Abstract
Background
The incidence of nonalcoholic fatty liver disease (NAFLD) has increased recently and is related to obesity and the associated surge in type 2 diabetes mellitus (DM) and metabolic syndrome diagnoses. We aim to compare the effectiveness of tofogliflozin and pioglitazone treatment on hepatic steatosis in patients with NAFLD with type 2 DM.
Methods
This is an open label, prospective, randomized exploratory study. Patients who meet the inclusion criteria and do not meet any exclusion criteria will undergo magnetic resonance imaging (MRI)-based proton density fat fraction (MRI-PDFF). Patients with ≥10% liver fat content on MRI-PDFF will be randomly assigned to receive tofogliflozin 20 mg per day (n = 20) or pioglitazone 15–30 mg per day (n = 20). MRI will be performed after 24 weeks following initiation of medication therapy. Then, patients will take tofogliflozin and pioglitazone in combination in both groups for 24 weeks. MRI will be performed again at 48 weeks (24 weeks after initiation medication in combination).
Results
Our study's primary endpoint will be change in hepatic steatosis measured by MRI-PDFF at 24 weeks after medication therapy. The secondary endpoint will be change in alanine aminotransferase at 24 weeks of medication therapy and the main exploratory endpoint will be changes in liver fat content and liver sclerosis at 48 weeks of medication.
Conclusions
We will compare the effectiveness of tofogliflozin and pioglitazone treatment using MRI for improving hepatic steatosis in patients with NAFLD complicated by DM and investigate if the combination of these two medications is effective for treating NAFLD.
Trial registration
This trial is registered in the Japan Registry of Clinical Trials (jRCTs031180159).
Protocol version
1.2, 14 December 2018.
Keywords: Non-alcoholic fatty liver disease, Tofogliflozin, Pioglitazone, Diabetes mellitus, Hepatic steatosis, MRI-Based proton density fat fraction
Abbreviations: NAFLD, non-alcoholic fatty liver disease; DM, diabetes mellitus; MRI-PDFF, magnetic resonance imaging-based proton density fat fraction; NASH, non-alcoholic steatohepatitis; ALT, alanine aminotransferase; HbA1c, glycated hemoglobin; SPIRIT, the Standard Protocol Items: Recommendations for Interventional Trials; jRCTs, the Japan Registry of Clinical Trials; AE, adverse event; CRF, case report form; FAS, full analysis set; PPS, per protocol set
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) has attracted attention because of the recent increase in incidence of obesity and associated surge in number of patients with type 2 diabetes mellitus (DM) and metabolic syndrome. NAFLD is a hepatic manifestation of metabolic syndrome and comprises NAFL, which refers to steatosis affecting hepatocytes, and non-alcoholic steatohepatitis (NASH), which is defined as the presence of ≥5% hepatic steatosis and inflammation with hepatocyte injury, with or without any fibrosis and possibly progress to liver cirrhosis and hepatocellular carcinoma [1,2]. Importantly, NAFLD is associated with obesity, and type 2 DM and insulin resistance are major risk factors for the development of NAFLD. It has been reported that 20–75% of adult patients with NASH have type 2 DM, hyperglycemia, and impaired glucose tolerance [3]; additionally, history of type 2 DM increases the prevalence of NASH by 2.6-fold [4]. Although the fundamental treatment of NAFLD/NASH complicated by type 2 DM is weight loss by diet and exercise therapy, the thiazolidinedione pioglitazone, which is expected to improve insulin resistance, is recommended as a drug therapy [1,2]. Several large-scale randomized controlled trials on pioglitazone have reported its effectiveness for treating NASH [[5], [6], [7]]; it has also been shown to improve markers of hepatic steatosis and fibrosis on liver histology [2]. However, pioglitazone treatment has side effects like weight gain, pedal edema, bone loss, and heart failure [8]. In recent studies, SGLT2 inhibitor treatment is expected to be effective in treating DM combined with NAFLD/NASH. Treatment with SGLT2 inhibitors improve general metabolism and aid in weight loss, blood pressure reduction, and alanine aminotransferase (ALT) reduction in addition to glycated hemoglobin (HbA1c) reduction; furthermore, research has shown improvement in insulin resistance and reduction in liver fat content through treatment [9,10].
In this study, we will compare the effectiveness of tofogliflozin and pioglitazone using magnetic resonance imaging (MRI) in improving hepatic steatosis of patients with NAFLD with type 2 DM. Additionally, we observed if treatment with combination of these two drugs is effective for improving NAFLD.
2. Materials and methods
2.1. Trial design
This study is an open label, prospective, randomized exploratory study comparing the effectiveness of tofogliflozin and pioglitazone on hepatic steatosis in patients with NAFLD with type 2 DM. Patients will take tofogliflozin 20 mg once per day or pioglitazone 15–30 mg once per day for 24 weeks. Patients with HbA1c ≥ 6.0% (≥6.5% in those older than 65 years and using sulfonylurea or rapid insulin secretagogue) after 24 weeks of medication will then take tofogliflozin and pioglitazone in combination in both groups for another 24 weeks.
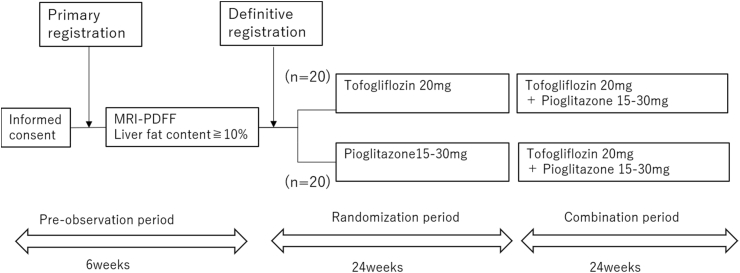
The flow chart of the study is shown in Fig. 1. MRI will be performed at baseline, 24 weeks, and 48 weeks after intervention and evaluated by blinded two liver specialists (KI and YO).
Fig. 1.
Study schedule.
The study plans to recruit 40 adult patients from Yokohama City University Hospital.
2.2. Ethical issues
The study will comply with the Declaration of Helsinki and Japanese ethical guidelines for clinical research. The study followed the Standard Protocol Items as follows: Recommendations for Interventional Trials (SPIRIT) and its checklists. This trial was registered in the Japan Registry of Clinical Trials (jRCTs, s031180159). Written informed consent for participation in the study will be obtained from all participants. The protocol and any information supplied to gain informed consent were approved by a qualified Institutional Review Board/Independent Ethics Committee of Yokohama City University before participant enrollment. All institutional review board and independent ethics committee requirements will be followed during the study and after its completion. Participant personal information will be kept in a locked storage and password-protected hard drive at the institution. Records will be retained for 5 years after study completion and then destroyed by the data center.
2.3. Study endpoints
Expected study endpoints are indicated in Table 1.
Table 1.
Study endpoints.
| Study endpoints |
|---|
| Primary endpoint |
|
| Key secondary endpoint |
|
| Other endpoint |
|
The primary endpoint is the comparison of change in hepatic steatosis on MRI-PDFF at 24 weeks between patients treated with tofogliflozin and those with pioglitazone. The key secondary endpoint is the change in ALT at 24 weeks of medication treatment, compared between two groups. The main exploratory endpoint will be changes in liver fat content and liver sclerosis measured by MRI-PDFF at 48 weeks of medication between two groups.
Other variables included adverse events, the results of standard laboratory analysis, physical examination, vital signs, and compliance rate. Physical assessments will be performed and analyzed using standard procedures at Yokohama City University.
2.4. Medication and treatment
Both the physician and patient will know the treatment allocation. The physician will prescribe tofogliflozin 20 mg/day or pioglitazone 15–30 mg/day according to the drug name provided by the patient enrollment center. Tofogliflozin will be prescribed from 15 mg/day in all female patients and male patients aged ≥65 years. The maximum dose of pioglitazone will be 30 mg/day in any case.
To confirm adherence to intervention protocols, the physician will ask patients if the medication routine was altered in any way.
2.5. Eligibility
The participants were adult patients with NAFLD with type 2 DM (age range, 20–74 years) with HbA1c ≥ 6.5% and ALT beyond the standard level who had undergone diet and exercise therapies before enrollment. Detailed inclusion and exclusion criteria are shown in Table 2.
Table 2.
Inclusion and exclusion criteria.
| Inclusion criteria |
|---|
|
|
| Description of inclusion criteria |
| Males and females; 20–74 years of age |
| Type 2 diabetes patients who have undergone diet and exercise therapies |
| Patients with HbA1c ≥ 6.5% at the inspection 90 days within the primary registration |
| Patients diagnosed NAFLD following the diagnostic criteria of medical guideline of NAFLD/NASH 2014 |
| Patients with ALT beyond the standard value at the inspection 90 days within the primary registration |
| Patients who have provided written consent to participate in this research |
| Exclusion criteria |
|
|
| Description of exclusion criteria |
| Patients in habit of drinking alcohol (male; more than 30g/day, female; more than 20g/day converting to ethanol) |
| Patients diagnosed viral hepatitis (including patients under treatment or career) |
| Patients with other types of hepatitis or liver disorder (e.g. drug-induced, autoimmune) |
| Patients diagnosed liver cirrhosis or patients with serious liver dysfunction (Child-Pugh B or C) |
| Patients with PLT <150,000 at the inspection 90 days within the primary registration |
| Patients with BMI <22 kg/m2 at the inspection 90 days within the primary registration |
| Patients with ALT ≥5 times the upper limit of the standard value at the inspection 90 days within the primary registration |
| Patients with serious renal dysfunction or with eGFR <60 mL/min/1.73 m2 at the inspection 90 days within the primary registration |
| Patients with type 1 diabetes mellitus or with HbA1c ≥ 9.0% at the inspection 90 days within the primary registration |
| Patients using SGLT2 inhibitor, Pioglitazone, insulin, or GLP-1 receptor agonists |
| Patients taking vitaminE formulation |
| Patients with any contradication of MRI (e.g. pacemaker) |
| Patients unable to take MRI (e.g. cannot hold a breath, patients with excessive iron deposition) |
| Patients with heart failure of more than NYHAIII at present or in the past |
In both groups, use of other SGLT2 inhibitors, thiazolidinedione, insulin, and GLP-1 receptor agonists will be prohibited during the observation period. Use of vitamin E will be prohibited during the randomization period. In principle, changes of medication for DM including adding, discontinuing, and changes of dosage will not be allowed throughout the study.
2.6. Randomization and masking
Patients who provide informed consent will first undergo screening to determine whether they fulfill the inclusion criteria and do not meet any exclusion criteria. The principal investigator or co-investigator will complete the Patient Enrollment Form for eligible patients (primary registration). After primary registration, patients with ≥10% or more liver fat content on MRI-PDFF will be randomly assigned to the tofogliflozin group or pioglitazone group, stratified by HbA1c (<7, ≥7.0%), ALT (<50, ≥50 IU/L), and MRI-PDFF (<20, ≥20%) (definitive registration).
Masking is not applicable because this is an open-label study.
2.7. Adverse events (AE) monitoring
All AE that occur during the study will be recorded in an electronic case report form, and include information about the symptom/disease and its onset and end date, severity and seriousness, investigator's opinion of the association with tofogliflozin or pioglitazone treatment, action taken regarding tofogliflozin or pioglitazone usage and AE treatment, cause of event, and information regarding the resolution or outcome. AE will be followed up until normalized or achieving levels not perceived as AE. When AE is organic (cerebral infarction and myocardial infarction) and irreversible, it will be followed up until symptoms are stable. If the investigator or co-investigator judges the AE as recovered or that there is no relationship between the study and AE, it will not be followed up in the same way and the reasons will be recorded on the medical record. The classifications of AE intensity are shown in Table 3.
Table 3.
Classifications of adverse events.
| Grade | Description |
|---|---|
| Grade 1 (mild) | Asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated |
| Grade 2 (moderate) | Minimal, local or non-invasive intervention indicated; limiting age-appropriate instrumental ADL |
| Grade 3 (severe) | Medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care ADL |
| Grade 4 (life-threatening) | Life-threatening consequences; urgent intervention indicated |
| Grade 5 (death) | Death related to AE |
2.8. Criteria for discontinuation of study treatment
Treatment will be discontinued if HbA1c increases to >1.0% after medication therapy initiation, AST/ALT increases > three times than that before medication therapy, a patient becomes ineligible for the trial, oral compliance is <75%, continuous medical examination becomes challenging due to severe AE, HbA1c <6.0% (<6.5% in those older than 65 years and using sulfonylurea or rapid insulin secretagogue) at the end of the randomization period (24 weeks of medication therapy) and in case of pregnancy. Treatment will also cease if the participant requests discontinuation, continuous medical examination becomes challenging, combined use of the prohibited medicine is required because of deterioration of the primary disease or its complication, or if the doctor decides discontinuation of the study is appropriate for other reasons.
2.9. Gene test
The investigators will collect blood samples at any time from definitive registration until 48 weeks after drug intervention to confirm if the patient has genetic polymorphism of PNPLA3 and/or TM6SF2. After analyzation, the patient will fill out the Case Report Form (CRF) and submit it to the data center within 5 days.
The gene test will not be necessary when the patient has previously undergone gene tests of PNPLA3 and TM6SF2.
2.10. Efficacy evaluation
Change in the primary endpoint (liver fat content) measured by MRI-PDFF compared between two groups will be calculated by the mean difference from baseline at 24 weeks.
2.11. Statistical analyses
We obtained the possible number of patients as this is an exploratory study.
The “full analysis set (FAS)” and “per protocol set (PPS)” will be performed to analyze the primary and secondary endpoints. The FAS is going to use the intention-to-treat population. In performing FAS, study participants will be patients who have been registered in this study and assigned the study drug, and patients with serious violations of the research plan (e.g., not obtaining consent and registration date out of the registration period) will be excluded. In performing PPS, we excluded patients with serious violations of inclusion and exclusion criteria of the research plan for study drugs and combination therapies, patients using prohibited drugs, and compliance rate of >120% or <75% from the FAS population. The remaining patients will be enrolled in the study.
Regarding safety, patients who have registered in the study, started treatment as assigned, and undergone part of or all treatment will be defined as the target population for safety analysis and analysis will be performed. The statistical significance of the change between each group will be evaluated for the primary endpoint. The null hypothesis is an equal difference in changes in liver fat content in both groups. This will be tested using the analysis of covariance defining the groups as fixed effects and stratified factors (HbA1c, ALT, and liver fat content) as covariates. In addition, summary statistics (number of cases, average value, standard deviation, minimum value, median value, and maximum value) of changes at 24 weeks of medication will be calculated. The two-sided level of significance is defined as 5%.
Medical statistics specialists will develop a statistical analysis plan and specify details of statistical methods including data handling. The plan will be prepared before the data is fixed.
2.12. The data monitoring committee
The data monitoring committee will be located at the Department of Clinical Examination, Yokohama City University School of Medicine. The patient will be monitored for his or her eligibility, how to provide informed consent, and how to register. If there are any problems with this process, corrective action will be taken. The management team will meet the facility person in charge (investigator or co-investigator) when necessary. Any visit to the facility will be reported in the monitoring report and given to the investigator within 2 weeks of the visit. The monitoring will be completed at the first medical intake and at the end of the study if there are no problems. When unexpected severe AE or an event that greatly affects the study occurs, monitoring will be adjusted appropriately. On-site monitoring will be performed to confirm the necessary documents and, if there are any problems, corrective action will be taken. Results will be conveyed in the monitoring report and given to the investigator within 2 weeks.
3. Discussion
Pioglitazone is a type of thiazolidinedione that ameliorates insulin resistance and improves glucose and lipid metabolism in type 2 DM, and is recommended for patients with NAFLD with type 2 DM [1,2]. However, treatment has side effects such as weight gain, pedal edema, bone loss, and heart failure [8].
SGLT2 inhibitors are a relatively new class of antidiabetic drugs that inhibit reabsorption of glucose in the kidney and therefore lower blood sugar [11]. They can improve insulin resistance/hyperglycemia and simultaneously efficiently decrease visceral fat [12]. Thus, they are expected to help in treating patients with NAFLD complicated by type 2 DM. A Japanese trial comparing pioglitazone and ipragliflozin reported that they have equally beneficial effects on NAFLD and glycemic control in type 2 DM with NAFLD, and patients under ipragliflozin treatment showed significant decreases in body weight and visceral/subcutaneous fat area compared to those of the group undergoing pioglitazone treatment [12]. In our study, we chose tofogliflozin as the comparison SGLT2 inhibitor. The half-life of tofogliflozin and ipragliflozin is different. The half-life of tofogliflozin (5.4 h) is shorter than that of ipragliflozin (14.97 h), which leads to quicker effects and less side effects at night such as hypoglycemia at night when used together with insulin glargine [13]. It is previously reported that tofogliflozin reduced plasma glucose level and body weight gain with a reduction in liver weight and triglyceride content in mice [14].
Presently, the only way to diagnose NASH is through liver biopsy as described in the guidelines (“the gold standard”). However, performing liver biopsy in all patients with NAFLD is impossible as there are more than 10 million patients in Japan. Additionally, there are various problems associated with liver biopsy such as invasiveness, cost, or misinterpretation between pathologists.
Recently, significant advances have been made in MRI techniques and hepatic steatosis and fibrosis can be diagnosed with extremely high sensitivity and specificity by using magnetic resonance (MR) elastography [15,16]. It is also possible to quantify the fat ratio by MRI by measuring the proton density fat fraction value using the iterative decomposition of water and fat with echo asymmetry and least squares estimation sequence (IDEAL IQ) method [17]. MR elastography and MRI-PDFF can be performed simultaneously in one imaging session, and, by combining their results, hepatic steatosis and hepatic fibrosis can be evaluated.
MRI-based noninvasive assessment of liver fibrosis and steatosis would be a potential alternative to liver biopsy in clinical practice [18]. For these reasons, we chose MRI for noninvasive assessment of hepatic steatosis in this study.
4. Conclusion
This study will compare the effectiveness of tofogliflozin and pioglitazone in improving hepatic steatosis in patients with NAFLD complicated by DM using MRI. In addition, we will investigate whether a combination of both drugs is effective for NAFLD.
Declarations
Ethics approval and consent to participate
The protocol was approved by the Ethics Committee of Yokohama City University Hospital. Patient consent to participate in the trial will be obtained from all patients.
Consent for publication
Consent for publication will be obtained from all participants.
Funding
This trial was supported by Kowa Company, Ltd. The funder had no role in the study design, data collection, or data analysis.
Author's contributions
AO, MY, and AN conceived the study. MT and TY conducted feasibility phase work. Recruitment of participants and follow-up will be performed by MI, T. Kobayashi, YH, T. Kessoku, ES, TI, T. Kurihashi, and SS. Reading of MRI will be done by KI and YO. All authors contributed to writing, and all read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have competing interests. This study is funded by Kowa Company, Ltd. (Nagoya, Japan).
Acknowledgements
We thank all the patients and investigators, and the institution involved in this study.
References
- 1.Evidence-based Clinical Practice Guidelines for Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. 2014. [Google Scholar]
- 2.Chalasani N. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 3.Reid A.E. Nonalcoholic steatohepatitis. Gastroenterology. 2001;121(3):710–723. doi: 10.1053/gast.2001.27126. [DOI] [PubMed] [Google Scholar]
- 4.Wanless I.R., Lentz J.S. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12(5):1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 5.Belfort R. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N. Engl. J. Med. 2006;355(22):2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 6.Sanyal A.J. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010;362(18):1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He L. Thiazolidinediones for nonalcoholic steatohepatitis: a meta-analysis of randomized clinical trials. Medicine (Baltim.) 2016;95(42):e4947. doi: 10.1097/MD.0000000000004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah P., Mudaliar S. Pioglitazone: side effect and safety profile. Expert Opin. Drug Saf. 2010;9(2):347–354. doi: 10.1517/14740331003623218. [DOI] [PubMed] [Google Scholar]
- 9.Obata A. Tofogliflozin improves insulin resistance in skeletal muscle and accelerates lipolysis in adipose tissue in male mice. Endocrinology. 2016;157(3):1029–1042. doi: 10.1210/en.2015-1588. [DOI] [PubMed] [Google Scholar]
- 10.Honda Y. The selective SGLT2 inhibitor ipragliflozin has a therapeutic effect on nonalcoholic steatohepatitis in mice. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0146337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu H., Novikov A., Vallon V. Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: basic mechanisms and therapeutic perspectives. Diabetes Metab. Res. Rev. 2017;33(5) doi: 10.1002/dmrr.2886. [DOI] [PubMed] [Google Scholar]
- 12.Ito D. Comparison of ipragliflozin and pioglitazone effects on nonalcoholic fatty liver disease in patients with type 2 diabetes: a randomized, 24-week, open-label, active-controlled trial. Diabetes Care. 2017;40(10):1364–1372. doi: 10.2337/dc17-0518. [DOI] [PubMed] [Google Scholar]
- 13.Takeishi S., Tsuboi H., Takekoshi S. Comparison of tofogliflozin 20 mg and ipragliflozin 50 mg used together with insulin glargine 300 U/mL using continuous glucose monitoring (CGM): a randomized crossover study. Endocr. J. 2017;64(10):995–1005. doi: 10.1507/endocrj.EJ17-0206. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki M. Tofogliflozin, a sodium/glucose cotransporter 2 inhibitor, attenuates body weight gain and fat accumulation in diabetic and obese animal models. Nutr. Diabetes. 2014;4(7):e125. doi: 10.1038/nutd.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dulai P.S., Sirlin C.B., Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J. Hepatol. 2016;65(5):1006–1016. doi: 10.1016/j.jhep.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa-Silva L. MR elastography is effective for the non-invasive evaluation of fibrosis and necroinflammatory activity in patients with nonalcoholic fatty liver disease. Eur. J. Radiol. 2018;98:82–89. doi: 10.1016/j.ejrad.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Reeder S.B. Quantification of hepatic steatosis with MRI: the effects of accurate fat spectral modeling. J. Magn. Reson. Imaging. 2009;29(6):1332–1339. doi: 10.1002/jmri.21751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imajo K. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016;150(3):626–637 e7. doi: 10.1053/j.gastro.2015.11.048. [DOI] [PubMed] [Google Scholar]