Abstract
Background
There is accumulating evidence that steroid sex hormones have a beneficial effect on a number of risk factors for peripheral arterial disease.
Objectives
The objective of this review was to determine whether exogenous steroid sex hormones are an effective treatment for patients with lower limb atherosclerosis.
Search methods
For this update the Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator searched the Specialised Register (last searched August 2012) and CENTRAL 2012, Issue 7. There were no language restrictions.
Selection criteria
We selected randomised or quasi‐randomised controlled trials of steroid sex hormones in patients with lower limb atherosclerosis.
Data collection and analysis
Both authors extracted data and assessed trial quality independently. Whenever possible investigators were contacted to obtain information needed for the review that could not be found in published reports.
Main results
Four trials appeared to meet the inclusion criteria, but one was excluded because of poor methodology. The three remaining trials compared testosterone treatment with placebo in a total of 109 subjects with intermittent claudication or critical leg ischaemia. The most recent trial to meet the inclusion criteria dated from 1971. No trials were available which investigated the potentially beneficial effects of oestrogenic hormones in women with lower limb atherosclerosis.
Testosterone therapy produced no significant improvement in tests of walking distance or in a variety of other objective tests for peripheral arterial disease, including venous filling time, muscle blood flow and plethysmography. The relative risk for subjective improvement in symptoms using the combined trial results was also non‐significant (relative risk (RR) 1.10, 95% confidence interval (CI) 0.81 to 1.48).
Authors' conclusions
There is no evidence to date that short‐term testosterone treatment is beneficial in subjects with lower limb atherosclerosis. However, this might reflect limited data rather than the lack of a real effect.
Plain language summary
Testosterone and oestrogen steroid sex hormones for lower limb atherosclerosis
Atherosclerosis of the arteries of the legs can become symptomatic as people age. People affected may experience discomfort and cramping pain in the legs that is triggered by exercise and relieved with rest, termed intermittent claudication. Some people with claudication go on to require reconstructive surgery and even amputation of a leg. Risk factors for peripheral arterial disease include cigarette smoking, high blood pressure, high cholesterol, low levels of high density lipoprotein (HDL) cholesterol and blood flow problems. The steroid sex hormones oestrogens and testosterone affect a number of these risk factors, particularly cholesterol and blood clotting, and may be helpful in peripheral vascular disease.
Although four randomised controlled trials met the inclusion criteria, one was excluded because of poor methodology. The three remaining trials compared testosterone treatment with placebo in a total of 109 middle‐aged to elderly people, predominantly men. The participants had symptoms of lower limb atherosclerosis, predominantly intermittent claudication. The trials were published from 1967 to 1971 and all took place in Denmark. Testosterone did not provide any clear improvement in the symptoms reported by the participants, walking distance or other objective tests for peripheral arterial disease including leg muscle blood flow. The dose of testosterone in the trials varied between 300 mg taken by mouth every two weeks for three months to a lower (100 mg) oral dose taken more often and 200 mg given by intramuscular injection, first weekly then every two weeks for six months. Side effects were poorly reported except for subjective sexual functioning, which did seem to improve with testosterone treatment. No trials investigated oestrogens in women with lower limb atherosclerosis.
Trials by the Women's Health Initiative (published in 2004) have shown that oestrogen and progestin does not confer any protection against peripheral arterial disease in healthy postmenopausal women or reduce the risk of coronary events in postmenopausal women with coronary heart disease.
Background
In Western societies, 8% of the population in late middle age demonstrate asymptomatic atherosclerosis of the lower limb and 5% experience intermittent claudication. Up to 20% of claudicants go on to require reconstructive surgery and 1% to 2% eventually undergo amputation. Follow‐up studies have shown that claudicants have mortality rates three times that of the general population, primarily due to cardiovascular disease (Leng 1993).
Although its full aetiology is unknown, several risk factors for peripheral arterial disease have been identified, including cigarette smoking, hypertension, total cholesterol, low levels of high density lipoprotein (HDL) cholesterol and haemostatic factors (Leng 1993). There is accumulating evidence that steroid sex hormones affect a number of these risk factors. For example, exogenous oestrogens improve the lipid profile and fibrinogen levels of postmenopausal women (Manolio 1993; Nabulsi 1993; PEPI Trial 1995). They may also increase HDL cholesterol and reduce low density lipoprotein (LDL) cholesterol levels in men (Oliver 1956). In males, endogenous testosterone levels have been associated with raised HDL cholesterol and a reduction in many of the haemostatic factors (Bonithon‐Kopp 1988; Khaw 1991).
Their advantageous effect on risk factor profiles provides a plausible mechanism by which steroid sex hormones may influence the progression of peripheral arterial disease. Indeed, early trials of testosterone in lower limb atherosclerosis were prompted by its observed activation of the blood fibrinolytic system (Fearnley 1962). More recently, an estimated 50% reduction in the incidence of coronary artery disease in postmenopausal women taking exogenous oestrogens compared with non‐oestrogen users (Stampfer 1991) stimulated interest in a potential protective effect for female sex hormones in cardiovascular disease. Clinical trials of hormone therapy in the prevention of coronary disease in women have been undertaken in the UK and the USA, including ESPRIT (Khan 2000), the Women's Health Initiative (WHI Study Group 1998), the Heart and Estrogen/Progestin Replacement Study (Hulley 1998) and the Estrogen Replacement and Atherosclerosis Trial (Herrington 2000). Two of these trials have shown that oestrogen/progestin does not confer any protection against peripheral arterial disease in healthy postmenopausal women (Hsia 2004) or reduce the risk of coronary events in postmenopausal women with coronary heart disease (Grady 2002).
We aim to determine whether there is evidence that steroid sex hormones are beneficial in the treatment of lower limb atherosclerosis.
Objectives
To determine the effectiveness and safety of steroid sex hormones in the treatment of lower limb atherosclerosis in terms of alleviating symptoms, preventing deterioration of underlying disease and reducing overall mortality.
Methods
Criteria for considering studies for this review
Types of studies
All randomised and quasi‐randomised controlled trials of steroid sex hormones in the treatment of lower limb atherosclerosis were considered. As there is currently little information available, trials in which allocation to different treatment regimes was not adequately concealed were included.
Types of participants
Patients of either sex with lower limb atherosclerosis, including those suffering from intermittent claudication (diagnosed either clinically or by questionnaire), critical leg ischaemia or with asymptomatic lower limb atherosclerosis were eligible for inclusion.
Types of interventions
Any preparation of a steroid sex hormone, either oestrogenic or androgenic, given either singly or in combination, by any route, and over any time period.
Types of outcome measures
We considered several outcome measures:
outcomes which measure lower limb atherosclerosis, including subjective improvement in symptoms and objective measurement of the condition using established tests such as the ankle brachial pressure index, walking distance and angiography as well as tests of muscle blood flow and plethysmography;
overall mortality;
cardiovascular events;
drug side effects.
Search methods for identification of studies
There were no restrictions on language.
Electronic searches
For this update the Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched August 2012) and the Cochrane Central Register of Controlled Trials (CENTRAL) 2012, Issue 7, part of The Cochrane Library, www.thecochranelibrary.com . See Appendix 1 for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used are described in the Specialised Register section of the Cochrane Peripheral Vascular Diseases Group module in The Cochrane Library (www.thecochranelibrary.com).
Searching other resources
We searched bibliographies of trials and other relevant identified articles.
Data collection and analysis
Selection of trials
One of the authors (JFP) identified possible trials and their eligibility for inclusion in the review was checked by GCL. Additional information was sought from the investigators of all trials that appeared to meet the inclusion criteria.
Assessment of trial quality
Quality of each included trial was assessed using a standard checklist developed by the Cochrane PVD Group. This included collecting information on method of randomisation and blinding for each trial. All trials were assessed by JFP and cross‐checked by GCL.
Data extraction
Both authors independently extracted data. Disagreements were resolved by discussion and the final results are included in the review.
Statistical analysis
Heterogeneity between trial results was tested subjectively by clinical judgement of differences in patient populations and interventions (including the use of oestrogenic, androgenic or combined hormone preparations), and objectively using appropriate statistical tests. Where possible, trial results were pooled by meta‐analysis.
Results
Description of studies
Summary details of included studies are given in the (Characteristics of included studies) table. Four trials were identified which met the inclusion criteria, but one of these was subsequently excluded as there was no adequate information on whether or not it was randomised, or on the type of clinical tests used, and the results could not be obtained (Albrechtsen 1972; see Characteristics of excluded studies). The three remaining trials, all undertaken in Denmark, investigated testosterone versus placebo in a total of 109 subjects suffering from the symptoms of lower limb atherosclerosis ‐ predominantly intermittent claudication (Dohn 1968; Genster 1971; Hentzer 1967). The most recent trial to meet the inclusion criteria dated from 1971. No trials on the effects of oestrogens were identified.
In all trials, the diagnosis of intermittent claudication was confirmed by clinical examination and, in the Hentzer trial, by a reduced muscle blood flow (Hentzer 1967). Clinical judgement of heterogeneity indicated that the populations involved in the trials were similar (middle‐aged to elderly subjects, predominantly men), and that all trials used the same sex hormone (testosterone). However, the interventions varied by dose of testosterone and the route and duration of administration. Hentzer used 200 mg testosterone (intramuscularly) weekly for three weeks and once every two weeks thereafter, for a total of six months (Hentzer 1967). Dohn administered 300 mg testosterone (orally) once every two weeks, for a total of three months (Dohn 1968). The latter was a cross‐over study (testosterone for three months followed by placebo for three months or vice versa) (Dohn 1968). Genster used 100 mg testosterone three times per week for two to six months (Genster 1971).
A variety of tests were performed in each of the trials but detailed results were available for only a proportion of these. None of the trials included mortality or cardiovascular event outcomes. The only disease outcome reported in all trials was subjective change in symptoms.
Results of the search
For this update there were no additional published studies identified. The author of one unpublished study (NCT00504712) was contacted but we were unable to obtain data.
Risk of bias in included studies
The methods used in the included trials are described in detail in the table of 'Characteristics of included studies'. Patients were allocated by alternation in one trial (Hentzer 1967) and it was stated that 'drugs were coded' in another (Dohn 1968). In the third trial (Genster 1971), one of the authors confirmed that the trial was randomised. All trials were double blind.
Effects of interventions
Walking tests Hentzer recorded the time until onset of claudication while patients walked up and down the same five steps at their own constant tempo (Hentzer 1967). After six months, there was no significant difference in the number of subjects who improved on this test between the treatment and placebo groups (16 of 19 in the experimental group and 15 of 17 in the control group; Chi‐squared, P > 0.1).
In the Dohn trial, change in performance of a metronome walking test was recorded, with the mean walking test result at baseline given a value of 1.00 (Dohn 1968). There was no significant difference between the mean change in performance in the experimental group (1.06, standard deviation (SD) = 0.65) and the corresponding change in the control group (1.07, SD = 0.68), after the first 12 week period of the cross‐over trial. It was not possible to extract further results for this test from the data presented.
Venous filling time Hentzer monitored venous filling time in all the legs of his subjects which demonstrated arterial insufficiency (Hentzer 1967). This was done with the leg hanging vertically but no other details of the measurement were given. The venous filling time improved, as defined by a change from > 15 seconds to < 15 seconds in at least one leg, in one of 19 subjects in the experimental group and three of 17 subjects in the control group. This difference was not statistically significant (Chi‐squared, P > 0.1).
Muscle blood flow Hentzer (Hentzer 1967) also measured muscle blood flow through the muscles of all the affected legs, using the clearance of Xe‐133 injected into the anterior tibialis muscle by the method described by Lassen and colleagues (Lassen 1964). There was no difference in the number of legs with an increase in muscle blood flow between the experimental group (19 of 32) and the control group (15 of 30; Chi‐squared, P > 0.1). However, mean muscle blood flow increased by 3.3 ml/100 g/min in the experimental group and decreased by 0.7 ml/100 g/min in the control group (P = 0.025 quoted in the original paper).
Plethysmography In the Dohn trial (Dohn 1968), plethysmographic measurements of peripheral pulse volume, blood flow at rest, reactive hyperaemia after five minutes of arterial compression and leg circulation after walking and exercise were taken. Improvement in these measurements was reported in 10 of 36 patients in the experimental group and nine of 38 patients in the control group (Chi‐ squared, P > 0.1).
Symptomatic change All trials recorded symptoms of intermittent claudication and reported the number of subjects in each treatment group with subjective improvement at the end of the trial. However, the method used to collect this information was not described. The overall odds ratio for symptomatic improvement was not significant (relative risk (RR) 1.10, 95% confidence interval (CI) 0.81 to 1.48).
Side effects In Hentzer's study, there was no significant difference in muscle strength according to a hand dynamometer between the experimental (37.7 kg, SD = 2.1) and control (37 kg, SD = 2.8) groups at the end of the trial (Hentzer 1967). However, a significantly higher number of subjects reported a subjective increase in sexual functioning in the experimental group (21 out of 62) compared with the control group (6 of 60) when the results of the two trials were combined (Chi‐squared, P = 0.01).
Discussion
The only available randomised or quasi‐randomised controlled trials of steroid sex hormones in peripheral arterial disease involved the use of testosterone in predominantly male populations. Even in this area the data are extremely limited. Only three, small‐scale trials are available, involving the use of testosterone over relatively short periods of time and variable methods of measuring peripheral arterial disease. No trials are available which investigated oestrogenic hormones.
Overall, testosterone treatment was found to have no significant effect on disease outcomes, including subjective improvement in symptoms and tests of walking distance. It is still possible that testosterone could affect the progression of peripheral arterial disease but at present there are insufficient data to support this notion.
Side effects were poorly reported except for subjective sexual functioning, which did seem to be improved by testosterone treatment.
Authors' conclusions
Implications for practice.
There is currently no evidence that patients with peripheral arterial disease might benefit from treatment with testosterone, although this may reflect a lack of data rather than the absence of a real effect. Moreover, long‐term use of testosterone would be difficult to justify in view of the potential side effects of anabolic steroids.
Implications for research.
Although it is still possible that testosterone could affect the progression of peripheral arterial disease, further trials would be difficult to justify at the present time. Firstly, observational studies on the association between testosterone and coronary artery disease are inconclusive (Kalin 1990). Secondly, few studies have carefully assessed the adverse effects of prolonged exogenous testosterone administration in non‐hormone deficient men (Matsumoto 1990).
What's new
| Date | Event | Description |
|---|---|---|
| 19 September 2012 | Review declared as stable | This Cochrane review has been marked stable and will only be updated when new studies are identified. |
History
Protocol first published: Issue 2, 1996 Review first published: Issue 1, 1997
| Date | Event | Description |
|---|---|---|
| 7 August 2012 | New citation required but conclusions have not changed | Searches were re‐run. One study added to 'Studies awaiting classification'. Minor copy edits made. The review was assessed as up‐to‐date. Conclusions not changed. |
| 7 August 2012 | New search has been performed | Searches were re‐run. One study added to 'Studies awaiting classification'. Minor copy edits made. The review was assessed as up‐to‐date. Conclusions not changed |
| 11 February 2010 | New search has been performed | New searches carried out but no additional studies found. |
| 12 May 2008 | Amended | Converted to new review format. |
| 15 November 2007 | New search has been performed | No new trials found. Review updated (issue 1, 2008). Conclusions remain unchanged. |
| 8 November 2006 | New search has been performed | Plain Language Summary added. No new trials found. Review updated (Issue 1, 2007). Conclusions remain unchanged. |
| 22 February 2005 | New search has been performed | No new studies found. Review updated without change. |
| 19 February 2003 | New search has been performed | No new studies found. Review updated without change. |
| 2 October 2001 | New search has been performed | No new studies found. Review updated to include data from previously untranslated trial, resulting in no major change to findings. |
Acknowledgements
We are very grateful to Dr Oram who responded to our request for additional information about included trials and to Mr J Laustsen and Dr J Bismuth for assistance with translation of articles from Danish into English. We would also like to thank the Cochrane Consumer Network for providing a Plain Language Summary.
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MeSH descriptor Arteriosclerosis, this term only | 893 |
| #2 | MeSH descriptor Arteriolosclerosis, this term only | 0 |
| #3 | MeSH descriptor Arteriosclerosis Obliterans, this term only | 71 |
| #4 | MeSH descriptor Atherosclerosis, this term only | 376 |
| #5 | MeSH descriptor Arterial Occlusive Diseases, this term only | 753 |
| #6 | MeSH descriptor Intermittent Claudication, this term only | 707 |
| #7 | MeSH descriptor Ischemia, this term only | 746 |
| #8 | MeSH descriptor Peripheral Vascular Diseases explode all trees | 2134 |
| #9 | MeSH descriptor Vascular Diseases, this term only | 381 |
| #10 | (atherosclero* or arteriosclero* or PVD or PAOD or PAD) | 16498 |
| #11 | (arter*) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) | 5218 |
| #12 | (vascular) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) | 1409 |
| #13 | (vein*) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) | 761 |
| #14 | (veno*) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) | 1032 |
| #15 | (peripher*) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) | 1447 |
| #16 | peripheral near3 dis* | 3148 |
| #17 | arteriopathic | 7 |
| #18 | (claudic* or hinken*) | 1424 |
| #19 | (isch* or CLI) | 16626 |
| #20 | dysvascular* | 16 |
| #21 | leg near4 (obstruct* or occlus* or steno* or block* or obliter*) | 181 |
| #22 | limb near4 (obstruct* or occlus* or steno* or block* or obliter*) | 230 |
| #23 | (lower near3 extrem*) near4 (obstruct* or occlus* or steno* or block* or obliter*) | 134 |
| #24 | (aort* or iliac or femoral or popliteal or femoropop* or fempop* or crural) near3 (obstruct* or occlus*) | 320 |
| #25 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24) | 38572 |
| #26 | MeSH descriptor Androgens explode all trees | 500 |
| #27 | MeSH descriptor Estrogens explode all trees | 1240 |
| #28 | sex near hormon* | 1358 |
| #29 | (*testosterone or *estrogen or nandrolone or oxandrolone or oxymetholone or stanozolol ) | 10480 |
| #30 | (#26 OR #27 OR #28 OR #29) | 11293 |
| #31 | (#25 AND #30) | 456 |
Data and analyses
Comparison 1. Testosterone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subjective improvement in symptoms of intermittent claudication | 3 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.81, 1.48] |
1.1. Analysis.
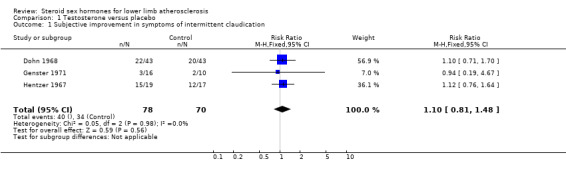
Comparison 1 Testosterone versus placebo, Outcome 1 Subjective improvement in symptoms of intermittent claudication.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dohn 1968.
| Methods | Study design: Randomised by 'drug coding', double blinded, cross‐over trial. Withdrawals post‐randomisation: 2 subjects completed first 12 week period only. |
|
| Participants | Country: Denmark. No. of patients: 44 randomised. Age: 5 subjects < 50 years, 34 subjects 50 to 69 years, 5 subjects > 70 years. Gender: male. Inclusion criteria: Intermittent claudication, "43 due to arteriosclerosis, 1 due to Buerger's disease'". Exclusion criteria: None mentioned. |
|
| Interventions | Treatment: 300 mg testosterone isobutyrate (orally), once every 24 days. Control: 50 mg meprobromate. Duration: 12 weeks treatment followed by 12 weeks control or vice versa. |
|
| Outcomes | Subjective effects, metronome walking test, plethysmography, side effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Genster 1971.
| Methods | Study design: Randomised, double‐blind trial. Withdrawals post randomisation: not mentioned. |
|
| Participants | Country: Denmark. No. of patients: 26 randomised. Age: over 40 years. Gender: male and female. Inclusion criteria: intermittent claudication/critical limb ischaemia. Exclusion criteria: not reported. |
|
| Interventions | Treatment: 100 mg testosterone, 3 times/week. Control: Sesame oil under same regime as treatment group. Duration: two to six months. |
|
| Outcomes | Subjective effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Hentzer 1967.
| Methods | Study design: quasi‐randomised by alternation, double‐blind trial. Withdrawals post‐randomisation: 1 drop‐out and 2 deaths not included in analysis. |
|
| Participants | Country: Denmark. No. of patients: 39 randomised, 36 completed study. Age: mean 59.4 years experimental group, 63.1 years control group. Gender: male. Inclusion criteria: intermittent claudication, absent peripheral pulses and reduced muscle blood flow. Exclusion criteria: mild symptoms of intermittent claudication only and patients with ulceration. |
|
| Interventions | Treatment: 19 patients received 200 mg testosterone (intramuscularly), once weekly for 3 weeks, once every 2 weeks thereafter. Control: 17 patients received 2 ml sesame oil. Duration: 6 months |
|
| Outcomes | Subjective effects, walking distance (time to onset of intermittent claudication when stepping), venous filling time, muscle blood flow, side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albrechtsen 1972 | No information was available on whether or not this trial was randomised. Furthermore, the data on the types of clinical tests used to assess disease outcome and their results were inadequate. No further information could be obtained from the authors, probably due to the length of time since publication. |
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00504712.
| Methods | Randomised, double blind study. |
| Participants | Male, 18 years and older Type 2 diabetes mellitus. Serum testosterone 12 nmol/L or less on two consecutive samples taken on different days and symptoms compatible with hypogonadism. Peripheral vascular disease as defined by previous diagnosis by a specialist vascular surgeon or ABPI less than 0.92 and ischaemic leg pain (claudication or rest pain) or distal complications (non‐healing arterial foot ulcer or gangrene). |
| Interventions | Testosterone Sustanon, 200 mg intramuscular testosterone every 2 weeks Placebo 0.9% saline injection every two weeks |
| Outcomes | Primary outcome measures: change in arterial stiffness (time frame: 3 months) |
| Notes | Author was contacted, not yet published but forms part of MD thesis. |
Contributions of authors
Jackie Price: identified possible trials, and their eligibility for inclusion; sought additional information from the investigators of trials that appeared to meet the inclusion criteria; assessed trial quality; extracted data; wrote text.
Gillian Leng: checked trial eligibility; cross‐checked trial quality assessment and data.
Sources of support
Internal sources
University of Edinburgh, UK.
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The PVD Group editorial base is supported by the Chief Scientist Office.
British Heart Foundation, UK.
Declarations of interest
JF Price: None known GC Leng: None known
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Dohn 1968 {published data only}
- Dohn K, Hvidt V, Nielsen J, Palm L. Testosterone therapy in obliterating arterial lesions in the lower limbs. Angiology 1968;19(6):342‐50. [DOI] [PubMed] [Google Scholar]
- Hvidt V, Dohn K, Lund J, Nielsen J, Palm L. Testosterone treatment of obliterative arterial lesions in the legs [Testosteronbehandling af obliterende arterielidelser pa underekstremiteterne]. Ugeskrift för Laeger 1965;127(36):1116‐23. [PubMed] [Google Scholar]
Genster 1971 {published data only}
- Genster HG, Oram V. Arterial insufficiency in the lower extremities treated with drugs [Arteriel insufficiens i underekstremiteterne behandlet medikamentelt]. Ugeskrift för Laeger 1971;133:244‐6. [PubMed] [Google Scholar]
Hentzer 1967 {published data only}
- Hentzer E, Madsen PC. Testosterone in the treatment of arterial insufficiency of the lower limbs [Testosteron ved arteriel insufficiens i underekstremiteterne]. Nordisk Medicin 1966;76(45):1307‐112. [PubMed] [Google Scholar]
- Hentzer E, Madsen PC. Testosterone in the treatment of arterial insufficiency of the lower limbs. Scandinavian Journal of Clinical & Laboratory Investigations 1967;Supplement 99:198‐206. [PubMed] [Google Scholar]
References to studies excluded from this review
Albrechtsen 1972 {published data only}
- Albrechtsen O, Barfod B, Barfod E, Laursen NPR, Nordentoft B, Yde H. Hormonal treatment of arterial insufficiency in the lower extremities. Danish Medical Bulletin 1972;19:157‐9. [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00504712 {published data only (unpublished sought but not used)}
- Stanworth R. Testosterone for Peripheral Vascular Disease [A randomised, double blind, placebo controlled, parallel pilot study to test the effect of testosterone treatment on peripheral vascular disease in hypogonadal men with type 2 Diabetes Mellitus]. http://clinicaltrials.gov/show/NCT00504712 2007. [NCT00504712]
Additional references
Bonithon‐Kopp 1988
- Bonithon‐Kopp C, Scarabin P‐Y, Bara L, Castanier M, Jacqueson A, Roger M. Relationship between sex hormones and haemostatic factors in healthy middle‐aged men. Atherosclerosis 1988;71(1):71‐6. [DOI] [PubMed] [Google Scholar]
Fearnley 1962
- Fearnley GR, Chakrabarti R. Increase of blood fibrinolytic activity by testosterone. Lancet 1962;1:128‐32. [DOI] [PubMed] [Google Scholar]
Grady 2002
- Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow‐up (HERS II). JAMA 2002;288(1):99‐101. [DOI] [PubMed] [Google Scholar]
Herrington 2000
- Herrington DM, Reboussin DM, Brosnihan KB, Sharp PC, Shumaker SA, Snyder TE, et al. Effects of estrogen replacement on the progression of coronary‐artery atherosclerosis. New England Journal of Medicine 2000;343(8):522‐9. [DOI] [PubMed] [Google Scholar]
Hsia 2004
- Hsia J, Criqui MH, Rodabough RJ, Langer RD, Phillips LS, Allison M, et al. Estrogen plus progestin and the risk of peripheral arterial disease: the Women's Health Initiative. Circulation 2004;109(5):620‐6. [DOI] [PubMed] [Google Scholar]
Hulley 1998
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomised trial of oestrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA 1998;280(7):605‐13. [DOI] [PubMed] [Google Scholar]
Kalin 1990
- Kalin MF, Zumoff B. Sex hormones and coronary disease: a review of the clinical studies. Steroids 1990;55(8):330‐52. [DOI] [PubMed] [Google Scholar]
Khan 2000
- Khan MA, Heagerty AM, Kitchener H, McNamee R, Cherry NM, Hannaford P. Oestrogen and women's heart disease: ESPRIT‐UK. QJM 2000;93:699‐700. [DOI] [PubMed] [Google Scholar]
Khaw 1991
- Khaw KT, Barrett‐Connor E. Endogenous sex hormones, high density lipoprotein cholesterol, and other lipoprotein fractions in men. Arteriosclerosis and Thrombosis 1991;11(3):489‐94. [DOI] [PubMed] [Google Scholar]
Lassen 1964
- Lassen NA, Lindbjerg IF, Munck O. Measurement of blood‐flow through skeletal muscle by intramuscular injection of Xenon‐133. Lancet 1964;1:686‐9. [DOI] [PubMed] [Google Scholar]
Leng 1993
- Leng GC, Fowkes FGR. The epidemiology of peripheral arterial disease. Vascular Medicine Reviews 1993;4:5‐18. [Google Scholar]
Manolio 1993
- Manolio TA, Furberg CD, Shemanski L, Psaty BM, O'Leary DH, Tracy RP, et al. Associations of postmenopausal estrogen use with cardiovascular disease and its risk factors in older women. The CHS Collaborative Research Group. Circulation 1993;88(5 part 1):2163‐71. [DOI] [PubMed] [Google Scholar]
Matsumoto 1990
- Matsumoto AM. Effects of chronic testosterone administration in normal men: safety and efficacy of high dosage testosterone and parallel dose‐dependent supression of luteinizing hormone, follicle‐stimulating hormone, and sperm production. Journal of Clinical Endocrinology and Metabolism 1990;70(1):282‐7. [DOI] [PubMed] [Google Scholar]
Nabulsi 1993
- Nabulsi AA, Folsom AR, White A, Patsch W, Heiss G, Wu KK, et al. Association of hormone‐replacement therapy with various cardiovascular risk factors in postmenopausal women. The Atherosclerosis Risk in Communities Study Investigators. New England Journal of Medicine 1993;328(15):1069‐75. [DOI] [PubMed] [Google Scholar]
Oliver 1956
- Oliver MF, Boyd GS. Endocrine aspects of coronary sclerosis. Lancet 1956;2:1273‐6. [DOI] [PubMed] [Google Scholar]
PEPI Trial 1995
- Anonymous. Effects of estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA 1995;273(3):199‐208. [PubMed] [Google Scholar]
Stampfer 1991
- Stampfer MJ, Colditz GA. Estrogen replacement therapy and coronary heart disease: a quantitative assessment of the epidemiologic evidence. Preventive Medicine 1991;20(1):47‐63. [DOI] [PubMed] [Google Scholar]
WHI Study Group 1998
- Anonymous. Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Controlled Clinical Trials 1998;19(1):61‐109. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Price 1997
- Price JF, Leng GC. Steroid sex hormones for lower limb atherosclerosis. Cochrane Database of Systematic Reviews 1997, Issue 1. [DOI: 10.1002/14651858.CD000188] [DOI] [PubMed] [Google Scholar]
Price 2001
- Price J, Leng GC. Steroid sex hormones for lower limb atherosclerosis. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD000188] [DOI] [Google Scholar]


