TO THE EDITOR:
22q11.2 deletion syndrome (22q11.2del) is a chromosomal microdeletion with a myriad of potential features, most commonly including developmental delay including speech and language deficits, conotruncal cardiac anomalies, hypocalcemia, palatal abnormalities, and thymic hypoplasia.1 This syndrome has an incidence of approximately 1:3000 live births.2 A previous study found that the features of 22q11.2del generally sort independently.3 That study of 60 children older than 6 months examined T-cell lymphopenia in children with 5 distinct cardiac anomalies and found no association between specific cardiac anomaly and T-cell numbers. This study was developed to examine the question in more detail to improve risk stratification of patients.
One of the most common features of 22q11.2del is T-cell lymphopenia due to thymic hypoplasia. T-cell lymphopenia is a risk factor for prolonged viral infections and subsequent bacterial superinfection. Recently, a study demonstrated that significant T-cell lymphopenia could occur in nonsyndromic cases of single ventricle anatomy.4 Surgical removal of the thymus, structural damage to the thoracic duct, and altered lymphatic flow have been proposed as potential mechanisms. We wished to examine whether the presence of a significant cardiac anomaly impacted T-cell counts specifically in 22q11.2del. T-cell counts predict health care utilization and can be used to risk stratify patients with 22q11.2del.5 A better understanding of whether there are multifactorial contributions to the observed T-cell count would improve patient care.
We collected electronic medical record data on 57 toddlers (age 1–3 years) and 115 infants (≤12 months) who had a complete data set (age of T-cell count, T-cell count, type of cardiac anomaly) in the electronic medical record. Longitudinal data on a subset of 119 patients were also analyzed. This study was approved by the Children’s Hospital Institutional Review Board. When multiple data points were available on a patient, the earliest one was used for the cross-sectional analysis. We classified T-cell counts as follows: less than 700 cells/mm3, Very Low; 700 to 1500 cells/mm3, Low; and more than 1500 cells/mm3, Normal T-cell counts. We used 4 categories of congenital heart disease previously assigned in our database on the basis of timing of intervention.6 No congenital heart defect was defined as a spontaneously closing ventricular septal defect or no identified structural abnormality. A simple anomaly was defined as a ventricular septal defect, atrial septal defect, or mild pulmonary stenosis. These conditions generally do not require surgery. A moderate cardiac anomaly was defined as tetralogy of Fallot, pulmonary stenosis, or partial atrioventricular septal defect. These conditions typically require surgery in early childhood but not usually in the newborn period. A complex cardiac anomaly was defined as pulmonary atresia, complete atrioventricular septal defect, interrupted aortic arch, or truncus arteriosus. These typically require surgery in the neonatal period. The demographic data demonstrated that 46% were female and 68% were white; 116 members of the cohort had deletion sizing that revealed LCR22A-LCR22D deletions and 28 had atypical nested deletions within the LCR22A-LCR22D region, also known as the DiGeorge critical region. The remainder did not have deletion sizing performed because they were identified using only fluorescent in situ hybridization.
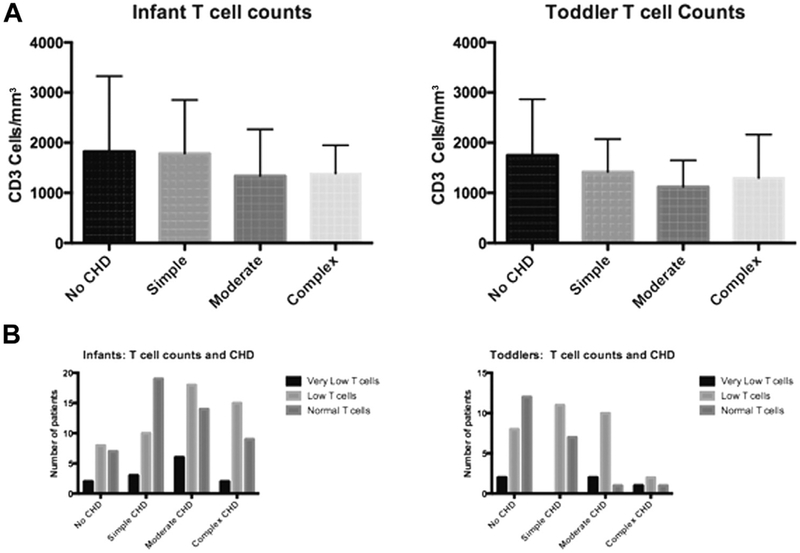
We examined the means of T-cell counts across the different types of cardiac anomalies. ANOVA revealed no significant association of T-cell counts with category of cardiac anomaly (Figure 1, A). We then examined the distribution of T-cell counts as categorical variables using Very Low, Low, and Normal categories as defined above (Figure 1, B). Although normal T-cell counts were most common in toddlers who had no cardiac disease, there was no overall association of T-cell count category and cardiac anomaly category by chi-square analysis. Interrupted aortic arch and tetralogy of Fallot were examined as distinct anomalies and there was no difference in T-cell counts when examined as individual diagnoses. To gain further insight into whether the complexity of the surgical procedure was associated with low T-cell counts, we used the Society of Thoracic Surgeons-European Association for Cardiothoracic Surgery (STS-EACTS) score for patients when available.7 This is a 1 to 5 scoring system that has been demonstrated to predict mortality, with higher numbers having worse outcomes.8 A total of 59% of toddlers had this information available and there was no significant association of STS-EACTS scores with T-cell counts. We additionally defined the slope of change in T-cell counts in the 3 cardiac groups using a subset of 119 patients with longitudinal data. Decline in T-cell counts in humans is thought to be primarily driven by thymic involution. We examined the rate of decline by measuring the mean slope for each cardiac group to determine whether there were significant differences. There were no differences in the slope of decline for the 3 cardiac groups. Therefore, by multiple lines of analysis, we saw no association between severity of cardiac anomaly or complexity of surgical procedure and T-cell counts. Although our sample size was large, it is possible that there could be a small effect of cardiac anomaly on T-cell counts that would be revealed by a larger sample size.
FIGURE 1.
T-cell counts in patients with 22q11.2del. A, Patients were stratified according to type of cardiac anomaly. Within each category of cardiac anomaly, the mean T-cell counts are displayed with SDs. B, Patients were stratified according to cardiac anomaly and the category of T-cell count. The differences for both analyses did not reach significance.
There are some other limitations to this study. It was performed at a single center using extracted data from medical records. No effort was made to assess infection burden or other potential consequences of immune deficiency. Our data on complexity of the surgery were limited and thymectomy might be an influence on ultimate T-cell counts that we were not able to analyze as a separate variable. These limitations are balanced by strengths to the study. The data set is large and the T-cell assessments were performed in the same hospital laboratory ensuring consistency. We have a standard nomenclature for cardiac anomalies so that within the study cohort, the diagnoses are comparable. In conclusion, this large study found that T-cell counts comprise a spectrum across patients with 22q11.2del and various cardiac anomalies with no strong association between cardiac anomaly and T-cell count. These data support universal examination of T cells in patients with 22q11.2del.
Clinical Implications.
T-cell numbers may be influenced by cardiac anomalies in the general population. This study examined the severity of the cardiac anomaly with respect to T-cell count and found no statistical association.
Footnotes
Conflicts of interest: K. E. Sullivan has received consultancy fees from the Immune Deficiency Foundation; is employed by the National Institutes of Health (NIH) and the Children’s Hospital of Philadelphia; has received research support from the NIH; has received lecture fees for various meetings; and receives royalties from UpToDate. E. Goldmuntz, J. W. Gaynor, E. Zackai, and D. McDonald-McGinn (presented lectures on 22q11.2del) have received lecture fees from Natera. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.McDonald-McGinn DM, Sullivan KE, Marino B, Philip N, Swillen A, Vorstman JA, et al. 22q11.2 deletion syndrome. Nat Rev Dis Primers 2015;1: 15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grati FR, Molina Gomes D, Ferreira JC, Dupont C, Alesi V, Gouas L, et al. Prevalence of recurrent pathogenic microdeletions and microduplications in over 9500 pregnancies. Prenat Diagn 2015;35:801–9. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan KE, Jawad AF, Randall P, Driscoll DA, Emanuel BS, McDonald-McGinn DM, et al. Lack of correlation between impaired T cell production, immunodeficiency and other phenotypic features in chromosome 22q11.2 deletions syndrome (DiGeorge syndrome/velocardiofacial syndrome). Clin Immunol Immunopathol 1998;84:141–6. [DOI] [PubMed] [Google Scholar]
- 4.Morsheimer MM, Rychik J, Forbes L, Dodds K, Goldberg DJ, Sullivan K, et al. Risk factors and clinical significance of lymphopenia in survivors of the Fontan procedure for single-ventricle congenital cardiac cisease. J Allergy Clin Immunol Pract 2016;4:491–6. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan KE, Burrows E, McDonald McGinn DM. Healthcare utilization in chromosome 22q11.2 deletion patients with cardiac disease and low T cell counts. Am J Med Genet A 2016;170:1630–4. [DOI] [PubMed] [Google Scholar]
- 6.Billett J, Cowie MR, Gatzoulis MA, Vonder Muhll IF, Majeed A. Comorbidity, healthcare utilisation and process of care measures in patients with congenital heart disease in the UK: cross-sectional, population-based study with case-control analysis. Heart 2008;94:1194–9. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien SM, Clarke DR, Jacobs JP, Jacobs ML, Lacour-Gayet FG, Pizarro C, et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg 2009;138:1139–53. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs JP, Jacobs ML, Maruszewski B, Lacour-Gayet FG, Tchervenkov CI, Tobota Z, et al. Initial application in the EACTS and STS Congenital Heart Surgery Databases of an empirically derived methodology of complexity adjustment to evaluate surgical case mix and results. Eur J Cardiothorac Surg 2012;42:775–9. discussion 779–80. [DOI] [PMC free article] [PubMed] [Google Scholar]