Abstract
Purpose:
Radiation-induced cardiac toxicity (RICT) is an increasingly well-appreciated source of morbidity and mortality in patients receiving thoracic radiotherapy (RT). Currently available methods to predict RICT are suboptimal. We investigated circulating microRNAs (c-miRNAs) as potential biomarkers of RICT in patients undergoing definitive RT for non-small cell lung cancer (NSCLC).
Methods:
Data from 63 patients treated on institutional trials were analyzed. Prognostic models of grade 3 or greater (G3+) RICT based on pre-treatment c-miRNA levels (‘c-miRNA’), mean heart dose (MHD) and pre-existing cardiac disease (PCD) (‘clinical’), and a combination of these (‘c-miRNA + clinical’) were developed. Elastic net Cox regression and full cross-validation were used for variable selection, model building, and model evaluation. Concordance statistic (c-index) and integrated Brier score (IBS) were used to evaluate model performance.
Results:
MHD, PCD, and serum levels of 14 c-miRNA species were identified as jointly prognostic for G3+ RICT. The ‘c-miRNA and ‘clinical’ models yielded similar cross-validated c-indices (0.70 and 0.72, respectively) and IBSs (0.26 and 0.28, respectively). However, prognostication was not improved by combining c-miRNA and clinical factors (c-index 0.70, IBS 0.28). The ‘c-miRNA’ and ‘clinical’ models were able to significantly stratify patients into high- and low-risk groups of developing G3+ RICT. Chi-square testing demonstrated a marginally significantly higher prevalence of PCD in patients with high- compared to low-risk c-miRNA profile (p=0.09), suggesting an association between some c-miRNAs and PCD.
Conclusions:
We identified a pre-treatment c-miRNA signature prognostic for G3+ RICT. With further development, pre- and mid-treatment c-miRNA profiling could contribute to multiparametric, patient-specific dose-selection and treatment-adaptation.
Keywords: Non-small cell lung cancer, Radiotherapy, Cardiac toxicity, Biomarker, MicroRNA
Introduction
Definitive thoracic radiotherapy (RT) for non-small cell lung cancer (NSCLC) has been linked to an increased risk of cardiac-related morbidity and mortality (Bradley et al. 2015; Chun et al. 2017; Dess et al. 2017; Wang et al. 2017). Current methods to predict RICT rely on dosimetric parameters, such as mean heart dose (MHD), and clinical factors, such as preexisting cardiac disease (PCD) (Dess et al. 2017; Wang et al. 2017). However, prediction could potentially be improved by the identification of additional biomarkers. Improved risk-stratification could be useful for guiding personalized treatment planning and dose-selection.
MicroRNAs (miRNAs) are small, non-coding RNAs that post-transcriptionally regulate gene expression (Ambros 2004). MiRNAs have been implicated in positively and negatively regulating numerous physiologic and pathologic processes, including oncogenesis and heart disease (Esquela-Kerscher and Slack 2006; Quiat and Olson 2013). MiRNAs have been shown to modulate the effects of therapeutic radiation (Cho et al. 2014; Jiang et al. 2017; Koo et al. 2017; Lan et al. 2015; Weidhaas et al. 2007; Zhai et al. 2016; Zhen et al. 2016), and in animal myocardial tissue, certain miRNA species have been implicated in promoting or protecting against RT-induced cardiac damage (Kura et al. 2016; Viczenczova et al. 2016).
While miRNAs were initially characterized in terms of their intracellular functions, extracellular, circulating miRNAs (c-miRNAs) have recently been identified in blood and other bodily fluids (Valadi et al. 2007). Like tissue miRNAs, c-miRNAs demonstrate variable expression patterns in the presence and absence of cancer, and are remarkably stable (Mitchell et al. 2008; Yuan et al. 2016). Unlike tissue miRNAs, however, c-miRNAs are readily obtainable in blood and do not require invasive tissue sampling. Given these features, c-miRNAs represent attractive candidates for biomarker development (Kong et al. 2012). While c-miRNAs have shown promise as biomarkers of cardiac disease (Matsumoto et al. 2013; Wang et al. 2016; Zampetaki et al. 2012), as well as response to and survival following RT for NSCLC (Chen et al. 2016; Sun et al. 2017), their utility in predicting RICT has not been explored.
Due to the clinical challenge posed by RICT and the lack of patient-specific biomarkers for risk stratification, we sought to identify a pre-treatment c-miRNA signature for prediction of RICT in patients treated with definitive RT for locally advanced and medically inoperable NSCLC.
Materials and methods
Patient cohort
We analyzed data from 63 patients treated on four prospective Institutional Review Board-approved lung-cancer studies: (1) a phase 1–2 study of radiation dose-escalation with concurrent chemotherapy (NCT not available), (2–3) two consecutive studies using functional imaging and biomarkers to assess patient outcome (NCT not available and NCT00603057), and (4) a study using mid-treatment positron emission tomography (PET) to guide individualized dose-escalation (NCT01190527). NCT numbers are not available for the earliest two trials as they were not registered with clinicaltrials.gov. Primary trial outcomes are not reported in this manuscript, but data from patients treated on these protocols were included in this analysis. Patients with stage II-III NSCLC and a Karnofsky performance status (KPS) of at least 60 were included in this study, whereas patients with a component of small-cell lung cancer and those treated with stereotactic body RT (SBRT) were excluded.
Treatment regimen
All patients were treated with definitive RT, with or without concurrent chemotherapy. After completion of RT, some patients were also treated with consolidative chemotherapy, depending on their respective protocol and physician preference. Target radiation doses ranged from 66 to 86 Gy in daily fractions, as directed by the respective protocols. Radiation was delivered using three-dimensional conformal RT (3DCRT) as previously described (Kong et al. 2005). Gross tumor volume included the primary tumor and any involved regional lymph nodes, as determined by tissue diagnosis and/or positron emission tomography (PET). Uninvolved lymph node regions were not included in the clinical target volume. Tissue inhomogeneity corrections were applied for all plans. Heart constraints employed in these trials included volume receiving 40 Gy < 100% (V40Gy < 100%) and V65Gy < 33%.
As dose and fractionation varied among patients, we standardized values to equivalent doses in 2-Gy fractions (EQD2) (Fowler 1989). EQD2 values were calculated using the linear-quadratic formula with an alpha-beta ratio of 10 Gy for tumor and 2.5 Gy for heart (Hall and Giaccia 2006).
Sample collection, RNA isolation, and miRNA profiling
Blood samples were collected using red-top tubes with no use of anticoagulant within one week prior to initiation of thoracic radiation, following which they were processed as previously described (Sun et al. 2017). Total RNA was isolated from serum samples using the miRNeasy Mini Kit (Qiagen, Hilden Germany) following the manufacturer’s protocol with slight modifications, as previously described (Sun et al. 2017). Post-isolation, RNA concentration and quality were determined using a Nanodrop 2000 instrument (Thermo Scientific, Waltham, Massachusetts).
Serum RNA was reverse transcribed to complementary DNA (cDNA) using the miScript II Reverse Transcription Kit (Qiagen, Hilden Germany). Reverse-transcription products were analyzed for the presence and differential expression of a panel of 62 miRNAs detectable in serum, plasma, and other bodily fluids using Human Serum & Plasma miRNA PCR Arrays (Cat. No MIHS-106Z, Qiagen, Hilden Germany) as previously described (Philippidou et al. 2010; Ryu et al. 2011). A list of all miRNA species assayed is found in Supplemental Table 1. In order to eliminate potential variation introduced during the isolation and RNA quantification processes, the raw Ct value for each miRNA was normalized to the raw Ct value for spike-in cel-miR-39 obtained from each individual sample using the 2−ΔΔCt method (Livak and Schmittgen 2001). C-miRNA levels are described as elevated or depressed relative to the mean value of each cmiRNA calculated from the entire patient population.
Outcome definitions
The primary endpoint was grade 3 or greater (G3+) RICT, modeled as a survival-type endpoint to account for differential follow-up. Patients alive at their last follow-up or who died of causes unrelated to RICT were censored at those dates. Radiation-induced cardiac toxicity was initially graded per CTCAE v.3.0 and then for this analysis reviewed, confirmed, and updated to CTCAE v4.03. In addition, cardiac events not previously attributed to radiation were documented, graded, and included in the analysis. All events were confirmed by two independent physicians without knowledge of the treatment plan or cardiac radiation dose.
Statistical methods
We investigated mean heart dose (MHD), pre-existing cardiac disease (PCD), and pre-treatment serum c-miRNA levels as potential prognostic biomarkers of G3+ RICT. Mean heart dose and PCD were selected as they had been previously identified by our group as significant contributors to the development of RICT (Dess et al. 2017). Pre-existing cardiac disease was defined as the presence of either ischemic heart disease or congestive heart failure (CHF). Ischemic heart disease was defined as acute myocardial infarction (MI); coronary artery bypass grafting procedure, angioplasty or stent placement; or a diagnosis of coronary artery disease (CAD).
Cox elastic net regression was used to model the risk of G3+ RICT as a function of covariates. Elastic net is a hybrid of LASSO and RIDGE regression techniques and retains the good prediction performance of RIDGE while also enforcing sparsity (Zou and Hastie 2005). All variables were modeled as main effects. Three separate models were fit: one consisting of cmiRNA signature (‘c-miRNA’), one consisting of MHD and PCD (‘clinical’), and one consisting of a combination of these (‘c-miRNA + clinical’). Factors were combined into these models using Cox elastic net multivariable regression with the relative weight of each factor being related to their respective coefficients derived from model fitting. The c-miRNA signature combined the effects of each selected c-miRNA into an aggregate estimate of c-miRNA-attributable risk. Model performance was assessed by concordance statistic (c-index) (Heagerty and Zheng 2005) and integrated Brier score (IBS) (Graf et al. 1999). The c-index describes a model’s discriminatory ability in a manner similar to area under the receiver operating characteristic curve (AUC), but differs from AUC in that it considers the time to an event instead of cumulative incidence at a specified time point. A higher c-index indicates superior discriminatory ability. The Brier score measures the squared difference between predicted and observed survival with censorship. The integrated Brier Score (IBS) is a summary of the cumulative prediction error integrated over a time period. A lower IBS denotes superior model performance, with an IBS of 0 indicating perfect model predictions over the period of interest. IBS is commonly used as a performance score to evaluate the predictive performance of survival models (Rufibach 2010; Schumacher et al. 2003).
Model assessment and tuning parameter selection were performed using 5-fold cross validation repeated 20 times (to minimize variability associated with fold choice). Each time, the four training-fold dataset was used to build the prognostic model (including data normalization, fitting Cox elastic net), following which the corresponding test-fold dataset was used for cross-validated c-index and IBS estimation.
In order to investigate the ability of these models to risk-stratify in terms of RICT, we calculated Kaplan-Meier estimates of the proportion of patients experiencing G3+ RICT over time in low- versus high-risk patient groups. Risk grouping was determined from repeated stratified 5-fold cross validation with the resultant median being used as dichotomization point. To assess the relationship between c-miRNA group and PCD, we performed two way table chi-square testing, using Monte Carlo simulation to calculate the corresponding p-value. All statistical analyses were performed with R version 3.2.1.
Results
Characteristics of the 63 patients analyzed in this study are found in Table 1. Of all patients, 11 developed G3+ RICT, corresponding to a cumulative rate of 16.9%. Six of these events were acute coronary syndrome (ACS), two were CHF, two were cardiac arrest, and one was arrhythmia.
Table 1.
Demographic, disease, and treatment characteristics of study participants.
| Characteristic | Value |
|---|---|
| Median age, years (range) | 65.7 (45.3 – 84.6) |
| Sex, n (%) | |
| Female | 15 (23.8%) |
| Male | 48 (76.2%) |
| KPS, n (%) | |
| 81 – 100 | 32 (50.8%) |
| 71 −80 | 21 (33.3%) |
| 60–70 | 10 (15.9%) |
| Smoking status, n (%) | |
| Current | 32 (50.8%) |
| Former | 29 (46.0%) |
| Never | 2 (3.2%) |
| Unknown | 0 |
| PCD, n (%) | |
| Yes | 19 (30.2%) |
| No | 44 (69.8%) |
| Tumor histology, n (%) | |
| Adenocarcinoma | 20 (31.7%) |
| Squamous cell carcinoma | 21 (33.3%) |
| NSCLC NOS | 22 (34.9%) |
| Group stage, n (%) | |
| 11A | 2 (3.2%) |
| MB | 5 (7.9%) |
| IIIA | 24 (38.1%) |
| IIIB | 32 (50.8%) |
| Concurrent chemotherapy, n (%) | |
| Yes | 53 (84.1%) |
| No | 10 (15.9%) |
| Mean radiation dose, Gy (EQD2) | |
| Mean target dose (Std Dev) | 74.4 (8.7) |
| Mean MHD (Std Dev) | 13.7 (8.8) |
KPS = Karnofsky performance status. PCD = pre-existing cardiac disease. NSCLC = non-small cell lung cancer. NOS = not otherwise specified. Gy = Gray. EQD2 = equivalent dose in 2-Gy fractions. Std Dev = standard deviation. MHD = mean heart dose.
In addition to MHD and PCD, multivariable elastic net analysis identified 14 c-miRNA species for which variations in serum concentrations correlated with subsequent development of G3+ RICT (Table 2). Of these, higher levels of five were associated with increased risk, while higher levels of 9 were associated with decreased risk. The c-miRNA associated with the greatest hazard ratio (HR) for G3+ RICT was miR-574–3p, for which an elevation in serum levels one standard deviation above the mean was associated with a HR of 1.85. MiR-15b and miR-21, two miRNA species previously implicated in regulating cardiac response to RT in animal studies (Kura et al. 2016; Viczenczova et al. 2016), were not significantly correlated with G3+ RICT in this analysis and were therefore not included in the subsequent modeling (Supplemental Table 2).
Table 2.
Coefficients and hazard ratios (HRs) associated with the 16 variables selected by elastic net as prognostic for G3+ RICT.
| Variable | Coefficient (log(HR)) | HR |
|---|---|---|
| PCD | 1.113 | 3.043 |
| MHD (per 10 Gy) | 0.180 | 1.197 |
| miR-100–5p | −0.006 | 0.994 |
| miR-106b-5p | −0.080 | 0.924 |
| miR-145–5p | −0.176 | 0.839 |
| miR-146a-5p | −0.598 | 0.550 |
| miR-192–5p | −0.282 | 0.754 |
| miR-195–5p | −0.150 | 0.861 |
| miR-223–3p | −0.033 | 0.968 |
| miR-25–3p | −0.191 | 0.826 |
| miR-34a-5p | −0.325 | 0.722 |
| miR-574–3p | 0.616 | 1.851 |
| miR-885–5p | 0.499 | 1.647 |
| Iet-7c | 0.284 | 1.328 |
| miR-200b-3p | 0.501 | 1.651 |
| miR-134 | 0.086 | 1.089 |
HR for miRNAs represents change in risk associated with in 1 crease in serum concentration 1 standard deviation above the mean value for that miRNA among all patients.
HR = hazard ratio. PCD = pre-existing cardiac disease. MHD = mean heart dose.
We next generated three models for prognostication of G3+ RICT based on c-miRNA signature (‘c-miRNA’), MHD and PCD (‘clinical’), and a combination of these (‘c-miRNA + clinical’). Table 3 lists the cross-validated c-index and IBS associated with each model. The highest c-index, corresponding to superior discriminatory ability, was obtained using the ‘clinical’ model, and the lowest IBS, corresponding to superior overall performance, was obtained using the ‘c-miRNA’ model, although differences in these values among models were modest. In spite of the fact that multivariable elastic net analysis identified all of these factors as adding value to G3+ RICT prediction, combining c-miRNA and clinical factors did not improve model performance.
Table 3.
C-indices for prognostication of G3+ RICT using indicated models.
| c-index | IBS | |
|---|---|---|
| C-miRNA | 0.70 | 0.26 |
| Clinical Factors | 0.72 | 0.28 |
| C-miRNA + Clinical Factors | 0.70 | 0.28 |
C-index = concordance statistic. IBS = integrated Briar score.
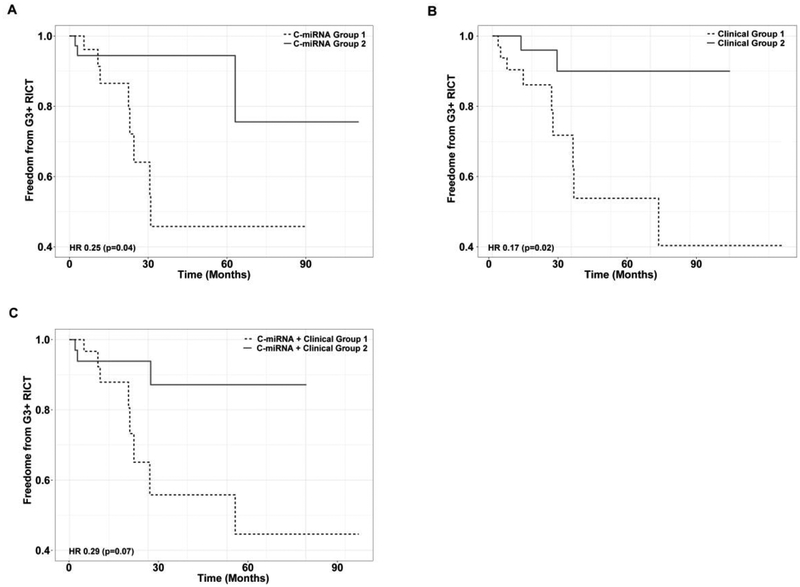
As no p-values are available from penalized regression techniques such as elastic net, we next sought to empirically test whether the models could distinguish between patients who are at lower or higher risk of G3+ RICT. To do this, we used each model to divide patients into low- and high-risk groups and analyzed Kaplan-Meier estimates of G3+ RICT in each group. Hazard ratios of G3+ RICT in low- versus high-risk patients were 0.25 (p=0.04, 95%CI 0.06–0.94), 0.17 (p=0.02, 95%CI 0.04–0.79), and 0.29 (p=0.07, 95%CI 0.08–1.12) for the ‘c-miRNA,’ ‘clinical,’ and ‘c-miRNA + clinical’ models, respectively, indicating significant risk-stratification by the ‘c-miRNA’ and ‘clinical’ models. The corresponding Kaplan Meier curves are shown in Figure 1.
Figure 1. Kaplan-Meier estimates of G3+ RICT in low- and high-risk patients as determined by c-miRNA (A), clinical (B), and c-miRNA + clinical (C) models.
G3+ = grade 3 or greater. RICT = radiation-induced cardiac toxicity. HR = hazard ratio.
Because adding c-miRNA data to clinical factors did not result in an improved c-index or IBS, we hypothesized that the c-miRNA signature may to some degree be correlated with PCD. To investigate this, we performed chi-square comparison of the prevalence of PCD in patients designated high- vs. low risk by the ‘c-miRNA’ model (Table 4). This resulted in a p-value of 0.09, indicating a marginally significant association between pre-treatment c-miRNA signature and PCD, and suggesting that this association may partially account for the prognostic ability of the c-miRNA signature.
Table 4.
Chi-square table of PCD in patients with high- vs. low-risk c-miRNA profile.
| PCD | p value | ||
|---|---|---|---|
| Yes | No | ||
| c-miRNA Group 1 | 13 | 19 | 0.09 |
| c-miRNA Group 2 | 6 | 25 | |
PCD = pre-existing cardiac disease.
Discussion
In this work, we identified a pre-treatment c-miRNA signature prognostic for G3+ RICT in patients treated with definitive RT for stages II-III NSCLC. This signature was comprised of 14 cmiRNA species for which variations in serum concentrations correlated with increased or decreased risk of G3+ RICT. A model based on this c-miRNA signature performed as well as a model based on MHD and PCD. Although multivariable analysis indicated that this c-miRNA signature added prognostic value to MHD and PCD, combining these factors into a ‘c-miRNA + clinical’ model did not improve accuracy. Both the ‘c-miRNA’ and ‘clinical’ models were able to significantly stratify patients as low- or high-risk for development of G3+ RICT.
The incidence of G3+ RICT in this study was 16.9%, which is higher than reported in many previous trials. For example, the rates of acute and late G3+ cardiac events among all patients in RTOG 9410 were approximately 2% and 2.5%, respectively (Curran et al. 2011). The relatively high incidence in the present study is likely due to multiple factors. One important consideration is that many of these patients were treated with dose-escalated RT. In other recent analyses of dose-escalated RT for NSCLC, rates of cardiac toxicity have been comparable. For example, in RTOG 0617, the rate of G3+ cardiac toxicity was 24% (Speirs et al. 2017). In a retrospective analysis of dose-escalated RT from the University of North Carolina, the rate of symptomatic cardiac events was 23% (Wang et al. 2017). Multiple studies have shown a correlation between cardiac dose and toxicity (Dess et al. 2017; Wang et al. 2017). Another contributing factor may have been accuracy of toxicity reporting. Some have suggested that cardiac toxicity is commonly under-reported in clinical trials employing thoracic RT (Faivre-Finn 2015; Gaya and Ashford 2005). In this study, thorough review medical records disclosed additional cardiac events beyond those captured during protocol-driven data collection.
The two factors incorporated in our clinical model, MHD and PCD, were selected because they have been previously shown by our group to be highly prognostic for cardiac-related morbidity and mortality following RT for NSCLC (Dess et al. 2017). In this analysis, these factors were again prognostic of G3+ RICT. The observation that combining c-miRNA data with these variables did not improve prognostication suggests that certain of these factors may be correlated with each other. As a correlation between MHD and pre-treatment c-miRNA profile seemed unlikely, we hypothesized that at least some of the c-miRNAs identified may be associated with PCD. Chi-square testing of observed versus expected prevalence of PCD in patients defined as high-risk by c-miRNA profile yielded a marginally significant p-value of 0.09. This result suggests that the mechanism by which some, but not all, of the c-miRNA species prognosticate for G3+ RICT is through their association with PCD.
The hypothesis that a c-miRNA signature could be associated with PCD is supported by previously published studies. There are numerous reports of various miRNA species functioning as biomarkers of cardiac disease, including some investigating the same miRNAs identified in this work. For example, circulating and/or tissue levels of miR-100, −106b, −145, −146, and −223, have been observed to inversely correlate with MI, CAD, CHF, atherosclerosis, type 2 diabetes, obesity, and atrial fibrillation (Chiang et al. 2014; Faccini et al. 2017; Liu et al. 2012; Pek et al. 2016; Ramkaran et al. 2014; Weber et al. 2011; Yang et al. 2011; Zampetaki et al. 2012). In the current study, higher serum levels of these c-miRNAs were associated with lower risk of G3+ RICT, which is consistent with the referenced works. In addition, miR-134, −200b, −574, −858, and let-7c levels have been correlated with ACS, MI, CAD, atherosclerosis, CHF, and cardiomyopathy (Ghosh et al. 2012; He et al. 2014; Ikeda et al. 2007; Lan et al. 2016; Vogel et al. 2013; Wang et al. 2016; Zhou et al. 2015). In this study, higher serum levels of these miRNAs were associated with higher risk of G3+ RICT, which is also consistent with the cited works. However, the relationships of some of the c-miRNAs identified do not coincide with published studies. These include miR-192 and −195, higher levels of which have been correlated with heart failure and MI (Long et al. 2012; Matsumoto et al. 2013; van Rooij et al. 2006). In our study, however, higher levels of these c-miRNAs were associated with lower risk of G3+ RICT. Taken together, these comparisons suggest that the prognostic utility of some of the identified c-miRNA species may be related to their correlation with PCD, but that others may be associated with RICT through different mechanisms. Further study of these miRNA species is needed in order to more fully understand how they may function as biomarkers of RICT or cardiac disease of other etiologies.
Although the c-miRNA model was associated with the lowest cross-validated IBS, it only slightly outperformed the clinical model in this regarded and was associated with a slightly inferior cross-validated c-index. As such, the clinical utility of this c-miRNA signature in its current form is likely limited. However, there are several important implications of this work. For example, many of the miRNA species identified in this study appear to be involved in promoting or protecting against cardiac disease. It should be noted, however, that it is difficult to draw conclusions regarding the biologic function of miRNAs from biomarker studies. For example, in a hypothetical patient with cardiac disease, serum levels of a given miRNA could be elevated either because it is directly contributing to cardiac damage or because its expression has been induced as a compensatory mechanism of attempted repair. Further investigation of these miRNAs may expand our understanding of the roles miRNAs play in cardiac disease of multiple etiologies and uncover targets for therapeutic intervention. In addition, while in this study we investigated pre-treatment c-miRNA data, it is possible that mid-treatment changes in c-miRNA levels may provide additional prognostic information. Cardiac irradiation may elicit miRNA-mediated responses that vary among patients, with up- or down-regulation of various species being associated with lower or higher risk of RICT. These changes may be detectable prior to the corresponding clinical manifestations of RICT. The c-miRNAs identified in this study represent attractive candidates for study as mid-treatment biomarkers of cardiac damage. With further study, a mid-treatment c-miRNA profile could be identified that could guide adaptation of RT and/or inform post-RT optimization of cardiac health.
There are limitations to this study that should be considered. While we performed rigorous cross-validation of parameter selection and model performance, we have not externally validated our findings. The study was also constrained by the limited number of c-miRNA species measured. Samples were analyzed at a time when miRNA profiling technologies were less comprehensive than those currently available. In addition, the miRNAs measured were not selected based on possible roles in cardiac function. As a result, certain miRNAs implicated by others in cardiac disease were not evaluated, such as miRNA-1 (Kura et al. 2016; Viczenczova et al. 2016). Future studies should evaluate broader panels of miRNAs in order to increase chances of identifying useful prognostic factors.
Radiotherapy for locally-advanced NSCLC is complicated by competing goals to maximize disease control while minimizing toxicity. This challenge is particularly evident when selecting target dose and normal-tissue constraints during RT planning. An ideal method to guide this process would employ multiple patient-specific factors, as opposed to parameters derived from population-based studies. To develop an individualized, multiparametric approach, clinical, biological, imaging, and other biomarkers of response and toxicity must be characterized. This work represents significant progress toward this aim. With further improvement, the c-miRNA signature described here could contribute to a powerful model for prediction of RICT. Such a model could, in turn, be utilized in conjunction with additional factors predictive of other outcomes to comprehensively direct personalized treatment planning. In addition, study of mid-treatment changes in these and other biomarkers could allow for treatment adaptation to further individualize RT.
Supplementary Material
Acknowledgments
Funding: This work was supported in part by R0 1CA142840 (Kong) and P01 CA059827 (Ten Haken and Lawrence).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest: The authors declare no conflicts of interest relevant to this work.
Ethics approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Institutional Review Board of the University of Michigan. This article does not contain studies with animals performed by any of the authors.
Informed consent: Informed consent Informed consent was obtained from all individual participants included in the study.
References
- Ambros V (2004) The functions of animal microRNAs Nature 431:350–355 doi: 10.1038/nature02871 [DOI] [PubMed] [Google Scholar]
- Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S et al. (2015) Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study Lancet Oncol 16:187–199 doi: 10.1016/S1470-2045(14)71207-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Xu Y, Liao X, Liao R, Zhang L, Niu K et al. (2016) Plasma miRNAs in predicting radiosensitivity in non-small cell lung cancer Tumour Biol 37:11927–11936 doi: 10.1007/s13277-016-5052-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang DY, Kongchan N, Beavers DL, Alsina KM, Voigt N, Neilson JR et al. (2014) Loss of microRNA-106b-25 cluster promotes atrial fibrillation by enhancing ryanodine receptor type-2 expression and calcium release Circ Arrhythm Electrophysiol 7:1214–1222 doi: 10.1161/CIRCEP.114.001973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho BJ, Kim HH, Lee DJ, Choi EJ, Hwang YH, Chun SH et al. (2014) MicroRNA-21 inhibitor potentiates anti-tumor effect of radiation therapy in vitro and in vivo Tumor Microenvironment and Therapy 2:1–13 [Google Scholar]
- Chun SG, Hu C, Choy H, Komaki RU, Timmerman RD, Schild SE et al. (2017) Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial J Clin Oncol 35:56–62 doi: 10.1200/JCO.2016.69.1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran WJ Jr., Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S et al. (2011) Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410 J Natl Cancer Inst 103:1452–1460 doi: 10.1093/jnci/djr325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dess RT, Sun Y, Matuszak MM, Sun G, Soni PD, Bazzi L et al. (2017) Cardiac Events After Radiation Therapy: Combined Analysis of Prospective Multicenter Trials for Locally Advanced Non-Small-Cell Lung Cancer J Clin Oncol 35:1395–1402 doi: 10.1200/JCO.2016.71.6142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquela-Kerscher A, Slack FJ (2006) Oncomirs - microRNAs with a role in cancer Nat Rev Cancer 6:259–269 doi: 10.1038/nrc1840 [DOI] [PubMed] [Google Scholar]
- Faccini J, Ruidavets JB, Cordelier P, Martins F, Maoret JJ, Bongard V et al. (2017) Circulating miR-155, miR-145 and let-7c as diagnostic biomarkers of the coronary artery disease Sci Rep 7:42916 doi: 10.1038/srep42916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre-Finn C (2015) Dose escalation in lung cancer: have we gone full circle? Lancet Oncol 16:125–127 doi: 10.1016/S1470-2045(15)70001-X [DOI] [PubMed] [Google Scholar]
- Fowler JF (1989) The linear-quadratic formula and progress in fractionated radiotherapy Br J Radiol 62:679–694 doi: 10.1259/0007-1285-62-740-679 [DOI] [PubMed] [Google Scholar]
- Gaya AM, Ashford RF (2005) Cardiac complications of radiation therapy Clin Oncol (R Coll Radiol) 17:153–159 [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Nagpal V, Covington JW, Michaels MA, Vaughan DE (2012) Molecular basis of cardiac endothelial-to-mesenchymal transition (EndMT): differential expression of microRNAs during EndMT Cell Signal 24:1031–1036 doi: 10.1016/j.cellsig.2011.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf E, Schmoor C, Sauerbrei W, Schumacher M (1999) Assessment and comparison of prognostic classification schemes for survival data Stat Med 18:2529–2545 [DOI] [PubMed] [Google Scholar]
- Hall EJ, Giaccia AJ (2006) Radiobiology for the Radiologist vol 6 Lippincott Williams & Wilkins; Philadelphia:, [Google Scholar]
- He F, Lv P, Zhao X, Wang X, Ma X, Meng W et al. (2014) Predictive value of circulating miR-328 and miR-134 for acute myocardial infarction Mol Cell Biochem 394:137–144 doi: 10.1007/s11010-014-2089-0 [DOI] [PubMed] [Google Scholar]
- Heagerty PJ, Zheng Y (2005) Survival model predictive accuracy and ROC curves Biometrics 61:92–105 [DOI] [PubMed] [Google Scholar]
- Ikeda S, Kong SW, Lu J, Bisping E, Zhang H, Allen PD et al. (2007) Altered microRNA expression in human heart disease Physiol Genomics 31:367–373 doi: 10.1152/physiolgenomics.00144.2007 [DOI] [PubMed] [Google Scholar]
- Jiang LP, He CY, Zhu ZT (2017) Role of microRNA-21 in radiosensitivity in non-small cell lung cancer cells by targeting PDCD4 gene Oncotarget 8:23675–23689 doi: 10.18632/oncotarget.15644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong FM, Ten Haken RK, Schipper MJ, Sullivan MA, Chen M, Lopez C et al. (2005) High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study Int J Radiat Oncol Biol Phys 63:324–333 doi: 10.1016/j.ijrobp.2005.02.010 [DOI] [PubMed] [Google Scholar]
- Kong YW, Ferland-McCollough D, Jackson TJ, Bushell M (2012) microRNAs in cancer management Lancet Oncol 13:e249–258 doi: 10.1016/S1470-2045(12)70073-6 [DOI] [PubMed] [Google Scholar]
- Koo T, Cho BJ, Kim DH, Park JM, Choi EJ, Kim HH et al. (2017) MicroRNA-200c increases radiosensitivity of human cancer cells with activated EGFR-associated signaling Oncotarget 8:65457–65468 doi: 10.18632/oncotarget.18924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kura B, Yin C, Frimmel K, Krizak J, Okruhlicova L, Kukreja RC et al. (2016) Changes of microRNA-1, −15b and −21 levels in irradiated rat hearts after treatment with potentially radioprotective drugs Physiol Res 65 Suppl 1:S129–137 [DOI] [PubMed] [Google Scholar]
- Lan F, Yue X, Ren G, Li H, Ping L, Wang Y et al. (2015) miR-15a/16 enhances radiation sensitivity of non-small cell lung cancer cells by targeting the TLR1/NF-kappaB signaling pathway Int J Radiat Oncol Biol Phys 91:73–81 doi: 10.1016/j.ijrobp.2014.09.021 [DOI] [PubMed] [Google Scholar]
- Lan G, Xie W, Li L, Zhang M, Liu D, Tan YL et al. (2016) MicroRNA-134 actives lipoprotein lipase-mediated lipid accumulation and inflammatory response by targeting angiopoietin-like 4 in THP-1 macrophages Biochem Biophys Res Commun 472:410–417 doi: 10.1016/j.bbrc.2015.10.158 [DOI] [PubMed] [Google Scholar]
- Liu Z, Yang D, Xie P, Ren G, Sun G, Zeng X et al. (2012) MiR-106b and MiR-15b modulate apoptosis and angiogenesis in myocardial infarction Cell Physiol Biochem 29:851–862 doi: 10.1159/000258197 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method Methods 25:402–408 doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Long G, Wang F, Duan Q, Yang S, Chen F, Gong W et al. (2012) Circulating miR-30a, miR-195 and let-7b associated with acute myocardial infarction PLoS One 7:e50926 doi: 10.1371/journal.pone.0050926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Sakata Y, Suna S, Nakatani D, Usami M, Hara M et al. (2013) Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction Circ Res 113:322–326 doi: 10.1161/CIRCRESAHA.113.301209 [DOI] [PubMed] [Google Scholar]
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL et al. (2008) Circulating microRNAs as stable blood-based markers for cancer detection Proc Natl Acad Sci U S A 105:10513–10518 doi: 10.1073/pnas.0804549105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pek SL, Sum CF, Lin MX, Cheng AK, Wong MT, Lim SC et al. (2016) Circulating and visceral adipose miR-100 is down-regulated in patients with obesity and Type 2 diabetes Mol Cell Endocrinol 427:112–123 doi: 10.1016/j.mce.2016.03.010 [DOI] [PubMed] [Google Scholar]
- Philippidou D, Schmitt M, Moser D, Margue C, Nazarov PV, Muller A et al. (2010) Signatures of microRNAs and selected microRNA target genes in human melanoma Cancer Res 70:4163–4173 doi: 10.1158/0008-5472.CAN-09-4512 [DOI] [PubMed] [Google Scholar]
- Quiat D, Olson EN (2013) MicroRNAs in cardiovascular disease: from pathogenesis to prevention and treatment J Clin Invest 123:11–18 doi: 10.1172/JCI62876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramkaran P, Khan S, Phulukdaree A, Moodley D, Chuturgoon AA (2014) miR-146a polymorphism influences levels of miR-146a, IRAK-1, and TRAF-6 in young patients with coronary artery disease Cell Biochem Biophys 68:259–266 doi: 10.1007/s12013-013-9704-7 [DOI] [PubMed] [Google Scholar]
- Rufibach K (2010) Use of Brier score to assess binary predictions J Clin Epidemiol 63:938–939; author reply 939 doi: 10.1016/j.jclinepi.2009.11.009 [DOI] [PubMed] [Google Scholar]
- Ryu MS, Langkamp-Henken B, Chang SM, Shankar MN, Cousins RJ (2011) Genomic analysis, cytokine expression, and microRNA profiling reveal biomarkers of human dietary zinc depletion and homeostasis Proc Natl Acad Sci U S A 108:20970–20975 doi: 10.1073/pnas.1117207108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M, Graf E, Gerds T (2003) How to assess prognostic models for survival data: a case study in oncology Methods Inf Med 42:564–571 [PubMed] [Google Scholar]
- Speirs CK, DeWees TA, Rehman S, Molotievschi A, Velez MA, Mullen D et al. (2017) Heart Dose Is an Independent Dosimetric Predictor of Overall Survival in Locally Advanced Non-Small Cell Lung Cancer J Thorac Oncol 12:293–301 doi: 10.1016/j.jtho.2016.09.134 [DOI] [PubMed] [Google Scholar]
- Sun Y, Hawkins PG, Bi N, Dess RT, Tewari M, Hearn JWD et al. (2017) Serum MicroRNA Signature Predicts Response to High-Dose Radiation Therapy in Locally Advanced Non-Small Cell Lung Cancer Int J Radiat Oncol Biol Phys doi: 10.1016/j.ijrobp.2017.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells Nat Cell Biol 9:654–659 doi: 10.1038/ncb1596 [DOI] [PubMed] [Google Scholar]
- van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD et al. (2006) A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure Proc Natl Acad Sci U S A 103:18255–18260 doi: 10.1073/pnas.0608791103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viczenczova C, Szeiffova Bacova B, Egan Benova T, Kura B, Yin C, Weismann P et al. (2016) Myocardial connexin-43 and PKC signalling are involved in adaptation of the heart to irradiation-induced injury: Implication of miR-1 and miR-21 Gen Physiol Biophys 35:215–222 doi: 10.4149/gpb_2015038 [DOI] [PubMed] [Google Scholar]
- Vogel B, Keller A, Frese KS, Leidinger P, Sedaghat-Hamedani F, Kayvanpour E et al. (2013) Multivariate miRNA signatures as biomarkers for non-ischaemic systolic heart failure Eur Heart J 34:2812–2822 doi: 10.1093/eurheartj/eht256 [DOI] [PubMed] [Google Scholar]
- Wang K, Eblan MJ, Deal AM, Lipner M, Zagar TM, Wang Y et al. (2017) Cardiac Toxicity After Radiotherapy for Stage III Non-Small-Cell Lung Cancer: Pooled Analysis of Dose-Escalation Trials Delivering 70 to 90 Gy J Clin Oncol 35:1387–1394 doi: 10.1200/JCO.2016.70.0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KJ, Zhao X, Liu YZ, Zeng QT, Mao XB, Li SN et al. (2016) Circulating MiR-19b-3p, MiR-134–5p and MiR-186–5p are Promising Novel Biomarkers for Early Diagnosis of Acute Myocardial Infarction Cell Physiol Biochem 38:1015–1029 doi: 10.1159/000443053 [DOI] [PubMed] [Google Scholar]
- Weber M, Baker MB, Patel RS, Quyyumi AA, Bao G, Searles CD (2011) MicroRNA Expression Profile in CAD Patients and the Impact of ACEI/ARB Cardiol Res Pract 2011:532915 doi: 10.4061/2011/532915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidhaas JB, Babar I, Nallur SM, Trang P, Roush S, Boehm M et al. (2007) MicroRNAs as potential agents to alter resistance to cytotoxic anticancer therapy Cancer Res 67:11111–11116 doi: 10.1158/0008-5472.CAN-07-2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, He YS, Wang XQ, Lu L, Chen QJ, Liu J et al. (2011) MiR-146a inhibits oxidized low-density lipoprotein-induced lipid accumulation and inflammatory response via targeting toll-like receptor 4 FEBS Lett 585:854–860 doi: 10.1016/j.febslet.2011.02.009 [DOI] [PubMed] [Google Scholar]
- Yuan T, Huang X, Woodcock M, Du M, Dittmar R, Wang Y et al. (2016) Plasma extracellular RNA profiles in healthy and cancer patients Sci Rep 6:19413 doi: 10.1038/srep19413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampetaki A, Willeit P, Tilling L, Drozdov I, Prokopi M, Renard JM et al. (2012) Prospective study on circulating MicroRNAs and risk of myocardial infarction J Am Coll Cardiol 60:290–299 doi: 10.1016/j.jacc.2012.03.056 [DOI] [PubMed] [Google Scholar]
- Zhai G, Li G, Xu B, Jia T, Sun Y, Zheng J et al. (2016) miRNA-148b regulates radioresistance in non-small lung cancer cells via regulation of MutL homologue 1 Biosci Rep 36 doi: 10.1042/BSR20150300 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhen L, Li J, Zhang M, Yang K (2016) MiR-10b decreases sensitivity of glioblastoma cells to radiation by targeting AKT J Biol Res (Thessalon) 23:14 doi: 10.1186/s40709-016-0051-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Shao G, Chen X, Yang X, Huang X, Peng P et al. (2015) miRNA 206 and miRNA 574–5p are highly expression in coronary artery disease Biosci Rep 36:e00295 doi: 10.1042/BSR20150206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Hastie T (2005) Regularization and variable selection via the elastic net Journal of the Royal Statistical Society: Series B (Statistical Methodology) 67:301–320 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.