Abstract
Aim:
The aim of the study is to evaluate percent fall in CA-125 levels after neoadjuvant chemotherapy (NAC) and preoperative CA-125 value to predict surgical and survival outcomes in women with advanced-stage epithelial ovarian cancer (EOC).
Methods:
A retrospective review of 406 women receiving NAC for advanced-stage EOC from January 2012 to July 2015 was conducted. Data were collected for demography, radiographic profile, CA-125 levels before and after NAC, chemotherapy, and surgicopathological information. Percent fall in CA-125 was categorized into two groups: <95% (R < 95) and >95% (R > 95) fall from prechemotherapy to preoperative levels. Similarly, women were also categorized using preoperative CA-125 levels of <100 and >100 U/ml. A subset of women from January 2012 to December 2013 was followed to June 2015 for evidence of any recurrence to determine survival outcomes.
Results:
About 56% women had R > 95 and 44% had R < 95. As compared to R < 95, R > 95 group was more likely to have complete cytoreduction (P = 0.00). Furthermore, women with R > 95 had significant better progression-free survival (PFS) as compared to women with R < 95 (P = 0.009) but no difference in overall survival (OS) (P = 0.28). Women with preoperative CA-125 <100 had significant higher number of complete cytoreduction (55% vs. 40%; P = 0.00) and were associated with both PFS (P = 0.007) and OS benefit (P = 0.02).
Conclusion:
Our data showed that >95% fall in CA-125 and an absolute preoperative CA-125 value of <100 U/ml is associated with better surgical and survival outcome in women with advanced EOC. These data are important in patient counseling and treatment planning.
Keywords: Interval debulking surgery, neoadjuvant chemotherapy, percent fall
Introduction
Epithelial ovarian cancer (EOC) is one of the leading causes of death due to gynecologic malignancy in India. According to the WHO, there were approximately 26,834 new ovarian cancer cases and 19,549 deaths in 2012 in India, with over 70% of women presenting with advanced-stage disease at the time of diagnosis.[1] Standard treatment for ovarian cancer consists of primary debulking surgery followed by platinum-based chemotherapy.[2,3,4] Moreover, primary debulking surgery for an advanced ovarian cancer is much more morbid as compared to interval debulking surgery (IDS), requiring extensive bowel resections and multiple upper abdominal procedures. Cytoreduction (either primary or interval) to either no gross residual (complete) or <1 cm residual disease (optimal) has a well-established survival benefit.[2,5] Thus, patients who are poor candidates for an optimal cytoreduction during primary surgery are given 3–4 cycles of platinum-based Neo-adjuvant chemotherapy (NACT) with a goal of optimal interval cytoreduction.[6,7]
However, many queries need to be resolved regarding the use of NACT such as the optimal way to monitor the tumor response to neoadjuvant chemotherapy (NAC), the optimal number of NACT cycles to be given, and the optimal timing of surgery. Computed tomography (CT) scan is the standard imaging modality to monitor the tumor response and to predict the extent of cytoreduction after NACT. However, the sensitivity of CT scan for peritoneal metastasis of 1 cm or smaller in maximum diameter is only 25%–50%,[8] concluding that in some patients, optimal cytoreduction may not be achieved during IDS, even if it is predicted by the CT scan.
Ca-125 has got a well-established role in making the diagnosis as well as for monitoring the response after treatment during follow-up of the patients. Furthermore, it has been shown to have prognostic significance in adjuvant setting.
However, its prognostic role as a single pretreatment cutoff value is controversial as it loosely correlates with the volume of the disease. Many studies have shown that CA-125 is a surrogate marker for in vivo chemosensitivity of the disease; and thus, recently, studies have started evaluating its prognostic significance in neoadjuvant setting. Multiple studies have tried to correlate the preoperative (after NACT) CA-125 levels with both surgical (complete or optimal) and survival progression-free survival (PFS) and overall survival (OS) outcomes.[9,10,11,12] In a study by Rodriguez et al.,[10] preoperative CA-125 levels of <100 U/ml were highly likely to be cytoreduced to no gross residual disease. Another similar study[11] has concluded that preoperative CA-125 level of <20 U/ml is an independent predictor of complete cytoreduction. Thus, the results have been inconsistent regarding prognostic value of preoperative CA-125 levels. Recently, a study by Mahdi et al.[13] has shown that percent reduction of at least 90% in CA-125 levels after NACT is associated with more number of complete IDS, favorable pathological response, and fewer bowel resections but not with improved survival outcome.
With this background, we evaluate the role of percent fall in CA-125 levels after NACT to predict complete or optimal cytoreduction and need for bowel resections and upper abdominal procedures. Second, we evaluate whether percent fall in CA-125 levels correlates with patient survival outcomes (PFS and OS). At the same time, we also determine whether preoperative CA-125 cutoff value of <100 U/ml correlates with the same endpoints.
Methods
All women who received platinum-based NAC for Stages III and IV EOC and for PPC between January 2012 and July 2015 in gynecologic oncology department of Gujarat Cancer Research Institute (GCRI) were identified. The diagnosis was made on biopsy or cytology report, and staging was done based on the extent of disease on imaging. Approval to conduct the study was taken from GCRI institutional review board. Then, the women who underwent IDS and have known prechemotherapy and preoperative CA-125 values were recruited for the study. All women with mucinous carcinoma on final histopathology were excluded because of their different clinical behavior and distinct molecular profile and also, as they are less chemosensitive compared to their serous counterparts and generally associated with lower CA-125 levels.
Data were collected in a retrospective manner for demographic profile, clinical profile, chemotherapy details, and surgical outcomes. All women had undergone IDS with an aim to achieve complete cytoreduction (no gross residual disease). Cytoreduction was considered optimal if woman had <1 cm residual disease in maximum diameter and was considered suboptimal if >1 cm disease was left in situ.
For statistical analysis, percent fall in CA-125 levels from prechemotherapy to preoperative value was calculated as <95% and >95%, and women were categorized accordingly: R < 95 and R > 95. Similarly, based on previous studies, we selected 100 U/ml as cutoff value for preoperative CA-125 level to see whether it correlates with surgical as well as survival outcomes of the women.
Standard univariable analyses were performed, as were multivariable analysis with logistic regression to control for potential confounding variables. The Kaplan–Meier method and Cox proportional hazards regression modeling were used to estimate hazard ratios (HRs) related to PFS and OS. OS was calculated from the date of diagnosis to the date of death or last follow-up. Patients who are alive at the time of analysis were censored. PFS was calculated from the date of diagnosis to the date of progression/recurrence or last follow-up. Similarly, patients with no recurrence were censored. Cox proportional hazards regression was used to identify independent predictors of OS and PFS. Factors entered in the multivariable model included age at diagnosis, stage, and extent of tumor cytoreduction. Finally, we performed an interaction testing to see if percent reduction in CA-125 has a differential impact on those who received at least three cycles and those who received <3 cycles.
A subset of women from January 2012 to December 2013 was followed up to July 2015 at regular intervals with clinical examination, CA-125 levels, and USG (abdomen and pelvis) to determine their survival outcomes (PFS and OS). Recurrence was diagnosed based on rising CA-125 levels along with clinical/radiological evidence of disease or histological diagnosis of recurrence.
Results
A total of 433 women underwent IDS from January 2012 to July 2015 at our institution. Of them, 27 women were excluded based on missing data and mucinous histology. Finally, 406 women were analyzed in the study. Overall, 67% (273/406) of the women underwent complete and optimal cytoreduction and 33% (133/406) had sub-optimal cytoreduction. The median age of the entire cohort was 57 years. About 71.5% of the women were in Stage III and 28.5% were in Stage IV. The most common histology was serous accounting for 95.5% and the rest were comprised of endometrioid, clear cell, and other rare tumors.
Of 406 women, 57% (230/406) had >95% reduction (R > 95) and 43% (176/406) had equal or <95% reduction (R < 95) in CA-125 levels after receiving NACT. The distribution of clinical characteristics between the two study groups is listed in Table 1.
Table 1.
Distribution of clinical and surgical characteristics by percent fall in CA-125 levels after neoadjuvant chemotherapy (n=406)
| Variable | R > 95 (n=230), n (%) | R < 95 (n=176), n (%) | P |
|---|---|---|---|
| Age (median), years | 50 (45-54) | 53 (40-63) | 0.62 |
| Histology | |||
| Serous | 218 (94.7) | 170 (96.5) | 0.77 |
| Endometrioid | 8 (3.4) | 4 (2.2) | |
| Clear cell | 3 (1.3) | 1 (0.5) | |
| Others | 1 (0.4) | 1 (0.5) | |
| Stage | |||
| III | 156 (67.8) | 118 (67) | 0.57 |
| IV | 74 (32.1) | 58 (33) | |
| Grade | |||
| High grade | 103 (44.7) | 68 (38.6) | 0.91 |
| Not specified | 127 (55.2) | 108 (61.3) | |
| Preoperative fall in CA-125 levels (median) | 97.9 | 87.3 | 0.00 |
| Preoperative CA-125 levels (median) U/ml | 24 | 107 | 0.00 |
| Extent of cytoreduction | |||
| Complete | 152 (66) | 52 (59.5) | 0.00 |
| Optimal | 51 (22.1) | 18 (10.2) | |
| Suboptimal | 24 (10) | 93 (52.8) | |
| Inoperable | 03 (1.3) | 13 (7.3) | |
| Bowel and upper abdominal surgeries | 20 (8.6) | 09 (5.1) | 0.69 |
| Number of NACT cycles | |||
| <3 | 143 (62.1) | 110 (62.5) | 1.0 |
| ≥3 | 87 (37.8) | 66 (37.5) |
NACT=Neo-adjuvant chemotherapy
The rate of optimal/complete cytoreduction was significantly higher in R > 95 group compared to R < 95 group (89% vs. 40%, P = 0.00). The number of bowel resections and upper abdominal surgeries was similar between both the groups (4.7% vs. 4% and 1.1% vs. 1%, P = 0.69). Here, it should be noted that both the groups received similar number of NACT cycles (P = 1).
Similarly, based on single preoperative levels of CA-125, women were categorized as having ≥100 U/ml of levels. Table 2 shows the study characteristics between both the groups.
Table 2.
Distribution of clinical and surgical characteristics by preoperative CA-125 levels (n=406)
| Variable | CA-125 ≤100 U/ml (n=275), n (%) | CA-125 >100 U/ml (n=131), n (%) | P |
|---|---|---|---|
| Age (median), years | 52 (25-74) | 50 (27-70) | 0.99 |
| Histology | |||
| Serous | 261 (97) | 127 (96.9) | 0.71 |
| Endometrioid | 9 (3.2) | 3 (2.2) | |
| Clear cell | 3 (1.0) | 1 (0.7) | |
| Others | 2 (0.7) | 0 | |
| Stage | |||
| III | 195 (71.0) | 91 (67) | 0.91 |
| IV | 80 (29.0) | 40 (33) | |
| Grade | 0.45 | ||
| High grade | 114 (41.4) | 50 (38.1) | |
| Not specified | 161 (58.5) | 81 (61.8) | |
| Preoperative fall in CA-125 levels (median) | 96.9 | 89.1 | 0.61 |
| Preoperative CA-125 levels (median) U/ml | 28 (13-100) | 251 (104-5859) | 0.00 |
| Extent of cytoreduction | 0.00 | ||
| Complete | 152 (55.2) | 52 (39.6) | |
| Optimal | 57 (20.7) | 12 (9.1) | |
| Suboptimal | 58 (21.0) | 57 (43.5) | |
| Inoperable | 6 (2.1) | 10 (7.6) | |
| Bowel and upper abdominal surgeries | 20 (7.2) | 4 (3.0) | 0.69 |
Women with preoperative CA-125 levels <100 U/ml were more likely to have complete/optimal cytoreduction (76% vs. 49%, P = 0.00). Although the rate of bowel resections and upper abdominal surgeries was slightly higher in women with preoperative CA-125 levels <100 U/ml (5.4% vs. 2.2% and 1.8% vs. 0.7%), the difference was not statistically significant (P = 0.148).
A total of 203 women from January 2012 to December 2013 were followed up to July 2015 to determine PFS and OS. Of them, 26 women had lost to follow-up, and thus, survival analysis was done for 177 women. The median follow-up time was 30 months. The median PFS and OS for the entire cohort were 9 months and 20 months, respectively. Correlation of percent fall in CA-125 levels and single preoperative cutoff value with survival outcomes has been shown in Table 3.
Table 3.
Correlation with survival outcomes with the two study groups (n=177)
| PFS | OS | |||||
|---|---|---|---|---|---|---|
| Median | HR (95% CI) | P | Median | HR (95% CI) | P | |
| R > 95 | 9.0 | 7.1-10.8 | 0.009 | 21.0 | 18.7-23.2 | 0.28 |
| R ≤ 95 | 5.0 | 3.5-6.4 | 18.0 | 15.1-20.8 | ||
| CA-125<100 U/ml | 8.0 | 6.2-9.7 | 0.007 | 21.0 | 19-22.9 | 0.02 |
| CA-124>100 U/ml | 5.0 | 3.4-6.5 | 17.0 | 15.8-18.1 | ||
CI=Confidence interval, HR=Hazard ratio, OS=Overall survival, PFS=Progression-free survival
Women with >95% reduction in CA-125 levels after NACT had a significant improvement in PFS (median 9 vs. 5 months, P = 0.009) but no difference in OS (median 21 vs. 18 months, P = 0.28) [Figure 1]. However, women with preoperative CA-125 levels of <100 U/ml had a significantly longer PFS (median 8 vs. 5 months, P = 0.007) as well as OS (median 21 vs. 17 months, P = 0.02) [Figure 2].
Figure 1.
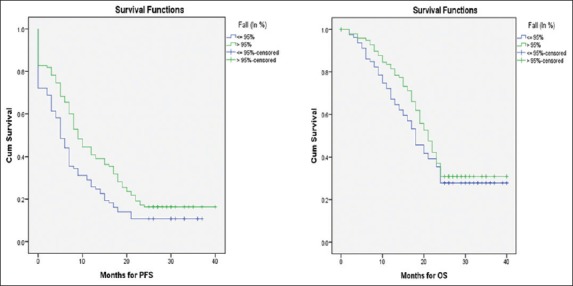
Kaplan–Meier curves for progression-free survival and overall survival by percent fall in CA-125 levels after NACT >95% versus <95% fall
Figure 2.
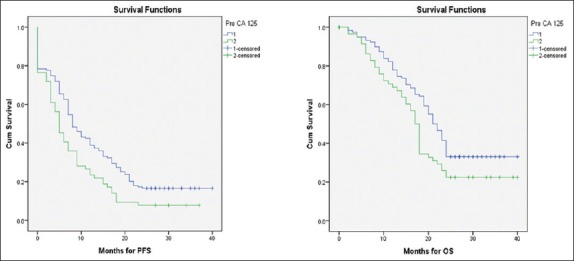
Kaplan–Meier curves of progression-free survival and overall survival by preoperative CA-125 cutoff value (<100 U/ml vs. >100 U/ml)
Discussion
Despite the debate about equivalence with regard to OS between primary debulking surgery and interval cytoreduction for advanced-stage ovarian cancer, women who are inappropriate for primary surgery due to various reasons will continue to receive NACT followed by IDS. In our study, 67% of women underwent optimal and complete cytoreduction; the rate is lower than those reported by Rodriguez et al.[10] and Tate et al.[12] (both 96%) but higher than that reported by Le et al.[9] (54%) and Furukawa et al.[11] (61.3%). This difference can be explained on the basis of (i) different protocols to select patients for primary surgery vs IDS at different centres and (ii) whether the surgery is being performed by a Gynecologist or Gyne-Oncologist. Furthermore, different surgical aggressiveness by various surgeons also explains the difference.
Gynecologic oncology group (GOG) has defined optimal cytoreduction as <1 cm gross residual in maximum diameter being left in situ. In 2006, Bristow and Chi[14] further demonstrated that HR of >1 cm gross residual was almost double as compared to <1 cm (3.98 vs. 2.09). Recently, a GOG trial (GOG 182) has shown a further survival advantage for those with no gross residual disease; thus, the current goal of cytoreductive surgery is complete cytoreduction. Till date, the current standard to predict whether a patient will undergo complete cytoreduction after NACT is imaging along with CA-125 response. Our study has provided an objective, noninvasive, and simple criteria to predict the extent of cytoreduction. In our study, women with >95% fall in CA-125 levels were more likely to have complete cytoreduction with a positive predictive value (PPV) of 74.5% as compared to only 30% in women with <95% reduction. Furthermore, women with >95% reduction tend to have longer PFS but not OS. Thus, percent fall in CA-125 levels could help predict the probability of complete cytoreduction in neoadjuvant setting and also to counsel the patients regarding improved survival outcome.
Multiple studies[9,10,11,12] have evaluated the role of a single preoperative CA-125 level (after receiving NACT) to predict complete cytoreduction and survival outcomes. In our series, preoperative CA-125 levels of <100 U/ml help to predict complete cytoreduction with PPV of 75% as compared to 40% with CA-125 levels of >100 U/ml. Furthermore, it was associated with improved PFS and OS on univariate analysis.
It should be noted that in our study, both the groups (R > 95 and R < 95) are similar regarding the number of NACT cycles received (P = 1.0) [Table 1]. Therefore, giving additional cycles of NACT did not result in further significant fall in CA-125 levels in our study. Bristow and Chi[14] have reported in a meta-analysis that each additional cycle of NAC is associated with a 4.1-month reduction in median cohort survival. Hence, having an objective preoperative predictor of complete cytoreduction such as >95% fall in CA-125 levels along with preoperative CA-125 cutoff value <100 U/ml might help to plan for an earlier surgery to improve the survival. On the other hand, in nonresponders, the role of salvage chemotherapy or second-line chemotherapy might be explored, so that morbidity associated with a suboptimal laparotomy might be avoided.
There are multiple strengths to our study including a larger sample size and ability to correlate both CA-125 percent fall and preoperative CA-125 levels with surgical and survival outcomes of women receiving NAC for advanced-stage ovarian cancer. However, limitations of our study involve its retrospective designs.
To conclude, our study has shown that both >95% percent fall and preoperative CA-125 levels of <100 U/ml predict complete cytoreduction with PPV of 75% and more importantly can predict improved survival outcome in women with advanced-stage ovarian cancer. These findings are important in patient counseling and further treatment planning.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.GLOBOCAN 2012: Estimated Cancer Incidence, Mortality, Prevalence Worldwide in 2012. IARC Cancer Base No 11 [Google Scholar]
- 2.Chi DS, Liao JB, Leon LF, Venkatraman ES, Hensley ML, Bhaskaran D, et al. Identification of prognostic factors in advanced epithelial ovarian carcinoma. Gynecol Oncol. 2001;82:532–7. doi: 10.1006/gyno.2001.6328. [DOI] [PubMed] [Google Scholar]
- 3.Hoskins WJ, McGuire WP, Brady MF, Homesley HD, Creasman WT, Berman M, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170:974–9. doi: 10.1016/s0002-9378(94)70090-7. [DOI] [PubMed] [Google Scholar]
- 4.Shih KK, Chi DS. Maximal cytoreductive effort in epithelial ovarian cancer surgery. J Gynecol Oncol. 2010;21:75–80. doi: 10.3802/jgo.2010.21.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winter WE, 3rd, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, et al. Prognostic factors for stage III epithelial ovarian cancer: A gynecologic oncology group study. J Clin Oncol. 2007;25:3621–7. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 6.Vergote I, van Gorp T, Amant F, Neven P, Berteloot P. Neoadjuvant chemotherapy for ovarian cancer. Oncology (Williston Park) 2005;19:1615–22. [PubMed] [Google Scholar]
- 7.Vergote I, Amant F, Kristensen G, Ehlen T, Reed NS, Casado A, et al. Primary surgery or neoadjuvant chemotherapy followed by interval debulking surgery in advanced ovarian cancer. Eur J Cancer. 2011;47(Suppl 3):S88–92. doi: 10.1016/S0959-8049(11)70152-6. [DOI] [PubMed] [Google Scholar]
- 8.Ushijima K, Ota S, Komai K, Matsuo G, Motoshima S, Honda S, et al. Clinical assessment of neoadjuvant chemotherapy and interval cytoreductive surgery for unresectable advanced ovarian cancer. Int Surg. 2002;87:185–90. [PubMed] [Google Scholar]
- 9.Le T, Faught W, Hopkins L, Fung-Kee-Fung M. Importance of CA125 normalization during neoadjuvant chemotherapy followed by planned delayed surgical debulking in patients with epithelial ovarian cancer. J Obstet Gynaecol Can. 2008;30:665–70. doi: 10.1016/S1701-2163(16)32914-0. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez N, Rauh-Hain JA, Shoni M, Berkowitz RS, Muto MG, Feltmate C, et al. Changes in serum CA-125 can predict optimal cytoreduction to no gross residual disease in patients with advanced stage ovarian cancer treated with neoadjuvant chemotherapy. Gynecol Oncol. 2012;125:362–6. doi: 10.1016/j.ygyno.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Furukawa N, Sasaki Y, Shigemitsu A, Akasaka J, Kanayama S, Kawaguchi R, et al. CA-125 cut-off value as a predictor for complete interval debulking surgery after neoadjuvant chemotherapy in patients with advanced ovarian cancer. J Gynecol Oncol. 2013;24:141–5. doi: 10.3802/jgo.2013.24.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tate S, Hirai Y, Takeshima N, Hasumi K. CA125 regression during neoadjuvant chemotherapy as an independent prognostic factor for survival in patients with advanced ovarian serous adenocarcinoma. Gynecol Oncol. 2005;96:143–9. doi: 10.1016/j.ygyno.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Mahdi H, Maurer KA, Nutter B, Rose PG. The impact of percent reduction in Ca-125 levels on prediction of theextent of interval cytoreduction and outcome in patients with advanced-stage cancer of mullerian origin treated with neoadjuvant chemotherapy. Int J Gynecol Cancer. 2015;25:823–9. doi: 10.1097/IGC.0000000000000434. [DOI] [PubMed] [Google Scholar]
- 14.Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: A meta-analysis. Gynecol Oncol. 2006;103:1070–6. doi: 10.1016/j.ygyno.2006.06.025. [DOI] [PubMed] [Google Scholar]


