Abstract
Background
Acute lower respiratory tract infections (LRTI) range from acute bronchitis and acute exacerbations of chronic bronchitis to pneumonia. Approximately five million people die from acute respiratory tract infections annually. Among these, pneumonia represents the most frequent cause of mortality, hospitalisation and medical consultation. Azithromycin is a macrolide antibiotic, structurally modified from erythromycin and noted for its activity against some gram‐negative organisms associated with respiratory tract infections, particularly Haemophilus influenzae (H. influenzae).
Objectives
To compare the effectiveness of azithromycin to amoxycillin or amoxycillin/clavulanic acid (amoxyclav) in the treatment of LRTI, in terms of clinical failure, incidence of adverse events and microbial eradication.
Search methods
We searched CENTRAL (2014, Issue 10), MEDLINE (January 1966 to October week 4, 2014) and EMBASE (January 1974 to November 2014).
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs, comparing azithromycin to amoxycillin or amoxycillin/clavulanic acid in participants with clinical evidence of an acute LRTI, such as acute bronchitis, pneumonia and acute exacerbation of chronic bronchitis.
Data collection and analysis
The review authors independently assessed all potential studies identified from the searches for methodological quality. We extracted and analysed relevant data separately. We resolved discrepancies through discussion. We initially pooled all types of acute LRTI in the meta‐analyses. We investigated the heterogeneity of results using the forest plot and Chi2 test. We also used the index of the I2 statistic to measure inconsistent results among trials. We conducted subgroup and sensitivity analyses.
Main results
We included 16 trials involving 2648 participants. We were able to analyse 15 of the trials with 2496 participants. The pooled analysis of all the trials showed that there was no significant difference in the incidence of clinical failure on about days 10 to 14 between the two groups (risk ratio (RR), random‐effects 1.09; 95% confidence interval (CI) 0.64 to 1.85). A subgroup analysis in trials with acute bronchitis participants showed significantly lower clinical failure in the azithromycin group compared to amoxycillin or amoxyclav (RR random‐effects 0.63; 95% CI 0.45 to 0.88). A sensitivity analysis showed a non‐significant reduction in clinical failure in azithromycin‐treated participants (RR 0.55; 95% CI 0.25 to 1.21) in three adequately concealed studies, compared to RR 1.32; 95% CI 0.70 to 2.49 in 12 studies with inadequate concealment. Twelve trials reported the incidence of microbial eradication and there was no significant difference between the two groups (RR 0.95; 95% CI 0.87 to 1.03). The reduction of adverse events in the azithromycin group was RR 0.76 (95% CI 0.57 to 1.00).
Authors' conclusions
There is unclear evidence that azithromycin is superior to amoxycillin or amoxyclav in treating acute LRTI. In patients with acute bronchitis of a suspected bacterial cause, azithromycin tends to be more effective in terms of lower incidence of treatment failure and adverse events than amoxycillin or amoxyclav. However, most studies were of unclear methodological quality and had small sample sizes; future trials of high methodological quality and adequate sizes are needed.
Keywords: Humans; Acute Disease; Amoxicillin; Amoxicillin/therapeutic use; Amoxicillin‐Potassium Clavulanate Combination; Amoxicillin‐Potassium Clavulanate Combination/therapeutic use; Anti‐Bacterial Agents; Anti‐Bacterial Agents/therapeutic use; Azithromycin; Azithromycin/therapeutic use; Bronchitis; Bronchitis/drug therapy; Drug Therapy, Combination; Pneumonia; Pneumonia/drug therapy; Randomized Controlled Trials as Topic; Respiratory Tract Infections; Respiratory Tract Infections/drug therapy; Treatment Failure
Plain language summary
Azithromycin for acute lower respiratory tract infections
Review question We conducted this review to compare azithromycin with amoxycillin or amoxyclav in treating acute lower respiratory tract infections (LRTI).
Background Acute lower respiratory tract infections (LRTI) are one of the most common diagnoses in outpatient settings. They range from acute bronchitis and acute exacerbations of chronic bronchitis to pneumonia. Azithromycin is a subclass of macrolide antibiotics and is used to treat certain bacterial infections.
Search date We searched for trials published and pending as at November 2014.
Study characteristics Randomised controlled trials (RCTs) and quasi‐RCTs, comparing azithromycin to amoxycillin or amoxycillin/clavulanic acid in participants with clinical evidence of an acute LRTI, such as acute bronchitis, pneumonia and acute exacerbation of chronic bronchitis.
Key results We analysed the results from 15 trials with 2496 participants. The effects of azithromycin on cure, improvement or failure were not better than those of amoxycillin or amoxyclav. However, azithromycin seems to have a lower incidence of adverse events than amoxycillin or amoxyclav but it is not significant.
Quality of evidence Overall the quality of the evidence for the main outcome is low as only three of 15 included trials showed adequate allocation concealment. Hence, currently, there is insufficient evidence to show conclusively that azithromycin is superior to amoxycillin or amoxyclav in treating acute LRTI.
Background
Description of the condition
The spectrum of acute lower respiratory tract infection (LRTI) ranges from acute bronchitis and acute exacerbations of chronic bronchitis to pneumonia. Annually approximately five million people die of acute respiratory tract infections. Among these, pneumonia represents the most frequent cause of mortality, hospitalisation and medical consultation (Bariffi 1995).
Acute bronchitis is one of the most common diagnoses in outpatients. The diagnosis of acute bronchitis is mainly based on the symptom of cough and it is usually mild and self limiting. Acute bronchitis with underlying pulmonary diseases or a prolonged cough of more than two weeks are considered for antibiotic therapy (Knutson 2002). A prospective multicentre study of 359 cases of community‐acquired pneumonia in the United States reported that 58.5% had identifiable pathogens, 32.9% had unknown aetiology and 8.6% had aspiration‐related and post‐obstructive pneumonia. The most frequent aetiologic agent was Streptococcus pneumoniae (S. pneumoniae) (15%), followed by Haemophilus influenzae (H. influenzae) (10.9%), Legionella spp (6.7%) and Chlamydia pneumoniae (C. pneumoniae) (6.1%) (Fang 1990). A study in The Netherlands of 145 adults with LRTI showed that a bacterial cause was found in 43 cases (30%) and a viral cause in 57 cases (39%). Influenza A virus was the most frequently diagnosed micro‐organism. The most frequently identified bacterial agents were H. influenzae (9%) and Mycoplasma pneumoniae (M. pneumoniae) (9%) followed by S. pneumoniae (6%) (Graffelman 2004).
Description of the intervention
Antimicrobial treatment in LRTI has to be effective, partly because of the need to reduce the cost and also due to the problem of increasing resistance to the commonly used antibiotics (Legnani 1997). It has also been suggested that the start of therapy should not be delayed for longer than six hours for diagnostic studies (Brown 1998). The importance of early antimicrobial treatment was supported by a study in elderly patients with pneumonia, which showed that 30‐day mortality was lower after administration of antibiotics within eight hours of arrival at hospital, than after delayed treatment (Meehan 1997). Compliance is also important, particularly in outpatients. A study related to medical compliance for the outpatient management of infectious diseases indicated that there was an inverse relationship between frequency of dose and compliance. A short‐term regimen, requiring administration once a day, was found to have the highest compliance rate ‐ 80% compared to 69% and 38% for administration twice a day and three times a day, respectively (Sclar 1994).
Amoxycillin, an oral antibiotic, constitutes extended spectrum penicillin and is active against many aerobic gram‐negative bacilli encountered in patients with pneumonia. By combining the beta‐lactamase inhibitor clavulanic acid with amoxycillin, the invitro spectrum of penicillin is expanded to include beta‐lactamase producing organisms, which would otherwise be resistant to this drug (Mandell 1994). Amoxycillin has been accepted as one of the first choice antibiotics in patients with community‐acquired LRTI. Amoxycillin‐clavulanic acid is recommended particularly in the high prevalence area of beta‐lactamase producing organisms, and also when an aetiologic agent is not identified (Bartlett 1998; Huchon 1998).
How the intervention might work
Azithromycin is a macrolide antibiotic structurally modified from erythromycin with an expanded spectrum of activity and improved tissue pharmacokinetic characteristics relative to erythromycin. The drug is noted for its activity against some gram‐negative organisms associated with respiratory tract infections, particularly H. influenzae. Azithromycin has similar properties to other macrolides against S. pneumoniae and Moraxella catarrhalis (M. catarrhalis), and is active against atypical pathogens such as Legionella pneumophilae (L. pneumophilae), C. pneumoniae and M. pneumoniae (Dunn 1996).
Why it is important to do this review
Over the past 30 years, strains of S. pneumoniae with diminished susceptibility to penicillin have emerged and spread worldwide (Austrian 1994). Cross‐resistance to other antibiotics has also been reported in many strains of S. pneumoniae that have diminished susceptibility to penicillin and cephalosporin (Goldstein 1996). A number of studies have indicated the importance of M. pneumoniae as the main aetiologic agent in ambulatory patients with pneumonia (Berntsson 1986; Langille 1993; Marrie 1996). Co‐infection by more than one pathogen was also reported, and ranged from less than 10% to 38.9% (Lieberman 1996). The value of routine microbial investigation in all patients with LRTI is uncertain (Woodhead 1991). A survey on the management of 2056 such infections, obtained from general practitioners in France, Germany, Italy, Spain and the UK, reported that microbiological examination was performed in only 7% of cases compared to 22% for chest radiography (Woodhead 1996).
This review compares the effects of azithromycin and amoxycillin or amoxycillin‐clavulanic acid in treating acute LRTI such as acute bronchitis, pneumonia and acute exacerbation of chronic bronchitis in terms of clinical failure, incidence of adverse events and microbial eradication.
Objectives
To compare the effectiveness of azithromycin to amoxycillin or amoxycillin/clavulanic acid (amoxyclav) in the treatment of LRTI, in terms of clinical failure, incidence of adverse events and microbial eradication.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs.
Types of participants
Participants of any age or gender, with clinical evidence of acute LRTI such as acute bronchitis, pneumonia and acute exacerbations of chronic bronchitis.
Types of interventions
Azithromycin of any dose or regimen compared to amoxycillin or amoxycillin/clavulanic acid (amoxyclav).
Types of outcome measures
Primary outcomes
Clinical failure (persistence or deterioration of symptoms, death or relapse assessed at about 10 to 14 days after therapy started).
Secondary outcomes
Incidence of serious complications.
Adverse drug events.
Eradication of organism (causative micro‐organism absent from the sputum culture after treatment).
Search methods for identification of studies
Electronic searches
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL 2014, Issue 10) (accessed 7 November 2014), which contains the Acute Respiratory Infections Group's Specialised Register, MEDLINE (June 2011 to October week 5, 2014) and EMBASE (June 2011 to November 2014). Previously we searched CENTRAL (2011, Issue 3), MEDLINE (July 2007 to July week 4, 2011) and Embase.com (July 2007 to August 2011). Details of the original search strategy are in Appendix 1.
We used the search strategy described in Appendix 2 to search CENTRAL and MEDLINE. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision), Ovid format (Lefebvre 2011). We adapted the search strategy to search EMBASE (see Appendix 3). There were no language or publication restrictions.
Searching other resources
We searched the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov trials registries (28 January 2015) for completed and ongoing trials. We reviewed the citations in the trials identified by the above searches. We contacted the organisations and individual researchers working in this field for unpublished data and missing data from published trials.
Data collection and analysis
Selection of studies
In the previous versions of this review three review authors (RP, PL and ML) independently screened the results of the searches for potentially relevant studies. In this 2014 update version, three review authors (ML, RP and KM) independently screened the potentially relevant studies. We used an eligibility form to assess these studies for inclusion in the review. We resolved disagreements by discussion.
Data extraction and management
We used a data extraction form to collect information from included trials regarding participants, methods, interventions and outcomes. One review author (RP) extracted data. Another review author (PL) independently cross‐checked the findings. We checked the data sources to avoid multiple publications based on the same data. Extracted data included:
the time period and geographical location of the study;
baseline characteristics of participants;
inclusion/exclusion criteria; and
preparation and dosing of treatment regime.
We extracted information on the main outcomes: clinical failure, microbial eradication and adverse events.
Assessment of risk of bias in included studies
In the original review, Panpanich 2004, two review authors (RP, PL) independently assessed trial quality under the following domains:
generation of allocation sequence;
concealment of treatment allocation;
blinding;
completeness of the trial.
For the previous update, two review authors (ML, RP) independently assessed the quality of studies included in the review using the 'Risk of bias' tool as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other sources of bias. We classified overall risk of bias as low, unclear or high. We resolved any disagreements between the authors by consensus.
Measures of treatment effect
We carried out statistical analysis using the Review Manager software (RevMan 2014). We reported risk ratio (RR) (95% confidence interval (CI)) of clinical failure, microbial eradication and adverse events for each trial. When trials were sufficiently homogeneous, we pooled the intervention effects.
Unit of analysis issues
We did not have any unit of analysis issues in our review. All of the included trials were of parallel design. They had random assignments and data analyses at the patient level.
Dealing with missing data
We excluded the trials with more than 10% of missing data in sensitivity analysis when assessing the influence of missing data on the overall results of clinical failure.
Assessment of heterogeneity
We assessed heterogeneity in the estimates of the treatment effects among the included trials through visual examination of the forest plot and the Chi2 test of heterogeneity, using a 10% level of statistical significance. We also used the I2 statistic to measure inconsistency in results among trials (Higgins 2003). We considered a value greater than 50% to represent substantial heterogeneity.
Assessment of reporting biases
We examined publication bias for clinical failure by visually inspecting a funnel plot.
Data synthesis
We planned to analyse the summary weighted risk ratio and 95% CI using fixed‐effect inverse variance meta‐analysis for combining data where we judged trials sufficiently similar. We used a random‐effects meta‐analysis where there was clinical or methodological heterogeneity between studies sufficient to suggest that treatment effects might differ between trials. We used a random‐effects meta‐analysis to pool the treatment effects where the heterogeneity between studies could not be explained by any potential factor.
Subgroup analysis and investigation of heterogeneity
We conducted a subgroup analysis according to the following prespecified factors:
age group;
types of respiratory tract infections such as acute bronchitis, acute exacerbation of chronic bronchitis and pneumonia;
dose regimen of azithromycin; and
type of antibiotic in control group.
Sensitivity analysis
We conducted sensitivity analyses to assess the impact of the potentially important factors on the overall results of clinical failure. The potential factors were:
trial quality according to allocation concealment; and
trials with more than 10% missing data.
Results
Description of studies
Results of the search
In our original published version of this review, Panpanich 2004, we identified 26 potentially relevant studies. Of these, we included 15 studies and details are presented in the Characteristics of included studies table. In the first update in 2008 we include one more trial (Kogan 2003), which was previously in the Studies awaiting classification section.
In the previous updated search in August 2011 we identified 43 records, of which we assessed three as potentially relevant for inclusion (Dimopoulos 2007; Maimon 2008; Morris 2010). However, we excluded all three studies because one was a trial comparing azithromycin versus amoxycillin in treating acute otitis media and the other two were meta‐analyses.
In the update search in November 2014 we identified 38 records. After considering their titles and abstracts we excluded all of them because they did not satisfy our specified inclusion criteria.
Included studies
We included a total of 16 trials involving 2648 participants. We did not include the Kogan 2003 results in the analyses because the outcomes of clinical response and radiological findings were not relevant to our criteria. Details of the included trials are provided in the Characteristics of included studies table.
All 15 trials in the original review reported numbers of participants cured, improvements, failures and relapses. Microbial eradication was reported in 12 trials (Balmes 1991; Beghi 1995; Daniel 1991; Gris 1996; Harris 1998; Hoepelman 1993; Hoepelman 1998; Mertens 1992; Sevieri 1993; Whitlock 1995; Zachariah 1996; Zheng 2002). No trial reported duration of fever. All trials reported failure at about 10 to 14 days after treatment started.
Study location
Fourteen trials were published in English, one trial was in Italian (Sevieri 1993) and one was in Chinese (Zheng 2002). The studies were conducted between 1991 and 2003 in France, Belgium, The Netherlands, Finland, Germany, UK, USA, Italy, Chile and China.
Participants
Twelve out of 16 trials were conducted in adults. Five trials recruited adult participants either with acute bacterial bronchitis or chronic bronchitis with acute exacerbation or pneumonia (Gris 1996; Hoepelman 1993; Hoepelman 1998; Kogan 2003; Zachariah 1996). Five trials recruited only participants with chronic bronchitis with acute exacerbation (Beghi 1995; Mertens 1992; Sevieri 1993; Whitlock 1995; Zheng 2002). Three trials were conducted in children aged six months to 16 years with community‐acquired pneumonia (Ferwerda 2001; Harris 1998; Wubbel 1999).
Interventions
Azithromycin was compared to amoxycillin‐clavulanic acid in 13 trials (Balmes 1991; Beghi 1995; Biebuyck 1996; Ferwerda 2001; Gris 1996; Harris 1998; Hoepelman 1993; Hoepelman 1998; Sevieri 1993; Whitlock 1995; Wubbel 1999; Zachariah 1996; Zheng 2002). Three trials compared azithromycin to amoxycillin (Daniel 1991; Kogan 2003; Mertens 1992).
There were two regimens of azithromycin in the adult trials:
azithromycin 500 mg single dose daily for three days (eight trials); and
azithromycin 500 mg single dose on day one followed by 250 mg single dose daily on days two to five (four trials).
The regimen of azithromycin in children in two trials was 10 mg/kg single dose on day one and followed by 5 mg/kg once daily on day two to five (Harris 1998; Wubbel 1999). Another trial used 10 mg/kg/day once daily for three days (Ferwerda 2001).
Two trials in children compared azithromycin to amoxycillin/clavulanic acid in participants aged up to five years; and erythromycin in older children (Harris 1998; Wubbel 1999). This review only included the data comparing amoxycillin/clavulanic acid.
Excluded studies
We excluded 13 studies. The main reason for exclusion seen in 10 studies was comparison of azithromycin with other antibiotics not related to amoxycillin. Two studies were meta‐analyses. For more details, see the Characteristics of excluded studies table.
Risk of bias in included studies
Details of the risk of bias of each study are given in the 'Risk of bias' tables in the Characteristics of included studies table. The overall risk of bias is presented graphically in Figure 1 and summarised in Figure 2.
1.
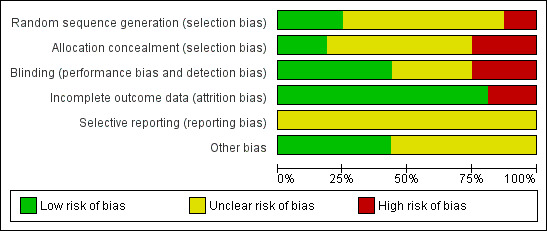
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
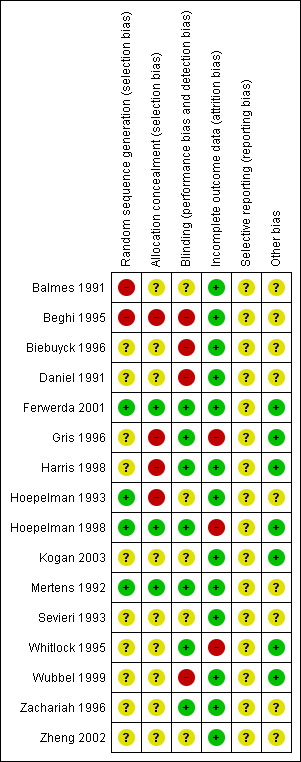
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Four out of 16 included studies had adequate sequence generation for randomisation (Ferwerda 2001; Hoepelman 1993; Hoepelman 1998; Mertens 1992). Three out of 16 included studies had adequate allocation concealment (Ferwerda 2001; Hoepelman 1998; Mertens 1992). We assessed these trials as low risk for selection bias.
Blinding
Seven included studies used double‐blinding by matched placebo in both treatment groups (Ferwerda 2001; Gris 1996; Harris 1998; Hoepelman 1998; Mertens 1992; Whitlock 1995; Zachariah 1996). We assessed them as having low risk of performance and detection biases.
Nine studies either had no description of blinding or unclear information relating to blinding (Balmes 1991; Beghi 1995; Biebuyck 1996; Daniel 1991; Hoepelman 1993; Kogan 2003; Sevieri 1993; Wubbel 1999; Zheng 2002). We assessed these studies to be potentially high risk of bias.
Incomplete outcome data
Three out of 16 included studies had more than 10% missing data for the clinical failure outcome (Gris 1996; Hoepelman 1998; Whitlock 1995). We assessed them to be at high risk of attrition bias and excluded them from the sensitivity analysis. The remaining 13 studies either had analyses of total randomised patients or had missing data of less than 10%.
Selective reporting
Since we did not have access to the protocols for any of the included studies, the risk of bias in selective reporting was unclear for all.
Other potential sources of bias
Six out of 16 included studies had balanced characteristics between the two treatment groups (Ferwerda 2001; Gris 1996; Harris 1998; Hoepelman 1998; Kogan 2003; Whitlock 1995). We assessed them as having a low risk of other potential sources of bias.
We considered that the majority of the included studies, 81.3% (13/16), had a low risk of attrition bias. Among the 16 included studies, only one study had a low risk of bias in five of the six risk of bias domains (Ferwerda 2001).
Effects of interventions
We included 15 trials involving 2496 participants in the analysis. There were 1388 participants who received azithromycin and 1108 who received amoxycillin or amoxyclav. All trials reported the incidence of clinical failure (persistence or deterioration of symptoms or relapse). Eleven trials reported the incidence of microbacterial eradication. There was no evidence of publication bias by visual inspection of the funnel plot (Figure 3).
3.
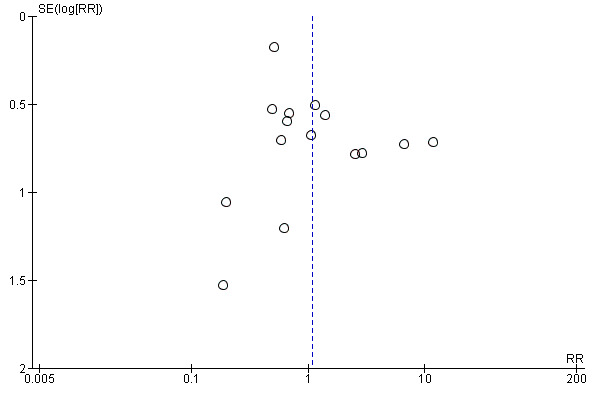
Funnel plot of comparison: 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, outcome: 1.1 Clinical failure.
Primary outcome
1. Clinical failure
The pooled analysis of all trials showed that the incidence of clinical failure on day 10 to 14 in the azithromycin group was 10.1% (140/1388) compared to 10.3% (114/1108) in the amoxycillin or amoxyclav group. There was no statistical significance in the difference in incidence of clinical failure between the two groups (risk ratio (RR) 1.09; 95% confidence interval (CI) 0.64 to 1.85, random‐effects model) (Analysis 1.1). However, the heterogeneity between trials was significant with an I2 statistic of 65.3% (P value = 0.0002).
1.1. Analysis.
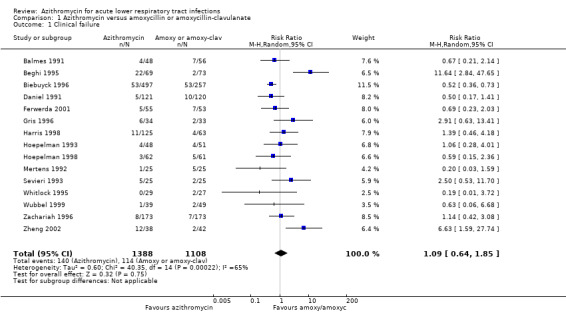
Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 1 Clinical failure.
Heterogeneity would be anticipated with the variation in age groups and types of diagnoses between trials. Subgroup analysis stratified by age groups showed no significant difference in treatment effects between the azithromycin group and the amoxycillin or amoxyclav group in either adults (RR 1.15; 95% CI 0.60 to 2.20, random‐effects model) or children (RR 0.93; 95% CI 0.45 to 1.94) (Analysis 1.3).
1.3. Analysis.
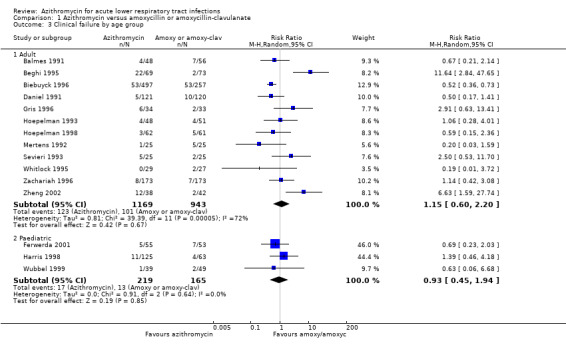
Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 3 Clinical failure by age group.
In a subgroup analysis of trials with acute bronchitis participants, the incidence of clinical failure was significantly lower in the azithromycin group compared to amoxycillin or amoxyclav (RR 0.63; 95% CI 0.45 to 0.88, random‐effects model) (Analysis 1.2). In analysis of trials with acute exacerbation of chronic bronchitis participants, there was significant heterogeneity between trials with an I2statistic of 75.5% (P value = 0.0001) and clinical failure was not significantly different between the groups.
1.2. Analysis.
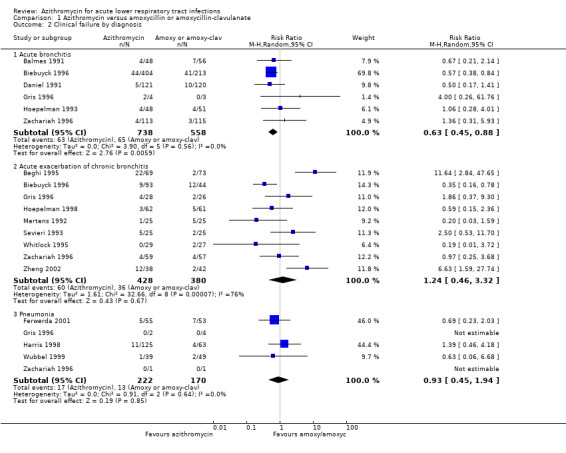
Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 2 Clinical failure by diagnosis.
We considered the impact of study quality (according to adequate concealment) on the pooled results for clinical failure in a sensitivity analysis. The reduction of clinical failure in azithromycin‐treated participants was RR 0.55 (95% CI 0.25 to 1.21) in three adequately concealed studies, compared to RR 1.32 (95% CI 0.70 to 2.49), restricted to 12 studies with inadequate concealment.
We also performed a sensitivity analysis by excluding the largest trial (Biebuyck 1996). The result showed that the overall effect of azithromycin compared to amoxycillin or amoxyclav for reducing clinical failure was a RR of 1.20 (95% CI 0.69 to 2.09). This figure was quite similar to the result for the total of 15 trials (RR 1.09; 95% CI 0.64 to 1.85) (Analysis 1.5).
1.5. Analysis.
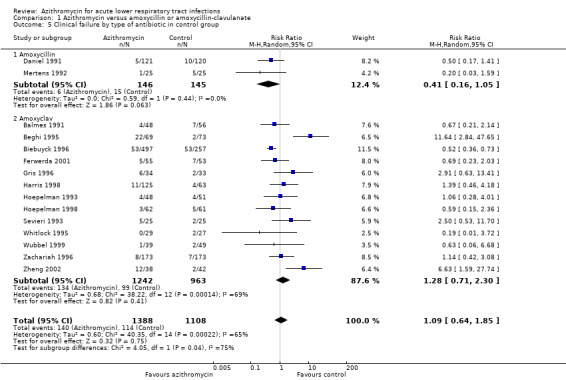
Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 5 Clinical failure by type of antibiotic in control group.
When excluding the three trials with more than 10% missing data (Gris 1996; Hoepelman 1998; Whitlock 1995), a sensitivity analysis of the remaining 12 trials showed that the overall effect of azithromycin compared to amoxycillin or amoxyclav on reducing clinical failure was a RR of 1.13 (95% CI 0.62 to 2.03). This figure was quite similar to the result for the total of 15 trials (RR 1.09; 95% CI 0.64 to 1.85).
Secondary outcomes
1. Incidence of serious complications
No trials reported death.
2. Adverse drug events
Twelve trials reported adverse events. The most frequent adverse events were mild to moderate gastrointestinal symptoms, nausea, vomiting and diarrhoea. The others reported were headache, insomnia, rash and transient laboratory liver function changes. One large trial reported a higher number of participants discontinuing amoxyclav treatment because of adverse events compared to the azithromycin group: 7% compared to 1.2% respectively (Biebuyck 1996). The overall incidence of adverse events in the azithromycin group was 17.9% (244/1363) compared to 23.6% (246/1043) in the amoxycillin or amoxyclav group. The reduction of adverse events in the azithromycin group was a RR of 0.76 (95% CI 0.57 to 1.00) (Analysis 1.10).
1.10. Analysis.
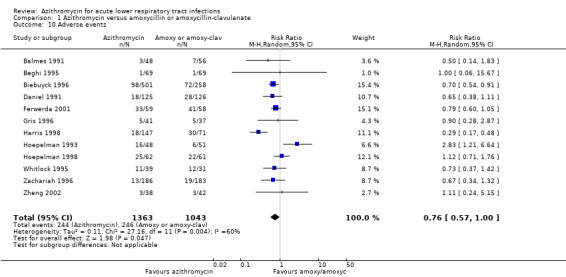
Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 10 Adverse events.
3. Eradication of organism
Twelve trials reported the incidence of microbial eradication. The pooled analysis showed that the incidence of microbial eradication in the azithromycin group was 66.4% (326/491) compared to 67.6% (318/470) in the amoxicillin or amoxyclav group. There was no significant difference between the two groups (RR 0.95; 95% CI 0.87 to 1.03, fixed‐effect model) (Analysis 1.9).
1.9. Analysis.
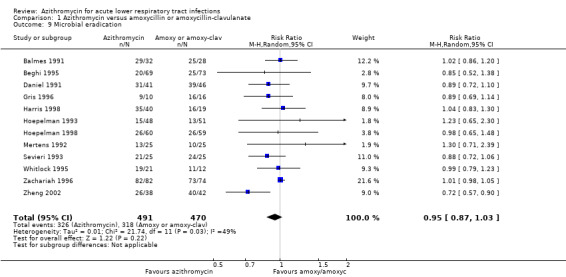
Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 9 Microbial eradication.
Discussion
Summary of main results
The results of this review showed that the effect of azithromycin compared to amoxycillin or amoxyclav on clinical failure, microbial eradication and adverse events measured at about day 10 to 14 was not statistically significant. However, in a group of participants with acute bronchitis of a suspected bacterial cause, the incidence of clinical failure was significantly lower in the azithromycin group.
Overall completeness and applicability of evidence
The 15 analysed studies were published over a period of 11 years (1991 to 2002). Fourteen out of the 15 included studies were from a wide range of high‐income countries. Eighty per cent (12/15) of the included studies were conducted in adults. There were few differences in the doses of azithromycin and the context of each study. The pooled results may be applied to similar settings.
Quality of the evidence
There were some limitations that related to the quality of the included trials (Figure 1; Figure 2). The most important was that adequately concealed treatment allocation was performed in only three trials and 53% of the studies (8/15) either did not report this information or reported insufficient information about blinding. The results of these studies should be interpreted with caution.
Potential biases in the review process
We followed the Cochrane Acute Respiratory Infections Group's guidelines for conducting the review. However, publication bias may remain a possible (but unknown) source of important bias.
Agreements and disagreements with other studies or reviews
There are no other systematic reviews on this topic.
Authors' conclusions
Implications for practice.
There is unclear evidence that azithromycin is superior to amoxycillin or amoxyclav in treating acute lower respiratory tract infection. However, in patients with acute bronchitis of a suspected bacterial cause, azithromycin is more effective in lowering the incidence of clinical failure than amoxycillin or amoxyclav. Azithromycin seems to have a lower incidence of adverse events than amoxycillin or amoxyclav. In clinical practice, the choice between azithromycin and amoxycillin or amoxyclav could be based on other considerations such as cost, convenience and adherence to treatment.
Implications for research.
A high‐quality and adequate sized trial is needed to clarify whether azithromycin is better than amoxycillin or amoxyclav in treating acute lower respiratory tract infection.
Feedback
Less adverse events?
Summary
While informative, this systematic review leaves at least one unanswered question. Namely, with regards to the outcome of adverse events which seems to favour azithromycin, the severity of these complications are not well described. Another important consideration in the interpretation of this data is that the absence of difference between azithromycin and amoxi/clavulin is far more robust than with the comparison to Amoxil. As a result, clinicians might be tempted to equate all three drugs when reading this systematic review when in fact, the demonstration of equivalence is more convincing between azithromycin and amoxi/clavulin and not amoxycillin and azithromycin. It is also quite interesting to note that the authors conclude a possible benefit in acute bronchitis when a Cochrane review concludes that there is no net benefit associated with the use of antibiotics in acute bronchitis.
I certify that I have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms.
Reply
I have revised the review and added details about adverse events in paragraph 6 of the RESULTS section. There is information regarding this in the last sentence of paragraph 3 of the RESULTS section, reporting the risk ratios of two comparisons; azithromycin versus amoxyclav and azithromycin versus amoxycillin.
In the DISCUSSION section we stated that the effect of azithromycin in reducing clinical failure was shown to be much stronger when compared to amoxycillin than to amoxyclav. The evidence was not clear because there were only two trials for the control group of amoxycillin.
Acute bronchitis in this review refers to acute bronchitis with suspected bacterial cause. The review focuses on comparison of effects of azithromycin to amoxyclav or amoxycillin. I understand that the other Cochrane review you mentioned compared antibiotics with placebo. The conclusions of review could be different
Ratana Panpanich Peerasak Lerttrakarnnon Malinee Laopaiboon
Contributors
Eddy Lang Comment posted 20 March 2005
What's new
| Date | Event | Description |
|---|---|---|
| 7 November 2014 | New citation required but conclusions have not changed | Our conclusions remain unchanged. A new author joined the review team. |
| 7 November 2014 | New search has been performed | Searches conducted. We did not identify any new trials for inclusion or exclusion. |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 8 August 2011 | New search has been performed | Searches conducted. We included one trial, which was previously awaiting classification (Kogan 2003) and excluded three new trials (Dimopoulos 2007; Maimon 2008; Morris 2010). Our conclusions remain unchanged. |
| 11 June 2008 | Amended | Converted to new review format. |
| 17 July 2007 | New search has been performed | Searches conducted. |
| 19 March 2005 | Feedback has been incorporated | Feedback comment and reply added. |
| 9 January 2004 | New search has been performed | Searches conducted. |
Acknowledgements
We thank Professor Paul Garner for his kind advice on the protocol development and plan for data analysis. We are grateful to Dr. Shi Luming for her help in extracting data from the Chinese paper included in the review. We also thank the following people for commenting on the draft review: Gustav Malangu, Chantal Raherison, Mark Jones and Diederik van de Beek. We thank Liz Dooley and Sarah Thorning from the Cochrane Acute Respiratory Infections Group for their assistance with the preparation of this systematic review. The data presented and the views expressed are the responsibility of the review authors.
Appendices
Appendix 1. Original search strategy
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2007, Issue 2), MEDLINE (January 1966 to July 2007) and EMBASE (January 1974 to July 2007).
We combined the following search strategy with the Cochrane highly sensitive search strategy phases one and two as published in appendix 5c of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). The search terms were also run over CENTRAL. See Appendix 3 for the EMBASE search strategy.
MEDLINE (OVID) 1 exp Azithromycin/ 2 (azithromycin or azithromicin).mp. 3 or/1‐2 4 exp Amoxicillin/ 5 exp Amoxicillin‐Potassium Clavulanate Combination/ 6 (amoxicillin or amoxycillin).mp. 7 amoxicillin clavula$.mp. 8 or/4‐7 9 exp Pneumonia/ 10 pneumonia.mp. 11 exp Bronchitis/ 12 bronchitis.mp. 13 (lower respiratory tract infection$ or lower respiratory infection$ or LTRI$).mp. 14 or/9‐13 15 and/3,8,14
EMBASE (WebSPIRS) #1 explode 'azithromycin‐' / all subheadings in DEM,DER,DRM,DRR #2 (azithromycin or azithromicin) in ti #3 (azithromycin or azithromicin) in ab #4 #1 or #2 or #3 #5 explode 'amoxicillin‐' / all subheadings in DEM,DER,DRM,DRR #6 explode 'amoxicillin‐plus‐clavulanic‐acid' / all subheadings in DEM,DER,DRM,DRR #7 (amoxicillin or amoxycillin) in ti #8 (amoxicillin or amoxycillin) in ab #9 (amoxicillin clavula* in ti) or (amoxicillin clavula* in ab) #10 #5 or #6 or #7 or #8 or #9 #11 explode 'pneumonia‐' / all subheadings in DEM,DER,DRM,DRR #12 (pneumonia in ti) or (pneumonia in ab) #13 explode 'bronchitis‐' / all subheadings in DEM,DER,DRM,DRR #14 (bronchitis in ti) or (bronchitis in ab) #15 explode 'lower‐respiratory‐tract‐infection' / all subheadings in DEM,DER,DRM,DRR #16 (lower respiratory tract infection$ or lower respiratory infection$ or LTRI$)in ti #17 (lower respiratory tract infection$ or lower respiratory infection$ or LTRI$)in ab #18 #11 or #12 or #13 or #14 or #15 or #16 or #17 #19 #4 and #10 and #18
Appendix 2. MEDLINE (Ovid) search strategy
1 exp Pneumonia/ 2 (pneumon* or bronchopneumon* or pleuropneumon*).tw. 3 exp Bronchitis/ 4 (bronchit* or bronchiol*).tw. 5 (infect* adj2 lower respiratory).tw. 6 lrti.tw. 7 ((chest or lung) adj2 infect*).tw. 8 or/1‐7 9 Azithromycin/ 10 (azithromycin or azithromicin).tw,nm. 11 9 or 10 12 exp Amoxicillin/ 13 (amoxicillin* or amoxycillin*).tw,nm. 14 (amoxyclav* or amoxy‐clav* or co‐amoxyclav* or augmentin).tw,nm. 15 or/12‐14
Appendix 3. EMBASE (Elsevier) search strategy
#2.8 #2.3 NOT #2.7805950 #2.7 #2.4 NOT #2.6 #2.6 #2.4 AND #2.5 #2.5 'human'/de AND [embase]/lim #2.4 'animal'/de OR 'nonhuman'/de OR 'animal experiment'/de AND [embase]/lim #2.3 #2.1 OR #2.2 #2.2 random*:ab,ti OR placebo*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR allocat*:ab,ti OR trial:ti OR (doubl* NEXT/1 blind*):ab,ti AND [embase]/lim #2.1 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp AND [embase]/lim #1 2066 #1.16 #1.7 AND #1.10 AND #1.152066 #1.15 #1.11 OR #1.12 OR #1.13 OR #1.14 #1.14 amoxyclav*:ab,ti OR 'co‐amoxyclav':ab,ti OR augmentin:ab,ti AND [embase]/lim #1.13 'amoxicillin plus clavulanic acid'/de AND [embase]/lim #1.12 amoxycillin*:ab,ti OR amoxicillin*:ab,ti AND [embase]/lim #1.11 'amoxicillin'/de AND [embase]/lim #1.10 #1.8 OR #1.9 #1.9 aziththromycin*:ab,ti OR azythromycin*:ab,ti OR azithromicin*:ab,ti AND [embase]/lim #1.8 'azithromycin'/de AND [embase]/lim #1.7 #1.1 OR #1.2 OR #1.3 OR #1.4 OR #1.5 OR #1.6 #1.6 (infection* NEAR/2 'lower respiratory'):ab,ti AND [embase]/lim #1.5 'lower respiratory tract infection'/de OR 'chest infection'/de OR 'lung infection'/de AND [embase]/lim #1.4 bronchit*:ab,ti OR bronchiolit*:ab,ti AND [embase]/lim #1.3 'bronchitis'/exp AND [embase]/lim #1.2 pneumon*:ab,ti OR bronchopneumon*:ab,ti OR pleuropneumon*:ab,ti AND [embase]/lim #1.1 'pneumonia'/exp AND [embase]/lim
Data and analyses
Comparison 1. Azithromycin versus amoxycillin or amoxycillin‐clavulanate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure | 15 | 2496 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.85] |
| 2 Clinical failure by diagnosis | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Acute bronchitis | 6 | 1296 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.45, 0.88] |
| 2.2 Acute exacerbation of chronic bronchitis | 9 | 808 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.46, 3.32] |
| 2.3 Pneumonia | 5 | 392 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.45, 1.94] |
| 3 Clinical failure by age group | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Adult | 12 | 2112 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.60, 2.20] |
| 3.2 Paediatric | 3 | 384 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.45, 1.94] |
| 4 Clinical failure by dose regimen of azithromycin | 12 | 2112 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.60, 2.20] |
| 4.1 500 mg once daily x 3 | 8 | 1631 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.55, 2.83] |
| 4.2 500 mg single dose followed by 250 mg on day 2 to 5 | 4 | 481 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.25, 3.62] |
| 5 Clinical failure by type of antibiotic in control group | 15 | 2496 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.85] |
| 5.1 Amoxycillin | 2 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.16, 1.05] |
| 5.2 Amoxyclav | 13 | 2205 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.71, 2.30] |
| 6 Sensitivity analysis excluding one large trial | 14 | 1742 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.69, 2.09] |
| 7 Sensitivity analysis excluding three trials with missing data > 10% | 12 | 2250 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.62, 2.03] |
| 8 Sensitivity analysis with the condition of concealment | 15 | 2496 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.85] |
| 8.1 Adequately concealed studies | 3 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.25, 1.21] |
| 8.2 Inadequately or unclearly concealed studies | 12 | 2215 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.70, 2.49] |
| 9 Microbial eradication | 12 | 961 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.87, 1.03] |
| 10 Adverse events | 12 | 2406 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.57, 1.00] |
1.4. Analysis.
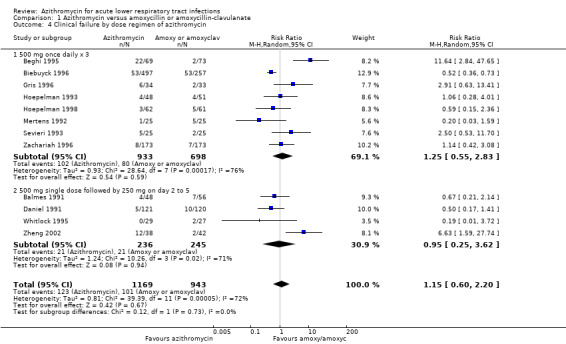
Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 4 Clinical failure by dose regimen of azithromycin.
1.6. Analysis.
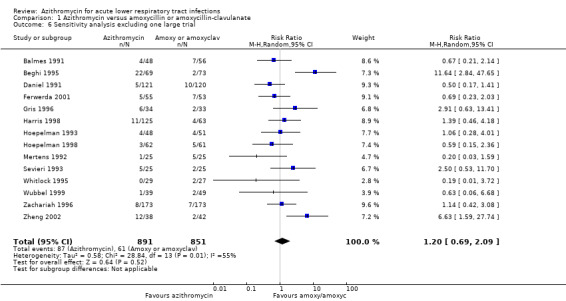
Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 6 Sensitivity analysis excluding one large trial.
1.7. Analysis.
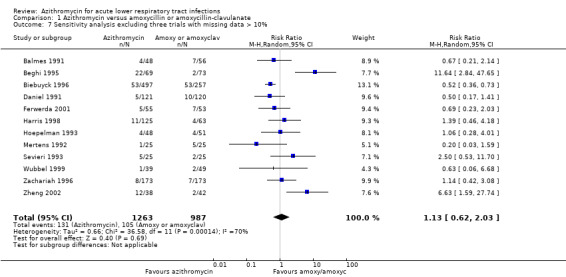
Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 7 Sensitivity analysis excluding three trials with missing data > 10%.
1.8. Analysis.
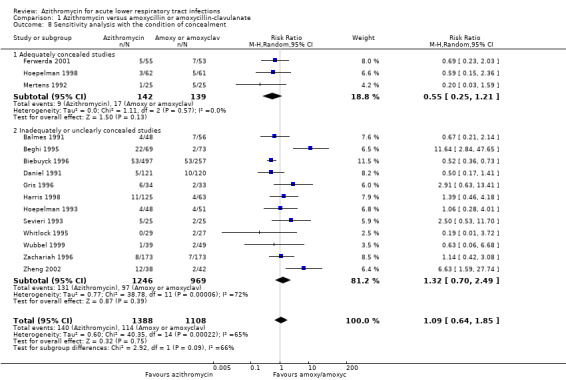
Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 8 Sensitivity analysis with the condition of concealment.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Balmes 1991.
| Methods | Location: France Participants were randomly assigned to treatment. No description of blinding. Efficacy was evaluated at 10 to 15 days after the therapy started | |
| Participants | 110 adults with acute lower respiratory tract infection, either acute bacterial bronchitis or pneumonia. Acute bronchitis was defined as bacterial bronchial or bronchopulmonary infection accompanied by the production of purulent sputum. Participants with infectious mononucleosis, chronic or chronic obstructive pulmonary disease without acute infection, or who had received antibiotics within 48 hours prior to the study were excluded Participants: azithromycin group N = 52 (acute bronchitis 48, pneumonia 4), amoxycillin/clavulanic acid group N = 58 (acute bronchitis 54, pneumonia 4) | |
| Interventions | 1. Azithromycin 500 mg single dose on day 1 followed by a single dose of 250 mg daily on day 2 to 5 2. Amoxycillin/clavulanic acid 625 mg (amoxycillin 500 mg, clavulanic 125 mg) 3 times daily for 10 days | |
| Outcomes | Cure Improvement Failure Adverse events Pathogen eradication | |
| Notes | Of the bronchitis cases, 20/48 in the azithromycin group and 19/54 in the amoxycillin/clavulanic acid group were described as acute exacerbation of chronic bronchitis Of 110 randomised patients, 104 were assessed and included in analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The study did not report how randomisation was done |
| Allocation concealment (selection bias) | Unclear risk | The concealment process was not reported. Quote: "Of this total, 52 (30 males, 22 females) were randomized to receive oral azithromycin and 58 (39 males, 19 females) to receive oral amoxycillin/CA." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study did not provide blinding information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasonable, as 6 patients not having clinical assessment were clearly reported. 4 participants were in the azithromycin group (7.7%; 4/52) and 2 participants were in the amoxycillin group (3.5%; 2/58) |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Unclear risk | Baseline characteristics of the 2 treatment groups were not addressed |
Beghi 1995.
| Methods | Multicentre study, participants were randomised to receive either azithromycin or amoxycillin/clavulanic acid. No blinding. Efficacy was evaluated 10 days after the therapy started | |
| Participants | 142 hospitalised or outpatients aged 18 years or more with acute purulent exacerbation of chronic bronchitis. Exclusion criteria: participants treated with other antibiotics 48 hours prior to the study, leucopenia, coagulation disorders, renal dysfunction, HIV/AIDS on immunosuppressive drugs, suspected pneumonia with lung abscess, pleuritis, empyema or active tuberculosis, pregnancy and lactation. Participants: azithromycin group N = 69, amoxycillin/clavulanic acid group N = 73 | |
| Interventions | 1. Azithromycin (Pfizer) 500 mg single dose daily for 3 days 2. Amoxycillin/clavulanic acid SmithKline Beecham (amoxycillin 875 mg + clavulanic acid 125 mg) twice daily for 8 days | |
| Outcomes | Cure (disappearance of all signs and clinical symptoms of infection by day 10) Improvement (disappearance of only a few signs and/or clinical symptoms) Failure (persistence or worsening of signs and symptoms at days 4 and 10) | |
| Notes | Corticosteroids were allowed, provided this did not exceed 25 mg for prednisolone or its equivalent in both groups. Of the 142 participants, 2 participants dropped out and were not included in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | High risk | Not mentioned in the article |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants randomised were analysed. 69 in the azithromycin group and 73 in the amoxycillin group |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Baseline characteristics of the 2 treatment groups were not addressed |
Biebuyck 1996.
| Methods | Participants were randomised in a 2:1 ratio to receive either azithromycin or amoxycillin/clavulanic acid. No blinding. Efficacy was evaluated at 8 to 10 days after the therapy started | |
| Participants | 759 adult participants aged between 18 to 75 years were recruited; 620 had acute tracheobronchitis and 139 had acute exacerbations of chronic bronchitis. A diagnosis of acute tracheobronchitis was based on the presence of at least 2 of the following signs and symptoms: cough, fever 38 ºC or higher, purulent sputum and rhonchi/rales. Participants: azithromycin group N = 501, amoxycillin/clavulanic acid group N = 258 | |
| Interventions | 1. Azithromycin 500 mg once daily for 3 days (2 x 250 mg capsules taken at least 1 hour before or 2 hours after meals) 2. Amoxicillin/clavulanic acid 625 mg (amoxycillin 500 mg + clavulanate 125 mg) 3 times daily for 5 to 10 days, taken during or shortly after meals | |
| Outcomes | Cure Improvement Failure Adverse events | |
| Notes | Of 759 participants, 31 participants with various reasons (adverse events, lack of efficacy and lost to follow‐up) discontinued treatment; 9 in the azithromycin group and 22 in the amoxycillin/clavulanate group. 26 out of 31 who dropped out were followed and evaluated. In the analysis, 754 participants were included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. Quote: "Patients were randomized in a 2:1 ratio to receive either azithromycin or amoxicillin/clavulanic acid" |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 754 participants (99%; 754/759) were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Baseline characteristics of the 2 treatment groups were not addressed |
Daniel 1991.
| Methods | Multicentre study, 9 study centres in 4 European countries (Belgium, Finland, Germany and UK). Participants were allocated to either treatment group using a randomisation list. No blinding. Efficacy was evaluated at 10 to 15 days after the therapy started | |
| Participants | 251 adult participants aged 18 years or older were recruited, diagnosed by clinical criteria as having acute bronchitis or pneumonia. Participants with life‐threatening conditions, cystic fibrosis or who had received antibiotics in the 48 hours preceding the study were excluded. Participants: azithromycin group N = 125, amoxycillin group N = 126 | |
| Interventions | 1. Azithromycin 500 mg single dose on day 1 followed by 250 mg daily on days 2 to 5 2. Amoxicillin 500 mg orally 3 times daily for 7 days | |
| Outcomes | Cure Adverse events Pathogen eradication | |
| Notes | Of 251 randomised participants, 241 were assessed and included in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information for judgement. Quote: "Patients were allocated to either treatment group using a randomizations list" |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 96% (241/251) of the 251 randomised participants were included in analysis. 121 out of 125 in the azithromycin group and 120 of 126 in the amoxycillin group were assessed |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Baseline characteristics of the 2 treatment groups were not addressed |
Ferwerda 2001.
| Methods | Location: The Netherlands Multicentre, randomised, double‐blind, double‐dummy study. Randomisation was done in blocks of 6 at a research centre. Blinding was maintained by matched placebo. Clinical evaluation was done on days 3 to 5, days 10 to 13 and days 25 to 30 | |
| Participants | 118 participants aged 3 months to 12 years with community‐acquired lower respiratory tract infection were recruited. The diagnosis was based on the presence of respiratory signs and symptoms in combination with a positive chest radiograph or clinical evidence of a temperature 38 ºC or higher, cough, leucocytosis > 10,000 cells/mm³. Participants with symptoms for longer than 1 week, weight > 40 kg, or need for parenteral therapy were excluded. Azithromycin group N = 56, co‐amoxyclav group N = 54 | |
| Interventions | 1. Azithromycin suspension 10 mg/kg/day single dose for 3 days 2. Co‐amoxyclav suspension 45/11.25 mg/kg/day 3 times a day for 10 days | |
| Outcomes | Cure Improvement Failure Adverse events | |
| Notes | Of 118 randomised participants, 110 were clinically evaluated. 8 were excluded; 7 of them did not meet the inclusion criteria, and for 1 participant the informed consent was withdrawn. Compliance was measured by diary card, registered by parents | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using blocks of 6 at Imro Tarmarko Berghem, The Netherlands |
| Allocation concealment (selection bias) | Low risk | Treatments were provided by the study sponsor in matched placebo suspensions. Randomisation was done using blocks of 6 at Imro Tarmarko Berghem, The Netherlands Quote: "Patients were assigned randomly to treatment with oral azithromycin suspension (10 mg/kg/24 hours) in a single dose for 3 days or co‐amoxiclav suspension (45/11.25 mg/kg/24 h) tds for 10 days" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Matched placebo suspensions were used in the 2 treatment groups. Each participant was equally treated for 13 days |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At visit 3 (days 10 to 13) 1 patient was lost in each treatment group (azithromycin group N = 55, co‐amoxyclav N = 53) |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Low risk | Baseline characteristics were comparable between the 2 treatment groups |
Gris 1996.
| Methods | Location: Belgium, multicentre study Participants were randomly assigned to receive either azithromycin or amoxycillin/clavulanic acid. Double‐blinding was performed with matched placebo tablets. Efficacy was evaluated 14 days after the therapy started | |
| Participants | 78 adult participants aged 18 years or older with acute bronchitis, acute exacerbations of chronic bronchitis or pneumonia were recruited. Diagnosis was made based on the clinical signs and symptoms and chest radiology. Participants who received antibiotics in the 48 hours preceding the study were excluded. Participants: azithromycin group N = 41, co‐amoxyclav N = 37 | |
| Interventions | 1. Azithromycin 500 mg (Pfizer) once daily for 3 days 2. Co‐amoxyclav 625 mg (amoxycillin 500 mg + clavulanate 125 mg) 3 times daily for 10 days | |
| Outcomes | Cure Improvement Failure Adverse events Pathogen eradication | |
| Notes | 11 out of 78 participants were not clinically evaluated for the following reasons: failure to meet entry criteria, failure to comply with the protocol and adverse events (7 in the azithromycin group and 4 in the co‐amoxyclav group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study did not report how randomisation was done. Quote: "Patients were randomly assigned to treatment with azithromycin (Pfizer), one 500 mg tablet once daily on 3 consecutive days, or co‐amoxiclav (Augmentin, Beecham Research) 625 mg 3 times daily for 10 days" |
| Allocation concealment (selection bias) | High risk | Information about concealment was not provided. Quote: "Participants were randomly assigned to treatment with azithromycin (Pfizer), one 500 mg tablet once daily on 3 consecutive days, or co‐amoxiclav (Augmentin, Beecham Research) 625 mg 3 times daily for 10 days" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of the study was maintained with matched placebo tablets |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 17.1% (7/41) of the azithromycin group and 10.8% (4/37) of the amoxycillin/clavulanate group were not clinically evaluable. Quote: "Reasons for exclusion of patients from clinical evaluation were as follows: failure to meet entry criteria (one patient in each treatment group); failure to observe the protocol (3 azithromycin and 2 co‐amoxiclav patients); and treatment incomplete due to an adverse event not necessarily related to treatment (three azithromycin and one co‐amoxiclav patients)" Comment: even though clear explanation was given, the amount of incomplete data was high and not comparable between the 2 groups |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Low risk | Baseline characteristics were comparable between the 2 treatment groups |
Harris 1998.
| Methods | Location: US, multicentre study Participants were randomised 2:1 to receive either azithromycin or amoxycillin/clavulanate in those aged 6 months to 5 years, and erythromycin in children aged older than 5 years. Double‐blinding was performed. Participants were evaluated at 4 clinic visits: baseline, days 2 to 5, days 15 to 19, and 4 to 6 weeks after treatment | |
| Participants | Participants with community‐acquired pneumonia at 23 centres in the US, aged 6 months to 16 years. Pneumonia was diagnosed by chest X‐ray of acute infiltration and the presence of tachypnoea, with at least 1 of the following: fever, cough, white blood count 12,000/mm³ or more, and respiratory signs of suggestive of pneumonia. Participants with severe or multilobar pneumonia, with evidence of haematologic, renal, hepatic or cardiovascular disease, chronic steroid use or concomitant treatment with other drugs were excluded. Participants aged less than 5 years: azithromycin group N = 129, amoxy‐clavulanic acid group N = 66 | |
| Interventions | 1. Azithromycin oral suspension 10 mg/kg (maximum 500 mg) once on day 1, followed by 5 mg/kg (maximum 250 mg) once daily on days 2 to 5 2. Conventional therapy, 3 times daily for 10 days (amoxycillin/clavulanic acid 40 mg/kg/day for participants aged 6 months to 5 years, and erythromycin estolate 40 mg/kg/day for children aged 5 to 16 years) | |
| Outcomes | Cure Improvement Failure Adverse events Eradication of pathogen | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study did not report how randomisation was done. Quote: "Patients were randomized 2:1 to receive either azithromycin...." |
| Allocation concealment (selection bias) | High risk | No concealment information was available. Quote: "Patients were randomized 2:1 to receive either azithromycin...." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Matched placebo was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8.1% (25/310) of the azithromycin group and 7.5% (11/146) of the amoxycillin/clavulanate group were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Low risk | Baseline characteristics were comparable between the 2 treatment groups |
Hoepelman 1993.
| Methods | Location: The Netherlands, multicentre study Participants were randomly assigned to treatment. Single‐blinding was performed. All 99 randomised participants were clinically evaluated on days 3 to 7 and days 12 to 16 | |
| Participants | 99 outpatients from 4 centres in The Netherlands, with clinical evidence of lower respiratory tract infection, either pneumonia or purulent bronchitis or acute exacerbation of chronic bronchitis, were recruited. Participants with a terminal illness, concomitant use of other antibiotics, or with infectious mononucleosis, cystic fibrosis and gastrointestinal absorption abnormality were excluded. Azithromycin group N = 48, co‐amoxyclav group N = 51 | |
| Interventions | 1. Azithromycin 500 mg once daily for 3 days 2. Co‐amoxyclav 625 mg 3 times a day for 10 days | |
| Outcomes | Cure Improvement Failure Adverse events Eradication of pathogen | |
| Notes | Medication (bronchodilators, adrenergic stimulators or corticosteroids) was given in addition to the study drug to 83% of participants in the azithromycin group and 82% in the co‐amoxyclav group. Compliance was measured by pill count. All 99 randomised participants were evaluated for clinical efficacy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by the Department of Pharmacy at the University Hospital, Utrecht |
| Allocation concealment (selection bias) | High risk | The information about concealment is not provided. Quote: "Patients were randomized to receive either azithromycin as a once‐daily dose of 500 mg (two 250 mg capsules) for three days, or co‐amoxiclav (625 mg capsules) tid for ten days. Randomization was performed by the Department of Pharmacy at the University Hospital, Utrecht." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "We report the results of a single‐blind comparison of azithromycin with a ten‐day course of coamoxiclav (amoxycillin/clavulanic acid) in patients with acute lower respiratory tract infections". However, it is not clear to whom the single‐blind was applied and no information was available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis of clinical efficacy was performed using data provided from a total of 99 randomised participants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Unclear risk | Baseline characteristics of the 2 treatment groups were not available. However, the authors mentioned that "The two treatment groups were comparable with respect to age, sex ratio, and underlying diseases (not shown)" |
Hoepelman 1998.
| Methods | Location: The Netherlands, multicentre study Participants were randomised to receive either azithromycin or co‐amoxyclav. Double‐blinding was performed with matched placebo tablets. Clinical outcomes were evaluated on days 12 to 16 | |
| Participants | 144 outpatients were recruited. 123 of them had type I acute exacerbation of chronic bronchitis, 18 had acute purulent bronchitis and 3 had pneumonia. Participants with terminal illness, who were pregnant or lactating, were receiving concomitant antibiotics or had used antibiotics within 48 hours prior to the study treatment, or had infectious mononucleosis, cystic fibrosis or gastrointestinal abnormality that could affect absorption, were excluded. Participants: azithromycin group N = 72, co‐amoxyclav group N = 72 | |
| Interventions | 1. Azithromycin 500 mg once daily for 3 days 2. Co‐amoxyclav 625 mg 3 times daily for 10 days | |
| Outcomes | Clinical: cure, improvement, failure, relapse Microbiological: eradication, persistence, recurrence | |
| Notes | Medication (bronchodilators, adrenergic stimulators, corticosteroids) was given to 94% of participants in the azithromycin group and 97% in the co‐amoxyclav group. Of 144 randomised participants, only participants diagnosed with type I acute exacerbation of chronic bronchitis (N = 123) were analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by the Department of Pharmacy, University Hospital, Utrecht |
| Allocation concealment (selection bias) | Low risk | Quote: "The enrolled patients were randomized to receive either azithromycin, given as a single dose of 500 mg (two 250‐mg tablets) once daily for 3 days, or co‐amoxiclav 500 mg:125 mg (625‐mg tablets), administered three times daily for 10 days. Randomization was performed by the Department of Pharmacy, University Hospital, Utrecht" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "both antibiotics and matching placebos were supplied in a blister pack, which indicated the time of day when each of the tablets was to be taken." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Clinical response was analysed from the majority of enrolled participants: 85.4% (123/144) with diagnosis of type I acute exacerbation of chronic bronchitis (azithromycin group 86.1%; 62/72, co‐amoxyclav group 84.7%; 61/72). However, no reason for the missing data was provided |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Low risk | Baseline characteristics were comparable between the 2 treatment groups |
Kogan 2003.
| Methods | Location: Exequiel Gonzales Cortes Children's Hospital, Santiago, Chile Children who presented with signs of classic bacterial pneumonia were randomly assigned to receive oral amoxycillin or azithromycin |
|
| Participants | 48 children aged 1 month to 14 years were enrolled with classic bacterial pneumonia, with high fever and chest findings of crackles or signs of consolidation, and chest X‐rays with segmental, alveolar or lobar consolidation. 1 patient developed serious pneumonia in the first 12 hours of enrolment and was excluded from the study. The remaining 47 completed the study with 23 receiving azithromycin and 24 receiving amoxycillin. The number of children with M. pneumoniae was 8, with 5 in the azithromycin group and 3 in the amoxycillin‐clavulanate group | |
| Interventions | 1. Azithromycin 10 mg/kg once daily for 3 days 2. Amoxicillin 75 mg/kg/day in 3 divided doses for 7 days | |
| Outcomes | 1. Clinical response: fever (> 38 ºC) at 3, 7 and 14 days after intervention 2. Radiological findings | |
| Notes | Outcomes of this study were not relevant to our criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was available |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients from the classic pneumonia group were randomly assigned to receive oral amoxicillin...." Comment: the study did not report the concealment process |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Methods of blinding were not specified. Participants and caregivers may have been aware of their treatment group because the frequency and duration of drug administration was different between the groups. Radiology assessment was blinded Quote: "All chest X‐rays done ... were seen by the same radiologist, who was not familiar with the patients' clinical history and treatment group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The total randomised participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Low risk | Baseline characteristics were comparable between the 2 treatment groups |
Mertens 1992.
| Methods | Location: The Netherlands. This study was a part of unpublished international multicentre study Participants were randomised to receive either azithromycin or amoxycillin. Block randomisation was done by Pfizer‐Euroclin, Brussels, Belgium. Double‐blinding was performed with matched placebo tablets. Participants were clinically evaluated on days 5 to 7 and 12 to 15 | |
| Participants | 50 in‐ and outpatients aged 18 years or older with acute exacerbation of chronic bronchitis were recruited. Chronic bronchitis was clinically defined as having 3 levels of severity. Type I exacerbation (most severe grade), type II exacerbation (less severe grade) and type III exacerbation (least severe grade). Participants with a terminal illness or concomitant use of antibiotics within 48 hours prior to treatment were excluded. Participants: azithromycin group N = 25, amoxycillin group N = 25 | |
| Interventions | 1. Azithromycin 500 mg once daily for 3 days 2. Amoxicillin 500 mg 3 times daily for 5 days | |
| Outcomes | Cure Improvement Failure Pathogen eradication | |
| Notes | All 50 randomised participants were analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was done at Pfizer‐Euroclin, Brussels, Belgium |
| Allocation concealment (selection bias) | Low risk | Quote: "In this double‐blind study, patients were randomized to receive either azithromycin at a dosage of 500 mg (two 250‐mg capsules) once daily for 3 days or amoxicillin at a dosage of 500 mg (two 250‐mg capsules) three times daily for 5 days." Block randomisation was done at Pfizer‐Euroclin, Brussels, Belgium |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Matched placebo was used. Quote: "Each patient received six capsules per day (six amoxicillin capsules or two azithromycin capsules plus four placebos)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 50 randomised participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Unclear risk | Baseline characteristics were comparable between the 2 treatment groups |
Sevieri 1993.
| Methods | Location: Italy Participants were randomly assigned to treatment. The actual randomisation is not clear. No description of blinding | |
| Participants | 50 adult participants with acute purulent exacerbation of chronic bronchitis caused by H. influenzae were recruited. Participants: azithromycin group N = 25, amoxycillin/clavulanic acid group N = 25 | |
| Interventions | 1. Azithromycin 500 mg once daily for 3 days 2. Amoxicillin/clavulanic acid 1 g twice daily for 6 days | |
| Outcomes | Cure Pathogen eradication | |
| Notes | All 50 randomised participants were clinically and bacteriological evaluated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to treatment. The actual randomisation is not clear |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation method |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No description of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were evaluated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Unclear risk | Baseline characteristics of the 2 treatment groups were not addressed |
Whitlock 1995.
| Methods | Location: USA, multicentre study Participants were randomly assigned to treatment. Investigator‐blinded, parallel‐group study. Clinical evaluation was performed at 3 visits, days 5 to 7, days 11 to 14 and days 26 to 30 | |
| Participants | 70 outpatients aged between 35 and 75 years with a clinical diagnosis of acute bacterial exacerbation of chronic bronchitis were recruited. Participants with pneumonia, bronchitis with concurrent bronchiectasis or active bronchial asthma, or use of antibiotics within 72 hours of enrolment were excluded. Participants: azithromycin group N = 39, amoxycillin/clavulanate group N = 31 | |
| Interventions | 1. Azithromycin 500 mg once on day 1, followed by 250 mg daily on days 2 to 5 2. Amoxycillin/clavulanate 500 mg 3 times a day for 10 days | |
| Outcomes | Cure (complete resolution of resolution of acute exacerbation of COPD on day 11) Improvement (incomplete resolution) Failure Relapse (day 28) Adverse events Eradication of pathogen (day 11) Recurrence of pathogen (day 28) | |
| Notes | 14 participants were excluded from clinical outcome analysis; 8 of 14 had a resistant pathogen (azithromycin 6, amoxycillin/clavulanate 2), 6 had protocol violations (azithromycin 4, amoxycillin/clavulanate 2). Bacteriologic evaluation was performed in 37 participants who had baseline pathogen reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was available |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to receive either azithromycin once daily (two 250‐mg capsules together on day 1, followed by one 250‐mg capsule a day on days 2 through 5) or amoxicillin/clavulanate three times daily (one 500‐mg tablet three times a day for 10 days)" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "To maintain investigator blinded conditions, study medication was assigned and dispensed by an individual other than the investigator responsible for clinical assessments" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 25.6% (10/39) of the azithromycin group and 12.9% (4/31) of the amoxycillin/clavulanate group were excluded from evaluation of clinical response at the day 11 end of therapy visit with the reasons explained in the paper: isolation of a resistant pathogen at baseline (6 azithromycin; 2 amoxycillin/clavulanate) or miscellaneous protocol violations, including failure to meet the inclusion criteria, concurrent treatment with another antibiotic, irregular visits or loss to follow‐up (4 azithromycin, 2 amoxycillin/clavulanate) |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Low risk | Baseline characteristics were comparable between the 2 treatment groups |
Wubbel 1999.
| Methods | Location: USA, randomised, non‐blinded trial Participants were randomised to receive either azithromycin or amoxycillin/clavulanate in those aged 6 months to 5 years, and erythromycin in children aged older than 5 years. Participants were evaluated at enrolment and again at 2 to 3 days and 10 to 37 days after the treatment started | |
| Participants | 88 participants with community‐acquired pneumonia at the Children's Medical Center of Dallas aged 6 months to 16 years were enrolled. Participants aged 6 months to 5 years: azithromycin group N = 39, amoxy‐clavulanic acid group N = 49 | |
| Interventions | 1. Azithromycin oral suspension 10 mg/kg (maximum 500 mg) once on day 1, followed by 5 mg/kg (maximum 250 mg) once daily for 4 days 2. Conventional therapy, 3 times daily for 10 days (amoxycillin/clavulanic acid 40 mg/kg/day for participants aged 6 months to 5 years, and erythromycin estolate 40 mg/kg/day for children aged 5 to 16 years) | |
| Outcomes | Cure Improvement Failure Adverse events | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about how the list of randomised therapy assignments was generated |
| Allocation concealment (selection bias) | Unclear risk | No information was available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 147 were randomised, 69 to the azithromycin group and 78 to the amoxycillin group. All were analysed |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Low risk | Baseline characteristics were comparable between the 2 treatment groups |
Zachariah 1996.
| Methods | Multicentre, double‐blinded trial. Participants were randomly assigned to treatment. Matched placebo tablets were given. Participants were assessed clinically on days 5 and 14 | |
| Participants | 369 participants aged 18 years or more diagnosed with acute bronchitis, or acute infectious exacerbation of chronic bronchitis, or community‐acquired pneumonia were recruited. Acute bronchitis was defined as the presence of purulent sputum together with fever, leucocytosis and/or symptoms suggestive of lower respiratory tract infection. Pregnant and lactating women, participants with a terminal illness, gastrointestinal or hepatic disorders, infectious mononucleosis, or those who had received prior antimicrobial treatment were excluded. Participants: azithromycin group N = 186, co‐amoxyclav group N = 183 | |
| Interventions | 1. Azithromycin (Pfizer) 500 mg once daily for 3 days 2. Co‐amoxyclav (Augmentin; Smithkline Beecham) 375 mg 3 times daily for 10 days | |
| Outcomes | Cure Improvement Failure Relapse Adverse events Eradication of pathogen | |
| Notes | Of 369 randomised participants, 346 were clinically evaluated; 173 were in the azithromycin group and 173 were in the co‐amoxyclav group. 193 participants who had baseline pathogen were bacteriologically evaluated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about how the randomised assignments were generated |
| Allocation concealment (selection bias) | Unclear risk | No information about concealment was available. Quote: "After enrollment, patients were randomly assigned to one of the treatment groups..." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Matched placebo tablets were employed to maintain blinding of the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7% (13/186) of the azithromycin group and 5.5 % (10/183) of the amoxycillin/clavulanate group were excluded from the evaluation of clinical outcome. Quote: "Reasons for exclusion were as follows: failure to meet entry criteria (four azithromycin and one co‐amoxiclav‐treated patients); protocol violation (two azithromycin‐treated patients); lost to follow‐up (three patients in each treatment group), adverse events (three azithromycin‐ and six co‐amoxiclav treated patients); and withdrawal from study (one azithromycin‐treated patient)" |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Unclear risk | Baseline characteristics were comparable between the 2 treatment groups |
Zheng 2002.
| Methods | Location: China Participants were assigned to treatments. The information about randomisation sequence generation, allocation concealment and blinding is not clear | |
| Participants | 80 hospitalised participants with acute purulent exacerbation of chronic bronchitis and aged more than 30 years. Participants having antibiotics within 48 hours and with known allergy to beta‐lactam antibiotics, beta‐lactamase inhibitors, serum creatinine > 200 mg/L and immunosuppressant users were excluded Participants: azithromycin group N = 38, amoxycillin/clavulanic group N = 42 | |
| Interventions | 1. Azithromycin iv administration for 5 days, day 1 500 mg and days 2 to 5 250 mg 4 times a day 2. Amoxicillin/clavulanic acid iv administration for 7 days with 1.2 bid | |
| Outcomes | Cure Improved Failure Adverse effect | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were assigned to treatments. The method of randomisation was not clear |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation method |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were evaluated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Unclear risk | Baseline characteristics of the 2 treatment groups were not addressed |
iv: intravenously N: number bid: two times a day tds: three times a day
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berry 1998 | The in vitro study compared the efficacy of azithromycin and other macrolides with amoxycillin‐clavulanate against Streptococcus pneumoniae and Haemophilus influenzae |
| Bohte 1995 | The study compared azithromycin with benzyl penicillin or erythromycin in community‐acquired pneumonia |
| Bradbury 1993 | The study compared azithromycin with clarithromycin |
| Dimopoulos 2007 | A meta‐analysis of various antibiotic comparators including azithromycin versus amoxycillin |
| Ficnar 1997 | The study compared different doses of azithromycin in the treatment of upper and lower respiratory tract infections |
| Gomez 1996 | The comparators were amoxycillin or erythromycin. The data were analysed in overall results that meant we were not able to get the information specific to amoxycillin |
| Laurent 1996 | The study compared azithromycin with roxithromycin |
| Lauvau 1997 | The study included participants with upper respiratory infections |
| Maimon 2008 | A meta‐analysis of various antibiotic comparators not relevant to our compared interventions (azithromycin versus amoxycillin) |
| Morandini 1993 | The study compared azithromycin with roxithromycin |
| Morris 2010 | RCT of single‐dose azithromycin versus 7 days of amoxycillin in treating acute otitis media in Aboriginal children |
| Rahav 2004 | The study compared azithromycin with other antibiotics |
| Roord 1996 | The study compared azithromycin with erythromycin |
RCT: randomised controlled trial
Contributions of authors
In the original review Ratana Panpanich (RP) designed the protocol, identified studies, extracted data from the included studies and co‐wrote the review. Peerasak Lerttrakarnnon (PL) co‐wrote the review and extracted data from the included studies. Malinee Laopaiboon (ML) helped analyse data and prepared the final draft of this review. In the 2011 update ML contributed to all processes. RP contributed to 'Risk of bias' assessment and RP and PL also prepared the final draft of the updated review. In this 2014 update ML contributed to all processes. RP and KW contributed to screening of studies and the final draft of the updated review.
Sources of support
Internal sources
Khon Kaen University, Faculty of Public Health, Thailand.
Chiang Mai University, Faculty of Medicine, Thailand.
External sources
Effective Health Care Alliance Program, Liverpool School of Tropical Medicine, Liverpool, UK.
Thailand Research Fund (Distinguished Research Professor Award), Thailand.
Thai Cochrane Network, Thailand.
Declarations of interest
Malinee Laopaiboon: I received an honorarium from the Thailand Research Fund, which is a non‐profit organisation. Ratana Panpanich: none known. Kyaw Swa Mya: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Balmes 1991 {published data only}
- Balmes P, Clerc G, Dupont B, Labram C, Pariente R, Poirier R. Comparative study of azithromycin and amoxycillin/clavulanic acid in the treatment of lower respiratory tract infections. European Journal of Clinical Microbiology & Infectious Diseases 1991;10(5):437‐9. [CN‐00077568] [DOI] [PubMed] [Google Scholar]
Beghi 1995 {published data only}
- Beghi G, Berni F, Carratu L, Casalini A, Consigli G, D'Antò M, et al. Efficacy and tolerability of azithromycin versus amoxicillin/clavulanic acid in acute purulent exacerbation of chronic bronchitis. Journal of Chemotherapy 1995;7(2):146‐52. [CN‐00117950] [DOI] [PubMed] [Google Scholar]
Biebuyck 1996 {published data only}
- Biebuyck XA. Comparison of azithromycin and co‐amoxyclav in the treatment of acute tracheobronchitis and acute infectious exacerbations of chronic bronchitis in adults. Journal of Internal Medical Research 1996;24(5):407‐18. [CN‐00168989] [DOI] [PubMed] [Google Scholar]
Daniel 1991 {published data only}
- Daniel R. Simplified treatment of acute lower respiratory tract infection with azithromycin: a comparison with erythromycin and amoxycillin. European Azithromycin Study Group. Journal of International Medical Research 1991;19(5):373‐83. [CN‐00080152] [DOI] [PubMed] [Google Scholar]
Ferwerda 2001 {published data only}
- Ferwerda A, Moll HA, Hop WCJ, Kouwenberg JM, Tjon Pian Gi CV, Robben SG, et al. Efficacy, safety and tolerability of 3 day azithromycin versus 10 day co‐amoxiclav in the treatment of children with acute lower respiratory tract infections. Journal of Antimicrobial Chemotherapy 2001;47:441‐6. [DOI] [PubMed] [Google Scholar]
Gris 1996 {published data only}
- Gris P. Once‐daily, 3 day azithromycin versus a three‐times‐daily, 10‐day course of co‐amoxyclav in the treatment of adults with lower respiratory tract infections: results of a randomized, double‐blind comparative study. Journal of Antimicrobial Chemotherapy 1996;37:93‐101. [DOI] [PubMed] [Google Scholar]
Harris 1998 {published data only}
- Harris JA, Kolokathis A, Campbell M, Cassell GH, Hammerschlag MR. Safety and efficacy of azithromycin in the treatment of community‐acquired pneumonia in children. Pediatric Infectious Diseases Journal 1998;17:865‐71. [DOI] [PubMed] [Google Scholar]
Hoepelman 1993 {published data only}
- Hoepelman AIM, Sips AP, Helmond JLM, Barneveld PWC, Neve AJ, Zwinkels M, et al. A single‐blind comparison of three‐day azithromycin and ten‐day co‐amoxiclav treatment of acute lower respiratory tract infections. Journal of Antimicrobial Chemotherapy 1993;31(Suppl E):147‐52. [DOI] [PubMed] [Google Scholar]
Hoepelman 1998 {published data only}
- Hoepelman IM, Mollers MJ, Schie MH, Greefhorst AP, Schlösser NJ, Sinninghe Damsté EJ, et al. A short (3‐day) course of azithromycin tablets versus a 10‐day course of amoxycillin‐clavulanic acid (co‐amoxiclav) in the treatment of adults with lower respiratory tract infections and effects on long‐term outcome. International Journal of Antimicrobial Agents 1998;9:141‐6. [DOI] [PubMed] [Google Scholar]
Kogan 2003 {published data only}
- Kogan R, Martinez MA, Rubilar L, Paya E, Quevedo I, Puppo H, et al. Comparative randomized trial of azithromycin versus erythromycin and amoxicillin for treatment of community‐acquired pneumonia in children. Pediatric Pulmonology 2003;35(2):91‐8. [DOI] [PubMed] [Google Scholar]
Mertens 1992 {published data only}
- Mertens JC, Barneveld PW, Asin HR, Ligtvoet E, Visser MR, Branger T, et al. Double‐blind randomized study comparing the efficacies and safeties of a short (3 day) course of azithromycin and a 5 day course of amoxycillin in patients with acute exacerbations of chronic bronchitis. Antimicrobial Agents and Chemotherapy 1992;36(7):1456‐9. [CN‐00086554] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sevieri 1993 {published data only}
- Sevieri G, Roggi G, Monacci A. A comparison between amoxycillin clavulanic acid and azithromycin in exacerbations of chronic bronchitis sustained by Haemophilus influenzae [Confronto Fra Amoxycillina‐Acido Clavulanico E Azitromicina Nelle Riacutizzazione Di Bronchite Cronica Sostenuta Da Haemofilus Influenzae]. Minerva Pneumologica 1993;32(2):67‐70. [Google Scholar]
Whitlock 1995 {published data only}
- Whitlock W. Multicenter comparison of azithromycin and amoxycillin/clavulanate in the treatment of patients with acute exacerbations of chronic obstructive pulmonary disease. Current Therapeutic Research Clinical and Experimental 1995;56(10):985‐95. [CN‐00174186] [Google Scholar]
Wubbel 1999 {published data only}
- Wubbel L, Muniz L, Ahmed A, Trujillo M, Carubelli C, Abramo T, et al. Etiology and treatment of community acquired pneumonia in ambulatory children. Pediatric Infectious Disease Journal 1999;18(2):98‐104. [DOI] [PubMed] [Google Scholar]
Zachariah 1996 {published data only}
- Zachariah J. A randomized, comparative study to evaluate the efficacy and tolerability of a 3‐day course of azithromycin versus a 10‐day course of co‐amoxiclav as treatment of adult patients with lower respiratory tract infections. Journal of Antimicrobial Chemotherapy 1996;37(Suppl):103‐13. [DOI] [PubMed] [Google Scholar]
Zheng 2002 {published data only}
- Zheng S, Li X. Research of amoxicillin/clavulanic in acute purulent exacerbation of chronic bronchitis compare with azithromycin. Journal of Clinical Pulmonary Medicine 2002;7(3):5‐6. [Google Scholar]
References to studies excluded from this review
Berry 1998 {published data only}
- Berry V, Rhorburn CE, Knott SJ, Woodnutt G. Bacteriological efficacies of three macrolides compared with those of amoxycillin‐clavulanate against Streptococcus pneumoniae and Haemophilus influenza. Antimicrobial Agents and Chemotherapy 1998;42(12):3193‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bohte 1995 {published data only}
- Bohte R, van't Wout JW, Lobatto S, Blusse van Oud Alblas A, Boekhout M, Nauta EH. Efficacy and safety of azithromycin versus benzylpenicillin or erythromycin in community acquired pneumonia. European Journal of Clinical Microbiology & Infectious Diseases 1995;14(3):182‐7. [DOI] [PubMed] [Google Scholar]
Bradbury 1993 {published data only}
- Bradbury F. Comparison of azithromycin versus clarithromycin in the treatment of patients with lower respiratory tract infection. Journal of Antimicrobial Chemotherapy 1993;31(Suppl E):153‐62. [DOI] [PubMed] [Google Scholar]
Dimopoulos 2007 {published data only}
- Dimopoulos G, Siempos II, Korbila IP, Manta KG, Falagas ME. Comparison of first‐line with second‐line antibiotics for acute exacerbations of chronic bronchitis: a metaanalysis of randomized controlled trials. Chest 2007;132(2):447‐55. [DOI] [PubMed] [Google Scholar]
Ficnar 1997 {published data only}
- Ficnar B, Huzjak N, Oreskovic K, Matrapazovski M, Kilnar I. Azithromycin: 3 day versus 5 day course in the treatment of respiratory tract infections in children. Journal of Chemotherapy 1997;9(1):38‐43. [DOI] [PubMed] [Google Scholar]
Gomez 1996 {published data only}
- Gomez Campdera JA, Navarro Gomez ML, et al. Azithromycin in the treatment of ambulatory pneumonia in children [Azitromycina en el tratamiento de las neumonias ambulatorias en la infancia]. Acta Pediatrica Espanola 1996;54(8):554‐62. [Google Scholar]
Laurent 1996 {published data only}
- Laurent K. Efficacy, safety and tolerability of azithromycin versus roxithromycin in the treatment of acute lower respiratory tract infections. Journal of Antimicrobial Chemotherapy 1996;37(Suppl C):115‐24. [DOI] [PubMed] [Google Scholar]
Lauvau 1997 {published data only}
- Lauvau D, Verbist L. An open, multicentre, comparative study of the efficacy and safety of azithromycin and co‐amoxyclav in the treatment of upper and lower respiratory tract infections in children. Journal of International Medical Research 1997;25(5):285‐95. [CN‐00197916] [DOI] [PubMed] [Google Scholar]
Maimon 2008 {published data only}
- Maimon N, Nopmaneejumruslers C, Marras C. Antibacterial class is not obviously important in outpatient pneumonia: a meta‐analysis. European Respiratory Journal 2008;31:1068‐76. [DOI] [PubMed] [Google Scholar]
Morandini 1993 {published data only}
- Morandini G, Perduca M, Zannini G, Foschino MP, Miragliotta G, Carnimeo NS. Clinical efficacy of azithromycin in lower respiratory tract infections. Journal of Chemotherapy 1993;5(1):32‐6. [DOI] [PubMed] [Google Scholar]
Morris 2010 {published data only}
- Morris PS, Gadil G, McCallum GB, Wilson CA, Smith‐Vaughan HC, Torzillo P, et al. Single‐dose azithromycin versus seven days of amoxycillin in the treatment of acute otitis media in Aboriginal children (AATAAC): a double blind, randomised controlled trial. Medical Journal of Australia 2010;192(1):24‐9. [DOI] [PubMed] [Google Scholar]
Rahav 2004 {published data only}
- Rahav G, Fidel J, Gibor Y, Shapiro M. Azithromycin versus comparative therapy for the treatment of community acquired pneumonia. International Journal of Antimicrobial Agents 2004;24(2):181‐4. [DOI] [PubMed] [Google Scholar]
Roord 1996 {published data only}
- Roord J, Wolf BHM, Goossens MMHT, Kimpen JLL. Prospective open randomized study comparing efficacies and safeties of a 3 day course of azithromycin and a 10 day course of erythromycin in children with community acquired acute lower respiratory tract infections. Antimicrobial Agents and Chemotherapy 1996;40(12):2765‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Austrian 1994
- Austrian R. Confronting drug‐resistant pneumococci. Annals of Internal Medicine 1994;121:807‐9. [DOI] [PubMed] [Google Scholar]
Bariffi 1995
- Bariffi F, Sanduzzi A, Ponticiello A. Epidemiology of lower respiratory tract infections. Journal of Chemotherapy 1995;7(4):263‐76. [DOI] [PubMed] [Google Scholar]
Bartlett 1998
- Bartlett JG, Breiman RF, Mandell LA, File TM. Community‐acquired pneumonia in adults: guidelines for management. The Infectious Diseases Society of America. Clinical Infectious Diseases 1998;26:811‐38. [DOI] [PubMed] [Google Scholar]
Berntsson 1986
- Berntsson E, Lagergard T, Strannegard O, Trollfors B. Etiology of community‐acquired pneumonia in outpatients. European Journal of Clinical Microbiology 1986;5:446. [DOI] [PubMed] [Google Scholar]
Brown 1998
- Brown PD, Lerner SA. Community‐acquired pneumonia. Lancet 1998;352:1295‐302. [DOI] [PubMed] [Google Scholar]
Dunn 1996
- Dunn CJ, Barradell LB. Azithromycin: a review of its pharmacological properties and use as 3‐day therapy in respiratory tract infections. Drug 1996;51(3):483‐505. [DOI] [PubMed] [Google Scholar]
Fang 1990
- Fang GD, Fine M, Orloff J, Arisumi D, Yu VL, Kapoor W, et al. New and emerging etiology for community‐acquired pneumonia with implications for therapy: a prospective multicenter study of 359 cases. Medicine 1990;69(5):307‐16. [DOI] [PubMed] [Google Scholar]
Goldstein 1996
- Goldstein FW, Acar JF. Antimicrobial resistance among lower respiratory tract isolates of Streptococcus pneumoniae: results of a 1992‐93 Western Europe and USA Collaborative Surveillance Study: The Alexandes Project Collaborative Groups. Journal of Antimicrobial Chemotherapy 1996;38(Suppl A):71‐84. [DOI] [PubMed] [Google Scholar]
Graffelman 2004
- Graffelman AW, Neven AK, Cessie SI, Kroes ACM, Springer MP, Peterhans Broek PJ. Pathogens involved in lower respiratory tract infections in general practice. British Journal of General Practice 2004;54:15‐9. [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org. Available from www.cochrane‐handbook.org, 2011.
Huchon 1998
- Huchon G, Woodhead M, Gialdroni‐Grassi G, et al. Guidelines for management of adult community‐acquired lower respiratory tract infections. European Respiratory Journal 1998;11:986‐91. [DOI] [PubMed] [Google Scholar]
Knutson 2002
- Knutson D, Braun C. Diagnosis and management of acute bronchitis. American Family Physician 2002;65(10):2039‐44. [PubMed] [Google Scholar]
Langille 1993
- Langille DB, Yates L, Marrie TJ. Serological investigation of pneumonia as it presents to the physician's office. Canadian Journal of Infectious Diseases 1993;4:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
Legnani 1997
- Legnani D. Role of oral antibiotics in treatment of community‐acquired lower respiratory tract infections. Diagnostic Microbiology and Infectious Disease 1997;27:41‐7. [DOI] [PubMed] [Google Scholar]
Lieberman 1996
- Lieberman D, Schlaeffer F, Bolden I, Lieberman D, Horowitz S, Friedman MG, et al. Multiple pathogen in adult patients admitted with community‐acquired pneumonia: a one year prospective study of 346 consecutive patients. Thorax 1996;51(2):179‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mandell 1994
- Mandell LA. Antibiotics for pneumonia therapy. Medical Clinics of North America 1994;78(5):997‐1014. [DOI] [PubMed] [Google Scholar]
Marrie 1996
- Marrie TJ, Peeling RW, Fine MJ, Singer DE, Coley CM, Kapoor WN. Ambulatory patients with community‐acquired pneumonia: the frequency of atypical agents and clinical course. American Journal of Medicine 1996;101:508‐15. [DOI] [PubMed] [Google Scholar]
Meehan 1997
- Meehan TP, Fine Mj, Krunholz HM, Scinto JD, Galusha DH, Mockalis JT, et al. Quality of care, process and outcomes in elderly patients with pneumonia. JAMA 1997;278:2080‐4. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sclar 1994
- Sclar DA, Tartaglione TA, Fine MJ. Overview of issues related to medical compliance with implications for the outpatient management of infectious diseases. Infectious Agents and Disease 1994;3(5):266‐73. [PubMed] [Google Scholar]
Woodhead 1991
- Woodhead MA, Arrowsmith J, Chamberlain‐Webber R, Wooding S, Williams I. The value of routine microbial investigation in community‐acquired pneumonia. Respiratory Medicine 1991;85:313‐7. [DOI] [PubMed] [Google Scholar]
Woodhead 1996
- Woodhead M, Gialdroni‐Grassi G, Huchon GL, Leophonte P, Manresa F, Schaberg T. Use of investigations in lower respiratory tract infection in the community: a European survey. European Respiratory Journal 1996;9(8):1596‐600. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Laopaiboon 2011
- Laopaiboon M, Panpanich R, Lerttrakarnnon P. Azithromycin for acute lower respiratory tract infections. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD001954.pub3] [DOI] [Google Scholar]
Panpanich 2000
- Panpanich R, Lerttrakarnnon P. Azithromycin for acute lower respiratory tract infections. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD001954] [DOI] [PubMed] [Google Scholar]
Panpanich 2004
- Panpanich R, Lerttrakarnnon P, Laopaiboon M. Azithromycin for acute lower respiratory tract infections. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD001954.pub2] [DOI] [PubMed] [Google Scholar]
Panpanich 2008
- Panpanich R, Lerttrakarnnon P, Laopaiboon M. Azithromycin for acute lower respiratory tract infections. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD001954.pub3] [DOI] [PubMed] [Google Scholar]


