Abstract
Background
Angiotensin converting enzyme inhibitors (ACEi) and angiotensin II receptor antagonists (AIIRA) are considered to be equally effective for patients with diabetic kidney disease (DKD), but renal and not mortality outcomes have usually been considered.
Objectives
To evaluate the benefits and harms ACEi and AIIRA in patients with DKD.
Search methods
We searched MEDLINE (1966 to December 2005), EMBASE (1980 to December 2005), the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library issue 4 2005) and contacted known investigators.
Selection criteria
Studies comparing ACEi or AIIRA with placebo or each other in patients with DKD were included.
Data collection and analysis
Two authors independently assessed trial quality and extracted data. Statistical analyses were performed using the random effects model and results expressed as risk ratio (RR) with 95% confidence intervals (CI). Heterogeneity among studies was explored using the Cochran Q statistic and the I² test, subgroup analyses and random effects meta‐regression.
Main results
Forty nine studies (12,067 patients) were identified. Thirty eight compared ACEi with placebo, four compared AIIRA with placebo and seven compared ACEi and AIIRA directly. There was no significant difference in the risk of all‐cause mortality for ACEi versus placebo (RR 0.91, 95% CI 0.71 to 1.17) and AIIRA versus placebo (RR 0.99, 95% CI 0.85 to 1.17). A subgroup analysis of studies using full‐dose ACEi versus studies using half or less than half the maximum tolerable dose of ACEi showed a significant reduction in the risk of all‐cause mortality with the use of full‐dose ACEi (RR 0.78, 95% CI 0.61 to 0.98). Baseline mortality rates were similar in the ACEi and AIIRA studies. The effects of ACEi and AIIRA on renal outcomes (ESKD, doubling of creatinine, prevention of progression of micro‐ to macroalbuminuria, remission of micro‐ to normoalbuminuria) were similarly beneficial. Reliable estimates of effect of ACEi versus AIIRA could not be obtained from the three studies in which they were compared directly because of their small sample size.
Authors' conclusions
Although the survival benefits of ACEi are known for patients with DKD, the relative effects on survival of ACEi with AIIRA are unknown due to the lack of adequate direct comparison studies. In placebo controlled studies, only ACEi (at the maximum tolerable dose, but not lower so‐called renal doses) were found to significantly reduce the risk of all‐cause mortality. Renal and toxicity profiles of these two classes of agents were not significantly different.
Plain language summary
Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease
Kidney disease develops in 25% to 40% of diabetic patients, usually 20 to 25 years after the onset of diabetes. Approximately one third of those with diabetic kidney disease (DKD) will progress to end‐stage kidney disease (ESKD) and will require long‐term dialysis or possibly receive a kidney transplant. Many patients however may die from associated coronary artery disease or other cardiovascular causes before the onset of ESKD. Antihypertensive drugs have been shown to not only be of benefit to the heart but to also provide kidney protection by slowing the progression of DKD to ESKD. Two drugs in particular have been considered equally effective for patients with DKD ‐ these are angiotensin converting enzyme inhibitors (ACEi) and angiotensin II receptor antagonists (AIIRA). However studies have focused on kidney protection rather than over mortality. The aim of this review was to assess the benefits and harms or ACEI and AIIRA therapy in patients with DKD. Fifty studies (13,215 patients) were identified comparing ACEi to placebo, AIIRA to placebo and ACEi to AIIRA. The risk of death from any cause was not significantly reduced with the use of ACEi versus placebo, AIIRA versus placebo or ACEi versus AIIRA. However when we looked at the studies which used the maximum dose tolerated of ACEi rather than the lower, so‐called renal doses, there was a significant reduction in the risk of death due to any cause. We were unable to determine which drug provides better protection due to the lack of head‐to‐head trials.
Background
Diabetic kidney disease (DKD), defined as the presence of micro‐ or macroalbuminuria in patients with diabetes, occurs in 25% to 40% of type 1 and 2 diabetic patients within 20 to 25 years of the onset of diabetes (Ritz 1999). Both types of patients probably share the same pathogenetic and clinical stages of renal damage, including renal hypertrophy, incipient (microalbuminuria: urine albumin excretion 30‐300 mg/d) nephropathy, overt (macroalbuminuria: > 300 mg/d) nephropathy and, finally, the presence of impairment of glomerular filtration rate (GFR) up to end‐stage kidney disease (ESKD) (Mogensen 1995; Mogensen 1999) About one third of patients with DKD progress to ESKD (Ritz 1999).
Agents used to delay the progression of DKD include beta‐blockers, calcium channel blockers, diuretics, angiotensin converting enzyme inhibitors (ACEi), and angiotensin II receptor antagonists (AIIRA). Since large scale randomised controlled trials (RCTs) have shown that ACEi and AIIRA slow the deterioration of renal function and reduce proteinuria, these have become the most broadly used agents in diabetic patients with nephropathy and major international guidelines (Arauz‐Pacheo 2003; JNC 7 2003) advocate for their equivalent use as first line agents in these populations (CAPTOPRIL 1993; HOPE 2000; IDNT 2001; Kasiske 1993; RENAAL 2001). If a patient has diabetes and nephropathy, mortality is reported to be 10% to 40% within 10 years of diagnosis, depending on cardiovascular comorbidities, with the primary cause of early death being fatal cardiovascular events. The presence of micro‐ or macroalbuminuria has been shown to be an independent risk factor for early death due to cardiovascular events in diabetic patients over and above the increased risk conferred by the diabetic status (Dinneen 1997). Microalbuminuria is associated with a two‐ to fourfold increase in the risk of death whereas macroalbuminuria/overt proteinuria and hypertension are associated with an even higher risk when present together.
In view of current guidelines recommendations on equivalent use of ACEi and AIIRA in these populations, we performed a systematic review of RCTs of ACEi and AIIRA used in patients with DKD to evaluate the evidence basis supporting these statements, with particular focus on their effects on renal and cardiovascular outcomes.
Objectives
To evaluate the benefits and harms of ACEi and AIIRA in patients with DKD, with major focus on renal and cardiovascular outcomes.
Methods
Criteria for considering studies for this review
Types of studies
RCTs of at least six months duration in which ACEi or AIIRA were compared with placebo or no treatment or in which the relative effects of the agents were compared directly, head‐to‐head, in patients with DKD, were included in this systematic review.
Types of participants
RCTs of patients with DKD were included, independent of stage of nephropathy, either microalbuminuria (albumin excretion 30‐300 mg/d) or macroalbuminuria (albumin excretion >300 mg/d).
Types of interventions
ACEi versus placebo or no treatment
AIIRA versus placebo or no treatment
Head‐to‐head comparative RCTs of ACEi versus AIIRA
Types of outcome measures
All‐cause mortality
ESKD
Doubling of serum creatinine concentration
Progression from micro‐ to macroalbuminuria
Regression from micro‐ to normoalbuminuria
Drug related toxicity, including cough, headache, hyperkalaemia, impotence and pedal oedema
Search methods for identification of studies
Search strategies were independently designed and performed by two separate investigators (GFMS, MC) in collaboration with the Cochrane Renal Group's Trial Search Coordinators at various stages from 2003 with a final update in December 2005.
The following electronic biomedical databases were searched
Cochrane Renal Groups studies register and the Cochrane Central Register of Controlled Trials (CENTRAL, in The Cochrane Library issue 4 2005)
MEDLINE(1966 ‐ December 2005)
EMBASE (1988 ‐ December 2005)
We performed a combined search to identify studies for this review and the review Antihypertensive agents for preventing diabetic kidney disease (Strippoli 2005) and screened as described below.
CENTRAL and the Renal Group's specialised register contain the handsearched results of conference proceedings from general and speciality meetings. This is an ongoing activity across the Cochrane Collaboration and is both retrospective and prospective (http://www.cochrane.us/masterlist.asp). Therefore we did not specifically search conference proceedings. Please refer to The Cochrane Renal Review Group's Module in The Cochrane Library for the most up‐to‐date list of conference proceedings.
Data collection and analysis
This review was undertaken by six authors (GFMS, MC, JD, CB, SDN, JCC). The search strategies described were used to obtain titles and abstracts of studies that might be relevant to the review. The titles and abstracts were screened independently by (GS, MC, SDN and CB), who discarded studies that were not applicable based on the inclusion criteria for this review, however studies and reviews that might include relevant data or information on studies were retained initially and their full‐text version was analysed. Authors GS, MC, SDN and CB independently assessed abstracts of all citations retrieved by the searches and, if necessary, the full text of these studies to determine study eligibility. Data extraction was carried out independently by the same reviewers using standard data extraction forms. Where more than one publication of one trial existed, only the publication with the most complete data was used. Any further information or clarification required from the authors was requested by written or electronic correspondence and relevant information obtained in this manner was included in the review. Disagreements in data extraction were resolved by discussion among authors.
Study quality
The methodological quality of included studies was assessed independently by GFMS, MC, SDN and CB without blinding to authorship or journal using the checklist developed by the Cochrane Renal Group. Discrepancies were resolved by discussion among authors. The quality items assessed were allocation concealment, blinding of investigators, participants outcome assessors, data assessors, intention‐to‐treat analysis, and the completeness to follow‐up.
Quality checklist
Allocation concealment
Adequate (A): Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study
Unclear (B): Randomisation stated but no information on method used is available
Inadequate (C): Method of randomisation used such as alternate medical record numbers or unsealed envelopes; any information in the study that indicated that investigators or participants could influence intervention group
Blinding
Blinding of investigators: Yes/no/not stated
Blinding of participants: Yes/no/not stated
Blinding of outcome assessor: Yes/no/not stated
Blinding of data analysis: Yes/no/not stated
Intention‐to‐treat analysis
Yes: Specifically reported by authors that intention‐to‐treat analysis was undertaken and this was confirmed on study assessment.
Yes: Not specifically stated but confirmed upon study assessment
No: Not reported and lack of intention‐to‐treat analysis confirmed on study assessment (patients who were randomised were not included in the analysis because they did not receive the study intervention, they withdrew from the study or were not included because of protocol violation).
No: Stated, but not confirmed upon study assessment
Not stated
Completeness to follow‐up
Per cent of participants excluded or lost to follow‐up.
Statistical assessment
The effect of ACEi or AIIRA in individual studies were summarised using risk ratio (RR) with 95% confidence intervals (CI). Data of all studies comparing the same interventions (ACEi versus placebo, AIIRA versus placebo, ACEi versus AIIRA) were pooled in meta‐analyses using the random effects model. Heterogeneity of treatment effects between studies was examined using the Cochran Q and I² statistic (Higgins 2003). Subgroup analysis and random effects meta‐regression were performed as applicable to explore the influence of the following sources of heterogeneity on treatment effect: duration of follow up, type of diabetes (type 1, type 2 or studies including mixed populations of type 1 and type 2 diabetic patients), type of drug (different agents of the same class), presence or absence of hypertension at baseline, stage of DKD (studies enrolling patients with microalbuminuria, macroalbuminuria or mixed populations of patients with micro‐ and macroalbuminuria), and specific quality items (allocation concealment, blinding, use of intention ‐to‐treat analysis). meta‐regression analyses were undertaken in STATA version 8.0.
Results
Description of studies
The combined search identified a total of 5701 citations, of which 5376 were excluded after title and abstract review. The major reasons for exclusion were a non‐randomised design, non‐antihypertensive interventions, study populations with non‐DKD, and duplicate publications. Full text analysis of 325 articles lead to the final inclusion of 49 studies (73 publications) which met the inclusion criteria (Figure 1‐ Flow chart of study identification) (ABCD 1996; Ahmad 1997; Ahmad 2003; AIPRI 1996; ATLANTIS SG 2000; Bakris 1994; Bauer 1992; Bojestig 2001; Capek 1994; CAPTOPRIL 1993; Carella 1999; Chase 1993; Cordonnier 1999; Crepaldi 1998; DETAIL 2004; DIABHYCAR 2004; ESPRIT 2001; EUCLID 1997; Garg 1998; Hansen 1994; HOPE 2000; IDNT 2001; IRMA‐2 2001; JAPAN‐IDDM 2002; Jerums 2001; Jerums 2004; Ko 2005; Lacourciere 2000; Laffel 1995; Lebovitz 1994; Marre 1987; Mathiesen 1999; Muirhead 1999; Nankervis 1998; O'Donnell 1993; Parving 1989; Parving 2001a; Phillips 1993; Poulsen 2001; Ravid 1993; RENAAL 2001; Rizzoni 2005; Romero 1993; Sano 1994; Sato 2003; Stornello 1992; Tan 2002; Trevisan 1995; Tutuncu 2001). Data on characteristics of the populations, interventions and outcomes were extracted from all studies and supplemental data on design features and outcomes were obtained from the authors of ten studies or from duplicate publications relating to the primary trial.
1.
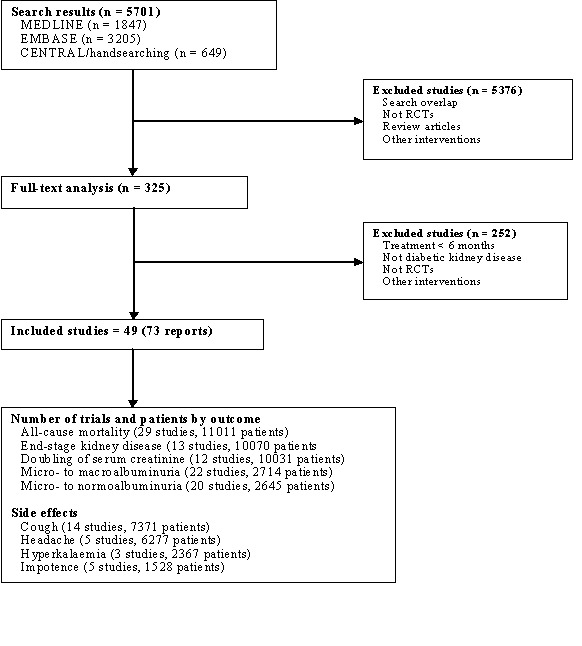
Flow chart showing number of citations retrieved by individual searches, number of trials included in the systematic review and number of trials reporting each outcome.
Of the 49 studies, 38 (8970 patients) compared ACEi with placebo (ABCD 1996; Ahmad 1997; Ahmad 2003; AIPRI 1996; ATLANTIS SG 2000; Bakris 1994; Bauer 1992; Bojestig 2001; Capek 1994; CAPTOPRIL 1993; Carella 1999; Chase 1993; Cordonnier 1999; Crepaldi 1998; DIABHYCAR 2004; ESPRIT 2001; EUCLID 1997; Garg 1998; Hansen 1994; HOPE 2000; JAPAN‐IDDM 2002; Jerums 2001; Jerums 2004; Laffel 1995; Lebovitz 1994; Marre 1987; Mathiesen 1999; Nankervis 1998; O'Donnell 1993; Parving 1989; Parving 2001a; Phillips 1993; Poulsen 2001; Ravid 1993; Romero 1993; Sano 1994; Stornello 1992; Trevisan 1995), four (2540 patients) compared AIIRA with placebo (Lacourciere 2000; Muirhead 1999; RENAAL 2001; Tutuncu 2001), and seven (557 patients) compared ACEi with AIIRA (DETAIL 2004; IDNT 2001; IRMA‐2 2001; Ko 2005; Rizzoni 2005; Sato 2003; Tan 2002).
Of studies comparing ACEi with placebo, 20 enrolled patients with type 1 diabetes, 13 enrolled patients with type 2 diabetes, and five enrolled mixed populations of type 1 and type 2 diabetic patients. Seventeen studies enrolled patients with hypertension at baseline, the remaining enrolled normotensive patients. In 20 studies, other antihypertensive agents were administered beyond the randomised interventions (ACEi or AIIRA) in a non‐randomised fashion to equalise blood pressure in both groups, minimise the confounding effect of blood pressure. Twenty five studies enrolled patients with microalbuminuria, eight enrolled patients with macroalbuminuria, and five enrolled mixed populations of micro‐ and macroalbuminuric patients. Three studies also enrolled minimal proportions of patients with normoalbuminuria and were therefore included.
The four studies that compared AIIRA with placebo all enrolled hypertensive patients with type 2 diabetes. Antihypertensive co‐interventions were given in all four studies. Two studies enrolled patients with microalbuminuria and the other two studies enrolled patients with macroalbuminuria. Of the seven studies comparing ACEi with AIIRA directly, six enrolled microalbuminuric patients and one enrolled mixed populations of micro‐ and macroalbuminuric patients. Six studies enrolled patients with type 2 diabetes and one study enrolled patients with both type 1 and type 2 diabetes. Six studies enrolled hypertensive patients and one trial enrolled normotensive participants. Antihypertensive co‐interventions were given in two studies.
Risk of bias in included studies
By current standards, trial methodological quality was suboptimal.
Allocation concealment
Allocation concealment was unclear in 40/49 (82%) studies, inadequate in 1/49 (2%) trial, and adequate in 8/49 (16%) studies.
Blinding
Blinding occurred in the participants in 36/49 (73%) studies, investigators in 32/49 (65%) studies, and outcome assessors in 4/49 (8%) studies.
Reported intention‐to‐treat analysis
An intention‐to‐treat analysis was used in 15/49 (31%) studies.
Completeness of follow‐up
Between 0% and 20% of patients were lost to follow‐up in 45/49 (92%) studies and between 21% and 41% were lost to follow‐up in 4/49 (8%) studies.
Effects of interventions
All‐cause mortality
ACEi versus placebo/no treatment
There was no significant reduction in the risk of all‐cause mortality with ACEi compared with placebo/no treatment (Analysis 1.1 (21 studies, 7295 patients): RR 0.91, 95% CI 0.71 to 1.17). This analysis was dominated by two studies, which contributed 49.68% and 37.78% of the weight to the summary estimate (DIABHYCAR 2004; HOPE 2000) but there was no significant heterogeneity between the studies (heterogeneity χ² = 11.04, I² = 27.6 %). A subgroup analysis of studies which used ACEi at the maximum tolerable dose compared with placebo/no treatment, there was a significant reduction in the risk of all‐cause mortality (Analysis 1.02 (5 studies, 2034 patients): RR 0.78, 95% CI 0.61 to 0.98) while this was not found in studies using half or less than half the maximum tolerable dose of these agents (Analysis 1.1.1 (4 studies, 5261 patients): RR 1.18, 95% CI 0.41 to 3.44). There was no significant heterogeneity in any of these analyses.
1.1.
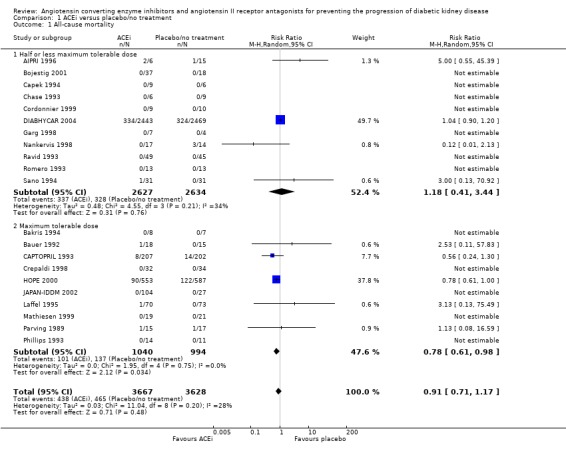
Comparison 1 ACEi versus placebo/no treatment, Outcome 1 All‐cause mortality.
AIIRA versus placebo/no treatment
No statistically significant reduction in the risk of all‐cause mortality was found in the five studies (3409 patients) of AIIRA versus placebo/no treatment (Analysis 2.1: RR 0.99, 95% CI 0.85 to 1.17). This analysis was dominated by two studies, which contributed 64.6% and 34.9% of the weight to the summary estimate (IDNT 2001; RENAAL 2001). There was no significant heterogeneity between the studies (χ² = 0.63 , I² = 0%).
2.1.
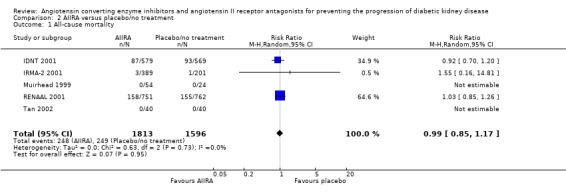
Comparison 2 AIIRA versus placebo/no treatment, Outcome 1 All‐cause mortality.
ACEi versus AIIRA
No statistically significant reduction in the risk of all‐cause mortality was found in the only three studies (307 patients) that compared ACEi with AIIRA (Analysis 3.1: RR 0.92, 95% CI 0.31 to 2.78).
3.1.
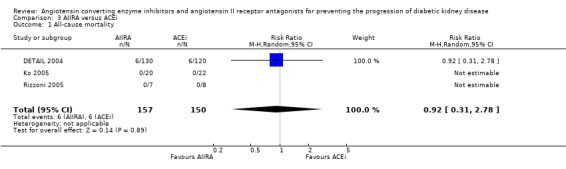
Comparison 3 AIIRA versus ACEi, Outcome 1 All‐cause mortality.
ESKD and doubling of serum creatinine
ACEi versus placebo/no treatment
There was a significant reduction in the risk of ESRD with ACEi compared to placebo/no treatment (Analysis 1.4 (10 studies, 6819 patients): RR 0.60, 95% CI 0.39 to 0.93) with no significant heterogeneity between the studies (χ² = 1.71, I² = 0%).
1.4.
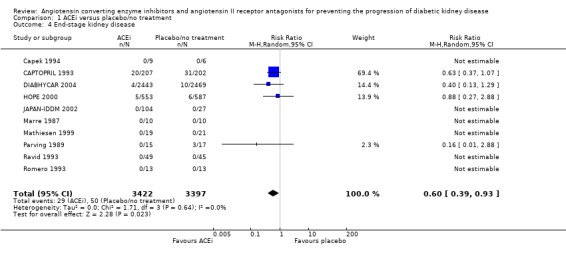
Comparison 1 ACEi versus placebo/no treatment, Outcome 4 End‐stage kidney disease.
There was some evidence of reduction of the risk of doubling of serum creatinine concentration with ACEi compared to placebo/no treatment (Analysis 1.3 (9 studies, 6780 patients): RR 0.68, 95% CI 0.47 to 1.00). There was no significant heterogeneity between the studies (χ² = 9.52, I² = 37.0%).
1.3.
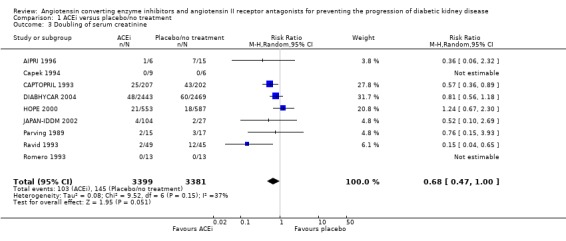
Comparison 1 ACEi versus placebo/no treatment, Outcome 3 Doubling of serum creatinine.
AIIRA versus placebo/no treatment
There was a significant reduction in the risk of ESKD with AIIRA compared to placebo/no treatment (Analysis 2.4 (3 studies, 3251 patients): RR 0.78, 95% CI 0.67 to 0.91) with no significant heterogeneity between the studies (χ² = 0.05, I² = 0%).
2.4.
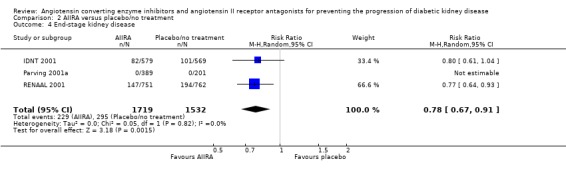
Comparison 2 AIIRA versus placebo/no treatment, Outcome 4 End‐stage kidney disease.
There was also a significant reduction in the risk of doubling of serum creatinine concentration with AIIRA compared to placebo/no treatment (Analysis 2.3 (3 studies, 3251 patients), RR 0.79, 95% CI 0.67 to 0.93), with no significant heterogeneity between the studies (χ² = 1.22, I² = 18.2%).
2.3.
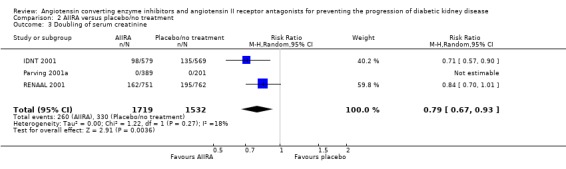
Comparison 2 AIIRA versus placebo/no treatment, Outcome 3 Doubling of serum creatinine.
ACEi versus AIIRA
The seven studies that compared ACEi with AIIRA did not report the outcome of ESKD or doubling of serum creatinine, and we were unable to obtain these data from the authors.
Progression from micro‐ to macroalbuminuria
ACEi versus placebo/no treatment
ACEi significantly reduced the risk of progression from micro‐ to macroalbuminuria (Analysis 1.5 (17 studies, 2036 patients): RR 0.45, 95% CI 0.29 to 0.69).There was no significant heterogeneity between the studies (χ² = 26.48, I² = 47.1%).
1.5.

Comparison 1 ACEi versus placebo/no treatment, Outcome 5 Micro‐ to macroalbuminuria.
AIIRA versus placebo/no treatment
The use of AIIRA versus placebo/no treatment was also associated with a significant reduction in the risk of progression from micro‐ to macroalbuminuria (Analysis 2.5 (3 studies, 761 patients): RR 0.49, 95% CI 0.32 to 0.75), with no significant heterogeneity between the studies (χ² = 1.10, I² = 0%).
2.5.
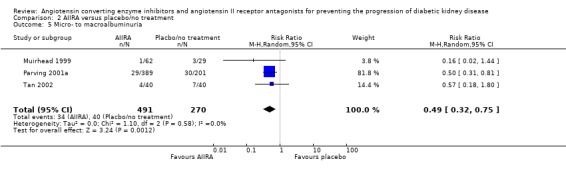
Comparison 2 AIIRA versus placebo/no treatment, Outcome 5 Micro‐ to macroalbuminuria.
ACEi versus AIIRA
Progression from micro to macroalbuminuria was reported in one trial (41 patients) only, which showed no significant difference in risk (Ko 2005).
Regression for micro‐ to normoalbuminuria
ACEi versus placebo/no treatment
There was a significant increase in regression from micro‐ to normoalbuminuria with ACEi versus placebo/no treatment (Analysis 1.6 (16 studies, 1910 patients): RR 3.06, 95% CI 1.76 to 5.35), with no significant heterogeneity between the studies (χ² = 23.91, I² = 45.6%).
1.6.
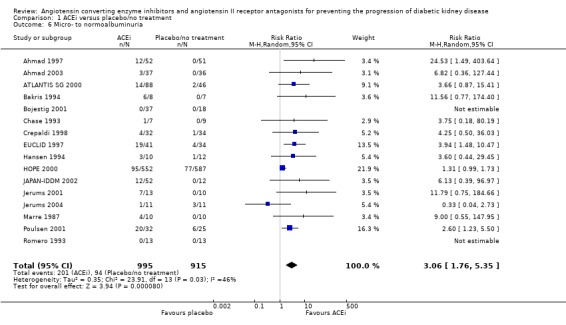
Comparison 1 ACEi versus placebo/no treatment, Outcome 6 Micro‐ to normoalbuminuria.
AIIRA versus placebo/no treatment
There was a significant increase in regression from micro‐ to normoalbuminuria with AIIRA versus placebo/no treatment (Analysis 2.6 (2 studies, 670 patients): RR 1.42, 95% CI 1.05 to 1.93) with no significant heterogeneity between the studies (χ² = 0.51, I² = 0%).
2.6.
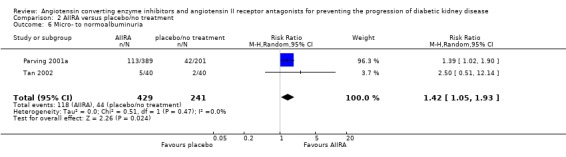
Comparison 2 AIIRA versus placebo/no treatment, Outcome 6 Micro‐ to normoalbuminuria.
ACEi versus AIIRA
Regression from micro‐ to normoalbuminuria was reported in only one head‐to‐head study and showed a non‐significant difference in the risk (Tutuncu 2001).
Toxicity
ACEi versus placebo/no treatment
Cough
The use of ACEi was associated with a significant increase in the risk of cough (Analysis 1.7 (10 studies, 7087 patients): RR 3.17, 95% CI 2.29 to 4.38), with no significant heterogeneity between the studies (χ² = 4.30, I² = 0%).
1.7.
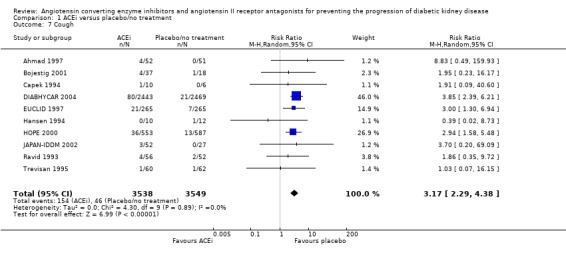
Comparison 1 ACEi versus placebo/no treatment, Outcome 7 Cough.
Hyperkalaemia
The use of ACEi was not found to be associated with a significant increase in the risk of hyperkalaemia (Analysis 1.10 (2 studies, 1219 patients): RR 0.85, 95% CI 0.32 to 2.21), with no significant heterogeneity between the studies (χ² = 0.05, I² = 0%).
1.10.

Comparison 1 ACEi versus placebo/no treatment, Outcome 10 Hyperkalaemia.
Headache
Four studies (6186 patients) reported headaches and there was no significant increase in the risk of headache with ACEi compared to placebo/no treatment (Analysis 1.8: RR 0.92, 95% CI 0.33 to 2.53). Also in this analysis, there was no significant heterogeneity between the studies (χ² = 3.20, I² = 6.2%).
1.8.
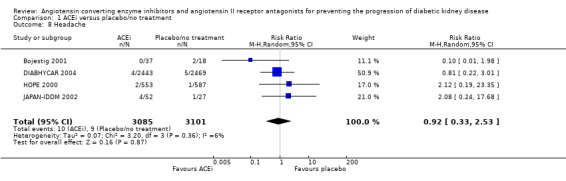
Comparison 1 ACEi versus placebo/no treatment, Outcome 8 Headache.
Impotence
Five studies (1528 patients) reported on impotence, and there was no evidence of a significant difference in the risk with ACEi compared to placebo/no treatment (Analysis 1.9: RR 1.02, 95% CI 0.26 to 3.91), and there was no significant heterogeneity between the studies (χ² = 0.61, I² = 0%).
1.9.
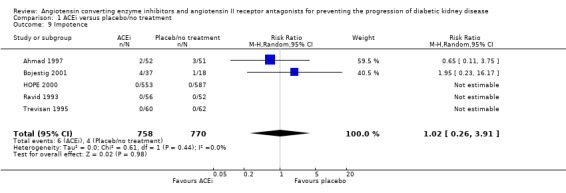
Comparison 1 ACEi versus placebo/no treatment, Outcome 9 Impotence.
AIIRA versus placebo/no treatment
Cough
AIIRA were not found to be associated with an increased risk of cough compared to placebo/no treatment (Analysis 2.07 (2 studies, 194 patients): RR 4.93, 95% CI 1.00 to 24.35). There was no significant heterogeneity between the studies (χ² = 1.43, I² = 30.0%).
Hyperkalaemia
There was a significant increase in the risk of hyperkalaemia with AIIRA compared to placebo/no treatment (Analysis 2.10 (1study, 1148 patients): RR 5.41, 95% CI 1.20 to 24.28).
2.10.
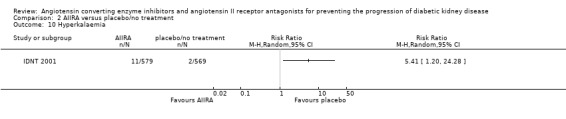
Comparison 2 AIIRA versus placebo/no treatment, Outcome 10 Hyperkalaemia.
Headache
One trial (91 patients) of AIIRA versus placebo/no treatment reported the outcome of headache and found no significant increase in risk with AIIRA (Analysis 2.8: RR 0.70, 95% CI 0.03 to 16.68).
2.8.
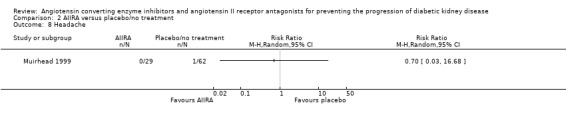
Comparison 2 AIIRA versus placebo/no treatment, Outcome 8 Headache.
Impotence
There were no studies of AIIRA versus placebo/no treatment reporting the outcome of impotence.
Investigation of sources of heterogeneity
Metaregression and subgroup analyses for sources of heterogeneity were only possible in studies of ACEi versus placebo/no treatment given the small number of studies evaluating AIIRA versus placebo/no treatment or ACEi versus AIIRA directly. There was no evidence that the effect of ACEi on all‐cause mortality (interaction P value = 0.84), doubling of serum creatinine (interaction P value = 0.56), ESKD (interaction P value = 0.05) and progression from micro‐ to macroalbuminuria (interaction P value = 0.12) varied according to type of diabetes. On the contrary, the rate of regression from micro‐ to normoalbuminuria was significantly higher in patients with type 2 diabetes (interaction P value < 0.001).
The presence (versus absence) of hypertension in the enrolled populations did not significantly impact on the effect of ACEi compared to placebo/no treatment on all‐cause mortality (interaction P value = 0.45), doubling of serum creatinine (interaction P value = 0.22), ESKD (interaction P value = 0.34), but normotensive patients had a significantly higher rate of progression from micro‐ to macroalbuminuria (interaction P value = 0.001) and significantly higher rate of regression from micro‐ to normoalbuminuria (interaction P value = 0.01).
The stage of nephropathy in enrolled populations (microalbuminuria versus macroalbuminuria or mixed populations with micro‐ or macroalbuminuria) did not significantly affect any of these outcomes in patients treated with ACEi compared to placebo/no treatment (all‐cause mortality, interaction P value = 0.92; doubling of serum creatinine, interaction P value = 0.88, ESKD, interaction P value = 0.55, progression from micro‐ to macroalbuminuria, interaction P value = 0.86, regression from micro‐ to normoalbuminuria, interaction P value = 0.80). There were also no significant variations in the effect of ACEi versus placebo/no treatment on any of these outcomes by any trial quality indicators (allocation concealment, blinding, use of intention‐to‐treat analysis and proportions of patients lost to follow‐up). The few significant differences observed in these analyses were all explained by the results of the large HOPE 2000 study. When we excluded this study from our analyses, results were homogeneous for all outcomes.
Discussion
Key findings
Placebo controlled studies have shown a survival advantage for ACEi when used at the maximum tolerable dose but not at "renal doses" (half or less than half the maximum tolerable dose) in patients with DKD. On the other hand, there has been no data showing a survival advantage with AIIRA versus placebo/no treatment, in patients with DKD. The relative survival advantage of one class of antihypertensives over the other in this population is, however, still unknown because only indirect comparisons based on small studies are available. ACEi, used at the maximum tolerable dose, significantly reduced the risk of all‐cause mortality (mainly cardiovascular) by about 20% and progression from micro‐ to macroalbuminuria by about 55%. They also increased the rate of regression from micro‐ to normoalbuminuria by about threefold. We found no evidence that these effects are related to baseline hypertension, type of diabetes, stage of DKD, and duration of treatment. In comparison, current studies of AIIRA in patients with DKD have not shown a reduction in all‐cause mortality, with a RR of 0.99 and narrow 95% CI (0.85 to 1.17), which is unlikely to be explained by chance alone. There is strong evidence that AIIRA are beneficial for renal outcomes, with a reduction in risk of ESKD and doubling of serum creatinine of about 22%, a reduction in progression rates from micro‐ to macroalbuminuria by around 51%, and an increase in the regression from micro‐ to normoalbuminuria of about 42%. Three potential explanations for these apparent different effects between the two classes of antihypertensives are chance, confounding, and true differences. The usual 5% level for statistical significance was reached for all renal outcomes for AIIRA versus ACEi,and this threshold was reached for ESKD, the prevention of progression from micro‐ to macroalbuminuria, and regression of micro‐ to normoalbuminuria, but not doubling of serum creatinine. The point estimates of effect for all renal outcomes favoured ACEi versus AIIRA, but there was some imprecision surrounding these summary point estimates for ACEi due to lower event rates and because of the heterogeneity in study results due to one large study (HOPE 2000). For all cause mortality, the absence of benefit shown by AIIRA is unlikely to be due to chance alone because the summary point estimate is close to unity (0.99) and the 95% CIs are relatively narrow. We did not formally test differences in ACEi and AIIRA through indirect comparison since there are clear differences in the design and conduct of the ACEi and AIIRA studies, which may explain apparent differences in results if such non randomised comparison was performed. In particular, micro‐HOPE primarily included high cardiac risk patients with relatively low renal risk, and although end of treatment blood pressure was not different between the two groups (possibly due to survival bias), equalisation of blood pressure was not targeted and so may have confounded the observed benefit of ramipril. True differences in the relative effects of ACEi and AIIRA can only be established by adequately powered studies that directly compare the two agents, which unfortunately are currently not available.
Comparison with existing knowledge
In studies that enrolled patients with diabetes without nephropathy (which have not been included in this review), it has been shown that intensive control of blood pressure with any agent reduced cardiovascular morbidity and mortality, independent of type of agent used (Grossman 2001). In addition, in one study in which losartan was compared to atenolol in hypertensive patients with diabetes, losartan significantly reduced the risk of all‐cause mortality (Wachtell 2003). Following myocardial infarction, ACEi have been shown to reduce all‐cause mortality (Zunetti 1996), whereas no relevant information with AIIRA are available. Our findings are consistent with other large meta‐analyses in patients with congestive heart failure, which showed a significant reduction in the risk of all cause mortality with ACEi versus placebo/no treatment but not for AIIRA (Cohn 2001; Garg 1995). Previous studies have already analysed the role of various antihypertensive agents, including ACEi and AIIRA, in patients affected by DKD. Particular focus was on the effect of ACEi in specific categories of patients (e.g. only type 1 diabetics). A recent meta‐analysis of individual patient data from the ACEi in DKD Triallist Group concluded that in normotensive patients with type 1 diabetes and microalbuminuria, ACEi significantly reduced progression to macroalbuminuria and increased the chances of regression to normoalbuminuria (ACEIDN 2001). An earlier metaregression analysis indicated that ACEi reduced proteinuria and preserved GFR in patients with diabetes, independent of changes in systemic blood pressure (Kasiske 1993). The main difference with our study is that we included both ACEi and AIIRA studies, obtained additional data from the authors when possible, and evaluated all outcomes of interest, including all‐cause mortality, and not simply the traditional renal outcomes. Another meta‐analysis on the effects of inhibitors of the renin‐angiotensin system and other antihypertensive agents on renal outcomes has been recently published (Casas 2005). This analysis, which included small studies conducted in high‐risk patients with diabetic and non‐diabetic chronic nephropathies, concluded that there are no superior effects of renin‐angiotensin‐system blockers over other antihypertensive agents in chronic nephropathies. Comparison between these data and ours is difficult because of different inclusion criteria and research questions.
Strengths and limitations
The strength of this investigation is that it represents a comprehensive systematic review with rigid inclusion criteria for RCTs only; and a comprehensive search strategy. Data extraction, data analysis, and methodological quality assessments were performed by two or more independent investigators, and consistency was checked with all six authors. The major limitation of our study is the lack of direct comparative data of ACEi and AIIRA. Studies directly comparing the two agents were only few and small and did not report outcomes relevant to patients, therefore they were largely uninformative. Other limitations include the small number and suboptimal quality of included studies and the potential for publication bias. These issues are unlikely to be influential as the review is dominated by a few larger studies.
Authors' conclusions
Implications for practice.
The role of ACEi in the management of patients with DKD is well established. Recently, equivalence of the newer and more expensive class of antihypertensive agents, AIIRA, has been widely advocated and is accepted in current practice. For example, the Joint National Committee on Prevention, Diagnosis and Management of Hypertension (JNC 7 2003) and the guidelines of the American Diabetes Association (ADA, Arauz‐Pacheo 2003), suggest that ACEi and AIIRA can be used interchangeably. Our study shows that there is randomised trial evidence that ACEi versus placebo/no treatment used at their maximum tolerable dose prevent death in patients with DKD, but not that AIIRA versus placebo/no treatment do. Both agents prevent progression of nephropathy and promote regression to a more favourable clinical pattern of normoalbuminuria. The relative effects of ACEi and AIIRA are uncertain. These data suggest that outside of a comparative RCT, ACEi, the cheaper class of agent with proven survival benefit, should be used as first line treatment.
Implications for research.
The findings of this study mandate an adequately powered comparative trial of ACEi versus AIIRA with renal and all‐cause mortality as measured outcomes. In general studies of the newer pharmacological agents (AIIRA) have been designed as placebo‐controlled rather than direct comparisons with existing agents (ACEi). This clearly makes it easier to prove that there is a benefit with the new agent, but harder to prove differential advantage compared to existing ones, as this may be only done by indirect comparison. It should be therefore recommended that future studies compare these agents directly. Given the recent promising results of combination therapy, a factorial trial may be the preferred design. Meanwhile, undertaking an individual patient data meta‐analysis may allow the effects of baseline cardiac and renal disease to be better understood and accounted for through subgroup analysis.
What's new
| Date | Event | Description |
|---|---|---|
| 18 March 2010 | Amended | Contact details updated. |
History
Protocol first published: Issue 4, 2006 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 13 May 2009 | Amended | Contact details updated. |
| 26 August 2008 | Amended | Converted to new review format. |
Acknowledgements
We are indebted to Narelle Willis for editorial support and Sandra Puckeridge for administrative support. Ruth Mitchell, Linda Heslop and Gail Higgins provided search strategies for this review. We are also indebted to Janice Pogue and the HOPE triallists, Drs M Ravid, PJ Phillips, HH Parving, R Romero, S Katayama, EM Mathiesen, BR Brenner, and KC Tan who provided data of their studies upon request. This study was partly funded by a "2002 Young Investigator Scholarship" awarded to Giovanni FM Strippoli by the Italian Society of Nephrology, and by a University of Sydney School of Public Health non‐established PhD scholarship.
Data and analyses
1.
ACEi versus placebo/no treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 21 | 7295 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.71, 1.17] |
| 1.1 Half or less maximum tolerable dose | 11 | 5261 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.41, 3.44] |
| 1.2 Maximum tolerable dose | 10 | 2034 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.61, 0.98] |
| 2 Cardiovascular mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Doubling of serum creatinine | 9 | 6780 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.47, 1.00] |
| 4 End‐stage kidney disease | 10 | 6819 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.39, 0.93] |
| 5 Micro‐ to macroalbuminuria | 17 | 2036 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.29, 0.69] |
| 6 Micro‐ to normoalbuminuria | 16 | 1910 | Risk Ratio (M‐H, Random, 95% CI) | 3.06 [1.76, 5.35] |
| 7 Cough | 10 | 7087 | Risk Ratio (M‐H, Random, 95% CI) | 3.17 [2.29, 4.38] |
| 8 Headache | 4 | 6186 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.33, 2.53] |
| 9 Impotence | 5 | 1528 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.91] |
| 10 Hyperkalaemia | 2 | 1219 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.32, 2.21] |
1.2.
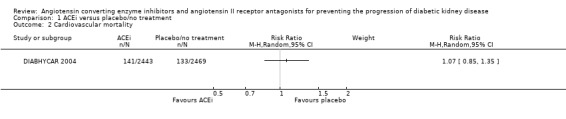
Comparison 1 ACEi versus placebo/no treatment, Outcome 2 Cardiovascular mortality.
2.
AIIRA versus placebo/no treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 5 | 3409 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.85, 1.17] |
| 2 Cardiovascular mortality | 0 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Doubling of serum creatinine | 3 | 3251 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.67, 0.93] |
| 4 End‐stage kidney disease | 3 | 3251 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.67, 0.91] |
| 5 Micro‐ to macroalbuminuria | 3 | 761 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.32, 0.75] |
| 6 Micro‐ to normoalbuminuria | 2 | 670 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.05, 1.93] |
| 7 Cough | 2 | 194 | Risk Ratio (M‐H, Random, 95% CI) | 4.93 [1.00, 24.35] |
| 8 Headache | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Impotence | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Hyperkalaemia | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
2.7.

Comparison 2 AIIRA versus placebo/no treatment, Outcome 7 Cough.
3.
AIIRA versus ACEi
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 3 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.31, 2.78] |
| 2 Cardiovascular mortality | 3 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.10, 3.62] |
| 3 Doubling of serum creatinine | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 End‐stage kidney disease | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Micro‐ to macroalbuminuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Micro‐ to normoalbuminuria | 2 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.76, 1.94] |
| 7 Cough | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.01, 48.70] |
| 8 Headache | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Hyperkalaemia | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Impotence | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
3.2.
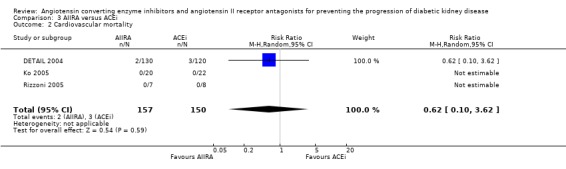
Comparison 3 AIIRA versus ACEi, Outcome 2 Cardiovascular mortality.
3.5.

Comparison 3 AIIRA versus ACEi, Outcome 5 Micro‐ to macroalbuminuria.
3.6.

Comparison 3 AIIRA versus ACEi, Outcome 6 Micro‐ to normoalbuminuria.
3.7.
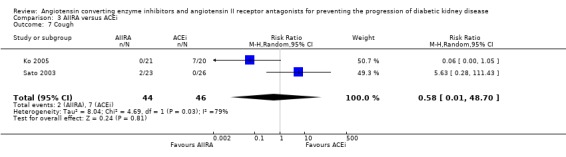
Comparison 3 AIIRA versus ACEi, Outcome 7 Cough.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Country: USA Setting/Design: Multicentre Time frame: NS Randomisation method: Permuted block randomisation within strata Blinding ‐ Participants: No ‐ Investigators: No ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 5‐7 years Loss to follow‐up: NS | |
| Participants |
Inclusion criteria
Enalapril group 1
Placebo group
Exclusion criteria
|
|
| Interventions |
Enalapril group 1
Control group
Co‐interventions If study intervention alone did not achieve the target BP, then open‐labeled antihypertensive medication were added in a step‐wise manner. Additional antihypertensive agents added at the descretion of tne medical director but did not include a CCB or ACEi |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Country: India Setting/Design: University Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: No ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 5 years Loss to follow‐up: 13 | |
| Participants |
Inclusion criteria
Enalapril group 1
Enalapril group 2
Exclusion criteria: NS |
|
| Interventions |
Enalapril group 1
10 mg/d Enalapril group 2 Moderate treatment Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: India Setting/Design: University Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: No ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 5 years Loss to follow‐up: 13 | |
| Participants |
Inclusion criteria
Enalapril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Enalapril group
10 mg/d Placebo group Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: Italy Setting/Design: Multicentre Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 36 months Loss to follow‐up: 0 | |
| Participants |
Inclusion criteria
Benazepril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Benazepril group
0 mg/d Placebo group Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: UK Setting/Design: Multicentre Time frame: NS Randomisation method: NS Blinding ‐ Participants: No ‐ Investigators: No ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: Yes Follow‐up period: 24 months Loss to follow‐up: 42 | |
| Participants |
Inclusion criteria
Ramipril group 1
Ramipril group 2
Placebo group
Exclusion criteria
|
|
| Interventions |
Ramipril group 1
25 mg/d Ramipril group 2 5 mg/d Placebo group Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Country: USA Setting/Design: Hospital Time frame: NS Randomisation method: NS Blinding ‐ Participants: No ‐ Investigators: No ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 18 months Loss to follow‐up: 7 | |
| Participants |
Inclusion criteria
Lisinopril group
Placebo group
Exclusion criteria: NS |
|
| Interventions |
Lisinopril group
75 mg/d Placebo group Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Country: USA Setting/Design: University Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 18 months Loss to follow‐up: 9 | |
| Participants |
Inclusion criteria
Enalapril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Enalapril group
5‐40 mg/d Placebo group Co‐interventions Conventional antihypertensive drugs |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: Sweden Setting/Design: Multicentre Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: Yes Follow‐up period: 24 months Loss to follow‐up: 1 | |
| Participants |
Inclusion criteria
Ramipril group 1
Ramipril group 2
Placebo group
Exclusion criteria
|
|
| Interventions |
Ramipril group 1
1.25 mg/d Ramipril group 2 5 mg/d Placebo group Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: Austria Setting/Design: Multicentre Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: No ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 12 months Loss to follow‐up: 5 | |
| Participants |
Inclusion criteria
Captopril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Captopril group
37.5 mg/d Placebo group Co‐interventions: NS |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: USA Setting/Design: Multicentre Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: Yes Follow‐up period: 36 months Loss to follow‐up: 108 | |
| Participants |
Inclusion criteria
Captopril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Captopril group
75 mg/d Placebo group Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: USA Setting/Design: University hospital Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 24 months Loss to follow‐up: 1 | |
| Participants |
Inclusion criteria
Fosinopril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Fosinopril group
10 mg/d Placebo group Co‐interventions: NS |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: USA Setting/Design: University Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 24 months Loss to follow‐up: 0 | |
| Participants |
Inclusion criteria
Captopril group
Placebo group
Exclusion criteria: NS |
|
| Interventions |
Ramipril group
5.0 mg/d Placebo group Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: France Setting/Design: Multicentre Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 24 months Loss to follow‐up: 3 | |
| Participants |
Inclusion criteria
Perindopril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Perindopril group
4 mg/d Placebo group Co‐interventions Prazosin/diuretics |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: Italy Setting/Design: University Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 36 months Loss to follow‐up: 10 | |
| Participants |
Inclusion criteria
Lisinopril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Lisinopril group
2.5‐20 mg/d Placebo group Co‐interventions Atenolol |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: USA Setting/Design: Multicentre Time frame: NS Randomisation method: Central location‐based on permuted blocks Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 5 years Loss to follow‐up: 2 | |
| Participants |
Inclusion criteria
Enalapril group
Telmiosartan group
Exclusion criteria
|
|
| Interventions |
Enalapril group
20 mg/d Telmiosartan group 80 mg/d Co‐interventions Others antihypertensive agents (except ACEi or AIRA) were allowed after 2 months. |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Country: France Setting/Design: Multicentre Time frame: 1995‐2001 Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: Yes Follow‐up period: 3‐6 months Loss to follow‐up: 0 | |
| Participants |
Inclusion criteria
Ramipril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Ramipril group
1.25 mg/d Placebo group Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Country: UK Setting/Design: Multicentre Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 3 years Loss to follow‐up: 4 | |
| Participants |
Inclusion criteria
Enalapril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Enalapril group
10 mg/d Placebo group Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: UK Setting/Design: University Time frame: NS Randomisation method: Yes Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: Yes Follow‐up period: 24 months Loss to follow‐up: 82 | |
| Participants |
Inclusion criteria
Lisinopril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Lisinopril group
10‐20 mg/d Placebo group Co‐interventions: NS |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Country: USA Setting/Design: University Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: Yes ‐ Data analysis: No Intention‐to‐treat: Yes Follow‐up period: 12 months Loss to follow‐up: 1 | |
| Participants |
Inclusion criteria
Ramipril group
Ramipril + pentoxifylline group
Placebo group
Exclusion criteria: NS |
|
| Interventions |
Ramipril group
5 mg/d Ramipril + pentoxifylline group 5 mg/d + 400 mg 3x/d Placebo group Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: Denmark Setting/Design: Multicentre Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 24 months Loss to follow‐up: 1 | |
| Participants |
Inclusion criteria
Captopril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Captopril group
100 mg/d Placebo group Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: Canada Setting/Design: Multicentre Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: Yes ‐ Data analysis: No Intention‐to‐treat: Yes Follow‐up period: mean 4.5 years Loss to follow‐up:13 | |
| Participants |
Inclusion criteria
Ramipril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Ramipril group
10 mg/d Placebo group Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Country: USA Setting/Design: Multicentre Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: Yes Follow‐up period: 30 months Loss to follow‐up: 11 | |
| Participants |
Inclusion criteria
Irbesartan group
Placebo group
Exclusion criteria: NS |
|
| Interventions |
Irbesartan group
75‐300 mg/d Placebo group Co‐interventions Antihypertensive agents other than ACEi, AIIRA and CCB |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Country: Denmark Setting/Design: Muticentre Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: Yes Follow‐up period: 2 years Loss to follow‐up: 77 | |
| Participants |
Inclusion criteria
Irbesartan group 1
Irbesartan group 2
Placebo group
Exclusion criteria
|
|
| Interventions |
Irbesartan group 1
150 mg/d Irbesartan group 2 300 mg/d Placebo group Co‐interventions Diuretics, beta‐blockers, CCB (except dihydropyridines), and alpha blockers |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: Japan Setting/Design: Multicentre Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: mean 1.5years Loss to follow‐up: 22 | |
| Participants |
Inclusion criteria
Imidapril group
Captopril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Imidapril group
5 mg/d Captopril group 37.5 mg/d Placebo group Co‐interventions Antihypertensive agents other than ACEi, CCB and AIIRA |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Country: Australia Setting/Design: Multicentre Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: Yes Follow‐up period: 24‐36 months Loss to follow‐up: 9 | |
| Participants |
Inclusion criteria
Perindopril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Perindopril group
2‐8 mg/d Placebo group Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: Australia Setting/Design: Multicentre Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: Yes Follow‐up period: 66 months(median) Loss to follow‐up: 32 | |
| Participants |
Inclusion criteria
Perindopril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Perindopril group
2‐8 mg/d Placebo group Co‐interventions Diuretics, CCB, beta‐blockers if blood pressure remained uncontrolled. |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: Hong Kong Setting/Design: Teaching hospitals Time frame: NS Randomisation method: NS Blinding ‐ Participants: No ‐ Investigators: No ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: Yes Follow‐up period: 1 year Loss to follow‐up: 1 | |
| Participants |
Inclusion criteria
Enalapril group
Valsartan group
Exclusion criteria
|
|
| Interventions |
Enalapril group
5‐10 mg/d Valsartan group 80‐160 mg/d Co‐interventions Other antihypertensive agents |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: Canada Setting/Design: Multicentre Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 12 months Loss to follow‐up: 11 | |
| Participants |
Inclusion criteria
Losartan group
Enalapril group
Exclusion criteria
|
|
| Interventions |
Losartan group
50 mg/d Enalapril group 5‐10 mg/d Co‐interventions Antihypertensive agents other than ACEi, AIIRA and CCB |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: USA Setting/Design: University Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: Yes Follow‐up period: 24 months Loss to follow‐up: 43 | |
| Participants |
Inclusion criteria
Captopril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Captopril group
100 mg/d Placebo group Co‐interventions Prazosin‐clonidine |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: USA Setting/Design: Multicentre Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 36 months Loss to follow‐up: 44 | |
| Participants |
Inclusion criteria
Enalapril group
Placebo group
Exclusion criteria: NS |
|
| Interventions |
Enalapril group
5‐40 mg/d Placebo group Co‐interventions Alpha and beta adrenergic antagonist, diuretics and CCB |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: France Setting/Design: Hospital Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 6 months Loss to follow‐up: 0 | |
| Participants |
Inclusion criteria
Enalapril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Enalapril group
20 mg/d Placebo group Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Country: Denmark Setting/Design: Hospital Time frame: NS Randomisation method: NS Blinding ‐ Participants: No ‐ Investigators: No ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 4 years (8 years for GFR) Loss to follow‐up: 4 | |
| Participants |
Inclusion criteria
Captopril + bendrofluazide group
Placebo group
Exclusion criteria: NS |
|
| Interventions |
Captopril + bendrofluazide group
Captopril (100 mg/d) + bendrofluazide (2.5 mg/d) Placebo group Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: Canada Setting/Design: Multicentre Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: Yes Follow‐up period: 13 months Loss to follow‐up: 19 | |
| Participants |
Inclusion criteria
Valsartan group 1
Valsartan group 2
Captopril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Valsartan group 1
80 mg/d Valsartan group 2 160 mg/d Captopril group 75 mg/d Placebo group Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: Australia Setting/Design: Hospital Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 36 months Loss to follow‐up: 9 | |
| Participants |
Inclusion criteria
Perindopril group
Placebo group
Exclusion criteria Non‐diabetic renal disease or other major disease |
|
| Interventions |
Perindopril group
4 mg/d Placebo group Co‐interventions CCB, beta‐blockers, alpha‐blockers or diuretic |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: UK Setting/Design: University Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 48 weeks Loss to follow‐up: 9 | |
| Participants |
Inclusion criteria
Lisinopril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Lisinopril group
10 mg/d Placebo group Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: Denmark Setting/Design: Hospital Time frame: NS Randomisation method: NS Blinding ‐ Participants: No ‐ Investigators: No ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 12 months Loss to follow‐up: 1 | |
| Participants |
Inclusion criteria
Captopril group
Placebo group
Exclusion criteria: NS |
|
| Interventions |
Captopril group
25‐100 mg/d Placebo group Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: Denmark Setting/Design: Hospital Time frame: NS Randomisation method: Yes Blinding ‐ Participants: No ‐ Investigators: No ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: Yes Follow‐up period: 96 months Loss to follow‐up: 18 | |
| Participants |
Inclusion criteria
Captopril group
Control group
Exclusion criteria
|
|
| Interventions |
Captopril group
12.5‐125 mg/d Control group Co‐interventions Diuretics, dihydropyridine, CCB, beta‐blocker |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: USA Setting/Design: Hospital Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 24 months Loss to follow‐up: 4 | |
| Participants |
Inclusion criteria
Cilaxapril group
Control group
Exclusion criteria
|
|
| Interventions |
Cilaxapril group
2.5 or 5 mg/d (DBP ≥ 85 mm Hg) for 24 weeks Control group Matched placebo Co‐interventions: NS |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: Denmark Setting/Design: University Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: Yes Follow‐up period: 24 months Loss to follow‐up: 0 | |
| Participants |
Inclusion criteria
Lisinopril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Lisinopril group
40 mg/d Placebo group Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: Israel Setting/Design: Multicentre Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 5 years Loss to follow‐up: 8 | |
| Participants |
Inclusion criteria
Enalapril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Enalapril group
10 mg/d Placebo group Co‐interventions Long‐acting nifedipine |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: USA Setting/Design: Multicentre Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: Yes ‐ Data analysis: No Intention‐to‐treat: Yes Follow‐up period: mean 3.4 years Loss to follow‐up: 3 | |
| Participants |
Inclusion criteria
Losartan group
Placebo group
Exclusion criteria
|
|
| Interventions |
Losartan group
50‐100 mg/d Placebo group Co‐interventions CCB, diuretics, alpha‐blockers, beta‐blockers and centrally acting agents |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: Italy Setting/Design: Multicentre Time frame: NS Randomisation method: NS Blinding ‐ Participants: No ‐ Investigators: No ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 1 year Loss to follow‐up: 0 | |
| Participants |
Inclusion criteria
Enalapril group
Candesartan group
Exclusion criteria
|
|
| Interventions |
Enalapril group
10‐20 mg/d Candesartan group 8‐16 mg/d Co‐interventions Diuretics if BP not controlled in 12 weeks |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: Spain Setting/Design: University‐Hospital Time frame: NS Randomisation method: NS Blinding ‐ Participants: No ‐ Investigators: No ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 6 months Loss to follow‐up: 0 | |
| Participants |
Inclusion criteria
Captopril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Captopril group
Initial dose: 25 mg/d (mean 61 ±19 mg/d) Placebo group Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: Japan Setting/Design: University Time frame: NS Randomisation method: NS Blinding ‐ Participants: No ‐ Investigators: No ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 48 months Loss to follow‐up: 4 | |
| Participants |
Inclusion criteria
Enalapril group 1
Enalapril group 2
Control group 1
Control group 2
|
|
| Interventions |
Enalapril groups
Enalapril 5 mg/d Control group Co‐interventions Nifedipine (30 mg/d) for well controlled hypertensive patients |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: Japan Setting/Design: Teaching hospital Time frame: NS Randomisation method: NS Blinding ‐ Participants: No ‐ Investigators: No ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 11 ± months Loss to follow‐up: 0 | |
| Participants |
Inclusion criteria
ACEi group
Candesartan group
Exclusion criteria: NS |
|
| Interventions |
ACEi group
Enalapril or trandolapril (not specified) Candesartan group Not specified Co‐interventions CCB, alpha 1 blocker and central acting alpha 2 stimulant, nifedipine(30 mg/d) |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: Italy Setting/Design: University Time frame: NS Randomisation method: Yes Blinding ‐ Participants: No ‐ Investigators: No ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: Yes Follow‐up period: 6 months x 2 Loss to follow‐up: 0 | |
| Participants |
Inclusion criteria
Enalapril group
Atenolol group
Exclusion criteria
|
|
| Interventions |
Enalapril group
5 mg/d Atenolol group 50 mg/d Co‐interventions: NS |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: China Setting/Design: Hospital Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: Yes Follow‐up period: 6 months Loss to follow‐up: 0 | |
| Participants |
Inclusion criteria
Losartan group
Placebo group
Exclusion criteria
|
|
| Interventions |
Losartan group
50 mg/d Placebo group Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: Italy Setting/Design: Multicentre Time frame: NS Randomisation method: NS Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 6 months Loss to follow‐up: 14 | |
| Participants |
Inclusion criteria
Ramipril group
Placebo group
Exclusion criteria
|
|
| Interventions |
Ramipril group
1.25 mg/d Placebo group Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Country: Turkey Setting/Design: University Time frame: NS Randomisation method: NS Blinding ‐ Participants: No ‐ Investigators: No ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 12 months Loss to follow‐up: 3 | |
| Participants |
Inclusion criteria
Enalapril group
Losartan group
Enalapril + Losartan group
Exclusion criteria
|
|
| Interventions |
Enalapril group
5 mg/d Losartan group 50 mg/d Enalapril + Losartan group Co‐interventions: No |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
ACEi ‐ angiotensin converting enzyme inhibitor; AER ‐ albumin excretion rate; AIIRA ‐ angiotensin II receptor antagonist; BMI ‐ body mass index; CAB ‐ coronary artery bypass; CCB ‐ calcium channel blocker; CrCl ‐ creatinine clearance; CHF ‐ congestive heart failure; CVA ‐ cerebrovascular accident; CVD ‐ cerebrovascular disease; CVE ‐ cardiovascular event; DBP ‐ diastolic blood pressure; DM ‐ diabetes mellitus; ESKD ‐ end‐stage kidney disease; GFR ‐ glomerular filtration rate; HD ‐ haemodialysis; MAP ‐ mean arterial pressure; MI ‐ myocardial infarction; NS ‐ not stated; NSAIDs ‐ nonsteroidal anti‐inflammatory drugs; PD ‐ peritoneal dialysis; SBP ‐ systolic blood pressure; SCr ‐ serum creatinine; UAE ‐ urinary albumin excretion; UTI ‐ urinary tract infection
Contributions of authors
Strippoli GFM: Design, conduct, data‐extraction, data‐analysis, data interpretation, writing the review
Bonifati C: Data‐extraction, data entry and interpretation, writing the review
Craig M: Data‐extraction, writing the review
Navaneethan S: Data‐extraction, data entry and interpretation, writing of review
Craig JC: Design, conduct, data‐analysis, data interpretation, writing the review.
Sources of support
Internal sources
University of Sydney School of Public Health, non‐established PhD scholarship, Australia.
External sources
2002‐03 Young Investigator Scholarship, Italian Society of Nephrology, Italy.
Declarations of interest
None declared
Edited (no change to conclusions)
References
References to studies included in this review
ABCD 1996 {published data only}
- Estacio RO, Jeffers B, Biggerstaff S, Schrier RW. Effects of a calcium channel antagonist versus an ace inhibitor on diabetic nephropathy [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):114A. [CENTRAL: CN‐00445260] [Google Scholar]
- Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care 23;Suppl 2:B54‐64. [MEDLINE: ] [PubMed] [Google Scholar]
- Estacio RO, Jeffers BW, Hiatt WR, Biggerstaff SL, Gifford N, Schrier RW. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non‐insulin‐dependent diabetes and hypertension. New England Journal of Medicine 1998;338(10):645‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Estacio RO, Mehler P, Esler A, Schrier RW. Aggressive lowering of blood pressure in normotensive type 2 diabetic patients: beneficial effects on stroke, progression of retinopathy and nephropathy [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):146A. [CENTRAL: CN‐00445261] [Google Scholar]
- Estacio RO, Savage S, Nagel NJ, Schrier RW. Baseline characteristics of participants in the Appropriate Blood Pressure Control in Diabetes trial. Controlled Clinical Trials 1996;17(3):242‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Estacio RO, Schrier RW. Antihypertensive therapy in type 2 diabetes: implications of the appropriate blood pressure control in diabetes (ABCD) trial. American Journal of Cardiology 1998;82(9B):9R‐14R. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mehler PS, Coll JR, Estacio R, Esler A, Schrier RW, Hiatt WR. Intensive blood pressure control reduces the risk of cardiovascular events in patients with peripheral arterial disease and type 2 diabetes. Circulation 2003;107(5):753‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Savage S, Estacio RO, Jeffers B, Schrier RW. Urinary albumin excretion as a predictor of diabetic retinopathy, neuropathy, and cardiovascular disease in NIDDM. Diabetes Care 1996;19(11):1243‐48. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney International 2002;61(2):1086‐97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia 1996;39(12):1646‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ahmad 1997 {published data only}
- Ahmad J, Siddiqui MA, Ahmad H. Effective postponement of diabetic nephropathy with enalapril in normotensive type 2 diabetic patients with microalbuminuria. Diabetes Care 1997;20(10):1576‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ahmad 2003 {published data only}
- Ahmad J, Shafique S, Abidi SM, Parwez I. Effect of 5‐year enalapril therapy on progression of microalbuminuria and glomerular structural changes in type 1 diabetic subjects. Diabetes Research & Clinical Practice 2003;60(2):131‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
AIPRI 1996 {published data only}
- Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, et al. Effect of the angiotensin‐converting‐enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin‐Converting‐Enzyme Inhibition in Progressive Renal Insufficiency Study Group. New England Journal of Medicine 1996;334(15):939‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ATLANTIS SG 2000 {published data only}
- O'Hare P, Bilbous R, Mitchell T, O'Callaghan CJ, Viberti GC. Ace‐Inhibitor Trial to Lower Albuminuria in Normotensive Insulin‐Dependent Subjects Study Group. Low‐dose ramipril reduces microalbuminuria in type 1 diabetic patients without hypertension: results of a randomized controlled trial. Diabetes Care 2000;23(12):1823‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bakris 1994 {published data only}
- Bakris GL, Slataper R, Vicknair N, Sadler R. ACE inhibitor mediated reductions in renal size and microalbuminuria in normotensive, diabetic subjects. Journal of Diabetes & its Complications 1994;8(1):2‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bauer 1992 {published data only}
- Bakris GL, Barnhill BW, Sadler R. Treatment of arterial hypertension in diabetic humans: importance of therapeutic selection. Kidney International 1992;41(4):912‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bojestig 2001 {published data only}
- Bojestig M, Karlberg BE, Lindstrom T, Nystrom FH. Reduction of ACE activity is insufficient to decrease microalbuminuria in normotensive patients with type 1 diabetes. Diabetes Care 2001;24(5):919‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Capek 1994 {published data only}
- Capek M, Schnack C, Ludvik B, Kautzky‐Willer A, Banyai M, Prager R. Effects of captopril treatment versus placebo on renal function in type 2 diabetic patients with microalbuminuria: a long‐term study. Clinical Investigator 1994;72(12):961‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CAPTOPRIL 1993 {published data only}
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin‐converting‐enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. New England Journal of Medicine 1993;329(20):1458‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carella 1999 {published data only}
- Carella MJ, Gossain VV, Jones J. The effects of a low‐dose regimen of fosinopril on elevated urinary albumin excretion in normotensive type 1 diabetic patients. Journal of Medicine 1999;30(5‐6):305‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Chase 1993 {published data only}
- Chase HP, Garg SK, Harris S, Hoops S, Jackson WE, Holmes DL. Angiotensin‐converting enzyme inhibitor treatment for young normotensive diabetic subjects: a two‐year trial. Annals of Ophthalmology 1993;25(8):284‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Cordonnier 1999 {published data only}
- Cordonnier DJ, Pinel N, Barro C, Maynard M, Zaoui P, Halimi S, et al. Expansion of cortical interstitium is limited by converting enzyme inhibition in type 2 diabetic patients with glomerulosclerosis. The Diabiopsies Group. Journal of the American Society of Nephrology 1999;10(6):1253‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Crepaldi 1998 {published data only}
- Crepaldi G, Carta Q, Deferrari G, Mangili R, Navalesi R, Santeusanio F, et al. Effects of lisinopril and nifedipine on the progression to overt albuminuria in IDDM patients with incipient nephropathy and normal blood pressure. The Italian Microalbuminuria Study Group in IDDM. Diabetes Care 1998;21(1):104‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
DETAIL 2004 {published data only}
- Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, et al. Angiotensin‐receptor blockade versus converting‐enzyme inhibition in type 2 diabetes and nephropathy. New England Journal of Medicine 2004;351(19):1952‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
DIABHYCAR 2004 {published data only}
- Marre M, Lievre M, Chatellier G, Mann J, Passa P, Menard J. Effects of low dose ramipril on cardiovascular and renal outcomes in patients with type 2 diabetes and raised excretion of urinary albumin: randomised, double blind, placebo controlled trial (the DIABHYCAR study). BMJ 2004;328(7438):495‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ESPRIT 2001 {published data only}
- Baines LA. Effect of 3 years of antihypertensive therapy on renal structure in type 1 diabetic patients with albuminuria: the European Study for the Prevention of Renal Disease in Type 1 Diabetes (ESPRIT). Diabetes 2001;50(4):843‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
EUCLID 1997 {published data only}
- Chaturvedi N, Stevenson J, Fuller JH, Rottiers R, Ferriss B, Karamanos B, et al. Randomised placebo‐controlled trial of lisinopril in normotensive patients with insulin‐dependent diabetes and normoalbuminuria or microalbuminuria. The EUCLID Study Group. Lancet 1997;349(9068):1787‐92. [MEDLINE: ] [PubMed] [Google Scholar]
- Penno G, Chaturvedi N, Talmud PJ, Cotroneo P, Manto A, Nannipieri M, et al. Effect of angiotensin‐converting enzyme (ACE) gene polymorphism on progression of renal disease and the influence of ACE inhibition in IDDM patients: findings from the EUCLID Randomized Controlled Trial. EURODIAB Controlled Trial of Lisinopril in IDDM. Diabetes 1998;47(9):1507‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garg 1998 {published data only}
- Garg SK, Chase HP, Jackson WE, Harris S, Carmain JA, Hansen MH, et al. Renal and retinal changes after treatment with Ramipril and pentoxifylline in subjects with IDDM. Annals of Ophthalmology‐Glaucoma 1998;30(1):33‐7. [EMBASE: 1998093196] [Google Scholar]
Hansen 1994 {published data only}
- Hansen KW, Klein F, Christensen PD, Sorensen K, Andersen PH, Moller J, et al. Effects of captopril on ambulatory blood pressure, renal and cardiac function in microalbuminuric type 1 diabetic patients. Diabete et Metabolisme 1994;20(5):485‐93. [MEDLINE: ] [PubMed] [Google Scholar]
- Viberti G, Mogensen CE, Groop LC, Pauls JF. Effect of captopril on progression to clinical proteinuria in patients with insulin‐dependent diabetes mellitus and microalbuminuria. European Microalbuminuria Captopril Study Group. JAMA 1994;271(4):275‐9. [MEDLINE: ] [PubMed] [Google Scholar]
HOPE 2000 {published data only}
- Gerstein HC, Yusuf S, Mann JF, Hoogwerf B, Zinman B, Held C, et al. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO‐HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000;355(9200):253‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Annals of Internal Medicine 2001;134(8):629‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
IDNT 2001 {published data only}
- Berl T, Hunsicker LG, Lewis JB, Pfeffer MA, Porush JG, Rouleau JL, et al. Cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial of patients with type 2 diabetes and overt nephropathy. Annals of Internal Medicine 2003;138(7):542‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. New England Journal of Medicine 2001;345(12):851‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
IRMA‐2 2001 {published data only}
- Parving HH, Lehnert H, Brochner‐Mortensen J, Gomis R, Andersen S, Arner P, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. New England Journal of Medicine 2001;345(12):870‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
JAPAN‐IDDM 2002 {published data only}
- Katayama S, Kikkawa R, Isogai S, Sasaki N, Matsuura N, Tajima N, et al. Effect of captopril or imidapril on the progression of diabetic nephropathy in Japanese with type 1 diabetes mellitus: a randomized controlled study (JAPAN‐IDDM). Diabetes Research & Clinical Practice 2002;55(2):113‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jerums 2001 {published data only}
- Jerums G, Allen TJ, Campbell DJ, Cooper ME, Gilbert RE, Hammond JJ, et al. Long‐term comparison between perindopril and nifedipine in normotensive patients with type 1 diabetes and microalbuminuria. American Journal of Kidney Diseases 2001;37(5):890‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jerums 2004 {published data only}
- Jerums G, Allen TJ, Campbell DJ, Cooper ME, Gilbert RE, Hammond JJ, et al. Long‐term renoprotection by perindopril or nifedipine in non‐hypertensive patients with type 2 diabetes and microalbuminuria. Diabetic Medicine 2004;21(11):1192‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ko 2005 {published data only}
- Ko GT, Tsang CC, Chan HC. Stabilization and regression of albuminuria in chinese patients with type 2 diabetes: a one‐year randomized study of valsartan versus enalapril. Advances in Therapy 2005;22(2):155‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lacourciere 2000 {published data only}
- Lacourciere Y, Belanger A, Godin C, Halle JP, Ross S, Wright N, et al. Long‐term comparison of losartan and enalapril on kidney function in hypertensive type 2 diabetics with early nephropathy. Kidney International 2000;58(2):762‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Laffel 1995 {published data only}
- Laffel LM, McGill JB, Gans DJ. The beneficial effect of angiotensin‐converting enzyme inhibition with captopril on diabetic nephropathy in normotensive IDDM patients with microalbuminuria. North American Microalbuminuria Study Group. American Journal of Medicine 1995;99(5):497‐504. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lebovitz 1994 {published data only}
- Lebovitz HE, Wiegmann TB, Cnaan A, Shahinfar S, Sica DA, Broadstone V, et al. Renal protective effects of enalapril in hypertensive NIDDM: role of baseline albuminuria. Kidney International ‐ Supplement 1994;45:S150‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Marre 1987 {published data only}
- Marre M. Microalbuminuria and ACE inhibition in non‐hypertensive diabetics. Journal of Diabetic Complications 1990;4(2):84‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Marre M, Chatellier G, Leblanc H, Guyene TT, Menard J, Passa P. Prevention of diabetic nephropathy with enalapril in normotensive diabetics with microalbuminuria. BMJ 1988;297(6656):1092‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marre M, Leblanc H, Suarez L, Guyenne TT, Menard J, Passa P. Converting enzyme inhibition and kidney function in normotensive diabetic patients with persistent microalbuminuria. British Medical Journal Clinical Research Ed 1987;294(6585):1448‐52. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathiesen 1999 {published data only}
- Hansen PM, Mathiesen ER, Kofoed‐Enevoldsen A, Deckert T. Possible effect of angiotensin‐converting enzyme inhibition on glomerular charge selectivity. Journal of Diabetes & its Complications 1995;9(3):158‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mathiesen ER, Hommel E, Giese J, Parving HH. Efficacy of captopril in postponing nephropathy in normotensive insulin dependent diabetic patients with microalbuminuria. BMJ 1991;303(6794):81‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen ER, Hommel E, Hansen HP, Smidt UM, Parving HH. Randomised controlled trial of long term efficacy of captopril on preservation of kidney function in normotensive patients with insulin dependent diabetes and microalbuminuria. BMJ 1999;319(7201):24‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muirhead 1999 {published data only}
- Muirhead N, Feagan BF, Mahon J, Lewanczuk RZ, Rodger NW, Botteri F, et al. The effects of valsartan and captopril on reducing microalbuminuria in patients with type 2 diabetes mellitus: A placebo‐controlled trial. Current Therapeutic Research, Clinical & Experimental 1999;60(12):650‐60. [EMBASE: 2000024629] [Google Scholar]
Nankervis 1998 {published data only}
- Nankervis A, Nicholls K, Kilmartin G, Allen P, Ratnaike S, Martin FI. Effects of perindopril on renal histomorphometry in diabetic subjects with microalbuminuria: a 3‐year placebo‐controlled biopsy study. Metabolism: Clinical & Experimental 1998;47(12 Suppl 1):12‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
O'Donnell 1993 {published data only}
- O'Donnell MJ, Rowe BR, Lawson N, Horton A, Gyde OH, Barnett AH. Placebo‐controlled trial of lisinopril in normotensive diabetic patients with incipient nephropathy. Journal of Human Hypertension 1993;7(4):327‐32. [MEDLINE: ] [PubMed] [Google Scholar]
Parving 1989 {published data only}
- Parving HH, Hommel E, Damkjaer Nielsen M, Giese J. Effect of captopril on blood pressure and kidney function in normotensive insulin dependent diabetics with nephropathy. BMJ 1989;299(6698):533‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parving 2001a {published data only}
- Parving HH, Brenner BM, Cooper ME, Zeeuw D, Keane WF, Mitch WE, et al. Effect of losartan on renal and cardiovascular complications of patients with type 2 diabetes and nephropathy. Ugeskrift for Laeger 2001;163(40):5514‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Parving HH, Hommel E, Jensen BR, Hansen HP. Long‐term beneficial effect of ACE inhibition on diabetic nephropathy in normotensive type 1 diabetic patients. Kidney International 2001;60(1):228‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Phillips 1993 {published data only}
- Phillips PJ, Phillipou G, Bowen KM, Lowe J, Yue DK, Wischusen J, et al. Diabetic microalbuminuria and cilazapril. American Journal of Medicine 1993;94(4A):58S‐60S. [MEDLINE: ] [PubMed] [Google Scholar]
Poulsen 2001 {published data only}
- Poulsen PL, Ebbehoj E, Mogensen CE. Lisinopril reduces albuminuria during exercise in low grade microalbuminuric type 1 diabetic patients: a double blind randomized study. Journal of Internal Medicine 2001;249(5):433‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Poulsen PL, Ebbehoj E, Nosadini R, Fioretto P, Deferrari G, Crepaldi G, et al. Early ACE‐i intervention in microalbuminuric patients with type 1 diabetes: effects on albumin excretion, 24 h ambulatory blood pressure, and renal function. Diabetes & Metabolism 2001;27(2 Pt 1):123‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Ravid 1993 {published data only}
- Ravid M, Clutter WE. Long‐term effect of enalapril on normotensive type II diabetes mellitus. Annals of Internal Medicine 1993;119(Suppl 1):6. [EMBASE: 1993289276] [Google Scholar]
- Ravid M, Lang R, Rachmani R, Lishner M. Long‐term renoprotective effect of angiotensin‐converting enzyme inhibition in non‐insulin‐dependent diabetes mellitus. A 7‐year follow‐up study. Archives of Internal Medicine 1996;156(3):286‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Ravid M, Neumann L, Lishner M. Plasma lipids and the progression of nephropathy in diabetes mellitus type II: effect of ACE inhibitors. Kidney International 1995;47(3):907‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ravid M, Savin H, Jutrin I, Bental T, Katz B, Lishner M. Long‐term stabilizing effect of angiotensin‐converting enzyme inhibition on plasma creatinine and on proteinuria in normotensive type II diabetic patients. Annals of Internal Medicine 1993;118(8):577‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RENAAL 2001 {published data only}
- Brenner BM, Cooper ME, Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. New England Journal of Medicine 2001;345(12):861‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rizzoni 2005 {published data only}
- Rizzoni D, Portieri E, Ciuceis C, Sleiman I, Rodella L, Rezzani R, et al. Effect of treatment with candesartan or enalapril on subcutaneous small artery structure in hypertensive patients with noninsulin‐dependent diabetes mellitus. Hypertension 2005;45(4):659‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Romero 1993 {published data only}
- Romero R, Salinas I, Lucas A, Abad E, Reverter JL, Johnston S, et al. Renal function changes in microalbuminuric normotensive type II diabetic patients treated with angiotensin‐converting enzyme inhibitors. Diabetes Care 1993;16(4):597‐600. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sano 1994 {published data only}
- Sano T, Hotta N, Kawamura T, Matsumae H, Chaya S, Sasaki H, et al. Effects of long‐term enalapril treatment on persistent microalbuminuria in normotensive type 2 diabetic patients: results of a 4‐year, prospective, randomized study. Diabetic Medicine 1996;13(2):120‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sano T, Kawamura T, Matsumae H, Sasaki H, Nakayama M, Hara T, et al. Effects of long‐term enalapril treatment on persistent micro‐albuminuria in well‐controlled hypertensive and normotensive NIDDM patients. Diabetes Care 1994;17(5):420‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sato 2003 {published data only}
- Sato A, Tabata M, Hayashi K, Saruta T. Effects of the angiotensin II type 1 receptor antagonist candesartan, compared with angiotensin‐converting enzyme inhibitors, on the urinary excretion of albumin and type IV collagen in patients with diabetic nephropathy. Clinical & Experimental Nephrology 2003;7(3):215‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stornello 1992 {published data only}
- Stornello M, Valvo EV, Scapellato L. Hemodynamic, renal, and humoral effects of the calcium entry blocker nicardipine and converting enzyme inhibitor captopril in hypertensive type II diabetic patients with nephropathy. Journal of Cardiovascular Pharmacology 1989;14(6):851‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stornello M, Valvo EV, Scapellato L. Persistent albuminuria in normotensive non‐insulin‐dependent (type II) diabetic patients: comparative effects of angiotensin‐converting enzyme inhibitors and beta‐adrenoceptor blockers. Clinical Science 1992;82(1):19‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tan 2002 {published data only}
- Tan KC, Chow WS, Ai VH, Lam KS. Effects of angiotensin II receptor antagonist on endothelial vasomotor function and urinary albumin excretion in type 2 diabetic patients with microalbuminuria. Diabetes/Metabolism Research Reviews 2002;18(1):71‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Trevisan 1995 {published data only}
- Trevisan R, Tiengo A. Effect of low‐dose ramipril on microalbuminuria in normotensive or mild hypertensive non‐insulin‐dependent diabetic patients. North‐East Italy Microalbuminuria Study Group. American Journal of Hypertension 1995;8(9):876‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tutuncu 2001 {published data only}
- Tutuncu NB, Gurlek A, Gedik O. Efficacy of ACE inhibitors and ATII receptor blockers in patients with microalbuminuria: a prospective study. Acta Diabetologica 2001;38(4):157‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
REIN 1998 {published data only}
- Ruggenenti P, Perna A, Gherardi G, Gaspari F, Benini R, Remuzzi G. Renal function and requirement for dialysis in chronic nephropathy patients on long‐term ramipril: REIN follow‐up trial. Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN). Ramipril Efficacy in Nephropathy. Lancet 1998;352(9136):1252‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
ACEIDN 2001
- ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin‐converting‐enzyme inhibitors? A meta‐analysis of individual patient data. Annals of Internal Medicine 2001;134(5):370‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arauz‐Pacheo 2003
- Arauz‐Pacheo C, Parrott MA, Raskin P, American Diabetes Association. Treatment of hypertension in adults with diabetes. Diabetes Care 2003;26 Suppl 1:S80‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Casas 2005
- Casas JP, Chua W, Loukogeorgakis S, Vallance P, Smeeth L, Hingorani AD, et al. Effect of inhibitors of the renin‐angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta‐analysis. Lancet 2005;366(9502):2026‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cohn 2001
- Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin‐receptor blocker valsartan in chronic heart failure. New England Journal of Medicine 2001;345(23):1667‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dinneen 1997
- Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non‐insulin‐dependent diabetes mellitus. A systematic overview of the literature. Archives of Internal Medicine 1997;157(13):1413‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Garg 1995
- Garg R, Yusuf S. Overview of randomized trials of angiotensin‐converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA 1995;273(18):1450‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Grossman 2001
- Grossman E, Messerli FH, Goldbourt U. Intensive blood pressure control and drugs reduce morbidity and mortality in hypertension and diabetes mellitus. Evidence Based Medicine 2001;6(2):44. [EMBASE: 2001419226] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
JNC 7 2003
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC‐7 report. JAMA 2003;289(19):2560‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kasiske 1993
- Kasiske BL, Kalil RS, Ma JZ, Liao M, Keane WF. Effect of antihypertensive therapy on the kidney in patients with diabetes: a meta‐regression analysis. Annals of Internal Medicine 1993;118(2):129‐38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mogensen 1995
- Mogensen CE. Microalbuminuria in prediction and prevention of diabetic nephropathy in insulin‐dependent diabetes mellitus patients. Journal of Diabetes & its Complications 1995;9(4):337‐49. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mogensen 1999
- Mogensen CE. Drug treatment for hypertensive patients in special situations: diabetes and hypertension. Clinical & Experimental Hypertension (New York) 1999;21(5‐6):895‐906. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ritz 1999
- Ritz E, Ortho SR. Nephropathy in patients with type 2 diabetes mellitus. New England Journal of Medicine 1999;341(15):1127‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Strippoli 2005
- Strippoli GFM, Craig M, Craig JC. Antihypertensive agents for preventing diabetic kidney disease. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD004136.pub2] [DOI] [PubMed] [Google Scholar]
Wachtell 2003
- Wachtell K, Ibsen H, Olsen MH, Borch‐Johnsen K, Lindholm LH, Mogensen CE, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Annals of Internal Medicine 2003;139(11):901‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zunetti 1996
- Zuanetti G. Prognosis of diabetic patients post‐MI: the role of ACE inhibitor treatment. GISSI‐3 Investigators. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico. Journal of Diabetes & its Complications 1996;10(3):139‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Strippoli 2004
- Strippoli GF, Craig M, Deeks JJ, Schena FP, Craig JC. Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: systematic review. BMJ 2004;329(7470):828‐31. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]


