Abstract
Osteoarthritis (OA) pathogenesis is mediated largely through the actions of proteolytic enzymes such as matrix metalloproteinase (MMP) 13. The transcriptional regulator CITED2, which suppresses the expression of MMP13 in chondrocytes, is induced by interleukin (IL)-4 in T cells and macrophages, and by moderate mechanical loading in chondrocytes. We tested the hypothesis that CITED2 mediates cross-talk between IL-4 signaling and mechanical loading-induced pathways that result in chondroprotection, at least in part, by downregulating MMP13. IL-4 induced CITED2 gene expression in human chondrocytes in a dose- and time-dependent manner through JAK/STAT signaling. Mechanical loading combined with IL-4 resulted in additive effects on inducing CITED2 expression and downregulating of MMP13 in human chondrocytes in vitro. In vivo, IL-4 gene knockout (KO) mice exhibited reduced basal levels of CITED2 expression in chondrocytes. While moderate treadmill running induced CITED2 expression and reduced MMP13 expression in wild-type mice, these effects were blunted (for CITED2) or abolished (for MMP13) in chondrocytes of IL-4 gene KO mice. Moreover, intra-articular injections of mouse recombinant IL-4 combined with regular cage activity mitigated post-traumatic OA to a greater degree compared to immobilized mice treated with IL-4 alone. These data suggest that using moderate loading to enhance IL-4 may be a potential therapeutic strategy for chondroprotection in OA.
Keywords: CITED2, MMP13, IL-4, inflammation, cartilage degradation, osteoarthritis
Introduction
Mechanical loading within a physiological range of intensities and frequencies is required to maintain cartilage homeostasis and normal joint function.1 It has been long established that moderate mechanical loading by exercise benefits cartilage health and function by increasing cartilage thickness,2 proteoglycan content,2,3 and mechanical stiffness.4 However, nonphysiological loading such as overuse can lead to cartilage degradation.5–7 Proinflammatory cytokines and other mediators produced in joint tissues in response to nonphysiological loading play important roles in cartilage degradation and osteoarthritis (OA) pathogenesis.8–11 In particular, interleukin (IL)-1 and tumor necrosis factor-α can induce cartilage degradation12 by upregulating matrix metalloproteinase (MMP) 13 gene expression in articular chondrocytes.13,14
IL-4, produced mainly by T lymphocytes in the thymus, is an anti-inflammatory cytokine that reduces proinflammatory cytokine production and activity.15 A chondroprotective role of IL-4 has been proposed15 and recent studies further demonstrated that IL-4 protects cartilage from degradation.16 CBP/P300-interacting transactivator 2 (CITED2, also known as MRG1) is a multiple stimuli–responsive transcriptional regulator, originally identified as an IL-4-inducible molecule in T lymphocytes17 and macrophages.18 It is a critical chondroprotective mediator that downregulates MMPs during moderate loading.19–21 Collectively, these findings raised the following questions: (1) Is CITED2 inducible by IL-4 in chondrocytes? (2) Does CITED2 serve as a common mediator for both IL-4 and mechanical loading in chondroprotection? (3) Could cross-talk between the anti-inflammatory and mechanical loading pathways result in a synergy that could represent a new intervention for chondroprotection in articular cartilage? Accordingly, we carried out a series of in vitro and in vivo studies to address these questions.
Materials and methods
Cell culture and transfection
Immortalized human chondrocytes, the C28/I2 cell line, were cultured in DMEM/F12 (Life Technologies) supplemented with 10% FBS (Biowest). The C28/I2 cells were plated in 6-well plates and transfected with 10 pmol of STAT6-specific siRNA (Santa Cruz Biotechnology) or scrambled siRNA (Santa Cruz Biotechnology) using Lipofectamine transfection reagent (Life Technologies) and subjected to further treatments 24 h after transfection. Transfection efficiency was monitored based on quantification of transfected green fluorescence labeled irrelevant siRNA by FACS analysis in a parallel experiment. Primary human chondrocytes were isolated from cartilage discarded from knee replacement surgeries under IRB-approved protocols, cultured as above in the presence of IL-1β (10 ng/mL), and used for experiments within passages 1 or 2, as described.22
IL-4 treatment and moderate mechanical loading
Three different forms of physiologically relevant mechanical loading such as uniaxial strain (US), fluid shear stress (FSS), and intermittent hydrostatic pressure (IHP) were used in this study. For US loading, C28/I2 cells or human primary chondrocytes were cultured in poly-L-lysine–coated custom-designed cell culture and stretch wells. After overnight culture, the cells were treated with IL-4 (R&D) at 1–25 ng/mL and/or subjected to US at (0–10%, 1 Hz) using a custom-designed US loading device22 without or with 10 ng/mL IL-1β, as indicated in the specific experiments. The cells were then lysed for real-time PCR or western blot analysis. In order to dissect the signaling pathway of IL-4 in the induction of CITED2 gene expression, C28/I2 cells were treated without or with inhibitors of JAK3 (Ruxolitinib 300 μM Santa Cruz Biotechnology) or transfected with siSTAT6 (Santa Cruz Biotechnology) with approximately 70% efficiency based on quantification by FACS analysis (see above). For FSS loading, the C28/I2 cells were plated in iBidi flow chambers (μ–slide VI0.4 chamber, ibidi GmbH, Germany). The cells were cultured in serum starvation medium for 16–18 h, and then subjected to oscillatory FSS of ±5 dyn/cm2 at 1 Hz using the Legato ® 200 syringe pump (KD Scientific), as described.23 For IHP loading, C28/I2 cells or primary chondrocytes were plated in 35-mm dishes, starved overnight (16–18 h) and then loaded with 5 MPa pressure at 1 Hz for 1 h on a custom-designed hydrostatic pressure mechanical loading device, as described.19 After mechanical loading, total RNA or protein was isolated for analysis using real-time qPCR or western blotting, respectively.
Mouse model and treadmill running
IL-4 gene knockout (KO) mice (Jackson Laboratories) or wild-type (WT) controls (5- to 6-month-old, male) were subjected to moderate treadmill running at 10 m/min24 for 45 minutes. Articular cartilage from one knee was dissected from the mice 3 h after treadmill running, total RNA was extracted, and Cited2 and Mmp13 mRNA levels were analyzed by real-time PCR with Gapdh as an internal control. The other articular joint was fixed in formalin, decalcified, and embedded in paraffin.
Destabilization of the medial meniscus and treatment
Five- to six-month-old mice (male, C57BL/6, Jackson Labs) were subjected to destabilization of the medial meniscus (DMM).25 The mice were then randomly assigned into three groups/treatments starting immediately after DMM surgery: (1) weekly intra-articular injections of PBS (10 μL, vehicle control) and normal cage activity; (2) weekly intra-articular injections of recombinant mouse IL-4 (100 pg in 10 μL PBS) and hind limb immobilization; and (3) weekly intra-articular injections of recombinant mouse IL-4 (100 pg in 10 μL PBS) and normal cage activity. Four weeks after DMM surgery, the mice were sacrificed and the knee joints were dissected, fixed in formalin, and embedded in paraffin. Histologic analysis was performed on the Safranin O/Fast green-stained slides using the OARSI scoring system.26
Immunohistochemistry and immunofluorescence staining
Paraffin-embedded tissues were sectioned at 5–7 μm thickness. Sections were deparaffinized, and incubated overnight at 4 °C with antibodies against CITED2 (Abcam) and MMP13 (Abcam). For immunohistochemistry, sections were incubated with anti-rabbit secondary antibody (Biocare Medical), and visualized with DAB chromagen (Vector Laboratories). For immunofluorescence staining, sections were incubated with an anti-rabbit secondary antibody conjugated with Alexa Fluor® 647 (Cell Signaling) and a DAPI counterstain (Cell Signaling).
Western blot
IL-4– and/or mechanical loading-treated human chondrocytes were lysed for western blot analysis. Approximately 20 μg of total protein extracted from cell lysates was separated by electrophoresis (SDS-PAGE) in a 10% polyacrylamide gel, and the separated proteins were transferred to Immun-Blot PVDF membranes (Bio-Rad, Hercules, CA). Membranes were incubated with primary antibodies specific for JAK3, STAT6, phosphorylated JAK3, and STAT6 (all from Cell Signaling), and for MMP13 (Abcam) and CITED2 (Santa Cruz), followed by incubation with secondary antibodies conjugated to horseradish peroxidase, and detected by an ECL western blotting analysis system (GE Life Sciences, Piscataway, NJ). A rabbit antibody specific for human β-actin (Sigma-Aldrich, St. Louis, MO) was used as a loading control.
RNA isolation and real-time PCR
Total RNA was isolated from chondrocytes using the GeneJET RNA kit (Thermo Scientific) following manufacturer’s instructions. First strand cDNA was synthesized with iScript RT supermix (Bio-Rad). Real-time PCR was performed to determine relative gene expression using SYBR green (Bio-Rad) and gene-specific primers, as follows: CITED2-F: TGGGCGAGCACATACACTAC, CITED2-R: GGTAGGGGTGATGGTTGAAA; MMP13-F: ACTGAGAGGCTCCGAGAAATG, MMP13-R: GAACCCCGCATCTTGGCTT; GAPDH-F: CATGGAGAAGGCTGGGGCTCATTTG, GAPDH-R: GGGGTGCTAAGCAGTTGGTGGT. Relative gene expression was calculated using the ΔΔCT method.19,22
Statistical analysis
Differences were tested for statistical significance using a Student’s t-test for pairwise comparisons and ANOVA followed by Tukey’s posthoc test for multiple comparisons. All data sets were measured at least in triplicate. Data are plotted as mean ± S.E.M using column charts.
Results
IL-4 induces CITED2 expression in human chondrocytes through the JAK/STAT pathway
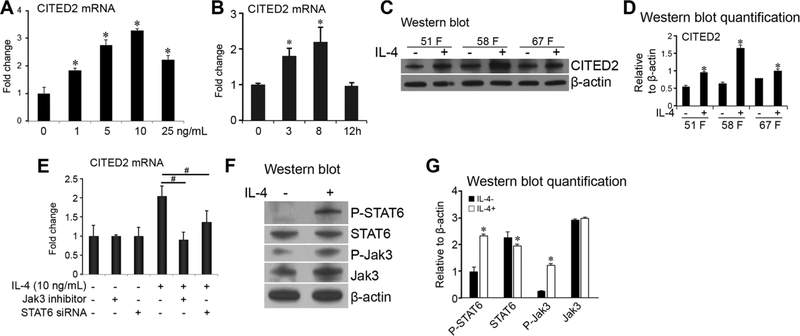
Human chondrocytic C28/I2 cells were treated with recombinant human IL-4 at the indicated concentrations (Fig. 1A). CITED2 gene expression was inducible by IL-4 in a dose-dependent manner (Fig. 1A). Furthermore, in C28/I2 cells treated with 10 ng/mL IL-4, CITED2 mRNA levels were upregulated at 3 h, peaked at 8 h, and declined to a basal level by 12 h (Fig. 1B). Western blot analysis showed higher levels of CITED2 in all three samples of human primary OA chondrocytes after treatment with human recombinant IL-4 compared to untreated cultures (Fig. 1C and D). Previous studies demonstrated that the JAK/STAT signaling pathways play a critical role in mediating IL-4 signaling.27,28 We further examined the role of these pathways in IL-4–induced CITED2 in chondrocytes by blocking JAK3 and STAT6. Our results revealed that treatment with JAK3-specific inhibitors abolished and STAT6 mRNA knockdown reduced induction of CITED2 by IL-4 in human chondrocytes (Fig. 1E). The reduction, instead of abolishment, of IL-4–induced CITED2 expression by STAT6-specific siRNA may be due to the incomplete knockdown of STAT6; STAT6 siRNA had a transfection efficiency of 70%. Moreover, western blotting showed that IL-4–upregulated CITED2 was associated with increased phosphorylation of JAK3 and STAT6 (Fig. 1F and G). These results indicate that IL-4–induced CITED2 expression is mediated, at least in part, by JAK/STAT signaling.
Figure 1.
IL-4 induces CITED2 gene expression in human chondrocytes. (A) Relative fold change of CITED2 mRNA in C28/I2 chondrocytes in response to 0–25 ng/mL of IL-4 for 3 h or (B) 10 ng/mL IL-4 for 0, 3, 8, or 12 hours. (C) Representative western blot of CITED2 and β-actin. (D) CITED2 protein expression relative to β-actin based on quantification of western blots in primary human OA chondrocytes from three patients (51-year-old female, 58-year-old female, and 67-year-old female) undergoing total knee replacement with and without IL-4 treatment (10 ng/mL) for 3 hours. (E) Relative CITED2 mRNA expression in C28/I2 chondrocytes without or with 10 ng/mL IL-4 in the presence or absence of JAK3 inhibitor or STAT6 siRNA. (F) Representative western blot of phosphorylated and total STAT6, phosphorylated and total JAK3, and β-actin after 10 ng/mL IL-4 treatment. (G) Expression of phosphorylated and total STAT6, and phosphorylated and total JAK3 relative to β-actin based on quantification of western blots in C28/I2 chondrocytes. *P < 0.05 for experimental treatment versus control, #P < 0.05 between the indicated experimental groups, based on one-way ANOVA with Tukey posthoc test.
Mechanical loading amplifies the induction of CITED2 in chondrocytes treated with a low dose of IL-4
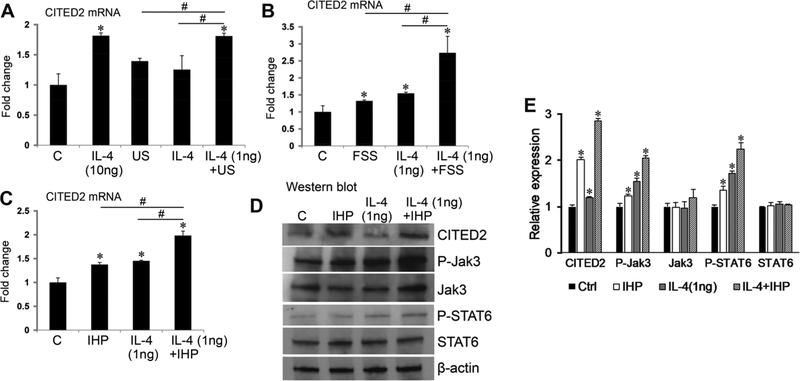
Our previous findings showed that moderate loading, such as IHP,19 FSS,21 and US,22 induces CITED2 expression in human chondrocytes. Having shown in Figure 1 that CITED2 is inducible by IL-4 in chondrocytes, we asked whether moderate mechanical loading and IL-4 exert an additive or synergistic effect on CITED2 expression. CITED2 gene expression was induced by moderate US at 5%, 1 Hz for 1 h (Fig. 2A). Interestingly, CITED2 mRNA levels were significantly higher in the cells treated with both moderate loading (5% US, 1 Hz, 1 h) and a low concentration of IL-4 (1 ng/mL) than in the cells treated with loading or IL-4 at low concentrations alone. The level of CITED2 mRNA expression increased when IL-4 treatment was combined with moderate loading, which was comparable to that achieved with a high dose of IL-4 (10 ng/mL). Similarly, FSS at 5 dyn/cm2 (1 Hz for 1 h, Fig. 2B) or IHP at 5 MPa (1 Hz for 1 h, Fig. 2C) increased CITED2 mRNA expression, and amplified the CITED2 mRNA level in chondrocytes treated with a low level of IL-4.
Figure 2.
Mechanical loading and IL-4 additively increase CITED2 gene and protein expression in human chondrocytes. CITED2 mRNA levels were analyzed in human C28/I2 chondrocytes subjected to the indicated concentrations of IL-4 and/or (A) uniaxial stretch (US), 5%, 1 Hz, 1 h; (B) fluid shear stress (FSS), 5 dyn/cm2, 1 Hz, 1 h; or (C) intermittent hydrostatic pressure (IHP),5 MPa, 1 Hz, 1 hour. (D and E) Western blotting of CITED2, phosphorylated and total JAK3, and phosphorylated and total STAT6 in chondrocytes treated with IL-4 and/or IHP loading (5 MPa, 1 Hz, 1 h). The internal control was β-actin. Results represent three parallel experiments. A representative western blot is shown in D. Quantification of target protein/β-actin ratio from three western blots is shown in E. *P < 0.05 for experimental treatment versus control, #P < 0.05 between the indicated experimental groups, based on one-way ANOVA with Tukey posthoc test.
We next examined whether the JAK/STAT pathway mediates the cross-talk between mechanical loading and IL-4 in the induction of CITED2 in human chondrocytes. Human C28/I2 chondrocytes were treated with 5 MPa IHP (1 Hz for 1 h) and/or 1 ng/mL of IL-4. Western blotting showed increased levels of phosphorylated JAK3 and STAT6 and CITED2 protein in cells treated with mechanical loading or IL-4, and we observed further upregulation in cells subjected to both mechanical loading and IL-4 treatment (Fig. 2D and E). These data indicate that the cross-talk between mechanical loading and IL-4 in upregulation of CITED2 is mediated, at least in part, through the JAK/STAT pathway.
Mechanical loading and a low dose of IL-4 exert an amplified inhibitory effect on MMP13 in human OA chondrocytes
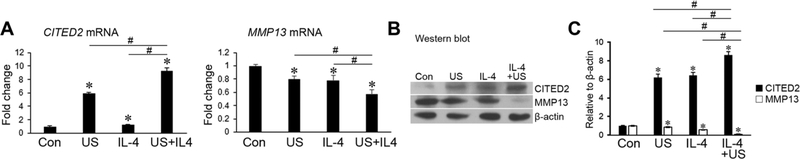
We have previously demonstrated that moderate loading induces CITED2 gene expression and exerts a chondroprotective effect, at least in part, through repressing MMPs such as MMP13 in vitro19,21,22 and in vivo.20 We therefore examined the effect of mechanical loading in combination with IL-4 on MMP13 expression in human OA primary chondrocytes in the presence of IL-1β. US was used as a representative loading model in this experiment because US, IHP, and FSS at moderate levels or intensities have been shown to similarly enhance the effect of IL-4 in inducing CITED2 (Fig. 2). MMP13 expression was mitigated by mechanical loading and IL-4 alone at the mRNA (Fig. 3A) and protein levels (Fig. 3B and C). Interestingly, treatment of OA primary chondrocytes with mechanical loading in the presence of IL-4 intensified the inhibitory effects of either treatment alone (Fig. 3A–C). The effect of IL-4 and loading on CITED2 induction, alone and in combination, in human OA primary chondrocytes (Fig. 3B and C) mirrored the effect seen in human C28/I2 cells (Fig. 2).
Figure 3.
Mechanical loading and IL-4 additively decrease the expression of MMP13 mRNA in human chondrocytes. (A) MMP13 mRNA and (B) MMP13 protein levels were analyzed by real-time qPCR and western blotting, respectively. Human OA primary chondrocytes were subjected to IL-4 treatment (1 ng/mL) and/or uniaxial stretch (US) at 5%, 1 Hz, 1 h in the presence of human recombinant IL-1 β. Results represent three parallel experiments. A representative western blot is shown in B. Quantification of target protein/β-actin ratio from three western blots is shown in C. *P < 0.05 for experimental treatment versus control, #P < 0.05 between the indicated experimental groups, based on one-way ANOVA with Tukey posthoc test.
IL-4 and mechanical loading exert an additive effect in chondroprotection in vivo
We next examined the effects of IL-4 deficiency on treadmill running–induced CITED2 and suppression of MMP13 using IL-4 gene KO mice. Real-time PCR analysis revealed that Cited2 mRNA levels in articular cartilage of IL-4 gene KO mice were more than 30% lower than in those of WT mice (P < 0.05, Fig. 4A). However, treadmill running induced Cited2 mRNA expression in both WT and IL-4 KO mice at similar proportions (1.71-fold in WT versus 1.69-fold in IL-4 KO mice). In contrast, the basal Mmp13 mRNA expression in IL-4 KO mice was slightly higher than that in WT mice (Fig. 4A). However, treadmill running significantly reduced Mmp13 mRNA in WT mice, while it showed no significant effect on that in IL-4 KO mice. Similar effects of IL-4 deficiency on loading-induced CITED2 and suppression of MMP13 were observed at the protein level, as detected by immunofluorescence staining (Fig. 4B and C). These data suggest that IL-4 plays a role in maintaining CITED2 expression and suppressing MMP13 in mouse chondrocytes at the basal level in vivo, and IL-4 plays a significant role in loading-induced suppression of MMP13 expression, partially through induction of CITED2 expression.
Figure 4.
Treadmill running–induced downregulation of Mmp13 is diminished in IL-4 KO mice. (A) Relative mRNA levels of Cited2 and Mmp13, (B) immunofluorescence staining, and (C) quantification of positive cells positive for CITED2 and MMP13 in wild-type and IL-4 KO mice with and without treadmill running. n = 3 mice per group. *P < 0.05 for experimental treatment versus control, #P < 0.05 between the indicated experimental groups, based on one-way ANOVA with Tukey posthoc test. Scale bar = 10 μm.
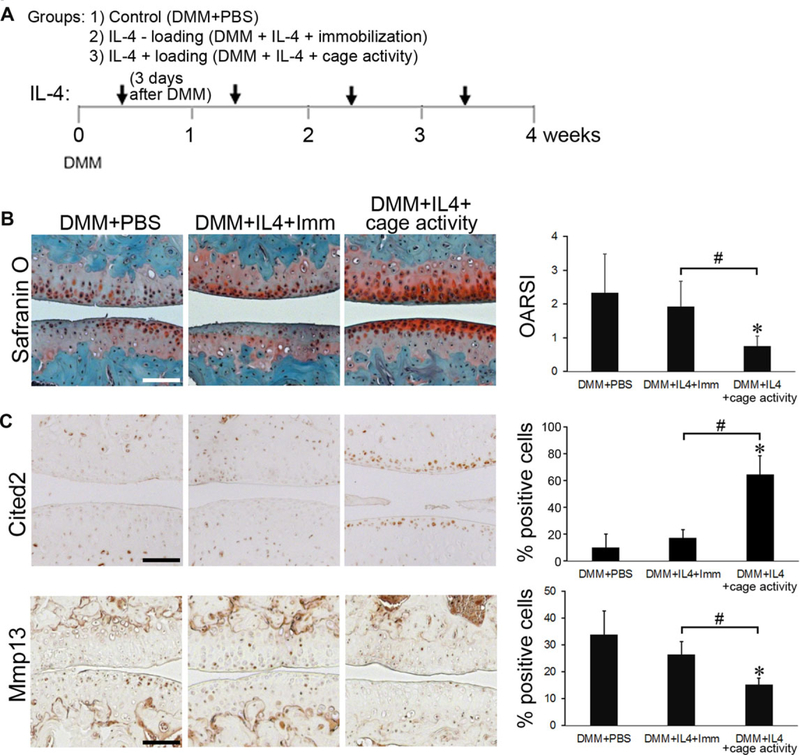
Based on the above findings, we anticipated that the combination of IL-4 and moderate mechanical loading through cage activity would exert chondroprotection in vivo and allow administration of a lower dose of IL-4 and reduction of the duration and/or intensity of loading with efficacy similar to either treatment alone. To test this notion, mice were subjected to DMM, and followed with the following treatments: (1) weekly intra-articular injections of PBS with normal cage activity; (2) weekly intra-articular injections of IL-4 with hind limb immobilization; and (3) weekly intra-articular injections of IL-4 with regular cage activity (Fig. 5A). The dose of IL-4 used was 100 pg, and moderate loading was maintained by keeping the mice at normal cage activity. As a control, immobilization was used to eliminate loading generated by normal cage activity. At 4 weeks after DMM surgery, injection of a low dose of IL-4 combined with cage activity significantly slowed OA progression, compared to PBS control or immobilization, as indicated by Safranin O staining and OARSI scoring (Fig. 5B). The improved pathology was associated with an increased number of chondrocytes positive for CITED2 and decreased number positive for MMP13 (Fig. 5C).
Figure 5.
IL-4 and moderate loading through cage activity exert additive chondroprotection in vivo. C57BL/6 mice (male, 10–12 weeks old, n = 3 mice per group) subjected to DMM surgery and further subjected to weekly intra-articular injections of: (1)PBS (DMM + PBS) with normal cage activity; (2) IL-4 combined with hind limb immobilization (DMM + IL4 + Imm); and (3)IL-4 combined with normal cage activity (DMM + IL4 + cage activity). (A) Treatment protocol. (B) Safranin O/Fast green staining and OARSI scores. (C) Immunohistochemistry and quantification of the number of chondrocytes positive for CITED2 and MMP13 staining. *P < 0.05 for experimental treatment versus control, #P < 0.05 between the indicated experimental groups, based on one-way ANOVA with Tukey posthoc test. Scale bar = 100 μm.
Discussion
CITED2 is a transcriptional regulator induced by IL-4 in T lymphocytes17 and macrophages.18 Our previous studies revealed that CITED2 plays a critical chondroprotective role by suppressing MMP13 at the gene and protein levels.19,21,22,29 CITED2 responds to multiple stimuli, including cytokines and mechanical stress.1,17,19,20 In this study, we provided evidence that the anti-inflammatory cytokine IL-4 induces CITED2 expression in human chondrocytes. While the level of CITED2 increase is modest (1.5- to 3-fold) in this study, this magnitude is consistent with our previous studies of CITED2 and is biologically significant.17,19,21,22 It has been reported that OA cartilage has significantly lower expression of IL-4, which has the ability to hamper IL-1β–induced release of MMP13.16 In addition to its systemic anti-inflammatory role, IL-4 is mechanically inducible30–33 and exerts an anti-inflammatory role in chondrocytes.34 However, while IL-4 has been used in clinical trials for cancer, it has not been well received due to adverse effects from high dosages.35,36
MMP13 is one of the major enzymes capable of degrading the extracellular matrix of articular cartilage.37–39 One of the mechanisms by which proinflammatory cytokines are involved in OA pathogenesis is through its action in the induction of proteolytic enzymes such as MMP13. Downregulation of MMPs in OA chondrocytes is a critical step for halting OA progression. Here, we demonstrated that moderate loading combined with a low dose of IL-4 exerts an intensified inhibitory effect on MMP13 gene expression. In our study, we demonstrated that mechanical loading at cage activity levels can amplify the effects of a low dose of IL-4 and suppress MMP13 in vivo, suggesting that levels of mechanical stimuli in combination with a low-dose of IL-4 may exert significant anti-inflammatory and antiarthritic effects, at least in part, by suppressing MMP13 gene and protein expression. The use of a low dose of IL-4 could prevent the side effects associated with high doses. Meanwhile, for OA treatment, by using a low-dose of IL-4 patients may require less physical exercise/activity for chondroprotection, which is particularly clinically important for the patients whose physical activities are restricted.
In this study, we demonstrated that CITED2 is a common mediator for both IL-4 and mechanical loading. Thus, we asked if there could be a common signaling pathway through which CITED2 expression was induced in human chondrocytes. Indeed, increased phosphorylated JAK3, consistent with the role of CITED2 as a downstream mediator of IL-4,17 and STAT6 were observed in both mechanically loaded and IL-4–treated chondrocytes.27 Phosphorylated JAK3 and STAT6 further increased in cells treated with the combination of IL-4 and mechanical loading. The activation of JAK3/STAT6 signaling was also reflected in the increased CITED2 in chondrocytes exposed to the combination of IL-4 and mechanical loading. Therefore, cross-talk between IL-4 and mechanical loading pathways is mediated, at least in part, through the JAK/STAT pathway. As a result, chondrocytes treated with the combination of IL-4 and mechanical loading expressed significantly higher CITED2 and lower MMP13, potentially exerting greater chondroprotection.
OA is a disease of the joint.40 We and others have shown that moderate loading exerts chondroprotection not only in cartilage,19,22,41 but also in the synovium.42–44 While this study focused on IL-4 and mechanical loading in chondrocytes, other joint tissues such as the synovium contribute to OA disease. Future studies elucidating the contributions of CITED2-mediated loading and IL-4 to alleviating disease in other joint tissues such as the synovium and subchondral bone will be of interest.
Of note, moderate exercise is beneficial in disease or aging conditions, such as that represented by the decline in IL-4 with age,45,46 and low-intensity exercise can produce analgesia mediated by IL-4.47 As age is a risk factor for OA and pain is the major symptom of OA, future studies elucidating the contribution of CITED2-mediated loading and IL-4 to pain relief will be of interest.
In conclusion, our studies suggest that two stimuli, IL-4 and mechanical loading, independently and collaboratively, activate the common mediator CITED2, and induce anticatabolic cellular responses leading to the downregulation of proteolytic enzymes such as MMP13. Our study provides evidence that the effects of IL-4 at low doses can be amplified significantly by mechanical loading, resulting in anticatabolic effects such as suppression of MMP13, and may provide a new intervention strategy to mitigate cartilage degradation for OA therapy.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Sun HB 2010. Mechanical loading, cartilage degradation, and arthritis. Ann. N.Y. Acad. Sci 1211: 37–50. [DOI] [PubMed] [Google Scholar]
- 2.Kiviranta I, Tammi M, Jurvelin J, et al. 1988. Moderate running exercise augments glycosaminoglycans and thickness of articular cartilage in the knee joint of young beagle dogs. J. Orthop. Res 6: 188–195. [DOI] [PubMed] [Google Scholar]
- 3.Saamanen AM, Tammi M, Kiviranta I, et al. 1988. Running exercise as a modulatory of proteoglycan matrix in the articular cartilage of young rabbits. Int. J. Sports Med. 9: 127–133. [DOI] [PubMed] [Google Scholar]
- 4.Jurvelin J, Kiviranta I, Tammi M, et al. 1986. Effect of physical exercise on indentation stiffness of articular cartilage in the canine knee. Int. J. Sports Med. 7: 106–110. [DOI] [PubMed] [Google Scholar]
- 5.Azadi M, Nia HT, Gauci SJ, et al. 2016. Wide bandwidth nanomechanical assessment of murine cartilage reveals protection of aggrecan knock-in mice from joint-overuse. J. Biomech 49: 1634–1640. [DOI] [PubMed] [Google Scholar]
- 6.Lotz MK & Kraus VB. 2010. New developments in osteoarthritis. posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res. Ther 12: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Punzi L, Galozzi P, Luisetto R, et al. 2016. Post-traumatic arthritis: overview on pathogenic mechanisms and role of inflammation. RMD Open 2: e000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attur M, Al-Mussawir HE, Patel J, et al. 2008. Prostaglandin E2 exerts catabolic effects in osteoarthritis cartilage: evidence for signaling via the EP4 receptor. J. Immunol 181: 5082–5088. [DOI] [PubMed] [Google Scholar]
- 9.Lianxu C, Hongti J & Changlong Y. 2006. NF-kappaBp65-specific siRNA inhibits expression of genes of COX-2, NOS-2 and MMP-9 in rat IL-1beta-induced and TNF-alpha-induced chondrocytes. Osteoarthritis Cartilage 14: 367–376. [DOI] [PubMed] [Google Scholar]
- 10.Pelletier JP, Martel-Pelletier J & Abramson SB. 2001. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 44: 1237–1247. [DOI] [PubMed] [Google Scholar]
- 11.Scher JU, Pillinger MH & Abramson SB. 2007. Nitric oxide synthases and osteoarthritis. Curr. Rheumatol. Rep 9: 9–15. [DOI] [PubMed] [Google Scholar]
- 12.van de Loo FA, Joosten LA, van Lent PL, et al. 1995. Role of interleukin-1, tumor necrosis factor alpha, and interleukin-6 in cartilage proteoglycan metabolism and destruction. Effect of in situ blocking in murine antigen- and zymosan-induced arthritis. Arthritis Rheum. 38: 164–172. [DOI] [PubMed] [Google Scholar]
- 13.Liacini A, Sylvester J, Li WQ, et al. 2003. Induction of matrix metalloproteinase-13 gene expression by TNF-alpha is mediated by MAP kinases, AP-1, and NF-kappaB transcription factors in articular chondrocytes. Exp. Cell Res. 288: 208–217. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto K, Otero M, Imagawa K, et al. 2013. Regulated transcription of human matrix metalloproteinase 13 (MMP13) and interleukin-1β (IL1B) genes in chondrocytes depends on methylation of specific proximal promoter CpG sites. J. Biol. Chem 288: 10061–10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Meegeren ME, Roosendaal G, Jansen NW, et al. 2012. IL-4 alone and in combination with IL-10 protects against blood-induced cartilage damage. Osteoarthritis Cartilage 20: 764–772. [DOI] [PubMed] [Google Scholar]
- 16.Assirelli E, Pulsatelli L, Dolzani P, et al. 2014. Human osteoarthritic cartilage shows reduced in vivo expression of IL-4, a chondroprotective cytokine that differentially modulates IL-1β-stimulated production of chemokines and matrix-degrading enzymes in vitro. PLoS One 9: e96925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun HB, Zhu YX, Yin T, et al. 1998. MRG1, the product of a melanocyte-specific gene related gene, is a cytokine-inducible transcription factor with transformation activity. Proc. Natl. Acad. Sci. USA 95: 13555–13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim GD, Das R, Rao X, et al. 2017. CITED2 restrains pro-inflammatory macrophage activation and response. Mol. Cell. Biol 10.1128/MCB.00452-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leong DJ, Li YH, Gu XI, et al. 2011. Physiological loading of joints prevents cartilage degradation through CITED2. FASEB J. 25: 182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun HB, Zhao L, Tanaka S, et al. 2012. Moderate joint loading reduces degenerative actions of matrix metalloproteinases in the articular cartilage of mouse ulnae. Connect. Tissue Res. 53: 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokota H, Goldring MB & Sun HB. 2003. CITED2-mediated regulation of MMP-1 and MMP-13 in human chondrocytes under flow shear. J. Biol. Chem 278: 47275–47280. [DOI] [PubMed] [Google Scholar]
- 22.He Z, Leong DJ, Zhuo Z, et al. 2016. Strain-induced mechanotransduction through primary cilia, extracellular ATP, purinergic calcium signaling, and ERK1/2 transactivates CITED2 and downregulates MMP-1 and MMP-13 gene expression in chondrocytes. Osteoarthritis Cartilage 24: 892–901. [DOI] [PubMed] [Google Scholar]
- 23.Seref-Ferlengez Z, Maung S, Schaffler MB, et al. 2016. P2X7R-Panx1 complex impairs bone mechanosignaling under high glucose levels associated with type-1 diabetes. PLoS One 11: e0155107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conner JD, Wolden-Hanson T & Quinn LS. 2014. Assessment of murine exercise endurance without the use of a shock grid: an alternative to forced exercise. J. Vis. Exp e51846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glasson SS, Blanchet TJ & Morris EA. 2007. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage 15: 1061–1069. [DOI] [PubMed] [Google Scholar]
- 26.Glasson SS, Chambers MG, Van Den Berg WB, et al. 2010. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage 18(Suppl. 3): S17–S23. [DOI] [PubMed] [Google Scholar]
- 27.Millward-Sadler SJ, Khan NS, Bracher MG, et al. 2006. Roles for the interleukin-4 receptor and associated JAK/STAT proteins in human articular chondrocyte mechanotransduction. Osteoarthritis Cartilage 14: 991–1001. [DOI] [PubMed] [Google Scholar]
- 28.Kurgonaite K, Gandhi H, Kurth T, et al. 2015. Essential role of endocytosis for interleukin-4-receptor-mediated JAK/STAT signalling. J. Cell Sci. 128: 3781–3795. [DOI] [PubMed] [Google Scholar]
- 29.Leong DJ, Majeska RJ, Xu L, et al. 2011. CITED2 prevents cartilage degradation in collagen-induced arthriticrats through suppression of matrix metalloproteinases. ORS Trans. Paper No 259. [Google Scholar]
- 30.Millward-Sadler SJ, Wright MO, Lee H, et al. 1999. Integrin-regulated secretion of interleukin 4: a novel pathway of mechanotransduction in human articular chondrocytes. J. Cell Biol. 145: 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee HS, Millward-Sadler SJ, Wright MO, et al. 2000. Integrin and mechanosensitive ion channel-dependent tyrosine phosphorylation of focal adhesion proteins and beta-catenin in human articular chondrocytes after mechanical stimulation. J. Bone Miner. Res 15: 1501–1509. [DOI] [PubMed] [Google Scholar]
- 32.Millward-Sadler SJ, Wright MO, Davies LW, et al. 2000. Mechanotransduction via integrins and interleukin-4 results in altered aggrecan and matrix metalloproteinase 3 gene expression in normal, but not osteoarthritic, human articular chondrocytes. Arthritis Rheum. 43: 2091–2099. [DOI] [PubMed] [Google Scholar]
- 33.Salter DM, Millward-Sadler SJ, Nuki G, et al. 2002. Differential responses of chondrocytes from normal and osteoarthritic human articular cartilage to mechanical stimulation. Biorheology 39: 97–108. [PubMed] [Google Scholar]
- 34.Chowdhury TT, Bader DL & Lee DA. 2006. Anti-inflammatory effects of IL-4 and dynamic compression in IL-1beta stimulated chondrocytes. Biochem. Biophys. Res. Commun 339: 241–247. [DOI] [PubMed] [Google Scholar]
- 35.Gilleece MH, Scarffe JH, Ghosh A, et al. 1992. Recombinant human interleukin 4 (IL-4) given as daily subcutaneous injections—a phase I dose toxicity trial. Br. J. Cancer 66: 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prendiville J, Thatcher N, Lind M, et al. 1993. Recombinant human interleukin-4 (rhu IL-4) administered by the intravenous and subcutaneous routes in patients with advanced cancer—a phase I toxicity study and pharmacokinetic analysis. Eur. J. Cancer 29A: 1700–1707. [DOI] [PubMed] [Google Scholar]
- 37.Goldring MB & Goldring SR. 2007. Osteoarthritis. J. Cell. Physiol 213: 626–634. [DOI] [PubMed] [Google Scholar]
- 38.Goldring MB & Goldring SR. 2010. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. N.Y. Acad. Sci 1192: 230–237. [DOI] [PubMed] [Google Scholar]
- 39.Goldring MB & Marcu KB. 2009. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. Ther 11: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loeser RF, Goldring SR, Scanzello CR, et al. 2012. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 64: 1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao L, Lin C, Shim J, et al. 2013. Knee loading reduces MMP13 activity in the articular cartilage of osteoarthritic mice. ORS Trans. Paper No 0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun HB & Yokota H. 2001. Messenger-RNA expression of matrix metalloproteinases, tissue inhibitors of metalloproteinases, and transcription factors in rheumatic synovial cells under mechanical stimuli. Bone 28: 303–309. [DOI] [PubMed] [Google Scholar]
- 43.Sun HB & Yokota H. 2001. Altered mRNA level of matrix metalloproteinase-13 in MH7A synovial cells under mechanical loading and unloading. Bone 28: 399–403. [DOI] [PubMed] [Google Scholar]
- 44.Sun HB & Yokota H. 2002. Reduction of cytokine-induced expression and activity of MMP-1 and MMP-13 by mechanical strain in MH7A rheumatoid synovial cells. Matrix Biol. 21: 263–270. [DOI] [PubMed] [Google Scholar]
- 45.Maher FO, Nolan Y & Lynch MA. 2005. Downregulation of IL-4-induced signalling in hippocampus contributes to deficits in LTP in the aged rat. Neurobiol. Aging 26: 717–728. [DOI] [PubMed] [Google Scholar]
- 46.Littlefield A & Kohman RA. 2017. Differential response to intrahippocampal interleukin-4/interleukin-13 in aged and exercise mice. Neuroscience 343: 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bobinski F, Teixeira JM, Sluka KA, et al. 2018. Interleukin-4 mediates the analgesia produced by low-intensity exercise in mice with neuropathic pain. Pain 159: 437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]