Abstract
Glucagon-like peptide 1 receptors (GLP-1R) are expressed in the lateral septum (LS) of rats and mice, and we have published that endogenous LS GLP-1 affects feeding and motivation for food in rats. Here we asked if these effects are also observed in mice. In separate dose-response studies using male C57Bl6J mice, intra-LS GLP-1 or the GLP-1R antagonist Exendin 9 (Ex9) was delivered shortly before dark onset, at doses subthreshold for effect when injected intracerebroventricularly (icv). Intra-LS GLP-1 significantly suppressed chow intake early in the dark phase and tended to reduce overnight intake. However, blockade of LS GLP-1R with Ex9 had no effect on ad libitum dark onset chow intake. We then asked if LS GLP-1R blockade blunts nutrient preload-induced intake suppression. Mice were trained to consume Ensure immediately before dark onset, which suppressed subsequent chow intake, and intra-LS Ex9 attenuated that preload-induced intake suppression. We also found that restraint stress robustly activates hindbrain GLP-1-producing neurons, and that LS GLP-1R blockade attenuates 30-min restraint stress-induced hypophagia in mice. Furthermore, we have reported that in the rat, GLP-1R in the dorsal subregion of the LS (dLS) affect motivation for food. We examined this in food-restricted mice responding for sucrose pellets on a progressive ratio (PR) schedule. Intra-dLS GLP-1R stimulation significantly suppressed, and Ex9 significantly increased, operant responding, and the Ex9 effect remained after mice returned to ad libitum conditions. Similarly, we found that stimulation of dLS GLP-1 suppressed licking for sucrose and conversely, Ex9 increased licking under ad libitum feeding conditions. Together, our data suggest that endogenous activation of LS GLP-1R plays a role in feeding in mice under some but not all conditions, and that these receptors strongly influence motivation for food.
1. Introduction
It is well established that central glucagon-like peptide 1 (GLP-1) plays a significant role in the control of feeding behavior [1–3]. Hindbrain GLP-1-producing (PPG) neurons project widely throughout the brain to many regions that express GLP-1 receptors (GLP-1R) [4–6]. Most research on the role of central GLP-1 in behavior has focused on its contribution to food intake control [7–10]. GLP-1 neurons are activated by feeding-relevant signals, such as the satiation signal cholecystokinin (CCK) and vagus nerve stimulation, and many studies have demonstrated that stimulation of GLP-1R in numerous brain regions suppresses food intake [9–12]. The results of a number of loss of function studies in which GLP-1R are blocked or their expression is reduced suggest that central GLP-1 is important for the physiologic control of energy balance [10,13–19]. Moreover, the central GLP-1 system appears to be involved in behavioral and endocrine stress responses [20–23]. GLP-1 neurons are potently activated by acute stress, and intracerebroventricular administration of a GLP-1R antagonist can block restraint stress-induced hypophagia in rats [2,24]. In studies using mice lacking GLP-1Rs in the paraventricular nucleus (PVN) of the hypothalamus, Ghosal and colleagues demonstrated that these receptors contribute to neuroendocrine and sympathetic nervous system responses to acute and chronic stress in addition to anxiety-like behavior [22].
Recently, our lab has focused on the role of lateral septum (LS) GLP-1R in feeding behavior in rats. In a series of studies, we demonstrated that pharmacologic stimulation of LS GLP-1R suppresses feeding, while blockade of these receptors significantly increases intake of a variety of foods, including chow, high-fat diet, sucrose solution, and corn oil emulsion; these results suggest that endogenous GLP-1 signaling in the LS plays a physiologic role in limiting food intake. We also reported that endogenous stimulation of GLP-1R in the dorsal subregion of the LS (dLS), in particular, influences motivation for food in rats [13]. Because the LS has a known role in stress responses [25,26], we investigated the contribution of LS GLP-1R. We reported that in rats, intra-dLS pretreatment with low-dose GLP-1R antagonist attenuated restraint stress-induced hypophagia [27].
Much of the research described above was conducted in rats, and the work that has been done in mice reveals some similarities and several notable species differences. For example, one study found that GLP-1R antagonism blocks aversive effects of LiCl in rats, but not mice, suggesting that GLP-1 is not required for mediating the effects of visceral illness in mice [28]. There are also known differences in the ability of GLP-1 neurons to detect leptin. In mice ~100% of GLP-1 neurons are directly responsive to leptin, whereas GLP-1 neurons show no response to leptin in the rat [29]. Moreover, a recent study using mice demonstrated that loss of central GLP-1 via selective ablation of NTS PPG neurons had no significant effect on ad libitum chow intake, body weight, or glucose tolerance. It was only when mice experienced a homeostatic challenge (i.e. restraint stress or nutrient preload) that PPG neurons appeared to be necessary for feeding control [11]. In contrast, data from studies using rats suggest that endogenous GLP-1 does in fact contribute to the normal control of feeding and glucose control [10,30,31]. In rats, both pharmacologic blockade or knockdown of GLP-1R in specific brain regions has been shown to increase ad libitum chow intake, and NTS GLP-1 mRNA knockdown also led to increased food intake and body weight [7,10].
These findings highlight the danger of assuming that findings in one animal model generalize across species. As our laboratory began to utilize mice, we undertook studies to determine whether LS GLP-1R play a role in the control of feeding behavior in mice as we have previously shown they do in the rat. Based on published anatomic data from transgenic mice expressing YFP in GLP-1 neurons, and others expressing RFP in GLP-1R neurons, there does appear to be a significant GLP-1 neuron projection to the LS and a large population of GLP-1-responsive neurons in this nucleus in the mouse [5,6,14,32]. Therefore, we hypothesized that LS GLP-R stimulation and blockade would have similar effects in the mouse as in the rat.
2. Methods
2.1. Subjects:
Naïve male and female C57Bl6J mice (Jackson Laboratories) or transgenic mice (described in 2.6. Study 3) were maintained individually in temperature-controlled vivariums on a 12:12-h light-dark cycle in plastic cages. Mice had ad libitum access to distilled water and chow (Purina 5001), except where otherwise noted. All experimental procedures were approved by the Florida State University Institutional Animal Care and Use Committee and conformed to the standard of the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
2.2. Surgery:
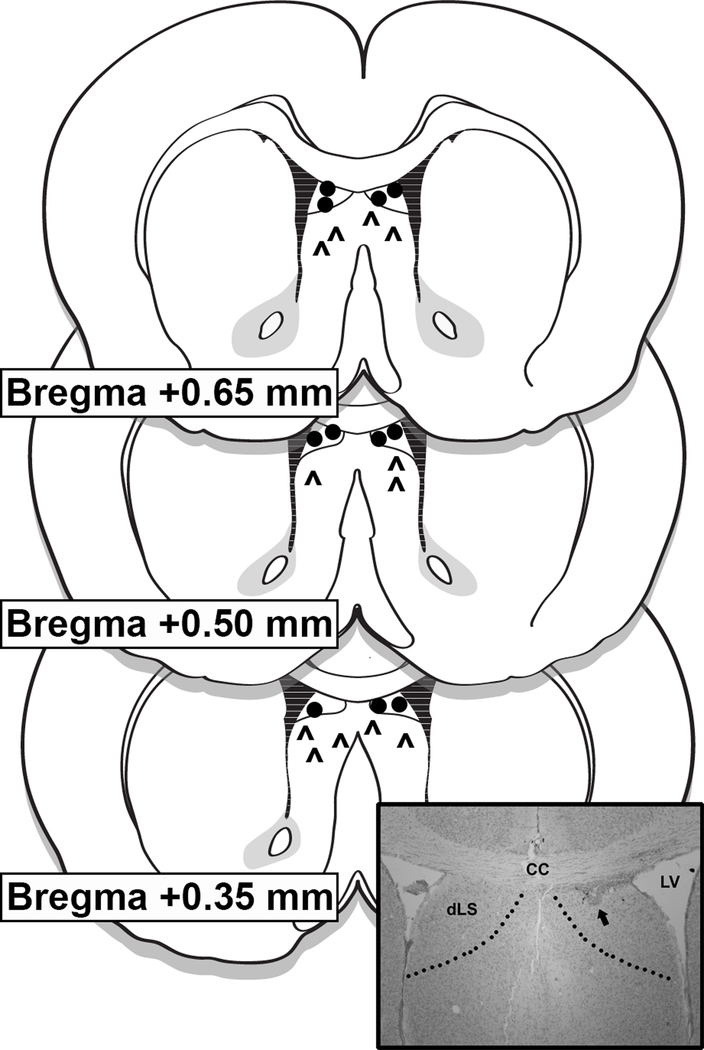
Mice were implanted with unilateral or bilateral 26 G guide cannulas (Plastics One, Roanoke, VA) under 2–4% isoflurane delivered at a rate of 1 l/min. Unilateral cannulas were implanted in the lateral ventricle using the following coordinates: 1.0 mm lateral to midline, 0.5 mm posterior to bregma, and 2.0 mm ventral to the skull surface. Due to cannulations being carried out by different surgeons, LS injection coordinates differed slightly between experiments. For GLP-1 and Ex9 dose-response experiments the coordinates for unilateral cannulas were: 0.26 mm lateral to midline, 1.0 mm rostral to bregma, and 2.0 mm ventral to the skull surface. For blockade of stress-induced hypophagia with intra-LS Ex9, unilateral cannulas targeting the dorsal subdivision of the LS (dLS) were implanted using the following coordinates: 0.26 mm lateral to midline, 0.35 mm rostral to bregma, and 1.6 mm ventral to the skull surface. For the progressive ratio and licking microstructure experiments, mice were implanted with bilateral cannulas targeting the dLS with the following coordinates: 0.3 mm lateral to midline, 0.8 mm rostral to bregma, and 1.6 mm ventral to the skull surface. In all cases injectors (33G) extended 1.5 mm below the end of the guide cannulas to target the LS or dLS. Correct placement of cannulas within the LS and dLS was verified histologically following behavioral experiments. Injection sites within the boundaries of the LS or dLS were considered correct, and only data from mice with accurate placements were included in analyses (71% hit rate) (Fig 1).
Figure 1.
Representative diagram of LS injection placements based on the atlas of Franklin and Paxinos [37]. Additional subjects’ injection sites were identified in similar locations at points between the anterior-posterior levels displayed here. Carets (^) represent LS placements, while circles represent dorsal LS (dLS) placements. The photomicrograph inset shows a representative injection site. CC = corpus callosum; LV = lateral ventricle; dLS = dorsal lateral septum.
2.3. General methods for behavioral experiments:
Before the start of testing, mice were habituated to all experimental procedures. For habituation to unilateral intra-LS injection procedures, mice received an intra-LS infusion of 0.5 μl sterile 0.9% saline. For habituation to bilateral intra-dLS injection procedures, mice received a 0.25 μl injection of sterile 0.9% saline, delivered to each hemisphere, for a total volume of 0.5 μl distributed across the two dLS sites; injections into each hemisphere were given simultaneously. For both unilateral and bilateral infusions, injectors were then left in place for an additional minute before removal. Body weights were recorded daily, and all drug treatments were separated by a minimum of 48 h.
2.4. Study 1: effects of LS GLP-1R stimulation or blockade on chow intake
Using within-subjects, counterbalanced designs, we determined the effect of LS GLP-1R stimulation or blockade on chow intake. Doses of GLP-1 and Ex9 (American Peptide, Vista, CA) were selected based on previously unpublished preliminary data (see Table 1) determining that they were below threshold for an effect on feeding when delivered to the lateral ventricle (LV). This dose range for GLP-1 is also supported by a recent publication in which 3rd ventricle treatment effects were assessed [33]. In the GLP-1 dose response study, mice (n = 6 males, mean body weight 25±0.1 g) received intra-LS injections of saline vehicle or GLP-1 (0.3 and 1.0 μg) in 0.5 μl of saline 45 min prior to dark onset, at which point chow was returned and subsequent intake was measured. Using the same design, in the Ex9 dose response, mice (n = 9 males, mean body weight 23±0.11 g) received LS injection of saline vehicle or Ex9 (3 and 10 μg) in 0.5 μl of saline. Injection conditions in each study were separated by 48–72 h, with all mice receiving all conditions.
Table 1: Chow intake after LV injections of GLP-1 or Ex9.
These pilot studies were within-subjects counterbalanced design performed male mice (n = 6) in the manner described for Study 1. Repeated measures 1-way ANOVA showed no effects.
| GLP-1 (μg): | 0 | 0.1 | 0.3 | 1 |
| 2-h chow intake mean (SEM) | 0.71 (0.07) | 0.60 (0.13) | 0.62 (0.15) | 0.55 (0.15) |
| Ex9 (μg): | 0 | 3 | 10 | 30 |
| 2-h chow intake mean (SEM) | 0.070 (0.14) | 0.80 (0.20) | 0.64 (0.11) | 0.54 (0.13) |
2.5. Study 2: effects of LS GLP-1R blockade on nutrient preload-induced intake suppression
GLP-1 neurons are known to be activated by large meals, and so in attempt to increase endogenous GLP-1 stimulation of the LS GLP-1R population, we trained a subset of the mice from the Ex9 dose response study (n=7 males, mean body weight 23±0.61 g) to consume a large meal of chocolate Ensure 15 min prior to dark onset. After 20 days of training, mice consumed 2.27 ± 0.08 grams of ensure. On experiment days mice received intra-LS injections of either saline vehicle or Ex9 (3 and 10 μg) in 0.5 μl of saline 30 min prior to dark onset, and then were given access to ensure for the 15 min just before lights out, at which point chow was returned and subsequent intake was measured. Injection conditions in each study were separated by 48–72 h, with all mice receiving all conditions
2.6. Study 3: effect of restraint stress on c-Fos responses of hindbrain PPG neurons
Here we utilized transgenic mice (n=6 males; n=3 females) that express the yellow fluorescent protein reporter (YFP) variant Venus [34] under the control of the glucagon promotor (mGLU-124 line) [35], on a C57Bl/6 background. The presence of YFP identifies preproglucagon (PPG), and therefore identifies GLP-1-producing neurons [36]. On the day of the experiment, chow was removed from mice 1 h prior to restraint stress or no stress conditions. Mice (n=3 males; n=2 females, mean body weight 24±1.87 g) were restrained for 30 min (Res) in a rodent restraint cone and then returned to their home cages for 60 min prior to perfusion. During this same time period, for the no stress condition (no Res), mice (n=3 males; n=1 females, mean body weight 23±1.92 g) were left undisturbed in their home cages. All mice were deeply anesthetized and transcardially perfused with 10mM PBS followed by 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA). Brains were removed, sunk in 30% sucrose, and then frozen in isopentane on dry ice. Coronal cryostat sections (20 μm) through the caudal brainstem were slide-mounted and stored at −80⁰ C to await immunohistochemical processing.
Anatomically matched sections from each mouse that included the AP to the caudal NTS (cNTS) in the brainstem were selected for c-Fos and YFP staining. For immunohistochemical processing, primary and secondary antisera were diluted in phosphate buffer saline containing 0.2% Triton X-100 and 5% normal donkey serum. Slide-mounted sections were washed with 10m Mphosphate buffered saline (PBS) at room temperature and incubated overnight at room temperature with rabbit anti-c-Fos primary antibody (Cell Signaling Technology; catalog # 2250) at 1:1000 and chicken anti-GFP for YFP (Abcam; catalog # ab13970) at 1:5000. Slides were then washed in 10 mM PBS, followed by a 2-h incubation at room temperature with donkey anti-rabbit IgG-Cy5 antibody (Jackson ImmunoResearch; catalog # 711–175-152) used at a 1:500 dilution and donkey anti-chicken IgG-Cy3 antibody (Jackson ImmunoResearch; catalog # 703–165-155) used at a 1:1000 dilution. Slides were washed in 10 mM PBS, then coverslipped using Aqua Polymount (Polysciences, Inc., Warrington, PA) mounting media.
From each mouse, we assessed a series of 12–14 alternating sections through the cNTS ~8.24 mm through 7.32 mm posterior to bregma [37]. Slides were examined with an Olympus BX41 fluorescence microscope and monochromatic digital images were acquired with a Retiga EXI Aqua camera and Q-Capture software (Hunt Optics). Adobe Photoshop CS4 was used to adjust contrast, add color, and merge images of cFos and GFP immunoreactivity. GFP-labeled cells and c-Fos-like immunoreactivity were counted by eye. We then calculated the average number of GFP- labeled cells and c-Fos-positive cell nuclei per section across all sections taken from the cNTS and reticular formation (RF).
2.7. Study 4: effects of dLS GLP-1R blockade on stress-induced hypophagia
In the rat, we have previously reported that GLP-1R blockade in the dorsal subregion of the LS (dLS) significantly attenuates stress-induced hypophagia [27]. Here, we utilized a mixed-model design to assess the feeding response to stress in intra-dLS saline and Ex9-treated mice. This design was utilized so that each animal was exposed to stress only once. dLS-cannulated mice were infused with either saline vehicle (n=5 males, mean body weight 25±0.5 g) or 10 μg Ex9 in 0.5 μl of saline (n=7 males, mean body weight 25±0.6 g). Fifteen mins later, the mice were restrained for 30 min (Res) and then returned to their home cages at dark onset or left undisturbed in their home cages (no Res) for the no stress condition. At dark onset, chow was returned, and subsequent intake was measured. Brain injections were separated by 48–72 h
2.8. Study 5: effects of dLS GLP-1R stimulation or blockade on operant responding
We have previously reported that in the rat, GLP-1R blockade in the dLS, but not elsewhere in the LS, increases motivation for food [13]. Therefore, mice (n=4 males; n=7 females, mean body weight 21±0.82 g) with cannulas targeting the dLS were trained to lever press for 20-mg sucrose pellets (TestDiet, Richmond, IN) on a progressive ratio schedule, where an increasing number of operant responses is required for each successive reinforcement. Here we used a within-subjects, counterbalanced design to determine the effect of dLS GLP-1R stimulation or blockade on operant responding. Training was conducted in operant conditioning chambers (Coulbourn Instruments, Allentown, PA). During training and initial testing, mice were maintained at 85% of their ad libitum body weights. Two levers were present in each chamber; presses on the active lever were reinforced, whereas inactive lever presses were not reinforced. For all training and testing sessions, a cue light was illuminated above the active lever and there was a 5-s timeout after each reinforcement. The positions of the active and inactive levers were counterbalanced across subjects.
Mice were initially trained on a fixed ratio one schedule (FR1), where each response resulted in delivery of one sucrose pellet. FR1 training was conducted for 7 days. Next, mice were moved to a FR3 schedule where three responses were required to achieve one sucrose pellet for 7 days; then mice were moved to a FR5 schedule where five responses were required to achieve one sucrose pellet for 10 days. The daily fixed ratio training sessions were all 1 h in duration. After this training, all mice were switched to a progressive ratio (PR) schedule that followed the algorithm of Richardson and Roberts [38]: 1, 2, 4, 6, 9, 12, 16, 20, 28, 36, 48, etc.,… lever presses for reinforcement. PR sessions ended when the mice failed to press the active lever for 20 min, with a maximum duration of 45 minutes. Mice were then returned to home cages and given their daily chow ration with ad libitum water access. Experimentation began after 12 days of PR training, at which point mice showed stable responding. On testing days, mice (still maintained at 85% of their ad libitum body weight) received bilateral intra-dLS injection of saline vehicle, GLP-1 (1.0 μg), or Ex9 (10 μg) 45 min before the start of the PR session. The dose of drug was evenly divided between the two hemispheres (i.e., 0.5 μg in 0.25 μl of saline on each side for 1.0 μg GLP-1).
After mice had received all three conditions, presented in counterbalanced order, ad libitum chow was returned on the home cages. Mice (n=3 males; n=7 females, mean body weight 21±0.91 g) were given one week to replete during which they continued to receive PR sessions. We then tested PR responding under ad libitum feeding conditions. On test days, bilateral dLS injections of saline vehicle or Ex9 (10 μg) were made 45 min before the PR session.
2.9. Study 6: effects of dLS GLP-1R stimulation or blockade on meal patterns and licking microstructure for sucrose
Utilizing a within-subjects, counterbalanced design, we determined the effect of dLS GLP-1R stimulation or blockade on meal patterns and licking microstructure for sucrose. All training and testing sessions were conducted in custom built lickometers. Each lickometer was equipped with a recessed drinking spout located 2 cm above the grid floor. Licks were detected as the tongue makes contact with the spout, completing a circuit allowing the computer to record the time of each lick. All licks were recorded in the software control program for later analysis. Licking data were then analyzed by a custom macro. A meal was defined as at least three licks, and the criterion for the end of a meal was a pause of 300 or more seconds [39]. Intermeal interval was defined as the time between the last lick of one meal and the first lick of the next. A licking burst, within each meal, was defined as series of licks separated by an interlick interval (ILI) of <1 s [39]. Variables obtained from the custom macro included meal duration, burst duration, within-meal burst number, mean number of licks per burst, and number of licks in the first minute of the meal, size, and average interburst interval. Within-burst interlick interval was calculated as an average of interlick intervals below 250 ms, because this captures more than 95% of interlick intervals [40].
All mice (n=20 males, mean body weight 26±0.43 g) were initially water-deprived (~20 h) and placed in lickometers for 30 min on four consecutive days to acquaint them to licking for fluid (dH20) at a stainless steel spout. Water bottles were returned on the home cages ~30 min after the fourth and final dH20 session. After one day to replete in the home cage, chow was removed for the second phase of training. Mice were gradually reduced to 85% of their ad libitum body weights by rationing their daily chow. For the remainder of the training and testing sessions, mice had ad libitum access to 0.25 M sucrose for 120 min in the lickometer chambers. No other food or water was present in the test chamber. Daily training continued for 12 days. On day 13 mice were habituated to bilateral intra- dLS injection procedures; mice received a 0.25 μl injection of sterile 0.9% saline delivered to each hemisphere, for a total volume of 0.5 μl distributed across the two dLS sites. Injections into each hemisphere were given simultaneously.
After habituation to injection procedures, we then began testing under food restriction (85% ad libitum body weight). On experiment days, mice received an injection of saline vehicle, GLP-1 (1.0 μg) or Ex9 (10 μg) 30 min prior to the test session. The total dose of both GLP-1 and Ex9 was evenly divided between the two hemispheres (i.e. 0.5 μg GLP-1 or 5 μg Ex9 on each side). All mice received all conditions in counterbalanced order with treatments separated by at least 48 h. On days that mice did not receive a brain injection, they still had daily 120-min lickometer sessions. After the test sessions, mice were returned to their home cages and given their daily chow ration.
After mice had received all conditions, presented in counterbalanced order, ad libitum chow was returned on the home cages. Mice were given one week to replete during which they continued to receive daily 120-min lickometer sessions. We then tested under ad libitum feeding conditions. On experimental test days, mice received bilateral dLS injections of saline vehicle, GLP-1 (1.0 μg), or Ex9 (10 μg) 30 min prior to the test session.
Statistical Analysis
Data are reported as mean ± SE. Statistical analyses were conducted using IBM SPSS Statistics 22, and figures were prepared using Graphpad Prism 6 and Adobe Photoshop CS6. Effects were evaluated by t-test or within-subjects one-way ANOVA where appropriate and post-hoc comparisons were adjusted using Holm-Bonferroni. Effects intra-LS Ex9 on stress-induced hypophagia were evaluated using two-way mixed-model ANOVA and Holm-Bonferroni for multiple comparisons test. P values of <0.05 were taken as significant.
3. Results
3.1. Study 1: effects of LS GLP-1R stimulation or blockade on chow intake.
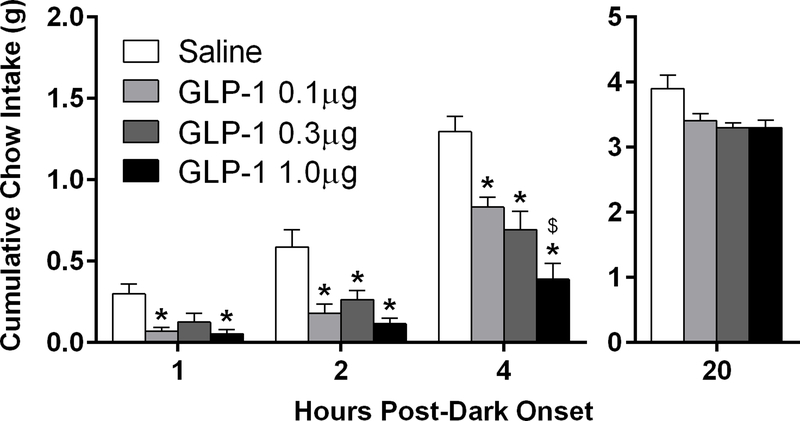
We first assessed whether GLP-1 in the LS is able to reduce chow intake in mice with intra-LS injections of GLP-1, at doses subthreshold for effect when delivered to the lateral ventricle. GLP-1 significantly reduced feeding at 1h [F(3,15) = 6.14, p<0.05], 2h [F(3,15) = 12.68, p<0.0001], and 4h [F(3,15) = 17.78, p<0.0001] with a significant dose-dependent effect at 4h, 0.1 μg vs. 1.0 μg GLP [t(5) = 4.0, p<0.005]; (Fig 2). Despite a main effect of GLP-1 on overnight intake measured at 20 h after dark onset [F(3,15) = 4.50, p<0.05], there were no differences between conditions in pairwise comparisons (Fig 2). There were no effects on body weight.
Figure 2.
Cumulative chow intake after intra-LS injection of GLP-1 is reduced during the first 4 h of the dark phase. Significant effects of intra-LS GLP-1 were seen at 1, 2 and 4 h, *p<0.05 relative to vehicle, $p<0.005 relative to 0.1 μg GLP-1. All data are shown as mean ± SEM. n=6.
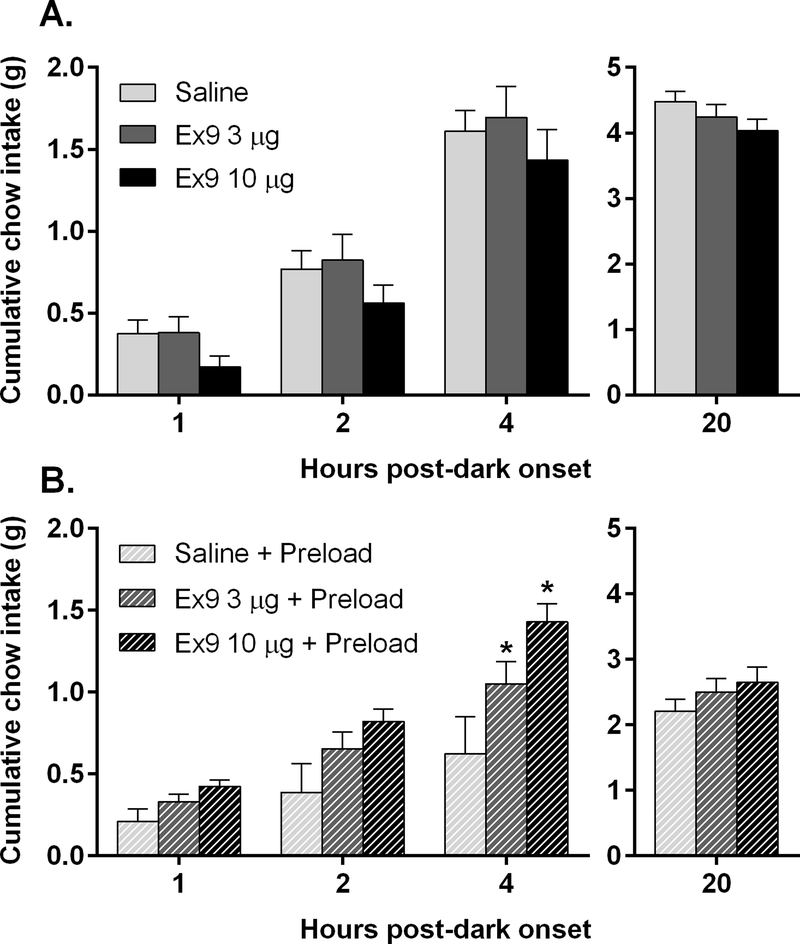
In contrast, despite a trend toward reduced feeding after Ex9, pairwise comparisons between vehicle and each dose of Ex9 revealed no significant differences at any time point (Fig 3A), nor was body weight affected.
Figure 3.
A: Cumulative chow intake is not affected by intra-LS injection of Ex9, n=9. B: After mice (n=7) consumed a large meal of chocolate Ensure, blockade of LS GLP-1R with Ex9 significantly increased chow intake at 4 hr after dark onset, *p<0.05. All data are shown as mean ± SEM.
3.2. Study 2: effects of LS GLP-1R blockade on nutrient preload-induced intake suppression
In contrast with the previous study’s results, blockade of LS GLP-1R with Ex9 significantly increased chow intake at 4h after dark onset [F(2,12) = 5.43, p<0.05] in mice that had consumed a large meal of chocolate Ensure as a preload (Fig 3B). There were no effects on body weight, and there were also no effects on the amount of Ensure consumed.
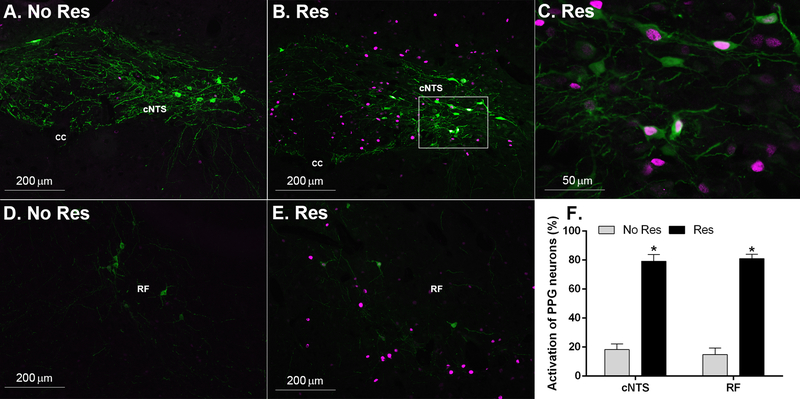
3.3. Study 3: effect of restraint stress on c-Fos responses of hindbrain PPG neurons
Neurons positive for GFP were observed throughout the cNTS. c-Fos-positive cells were found throughout the cNTS and co-localized with numerous GFP-labeled cells (Fig 4). Throughout the cNTS we counted 21.3±3.1 (no Res) and 16.5±2.9 (Res) GFP-labeled cells per section (not significantly different). We counted significantly more c-Fos-positive cell nuclei per section [t(7)=22.23, p<0.00001] throughout the cNTS in the mice that were stressed: 23.7± 2.4 (no Res) and 82.4±1.9 (Res). In the NTS, there were significantly more double labeled cells (both GFP and c-Fos-positive) in mice that were stressed [t(7)=4.74, p<0.01]: 3.8±0.9 (no Res) and 12.6±1.7 (Res). GFP-labeled neurons and c-Fos-positive cell nuclei were also observed in the reticular formation (RF). In the RF, there was no difference in the number of identified GFP-labeled cells per section: 10.0±2.1 (no Res) and 9.0±1.2 (Res). We counted significantly more c-Fos-positive cell nuclei per section in the RF in the mice that were stressed [t(7)=5.51, p<0.001]: 28.5± 5.9 (no Res) and 82.9±8.7 (Res). In the RF, there were significantly more double labeled cells (both GFP and c-Fos-positive) in mice that were stressed [t(7)=5.41, p<0.001]:1.5± 0.5 (no Res) and 7.3±0.9 (Res). Overall, we found significantly more GFP-labeled cells were c-Fos-positive after restraint stress in both the cNTS [t(7)=9.87, p<0.0001] and the RF [t(7)=12.58, p<0.0001] (Fig 4F).
Figure 4.
Effect of stress on c-Fos induction in hindbrain PPG neurons. Representative images of c-Fos induction responses in unstressed (A and D. No Res) and 30-min restraint stressed (B, C, and E. Res) YFP-PPG (mGLU-124 line) mice. C. Higher magnification image taken from the area inside the white box in panel B. F. Significantly more GFP-labeled PPG cells were c-Fos-positive after acute restraint stress in both the cNTS and RF relative to the no stress condition, *p<0.0001. Data are shown as mean ± SEM. n=4 No Res, n=5 Res.
3.4. Study 4: effects of LS GLP-1R blockade on stress-induced hypophagia
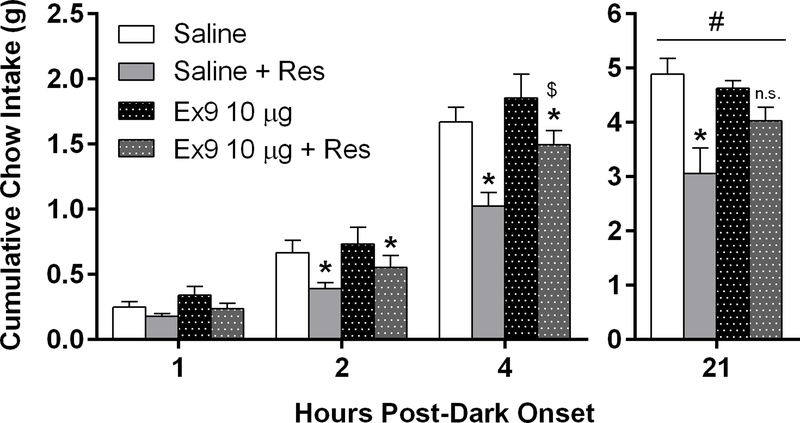
Having established that GLP-1R activation within the LS suppresses feeding and acute restraint stress activates PPG neurons, we assessed whether endogenous release of GLP-1 into the LS contributes to stress-induced hypophagia by blocking GLP-1Rs in the LS prior to exposure to acute restraint stress. Two-way mixed-model ANOVA revealed a main effect of stress at 1h [F(1,10) = 9.36, p<0.05], 2h [F(1,10) = 14.52, p<0.01], and 4h [F(1,10) = 29.65, p<0.0001] post-dark onset. At both 2h and 4h, pairwise comparisons demonstrated that 30 min of restraint stress significantly suppressed chow intake after both intra-dLS saline and Ex9 treatment (p’s<0.05) (Fig 5). At the 4h timepoint, mice in the Ex9 stressed condition ate significantly more than the saline-infused mice in the stressed condition at this timepoint (p<0.01) (Fig 5). For overnight chow intake (21h), two-way mixed-model ANOVA revealed a significant stress x drug interaction [F(1,10) = 6.94, p<0.05]. While acute stress significantly suppressed food intake in the saline group (p<0.01), there was no effect of stress in the Ex9 group (Fig 5).
Figure 5.
At 2 and 4 h post-dark onset, restraint stress (Res) significantly suppressed cumulative intake regardless of intra-LS treatment (*p<0.01 versus respective no stress condition; $p<0.05 relative to saline + Res mice). At 21 h, Ex9 treatment significantly attenuated the effect of stress-induced hypophagia. (#p<0.05 stress x drug interaction). Stress significantly suppressed 21 h intake relative to the saline no stress condition, *p < 0.01. All data are shown as mean ± SEM. n=5 Saline, n=7 Ex9 (10 μg).
3.5. Study 5: effects of dLS GLP-1R stimulation or blockade on operant responding
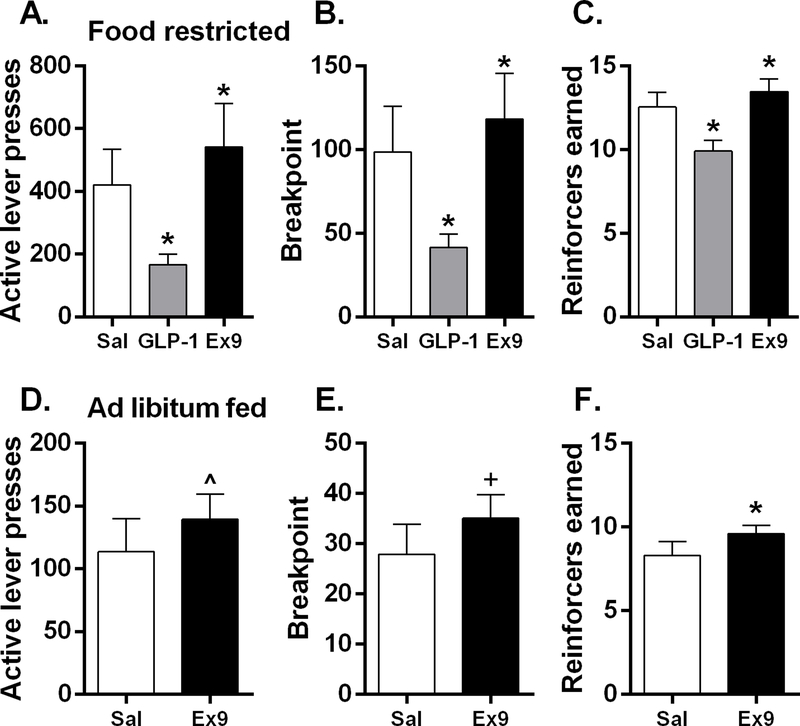
Whether stimulation or blockade of GLP-1R in the dLS is able to affect motivation for food reward was assessed with bilateral intra-LS injections of GLP-1 or Ex9 in mice trained on a PR schedule, where an increasing number of operant responses is required for each successive reinforcement. When mice were maintained at 85% of their ad libitum body weight, there was a significant main effect of drug on active lever presses [F(2,20) = 11.22, p<0.001], breakpoint [F(2,20) = 10.73, p<0.001], and reinforcers earned [F(2,20) = 25.97, p<0.001]. GLP-1 potently suppressed, whereas LS Ex9 significantly increased each of these measures (p’s<0.05) (Fig 6A–C). Under ad libitum feeding conditions, bilateral dLS Ex9 significantly increased reinforcers [t(9)=2.25, p<0.05] earned and tended to increase active lever presses (p=0.10) and breakpoint (p=0.07) (Fig 6D–F).
Figure 6.
In mice (n=4 male, n=7 female) maintained at 85% of ad libitum body weight, Bilateral dLS GLP-1 injection potently suppressed active lever presses (A), breakpoint (B), and reinforcers earned (B). Whereas LS Ex9 significantly increased each of these measures, *p<0.05. Under ad libitum feeding conditions, intra-dLS Ex9 significantly increased reinforcers earned (F) (*p<0.05) and tended to increase active lever presses (D) and breakpoint (E), ^p=0.10, +p=0.07. All data are shown as mean ± SEM.
3.6. Study 6: effects of LS GLP-1R stimulation or blockade on meal patterns and licking microstructure for sucrose
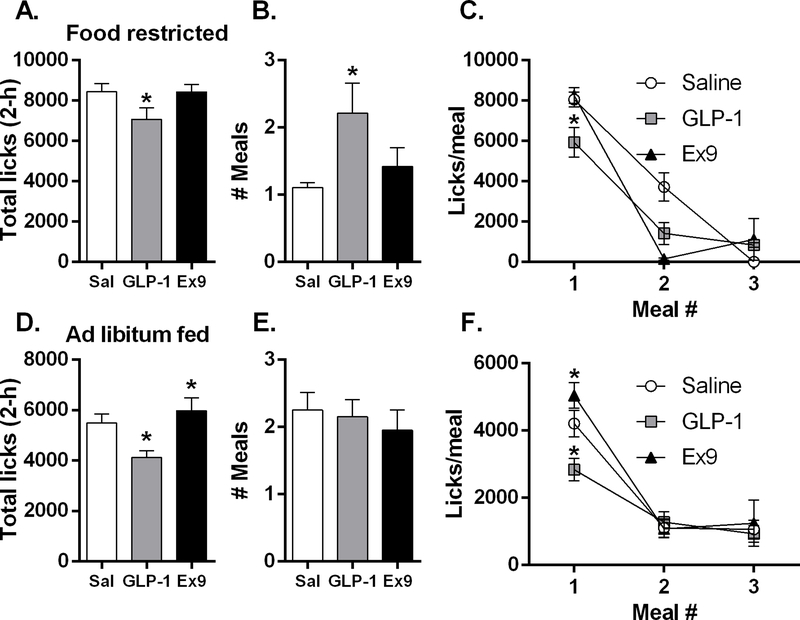
When mice were consuming 0.25 M sucrose under food restriction, there was a main effect of drug for both total number of licks during the 120-min session [F(2,36) = 9.69, p<0.01] and the size of the 1st meal [F(2,36) = 8.21, p<0.01], and planned comparisons revealed that bilateral dLS GLP-1 significantly suppressed these measures (p’s<0.01) (Fig 7A and Fig 7C). There was a main effect of drug for the number of meals consumed during the session [F(2,36) = 8.21, p<0.01], average burst duration (s) during the 1st meal [F(2,36) = 11.86, p<0.001], and for average burst size for the 1st meal (licks/burst) [F(2,36) = 9.94, p<0.001], and planned comparisons revealed that GLP-1 significantly increased all of these variables (p’s<0.01) (Fig 7B and Table 2). In food restricted mice, drug treatment significantly influenced duration of the 1st meal (meal duration (min) [F(2,36) = 4.26, p<0.05], burst number [F(2,36) = 22.09, p<0.0001], average ingestion rate (licks/min) [F(2,36) = 6.59, p<0.01], and 1st min lick rate [F(2,36) = 76.40, p<0.0001] (Table 2). Planned comparisons revealed that intra-dLS GLP-1 suppressed each of these variables (p’s<0.05). In contrast, pairwise comparisons between vehicle and Ex9 revealed no significant differences on any of these variables. There was a main effect of drug on average within-burst interlick interval (ILI) [F(2,36) = 6.04, p<0.05] (Table 2). For the food-deprived conditions, the data file for intra-dLS GLP-1 treatment for one mouse was corrupted, thus data from only 19 of the 20 mice could be used for analysis.
Figure 7.
In food restricted mice (n=19), intra-dLS GLP-1 significantly suppressed both total session licks (A) and the size of the 1st meal (C) while increasing meal number (B), *p<0.05. Under food restriction, 2 of 19 mice in the saline condition, 8 of 19 after GLP-1, and 3 of 19 mice following Ex9 took a second meal of 2 or more bursts; there were no mice that took a third meal after saline, while 3 of 19 after GLP-1 and 2 of 19 mice following Ex9 took a third meal. In ad libitum fed mice (n=20), GLP-1 potently suppressed total session licks (D) and 1st meal size (F) and conversely Ex9 increased both of these variables; there was no effect on meal frequency (E). When fed ad libitum, 13 of 20 mice in the saline condition, 13 of 20 after GLP-1, and 9 of 20 mice following Ex9 took a second meal of 2 or more bursts; there were 9 of 20 mice that took a third meal after saline, while 7 of 20 after GLP-1 and 5 of 19 following Ex9 took a third meal, *p<0.05. All data are shown as mean ± SEM.
Table 2.
Licking variables measured when determining the effects of dLS GLP-1R stimulation or blockade on licking for sucrose in mice maintained at 85% of ad lib body weight. Bolded values are significantly different from the saline condition (p < 0.05).
| Variable | Saline | GLP-1 | Ex9 |
|---|---|---|---|
| Burst duration (s) | 4.8 (0.68) | 6.6 (0.94) | 4.6 (0.58) |
| Burst size (licks/burst) | 32.3 (4.58) | 40.3 (5.57) | 29.6 (3.87) |
| Meal duration (min) | 110.9 (4.76) | 91.5 (8.39) | 112.5 (3.52) |
| Burst number | 324.2 (44.10) | 212.8 (46.05) | 362.2 (49.05) |
| Ingestion rate (licks/min) | 75.3 (5.18) | 63.4 (5.52) | 74.2 (3.59) |
| 1st min lick rate | 266.8 (11.90) | 127.6 (15.81) | 269.1 (11.31) |
| Average within-burst ILI | 144.6 (1.31) | 150.9 (2.34) | 150.0 (2.25) |
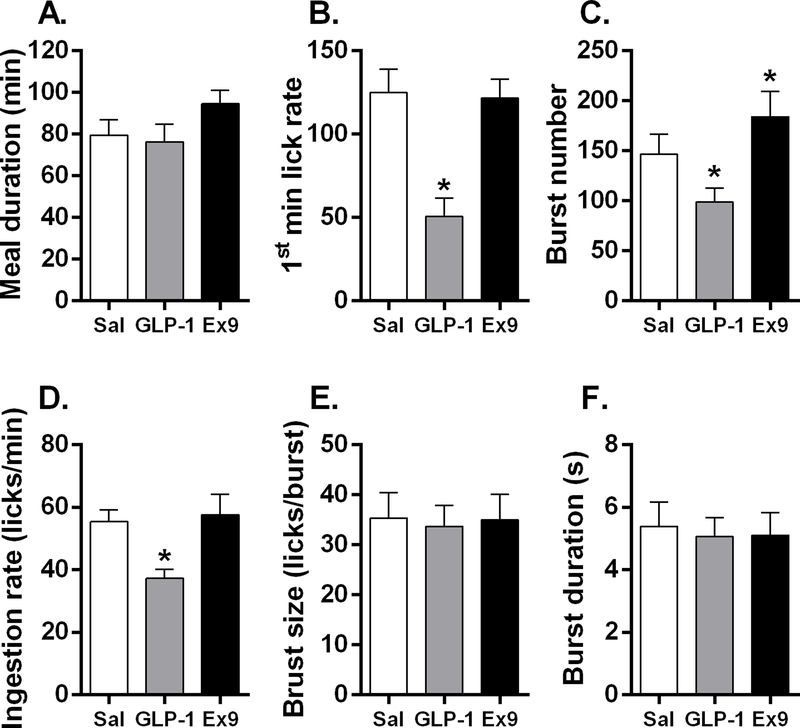
Under ad libitum feeding conditions, there was a main effect of drug on total session licks [F(2, 38) = 25.05, p<0.0001]; planned comparisons revealed that GLP-1 potently suppressed total session licks and conversely Ex9 increased total licks (p’s<0.05) (Fig 7D). Over the course of the 2 h session, mice were able to take several meals. The first meal was the primary meal, with all subsequent meals being much smaller (Fig 7F). Drug treatment significantly influenced 1st meal size [F(2, 38) = 14.53, p<0.0001]; after LS GLP-1, the 1st meal was significantly suppressed and Ex9 significantly increased 1st meal size (p’s<0.05) (Fig 7F). Only 13 of 20 mice took a 2nd meal following both saline and GLP-1 treatments, and 9 took a 2nd meal after Ex9 conditions. After saline, 9 mice took a 3rd meal; following GLP-1, 7 mice took a 3rd meal, and after Ex9, only 5 mice took a 3rd meal. These additional meals were not taken by enough mice to allow statistical analysis. Average number of meals taken during the session was not affected by GLP-1 or Ex9 (Fig. 7E). Because the 1st meal was the only meal that included all mice, we focused our licking microstructure analysis on this meal. There was no difference in 1st meal duration (min) following drug treatments (Fig 8A). There was a main effect of drug treatment on 1st min lick rate [F(2, 38) = 12.36, p<0.001]; planned comparisons revealed that mice licked significantly less in the 1st minute of the session after stimulation of LS GLP-1R (p<0.05) (Fig 8B). During the 1st meal, there was a significant main effect of drug on burst number [F(2, 38) = 6.85, p<0.01]; mice took significantly fewer bursts after LS GLP-1 and more bursts after Ex9 (p’s<0.05) (Fig 8C). Drug treatment also significantly influenced ingestion rate (licks/min) [F(2, 38) = 13.17, p<0.001] (Fig 8D). Planned comparisons revealed that bilateral dLS GLP-1 significantly suppressed average ingestion rate (licks/min) during meal 1 (p<0.05), but Ex9 did not affect this measure (Fig 8D). There was no difference in burst size (licks/burst) (Fig 8E), burst duration (s) (Fig 8F), or average within-burst interlick interval (ILI) during the 1st meal (Sal: 149.7±2.47, GLP-1: 147.9±1.69, Ex9: 145.3±1.76) after drug treatment. There were no effects on body weight.
Figure 8.
In ad libitum fed mice (n=20), meal duration was not affected by drug treatment (A). Intra-dLS GLP-1 suppressed lick rate during the first minute of the meal (B). Burst number was significantly suppressed after GLP-1 and increased after Ex9 (C). Ingestion rate was significantly decreased following dLS GLP-1 (D). There was no effect of drug treatment on burst size (E) or burst duration (F)., *p<0.05. All data are shown as mean ± SEM.
4. Discussion
Our behavioral data provide direct evidence that LS GLP-1R are involved in coordinating feeding behavior in mice. Pharmacological activation of LS GLP-1R, at doses that were ineffective when delivered to the LV, potently reduced chow intake. Surprisingly, intra-LS injection of LV-subthreshold doses of the GLP-1R antagonist Ex9 did not affect ad libitum chow intake, suggesting that in mice, normal, ad libitum feeding is not controlled by endogenous GLP-1 in the LS. Yet our findings demonstrate that endogenous release of GLP-1 into the LS does in fact play a role in suppressing chow intake after large meals and following restraint stress in mice. Furthermore, our data also show that endogenous dLS GLP-1R stimulation suppresses motivation and licking for sucrose. While we have previously demonstrated that LS GLP-1 plays a role in the control of feeding behavior in rats, this is the first demonstration for a role for this pathway in feeding behavior in mice. Overall our data suggest a similar role for LS GLP-1 in rats and mice, however, we do find important species differences.
We predicted an increase in chow intake following LS GLP-1R blockade, based on what we have previously seen in the rat, but here we found that in the mouse, Ex9 did not affect dark onset ad libitum chow intake at any timepoint. This lack of effect led us to hypothesize that under normal conditions of ad libitum chow feeding, the GLP-1 neuronal input to the LS is not sufficiently activated to cause substantial endogenous GLP-1R stimulation that our Ex9 injection would block. To explore this possibility, we trained mice to consume a large nutrient preload, expected to activate GLP-1 neurons and promote the release of endogenous GLP-1 in the LS at the time of our drug manipulation. Under these conditions, blockade of LS GLP-1 receptors did significantly increase chow intake, suggesting that endogenous GLP-1 in this brain area acts to suppress feeding following a large meal.
Acute restraint stress is known to activate GLP-1 neurons in rats, and food intake is significantly suppressed after that stressor [2,27]. Consistent with those findings, our data here show that in mice, PPG neurons are potently activated in response to 30 minutes of restraint stress; the majority of PPG cells within both the cNTS and RF were activated after restraint stress in mice. Previous studies using mice have demonstrated that GLP-1R in both the PVN and the bed nucleus of the stria terminalis (BNST) are critical for a number of physiological responses to stress [14,22]. Here, we found that blockade of dLS GLP-1R attenuated the suppression of chow intake following restraint stress in mice, consistent with our previous results in rats [27]. Together, our behavioral findings suggest that the GLP-1 pathway to the LS is activated both by large meals and by stress, and that endogenous GLP-1 in the LS acts to suppress feeding after either stimulus. Our findings here are consistent with our recent report in which we found that selective ablation of NTS PPG neurons in mice had no effect on ab libitum chow intake. However, following a large meal, both ablation or acute inhibition of PPG neurons increased food intake [11]. Furthermore, we demonstrated that stress-induced hypophagia requires PPG neurons, suggesting that in mice, PPG neurons play a role in suppressing feeding after a large meal and following restraint stress [11].
The LS was identified in Olds and Milner’s classic studies as an important site for motivation, [41] and in the rat, we have shown that GLP-1R in the dorsal subregion of the LS affect motivation for food [13]. We asked if the same is true for mice and found that pharmacologic activation of dLS GLP-1R potently suppressed active lever presses, breakpoint, and reinforcers earned in the operant responding progressive ratio task, whereas blockade of these receptors significantly increased performance on these measures. We found that both the agonist and antagonist effects on motivation for sucrose were still evident, though smaller in magnitude when mice were maintained on ad libitum chow access relative to when they were tested under chronic food restriction conditions. Together these findings suggest that endogenous GLP-1R activity in the dLS plays a significant role in motivation for food in mice.
We previously reported that in the rat, endogenous GLP-1 in the LS suppresses intake of 0.25 M sucrose solution [13]. To examine in what manner dLS GLP-1R affect sucrose intake in mice, we asked how pharmacologic stimulation or blockade of these receptors influences meal patterns and licking microstructure for sucrose. Conducting meal pattern analyses can offer insight into the behavioral mechanisms of the feeding effects of exogenous and endogenous GLP-1 in the LS. Total intake is the product of the number of meals taken and the size of those meals, and GLP-1 and Ex9 in the LS could be acting on either or both of those variables. Under chronic food restriction, GLP-1 potently suppressed total session licks, whereas there was no effect of Ex9. The lack of effect following dLS Ex9 is unsurprising because in this food-restricted state, mice were emitting over 8000 licks in the session after saline treatment, and it seems unlikely that Ex9 could increase licking above this already elevated baseline. We next asked if dLS GLP-1R stimulation or blockade would influence meal patterns and licking for sucrose after mice were returned to ad libitum feeding conditions. Again, we found that dLS GLP-1 significantly suppressed total session licks, and in this experiment, during which baseline licking was reduced compared with licking under chronic food restriction conditions, we found that Ex9 significantly increased total licks. Over the course of the 2-h session, mice usually took several meals. Whether food restricted or maintained on ad libitum chow, the first sucrose meal is the primary and largest meal, with all subsequent meals being much smaller. In the experiment conducted during food restriction, dLS GLP-1 reduced first meal size, and increased the number of meals taken in the session, which may be an attempt by hungry mice to compensate for the reduced size of the first meal. In the experiment conducted under ad libitum conditions, dLS GLP-1 suppressed and, conversely, Ex9 significantly increased first meal size. Here, neither drug treatment influenced meal frequency. These findings support the hypothesis that the dLS GLP-1R population plays a physiologic role in promoting satiation under these experimental conditions. Here mice were consuming a large amount of sucrose in a short session, which likely promotes the release of endogenous GLP-1, much like the Ensure nutrient preload we used in the dark phase chow intake experiment.
Detailed examination of the pattern of licking within the first meal provides further information about how dLS GLP-1R stimulation and blockade influence sucrose intake. Because the first meal was the primary meal and the only meal that included all mice, we focused our microstructural analyses on this meal for both food restricted and ad libitum feeding conditions. The initial lick rate (1st min lick rate) reflects the pre-ingestive evaluation of the tastant, as it is typically greater for more palatable solutions (i.e., higher concentrations of sucrose) and occurs prior to the accumulation of nutrients in the gut [39,42]. Here we found that under both restricted and fed feeding states, mice licked significantly less in the 1st minute of meal 1 after stimulation of dLS GLP-1R while Ex9 had no effect on this variable. Burst size represents the average number of licks occurring within each burst of licking and is also thought to reflect palatability of the ingested tastant, but this was not affected by either drug when mice had ad libitum chow access, while GLP-1 increased burst size under food restriction. Licking burst number, or the frequency of initiation of a new bout of licking, is often taken to reflect the potency of post-ingestive negative feedback [39,40]. Here we found that during the 1st meal, mice took significantly fewer bursts following dLS GLP-1 under both feeding conditions. When mice had ad libitum chow access, they took significantly more bursts after Ex9. Because LS GLP-1’s effects were evident during the 1st min of meal 1 and the reduction in sucrose intake during the 1st meal was primarily due to reduction in burst number, it is possible that GLP-1R stimulation suppresses sucrose intake by reducing the motivational value (i.e. palatability) as well as by enhancing post-ingestive negative feedback signals that act to suppress licking behavior. A suppression in motivation would be consistent with the effects observed in the progressive ratio experiments. On the contrary, Ex9 had no effect early in the meal, suggesting that endogenous LS GLP-1R stimulation likely does not influence palatability. Nonetheless, blockade of LS GLP-1R increased sucrose intake, primarily due to an increase in burst number, suggesting that Ex9 may increase meal size by attenuating post-ingestive negative feedback signals that would normally suppress licking during the first meal. This finding is consistent with our data that endogenous GLP-1 in the LS acts to suppress feeding after a large meal.
The lack of LS Ex9 effect on ad libitum dark phase chow intake seems inconsistent with the significant effects of LS Ex9 on licking for sucrose solution and lever pressing for sucrose in the PR task. It is possible that endogenous GLP-1 in the LS plays a more significant role in feeding for sucrose or for highly palatable food than for standard chow. However, we suggest that our demonstration that LS Ex9 could increase chow intake after restraint stress or nutrient preload renders this explanation less likely. Other differences in the test paradigm likely play a role. In the licking and PR experiments, mice received extensive training in non-home cage test chambers, and these conditioned eating situations, which also involved reward and palatability, may promote GLP-1 release in the LS to an extent that daily dark phase onset does not. Further research will be required to fully understand the conditions under which endogenous GLP-1 is most relevant.
In conclusion, our behavioral data show that exogenous GLP-1 in the LS suppresses feeding in mice, similar to its effects in rats. However, in striking contrast with the rat data, we found that endogenous GLP-1 in the LS does not seem to contribute to normal dark cycle ad libitum chow intake in mice [13]. Instead, we see an effect of LS GLP-1R blockade under other circumstances: after a large nutrient load, after restraint stress, and when mice are licking or lever-pressing for sucrose. These data provide a useful foundation for continuing to examine this pathway using mouse models and suggest that while endogenous GLP-1 action in the LS influences feeding in both species, the conditions under which these effects are most robust differs between mice and rats.
Acknowledgements
We thank Christine Jackson for technical assistance during portions of the behavioral experiments.
Grants
This work was funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIH R01-DK095757) to D.L.W. and (NIH F31-DK115102) to S.J.T., as well as a UCL Graduate Research Studentship to M.K.H. and a Bogue Travel Fellowship to M.K.H. and a MRC-UK grant (MR/N02589X/1) to S.T. The NIH program training grant T32 MH093311 (to P.K. Keel and L.A. Eckel) supported C.B.M. Research in the Reimann/Gribble laboratories is funded by the Wellcome Trust [106262/Z/14/Z, 106263/Z/14/Z] and MRC-UK [MRC_MC_UU_12012/3].
Footnotes
Disclosures
The authors have no conflicts of interest to declare.
References
- [1].Larsen PJ, Tang-Christensen M, Holst JJ, Ørskov C, Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem, Neuroscience. 77 (1997) 257–270. doi: 10.1016/S0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- [2].Maniscalco JW, Zheng H, Gordon PJ, Rinaman L, Negative Energy Balance Blocks Neural and Behavioral Responses to Acute Stress by “Silencing” Central Glucagon-Like Peptide 1 Signaling in Rats, J. Neurosci. 35 (2015) 10701–10714. doi: 10.1523/JNEUROSCI.3464-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gaykema RP, Newmyer BA, Ottolini M, Raje V, Warthen DM, Lambeth PS, Niccum M, Yao T, Huang Y, Schulman IG, Harris TE, Patel MK, Williams KW, Scott MM, Activation of murine pre-proglucagon-producing neurons reduces food intake and body weight, J. Clin. Invest 127 (2017) 1031–1045. doi: 10.1172/JCI81335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rinaman L, Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure., Brain Res. 1350 (2010) 18–34. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, Trapp S, Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain, Mol. Metab 4 (2015) 718–731. doi: 10.1016/j.molmet.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S, Preproglucagon neurons project widely to autonomic control areas in the mouse brain, Neuroscience. 180 (2011) 111–121. doi: 10.1016/j.neuroscience.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Barrera JG, Jones KR, Herman JP, a D’Alessio D, Woods SC, Seeley RJ, Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function., J. Neurosci 31 (2011) 3904–13. doi: 10.1523/JNEUROSCI.2212-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CMB, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JPH, Smith DM, Ghatei MA, Herbert J, Bloom SR, A role for glucagon-like peptide-1 in the central regulation of feeding, Nature. 379 (1996) 69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- [9].Skibicka KP, The central GLP-1: Implications for food and drug reward, Front. Neurosci 7 (2013) 181. doi: 10.3389/fnins.2013.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kanoski SE, Hayes MR, Skibicka KP, GLP-1 and weight loss: unraveling the diverse neural circuitry, Am. J. Physiol. - Regul. Integr. Comp. Physiol 310 (2016) R885–R895. doi: 10.1152/ajpregu.00520.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Holt MK, Richards JE, Cook DR, Brierley DI, Williams DL, Reimann F, Gribble FM, Trapp S, Preproglucagon Neurons in the Nucleus of the Solitary Tract are the Main Source of Brain GLP-1, Mediate Stress-Induced Hypophagia, and Limit Unusually Large Intakes of Food., Diabetes. 68 (2018) db180729. doi: 10.2337/db18-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Williams DL, Neural integration of satiation and food reward: Role of GLP-1 and orexin pathways, Physiol. Behav 136 (2014) 194–199. doi: 10.1016/j.physbeh.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Terrill SJ, Jackson CM, Greene HE, Lilly N, Maske CB, Vallejo S, Williams DL, Role of lateral septum glucagon-like peptide 1 receptors in food intake, Am. J. Physiol. - Regul. Integr. Comp. Physiol 311 (2016) R124–R132. doi: 10.1152/ajpregu.00460.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Williams DL, Lilly NA, Edwards IJ, Yao P, Richards JE, Trapp S, GLP-1 action in the mouse bed nucleus of the stria terminalis, Neuropharmacology. 131 (2018) 83–95. doi: 10.1016/j.neuropharm.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Alhadeff AL, Mergler BD, Zimmer DJ, Turner CA, Reiner DJ, Schmidt HD, Grill HJ, Hayes MR, Endogenous Glucagon-like Peptide-1 Receptor Signaling in the Nucleus Tractus Solitarius is Required for Food Intake Control, Neuropsychopharmacology. 42 (2017) 1471–1479. doi: 10.1038/npp.2016.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Alhadeff AL, Rupprecht LE, Hayes MR, GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake, Endocrinology. 153 (2012) 647–658. doi: 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dossat AM, Lilly N, Kay K, Williams DL, Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake., J. Neurosci 31 (2011) 14453–7. doi: 10.1523/JNEUROSCI.3262-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Richard JE, Farkas I, Anesten F, Anderberg RH, Dickson SL, Gribble FM, Reimann F, Jansson JO, Liposits Z, Skibicka KP, GLP-1 receptor stimulation of the lateral parabrachial nucleus reduces food intake: Neuroanatomical, electrophysiological, and behavioral evidence, Endocrinology. 155 (2014) 4356–4367. doi: 10.1210/en.2014-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hsu TM, Hahn JD, Konanur VR, Lam A, Kanoski SE, Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission, Neuropsychopharmacology. 40 (2015) 327–337. doi: 10.1038/npp.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ghosal S, Myers B, Herman JP, Role of central glucagon-like peptide-1 in stress regulation, Physiol. Behav 122 (2013) 201–207. doi: 10.1016/j.physbeh.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kinzig KP, a D’Alessio D, Herman JP, Sakai RR, Vahl TP, Figueiredo HF, Murphy EK, Seeley RJ, CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors., J. Neurosci 23 (2003) 6163–6170. doi:23/15/6163 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ghosal S, Packard AEB, Mahbod P, McKlveen JM, Seeley RJ, Myers B, Ulrich-Lai Y, Smith EP, D’Alessio DA, Herman JP, Disruption of Glucagon-Like Peptide 1 Signaling in Sim1 Neurons Reduces Physiological and Behavioral Reactivity to Acute and Chronic Stress, J. Neurosci 37 (2017) 184–193. doi: 10.1523/JNEUROSCI.1104-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Holt MK, Trapp S, The physiological role of the brain GLP-1 system in stress., Cogent Biol 2 (2016) 1229086. doi: 10.1080/23312025.2016.1229086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Maniscalco JW, Kreisler AD, Rinaman L, Satiation and Stress-Induced Hypophagia: Examining the Role of Hindbrain Neurons Expressing Prolactin-Releasing Peptide or Glucagon-Like Peptide 1, Front. Neurosci 6 (2013) 1–17. doi: 10.3389/fnins.2012.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Anthony TE, Dee N, Bernard A, Lerchner W, Heintz N, Anderson DJ, Control of stress-induced persistent anxiety by an extra-amygdala septohypothalamic circuit, Cell. 156 (2014) 522–536. doi: 10.1016/j.cell.2013.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Singewald GM, Rjabokon A, Singewald N, Ebner K, The modulatory role of the lateral septum on neuroendocrine and behavioral stress responses., Neuropsychopharmacology. 36 (2011) 793–804. doi: 10.1038/npp.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Terrill SJ, Maske CB, Williams DL, Endogenous GLP-1 in lateral septum contributes to stress-induced hypophagia, Physiol. Behav 192 (2018) 17–22. doi: 10.1016/j.physbeh.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lachey JL, D’Alessio DA, Rinaman L, Elmquist JK, Drucker DJ, Seeley RJ, The role of central glucagon-like peptide-1 in mediating the effects of visceral illness: Differential effects in rats and mice, Endocrinology. 146 (2005) 458–462. doi: 10.1210/en.2004-0419. [DOI] [PubMed] [Google Scholar]
- [29].Huo L, Gamber KM, Grill HJ, Bjørbæk C, Divergent leptin signaling in proglucagon neurons of the nucleus of the solitary tract in mice and rats, Endocrinology. 149 (2008) 492–497. doi: 10.1210/en.2007-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sandoval DA, D’Alessio DA, Physiology of Proglucagon Peptides: Role of Glucagon and GLP-1 in Health and Disease, Physiol. Rev 95 (2015) 513–548. doi: 10.1152/physrev.00013.2014. [DOI] [PubMed] [Google Scholar]
- [31].Jessen L, Smith EP, Ulrich-Lai Y, Herman JP, Seeley RJ, Sandoval D, D’Alessio D, Central nervous system GLP-1 receptors regulate islet hormone secretion and glucose homeostasis in male rats, Endocrinology. 158 (2017) 2124–2133. doi: 10.1210/en.2016-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Llewellyn-Smith IJ, Gnanamanickam GJE, Reimann F, Gribble FM, Trapp S, Preproglucagon (PPG) neurons innervate neurochemicallyidentified autonomic neurons in the mouse brainstem, Neuroscience. 229 (2013) 130–143. doi: 10.1016/J.NEUROSCIENCE.2012.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cho Y-M, Choi HJ, Lee YH, Jang B-T, NamKoong C, Kim MS, Central administration of GLP-1 and GIP decreases feeding in mice, Biochem. Biophys. Res. Commun 490 (2017) 247–252. doi: 10.1016/j.bbrc.2017.06.031. [DOI] [PubMed] [Google Scholar]
- [34].Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A, A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications., Nat. Biotechnol 20 (2002) 87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- [35].Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM, Glucose Sensing in L Cells: A Primary Cell Study, Cell Metab. 8 (2008) 532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Thiebaud N, Llewellyn-Smith IJ, Gribble F, Reimann F, Trapp S, Fadool DA, The incretin hormone glucagon-like peptide 1 increases mitral cell excitability by decreasing conductance of a voltage-dependent potassium channel, J. Physiol 594 (2016) 2607–2628. doi: 10.1113/JP272322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Franklin KBJ, Paxinos G, The mouse brain in stereotaxic coordinates, 3rd ed., Boston, 2008. [Google Scholar]
- [38].Richardson NR, Roberts DC, Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy., J. Neurosci. Methods 66 (1996) 1–11. [DOI] [PubMed] [Google Scholar]
- [39].Spector AC, Klumpp PA, Kaplan JM, Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat, Behav. Neurosci 112 (1998) 678–694. doi: 10.1037/0735-7044.112.3.678. [DOI] [PubMed] [Google Scholar]
- [40].Davis JD, Gerard SP, Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions, Behav. Neurosci 106 (1992) 217–228. [PubMed] [Google Scholar]
- [41].Olds J, Milner P, Positive Reinforcement Produced By Electrical Stimulation of Septal Area and Other Regions of Rat Brain, J. Comp. Physiol. Psychol 47 (1954) 419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- [42].Davis JD, The effectiveness of some sugars in stimulating licking behavior in the rat, Physiol. Behav 11 (1973) 39–45. doi: 10.1016/0031-9384(73)90120-0. [DOI] [PubMed] [Google Scholar]