (Table 6; Analysis 1.12; Analysis 1.13).
1.12. Analysis.
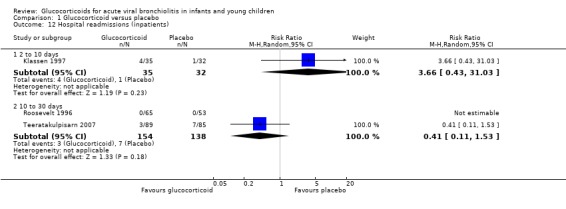
Comparison 1 Glucocorticoid versus placebo, Outcome 12 Hospital readmissions (inpatients).
1.13. Analysis.
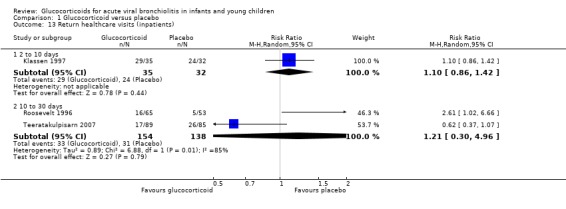
Comparison 1 Glucocorticoid versus placebo, Outcome 13 Return healthcare visits (inpatients).
Three inpatient trials reported data on parent‐reported symptoms (Table 7). Different sets of symptoms were measured at distinct time points, and methods of measurement and analysis varied. In Teeratakulpisarn 2007 time to being symptom free was significantly shorter in the glucocorticoid group, while Cade 2000 used a different analysis and did not shown any statistically significant differences. There were no differences regarding respiratory symptoms and feeding in both Cade 2000 and Roosevelt 1996. No inpatient trials assessed or reported quality of life outcomes.
De Boeck 1997 reported results from pulmonary function tests on day three. No differences were found in minute ventilation, dynamic lung compliance, and inspiratory and expiratory pulmonary resistance, both before and after nebulised bronchodilator.
All patients
Adverse events
Six trials reported adverse events. Five assessed specific glucocorticoid‐related harms including the two largest studies (Table 8). We considered all harms data together regardless of patient setting in order to adequately assess the safety profile of glucocorticoids. Data were available from 600 to 1579 participants for each safety outcome. We did not pool results given the heterogeneity in definitions, methods and timings of assessment. Individual trial analysis did not show significant differences between glucocorticoids and placebo regarding the occurrence of vomiting, gastrointestinal bleeding, hypertension, pneumonia or varicella.
6. Harms ‐ adverse events.
| Adverse event | Number of participants | Study | Glucocorticoid‐including group, N (%) | Placebo or comparator group, N (%) | Notes | |
| GLUCOCORTICOID versus PLACEBO, GLUCOCORTICOID versus EPINEPHRINE, GLUCOCORTICOID AND EPINEPHRINE versus PLACEBO | ||||||
| Gastrointestinal | Vomiting | 1466 | Plint 2009* | D + E: 2/199 (1) D + P: 5/199 (2.5) | P + E: 4/198 (2) P + P: 3/201 (1.5) | Observed in the emergency department by research nurse |
| Kuyucu 2004* | No events in either group (D + E, D + S, P + E, P + S) | Methods/timings NR | ||||
| Corneli 2007 | 16/305 (5.2) | 14/295 (4.7) | Within 20 minutes after administration of the study medication | |||
| Bleeding | 1576 | Corneli 2007 | No events in either group | # | ||
| Teeratakulpisarn 2007 | 2/90 (2) | 1/89 (1) | Occult blood; also assessed diarrhoea separately. Methods/timings NR | |||
| Plint 2009 | D + E: 17/199 (8.5) D + P: 12/199 (6) | P + E: 14/198 (7) P+P: 16/201 (8) | Dark stools; reported by families during the 22‐day telephone follow‐up. No patient had more than 1 episode | |||
| Endocrine | Hypertension | 1397 | Plint 2009 | D + E: 0/199 (0) D + P: 1/199 (0.5) | P + E: 1/198 (0.5) P + P: 0/201 (0) | Observed in infants admitted to hospital |
| Corneli 2007 | No events in either group | # | ||||
| Infectious | Pneumonia | 851 | Corneli 2007 | 1/305 (3.3) | 2/295 (7) | Also assessed empyema separately |
| Teeratakulpisarn 2007 | 0/90 (0) | 3/89 (3.4) | Methods/timings NR | |||
| Klassen 1997 | 1/35 (3) | 1/37 (3) | Methods/timings NR | |||
| Varicella | 1397 | Corneli 2007 | No events in either group | # | ||
| Plint 2009 | No events in either group | Reported by families during the 22‐day telephone follow‐up | ||||
| General | Tremor | 866 | Kuyucu 2004 | No events in either group | Methods/timings NR | |
| Plint 2009 | D + E: 4/199 (2) D + P: 5/199 (2.5) | P + E: 4/198 (2) P + P: 2/201 (1) | Observed in the emergency department by research nurse | |||
| Pallor/flushing | 866 | Kuyucu 2004 | No events in either group | Methods/timings NR | ||
| Plint 2009 | D + E: 23/199 (11.5) D + P: 15/199 (7.5) | P + E: 22/198 (11.1) P + P: 16/201 (8) | Observed in the emergency department by research nurse | |||
Additional reported adverse events: Goebel 2000 reported toxicity data: one patient was "jittery"; no evidence of further treatment complications. Plint 2009 also reported hyperkalaemia observed in infants admitted to hospital (only one case was noted in the dexamethasone group).
*epinephrine ‐ E; dexamethasone ‐ D; salbutamol ‐ S; placebo ‐ P
#Corneli 2007: Study clinicians and research assistants monitored the infants for adverse events during observation in the emergency department. Subsequent adverse events were determined at follow‐up. A patient safety committee, made up of people not involved with patient enrolment, tracked all adverse events.
Glucocorticoid and bronchodilator (epinephrine or salbutamol) versus placebo
Both outpatient trials assessing either of these comparisons used different severity thresholds for patient inclusion: Respiratory Distress Assessment Instrument (RDAI) score above four in Plint 2009 (moderate disease), and scores between 4 and 10 using a trial‐specific clinical scale in Barlas 1998 (mild to moderate disease).
Primary outcomes
The factorial trial Plint 2009 included a comparison of oral dexamethasone and nebulised epinephrine against double placebo (399 analysed participants). This was the largest trial included in the review, with low overall risk of bias. The RRs for admissions by days 1 and 7 were 0.65 (95% CI 0.40 to 1.05) and 0.65 (95% CI 0.44 to 0.95), respectively (Analysis 2.1). There was a statistically significant reduction in admissions by day 7, with a relative risk reduction estimate of 35%. Absolute risk reduction was 9% (95% CI 1 to 17), and the number needed to treat to benefit (NNTB) to reduce one admission by day 7 was 11 (95% CI 7 to 76); these results were obtained through unadjusted analysis. However, the factorial trial design requires special methodological considerations, since this was not the study's main comparison, and there was an unanticipated additive/synergistic effect between epinephrine and dexamethasone. Reported analyses adjusted for multiple comparisons were above the threshold for statistical significance (RR 0.65; 95% CI 0.41 to 1.03). We graded the overall strength of evidence as low for these results given their imprecision and the fact that they were obtained from a single trial (Table 5; Table 2).
2.1. Analysis.
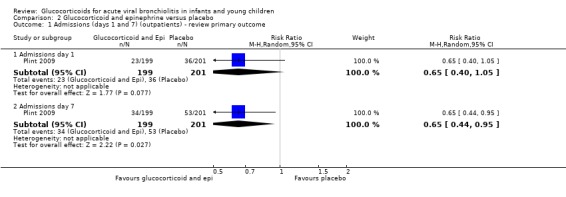
Comparison 2 Glucocorticoid and epinephrine versus placebo, Outcome 1 Admissions (days 1 and 7) (outpatients) ‐ review primary outcome.
Barlas 1998, a small high risk of bias trial, compared intravenous prednisolone and nebulised salbutamol versus placebo. Admissions by day 1 (30 participants) showed no statistically significant differences between groups (RR 0.67; 95% CI 0.13 to 3.44) (Analysis 3.1).
3.1. Analysis.
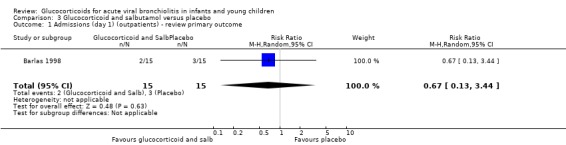
Comparison 3 Glucocorticoid and salbutamol versus placebo, Outcome 1 Admissions (day 1) (outpatients) ‐ review primary outcome.
Secondary outcomes
Clinical score results at 60 minutes favoured glucocorticoid and epinephrine (SMD ‐0.34; 95% CI ‐0.54 to ‐0.14) (Analysis 2.2), while having an increased heart rate (MD 8.44; 95% CI 4.85 to 12.03) (Analysis 2.5). No differences were found between groups regarding oxygen saturation and respiratory rate (Analysis 2.3; Analysis 2.4). There were also no differences regarding return healthcare visits for bronchiolitis symptoms (RR 1.11; 95% CI 0.89 to 1.38) (Table 6; Analysis 2.6). Symptom results showed reduced time to normal feeding and quiet breathing in the glucocorticoid and epinephrine group (mean symptom duration ratios: 0.63; 95% CI 0.5 to 0.8 and 0.83; 95% CI 0.69 to 1.00) (Table 7). No differences were found in time to normal sleeping and time to no coughing.
2.2. Analysis.
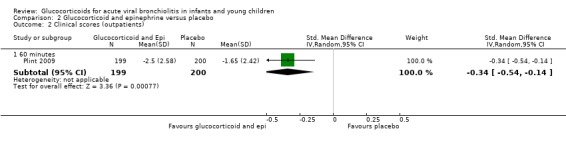
Comparison 2 Glucocorticoid and epinephrine versus placebo, Outcome 2 Clinical scores (outpatients).
2.5. Analysis.
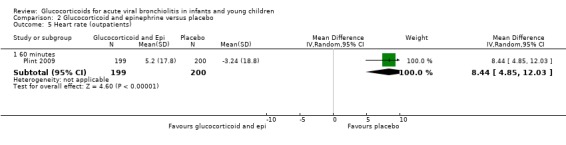
Comparison 2 Glucocorticoid and epinephrine versus placebo, Outcome 5 Heart rate (outpatients).
2.3. Analysis.
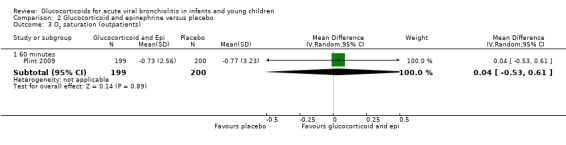
Comparison 2 Glucocorticoid and epinephrine versus placebo, Outcome 3 O2 saturation (outpatients).
2.4. Analysis.
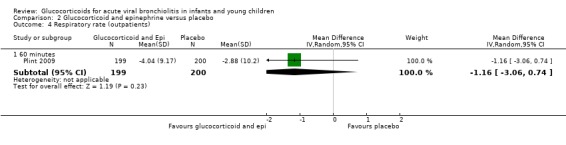
Comparison 2 Glucocorticoid and epinephrine versus placebo, Outcome 4 Respiratory rate (outpatients).
2.6. Analysis.
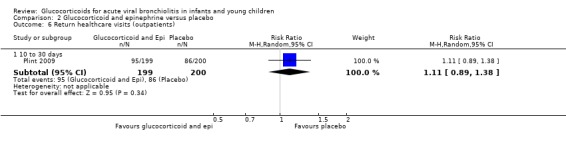
Comparison 2 Glucocorticoid and epinephrine versus placebo, Outcome 6 Return healthcare visits (outpatients).
Results for clinical scores, oxygen saturation and heart rate at 60 minutes, 120 minutes and three to six hours did not show any differences between groups in the single trial comparing glucocorticoid and salbutamol versus placebo (Analysis 3.2; Analysis 3.3; Analysis 3.4). No further secondary outcomes were assessed in this comparison.
3.2. Analysis.
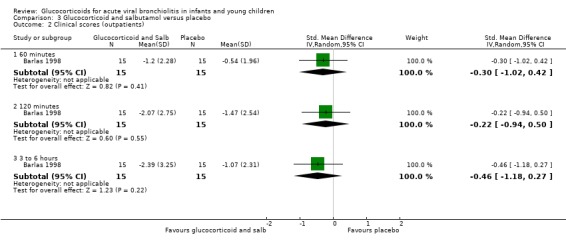
Comparison 3 Glucocorticoid and salbutamol versus placebo, Outcome 2 Clinical scores (outpatients).
3.3. Analysis.
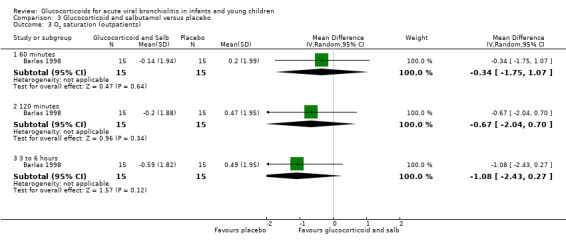
Comparison 3 Glucocorticoid and salbutamol versus placebo, Outcome 3 O2 saturation (outpatients).
3.4. Analysis.
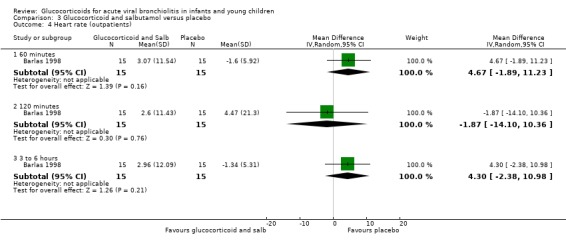
Comparison 3 Glucocorticoid and salbutamol versus placebo, Outcome 4 Heart rate (outpatients).
Other comparisons
These included glucocorticoid versus bronchodilator (epinephrine or salbutamol), glucocorticoid and bronchodilator (epinephrine or salbutamol) versus different bronchodilator (epinephrine or salbutamol), and direct comparisons between different types of glucocorticoid (prednisolone versus budesonide). All trials were performed in the outpatient setting, and all except one were small‐sized and had a high risk of bias.
Primary outcomes
The glucocorticoid versus epinephrine comparison included data from two trials (444 participants) for admissions by day 1, and one trial by day 7 (399 participants). Risk of bias was low for one trial and high for the other. There were no significant differences between groups at both time points (Analysis 4.1). Only one small high risk of bias trial included data on day 1 admissions for both glucocorticoid versus salbutamol (45 participants) and glucocorticoid and salbutamol versus epinephrine comparisons (30 participants), with no differences between arms (Analysis 5.1; Analysis 7.1). There were no admissions in another trial including the latter comparison, as well as glucocorticoid and epinephrine versus salbutamol (Analysis 6.1; Analysis 7.1).
4.1. Analysis.
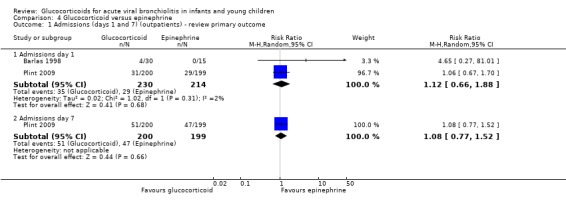
Comparison 4 Glucocorticoid versus epinephrine, Outcome 1 Admissions (days 1 and 7) (outpatients) ‐ review primary outcome.
5.1. Analysis.
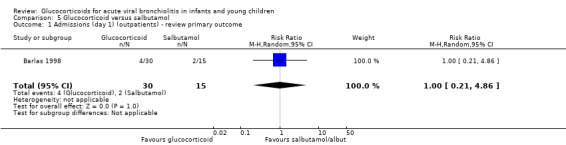
Comparison 5 Glucocorticoid versus salbutamol, Outcome 1 Admissions (day 1) (outpatients) ‐ review primary outcome.
7.1. Analysis.
Comparison 7 Glucocorticoid and salbutamol versus epinephrine, Outcome 1 Admissions (day 1) (outpatients) ‐ review primary outcome.
6.1. Analysis.
Comparison 6 Glucocorticoid and epinephrine versus salbutamol, Outcome 1 Admissions (day 1) (outpatients) ‐ review primary outcome.
Barlas 1998 multi‐arm trial also performed an unblinded comparison between systemic prednisolone and inhaled budesonide, with no statistically significant differences in admissions by day 1 (Analysis 8.1).
8.1. Analysis.
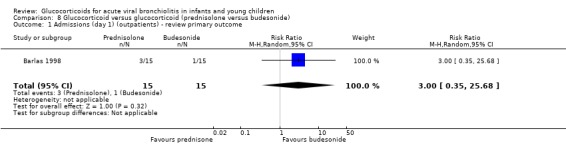
Comparison 8 Glucocorticoid versus glucocorticoid (prednisolone versus budesonide), Outcome 1 Admissions (day 1) (outpatients) ‐ review primary outcome.
Secondary outcomes
When compared to glucocorticoid at 60 minutes, epinephrine use was associated with lower clinical scores (SMD 0.31; 95% CI 0.12 to 0.50) and higher oxygen saturation (MD ‐0.99; 95% CI ‐1.46 to ‐0.52; units: %) (two trials, 442 participants), while heart rate was lower with glucocorticoids (MD ‐7.56 bpm; 95% CI ‐11.34 to ‐3.79) and there were no differences in respiratory rate (Analysis 4.2; Analysis 4.3; Analysis 4.4; Analysis 4.5). There were no differences in the single trial assessing clinical scores and heart rate at later time points.
4.2. Analysis.
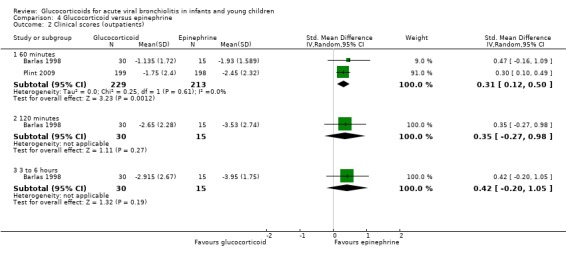
Comparison 4 Glucocorticoid versus epinephrine, Outcome 2 Clinical scores (outpatients).
4.3. Analysis.
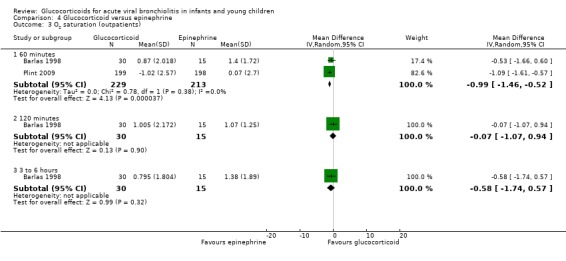
Comparison 4 Glucocorticoid versus epinephrine, Outcome 3 O2 saturation (outpatients).
4.4. Analysis.
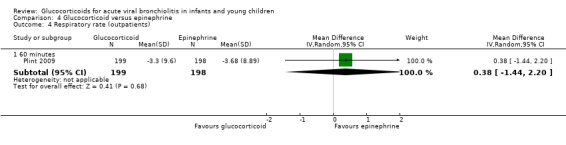
Comparison 4 Glucocorticoid versus epinephrine, Outcome 4 Respiratory rate (outpatients).
4.5. Analysis.
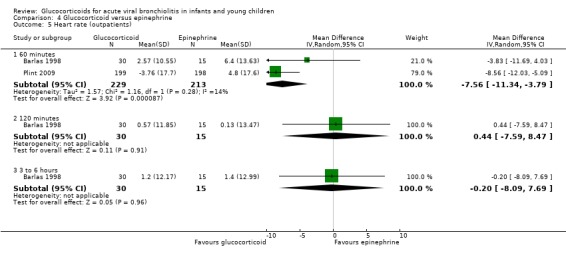
Comparison 4 Glucocorticoid versus epinephrine, Outcome 5 Heart rate (outpatients).
Salbutamol was also favoured when compared to glucocorticoids, in clinical scores at 60 minutes and three to six hours (SMD 0.65; 95% CI 0.01 to 1.28; and SMD 0.70; 95% CI 0.06 to 1.34, respectively), but not at 120 minutes (Analysis 5.2). Heart rate at 120 minutes was lower in glucocorticoid group (MD ‐7.53 bpm; 95% CI ‐14.28 to ‐0.78) and there were no differences in oxygen saturation at any time point (Analysis 5.3; Analysis 5.4).
5.2. Analysis.
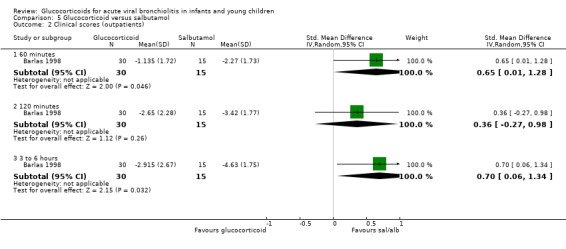
Comparison 5 Glucocorticoid versus salbutamol, Outcome 2 Clinical scores (outpatients).
5.3. Analysis.
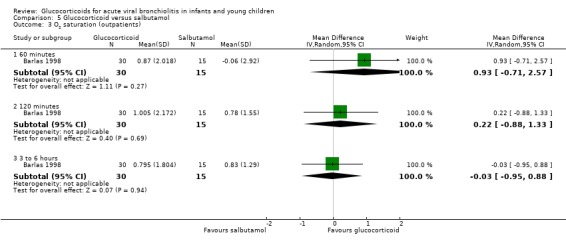
Comparison 5 Glucocorticoid versus salbutamol, Outcome 3 O2 saturation (outpatients).
5.4. Analysis.
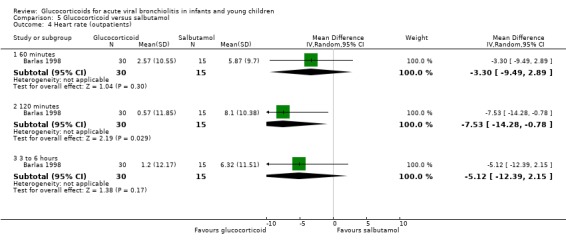
Comparison 5 Glucocorticoid versus salbutamol, Outcome 4 Heart rate (outpatients).
At 3 to 10 days, clinical scores and respiratory rate results favoured glucocorticoids and epinephrine as compared to salbutamol (SMD ‐1.22; 95% CI ‐1.98 to ‐0.46, and MD ‐13.70; 95% CI ‐20.56 to ‐6.84, respectively) (Analysis 6.2; Analysis 6.3; Analysis 6.4). There were no other differences at earlier time points and regarding heart rate.
6.2. Analysis.
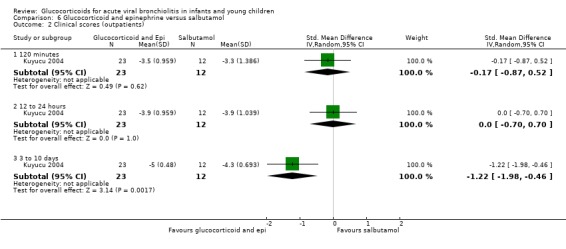
Comparison 6 Glucocorticoid and epinephrine versus salbutamol, Outcome 2 Clinical scores (outpatients).
6.3. Analysis.
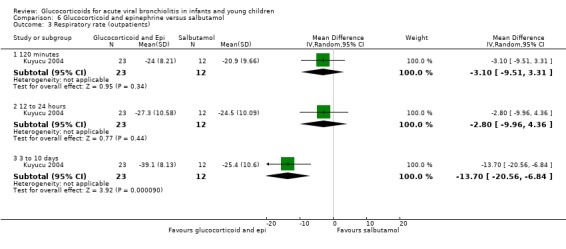
Comparison 6 Glucocorticoid and epinephrine versus salbutamol, Outcome 3 Respiratory rate (outpatients).
6.4. Analysis.
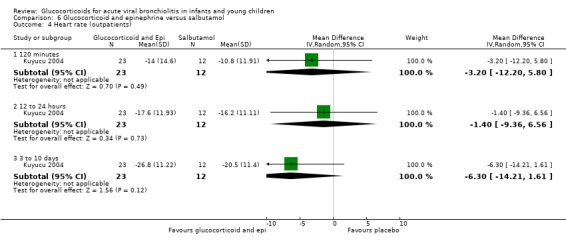
Comparison 6 Glucocorticoid and epinephrine versus salbutamol, Outcome 4 Heart rate (outpatients).
Oxygen saturation at 60 and 120 minutes was higher in the epinephrine group when compared to glucocorticoid and salbutamol (MD ‐1.54; 95% CI ‐2.85 to ‐0.23, and MD ‐1.27; 95% CI ‐2.41 to ‐0.13, respectively) (Analysis 7.2; Analysis 7.3; Analysis 7.4; Analysis 7.5). No other statistically significant differences were found in clinical scores, oxygen saturation or respiratory or heart rate at other time points.
7.2. Analysis.
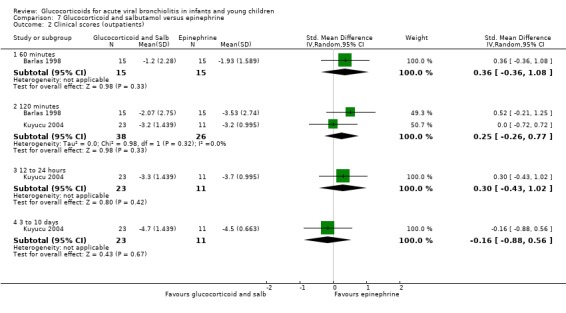
Comparison 7 Glucocorticoid and salbutamol versus epinephrine, Outcome 2 Clinical scores (outpatients).
7.3. Analysis.
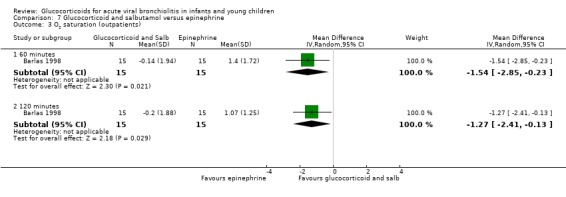
Comparison 7 Glucocorticoid and salbutamol versus epinephrine, Outcome 3 O2 saturation (outpatients).
7.4. Analysis.
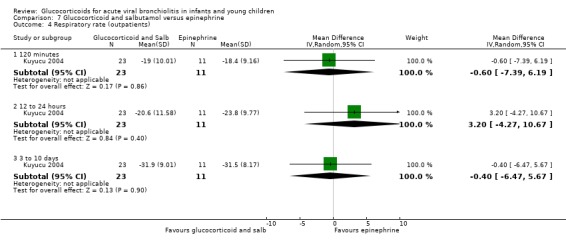
Comparison 7 Glucocorticoid and salbutamol versus epinephrine, Outcome 4 Respiratory rate (outpatients).
7.5. Analysis.
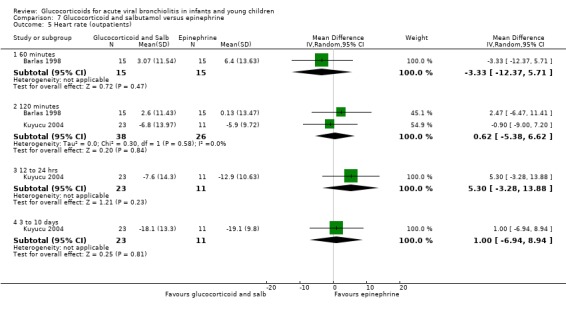
Comparison 7 Glucocorticoid and salbutamol versus epinephrine, Outcome 5 Heart rate (outpatients).
When comparing systemic prednisolone and inhaled budesonide, oxygen saturation results favoured budesonide at 60 minutes and 120 minutes (MD ‐1.46; 95% CI ‐2.74 to ‐0.18, and MD ‐1.73; 95% CI ‐3.06 to ‐0.40, respectively), and heart rate was lower with prednisolone at three to six hours (Analysis 8.3; Analysis 8.4). No differences were found in all other outcomes and time points (Analysis 8.2).
8.3. Analysis.
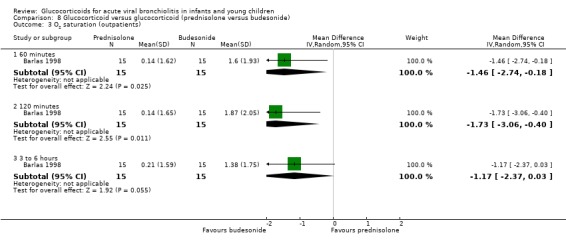
Comparison 8 Glucocorticoid versus glucocorticoid (prednisolone versus budesonide), Outcome 3 O2 saturation (outpatients).
8.4. Analysis.
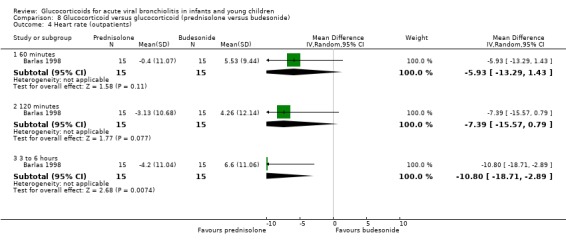
Comparison 8 Glucocorticoid versus glucocorticoid (prednisolone versus budesonide), Outcome 4 Heart rate (outpatients).
8.2. Analysis.
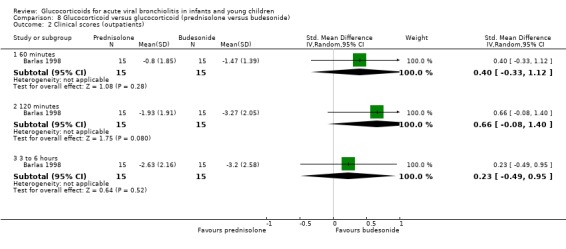
Comparison 8 Glucocorticoid versus glucocorticoid (prednisolone versus budesonide), Outcome 2 Clinical scores (outpatients).
Plint 2009 reported safety assessments comparing glucocorticoid and epinephrine (Table 8). Pallor was observed in 7.5% of participants in the glucocorticoid group, compared to 11.1% in the epinephrine group. There were no significant differences in vomiting, bleeding, hypertension, varicella and tremor between glucocorticoids and epinephrine. No other trial from any of the other comparisons reported adverse events data.
Discussion
Summary of main results
Results from this review do not suggest a clinically relevant stand‐alone effect of systemic or inhaled glucocorticoids in either outpatient and inpatient settings (Table 1). There were no statistically significant differences in outpatient admissions by days 1 and 7, and pooled RR estimates favouring glucocorticoids versus placebo were below commonly used thresholds for clinical relevance. Strength of evidence was moderate to high, indicating our confidence in these effect estimates. There were also no differences in secondary outcomes, particularly clinical scores, oxygen saturation and respiratory symptoms. For inpatient trials, precise and consistent results did not show differences in length of stay (LOS) as compared to placebo. The lower boundary of the pooled estimate confidence interval (CI) was about nine hours, likely excluding a clinically relevant benefit from glucocorticoids. While clinical score results were superior during the first day of treatment, no consistent differences were found at later time points or in any other secondary outcomes. Subgroup analyses according to age and respiratory syncytial virus (RSV) status did not suggest effect modification by these factors; heterogeneity did not allow adequate analysis of atopy and glucocorticoid type or dose.
Exploratory evidence suggests that combined glucocorticoids and bronchodilators may have clinically relevant benefits. A large factorial trial with low risk of bias found that high‐dose dexamethasone with epinephrine reduced admissions by day 7 when compared to placebo, in outpatients with moderately severe bronchiolitis (Table 2). The unadjusted risk ratio (RR) reduction estimate was 36%, and 11 children with bronchiolitis had to be treated to reduce one admission given the study's baseline risk. Clinical scores and symptoms results supported this benefit. However, these are the findings of a single study and should be interpreted cautiously. There were methodological issues with trial design and results may have arisen by chance. Further evidence regarding combined therapy is scarce and imprecise, and exploratory subgroup analysis was not conclusive as to an additive/synergistic effect of glucocorticoids combined with bronchodilators.
No relevant differences were found in short‐term general and intervention‐specific adverse effects for these comparisons. However, balancing harms and benefits of glucocorticoids alone or combined was hampered by the lack of long‐term safety data.
Overall completeness and applicability of evidence
The heterogeneous definition of bronchiolitis is often a motive for controversy when interpreting trial and review results (DiTraglia 2004; Weinberger 2003; Weinberger 2007). There is no international consensus due to variation in semantics and clinical findings (for example, in the UK, 'crackles' are often key to diagnosis, as opposed to 'wheeze' in North America) (Everard 2009). A first episode of wheezing may be a manifestation of wheezing phenotypes with heterogeneous biological, genetic, viral or environmental determinants, and distinct prognosis (Brand 2008; Martinez 2005; Sly 2008). However, research is still ongoing to identify simple, valid and universal discriminative and prognostic tools to prospectively distinguish between them (Brand 2008; Schultz 2010; Sly 2008). We used a pragmatic definition and focused on first time wheezing so results could be directly pertinent to infants with 'typical' viral bronchiolitis, as opposed to those with acute recurrent wheezing or asthma.
We found variability in both bronchiolitis severity and glucocorticoids schemes, but this did not affect the consistency of results. Baseline disease in outpatients was often moderate, but the use of different clinical criteria and scales limited the comparison between trials, particularly for inpatients. The wide range of control group admission rates and LOS can be partially explained by differing disease severity, but it also reflects variation in bronchiolitis management, for example, different admission/discharge criteria and standards of care (Babl 2008; Barben 2003; Brand 2000; Christakis 2005; Gonzalez 2010; Mallory 2003; Mansbach 2005). Our findings were consistent in trials performed worldwide, and results likely apply to settings with different resources and management strategies.
Most studies were restricted to healthy infants, often excluding children with chronic conditions and prematurity. Lack of evidence for this subset of patients is problematic, since many are particularly at risk of adverse outcomes (Damore 2008; Figueras‐Aloy 2008; Meissner 2003). Epidemiological studies have highlighted the short‐ and long‐term impact of RSV disease in prematurity (Figueras‐Aloy 2008; Simoes 2008), and underlying changes in respiratory pathophysiology may limit the external validity of our results in these populations.
Results from subgroup analyses did not identify any subset of participants with a different response to glucocorticoids. Older aged and atopic children are at higher risk of recurrent wheezing and asthma (Castro‐Rodriguez 2000), and both factors have been traditionally proposed as markers of underlying glucocorticoid‐responsive phenotypes in first‐time wheezers (Weinberger 2007). We found no conclusive evidence of such effect with age. We were unable to study atopy, but subgroup analyses from individual studies did not identify any significant differences. Specific viruses may also modulate response, as RSV and rhinovirus infections are associated with recurrent wheezing and the latter is a stronger predictor and possibly more responsive to glucocorticoids (Jackson 2008; Korppi 2007; Lehtinen 2007; Stein 1999). We found no differences according to RSV status, while other viral aetiologies were not reported. Accumulating evidence shows that glucocorticoids have reduced effectiveness in later acute recurrent wheezing (Bush 2009; Panickar 2009). Further, each of these factors per se has limited prognostic accuracy in defining stable wheezing phenotypes (Brand 2008; Simpson 2010; Sly 2008). Our results suggest that 'typical' viral bronchiolitis is not glucocorticoid‐responsive. Potential methodological limitations include the use of aggregated data and heterogeneity in definition, ascertainment and reporting of subgroups.
We found promising exploratory results from one large trial using combined dexamethasone with epinephrine for moderately ill outpatients. Although reliance on findings from single precise well‐conducted trials is often reasonable (Glasziou 2010), in this factorial trial the additive interaction between treatments was unanticipated, and this limits the interpretation of its results (McAlister 2003; Montgomery 2003). Our observational and exploratory subgroup analyses of protocolised bronchodilators may indirectly support an additive effect, but findings were not conclusive for both outpatients and inpatients. The latter are often a separate population due to differences in severity, duration of symptoms or non‐response to initial bronchodilators, and these may affect response to therapy. Replication is therefore needed to improve our confidence in the direction, precision and magnitude of the effect estimates for outpatients, and its applicability for inpatients.
Whether results from combination therapy can be generalisable to different glucocorticoid or bronchodilator schemes is also not known. Systemic dexamethasone is favoured in another common viral respiratory disorder, croup (Bjornson 2008). Its long half‐life and stronger potency may account for its effect, but underlying pathological changes are distinct between these two conditions. Plint 2009 used multiple high doses of dexamethasone. A previous dose‐finding trial suggested similar results with a single high dose, although there was no placebo comparator (Schuh 2008); the lowest efficacious dose remains unknown. The choice of bronchodilator is also undecided. A recently updated Cochrane review on epinephrine in bronchiolitis showed a reduction in first day outpatient admissions, as well as other short‐term severity outcomes (Hartling 2011a). This might explain part of the early benefit of combined therapy seen in Plint 2009. Further research is needed to clarify whether combined epinephrine is superior to combined salbutamol, particularly given the variation in bronchodilator choice in practice.
Evidence from basic and translational research may support a synergistic effect of combined therapy, but it is not clear how this reconciles with the limited effect of glucocorticoids alone. Inflammation pathways and mediators involved in bronchiolitis seem to be distinct from those in glucocorticoid‐sensitive asthma. Innate immunity, specific cytokine dysregulation patterns and neutrophilic inflammation may be relevant for some early wheezing phenotypes (Bont 2009; Halfhide 2008), which could explain the limited biological action of glucocorticoids alone (Buckingham 2002; Lehtinen 2007; Somers 2009). Paradoxically, clinical and biological synergism between glucocorticoids and bronchodilators has been a major topic in asthma treatment (Giembycz 2008). Two‐way molecular interactions exist, including beta2‐agonist‐stimulated glucocorticoid‐mediated gene transcription (Kaur 2008) and glucocorticoid‐induced increase in the transcription of the ß2‐receptor gene (Black 2009). Epinephrine’s α‐adrenergic vasoconstricting and oedema‐reducing activity could confer an additional short‐term benefit. Whether these mechanisms are involved in acute bronchiolitis therapy, and the role of specific types and doses of bronchodilators and glucocorticoids, is unknown.
These positive results should be balanced against incomplete data on harms. Safety concerns are expected when considering the widespread use of epinephrine and glucocorticoids in young children with viral wheezing, particularly with repeated high glucocorticoid doses (Bush 2009; Frey 2009). Current data from RCTs and observational studies in croup suggest a favourable short‐term safety profile from both dexamethasone and epinephrine (Bjornson 2008;Zhang 2005). Considering all trials, our results do not suggest any serious or frequent short‐term expected or unexpected harms from glucocorticoids in the absence of co‐morbidities. However, the power to detect important differences was limited due to the infrequent occurrence of events, and adverse event detection was heterogeneous. Glucocorticoids also raises long‐term safety issues. Their use in prematurity for neonatal respiratory distress has been associated with effects on adrenal function, cardiovascular responses, somatic and lung growth, and neurodevelopment (Doyle 2010; Karemaker 2008a; Karemaker 2008b; Onland 2008; Wilson‐Costello 2009). Evidence is scarce, however, regarding effects of short‐term use in otherwise healthy term infants, and none of these were studied in included trials. Further pharmacoepidemiologic data are needed to permit adequate short and long‐term risk‐benefit assessments.
Quality of the evidence
Two key factors affected the strength of evidence: potential risk of bias in the included studies, and sparsity of data for many of the outcomes and comparisons, with imprecise estimates and unknown consistency across studies.
A majority of trials had unclear risk of bias, usually due to incomplete or inadequate reporting, and many comparisons only included small trials at high risk of bias. Inadequate allocation concealment and blinding were likely to be relevant given the nature of interventions (for example, inhaled versus systemic administration) and outcome assessments (for example, physician‐based admissions or discharge decisions). Incomplete outcome data were often found, with losses of follow‐up in outpatient trials. However, for the main glucocorticoid versus placebo comparison, sensitivity analyses restricted to low risk of bias trials did not change the direction or magnitude of results for primary outcomes, highlighting their consistency.
Sparsity of data was a result of a large number of comparisons as well as variability in the choice of outcomes and timing of assessments. Within trials, this also led to frequent uncertainties regarding selective outcome reporting. The message around consistency and relevance of outcomes is not new to this field (Flores 1997; King 2004; Klassen 1996). The absence of standardised, validated and patient‐important outcome measures has been a serious threat to bronchiolitis trial validity. Our primary outcomes focused on hospital use, which has clear implications for patients, families and health services. However, there is no guidance supporting the choice of methodologically sound and patient‐important outcomes. Lack of reporting of admission and discharge criteria is also problematic given the wide variation in bronchiolitis management. Additionally, the choice of clinical scales was inconsistent. The Respiratory Distress Assessment Instrument (RDAI) was used in a considerable number of trials, but its clinimetric properties ‐ for example, responsiveness and interpretability ‐ are not well known, which limits the interpretation of findings. This was compounded by the absence of quality of life measures. Further work is needed to define a core set of clinically important efficacy and safety outcome measures and timing of assessments, for trials and systematic reviews in this field.
Potential biases in the review process
Some limitations have already been described, others should also be highlighted. We did not obtain further data from authors of included studies, which might have clarified 'Risk of bias' assessments and further added to reported trial characteristics and secondary outcome results. There is scarce guidance on how to investigate synergism/antagonism at a systematic review level, therefore our approach should be considered exploratory, including our use of factorial trial results. However, we performed sensitivity analyses of different analysis methods and these did not show a change in the direction of results. Our choice of outcome time intervals may have been a source of heterogeneity, although it was limited by the sparsity of reported data. Limitations of subgroup analyses are well known and have been addressed. Grading of evidence was limited by the lack of guidance regarding clinically relevant differences in studied outcomes.
Agreements and disagreements with other studies or reviews
Two previous non‐Cochrane systematic reviews assessed the use of glucocorticoids in acute bronchiolitis, one of which also performed meta‐analysis (Garrison 2000; King 2004). None of the reviews included data from the two recent large glucocorticoid outpatient trials. There was some discordance in inclusion criteria regarding population and interventions: Garrison 2000 only included inpatient trials and was restricted to systemic glucocorticoids, and no review excluded previous wheezing. The choice of primary outcomes and their definitions, timings and analysis also differed. While Garrison 2000 highlighted a statistically significant reduction in LOS for inpatients, this analysis used a modified outcome definition. When comparing similar analyses for this outcome, quantitative results were comparable between all reviews, including ours, and suggest no relevant benefit from glucocorticoids in inpatients. Outpatient descriptive and quantitative results from King 2004 also found no difference in admissions. No previous review assessed the hypothesis of synergism between glucocorticoids and bronchodilators at an analysis level, while subgroup analyses assessing possible dose‐response and effect modifiers like age and RSV status showed similar negative results.
Authors' conclusions
Implications for practice.
Current evidence does not support a clinically relevant effect of systemic or inhaled glucocorticoids on admissions or length of stay, when used alone in infants with bronchiolitis defined as a first episode of wheezing. Clinical score results suggest some short‐term benefit of glucocorticoids for inpatients, but no differences were found in other secondary outcomes. Absence of treatment effects was consistent throughout studies despite substantial heterogeneity regarding included populations, interventions and outcomes, and this finding is likely to be applicable in diverse settings.
Exploratory results from a single large trial suggest combined high‐dose systemic dexamethasone and epinephrine may reduce outpatient admissions in moderately severe bronchiolitis. These findings should be interpreted cautiously and may have arisen by chance. While no relevant differences were reported in short‐term adverse events, long‐term safety data were missing. Efficacy, harms and applicability of combined therapy need to be clarified further.
Implications for research.
A large randomised controlled trial is needed to replicate and complement findings from combination therapy with glucocorticoid and bronchodilator for outpatients. Additional aims could include assessing the minimum efficacious glucocorticoid dose and the most adequate co‐intervention. This strategy could also be tested in inpatient settings. Choice of comparators should take into account the wide variability in bronchodilator use, so that valid results may be more easily implemented. Further investigation of parent‐reported outcomes is needed, as well as data to assess the long‐term safety of this association. Future trials should use standardised sets of outcome measures in this field.
What's new
| Date | Event | Description |
|---|---|---|
| 21 January 2013 | New citation required but conclusions have not changed | Our conclusions remain unchanged |
| 21 January 2013 | New search has been performed | Searches conducted. No new trials were included in this update. We excluded 10 new potentially relevant publications after full‐text review (Bai 2010; Gerasymov 2010; Jartti 2011; Karaatmaca 2010; Lukkarinen 2011; Martini 2009; Principi 2011; Smart 2009; van Woensel 2011; Zhu 2009) |
History
Protocol first published: Issue 1, 2001 Review first published: Issue 3, 2004
| Date | Event | Description |
|---|---|---|
| 16 September 2010 | Amended | Corrected references and text in Results ‐ Effects of interventions ‐ Glucocorticoid and bronchodilator (epinephrine or salbutamol) versus placebo. |
| 1 May 2010 | New citation required and conclusions have changed | A new team of authors have updated this previously withdrawn review. Current evidence suggests combined glucocorticoids and epinephrine may be effective in reducing outpatient admissions in this patient group. |
| 25 November 2009 | New search has been performed | Searches conducted. Eleven new trials (Barlas 1998; Bentur 2005; Cade 2000; Corneli 2007; Gomez 2007; Kuyucu 2004; Mesquita 2009; Plint 2009; Richter 1998; Teeratakulpisarn 2007; Zhang 2003) have been included and 61 new trials have been excluded in this update. |
| 9 January 2008 | Amended | Converted to new review format. |
| 4 January 2007 | Feedback has been incorporated | Feedback added. |
| 26 May 2004 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
The review authors gratefully acknowledge the following individuals for their contributions to this project: Ms. Andrea Milne (data extraction), Ms. Annabritt Chisholm (article retrieval), Ms. Heather McPhee (data extraction), Ms. Nicola Hooton (protocol development, study selection), Dr. Özge Tunçalp (Turkish translation) and Dr. João Franco (Spanish translation and data extraction). We acknowledge Robert Platt and Juan Manuel Lozano, who authored the previous version of this review but were not involved in the current update. We would also like to thank the Acute Respiratory Infections Group editorial team, particularly Liz Dooley (Managing Editor) and Sarah Thorning (Trials Search Co‐ordinator) for their invaluable review and editing of the manuscript. We also wish to thank the following people for commenting on the 2010 updated review: Janet Wale, Hesham El‐Sayed, Teresa Neeman and Peter Morris.
RF was supported by the Programme for Advanced Medical Education, sponsored by Fundação Calouste Gulbenkian, Fundação Champalimaud, Ministério da Saúde e Fundação para a Ciência e Tecnologia, Portugal.
Appendices
Appendix 1. Search strategy: Cochrane Central Register of Controlled Trials ‐ Ovid version
1. exp BRONCHIOLITIS/ 2. (bronchiolitis or wheez*).mp. [mp = title, original title, abstract, MeSH headings, heading words, keyword] 3. exp Respiratory Syncytial Viruses/ or exp exp Respiratory Syncytial Virus Infections/ 4. Respiratory Syncytial Virus$.mp. 5. or/1‐4 6. exp Bronchodilator Agents/ 7. exp Adrenergic Agents/ 8. exp Glucocorticoids/ or exp Adrenal Cortex Hormones/ 9. (Glucocorticoid* or Corticoglucocorticoid*).mp. 10. exp Anti‐Inflammatory Agents/ 11. exp Drug Therapy, combination/ 12. exp Epinephrine/ 13. adrenal cortex hormone*.ti,ab. 14. (epinephrine or adrenalin*).mp. 15. albuterol.mp. 16. beclomet?asone.mp. 17. betamet?asone.mp. 18. budesonide.mp. 19. dexamet?asone.mp. 20. salbutamol.mp. 21. ipratropium.mp. 22. prednisolone.mp. 23. prednisone.mp. 24. methylprednisone.mp. 25. terbutaline.mp. 26. fluticasone.mp. 27. exp Orciprenaline/ or (orciprenaline or fenoterol).mp. 28. aminophylline.mp. 29. androstadienes.mp. 30. hydrocortisone.mp. 31. or/6‐30 32. 5 and 31 33. exp Infant/ 34. (Infant* or infancy or Newborn* or Baby* or Babies or Neonat* or Preterm* or Prematur* or Postmatur*).mp. 35. or/33‐34 36. 32 and 35
Appendix 2. Search strategy: EMBASE ‐ Ovid version
1. exp BRONCHIOLITIS/ 2. (bronchiolitis or wheez*).mp. 3. exp Respiratory Syncytial Pneumovirus/ 4. Respiratory Syncytial Virus$.mp. 5. or/1‐4 6. exp Bronchodilating Agents/ 7. exp Adrenergic Receptor Stimulating Agents/ 8. exp Glucocorticoid/ or exp corticoglucocorticoid/ 9. (glucocorticoid* or corticoglucocorticoid*).mp. 10. exp Anti‐Inflammatory Agent/ 11. exp Drug combination/ 12. exp Adrenalin/ 13. adrenal cortex hormone*.ti,ab. 14. (epinephrine or adrenalin*).mp. 15. albuterol.mp. 16. betamet?asone.mp. 17. beclomet?asone.mp. 18. budesonide.mp. 19. exp Dexamethasone/ or dexametha?one.mp. 20. salbutamol.mp. 21. ipratropium.mp. 22. exp Prednisolone/ or prednisolone.mp. 23. exp Prednisone/ or prednisone.mp. 24. methylprednisone.mp. 25. terbutaline.mp. 26. fluticasone.mp. 27. Orciprenaline/ or Fenoterol/ or (orciprenaline or fenoterol).mp. 28. aminophylline.mp. 29. androstadienes.mp. 30. exp hydrocortisone/ 31. hydrocortisone.mp. 32. or/6‐31 33. 5 and 32 34. exp clinical trial/ 35. randomi?ed.ti,ab. 36. placebo.ti,ab. 37. dt.fs. 38. randomly.ti,ab. 39. trial.ti,ab. 40. groups.ti,ab. 41. or/34‐40 42. animal/ 43. human/ 44. 42 not (42 and 43) 45. 41 not 44 46. 33 and 45 47. limit 46 to (child or preschool child <1 to 6 years>) 48. exp Infant/ 49. (Infant* or infancy or Newborn* or Baby* or Babies or Neonat* or Preterm* or Prematur* or Postmatur*).mp. 50. 48 or 49 51. 46 and 50 52. 47 or 51
Appendix 3. Search strategy: IRAN MedEx
(Bronchiolitis or bronquiolitis or broncho‐alveolites virales or bronchiolite*)
Appendix 4. Search strategy: LILACS BIREME/OPAS/OMS ‐ Latin American and Caribbean Center on Health Sciences Information
wheeze OR Sibilancias OR bronquiolitis OR bronchiolitis OR bronquiolite [Words] and infant OR pediatric OR newborn OR nacidos OR Lactentes OR lactantes OR pediátrica [Words]
Appendix 5. Search strategy: MEDLINE ‐ Ovid version
1. exp BRONCHIOLITIS/ 2. (bronchiolitis or wheez*).mp. 3. exp Respiratory Syncytial Viruses/ or exp Respiratory Syncytial Virus Infections/ 4. Respiratory Syncytial Virus$.mp. 5. or/1‐4 6. exp Bronchodilator Agents/ 7. exp Adrenergic Agents/ 8. exp Glucocorticoids/ or exp Adrenal Cortex Hormones/ 9. (Glucocorticoid* or Corticoglucocorticoid*).mp. 10. exp Anti‐Inflammatory Agents/ 11. exp Drug Therapy, combination/ 12. exp Epinephrine/ 13. (epinephrine or adrenalin*).mp. 14. albuterol.mp. 15. betamet?asone.mp. 16. beclomet?asone.mp. 17. budesonide.mp. 18. dexamet?asone.mp. 19. salbutamol.mp. 20. ipratropium.mp. 21. prednisolone.mp. 22. prednisone.mp. 23. methylprednisone.mp. 24. terbutaline.mp. 25. fluticasone.mp. 26. exp Orciprenaline/ or (orciprenaline or fenoterol).mp. 27. aminophylline.mp. 28. androstadienes.mp. 29. hydrocortisone.mp. 30. or/6‐29 31. 5 and 30 32. randomised controlled trial.pt. 33. clinical trial.pt. 34. randomi?ed.ti,ab. 35. placebo.ti,ab. 36. dt.fs. 37. randomly.ti,ab. 38. trial.ti,ab. 39. groups.ti,ab. 40. or/32‐39 41. animals/ 42. humans/ 43. 41 not (41 and 42) 44. 40 not 43 45. 44 and 31 46. exp Infant/ 47. (Infant* or infancy or Newborn* or Baby* or Babies or Neonat* or Preterm* or Prematur* or Postmatur*).mp. 48. or/46‐47 49. 45 and 48
Appendix 6. Scopus ‐ Elsevier B.V.
(((TITLE(bronchiolitis OR wheez*) AND TITLE‐ABS‐KEY(glucocorticoid* OR glucocorticoid* OR corticoglucocorticoid*))) AND KEY("epinephrine" OR "adrenaline" OR "albuterol" OR "corticoglucocorticoids" OR "hydrocortisone" OR "glucocorticoids" OR ("inhaled glucocorticoids") OR "salbutamol" OR "betamethasone" OR "beclomethasone" OR "dexamethasone" OR "glucocorticoid" OR ("inhaled budesonide") OR "glucocorticoids" OR "bronchodilator" OR ("glucocorticoid use") OR "prednisolone" OR "methylprednisone" OR ("oral prednisolone") OR "prednisone" OR "ipratropium" OR "terbutaline" OR "orciprenaline" OR "fenoterol" OR "aminophylline" OR "androstadienes" OR "hydrocortisone")) AND (TITLE‐ABS‐KEY("Clinical Trial" OR "Clinical Trials" OR "Randomised Controlled Trial*" OR "Random Allocation" OR "double‐blind method" OR "single‐blind method" OR placebos OR research design OR comparative study OR evaluation studies OR follow‐up studies OR prospective)) AND (infan* OR newborn* OR neonat* OR baby OR babies)
(((TITLE(bronchiolitis) AND TITLE‐ABS‐KEY(glucocorticoid* OR glucocorticoid*OR corticoglucocorticoid*))) AND KEY("epinephrine" OR "albuterol" OR "corticoglucocorticoids" OR "hydrocortisone" OR "glucocorticoids" OR ("inhaled glucocorticoids") OR "salbutamol" OR "dexamethasone" OR "glucocorticoid" OR ("inhaled budesonide") OR "glucocorticoids"OR "bronchodilator"OR ("glucocorticoid use") OR "prednisolone" OR ("oral prednisolone") OR "prednisone")) AND (TITLE‐ABS‐KEY("Clinical Trial" OR "Clinical Trials" OR "Randomised Controlled Trial*" OR "Random Allocation" OR "double‐blind method" OR "single‐blind method" OR placebosOR research design OR comparativestudy OR evaluationstudies OR follow‐up studies OR prospective))
Appendix 7. Search details 2013 update
Details of the MEDLINE, CENTRAL, EMBASE, SCOPUS and LILACS 2013 update searches.
We used the search strategy below to search CENTRAL and MEDLINE. To identify child studies the search strategy was combined with a filter based on the work of Boluyt (Boluyt 2008). The MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). The search strategy was adapted to search EMBASE , LILACS and Scopus (all listed below).
MEDLINE (Ovid)
1 exp Bronchiolitis/ 2 (bronchiolit* or wheez*).mp. 3 respiratory syncytial viruses/ or respiratory syncytial virus, human/ 4 Respiratory Syncytial Virus Infections/ 5 respiratory syncytial virus*.mp. or rsv.tw. 6 or/1‐5 7 exp Bronchodilator Agents/ 8 exp Adrenergic Agents/ 9 exp Glucocorticoids/ 10 exp Adrenal Cortex Hormones/ 11 (glucocorticoid* or corticoglucocorticoid*).mp. 12 exp Anti‐Inflammatory Agents/ 13 Drug Therapy, Combination/ 14 exp Epinephrine/ 15 (epinephrine or adrenalin*).mp. 16 albuterol.mp. 17 betamet?asone.mp. 18 beclomet?asone.mp. 19 budesonide.mp. 20 dexamet?asone.mp. 21 salbutamol.mp. 22 ipratropium.mp. 23 prednisolone.mp. 24 prednisone.mp. 25 methylprednisone.mp. 26 terbutaline.mp. 27 fluticasone.mp. 28 exp Metaproterenol/ 29 (orciprenaline or fenoterol or metaproterenol).mp. 30 aminophylline.mp. 31 (androstadiene or androstadienes).mp. 32 hydrocortisone.mp. 33 or/7‐32 34 6 and 33
Embase.com search strategy
#30 #22 AND #29 #29 #25 NOT #28 #28 #27 NOT #26 #27 'animal'/de OR 'nonhuman'/de OR 'animal experiment'/de #26 'animal'/de OR 'nonhuman'/de OR 'animal experiment'/de AND 'human'/exp #25 #23 OR #24 #24 random*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR placebo*:ab,ti OR allocat*:ab,ti OR (doubl* NEXT/1 blind*):ab,ti OR trial:ti #23 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp #22 #18 AND #21 #21 #19 OR #20 #20 infant*:ab,ti OR infancy:ab,ti OR newborn*:ab,ti OR baby*:ab,ti OR babies:ab,ti OR neonat*:ab,ti OR preterm*:ab,ti OR prematur*:ab,ti OR postmatur*:ab,ti OR child*:ab,ti OR schoolchild*:ab,ti OR preschool*:ab,ti OR kid:ab,ti OR kids:ab,ti OR toddler*:ab,ti OR adoles*:ab,ti OR teen*:ab,ti OR boy*:ab,ti OR girl*:ab,ti OR minor*:ab,ti OR pubert*:ab,ti OR pubescen*:ab,ti OR pediatric*:ab,ti OR paediatric*:ab,ti OR kindergar*:ab,ti OR highschool*:ab,ti OR ((nursery OR primary OR secondary OR elementary OR high) NEXT/1 school*):ab,ti AND [embase]/lim1476119 #19 'infant'/exp OR 'child'/exp OR 'adolescent'/exp OR 'puberty'/exp OR 'pediatrics'/exp OR 'kindergarten'/de OR 'nursery school'/de OR 'primary school'/de OR 'middle school'/de OR 'high school'/de #18 #6 AND #17 #17 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 #16 albuterol:ab,ti OR betamethasone:ab,ti OR betametasone:ab,ti OR beclometasone:ab,ti OR beclomethosone:ab,ti OR budesonide:ab,ti OR dexamethasone:ab,ti OR salbutamol:ab,ti OR ipratropium:ab,ti OR prednisolone:ab,ti OR prednisone:ab,ti OR methylprednisone:ab,ti OR terbutaline:ab,ti OR fluticasone:ab,ti OR orciprenaline:ab,ti OR fenoterol:ab,ti OR metaproterenol:ab,ti OR aminophylline:ab,ti OR androstadiene*:ab,ti OR hydrocortisone:ab,ti #15 adrenalin*:ab,ti OR epinephrine:ab,ti #14 'adrenalin'/de #13 'drug combination'/de #12 'antiinflammatory agent'/exp #11 'corticosteroid'/exp #10 glucocorticoid*:ab,ti OR corticoglucocorticoid*:ab,ti #9 'glucocorticoid'/exp #8 'adrenergic receptor stimulating agent'/exp #7 'bronchodilating agent'/exp #6 #1 OR #2 OR #3 OR #4 OR #5 #5 'respiratory syncytial virus':ab,ti OR 'respiratory syncytial viruses':ab,ti OR rsv:ab,ti #4 'respiratory syncytial virus infection'/de #3 'respiratory syncytial pneumovirus'/de #2 bronchiolit*:ab,ti OR wheez*:ab,ti #1 'bronchiolitis'/exp
Scopus (Elsevier) search strategy
(((TITLE‐ABS‐KEY(bronchiolitis OR wheez*) AND SUBJAREA(mult OR agri OR bioc OR immu OR neur OR phar OR mult OR medi OR nurs OR vete OR dent OR heal)) AND ((TITLE‐ABS‐KEY(glucocorticoid* OR corticoglucocorticoid* OR epinephrine OR adrenalin*) OR TITLE‐ABS‐KEY(albuterol OR betametasone OR betamethasone OR beclomethasone OR beclometasone OR budesonide OR dexamethasone OR dexamethasone) OR TITLE‐ABS‐KEY(salbutamol OR ipratropium OR prednisolone OR prednisone OR methylprednisone OR terbutaline OR fluticasone) OR TITLE‐ABS‐KEY (orciprenaline OR fenoterol OR metaproterenol OR aminophylline OR androstadiene* OR hydrocortisone)) AND SUBJAREA(mult OR agri OR bioc OR immu OR neur OR phar OR mult OR medi OR nurs OR vete OR dent OR heal))) AND (TITLE‐ABS‐KEY(infant* OR infancy OR newborn* OR baby* OR babies OR neonat* OR preterm* OR postmatur* OR child* OR toddler* OR preschool* OR pediatric* OR paediatric*) AND SUBJAREA(mult OR agri OR bioc OR immu OR neur OR phar OR mult OR medi OR nurs OR vete OR dent OR heal))) AND (TITLE‐ABS‐KEY("clinical trial" OR "clinical trials" OR random* OR placebo* OR "double‐blind" OR "single‐blind" OR "research design" OR "comparative study" OR "evaluation studies" OR "follow‐up studies" OR prospective) AND SUBJAREA(mult OR agri OR bioc OR immu OR neur OR phar OR mult OR medi OR nurs OR vete OR dent OR heal))
LILACS (BIREME VHL) search strategy
(MH:Bronchiolitis OR bronchiolit$ OR Bronquiolitis OR Bronquiolite OR MH:C08.127.446.135$ OR MH:C08.381.495.146.135$ OR C08.730.099.135$ OR MH:Bronchopneumonia OR bronchopneumon$ OR Bronconeumonía OR MH:"Respiratory Syncytial Virus Infections" OR "Infecciones por Virus Sincitial Respiratorio" OR "Infecções por Vírus Respiratório Sincicial" OR MH:C02.782.580.600.620.750 OR "respiratory syncytial virus" OR "respiratory syncytial viruses" OR rsv OR MH:"Respiratory Syncytial Viruses" OR "Virus Sincitiales Respiratorios" OR "Vírus Sinciciais Respiratórios" OR "Virus Sincitial Respiratorio" OR MH:B04.820.455.600.670.600.750 OR MH:B04.909.777.455.600.670.600.750 OR MH:"Respiratory Syncytial Virus, Human" OR "Virus Sincitial Respiratorio Humano" OR "Vírus Sincicial Respiratório Humano" OR MH:B04.820.455.600.670.600.750.730 OR MH:B04.909.777.455.600.670.600.750.730 OR Sibilancias OR wheez$ OR Sibiação) AND (MH:"adrenal cortex hormones" OR MH:D06.472.040$ OR Corticoesteroide$ OR Corticosteróide$ OR Corticoid$ OR corticosteroid$ OR MH:glucocorticoids OR MH:D06.472.040.543$ OR MH:D27.505.696.399.472.788$ OR glucocortic$ OR MH:steroids OR steroid$ OR Esteroide$ OR Esteróide$ OR MH:D04.808$ OR MH:epinephrine OR MH:D02.092.211.215.311.461$ OR MH:D02.092.311.461 OR Epinefrina OR adrenalin$ MH:dexamethsone OR dexamethason$ OR dexametason$ OR MH:prednisolone OR prednisol$ OR MH:methylprednisone OR methylprednison$ OR metilprednisol$ OR MH:betamethasone OR betamethason$ OR betametason$ OR MH:hydrocortisone OR hydrocortison$ OR hidrocortison$ OR albuterol OR budesonide OR salbutamol OR ipratropium OR terbutaline OR fluticasone OR MH:metaproterenol OR MH:fenoterol OR metaproterenol OR fenoterol OR orciprenaline OR aminophylline OR androstadiene$) > clinical_trials
Data and analyses
Comparison 1. Glucocorticoid versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Admissions (days 1 and 7) (outpatients) ‐ review primary outcome | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Admissions day 1 | 10 | 1762 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.78, 1.08] |
| 1.2 Admissions day 7 | 6 | 1530 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.06] |
| 2 Length of stay (inpatients) ‐ review primary outcome | 8 | 633 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.39, 0.04] |
| 3 Length of stay (outpatients) | 3 | 255 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.81, 1.01] |
| 4 Clinical scores (outpatients) | 11 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 60 minutes | 6 | 1006 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.16, 0.09] |
| 4.2 120 minutes | 5 | 214 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.55, 0.21] |
| 4.3 3 to 6 hours | 5 | 808 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.50, 0.21] |
| 4.4 12 to 24 hours | 2 | 69 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.51, 0.76] |
| 4.5 3 to 10 days | 5 | 224 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.61, 0.21] |
| 5 Clinical scores (inpatients) | 4 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 5.1 3 to 6 hours | 1 | 174 | Std. Mean Difference (Random, 95% CI) | ‐1.03 [‐1.87, ‐0.19] |
| 5.2 6 to 12 hours | 3 | 269 | Std. Mean Difference (Random, 95% CI) | ‐0.62 [‐1.00, ‐0.23] |
| 5.3 12 to 24 hours | 3 | 264 | Std. Mean Difference (Random, 95% CI) | ‐0.28 [‐0.66, 0.09] |
| 5.4 24 to 72 hours | 4 | 271 | Std. Mean Difference (Random, 95% CI) | ‐0.53 [‐1.14, 0.08] |
| 6 O2 saturation (outpatients) | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 60 minutes | 5 | 936 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.73, 0.19] |
| 6.2 120 minutes | 2 | 75 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.56, 1.37] |
| 6.3 3 to 6 hours | 5 | 808 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.84, ‐0.02] |
| 6.4 24 to 72 hours | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.01, 1.41] |
| 7 O2 saturation (inpatients) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 6 to 12 hours | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐0.7 [‐1.98, 0.58] |
| 7.2 12 to 24 hours | 2 | 116 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐2.04, 1.16] |
| 7.3 24 to 72 hours | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐0.77, 2.97] |
| 8 Respiratory rate (outpatients) | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 60 minutes | 3 | 861 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐1.51, 1.03] |
| 8.2 120 minutes | 2 | 69 | Mean Difference (IV, Random, 95% CI) | ‐1.95 [‐9.30, 5.39] |
| 8.3 3 to 6 hours | 3 | 733 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐3.07, 0.82] |
| 8.4 12 to 24 hours | 2 | 69 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐7.10, 7.40] |
| 8.5 3 to 10 days | 4 | 174 | Mean Difference (IV, Random, 95% CI) | ‐1.64 [‐7.89, 4.61] |
| 9 Respiratory rate (inpatients) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 6 to 12 hours | 1 | 66 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐11.45, 3.45] |
| 9.2 12 to 24 hours | 2 | 110 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐5.08, 2.64] |
| 9.3 24 to 72 hours | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐15.37, 11.57] |
| 10 Heart rate (outpatients) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 60 minutes | 5 | 936 | Mean Difference (IV, Random, 95% CI) | 0.46 [‐1.62, 2.55] |
| 10.2 120 minutes | 4 | 144 | Mean Difference (IV, Random, 95% CI) | ‐3.54 [‐8.83, 1.75] |
| 10.3 3 to 6 hours | 5 | 808 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐7.01, 5.71] |
| 10.4 12 to 24 hours | 2 | 69 | Mean Difference (IV, Random, 95% CI) | 1.85 [‐11.18, 14.88] |
| 10.5 3 to 10 days | 3 | 136 | Mean Difference (IV, Random, 95% CI) | 0.43 [‐8.32, 9.18] |
| 11 Heart rate (inpatients) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 12 to 24 hours | 1 | 49 | Mean Difference (IV, Random, 95% CI) | ‐9.0 [‐18.99, 0.99] |
| 12 Hospital readmissions (inpatients) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 2 to 10 days | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 3.66 [0.43, 31.03] |
| 12.2 10 to 30 days | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.11, 1.53] |
| 13 Return healthcare visits (inpatients) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 2 to 10 days | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.86, 1.42] |
| 13.2 10 to 30 days | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.30, 4.96] |
| 14 Return healthcare visits (outpatients) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 10 to 30 days | 3 | 863 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.80, 1.35] |
| 15 Admissions at day 1 (outpatients) ‐ subgroup analysis protocolised use of bronchodilator | 10 | 1762 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.78, 1.08] |
| 15.1 Protocolised use of bronchodilator | 7 | 717 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.56, 1.29] |
| 15.2 No protocolised use of bronchodilator | 3 | 1045 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.79, 1.13] |
| 16 Admissions within 7 days (outpatients) ‐ subgroup analysis protocolised use of bronchodilator | 6 | 1530 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.06] |
| 16.1 Protocolised use of bronchodilator | 4 | 581 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.44, 1.05] |
| 16.2 No protocolised use of bronchodilator | 2 | 949 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.82, 1.11] |
| 17 Admissions at day 1 (outpatients) ‐ subgroup analysis age | 10 | 1762 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.78, 1.08] |
| 17.1 All < 12 months | 3 | 1397 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.79, 1.10] |
| 17.2 Including > 12 months | 7 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.51, 1.49] |
| 18 Admissions within 7 days (outpatients) ‐ subgroup analysis age | 6 | 1530 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.06] |
| 18.1 All < 12 months | 3 | 1346 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.80, 1.06] |
| 18.2 Including > 12 months | 3 | 184 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.25, 1.83] |
| 19 Length of stay (inpatients) ‐ subgroup analysis protocolised use of bronchodilator | 8 | 633 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.39, 0.04] |
| 19.1 Protocolised use of bronchodilator | 4 | 206 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.23, ‐0.00] |
| 19.2 No protocolised use of bronchodilator | 4 | 427 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.83, 0.20] |
| 20 Length of stay (inpatients) ‐ subgroup analysis age | 8 | 633 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.39, 0.04] |
| 20.1 All < 12 months | 4 | 314 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.65, 0.45] |
| 20.2 Including > 12 months | 4 | 319 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.53, 0.12] |
| 21 Length of stay (inpatients) ‐ subgroup analysis RSV status | 8 | 633 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.39, 0.04] |
| 21.1 All RSV‐positive | 3 | 251 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.63, 0.45] |
| 21.2 Not all RSV‐positive, or NR | 5 | 382 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.57, 0.17] |
| 22 Admissions (days 1 and 7) (outpatients) ‐ sensitivity analysis with only low overall RoB | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 22.1 Admissions day 1 | 3 | 1397 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.79, 1.10] |
| 22.2 Admissions day 7 | 3 | 1346 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.80, 1.06] |
| 23 Length of stay (inpatients) ‐ sensitivity analysis with only low overall RoB | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.46, 1.21] |
1.23. Analysis.
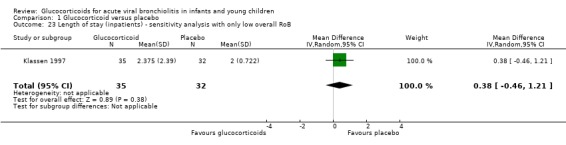
Comparison 1 Glucocorticoid versus placebo, Outcome 23 Length of stay (inpatients) ‐ sensitivity analysis with only low overall RoB.
Comparison 2. Glucocorticoid and epinephrine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Admissions (days 1 and 7) (outpatients) ‐ review primary outcome | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Admissions day 1 | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.40, 1.05] |
| 1.2 Admissions day 7 | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.44, 0.95] |
| 2 Clinical scores (outpatients) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 60 minutes | 1 | 399 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.54, ‐0.14] |
| 3 O2 saturation (outpatients) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 60 minutes | 1 | 399 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.53, 0.61] |
| 4 Respiratory rate (outpatients) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 60 minutes | 1 | 399 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐3.06, 0.74] |
| 5 Heart rate (outpatients) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 60 minutes | 1 | 399 | Mean Difference (IV, Random, 95% CI) | 8.44 [4.85, 12.03] |
| 6 Return healthcare visits (outpatients) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 10 to 30 days | 1 | 399 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.89, 1.38] |
Comparison 3. Glucocorticoid and salbutamol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Admissions (day 1) (outpatients) ‐ review primary outcome | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.13, 3.44] |
| 2 Clinical scores (outpatients) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 60 minutes | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.02, 0.42] |
| 2.2 120 minutes | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.94, 0.50] |
| 2.3 3 to 6 hours | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐1.18, 0.27] |
| 3 O2 saturation (outpatients) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 60 minutes | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.75, 1.07] |
| 3.2 120 minutes | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐2.04, 0.70] |
| 3.3 3 to 6 hours | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐1.08 [‐2.43, 0.27] |
| 4 Heart rate (outpatients) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 60 minutes | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 4.67 [‐1.89, 11.23] |
| 4.2 120 minutes | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐1.87 [‐14.10, 10.36] |
| 4.3 3 to 6 hours | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 4.3 [‐2.38, 10.98] |
Comparison 4. Glucocorticoid versus epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Admissions (days 1 and 7) (outpatients) ‐ review primary outcome | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Admissions day 1 | 2 | 444 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.66, 1.88] |
| 1.2 Admissions day 7 | 1 | 399 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.77, 1.52] |
| 2 Clinical scores (outpatients) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 60 minutes | 2 | 442 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [0.12, 0.50] |
| 2.2 120 minutes | 1 | 45 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.27, 0.98] |
| 2.3 3 to 6 hours | 1 | 45 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.20, 1.05] |
| 3 O2 saturation (outpatients) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 60 minutes | 2 | 442 | Mean Difference (IV, Random, 95% CI) | ‐0.99 [‐1.46, ‐0.52] |
| 3.2 120 minutes | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐1.07, 0.94] |
| 3.3 3 to 6 hours | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.74, 0.57] |
| 4 Respiratory rate (outpatients) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 60 minutes | 1 | 397 | Mean Difference (IV, Random, 95% CI) | 0.38 [‐1.44, 2.20] |
| 5 Heart rate (outpatients) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 60 minutes | 2 | 442 | Mean Difference (IV, Random, 95% CI) | ‐7.56 [‐11.34, ‐3.79] |
| 5.2 120 minutes | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐7.59, 8.47] |
| 5.3 3 to 6 hours | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐8.09, 7.69] |
Comparison 5. Glucocorticoid versus salbutamol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Admissions (day 1) (outpatients) ‐ review primary outcome | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.21, 4.86] |
| 2 Clinical scores (outpatients) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 60 minutes | 1 | 45 | Std. Mean Difference (IV, Random, 95% CI) | 0.65 [0.01, 1.28] |
| 2.2 120 minutes | 1 | 45 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.27, 0.98] |
| 2.3 3 to 6 hours | 1 | 45 | Std. Mean Difference (IV, Random, 95% CI) | 0.70 [0.06, 1.34] |
| 3 O2 saturation (outpatients) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 60 minutes | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.93 [‐0.71, 2.57] |
| 3.2 120 minutes | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.88, 1.33] |
| 3.3 3 to 6 hours | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.95, 0.88] |
| 4 Heart rate (outpatients) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 60 minutes | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐3.30 [‐9.49, 2.89] |
| 4.2 120 minutes | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐7.53 [‐14.28, ‐0.78] |
| 4.3 3 to 6 hours | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐5.12 [‐12.39, 2.15] |
Comparison 6. Glucocorticoid and epinephrine versus salbutamol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Admissions (day 1) (outpatients) ‐ review primary outcome | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Clinical scores (outpatients) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 120 minutes | 1 | 35 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.87, 0.52] |
| 2.2 12 to 24 hours | 1 | 35 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.70, 0.70] |
| 2.3 3 to 10 days | 1 | 35 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐1.98, ‐0.46] |
| 3 Respiratory rate (outpatients) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 120 minutes | 1 | 35 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐9.51, 3.31] |
| 3.2 12 to 24 hours | 1 | 35 | Mean Difference (IV, Random, 95% CI) | ‐2.80 [‐9.96, 4.36] |
| 3.3 3 to 10 days | 1 | 35 | Mean Difference (IV, Random, 95% CI) | ‐13.70 [‐20.56, ‐6.84] |
| 4 Heart rate (outpatients) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 120 minutes | 1 | 35 | Mean Difference (IV, Random, 95% CI) | ‐3.20 [‐12.20, 5.80] |
| 4.2 12 to 24 hours | 1 | 35 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐9.36, 6.56] |
| 4.3 3 to 10 days | 1 | 35 | Mean Difference (IV, Random, 95% CI) | ‐6.30 [‐14.21, 1.61] |
Comparison 7. Glucocorticoid and salbutamol versus epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Admissions (day 1) (outpatients) ‐ review primary outcome | 2 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [0.26, 96.13] |
| 2 Clinical scores (outpatients) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 60 minutes | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.36, 1.08] |
| 2.2 120 minutes | 2 | 64 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.26, 0.77] |
| 2.3 12 to 24 hours | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.43, 1.02] |
| 2.4 3 to 10 days | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.88, 0.56] |
| 3 O2 saturation (outpatients) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 60 minutes | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐1.54 [‐2.85, ‐0.23] |
| 3.2 120 minutes | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐1.27 [‐2.41, ‐0.13] |
| 4 Respiratory rate (outpatients) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 120 minutes | 1 | 34 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐7.39, 6.19] |
| 4.2 12 to 24 hours | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 3.20 [‐4.27, 10.67] |
| 4.3 3 to 10 days | 1 | 34 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐6.47, 5.67] |
| 5 Heart rate (outpatients) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 60 minutes | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐3.33 [‐12.37, 5.71] |
| 5.2 120 minutes | 2 | 64 | Mean Difference (IV, Random, 95% CI) | 0.62 [‐5.38, 6.62] |
| 5.3 12 to 24 hrs | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 5.30 [‐3.28, 13.88] |
| 5.4 3 to 10 days | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐6.94, 8.94] |
Comparison 8. Glucocorticoid versus glucocorticoid (prednisolone versus budesonide).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Admissions (day 1) (outpatients) ‐ review primary outcome | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.35, 25.68] |
| 2 Clinical scores (outpatients) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 60 minutes | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.33, 1.12] |
| 2.2 120 minutes | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [‐0.08, 1.40] |
| 2.3 3 to 6 hours | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.49, 0.95] |
| 3 O2 saturation (outpatients) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 60 minutes | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐1.46 [‐2.74, ‐0.18] |
| 3.2 120 minutes | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐3.06, ‐0.40] |
| 3.3 3 to 6 hours | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐2.37, 0.03] |
| 4 Heart rate (outpatients) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 60 minutes | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐5.93 [‐13.29, 1.43] |
| 4.2 120 minutes | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐7.39 [‐15.57, 0.79] |
| 4.3 3 to 6 hours | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐10.8 [‐18.71, ‐2.89] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barlas 1998.
| Methods | Parallel design, multi‐arm (6) Single‐centre, conducted in Turkey | |
| Participants | Outpatients (emergency department/outpatient clinic) Inclusion/exclusion criteria Inclusion criteria: age < 24 months; 1st episode of wheezing; clinical score between 4 to 10 (mild‐moderate) Exclusion criteria: patients with history of premature heart disease, chronic heart and lung problems, prior diagnosis of bronchial asthma, used bronchodilators and anti‐inflammatory medications Participant characteristics (all groups) Sample size: randomised (N): 90, analysed ‐ all outcomes (N): 90 (unclear what type of analysis was performed regarding ITT and missing data) Age, mean ± SD: 8.52 ± 0.59 Males, N (%): 50 (56) RSV status: 19/57 positive Atopic status: 4/86 (family) |
|
| Interventions |
GROUP 1
Drug name: placebo ‐ mist tent
Dose: NR
Mode of administration: nebulised
Timing/duration: NR GROUP 2 Drug name: albuterol Dose: 0.15 mg/kg Mode of administration: nebulised Timing/duration: every hour during the first 4h GROUP 3 (with glucocorticoid) Drug name: prednisolone Dose: 2 mg/kg Mode of administration: IV Timing/duration: single dose GROUP 4 (with glucocorticoid) Drug name: albuterol + prednisolone Dose: 0.15 mg/kg (alb) + 2 mg/kg (pre) Mode of administration: nebulised + IV Timing/duration: single dose for both interventions GROUP 5 Drug name: racaemic adrenaline (epinephrine) Dose: 0.1 mL/kg Mode of administration: nebulised Timing/duration: every 2h during the first 4h GROUP 6 (with glucocorticoid) Drug name: budesonide Dose: 0.5 mg Mode of administration: nebulised Timing/duration: single dose Additional co‐interventions for all groups: NR Protocolised use of bronchodilators with glucocorticoids: yes (Group 4 ‐ salbutamol) |
|
| Outcomes |
Primary outcome/outcome used to calculate sample size
NR Secondary outcomes Hospital admission by day 1; SaO2*; heart rate*; clinical scale*: developed for this trial ‐ 15‐point score, based on respiratory rate, wheezing, retractions, nostril movement and general patient condition; length of observation period; improvement with initial therapy; additional therapy *time points: baseline, 60, 120 minutes, 4 hours |
|
| Funding | NR | |
| Notes | Language of publication: Turkish Study did not report any study‐level subgroup analyses Results from groups 3 and 6 were combined for some analyses This study contributed to the following comparisons in this review: steroid versus placebo (with 2 comparisons: steroid versus placebo ‐ 'Barlas 1998 (G versus P)'; and steroid + salbutamol versus salbutamol ‐ 'Barlas 1998 (G + S versus S)'); steroid versus epinephrine; steroid versus salbutamol; prednisolone versus budesonide; steroid + salbutamol versus. Placebo; steroid + salbutamol versus epinephrine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors mention that the patients were assigned to groups randomly |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Health care use ((re)admissions, LOS, return visits) | High risk | Different routes of administration for different interventions (e.g. nebulised versus IV), without blinding; no information provided regarding blinding of interventions with the same route of administration. Outcome likely to be influenced by lack of blinding |
| Blinding (performance bias and detection bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | High risk | Different routes of administration for different interventions (e.g. nebulised versus IV), without blinding; no information provided regarding blinding of interventions with the same route of administration. Outcome likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Health care use ((re)admissions, LOS, return visits) | Low risk | No missing outcome data reported |
| Incomplete outcome data (attrition bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | No missing outcome data reported |
| Selective reporting (reporting bias) | Low risk | Published report includes all pre‐specified and expected outcomes; the study protocol was not available |
| Other bias | Unclear risk | No information provided |
| Overall risk of bias | High risk | |
Barlas 1998 (G vs P).
| Methods | (see Barlas 1998) | |
| Participants | (see Barlas 1998) | |
| Interventions | (see Barlas 1998) This glucocorticoid versus placebo comparison includes pooled data from Groups 3 and 6 (prednisolone and budesonide) versus Group 1 (placebo) |
|
| Outcomes | (see Barlas 1998) | |
| Funding | (see Barlas 1998) | |
| Notes | (see Barlas 1998) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors mention that the patients were assigned to groups randomly |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Health care use ((re)admissions, LOS, return visits) | High risk | Different routes of administration for different interventions (e.g. nebulised versus IV), without blinding; no information provided regarding blinding of interventions with the same route of administration. Outcome likely to be influenced by lack of blinding |
| Blinding (performance bias and detection bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | High risk | Different routes of administration for different interventions (e.g. nebulised versus IV), without blinding; no information provided regarding blinding of interventions with the same route of administration. Outcome likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Health care use ((re)admissions, LOS, return visits) | Low risk | No missing outcome data reported |
| Incomplete outcome data (attrition bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | No missing outcome data reported |
| Selective reporting (reporting bias) | Low risk | Published report includes all pre‐specified and expected outcomes; the study protocol was not available |
| Other bias | Unclear risk | No information provided |
| Overall risk of bias | High risk | |
Barlas 1998 (G+S vs S).
| Methods | (see Barlas 1998) | |
| Participants | (see Barlas 1998) | |
| Interventions | (see Barlas 1998) This glucocorticoid + salbutamol versus salbutamol comparison includes data from Group 4 (prednisolone and albuterol) versus Group 2 (albuterol) |
|
| Outcomes | (see Barlas 1998) | |
| Funding | (see Barlas 1998) | |
| Notes | (see Barlas 1998) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors mention that the patients were assigned to groups randomly |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Health care use ((re)admissions, LOS, return visits) | High risk | Different routes of administration for different interventions (e.g. nebulised versus IV), without blinding; no information provided regarding blinding of interventions with the same route of administration. Outcome likely to be influenced by lack of blinding |
| Blinding (performance bias and detection bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | High risk | Different routes of administration for different interventions (e.g. nebulised versus IV), without blinding; no information provided regarding blinding of interventions with the same route of administration. Outcome likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) Health care use ((re)admissions, LOS, return visits) | Low risk | No missing outcome data reported |
| Incomplete outcome data (attrition bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | No missing outcome data reported |
| Selective reporting (reporting bias) | Low risk | Published report includes all pre‐specified and expected outcomes; the study protocol was not available |
| Other bias | Unclear risk | No information provided |
| Overall risk of bias | High risk | > 1 domain as high risk of bias |
Bentur 2005.
| Methods | Parallel design, 2‐arm Centres: NR, conducted in Israel |
|
| Participants | Inpatients Inclusion/exclusion criteria Inclusion criteria: age 3 to 12 months; 1st episode wheezing/dyspnoea; RSV present; parental consent Exclusion criteria: previous therapy with systemic glucocorticoids; inhaled β2‐agonists prior to admission; other chronic diseases Participant characteristics All groups Sample size: randomised (N): NR, analysed ‐ trial/review primary outcomes (N): 61 (unclear what type of analysis was performed regarding ITT and missing data) GROUP 1 Sample size: randomised (N): NR, analysed ‐ trial/review primary outcomes (N): 29 Age, mean ± SD: 3.3 ± 2.5 Males, N (%): 14 (48.3) RSV status: all positive GROUP 2 Sample size: randomised (N): NR, analysed ‐ trial/review primary outcomes (N): 32 Age, mean ± SD: 3.8 ± 2.0 Males, N (%): 14 (43.8) RSV status: all positive Atopic status: NR |
|
| Interventions |
GROUP 1 (with glucocorticoid)
Drug name: dexamethasone + epinephrine
Dose: 0.25 mg (dex) + 1 mL (epi)
Mode of administration: nebulised in 5 L/minute pre‐specified 100% O2
Timing/duration: every 6h until discharge GROUP 2 Drug name: placebo ‐ 0.9% saline + epinephrine Dose: 0.5 mL (0.9% sal) + 1 mL (epi) Mode of administration: nebulised in 5 L/minute pre‐specified 100% O2 Timing/duration: every 6h until discharge Additional co‐interventions for all groups: O2 therapy if SaO2 < 92%; IV fluids if respiratory rate > 60 bpm Protocolised use of bronchodilators with glucocorticoids: yes (epinephrine) |
|
| Outcomes |
Primary outcome NR Outcome used to calculate sample size Clinical scale developed for this trial ‐ 10‐point score, based on respiratory rate, wheezing, retraction, general condition, oxygen saturation Secondary outcomes Length of stay (and time‐to‐discharge analysis); SaO2*; respiratory rate*; heart rate*; duration of O2 and IV fluids; clinical status#; hospital re‐admissions#; wheezing exacerbations# *time points: baseline, every 8 hours #time points: 1 week, 1 mo., 3 mo. post discharge |
|
| Funding | NR | |
| Notes | Study reported stratified results for premature and term infants; no specific interaction term This study contributed to the following comparisons in this review: steroid versus placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was in blocks of 10 (five saline/five dexamethasone)." Sequence generation probably adequate |
| Allocation concealment (selection bias) | Low risk | "The hospital pharmacist prepared the treatment and placebo solutions, both of which were supplied in identical containers and were indistinguishable to the researchers." Allocation concealment probably adequate |
| Blinding (performance bias and detection bias) Health care use ((re)admissions, LOS, return visits) | Low risk | Double‐blind; "treatment and placebo solutions (...) were supplied in identical containers and were indistinguishable to the researchers." |
| Blinding (performance bias and detection bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | Double‐blind; "treatment and placebo solutions (...) were supplied in identical containers and were indistinguishable to the researchers." |
| Blinding (performance bias and detection bias) Other outcomes (adverse events, others) | Low risk | Double‐blind; "treatment and placebo solutions (...) were supplied in identical containers and were indistinguishable to the researchers." |
| Incomplete outcome data (attrition bias) Health care use ((re)admissions, LOS, return visits) | Low risk | No missing outcome data reported |
| Incomplete outcome data (attrition bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | No missing outcome data reported |
| Incomplete outcome data (attrition bias) Other outcomes (adverse events, others) | Low risk | No missing outcome data reported |
| Selective reporting (reporting bias) | High risk | Some outcomes specified in methods not reported in results; LOS not prespecified |
| Other bias | Low risk | No significant baseline imbalances; no other sources of bias |
| Overall risk of bias | High risk | > 1 domain as high risk of bias |
Berger 1998.
| Methods | Parallel design, 2‐arm Single‐centre, conducted in Israel (affiliations: Shaare Zedek Medical Center, Jerusalem) |
|
| Participants | Outpatients (emergency department) Inclusion/exclusion criteria Inclusion criteria: age ≤ 18 months; 1st episode of wheezing associated with low‐grade fever, rhinitis, tachypnoea and increased respiratory effort; otherwise healthy infant Exclusion criteria: chronic cardiopulmonary disease; asthma; proven or suspected acute bacterial infection; previous therapy with glucocorticoids; symptoms > 7d; fever > 38.5°C; severe bronchiolitis (clinical score > 7) Participant characteristics All groups Sample size: randomised (N): 42, analysed ‐ trial/review primary outcomes (N): 38 (per protocol analysis was used) GROUP 1 Sample size: randomised (N): NR, analysed ‐ trial/review primary outcomes (N): 20 Age, mean ± SD: 5.2 ± 0.7 Males, N (%): NR RSV status: 50% positive Atopic status: 1/20 (infant), 3/20 (family) GROUP 2 Sample size: randomised (N): NR, analysed ‐ trial/review primary outcomes (N): 18 Age, mean ± SD: 4.8 ± 0.9 Males, N (%): NR RSV status: 50% positive Atopic status: 0 (infant), 4/20 (family) |
|
| Interventions |
GROUP 1 (with glucocorticoid)
Drug name: prednisone
Dose: 1 mg/kg
Mode of administration: oral
Timing/duration: twice daily, 3 days GROUP 2 Drug name: placebo (NR) Dose: 1 mg/kg Mode of administration: oral Timing/duration: twice daily, 3 days Additional co‐interventions for all groups: inhaled albuterol solution 0.03 mL/kg/dose (0.15 mg/kg/dose) every 4 to 6 hours; O2 and hydration as needed Protocolised use of bronchodilators with glucocorticoids: yes (salbutamol) |
|
| Outcomes |
Primary outcome
NR Outcome used to calculate sample size Clinical scale developed for this trial ‐ 9‐point score, based on respiratory rate, wheezing, accessory muscle use Secondary outcomes Initial hospital admission (usually within 4 h); SaO2*; respiratory rate*; well‐being#; return healthcare visits#; medications#; recurrent symptoms (by 2 years) *time points: baseline, 3 days #time points: 7 days |
|
| Funding | NR | |
| Notes | Study did not report any study‐level subgroup analyses This study contributed to the following comparisons in this review: steroid versus placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization was made according to a standardized statistical method" |
| Allocation concealment (selection bias) | Low risk | "Upon enrolment, each patient was randomly assigned by a research pharmacist"; "neither the investigators nor the families were aware of treatment assignments." |
| Blinding (performance bias and detection bias) Health care use ((re)admissions, LOS, return visits) | Low risk | "The solutions provided by the pharmacy appeared identical, and neither the investigators nor the families were aware of treatment assignments." "The examiner was blind to what treatment the patient had received." |
| Blinding (performance bias and detection bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | "The solutions provided by the pharmacy appeared identical, and neither the investigators nor the families were aware of treatment assignments." "The examiner was blind to what treatment the patient had received." |
| Blinding (performance bias and detection bias) Patient/parent‐reported outcomes (symptoms, QoL) | Low risk | "The solutions provided by the pharmacy appeared identical, and neither the investigators nor the families were aware of treatment assignments." "The examiner was blind to what treatment the patient had received." |
| Incomplete outcome data (attrition bias) Health care use ((re)admissions, LOS, return visits) | Unclear risk | Number completing treatment specified for each group; total of 4 drop‐outs, unclear what were the specific motives and to which arm they were assigned to |
| Incomplete outcome data (attrition bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Unclear risk | Number completing treatment specified for each group; total of 4 drop‐outs, unclear what were the specific motives and to which arm they were assigned to |
| Incomplete outcome data (attrition bias) Patient/parent‐reported outcomes (symptoms, QoL) | Unclear risk | Number completing treatment specified for each group; total of 4 drop‐outs, unclear what were the specific motives and to which arm they were assigned to |
| Selective reporting (reporting bias) | Low risk | Published report includes all pre‐specified and expected outcomes; the study protocol was not available |
| Other bias | Low risk | No significant baseline imbalances; no other sources of bias |
| Overall risk of bias | Unclear risk | > 1 domain as unclear risk of bias |
Cade 2000.
| Methods | Parallel design, 2‐arm Multi‐centre (5), conducted in the UK (West Yorkshire hospitals) |
|
| Participants | Inpatients Inclusion/exclusion criteria Inclusion criteria: age < 12 mo.; confirmed RSV; informed consent; randomised within 12 hours admission Exclusion criteria: history of hospitalisation with respiratory tract illness; chronic respiratory illness; congenital heart disease; prematurity; pre‐existing immunodeficiencies; recent exposure to varicella or tuberculosis; prolonged exposure to systemic glucocorticoids Participant characteristics All groups Sample size: randomised (N): 165, analysed ‐ trial primary outcome (N): 155 (ITT with available case analysis was used), analysed ‐ review primary outcome (N): 161 (ITT with available case analysis was used) GROUP 1 Sample size: randomised (N): 83, analysed ‐ trial primary outcome (N): 79, analysed ‐ review primary outcome (N): 82 Age, mean ± SD: 4.3 ± 2.8 Males, N (%): 45 (54.9) RSV status: all positive Atopic status: 43/82 present (infant) GROUP 2 Sample size: randomised (N): 82, analysed ‐ trial primary outcome (N): 76, analysed ‐ review primary outcome (N): 79 Age, mean ± SD: 4.0 ± 2.8 Males, N (%): 47 (59.5) RSV status: all positive Atopic status: 38/79 present (infant) |
|
| Interventions |
GROUP 1 (with glucocorticoid)
Drug name: budesonide
Dose: 1 mg
Mode of administration: nebulised 10 minutes
Timing/duration: twice daily, 14 to 21 days GROUP 2 Drug name: placebo (NR) Dose: NR Mode of administration: nebulised 10 minutes Timing/duration: twice daily, 14 to 21 days Additional co‐interventions for all groups: 6.5 L/minute O2 therapy; reported use of ipratropium bromide, B2 agonists, oral/IV glucocorticoids, antibiotics Protocolised use of bronchodilators with glucocorticoids: no |
|
| Outcomes |
Primary outcome/outcome used to calculate sample size
Coughing or wheezing episodes (within 12 mo.; proportion with at least 1 episode) Secondary outcomes LOS; clinical scale developed for this trial ‐ 11‐point score, based on heart rate, respiratory rate, supplemental oxygen requirements, and the presence or absence of chest wall retractions*; additional medication use*#; hospital readmission#; return healthcare visits#; respiratory symptoms# *time points: during hospitalisation #time points: first 28 days, by 12 months (personal diaries, nurse visits, medical records) |
|
| Funding | Astra Foundation ‐ full financial support grant | |
| Notes | Study reported analyses of some outcomes by initial severity score, duration of symptoms at presentation, atopic history and exposure to cigarette smoke or damp in the household; no specific interaction term This study contributed to the following comparisons in this review: steroid versus placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...infants were randomised to receive either treatment or placebo. Randomisation was stratified by sex and centre." No further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Health care use ((re)admissions, LOS, return visits) | Unclear risk | "the trial solution and side stream nebulisers were manufactured and packaged to ensure the double‐blind nature of the study"; as no further information provided, and both random sequence generation and allocation concealment were unclear, we considered blinding unclear |
| Blinding (performance bias and detection bias) Patient/parent‐reported outcomes (symptoms, QoL) | Unclear risk | "the trial solution and side stream nebulisers were manufactured and packaged to ensure the double‐blind nature of the study"; as no further information provided, and both random sequence generation and allocation concealment were unclear, we considered blinding unclear |
| Blinding (performance bias and detection bias) Other outcomes (adverse events, others) | Unclear risk | "the trial solution and side stream nebulisers were manufactured and packaged to ensure the double‐blind nature of the study"; as no further information provided, and both random sequence generation and allocation concealment were unclear, we considered blinding unclear |
| Incomplete outcome data (attrition bias) Health care use ((re)admissions, LOS, return visits) | Low risk | 3 exclusions post‐randomisation, motives reported, balanced between groups |
| Incomplete outcome data (attrition bias) Patient/parent‐reported outcomes (symptoms, QoL) | Unclear risk | Data on parental diaries reported in 79/83 and 76/82 participants, of which 96% and 98% were complete, respectively; apparently there is a mismatch with the absolute numbers presented; no motives for incomplete outcome data reported |
| Incomplete outcome data (attrition bias) Other outcomes (adverse events, others) | Low risk | 3 exclusions post‐randomisation, motives reported, balanced between groups |
| Selective reporting (reporting bias) | High risk | Published report includes all pre‐specified and expected outcomes; however, "length of time until symptom free" definition was changed due to incomplete outcome data; the study protocol was not available |
| Other bias | Low risk | No significant baseline imbalances; no other sources of bias |
| Overall risk of bias | High risk | > 1 domain as high risk of bias |
Corneli 2007.
| Methods | Parallel design, 2‐arm Multi‐centre (20), conducted in the US (centres from the Pediatric Emergency Care Applied Research Network ‐ PECARN) |
|
| Participants | Outpatients (emergency department) Inclusion/exclusion criteria Inclusion criteria: age 2 to 12 mo.; 1st episode of bronchiolitis (no wheezing, asthma, no previous use of bronchodilators); within 7 days onset; moderate to severe (RDAI ≥ 6) Exclusion criteria: prior adverse event to dexamethasone; heart or lung disease; premature birth (< 36 weeks); immunosuppression or immunodeficiency; therapy with glucocorticoids in previous 14 d; active or recent exposure to varicella; critically ill; parent inability to speak English/Spanish Participant characteristics All groups Sample size: randomised (N): 600, analysed ‐ review/trial primary outcome (N): 600 (ITT with all data used; also performed per protocol analysis) GROUP 1 Sample size: randomised (N): 305, analysed ‐ review/trial primary outcome (N): 305 Age, mean ± SD: 5.1 ± 2.6 months Males, N (%): 190 (62.5) RSV status: 85/127 positive GROUP 2 Sample size: randomised (N): 295, analysed ‐ review/trial primary outcome (N): 295 Age, mean ± SD: 5.1 ± 2.8 months Males, N (%): 178 (60.5) RSV status: 81/142 positive Atopic status: NR (reported family history of wheezing) |
|
| Interventions |
GROUP 1 (with glucocorticoid)
Drug name: dexamethasone
Dose: 1 mL/kg (max 12 mg); oral solution = 1 mg/mL of liquid from generic dexamethasone phosphate injection solution
Mode of administration: oral
Timing/duration: 1 dose GROUP 2 Drug name: placebo (NR) Dose: 1 mL/kg (max 12 mg) Mode of administration: oral Timing/duration: 1 dose Additional co‐interventions for all groups: reported use of albuterol, epinephrine Protocolised use of bronchodilators with glucocorticoids: no |
|
| Outcomes |
Primary outcome/outcome used to calculate sample size
Hospital admission (at 4 hours) Secondary outcomes Length of stay for admitted patients; SaO2*; respiratory rate *; heart rate *; temperature*; clinical scale: Respiratory Assessment Change Score, a change score based on RDAI and respiratory rate change from baseline*; hospital re‐admission#; return healthcare visits#; adverse events# *time points: 4 hours #time points: within 7 to 10 days |
|
| Funding | Grant from the Maternal and Child Health Research program and co‐operative agreements with the Emergency Medical Services for Children program of the Maternal and Child Health Bureau, Health Resources and Services Administration | |
| Notes | Study reported subgroup analyses of patients with eczema or a family history of asthma (pre‐specified), RSV positive and aged < 6 months; adjusted analysis plan with interaction terms This study contributed to the following comparisons in this review: steroid versus placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computerized randomization"; "random permuted blocks stratified by center" |
| Allocation concealment (selection bias) | Low risk | "randomization by telephone, using the keypad for data entry" |
| Blinding (performance bias and detection bias) Health care use ((re)admissions, LOS, return visits) | Low risk | "emergency department staff, study personnel, and parents and guardians were unaware of the group assignments."; "randomization codes were secured until all data entry was complete."; "research pharmacies prepared oral dexamethasone solutions ... and identical oral placebo solutions. The preparations were packaged in identical clear plastic vials labeled only with the randomization numbers." |
| Blinding (performance bias and detection bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | "emergency department staff, study personnel, and parents and guardians were unaware of the group assignments."; "randomization codes were secured until all data entry was complete."; "research pharmacies prepared oral dexamethasone solutions ... and identical oral placebo solutions. The preparations were packaged in identical clear plastic vials labeled only with the randomization numbers." |
| Blinding (performance bias and detection bias) Other outcomes (adverse events, others) | Low risk | "emergency department staff, study personnel, and parents and guardians were unaware of the group assignments."; "randomization codes were secured until all data entry was complete."; "research pharmacies prepared oral dexamethasone solutions ... and identical oral placebo solutions. The preparations were packaged in identical clear plastic vials labeled only with the randomization numbers." |
| Incomplete outcome data (attrition bias) Health care use ((re)admissions, LOS, return visits) | Low risk | Primary outcome ITT, all analysed; no missing primary outcome data. For secondary outcomes, less than 10% follow‐up data lost; balanced between groups; motives reported |
| Incomplete outcome data (attrition bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | Secondary outcomes were also assessed per protocol; less than 10% follow‐up data lost; balanced between groups; motives reported |
| Incomplete outcome data (attrition bias) Other outcomes (adverse events, others) | Low risk | Secondary outcomes were also assessed per protocol; less than 10% follow‐up data lost; balanced between groups; motives reported |
| Selective reporting (reporting bias) | Low risk | Published report includes all pre‐specified and expected outcomes; study protocol was available |
| Other bias | Low risk | No significant baseline imbalances; no other sources of bias |
| Overall risk of bias | Low risk | All applicable domains low risk of bias |
De Boeck 1997.
| Methods | Parallel design, 2‐arm Single‐centre, conducted in Belgium (affiliation: University Hospital Leuven) |
|
| Participants | Inpatients Inclusion/exclusion criteria Inclusion criteria: age < 24 months; detection of RSV; 1st episode of wheezing or shortness of breath; onset of illness within the previous 5 days; informed consent Exclusion criteria: heart, lung or immune disorder; premature infants born before 34 weeks Participant characteristics All groups Sample size: randomised (N): 32, analysed ‐ all trial outcomes (N): 29 (per protocol analysis was used) GROUP 1 Sample size: randomised (N): NR, analysed ‐ all trial outcomes (N): 14 Age: 6.2 (median), 3.7 to 7.5 (IQR) months RSV status: all positive GROUP 2 Sample size: randomised (N): NR, analysed ‐ all trial outcomes (N): 15 Age: 7.1 (median), 4.4 to 8.9 (IQR) RSV status: all positive Males, N (%): NR Atopic status: NR |
|
| Interventions |
GROUP 1 (with glucocorticoid)
Drug name: dexamethasone
Dose: 0.6 mg/kg
Mode of administration: IV
Timing/duration: day 1, 2 doses of 0.6 mg/kg; days 2 and 3, 0.15 mg/kg GROUP 2 Drug name: placebo (NR) Dose: NR Mode of administration: IV Timing/duration: day 1, 2 doses of 0.6 mg/kg; days 2 and 3, 0.15 mg/kg Additional co‐interventions for all groups: salbutamol (0.5%); 0.25 mL, ipratropium bromide (0.025%), 0.5 mL; both aerosolised every 6 h; also reported use of antibiotics Protocolised use of bronchodilators with glucocorticoids: yes (salbutamol + ipratropium) |
|
| Outcomes |
Primary outcome/outcome used to calculate sample size
NR Secondary outcomes LOS; SaO2*; respiratory rate*; clinical scale modified from Tal et al ‐ 12‐point score; pulmonary function tests (minute ventilation, dynamic lung compliance, and airway resistance ‐ PEDS, MAS, Inc., Hatfield, Pa.) (before/after aerosol and day 3) *time points: every 12 hours until day 3 |
|
| Funding | NR | |
| Notes | Study did not report any study‐level subgroup analyses This study contributed to the following comparisons in this review: steroid versus placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomised"; no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Health care use ((re)admissions, LOS, return visits) | Unclear risk | "double‐blind"; no further information provided |
| Blinding (performance bias and detection bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Unclear risk | "double‐blind"; no further information provided |
| Blinding (performance bias and detection bias) Pulmonary function | Unclear risk | "double‐blind"; no further information provided |
| Incomplete outcome data (attrition bias) Health care use ((re)admissions, LOS, return visits) | Unclear risk | 3 participants did not complete the study and were not included in the analysis; no report of motives or arm to which they had been assigned |
| Incomplete outcome data (attrition bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Unclear risk | 3 participants did not complete the study and were not included in the analysis; no report of motives and arm to which they had been assigned |
| Incomplete outcome data (attrition bias) Pulmonary function | Unclear risk | 3 participants did not complete the study and were not included in the analysis; no report of motives and arm to which they had been assigned |
| Selective reporting (reporting bias) | Unclear risk | Not all time points were reported and some outcomes with reported results were not mentioned in methods |
| Other bias | Unclear risk | Few details on baseline characteristics |
| Overall risk of bias | Unclear risk | > 1 domain as unclear risk of bias |
Goebel 2000.
| Methods | Parallel design, 2‐arm Multi‐centre (2), conducted in the US (University of South Alabama) |
|
| Participants | Outpatients (paediatric emergency department/children's clinic) Inclusion/exclusion criteria Inclusion criteria: age 23 months of age or younger; viral respiratory tract infection; 1st time wheeze that did not clear completely after 1 dose of nebulised albuterol Exclusion criteria: history of immune defect; neurological disease with possible aspiration; gastroesophageal reflux; congenital or acquired chronic heart or lung disease; mechanical ventilation; birth < 36 weeks; temp > 38.5°C (rectal); antibiotic therapy < 1 week or antipyretic therapy < 8 hours before enrolment; concomitant bacterial infection; emesis precluding oral medications; initial bronchiolitis score < 2 or > 9 Participant characteristics All groups Sample size: randomised (N): 51, analysed ‐ primary trial outcome (N): 32 (per protocol analysis was used), analysed ‐ review primary outcome (N): 48 (per protocol analysis was used) GROUP 1 Sample size: randomised (N): NR, analysed ‐ primary trial outcome (N): 17, analysed ‐ review primary outcome (N): 24 Age: 4.0 (median); 0 to 13 (range) months Males, N (%): 6 (25) RSV status: 11 positive GROUP 2 Sample size: randomised (N): NR, analysed ‐ primary trial outcome (N): 15, analysed ‐ review primary outcome (N): 24 Age: 4.5 (median); 0 to 16 (range) Males, N (%): 8 (33.3) RSV status: 15 positive Atopic status: NR |
|
| Interventions |
GROUP 1 (with glucocorticoid)
Drug name: prednisolone
Dose: 2 mg/kg/day
Mode of administration: oral
Timing/duration: twice per day for 5 days GROUP 2 Drug name: placebo ‐ similar in appearance and taste, 100 mL each of water and glycerin with 5 mL of cherry‐flavoured Kool‐Aid and 100 mg of quinine Dose: equal volume per body weight Mode of administration: oral Timing/duration: twice per day for 5 days Additional co‐interventions for all groups: albuterol initially 1 dose (0.15 mg/kg), continued at 0.3 mg/kg/d 3 times a day by mouth or 0.15 mg/kg/dose qid by nebuliser Protocolised use of bronchodilators with glucocorticoids: yes (salbutamol) |
|
| Outcomes |
Primary outcome
Clinical scale modified from Tal et al: 12‐point score, based on respiratory rate, flaring or retractions, oxygen saturation on room air, and wheezing* Outcome used to calculate sample size NR Secondary outcomes Hospital admission ‐ initial and later; adverse events *time points: days 2, 3, 6, full convalescence |
|
| Funding | NR | |
| Notes | Study did not report any study‐level subgroup analyses This study contributed to the following comparisons in this review: steroid versus placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization list" |
| Allocation concealment (selection bias) | Low risk | Treatment formulated by the hospital pharmacy; "identical medication bottles containing either prednisolone or placebo had been previously prepared and numbered consecutively according to the randomization list." |
| Blinding (performance bias and detection bias) Health care use ((re)admissions, LOS, return visits) | Low risk | "similar in appearance and taste"; "all study physicians, patients, and caregivers were blinded" |
| Blinding (performance bias and detection bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | "similar in appearance and taste"; "all study physicians, patients, and caregivers were blinded" |
| Incomplete outcome data (attrition bias) Health care use ((re)admissions, LOS, return visits) | High risk | 3 post‐randomisation exclusions from all analyses with motives reported; 16 patients with missing primary outcome data |
| Incomplete outcome data (attrition bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | High risk | 3 post‐randomisation exclusions from all analyses with motives reported; 16 patients with missing primary outcome data |
| Selective reporting (reporting bias) | Low risk | Published report includes all pre‐specified and expected outcomes; study protocol was not available |
| Other bias | Low risk | No significant baseline imbalances; no other sources of bias |
| Overall risk of bias | High risk | > 1 domain as high risk of bias |
Gomez 2007.
| Methods | Parallel design, 2‐arm Single‐centre, conducted in Mexico (Hospital General de Zona 1, San Luis Potosi) |
|
| Participants | Inpatients (emergency department and infant paediatric department) Inclusion/exclusion criteria Inclusion criteria: age 1 to 18 mo.; observed in the ED of the centre; clinical and radiological diagnosis of bronchiolitis; < 72 hours of evolution of symptoms; RDAI score > 2, Silvermann‐Andersen score > 0; informed consent Exclusion criteria: previous bronchospasm/bronchiolitis; congenital heart disease; chronic lung disease; possible bronchopneumonia; children treated with salbutamol/dexamethasone in the previous 48 hours Participant characteristics All groups Sample size: randomised (N): NR, analysed ‐ trial primary outcomes (N): 49 (unclear what type of analysis was performed regarding ITT and missing data) GROUP 1 Sample size: randomised (N): NR, analysed ‐ all trial outcomes (N): 24 Age, mean ± SD: 5.7 ± 1.3 months Males, N (%): 12 (50) GROUP 2 Sample size: randomised (N): NR, analysed ‐ primary trial outcome (N): 25 Age, mean ± SD: 5.22 ± 1.6 months Males, N (%): 13 (52) RSV status: NR Atopic status: NR |
|
| Interventions |
GROUP 1
Drug name: salbutamol
Dose: 0.3 mL/1.5 mg
Mode of administration: nebulised in 5 L/minute of O2
Timing/duration: every 4 hours for 24 hours (total 6 doses) GROUP 2 (with glucocorticoid) Drug name: salbutamol + dexamethasone Dose: 0.3 mL/1.5 mg (salb) + dexamethasone: 0.5 mL/2 mg Mode of administration: nebulised Timing/duration: every 4 hours for 24 hours (total 6 doses) Additional co‐interventions for all groups: NR Protocolised use of bronchodilators with glucocorticoids: yes (salbutamol) |
|
| Outcomes |
Primary outcome/outcome used to calculate sample size
NR Secondary outcomes LOS; SaO2*; respiratory rate*; heart rate*; clinical scale: RDAI, 17‐point score based on wheezing and retractions* *time points: every 4 hours until 24 hours |
|
| Funding | NR | |
| Notes | Study did not report any study‐level subgroup analyses This study contributed to the following comparisons in this review: steroid versus placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation (blocks of 4). Sequence generation probably adequate |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Health care use ((re)admissions, LOS, return visits) | Low risk | Patient, nurse and physician blinded to treatment group; drugs in opaque containers, similar organoleptic properties |
| Blinding (performance bias and detection bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | Patient, nurse and physician blinded to treatment group; drugs in opaque containers, similar organoleptic properties |
| Incomplete outcome data (attrition bias) Health care use ((re)admissions, LOS, return visits) | Unclear risk | No information reported on exclusions pre‐ or post‐randomisation, nor on losses to follow‐up |
| Incomplete outcome data (attrition bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Unclear risk | No information reported on exclusions pre‐ or post‐randomisation, nor on losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Hospitalisation‐related outcomes not mentioned in methods |
| Other bias | Low risk | No significant baseline imbalances; no other sources of bias |
| Overall risk of bias | Unclear risk | > 1 domain as unclear risk of bias |
Klassen 1997.
| Methods | Parallel design, 2‐arm Single‐centre, conducted in Canada (Children's Hospital of Eastern Ontario) |
|
| Participants | Inpatients (inpatient wards, paediatric tertiary hospital) Inclusion/exclusion criteria Inclusion criteria: age > 6 weeks < 15 mo.; 1st time wheeze; evidence of viral infection (rhinorrhoea/ temp > 37.5°C); admitted to inpatient ward; SaO2 < 95%; RDAI score > 6 Exclusion criteria: underlying disease that might affect cardiopulmonary status; asthma; wheezing/cough previously treated with bronchodilators; therapy with glucocorticoids within the past 2 weeks; history of adverse events with glucocorticoids Participant characteristics All groups Sample size: randomised (N): 72 (5 ineligible), analysed ‐ trial/review primary outcome (N): 67 (ITT with available case analysis was used) GROUP 1 Sample size: randomised (N): 35, analysed ‐ trial/review primary outcomes (N): 35 Age, mean: 4.68; 3.6 to 5.76 (95% CI) Males, N (%): 22 (63) RSV status: 30 (86%) positive GROUP 2 Sample size: randomised (N): 37, analysed ‐ trial/review primary trial outcome (N): 32 Age, mean: 4.68; 3.6 to 5.64 (95% CI) Males, N (%): 15 (47) RSV status: 28 (88%) positive Atopic status: NR |
|
| Interventions |
GROUP 1 (with glucocorticoid)
Drug name: dexamethasone (clear 70% sucrose solution + dex sodium phosphate)
Dose: 1st: 0.5 mg/kg; 2nd and others: 0.3 mg/kg
Mode of administration: oral
Timing/duration: 3 doses max: at admission, once each of the following mornings or until discharge (if before) GROUP 2 Drug name: placebo (70% sucrose solution) Dose: NR Mode of administration: oral Timing/duration: 3 doses max: at admission, once each of the following mornings or until discharge (if before) Additional co‐interventions for all groups: salbutamol by nebulisation, 0.15 mg/kg every 4 hours for first 24 hours, O2 concentration of 35% in a plastic tent; reported use of additional bronchodilators and antibiotics Protocolised use of bronchodilators with glucocorticoids: yes (salbutamol) |
|
| Outcomes |
Primary outcome/outcome used to calculate sample size
Clinical scale: RDAI, 17‐point score based on wheezing and retractions (at 24 h; other time points*) Secondary outcomes LOS; SaO2*; respiratory rate*; heart rate*; hospital readmission (1 week); return healthcare visits; adverse events, number of nebulisation; additional medications *time points: 12, 24, 36, 48, 60 hours |
|
| Funding | Grant from Physicians Services Inc, Toronto, Ontario, Canada | |
| Notes | Study did not report any study‐level subgroup analyses This study contributed to the following comparisons in this review: steroid versus placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation, stratified by age |
| Allocation concealment (selection bias) | Low risk | Randomisation performed by pharmacy; all packages of study medications were prepared and labelled with a study number; concealed until study complete and data analysis had begun |
| Blinding (performance bias and detection bias) Health care use ((re)admissions, LOS, return visits) | Low risk | Dexamethasone and placebo with identical appearance; research assistants, treating physicians, and parents were masked to the treatment allocation |
| Blinding (performance bias and detection bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | Dexamethasone and placebo with identical appearance; research assistants, treating physicians, and parents were masked to the treatment allocation |
| Blinding (performance bias and detection bias) Other outcomes (adverse events, others) | Low risk | Dexamethasone and placebo with identical appearance; research assistants, treating physicians, and parents were masked to the treatment allocation |
| Incomplete outcome data (attrition bias) Health care use ((re)admissions, LOS, return visits) | Low risk | 5 post‐randomisation exclusions, motives reported; 1 patient discharged with missing outcome data |
| Incomplete outcome data (attrition bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | 5 post‐randomisation exclusions, motives reported; 1 patient discharged with missing outcome data |
| Incomplete outcome data (attrition bias) Other outcomes (adverse events, others) | Low risk | 5 post‐randomisation exclusions, motives reported; 1 patient discharged with missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published report includes all pre‐specified and expected outcomes; study protocol was not available |
| Other bias | Low risk | No significant baseline imbalances; no other sources of bias |
| Overall risk of bias | Low risk | All applicable domains low risk of bias |
Kuyucu 2004.
| Methods | Double‐randomisation/factorial design, 4 arms Single‐centre, conducted in Turkey (Faculty of Medicine, Mersin University) |
|
| Participants | Outpatients (paediatric outpatient clinics and emergency department) Inclusion/exclusion criteria Inclusion criteria: age 2 to 21 mo.; admitted with 1st episode of wheezing; clinical findings compatible with acute bronchiolitis; RDAI ≥ 4 Exclusion criteria: history of wheezing; previous therapy with bronchodilators; previous diagnosis of asthma or allergic bronchitis; personal history of atopic dermatitis or allergic rhinitis; chronic cardiac or pulmonary disease; any glucocorticoid therapy in the previous 2 week; signs of severe respiratory disease; bacterial infection; parental history of asthma or atopic disease Participant characteristics All groups Sample size: randomised (N): 90, analysed ‐ trial/review primary outcome (N): 69 (ITT with available case analysis was used) GROUP 1 Sample size: randomised (N): 26, analysed ‐ trial/review primary outcomes (N): 23 Age, mean ± SD: 7.2 ± 0.8 months GROUP 2 Sample size: randomised (N): 24, analysed ‐ trial/review primary trial outcome (N): 23 Age, mean ± SD: 7.9 ± 1.0 months GROUP 3 Sample size: randomised (N): 19, analysed ‐ trial/review primary trial outcome (N): 11 Age, mean ± SD: 9.6 ± 1.3 months GROUP 4 Sample size: randomised (N): 21, analysed ‐ trial/review primary trial outcome (N): 12 Age, mean ± SD: 9.9 ± 1.7 months Males, N (%): NR RSV status NR Atopic status: NR |
|
| Interventions |
GROUP 1 (with glucocorticoid)
Drug name: epinephrine + dexamethasone
Dose: 3 mL (3 mg) of 1:1000 L‐epinephrine + 0.6 mg/kg (dex)
Mode of administration: nebulised with O2, flow 5 to 6 L/minute for 10 minutes (epi) + IM (dex)
Timing/duration: epinephrine ‐ initial dose, if no improvement at 120 minutes, then 2nd dose given; dexamethasone single dose GROUP 2 (with glucocorticoid) Drug name: salbutamol + dexamethasone Dose: 0.15 mg/kg of 1 mg/mL solution of salbutamol added to 0.9% saline to total 3 mL+ 0.6 mg/kg (dex) Mode of administration: nebulised with O2, flow 5 to 6 L/minute for 10 minutes (epi) + IM (dex) Timing/duration: salbutamol ‐ initial dose, if no improvement at 120 minutes, then 2nd dose given; dexamethasone single dose GROUP 3 Drug name: epinephrine + placebo (NR) Dose: 3 mL (3 mg) of 1:1000 L‐epinephrine Mode of administration: nebulised with O2, flow 5 to 6 L/minute for 10 minutes (epi) + IM (pla) Timing/duration: epinephrine ‐ initial dose, if no improvement at 120 minutes, then 2nd dose given; placebo single dose GROUP 4 Drug name: salbutamol + placebo (NR) Dose: 0.15 mg/kg of 1 mg/mL solution of salbutamol added to 0.9% saline solution to make a total of 3 mL Mode of administration: nebulised with O2, flow 5 to 6 L/minute for 10 minutes (epi) + IM (pla) Timing/duration: salbutamol ‐ initial dose, if no improvement at 120 minutes, then 2nd dose given; placebo single dose Additional co‐interventions for all groups: NR Protocolised use of bronchodilators with glucocorticoids: yes (epinephrine Group 1, salbutamol Group 2) |
|
| Outcomes |
Primary outcome
Respiratory rate*; heart rate*; clinical scale*: RDAI, 17‐point score based on wheezing and retractions Outcome used to calculate sample size NR Secondary outcomes Admissions; additional medication; adverse events; respiratory complaints (2 months) *time points: 30, 60, 90, 120 minutes, 24 hours, 5 days |
|
| Funding | NR | |
| Notes | Study did not report any study‐level subgroup analyses This study contributed to the following comparisons in this review: steroid versus. Placebo, steroids + epinephrine versus salbutamol, steroids + salbutamol versus epinephrine Factorial design not reported explicitly; analysis was "inside the table", and did not aggregate group results |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised fashion"; randomised to the first treatment, "randomised fashion independent of the first randomization" for the second treatment; no further information provided |
| Allocation concealment (selection bias) | Unclear risk | "Preparation and administration of nebulized solutions were performed by a trained emergency department nurse." |
| Blinding (performance bias and detection bias) Health care use ((re)admissions, LOS, return visits) | Unclear risk | Double‐blind; "parents and investigators remained blinded to administered medications throughout study period" |
| Blinding (performance bias and detection bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Unclear risk | Double‐blind; "parents and investigators remained blinded to administered medications throughout study period" |
| Blinding (performance bias and detection bias) Other outcomes (adverse events, others) | Unclear risk | Double‐blind; "parents and investigators remained blinded to administered medications throughout study period" |
| Incomplete outcome data (attrition bias) Health care use ((re)admissions, LOS, return visits) | High risk | 21 patients with missing outcome data, imbalanced between groups; no motives reported |
| Incomplete outcome data (attrition bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | High risk | 21 patients with missing outcome data, imbalanced between groups; no motives reported |
| Incomplete outcome data (attrition bias) Other outcomes (adverse events, others) | High risk | 21 patients with missing outcome data, imbalanced between groups; no motives reported |
| Selective reporting (reporting bias) | Low risk | Published report includes all pre‐specified and expected outcomes; study protocol was not available |
| Other bias | Unclear risk | Duration of illness was significantly different in G + S group |
| Overall risk of bias | High risk | > 1 domain as high risk of bias |
Kuyucu 2004 (G+E vs P+E).
| Methods | (see Kuyucu 2004) | |
| Participants | (see Kuyucu 2004) | |
| Interventions | (see Kuyucu 2004) This glucocorticoid versus placebo comparison includes data from Group 1 (epinephrine + dexamethasone) versus Group 3 (epinephrine + placebo) |
|
| Outcomes | (see Kuyucu 2004) | |
| Funding | (see Kuyucu 2004) | |
| Notes | (see Kuyucu 2004) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised fashion"; randomised to the first treatment, "randomised fashion independent of the first randomization" for the second treatment; no further information provided |
| Allocation concealment (selection bias) | Unclear risk | "Preparation and administration of nebulized solutions were performed by a trained emergency department nurse." |
| Blinding (performance bias and detection bias) Health care use ((re)admissions, LOS, return visits) | Unclear risk | Double‐blind; "parents and investigators remained blinded to administered medications throughout study period" |
| Blinding (performance bias and detection bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Unclear risk | Double‐blind; "parents and investigators remained blinded to administered medications throughout study period" |
| Blinding (performance bias and detection bias) Other outcomes (adverse events, others) | Unclear risk | Double‐blind; "parents and investigators remained blinded to administered medications throughout study period" |
| Incomplete outcome data (attrition bias) Health care use ((re)admissions, LOS, return visits) | High risk | 21 patients with missing outcome data, imbalanced between groups; no motives reported |
| Incomplete outcome data (attrition bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | High risk | 21 patients with missing outcome data, imbalanced between groups; no motives reported |
| Incomplete outcome data (attrition bias) Other outcomes (adverse events, others) | High risk | 21 patients with missing outcome data, imbalanced between groups; no motives reported |
| Selective reporting (reporting bias) | Low risk | Published report includes all pre‐specified and expected outcomes; study protocol was not available |
| Other bias | Unclear risk | Duration of illness was significantly different in G + S group |
| Overall risk of bias | High risk | > 1 domain as high risk of bias |
Kuyucu 2004 (G+S vs P+S).
| Methods | (see Kuyucu 2004) | |
| Participants | (see Kuyucu 2004) | |
| Interventions | (see Kuyucu 2004) This glucocorticoid versus placebo comparison includes data from Group 1 (salbutamol + dexamethasone) versus Group 3 (salbutamol + placebo) |
|
| Outcomes | (see Kuyucu 2004) | |
| Funding | (see Kuyucu 2004) | |
| Notes | (see Kuyucu 2004) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised fashion"; randomised to the first treatment, "randomised fashion independent of the first randomization" for the second treatment; no further information provided |
| Allocation concealment (selection bias) | Unclear risk | "Preparation and administration of nebulized solutions were performed by a trained emergency department nurse." |
| Blinding (performance bias and detection bias) Health care use ((re)admissions, LOS, return visits) | Unclear risk | Double‐blind; "parents and investigators remained blinded to administered medications throughout study period" |
| Blinding (performance bias and detection bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Unclear risk | Double‐blind; "parents and investigators remained blinded to administered medications throughout study period" |
| Blinding (performance bias and detection bias) Other outcomes (adverse events, others) | Unclear risk | Double‐blind; "parents and investigators remained blinded to administered medications throughout study period" |
| Incomplete outcome data (attrition bias) Health care use ((re)admissions, LOS, return visits) | High risk | 21 patients with missing outcome data, imbalanced between groups; no motives reported |
| Incomplete outcome data (attrition bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | High risk | 21 patients with missing outcome data, imbalanced between groups; no motives reported |
| Incomplete outcome data (attrition bias) Other outcomes (adverse events, others) | High risk | 21 patients with missing outcome data, imbalanced between groups; no motives reported |
| Selective reporting (reporting bias) | Low risk | Published report includes all pre‐specified and expected outcomes; study protocol was not available |
| Other bias | Unclear risk | Duration of illness was significantly different in G + S group |
| Overall risk of bias | High risk | > 1 domain as high risk of bias |
Mesquita 2009.
| Methods | Parallel design, 2 arms Single‐centre, conducted in Paraguay (Hospital General Pediátrico “Niños de Acosta Ñu”, Asunción) |
|
| Participants | Outpatients (paediatric emergency department) Inclusion/exclusion criteria Inclusion criteria: age 2 to 24 mo.; 1st episode of bronchiolitis defined as respiratory distress, respiratory rate 40 to 80 bpm, wheezing; < 7 d after onset of cold Exclusion criteria: clinical or radiological pneumonia; cardiopulmonary congenital malformations; bronchopulmonary dysplasia; cystic fibrosis; foreign body aspirations; neurological alteration; previous wheezing or asthma episode; inhaled or systemic glucocorticoid < 15 d; β2‐agonists < 4 hours; history of atopy in the child (dermatitis or allergic rhinitis) or parental asthma; severe wheezing attack (respiratory rate ≥ 100/minute and/or heart rate ≥ 200/minute and/or shock or lethargy) Participant characteristics All groups Sample size: randomised (N): 80, analysed ‐ trial/review primary outcomes (N): 65 (per protocol analysis was used) GROUP 1 Sample size: randomised (N): NR, analysed ‐ trial/review primary outcomes (N): 33 Age, mean ± SD: 7.3 ± 4 months Males, N (%): 19 (58) RSV status: 17/29 positive GROUP 2 Sample size: randomised (N): NR, analysed ‐ trial/review primary trial outcome (N): 32 (available case analysis was used) Age, mean ± SD: 5.9 ± 3 months Males, N (%): 15 (47) RSV status: 19/23 positive Atopic status: NR |
|
| Interventions |
GROUP 1 (with glucocorticoid)
Drug name: dexamethasone
Dose: 0.5 mg/kg (1 mL/kg)
Mode of administration: oral
Timing/duration: 1 dose
GROUP 2
Drug name: placebo (NR)
Dose: 1 mL/kg
Mode of administration: oral
Timing/duration: 1 dose
Co‐interventions: Additional co‐interventions for all groups: all patients received 4 mL physiological solution during a 6‐minute nebulisation with 02 flow of 6 L/minute; after 30 minutes, a dose of 1 mL L‐adrenaline solution (1:1000, 1 mL = 1 mg) was received by nebulisation Protocolised use of bronchodilators with glucocorticoids: yes (epinephrine) |
|
| Outcomes |
Primary outcome/outcome used to calculate sample size
Clinical scale*: RDAI, 17‐point score based on wheezing and retractions (at 4 h; other time points*) Secondary outcomes Hospital admission (at 4 hours); SaO2*; respiratory rate*; heart rate* *time points: 60 minutes, 4 hours |
|
| Funding | NR (Lab. Formula Magistral, Asunción, Paraguay provided drugs) | |
| Notes | Study did not report any study‐level subgroup analyses This study contributed to the following comparisons in this review: steroid versus placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients were randomised to a double‐blind, placebo‐control study using a table of random numbers" |
| Allocation concealment (selection bias) | Low risk | "The research pharmacy prepared the active drug ... and the placebo ...; their bottles were labelled only with the randomised numbers." |
| Blinding (performance bias and detection bias) Health care use ((re)admissions, LOS, return visits) | Low risk | Double‐blind; "The research pharmacy prepared the active drug (dexamethasone, Lab. Formula Magistral, Paraguay) and the placebo in identical sweet syrups and their bottles were labelled only with the randomised numbers."; in the whole period of the trial, the investigators were blinded of the treatment administered." |
| Blinding (performance bias and detection bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | Double‐blind; "The research pharmacy prepared the active drug (dexamethasone, Lab. Formula Magistral, Paraguay) and the placebo in identical sweet syrups and their bottles were labelled only with the randomised numbers."; in the whole period of the trial, the investigators were blinded of the treatment administered." |
| Incomplete outcome data (attrition bias) Health care use ((re)admissions, LOS, return visits) | Low risk | 15 children excluded post‐enrollment, motives reported (2 children quit the protocol before the first hour, 3 post‐treatment) |
| Incomplete outcome data (attrition bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | 15 children excluded post‐enrollment, motives reported (2 children quit the protocol before the first hour, 3 post‐treatment) |
| Selective reporting (reporting bias) | Unclear risk | Subgroups not defined in methods; published report includes all pre‐specified and expected outcomes; study protocol was not available |
| Other bias | Low risk | No significant baseline imbalances; no other sources of bias |
| Overall risk of bias | Unclear risk | Unclear |
Plint 2009.
| Methods | Factorial design, 4 arms Multi‐centre (8), conducted in Canada (hospitals are members of the research group Paediatric Emergency Research Canada) |
|
| Participants | Outpatients (paediatric emergency department) Inclusion/exclusion criteria Inclusion criteria: age 6 to 12 mo.; RDAI: 4 to 15; 1st episode wheezing associated with upper respiratory tract infection; presenting bronchiolitis Exclusion criteria: prior bronchodilator treatment in the emergency department; oral or inhaled glucocorticoid during previous 2 weeks; previous episode of wheezing or history of asthma; previous bronchodilator use; chronic cardiopulmonary disease; immunodeficiency; serious distress (defined as a pulse rate > 200 beats per minute, a respiratory rate > 80 breaths per minute, or an RDAI score > 15); lethargy; exposed to varicella < 3 weeks; < 37‐week gestation who had a corrected age of less than 6 weeks at presentation; communication barriers with family Participant characteristics All groups Sample size: randomised (N): 800, analysed ‐ trial/review primary outcome (N): 797 (ITT with available case analysis was performed) GROUP 1 Sample size: randomised (N): 200, analysed ‐ trial/review primary outcomes (N): 199 Age: 5 (median) 3 to 7 (interquartile range) months Males, N (%): 124 (62) RSV status: 128 (64) positive Atopic status: 28 (14) present (infant) GROUP 2 Sample size: randomised (N): 199, analysed ‐ trial/review primary trial outcome (N): 198 Age: 5 (median) 3 to 7 (interquartile range) months Males, N (%): 122 (61) RSV status: 129 (65) positive Atopic status: 20 (10) present (infant) GROUP 3 Sample size: randomised (N): 200, analysed ‐ trial/review primary trial outcome (N): 199 Age: 5 (median) 3 to 7 (interquartile range) months Males, N (%): 127 (64) RSV status: 127 (64) positive Atopic status: 19 (9.5) present (infant) GROUP 4 Sample size: randomised (N): 201, analysed ‐ trial/review primary trial outcome (N): 201 Age: 5 (median) 3 to 7 (interquartile range) months Males, N (%): 120 (60) RSV status: 136 (68) positive Atopic status: 22 (10.9) present (infant) |
|
| Interventions |
GROUP 1 (with glucocorticoid)
Drug name: epinephrine + dexamethasone (generic dexamethasone phosphate injection solution mixed with Ora‐Plus and Ora‐Sweet ‐ Paddock Laboratories)
Dose: 3 mL 1:1000 solution (epi)+ 1.0 mg/kg weight (max 10 mg) then 0.6 mg/kg (max 10 mg) after ED (dex)
Mode of administration: nebulised in O2 flow 8L/minute (epi) + oral (dex)
Timing/duration: nebulise 2 doses 30 minutes apart (epi); oral after 1st nebulisation in ED, followed by 5, 1 x daily doses after leaving ED (dex) GROUP 2 Drug name: epinephrine + placebo (Ora‐Plus and Ora‐Sweet) Dose: 3 mL 1:1000 solution (epi) + placebo Mode of administration: nebulised in O2 flow 8 L/minute (epi) + oral (pla) Timing/duration: nebulise 2 doses 30 minutes apart (epi); oral after 1st nebulisation in ED, followed by 5, 1 x daily doses after leaving ED (pla) GROUP 3 (with glucocorticoid) Drug name: dexamethasone + placebo (saline) Dose: 1.0 mg/kg weight (max 10 mg) then 0.6 mg/kg (max 10 mg) after ED (dex) + 3 mL (pla) Mode of administration: oral (dex) + nebulised in O2 flow 8L/minute (pla) Timing/duration: nebulise 2 doses 30 minutes apart (pla); oral after 1st nebulisation in ED, followed by 5, 1 x daily doses after leaving ED (dex) GROUP 4 Drug name: placebo (saline) + placebo (Ora‐Plus and Ora‐Sweet) Dose: 3 mL + oral Mode of administration: nebulised in O2 flow 8 L/minute + oral Timing/duration: nebulise 2 doses 30 minutes apart; oral after 1st nebulisation in ED, followed by 5, 1 x daily doses after leaving ED Additional co‐interventions for all groups: O2 if SaO2 < 92%; acetaminophen (15 mg/kg) if fever; additional interventions allowed after 90' ‐ reported use of other bronchodilators and antibiotics Protocolised use of bronchodilators with glucocorticoids: yes (epinephrine Group 1) |
|
| Outcomes |
Primary outcome/outcome used to calculate sample size
Hospital admission by day 7 Secondary outcomes Hospital admission by day 1 and day 22; SaO2*; respiratory rate*; heart rate*, temperature*; RDAI: 17‐point score based on wheezing and retractions*; time to discharge (time between the triage time at the enrolment and the time of discharge from the last emergency department visit or from the last hospitalisation for each patient within the next 7 days); return healthcare visits#; symptoms (length/severity)#; adverse events *time points: 30, 60, 120, 240 minutes #time points: within 22 d |
|
| Funding | Grants from the Canadian Institutes of Health Research and Alberta Children’s Hospital Foundation | |
| Notes | Study reported a priori subgroup analyses of presence or absence of atopy, RSV status and duration of illness at presentation; adjusted analysis plan with interaction terms This study contributed to the following comparisons in this review: steroid versus placebo, steroids + epinephrine versus placebo Analysis of factorial design was "inside the table", due to results suggesting unanticipated synergism between epinephrine and dexamethasone |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization sequence, stratified by center, used randomised permuted blocks of 8 and 12." |
| Allocation concealment (selection bias) | Low risk | "Codes were secured at each center’s pharmacy until enrolment and data en‐ try were complete. In order to conceal the allocation sequence, the pharmacy at each site prepared the study drugs in sequentially numbered, visually identical packets." |
| Blinding (performance bias and detection bias) Health care use ((re)admissions, LOS, return visits) | Low risk | Double‐blind; "The active drugs and placebo were identical in appearance, volume, weight, odor, and taste." |
| Blinding (performance bias and detection bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | Double‐blind; "The active drugs and placebo were identical in appearance, volume, weight, odor, and taste." |
| Blinding (performance bias and detection bias) Patient/parent‐reported outcomes (symptoms, QoL) | Low risk | Double‐blind; "The active drugs and placebo were identical in appearance, volume, weight, odor, and taste." |
| Blinding (performance bias and detection bias) Other outcomes (adverse events, others) | Low risk | Double‐blind; "The active drugs and placebo were identical in appearance, volume, weight, odor, and taste." |
| Incomplete outcome data (attrition bias) Health care use ((re)admissions, LOS, return visits) | Low risk | 3 patients with missing outcome data |
| Incomplete outcome data (attrition bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | 3 patients with missing outcome data |
| Incomplete outcome data (attrition bias) Patient/parent‐reported outcomes (symptoms, QoL) | Low risk | 3 patients with missing outcome data |
| Incomplete outcome data (attrition bias) Other outcomes (adverse events, others) | Low risk | 3 patients with missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published report includes all pre‐specified and expected outcomes; study protocol was available |
| Other bias | Low risk | No significant baseline imbalances; no other sources of bias |
| Overall risk of bias | Low risk | All applicable domains low risk of bias |
Plint 2009 (G+E vs P+E).
| Methods | See Plint 2009 | |
| Participants | See Plint 2009 | |
| Interventions | See Plint 2009 This glucocorticoid and epinephrine versus placebo and epinephrine comparison includes data from Group 1 (glucocorticoid and epinephrine) versus Group 2 (placebo and epinephrine) |
|
| Outcomes | See Plint 2009 | |
| Funding | See Plint 2009 | |
| Notes | See Plint 2009 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization sequence, stratified by center, used randomised permuted blocks of 8 and 12." |
| Allocation concealment (selection bias) | Low risk | "Codes were secured at each center’s pharmacy until enrolment and data en‐ try were complete. In order to conceal the allocation sequence, the pharmacy at each site prepared the study drugs in sequentially numbered, visually identical packets." |
| Blinding (performance bias and detection bias) Health care use ((re)admissions, LOS, return visits) | Low risk | Double‐blind; "The active drugs and placebo were identical in appearance, volume, weight, odor, and taste." |
| Blinding (performance bias and detection bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | Double‐blind; "The active drugs and placebo were identical in appearance, volume, weight, odor, and taste." |
| Blinding (performance bias and detection bias) Patient/parent‐reported outcomes (symptoms, QoL) | Low risk | Double‐blind; "The active drugs and placebo were identical in appearance, volume, weight, odor, and taste." |
| Blinding (performance bias and detection bias) Other outcomes (adverse events, others) | Low risk | Double‐blind; "The active drugs and placebo were identical in appearance, volume, weight, odor, and taste." |
| Incomplete outcome data (attrition bias) Health care use ((re)admissions, LOS, return visits) | Low risk | 3 patients with missing outcome data |
| Incomplete outcome data (attrition bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | 3 patients with missing outcome data |
| Incomplete outcome data (attrition bias) Patient/parent‐reported outcomes (symptoms, QoL) | Low risk | 3 patients with missing outcome data |
| Incomplete outcome data (attrition bias) Other outcomes (adverse events, others) | Low risk | 3 patients with missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published report includes all pre‐specified and expected outcomes; study protocol was available |
| Other bias | Low risk | No significant baseline imbalances; no other sources of bias |
| Overall risk of bias | Low risk | All applicable domains low risk of bias |
Plint 2009 (G+P vs P+P).
| Methods | See Plint 2009 | |
| Participants | See Plint 2009 | |
| Interventions | See Plint 2009 This glucocorticoid versus placebo comparison includes data from Group 3 (dexamethasone + placebo) versus Group 4 (placebo and placebo) |
|
| Outcomes | See Plint 2009 | |
| Funding | See Plint 2009 | |
| Notes | See Plint 2009 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization sequence, stratified by center, used randomised permuted blocks of 8 and 12." |
| Allocation concealment (selection bias) | Low risk | "Codes were secured at each center’s pharmacy until enrolment and data entry were complete. In order to conceal the allocation sequence, the pharmacy at each site prepared the study drugs in sequentially numbered, visually identical packets." |
| Blinding (performance bias and detection bias) Health care use ((re)admissions, LOS, return visits) | Low risk | Double‐blind; "The active drugs and placebo were identical in appearance, volume, weight, odor, and taste." |
| Blinding (performance bias and detection bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | Double‐blind; "The active drugs and placebo were identical in appearance, volume, weight, odor, and taste." |
| Blinding (performance bias and detection bias) Patient/parent‐reported outcomes (symptoms, QoL) | Low risk | Double‐blind; "The active drugs and placebo were identical in appearance, volume, weight, odor, and taste." |
| Blinding (performance bias and detection bias) Other outcomes (adverse events, others) | Low risk | Double‐blind; "The active drugs and placebo were identical in appearance, volume, weight, odor, and taste." |
| Incomplete outcome data (attrition bias) Health care use ((re)admissions, LOS, return visits) | Low risk | 3 patients with missing outcome data |
| Incomplete outcome data (attrition bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | 3 patients with missing outcome data |
| Incomplete outcome data (attrition bias) Patient/parent‐reported outcomes (symptoms, QoL) | Low risk | 3 patients with missing outcome data |
| Incomplete outcome data (attrition bias) Other outcomes (adverse events, others) | Low risk | 3 patients with missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published report includes all pre‐specified and expected outcomes; study protocol was available |
| Other bias | Low risk | No significant baseline imbalances; no other sources of bias |
| Overall risk of bias | Low risk | All applicable domains low risk of bias |
Richter 1998.
| Methods | Parallel design, 2 arms Single‐centre, conducted in the UK (affiliation: Royal Alexandra Children's Hospital Brighton) |
|
| Participants | Inpatients Inclusion/exclusion criteria Inclusion criteria: age < 12 mo.; no history of wheezing; hospitalised with clinical features of bronchiolitis (tachypnoea, recession, wheezing, crepitations) Exclusion criteria: congenital abnormality; pre‐existing pulmonary disease; immune deficiency; need for assisted ventilation Participant characteristics All groups Sample size: randomised (N): 40, analysed ‐ trial primary outcomes (N): 39 (ITT with available case analysis was performed), analysed ‐ review primary outcomes (N): 40 (ITT with all data was performed) GROUP 1 Sample size: randomised (N): 21, analysed ‐ trial primary outcomes (N): 20, analysed ‐ review primary outcomes (N): 21 Age: 4.08 (median); 1.1 to 10.15 (range) months Males, N (%): 12 (57) RSV status: 16 (76) positive Atopic status: 18 (86) present (family) GROUP 2 Sample size: randomised (N): 19, analysed ‐ trial primary outcomes (N): 19, analysed ‐ review primary outcomes (N): 19 Age: 2.7 (median); 0.9 to 7.82 (range) months Males, N (%): 10 (52.6) RSV status: 17 (89) positive Atopic status: 12 (63) present (family) |
|
| Interventions |
GROUP 1 (with glucocorticoid)
Drug name: budesonide
Dose: 1 mg in 2 mL then 0.5 mg in 2 mL
Mode of administration: nebulised with O2, flow 6 L/minute
Timing/duration: 1 mg/2mL ‐ twice daily for 5 d; 0.5 mg/2 mL ‐ 2 x daily for 6 weeks GROUP 2 Drug name: placebo Dose: 2 mL 0.9% saline Mode of administration: nebulised with O2, flow 6 L/minute Timing/duration: twice daily for 6 weeks Additional co‐interventions for all groups: no restrictions on use of other drug treatments Protocolised use of bronchodilators with glucocorticoids: no |
|
| Outcomes |
Primary outcome
Clinical scale adapted from Wesley et al, based on respiratory rate, O2 concentration required to keep O2 > 92%, wheeze, degree of recession, and need for IV fluids or nasogastric tube feeding (at 48 hours; other time points*) Clinical scale*: RDAI, 17‐point score based on wheezing and retractions (at 4 hours; other time points*) Outcome used to calculate sample size Wheezing episodes in the early months after bronchiolitis (no specific time point or definition) Secondary outcomes Duration and maximum requirements of O2 therapy; LOS; hospital re‐admission (6 mo.); symptoms (diary; based on Noble et al, daytime and nighttime cough and wheeze)#¶; inhaled bronchodilators#¶; length and growth rate# *time points: twice daily until discharge, 48 hours #time points: daily until 6 weeks ¶time points: until 6 months, when symptomatic and by 6‐week periods |
|
| Funding | Astra Clinical Research Unit and Rockinghorse Appeal | |
| Notes | Study did not report any study‐level subgroup analyses This study contributed to the following comparisons in this review: steroid versus placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Randomisation details held by hospital pharmacy |
| Blinding (performance bias and detection bias) Health care use ((re)admissions, LOS, return visits) | Unclear risk | Double‐blind; "Throughout the study, the investigators, nursing and medical staff, and parents were unaware to which treatment group infants had been assigned"; "both budesonide and placebo were supplied in plastic repsules prepared by Astra pharmaceuticals" |
| Blinding (performance bias and detection bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Unclear risk | Double‐blind; "Throughout the study, the investigators, nursing and medical staff, and parents were unaware to which treatment group infants had been assigned"; "both budesonide and placebo were supplied in plastic repsules prepared by Astra pharmaceuticals" |
| Blinding (performance bias and detection bias) Other outcomes (adverse events, others) | Unclear risk | Double‐blind; "Throughout the study, the investigators, nursing and medical staff, and parents were unaware to which treatment group infants had been assigned"; "both budesonide and placebo were supplied in plastic repsules prepared by Astra pharmaceuticals" |
| Incomplete outcome data (attrition bias) Health care use ((re)admissions, LOS, return visits) | Low risk | 1 participant with missing outcome data |
| Incomplete outcome data (attrition bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | 1 participant with missing outcome data |
| Incomplete outcome data (attrition bias) Other outcomes (adverse events, others) | Low risk | 1 participant with missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Not all outcomes reported are specified in methods |
| Other bias | Low risk | No significant baseline imbalances; no other sources of bias |
| Overall risk of bias | Unclear risk | > 1 domain as unclear risk of bias |
Roosevelt 1996.
| Methods | Parallel design, 2 arms Single‐centre, conducted in the US (The Children's Memorial Hospital, Chicago) |
|
| Participants | Inpatients Inclusion/exclusion criteria Inclusion criteria: age < 12 months; bronchiolitis (lower respiratory tract infection characterised by wheezing); 1st episode of wheezing; requiring inpatient management; examined in ED Exclusion criteria: age: < 4 weeks old; needing admission to ICU; history of congenital heart disease; history of intubation, ventilation, or O2 therapy Participant characteristics All groups Sample size: randomised (N): 122, analysed ‐ trial primary outcomes (N): 118 (per protocol analysis was performed) GROUP 1 Sample size: randomised (N): NR, analysed ‐ trial primary outcomes (N): 65 Age, mean ± SD: 5.3 ± 3.7 months Males, N (%): 41 (63) RSV status: 39 (60) positive Atopic status: 26 (40) present (family) GROUP 2 Sample size: randomised (N): NR, analysed ‐ trial primary outcomes (N): 53 Age, mean ± SD: 5.0 ± 2.5 months Males, N (%): 33 (62) RSV status: 40 (76) positive Atopic status: 23 (43) present (family) |
|
| Interventions |
GROUP 1 (with glucocorticoid)
Drug name: dexamethasone
Dose: 1 mg/kg
Mode of administration: IM
Timing/duration: every 24 hours for max 3 doses GROUP 2 Drug name: placebo (saline) Dose: equivalent volume Mode of administration: IM Timing/duration: every 24 hours for max 3 doses Additional co‐interventions for all groups: left at the discretion of physician Protocolised use of bronchodilators with glucocorticoids: no |
|
| Outcomes |
Primary outcome
Time to resolution (number of 12‐hour periods needed for the following criteria to be met: SaO2 > 95% while receiving no supplemental oxygen, accessory muscle score of 0, a wheeze of 0 or 1, and resumption of normal feeding) Outcomes used to calculate sample size Time to resolution; duration of O2 therapy Secondary outcomes Use of co‐interventions; clinical scale adapted from Schuh et al: 6‐point score based on accessory muscle use and wheeze*; SaO2*; respiratory rate*; heart rate*; blood pressure*; temperature*; occult blood in the stool; return healthcare visits#; hospital re‐admissions#; symptoms# *time points: every 12 hours until resolution #time points: 10 to 14 d |
|
| Funding | Green Bay Foundation ‐ James P Gorter Family Fund | |
| Notes | Study did not report any study‐level subgroup analyses This study contributed to the following comparisons in this review: steroid versus placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; no further information provided |
| Allocation concealment (selection bias) | Low risk | "The hospital pharmacy prepared and coded drug and placebo." |
| Blinding (performance bias and detection bias) Health care use ((re)admissions, LOS, return visits) | Unclear risk | Double‐blind; "investigators were unaware of treatment allocation" |
| Blinding (performance bias and detection bias) Patient/parent‐reported outcomes (symptoms, QoL) | Unclear risk | Double‐blind; "investigators were unaware of treatment allocation" |
| Blinding (performance bias and detection bias) Other outcomes (adverse events, others) | Unclear risk | Double‐blind; "investigators were unaware of treatment allocation" |
| Incomplete outcome data (attrition bias) Health care use ((re)admissions, LOS, return visits) | Low risk | Four participant exclusions, motives reported |
| Incomplete outcome data (attrition bias) Patient/parent‐reported outcomes (symptoms, QoL) | High risk | Patient‐reported data missing for 29 participants, no motives reported |
| Incomplete outcome data (attrition bias) Other outcomes (adverse events, others) | Low risk | Four participant exclusions, motives reported |
| Selective reporting (reporting bias) | Low risk | Published report includes all pre‐specified and expected outcomes; study protocol was not available |
| Other bias | Low risk | No significant baseline imbalances; no other sources of bias |
| Overall risk of bias | High risk | > 1 domain as high risk of bias |
Schuh 2002.
| Methods | Parallel design, 2 arms Single‐centre, conducted in Canada (Hospital for Sick Children, University of Toronto) |
|
| Participants | Outpatients (paediatric emergency department) Inclusion/exclusion criteria Inclusion criteria: age 8 weeks to 23 months, 1st wheezing episode associated with respiratory distress and URTI; RDAI ≥ 6 at baseline Exclusion criteria: history of wheezing or bronchodilator therapy; prematurity; neonatal ventilation; chronic lung/cardiac disease; aspiration, neurologic/neuromuscular problems; immunodeficiency; critically ill infants requiring immediate airway stabilisation; previous oral or inhaled glucocorticoids; exposed to varicella < 21 days of arrival Participant characteristics All groups Sample size: randomised (N): 71, analysed ‐ trial primary outcomes (N): 70 (ITT with available case analysis was performed), analysed ‐ review primary outcomes (N): 67 (ITT with available case analysis was performed) GROUP 1 Sample size: randomised (N): NR, analysed ‐ trial primary outcomes (N): 36, analysed ‐ review primary outcomes (N): 35 Age, mean ± SD: 6.1 ± 3.5 months Males, N (%): 20 (56) RSV status: 15/28 positive Atopic status: 30 (83) present (infant) GROUP 2 Sample size: randomised (N): NR, analysed ‐ trial primary outcomes (N): 34, analysed ‐ review primary outcomes (N): 32 Age, mean ± SD: 6.9 ± 3.9 months Males, N (%): 23 (68) RSV status: 15/30 positive Atopic status: 18 (53) present (infant) |
|
| Interventions |
GROUP 1 (with glucocorticoid)
Drug name: dexamethasone (prepared from the intravenous dexamethasone solution flavoured with wild cherry syrup)
Dose: 1 mg/kg (first dose) and then 0.6 mg/kg/day (if discharged)
Mode of administration: oral
Timing/duration: single dose if admitted, 5 days if discharged GROUP 2 Drug name: placebo Dose: identical colour, texture, taste and smell Mode of administration: oral Timing/duration: single dose if admitted, 5 days if discharged Additional co‐interventions for all groups: nebulised albuterol 2.5 mg/dose in 3 mL normal saline with oxygen flow of 6 to 7 L/minute at 0, 30, 60 and 120 minutes during the observation period; albuterol (1.5 mg to 0.3 mL) 4 times daily with the same nebuliser if discharged home. All decisions regarding the need for further treatment and hospitalisation were made by the attending physicians not involved in the study; they were requested not to administer additional therapy (other than acetaminophen for fever) unless the patient’s condition deteriorated significantly; use of bronchodilators is reported. Hospitalised patients were given nebulised albuterol only and supportive treatment as indicated Protocolised use of bronchodilators with glucocorticoids: yes (salbutamol) |
|
| Outcomes |
Primary outcome/outcome used to calculate sample size
Clinical scale: Respiratory Assessment Change Score, a change score based on RDAI and respiratory rate change from baseline (at 240'; other time points*) Secondary outcomes Hospital admission (at 4 hours, 7 days and 28 days); RDAI: 17‐point score based on wheezing and retractions*; respiratory rate*; SaO2 (4 hours); heart rate (4 hours); additional treatments#; return healthcare visits# *time points: 60', 120', 180', 240', 7 days #time points: 7 days, 28 days |
|
| Funding | Grants from the Medical Research Council of Canada and Merck Frosst, Canada | |
| Notes | Study did not report any study‐level subgroup analyses This study contributed to the following comparisons in this review: steroid versus placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A blocked randomization code was prepared by our pharmacy from a computer generated list of random numbers." |
| Allocation concealment (selection bias) | Low risk | "The pharmacy prepared sequential sealed packets containing the experimental drugs. The randomization code was revealed only after all patients had completed the study." |
| Blinding (performance bias and detection bias) Health care use ((re)admissions, LOS, return visits) | Low risk | "The experimental dexamethasone syrup was prepared from the intravenous dexamethasone solution flavored with wild cherry syrup; the latter flavoring was also given to the group taking the placebo. The active therapy and placebo were of identical color, texture, taste, and smell. The identity of the treatment assignment was completely masked to patients, family, clinicians, and research personnel with the exception of the research pharmacists." |
| Blinding (performance bias and detection bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | "The experimental dexamethasone syrup was prepared from the intravenous dexamethasone solution flavored with wild cherry syrup; the latter flavoring was also given to the group taking the placebo. The active therapy and placebo were of identical color, texture, taste, and smell. The identity of the treatment assignment was completely masked to patients, family, clinicians, and research personnel with the exception of the research pharmacists." |
| Blinding (performance bias and detection bias) Patient/parent‐reported outcomes (symptoms, QoL) | Low risk | "The experimental dexamethasone syrup was prepared from the intravenous dexamethasone solution flavored with wild cherry syrup; the latter flavoring was also given to the group taking the placebo. The active therapy and placebo were of identical color, texture, taste, and smell. The identity of the treatment assignment was completely masked to patients, family, clinicians, and research personnel with the exception of the research pharmacists." |
| Incomplete outcome data (attrition bias) Health care use ((re)admissions, LOS, return visits) | Low risk | 1 participant exclusion, 3 with missing outcome data, motives reported |
| Incomplete outcome data (attrition bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | 1 participant exclusion, 3 with missing outcome data, motives reported |
| Incomplete outcome data (attrition bias) Patient/parent‐reported outcomes (symptoms, QoL) | Unclear risk | Missing symptom data by day 28 for 6 participants, imbalanced, no motives for attrition reported |
| Selective reporting (reporting bias) | Unclear risk | Some outcomes specified in methods not reported in results |
| Other bias | Unclear risk | Significant difference in baseline for family history of atopy; contamination ‐ 22% of placebo received corticosteroids; 9 protocol violations (did not pursue therapy at home) |
| Overall risk of bias | Unclear risk | > 1 domain as unclear risk of bias |
Teeratakulpisarn 2007.
| Methods | Parallel design, 2 arms Multi‐centre (2), conducted in Thailand (2 tertiary hospitals in the northeast) |
|
| Participants | Inpatients Inclusion/exclusion criteria Inclusion criteria: age 4 week to 24 months; 1st episode wheezing with tachypnoea; increased respiratory effort; URTI; criteria for hospitalisation: < 3 months, respiratory rate > 60 bpm (< 12 months) or > 50 bpm (≥ 12 months), SaO2 < 95%, apathy/refusal to eat Exclusion criteria: symptoms > 7 d; admission to ICU with intubation; history of: intubation, asthma, atopy with good response to 1st dose β2‐agonist; therapy with glucocorticoid < 2 weeks; contraindication to glucocorticoid therapy; premature birth Participant characteristics All groups Sample size: randomised (N): 179, analysed ‐ trial/review primary outcomes (N): 174 (per protocol analysis was performed) GROUP 1 Sample size: randomised (N): 90, analysed ‐ trial/review primary outcomes (N): 89 Age, mean ± SD: 10.2 ± 5.5 months Males, N (%): 55 (62) Atopic status: 26 (29) present (family) GROUP 2 Sample size: randomised (N): 89, analysed ‐ trial/review primary outcomes (N): 85 Age, mean ± SD: 11.2 ± 5.9 months Males, N (%): 55 (65) Atopic status: 24 (28) present (family) RSV status: NR |
|
| Interventions |
GROUP 1 (with glucocorticoid)
Drug name: dexamethasone
Dose: 0.6 mg/kg
Mode of administration: intramuscular injection
Timing/duration: 1 dose GROUP 2 Drug name: placebo Dose: equivalent volume of saline Mode of administration: intramuscular injection Timing/duration: 1 dose Additional co‐interventions for all groups: use of epinephrine, β2‐agonist nebulisation, O2 permitted and reported (both study groups were similarly treated following the National Treatment Guidelines for Acute Respiratory Infection in Children, Thailand); also reported use of antibiotics. The investigators monitored the treatment regimens in order to avoid any additional form of glucocorticoid being added to either group until the study endpoint was reached Protocolised use of bronchodilators with glucocorticoids: no |
|
| Outcomes |
Primary outcome/outcome used to calculate sample size
Time from the study entry to resolution of respiratory distress, recognised by a total clinical score of 3 and an oxygen saturation 95% at room air together with a respiratory rate score of 0 or 1, a wheezing score of 0 or 1, and a retraction muscle score of 0 or 1. Clinical scale developed for this trial, modified from De Boeck et al. and Tal et al ‐ 12‐point score based on respiratory rate, wheezing, accessory respiratory muscle retraction and oxygen saturation* Secondary outcomes Duration of O2 therapy; LOS; hospital re‐admission#, return healthcare visits#; duration of symptoms#; adverse events; additional medications *time points: every 6 hours until the study endpoint was reached #time points: 2‐week intervals, until 1 month |
|
| Funding | Grant sponsor: The National Research Council of Thailand | |
| Notes | Study reported subgroup analysis in children under 12 months; no specific interaction term This study contributed to the following comparisons in this review: steroid versus placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Mixed, permuted, block randomization, using a computer‐generated number, was performed for each site and prepared by an off‐site investigator." |
| Allocation concealment (selection bias) | Low risk | "...randomization ... prepared by an off‐site investigator. The study vials were prepared, numbered, and sealed by a pharmacist according to the randomization numbers. The treatment allocation was concealed from the investigators, the attending pediatricians and all of the health personnel involved in patient care." |
| Blinding (performance bias and detection bias) Health care use ((re)admissions, LOS, return visits) | Low risk | Double‐blind; "container of identical appearance" |
| Blinding (performance bias and detection bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | Double‐blind; "container of identical appearance" |
| Blinding (performance bias and detection bias) Patient/parent‐reported outcomes (symptoms, QoL) | Low risk | Double‐blind; "container of identical appearance" |
| Blinding (performance bias and detection bias) Other outcomes (adverse events, others) | Low risk | Double‐blind; "container of identical appearance" |
| Incomplete outcome data (attrition bias) Health care use ((re)admissions, LOS, return visits) | Low risk | Five participants with missing outcome data; some motives reported |
| Incomplete outcome data (attrition bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | Five participants with missing outcome data; some motives reported |
| Incomplete outcome data (attrition bias) Patient/parent‐reported outcomes (symptoms, QoL) | Low risk | Five participants with missing outcome data; some motives reported |
| Incomplete outcome data (attrition bias) Other outcomes (adverse events, others) | Low risk | Five participants with missing outcome data; some motives reported |
| Selective reporting (reporting bias) | Unclear risk | All outcomes specified in methods and results, subgroups not mentioned in methods |
| Other bias | Low risk | No significant baseline imbalances; no other sources of bias |
| Overall risk of bias | Unclear risk | > 1 domain as unclear risk of bias |
Zhang 2003.
| Methods | Parallel design, 2 arms Single‐centre, conducted in Brazil (teaching hospital of the Federal University of Rio Grande, Rio Grande) |
|
| Participants | Inpatients (30‐bed paediatric inpatient ward) Inclusion/exclusion criteria Inclusion criteria: age < 12 months, diagnosis of bronchiolitis, 1st episode of wheezing with respiratory distress, history of upper respiratory tract infection Exclusion criteria: age < 4 weeks; any chronic cardiac or pulmonary disease; congenital abnormality; immediate favourable response to administration of single dose nebulised fenoterol; received glucocorticoids < 4 weeks; severe initial disease requiring intensive care Participant characteristics All groups Sample size: randomised (N): 52, analysed ‐ trial primary outcomes (N): 50 (ITT with available case analysis was performed), analysed ‐ review primary outcomes (N): 52 (ITT with all data was performed) GROUP 1 Sample size: randomised (N): 28, analysed ‐ trial primary outcomes (N): 26 , analysed ‐ review primary outcomes (N): 28 Age, mean ± SD: 4.0 ± 2.5 months Males, N (%): 21 (75) Atopic status: 23 (82.1) present (family) GROUP 2 Sample size: randomised (N): 24, analysed ‐ trial/review primary outcomes (N): 24 Age, mean ± SD: 3.4 ± 1.8 months Males, N (%): 20 (83.3) Atopic status: 21 (87.5) present (family) RSV status: NR |
|
| Interventions |
GROUP 1 (with glucocorticoid)
Drug name: prednisolone + standard care (see below)
Dose: 1 mg/kg
Mode of administration: oral
Timing/duration: 1st at enrolment; once daily at 8:00 am for 4 days (total 5 days of treatment); if hospital stay < 5 d, remaining doses given at home GROUP 2 Drug name: standard care Dose: judged by attending physician based on standard protocol: O2 therapy, fluid replacement, nebulised fenoterol Mode of administration: NR Timing/duration: NR Additional co‐interventions for all groups: standard care as stated above; attending paediatricians were advised against prescribing any glucocorticoid for recruited patients (use of IV hydrocortisone was reported) Protocolised use of bronchodilators with glucocorticoids: no |
|
| Outcomes |
Primary outcome/outcome used to calculate sample size
prevalence of post bronchiolitic wheeze (at 1, 3, 6, 12 mo) Secondary outcomes LOS; duration of O2 therapy; time to clinical resolution ‐ defined as the days needed for the following criteria to be met: pulse blood oxygen saturation above 95% without supplemental oxygen, absence of chest retractions and respiratory rate less than upper limits for age (< 2 months 60 bpm; 2 to 12 months 50 bpm) |
|
| Funding | Research Support Foundation of Rio Grande do Sul | |
| Notes | Study did not report any study‐level subgroup analyses This study contributed to the following comparisons in this review: steroid versus placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random‐number table generated randomization list" |
| Allocation concealment (selection bias) | Low risk | "The independent pharmacy staff members were responsible for group assignment and distribution of prednisolone"; "The randomization list was concealed until the study was complete." |
| Blinding (performance bias and detection bias) Health care use ((re)admissions, LOS, return visits) | High risk | Authors report that "All study investigators were blinded to treatment assignment throughout the study." However, no blinded placebo comparator is used |
| Blinding (performance bias and detection bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | High risk | Authors report that "All study investigators were blinded to treatment assignment throughout the study." However, no blinded placebo comparator is used |
| Blinding (performance bias and detection bias) Patient/parent‐reported outcomes (symptoms, QoL) | High risk | Authors report that "All study investigators were blinded to treatment assignment throughout the study." However, no blinded placebo comparator is used |
| Blinding (performance bias and detection bias) Other outcomes (adverse events, others) | High risk | Authors report that "All study investigators were blinded to treatment assignment throughout the study." However, no blinded placebo comparator is used |
| Incomplete outcome data (attrition bias) Health care use ((re)admissions, LOS, return visits) | Low risk | No missing outcome data |
| Incomplete outcome data (attrition bias) Clinical parameters (severity scales, SpO2, respiratory and heart rate) | Low risk | No missing outcome data |
| Incomplete outcome data (attrition bias) Patient/parent‐reported outcomes (symptoms, QoL) | Low risk | Two participants with missing long‐term symptom outcome data |
| Incomplete outcome data (attrition bias) Other outcomes (adverse events, others) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published report includes all pre‐specified and expected outcomes; study protocol was not available |
| Other bias | Low risk | No significant baseline imbalances; no other sources of bias |
| Overall risk of bias | High risk | > 1 domain as high risk of bias |
(Support for 'Risk of bias' judgement is shown for domains with unclear risk)
bpm = beats per minute d = day ED = emergency department h = hour ICU = intensive care unit IM = intramuscular IQR = interquartile range ITT = intention‐to‐treat IV = intravenous LOS = length of stay mo = month NR = not reported qid = four times a day RDAI = Respiratory Distress Assessment Instrument RSV = respiratory syncytial virus SD = standard deviation SaO2 = oxygen saturation tx = treatment URTI = upper respiratory tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anonymous 1989 | Publication type: commentary |
| Bacharier 2008 | Population: children aged 12 to 59 months with moderate‐to‐severe recurrent intermittent wheezing |
| Bai 2010 | Outcome: reported outcomes were recurrent wheezing and asthma |
| Berger 1996 | Publication type and duplicate: abstract of later published trial |
| Bibi 2004 | Population: clinical definition of bronchiolitis uncertain |
| Blom 2007 | Publication type: Cochrane systematic review |
| Bont 2006 | Publication type: letter to editor |
| Buckingham 2002 | Population: intensive care unit patients who were intubated and mechanically ventilated |
| Bülow 1999 | Population: clinical definition of bronchiolitis uncertain |
| Callen Blecua 2000 | Intervention:inhaled glucocorticoid therapy given for 3 months after mild bronchiolitis |
| Chao 2003 | Population: clinical definition of bronchiolitis uncertain |
| Chipps 2008 | Publication type: letter to editor |
| Connolly 1969 | Population: clinical definition of bronchiolitis uncertain |
| Cornell 2007 | Publication type: review |
| Csonka 2003 | Population: children aged 6 to 35 months, clinical definition of bronchiolitis uncertain and recurrent wheezing |
| da Silva 2007 | Article pending retrieval |
| Dabbous 1966 | Population: clinical definition of bronchiolitis uncertain |
| Daugbjerg 1993 | Population: history of recurrent wheezing |
| Dennis 1963 | Study design: not randomised |
| Doornebal 2009 | Study design: not randomised |
| Ermers 2008 | Population: clinical definition of bronchiolitis uncertain and some participants required mechanical ventilation |
| Ermers 2009 | Intervention: 3 months inhaled glucocorticoids |
| Filippskii 1983 | Population: children aged 0 months to 3 years |
| Fox 1999 | Population: clinical definition of bronchiolitis uncertain and history of recurrent wheezing |
| Garrison 2000 | Publication type: meta‐analysis |
| Gerasymov 2010 | Intervention: same glucocorticoid, dose‐comparison |
| Hall 2008 | Publication type: commentary |
| Hoekstra 2004 | Publication type: letter to editor |
| Jartti 2002 | Publication type: review |
| Jartti 2006 | Population: first or second episode of wheezing |
| Jartti 2007 | Population: children aged 3 months to 16 years, clinical definition of bronchiolitis uncertain and recurrent wheezing |
| Jartti 2011 | Study design: post hoc analysis of previous RCT |
| Kajosaari 2000 | Population: clinical definition of bronchiolitis uncertain |
| Karaatmaca 2010 | Population: first or second episode of wheezing |
| Kelm‐Kahl 2008 | Publication type: review |
| Kitowicz 2007 | Publication type: review |
| Koumbourlis 2009 | Publication type: letter to editor |
| Leer 1969a | Population: children aged 0 to 30 months, clinical definition of bronchiolitis uncertain and recurrent wheezing |
| Leer 1969b | Publication type: letter to editor |
| Lin 1991 | Population: ages below 36 months |
| Lukkarinen 2011 | Study design: post hoc analysis of previous RCT |
| Mallol 1987 | Population: clinical definition of bronchiolitis uncertain |
| Martini 2009 | Unobtainable |
| Merkus 2005 | Publication type: letter to editor |
| Milner 1997 | Publication type: letter to editor |
| O'Callaghan 1989 | Publication type: letter to editor |
| Oommen 2003 | Publication type: children aged 1 to 5 years with recurrent episodic viral wheeze |
| Oski 1961 | Study design: not randomised |
| Panickar 2009 | Population: children between the ages of 10 months and 60 months, possibly with recurrent wheezing |
| Park 1997 | Population: history of recurrent wheezing |
| Patel 2004 | Publication type: Cochrane systematic review |
| Patel 2008 | Duplicate: abstract of RCT by Plint et al (included) |
| Plint 2008 | Duplicate: abstract of RCT by Plint et al (included) |
| Poets 2005 | Publication type: review |
| Principi 2011 | Publication type: commentary |
| Rajeshwari 2006 | Publication type: commentary |
| Ranganathan 2003 | Publication type: review |
| Renzi 2003 | Unobtainable (abstract from conference proceedings) |
| Sammartino 1995 | Publication type: letter to editor |
| Sano 2000 | Population: history of recurrent wheeze |
| Schuh 2004 | Publication type: commentary |
| Schuh 2008 | Intervention: single versus multiple doses of the same glucocorticoid (dexamethasone) |
| Smart 2009 | Publication type: commentary |
| Smith 2008 | Publication type: review |
| Spencer 1989 | Publication type: letter to editor |
| Springer 1990 | Study design: not randomised |
| Sussman 1964 | Study design: not randomised |
| Tal 1982 | Population: history of recurrent wheezing |
| Tofts 2009 | Publication type: letter to editor |
| Uhereczky 2001 | Population: children aged 3 to 36 months, history of recurrent wheezing |
| Van Bever 1996 | Publication type: letter to editor |
| van Woensel 1997 | Publication type: letter to editor |
| van Woensel 2000 | Population and intervention: not a study of acute effects of glucocorticoids; this study measured the follow‐up incidence of recurrent wheezing in treated infants |
| van Woensel 2003a | Population: involved intensive care unit patients who were intubated and mechanically ventilated |
| van Woensel 2003b | Population: involved intensive care unit patients who were intubated and mechanically ventilated |
| van Woensel 2011 | Population: involved intensive care unit patients who were intubated and mechanically ventilated |
| Wardrope 2000 | Duplicate: RCT by Cade et al (included) |
| Webb 1986 | Publication type: history of recurrent wheezing |
| Weinberger 2004 | Publication type: letter to editor |
| Weinberger 2007 | Publication type: letter to editor |
| Wong 2000 | Intervention: 3 months inhaled glucocorticoids |
| Yaffe 1970 | Publication type: commentary |
| Zhu 2009 | Study design: uncertain |
| Zuerlein 1990 | Population: children aged 5 to 27 months, clinical definition of bronchiolitis uncertain |
RCT = randomised controlled trial
Differences between protocol and review
The protocol limited the selection to studies testing systemic glucocorticoids only; we later decided to also include studies with inhaled glucocorticoids. Not all planned subgroup analyses were performed due to the reduced number of trials and data heterogeneity.
Contributions of authors
Ricardo M Fernandes (RF): guarantor of the review, involved at all phases. Contribution: review update design and implementation, search strategy, screening of search results, data extraction and entry, 'Risk of bias' and GRADE assessments, data analysis, interpretation of results, manuscript writing and revision. Liza M Bialy (LB): screening results, data extraction and entry, 'Risk of bias' assessments, manuscript writing and revision. Ben Vandermeer (BV): review update design, data entry and analysis, manuscript revision. Lisa Tjosvold (LT): search strategy and implementation, article retrieval, manuscript revision. Amy C Plint (AC): review update, screening of search results, interpretation of results, manuscript revision. Hema Patel (HP): protocol design, screening of search results, interpretation of results, manuscript revision. Responsible for the previous Cochrane review (2004). David W Johnson (DJ): review update, screening of search results, interpretation of results, manuscript revision. Terry P Klassen (TK): review update, interpretation of results, manuscript revision. Lisa Hartling (LH): review update and implementation, screening of search results, 'Risk of bias' and GRADE assessments, interpretation of results, manuscript writing and revision.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Canadian Institutes of Health Research, Canada.
Knowledge Synthesis Grant (FRN 91767)
-
Programme for Advanced Medical Education (Fundação Calouste Gulbenkian, Fundação Champalimaud, Ministério da Saúde and Fundação para a Ciência e Tecnologia), Portugal.
Fellowship (Ricardo M Fernandes)
-
Cochrane Incentives Funding Scheme, UK.
Grant
Declarations of interest
AP, HP, DJ and TK are authors of one or more RCTs included in this review. Assessment of eligibility, risk of bias and strength of evidence of these trials were performed by other review authors. The review authors declare no other real or perceived conflicts of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Barlas 1998 {published data only}
- Barlas C, Kiper N, Göçmen A, Ozçelik U, Dilber E, Anadol D, et al. Racemic adrenaline and other treatment regimens in mild and moderate bronchiolitis. Cocuk Sagligi Ve Hastaliklari Dergisi 1998;41(2):155‐66. [Google Scholar]
Barlas 1998 (G+S vs S) {published data only}
- Barlas C, Kiper N, Göçmen A, Ozçelik U, Dilber E, Anadol D, et al. Racemic adrenaline and other treatment regimens in mild and moderate bronchiolitis. Cocuk Sagligi Ve Hastaliklari Dergisi 1998;41(2):155‐66. [Google Scholar]
Barlas 1998 (G vs P) {published data only}
- Barlas C, Kiper N, Göçmen A, Ozçelik U, Dilber E, Anadol D, et al. Racemic adrenaline and other treatment regimens in mild and moderate bronchiolitis. Cocuk Sagligi Ve Hastaliklari Dergisi 1998;41(2):155‐66. [Google Scholar]
Bentur 2005 {published data only}
- Bentur L, Shoseyov D, Feigenbaum D, Gorichovsky Y, Bibi H. Dexamethasone inhalations in RSV bronchiolitis: a double‐blind, placebo‐controlled study. Acta Paediatrica 2005;94(7):866‐71. [DOI] [PubMed] [Google Scholar]
Berger 1998 {published data only}
- Berger I, Argaman Z, Schwartz SB, Segal E, Kiderman A, Branski D, et al. Efficacy of glucocorticoids in acute bronchiolitis: short‐term and long‐term follow‐up. Pediatric Pulmonology 1998;26:162‐6. [DOI] [PubMed] [Google Scholar]
Cade 2000 {published data only}
- Cade A, Brownlee KG, Conway SP, Haigh D, Short A, Brown J, et al. Randomised placebo controlled trial of nebulised glucocorticoids in acute respiratory syncytial viral bronchiolitis. Archives of Disease in Childhood 2000;82(2):126‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corneli 2007 {published data only}
- Corneli HM, Zorc JJ, Mahajan P, Shaw KN, Holubkov R, Reeves SD, et al. A multicenter, randomized, controlled trial of dexamethasone for bronchiolitis. New England Journal of Medicine 2007;357(4):331‐9. [DOI] [PubMed] [Google Scholar]
De Boeck 1997 {published data only}
- Boeck K, Aa N, Lierde S, Corbeel L, Eeckels R. Respiratory syncytial virus bronchiolitis: a double‐blind dexamethasone efficacy study. Journal of Pediatrics 1997;131(6):919‐21. [DOI] [PubMed] [Google Scholar]
Goebel 2000 {published data only}
- Goebel J, Estrada B, Quinonez J, Nagi N, Sanford D, Boerth RC. Prednisolone plus albuterol versus albuterol alone in mild to moderate bronchiolitis. Clinical Pediatrics 2000;39(4):213‐20. [DOI] [PubMed] [Google Scholar]
Gomez 2007 {published data only}
- Gomez‐y‐Lopez RE, Hernandez‐Sierra JF, Torres‐Ruvalcaba BA, Martinez‐Puente E, Carmen Martinez‐Garcia M. Comparative clinical study of dexamethasone vs. nebulized salbutamol in acute bronchiolitis. Gaceta Medica de Mexico 2007;143(3):189‐92. [PubMed] [Google Scholar]
Klassen 1997 {published data only}
- Klassen TP, Sutcliffe T, Watters LK, Wells GA, Allen UD, Li MM. Dexamethasone in salbutamol‐treated inpatients with acute bronchiolitis: a randomized, controlled trial. Journal of Pediatrics 1997;130(2):191‐6. [DOI] [PubMed] [Google Scholar]
Kuyucu 2004 {published data only}
- Kuyucu S, Unal S, Kuyucu N, Yilgor E. Additive effects of dexamethasone in nebulized salbutamol or L‐epinephrine treated infants with acute bronchiolitis. Pediatrics International 2004;46(5):539‐44. [DOI] [PubMed] [Google Scholar]
Kuyucu 2004 (G+E vs P+E) {published data only}
- Kuyucu S, Unal S, Kuyucu N, Yilgor E. Additive effects of dexamethasone in nebulized salbutamol or L‐epinephrine treated infants with acute bronchiolitis. Pediatrics International 2004;46(5):539‐44. [DOI] [PubMed] [Google Scholar]
Kuyucu 2004 (G+S vs P+S) {published data only}
- Kuyucu S, Unal S, Kuyucu N, Yilgor E. Additive effects of dexamethasone in nebulized salbutamol or L‐epinephrine treated infants with acute bronchiolitis. Pediatrics International 2004;46(5):539‐44. [DOI] [PubMed] [Google Scholar]
Mesquita 2009 {published data only}
- Mesquita M, Castro‐Rodriguez JA, Heinichen L, Fariña E, Iramain R. Single oral dose of dexamethasone in outpatients with bronchiolitis: a placebo controlled trial. Allergologia et Immunopathologia 2009;37(2):63‐7. [DOI] [PubMed] [Google Scholar]
Plint 2009 {published data only}
- Plint AC, Johnson DW, Patel H, Wiebe N, Correll R, Brant R, et al. Epinephrine and dexamethasone in children with bronchiolitis. New England Journal of Medicine 2009;360(1533‐4406):2079‐89. [DOI] [PubMed] [Google Scholar]
Plint 2009 (G+E vs P+E) {published data only}
- Plint AC, Johnson DW, Patel H, Wiebe N, Correll R, Brant R, et al. Epinephrine and dexamethasone in children with bronchiolitis. New England Journal of Medicine 2009;360(1533‐4406):2079‐89. [DOI] [PubMed] [Google Scholar]
Plint 2009 (G+P vs P+P) {published data only}
- Plint AC, Johnson DW, Patel H, Wiebe N, Correll R, Brant R, et al. Epinephrine and dexamethasone in children with bronchiolitis. New England Journal of Medicine 2009;360(1533‐4406):2079‐89. [DOI] [PubMed] [Google Scholar]
Richter 1998 {published data only}
- Richter H, Seddon P. Early nebulized budesonide in the treatment of bronchiolitis and the prevention of postbronchiolitic wheezing. Journal of Pediatrics 1998;132(5):849‐53. [DOI] [PubMed] [Google Scholar]
Roosevelt 1996 {published data only}
- Roosevelt G, Sheehan K, Grupp‐Phelan J, Tanz RR, Listernic R. Dexamethasone in bronchiolitis: a randomised controlled trial. Lancet 1996;348(9023):292‐305. [DOI] [PubMed] [Google Scholar]
Schuh 2002 {published data only}
- Schuh S, Coates AL, Binnie R, Allin T, Goia C, Corey M, et al. Efficacy of oral dexamethasone in outpatients with acute bronchiolitis. Journal of Pediatrics 2002;140:27‐32. [DOI] [PubMed] [Google Scholar]
Teeratakulpisarn 2007 {published data only}
- Teeratakulpisarn J, Limwattananon C, Tanupattarachai S, Limwattananon S, Teeratakulpisarn S, Kosalaraksa P. Efficacy of dexamethasone injection for acute bronchiolitis in hospitalized children: a randomized, double‐blind, placebo‐controlled trial. Pediatric Pulmonology 2007;42(5):433‐9. [DOI] [PubMed] [Google Scholar]
Zhang 2003 {published data only}
- Zhang L, Ferruzzi E, Bonfanti T, Auler MI, D'Avila NE, Faria CS, et al. Long and short‐term effect of prednisolone in hospitalized infants with acute bronchiolitis. Journal of Paediatrics and Child Health 2003;39(7):548‐51. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anonymous 1989 {published data only}
- Anonymous. Inhaled steroids and recurrent wheeze after bronchiolitis. Lancet 1989;1(8645):999‐1000. [PubMed] [Google Scholar]
Bacharier 2008 {published data only}
- Bacharier LB, Phillips BR, Zeiger RS, Szefler SJ, Martinez FD, Lemanske RFJr, et al. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate‐to‐severe intermittent wheezing. Journal of Allergy & Clinical Immunology 2008;122(6):1127‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bai 2010 {published data only}
- Bai J, Xu PR. Montelukast in the treatment of bronchiolitis, a multi‐center, randomized, three‐blind, placebo‐controlled trial. Chinese Journal of Evidence‐Based Medicine 2010;10:1011‐5. [Google Scholar]
Berger 1996 {published data only}
- Berger I. A double‐blind placebo‐controlled study on the efficacy of corticosteroids in infants with bronchiolitis. European Respiratory Journal 1996;9(Suppl 23):246. [Google Scholar]
Bibi 2004 {published data only}
- Bibi H. A double blind study to determine the benefit of inhaled corticosteroids in bronchiolitis. European Respiratory Journal 2004;24(Suppl 48):A282. [Google Scholar]
Blom 2007 {published data only}
- Blom D, Ermers M, Bont L, Aalderen WM, Woensel JB. Inhaled corticosteroids during acute bronchiolitis in the prevention of post‐bronchiolitic wheezing. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD004881.pub3] [DOI] [PubMed] [Google Scholar]
Bont 2006 {published data only}
- Bont L, Kimpen JL, Ermers MJ. Inhaled corticosteroids and children. New England Journal of Medicine 2006;355(6):624‐5. [PubMed] [Google Scholar]
Buckingham 2002 {published data only}
- Buckingham SC, Jafri HS, Bush AJ, Carubelli CM, Sheeran P, Hardy RD, et al. A randomized, double‐blind, placebo‐controlled trial of dexamethasone in severe respiratory syncytial virus (RSV) infection: effects on RSV quantity and clinical outcome. Journal of Infectious Diseases 2002;185(9):1222‐8. [DOI] [PubMed] [Google Scholar]
Bülow 1999 {published data only}
- Bülow SM, Nir M, Levin E, Friis B, Thomsen LL, Nielson JE, et al. Prednisolone treatment of respiratory syncytial virus infection: a randomized controlled trial of 147 infants. Pediatrics 1999;104(6):e77. [DOI] [PubMed] [Google Scholar]
Callen Blecua 2000 {published data only}
- Callen Blecua M, Aizpurua Galdeano P, Ozcoidi Erro I, Mancisidor Aguinagalde L, Guedea Adiego C, Busselo Ortega E, et al. Inhaled corticosteroids and wheezing post‐bronchiolitis. Anales Españoles de Pediatría 2000;52(4):351‐5. [PubMed] [Google Scholar]
Chao 2003 {published data only}
- Chao LC, Lin YZ, Wu WF, Huang FY. Efficacy of nebulized budesonide in hospitalized infants and children younger than 24 months with bronchiolitis. Acta Paediatrica Taiwan 2003;44(6):332‐5. [PubMed] [Google Scholar]
Chipps 2008 {published data only}
- Chipps BE. A multicenter, randomized, controlled trial of dexamethasone for bronchiolitis. Pediatrics 2008;122(Suppl 4):215. [Google Scholar]
Connolly 1969 {published data only}
- Connolly JH, Field CMB, Glasgow JFT, Slattery CM, MacLynn DM. A double blind trial of prednisolone in epidemic bronchiolitis due to respiratory syncytial virus. Acta Paediatrica Scandinavica 1969;58(2):116‐20. [DOI] [PubMed] [Google Scholar]
Cornell 2007 {published data only}
- Cornell HM, Zorc JJ, Majahan P, Shaw KN, Holubkov R, Reeves SD, et al. Does dexamethasone improve bronchiolitis in infants?. Journal of Family Practice 2007;56(11):890. [PubMed] [Google Scholar]
Csonka 2003 {published data only}
- Csonka P, Kaila M, Laippala P, Iso‐Mustajarvi M, Vesikari T, Ashorn P. Oral prednisolone in the acute management of children age 6 to 35 months with viral respiratory infection‐induced lower airway disease: a randomized, placebo‐controlled trial. Journal of Pediatrics 2003;143(6):725‐30. [DOI] [PubMed] [Google Scholar]
Dabbous 1966 {published data only}
- Dabbous IA, Tkachyk JS, Stamm SJ. A double blind study on the effects of corticosteroids in the treatment of bronchiolitis. Pediatrics 1966;37(3):477‐84. [PubMed] [Google Scholar]
da Silva 2007 {published data only}
- Silva ML. Effectiveness and Safety of Budesonide Nebulised in Control of Acute Crisis of Wheezing in Children Under Three Years not Responsive to Fenoterol [Dissertation]. São Paulo, SP: Universidade Estadual Paulista, 2007. [Google Scholar]
Daugbjerg 1993 {published data only}
- Daugbjerg P, Brenoe E, Forchhammer H, Frederiksen B, Glazowski MJ, Ibsen KK, et al. A comparison between nebulized terbutaline, nebulized corticosteroid and systemic corticosteroid for acute wheezing in children up to 18 months of age. Acta Paediatrica 1993;82(6‐7):547‐51. [DOI] [PubMed] [Google Scholar]
Dennis 1963 {published data only}
- Dennis J. Steroids in the treatment of obstructive bronchiolitis. Southern Medical Journal 1963;56:1436. [Google Scholar]
Doornebal 2009 {published data only}
- Doornebal N, Brand PL. Children younger than 1 year with acute dyspnea and wheezing: don't prescribe oral corticosteroids. Nederlands Tijdschrift voor Geneeskunde 2009;153(10):440‐2. [PubMed] [Google Scholar]
Ermers 2008 {published data only}
- Ermers M, Rovers M, Woensel J, Kimpen J, Bont L. High‐dose inhaled corticosteroids prevent wheeze following respiratory syncytial virus infection: a randomized double‐blind placebo‐controlled trial. European Respiratory Journal 2008;32(Suppl 52):720. [Google Scholar]
Ermers 2009 {published data only}
- Ermers MJJ, Rovers MM, Woensel JB, Kimpen JLL, Bont LJ. The effect of high dose inhaled corticosteroids on wheeze in infants after respiratory syncytial virus infection: randomised double blind placebo controlled trial. BMJ 2009;338:996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Filippskii 1983 {published data only}
- Filippskii GK, Tkacheva NV, Raozhina VI, Ivanov NN, Filimonova II. Possibilities of using glucocorticoid therapy for acute bronchiolitis in very young children. Pediatriia 1983;8:71‐3. [PubMed] [Google Scholar]
Fox 1999 {published data only}
- Fox GF, Everard ML, Marsh MJ, Milner AD. Randomised controlled trial of budesonide for the prevention of post‐bronchiolitis wheezing. Archives of Disease in Childhood 1999;80(4):343‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garrison 2000 {published data only}
- Garrison MM, Christakis DA, Harvey E, Cummings P, Lavis RL. Systemic corticosteroids in infant bronchiolitis: a meta‐analysis. Pediatrics 2000;105(4):e44. [DOI] [PubMed] [Google Scholar]
Gerasymov 2010 {published data only}
- Gerasymov S. High‐dose vs low‐dose nebulized budesonide suspension in a sleeping infant with bronchiolitis [Abstract]. European Respiratory Society Annual Congress Abstract Book. 2010:[E3461].
Hall 2008 {published data only}
- Hall CB. Dexamethasone of no benefit in moderate‐to‐severe bronchiolitis. Journal of Pediatrics 2008;152(1):143‐4. [DOI] [PubMed] [Google Scholar]
Hoekstra 2004 {published data only}
- Hoekstra MO, Kapitein B, Ent CK, Arets HGM. Are inhaled corticosteroids safe and effective in infants with asthma‐like symptoms?. Pediatrics 2004;114(1):325. [DOI] [PubMed] [Google Scholar]
Jartti 2002 {published data only}
- Jartti T, Vanto T, Heikkinen T, Ruuskanen O. Systemic glucocorticoids in childhood expiratory wheezing: relation between age and viral etiology with efficacy. Pediatric Infectious Disease Journal 2002;21(9):873‐8. [DOI] [PubMed] [Google Scholar]
Jartti 2006 {published data only}
- Jartti T, Lehtinen P, Vanto T, Hartiala J, Vuorinen T, Makela MJ, et al. Evaluation of the efficacy of prednisolone in early wheezing induced by rhinovirus or respiratory syncytial virus. Pediatric Infectious Disease Journal 2006;25(6):482‐8. [DOI] [PubMed] [Google Scholar]
Jartti 2007 {published data only}
- Jartti T, Lehtinen P, Vanto T, Vuorinen T, Hiekkanen H, Hartiala J, et al. Atopic characteristics of wheezing children and responses to prednisolone. Pediatric Pulmonology 2007;42(12):1125‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jartti 2011 {published data only}
- Jartti T, Soderlund‐Venermo M, Allander T, Vuorinen T, Hedman K, Ruuskanen O. No efficacy of prednisolone in acute wheezing associated with human bocavirus infection. Pediatric Infectious Disease Journal 2011;30:521‐3. [DOI] [PubMed] [Google Scholar]
Kajosaari 2000 {published data only}
- Kajosaari M, Syvanen P, Forars M, Juntunen‐Backman K. Inhaled corticosteroids during and after respiratory syncytial virus‐bronchiolitis may decrease subsequent asthma. Pediatric Allergy and Immunology 2000;11(3):198‐202. [DOI] [PubMed] [Google Scholar]
Karaatmaca 2010 {published data only}
- Karaatmaca B, Aydoan M, Piraneci R, Bicnuller S. Comparison of the effectiveness of nebulized budesonide, salbutamol and adrenaline in the treatment of acute bronchiolitis: a randomized double blind placebo controlled clinical trial. Allergy: European Journal of Allergy and Clinical Immunology 2010;65(Suppl 92):716‐7. [Google Scholar]
Kelm‐Kahl 2008 {published data only}
- Kelm‐Kahl I. A single dose of corticosteroids has no influence on the rate of hospital admission for children with bronchiolitis. Pneumologie 2008;62(2):63‐4. [Google Scholar]
Kitowicz 2007 {published data only}
- Kitowicz A, Criswell DF. Do inhaled corticosteroids improve oxygen saturation in infants with respiratory syncytial virus (RSV) bronchiolitis?. Journal of the Oklahoma State Medical Association 2007;100(7):266. [PubMed] [Google Scholar]
Koumbourlis 2009 {published data only}
- Koumbourlis AC. Oral corticosteroids in children with wheezing. New England Journal of Medicine 2009;360(16):1673. [DOI] [PubMed] [Google Scholar]
Leer 1969a {published data only}
- Leer JA, Bloomfield N, Green JL, Heimlick EM, Hyde JS, Moffett HL, et al. Corticosteroids treatment in bronchiolitis. American Journal of Diseases of Children 1969;117(5):495‐503. [PubMed] [Google Scholar]
Leer 1969b {published data only}
- Leer JA. Corticosteroids in bronchiolitis. JAMA 1969;208(6):1016‐7. [PubMed] [Google Scholar]
Lin 1991 {published data only}
- Lin YZ, Hsieh KH, Chen W, Wu KW. Clinical trial of corticosteroid and beta‐2 bronchodilator in acute wheezing infants. Chung‐Hua Min Kuo Hsiao Erh Ko i Hsueh Hui Tsa Chih 1991;32(6):333‐40. [PubMed] [Google Scholar]
Lukkarinen 2011 {published data only}
- Lukkarinen M, Lukkarinen H, Vuorinen T, Tuomas J. Prednisolone treatment of the first wheezing episode associated with rhinovirus infection reduces asthma in a 5‐year follow‐up. Allergy: European Journal of Allergy and Clinical Immunology 2011;66(Suppl 94):157. [Google Scholar]
Mallol 1987 {published data only}
- Mallol J, Munoz R, Puppo H, Ulloa V, Toro O, Girardi G, et al. Effects of nebulized fenoterol, associated with ipratropium or glucocorticoids, on the heart rate of infants under one year of age with acute wheezing. Pediatric Pulmonology 1987;3(2):83‐5. [DOI] [PubMed] [Google Scholar]
Martini 2009 {published data only}
- Martini ABC. Viral respiratory tract infection: oral glucocorticoids not effective in wheezing children. Virale Atemwegsinfektioneh: orale glucocorticoide bei kindern mit giemen nicht effektiv 2009;149:40‐1. [Google Scholar]
Merkus 2005 {published data only}
- Merkus PJFM, Jongste JC, Teper AM, Kofman CD, Vidaurreta SM. Inhaled corticosteroids in wheezy infants. American Journal of Respiratory and Critical Care Medicine 2005;172(8):1058‐9. [DOI] [PubMed] [Google Scholar]
Milner 1997 {published data only}
- Milner AD. The role of corticosteroids in bronchiolitis and croup. Thorax 1997;52(7):595‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Callaghan 1989 {published data only}
- O'Callaghan C, Milner AD. Inhaled glucocorticoids and recurrent wheeze after bronchiolitis. Lancet 1989;333(8652):1458. [DOI] [PubMed] [Google Scholar]
Oommen 2003 {published data only}
- Oommen A, Lambert PC, Grigg J. Efficacy of a short course of parent‐initiated oral prednisolone for viral wheeze in children aged 1‐5 years: randomised controlled trial. Lancet 2003;362(9394):1433‐8. [DOI] [PubMed] [Google Scholar]
Oski 1961 {published data only}
- Oski FA, Salitsky S, Barness LA. Steroid therapy in bronchiolitis: a double‐blind study. American Journal of Diseases of Children 1961;102:759‐64. [Google Scholar]
Panickar 2009 {published data only}
- Panickar J, Lakhanpaul M, Lambert PC, Kenia P, Stephenson T, Smyth A, et al. Oral prednisolone for preschool children with acute virus‐induced wheezing. New England Journal of Medicine 2009;360(4):329‐38. [DOI] [PubMed] [Google Scholar]
Park 1997 {published data only}
- Park J. The effect of salbutamol and budesonide inhalation therapy in infants with bronchiolitis. Journal of the Korean Pediatric Society 1997;40(1):45‐54. [Google Scholar]
Patel 2004 {published data only}
- Patel H, Platt R, Lozano JM, Wang EE. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004878.pub2] [DOI] [PubMed] [Google Scholar]
Patel 2008 {published data only}
- Patel H, Plint AC, Johnson DW, Wiebe N, Correll R, Brant R, et al. A multicenter randomized controlled trial of nebulized epinephrine and dexamethasone in outpatients with bronchiolitis. Paediatrics and Child Health 2008;13(Suppl A):Abstract 136. [Google Scholar]
Plint 2008 {published data only}
- Plint AC, Johnston DM, Patel H, Wiebe N, Correll R, Mitton C, et al. A multicenter randomized controlled trial of nebulized epinephrine and dexamethasone in outpatients with bronchiolitis. Academic Emergency Medicine 2008;15(5 Suppl 1):37. [Google Scholar]
Poets 2005 {published data only}
- Poets CF. Review: corticosteroids do not reduce hospital length of stay or respiratory distress in infantile acute viral bronchiolitis. Evidence‐Based Medicine 2005;10(1):20. [Google Scholar]
Principi 2011 {published data only}
- Principi T, Komar L. KiDrug alert journal club. Canadian Journal of Clinical Pharmacology 2011;18:e273‐4. [PubMed] [Google Scholar]
Rajeshwari 2006 {published data only}
- Rajeshwari K. Corticosteroid therapy for wheezy infants. Indian Pediatrics 2006;4(7):662. [Google Scholar]
Ranganathan 2003 {published data only}
- Ranganathan SC, McKenzie SA. The use of corticosteroids in syntomathic asthma in childhood. Minerva Pediatrica 2003;55(4):357‐67. [PubMed] [Google Scholar]
Renzi 2003 {published data only}
- Renzi PM, Marcotte JE, Berube D, Bjornson C, Spier S. Effect of nebulized budesonide for 10 weeks on the development of asthma after acute bronchiolitis in infants. Proceedings of the American Thoracic Society 99th International Conference, 2003 May 16‐21, Seattle WA. 2003:B012.
Sammartino 1995 {published data only}
- Sammartino L. Budesonide in acute bronchiolitis. Journal of Paediatrics and Child Health 1995;31(1):61‐2. [PubMed] [Google Scholar]
Sano 2000 {published data only}
- Sano F, Cortez GK, Sole D, Naspitz CK. Inhaled budesonide for the treatment of acute wheezing and dyspnea in children up to 24 months old receiving intravenous hydrocortisone. Journal of Allergy & Clinical Immunology 2000;105(4):699‐703. [DOI] [PubMed] [Google Scholar]
Schuh 2004 {published data only}
- Schuh S, Coates AL, Binnie R. Efficacy of oral dexamethasone in outpatients with acute bronchiolitis. In: Paediatric emergency research in Canada: using the iterative loop as a research paradigm for advancing the field. Paediatrics and Child Health 2004;9(6):395‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schuh 2008 {published data only}
- Schuh S, Coates AL, Dick P, Stephens D, Lalani A, Nicota E, et al. A single versus multiple doses of dexamethasone in infants wheezing for the first time. Pediatric Pulmonology 2008;43(9):844‐50. [DOI] [PubMed] [Google Scholar]
Smart 2009 {published data only}
- Smart BA. Oral prednisolone for preschool children with acute virus‐induced wheezing. Pediatrics 2009;124(Suppl):151. [DOI] [PubMed] [Google Scholar]
Smith 2008 {published data only}
- Smith J, Salinas R. Do systemic corticosteroids improve acute outcomes in infants with RSV bronchiolitis?. Journal of the Oklahoma State Medical Association 2008;101(1):14. [PubMed] [Google Scholar]
Spencer 1989 {published data only}
- Spencer DA, Gleeson JG, Price JF. Inhaled budesonide for acute wheeze in infants. Lancet 1989;1(8639):665. [DOI] [PubMed] [Google Scholar]
Springer 1990 {published data only}
- Springer C, Bar‐Yishay E, Uwayyed K, Avital A, Vilozni D, Godfrey S. Corticosteroids do not affect the clinical or physiological status of infants with bronchiolitis. Pediatric Pulmonology 1990;9:181‐5. [DOI] [PubMed] [Google Scholar]
Sussman 1964 {published data only}
- Sussman S, Grossman M, Magoffin R, Schieble J. Dexamethasone (16‐alpha‐methyl, 9‐alpha‐flouroprednisolone) in obstructive respiratory tract infections in children. A controlled study. Pediatrics 1964;34:851‐5. [PubMed] [Google Scholar]
Tal 1982 {published data only}
- Tal A, Bavilski C, Yohai D, Bearman JE, Gorodischer R, Moses SW. Dexamethasone and salbutamol in the treatment of acute wheezing in infants. Pediatrics 1983;71(1):13‐8. [PubMed] [Google Scholar]
Tofts 2009 {published data only}
- Tofts RPH. Steroids do not improve RSV‐related wheeze in children. Thorax 2009;64(9):769. [Google Scholar]
Uhereczky 2001 {published data only}
- Uhereczky G, Gács É, Jákly A, Göndöcs R. Inhaled corticosteroids in atopic wheezy infants. Proceedings of the International Paediatric Respiratory and Allergy Congress 2001, April 1‐4, Prague. 2001:84.
Van Bever 1996 {published data only}
- Bever HP. Corticosteroids for bronchiolitis. Lancet 1996;348(9029):753‐4. [DOI] [PubMed] [Google Scholar]
van Woensel 1997 {published data only}
- Woensel JBM, Wolfs TFW, Aalderen WMC, Brand PLP, Kimpen JLL. Randomised double blind placebo controlled trial of prednisolone in children admitted to hospital with respiratory syncytial virus bronchiolitis. Thorax 1997;52:634‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Woensel 2000 {published data only}
- Woensel JB, Kimpen JL, Sprikkelman AB, Ouwehand A, Aalderen WM. Long‐term effects of prednisolone in the acute phase of bronchiolitis caused by respiratory syncytial virus. Pediatric Pulmonology 2000;30(2):92‐6. [DOI] [PubMed] [Google Scholar]
van Woensel 2003a {published data only}
- Woensel JBM, Van‐Aalderen WMC, DeWeerd W, Jansen NJG, Van‐Gestel JPJ, Markhorst DG, et al. Dexamethasone for treatment of patients mechanically ventilated for lower respiratory tract infection caused by respiratory syncytial virus. Thorax 2003;58(5):383‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Woensel 2003b {published data only}
- Woensel JBM, Lutter R, Biezeveld MH, Dekker T, Nijhuis M, Aalderen WMC, et al. Effect of dexamethasone on tracheal viral load and interleukin‐8 tracheal concentration in children with respiratory syncytial virus infection. Pediatric Infectious Disease Journal 2003;22(8):721‐6. [DOI] [PubMed] [Google Scholar]
van Woensel 2011 {published data only}
- Woensel JBM, Vyas H, Group Star Trial. Dexamethasone in children mechanically ventilated for lower respiratory tract infection caused by respiratory syncytial virus: a randomized controlled trial. Critical Care Medicine 2011;39:1779‐83. [DOI] [PubMed] [Google Scholar]
Wardrope 2000 {published data only}
- Wardrope J. Journal scan: randomised placebo controlled trial of nebulised corticosteroids in acute respiratory syncytial viral bronchiolitis. Journal of Accident and Emergency Medicine 2000;17(5):368‐70. [Google Scholar]
Webb 1986 {published data only}
- Webb MS, Henry RL, Milner AD. Oral corticosteroids for wheezing attacks under 18 months. Archives of Disease in Childhood 1986;61(1):15‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinberger 2004 {published data only}
- Weinberger M, Ahrens R. Oral prednisolone for viral wheeze in young children. Lancet 2004;363(9405):330‐1. [DOI] [PubMed] [Google Scholar]
Weinberger 2007 {published data only}
- Weinberger MM. High‐dose systemic corticosteroids may be effective early in the course of bronchiolitis. Pediatrics 2007;119(4):864‐5. [DOI] [PubMed] [Google Scholar]
Wong 2000 {published data only}
- Wong JY, Moon S, Beardsmore C, O'Callaghan C, Simpson H. No objective benefit from glucocorticoids inhaled via a spacer in infants recovering from bronchiolitis. European Respiratory Journal 2000;15(2):388‐94. [DOI] [PubMed] [Google Scholar]
Yaffe 1970 {published data only}
- Yaffe SJ, Weiss CF. Committee on drugs. Should glucocorticoids be used in treating bronchiolitis?. Pediatrics 1970;46(4):640‐2. [PubMed] [Google Scholar]
Zhu 2009 {published data only}
- Zhu YF, Cai XH, Zhu JY, Jiang WP, Lan JH, Liu S. Study of CD4+ CD25+ regulatory T cells and expression of Foxp3 mRNA in bronchiolitis and glucocorticoid regulation. Chung‐Hua i Hsueh Tsa Chih [Chinese Medical Journal] 2009;89:1563‐6. [PubMed] [Google Scholar]
Zuerlein 1990 {published data only}
- Zuerlein N. Efficacy of combined corticosteroid (CS) and beta‐agonist (BA) therapy for treatment of bronchiolitis in infants. Annals of Allergy 1990;64:85. [Google Scholar]
Additional references
AAP 2006
- American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics 2006;118:1774‐93. [DOI] [PubMed] [Google Scholar]
Babl 2008
- Babl FE, Sheriff N, Neutze J, Borland M, Oakley E. Bronchiolitis management in pediatric emergency departments in Australia and New Zealand: a PREDICT study. Pediatric Emergency Care 2008;24:656‐8. [DOI] [PubMed] [Google Scholar]
Barben 2000
- Barben JU, Robertson CF, Robinson PJ. Implementation of evidence‐based management of acute bronchiolitis. Journal of Paediatrics and Child Health 2000;36:491‐7. [DOI] [PubMed] [Google Scholar]
Barben 2003
- Barben J, Hammer J. Current management of acute bronchiolitis in Switzerland. Swiss Medical Weekly 2003;133:9‐15. [DOI] [PubMed] [Google Scholar]
Barben 2008
- Barben J, Kuehni CE, Trachsel D, Hammer J. Management of acute bronchiolitis: can evidence based guidelines alter clinical practice?. Thorax 2008;63:1103‐9. [DOI] [PubMed] [Google Scholar]
Baumer 2007
- Baumer JH. SIGN guideline on bronchiolitis in infants. Archives of Disease in Childhood Education and Practice 2007;92:ep149‐51. [DOI] [PubMed] [Google Scholar]
Bialy 2011
- Bialy L, Foisy M, Smith M, Fernandes RM. The Cochrane Library and the treatment of bronchiolitis in children: an overview of reviews. Evidence‐Based Child Health: A Cochrane Review Journal 2011;6(1):258‐75. [Google Scholar]
Bjornson 2008
- Bjornson CL, Johnson DW. Croup. Lancet 2008;371(9609):329‐39. [PUBMED: 18295000] [DOI] [PMC free article] [PubMed] [Google Scholar]
Black 2009
- Black JL, Oliver BG, Roth M. Molecular mechanisms of combination therapy with inhaled corticosteroids and long‐acting beta‐agonists. Chest 2009;136(4):1095‐100. [PUBMED: 19809050] [DOI] [PubMed] [Google Scholar]
Boluyt 2008
- Boluyt N, Tjosvold L, Lefebvre C, Klassen TP, Offringa M. Usefulness of systematic review search strategies in finding child health systematic reviews in MEDLINE. Archives of Pediatrics & Adolescent Medicine 2008;162(2):111‐6. [DOI] [PubMed] [Google Scholar]
Bont 2009
- Bont L. Current concepts of the pathogenesis of RSV bronchiolitis. Advances in Experimental Medicine and Biology 2009;634:31‐40. [PUBMED: 19280846] [DOI] [PubMed] [Google Scholar]
Brand 2000
- Brand PL, Vaessen‐Verberne AA. Differences in management of bronchiolitis between hospitals in The Netherlands. Dutch Paediatric Respiratory Society. European Journal of Pediatrics 2000;159:343‐7. [DOI] [PubMed] [Google Scholar]
Brand 2008
- Brand PL, Baraldi E, Bisgaard H, Boner AL, Castro‐Rodriguez JA, Custovic A, et al. Definition, assessment and treatment of wheezing disorders in preschool children: an evidence‐based approach. European Respiratory Journal 2008;32:1096‐110. [DOI] [PubMed] [Google Scholar]
Bush 2007
- Bush A, Thomson AH. Acute bronchiolitis. BMJ 2007;335:1037‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bush 2009
- Bush A. Practice imperfect‐‐treatment for wheezing in preschoolers. New England Journal of Medicine 2009;360:409‐10. [DOI] [PubMed] [Google Scholar]
Calvo 2010
- Calvo C, Pozo F, Garcia‐Garcia M, Sanchez M, Lopez‐Valero M, Perez‐Brena P, et al. Detection of new respiratory viruses in hospitalized infants with bronchiolitis: a three‐year prospective study. Acta Paediatrica 2010;99:1651‐2227 (Electronic). [DOI] [PMC free article] [PubMed] [Google Scholar]
Carroll 2008
- Carroll KN, Gebretsadik T, Griffin MR, Wu P, Dupont WD, Mitchel EF, et al. Increasing burden and risk factors for bronchiolitis‐related medical visits in infants enrolled in a state health care insurance plan. Pediatrics 2008;122:58‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castro‐Rodriguez 2000
- Castro‐Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. American Journal of Respiratory and Critical Care Medicine 2000;162:1403‐6. [PUBMED: 11029352] [DOI] [PubMed] [Google Scholar]
Christakis 2005
- Christakis DA, Cowan CA, Garrison MM, Molteni R, Marcuse E, Zerr DM. Variation in inpatient diagnostic testing and management of bronchiolitis. Pediatrics 2005;115:878‐84. [DOI] [PubMed] [Google Scholar]
Collins 2008
- Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. Journal of Virology 2008;82(5):2040‐55. [PUBMED: 17928346] [DOI] [PMC free article] [PubMed] [Google Scholar]
Colosia 2012
- Colosia AD, Masaquel A, Hall CB, Barrett AM, Mahadevia PJ, Yogev R. Residential crowding and severe respiratory syncytial virus disease among infants and young children: a systematic literature review. BMC Infectious Diseases 2012;12:95. [PUBMED: 22520624] [DOI] [PMC free article] [PubMed] [Google Scholar]
Damore 2008
- Damore D, Mansbach JM, Clark S, Ramundo M, Camargo CA Jr. Prospective multicenter bronchiolitis study: predicting intensive care unit admissions. Academic Emergency Medicine 2008;15:887‐94. [DOI] [PubMed] [Google Scholar]
David 2010
- David M, Luc‐Vanuxem C, Loundou A, Bosdure E, Auquier P, Dubus JC. Assessment of the French Consensus Conference for Acute Viral Bronchiolitis on outpatient management: progress between 2003 and 2008. Archives Pediatrie 2010;17:125‐31. [DOI] [PubMed] [Google Scholar]
DiFranza 2012
- DiFranza JR, Masaquel A, Barrett AM, Colosia AD, Mahadevia PJ. Systematic literature review assessing tobacco smoke exposure as a risk factor for serious respiratory syncytial virus disease among infants and young children. BMC Pediatrics 2012;12:81. [PUBMED: 22721493] [DOI] [PMC free article] [PubMed] [Google Scholar]
DiTraglia 2004
Doyle 2010
- Doyle LW, Ehrenkranz RA, Halliday HL. Dexamethasone treatment after the first week of life for bronchopulmonary dysplasia in preterm infants: a systematic review. Neonatology 2010;98(4):289‐96. [PUBMED: 20453523] [DOI] [PubMed] [Google Scholar]
Ducharme 2009
- Ducharme FM, Lemire C, Noya FJ, Davis GM, Alos N, Leblond H, et al. Preemptive use of high‐dose fluticasone for virus‐induced wheezing in young children. New England Journal of Medicine 2009;360:339‐53. [DOI] [PubMed] [Google Scholar]
Everard 2009
- Everard ML. Acute bronchiolitis and croup. Pediatric Clinics of North America 2009;56:119‐xi. [DOI] [PubMed] [Google Scholar]
Figueras‐Aloy 2008
- Figueras‐Aloy J, Carbonell‐Estrany X, Quero‐Jimenez J, Fernandez‐Colomer B, Guzman‐Cabanas J, Echaniz‐Urcelay I, et al. FLIP‐2 Study: risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born in Spain at a gestational age of 32 to 35 weeks. Pediatric Infectious Disease Journal 2008;27:788‐93. [DOI] [PubMed] [Google Scholar]
Flores 1997
- Flores G, Horwitz RI. Efficacy of beta2‐agonists in bronchiolitis: a reappraisal and meta‐analysis. Pediatrics 1997;100:233‐9. [PUBMED: 9240805] [DOI] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45:769‐73. [DOI] [PubMed] [Google Scholar]
Frey 2009
Giembycz 2008
- Giembycz MA, Kaur M, Leigh R, Newton R. A holy grail of asthma management: toward understanding how long‐acting beta(2)‐adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids. British Journal of Pharmacology 2008;153(6):1090‐104. [PUBMED: 18071293] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94:130‐6. [PMC free article] [PubMed] [Google Scholar]
Glasziou 2010
- Glasziou PP, Shepperd S, Brassey J. Can we rely on the best trial? A comparison of individual trials and systematic reviews. BMC Medical Research Methodology 2010;10:23. [PUBMED: 20298582] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzalez 2010
- Gonzalez de Dios J, Ochoa Sangrador C. Study of variability in the management of acute bronchiolitis in Spain in relation to age of patients. National multicenter study (aBREVIADo project). Anales de Pediatría (Barcelona, Spain: 2003) 2010;72:4‐18. [DOI] [PubMed] [Google Scholar]
GRADE 2009
- The GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendation Version 3.2. Available from www.gradeworkinggroup.org 2009.
Guilbert 2011
- Guilbert TW, Bacharier LB. Controversies in the treatment of the acutely wheezing infant. American Journal of Respiratory and Critical Care Medicine 2011;183(10):1284‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurusamy 2009
Halfhide 2008
- Halfhide C, Smyth RL. Innate immune response and bronchiolitis and preschool recurrent wheeze. Paediatric Respiratory Reviews 2008;9(4):251‐62. [PUBMED: 19026366] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2007
- Hall CB. Therapy for bronchiolitis: when some become none. New England Journal of Medicine 2007;357:402‐4. [DOI] [PubMed] [Google Scholar]
Hartling 2011a
- Hartling L, Bialy LM, Vandermeer B, Tjosvold L, Johnson DW, Plint AC, et al. Epinephrine for bronchiolitis. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD003123.pub3] [DOI] [PubMed] [Google Scholar]
Hartling 2011b
- Hartling L, Fernandes RM, Bialy L, Milne A, Johnson D, Plint A, et al. Steroids and bronchodilators for acute bronchiolitis in the first two years of life: systematic review and meta‐analysis. BMJ 2011;342:d1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
Jackson 2008
- Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high‐risk children. American Journal of Respiratory and Critical Care Medicine 2008;178(7):667‐72. [PUBMED: 18565953] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karemaker 2008a
- Karemaker R, Karemaker JM, Kavelaars A, Tersteeg‐Kamperman M, Baerts W, Veen S, et al. Effects of neonatal dexamethasone treatment on the cardiovascular stress response of children at school age. Pediatrics 2008;122(5):978‐87. [PUBMED: 18977976] [DOI] [PubMed] [Google Scholar]
Karemaker 2008b
- Karemaker R, Kavelaars A, ter Wolbeek M, Tersteeg‐Kamperman M, Baerts W, Veen S, et al. Neonatal dexamethasone treatment for chronic lung disease of prematurity alters the hypothalamus‐pituitary‐adrenal axis and immune system activity at school age. Pediatrics 2008;121(4):e870‐8. [PUBMED: 18381516] [DOI] [PubMed] [Google Scholar]
Kaur 2008
- Kaur M, Chivers JE, Giembycz MA, Newton R. Long‐acting beta2‐adrenoceptor agonists synergistically enhance glucocorticoid‐dependent transcription in human airway epithelial and smooth muscle cells. Molecular Pharmacology 2008;73(1):203‐14. [PUBMED: 17901197] [DOI] [PubMed] [Google Scholar]
King 2004
- King VJ, Viswanathan M, Bordley WC, Jackman AM, Sutton SF, Lohr KN, et al. Pharmacologic treatment of bronchiolitis in infants and children: a systematic review. Archives of Pediatrics & Adolescent Medicine 2004;158:127‐37. [DOI] [PubMed] [Google Scholar]
Klassen 1996
Koehoorn 2008
- Koehoorn M, Karr CJ, Demers PA, Lencar C, Tamburic L, Brauer M. Descriptive epidemiological features of bronchiolitis in a population‐based cohort. Pediatrics 2008;122:1196‐203. [DOI] [PubMed] [Google Scholar]
Korppi 2007
Kusel 2006
- Kusel MM, Klerk NH, Holt PG, Kebadze T, Johnston SL, Sly PD. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatric Infectious Disease Journal 2006;25:680‐6. [DOI] [PubMed] [Google Scholar]
Langley 1997
- Langley JM, Wang EE, Law BJ, Stephens D, Boucher FD, Dobson S, et al. Economic evaluation of respiratory syncytial virus infection in Canadian children: a Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) study. Journal of Pediatrics 1997;131:113‐7. [DOI] [PubMed] [Google Scholar]
Langley 2003
- Langley JM, LeBlanc JC, Smith B, Wang EE. Increasing incidence of hospitalization for bronchiolitis among Canadian children, 1980‐2000. Journal of Infectious Diseases 2003;188:1764‐7. [DOI] [PubMed] [Google Scholar]
Leer 1969
- Leer JA Jr, Green JL, Heimlich EM, Hyde JS, Moffet HL, Young GA, et al. Corticoglucocorticoid treatment in bronchiolitis. A controlled, collaborative study in 297 infants and children. American Journal of Diseases of Children 1969;117:495‐503. [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org. Chichester, UK: Wiley‐Blackwell, 2011. [Google Scholar]
Lehtinen 2007
- Lehtinen P, Ruohola A, Vanto T, Vuorinen T, Ruuskanen O, Jartti T. Prednisolone reduces recurrent wheezing after a first wheezing episode associated with rhinovirus infection or eczema. Journal of Allergy and Clinical Immunology 2007;119(3):570‐5. [PUBMED: 17196244] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lowell 1987
- Lowell DI, Lister G, Koss H, McCarthy P. Wheezing in infants: the response to epinephrine. Pediatrics 1987;79(6):939‐45. [PUBMED: 3295741] [PubMed] [Google Scholar]
Mallory 2003
- Mallory MD, Shay DK, Garrett J, Bordley WC. Bronchiolitis management preferences and the influence of pulse oximetry and respiratory rate on the decision to admit. Pediatrics 2003;111:e45‐51. [DOI] [PubMed] [Google Scholar]
Mansbach 2005
- Mansbach JM, Emond JA, Camargo CA Jr. Bronchiolitis in US emergency departments 1992 to 2000: epidemiology and practice variation. Pediatric Emergency Care 2005;21:242‐7. [DOI] [PubMed] [Google Scholar]
Mansbach 2007
- Mansbach JM, Pelletier AJ, Camargo CA Jr. US outpatient office visits for bronchiolitis, 1993‐2004. Ambulatory Pediatrics 2007;7:304‐7. [DOI] [PubMed] [Google Scholar]
Mansbach 2008a
- Mansbach JM, Camargo CA Jr. Bronchiolitis: lingering questions about its definition and the potential role of vitamin D. Pediatrics 2008;122:177‐9. [DOI] [PubMed] [Google Scholar]
Mansbach 2008b
- Mansbach JM, McAdam AJ, Clark S, Hain PD, Flood RG, Acholonu U, et al. Prospective multicenter study of the viral etiology of bronchiolitis in the emergency department. Academic Emergency Medicine 2008;15(2):111‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mansbach 2012
- Mansbach JM, Piedra PA, Teach SJ, Sullivan AF, Forgey T, Clark S, et al. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Archives of Pediatrics & Adolescent Medicine 2012; Vol. 166:8. [DOI] [PMC free article] [PubMed]
Martinez 2005
- Martinez FD. Heterogeneity of the association between lower respiratory illness in infancy and subsequent asthma. Proceedings of the American Thoracic Society 2005;2:157‐61. [DOI] [PubMed] [Google Scholar]
McAlister 2003
- McAlister FA, Straus SE, Sackett DL, Altman DG. Analysis and reporting of factorial trials: a systematic review. JAMA 2003;289:2545‐53. [DOI] [PubMed] [Google Scholar]
Meissner 2003
- Meissner HC. Selected populations at increased risk from respiratory syncytial virus infection. Pediatric Infectious Disease Journal 2003;22(Suppl):40‐4. [DOI] [PubMed] [Google Scholar]
Miyairi 2008
- Miyairi I, DeVincenzo JP. Human genetic factors and respiratory syncytial virus disease severity. Clinical Microbiology Reviews 2008;21:686‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Montgomery 2003
- Montgomery AA, Peters TJ, Little P. Design, analysis and presentation of factorial randomised controlled trials. BMC Medical Research Methodology 2003;3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nair 2010
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta‐analysis. Lancet 2010;375:1545‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Onland 2008
- Onland W, Jaegere AP, Offringa M, Kaam AH. Effects of higher versus lower dexamethasone doses on pulmonary and neurodevelopmental sequelae in preterm infants at risk for chronic lung disease: a meta‐analysis. Pediatrics 2008;122(1):92‐101. [PUBMED: 18595991] [DOI] [PubMed] [Google Scholar]
Owens 2010
- Owens DK, Lohr KN, Atkins D, Treadwell JR, Reston JT, Bass EB, et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions‐agency for healthcare research and quality and the effective health‐care program. Journal of Clinical Epidemiology 2010;63:513‐23. [DOI] [PubMed] [Google Scholar]
Papadopoulos 2002
- Papadopoulos NG, Moustaki M, Tsolia M, Bossios A, Astra E, Prezerakou A, et al. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. American Journal of Respiratory and Critical Care Medicine 2002;165(9):1285‐9. [DOI] [PubMed] [Google Scholar]
Paramore 2004
- Paramore LC, Ciuryla V, Ciesla G, Liu L. Economic impact of respiratory syncytial virus‐related illness in the US: an analysis of national databases. Pharmacoeconomics 2004;22:275‐84. [DOI] [PubMed] [Google Scholar]
Pelletier 2006
- Pelletier AJ, Mansbach JM, Camargo CA Jr. Direct medical costs of bronchiolitis hospitalizations in the United States. Pediatrics 2006;118:2418‐23. [DOI] [PubMed] [Google Scholar]
Perez‐Yarza 2007
- Perez‐Yarza EG, Moreno A, Lazaro P, Mejias A, Ramilo O. The association between respiratory syncytial virus infection and the development of childhood asthma: a systematic review of the literature. Pediatric Infectious Disease Journal 2007;26:733‐9. [DOI] [PubMed] [Google Scholar]
Plint 2004
- Plint AC, Johnson DW, Wiebe N, Bulloch B, Pusic M, Joubert G, et al. Practice variation among pediatric emergency departments in the treatment of bronchiolitis. Academic Emergency Medicine 2004;11:353‐60. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Robbins 2006
- Robbins JM, Kotagal UR, Kini NM, Mason WH, Parker JG, Kirschbaum MS. At‐home recovery following hospitalization for bronchiolitis. Ambulatory Pediatrics 2006;6:8‐14. [DOI] [PubMed] [Google Scholar]
Schuh 1990
- Schuh S, Canny G, Reisman JJ, Kerem E, Bentur L, Petric M, et al. Nebulized albuterol in acute bronchiolitis. Journal of Pediatrics 1990;117(4):633‐7. [PUBMED: 2213394] [DOI] [PubMed] [Google Scholar]
Schultz 2010
- Schultz A, Devadason SG, Savenije OE, Sly PD, Souef PN, Brand PL. The transient value of classifying preschool wheeze into episodic viral wheeze and multiple trigger wheeze. Acta Paediatrica 2010;99(1):56‐60. [PUBMED: 19764920] [DOI] [PubMed] [Google Scholar]
Shay 1999
- Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis‐associated hospitalizations among US children, 1980‐1996. JAMA 1999;282:1440‐6. [DOI] [PubMed] [Google Scholar]
Simoes 2003
- Simoes EA. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. Journal of Pediatrics 2003;143:S118‐26. [DOI] [PubMed] [Google Scholar]
Simoes 2008
- Simoes EA. RSV disease in the pediatric population: epidemiology, seasonal variability, and long‐term outcomes. Managed Care 2008;17:3‐6, discussion. [PubMed] [Google Scholar]
Simpson 2010
- Simpson A, Tan VY, Winn J, Svensen M, Bishop CM, Heckerman DE, et al. Beyond atopy: multiple patterns of sensitization in relation to asthma in a birth cohort study. American Journal of Respiratory and Critical Care Medicine 2010;181(11):1200‐6. [PUBMED: 20167852] [DOI] [PubMed] [Google Scholar]
Singh 2007
- Singh AM, Moore PE, Gern JE, Lemanske RF Jr, Hartert TV. Bronchiolitis to asthma: a review and call for studies of gene‐virus interactions in asthma causation. American Journal of Respiratory and Critical Care Medicine 2007;175:108‐19. [DOI] [PubMed] [Google Scholar]
Sly 2008
- Sly PD, Boner AL, Bjorksten B, Bush A, Custovic A, Eigenmann PA, et al. Early identification of atopy in the prediction of persistent asthma in children. Lancet 2008;372(9643):1100‐6. [PUBMED: 18805338] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sly 2010
- Sly PD, Kusel M, Holt PG. Do early‐life viral infections cause asthma?. Journal of Allergy and Clinical Immunology 2010;125:1202‐5. [DOI] [PubMed] [Google Scholar]
Smyth 2006
- Smyth RL, Openshaw PJ. Bronchiolitis. Lancet 2006;368:312‐22. [DOI] [PubMed] [Google Scholar]
Somers 2009
- Somers CC, Ahmad N, Mejias A, Buckingham SC, Carubelli C, Katz K, et al. Effect of dexamethasone on respiratory syncytial virus‐induced lung inflammation in children: results of a randomized, placebo controlled clinical trial. Pediatric Allergy and Immunology 2009;20(5):477‐85. [PUBMED: 19397752] [DOI] [PubMed] [Google Scholar]
Stein 1999
- Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999;354(9178):541‐5. [PUBMED: 10470697] [DOI] [PubMed] [Google Scholar]
Tal 1983
- Tal A, Bavilski C, Yohai D, Bearman JE, Gorodischer R, Moses SW. Dexamethasone and salbutamol in the treatment of acute wheezing in infants. Pediatrics 1983;71(1):13‐8. [PUBMED: 6129609] [PubMed] [Google Scholar]
Taussig 2008
- Taussig LM, Landau LI. Pediatric Respiratory Medicine. Philadelphia: Mosby ‐ Elsevier, 2008. [Google Scholar]
Turner 2008
- Turner T, Wilkinson F, Harris C, Mazza D. Evidence based guideline for the management of bronchiolitis. Australian Family Physician 2008;37:6‐13. [PubMed] [Google Scholar]
van Woensel 2002
- Woensel JB, Aalderen WM, Kneyber MC, Heijnen ML, Kimpen JL. Bronchiolitis hospitalisations in the Netherlands from 1991 to 1999. Archives of Disease in Childhood 2002;86:370‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinberger 2003
Westley 1978
- Westley CR, Cotton EK, Brooks JG. Nebulized racemic epinephrine by IPPB for the treatment of croup: a double‐blind study. American Journal of Diseases of Children 1978;132(5):484‐7. [PUBMED: 347921] [DOI] [PubMed] [Google Scholar]
Wiebe 2006
- Wiebe N, Vandermeer B, Platt RW, Klassen TP, Moher D, Barrowman NJ. A systematic review identifies a lack of standardization in methods for handling missing variance data. Journal of Clinical Epidemiology 2006;59:342‐53. [DOI] [PubMed] [Google Scholar]
Wilson‐Costello 2009
- Wilson‐Costello D, Walsh MC, Langer JC, Guillet R, Laptook AR, Stoll BJ, et al. Impact of postnatal corticosteroid use on neurodevelopment at 18 to 22 months' adjusted age: effects of dose, timing, and risk of bronchopulmonary dysplasia in extremely low birth weight infants. Pediatrics 2009;123(3):e430‐7. [PUBMED: 19204058] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 1989
- Wright AL, Taussig LM, Ray CG, Harrison HR, Holberg CJ. The Tucson Children's Respiratory Study. II. Lower respiratory tract illness in the first year of life. American Journal of Epidemiology 1989;129:1232‐46. [DOI] [PubMed] [Google Scholar]
Yusuf 2007
- Yusuf S, Piedimonte G, Auais A, Demmler G, Krishnan S, Caeseele P, et al. The relationship of meteorological conditions to the epidemic activity of respiratory syncytial virus. Epidemiology and Infection 2007;135:1077‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2005
- Zhang L, Sanguebsche LS. The safety of nebulization with 3 to 5 ml of adrenaline (1:1000) in children: an evidence based review [Seguranca de nebulizacao com 3 a 5 ml de adrenalina (1:1000) em criancas: uma revisao baseada em evidencia]. Jornal de Pediatria 2005;81(3):193‐7. [PUBMED: 15951902] [PubMed] [Google Scholar]
Zhang 2011
- Zhang L, Mendoza‐Sassi RA, Wainwright C, Klassen TP. Nebulized hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD006458.pub2] [DOI] [Google Scholar]
References to other published versions of this review
Fernandes 2010
- Fernandes RM, Bialy LM, Vandermeer B, Tjosvold L, Plint AC, Patel H, et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD004878.pub3] [DOI] [PubMed] [Google Scholar]
Patel 2004
- Patel H, Platt R, Lozano JM, Wang EEL. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004878.pub2] [DOI] [PubMed] [Google Scholar]


