Abstract
Background
Lung cancer is the most commonly diagnosed cancer and the leading cause of cancer death in both sexes worldwide. Approximately 50% of those diagnosed with lung cancer will have locally advanced or metastatic disease and will be treated in a palliative setting. Platinum‐based combination chemotherapy has benefits in terms of survival and symptom control when compared with best supportive care.
Objectives
To assess the effectiveness and safety of carboplatin‐based chemotherapy when compared with cisplatin‐based chemotherapy, both in combination with a third‐generation drug, in people with advanced non‐small cell lung cancer (NSCLC). To compare quality of life in people with advanced NSCLC receiving chemotherapy with cisplatin and carboplatin combined with a third‐generation drug.
Search methods
We searched the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL; 13 January 2019), MEDLINE (via PubMed) (1966 to 13 January 2019), and Embase (via Ovid) (1974 to 13 January 2019). In addition, we handsearched the proceedings of the American Society of Clinical Oncology Meetings (January 1990 to September 2018) and reference lists from relevant resources.
Selection criteria
Randomised clinical trials (RCTs) comparing regimens with carboplatin or cisplatin combined with a third‐generation drug in people with locally advanced or metastatic NSCLC. We accepted any regimen and number of cycles that included these drugs, since there is no widely accepted standard regimen.
Data collection and analysis
Two review authors independently assessed the search results, and a third review author resolved any disagreements. The primary outcomes were overall survival and health‐related quality of life. The secondary outcomes were one‐year survival rate, objective response rate and toxicity.
Main results
In this updated review, we located one additional RCT, for a total of 11 included RCTs (5088 participants, 4046 of whom were available for meta‐analysis). There was no difference in overall survival (hazard ratio (HR) 0.99, 95% confidence interval (CI) 0.82 to 1.20; 10 RCTs; 2515 participants; high‐quality evidence); one‐year survival rate (risk ratio (RR) 0.98, 95% CI 0.89 to 1.08; I2 = 17%; 4004 participants; all 11 RCTs; high‐quality evidence); or response rate (RR 0.89, 95% CI 0.79 to 1.00; I2 = 12%; all 11 RCTs; 4020 participants; high‐quality evidence). A subgroup analysis comparing carboplatin with different doses of cisplatin found an overall survival benefit in favour of carboplatin‐based regimens when compared to cisplatin at lower doses (40 to 80 mg/m2) (HR 1.15, 95% CI 1.03 to 1.28; 6 RCTs; 2508 participants), although there was no overall survival benefit when carboplatin‐based chemotherapy was compared to cisplatin at higher doses (80 to 100 mg/m2) (HR 0.93, 95% CI 0.83 to 1.04; I2 = 0%; 4 RCTs; 1823 participants). Carboplatin caused more thrombocytopenia (RR 2.46, 95% CI 1.49 to 4.04; I2 = 68%; 10 RCTs; 3670 participants) and was associated with more neurotoxicity (RR 1.42, 95% CI 0.91 to 2.23; I2 = 0%, 5 RCTs; 1489 participants), although we believe this last finding is probably related to a confounding factor (higher dose of paclitaxel in the carboplatin‐containing treatment arm of a large study included in the analysis). There was no statistically significant difference in renal toxicity (RR 0.52, 95% CI 0.19 to 1.45; I2 = 3%; 3 RCTs; 1272 participants); alopecia (RR 1.11, 95% CI 0.73 to 1.68; I2 = 0%; 2 RCTs; 300 participants); anaemia (RR 1.37, 95% CI 0.79 to 2.38; I2 = 77%; 10 RCTs; 3857 participants); and neutropenia (RR 1.18, 95% CI 0.85 to 1.63; I2 = 94%; 10 RCTs; 3857 participants) between cisplatin‐based chemotherapy and carboplatin‐based chemotherapy regimens. Two RCTs performed a health‐related quality of life analysis; however, as they used different methods of measurement we were unable to perform a meta‐analysis. One RCT reported comparative health‐related quality of life data between cisplatin and carboplatin‐containing arms but found no significant differences in global indices of quality of life, including global health status or functional scales.
In this Cochrane review, we found that the quality of evidence was high for overall survival, one‐year survival rate and response rate but moderate quality evidence for the other outcomes measured.
Authors' conclusions
Advanced NSCL patients treated with carboplatin or cisplatin doublet with third‐generation chemotherapy drugs showed equivalent overall survival, one‐year survival, and response rate. Regarding adverse events, carboplatin caused more thrombocytopenia, and cisplatin caused more nausea/vomiting. Therefore, in this palliative therapeutic intent, the choice of the platin compound should take into account the expected toxicity profile, patient's comorbidities and preferences.
Plain language summary
Comparing carboplatin‐based chemotherapy with cisplatin‐based chemotherapy in the treatment of people with advanced lung cancer
Review question
Is carboplatin more effective and less toxic than cisplatin in the treatment of people with advanced non‐small cell lung cancer?
Background
Lung cancer is the leading cause of cancer death, and more than a half of people found to have the cancer have incurable disease at diagnosis. Non‐small cell is the most common type of lung cancer, corresponding to approximately 85% of all lung cancer cases. Despite recent advances in targeted therapies and immunotherapy, platinum‐based chemotherapy remains a useful and accessible option with well‐established survival benefits for people with advanced non‐small cell lung cancer. Although treatment regimens including cisplatin or carboplatin plus another agent are widely used, they can be associated with undesirable side effects. The aim of this systematic review was to determine which of these two frequently used drugs was more effective and caused fewer side effects.
Study characteristics
We found 11 trials (including 4046 people) that compared cisplatin with carboplatin, both combined with another modern drug, called a third‐generation drug.
Key results
The drugs were equally effective at prolonging survival, but the toxicity profile was different. Carboplatin caused a greater decrease in the number of platelets (which control clotting) in the blood.
Quality of evidence
In this Cochrane review, we found that the quality of evidence was high for overall survival, one‐year survival rate and response rate but moderate quality evidence for the other outcomes measured.
Summary of findings
Summary of findings for the main comparison. Carboplatin compared with cisplatin chemotherapy in combination with third‐generation drugs for advanced non‐small cell lung cancer.
| Carboplatin compared with cisplatin chemotherapy in combination with third‐generation drugs for advanced non‐small cell lung cancer | ||||
| Patient or population: combination with third‐generation drugs for advanced non‐small cell lung cancer Setting: outpatient Intervention: carboplatin‐containing chemotherapy Comparison: cisplatin‐containing chemotherapy | ||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) |
| Risk with carboplatin | ||||
| Overall survival (baseline risks for low‐ and high‐risk groups in the control arm were estimated at 1 year) |
Low risk of death | HR 0.99 (0.82 to 1.2) | 2515 (10 RCTs) | ⊕⊕⊕⊕ HIGH |
| 297 per 1000 (254 to 348) | ||||
| High risk of death | ||||
| 447 per 1000 (388 to 512) | ||||
| 1‐year survival rate | Study population | RR 0.98 (0.89 to 1.08) | 4004 (11 RCTs) | ⊕⊕⊕⊕ HIGH |
| 363 per 1000 (330 to 400) | ||||
| Response rate | Study population | RR 0.89 (0.79 to 1.00) | 4020 (11 RCTs) | ⊕⊕⊕⊕ HIGH |
| 246 per 1000 (219 to 277) | ||||
| Grade III or IV neurotoxicity | Study population | RR 1.42 (0.91 to 2.23 | 3857 (10 RCTs) | ⊕⊕⊕⊝ MODERATE 1 |
| 83 per 1000 (57 to 122) | ||||
| Grade III or IV thrombocytopenia | Study population | RR 2.46 (1.49 to 4.04) | 3857 (10 RCTs) | ⊕⊕⊕⊝ MODERATE 2 |
| 109 per 1000 (66 to 180) | ||||
| Grade III or IV renal toxicity | Study population | RR 0.52 (0.19 to 1.45) | 1272 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 3 |
| 10 per 1000 (4 to 27) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; RCT: randomised clinical trial; RR: risk ratio | ||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||
1Renal toxicity rates were only reported by five included trials. 2The heterogeneity found in this analysis was due to data from the Ferry 2017 trial, so that when data from this trial were excluded, the result remained similar, but without significant heterogeneity (RR 2.00, 95% CI 1.37 to 2.91; I2 = 21%). 3Renal toxicity rates were only reported by three included trials.
Background
Description of the condition
Lung cancer is the most commonly diagnosed cancer (11.6% of total cases) and the leading cause of cancer death (18.4% of total cancer deaths) in both sexes worldwide (GLOBOCAN 2018). The vast majority (about 85%) of lung cancers are pathologic classified as non‐small cell type, and more than a half of patients are diagnosed with incurable locally advanced or metastatic disease (Noone 2018).
Description of the intervention
Chemotherapy in advanced non‐small cell lung cancer (NSCLC) has been integrative part of treatment for several decades. In the 1990s, a meta‐analysis of 52 randomised clinical trials (RCTs) showed that treatment with cisplatin‐based chemotherapy resulted in an increased median survival by six weeks compared with best supportive care in people with NSCLC (NSCLC Collaborative Group 1995). Evidence accumulated since then has indicated that a platinum agent in combination with a third‐generation chemotherapy agent is the standard first‐line treatment (Hanna 2017).
More recently, the development of effective targeted therapies and immune checkpoint inhibitors has revolutionised the management of advanced solid tumours, including NSCLC treatment (Vasconcellos 2018). However, cisplatin‐based chemotherapy still has a main role in the first and subsequent lines of therapy (Lisberg 2019), and modern therapies are not available worldwide because of global variation in access to cancer treatment (de Souza 2016).
It is well known that cisplatin causes a number of significant side effects including nausea and vomiting, alopecia, neutropenia, ototoxicity, neurotoxicity, and renal function impairment (O'Dwyer 2015). Despite efforts to identify genetic predictors of the effectiveness and toxicity of cytotoxic therapies, to this point there are no robust data that can be used in clinical practice to guide the best subgroup of patients to receive cisplatin (Perez‐Ramirez 2017). As a consequence, carboplatin has emerged as a reasonable and potentially more tolerable alternative, since it has been associated with myelotoxicity (particularly thrombocytopenia) but less nephrotoxicity and neurotoxicity (Ho 2016).
Since 1990, more than 20 trials have compared cisplatin with carboplatin in individuals with advanced NSCLC, whilst only a few trials have compared regimens containing a third‐generation drug such as irinotecan, paclitaxel, docetaxel, gemcitabine, and vinorelbine. For example, Schiller and colleagues published a landmark phase III trial that compared cisplatin plus paclitaxel versus carboplatin plus paclitaxel. They found similar survival and response rates but less toxicity with carboplatin plus paclitaxel, although quality of life (QoL) was not assessed (Schiller 2002).
Previous systematic reviews have not found an overall survival difference between platinum analogs used in modern doublet‐regimens in people with advanced NSCLC, although some contradictory results regarding overall response rate and safety profile have been reported (Ardizzoni 2007; Baggstrom 2007; Hotta 2004; Jiang 2007; de Castria 2013). Another important limitation of these previous studies is the inadequate QoL assessment due to the low number and poor quality of trials reporting QoL analysis (Fossella 2003; Paccagnella 2004; Rosell 2002).
Why it is important to do this review
In view of the still important role of platinum doublet‐based chemotherapy in palliative advanced NSCLC patients, we aimed to update our previous Cochrane meta‐analysis in order to better elucidate and clarify the requirement to use platinum salts content regimens with high efficiency and better safety profile.
Objectives
To assess the effectiveness and safety of carboplatin‐based chemotherapy compared with cisplatin‐based chemotherapy, both in combination with a third‐generation drug, in people with advanced NSCLC.
To compare the QoL of people with advanced NSCLC receiving chemotherapy with cisplatin and carboplatin combined with a third‐generation drug.
Methods
Criteria for considering studies for this review
Types of studies
RCTs that compared regimens with cisplatin or carboplatin in combination with a third‐generation drug (i.e. docetaxel, paclitaxel, vinorelbine, gemcitabine, or irinotecan) in people with advanced NSCLC. We excluded non‐randomised and quasi‐randomised studies.
Types of participants
People with pathologically confirmed NSCLC, with metastatic disease, or pleural or pericardial effusion (stage IIIB or IV; Sobin 2002).
Types of interventions
Cisplatin plus gemcitabine versus carboplatin plus gemcitabine.
Cisplatin plus docetaxel versus carboplatin plus docetaxel.
Cisplatin plus paclitaxel versus carboplatin plus paclitaxel.
Cisplatin plus vinorelbine versus carboplatin plus vinorelbine.
Cisplatin plus irinotecan versus carboplatin plus irinotecan.
We included trials comparing these compounds for any number of cycles or treatment schedules.
Types of outcome measures
Primary outcomes
Overall survival.
Health‐related quality of life (HRQoL), assessed by a validated scale.
Secondary outcomes
One‐year survival rate.
Objective response rate, classified according to the Response Evaluation Criteria in Solid Tumors (RECIST) (Eisenhauer 2009).
Drug toxicities (according to the National Cancer Institute Common Toxicity Criteria v2.0) (NCI Common Toxicity Criteria). We analysed trials reporting toxicity data as events per cycle or events per participant.
Search methods for identification of studies
Our search for trials was performed in accordance with the Cochrane Lung Cancer Review Group recommendations, and there were no limits regarding study publication date or language.
Electronic searches
We performed electronic searches of the following databases:
The Cochrane Central Register of Controlled Trials (CENTRAL) (issue 1, 2019).
MEDLINE (via PubMed) (1966 to 13 January 2019);
Embase (via Ovid) (1974 to 13 January 2019);
The search strategies used for each database are presented in Appendix 1.
Searching other resources
We carried out a manual search of the Proceedings of the American Society of Clinical Oncology Meetings (1990 to 2018). We searched the reference lists of relevant studies and contacted study authors to obtain information about ongoing or non‐published studies.
Data collection and analysis
Selection of studies
Two review authors (GNM and VFV) independently examined the abstracts of studies identified by the search. We obtained full‐text versions of all those articles deemed potentially relevant. A third review author (TBC) resolved any disagreements.
Data extraction and management
We extracted and recorded data on data extraction forms. Two review authors (GNM and VFV) independently developed and piloted the forms and conducted full data extraction. A third review author (TBC) resolved any disagreements. We included the following information from individual studies on the data extraction forms:
publication details;
study design, setting, inclusion/exclusion criteria, method of allocation, allocation concealment, blinding, risk of bias;
participant population (e.g. age, type of surgical procedure, type of tumour);
details of intervention: doses, regimen, scheme, duration;
outcome measures;
withdrawals, duration and method of follow‐up, proportion of follow‐up;
type of analyses (e.g. intention‐to‐treat, modified intention‐to‐treat).
Assessment of risk of bias in included studies
Two review authors (GNM and VFV) independently assessed risk of bias for each study using the Cochrane 'Risk of bias' tool (Higgins 2011). We assigned 'low risk of bias', 'high risk of bias', or 'unclear risk of bias' for each 'Risk of bias' domain using the specific questions detailed below and in (Figure 1).
1.
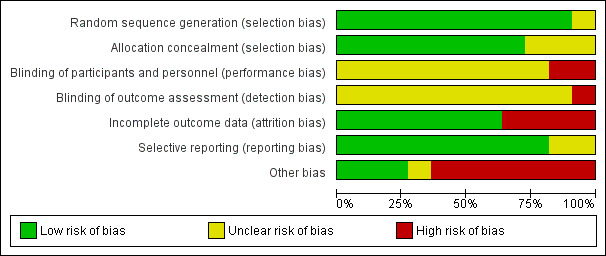
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Sequence generation: was the allocation sequence adequately generated?
Allocation concealment: was allocation adequately concealed?
Blinding of participants, personnel, and outcome assessors: was knowledge of the allocated interventions adequately prevented during the study?
Incomplete outcome data: were outcome data adequately assessed and accounted for?
Selective outcome reporting: were reports of the study free of the suggestion of selective outcome reporting?
Other potential threats to validity: was the study apparently free from other problems that could put it at risk of bias?
Measures of treatment effect
We calculated risk ratios (RRs) and 95% confidence intervals (CI) for dichotomous outcomes. When focusing on grade III or IV toxic events (NCI Common Toxicity Criteria), an RR value greater than one indicated that the carboplatin‐based regimen was more toxic than the cisplatin‐based regimen.
For time‐to‐event outcomes, we presented hazard ratio (HR) with 95% CIs where appropriate. For continuous outcomes we planned to use mean difference (MD).
Unit of analysis issues
The unit of analysis was the individual. We only included RCTs in this review. We found no cross‐over or cluster‐randomised trials.
Dealing with missing data
If the data were missing to the extent that we could not add the study to the meta‐analysis and we were unable to obtain the data, we presented the findings and discussed them in the main text of the review.
Assessment of heterogeneity
We evaluated statistical heterogeneity amongst studies using the Chi2 test and the I2 statistic. We considered an I2 value greater than 50% as substantial heterogeneity.
Assessment of reporting biases
We contacted study authors to request full data sets or to establish reasons for the non‐reporting of some data outcomes. We performed searches for the protocols of included trials.
We planned to create and assess a funnel plot to investigate small‐study biases. In interpreting funnel plots, we planned to investigate possible reasons for funnel plot asymmetry as outlined in Section 10.4 of the Cochrane Handbook for Systematic Reviews of Interventions and relate this to the findings of our review.
Data synthesis
We summarised data through the forest plot graphics produced by Review Manager 5 (Review Manager 2014), using a random‐effects model by default, since clinical or methodological diversity, or both, amongst studies was expected.
We presented a narrative summary of the results of individual studies and discussed the results where data aggregation was not possible.
GRADE and 'Summary of findings' tables
For this update, we created a 'Summary of findings' table using all the outcomes stated in the Types of outcome measures section.
Two review authors (VFV and GNM) independently assessed the certainty of the evidence. We used the GRADE criteria (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to evaluate the certainty of the evidence of the studies that contribute data to the meta‐analyses of outcomes. We assessed the certainty of the evidence as either high, moderate, low, or very low. We used the methods and recommendations described in Section 8.5 and 8.7 and Chapters 11 and 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011; Schünemann 2011). We used GRADEpro GDT software to create the 'Summary of findings' tables (GRADEpro GDT). We explained all decisions to downgrade the certainty of the evidence using footnotes and provided comments to aid in the reader's understanding of the review where needed.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analysis for the primary outcomes:
different combined drug (gemcitabine, paclitaxel, and docetaxel, since we expected different effects when different drugs were combined with cisplatin or carboplatin);
different dose ranges of cisplatin: lower (40 to 80 mg/m2) and higher (80 to 100 mg/m2). These subgroups and dose limits were proposed by the authors before the search and were based on the most frequently used dose of cisplatin in current trials (75 to 80 mg/mL).
Sensitivity analysis
We planned to perform the following sensitivity analyses for the primary outcomes to assess the robustness of the overall results:
excluding trials with high risk of detection or selection bias;
random‐effects versus fixed‐effect model;
only phase III trials: since response to therapy was previously overestimated in phase II trials, analysis of efficacy (response rate and survival data) should be considered exploratory in such trials (Green 2003). We carried out a sensitivity analysis excluding phase II trials.
Interpreting results and reaching conclusions
We followed the recommendations proposed by Schünemann 2011 for interpreting findings and carefully distinguished between lack of evidence of effect and lack of effect. We based our conclusions only on the results from the quantitative or narrative synthesis of the studies included in this review. We did not draw recommendations for practice. Our suggestions for further studies were based on the remaining uncertainties in the area.
Results
Description of studies
Results of the search
Our updated search strategy found 1750 new records: 257 in CENTRAL, 294 in MEDLINE, and 1199 in Embase. We considered nine records to be potentially eligible for our systematic review. We excluded a further seven manuscripts after full‐text analysis. A summary of the search process is provided in Figure 2.
2.
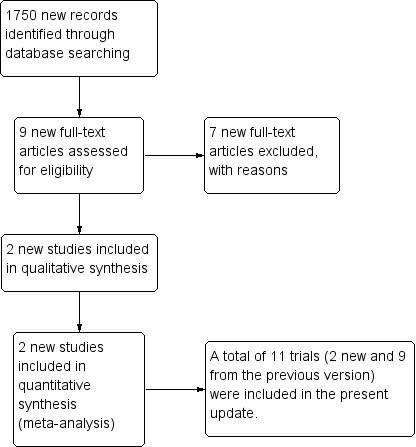
Updated meta‐analysis study flow diagram.
Included studies
In addition to the 10 RCTs included in the previous version of this meta‐analysis (de Castria 2013), we included one new RCT, Saad 2017, and the final publication of the British Thoracic Oncology Group (BTOG2) trial, whose data had been included in the original version of this meta‐analysis (Ferry 2017). Data from 71 participants were thus added to the 10 RCTs included in the original version of the review, totalling 5088 participants, of which data from 4046 were available for meta‐analysis.
All trials were conducted in people with locally advanced or metastatic NSCLC and no important comorbidities. The included trials used gemcitabine (Cai 2002; Ferry 2017; Mazzanti 2003; Saad 2017; Zatloukal 2003), paclitaxel (Chen 2006; Rosell 2002; Schiller 2002; Sweeney 2001; Yan 2001), or docetaxel (Fossella 2003), in combination with a platinum compound.
All of the study authors specified inclusion criteria, but exclusion criteria were not cited in two Chinese trials (Cai 2002; Yan 2001). Exclusion criteria included pregnancy, current organ dysfunction, and symptomatic central nervous system metastases (asymptomatic was allowed in some trials). Details of the included trials are provided in the Characteristics of included studies tables.
In a controlled, open‐label trial, Saad 2017 randomised 71 people with squamous NSCLC and with Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0 to 2 to receive either gemcitabine (1000 mg/m2) on days one and eight plus carboplatin (area under the curve (AUC) 5 mg/mL X min) on day one or gemcitabine (1000 mg/m2) on days one and eight plus cisplatin (40 mg/m2) on days one and eight. The treatments were repeated every three weeks for up to six cycles.
Ferry 2017 randomised 1363 people with ECOG PS 0 to 2 to receive gemcitabine (1250 mg/m2) on days one and eight and cisplatin (80 mg/m2) on day one, every three weeks or gemcitabine (1250 mg/m2) and cisplatin (50 mg/m2) on day one, every three weeks or gemcitabine (1250 mg/m2) and carboplatin (AUC 6 mg/mL X min) on day one, every three weeks. This was a phase III trial with three arms (one arm with carboplatin and the other two arms with cisplatin at different doses). For the purpose of the analysis, dichotomous data (such as toxicity, response rate, and overall survival in one year) from cisplatin‐based arms were combined to create a single group of cisplatin and thus a single pair‐wise comparison. Since combining data from the arms with different doses of cisplatin was not possible for overall survival analysis, data from this study were excluded from the main overall survival analysis (Analysis 1.1), but data from each arm were included in the overall survival subgroup analysis that evaluated cisplatin at different doses (Analysis 2.1).
1.1. Analysis.
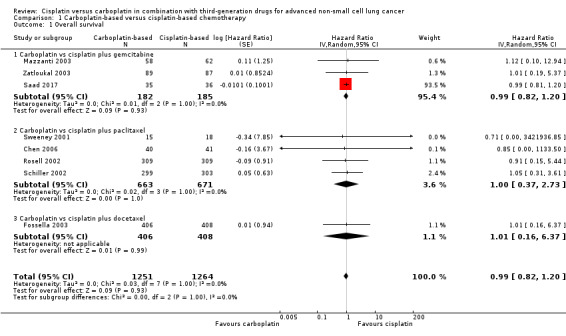
Comparison 1 Carboplatin‐based versus cisplatin‐based chemotherapy, Outcome 1 Overall survival.
2.1. Analysis.
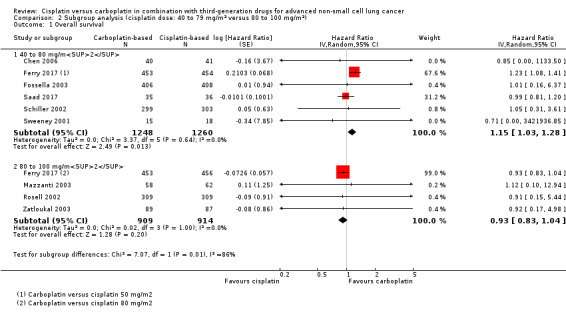
Comparison 2 Subgroup analysis (cisplatin dose: 40 to 79 mg/m2 versus 80 to 100 mg/m2), Outcome 1 Overall survival.
In a phase II trial, Cai 2002 randomised 40 people to receive gemcitabine (1000 mg/m2) on days one and eight and carboplatin (AUC 4 to 6 mg/mL X min) on day one or gemcitabine (1000 mg/m2) on days one and eight and cisplatin (30 to 40 mg/m2) on days one to three. Treatments were repeated every three weeks. The study authors included people with a Karnofsky performance status (PS) of 40 or higher.
Chen 2006 studied 81 people aged 70 years or older, PS 0 to 2 on the World Health Organization (WHO) scale and with no signs or symptoms of brain metastases. Participants were randomised to paclitaxel (160 mg/m2) on day one and carboplatin (AUC 6 mg/mL X min) on day one or paclitaxel (160 mg/m2) on day one and cisplatin (60 mg/m2) on day one. Treatments were repeated every three weeks.
Fossella 2003 published results of a phase III trial comparing regimens of chemotherapy in 1218 participants with locally advanced or metastatic NSCLC with a Karnofsky PS of 70 or higher and no significant comorbidities. Participants were randomised to receive docetaxel (75 mg/m2) on day one and cisplatin (75 mg/m2) on day one or docetaxel (75 mg/m2) on day one and intravenous carboplatin (AUC 6 mg/mL X min) on day one. Treatments were repeated every three weeks. We did not use a third treatment arm (vinorelbine plus cisplatin) in the analysis because there was no parallel arm with vinorelbine and carboplatin.
In Mazzanti 2003, 120 people with ECOG PS 0 to 2 and a life expectancy greater than 12 weeks were eligible to receive gemcitabine (1200 mg/m2) on days one and eight and cisplatin (80 mg/m2) on day two or gemcitabine (1200 mg/m2) on days one and eight and carboplatin (AUC 5 mg/mL X min) on day two. Treatments were repeated every three weeks. People with symptomatic central nervous system metastases were excluded.
Rosell 2002 published the results of a phase III trial of 618 people with a ECOG PS 0 to 2 who were able to understand the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ‐C30). Participants received paclitaxel (200 mg/m2) and cisplatin (80 mg/m2) or paclitaxel (200 mg/m2) and carboplatin (AUC 6 mg/mL X min). Treatments were repeated every three weeks. The primary outcome was response rate and the secondary outcomes included the EORTC QLQ‐C30 and the EORTC Quality of Life Lung Cancer supplement 13 (QLQ‐LC13).
Schiller 2002 published the results of a phase III trial with 1207 participants. Initially people with an ECOG PS 0 to 2 were eligible for enrolment, but the study design was amended after the enrolment of 66 people with PS 2 to exclude those participants due to the high rate of serious adverse events. Participants were randomised to receive paclitaxel (135 mg/m2 infusion over 24 hours) on day one and cisplatin (75 mg/m2) on day two or paclitaxel (225 mg/m2 infusion over three hours) on day one and carboplatin (AUC 6 mg/mL X min) on day one. Treatments were repeated every three weeks. We did not analyse arms two and three of the study in this review because the regimens used did not fulfil our inclusion criteria. A total of 1155 people were analysed.
Sweeney 2001 performed a phase II trial with 68 people, all of whom had an ECOG PS 2, randomising them to paclitaxel (135 mg/m2 infusion over 24 hours) on day one and cisplatin (75 mg/m2) on day two or paclitaxel (225 mg/m2 infusion over three hours) on day one and carboplatin (AUC 6 mg/mL X min) on day one. Treatments were repeated every three weeks. We excluded arms two and three of this trial because the regimens used did not fulfil our inclusion criteria. The primary outcomes of this trial were toxicity and adverse events.
Yan 2001 randomised 126 people with a Karnofsky PS of 60 or higher to receive paclitaxel (175 mg/m2) on day one and carboplatin (350 mg/m2) on day one or paclitaxel (175 mg/m2) on days one and eight and cisplatin (100 mg/m2) on day one. Treatments were repeated every four weeks.
Zatloukal 2003 randomised 176 people with a Karnofsky PS of 70 or higher to receive gemcitabine (1200 mg/m2) on days one and eight and intravenous cisplatin (80 mg/m2) on day one, or gemcitabine (1200 mg/m2) and carboplatin (AUC 5 mg/mL X min) on day one. Treatments were repeated every three weeks. The primary outcome of the trial was toxicity.
Excluded studies
In this update, we excluded seven publications from the first selection after full‐text analysis due to the following reasons: three were not RCTs (Sun 2014; Tiseo 2014; von Verschuer 2017); one presented a post hoc analysis of an RCT (Smit 2016); one evaluated platinum versus non‐platinum chemotherapy (Moro‐Sibilot 2015); one presented a quality of life analysis of another trial (Billingham 2011); and one was neither designed nor powered to perform a direct comparison between different platinum regimens (Schuette 2013).
Risk of bias in included studies
Allocation
Allocation was adequately concealed in 8 of 11 included trials (Chen 2006; Ferry 2017; Fossella 2003; Mazzanti 2003; Rosell 2002; Schiller 2002; Sweeney 2001; Zatloukal 2003). The information about allocation concealment in two Chinese RCTs was unclear, and we could not contact the authors (Cai 2002; Yan 2001). Saad 2017 was an open‐label study.
Blinding
Blinding of participants (performance bias)
None of the 11 RCTs reported complete information about the blinding processes. Saad 2017 and Fossella 2003 were open‐label studies and were considered to have high risk of performance bias related to the blinding of participants. Nine trials reported no information related to blinding processes (Cai 2002; Chen 2006; Ferry 2017; Mazzanti 2003; Rosell 2002; Schiller 2002; Sweeney 2001; Yan 2001; Zatloukal 2003), and we were unable to obtain details about blinding through contact with the authors; we therefore judged these trials as at unclear risk for blinding of participants.
Blinding of outcome assessment (detection bias)
None of the 11 RCTs reported complete information about the blinding processes. Saad 2017 and Fossella 2003 were open‐label studies and were considered to have high risk of detection bias related to the blinding of the outcome assessment process. Nine trials reported no information related to blinding processes (Cai 2002; Chen 2006; Ferry 2017; Mazzanti 2003; Rosell 2002; Schiller 2002; Sweeney 2001; Yan 2001; Zatloukal 2003), and we were unable to obtain details about blinding through contact with the authors; we therefore judged these trials as at unclear risk for blinding of outcome assessment.
Incomplete outcome data
Fifteen of 1218 participants in Fossella 2003 did not receive treatment (nine were ineligible, four withdrew consent, and two died of malignant disease before the first drug infusion), thus we excluded these participants from the safety analysis.
In Schiller 2002, the study design was amended to include only participants with an ECOG PS 0 or 1 after the enrolment of 66 participants with an ECOG PS 2. These participants were not included in the final analysis, which could have affected the results since people with a ECOG PS 2 have a poorer prognosis, and the numbers of these participants in each arm were not reported.
Of the 618 participants randomised in Rosell 2002, 10 (2%) did not receive a study drug (three in the carboplatin arm and seven in the cisplatin arm), but we considered this unlikely to result in a significant bias.
Selective reporting
Cai 2002 reported no survival data and was excluded from the analysis of overall survival and one‐year survival rate.
Yan 2001 reported no overall survival data but did provide one‐year survival rate.
The remaining studies reported overall survival data only as median and confidence intervals, therefore we converted them to hazard ratios, according to the method proposed by Parmar 1998.
In the analysis of adverse effects that were measured as number of events per cycle, alopecia was not mentioned in Mazzanti 2003 and renal toxicity analysis was not performed by Ferry 2017.
In the analysis of adverse effects that were measured as events per participant, nausea, vomiting, or both were not evaluated by Cai 2002, which was the only RCT to describe the incidence of skin rash. Only Yan 2001 and Zatloukal 2003 analysed the incidence of alopecia. Five studies evaluated neurotoxicity (Chen 2006; Rosell 2002; Schiller 2002; Sweeney 2001; Zatloukal 2003).
Other potential sources of bias
It is important to note that Ferry 2017 used the Wright formula for calculation of creatinine clearance, which usually results in about 10% higher doses of carboplatin than with the use of the Cockcroft‐Gault formula (Wright 2001).
Six trials were planned as randomised phase II studies, therefore the findings obtained from the treatment arm comparisons should be considered exploratory (Cai 2002; Chen 2006; Mazzanti 2003; Saad 2017; Sweeney 2001; Yan 2001).
In Rosell 2002, a reduction of carboplatin dose was necessary for 96 of 279 (34%) participants randomised to the drug, and the mean dose AUC for them was 4.9 mg/mL X min. This dose could be associated with a lower effectiveness.
In Schiller 2002, the paclitaxel dose (135 mg/m2and 225 mg/m2) and length of infusion (24 and 3 hours) were different, which could have compromised the comparison in efficacy and toxicity.
Effects of interventions
See: Table 1
Carboplatin‐based versus cisplatin‐based chemotherapy
The table 1 summarize the main results regarding the comparison of carboplatin with cisplatin chemotherapy in combination with third‐generations drugs for advanced non‐small cell lung cancer (Table 1).
Overall survival (Analysis 1.1)
Overall survival was evaluated by 10 RCTs, and the meta‐analysis found no difference between cisplatin‐ and carboplatin‐based chemotherapy (hazard ratio (HR) 0.99, 95% confidence interval (CI) 0.82 to 1.20; 10 RCTs; 2515 participants;; high‐quality evidence) (Analysis 1.1, Figure 3). There was no significant heterogeneity amongst RCTs.
3.
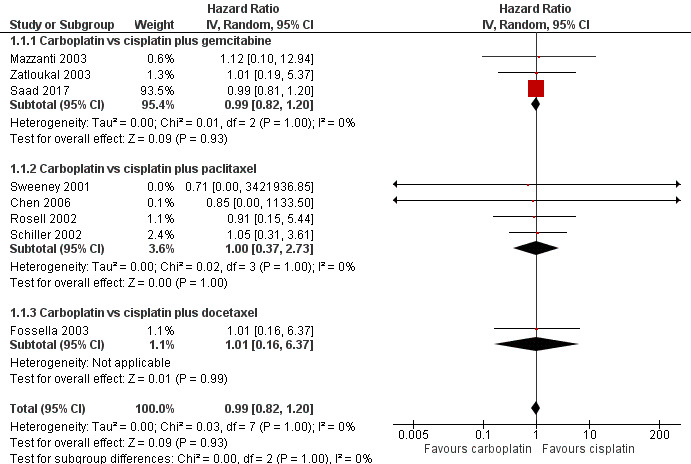
Forest plot of comparison: 1 Carboplatin‐based versus cisplatin‐based chemotherapy, outcome: 1.1 Overall survival.
Subgroup analysis
The subgroup analysis considering the combined drug was consistent with the main result for gemcitabine (HR 0.99, 95% CI 0.82 to 1.20; 5 RCTs; 367 participants; ); paclitaxel (HR 1.00, 95% CI 0.37 to 2.73; 4 RCTS;1334 participants; ); and docetaxel (HR 1.01, 95% CI 0.16 to 6.37; 1 RCT; 814 participants).
Quality of life analysis
Two RCTs performed a QoL analysis. Fossella 2003 evaluated QoL using the EORTC QLQ‐LC13 and the Lung Cancer Symptom Scale (LCSS) questionnaires but did not compare cisplatin and carboplatin arms directly. Rosell 2002 applied the QLQ‐C30 and QOL‐LC13 questionnaires to compare the two drugs and found no significant differences in global health status or in functional scales. Because of this paucity of QoL data, we could not perform a meta‐analysis. Rosell 2002 reported comparative QoL data between the study arms, but found no significant differences in global indices of quality of life, including global health status or the functional scales. However, the cisplatin‐containing arm was associated with a higher rate of appetite loss (P = 0.084), whilst participants in the arm containing carboplatin reported haemoptysis more frequently (P = 0.048); pain and chest pain (P = 0.058 and P = 0.046, respectively); and required greater pain medication consumption (P = 0.054).
One‐year survival rate (Analysis 1.2)
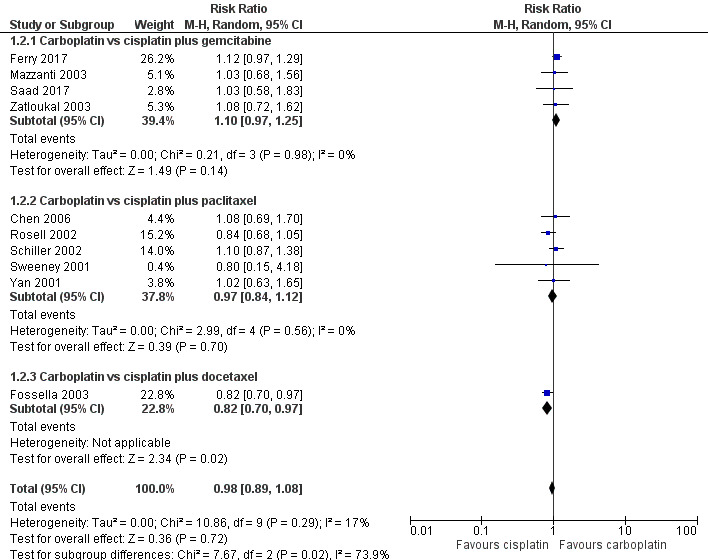
One‐year survival rate was evaluated in 11 RCTs, and no difference was found between cisplatin‐ and carboplatin‐based chemotherapy (risk ratio (RR) 0.98, 95% CI 0.89 to 1.08; 11 RCTS; 4004 participants; high‐quality evidence) (Analysis 1.2, Figure 4). No significant heterogeneity was detected amongst RCTs.
1.2. Analysis.
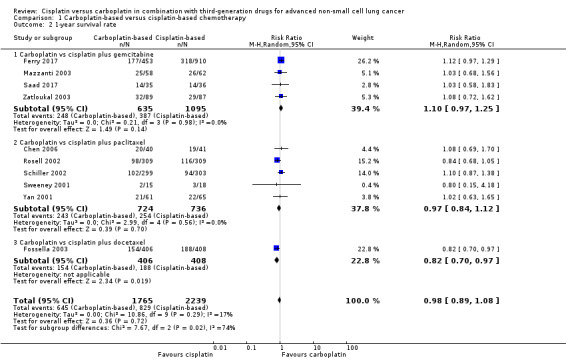
Comparison 1 Carboplatin‐based versus cisplatin‐based chemotherapy, Outcome 2 1‐year survival rate.
4.
Forest plot of comparison: 1 Carboplatin‐based versus cisplatin‐based chemotherapy, outcome: 1.2 1‐year survival rate.
Subgroup analysis
The subgroup analysis considering the companion drugs was consistent with the main result for paclitaxel (RR 0.97, 95% CI 0.84 to 1.12; 5 RCTS; 1460 participants; I2 = 0%) and gemcitabine (RR 1.10, 95% CI 0.97 to 1.25; 5 RCTS; 1730 participants; I2 = 0%). However, one RCT found benefit with the cisplatin‐based regimen with docetaxel (RR 0.82, 95% CI 0.70 to 0.97; 1 RCT; 814 participants) (Fossella 2003).
Response rate (Analysis 1.3)
All 11 RCTs evaluated response rate (5088 participants; 4020 pooled for meta‐analysis). Meta‐analysis showed that there was no difference in response rate between cisplatin‐ and carboplatin‐based chemotherapy (RR 0.89, 95% CI 0.79 to 1.00; I2 = 12%) (Analysis 1.3, Figure 5).
1.3. Analysis.
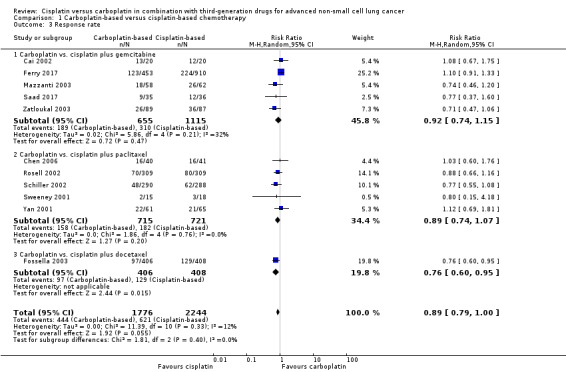
Comparison 1 Carboplatin‐based versus cisplatin‐based chemotherapy, Outcome 3 Response rate.
5.
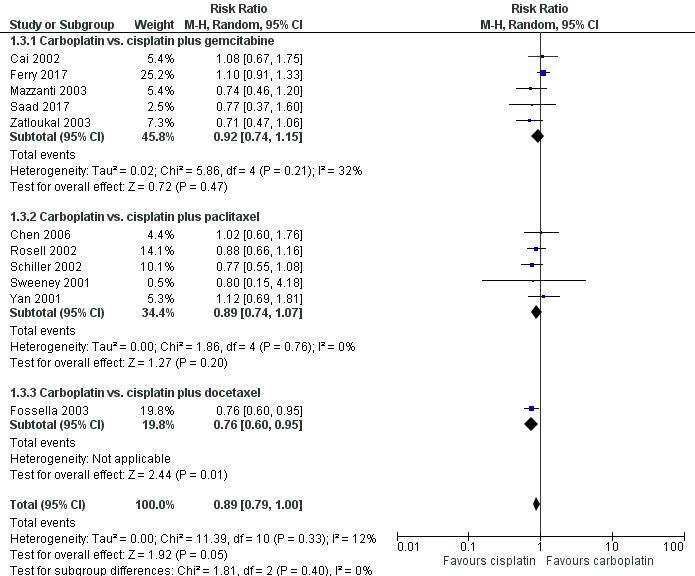
Forest plot of comparison: 1 Carboplatin‐based versus cisplatin‐based chemotherapy, outcome: 1.3 Response rate.
Subgroup analysis
Overall, the subgroup analyses according to different third‐generation drugs used did not showed superiority regarding the platinum agent used. The response rates in five trials (1436 participants) with cisplatin or carboplatin combined with paclitaxel (RR 0.89, 95% CI 0.74 to 1.07; I2 = 0%) as also in five trials (1770 available participants) combined with gemcitabine (RR 0.92, 95% CI 0.74 to 1.15; I2 = 32%) did not demonstraded statistical significant difference between cisplatin or carboplatin. One exception was a cisplatin superiority over carboplatin regarding docetaxel doublet (RR 0.76, 95% CI 0.60 to 0.95) in the only trial (814 participants) that used docetaxel (Fossella 2003).
Drug toxicity ‐ grade III or IV toxicity as events per cycle (Analysis 1.4)
Eleven RCTs assessed this outcome. The rates of adverse effects were reported as number of events per participant or events per cycle. Since nine RCTs reported data as per participant and two trials as per treatment cycle, we analysed them separately and only considered grade III and IV toxicities (Analysis 1.4)
1.4. Analysis.
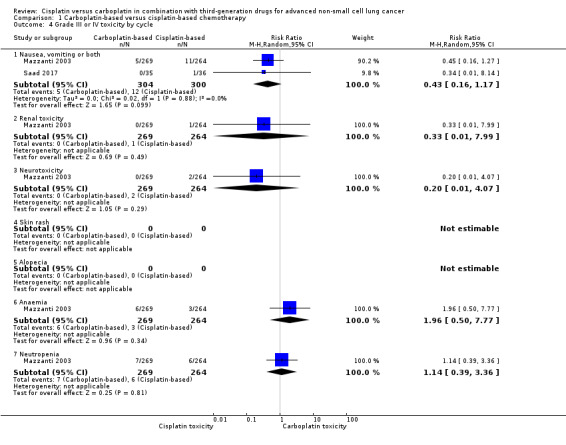
Comparison 1 Carboplatin‐based versus cisplatin‐based chemotherapy, Outcome 4 Grade III or IV toxicity by cycle.
A meta‐analysis of two trials that evaluated toxicity as events per cycle found a higher incidence of anaemia (RR 4.51, 95% CI 2.96 to 6.89; I2 = 0%) and neutropenia (RR 4.22, 95% CI 1.56 to 11.37; I2 = 87%) in the carboplatin arm. There was no difference in the incidence of nausea or vomiting (or both) (RR 0.77, 95% CI 0.28 to 2.12; I2 = 81%); renal toxicity (RR 0.33, 95% CI 0.01 to 7.99); or neurotoxicity (RR 0.20, 95% CI 0.01 to 4.07). The heterogeneity found in the neutropenia and nausea/vomiting analysis could be explained by the difference in the risk of neutropenia and the large differences in sample sizes.
Drug toxicity ‐ grade III or IV toxicity as events per participant (Analysis 1.5)
We performed a meta‐analysis of 10 RCTs that evaluated toxicity as events per participant and found a higher incidence of thrombocytopenia (RR 2.46, 95% CI 1.49 to 4.04; I2 = 68%) and neurotoxicity (RR 1.42, 95% CI 0.91 to 2.23; I2 = 0%) in the carboplatin‐based treatment arms. The heterogeneity found in this analysis was due to data from the Ferry 2017 trial; when data from this trial were excluded, the result remained similar, but without significant heterogeneity (RR 2.00, 95% CI 1.37 to 2.91; I2 = 21%).
There was no significant difference in the incidence of nausea or vomiting (or both) (RR 0.59, 95% CI 0.31 to 1.11; I2 = 86%); renal toxicity (RR 0.52, 95% CI 0.19 to 1.45; I2 = 3%); skin rash (RR 3.00, 95% CI 0.13 to 69.52; I2 = 0%); alopecia (RR 1.11, 95% CI 0.73 to 1.68; I2 = 0%); anaemia (RR 1.37, 95% CI 0.79 to 2.38; I2 = 77%); and neutropenia (RR 1.18, 95% CI 0.85 to 1.63; I2 = 94%) between cisplatin‐ and carboplatin‐based chemotherapy regimens. In this context, the heterogeneity found could be due to the different toxicity profiles of the other drugs used in association with the platinum compounds.
Subgroup analysis
When considering only trials using paclitaxel, the cisplatin‐based treatment arms showed a higher incidence of nausea or vomiting (or both) (RR 0.45, 95% CI 0.26 to 0.77; I2 = 53%). Heterogeneity in nausea or vomiting (or both) was due mainly to Yan 2001, the only trial that had a greater incidence of nausea or vomiting (or both) in the carboplatin arm. We performed a sensitivity analysis excluding Yan 2001 and obtained a similar estimate of effect but with no heterogeneity (RR 0.36, 95% CI 0.28 to 0.46; I2 = 0%). Historically, cisplatin has been associated with a higher rate of nausea and vomiting when compared with carboplatin, but in Yan 2001 carboplatin caused more nausea or vomiting (or both). There was no specific reason for this, but one hypothesis is the use of carboplatin in a fixed dose (300 mg/m2) rather than an AUC dose.
Subgroup analysis including trials with paclitaxel showed a higher incidence of thrombocytopenia (RR 2.34, 95% CI 1.51 to 3.63; I2 = 0%), but no significant difference in neutropenia (RR 0.91, 95% CI 0.73 to 1.15; I2 = 62%) or anaemia (RR 0.82, 95% CI 0.60 to 1.14; I2 = 0%) between treatment arms. The significant heterogeneity in analysis of neutropenia may be explained by the fact that the two larger trials had opposite estimates of effect. After excluding only Rosell 2002, we found a similar incidence of neutropenia (RR 0.83, 95% CI 0.58 to 1.18; I2 = 30%; 4 trials) as well as after removing only Schiller 2002 from the analysis (RR 0.94, 95% CI 0.60 to 1.46; I2 = 43%; 4 trials).
Subgroup analysis (cisplatin dose)
* Carboplatin‐based versus cisplatin‐based chemotherapy administred in low dose (40 to 80mg/m2) or high dose (80 to 100mg/m2)
Overall survival (Analysis 2.1)
Carboplatin versus cisplatin (40 to 80 mg/m2)
Meta‐analysis of six RCTs (4008 participants; 2508 available for pooling) showed a significant difference between carboplatin and the lower dose of cisplatin (40 to 80 mg/m2) in terms of overall survival (HR 1.15, 95% CI 1.03 to 1.28; I2 = 0%), favouring carboplatin‐based therapy (Analysis 2.1, Figure 6).
6.
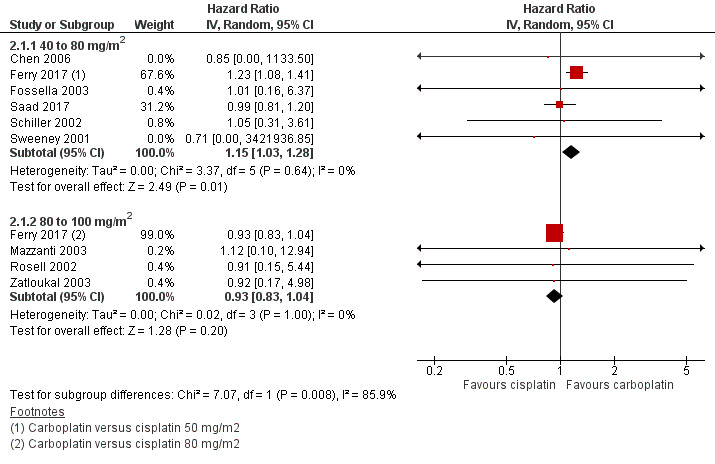
Forest plot of comparison: 2 Subgroup analysis (cisplatin dose: 40 to 79 mg/m2 versus 80 to 100 mg/m2), outcome: 2.1 Overall survival.
Carboplatin versus cisplatin (80 to 100 mg/m2)
Meta‐analysis of four RCTs (2277 participants; 1823 available for pooling) comparing carboplatin and the higher dose of cisplatin (80 to 100 mg/m2) showed no difference in overall survival (HR 0.93, 95% CI 0.83 to 1.04; I2 = 0%) (Analysis 2.1).
One‐year survival (Analysis 2.2)
Carboplatin versus cisplatin (40 to 80 mg/m2)
Meta‐analysis of six RCTs (4008 participants; 2508 available for pooling) showed no difference between carboplatin and the lower dose of cisplatin in one‐year survival rates (RR 1.04, 95% CI 0.85 to 1.27; I2 = 61%) (Analysis 2.2).
2.2. Analysis.
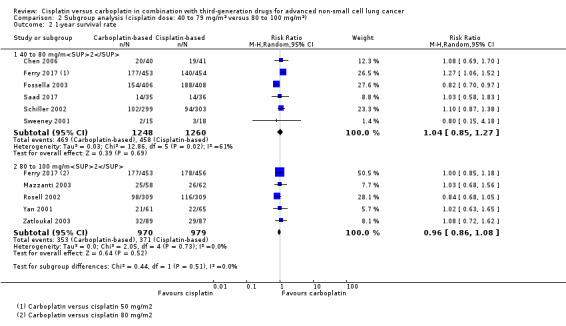
Comparison 2 Subgroup analysis (cisplatin dose: 40 to 79 mg/m2 versus 80 to 100 mg/m2), Outcome 2 1‐year survival rate.
Since Fossella 2003 was the only trial with a higher one‐year survival rate in the cisplatin arm and was the only trial using docetaxel in doublet, we performed an analysis excluding this trial and obtained a higher one‐year survival in the carboplatin arm without heterogeneity (RR 1.18, 95% CI 1.03 to 1.34; I2 = 0%).
Carboplatin versus cisplatin (80 to 100 mg/m2)
A meta‐analysis of five RCTs (2403 participants; 1949 available for pooling) found no statistically significant differences between carboplatin and the higher dose of cisplatin in one‐year survival rate (RR 0.96, 95% CI 0.86 to 1.08; I2 = 0%) (Analysis 2.2).
Response rate (Analysis 2.3)
Carboplatin versus cisplatin (40 to 80 mg/m2)
Meta‐analysis of seven RCTs (4048 participants; 2524 available for pooling) showed no statistically significant differences between carboplatin and the lower dose of cisplatin in response rate (RR 0.94, 95% CI 0.74 to 1.20; I2 = 58%). The heterogeneity found in this analysis was due to data from the lower dose cisplatin (50 mg/m2) arm of the Ferry 2017 trial. Re‐analysis after exclusion of these data demonstrated a higher response rate favouring cisplatin‐based chemotherapy without significant heterogeneity (RR 0.81, 95% CI 0.69 to 0.96; I2 = 0%) (Analysis 2.3).
2.3. Analysis.
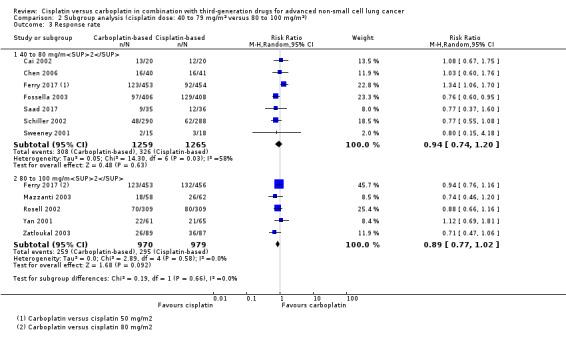
Comparison 2 Subgroup analysis (cisplatin dose: 40 to 79 mg/m2 versus 80 to 100 mg/m2), Outcome 3 Response rate.
Carboplatin versus cisplatin (80 to 100 mg/m2)
Meta‐analysis of five trials (2403 participants; 1949 available for pooling) comparing carboplatin to the higher dose of cisplatin (80 to 100 mg/m2) found no difference in response rate (RR 0.89, 95% CI 0.77 to 1.02; I2 = 0%) (Analysis 2.3).
Sensitivity analysis
Fixed‐effect model (Analysis 3.1; Analysis 3.2; Analysis 3.3)
When we performed fixed‐effect analyses, we found no significant differences in overall survival (HR 0.99, 95% CI 0.82 to 1.20) (Analysis 3.1) or one‐year survival rates (RR 0.98, 95% CI 0.91 to 1.07; I2 = 17%) (Analysis 3.2), although there was a marginally positive benefit in response rates favouring cisplatin‐based treatments (RR 0.89, 95% CI 0.81 to 0.99; I2 = 12%) (Analysis 3.3).
3.1. Analysis.
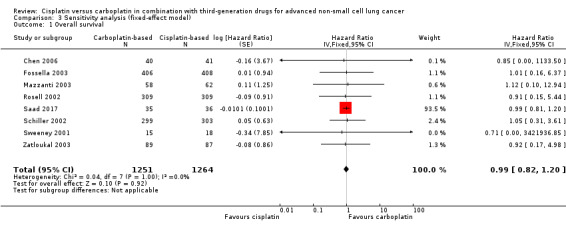
Comparison 3 Sensitivity analysis (fixed‐effect model), Outcome 1 Overall survival.
3.2. Analysis.
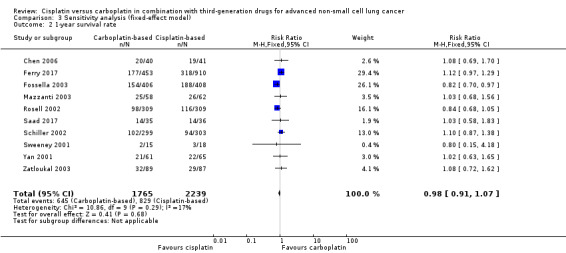
Comparison 3 Sensitivity analysis (fixed‐effect model), Outcome 2 1‐year survival rate.
3.3. Analysis.
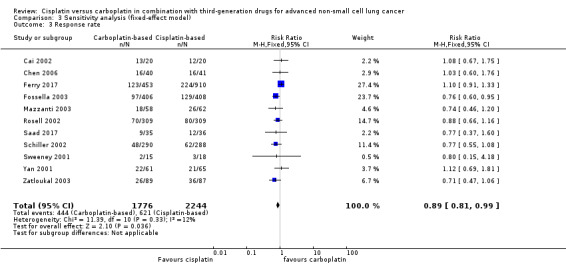
Comparison 3 Sensitivity analysis (fixed‐effect model), Outcome 3 Response rate.
Phase III trials (Analysis 4.1; Analysis 4.2; Analysis 4.3)
When we limited the analysis to phase III trials, there were minimal changes in the results and no significant differences in terms of overall survival (HR 0.99, 95% CI 0.45 to 2.17) (Analysis 4.1); one‐year survival rates (RR 0.97, 95% CI 0.84 to 1.13) (Analysis 4.2); and response rates (RR 0.86, 95% CI 0.71 to 1.03) (Analysis 4.3).
4.1. Analysis.
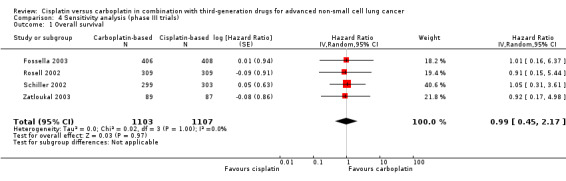
Comparison 4 Sensitivity analysis (phase III trials), Outcome 1 Overall survival.
4.2. Analysis.
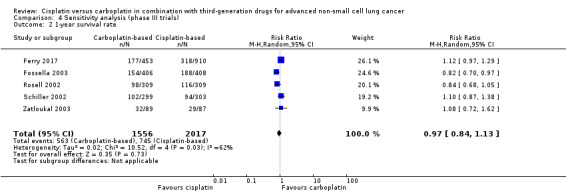
Comparison 4 Sensitivity analysis (phase III trials), Outcome 2 1‐year survival rate.
4.3. Analysis.
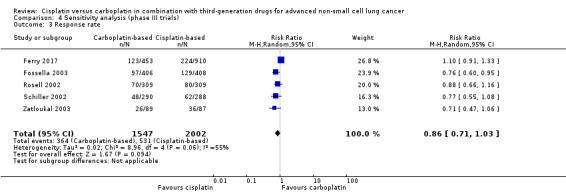
Comparison 4 Sensitivity analysis (phase III trials), Outcome 3 Response rate.
Discussion
Since the 1990s, many trials have been published comparing chemotherapy with best supportive care in people with advanced NSCLC and have consolidated the role of chemotherapy as a means of increasing survival and improving quality of life (NSCLC Collaborative Group 1995). Since then, cumulative histopathological advances have allowed a first subdivision with therapeutic implication of NSCLC: squamous and non‐squamous NSCLC. Furthermore, a deeper understanding of the molecular pathways involved in the pathogenesis of NSCLC has permitted the expansion of the number of molecular alterations/driver genes predictive of response to targeted therapy, including epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), ROS proto‐oncogene 1, receptor tyrosine kinase (ROS1), MET proto‐oncogene, receptor tyrosine kinase (MET), ret proto‐oncogene (RET), B‐Raf proto‐oncogene, serine/threonine kinase (BRAF), neurotrophic tyrosine receptor kinase (NTRK), and human epidermal growth factor receptor 2 (HER‐2) (Gridelli 2015). In the presence of these biomarkers, target therapies have been established as standard first‐line treatment options. More recently, immune checkpoint inhibitors have been incorporated into first‐line systemic therapy of patients with advanced NSCLC, and some patients have sustained a clinical response.) As a way of optimising the results obtained with immunotherapy, combinations of cytotoxic chemotherapy and immune checkpoints inhibitors have already been approved for some specific subgroups, reinforcing the role of chemotherapy in the treatment of these patients, either alone or in combination with other therapeutic modalities (Keynote‐024; Keynote‐189). In view of the palliative intent of the treatment of patients with metastatic NSCLC, the challenge thus remains to find the most effective therapies with the lowest risk of serious adverse effects.
We performed an update of a previously published meta‐analysis of trials comparing regimens including cisplatin plus a third‐generation drug with regimens including carboplatin plus a third‐generation drug (de Castria 2013). In addition to the 10 previously included studies, we added data from one more study, Saad 2017, and the final data from the publication of the British Thoracic Oncology Group (BTOG2) trial (Ferry 2017).
We found no significant difference between cisplatin‐ and carboplatin‐based regimens in terms of overall survival, one‐year survival, or response rate. As in the previous version of this review (de Castria 2013), other meta‐analyses have shown that cisplatin‐based regimens could be slightly more effective in terms of response rate (Ardizzoni 2007; Hotta 2004). However, this finding was not reproduced in the current update. Of note, the improved response rate previously demonstrated could be attributed mainly to one trial, which was the only trial with a significantly higher response rate for cisplatin (Fossella 2003). Docetaxel was used in both arms of this trial, even though paclitaxel and gemcitabine are generally preferred because of their better tolerability and are used in almost all modern trials.
Interestingly, a subgroup analysis investigating the different doses of cisplatin used showed an overall survival benefit in favour of carboplatin‐based regimens when compared to cisplatin at low doses (40 to 80 mg/m2). However, this benefit was not confirmed when carboplatin‐based regimens were compared with schedules using higher doses of cisplatin (80 to 100 mg/m2). No differences in one‐year survival rates or response rates were demonstrated when comparing carboplatin‐based regimens with different doses of cisplatin.
Since only two RCTs evaluated QoL, a meta‐analysis was not possible (Fossella 2003; Rosell 2002). This was also a challenge to the authors of previous meta‐analyses because different scores were used (Ardizzoni 2007; Hotta 2004; Jiang 2007), and some questionnaires could be used only in the countries in which a translated version of the QoL tool with validation was available. Moreover, no trial has compared QoL with cisplatin and carboplatin directly.
Different criteria were applied to evaluate response rate, including RECIST, Southwest Oncology Group (SWOG), WHO, and ECOG criteria. Furthermore, different doses of drugs were used in these trials, which could have modified the assessment of effect.
Nowadays, the role of histology as a predictor of response and toxicity to different therapies is well recognised (e.g. non‐squamous histology and pemetrexed treatment) (Scagliotti 2008). However, it was not possible to assess the role of histology or other molecular biomarkers in our analysis, since these factors were not evaluated by the majority of the included studies.
In this review, carboplatin‐based chemotherapy appeared to be associated with a higher incidence of neurotoxicity. However, there was only one trial that showed a significantly higher incidence of neurotoxicity in the carboplatin arm (Schiller 2002). This is almost certainly because participants in the carboplatin arm received 225 mg/m2 of paclitaxel, whereas participants in the cisplatin arm received only 135 mg/m2 of paclitaxel. Since paclitaxel is known to cause dose‐related neurotoxicity, this is likely to be a confounding factor in the final analysis.
Although we did not obtain data on second‐line therapies, it is possible that some of the included participants crossed over to another therapy when the disease progressed. The effect of such a cross‐over on the results of this systematic review is unknown and may have affected survival results.
Based on results from trials with combination therapies, many patients with advanced NSCLC are currently being treated with cytotoxic chemotherapy in combination with other types of systemic treatments, including immune checkpoint inhibitors and bevacizumab, a recombinant humanised monoclonal antibody that binds vascular endothelial growth factor (Sandler 2006; Reck 2010). Since our study was not designed to evaluate the interaction between cytotoxic chemotherapy and other therapies, the question remains as to whether there would be any advantage in favour of some of the platinum salts as a better companion therapy in combination with anti‐angiogenics or immunotherapy in the treatment of advanced NSCLC.
Summary of main results
We obtained data on 4046 participants in 11 RCTs. The included trials had at least one treatment arm with cisplatin and one treatment arm with carboplatin, both combined with paclitaxel (five trials), gemcitabine (five trials), or docetaxel (one trial).
There was no difference in overall survival (HR 0.99, 95% CI 0.82 to 1.20); one‐year survival rate (RR 0.98, 95% CI 0.89 to 1.08; I2 = 17%); or response rate (RR 0.89, 95% CI 0.79 to 1.00; I2 = 12%).
For grade III to IV toxicity measured as events per participant, we detected a higher incidence of thrombocytopenia (RR 2.46, 95% CI 1.49 to 4.04; I2 = 68%) and neurotoxicity (RR 1.42, 95% CI 0.91 to 2.23; I2 = 0%) in the carboplatin‐based treatment arms, although the neurotoxicity results were likely related to a confounding factor (higher dose of paclitaxel in the carboplatin‐based treatment group in a large study).
We also performed a subgroup analysis comparing carboplatin with different doses of cisplatin: lower dose (40 to 80 mg/m2) and higher dose (80 to 100 mg/m2). We found an overall survival benefit in favour of carboplatin‐based regimens when compared to cisplatin at lower doses (HR 1.15, 95% CI 1.03 to 1.28), although there was no overall survival benefit when carboplatin‐based chemotherapy was compared to cisplatin at higher doses (HR 0.93, 95% CI 0.83 to 1.04). There was no statistically significant difference in terms of one‐year survival rate or response rate between carboplatin and both doses of cisplatin.
We could not perform an analysis of QoL in our review because data were provided by only two trials (Fossella 2003; Rosell 2002).
Overall completeness and applicability of evidence
With regard to external validation, the doses of drugs varied amongst analysed trials, which should be considered when selecting treatment. It is also important to note that the trials analysed in our review did not take into account the status of driver gene mutations and predictive factors of benefit to immune checkpoint inhibitors, such as tumour mutational burden and programmed cell death‐ligant 1 (PD‐L1) expression, which are critical in deciding the initial approach in advanced disease.
Quality of the evidence
The categorisation of the quality of the evidence (into high, moderate, low, or very low) reflects the quality of the evidence available for our chosen outcomes in our defined populations of interest.
Our review included 11 RCTs. We considered the evidence from the available data to be of high quality for response rate, one‐year survival rate, and overall survival since no significant random sequence generation bias was detected. However, as different doses of drugs were used, and some adverse effects were omitted from analysis in the original trials, we also considered the evidence from the data for adverse effects to be of moderate quality and note that this information should be interpreted cautiously.
Potential biases in the review process
We performed an electronic search of the main databases and extended our search to include meetings of the American Society of Clinical Oncology. It is unknown whether there are other reports of unpublished trials in different languages or presented at different meetings.
We found two Chinese trials (Cai 2002; Yan 2001). We could not obtain data for overall survival or one‐year survival rate for Cai 2002, and there was no information on overall survival in Yan 2001. Since both trials recruited small numbers of participants, we concluded that they did not cause a significant bias in survival analysis.
We identified no more significant potential biases.
Agreements and disagreements with other studies or reviews
We found no significant difference between cisplatin‐ and carboplatin‐based regimens in terms of overall survival, one‐year survival rate, or response rate. As in the previous version of this review (de Castria 2013), other meta‐analyses have shown that cisplatin‐based regimens could be slightly more effective in terms of response rate (Ardizzoni 2007; Hotta 2004; Jiang 2007). However, this finding was not reproduced in the current update. As in our review, none of these meta‐analyses found that the benefit in favour of cisplatin in terms of response rate translated into overall survival gain.
In 2004, Hotta and colleagues published a meta‐analysis that included eight trials comparing doublets of cisplatin or carboplatin plus another drug (Hotta 2004). We included only five of these trials in our analysis because the other three studies used older agents combined with platin. In a subset analysis of trials consisting of a platin plus a third‐generation drug, Hotta 2004 found superior survival in the cisplatin arm, and Ardizzoni 2007 demonstrated a superior HR for mortality in the carboplatin arm.
Authors' conclusions
Implications for practice.
Our findings suggest several implications for practice. In our meta‐analysis, carboplatin and cisplatin had equivalent overall survival, one‐year survival, and response rate. When combined with gemcitabine or paclitaxel, carboplatin had the same response rate as cisplatin. When platinum compounds were combined with docetaxel, a higher response rate for cisplatin‐containing arms was demonstrated in one of the studies. The clinical decision about which platinum compound to use should thus take into account its adverse effects profile as well as the drug with which it will be combined.
With respect to toxicity, carboplatin caused more thrombocytopenia, and cisplatin caused more nausea/vomiting, as found in previous meta‐analyses. Since health‐related quality of life was not directly compared between cisplatin and carboplatin treatment, the approach for these patients has to be individualised.
Implications for research.
As previous review authors have found, we could not perform a meta‐analysis of quality of life data. Many trials have evaluated quality of life, and the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ‐C30) score has been the most popular score in some of these trials. It is crucial to consider quality of life in future randomised clinical trials.
Finally, although our review has shown that carboplatin has at least equivalent effectiveness to cisplatin, it is important to define the role of both drugs combined with a third‐generation drug plus new drugs, such as monoclonal antibodies and immune checkpoint inhibitors.
What's new
| Date | Event | Description |
|---|---|---|
| 18 February 2019 | New search has been performed | Two new authors. Background updated. Outcomes reorganised in other to limit the primary outcomes to two. We conducted a new search for clinical trials from 2013 to January 2019. A new study was included in the analysis (Saad 2017), and the data from the final publication of another trial were updated (Ferry 2017). We added a 'Summary of findings' table containing the main efficacy and safety data to the meta‐analysis. We assessed the certainty of the evidence using the GRADE approach. |
| 18 February 2019 | New citation required and conclusions have changed | A marginal benefit in favour of cisplatin‐containing treatments in terms of response rate seen in the initial version of this meta‐analysis was not verified in the current update. |
Acknowledgements
The authors would like to thank Sera Tort, Joint Co‐ordinating Editor of the Cochrane Lung Cancer Review Group; Marta Roqué, Statistical Editor; Desiree West, consumer of the Lung Cancer Group; and the Brazilian Cochrane Center team for their support.
Appendices
Appendix 1. Search strategies
| MEDLINE (via PubMed) | #1 (Cisplatin [mh]) OR Cisplatin OR (cis‐Diamminedichloroplatinum(II)) OR (Platinum Diamminodichloride) OR (Diamminodichloride, Platinum) OR cis‐Platinum OR (cis Platinum) OR Cisplatinum OR (Dichlorodiammineplatinum) OR (cis‐Diamminedichloroplatinum) OR (cis Diamminedichloroplatinum) OR (cis‐Dichlorodiammineplatinum(II)) OR ( Platinol) OR Platidiam OR Platino OR (NSC‐119875) OR Biocisplatinum #2 (Carboplatin [mh]) OR Carboplatin OR (cis‐Diammine(cyclobutanedicarboxylato)platinum II) OR CBDCA OR Ribocarbo OR (ribosepharm Brand of Carboplatin) OR Nealorin OR (Prasfarma Brand of Carboplatin) OR Neocarbo OR ( Neocorp Brand of Carboplatin) OR Paraplatin OR Carboplat OR Paraplatine OR ( Bristol‐Myers Squibb Brand of Carboplatin) OR Carbosin OR (Pharmachemie Brand of Carboplatin) OR Carbotec OR (Columbia Brand of Carboplatin) OR Ercar OR (Almirall Brand of Carboplatin) OR JM‐8 or (JM 8) OR JM8 OR NSC‐241240 OR (NSC 241240) OR NSC241240 OR Platinwas OR (Chiesi Brand of Carboplatin) OR Blastocarb OR (Lemery Brand of Carboplatin) #3 (Lung Neoplasms [mh]) OR (Lung Neoplasms) OR (Neoplasms, Lung) OR (Lung Neoplasm) OR (Neoplasm, Lung) OR (Neoplasms, Pulmonary) OR (Neoplasm, Pulmonary) OR (Pulmonary Neoplasm) OR (Pulmonary Neoplasms) OR (Lung Cancer) OR (Cancer, Lung) OR (Cancers, Lung) OR (Lung Cancers) OR (Pulmonary Cancer) OR (Cancer, Pulmonary) OR (Cancers, Pulmonary) OR (Pulmonary Cancers) OR (Cancer of the Lung) OR (Cancer of Lung) OR (Carcinoma, Non‐Small‐Cell [mh]) OR (Carcinoma, Non‐Small‐Cell) OR (Carcinoma, Non Small Cell Lung) OR (Carcinomas, Non‐Small‐Cell Lung) OR (Lung Carcinoma, Non‐Small‐Cell) OR (Lung Carcinomas, Non‐Small‐Cell) OR (Non‐Small‐Cell Lung Carcinomas) OR (Non‐Small‐Cell Lung Carcinoma) OR (Non Small Cell Lung Carcinoma) OR (Carcinoma, Non‐Small Cell Lung) OR (Non‐Small Cell Lung Cancer) #4 #1 AND #2 AND #3 #5 randomized controlled trial [pt] #6 controlled clinical trial [pt] #7 randomized [tiab] #8 placebo [tiab] #9 drug therapy [sh] #10 randomly [tiab] #11 trial [tiab] #12 groups [tiab] #13 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 #14 animals [mh] NOT humans [mh] #15 #13 NOT #14 #16 #4 AND #15 |
| Embase via Ovid | 1 Clinical trial/ 2 Randomized controlled trial/ 3 Randomization/ 4 Single blind procedure/ 5 Double blind procedure/ 6 Crossover procedure/ 7 Placebo/ 8 Randomi?ed controlled trial$.tw. 9 Rct.tw. 10 Random allocation.tw. 11 Randomly allocated.tw. 12 Allocated randomly.tw. 13 (allocated adj2 random).tw. 14 Single blind$.tw. 15 Double blind$.tw. 16 ((treble or triple) adj (blind$).tw. 17 Placebo$.tw. 18 Prospective study/ 19 Or/1‐18 20 Case study/ 21 Case report.tw. 22 Abstract report/ or letter/ 23 Or/20‐22 24 19 not 23 25 exp Lung Cancer/ 26 exp Lung non Small Cell Cancer/ 27 non small cell.ti,ab. 28 NSCLC.ti,ab. 29 25 or 26 or 27 or 28 30 (Cisplatin [mh]) OR Cisplatin OR (cis‐Diamminedichloroplatinum(II)) OR (Platinum Diamminodichloride) OR (Diamminodichloride, Platinum) OR cis‐Platinum OR (cis Platinum) OR Cisplatinum OR (Dichlorodiammineplatinum) OR (cis‐Diamminedichloroplatinum) OR (cis Diamminedichloroplatinum) OR (cis‐Dichlorodiammineplatinum(II)) OR ( Platinol) OR Platidiam OR Platino OR (NSC‐119875) OR Biocisplatinum 31 (Carboplatin [mh]) OR Carboplatin OR (cis‐Diammine(cyclobutanedicarboxylato)platinum II) OR CBDCA OR Ribocarbo OR (ribosepharm Brand of Carboplatin) OR Nealorin OR (Prasfarma Brand of Carboplatin) OR Neocarbo OR ( Neocorp Brand of Carboplatin) OR Paraplatin OR Carboplat OR Paraplatine OR ( Bristol‐Myers Squibb Brand of Carboplatin) OR Carbosin OR (Pharmachemie Brand of Carboplatin) OR Carbotec OR (Columbia Brand of Carboplatin) OR Ercar OR (Almirall Brand of Carboplatin) OR JM‐8 or (JM 8) OR JM8 OR NSC‐241240 OR (NSC 241240) OR NSC241240 OR Platinwas OR (Chiesi Brand of Carboplatin) OR Blastocarb OR (Lemery Brand of Carboplatin) 32 30 and 31 33 29 and 32 34 24 and 33 |
| CENTRAL | #1 LUNG‐NEOPLASMS*:ME #2 CARCINOMA‐NON‐SMALL‐CELL‐LUNG*.ME #3 ((LUNG OR PULMON*) AND (NEOPLAS* OR CANCER OR CARCINOMA*)) #4 (#1 OR #2 OR #3) #5 CISPLATIN #6 CARBOPLATIN #7 (#5 AND #6) #8 (#4 AND #7) |
Data and analyses
Comparison 1. Carboplatin‐based versus cisplatin‐based chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 8 | 2515 | Hazard Ratio (Random, 95% CI) | 0.99 [0.82, 1.20] |
| 1.1 Carboplatin vs cisplatin plus gemcitabine | 3 | 367 | Hazard Ratio (Random, 95% CI) | 0.99 [0.82, 1.20] |
| 1.2 Carboplatin vs cisplatin plus paclitaxel | 4 | 1334 | Hazard Ratio (Random, 95% CI) | 1.00 [0.37, 2.73] |
| 1.3 Carboplatin vs cisplatin plus docetaxel | 1 | 814 | Hazard Ratio (Random, 95% CI) | 1.01 [0.16, 6.37] |
| 2 1‐year survival rate | 10 | 4004 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.89, 1.08] |
| 2.1 Carboplatin vs cisplatin plus gemcitabine | 4 | 1730 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.97, 1.25] |
| 2.2 Carboplatin vs cisplatin plus paclitaxel | 5 | 1460 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.84, 1.12] |
| 2.3 Carboplatin vs cisplatin plus docetaxel | 1 | 814 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.97] |
| 3 Response rate | 11 | 4020 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 1.00] |
| 3.1 Carboplatin vs. cisplatin plus gemcitabine | 5 | 1770 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.74, 1.15] |
| 3.2 Carboplatin vs. cisplatin plus paclitaxel | 5 | 1436 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.07] |
| 3.3 Carboplatin vs. cisplatin plus docetaxel | 1 | 814 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.60, 0.95] |
| 4 Grade III or IV toxicity by cycle | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Nausea, vomiting or both | 2 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.16, 1.17] |
| 4.2 Renal toxicity | 1 | 533 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.99] |
| 4.3 Neurotoxicity | 1 | 533 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.07] |
| 4.4 Skin rash | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Alopecia | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Anaemia | 1 | 533 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [0.50, 7.77] |
| 4.7 Neutropenia | 1 | 533 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.39, 3.36] |
| 5 Grade III or IV toxicity by participant | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Nausea, vomiting or both | 9 | 3819 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.31, 1.11] |
| 5.2 Renal toxicity | 3 | 1272 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.33, 3.10] |
| 5.3 Neurotoxicity | 5 | 1489 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.91, 2.23] |
| 5.4 Skin rash | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 69.52] |
| 5.5 Alopecia | 2 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.73, 1.68] |
| 5.6 Anaemia | 10 | 3857 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.79, 2.38] |
| 5.7 Thrombocytopenia | 10 | 3857 | Risk Ratio (M‐H, Random, 95% CI) | 2.46 [1.49, 4.04] |
| 5.8 Neutropenia | 10 | 3857 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.85, 1.63] |
1.5. Analysis.
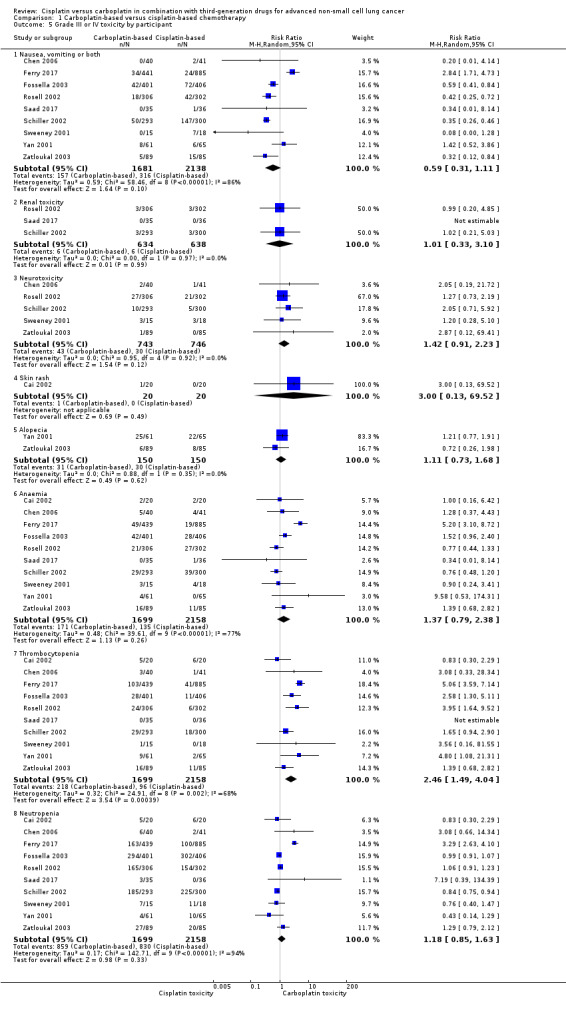
Comparison 1 Carboplatin‐based versus cisplatin‐based chemotherapy, Outcome 5 Grade III or IV toxicity by participant.
Comparison 2. Subgroup analysis (cisplatin dose: 40 to 79 mg/m2 versus 80 to 100 mg/m2).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 9 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 40 to 80 mg/m2 | 6 | 2508 | Hazard Ratio (Random, 95% CI) | 1.15 [1.03, 1.28] |
| 1.2 80 to 100 mg/m2 | 4 | 1823 | Hazard Ratio (Random, 95% CI) | 0.93 [0.83, 1.04] |
| 2 1‐year survival rate | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 40 to 80 mg/m2 | 6 | 2508 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.85, 1.27] |
| 2.2 80 to 100 mg/m2 | 5 | 1949 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.86, 1.08] |
| 3 Response rate | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 40 to 80 mg/m2 | 7 | 2524 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.74, 1.20] |
| 3.2 80 to 100 mg/m2 | 5 | 1949 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.77, 1.02] |
Comparison 3. Sensitivity analysis (fixed‐effect model).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 8 | 2515 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.82, 1.20] |
| 2 1‐year survival rate | 10 | 4004 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.07] |
| 3 Response rate | 11 | 4020 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.81, 0.99] |
Comparison 4. Sensitivity analysis (phase III trials).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 4 | 2210 | Hazard Ratio (Random, 95% CI) | 0.99 [0.45, 2.17] |
| 2 1‐year survival rate | 5 | 3573 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.84, 1.13] |
| 3 Response rate | 5 | 3549 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.71, 1.03] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cai 2002.
| Methods |
Inclusion: Eligible participants had to meet the following criteria:
Exclusion: There were no exclusion criteria specified for this study. |
|
| Participants | Arm I: 20 people Arm II: 20 people |
|
| Interventions | Arm I: gemcitabine (1000 mg/m2) iv on day 1 and 8 and carboplatin (AUC 4 to 6 mg/mL X minutes) on day 1, every 3 weeks Arm II: gemcitabine (1000 mg/m2) iv on day 1 and 8 and cisplatin (30 to 40 mg/m2) on days 1 to 3, every 3 weeks | |
| Outcomes | Primary outcome: response rate Secondary outcome: toxicity |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were stratified according to staging, sex, and histology. |
| Allocation concealment (selection bias) | Unclear risk | Not clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information about blinding process |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information about blinding process |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no evidence of incomplete outcome. |
| Selective reporting (reporting bias) | Unclear risk | Incidences of overall survival and 1‐year survival rate were not reported. The authors did not specify which efficacy outcomes would be analysed, therefore we assessed this trial as at unclear risk of reporting bias. |
| Other bias | High risk | Participants received carboplatin AUC 4 to 6 mg/mL X minute, which was inferior to the doses in almost all of the other included trials (AUC 6 mg/mL X minute). In addition, the analysis should be considered exploratory as this was a phase II trial. |
Chen 2006.
| Methods |
Inclusion: Eligible participants had to meet following criteria:
Exclusion: Patients were ineligible if they had:
|
|
| Participants | Arm I: 40 people Arm II: 41 people |
|
| Interventions | Arm I: paclitaxel (160 mg/m2) iv over 3 hours on day 1 and carboplatin (AUC 6 mg/mL X minutes) iv over 1 hour on day 1, every 3 weeks Arm II: paclitaxel (160 mg/m2) iv over 3 hours on day 1 and cisplatin (60 mg/m2) iv over 1 hour on day 1, every 3 weeks | |
| Outcomes | Primary outcome: response rate Secondary outcomes: time to progression; toxicity; overall survival |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were stratified according staging and PS. |
| Allocation concealment (selection bias) | Low risk | Participants were randomised into the paclitaxel plus carboplatin or paclitaxel plus cisplatin treatment arm by an outside centre not involved in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information about blinding process |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information about blinding process |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias |
| Other bias | High risk | Phase II trial and a study of elderly people, so could be associated with higher response rate |
Ferry 2017.
| Methods |
Inclusion: Eligible participants met criteria for histologically confirmed NSCLC, PS 0 to 2, life expectancy > 12 weeks, stage IIIB/IV disease, and had a GFR of > 60 mL/minute calculated using the Wright equation. Participant compliance and geographic proximity that allowed adequate follow‐up was required. Exclusion: Patients were ineligible if they had:
|
|
| Participants | Arm I: 456 people Arm II: 454 people Arm III: 453 people |
|
| Interventions | Arm I (GC80): gemcitabine (1250 mg/m2) iv over 30 minutes on days 1 and 8 and cisplatin (80 mg/m2) iv over 1 hour on day 1 (total time of infusion: 6 hours), every 3 weeks Arm II (GC50): gemcitabine (1250 mg/m2) iv over 30 minutes on days 1 and 8 and cisplatin (50 mg/m2) iv over 1 hour on day 1 (total time of infusion: 6 hours), every 3 weeks Arm III (GCb): gemcitabine (1250 mg/m2) iv over 30 minutes on days 1 and 8 and carboplatin (AUC 6 mg/mL X minutes) iv over 1 hour on day 1 (total time of infusion: 1.5 hours), every 3 weeks | |
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was stratified by PS (0, 1 and 2), stage (IIIB and IV), and centre to ensure balance between treatments within the strata defined by these key prognostic factors. |
| Allocation concealment (selection bias) | Low risk | Random assignment to treatment was conducted by a computer, based at the BTOG2 study office. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Since times to infusion differed (extra fluid administration in cisplatin arm), participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information about blinding of the assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Response rate could not be evaluated in several people (160 people in GC80, 152 people in GC50, and 151 people in GCb), which could have affected the final analysis of this endpoint. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias |
| Other bias | High risk | It is important to note that the Wright formula was used for calculation of creatinine clearance, which usually results in about 10% higher doses of carboplatin than with use of the Cockcroft‐Gault formula. |
Fossella 2003.
| Methods |
Inclusion: Participants with histological or cytological diagnosis of locally advanced or recurrent (stage IIIB ) or metastatic (stage IV) NSCLC who met the following criteria:
Exclusion: Patients were ineligible if they had:
|
|
| Participants | Arm I: 408 people Arm II: 406 people |
|
| Interventions | Arm I: docetaxel (75 mg/m2) iv over 1 hour on day 1 and cisplatin (75 mg/m2) iv over 1 hour on day 1, every 3 weeks Arm II: docetaxel (75 mg/m2) iv over 1 hour on day 1 and carboplatin (AUC 6 mg/mL X minutes) iv on day 1, every 3 weeks Arm III: vinorelbine and cisplatin (not used in this review) | |
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | Arm III (vinorelbine and cisplatin) was not used in this review. Arm III: vinorelbine (25 mg/m2) iv on days 1, 8, 15, and 22 plus cisplatin (100 mg/m2) iv on day 1, every 4 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Before random assignment to treatment, participants were stratified according to disease stage (IIIB vs IV) and geographic region (North America vs South Africa, New Zealand and Australia vs Europe, Lebanon and Israel vs South America). |
| Allocation concealment (selection bias) | Low risk | Random assignment to treatment was conducted by an independent research organisation using computer‐generated lists. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 1218 participants, 15 did not receive treatment (9 were ineligible, 4 withdrew consent, and 2 died of malignant disease before the first drug infusion) and were excluded from the safety analysis. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias |
| Other bias | Low risk | No other bias |
Mazzanti 2003.
| Methods |
Inclusion: Eligible participants had to meet the following criteria:
Participants who had received previous radiotherapy were included if their assessable disease was outside of the radiation field. Exclusion: Patients were ineligible if they had:
|
|
| Participants | Arm I: 58 people Arm II: 62 people |
|
| Interventions | Arm I: gemcitabine (1200 mg/m2) iv over 30 minutes on days 1 and 8 and cisplatin (80 mg/m2) iv over 45 minutes on day 2, every 3 weeks Arm II: gemcitabine (1200 mg/m2) iv over 30 minutes on days 1 and 8 and carboplatin (AUC 5 mg/mL X minutes) iv over 1 hour on day 2, every 3 weeks | |
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation algorithm, based on the Pocock and Simon method (Pocock 1975), included ECOG PS (0/1 vs 2) and disease stage (IIIB vs IV) as stratification factors. |
| Allocation concealment (selection bias) | Low risk | Eligible participants were randomised to 1 of 2 arms, GCb or GC, using a concealed list of random numbers. The randomisation algorithm was based on the Pocock and Simon method. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information about blinding process |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information about blinding process |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 participants were randomly assigned to the GC arm, but were ineligible to receive treatment (3 with an ECOG PS of 3 at baseline, 1 pretreated with chemotherapy, and 1 affected by a serious cardiac disease). |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias |
| Other bias | High risk | The trial was planned as a randomised phase II study to obtain information for further development in a controlled randomised phase III setting, thus the findings obtained from the treatment arm comparisons of this phase II study should be considered as exploratory. |
Rosell 2002.
| Methods |
Inclusion: Eligible participants were required to meet all of the following criteria:
Exclusion: Patients were ineligible if they had:
|
|
| Participants | Arm I: 309 people Arm II: 309 people |
|
| Interventions | Arm I: paclitaxel (200 mg/m2) iv over 3 hours and cisplatin (80 mg/m2) iv over 30 minutes every 3 weeks Arm II: paclitaxel (200 mg/m2) iv over 3 hours and carboplatin (AUC 6 mg/mL X minutes) iv over 30 minutes every 3 weeks | |
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | This procedure minimised imbalance in treatment assignment with respect to the following parameters: centre, PS (ECOG 0 or 1 vs 2), disease stage (IIIB vs IV), and histology (squamous cell vs non‐squamous cell carcinoma). |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed centrally by Bristol‐Myers Squibb Inc, Waterloo, Belgium, using a dynamic balancing algorithm of the Pocock‐Simon type. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information about blinding process |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information about blinding process |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 people (2%) never received a study drug (3 in carboplatin arm and 7 in cisplatin arm). This was considered unlikely to result in a significant bias. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias |
| Other bias | High risk | The only bias was that dose reduction of carboplatin was necessary for 96 of the 279 (34%) evaluable participants; this reduction occurred mainly during course 1, due to a miscalculation of AUC. The mean AUC for these 96 participants was 4.9 mg/mL X minutes. |
Saad 2017.
| Methods |
Inclusion: Eligible participants had to meet the following criteria:
Exclusion: Patients were ineligible if they had:
|
|
| Participants | Arm I: 36 people Arm II: 35 people |
|
| Interventions | Arm I: gemcitabine 1000 mg/m2 plus cisplatin 40 mg/m2 on days 1 and 8 of a 3‐week schedule for up to 6 cycles Arm II: gemcitabine 1000 mg/m2 iv on days 1 and 8 plus carboplatin at an AUC of 5 iv on day 1 of a 3‐week schedule for up to 6 cycles |
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided about randomisation methods. |
| Allocation concealment (selection bias) | Unclear risk | Eligible participants were simply randomised to either Gem/Cis group or Gem/Carb group. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Response evaluation was available for 60 and 40 participants after 3 and 6 cycles, respectively. |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting bias |
| Other bias | Unclear risk | No other bias |
Schiller 2002.
| Methods |
Inclusion: Eligible participants had confirmed NSCLC, measurable or non‐measurable, stage IIIB/IV or recurrent disease. Initially people with an ECOG PS of 0 to 2 were eligible for enrolment, but after the enrolment of 66 people with PS of 2, the study design was amended to exclude these participants due to the high rate of serious adverse events. Eligible participants also met the following criteria:
Exclusion: 52 people were ineligible for the following reasons (number of people):
|
|
| Participants | Arm I: 299 people Arm IV: 303 people |
|
| Interventions | Arm I: paclitaxel 135 mg/m2 over a 24‐hour period on day 1 and cisplatin, 75 mg/m2 on day 2 (3‐week cycle) Arm IV: paclitaxel 225 mg/m2 over a 3‐hour period on day 1 and carboplatin, AUC 6.0 mg/mL X minute on day 1 (3‐week cycle) | |
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | Arms II and III were not used in this review. Arm II: gemcitabine (1000 mg/m2) on days 1, 8, and 15 and cisplatin (100 mg/m2) on day 1, every 4 weeks Arm III: docetaxel (75 mg/m2) on day 1 and cisplatin (75 mg/m2) on day 1, every 3 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were stratified according to ECOG PS (0 or 1 vs 2, with higher scores indicating greater impairment), weight loss in the previous 6 months (< 5% vs > 5%), stage of disease (IIIB vs IV or recurrent disease), and the presence or absence of brain metastases. |
| Allocation concealment (selection bias) | Low risk | Participants were allocated using a computer‐generated random list into 1 of 4 arms. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information about blinding process |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information about blinding process |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "After 66 patients with a performance status of 2 had been enrolled, the study design was amended to include only patients with a performance status of 0 or 1 because of the high rate of serious adverse events in the patients with a performance status of 2" The authors considered only people with a PS of 0 or 1 in the final analysis. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias |
| Other bias | Low risk | No evidence of other bias |
Sweeney 2001.
| Methods |
Inclusion: Eligible participants had to meet the following criteria:
Participants with clinically stable brain metastases managed by surgery or radiotherapy (or both) were eligible. Exclusion: Patients were ineligible if they had:
|
|
| Participants | Arm I: 18 people Arm IV: 15 people |
|
| Interventions | Arm I: paclitaxel (135 mg/m2) iv over 24 hours on day 1 and cisplatin (75 mg/m2) on day 2, every 3 weeks Arm IV: paclitaxel (225 mg/m2) over 3 hours on day 1 and carboplatin (AUC 6 mg/mL X minutes) on day 1, every 3 weeks | |
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | Arms II and III were not used in this review. Arm II: gemcitabine (1 g/m2) on days 1, 8, and 15 and cisplatin (100 mg/m2) on day 1, every 4 weeks Arm III: docetaxel (75 mg/m2) on day 1 and cisplatin (75 mg/m2) on day 1, every 3 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants are stratified by weight loss within the past 6 months, disease stage, and presence of brain metastases. |
| Allocation concealment (selection bias) | Low risk | Participants were allocated using a computer‐generated random list into 1 of 4 arms. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information about blinding process |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information about blinding process |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias |
| Other bias | High risk | This report represents a final analysis of a subgroup of people with PS of 2 who seem to have a poorer prognosis compared with people with PS 0 or 1. |
Yan 2001.
| Methods |
Inclusion: Eligible participants had to meet the following criteria:
Exclusion: No exclusion criteria were specified for this study. |
|
| Participants | Arm I: 61 people Arm II: 65 people |
|
| Interventions | Arm I: paclitaxel (175 mg/m2) iv on day 1 and carboplatin (350 mg/m2) on day 1, every 4 weeks Arm II: paclitaxel (175 mg/m2) iv on days 1 and 8 and cisplatin (100 mg/m2) on day 1, every 4 weeks | |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were stratified according to sex and staging. |
| Allocation concealment (selection bias) | Unclear risk | No clear description of randomisation provided in the publication. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information about blinding process |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information about blinding process |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias |
| Other bias | High risk | The dose of carboplatin was 350 mg/m2 iv on day 1, which differs from the doses used in almost all the other included trials (AUC 4 to 6 mg/mL X minutes). |
Zatloukal 2003.
| Methods |
Inclusion: Chemo‐naive participants with histological or cytological diagnosis of stage IIIb or IV NSCLC who were not eligible for curative surgery or radiotherapy were enrolled. Participants had to meet the following criteria:
Exclusion: Patients were ineligible if they had:
|
|
| Participants | Arm I: 87 people Arm II: 89 people |
|
| Interventions | Arm I: gemcitabine (1200 mg/m2) iv over 30 minutes on days 1 and 8 and cisplatin (80 mg/m2) iv on day 1
Arm II: gemcitabine (1200 mg/m2) iv over 30 minutes and carboplatin (AUC 5 mg/mL X minutes) iv on day 1 2 weeks of treatment followed by 1 week of rest (a 21‐day period) was defined as a cycle of therapy for both arms. |
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was stratified by 4 factors: gender (male/female), disease stage (stage IIIb/stage IV), PS (≤ 80 and > 80), and investigational site (1 stratum per centre). |
| Allocation concealment (selection bias) | Low risk | Participants were balanced with respect to study treatment in each stratum and for each factor using the algorithm described by Pocock and Simon. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information about blinding process |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information about blinding process |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias |
| Other bias | Low risk | No evidence of other bias |
AUC: area under the curve; BTOG2: British Thoracic Oncology Group Trial; CTCAE: Common Terminology Criteria for Adverse Events; ECOG: Eastern Cooperative Oncology Group; EORTC: European Organisation for Research and Treatment of Cancer; GC: gemcitabine plus cisplatin; GC80: gemcitabine plus cisplatin (80 mg/m2); GC50: gemcitabine plus cisplatin (50 mg/m2); GCb: gemcitabine plus carboplatin; GFR: glomerular filtration rate; iv: intravenous; LCSS: Lung Cancer Symptom Scale; NSCLC: non‐small cell lung cancer; PS: performance status; QLQ‐C30: Quality of Life Questionnaire Core 30 Items; QoL: quality of life; QLQ‐LC13: Quality of Life Questionnaire Lung Cancer supplement 13; RECIST: Response Evaluation Criteria in Solid Tumors; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Billingham 2011 | Data from this study were included in Ferry 2017. |
| Moro‐Sibilot 2015 | No comparison between cisplatin and carboplatin |
| Schuette 2013 | No comparison between cisplatin and carboplatin |
| Smit 2016 | Post hoc analysis of another prospective trial |
| Sun 2014 | Not a randomised clinical trial |
| Tiseo 2014 | Not a randomised clinical trial |
| von Verschuer 2017 | Prospective cohort study |
All of the following publications are amongst the included trials, but were found in more than one database, as follows.
Saad 2017: found in PubMed, CENTRAL, and Embase databases; we excluded two of them.
Schuette 2013: found in PubMed, CENTRAL, and Embase databases; we excluded two of them.
Zatloukal 2003: found in PubMed, CENTRAL, and Embase databases; we excluded two of them.
Chen 2006: found in PubMed and CENTRAL databases; we excluded one of them.
Fossella 2003: found in PubMed and CENTRAL databases; we excluded one of them.
Cai 2002: found in PubMed and CENTRAL databases; we excluded one of them.
Yan 2001: found in PubMed and CENTRAL databases; we excluded one of them.
Schiller 2002: found in PubMed and CENTRAL databases; we excluded one of them.
Rosell 2002: found in PubMed and Embase databases; we excluded one of them.
Differences between protocol and review
We could not perform a quality of life analysis because only two trials evaluated this endpoint.
We took into account the wide range in doses of cisplatin and performed a separate analysis of 'higher' and 'lower' doses.
We had proposed in the protocol to use odds ratios to evaluate dichotomous outcomes; however, we reconsidered and used risk ratios to enable easier interpretation of the data to the reader.
Contributions of authors
Vitor Fiorin de Vasconcellos: selection of studies, data extraction, 'Risk of bias' assessment.
Guilherme Nader Marta: selection of studies, data extraction, 'Risk of bias' assessment, and review organisation in Review Manager 5.
Rachel Riera: methodological topics and review organisation in Review Manager 5.
Edina MK da Silva: review organisation in Review Manager 5, critical appraisal of the review.
Aecio FT Gois: review organisation in Review Manager 5, critical appraisal of the review.
Tiago Biachi de Castria: background, objectives and outcomes definitions, selection of studies, data extraction, 'Risk of bias' assessment and review organisation in Review Manager 5, critical appraisal of the last review.
Sources of support
Internal sources
Brazilian Cochrane Center, Brazil.
External sources
No sources of support supplied
Declarations of interest
Vitor Fiorin de Vasconcellos: none.
Guilherme Nader Marta: travel, accommodations, expenses: Bayer Schering Pharma and Roche.
Rachel Riera: none.
Edina MK da Silva: none.
Aecio FT Gois: none.
Tiago Biachi de Castria: travel, accommodations, expenses, lecture fees: F. Hoffmann‐La Roche.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Cai 2002 {published data only}
- Cai X, Chen P, Yin X, Li Q. Comparison of efficacy and toxicity between gemcitabine plus carboplatin and gemcitabine plus cisplatin in the treatment of advanced non‐small cell lung cancer. Zhongguo Fei Ai Za Zhi 2002;5(6):427‐8. [DOI] [PubMed] [Google Scholar]
Chen 2006 {published data only}
- Chen YM, Perng RP, Tsai CM, Whang‐Peng J. A phase II randomized study of paclitaxel plus carboplatin or cisplatin against chemo‐naive inoperable non‐small cell lung cancer in the elderly. Journal of Thoracic Oncology 2006;1(2):141‐5. [PubMed] [Google Scholar]
Ferry 2017 {published data only}
- Ferry D, Billingham L, Jarrett H, Dunlop D, Woll PJ, Nicolson M, et al. Carboplatin versus two doses of cisplatin in combination with gemcitabine in the treatment of advanced non‐small‐cell lung cancer: results from a British Thoracic Oncology Group randomised phase III trial. European Journal of Cancer 2017;83:302–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fossella 2003 {published data only}
- Fossella F, Pereira JR, Pawel J, Pluzanska A, Gorbounova V, Kaukel E, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non‐small‐cell lung cancer: the TAX 326 study group. Journal of Clinical Oncology 2003;21(16):3016‐24. [DOI] [PubMed] [Google Scholar]
Mazzanti 2003 {published data only}
- Mazzanti P, Massacesi C, Rocchi MB, Mattioli R, Lippe P, Trivisonne R, et al. Randomized, multicenter, phase II study of gemcitabine plus cisplatin versus gemcitabine plus carboplatin in patients with advanced non‐small cell lung cancer. Lung Cancer 2003;41(1):81‐9. [DOI] [PubMed] [Google Scholar]
Rosell 2002 {published data only}
- Rosell R, Gatzemeier U, Betticher DC, Keppler U, Macha HN, Pirker R, et al. Phase III randomised trial comparing paclitaxel/carboplatin with paclitaxel/cisplatin in patients with advanced non‐small‐cell lung cancer: a cooperative multinational trial. Annals of Oncology 2002;13(10):1539‐49. [DOI] [PubMed] [Google Scholar]
Saad 2017 {published data only}
- Saad AS, Ghali RR, Shawki MA. A prospective randomized controlled study of cisplatin versus carboplatin‐based regimen in advanced squamous nonsmall cell lung cancer. Journal of Cancer Research and Therapeutics 2017;13(2):198‐203. [DOI] [PubMed] [Google Scholar]
Schiller 2002 {published data only}
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non‐small‐cell lung cancer. New England Journal of Medicine 2002;346(2):92‐8. [DOI] [PubMed] [Google Scholar]
Sweeney 2001 {published data only}
- Sweeney CJ, Zhu J, Sandler AB, Schiller J, Belani CP, Langer C, et al. Outcome of patients with a performance status of 2 in Eastern Cooperative Oncology Group Study E1594: a phase II trial in patients with metastatic nonsmall cell lung carcinoma. Cancer 2001;92(10):2639‐47. [DOI] [PubMed] [Google Scholar]
Yan 2001 {published data only}
- Yan D, Wang G, Zhang G, Hu C. A randomized phase II trial of paclitaxel in combination chemotherapy with platinum in the treatment of non‐small cell lung cancer. Zhongguo Fei Ai Za Zhi 2001;4(3):188‐90. [DOI] [PubMed] [Google Scholar]
Zatloukal 2003 {published data only}
- Zatloukal P, Petruzelka L, Zemanová M, Kolek V, Skricková J, Pesek M, et al. Gemcitabine plus cisplatin vs. gemcitabine plus carboplatin in stage IIIb and IV non‐small cell lung cancer: a phase III randomized trial. Lung Cancer 2003;41(3):321‐31. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Billingham 2011 {unpublished data only}
- Billingham L, Gaunt P, Jarrett HW, Dunlop D, Thompson D, O’Byrne KJ, et al. Quality of life in advanced non‐small cell lung cancer, effects of cisplatin dose and carboplatin in combination with gemcitabine: results from BTOG2, a British Thoracic Oncology Group phase III trial in 1363 patients. Thorax 2011;66:A41. [Google Scholar]
Moro‐Sibilot 2015 {published data only}
- Moro‐Sibilot D, Audigier‐Valette C, Merle P, Quoix E, Souquet PJ, Barlesi F, et al. Non‐small cell lung cancer recurrence following surgery and perioperative chemotherapy: comparison of two chemotherapy regimens (IFCT‐0702: a randomized phase 3 final results study). Lung Cancer 2015;89(2):139‐45. [DOI] [PubMed] [Google Scholar]
Schuette 2013 {published data only}
- Schuette WH, Gröschel A, Sebastian M, Andreas S, Müller T, Schneller F, et al. A randomized phase II study of pemetrexed in combination with cisplatin or carboplatin as first‐line therapy for patients with locally advanced or metastatic non‐small‐cell lung cancer. Clinical Lung Cancer 2013;14(3):215‐23. [DOI] [PubMed] [Google Scholar]
Smit 2016 {published data only}
- Smit E, Moro‐Sibilot D, Castro Carpeño J, Lesniewski‐Kmak K, Aerts J, Villatoro R, et al. Cisplatin and carboplatin‐based chemotherapy in the first‐line treatment of non‐small cell lung cancer: analysis from the European FRAME study. Lung Cancer 2016;92:35‐40. [DOI] [PubMed] [Google Scholar]
Sun 2014 {published data only}
- Sun X, Zheng Y. Cisplatin or carboplatin for advanced non–small cell lung cancer?. Journal of Thoracic Oncology 2014;9(9):e70. [DOI] [PubMed] [Google Scholar]
Tiseo 2014 {published data only}
- Tiseo M, Ardizzoni A, Boni L. First‐line chemotherapy treatment of advanced non–small‐cell lung cancer. Does cisplatin versus carboplatin make a difference?. Journal of Thoracic Oncology 2014;9(11):e82. [DOI] [PubMed] [Google Scholar]
von Verschuer 2017 {published data only}
- Verschuer U, Schnell R, Tessen HW, Eggert J, Binninger A, Spring L, et al. Treatment, outcome and quality of life of 1239 patients with advanced non‐small cell lung cancer – final results from the prospective German TLK cohort study. Lung Cancer 2017;112:216‐24. [DOI] [PubMed] [Google Scholar]
Additional references
Ardizzoni 2007
- Ardizzoni A, Boni L, Tiseo M, Fossella FV, Schiller JH, Paesmans M, et al. Cisplatin versus carboplatin‐based chemotherapy in first‐line treatment of advanced non‐small‐cell lung cancer: an individual patient data meta‐analysis. Journal of the National Cancer Institute 2007;99(11):847‐57. [DOI] [PubMed] [Google Scholar]
Baggstrom 2007
- Baggstrom M, Stinchcombe T, Fried D, Poole C, Hensing T, Socinski M. Third‐generation chemotherapy agents in the treatment of advanced non‐small cell lung cancer: a meta‐analysis. Journal of Thoracic Oncology 2007;2(9):845‐53. [DOI] [PubMed] [Google Scholar]
de Souza 2016
- Souza JA, Hunt B, Asirwa FC, Adebamowo C, Lopes G. Global health equity: cancer care outcome disparities in high‐, middle‐, and low‐income countries. Journal of Clinical Oncology 2016;34(1):6‐13. [PUBMED: 26578608] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisenhauer 2009
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European Journal of Cancer 2009;45(2):228‐47. [DOI] [PubMed] [Google Scholar]
GLOBOCAN 2018
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro Guideline Development Tool. Hamilton (ON).: McMaster University (developed by Evidence Prime, Inc.), 2015. Available at gradepro.org.
Green 2003
- Green S, Benedetti J, Crowley J. Clinical trials in oncology. 2nd Edition. Boca Raton, FL: Chapman & Hall/CRC, 2003. [Google Scholar]
Gridelli 2015
- Gridelli C, Rossi A, Carbone DP, Guarize J, Karachaliou N, Mok T, et al. Non‐small‐cell lung cancer. Nature Reviews Disease Primers 2015;1(May 21;1:15009):1:16. [DOI: 10.1038/nrdp.2015.9] [DOI] [PubMed] [Google Scholar]
Hanna 2017
- Hanna N, Johnson D, Temin S, Baker S Jr, Brahmer J, Ellis PM, et al. Systemic therapy for stage IV non‐small‐cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. Journal of Clinical Oncology 2017;35(30):3484‐515. [PUBMED: 28806116] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ho 2016
- Ho GY, Woodward N, Coward JIG. Cisplatin versus carboplatin: comparative review of therapeutic management in solid malignancies. Critical Reviews in Oncology/Hematology 2016;102:37‐46. [DOI: 10.1016/j.critrevonc.2016.03.014] [DOI] [PubMed] [Google Scholar]
Hotta 2004
- Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Tanimoto M. Meta‐analysis of randomized clinical trials comparing cisplatin to carboplatin in patients with advanced non‐small‐cell lung cancer. Journal of Clinical Oncology 2004;22(19):3852‐9. [DOI] [PubMed] [Google Scholar]
Jiang 2007
- Jiang J, Liang X, Zhou X, Huang R, Chu Z. A meta‐analysis of randomized controlled trials comparing carboplatin‐based to cisplatin‐based chemotherapy in advanced non‐small cell lung cancer. Lung Cancer 2007;57(3):348‐58. [DOI] [PubMed] [Google Scholar]
Keynote‐024
- Reck M, Rodríguez‐Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. KEYNOTE‐024 Investigators. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. New England Journal of Medicine 2016;375(19):1823‐33. [DOI] [PubMed] [Google Scholar]
Keynote‐189
- Gandhi L, Rodríguez‐Abreu D, Gadgeel S, Esteban E, Felip E, Angelis F, et al. KEYNOTE‐189 Investigators. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. New England Journal of Medicine 2018;378(22):2078‐92. [DOI: 10.1056/NEJMoa1801005] [DOI] [PubMed] [Google Scholar]
Lisberg 2019
- Lisberg A, Garon EB. Does platinum‐based chemotherapy still have a role in first‐line treatment of advanced non‐small‐cell lung cancer?. Journal of Clinical Oncology 2019;37(7):529‐36. [PUBMED: 30676857] [DOI] [PubMed] [Google Scholar]
NCI Common Toxicity Criteria
- National Cancer Institute. Common toxicity criteria. ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcv20_4‐30‐992.pdf (accessed 26 June 2013).
Noone 2018
- Noone AM, Cronin KA, Altekruse SF, Howlader N, Lewis DR, Petkov VI, et al. Cancer incidence and survival trends by subtype using data from the Surveillance Epidemiology and End Results program, 1992‐2013. Cancer Epidemiology, Biomarkers & Prevention 2017; Vol. 26, issue 4:632‐41. [DOI: 10.1158/1055-9965.EPI-16-0520] [DOI] [PMC free article] [PubMed]
NSCLC Collaborative Group 1995
- NSCLC Collaborative Group. Chemotherapy in non‐small cell lung cancer: a meta‐analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 1995;311(7010):899‐909. [PMC free article] [PubMed] [Google Scholar]
O'Dwyer 2015
- O’Dwyer PJ, Calvert AH. Platinum analogs. In: DeVita VT, Lawrence TS, Rosenberg SA editor(s). Cancer: principles and practice of oncology. 10th Edition. Philadelphia: Lippincott Williams & Wilkins, 2015:199‐208. [Google Scholar]
Paccagnella 2004
- Paccagnella A, Favaretto A, Oniga F, Barbieri F, Ceresoli G, Torri W, et al. Cisplatin versus carboplatin in combination with mitomycin and vinblastine in advanced non small cell lung cancer. A multicenter, randomized phase III trial. Lung Cancer 2004;43(1):83‐91. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MKB, Valter T, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Perez‐Ramirez 2017
- Perez‐Ramirez C, Canadas‐Garre M, Molina MA, Robles AI, Faus‐Dader MJ, Calleja‐Hernandez MA. Contribution of genetic factors to platinum‐based chemotherapy sensitivity and prognosis of non‐small cell lung cancer. Mutation Research 2017;771:32‐58. [PUBMED: 28342452] [DOI] [PubMed] [Google Scholar]
Pocock 1975
- Pocock S, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975;31:103‐15. [PubMed] [Google Scholar]
Reck 2010
- Reck M, Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Overall survival with cisplatin‐gemcitabine and bevacizumab or placebo as first‐line therapy for nonsquamous non‐small‐cell lung cancer: results from a randomised phase III trial (AVAiL). Annals of Oncology 2010;21(9):1804‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sandler 2006
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel‐carboplatin alone or with bevacizumab for non‐small‐cell lung cancer. New England Journal of Medicine 2006;355(24):2542‐50. [DOI] [PubMed] [Google Scholar]
Scagliotti 2008
- Scagliotti V, Parikh P, Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy‐naive patients with advanced‐stage non‐small‐cell lung cancer. Journal of Clinical Oncology 2008;26:3543‐51. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. ..
Sobin 2002
- Sobin LH, Wittekind C. Union for International Cancer Control (UICC), TNM Classification of Malignant Tumours. 6th Edition. Vol. 1, New York: Wiley‐Liss, 2002. [Google Scholar]
Vasconcellos 2018
- Vasconcellos VF, Colli LM, Awada A, Castro Junior G. Precision oncology: as much expectations as limitations. ecancermedicalscience 2018; Vol. 12:ed86. [PUBMED: 30679954] [DOI] [PMC free article] [PubMed]
Wright 2001
- Wright J, Boddy A, Highley M, Fenwick J, McGill A, Calvert A. Estimation of glomerular filtration rate in cancer patients. British Journal of Cancer 2001;84(4):452‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
de Castria 2013
- Castria TB, Silva EM, Gois AF, Riera R. Cisplatin versus carboplatin in combination with third‐generation drugs for advanced non‐small cell lung cancer. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD009256.pub2] [DOI] [PubMed] [Google Scholar]


