Abstract
Background
Chronic suppurative otitis media (CSOM), sometimes referred to as chronic otitis media (COM), is a chronic inflammation and infection of the middle ear and mastoid cavity, characterised by ear discharge (otorrhoea) through a perforated tympanic membrane. The predominant symptoms of CSOM are ear discharge and hearing loss.
Topical antiseptics, one of the possible treatments for CSOM, inhibit the micro‐organisms that may be responsible for the infection. Antiseptics can be used alone or in addition to other treatments for CSOM, such as antibiotics or ear cleaning (aural toileting). Antiseptics or their application can cause irritation of the skin of the outer ear, manifesting as discomfort, pain or itching. Some antiseptics (such as alcohol) may have the potential to be toxic to the inner ear (ototoxicity), with a possible increased risk of causing sensorineural hearing loss, dizziness or tinnitus.
Objectives
To assess the effects of topical antiseptics for people with chronic suppurative otitis media.
Search methods
The Cochrane ENT Information Specialist searched the Cochrane ENT Register; Central Register of Controlled Trials (CENTRAL; 2019, Issue 4, via the Cochrane Register of Studies); Ovid MEDLINE; Ovid Embase; CINAHL; Web of Science; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 1 April 2019.
Selection criteria
We included randomised controlled trials (RCTs) with at least a one‐week follow‐up involving patients (adults and children) who had chronic ear discharge of unknown cause or CSOM, where the ear discharge had continued for more than two weeks.
The interventions were any single, or combination of, topical antiseptic agent of any class, applied directly into the ear canal as ear drops, powders or irrigations, or as part of an aural toileting procedure.
Two main comparisons were topical antiseptics compared to: a) placebo or no intervention; and b) another topical antiseptic (e.g. topical antiseptic A versus topical antiseptic B).
Within each comparison we separated studies where both groups of patients had received topical antiseptics a) alone or with aural toileting and b) on top of antibiotic treatment.
Data collection and analysis
We used the standard Cochrane methodological procedures. We used GRADE to assess the certainty of the evidence for each outcome.
Our primary outcomes were: resolution of ear discharge or 'dry ear' (whether otoscopically confirmed or not), measured at between one week and up to two weeks, two weeks to up to four weeks, and after four weeks; health‐related quality of life using a validated instrument; ear pain (otalgia) or discomfort or local irritation. Secondary outcomes included hearing, serious complications and ototoxicity measured in several ways.
Main results
Five studies were included. It was not possible to calculate the total number of participants as two studies only provided the number of ears included in the study.
A. Topical antiseptic (boric acid) versus placebo or no treatment (all patients had aural toileting)
Three studies compared topical antiseptics with no treatment, with one study reporting results we could use (254 children; cluster‐RCT). This compared the instillation of boric acid in alcohol drops versus no ear drops for one month (both arms used daily dry mopping). We made adjustments to the data to account for the intra‐cluster correlation. The very low certainty of the evidence means it is uncertain whether or not treatment with an antiseptic leads to an increase in resolution of ear discharge at both four weeks (risk ratio (RR) 1.94, 95% confidence interval (CI) 1.20 to 3.16; 174 participants) and at three to four months (RR 1.73, 95% CI 1.21 to 2.47; 180 participants). This study narratively described no differences in suspected ototoxicity or hearing outcomes between the arms (very low‐certainty evidence). None of the studies reported results for health‐related quality of life, adverse effects or serious complications.
B. Topical antiseptic A versus topical antiseptic B
Two studies compared different antiseptics but only one (93 participants), comparing a single instillation of boric acid powder with daily acetic acid ear drops, provided any information for this comparison. The very low certainty of the evidence means that it is uncertain whether more patients had resolution of ear discharge with boric acid powder compared to acetic acid at four weeks (RR 2.61, 95% CI 1.51 to 4.53; 93 participants), or whether there was a difference between the arms with respect to ear discomfort due to the low number of reported events (RR 0.10, 95% CI 0.01 to 1.81; 93 participants). Narratively, the study reported no difference in hearing outcomes between the groups. None of the included studies reported any of the other primary or secondary outcomes.
Authors' conclusions
Due to paucity of the evidence and the very low certainty of that which is available the effectiveness and safety profile of antiseptics in the treatment of CSOM is uncertain.
Plain language summary
Topical antiseptics for chronic suppurative otitis media
What is the aim of this review?
The aim of this Cochrane Review is to find out whether topical antiseptics are effective compared to placebo or no treatment in treating chronic suppurative otitis media (CSOM). The review also looked to see whether one topical antiseptic was more effective than the others. The Cochrane Review authors collected and analysed all relevant studies to answer this question.
Key messages
Due to a lack of trials and the very low certainty of the evidence that is available, the effectiveness of antiseptics in the treatment of CSOM is unclear. Adverse effects were not well reported in the studies.
What was studied in the review?
Chronic suppurative otitis media is a long‐term (chronic) swelling and infection of the middle ear, with ear discharge (otorrhoea) through a perforated tympanic membrane (eardrum). The main symptoms are ear discharge and hearing loss.
Topical antiseptics (antiseptics put directly into the ear as ear drops or as a powder) are sometimes used as a treatment for CSOM. Topical antiseptics kill or stop the growth of the micro‐organisms that may be responsible for the infection. Topical antiseptics can be used on their own or added to other treatments for CSOM, such as antibiotics or ear cleaning (aural toileting). Applying topical antiseptics can cause irritation of the skin within the outer ear, which may cause discomfort, pain or itching. Some antiseptics (such as alcohol) can be toxic to the inner ear (ototoxicity), which means they may cause irreparable hearing loss (sensorineural), dizziness or ringing in the ear (tinnitus).
What are the main results of the review?
We found five studies but it was not possible to tell how many participants were included as two studies only reported how many ears were treated. Different types of antiseptics were used: some used ear drops and some used powders.
Topical antiseptic (boric acid) versus no treatment (with a background treatment of ear cleaning)
One study (254 children) compared using boric acid in alcohol ear drops with no topical antiseptic treatment. All children had their ears cleaned daily using cotton wool sticks (dry mopping). The very low certainty of the evidence means that it is unclear whether or not treatment with an antiseptic leads to an increase in resolution of ear discharge at four weeks or at three to four months compared with the group who did not receive any topical antiseptic. The study reported that there was no difference between the two treatment groups in hearing or suspected ototoxicity. There was no information for any of the other outcomes.
Comparison of topical antiseptic agents
One study (93 participants) compared a single dose of boric acid powder with daily acetic acid ear drops. The very low certainty of the evidence means that it is unclear if boric acid leads to an increase in resolution of ear discharge compared to daily acetic acid drops at four weeks. It was uncertain if one group had more ear discomfort than the other group. There was no information for any of the other outcomes.
How up to date is the review?
The evidence is up to date to April 2019.
Summary of findings
Summary of findings for the main comparison. Topical antiseptic compared to no treatment for chronic suppurative otitis media.
| Topical antiseptic compared to no treatment for chronic suppurative otitis media | |||||||
| Patient or population: chronic suppurative otitis media Setting: community setting, Malawi Intervention: topical antiseptic (boric acid in alcohol ear drops and daily dry mopping) Comparison: no topical antiseptic (daily dry mopping alone) | |||||||
| Outcomes | Number of participants (studies) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| Without topical antiseptic | With topical antiseptic | Difference | |||||
| Resolution of ear discharge (between 1 week and up to 2 weeks) ‐ not reported | — | — | — | — | — | — | No study reported this outcome at this time point |
| Resolution of ear discharge (4 weeks or more) Follow‐up: 3 to 4 months |
180 (1 RCT) |
RR 1.73 (1.21 to 2.47) | Study population | ⊕⊝⊝⊝ very low1 | Boric acid in alcohol ear drops with dry mopping may help resolve ear discharge at 3 to 4 months, compared with dry mopping alone, but we are very uncertain | ||
| 31.5% | 54.5% (38.1 to 77.9) | 23.0% more (6.6 more to 46.3 more) | |||||
| Health‐related quality of life ‐ not reported | — | — | — | — | — | — | No study reported this outcome |
| Ear pain (otalgia) or discomfort or local irritation ‐ not reported | — | — | — | — | — | — | No study reported this outcome |
| Hearing Follow‐up: 4 months | 180 (1 RCT) |
One study stated that "there was no deterioration of hearing in groups 2 [boric acid] ... as compared to group 1 [no additional treatment]." | very low2 | We are very uncertain whether hearing is improved when boric acid in alcohol is used with dry mopping | |||
| Serious complications ‐ not reported | — | — | — | — | — | — | No study reported that any participant died or had any intracranial or extracranial complications |
| Suspected ototoxicity | 180 (1 RCT) |
One study stated that "there was no deterioration of hearing in groups 2 [boric acid] ...as compared to group 1 [no additional treatment]. Thus, no signs of ototoxicity could be found." | very low3 | — | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||||
1Downgraded to very low‐certainty: downgraded by two levels due to study limitations (risk of bias) because of concerns about randomisation, blinding, attrition bias and selective reporting. Downgraded by one level due to imprecision as there was one small study (180 participants) with a confidence interval crossing the line of minimally important benefit.
2Downgraded to very low‐certainty: downgraded by two levels due to study limitations (risk of bias) because of concerns about randomisation, blinding, attrition bias and selective reporting. Downgraded by one level due to imprecision as numeric results were not presented for this outcome.
3Downgraded to very low‐certainty: downgraded by two levels due to study limitations (risk of bias) because of concerns about randomisation, blinding, attrition bias and selective reporting. Downgraded by one level due to indirectness of the outcome: only hearing appears to have been considered for the outcome of ototoxicity and not other factors such as tinnitus or balance problems. Downgraded by one level due to imprecision, as numeric results were not presented for this outcome.
Background
This is one of a suite of Cochrane Reviews evaluating the comparative effectiveness of non‐surgical interventions for chronic suppurative otitis media using topical antibiotics, topical antibiotics with corticosteroids, systemic antibiotics, topical antiseptics and aural toileting (ear cleaning) methods (Table 2).
1. Table of Cochrane Reviews.
| Topical antibiotics with steroids | Topical antibiotics | Systemic antibiotics | Topical antiseptics | Aural toileting (ear cleaning) | |
| Topical antibiotics with steroids | Review CSOM‐4 | ||||
| Topical antibiotics | Review CSOM‐4 | Review CSOM‐1 | |||
| Systemic antibiotics | Review CSOM‐4 | Review CSOM‐3 | Review CSOM‐2 | ||
| Topical antiseptics | Review CSOM‐4 | Review CSOM‐6 | Review CSOM‐6 | Review CSOM‐5 | |
| Aural toileting | Review CSOM‐4 | Not reviewed | Not reviewed | Not reviewed | Review CSOM‐7 |
| Placebo (or no intervention) | Review CSOM‐4 | Review CSOM‐1 | Review CSOM‐2 | Review CSOM‐5 | Review CSOM‐7 |
CSOM‐1: Topical antibiotics for chronic suppurative otitis media (Brennan‐Jones 2018a).
CSOM‐2: Systemic antibiotics for chronic suppurative otitis media (Chong 2018a).
CSOM‐3: Topical versus systemic antibiotics for chronic suppurative otitis media (Chong 2018b).
CSOM‐4: Topical antibiotics with steroids for chronic suppurative otitis media (Brennan‐Jones 2018b).
CSOM‐5: Topical antiseptics for chronic suppurative otitis media (Head 2018a).
CSOM‐6: Antibiotics versus topical antiseptics for chronic suppurative otitis media (Head 2018b).
CSOM‐7: Aural toilet (ear cleaning) for chronic suppurative otitis media (Bhutta 2018).
This review compares the effectiveness of topical antiseptics (without corticosteroids) against other antiseptics or placebo/no treatment for chronic suppurative otitis media.
Description of the condition
Chronic suppurative otitis media (CSOM), which is also often referred to as chronic otitis media (COM), is a chronic inflammation and infection of the middle ear and mastoid cavity, characterised by ear discharge (otorrhoea) through a perforated tympanic membrane.
The predominant symptoms of CSOM are ear discharge and hearing loss. Ear discharge can be persistent or intermittent, and many sufferers find it socially embarrassing (Orji 2013). Some patients also experience discomfort or earache. Most patients with CSOM experience temporary or permanent hearing loss with average hearing levels typically between 10 and 40 decibels (Jensen 2013). The hearing loss can be disabling, and it can have an impact on speech and language skills, employment prospects, and on children’s psychosocial and cognitive development, including academic performance (Elemraid 2010; Olatoke 2008; WHO 2004). Consequently, quality of life can be affected. CSOM can also progress to serious complications in rare cases (and more often when cholesteatoma is present): both extracranial complications (such as mastoid abscess, postauricular fistula and facial palsy) and intracranial complications (such as otitic meningitis, lateral sinus thrombosis and cerebellar abscess) have been reported (Dubey 2007; Yorgancılar 2013).
CSOM is estimated to have a global incidence of 31 million episodes per year, or 4.8 new episodes per 1000 people (all ages), with 22% of cases affecting children under five years of age (Monasta 2012; Schilder 2016). The prevalence of CSOM varies widely between countries, but it disproportionately affects people at socio‐economic disadvantage. It is rare in high‐income countries, but common in many low‐ and middle‐income countries (Mahadevan 2012; Monasta 2012; Schilder 2016; WHO 2004).
Definition of disease
There is no universally accepted definition of CSOM. Some define CSOM in patients with a duration of otorrhoea of more than two weeks but others may consider this an insufficient duration, preferring a minimum duration of six weeks or more than three months (Verhoeff 2006). Some include diseases of the tympanic membrane within the definition of CSOM, such as tympanic perforation without a history of recent ear discharge, or the disease cholesteatoma (a growth of the squamous epithelium of the tympanic membrane).
In accordance with a consensus statement, here we use CSOM only to refer to tympanic membrane perforation, with intermittent or continuous ear discharge (Gates 2002). We have used a duration of otorrhoea of two weeks as an inclusion criterion, in accordance with the definition used by the World Health Organization, but we have used subgroup analyses to explore whether this is a factor that affects observed treatment effectiveness (WHO 2004).
Many people affected by CSOM do not have good access to modern primary healthcare, let alone specialised ear and hearing care, and in such settings health workers may be unable to view the tympanic membrane to definitively diagnose CSOM. It can also be difficult to view the tympanic membrane when the ear discharge is profuse. Therefore we have also included, as a subset for analysis, studies where participants have had chronic ear discharge for at least two weeks, but where the diagnosis is unknown.
At‐risk populations
Some populations are considered to be at high risk of CSOM. There is a high prevalence of disease among Indigenous people such as the Aboriginal and Torres Strait Islander Australian, Native American and Inuit populations. This is likely due to an interplay of factors, including socio‐economic deprivation and possibly differences resulting from population genetics (Bhutta 2016). Those with primary or secondary immunodeficiency are also susceptible to CSOM. Children with craniofacial malformation (including cleft palate) or chromosomal mutations such as Down syndrome are prone to chronic non‐suppurative otitis media ('glue ear'), and by extrapolation may also be at greater risk of suppurative otitis media. The reasons for this association with craniofacial malformation are not well understood, but may include altered function of the Eustachian tube, coexistent immunodeficiency, or both. These populations may be less responsive to treatment and more likely to develop CSOM, recurrence or complications.
Children who have a grommet (ventilation tube) in the tympanic membrane to treat glue ear or recurrent acute otitis media may be more prone to develop CSOM; however, their pathway to CSOM may differ and therefore they may respond differently to treatment. Children with grommets who have chronic ear discharge meeting the CSOM criteria are therefore considered to be a separate high‐risk subgroup (van der Veen 2006).
Treatment
Treatments for CSOM may include topical antibiotics (administered into the ear) with or without steroids, systemic antibiotics (given either by mouth or by injection), topical antiseptics and ear cleaning (aural toileting), all of which can be used on their own or in various combinations. Whereas primary healthcare workers or patients themselves can deliver some treatments (for example, some aural toileting and antiseptic washouts), in most countries antibiotic therapy requires prescription by a doctor. Surgical interventions are an option in cases where complications arise or in patients who have not responded to pharmacological treatment; however, there is a range of practice in terms of the type of surgical intervention that should be considered and the timing of the intervention. In addition, access to or availability of surgical interventions is setting‐dependent. This series of Cochrane Reviews therefore focuses on non‐surgical interventions. In addition, most clinicians consider cholesteatoma to be a variant of CSOM, but acknowledge that it will not respond to non‐surgical treatment (or will only respond temporarily) (Bhutta 2011). Therefore, people with cholesteatoma are not included in these reviews.
Description of the intervention
Antiseptics are substances that kill or inhibit the growth and development of micro‐organisms. Agents that have been used for treating CSOM include povidone iodine, aluminium acetate, boric acid, alcohol, acetic acid and hydrogen peroxide. Antiseptics can be delivered as drops or as washes using a syringe. The frequency of administration and duration of treatment can vary. Syringing may bring additional benefit by flushing out debris or pus, thus reducing the overall bacterial load. Antiseptics can be used alone or in addition to other treatments for CSOM, such as antibiotics or aural toileting.
How the intervention might work
CSOM is a chronic and often polymicrobial (involving more than one micro‐organism) infection of the middle ear. Topical antiseptics are administered to the ear to inhibit the micro‐organisms that may be responsible for the condition. Although the mechanism of action of most antiseptics is thought to relate to disruption of the bacterial cell wall followed by penetration into the cell and action at the target site(s), different groups of antiseptics have different properties (e.g. iodines, alcohols, acids) (Table 3). We therefore analysed these groups separately and pooling only occurred where there was no evidence of a difference in effect.
2. Antiseptics that have been used to treat CSOM.
| Antiseptic agent used aurally | Target and mechanism of action |
| Rubbing alcohol (ethanol, isopropanol) | Penetrating agents that cause loss of cellular membrane function, leading to release of intracellular components, denaturing of proteins, and inhibition of DNA, RNA, protein and peptidoglycan synthesis. |
| Povidone iodine | Highly active oxidising agents that destroy cellular activity of proteins. Disrupts oxidative phosphorylation and membrane‐associated activities. Iodine reacts with cysteine and methionine thiol groups, nucleotides and fatty acids, resulting in cell death. |
| Chlorhexidine | Membrane‐active agents that damage cell wall and outer membrane, resulting in collapse of membrane potential and intracellular leakage. Enhanced passive diffusion mediates further uptake, causing coagulation of cytosol. |
| Hydrogen peroxide | Produces hydroxyl free radicals that function as oxidants, which react with lipids, proteins and DNA. Sulfhydryl groups and double bonds are targeted in particular, thus increasing cell permeability. |
| Boric acid | It is likely that the change in the pH media of the ear canal interrupts the growth of bacteria by affecting the amino acid, which causes alteration in the three‐dimensional structure of bacterial enzymes. Extreme changes in pH cause protein denaturation. |
| Aluminium acetate/acetic acid | Acetic acid changes the pH media of the ear canal and interrupts the growth of bacteria by affecting the amino acid, which causes alteration in the three‐dimensional structure of bacterial enzymes. Extreme changes in pH cause protein denaturation. Aluminium acetate is an astringent that helps reduce itching, stinging and inflammation. |
Sources: Gupta 2015; McDonnell 1999; Sheldon 2005.
Antiseptics or their application can cause physical, chemical or allergic irritation of the skin of the outer ear, manifesting as discomfort/pain or itching. Some antiseptics (such as chlorhexidine or alcohol) can be toxic to the inner ear (ototoxicity), so there is a risk of causing sensorineural hearing loss, dizziness or tinnitus.
Why it is important to do this review
Antiseptic agents generally cost less than some of the other treatments available for the treatment of CSOM (in particular topical antibiotics). They are also more readily available, do not require prescription by a doctor and do not need refrigerated transport, which makes them attractive for use in resource‐constrained environments. Evidence‐based knowledge of their effectiveness and the relative effectiveness of the different types of antiseptic could help to optimise their use.
Objectives
To assess the effects of topical antiseptics for people with chronic suppurative otitis media.
Methods
Criteria for considering studies for this review
Types of studies
We included studies with the following design characteristics:
Randomised controlled trials (including cluster‐randomised trials where the unit of randomisation is the setting or operator) and quasi‐randomised trials.
Patients were followed up for at least one week.
We excluded studies with the following design characteristics:
Cross‐over trials, because CSOM is not expected to be a stable chronic condition. Unless data from the first phase were available, we excluded such studies.
Types of participants
We included studies with patients (adults and children) who had:
chronic ear discharge of unknown cause; or
chronic suppurative otitis media.
We defined patients with chronic ear discharge as patients with at least two weeks of ear discharge, where the cause of the discharge was unknown.
We defined patients with chronic suppurative otitis media (CSOM) as patients with:
chronic or persistent ear discharge for at least two weeks; and
a perforated tympanic membrane.
We did not exclude any populations based on age, risk factors (cleft palate, Down syndrome), ethnicity (e.g. Australian Aboriginal or Torres Strait Islanders) or the presence of ventilation tubes (grommets). Where available, we recorded these factors in the patient characteristics section during data extraction from the studies. If any of the included studies recruited these patients as a majority (80% or more), we analysed them in a subgroup analysis (see Subgroup analysis and investigation of heterogeneity).
We excluded studies where the majority (more than 50%) of participants:
had an alternative diagnosis to CSOM (e.g. otitis externa);
had underlying cholesteatoma;
had ear surgery within the last six weeks.
We did not include studies designed to evaluate interventions in the immediate peri‐surgical period, which were focused on assessing the impact of the intervention on the surgical procedure or outcomes.
Types of interventions
Intervention
Any single, or combination of, topical antiseptic agent of any class including but not limited to) povidone iodine, aluminium acetate, boric acid, alcohol and hydrogen peroxide. The topical antiseptics could be applied directly into the ear canal as ear drops, powders or irrigations, or as part of an aural toileting procedure.
Dose/duration
There was no limitation on the dose, duration or frequency of application.
Comparisons
The following were the comparators:
Placebo, no intervention (topical antiseptic versus placebo/no intervention).
Another topical antiseptic (topical antiseptic A versus topical antiseptic B).
There were two potential scenarios for analysis:
Topical antiseptics as a stand‐alone treatment: studies where all participants either received no treatment or only received aural toileting. This also included situations where the comparison group received a single administration of antiseptic (e.g. as part of microsuction at the start of treatment).
Topical antiseptics as an add‐on to topical/systemic antibiotics: studies where all participants received topical or systemic antibiotics, with or without aural toileting procedures.
Many comparison pairs were possible in this review. The main comparisons of interest that we have summarised and presented in the 'Summary of findings' table are:
topical antiseptics as a single therapy (main treatment) versus placebo/no intervention; and
topical antiseptics versus placebo/no intervention, where both arms also received topical or systemic antibiotics.
Types of outcome measures
We analysed the following outcomes in the review, but we did not use them as a basis for including or excluding studies.
We extracted and reported data from the longest available follow‐up for all outcomes.
Primary outcomes
-
Resolution of ear discharge or 'dry ear' (whether otoscopically confirmed or not), measured at:
between one week and up to two weeks;
two weeks to up to four weeks; and
after four weeks.
Health‐related quality of life using a validated instrument for CSOM (e.g. Chronic Otitis Media Questionnaire (COMQ)‐12 (Phillips 2014a; Phillips 2014b; van Dinther 2015), Chronic Otitis Media Outcome Test (COMOT)‐15 (Baumann 2011), Chronic Ear Survey (CES) (Nadol 2000)).
Ear pain (otalgia) or discomfort or local irritation.
Secondary outcomes
Hearing, measured as the pure‐tone average of air conduction thresholds across four frequencies tested (500 Hz, 1000 Hz, 2000 Hz and 4000 Hz) of the affected ear. If this was not available, we reported the pure‐tone average of the thresholds measured.
Serious complications, including intracranial complications (such as otitic meningitis, lateral sinus thrombosis and cerebellar abscess) and extracranial complications (such as mastoid abscess, postauricular fistula and facial palsy), and death.
-
Ototoxicity; this was measured as 'suspected ototoxicity' as reported by the studies where available, and as the number of people with the following symptoms that may be suggestive of ototoxicity:
sensorineural hearing loss;
balance problems/dizziness/vertigo;
tinnitus.
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 1 April 2019.
Electronic searches
The Information Specialist searched:
the Cochrane ENT Register (searched via the Cochrane Register of Studies to 1 April 2019);
the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 4) (searched via the Cochrane Register of Studies Web to1 April 2019);
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to 1 April 2019);
Ovid EMBASE (1974 to 1 April 2019);
EBSCO CINAHL (1982 to 1 April 2019);
LILACS (Latin American and Caribbean Health Science Information database), lilacs.bvsalud.org (search to 1 April 2019);
Web of Knowledge, Web of Science (1945 to 1 April 2019);
ClinicalTrials.gov, www.clinicaltrials.gov (search via the Cochrane Register of Studies to 1 April 2019);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (search to 1 April 2019).
We also searched:
IndMed (search to 22 March 2018);
African Index Medicus (search to 22 March 2018).
The search strategies for major databases are detailed in Appendix 1. The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. The strategies were designed to identify all relevant studies for a suite of reviews on various interventions for chronic suppurative otitis media (Bhutta 2018; Brennan‐Jones 2018a; Brennan‐Jones 2018b; Chong 2018a; Chong 2018b; Head 2018a; Head 2018b). Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011).
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched Ovid MEDLINE to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Information Specialist also ran non‐systematic searches of Google Scholar to retrieve grey literature and other sources of potential trials.
We did not perform a separate search for adverse effects. We considered adverse effects as described in the included studies only.
We contacted original authors for clarification and further data if trial reports were unclear and we arranged translations of papers where necessary.
Data collection and analysis
Selection of studies
At least two review authors (KH/LYC) independently screened all titles and abstracts of the references obtained from the database searches to identify potentially relevant studies. At least two review authors (KH/LYC) evaluated the full text of each potentially relevant study to determine whether it met the inclusion and exclusion criteria for this review.
We resolved any differences by discussion and consensus, with the involvement of a third author for clinical and methodological input where necessary.
Data extraction and management
At least two review authors (KH/LYC/CBJ/MB) independently extracted data from each study using a standardised data collection form (see Appendix 2). Whenever a study had more than one publication, we retrieved all publications to ensure complete extraction of data. Where there were discrepancies in the data extracted by different review authors, we checked these against the original reports and resolved any differences by discussion and consensus, with the involvement of a third author or a methodologist where appropriate. We contacted the original study authors for clarification or for missing data whenever possible. If differences were found between publications of a study, we contacted the original authors for clarification. We used data from the main paper(s) if no further information was found.
We included key characteristics of the included studies, such as study design, setting (including location), year of study, sample size, age and sex of participants, and how outcomes were defined or collected in the studies. In addition, we also collected baseline information on prognostic factors or effect modifiers (see Appendix 2). For this review, this included the following information whenever available:
duration of ear discharge at entry to the study;
diagnosis of ear discharge (where known);
number people who may have been at higher risk of CSOM, including those with cleft palate or Down syndrome;
ethnicity of participants including the number who were from Indigenous populations;
number who had previously had ventilation tubes (grommets) inserted (and, where known, the number who had tubes still in place);
number who had previous ear surgery;
number who had previous treatments for CSOM (non‐responders, recurrent versus new cases).
We recorded concurrent treatments alongside the details of the interventions used. See the 'Data extraction form' in Appendix 2 for more details.
For the outcomes of interest to the review, we extracted the findings of the studies on an available case analysis basis, i.e. we included data from all patients available at the time points based on the treatment randomised whenever possible, irrespective of compliance or whether patients had received the treatment as planned.
In addition to extracting pre‐specified information about study characteristics and aspects of methodology relevant to risk of bias, we extracted the following summary statistics for each trial and each outcome:
For continuous data: the mean values, standard deviations and number of patients for each treatment group. Where endpoint data were not available, we extracted the values for change from baseline. We analysed data from disease‐specific quality of life scales such as COMQ‐12, COMOT‐15 and CES as continuous data.
For binary data: the number of participants who experienced an event and the number of patients assessed at the time point.
For ordinal scale data: if the data appeared to be approximately normally distributed or if the analysis that the investigators performed suggested parametric tests were appropriate, then we treated the outcome measures as continuous data. Alternatively, if data were available, we converted it into binary data.
Time‐to‐event outcomes: we did not expect any outcomes to be measured as time‐to‐event data. However, if outcomes such as resolution of ear discharge were measured in this way, we reported the hazard ratios.
For resolution of ear discharge, we extracted the longest available data within the time frame of interest, defined as from one week up to (and including) two weeks (7 days to 14 days), from two weeks up to (and including) four weeks (15 to 28 days), and after four weeks (28 days or one month).
For other outcomes, we reported the results from the longest available follow‐up period.
Extracting data for pain/discomfort and adverse effects
For these outcomes, there were variations in how studies had reported the outcomes. For example, some studies reported both 'pain' and 'discomfort' separately whereas others did not. Prior to the commencement of data extraction, we agreed and specified a data extraction algorithm for how data should be extracted.
We extracted data for serious complications as a composite outcome. If a study reported more than one complication and we could not distinguish whether these occurred in one or more patients, we extracted the data with the highest incidence to prevent double counting.
Extracting data from figures
Where values for primary or secondary outcomes were shown as figures within the paper, we attempted to contact the study authors to try to obtain the raw values. When the raw values were not provided, we extracted information from the graphs using an online data extraction tool, using the best quality version of the relevant figures available.
Assessment of risk of bias in included studies
At least two review authors (KH/LYC/CBJ/MB) independently assessed the risk of bias of each included study. We followed the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), using the Cochrane 'Risk of bias' tool. With this tool we assessed the risk of bias as 'low', 'high' or 'unclear' for each of the following six domains:
sequence generation;
allocation concealment;
blinding of participants, personnel and outcome assessment;
incomplete outcome data;
selective reporting;
other sources of bias.
Measures of treatment effect
We summarised the effects of dichotomous outcomes (e.g. proportion of patients with complete resolution of ear discharge) as risk ratios (RR) with confidence intervals (CIs). For the key outcomes that are presented in the 'Summary of findings' table, we expressed the results as absolute numbers based on the pooled results and compared to the assumed risk. We also calculated the number needed to treat to benefit (NNTB) using the pooled results. The assumed baseline risk was typically either (a) the median of the risks of the control groups in the included studies, this being used to represent a 'medium‐risk population' or, alternatively, (b) the average risk of the control groups in the included studies, which is used as the 'study population' (Handbook 2011). If a large number of studies were available, and where appropriate, we also attempted to present additional data based on the assumed baseline risk in (c) a low‐risk population and (d) a high‐risk population.
For continuous outcomes, we expressed treatment effects as a mean difference (MD) with standard deviation (SD). If different scales were used to measure the same outcome, we used the standardised mean difference (SMD) and provided a clinical interpretation of the SMD values.
Unit of analysis issues
Cross‐over studies
This review did not use data from phase II of cross‐over studies.
The ear as the unit of randomisation: within‐patient randomisation in patients with bilateral ear disease
For data from studies where 'within‐patient' randomisation was used (i.e. studies where both ears (right versus left) were randomised) we adjusted the analyses for the paired nature of the data (Elbourne 2002; Stedman 2011), as outlined in section 16.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
The ear as the unit of randomisation: non‐paired randomisation in patients with bilateral ear disease
Some patients with bilateral disease may have received the same treatment in both ears, whereas others received a different treatment in each ear. We did not exclude these studies, but we only reported the data if specific pairwise adjustments were completed or if sufficient data were obtained to be able to make the adjustments.
The patient as the unit of randomisation
Some studies randomise by patient and those with bilateral CSOM received the same intervention for both ears. In some studies the results may be reported as a separate outcome for each ear (the total number of ears is used as the denominator in the analysis). The correlation of response between the left ear and right ear when given the same treatment was expected to be very high, and if both ears were counted in the analysis this was effectively a form of double counting, which may be especially problematic in smaller studies if the number of people with bilateral CSOM was unequal. We did not exclude these studies, but we only reported the results if the paper presented the data in such a way that we could include the data from each participant only once (one data point per participant) or if we had enough information to reliably estimate the effective sample size or inflated standard errors as presented in chapter 16.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). If this was not possible, we attempted to contact the authors for more information. If there was no response from the authors, then we did not include data from these studies in the analysis.
If we found cluster‐randomised trials by setting or operator, we analysed these according to the methods in section 16.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
Dealing with missing data
We attempted to contact the study authors via email whenever the outcome of interest was not reported but the methods of the study had suggested that the outcome had been measured. We did the same if not all of the data required for the meta‐analysis was reported, unless the missing data were standard deviations. If standard deviation data was not available, we approximated these using the standard estimation methods from P values, standard errors or 95% CIs if these were reported, as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). Where it was impossible to estimate these, we contacted the study authors.
Apart from imputations for missing standard deviations, we did not conduct any other imputations. We extracted and analysed data for all outcomes using the available case analysis method.
Assessment of heterogeneity
We assessed clinical heterogeneity (which may be present even in the absence of statistical heterogeneity) by examining the included studies for potential differences in the types of participants recruited, interventions or controls used, and the outcomes measured. We did not pool studies where the clinical heterogeneity made it unreasonable to do so.
We assessed statistical heterogeneity by visually inspecting the forest plots and by considering the Chi² test (with a significance level set at P value < 0.10) and the I² statistic, which calculates the percentage of variability that is due to heterogeneity rather than chance, with I² values over 50% suggesting substantial heterogeneity (Handbook 2011).
Assessment of reporting biases
We assessed reporting bias as within‐study outcome reporting bias and between‐study publication bias.
Outcome reporting bias (within‐study reporting bias)
We assessed within‐study reporting bias by comparing the outcomes reported in the published report against the study protocol, whenever this could be obtained. If the protocol was not available, we compared the outcomes reported to those listed in the methods section. If results were mentioned but not reported adequately in a way that allowed analysis (e.g. the report only mentioned whether the results were statistically significant or not), bias in a meta‐analysis was likely to occur. We tried to find further information from the study authors, but if no further information could be obtained, we noted this as being a high risk of bias. Where there was insufficient information to judge the risk of bias, we noted this as an unclear risk of bias (Handbook 2011).
Publication bias (between‐study reporting bias)
We intended to create funnel plots if sufficient studies (more than 10) were available for an outcome. If we observed asymmetry of the funnel plot, we would have conducted a more formal investigation using the methods proposed by Egger 1997.
Data synthesis
We conducted all meta‐analyses using Review Manager 5.3 (RevMan 2014). For dichotomous data, we analysed treatment differences as a risk ratio (RR) calculated using the Mantel‐Haenszel methods. We analysed time‐to‐event data using the generic inverse variance method.
For continuous outcomes, if all the data was from the same scale, we pooled the mean values obtained at follow‐up with change outcomes and reported this as a MD. However, if the SMD had to be used as an effect measurement, we did not pool change and endpoint data.
When statistical heterogeneity is low, random‐effects versus fixed‐effect methods yield trivial differences in treatment effects. However, when statistical heterogeneity is high, the random‐effects method provides a more conservative estimate of the difference.
Subgroup analysis and investigation of heterogeneity
We subgrouped studies where most participants (80% or more) met the criteria stated below in order to determine whether the effect of the intervention was different compared to other patients. Due to the risks of reporting and publication bias with unplanned subgroup analyses of trials, we only analysed subgroups reported in studies if these were prespecified and stratified at randomisation.
We planned to conduct subgroup analyses regardless of whether statistical heterogeneity was observed for studies that included patients identified as high‐risk (i.e. thought to be less responsive to treatment and more likely to develop CSOM, recurrence or complications) and patients with ventilation tubes (grommets). 'High‐risk' patients include Indigenous populations (e.g. Australian Aboriginal and Torres Strait Islanders, Native Americans and Inuit populations of Alaska, Canada and Greenland), people with craniofacial malformation (e.g. cleft palate), Down syndrome and people with known immunodeficiency.
We planned to present the main analyses of this review in the form of forest plots based on this main subgroup analysis.
For the high‐risk group, this applied to the outcomes resolution of ear discharge (dry ear), quality of life, pain/discomfort, development of complications and hearing loss.
For patients with ventilation tubes, this applied to the outcome resolution of ear discharge (dry ear) for the time point of four weeks or more because this group was perceived to be at lower risk of treatment failure and recurrence than other patient groups. If statistical heterogeneity was observed, we also conducted subgroup analysis for the effect modifiers below. If there were statistically significant subgroup effects, we presented these subgroup analysis results as forest plots.
For this review, effect modifiers included:
Diagnosis of CSOM: it was likely that some studies would include patients with chronic ear discharge but who had not had a diagnosis of CSOM. Therefore, we subgrouped studies where most patients (80% or more) met the criteria for CSOM diagnosis in order to determine whether the effect of the intervention was different compared to patients where the precise diagnosis was unknown and inclusion into the study was based purely on chronic ear discharge symptoms.
Duration of ear discharge: there is uncertainty about whether the duration of ear discharge prior to treatment has an impact on the effectiveness of treatment and whether more established disease (i.e. discharge for more than six weeks) is more refractory to treatment compared with discharge of a shorter duration (i.e. less than six weeks).
Patient age: patients who were younger than two years old versus patients up to six years old versus adults. Patients under two years are widely considered to be more difficult to treat.
We presented the results as subgroups regardless of the presence of statistical heterogeneity based on the type of antiseptics (e.g. iodines, alcohols, acids). This was because different types of antiseptics have different mechanisms of action and therefore the treatment effects and adverse effect profiles were likely to be different.
Sensitivity analysis
We planned to carry out sensitivity analyses to determine whether the findings were robust to the decisions made in the course of identifying, screening and analysing the trials. We planned to conduct sensitivity analysis for the following factors, whenever possible:
Impact of model chosen: fixed‐effect versus random‐effects model.
Risk of bias of included studies: excluding studies with high risk of bias (we defined these as studies that have a high risk of allocation concealment bias and a high risk of attrition bias (overall loss to follow‐up of 20%, differential follow‐up observed)).
Where there was statistical heterogeneity, studies that only recruited patients who had previously not responded to one of the treatments under investigation in the RCT. Studies that specifically recruited patients who did not respond to a treatment could potentially have reduced the relative effectiveness of an agent.
If any of these investigations found a difference in the size of the effect or heterogeneity, we mentioned this in the Effects of interventions section and/or presented the findings in a table.
GRADE and 'Summary of findings' table
Using the GRADE approach, at least two review authors (KH/LYC) independently rated the overall certainty of evidence using the GDT tool (http://www.guidelinedevelopment.org/) for the main comparison pairs listed in the Types of interventions section. The certainty of evidence reflects the extent to which we are confident that an estimate of effect is correct and we applied this in the interpretation of results. There were four possible ratings: 'high', 'moderate', 'low' and 'very low' (Handbook 2011). A rating of 'high' certainty evidence implies that we are confident in our estimate of effect and that further research is very unlikely to change our confidence in the estimate of effect. A rating of 'very low' certainty implies that any estimate of effect obtained is very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high certainty. However, several factors could lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading is determined by the seriousness of these factors:
study limitations (risk of bias);
inconsistency;
indirectness of evidence;
imprecision;
publication bias.
The 'Summary of findings' table presents the following outcomes:
-
resolution of ear discharge or 'dry ear':
at between one week and up to two weeks;
after four weeks;
health‐related quality of life;
ear pain (otalgia) or discomfort or local irritation;
hearing;
serious complications;
suspected ototoxicity.
Results
Description of studies
Results of the search
The searches retrieved a total of 7256 references and we identified five additional references from other sources. This reduced to 3147 after removal of duplicates. We screened the titles and abstracts and subsequently removed 2935 references. We assessed 212 full texts for eligibility of which we excluded 203 references; we excluded 82 of these references (54 studies) with reasons recorded in the review (see Excluded studies).
We included seven references (five studies). We identified one ongoing study (I‐HEAR‐BETAa; see Characteristics of ongoing studies) and there is one reference awaiting classification (Abdul 2005; see Characteristics of studies awaiting classification).
A flow chart of study retrieval and selection is provided in Figure 1.
1.
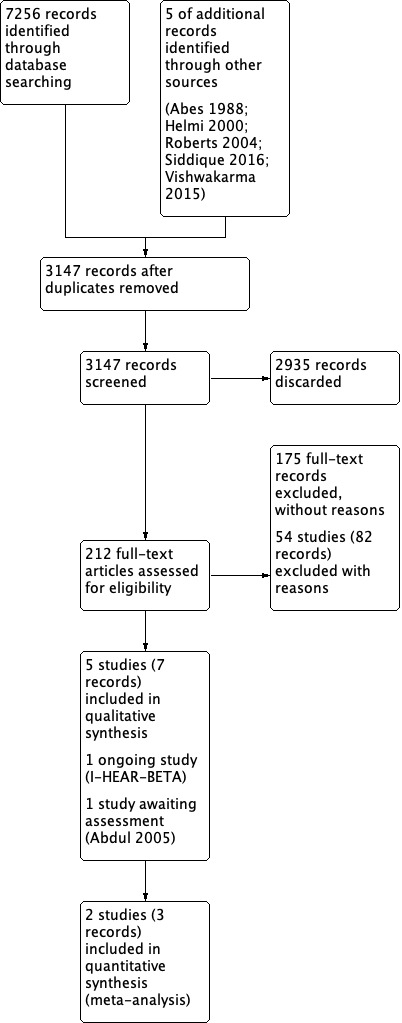
Study flow diagram.
Included studies
Five studies were included (Eason 1986; Loock 2012; Minja 2006; Papastavros 1989; Van Hasselt 1998b). Table 4 and the Characteristics of included studies table provide a summary of the included studies.
3. Summary of study characteristics.
|
Ref ID (no. participants) |
Setting | Population | Intervention 1 | Intervention 2 | Treatment duration | Follow‐up | Background Treatment | Notes |
| Topical antiseptics versus placebo/no treatment | ||||||||
|
Minja 2006 (n = 254 people) |
Tanzania, Schools (community) |
Children with CSOM for more than 3 months Mean age 11.8 years |
Boric acid in alcohol ear drops No further information |
No treatment | 1 month | 3 to 4 months | Daily aural toilet (dry mopping) | Cluster‐randomised trial by school Part of a 3‐arm trial |
|
Eason 1986 (n = 43 people) |
Solomon Islands, villages (community) | Children with CSOM for more than 3 months Mean age 5.4 years |
2% boric acid in 20% alcohol 3 ear drops/6 hours |
No treatment | Up to 6 weeks | 4 to 6 weeks | Daily aural toilet (dry mopping) | Part of a 5‐arm trial |
|
Van Hasselt 1998b (n = ? people, 174 ears) |
Malawi, villages (community) |
"CSOM" (no further definition) No age information |
1% povidone Iodine in 1.5% hypromellose (HPMC) – single application | HPMC alone | Single application | 1 week | Suction cleaning before application | Unpublished study Part of 3‐arm trial |
| Topical antiseptic A versus topical antiseptics B | ||||||||
|
Loock 2012 (n = 106 people) |
South Africa, City (secondary care) | Patients with otorrhoea because of active mucosal COM Age over 6 years (90% between 20 and 34 years) |
1% acetic acid 6 drops/12 hours |
Boric acid powder Single administration |
4 weeks (except for boric acid) | Up to 8 weeks | Aural cleaning at 1st visit | Part of a 3‐arm trial |
|
Papastavros 1989 (n = ?, 48 ears) |
Greece, city (secondary care) |
Patients with discharging ears 11 to 79 years |
Hydrogen peroxide, no further information | Borax powder insufflation, no further information | 10 days | 10 days | None | — |
Study design and sample size
Four studies were three‐arm trials (Loock 2012; Minja 2006; Papastavros 1989; Van Hasselt 1998b), and one study was part of a five‐arm trial (Eason 1986). In all cases only two study arms were relevant to this review. Details of the other study arms can be found in the Characteristics of included studies table.
All studies provided an indication that they were 'randomised'. Four were parallel‐group studies (Eason 1986; Loock 2012; Papastavros 1989; Van Hasselt 1998b). Minja 2006, indicates in the abstract that it was a randomised controlled trial but describes in the methods that "All children with CSOM attending the same school were included in the same treatment group" indicating that it was probably a cluster‐randomised trial.
Sample size
The sample size from the studies was difficult to interpret as some studies reported the number of participants and some only reported the number of ears included (Table 4).
Unit of randomisation
The unit of randomisation for each study is presented in Table 5. Minja 2006 states that children attending the same school were in the same treatment group and that there were 24 schools included in the study (although the number of children at each school is not provided). In order to adjust the results for intra‐cluster correlation we have re‐calculated the results to with a intra‐cluster correlation coefficient (ICC) of 0.015 (see Unit of analysis issues for more details). No estimates from the literature were available for this population, but in general for cluster‐randomised trials the ICC is between 0.01 and 0.02. We carried out sensitivity analyses to determine the impact of the ICC.
4. Resolution of ear discharge outcome.
| Reference | Unit of randomisation | Reported | Definition | Otoscopically confirmed? | Time points | mNotes |
| Eason 1986 | Person | Results reported by ear | "dry" or "not discharging" | Unclear | 2 to 4 weeks: 3 weeks 4+ weeks: 6 weeks |
Results not used as it was not possible to account for correlation between ears due to bilateral disease. |
| Loock 2012 | Person | Results reported by person | "inactive" ear (dry) | Otoscopically confirmed | 2 to 4 weeks: 4 weeks | Also measured patient satisfaction which asked patients whether their ears were 'completely dry', 'better but not completely dry', 'no better, still running' |
| Minja 2006 | School | Results reported by person. Only considered 'dry' if both ears were dry. | "Dry" ear | Otoscopically confirmed | 2 to 4 weeks: 4 weeks 4+ weeks: 3 to 4 months |
Intra‐cluster correlation coefficient (ICC) of 0.015 was used |
| Papastavros 1989 | Ear | Results reported by ear | "cure" ‐ co‐existence of all 3 of the following conditions: 1. Ear free of discharge 2. Colour of mucosa: light pink 3. Absence of mucosal oedema, granulations or polyps |
Unclear ‐ but probably | 1 to 2 weeks: 10 days | Results not used as it was not possible to account for correlation between ears due to bilateral disease |
| Van Hasselt 1998b | Unclear | Results reported by ear | 'dry ear' | Unclear | 1 to 2 weeks: 1 week | Results not used as it was not possible to account for correlation between ears due to bilateral disease |
Location
Three studies were conducted in different countries in Africa: South Africa (Loock 2012), Tanzania (Minja 2006) and Malawi (Van Hasselt 1998b). The remaining two studies were conducted in Greece (Papastavros 1989) and the Solomon Islands (Eason 1986).
Settings of trials
Three studies were community studies taking place in villages (Eason 1986; Van Hasselt 1998b) or primary schools (Minja 2006) in rural locations. The remaining two studies were based in secondary care from the ENT departments of hospitals in cities (Loock 2012; Papastavros 1989). The years in which the studies were conducted were not well reported: two studies were published in the 1980s (Eason 1986; Papastavros 1989), one unpublished study was probably conducted in the 1990s (Van Hasselt 1998b), and the last two were published post 2000 (Loock 2012; Minja 2006)
Population
Age and sex
The unpublished study did not provide any patient characteristics (Van Hasselt 1998b).
The ages of participants are reported in Table 4. Four studies reported that they included both males and females. The percentage of females in studies ranged from 36.6% to 55.3%.
High‐risk populations
Eason 1986 recruited participants from the Solomon Islands, which we considered to be a 'high‐risk' Indigenous group. The paper stated that the incidence of CSOM in the population was 3.8% for under 15‐year olds. None of the other studies reported the inclusion of any of the 'high‐risk' populations as defined in our inclusion criteria (cleft palate, Down syndrome, Indigenous groups, immunocompromised patients).
Diagnosis (confirmed tympanic membrane perforation/presence of micro‐purulent discharge)
Four of the studies included patients with CSOM (Eason 1986; Loock 2012; Minja 2006; Van Hasselt 1998b), although the definition was not clear in the unpublished study Van Hasselt 1998b.
Papastavros 1989 included patients with "discharging ears" where the discharge was either persistent for previous six months or those who had a history of at least three recurrences in the last 12 months. The alternative diagnoses for the ear discharge were not well described although it is mentioned that, of the 65 participants that subsequently underwent surgery, a diagnosis of cholesteatoma and/or osteitis was made in 28 participants (43%).
Duration of ear discharge
Two studies required participants to have had ear discharge for at least three months before starting the study (Eason 1986; Minja 2006). Papastavros 1989 required patients to either have had persistent ear discharge for six months or at least three recurrences in the last 12 months. The paper does not report the number of patients falling into each of these categories or the average duration of discharge for participants at the start of the trial.
Two studies did not have inclusion criteria or provide details of the average duration of ear discharge at the start of the study (Loock 2012; Van Hasselt 1998b)
Other important effect modifiers
Ventilation tubes
Loock 2012 excluded patients with ventilation tubes. No other studies reported whether any participants had previous or current ventilation tubes.
Previous ear surgery
Papastavros 1989 reported that 68 participants (75%) had undergone previous surgery but this did not occur within six weeks of starting the trial. Loock 2012 excluded patients who had previous surgery and this was not recorded in any of the other studies.
Interventions
Details of the interventions, background treatments and treatment durations for each of the included studies are summarised in Table 4.
Comparisons
Three studies compared the use of topical antiseptics with no treatment:
Minja 2006: boric acid in alcohol drops + dry mopping versus dry mopping alone.
Eason 1986: boric acid in alcohol drops + dry mopping versus dry mopping alone.
Van Hasselt 1998b: povidone Iodine + hydroxypropyl methyl‐cellulose (HPMC) versus HPMC alone.
Two studies compared two different topical antiseptics and these were analysed as separate comparisons due to the different antiseptic agents used:
Loock 2012: acetic acid ear drops versus boric acid powder.
Papastavros 1989: hydrogen peroxide ear drops versus boric acid powder.
Outcomes
Resolution of ear discharge
All five studies reported resolution of ear discharge as an outcome, although the definitions, methods and timing of assessment differed between studies. These are summarised in Table 5.
Health‐related quality of life using a validated instrument
No studies measured this outcome.
Ear pain (otalgia) or discomfort or local irritation
Loock 2012 gave the number of participants who reported unpleasant taste and burning sensation. No other studies reported the outcome.
Hearing
The methods for two studies indicated that hearing was measured (Loock 2012; Minja 2006). The results for Loock 2012 were presented as a narrative and Minja 2006 did not present the results by treatment group.
Serious complications (including intracranial complications, extracranial complications and death)
Serious complications were not consistently reported. One study reported that no serious complications occurred (Loock 2012) and the other studies did not report the outcome.
Suspected ototoxicity
This outcome was not consistently reported; only one study mentioned ototoxicity as an outcome with no cases identified (Minja 2006).
Excluded studies
We excluded 54 studies (82 records) after reviewing the full text. Further details for the reasons for exclusion can be found in the Characteristics of excluded studies table. The main reasons for exclusion were as follows:
We excluded 50 studies (78 records) as the comparisons were not appropriate for this review, but were relevant to another review in this suite:
Topical antibiotics (CSOM‐1): Asmatullah 2014; de Miguel 1999; Esposito 1990; Fradis 1997; Gyde 1978; Jamallulah 2016; Kasemsuwan 1997; Kaygusuz 2002; Liu 2003; Mira 1993; Nawasreh 2001; Ramos 2003; Siddique 2016; Tutkun 1995; van Hasselt 1998a.
Systemic antibiotics (CSOM‐2): de Miguel 1999; Eason 1986; Esposito 1990; Fliss 1990; Ghosh 2012; Legent 1994; Nwokoye 2015; Onali 2018; Picozzi 1983; Ramos 2003; Renuknanada 2014; Rotimi 1990; Sanchez Gonzales 2001; Somekh 2000; van der Veen 2007.
Topical versus systemic antibiotics (CSOM‐3): de Miguel 1999; Esposito 1990; Esposito 1992; Povedano 1995; Ramos 2003; Yuen 1994.
Topical antibiotics with steroids (CSOM‐4): Boesorire 2000; Browning 1988; Couzos 2003; Crowther 1991; Eason 1986; Gendeh 2001; Helmi 2000; Indudharan 2005; Kaygusuz 2002; Lazo Saenz 1999; Leach 2008; Miro 2000; Panchasara 2015; Ramos 2003; Subramaniam 2001; Tong 1996.
Antibiotics versus topical antiseptics (CSOM‐6): Fradis 1997; Gupta 2015; Jaya 2003; Macfadyen 2005; van Hasselt 1997; Vishwakarma 2015.
Aural toileting (CSOM‐7): Eason 1986; Kiris 1998; Smith 1996.
We excluded the remaining four studies (four records) for the following reasons:
Browning 1983: the comparison was antibiotics compared with topical antiseptics.
Clayton 1990: less than 20% of participants within the study had CSOM.
Roydhouse 1981: the intervention was a mucolytic agent (bromhexine), which was not classified as an antiseptic.
Thorpe 2000: compared three concentrations of the same topical antiseptic (aluminium acetate), which is not a question included in this review.
Risk of bias in included studies
See Figure 2 for the 'Risk of bias' graph (our judgements about each risk of bias item presented as percentages across all included studies) and Figure 3 for the 'Risk of bias' summary (our judgements about each risk of bias item for each included study).
2.
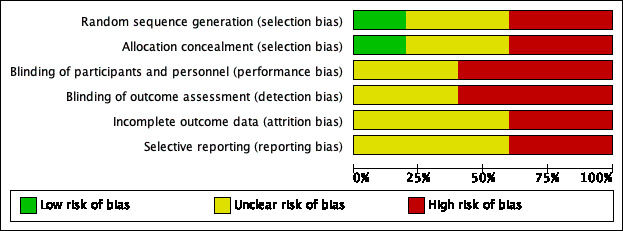
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
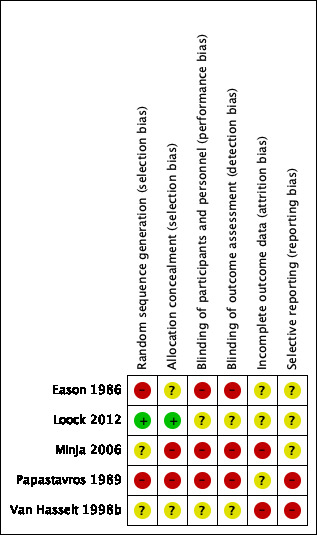
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
We judged two studies to be at high risk of selection bias with regards to randomisation. Papastavros 1989 did not provide details of how the randomisation schedule was produced and there were concerns about how patients were randomised. The paper reported that six participants were deliberately allocated to the systemic antibiotic group because they were suffering from more serious disease. Similarly, Eason 1986 did not provide information about sequence generation and there were unexplained imbalances between the groups. There were 1.6 times as many participants in the largest group compared to the smallest group, with the larger number of participants in the more effective treatment groups.
We assessed Minja 2006 and Van Hasselt 1998b as at 'unclear risk' because they did not provide enough information. We judged Loock 2012 to be at low risk of bias.
Allocation concealment
We assessed two studies to be at high risk of allocation concealment bias. Papastavros 1989 did not provide information regarding the method of allocation concealment but as the paper stated that six more severely affected participants were allocated to a specific treatment group it must be assumed that the allocation of participants to treatment groups was not well concealed. Minja 2006 indicated that the study was randomised but that participants from the same school were allocated to the same treatment group (i.e. a cluster‐randomised trial); it is not clear whether the people completing the allocation of schools knew to which group each school was going to be allocated.
Two studies did not provide enough information with regards to allocation concealment and so we assessed them as at unclear risk (Eason 1986; Van Hasselt 1998b). The remaining study was well reported and was at low risk (Loock 2012).
Blinding
Performance bias
We assessed three studies as high risk for performance bias due to a lack of blinding of participants and healthcare practitioners (Eason 1986; Minja 2006; Papastavros 1989).
We assessed two studies to be at unclear risk of performance bias. Loock 2012 made some attempts at blinding through the use of identical and unlabelled bottles, however one of the groups used a powder and the other used acetic acid ear drops, which have a characteristic smell, so it is likely that participants will have known to which group they were allocated. Van Hasselt 1998b indicated it was "double blinded" but as one of the treatments was iodine, these would have been different to the other solutions and so the effectiveness of blinding is not clear.
Detection bias
Similar to performance bias, we considered Eason 1986, Minja 2006 and Papastavros 1989 to be at high risk of bias due to the lack of blinding of outcome assessors, whereas Loock 2012 and Van Hasselt 1998b were at unclear risk as some attempts at blinding were made but there were doubts that these would have been successful.
Incomplete outcome data
We assessed two studies to be at high risk of attrition bias (Minja 2006; Van Hasselt 1998b). Minja 2006 noted a loss to follow‐up that was both high and uneven across the groups: 23% (17/74) in the group with amoxicillin (systemic antibiotics), 19% (25/130) in the group with boric acid ear drops and 11% (14/124) in the group with only dry mopping. The reasons for dropout were not well evaluated. Van Hasselt 1998b did not provide information regarding the number of people starting the trial so it is not possible to determine whether there was a dropout rate during the trial and whether that could have impacted the results.
We considered three studies to be at unclear risk of attrition bias (Eason 1986; Loock 2012; Papastavros 1989). Two studies did not provide information about any patients who were lost to follow‐up (Eason 1986; Papastavros 1989). Loock 2012 provided the loss to follow‐up rates in the three treatment groups as 5.8%, 15.1% and 18.5% but did not provide reasons within the paper.
Selective reporting
We assessed Papastavros 1989 and Van Hasselt 1998b to be at high risk of selective reporting bias. Papastavros 1989 described several important criteria and assumptions for the measurement of success/failures in the methods section, but no information was provided in the results section. In addition, not all criteria for responses to treatment were fully reported, i.e. recurrence. For Van Hasselt 1998b, the study was not published and was only reported as a conference presentation that we were not able to access. The information comes from a paragraph in the introduction of a separate paper and so it is not possible to evaluate the methods fully due to lack of information presented.
The remaining three studies were at unclear risk of selective reporting bias (Eason 1986; Loock 2012; Minja 2006). The main issue with Loock 2012 and Minja 2006 was the poor reporting of the audiometry results. The level of reporting in Eason 1986 was very low and the definition of "improved" for the primary outcome was not provided.
None of the studies had protocols identified through searches of clinical trials registries.
Other potential sources of bias
Funding
Three studies were sponsored through national research grants (Eason 1986: Medical Research Council of New Zealand; Loock 2012: ENT society of South Africa, National Health Laboratory Service of South Africa; Minja 2006: SAREC a department of the Swedish International Development Cooperation Agency) with Loock 2012 adding that "… the investigator received no sponsorship or incentive from manufacturers of any of the treatments used."
No information was provided in Papastavros 1989 or Van Hasselt 1998b.
Declarations of interest
Loock 2012 explicitly stated that "There was no conflict of interest …", whereas the remaining studies did not provide any information about conflicts of interest (Eason 1986; Minja 2006; Papastavros 1989; Van Hasselt 1998b).
Effects of interventions
See: Table 1
Comparison 1: Topical antiseptics versus placebo/no treatment
Three studies were included in this comparison:
Eason 1986 (43 children; 58 ears) compared boric acid in alcohol drops plus aural toileting with aural toileting alone.
Minja 2006 (254 children) compared boric acid in alcohol plus aural toileting with aural toileting alone.
Van Hasselt 1998b (unclear number of people, 174 ears) compared a single application of povidone iodine in hydroxypropyl methyl‐cellulose (HPMC) versus HMPC alone.
See also Table 1.
Primary outcomes
Resolution of ear discharge or 'dry ear'
Although results were reported at one week by Van Hasselt 1998b, and at three and six weeks by Eason 1986, all of these results were presented by ear rather than by person. It is not possible to determine how many people with bilateral and unilateral disease were included and therefore it is not possible to account for the within‐person correlation between ears. These results could not be included in the analyses.
Between one week and up to two weeks
No studies reported the results per person for this outcome at between one week and up to two weeks.
Two weeks to up to four weeks
Minja 2006 identified that more participants in the group receiving topical antiseptics had dry ear compared to the no topical antiseptics group at four weeks (risk ratio (RR) 1.94, 95% confidence interval (CI) 1.20 to 3.16; 1 study; 174 participants) (Analysis 1.1).
1.1. Analysis.
Comparison 1 Topical antiseptics versus no treatment, Outcome 1 Resolution of ear discharge (2 to 4 weeks).
After four weeks
Minja 2006 identified that more participants in the group receiving topical antiseptics had dry ear compared to the no topical antiseptics group at three to four months where the treatment duration was one month (RR 1.73, 95% CI 1.21 to 2.47; 1 study; 180 participants; very low‐certainty evidence) (Analysis 1.1).
Sensitivity analysis
As Minja 2006 appeared to be a cluster‐randomised controlled trial we adjusted the results using an intra‐cluster correlation coefficient (ICC) of 0.015 to account for the possible correlation of results within groups. We conducted a sensitivity analysis based on the ICC used and the results are available for two to four weeks in Analysis 2.1 and at three to four months in Analysis 2.2. The sensitivity analysis indicates that the choice of ICC does not influence the overall results greatly.
2.1. Analysis.
Comparison 2 Sensitivity analysis: topical antiseptics versus no treatment, Outcome 1 Resolution of ear discharge (2 to 4 weeks).
2.2. Analysis.
Comparison 2 Sensitivity analysis: topical antiseptics versus no treatment, Outcome 2 Resolution of ear discharge (3 to 4 months).
Health‐related quality of life using a validated instrument
None of the studies reported this outcome.
Ear pain (otalgia) or discomfort or local irritation
None of the studies reported this outcome.
Secondary outcomes
Hearing
Minja 2006 measured hearing but presented the numeric results according to the outcome of the ear and so it was not possible to use the results. However, the authors stated that "there was no deterioration of hearing in groups 2 [boric acid] ... as compared to group 1 [no additional treatment]" (very low‐certainty evidence).
Serious complications (including intracranial complications, extracranial complications and death)
No studies reported that any participant died or had any intracranial or extracranial complications.
Suspected ototoxicity
Minja 2006 stated that "there was no deterioration of hearing in groups 2 [boric acid] and 3 [systemic antibiotic PLUS boric acid], as compared to group 1 [no additional treatment]. Thus, no signs of ototoxicity could be found" (very low‐certainty evidence).
Subgroup analysis
Although we had planned to complete subgroup analyses, as only one study was included in the qualitative analysis these were not possible.
Comparison 2: Boric acid powder versus acetic acid ear drops
One study (106 participants) compared a single instillation of boric acid powder with daily instillation of acetic acid ear drops for four weeks (Loock 2012).
Primary outcomes
Resolution of ear discharge or 'dry ear'
Between one week and up to two weeks
The study did not present results for this outcome.
Two weeks to up to four weeks
Loock 2012 (93 participants) found that the use of boric acid powder may result in more dry ears at four weeks compared to the use of acetic acid ear drops (RR 2.61, 95% CI 1.51 to 4.53) (Analysis 3.1).
3.1. Analysis.
Comparison 3 Topical antibiotic A versus topical antibiotic B, Outcome 1 Resolution of ear discharge (2 to 4 weeks).
After four weeks
Loock 2012 provided results for those ears that were 'dry' at the four‐week follow‐up, but not for all randomised participants.
Health‐related quality of life using a validated instrument
The study did not measure this outcome.
Ear pain (otalgia) or discomfort or local irritation
Loock 2012 reported four cases of unpleasant taste and burning sensation with acetic acid but did not report any pain, discomfort or local irritation with boric acid powder (RR 0.10, 95% CI 0.01 to 1.81; 93 participants) (Analysis 3.2).
3.2. Analysis.
Comparison 3 Topical antibiotic A versus topical antibiotic B, Outcome 2 Ear pain, discomfort, irritation.
Secondary outcomes
Hearing
Loock 2012 measured hearing but only reported the results qualitatively in the paper, commenting "Audiometric tests showed no detectable overall, isolated nor idiosyncratic hearing loss from any treatment".
Serious complications (including intracranial complications, extracranial complications and death)
Loock 2012 did not report that any participant died or had any intracranial or extracranial complications.
Suspected ototoxicity
No cases of suspected ototoxicity were reported in Loock 2012.
Subgroup analysis
Although we had planned to complete subgroup analyses, as only one study was included in the comparison this was not possible.
Comparison 3: Boric acid versus hydrogen peroxide ear drops
One study (unclear number of participants, 48 ears) compared a single instillation of boric acid powder with daily instillation of hydrogen peroxide ear drops (Papastavros 1989).
Primary outcomes
Resolution of ear discharge or 'dry ear'
Papastavros 1989 presented the results by ear and there is no information to be able to account for the correlation between ears for participants with bilateral disease. Therefore the results are not presented.
Health‐related quality of life using a validated instrument
The study did not measure this outcome.
Ear pain (otalgia) or discomfort or local irritation
The study did not report this outcome.
Secondary outcomes
Hearing
The study did not report this outcome.
Serious complications (including intracranial complications, extracranial complications and death)
The study did not report that any participant died or had any intracranial or extracranial complications (Papastavros 1989).
Suspected ototoxicity
No cases of suspected ototoxicity were reported (Papastavros 1989).
Subgroup analysis
Although we had planned to complete subgroup analyses, as only one study was included in the comparison this was not possible.
Discussion
Summary of main results
We identified five studies for this review (Eason 1986; Loock 2012; Minja 2006; Papastavros 1989; Van Hasselt 1998b). Due to the limited number of studies, the methods used, the choice of outcome measures and the poor reporting of results there was a scarcity of evidence that we could include in the review. Adverse events in particular were not well reported.
See also Table 1.
Topical antiseptics versus placebo/no treatment
Although three studies compared topical antiseptics with no treatment, only one study reported results by person and could provide data on the primary outcome (Minja 2006; 254 children). This study appeared to be a cluster‐randomised controlled trial, randomised by school, and compared the instillation of boric acid in alcohol drops against no ear drops, with both arms using daily dry mopping. Although this study found that more children had resolution of ear discharge using boric acid compared to no treatment at both four weeks (risk ratio (RR) 1.94, 95% confidence interval (CI) 1.20 to 3.16; 174 participants) and three to four months (RR 1.73, 95% CI 1.21 to 2.47; 180 participants) we assessed the evidence to be very uncertain (very low‐certainty) due to the high risk of bias in the study, the imprecision of the results and the suspicion that there may be unpublished studies in this topic area that could influence the results (see Potential biases in the review process). There were narrative descriptions of no differences in suspected ototoxicity or hearing outcomes in this study. None of the studies reported results for health‐related quality of life, adverse effects or serious complications.
Boric acid powder versus acetic acid ear drops
One study compared boric acid powder instillation with acetic acid ear drops (Loock 2012; 93 participants). This study found that more participants had resolution of ear discharge with boric acid powder compared to acetic acid at four weeks (RR 2.61, 95% CI 1.51 to 4.53; 93 participants) but the certainty of the evidence is very low due to the risk of bias in the study not being blinded (for this comparison) and uncertainties about the impact of patients not included in the analysis. It is uncertain whether more patients experienced discomfort with acetic acid drops (RR 0.10, 95% CI 0.01 to 1.81; 93 participants), due to the low number of reported events. Narratively the study reported no difference in hearing outcomes between the groups. Serious complications or suspected ototoxicity were not reported.
Boric acid powder versus hydrogen peroxide ear drops
One study compared boric acid powder instillation with hydrogen peroxide ear drops (Papastavros 1989; unclear number of participants, 48 ears). This study measured the resolution of ear discharge after treatment but only presented the results by ear rather than by person. Data to allow us to account for the correlation were not provided so it was not possible to use the results. No other outcomes were reported.
Overall completeness and applicability of evidence
The overall completeness of the evidence base was lacking. Only five studies were available over the three comparisons and due to problems with how the results were reported, only one study for each comparison provided data for the primary outcome. Four different antiseptics were used across the studies (acetic acid, boric acid (as ear drops in alcohol or as powder), hydrogen peroxide and povidone iodine), which does not represent the full spectrum of different antiseptic agents available. The studies were conducted in community settings (three studies) and secondary care (two studies). Where given, all of the studies' inclusion criteria included children, although one study included both children and adults. The studies were mainly conducted in areas with an estimated high incidence of chronic suppurative otitis media (CSOM): more than four cases per thousand people (Malawi, South Africa, Tanzania and the Solomon Islands) (Monasta 2012).
There were very few data for outcomes other than resolution of ear discharge. No studies reported health‐related quality of life. Adverse events, suspected ototoxicity and serious complications were all poorly reported.
The ongoing I‐HEAR‐BETA trial should provide more information on this question as it compares a topical antiseptic with placebo ear drops (I‐HEAR‐BETA 2014). However, as all participants will also receive topical antibiotics, the study will only be able to identify whether topical antiseptics have any additional impact, over and above the topical antibiotic treatment. When included within this review, the study will feature as a separate comparison.
Quality of the evidence
Generally the included studies were small and not well reported. There were questions over whether the randomisation was adequate in all except one study. Studies were unblinded and suffered from possible selective reporting bias. This limits our ability to draw firm conclusions.
Potential biases in the review process
By only analysing the results from studies that provided the results by person, there were three studies (reporting results by ear) that we were not able to use for the primary outcome. This reduced the amount of data that we were able to analyse. However, as we know that the correlation of results between ears is likely to be high, we felt that the inclusion of the results of both ears in the analysis was likely to lead to double counting and results that could lead to spurious conclusions.
One of the included studies was only published as an (unobtainable) abstract and was identified only through reading the introduction of a separate paper (Van Hasselt 1998b). This raises a concern that there may have been other studies conducted where the results may not have been published, or published in a way that was not identified in the searches. We did attempt to review some regional medical databases (such as IndMed and the African Index Medicus) but there is still a concern that unpublished data may be an issue for this review.
Agreements and disagreements with other studies or reviews
This review is part of a series of reviews on CSOM (Bhutta 2018; Brennan‐Jones 2018a; Brennan‐Jones 2018b; Chong 2018a; Chong 2018b; Head 2018a; Head 2018b).
There are few previous reviews or guidelines for CSOM. The World Health Organization (WHO) in 2004 suggested that first‐line treatment of CSOM should comprise aural toilet and topical antibiotic drops, with second‐line treatment comprising an alternative topical antibiotic (guided by the results of microbiological culture) or parenteral antibiotics (WHO 2004). The Australian government recommendations from 2010 for the treatment of Aboriginal and Torres Strait islanders gave similar recommendations, with first‐line treatment comprising aural toilet (or antiseptic washout) followed by topical antibiotics, and second‐line treatment with parenteral antibiotics (Morris 2010). An expert panel of the American Academy of Otolaryngologists in 2000 came to a similar conclusion (Hannley 2000).
These reviews supersede a pair of previous Cochrane Reviews examining topical antibiotics for CSOM (Macfadyen 2005a; Macfadyen 2006).
The BMJ Best Evidence series on CSOM concluded that it was not possible to tell if topical antiseptics were more effective at resolving otorrhoea than placebo or no treatment (Morris 2012). They did not find evidence for adverse events. Different topical antiseptics were not compared against each other in this review.
One recent review investigating the use of boric acid in chronic suppurative otitis media, which included all types of studies, was consistent with our findings that instilling boric acid may result in fewer people with ear discharge (Adriztina 2018). However, they suggested that ototoxicity at higher concentrations (greater than 4%) should be considered.
Authors' conclusions
Implications for practice.
For consumers
We are uncertain whether topical antiseptics are better at resolving ear discharge in patients with chronic suppurative otitis media (CSOM) compared to placebo or no treatment, or when compared to other antiseptics. Adverse events from using topical antiseptics were not well reported.
For clinicians
It is very uncertain whether boric acid in alcohol drops combined with dry mopping may increase the proportion of patients with dry ears at four weeks and four months compared with dry mopping alone as the certainty of the evidence is very low. Adverse events from treatment were not well reported although the study authors did not find differences in hearing. There is a lack of information for direct comparisons of antiseptics. One study comparing boric acid with acetic acid ear drops found that boric acid (boric powder instillation) was more effective with respect to resolution of ear discharge at four weeks and there may be fewer adverse effects in the boric acid group, although the rate of adverse events was low. None of the studies reported suspected ototoxicity or serious complications.
Implications for research.
The results of this review, current to April 2019, show that there is very low‐certainty evidence that for people with CSOM topical boric acid may be beneficial in improving the resolution of ear discharge at four weeks and four months when compared to placebo. The low certainty of evidence for CSOM treatments in this review is common throughout this suite of seven reviews of CSOM treatments.
There is insufficient evidence to address the implications of topical antiseptics for high‐risk groups such as immunocompromised patients or Indigenous populations. Potential adverse effects and hearing outcomes were not well reported and the impact of background treatment with aural toileting and/or systemic antibiotics is also unclear.
Prior to commencing these reviews we conducted a scoping review that identified a key questions that clinicians, researchers and consumers would like to see answered:
Are topical antiseptics effective when added to other interventions (e.g. aural toileting, systematic antibiotics) compared with no treatment?
Due to the low certainty of the available evidence these questions cannot yet be addressed with any certainty. There is clearly room for more trials examining the impact of topical antiseptics for people with CSOM, including trials that assess the type and method of instillation of antiseptic.
Long‐term effects (effectiveness and harms) are also important. In addition to clinical trials, health services should establish prospective databases for patients with CSOM to record (long‐term) outcomes for resolution of discharge, adverse effects and hearing outcomes for people receiving treatment.
Suggestions for future trials
This review is one of a suite of reviews of treatments for CSOM, each of which features its own research recommendations. Across all reviews, key features of future research are as follows:
Design and methods
Where the intent is to assess the effectiveness of interventions, randomised controlled trials should be conducted. These trials (including those testing non‐systemic interventions), should randomise, analyse and report results by person (not ears).
In patients with bilateral CSOM, for outcomes that can be reported by ear, such as resolution of ear discharge or recurrence, only one finding should be analysed and reported per person. We suggest that a single ear be included in the trial (the decision on which ear is to be included and analysed must be made a priori, and the method or criteria for the decision must explicitly specified in the trial protocol and report). Since there are limited data on whether people with bilateral CSOM respond to treatment in the same way as people with unilateral CSOM, and whether both ears respond in the same way to treatment, reporting these factors would be useful.
Trials need to use appropriate methods for randomisation and allocation concealment to avoid selection bias, and they should be adequately powered.
Attempts should be made by the investigators to blind participants, healthcare professionals and study personnel to the treatment allocation. This could be through the use of a placebo and ensuring that the treatment regimens are the same between treatment arms. A double placebo design should be used where dosage form and/or regimen are different. Where it is not possible to blind participants and/or clinicians to the treatment received, efforts to blind the outcome assessment and analysis personnel should be made.
Population
Diagnosis of CSOM should be according to the World Health Organization (WHO) criteria, be otoscopically confirmed and include an assessment of hearing level.
Potentially important patient characteristics (such as existence of ear grommets) should be recorded and presented in the paper.
If patients from 'high‐risk' groups are included, these characteristics should be accounted for and explored in the design of the study.
Interventions
All interventions (adjunctive therapies and/or allowed treatment) should be the same apart from the treatments being evaluated.
Clear reporting of the therapies used, including dose, frequency and duration, and clear descriptions of any adjunctive therapies used across the treatment groups (including aural toileting), should be provided.
Outcomes
There is currently no core outcome set for CSOM, or a widely agreed set of priority outcomes and definitions for CSOM trials. The development of core outcome sets for CSOM, using established methods (Kirkham 2017), would be beneficial for future trials. This would help to ensure that trials are consistent, high‐quality and examine appropriate outcomes. The standardisation of outcomes allows for analysis and comparison of data across trials (and treatments) using network meta‐analysis or individual participant data meta‐analysis.
The assessment for adverse effects should be defined in the protocol and these should be systematically sought during the trial using explicit methods.
All outcomes (including hearing) should be measured and reported using valid and predefined methods.
A validated quality of life instrument should be used whenever possible.
Studies should follow‐up patients for at least six months and preferably over one year to identify the rate of recurrence of ear discharge, using a pre‐agreed definition of recurrence.
Trials should be registered in a regional or international clinical trials registry and, when published, adhere to reporting guidelines, such as CONSORT. Where publication in a peer‐reviewed journal is not possible, results should be included in the clinical trial report.
Acknowledgements
This project was funded by the NHMRC Centre of Research Excellence in Ear and Hearing Health of Aboriginal and Torres Strait Islander Children (NHMRC CRE_ICHEAR). The contents of the publications arising from this work are solely the responsibility of the authors and do not reflect the views of NHMRC.
We are grateful to Mr Iain Swan for peer reviewing the protocol for this review, and to consumer referee Joan Blakely for her helpful comments. We would also like to thank Dr. Adrian James, as Acting Co‐ordinating Editor for Cochrane ENT, for his insightful comments and advice, and the other members of the Cochrane ENT editorial board for their input and encouragement.
We would like to sincerely thank Jenny Bellorini and Samantha Cox from the Cochrane ENT team for their invaluable help, which has enabled the completion of this suite of reviews. In addition, we thank Professor Amanda Leach and Jessica Daw for their contributions towards the project.
We would also like to thank the following clinicians, scientists and consumers who provided comments on the initial scoping review and prioritisation exercise for this suite of reviews of CSOM: Amanda Leach, Chris Perry, Courtney McMahen, De Wet Swanepoel, Deborah Lehmann, Eka Dian Safitri, Francis Lannigan, Harvey Coates, Has Gunasekera, Ian Williamson, Jenny Reath, Kathy Brooker, Kathy Currie, Kelvin Kong, Matthew Brown, Pavanee Intakorn, Penny Abbot, Samantha Harkus, Sharon Weeks, Shelly Chadha, Stephen O'Leary, Victoria Stroud and Yupitri Pitoyo.
We are indebted to Therese Dalsbø, Artur Gevorgyan, Nathan Gonik, Anna Kashchuk, Esther Martin, Stefano Morettini, Jussi Mustonen, Irina Telegina, Yu‐Tian Xiao, Ibrahim Ethem Yayali, Francine Choi, Chiara Arienti, Maria Paula Garcia, Karen Sagomonyants and Elizabeth Weeda for translating and identifying primary studies for inclusion or exclusion for this suite of reviews. We are also indebted to Erika Ota from Cochrane Japan for organizing a group of MSc students, Shunka Cho, Kiriko Sasayama, Asuka Ohashi, Noyuri Yamaji, Mika Kato, to help with translating and identifying primary studies for inclusion or exclusion for this suite of reviews.
We thank Carolyn McFadyen for her help and support in providing documents from the previous Cochrane Reviews (Macfadyen 2005a; Macfadyen 2006).
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies
| CENTRAL (CRS Web) | MEDLINE (Ovid) | Embase (Ovid) |
| 1 MESH DESCRIPTOR Otitis Media EXPLODE ALL AND CENTRAL:TARGET1061 2 ("otitis media" or OME):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET2347 3 MESH DESCRIPTOR Tympanic Membrane Perforation EXPLODE ALL AND CENTRAL:TARGET71 4 MESH DESCRIPTOR Tympanic Membrane EXPLODE ALL AND CENTRAL:TARGET257 5 ("ear drum*" or eardrum* or tympanic):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET967 6 #4 OR #5 AND CENTRAL:TARGET967 7 (perforat* or hole or ruptur*):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET0 8 #6 AND #7 AND CENTRAL:TARGET0 9 #1 OR #2 OR #3 OR #8 AND CENTRAL:TARGET2386 10 MESH DESCRIPTOR Suppuration EXPLODE ALL AND CENTRAL:TARGET891 11 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or discomfort or earach* or mucopurulen*):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET90987 12 (pain):AB,TI,TO AND CENTRAL:TARGET87639 13 #10 or #11 or #12 AND CENTRAL:TARGET165103 14 MESH DESCRIPTOR Chronic Disease EXPLODE ALL AND CENTRAL:TARGET11305 15 MESH DESCRIPTOR Recurrence EXPLODE ALL AND CENTRAL:TARGET10431 16 (chronic* or persist* or recurr* or repeat*):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET182517 17 #14 OR #15 OR #16 AND CENTRAL:TARGET182523 18 #9 AND #17 AND #13 AND CENTRAL:TARGET378 19 ((chronic* or persist* or recurr* or repeat*) NEAR (ear or ears or aural) NEAR (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or mucopurulen* or pain* or discomfort or disease*)):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET0 20 ((earach* near (chronic or persist* or recurr* or repeat*))):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET3 21 MESH DESCRIPTOR Otitis Media, Suppurative EXPLODE ALL AND CENTRAL:TARGET104 22 (CSOM):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET88 23 #20 OR #21 OR #22 OR #18 OR #19 AND CENTRAL:TARGET418 | 1 exp Otitis Media/ 2 ("otitis media" or OME).ab,ti. 3 exp Tympanic Membrane Perforation/ 4 exp Tympanic Membrane/ 5 ("ear drum*" or eardrum* or tympanic).ab,ti. 6 4 or 5 7 (perforat* or hole or ruptur*).ab,ti. 8 6 and 7 9 1 or 2 or 3 or 4 or 8 10 exp Suppuration/ n 11 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or moist or wet or mucopurulen* or discomfort or pain* or earach*).ab,ti. 12 10 or 11 13 exp Chronic Disease/ 14 exp Recurrence/ 15 (chronic* or persist* or recurr* or repeat*).ab,ti. 16 13 or 14 or 15 17 9 and 12 and 16 18 ((chronic or persist*) adj3 (ear or ears or aural) adj3 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or mucopurulen* or pain* or discomfort)).ab,ti. 19 CSOM.ab,ti. 20 exp Otitis Media, Suppurative/ 21 (earach* adj6 (chronic or persist* or recurr* or repeat*)).ab,ti. 22 17 or 18 or 19 or 20 or 21 |
1 exp otitis media/ 2 ("otitis media" or OME).ab,ti. 3 exp eardrum perforation/ 4 exp eardrum/ 5 ("ear drum*" or eardrum* or tympanic).ab,ti. 6 4 or 5 7 (perforat* or hole or ruptur*).ab,ti. 8 6 and 7 9 1 or 2 or 3 or 8 10 exp suppuration/ 11 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or moist or wet or mucopurulen* or discomfort or pain* or earach*).ab,ti. 12 10 or 11 13 exp chronic disease/ 14 exp recurrent disease/ 15 (chronic* or persist* or recurr* or repeat*).ab,ti. 16 13 or 14 or 15 17 9 and 12 and 16 18 exp suppurative otitis media/ 19 CSOM.ab,ti. 20 ((chronic or persist*) adj3 (ear or ears or aural) adj3 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or mucopurulen* or pain* or discomfort or disease*)).ab,ti. 21 (earach* adj3 (chronic or persist* or recurr* or repeat*)).ab,ti. 22 17 or 18 or 19 or 20 or 21 |
| Web of Science (Web of Knowledge) | CINAHL (EBSCO) | Cochrane ENT Register (CRS Web) |
| #1 TOPIC: ("otitis media" or OME) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years #2 TOPIC: (("ear drum*" or eardrum* or tympanic) AND (perforat* or hole or ruptur*)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years #3 #2 OR #1 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years #4 TOPIC: ((suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or moist or wet or mucopurulen* or discomfort or pain* or earach*) AND (chronic* or persist* or recurr* or repeat*)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years #5 #4 AND #3 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years #6 TOPIC: (((chronic or persist*) NEAR/3 (ear or ears or aural) NEAR/3 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or mucopurulen* or pain* or discomfort))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years #7 TOPIC: ((earach* NEAR/3 (chronic or persist* or recurr* or repeat*))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years #8 #7 OR #6 OR #5 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
S21 S17 OR S18 OR S19 OR S20 S20 TX ((chronic or persist*) N3 (ear or ears or aural) N3 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or mucopurulen* or pain* or discomfort)) S19 TX (earach* N3 (chronic or persist* or recurr* or repeat*)) S18 TX csom S17 S9 AND S12 AND S16 S16 S13 OR S14 OR S15 S15 TX chronic* or persist* or recurr* or repeat* S14 (MH "Recurrence") S13 (MH "Chronic Disease") S12 S10 OR S11 S11 TX suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or moist or wet or mucopurulen* or discomfort or pain* or earach*) S10 (MH "Suppuration+") S9 S1 OR S2 OR S3 OR S8 S8 S6 AND S7 S7 TX perforat* or hole or ruptur* S6 S4 OR S5 S5 TX "ear drum*" or eardrum* or tympanic S4 (MH "Tympanic Membrane") S3 (MH "Tympanic Membrane Perforation") S2 TX "otitis media" or OME S1 (MH "Otitis Media+") |
1 ("otitis media" or OME):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 2 (("ear drum*" or eardrum* or tympanic)):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 3 (perforat* or hole or ruptur*):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 4 #2 AND #3 AND INREGISTER 5 #4 OR #1 AND INREGISTER 6 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or discomfort or earach* or mucopurulen*):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 7 (pain):AB,TI,TO AND INREGISTER 8 #6 OR #7 AND INREGISTER 9 (chronic* or persist* or recurr* or repeat*):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 10 #5 AND #8 AND #9 AND INREGISTER 11 (csom):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 12 (((chronic* or persist* or recurr* or repeat*) and (ear or ears or aural) and (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or mucopurulen* or pain* or discomfort or disease*))):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 13 ((earach* and (chronic or persist* or recurr* or repeat*))):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 14 #10 OR #11 OR #12 OR #13 AND INREGISTER |
| ClinicalTrials.gov (CRS Web) | ICTRP (WHO Portal) | Other |
|
Search 1: (chronic OR persistent OR recurrence OR recurrent) AND (suppuration OR pus OR discharge OR otorrhea or active OR mucopurulent) AND Condition: "Otitis Media" OR OME AND Study type: interventional Search 2: (chronic OR persistent OR recurrence OR recurrent) AND (earache OR "ear ache" OR "ear pain" OR "ear discharge" OR "wet ear" OR "moist ear" OR "weeping ear") AND Study type: interventional Search 3: ("ear drum" OR eardrum OR "tympanic membrane") AND (hole OR perforation OR rupture) AND Study type: interventional |
otitis media AND chronic OR ear discharge OR earache OR wet ear OR weeping ear OR moist ear OR CSOM OR OME AND chronic OR tympanic membrane AND perforation OR eardrum AND hole OR eardrum AND perforation |
LILACS TW:"otitis media" OR "TW:"ear discharge" OR TW:earache OR ((TW:eardrum OR TW:tympanic) AND (TW:perforation OR hole)) OR ((TW:wet OR moist OR weeping) AND TW:ear) AND: Filter: Controlled Clinical Trial IndMed otitis media OR ear discharge OR csom OR earache OR wet ear OR tympanic membrane perforation OR eardrum hole OR wet ear OR weeping ear or moist ear OR OME PakMediNet otitis media | ear discharge | csom | earache | wet ear | tympanic membrane perforation | eardrum hole | wet ear | weeping ear African Index Medicus "otitis media" OR "ear discharge" OR CSOM |
Appendix 2. Data extraction form
| REF ID: | Study title: |
| Date of extraction: | Extracted by: |
| Name and email address of correspondence authors: |
| General comments/notes (internal for discussion): |
FLOW CHART OF TRIAL:
|
Intervention (name the intervention) |
Comparison (name the intervention) |
|
| No. of people screened | ||
| No. of participants randomised ‐ all | ||
| No. randomised to each group | ||
| No. receiving treatment as allocated | ||
| No. not receiving treatment as allocated ‐ Reason 1 ‐ Reason 2 |
||
| No. that dropped out1 (no follow‐up data for any outcome available) |
||
| No. excluded from analysis2 (for all outcomes) ‐ Reason 1 ‐ Reason 2 |
||
1This includes patients who withdrew and provided no data, or did not turn up for follow‐up. 2This should be the people who were excluded from all analyses (e.g. because the data could not be interpreted or the outcome was not recorded for some reason). This is the number of people who dropped out, plus the people who were excluded by the authors for some reason (e.g. non‐compliant).
INFORMATION TO GO INTO THE 'CHARACTERISTICS OF INCLUDED STUDIES' TABLE:
| Methods | X arm, double‐/single‐/non‐blinded, [multicentre] parallel‐group/cross‐over/cluster RCT, with x duration of treatment and x duration of follow‐up |
| Participants |
Location: [country, rural?, no. of sites etc.] Setting of recruitment and treatment: [specialist hospital? general practice? school? state YEAR] Sample size:
Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria: |
| Interventions |
Intervention (n = x): drug name, method of administration, dose per day/frequency of administration, duration of treatment For aural toileting: who does it, methods or tools used, frequency, duration Comparator group (n = y): Concurrent treatment: Use of additional interventions (common to both treatment arms): |
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes
|
| Funding sources | "No information provided"/"None declared"/State source of funding |
| Declarations of interest | "No information provided"/"None declared"/State conflict |
| Notes |
Clinical trial registry no: (if available) Unit of randomisation: person/ears/other (e.g. cluster‐randomised by hospital/school) [In the case of randomisation by person]: Methods for including patients with bilateral disease, for example:
|
RISK OF BIAS TABLE:
(See table 8.5d in the Cochrane Handbook for Systematic Reviews of Interventions: http://handbook.cochrane.org/).
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High/low/unclear risk | Quote: "…" Comment: |
| Allocation concealment (selection bias) | High/low/unclear risk | Quote: "…" Comment: |
| Blinding of participants and personnel (performance bias) | High/low/unclear risk | Quote: "…" Comment: |
| Blinding of outcome assessment (detection bias) | High/low/unclear risk | Quote: "…" Comment: |
| Incomplete outcome data (attrition bias) | High/low/unclear risk | Quote: "…" Comment: |
| Selective reporting (reporting bias) | High/low/unclear risk | Quote: "…" Comment: |
FINDINGS OF STUDY
CONTINUOUS OUTCOMES
| Results (continuous data table) | |||||||
| Outcome | Intervention (name the intervention) |
Comparison (name the intervention) |
Other summary statistics/Notes | ||||
| Mean | SD | N | Mean | SD | N | Mean difference (95% CI), P values etc. | |
|
Disease‐specific health‐related quality of life (COMQ‐12, COMOT‐15, CES)1 Time point: (state) |
|||||||
|
Hearing: [Measurement method: include frequencies and report results separately if they are presented in the paper] Time point: [xx] |
|||||||
| Comments: [If there is no information apart from (vague) narration, quote here] [If information is in the form of graphs, used this software to read it: http://arohatgi.info/WebPlotDigitizer/app/, and save a copy of your charts in a folder] | |||||||
1State the measurement method: this will be instrument name/range for patient‐reported outcomes.
DICHOTOMOUS OUTCOMES
| Results (dichotomous data table) | ||||||
| Outcome | Applicable review/ Intervention1 |
Group A ‐ intervention arm | Group B – control | Other summary statistics/Notes | ||
| No. of people with events | No. of people analysed | No. of people with events | No. of people analysed | P values, RR (95% CI), OR (95% CI) | ||
|
Resolution of ear discharge or 'dry ear' at 1 to 2 weeks [Measurement method or definition used: not/unclear if/otoscopically confirmed]1 Time point: [State actual time point] |
||||||
|
Resolution of ear discharge or 'dry ear' at 2 to 4 weeks [Measurement method or definition used: not/unclear if/otoscopically confirmed] Time point: [xx] |
||||||
|
Resolution of ear discharge or 'dry ear' after 4 weeks [Measurement method or definition used: not/unclear if/otoscopically confirmed] Time point: [xx] |
||||||
|
Ear pain/discomfort/local irritation
[Measurement method or definition used e.g. patient‐reported] Time point: [xx] |
||||||
|
Suspected ototoxicity [Measurement method or definition used] Time point: [xx] |
||||||
|
Sensorineural hearing loss [Measurement method or definition used] Time point: [xx] |
||||||
|
Tinnitus [Measurement method or definition used] Time point: [xx] |
||||||
|
Dizziness/vertigo/balance [Measurement method or definition used] Time point: [xx] |
||||||
|
Serious complications:
[State whether the paper had prespecified looking for this event, how it was diagnosed] Time point: state length of follow‐up of the trial |
Note down the page number /table where info was found for ease of checking | |||||
|
Otitic meningitis [How was this diagnosed?] |
||||||
|
Lateral sinus thrombosis [How was this diagnosed?] |
||||||
|
Cerebellar abscess [How was this diagnosed?] |
||||||
|
Mastoid abscess/mastoiditis [How was this diagnosed?] |
||||||
|
Postauricular fistula [How was this diagnosed?] |
||||||
|
Facial palsy [How was this diagnosed?] |
||||||
|
Other complications [How was this diagnosed?] |
||||||
|
Death [How was this diagnosed?] |
||||||
|
Multiple serious complications [How was this diagnosed?] |
||||||
| Comment/additional notes: If any calculations are needed to arrive at the data above, note this down here. | ||||||
1State briefly how this was measured in the study, especially whether there was deviation from what was expected in the protocol.
For adverse events, note down how these were collected, e.g. whether the adverse event was one of the prespecified events that the study planned to collect, when it was collected and how/who measured it (e.g. as reported by patients, during examination and whether any scoring system was used).
Data and analyses
Comparison 1. Topical antiseptics versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Resolution of ear discharge (2 to 4 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 1 to 2 weeks | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 2 to 4 weeks | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.20, 3.16] |
| 1.3 3 to 4 months | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.21, 2.47] |
Comparison 2. Sensitivity analysis: topical antiseptics versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Resolution of ear discharge (2 to 4 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 No correction | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [1.28, 3.13] |
| 1.2 ICC used in primary analysis(0.015) | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.20, 3.16] |
| 1.3 High ICC (0.03) | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [1.18, 3.40] |
| 2 Resolution of ear discharge (3 to 4 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 No correction | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.26, 2.44] |
| 2.2 ICC used in primary analysis(0.015) | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.21, 2.47] |
| 2.3 High ICC (0.03) | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.17, 2.54] |
Comparison 3. Topical antibiotic A versus topical antibiotic B.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Resolution of ear discharge (2 to 4 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Boric acid powder versus acetic acid | 1 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [1.51, 4.53] |
| 2 Ear pain, discomfort, irritation | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.1 [0.01, 1.81] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Eason 1986.
| Methods | Five‐arm trial with 3 to 6 weeks duration of treatment and 6 weeks duration of follow‐up | |
| Participants |
Location: Solomon Islands, 15 villages around Munda Setting of recruitment and treatment: Helena Goldie Hospital, Munda; patients identified through community screening February 1985 to March 1986 Sample size:
Participant (baseline) characteristics:
High‐risk population: yes
Diagnosis method:
Other important effect modifiers:
Inclusion criteria: Children under 15 years old with CSOM (defined as presence of otorrhoea for more than 3 months and tympanic membrane perforation) living in Munda or principal villages Exclusion criteria: None listed |
|
| Interventions |
Group 1 (n = 31, 40 ears): Sofradex ear drops (0.5% w/v of framycetin sulphate, 0.050% w/v of dexamethasone and 0.005% w/v of gramicidin) (no details on volume or frequency of administration), PLUS oral clindamycin (15 mg/kg/day) into 3 divided oral daily doses,PLUS aural toilet 4 times per day using cotton wool wisps twisted on to orange sticks. Treatment duration = 4 to 6 weeks. Group 2 (n = 31, 41 ears): Sofradex ear drops (0.5% w/v of framycetin sulphate, 0.050% w/v of dexamethasone and 0.005% w/v of gramicidin) (no details on volume or frequency of administration), PLUS aural toilet 4 times per day using cotton wool wisps twisted on to orange sticks. Treatment duration = 4 to 6 weeks. Group 3 (n = 24, 32 ears): 2% boric acid in 20% alcohol (3 drops after cleaning using intermittent tragal depression to assist middle ear permeation) given 4 times per day, PLUS aural toilet using cotton wool wisps twisted on to orange sticks. Treatment duration = 4 to 6 weeks. Group 4 (n = 19, 26 ears): aural toilet 4 times per day using cotton wool wisps twisted on to orange sticks. Treatment duration = 4 to 6 weeks. Group 5 (n = 29, 41 ears): no treatment All treatments administered by parents. Concurrent treatment: Parents were instructed to encourage nose blowing, forbid swimming and insert cotton wool/Vaseline ear plugs before washing. For each child in groups 2 to 5 one of the authors stayed in the village for the first 3 days of treatment to provide parental tuition and supervision. This was continued by a nurse aid who remained until the medical team returned after 3 weeks. If the ear was then dry, the clinical response was judged good, ototopical solutions continued 1 further week only and aural toilet and clindamycin stopped. If the ear was still discharging, all treatment modalities were continued until the second assessment after 6 weeks. |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes: NR |
|
| Funding sources | "This study was made possible by a research grant from the Medical Research Council of New Zealand" | |
| Declarations of interest | No information provided | |
| Notes |
Unit of randomisation: person Methods for including patients bilateral disease: counting bilateral ears separately RCT was part of a larger epidemiological study. Hearing loss was measured for the epidemiological study but not specifically for the RCT. Results are not presented by those who have CSOM and those who do not. Only treatment groups 3 and 4 were relevant for this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk |
Quote: "Children from 15 villages with 184 diseased ears were randomly allocated into five treatment groups." Comment: insufficient information about sequence generation method. The largest group had 1.6 times (31 patients/41 ears) the number of participants compared to the number in the smallest group (19 patients/26 ears), with larger number of patients (31 each) in the more effective treatment groups. Unit of randomisation unclear although it is likely to be by person, results reported by percentage of affected ears. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details about allocation concealment are provided in the paper. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding is not specifically stated. The treatment arms involved different dosage forms (oral versus ear drops) – blinding of these interventions is impossible without the use of placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no clear information about who had assessed that the ears were "dry" versus "still discharging", whether this was done by patients or the medical team. No report of otoscopic examination for outcome. Therefore, in the absence of blinding, this is likely high risk. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no dropouts or missing data reported; no statements about missing data. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available on clinicaltrials.gov. The level of reporting is extremely low. Outcome was reported as two categories: "improved" versus "no change" as opposed to "dry ear" versus others. This definition was not provided, and it was unclear whether "improved" means "dry ear" or a reduction of discharge. Insufficient information to permit judgement of 'low risk' or 'high risk'. |
Loock 2012.
| Methods | Three‐arm, partially blinded, parallel‐group RCT, with up to 8 weeks duration of treatment and follow‐up | |
| Participants |
Location: South Africa, Cape Town, 1 site Setting of recruitment and treatment: otology clinic of the ENT outpatient clinic, Tygerberg Hospital; September 2007 to June 2010 Sample size: 159
Participant (baseline) characteristics:
High‐risk population: no
Diagnosis method:
Other important effect modifiers:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Topical antibiotics (n = 53): ciprofloxacin, ear drops, (no concentration given), 6 drops, 2 times per day for unspecified period (likely to be 4 weeks) Topical antiseptics (acetic acid) (n = 54): 1% acetic acid, ear drops, 6 drops, 2 times per day for unspecified period (likely to be 4 weeks) Topical antiseptics (boric acid) (n = 52): boric acid powder, single administration. After ear toilet and flushing of the middle ear and Eustachian tube with 6 drops of saline, the clinician 'tapped' boric acid powder into the external ear canal (EAC) using a 50 ml 'urological' syringe with a wide mouth, an aural speculum and ambient light and compacted the boric acid powder into the EAC using an 'earbud' until the EAC was filled with powder. The patient was instructed not to disturb the boric acid powder and to keep the ear dry. Concurrent treatment: aural toileting: at the first visit the clinician performed ear toilet by syringing the ear using a naked eye and ambient light only, a 50 ml syringe with a Luer lock and an angled 1 mm diameter suction tip, a clean technique and clean body‐temperature tap‐water, with or without dry mopping, until the perforation was clearly visible. Patients were advised not to get water into the ear. No details of other additional treatments were listed. In all cases, ear drops were 'pumped' down the Eustachian tube using tragal pressure, 6 drops/twice per day. |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes
|
|
| Funding sources | "Funding for purchase of the ciprofloxacin eardrops, audiological services and patient follow‐up visits was obtained through research funds generously provided by the ENT Society of South Africa. Funding for the microbiological investigations was generously sponsored by the National Health Laboratory Service of South Africa (NHLS)." "…the investigator received no sponsorship or incentive from manufacturers of any of the treatments used." |
|
| Declarations of interest | "There was no conflict of interest …" | |
| Notes |
Unit of randomisation: person Methods for including patients with bilateral disease: not stated This was a 3‐arm trial, but only 2 arms (acetic acid and boric acid) are relevant for this review. Although some results are given at 8 weeks, these are only for the people who have failed initial treatment. Therefore only the 4‐week results are presented. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated randomised series (Randomisation.com) generated for three groups in 30‐patient blocks …" Comment: appropriate sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "A computer‐generated randomised series …was kept by a pharmacist at a distant site. This pharmacist supplied sequential opaque dispensing envelopes, numbered in advance according to the randomised sequence, containing the allocated treatment. These envelopes were held by the research nurse, who gave the sealed envelope containing the allocated treatment to the investigator after the patient had been enrolled in the trial." Comment: allocation code only revealed after enrolment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The nurse would then supply the sequentially numbered envelope pre‐prepared by the pharmacist containing the allocated treatment. Each envelope contained an identical unlabelled bottle with one of: 1% acetic acid eardrops; ciprofloxacin eardrops; or normal saline with an added instruction to administer boric acid powder." Comment: although bottles were identical and unlabelled, it is possible to find out the allocated treatment because one of the groups had an additional powder, and it is possible that the acetic acid drops have a characteristic smell. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "At follow‐up, another clinician, unaware of the treatment allocation and hence 'blind' as far as possible, assessed the activity of the ear. Unavoidably, remnants of boric acid powder at times interfered with blinding of this clinician's assessment…. The main outcome measure was whether the clinician judged the perforation to be inactive (dry), active (wet) or 'moist'." Comment: blinding of outcome assessment was attempted, but it is possible that for some patients, the treatment used can be guessed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: loss to follow‐up was 10/54 (18.5%), 3/49 (5.8%) and 8/53 (15.1%) at the assessment at 4 weeks. The paper states that no patient withdrew but the reasons for loss to follow‐up were not provided. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available from clinicaltrial.gov or from the South African registry of clinical trials. The outcomes suggested in the methods section were presented in the results, even where there was a reason that the outcome was not possible to report. Results of the audiometric tests were not well presented. |
Minja 2006.
| Methods | Three‐arm, non‐blinded, cluster‐RCT (randomised by school), with 1 month duration of treatment and 3‐ to 4‐month duration of follow‐up | |
| Participants |
Location: Tanzania, 24 sites Setting of recruitment and treatment: 24 schools in 3 different socially comparable districts in the Dar Es Salaam region of Tanzania. Study published in 2006. Sample size:
Participant (baseline) characteristics:
Diagnosis method:
Other important effect modifiers:
Inclusion criteria:
Exclusion criteria: None listed |
|
| Interventions |
Systemic antibiotics + topical antiseptics (n = 74): amoxicillin (unspecified dose/body weight) for 10 days PLUS boric acid in alcohol ear drops (unspecified concentration) for 1 month. No further information about dosage or frequency of administration. Topical antiseptics (n = 130): boric acid in alcohol ear drops (unspecified concentration) for 1 month. No further information about dosage or frequency of administration. No additional treatment (n = 124): no additional treatment Concurrent treatment for all groups: dry mopping completed daily for 1 month, specific technique not specified. Dry mopping and instillation of boric acid ear drop done by "one teacher ... trained to dry mop the children's ear canal and instil the ear drops" in each school. |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes
|
|
| Funding sources | "SAREC/SIDA Sweden, who supported the study financially" (SAREC is a department of the Swedish International Development Cooperation Agency (SIDA)) | |
| Declarations of interest | Not specifically mentioned, although the paper does say "Dr. Leif Ingvarsson [one of the authors] was responsible for securing the funds." | |
| Notes |
Unit of randomisation: school Methods for including patients bilateral disease: 'dry ear' only counted if both ears were dry. Did not report how many patients had bilateral ear disease. There were a total of 371 non‐intact tympanic drums evaluated for hearing tests. Study did not report how many schools were using each treatment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomised controlled trial" (in abstract). Discussion section stated, "All the children with CSOM in the same school were put in the same treatment group (1, 2 or 3)". Comment: there is no information about randomised sequence generation |
| Allocation concealment (selection bias) | High risk | Quote: "Randomly selected primary schools…" "All the children with CSOM in the same school were put in the same treatment group (1, 2 or 3)". Comment: although the abstract indicates that the study was a randomised controlled trial it then goes on to state that the schools were "randomly selected primary schools", which provides doubt regarding whether the schools in the paper were randomised to treatment group (RCT) or just randomly selected from a list of schools (comparative cohort study). If the schools were randomly allocated to treatment group it is not clear whether the people completing the allocation knew to which group each school was going to be allocated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "All the children with CSOM in the same school were put in the same treatment group (1,2 or 3). Children in one school had no contacts with children from other schools included in the investigation." Comment: a single teacher administered all ear instillation and dry mopping in each school. Not clear if patients and teachers were aware of all treatment options. Unclear how this affects compliance with antibiotics etc. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "otoscopically examined". "Two ORL specialists … hearing pathologists". Comment: team composition described but no description of who evaluated the outcomes and whether they were aware of the treatment received. This is high‐risk as the whole school received the same treatment and patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: loss to follow‐up was uneven across groups, and higher for groups with more treatment. This was 17/74 (23%) in the group with amoxicillin (systemic antibiotics), 25/130 (19%) in the group with boric acid ear drops, and 14/124 (11%) in the group with only dry mopping. Reasons for dropout were not evaluated, but very similar for the 1 month and 3 to 4 months follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol for the trial could be found on the clinicaltrials.gov website. The primary outcome (number with discharging ears) is measured by the patient yet the hearing results are measured on a 'by ear' basis. |
Papastavros 1989.
| Methods | Three‐arm, non‐blinded, parallel‐group RCT, with 3‐week duration of treatment and follow‐up | |
| Participants |
Location: Greece, 1 site Setting of recruitment and treatment: Department of Otolaryngology, General Hospital, published in 1989 Sample size: for whole trial 90 patients (119 ears) = 60 patients with one discharging ear and 29 patients with discharge from both ears
Participant (baseline) characteristics: (only available for the whole study including the systemic antibiotics arm ‐ 90 participants)
High‐risk population: no
Diagnosis method:
Other important effect modifiers:
Inclusion criteria: Chronic suppurative otitis media, either with:
Exclusion criteria:
|
|
| Interventions |
Hydrogen peroxide (n = 21): hydrogen peroxide as ear drops. Dose and frequency of administration unknown. Duration: 10 days if unsuccessful or additional 10 days after successful outcome. Borax powder (n = 27): borax powder insufflation. Dose, frequency and method of insufflation were not given. Duration: 10 days if unsuccessful or additional 10 days after successful outcome The mean duration of treatment for treatment was approximately 19 days. Concurrent treatment: "toileting and debridement of ear was given as necessary" |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes |
Unit of randomisation: not specified Methods for including patients with bilateral disease: not specified. There were 90 patients (119 ears) but results were presented by ear. This was a 3‐arm trial designed to compare systemic antibiotics (based on culture and sensitivity) versus "ototopical agents" which were randomly divided between hydrogen peroxide and borax powder. The study is not included in the systemic antibiotics reviews because it allocated patients with high risk of complications to this group (i.e. not randomised). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "In the ototopical group, each patient was started either on instillation of H2O2 drops or insufflation of borax powder. The determination was again made at random… Some patients, assigned initially to one treatment group were transferred to the other as new cases if the therapeutic outcome was unfavourable." Comment: no information about method of sequence generation. However, there is clear evidence that the study did not follow the principle of randomisation by only allocating patients with more serious risk of complications to the systemic antibiotic group. The study also allowed patients who did not respond to be transferred to another group – which breaks randomisation. Unit of randomisation was not specified, and the number of patients in each intervention not specified. |
| Allocation concealment (selection bias) | High risk | Quote: "The determination was again made at random." "Unsuccessful outcomes: patients were either transferred to the other treatment modality or were released from the study." Comment: no information about method of allocation concealment in the initial treatment allocation, and how many patients were transferred to other treatment modality |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: the study was not blinded. No mention of placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: open‐label study. No details on assessor blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no statements about loss to follow‐up. Unclear whether there were losses ‐ number of patients per treatment arm not reported; number of ears between treatment arms not balanced. |
| Selective reporting (reporting bias) | High risk | Comment: no protocols were found on the World Health Organization clinical trials registry. The study stated several important criteria and assumptions for the measurement of success/failures in the methods section, but no information was provided in the results section. Not all criteria for responses to treatment were fully reported, i.e. recurrence. |
Van Hasselt 1998b.
| Methods | Three‐arm, double‐blind, parallel‐group RCT, with a single application treatment and 1‐week duration of follow‐up | |
| Participants |
Location: Malawi, rural, unclear no of sites Setting of recruitment and treatment: community‐based, study presented in 1998 Sample size:
Participant (Baseline) characteristics:
High‐risk population: not reported, but rural areas in Malawi
Diagnosis method:
Other important effect modifiers:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention 1 (n = unclear): 0.075% ofloxacin in hypromellose (hydroxypropyl methyl‐cellulose (HPMC)) 1.5%, single application (no details of quantity) Intervention 2 (n = unclear): 1% povidone Iodine in 1.5% hypromellose (HPMC), single application (no details of quantity) Intervention 3 (n = unclear): 1.5% hypromellose (HPMC), single application (no details of quantity) Concurrent treatment: suction cleaning in all groups (no information about methods used) before the first treatment. No other information. |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes: NR |
|
| Funding sources | No information provided – other studies by the authors were funded by Christian Blind Mission | |
| Declarations of interest | No information provided | |
| Notes |
Unit of randomisation: unclear, probably by ear Methods for reporting outcomes of patients with bilateral disease: counting bilateral ears separately, but number of patients per group was not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomised" Comment: no information about sequence generation. A lack of baseline characteristics makes it difficult to determine whether there was likely to be bias caused due to the random sequence generation. Unclear if this was randomised by person or by ear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information about allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Double‐blind" Comment: authors state that the trial was double‐blind and the treatment schedules were all the same. Blinding would be possible to do so with ofloxacin and hypromellose alone, however the iodine drops should look different. There is no mention how blinding was achieved. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Double‐blind" Comment: authors state that the trial was double‐blind and the treatment schedules were all the same. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there is no information regarding the number of people starting the trial and so it is not possible to determine whether there was a dropout rate during the trial and whether that could have impacted the results. |
| Selective reporting (reporting bias) | High risk | Comment: the study was not published and was only presented as a conference presentation which cannot be accessed. The information comes from a paragraph in the introduction of a separate paper and so it is not possible to evaluate the methods fully due to lack of information presented. No protocol was available on the WHO clinical trials registry. |
CSOM: chronic suppurative otitis media; F: female; HPMC: hydroxypropyl methyl‐cellulose; M: male; NR: not reported; RCT: randomised controlled trial; WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Asmatullah 2014 | COMPARISON: variety of topical antibiotics (see CSOM‐1) |
| Boesorire 2000 | COMPARISON: steroids added onto topical antibiotics (see CSOM‐4) |
| Browning 1983 | INTERVENTION: standard antibiotics were not given, the choice was dependent on cultures |
| Browning 1988 | COMPARISON: variety of topical antibiotics plus steroids (see CSOM‐4) |
| Clayton 1990 | POPULATION: less than 20% had otorrhoea with "central perforation"; others were patients with otitis externa and mastoid cavity problems INTERVENTION: topical antiseptic compared with topical antibiotics |
| Couzos 2003 | COMPARISON: steroids added onto topical antibiotics (see CSOM‐4) |
| Crowther 1991 | COMPARISON: topical antibiotic plus variety of steroids (see CSOM‐4) |
| de Miguel 1999 | COMPARISON: variety of topical antibiotics (see CSOM‐1), systemic antibiotics versus none (see CSOM‐2) and topical versus systemic antibiotics (see CSOM‐3) |
| Esposito 1990 | COMPARISON: systemic antibiotics versus none (see CSOM‐2), topical antibiotics versus none (see CSOM‐1), topical versus systemic antibiotic (see CSOM‐3) |
| Esposito 1992 | COMPARISON: topical versus systemic antibiotics (see CSOM‐3) |
| Fliss 1990 | COMPARISON: variety of systemic antibiotics (see CSOM‐2) |
| Fradis 1997 | COMPARISON: no comparison of interest; 3‐arm trial comparing 2 different topical antibiotics (see CSOM‐1) versus topical antiseptics (see CSOM‐6) |
| Gendeh 2001 | COMPARISON: steroids added onto topical antibiotics (see CSOM‐4) |
| Ghosh 2012 | COMPARISON: variety of systemic antibiotics (see CSOM‐2) |
| Gupta 2015 | COMPARISON: no comparison of interest; study compares topical antiseptics compared with topical antibiotics (see CSOM‐6) |
| Gyde 1978 | COMPARISON: variety of topical antibiotics (see CSOM‐1) |
| Helmi 2000 | COMPARISON: steroids added onto topical antibiotics (see CSOM‐4) |
| Indudharan 2005 | COMPARISON: steroids added onto topical antibiotics (see CSOM‐4) |
| Jamallulah 2016 | COMPARISON: variety of topical antibiotics (see CSOM‐1) |
| Jaya 2003 | COMPARISON: no comparison of interest; study compares topical antiseptics compared with topical antibiotics (see CSOM‐6) |
| Kasemsuwan 1997 | COMPARISON: topical antibiotic versus none (see CSOM‐1) |
| Kaygusuz 2002 | COMPARISON: topical antibiotics versus none (see CSOM‐1), variety of topical antibiotics plus steroids (see CSOM‐4) |
| Kiris 1998 | COMAPRISON: daily aural toilet versus singular aural toilet (see CSOM‐7) |
| Lazo Saenz 1999 | COMPARISON: steroids added onto topical antibiotics (see CSOM‐4) |
| Leach 2008 | COMPARISON: steroids added onto topical antibiotics (see CSOM‐4) |
| Legent 1994 | COMPARSION: variety of systemic antibiotics (see CSOM‐2) |
| Liu 2003 | COMPARISON: variety of topical antibiotics (see CSOM‐1) |
| Lorente 1995 | COMPARISON: variety of topical antibiotics (see CSOM‐1) |
| Macfadyen 2005 | COMPARISON: no comparison of interest; study compares topical antiseptics compared with topical antibiotics (see CSOM‐6) |
| Mira 1993 | COMPARISON: adding topical antibiotic to systemic antibiotic (see CSOM‐1) |
| Miro 2000 | COMPARISON: steroids added onto topical antibiotics (see CSOM‐4) |
| Nawasreh 2001 | COMPARISON: variety of topical antibiotics (see CSOM‐1) |
| Nwokoye 2015 | COMPARISON: variety of systemic antibiotics (see CSOM‐2) |
| Onali 2018 | COMPARISON: systemic antibiotic versus none (see CSOM‐2) |
| Panchasara 2015 | COMPARISON: steroids added onto topical antibiotics (see CSOM‐4) |
| Picozzi 1983 | COMPARISON: systemic metronidazole versus placebo in people who already had gentamicin plus hydrocortisone ear drops (see CSOM‐2) |
| Povedano 1995 | COMPARISON: systemic versus topical antibiotics (see CSOM‐3) |
| Ramos 2003 | COMPARISON: variety of topical antibiotics (see CSOM‐1), systemic antibiotics added onto topical antibiotics (see CSOM‐2), systemic versus topical antibiotics (see CSOM‐3) and topical antibiotics plus steroid (see CSOM‐4) |
| Renuknanada 2014 | COMPARISON: systemic antibiotics added onto topical antibiotics (see CSOM‐2) |
| Rotimi 1990 | COMPARISON: variety of systemic antibiotics (see CSOM‐2) |
| Roydhouse 1981 | INTERVENTION: intervention is not of interest for this review ‐ bromhexine (mucolytic agent) |
| Sanchez Gonzales 2001 | COMPARISON: variety of systemic antibiotics (see CSOM‐2) |
| Siddique 2016 | COMPARISON: variety of topical antibiotics (see CSOM‐1) |
| Smith 1996 | COMPARISON: aural toilet versus no treatment (see CSOM‐7) |
| Somekh 2000 | COMPARISON: variety of systemic antibiotics (see CSOM‐2) |
| Subramaniam 2001 | COMPARISON: steroids added onto topical antibiotics (see CSOM‐4) |
| Thorpe 2000 | COMPARISON: no comparison of interest; study compares 3 different concentrations of the same topical antibiotic |
| Tong 1996 | COMPARISON: steroids added onto topical antibiotics (see CSOM‐4) |
| Tutkun 1995 | COMPARISON: variety of topical antibiotics (see CSOM‐1) |
| van der Veen 2007 | COMPARISON: systemic antibiotics versus none (see CSOM‐2) |
| van Hasselt 1997 | COMPARISON: no comparison of interest; a 3‐arm trial comparing 2 different topical antibiotics (see CSOM‐1) versus topical antiseptics (see CSOM‐6) |
| van Hasselt 1998a | COMPARISON: variety of topical antibiotics (see CSOM‐1) |
| Vishwakarma 2015 | COMPARISON: no comparison of interest; study compares topical antiseptics versus topical antibiotics (see CSOM‐6) |
| Yuen 1994 | COMPARISON: systemic versus topical antibiotics (see CSOM‐3) |
CSOM‐1: Cochrane Review 'Topical antibiotics for chronic suppurative otitis media' (Brennan‐Jones 2018a). CSOM‐2: Cochrane Review 'Systemic antibiotics for chronic suppurative otitis media' (Chong 2018a). CSOM‐3: Cochrane Review 'Topical versus systemic antibiotics for chronic suppurative otitis media' (Chong 2018b). CSOM‐4: Cochrane Review 'Topical antibiotics with steroids for chronic suppurative otitis media' (Brennan‐Jones 2018b). CSOM‐6: Cochrane Review 'Antibiotics versus topical antiseptics for chronic suppurative otitis media' (Head 2018b). CSOM‐7: Cochrane Review 'Aural toilet (ear cleaning) for chronic suppurative otitis media' (Bhutta 2018).
Characteristics of studies awaiting assessment [ordered by study ID]
Abdul 2005.
| Methods | Unclear: "comparative study" |
| Participants | Active chronic suppurative otitis media |
| Interventions | Local ciprofloxacin versus aluminium acetate 3.5% |
| Outcomes | Unclear |
| Notes | Unable to locate paper It is not clear if there was a control arm from the title of the paper |
Characteristics of ongoing studies [ordered by study ID]
I‐HEAR‐BETA 2014.
| Trial name or title | I HEAR BETA |
| Methods | Multifactorial randomised controlled trial |
| Participants | Australian Aboriginal children (2 months of age and up to 17 years of age) with chronic suppurative otitis media |
| Interventions | All arms will receive standard recommended topical treatment (dry mopping with tissue spears and ciprofloxacin drops 5 drops twice a day) plus: Group 1: oral cotrimoxazole and topical povidone‐iodine ear washouts Group 2: oral cotrimoxazole and NO topical povidone‐iodine ear washouts Group 3: oral placebo and topical povidone‐iodine ear washouts Group 4: oral placebo and NO topical povidone‐iodine ear washouts |
| Outcomes | Presence of ear discharge in either ear, assessed by a trained research nurse using video‐otoscopy before cleaning the ear canal at the end of treatment (16 weeks) and at 1 year |
| Starting date | 2015 |
| Contact information | Prof Peter Morris (peter.morris@menzies.edu.au) and Prof Amanda Leach (amanda.leach@menzies.edu.au) |
| Notes | — |
Differences between protocol and review
There are no differences between the protocol and the review.
Contributions of authors
Karen Head: scoped review, designed and wrote protocol. Screened search results and selected studies, carried out data extraction and 'Risk of bias' assessment and statistical analyses, wrote the text of the review. Lee Yee Chong: scoped review, designed and wrote protocol. Screened search results and selected studies, carried out data extraction and 'Risk of bias' assessment and statistical analyses, reviewed and edited the text of the review. Mahmood F Bhutta: helped to scope, design and write the protocol; reviewed analyses of results and provided clinical guidance at all stages of the review. Reviewed and edited text of the review. Peter S Morris: clinical guidance at all stages of the review; reviewed analyses and reviewed and edited text of the review. Shyan Vijayasekaran: clinical guidance at all stages of the review; reviewed analyses and reviewed and edited text of the review. Martin J Burton: clinical guidance at all stages of the review; reviewed analyses and reviewed and edited text of the review. Wrote the abstract for the review. Anne GM Schilder: clinical guidance at all stages of the review; reviewed analyses and reviewed and edited text of the review. Christopher G Brennan‐Jones: clinical guidance at all stages of the review; reviewed analyses and reviewed and edited text of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
Infrastructure funding for Cochrane ENT
NHMRC Centre of Research Excellence in Ear and Hearing Health of Aboriginal and Torres Strait Islander Children, Australia.
Declarations of interest
Karen Head: none known. Lee Yee Chong: none known. Mahmood F Bhutta: Mahmood Bhutta has received an honorarium from Novus Therapeutics for advice on an experimental treatment for otitis media (not related to any treatment in this review). Peter S Morris: Peter Morris has contributed to an Expert Advisory Group on chronic suppurative otitis media and conjugate pneumococcal vaccines in Australia for Glaxo SmithKline. He has also been a Chief Investigator on project grants from National Health and Medical Research Council of Australia addressing treatments for chronic suppurative otitis media. Shyan Vijayasekaran: none known. Martin J Burton: Professor Martin Burton is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review. Anne GM Schilder: Professor Anne Schilder is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review. Her evidENT team at UCL is supported in part by the National Institute of Health Research (NIHR) University College London Hospitals Biomedical Research Centre. The research is funded by the NIHR and EU Horizon2020. She is the national chair of the NIHR Clinical Research Network ENT Specialty. She is the Surgical Specialty Lead for ENT for the Royal College of Surgeons of England's Clinical Trials Initiative. In her role as director of the NIHR UCLH BRC Deafness and Hearing Problems Theme, she acts as an advisor on clinical trial design and delivery to a range of biotech companies, most currently Novus Therapeutics. Christopher G Brennan‐Jones: none known.
New
References
References to studies included in this review
Eason 1986 {published data only}
- Eason RJ, Harding E, Nicholson R, Nicholson D, Pada J, Gathercole J. Chronic suppurative otitis media in the Solomon Islands: a prospective, microbiological, audiometric and therapeutic survey. New Zealand Medical Journal 1986;99:812‐5. [CENTRAL: CN‐00045614; CRS: 980668; PUBMED: 3466089] [PubMed] [Google Scholar]
Loock 2012 {published data only}
- Loock J. Strategies in the medical treatment of active mucosal chronic otitis media suitable for all levels of healthcare: a randomized controlled trial. Clinical Otolaryngology 2012;37(Suppl 1):165‐6. [CENTRAL: CN‐01008068; CRS: 1738685; EMBASE: 71023646] [Google Scholar]
- Loock JW. A randomised controlled trial of active chronic otitis media comparing courses of eardrops versus one‐off topical treatments suitable for primary, secondary and tertiary healthcare settings. Clinical Otolaryngology 2012;37(4):261‐70. [CENTRAL: CN‐00850193; CRS: 1626058; EMBASE: 365535141; PUBMED: 22804826] [DOI] [PubMed] [Google Scholar]
Minja 2006 {published data only}
- Minja BM, Moshi NH, Ingvarsson L, Bastos I, Grenner J. Chronic suppurative otitis media in Tanzanian school children and its effects on hearing. East African Medical Journal 2006;83(6):322‐5. [CENTRAL: CN‐00568108; CRS: 1397424; PUBMED: 16989377] [DOI] [PubMed] [Google Scholar]
Papastavros 1989 {published data only}
- Papastavros T, Giamarellou H, Varlejides S. Preoperative therapeutic considerations in chronic suppurative otitis media. Laryngoscope 1989;99(6 Pt 1):655‐9. [CENTRAL: CN‐00060222; CRS: 995265; PUBMED: 2725163] [DOI] [PubMed] [Google Scholar]
Van Hasselt 1998b {published and unpublished data}
- Hasselt P. A controlled trial of the treatment of CSOM in rural Malawi. Presentation at the Conference of the Pan‐African Federation of Otorhinolaryngological Societies (PAFOS) in Nairobi. 1998.
- Hasselt P, Kregten E. Treatment of chronic suppurative otitis media with ofloxacin in hydroxypropyl methylcellulose ear drops: a clinical/bacteriological study in a rural area of Malawi. International Journal of Pediatric Otorhinolaryngology 2002;63(1):49‐56. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Asmatullah 2014 {published data only}
- Asmatullah, Khan Q, Nawaz G, Ullah G, Iqbal J, Khan M, et al. Comparison of efficacy of topical ofloxacin and gentamycin in tubotympanic type of chronic suppurative otitis media. Medical Forum Monthly 2015;25(12):68‐71. [CENTRAL: CN‐01084518; CRS: 1815087; EMBASE: 2015070065] [Google Scholar]
Boesorire 2000 {published data only}
- Boesoirie T. A comparative study between ofloxacin ear drop and neomycin‐polymixin b‐hydrocortisone ear drops on the chronic suppurative otitis media. 9th ASEAN ORL Head and Neck Congress 31 March‐1 April, 2001. Singapore, 2001.
- Boesoirie T. A comparative study between ofloxacin ear drops and neomycin‐polymixinb‐hydrocortisone ear drops on the chronic suppurative otitis media. Department of ENT Head and Neck Surgery, University of Padjajaran, Bandung, Indonesia Unpublished, 2000.
Browning 1983 {published data only}
- Browning GG, Picozzi GL, Calder IT, Sweeney G. Controlled trial of medical treatment of active chronic otitis media. British Medical Journal (Clinical Research Ed.) 1983;287(6398):1024. [CENTRAL: CN‐00032195; CRS: 967258; PUBMED: 6412934] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picozzi CL, Calder I, Browning GG, Sweeney G. Controlled trial of medical treatment of active chronic otitis media. Clinical Otolaryngology and Allied Sciences 1982;7:137‐8. [CENTRAL: CN‐00262047; CRS: 1161168] [Google Scholar]
Browning 1988 {published data only}
- Browning GG, Gatehouse S, Calder IT. Medical management of active chronic otitis media: a controlled study. Journal of Laryngology and Otology 1988;102(6):491‐5. [CENTRAL: CN‐00054939; CRS: 989984; PUBMED: 3294318] [DOI] [PubMed] [Google Scholar]
Clayton 1990 {published data only}
- Clayton MI, Osborne JE, Rutherford D, Rivron RP. A double‐blind, randomized, prospective trial of a topical antiseptic versus a topical antibiotic in the treatment of otorrhoea. Clinical Otolaryngology and Allied Sciences 1990;15(1):7‐10. [CENTRAL: CN‐00066816; CRS: 1001848; PUBMED: 2323085] [DOI] [PubMed] [Google Scholar]
Couzos 2003 {published data only}
- Couzos S. The Naccho Ear Trial ‐ a community‐based RCT to improve chronic suppurative otitis media affecting Aboriginal children. 2005 International Meeting of Australian Society of Paediatric Oto‐rhino‐laryngology; 2005 Jul 11‐13; Denarau Island (Fiji). 2005. [CENTRAL: CN‐00614782; CRS: 1435671]
- Couzos S, Lea T, Mueller R, Coates H. A community‐based, multi‐centre, double‐blind randomized controlled trial comparing the effectiveness of topical ciprofloxacin and sofradex as treatment for chronic suppurative otitis media (CSOM) in aboriginal children. 8th International Symposium on Recent Advances in Otitis Media; 2003 Jun 3‐7; Ft. Lauderdale (FL). 2003. [CENTRAL: CN‐00449257; CRS: 1302426]
- Couzos S, Lea T, Mueller R, Murray R, Culbong M. Effectiveness of ototopical antibiotics for chronic suppurative otitis media in Aboriginal children: a community‐based, multicentre, double‐blind randomised controlled trial. Medical Journal of Australia 2003;179(4):185‐90. [CENTRAL: CN‐00439955; CRS: 1294300; EMBASE: 37038200; PUBMED: 12914507] [DOI] [PubMed] [Google Scholar]
- Couzos S, Lea T, Murray R, Culbong M. 'We are not just participants‐we are in charge': the NACCHO ear trial and the process for Aboriginal community‐controlled health research. Ethnicity & Health 2005;10(2):91‐111. [CENTRAL: CN‐00512515; CRS: 1355899; EMBASE: 2005‐04153‐001; PUBMED: 15804658] [DOI] [PubMed] [Google Scholar]
- Dugdale AE. Management of chronic suppurative otitis media. Medical Journal of Australia 2004;180(2):91. [CRS: 7773922] [DOI] [PubMed] [Google Scholar]
Crowther 1991 {published data only}
- Crowther JA, Simpson D. Medical treatment of chronic otitis media: steroid or antibiotic with steroid ear‐drops?. Clinical Otolaryngology and Allied Sciences 1991;16(2):142‐4. [CENTRAL: CN‐00076715; CRS: 1011630; PUBMED: 2070529] [DOI] [PubMed] [Google Scholar]
de Miguel 1999 {published data only}
- Miguel Martinez I, Vasallo Morillas JR, Ramos Macias A. Antimicrobial therapy in chronic suppurative otitis media [Terapeutica antimicrobiana en otitis media cronica supurada]. Acta Otorrinolaringologica Espanola 1999;50(1):15‐9. [CENTRAL: CN‐00161091; CRS: 1091988; PUBMED: 10091344] [PubMed] [Google Scholar]
Esposito 1990 {published data only}
- Esposito S, D'Errico G, Montanaro C. Topical and oral treatment of chronic otitis media with ciprofloxacin. A preliminary study. Archives of Otolaryngology‐Head & Neck Surgery 1990;116(5):557‐9. [CENTRAL: CN‐00067110; CRS: 1002139; PUBMED: 2328112] [DOI] [PubMed] [Google Scholar]
Esposito 1992 {published data only}
- Esposito S, Noviello S, D'Errico G, Montanaro C. Topical ciprofloxacin vs intramuscular gentamicin for chronic otitis media. Archives of Otolaryngology‐Head & Neck Surgery 1992;118(8):842‐4. [CENTRAL: CN‐00086057; CRS: 1020830; PUBMED: 1642836] [DOI] [PubMed] [Google Scholar]
Fliss 1990 {published data only}
- Fliss DM, Dagan R, Houri Z, Leiberman A. Medical management of chronic suppurative otitis media without cholesteatoma in children. Journal of Pediatrics 1990;116(6):991‐6. [CENTRAL: CN‐00067997; CRS: 1003023; PUBMED: 2189979] [DOI] [PubMed] [Google Scholar]
- Leiberman A, Fliss DM, Dagan R. Medical treatment of chronic suppurative otitis media without cholesteatoma in children‐‐a two‐year follow‐up. International Journal of Pediatric Otorhinolaryngology 1992;24(1):25‐33. [CRS: 7072188; PUBMED: 1399301] [DOI] [PubMed] [Google Scholar]
Fradis 1997 {published data only}
- Fradis M, Brodsky A, Ben‐David J, Srugo I, Larboni J, Podoshin L. Chronic otitis media treated topically with ciprofloxacin or tobramycin. Archives of Otolaryngology‐Head & Neck Surgery 1997;123(10):1057‐60. [CENTRAL: CN‐00144425; CRS: 1078973; PUBMED: 9339980] [DOI] [PubMed] [Google Scholar]
- Podoshin L, Brodzki A, Fradis M, Ben‐David J, Larboni J, Srugo I. Local treatment of purulent chronic otitis media with ciprofloxacin. Harefuah 1998;134(1):32‐6, 78. [CENTRAL: CN‐00682735; CRS: 1487878; PUBMED: 9517277] [PubMed] [Google Scholar]
Gendeh 2001 {published data only}
- Gendeh S. A comparative study of ofloxacin otic drops vs framycetin sulfate‐dexamethasone‐gramicidin otic drops in the medical treatment of otitis externa and chronic suppurative otitis media. 9th ASEAN ORL Head and Neck Congress, 31 March‐1 April, 2001. Singapore, 2001.
- Gendeh S. A comparative study of ofloxacinotic drops vs. framycetin sulfate‐dexamethasone‐gramicidin otic drops in the medical treatment of otitis externa and chronic suppurative otitis media. Department of ORL, Hospital University Kabangsaan Malaysia, Kuala Lumpur, Malaysia Unpublished, 2000.
Ghosh 2012 {published data only}
- CTRI/2011/10/002079. Comparison of effectiveness of ciprofloxacin and cefpodoxime in patients with acute attack of chronic middle ear infection. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=3489 (first received 19 October 2011). [CENTRAL: CN‐01013255; CRS: 1743872]
- Ghosh A, Jana U, Khaowas A, Das S, Mandal A, Das N. Comparison of the effectiveness and safety of cefpodoxime and ciprofloxacin in acute exacerbation of chronic suppurative otitis media: a randomized, open‐labeled, phase IV clinical trial. Journal of Pharmacology & Pharmacotherapeutics 2012;3(4):320‐4. [CENTRAL: CN‐00905716; CRS: 1676041; EMBASE: 2013360430; PUBMED: 23326103] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2015 {published data only}
- Gupta C, Agrawal A, Gargav ND. Role of acetic acid irrigation in medical management of chronic suppurative otitis media: a comparative study. Indian Journal of Otolaryngology and Head & Neck Surgery 2015;67(3):314‐8. [CENTRAL: CN‐01098823; CRS: 1829333; PUBMED: 26405670] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gyde 1978 {published data only}
- Gyde MC, Randall RF. Double‐blind comparative study of trimethroprim‐sulphacetamide‐polymyxin b and gentamicin in the treatment of otorrhoea [Etude comparative a double insu de la trimethorprime‐sulfacetamide=polymyxine B et de la gentamicine dans le traitement de l'otorrhee]. Annales d'Oto‐laryngologie et de Chirurgie Cervico Faciale 1978;95(1‐2):43‐55. [CENTRAL: CN‐00018235; CRS: 953322; PUBMED: 207210] [PubMed] [Google Scholar]
Helmi 2000 {published data only}
- Helmi A, Ratna D, Zainul A, Sosialisman E, Alfian FH, Bambang H. The efficacy and safety of ofloxacin otic solution for active suppurative otitis media. Faculty of Medicine, University of Indonesia, Jakarta, Indonesia Unpublished, 2000.
- Helmi A, et al. The efficacy and safety of ofloxacin otic solution for active suppurative otitis media. 9th ASEAN ORL Head and Neck Congress, 31 Mar‐1 Apr, 2001. Singapore, 2001.
Indudharan 2005 {published data only}
- Indudharan R, Valuyeetham KA, Raju SS. Role of glucocorticoids in ototopical antibiotic‐steroid preparations in the treatment of chronic suppurative otitis media. Archives of Medical Research 2005;36(2):154‐8. [CENTRAL: CN‐00512134; CRS: 1355519; EMBASE: 2005181801; PUBMED: 15847949] [DOI] [PubMed] [Google Scholar]
Jamallulah 2016 {published data only}
- Jamalullah M, Babat M, Choudhury IM. Comparison of efficacy of topical gentamycin 0.3% with topical ciprofloxacin 0.6% in patients with active tubotympanic type of chronic suppurative otitis media. Isra Medical Journal 2016;8(1):14‐8. [CENTRAL: CN‐01601161; CRS: 8519228] [Google Scholar]
Jaya 2003 {published data only}
- Jaya C, Job A, Mathai E, Antonisamy B. Evaluation of topical povidone‐iodine in chronic suppurative otitis media. Archives of Otolaryngology‐Head & Neck Surgery 2003;129(10):1098‐100. [CENTRAL: CN‐00452670; CRS: 1305097; EMBASE: 37248310; PUBMED: 14568795] [DOI] [PubMed] [Google Scholar]
Kasemsuwan 1997 {published data only}
- Kasemsuwan L, Clongsuesuek P. A double blind, prospective trial of topical ciprofloxacin versus normal saline solution in the treatment of otorrhoea. Clinical Otolaryngology and Allied Sciences 1997;22(1):44‐6. [CENTRAL: CN‐00138220; CRS: 1072793; PUBMED: 9088679] [DOI] [PubMed] [Google Scholar]
Kaygusuz 2002 {published data only}
- Kaygusuz I, Karlidag T, Gok U, Yalcin S, Keles E, Demirbag E, et al. Efficacy of topical ciprofloxacin and tobramycin in combination with dexamethasone in the treatment of chronic suppurative otitis media [Kronik supuratif otitis media tedavisinde topikal siprofloksasin ve tobramisinin deksametazon ile kullanimi]. Kulak Burun Bogaz Ihtisas Dergisi: KBB [Journal of Ear, Nose, and Throat] 2002;9(2):106‐11. [CENTRAL: CN‐00397704; CRS: 1259444; PUBMED: 12122630] [PubMed] [Google Scholar]
Kiris 1998 {published data only}
- Kiris M, Berktas M, Egeli E, Kutluhan A. The efficacy of topical ciprofloxacin in the treatment of chronic suppurative otitis media. Ear, Nose & Throat Journal 1998;77(11):904‐5, 909. [CENTRAL: CN‐00306946; CRS: 1191282; PUBMED: 9846467] [PubMed] [Google Scholar]
Lazo Saenz 1999 {published data only}
- Lazo Saenz GJ, Alonzo Rojo SE, Perez Blanco A. Topical treatment in chronic otitis media [Tratamiento atopico en otitis media cronica]. Annales de Otorinolaringologia Mexicana 1999;45(1):17‐9. [CENTRAL: CN‐00477445; CRS: 1325497] [Google Scholar]
Leach 2008 {published data only}
- Leach A, Wood Y, Gadil E, Stubbs E, Morris P. Topical ciprofloxin versus topical framycetin‐gramicidin‐dexamethasone in Australian aboriginal children with recently treated chronic suppurative otitis media: a randomized controlled trial. Pediatric Infectious Disease Journal 2008;27(8):692‐8. [CENTRAL: CN‐00650088; CRS: 1463553; EMBASE: 2009258884; PUBMED: 18664984] [DOI] [PubMed] [Google Scholar]
- Morris P, Leach A, Gadil E, Wood Y. Topical ciprofloxacin versus topical sofradex in children with persistent chronic suppurative otitis media: a randomized controlled trial. 8th International Symposium on Recent Advances in Otitis Media; 2003 Jun 3‐7; Fort Lauderdale (FL). 2003:289. [CENTRAL: CN‐00449347; CRS: 1302492]
Legent 1994 {published data only}
- Legent F, Bordure P, Beauvillain C, Berche P, Bordure PH. Controlled prospective study of oral ciprofloxacin versus amoxycillin/clavulanic acid in chronic suppurative otitis media in adults. Chemotherapy 1994;40(Suppl 1):16‐23. [CENTRAL: CN‐00108583; CRS: 1043205; EMBASE: 1994301734; PUBMED: 7805426] [DOI] [PubMed] [Google Scholar]
Liu 2003 {published data only}
- Liu J. The curative effect of Rifampicin solution in the treatment of chronic suppurative otitis media. Journal of Preclinical Medicine College of Shangdong University 2003;17(1):8‐9. [CENTRAL: CN‐00475923; CRS: 1324675] [Google Scholar]
Lorente 1995 {published data only}
- Lorente J, Sabater F, Maristany M, Jimenez R, Menem J, Vinas J, et al. Multicenter study comparing the efficacy and tolerance of topical ciprofloxacin (0.3%) versus topical gentamicin (0.3%) in the treatment of simple, non‐cholesteatomaous chronic otitis media in the suppurative phase [Estudio multicentrico comparativo de la eficacia y tolerancia de ciprofloxacino topico (0.3%) versus gentamicina topica (0.3%) en el tratamiento de la otitis media cronica simple no colesteatomatosa en fase supurativa]. Anales Otorrinolaringologicos Ibero‐americanos 1995;22(5):521‐33. [CENTRAL: CN‐00120014; CRS: 1054615; PUBMED: 7485860] [PubMed] [Google Scholar]
- Sabater F, Maristany M, Mensa J, Villar E, Traserra J. Prospective double‐blind randomized study of the efficacy and tolerance of topical ciprofloxacin vs topical gentamicin in the treatment of simple chronic otitis media and diffuse external otitis [Estudio prospectivo doble‐ciego randomizado de la eficacia y tolerancia de ciprofloxacino topico versus gentamicina topica en el tratamiento de la otitis media cronica supurada simple y de la otitis externa difusa]. Acta Otorrinolaringologica Espanola 1996;47(3):217‐20. [CENTRAL: CN‐00129550; CRS: 1064142; PUBMED: 8924287] [PubMed] [Google Scholar]
Macfadyen 2005 {published data only}
- Macfadyen C, Gamble C, Garner P, Macharia I, Mackenzie I, Mugwe P, et al. Topical quinolone vs. antiseptic for treating chronic suppurative otitis media: a randomized controlled trial. Clinical Otolaryngology 2005;30(2):193‐4. [CENTRAL: CN‐00521519; CRS: 1363906; EMBASE: 2005185914; PUBMED: 15839875] [DOI] [PubMed] [Google Scholar]
- Macfadyen C, Gamble C, Garner P, Macharia I, Mackenzie I, Mugwe P, et al. Topical quinolone vs. antiseptic for treating chronic suppurative otitis media: a randomized controlled trial. Tropical Medicine & International Health 2005;10(2):190‐7. [CENTRAL: CN‐00502876; CRS: 1347086; EMBASE: 2005088099; PUBMED: 15679563] [DOI] [PubMed] [Google Scholar]
Mira 1993 {published data only}
- Mira E, Benazzo M, Mira E, Benazzo M. Ceftizoxime as local therapy in the treatment of recurrences of chronic suppurative otitis media. Journal of Drug Development Supplement 1993;6(Suppl 2):39‐44. [CENTRAL: CN‐00362597; CRS: 1231417] [Google Scholar]
- Mira E, Benazzo M, Mira E, Benazzo M. Clinical evaluation of ceftizoxime (EposerinR) as local therapy in the treatment of recurrences of chronic suppurative otitis media [Uso topico delle cefalosporine nel trattamento delle otiti medie purulente: valutazione della ceftizoxima (eposerin R)]. Rivista Italiana di Otorinolaringologia Audiologia e Foniatria 1992;12(4):219‐25. [CENTRAL: CN‐00624623; CRS: 1442776] [Google Scholar]
Miro 2000 {published data only}
- Miro N. Controlled multicenter study on chronic suppurative otitis media treated with topical applications of ciprofloxacin 0.2% solution in single‐dose containers or combination of polymyxin B, neomycin, and hydrocortisone suspension. Otolaryngology‐Head & Neck Surgery 2000;123(5):617‐23. [CENTRAL: CN‐00331261; CRS: 1206633; PUBMED: 11077352] [DOI] [PubMed] [Google Scholar]
Nawasreh 2001 {published data only}
- Nawasreh O, Fraihat A. Topical ciprofloxacin versus topical gentamicin for chronic otitis media. La Revue de Sante de la Mediterranee Orientale / Al‐Majallah Al‐sihhiyah Li‐sharq Al‐mutawassit [Eastern Mediterranean Health Journal] 2001;7(1‐2):26‐30. [CENTRAL: CN‐00413339; CRS: 1273001; PUBMED: 12596948] [PubMed] [Google Scholar]
Nwokoye 2015 {published data only}
- Bakshi SS. Occurrence of otitis media in children and assessment of treatment options. Journal of Laryngology and Otology 2015;129(12):1253. [CRS: 8521489; PUBMED: 26429519] [DOI] [PubMed] [Google Scholar]
- Nwokoye NN, Egwari LO, Olubi OO. Occurrence of otitis media in children and assessment of treatment options. Journal of Laryngology and Otology 2015;129(8):779‐83. [CRS: 8519954; PUBMED: 26072993] [DOI] [PubMed] [Google Scholar]
Onali 2018 {published data only}
- Onali MA, Bareeqa SB, Zia S, Ahmed SI, Owais A, Ahmad AN. Efficacy of empirical therapy with combined ciprofloxacin versus topical drops alone in patients with tubotympanic chronic suppurative otitis media: a randomized double‐blind controlled trial. Clinical Medicine Insights. Ear Nose & Throat 2018;11:1179550617751907. [CENTRAL: CN‐01445979; CRS: 7538061; PUBMED: 29348711] [DOI] [PMC free article] [PubMed] [Google Scholar]
Panchasara 2015 {published data only}
- CTRI/2012/07/002784. Efficacy and safety of ofloxacin and its combination with dexamethasone in chronic suppurative otitis media ‐ a randomized, double blind, parallel group, comparative study. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=4129 (first received 11 July 2012). [CENTRAL: CN‐00867279; CRS: 1641742] [PMC free article] [PubMed]
- Panchasara A, Singh A, Mandavia D, Jha S, Tripathi C. Efficacy and safety of ofloxacin and its combination with dexamethasone in chronic suppurative otitis media. A randomised, double blind, parallel group, comparative study. Acta Otorhinolaryngologica Italica 2015;35(1):39‐44. [CENTRAL: CN‐01098783; CRS: 1829293; EMBASE: 615600505; PUBMED: 26015650] [PMC free article] [PubMed] [Google Scholar]
Picozzi 1983 {published data only}
- Picozzi G, Browning G, Calder I. Controlled trial of gentamicin and hydrocortisone ear drops with and without systemic metronidazole in the treatment of active chronic otitis media. Clinical Otolaryngology 1983;8:367‐8. [CENTRAL: CN‐00262065; CRS: 1161186] [Google Scholar]
- Picozzi GL, Browning GG, Calder IT. Controlled trial of gentamicin and hydrocortisone ear drops with and without systemic metronidazole in the treatment of active chronic otitis media. Clinical Otolaryngology and Allied Sciences 1984;9:305. [CENTRAL: CN‐00262101; CRS: 1161222] [Google Scholar]
Povedano 1995 {published data only}
- Povedano Rodriguez V, Seco Pinero MJ, Jurado Ramos A, Lopez Villarejo P. Efficacy of topical ciprofloxacin in the treatment of chronic otorrhea [Eficacia del ciprofloxacino topico en el tratamiento de la otorrea cronica]. Acta Otorrinolaringologica Espanola 1995;46(1):15‐8. [CENTRAL: CN‐00113534; CRS: 1048153; PUBMED: 7734157] [PubMed] [Google Scholar]
Ramos 2003 {published data only}
- Ramos A, Ayudarte F, Miguel I, Cuyas JM, Cenjor C. Use of topical ciprofloxacin in chronic suppurating otitis media [Utilizacion del ciprofloxacino topico en la otitis media cronica supurada]. Acta Otorrinolaringologica Espanola 2003;54(7):485‐90. [CENTRAL: CN‐00614808; CRS: 1435687; PUBMED: 14671920] [DOI] [PubMed] [Google Scholar]
Renuknanada 2014 {published data only}
- Renukananda GS, Santosh UP, George NM. Topical vs combination ciprofloxacin in the management of discharging chronic suppurative otitis media. Journal of Clinical and Diagnostic Research 2014;8(6):KC01‐4. [CENTRAL: CN‐00995335; CRS: 1725967; EMBASE: 2014430779; PUBMED: 25121008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rotimi 1990 {published data only}
- Rotimi VO, Olabiyi DA, Banjo TO, Okeowo PA. Randomised comparative efficacy of clindamycin, metronidazole, and lincomycin, plus gentamicin in chronic suppurative otitis media. West African Journal of Medicine 1990;9(2):89‐97. [CENTRAL: CN‐00072366; CRS: 1007294; PUBMED: 2268574] [PubMed] [Google Scholar]
Roydhouse 1981 {published data only}
- Roydhouse N. Bromhexine for otitis media with effusion. New Zealand Medical Journal 1981;94(696):373‐5. [CENTRAL: CN‐00026842; CRS: 961911; PUBMED: 7033848] [PubMed] [Google Scholar]
Sanchez Gonzales 2001 {published data only}
- Sanchez Gonzalez A, Gonzalez Galindo T. An open, comparative study of treatment of chronic middle ear otitis with levofloxacine vs amoxicillin/clavulanate. [Spanish] [Estudio abierto comparativo del tratamiento de otitis media cronica con levofloxacino vs amoxicilina/clavulanato]. Investigacion Medica Internacional 2001;28(1):33‐6. [CENTRAL: CN‐00425055; CRS: 1281854; EMBASE: 2001355708] [Google Scholar]
Siddique 2016 {published data only}
- Siddique W, Hakeem A, Ashfaq K, Khan M, Gul AA. Comparison between the efficacy of topical ciprofloxacin with neomycin in the management of chronic suppurative otitis media. Pakistan Armed Forces Medical Journal 2016;66(2):235‐9. [CENTRAL: CN‐01601188; CRS: 8527048] [Google Scholar]
Smith 1996 {published data only}
- Mackenzie IJ, Smith AW, Hatcher J, Machria I. Randomized controlled trial of treatment of chronic suppurative otitis media in Kenyan school children [abstract]. Clinical Otolaryngology 1997;22:81. [CENTRAL: CN‐00262473; CRS: 1161574] [Google Scholar]
- Smith AW, Hatcher J, Mackenzie IJ, Thompson S, Bal I, Macharia I, et al. Randomised controlled trial of treatment of chronic suppurative otitis media in Kenyan schoolchildren. Lancet 1996;348(9035):1128‐33. [CENTRAL: CN‐00132594; CRS: 1067178; EMBASE: 1996327505; PUBMED: 8888166] [DOI] [PubMed] [Google Scholar]
Somekh 2000 {published data only}
- Somekh E, Cordova Z. Ceftazidime versus aztreonam in the treatment of pseudomonal chronic suppurative otitis media in children. Scandinavian Journal of Infectious Diseases 2000;32(2):197‐9. [CENTRAL: CN‐00296955; CRS: 1183249; PUBMED: 10826908] [DOI] [PubMed] [Google Scholar]
Subramaniam 2001 {published data only}
- Subramaniam K, Jalaludin M, Krishnan G. Comparative study of ofloxacin otic drops versus neomycin‐polymixin b‐hydrocortisone in the medical management of chronic suppurative otitis media. Department of ORL, University Malaya Medical Center, Kuala Lumpur, Malaysia Unpublished, 2000.
- Subramaniam K, Jaludin M, Krishnan G. Comparative study of ofloxacin otic drops versus neomycin‐polymixin hydrocortisone in the medical management of chronic suppurative otitis media. 9th ASEAN ORL Head and Neck Congress, 31 March‐1 April, 2001. Singapore, 2001.
Thorpe 2000 {published data only}
- Thorp MA, Gardiner IB, Prescott CA. Burow's solution in the treatment of active mucosal chronic suppurative otitis media: determining an effective dilution. Journal of Laryngology and Otology 2000;114(6):432‐6. [CENTRAL: CN‐00299231; CRS: 1185520; PUBMED: 10962675] [DOI] [PubMed] [Google Scholar]
Tong 1996 {published data only}
- Tong MC, Woo JK, Hasselt CA. A double‐blind comparative study of ofloxacin otic drops versus neomycin‐polymyxin B‐hydrocortisone otic drops in the medical treatment of chronic suppurative otitis media. Journal of Laryngology & Otology 1996;1104:309‐14. [DOI] [PubMed] [Google Scholar]
Tutkun 1995 {published data only}
- Ozagar A, Koc A, Ciprut A, Tutkun A, Akdas F, Sehitoglu MA. Effects of topical otic preparations on hearing in chronic otitis media. Otolaryngology ‐ Head and Neck Surgery 1997;117(4):405‐8. [CENTRAL: CN‐00144419; CRS: 1078967; PUBMED: 9339804] [DOI] [PubMed] [Google Scholar]
- Tutkun A, Ozagar A, Koc A, Batman C, Uneri C, Sehitoglu MA. Treatment of chronic ear disease. Topical ciprofloxacin vs topical gentamicin. Archives of Otolaryngology‐Head & Neck Surgery 1995;121(12):1414‐6. [CENTRAL: CN‐00121174; CRS: 1055775; PUBMED: 7488373] [DOI] [PubMed] [Google Scholar]
van der Veen 2007 {published data only}
- Boonacker CW, Veen EL, Wilt GJ, Schilder AG, Rovers MM. Trimethoprim‐sulfamethoxazole in children with chronic otitis media: a randomized comparison of costs and effects. Otology & Neurotology 2008;29(7):961‐4. [CENTRAL: CN‐00666283; CRS: 1473130; PUBMED: 18758386] [DOI] [PubMed] [Google Scholar]
- Miller JL, Honey BL, Johnson PN, Hagemann TM. Effectiveness of trimethoprim/sulfamethoxazole for children with chronic active otitis media. Pediatrics 2007;120(6):1403. [CRS: 8527167; PUBMED: 18055693] [DOI] [PubMed] [Google Scholar]
- NCT00189098. Effectiveness of sulfamethoxazole‐trimethoprim in the treatment of chronic otitis media. Https://clinicaltrials.gov/show/nct00189098 (first received 16 September 2005). [CENTRAL: CN‐01039521; CRS: 1770119]
- Verhoeff M, Rovers MM, Sanders EAM, Schilder AGM. The COCO‐study: a randomized clinical trial of the efficacy of trimethoprim‐sulfamethoxazole (co‐trimoxazole) in children with chronic suppurative otitis media. Clinical Otolaryngology and Allied Sciences 2004;29(4):460. [CENTRAL: CN‐00874123; CRS: 1647964] [Google Scholar]
- Veen EL, Rovers MM, Albers FW, Sanders EA, Schilder AG. Effectiveness of trimethoprim/sulfamethoxazole for children with chronic active otitis media: a randomized, placebo‐controlled trial. Pediatrics 2007;119(5):897‐904. [CENTRAL: CN‐00588516; CRS: 1415321; PUBMED: 17473089] [DOI] [PubMed] [Google Scholar]
- Veen EL, Schilder AG, Timmers TK, Rovers MM, Fluit AC, Bonten MJ, et al. Effect of long‐term trimethoprim/sulfamethoxazole treatment on resistance and integron prevalence in the intestinal flora: a randomized, double‐blind, placebo‐controlled trial in children. Journal of Antimicrobial Chemotherapy 2009;63(5):1011‐6. [CENTRAL: CN‐00697186; CRS: 1500349; PUBMED: 19297377] [DOI] [PubMed] [Google Scholar]
van Hasselt 1997 {published data only}
- Hasselt P, Hasselt P. Pilot trial of treatment of chronic suppurative otitis media (CSOM) with several types of ear drops in Nkota Kota District, Malawi. Internal Report of the Christian Blind Mission International 1997. [CENTRAL: CN‐00519675; CRS: 1362364]
- Hasselt P, Kregten E. Treatment of chronic suppurative otitis media with ofloxacin in hydroxypropyl methylcellulose ear drops: a clinical/bacteriological study in a rural area of Malawi. International Journal of Pediatric Otorhinolaryngology 2002;63(1):49‐56. [CENTRAL: CN‐00519676; CRS: 1362365] [DOI] [PubMed] [Google Scholar]
van Hasselt 1998a {published data only}
- Hasselt P. A controlled trial of the treatment of CSOM in rural Malawi. Conference of the Pan‐African Federation of Otorhinolaryngological Societies (PAFOS); 1998; Nairobi. 1998. [CENTRAL: CN‐00519673; CRS: 1362362]
- Hasselt P, Kregten E. Treatment of chronic suppurative otitis media with ofloxacin in hydroxypropyl methylcellulose ear drops: a clinical/bacteriological study in a rural area of Malawi. International Journal of Pediatric Otorhinolaryngology 2002;63(1):49‐56. [CENTRAL: CN‐00519676; CRS: 1362365] [DOI] [PubMed] [Google Scholar]
Vishwakarma 2015 {published data only}
- Vishwakarma K, Khan FA, Nizamuddin S, Signh P, Yadav L. Role of topical acetic acid in comparison to gentamicin for the management of chronic suppurative otitis media. International Archives of Biomedical and Clinical Research 2015;1(1):13‐6. [CENTRAL: CN‐01601165; CRS: 8527169] [Google Scholar]
Yuen 1994 {published data only}
- Yuen PW, Lau SK, Chau PY, Hui Y, Wong SF, Wong S, et al. Ofloxacin eardrop treatment for active chronic suppurative otitis media: prospective randomized study. American Journal of Otology 1994;15(5):670‐3. [CENTRAL: CN‐00122894; CRS: 1057491; EMBASE: 1994288260; PUBMED: 8572070] [PubMed] [Google Scholar]
References to studies awaiting assessment
Abdul 2005 {published data only}
- Abdul ME, Shabana Y, Ghonim M. Comparative study of the efficacy of local ciprofloxacin versus aluminum acetate 3.5% in the management of active chronic suppurative otitis media [CSOM]. New Egyptian Journal of Medicine 2005;32:190‐3. [Google Scholar]
References to ongoing studies
I‐HEAR‐BETA 2014 {published data only}
- ACTRN12614000234617. Among Aboriginal children (2 months of age and up to 17 years of age) with chronic suppurative otitis media, is 4 months of povidone‐iodine ear wash and/or oral cotrimoxazole in addition to standard treatment (cleaning and dry mopping with tissue spears plus topical ciprofloxacin) superior to standard treatment alone for resolving ear discharge? A 2x2 factorial randomised controlled trial. www.anzctr.org.au (first received 25 March 2014). [ACTRN12614000234617]
Additional references
Adriztina 2018
- Adriztina I, Adenin LI, Lubis YM. Efficacy of boric acid as a treatment of choice for chronic suppurative otitis media and its ototoxicity. Korean Journal of Family Medicine 2018;39:2‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baumann 2011
- Baumann I, Gerendas B, Plinkert PK, Praetorius M. General and disease‐specific quality of life in patients with chronic suppurative otitis media‐‐a prospective study. Health and Quality of Life Outcomes 2011;9:48. [DOI: 10.1186/1477-7525-9-48] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhutta 2011
- Bhutta MF, Williamson IG, Sudhoff HH. Cholesteatoma. BMJ 2011;342:d1088. [DOI: 10.1136/bmj.d1088] [DOI] [PubMed] [Google Scholar]
Bhutta 2016
- Bhutta MF. Evolution and otitis media: a review, and a model to explain high prevalence in indigenous populations. Human Biology 2016;87(2):92‐108. [DOI] [PubMed] [Google Scholar]
Bhutta 2018
- Bhutta MF, Head K, Chong LY, Tu N, Schilder AGM, Burton MJ, et al. Aural toilet (ear cleaning) for chronic suppurative otitis media. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD013057] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brennan‐Jones 2018a
- Brennan‐Jones CG, Head K, Chong LY, Tu N, Burton MJ, Schilder AGM, et al. Topical antibiotics for chronic suppurative otitis media. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD013051.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brennan‐Jones 2018b
- Brennan‐Jones CG, Chong LY, Head K, Tu N, Burton MJ, Schilder AGM, et al. Topical antibiotics with steroids for chronic suppurative otitis media. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD013054] [DOI] [PubMed] [Google Scholar]
Chong 2018a
- Chong LY, Head K, Richmond P, Snelling T, Schilder AGM, Burton MJ, et al. Systemic antibiotics for chronic suppurative otitis media. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD013052] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chong 2018b
- Chong LY, Head K, Richmond P, Snelling T, Schilder AGM, Burton MJ, et al. Topical versus systemic antibiotics for chronic suppurative otitis media. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD013053] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dubey 2007
- Dubey SP, Larawin V. Complications of chronic suppurative otitis media and their management. Laryngoscope 2007;117(2):264‐7. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Elemraid 2010
- Elemraid MA, Brabin BJ, Fraser WD, Harper G, Faragher B, Atef Z, et al. Characteristics of hearing impairment in Yemeni children with chronic suppurative otitis media: a case‐control study. International Journal of Pediatric Otorhinolaryngology 2010;74(3):283‐6. [DOI] [PubMed] [Google Scholar]
Gates 2002
- Gates GA, Klein JO, Lim DJ, Mogi G, Ogra PL, Pararella MM, et al. Recent advances in otitis media. 1. Definitions, terminology, and classification of otitis media. Annals of Otology, Rhinology & Laryngology. Supplement 2002;188:8‐18. [DOI] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hannley 2000
- Hannley MT, Denneny JC, Holzer SS. Use of ototopical antibiotics in treating 3 common ear diseases. Otolaryngology—Head and Neck Surgery 2000;122(6):934‐40. [DOI] [PubMed] [Google Scholar]
Head 2018b
- Head K, Chong LY, Bhutta MF, Morris PS, Vijayasekaran S, Burton MJ, et al. Antibiotics versus topical antiseptics for chronic suppurative otitis media. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD013056.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 2013
- Jensen RG, Koch A, Homøe P. The risk of hearing loss in a population with a high prevalence of chronic suppurative otitis media. International Journal of Pediatric Otorhinolaryngology 2013;77(9):1530‐5. [DOI: 10.1016/j.ijporl.2013.06.025] [DOI] [PubMed] [Google Scholar]
Kirkham 2017
- Kirkham JJ, Davis K, Altman DG, Blazeby JM, Clarke M, Tunis S, Williamson PR. Core Outcome Set ‐ STAndards for Development: The COS‐STAD recommendations. PLoS Medicine 2017;14(11):e1002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahadevan 2012
- Mahadevan M, Navarro‐Locsin G, Tan HK, Yamanaka N, Sonsuwan N, Wang PC, et al. A review of the burden of disease due to otitis media in the Asia‐Pacific. International Journal of Pediatric Otorhinolaryngology 2012;76(5):623‐35. [DOI: 10.1016/j.ijporl.2012.02.031] [DOI] [PubMed] [Google Scholar]
McDonnell 1999
- McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clinical Microbiology Reviews 1999;12:147–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Monasta 2012
- Monasta L, Ronfani L, Marchetti F, Montico M, Vecchi Brumatti L, Bavcar A, et al. Burden of disease caused by otitis media: systematic review and global estimates. PloS One 2012;7(4):e36226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 2010
- Morris P, Leach A, Shah P, Nelson S, Anand A, Allnutt R, Bainbridge D, Edwards K, Patel H. Recommendations for Clinical Care Guidelines on the Management of Otitis Media: In Aboriginal and Torres Strait Islander Populations. Canberra: Office for Aboriginal and Torres Strait Islander Health, Australian Government, 2010. [Google Scholar]
Morris 2012
- Morris P. Chronic Suppurative Otitis Media. BMJ Clinical Evidence 6 Aug 2012;0507:1. [CENTRAL: PMC3412293; PUBMED: 23870746] [PMC free article] [PubMed] [Google Scholar]
Nadol 2000
- Nadol JB Jr, Staecker H, Gliklich RE. Outcomes assessment for chronic otitis media: the Chronic Ear Survey. Laryngoscope 2000;110(3 Pt 3):32‐5. [DOI: 10.1097/00005537-200003002-00009] [DOI] [PubMed] [Google Scholar]
Olatoke 2008
- Olatoke F, Ologe FE, Nwawolo CC, Saka MJ. The prevalence of hearing loss among schoolchildren with chronic suppurative otitis media in Nigeria, and its effect on academic performance. Ear, Nose, & Throat Journal 2008;87(12):E19. [PubMed] [Google Scholar]
Orji 2013
- Orji F. A survey of the burden of management of chronic suppurative otitis media in a developing country. Annals of Medical and Health Sciences Research 2013;4(3):598‐601. [DOI: 10.4103/2141-9248.122126] [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillips 2014a
- Phillips JS, Yung MW. COMQ‐12 scores in adult patients without chronic middle ear disease. Clinical Otolaryngology 2014;39(6):362‐7. [DOI: 10.1111/coa.12306] [DOI] [PubMed] [Google Scholar]
Phillips 2014b
- Phillips JS, Haggard M, Yung M. A new health‐related quality of life measure for active chronic otitis media (COMQ‐12): development and initial validation. Otology & Neurotology 2014;35(3):454‐8. [DOI: 10.1097/mao.0000000000000205] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schilder 2016
- Schilder AG, Chonmaitree T, Cripps AW, Rosenfeld RM, Casselbrant ML, Haggard MP, et al. Otitis media. Nature Reviews Disease Primers 2016;2:16063. [DOI: 10.1038/nrdp.2016.63] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheldon 2005
- Sheldon AT. Antiseptic "resistance": real or perceived threat?. Clinical Infectious Diseases 2005;40(11):1650‐6. [DOI] [PubMed] [Google Scholar]
Stedman 2011
- Stedman MR, Curtin F, Elbourne DR, Kesselheim AS, Brookhart MA. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2011;40(6):1732‐4. [DOI: 10.1093/ije/dyp345] [DOI] [PubMed] [Google Scholar]
van der Veen 2006
- Veen EL, Schilder AG, Heerbeek N, Verhoeff M, Zielhuis GA, Rovers MM. Predictors of chronic suppurative otitis media in children. Archives of Otolaryngology‐‐Head & Neck Surgery 2006;132(10):1115‐8. [DOI: 10.1001/archotol.132.10.1115] [DOI] [PubMed] [Google Scholar]
van Dinther 2015
- Dinther J, Droessaert V, Camp S, Vanspauwen R, Maryn Y, Zarowski A, et al. Validity and test‐retest reliability of the Dutch Version of the Chronic Otitis Media Questionnaire 12 (COMQ‐12). Journal of International Advanced Otology 2015;11(3):248‐52. [DOI: 10.5152/iao.2015.1701] [DOI] [PubMed] [Google Scholar]
Verhoeff 2006
- Verhoeff M, Veen EL, Rovers MM, Sanders EA, Schilder AG. Chronic suppurative otitis media: a review. International Journal of Pediatric Otorhinolaryngology 2006;70(1):1‐12. [DOI] [PubMed] [Google Scholar]
WHO 2004
- World Health Organization. Chronic Suppurative Otitis Media (CSOM): Burden of Illness and Management Options. Geneva, Switzerland: World Health Organization, 2004. [Google Scholar]
Yorgancılar 2013
- Yorgancılar E, Yildirim M, Gun R, Bakir S, Tekin R, Gocmez C, et al. Complications of chronic suppurative otitis media: a retrospective review. European Archives of Oto‐rhino‐laryngology 2013;270(1):69‐76. [DOI: 10.1007/s00405-012-1924-8] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Head 2018a
- Head K, Chong LY, Bhutta MF, Morris PS, Vijayasekaran S, Burton MJ, et al. Topical antiseptics for chronic suppurative otitis media. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD013055] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macfadyen 2005a
- Macfadyen CA, Acuin JM, Gamble CL. Topical antibiotics without steroids for chronically discharging ears with underlying eardrum perforations. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD004618.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macfadyen 2006
- Macfadyen CA, Acuin JM, Gamble CL. Systemic antibiotics versus topical treatments for chronically discharging ears with underlying eardrum perforations. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD005608] [DOI] [PubMed] [Google Scholar]


