Abstract
The pathophysiology of pulmonary hypertension (PH) and heart failure (HF) includes fibrogenic remodeling associated with loss of pulmonary arterial (PA) and cardiac compliance. We and others have previously identified that transglutaminase 2 (TG2) as a participant in adverse fibrogenic remodeling. However, little is known about the biologic mechanisms that regulate TG2 function. We examined physiological mouse models of experimental PH, HF and type 1 diabetes that are associated with altered glucose metabolism/ glycolysis and report here that TG2 expression and activity are elevated in pulmonary and cardiac tissues under all these conditions. We additionally used PA adventitial fibroblasts to test the hypothesis that TG2 is an intermediary between enhanced tissue glycolysis and fibrogenesis. Our in vitro results show that glycolytic enzymes and TG2 are up-regulated in fibroblasts exposed to high glucose, which stimulates cellular glycolysis as measured by Seahorse analysis. We examined the relationship of TG2 to a terminal glycolytic enzyme, pyruvate kinase M2 (PKM2), and found that PKM2 regulates glucose -induced TG2 expression and activity as well as fibrogenesis. Our studies further show that TG2 inhibition blocks glucose-induced fibrogenesis and cell proliferation. Our findings support a novel role for glycolysis -mediated TG2 induction and tissue fibrosis associated with experimental PH, HF and hyperglycemia.
Keywords: glycolysis, hyperglycemia, PKM2, TG2, tissue fibrosis
Introduction
Pulmonary hypertension (PH) and cardiac diseases such as aortic stenosis and those associated with diabetes mellitus causing diastolic dysfunction and heart failure (HF) are major causes of morbidity and mortality for millions of individuals worldwide. These diseases are associated with tissue fibrosis and loss of compliance (1–4). The pathogenesis and treatment of the tissue fibrosis remain largely unexplored. Recent studies indicate an increasing prevalence of PH and HF in patients in association with dysfunctional metabolic pathways (3, 4).
We recently reported a significant role for transglutaminase 2 (TG2) in the development of increased collagen synthesis and fibrosis in the lungs and right ventricle (RV) of the heart of a mouse model of experimental PH produced by chronic exposure to hypoxia following Sugen 5416 injection (SuHypoxia) (5). In this context, a previous publication of ours also supported the ability of chronic hypoxia alone to stimulate TG2 activity and expression in lungs (6) and PA smooth muscle cells (7). Similarly, Skill et al reported that high glucose concentrations in cell culture media stimulate TG2 expression and activity in renal epithelial cells (8). Because of an established role for stimulation of anaerobic glycolysis by exposure to hypoxia (9) we pursued other physiological experiments where glycolysis is enhanced to determine if glycolysis itself may be stimulatory for TG2 and fibrotic alterations of tissues. One such experiment is transverse aortic constriction (TAC) where chronic pressure overload enhances glycolytic activity (10–12) and fibrosis (13, 14) of the left ventricle (LV). We attempted to identify the influence of tissue pressure overload on TG2 expression and activity in LVs of the TAC experimental mouse model. Likewise, we also examined the in vivo influence of hyperglycemia on TG2 expression and activity in type 1 diabetic Akita mice with elevated blood glucose levels (15).
Glycolysis is stimulated by hypoxia and is also up-regulated in the presence of oxygen as in tumor biology where it has been referred to as the Warburg effect (aerobic glycolysis) (16). Glycolysis is a multiple step metabolic process in response to glucose uptake (17, 18) in which enzymatically catalyzed steps convert glucose to pyruvate molecules for cellular energy generation. In fact, the glycolytic pathway has recently been shown to be associated with the fibrogenic phenotype (19, 20). In the current study, we investigated the effects of hyperglycemia and aerobic glycolysis on TG2 and fibroblast cell signaling and function. Since the terminal glycolytic enzyme pyruvate kinase muscle isoform 2 (PKM2) has been reported to mediate metabolic and inflammatory dysfunction in cardiovascular disease (21), we specifically selected this enzyme for some of our studies. PKM2 has been previously shown to interact with TG2 under stressful cellular conditions (22). Additionally, PKM2 is a multifunctional protein (23, 24) and has received increasing attention as a regulatory target in metabolic reprogramming toward glycolysis in cancer biology (25, 26), tissue fibrosis (20) and the pathogenesis of PH and HF (19, 21, 27). However, downstream signal transduction pathways of glycolysis-mediated effects are not well-defined.
Our goal here was to first utilize experimental physiologic conditions of enhanced glycolysis to determine influences on tissue TG2. For these in vivo studies we chose: 1) hypoxia exposure that produces pulmonary hypertension (28); 2) pressure overload on the LV by TAC (14); and 3) hyperglycemia in the diabetic Akita mouse (15). With cellular studies we report associations among hyperglycemia, glycolysis, TG2 and tissue fibrosis. We show that the terminal glycolytic enzyme PKM2 has a regulatory function on TG2 of PA adventitial fibroblasts under hyperglycemia and glycolytic conditions resulting in myofibroblast transdifferentiation, synthesis of type 1 collagen, pro-fibrogenic signaling and fibroblast cell proliferation.
Materials and Methods
Reagents:
For in vitro studies, a pyruvate kinase isoform M2 inhibitor, Shikonin, was purchased from MilliporeSigma (Cat# S7576) and was dissolved in dimethyl sulfoxide (DMSO; MilliporeSigma). For in vivo studies, a small molecule and selective inhibitor of TG2 activity, ERW1041E was provided by Dr. Chaitan Khosla (Stanford University). For in vitro studies, ERW1041E was purchased from MilliporeSigma (Cat# 5095220001). The ERW1041E was dissolved in DMSO.
Animal models:
C57BL/6 wild-type (WT; Charles River Laboratories, Wilmington, MA) and type 1 diabetic heterozygous Akita (Ins2Akita/+ on C57BL/6j background; 6.4±1.8 weeks of age; The Jackson Laboratory, Bar Harbor, ME) mice were used for the study in accordance with a Tufts University Institutional Animal Care and Use Committee approved protocol. We used male mice for minimization of any variables in the studies. For induction of experimental pulmonary hypertension (PH), adult WT mice (6–8 weeks of age) were placed in hypoxic (10.5% O2) chambers (Biospherix, Parish, NY) for 3 weeks and administered 200 μL subcutaneous weekly injections of 20 mg/kg of Sugen 5416 (Tocris Bioscience, Minneapolis, MN) that enhances physiologic responses to hypoxia (29) and compared with similar mice placed in normoxia (room air) as previously reported (28). Mice were injected daily with vehicle (DMSO) or TG2 inhibitor ERW1041E as described (28). We have previously identified that ERW1041E is effective in inhibiting TG2 under these conditions (28). Cardiac (left and right ventricles) and lung tissue were also obtained from both WT and Akita mice. For a cardiac LV pressure overload model, LV tissues were isolated from adult male WT mice subjected to minimally invasive TAC surgery for 10 weeks and compared to Sham controls subjected to similar surgical procedures without aortic constriction as previously reported (14).
Cell culture:
Human pulmonary arterial (PA) adventitial fibroblasts used in these studies were purchased from a commercial supplier (Cat# 3120; ScienCell Research Laboratories, Carlsbad, CA). Cells were cultured in fibroblast medium supplemented with growth factors, fetal bovine serum and antibiotics (Cat# 2301; ScienCell Research Laboratories). Cell passages 2 – 5 were used and cellular purity was assessed by morphological appearance as previously described (5).
Cell treatment:
After reaching 70% confluence, fibroblasts were starved overnight with DMEM glucose-free media (Cat# 11966025; Thermo Fischer Scientific) supplemented with reduced (0.2%) fetal bovine serum (FBS; Atlanta Biologicals, GA) followed by culturing in normal glucose (5 mM) DMEM (Cat# 10567014; Thermo Fischer Scientific) media. Cells were pretreated for 1 hour with vehicle (DMSO), Shikonin or ERW1041E and then exposed to high glucose concentrations (Cat# A2494001; Thermo Fischer Scientific). Each treatment was replicated in at least 3 separate cell culture dishes.
Real-time glycolysis assay:
Fibroblasts were incubated with glucose-free Seahorse XF base medium without phenol-red and supplemented with 2 mM glutamine, 1 mM pyruvate and 5 mM HEPES (pH 7.4) at 37oC in a non-CO2 chamber for 1 hour prior to glycolytic Rate Assay (Cat# 103344–100; Seahorse XF glycolytic rate assay kit; Agilent Technologies, Billerica, MA). Quantitative real-time measurements of glycolytic rate and glycolytic capacity (extracellular acidification rate; ECAR) were assessed for fibroblasts using a Seahorse XFe96 Analyzer (Seahorse Core Facility, Tufts University, Boston, MA) according to manufacturer’s recommendations.
siRNA transfection:
Fibroblasts were grown to 70% confluence and transfected with 20 nM final concentration of PKM2 siRNA (Sense: 5’-CCAUAAUCGUCCUCACCAA dTdT-3’ and Antisense: 5’- UUGGUGAGGACGAUUAUGG dTdT-3’; Dharmacon, Lafayette, CO) and control siRNA (Thermo Fisher Scientific) using Lipofectamine 2000 reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. Fibroblasts were transfected with siRNA for 6 hours in growth media without antibiotics and then incubated overnight in normal glucose (5 mM) medium supplemented with reduced serum (0.2% FBS). Transfected cells were then exposed to 5 or 50 mM concentrations of glucose for 72 hours at 370C. Each siRNA transfection was replicated in at least 3 separate cell culture dishes.
RNA isolation and quantitative real-time polymerase chain reaction analysis:
After the experimental end points, total RNA was extracted using Trizol (Invitrogen) according to the manufacturer’s instructions. Total RNA was reverse transcribed using a High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Quantitative real-time polymerase chain reaction (RT-PCR) analyses were carried out using the SYBR Green Master Mix (Thermo Fisher Scientific) method on an ABI Prism 7900 Sequence Detection System (Thermo Fisher Scientific). Mouse and human primers (Table 1) were purchased from IDT Technologies (Coralville, IA). Samples were quantified in triplicates and the cycle threshold (Ct) values were normalized using 18S ribosomal RNA and analyzed as previously described (7).
Table 1.
RT-PCR primer sequence list.
| Target | Species | Forward primer | Reverse primer |
|---|---|---|---|
| 18s rRNA | Mouse | GGACAGGACTAGGCGGAACA | AGGGGAGAGCGGGTAAGAGA |
| HK2 | Mouse | TGGGTTTCACCTTCTCGTTC | TTCACCAGGATGAGTCTGAC |
| PFKFB3 | Mouse | GGAGGCTGTGAAGCAGTACA | CAGCTAAGGCACATTGCTTC |
| 18s rRNA | Human | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
| α-SMA | Human | CAAAGCCGGCCTTACAGAG | AGCCCAGCCAAGCACTG |
| TGFβ1 | Human | CGAGCCTGAGGCCGACTAC | TCGGAGCTCTGATGTGTTGAA |
| Col1 | Human | GTCGAGGGCCAAGACGAAG | CAGATCACGTCATCGCACAAC |
| Fn | Human | AAACCAATTCTTGGAGCAGG | CCATAAAGGGCAACCAAGAG |
18s rRNA, 18s ribosomal RNA; HK2, hexokinase 2; PFKFB3, 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase isoform 3; α–SMA, α–smooth muscle actin; TGFβ1, transforming growth factor β1; Col1, type 1 collagen; and Fn, Fibronectin.
Protein extraction and Western blot analysis:
After the experimental end points, tissues were homogenized and cells were lysed in NP-40 lysis buffer (Boston Bio Products, Ashland, MA) containing protease and phosphatase inhibitor cocktails (MilliporeSigma). Tissue and cell lysates were centrifuged, and the supernatants were quantified for protein content using a Bradford Assay (Bio-Rad Laboratories). Samples were then reduced in SDS sample buffer (Boston Bio Products) and processed for SDS-PAGE analysis as described previously (30). Immunoblotting was performed using antibodies against target protein transglutaminase 2 (TG2; Cat# sc-48387; Santa Cruz Biotechnology); serotonin (Cat# S5545, Sigma); pyruvate kinase M2 (PKM2; Cat# 3198; Cell Signaling Technology); hexokinase 2 (HK2; Cat# ab131196; Abcam, Cambridge, MA); 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase isoform 3 (PFKFB3; Cat# ab181861; Abcam); fibronectin (Fn; Cat# sc-8422, Santa Cruz Biotechnology); type 1 collagen (Col1; Cat# ab34710, Abcam,); α-smooth muscle actin (α-SMA; Cat# sc-32251; Santa Cruz Biotechnology) and β-actin (Cat# 4970, Cell Signaling Technology, Danvers, MA). Horseradish peroxidase (HRP) tagged secondary antibodies (Santa Cruz Biotechnology) were used for primary antibody target detection. ECL substrate (Thermo Fisher Scientific) was used to visualize the protein bands. Densitometry analysis was performed using Un-Scan-It gel analysis software (Silk Scientific, Orem, UT) as described previously (30).
Cell growth analysis:
Fibroblast cell growth was measured in 96-well plates using the CellTiter-Glo Luminescent Cell Viability Assay (Cat# G7570; Promega, Madison, WI) that detects metabolic activity based on quantitation of the amount of ATP present. Luminescent signal was recorded by a Luminometer (MTX Lab Systems, Bradenton, FL).
Statistical analysis:
Data are expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed using SigmaPlot 12.5 software (Systat Software, San Jose, CA). Unpaired 2-tailed Student’s t-test was performed for comparison between two groups. One-way ANOVA was performed for comparisons among multiple groups. A P value of < 0.05 was considered statistically significant.
Results
Up-regulation of glycolytic markers in the chronic SuHypoxia mouse model.
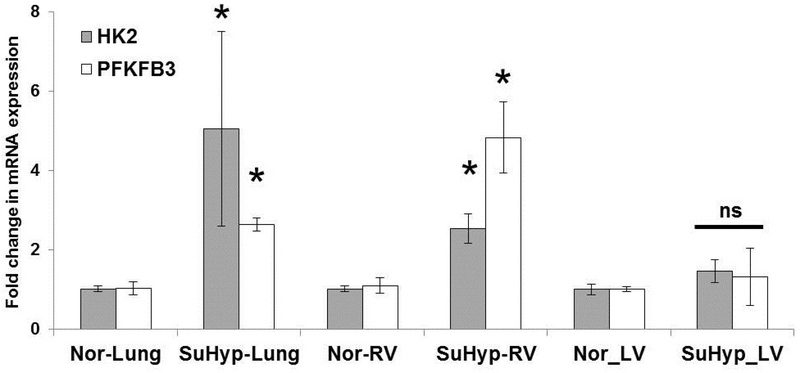
We analyzed lung and cardiac tissues to assess whether glycolysis-related changes occur in mice exposed to SuHypoxia. In addition to previously published findings of SuHypoxia –induced PH and expression of TG2 and fibrogenic markers, (5, 28), we now report that mRNA expression of rate-limiting glycolytic enzymes hexokinase 2 (HK2) and 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) are increased in lungs and RVs of 3-week SuHypoxia mice compared to room air controls (Figure 1). Of note was the lack of a statistical difference between SuHypoxia -exposed mouse LVs compared to normoxia control mouse LVs as compared to RVs and lungs (Figure 1), suggesting that glycolysis, perhaps induced by pressure alterations, rather than hypoxia-itself, may be the driving factor for these tissue-specific differences noted in the RV and lungs as compared to the LVs.
Figure 1. Glycolytic enzymes are elevated in a SuHypoxia mouse model of experimental PH.
Wild-type mice were exposed to 3-week normoxia (room air) or Sugen+Hypoxia (10.5% O2; SuHypoxia). mRNA expression levels as assessed by real-time RT-PCR analysis demonstrate the effects on hexokinase 2 (HK2) and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase isoform 3 (PFKFB3) levels in mouse lung, right ventricular and left ventricular tissues. Bar graph demonstrating fold change in mRNA expression normalized to 18s. n=3–4 mice/ group; mean±SEM. Statistical analysis was performed by t-test. *p<0.05 compared to normoxic control; ns = not significant.
Glycolytic enzyme regulation is not mediated by TG2 activity.
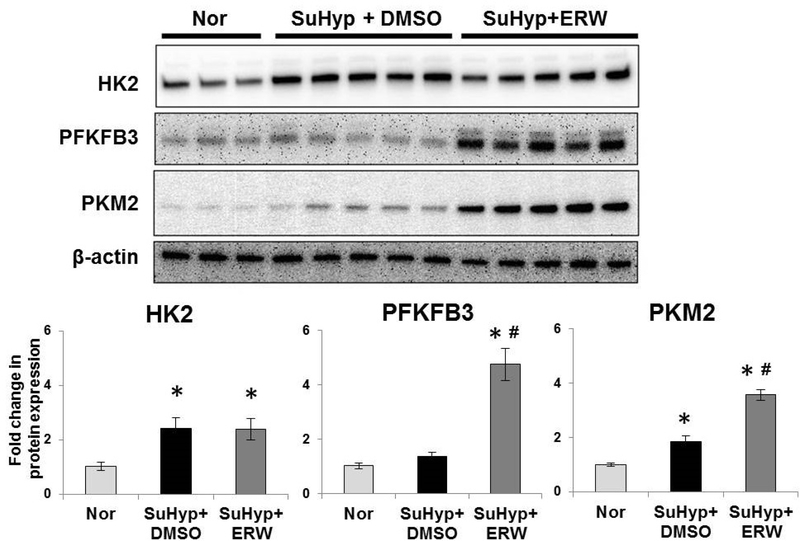
Prior studies from our lab found that pharmacological inhibition of TG2 activity blocked fibrogenic signaling in the SuHypoxia mouse model (5). To further examine a role for glycolysis and TG2, we compared the expression of key glycolytic enzymes in this mouse model. Similar to our previous reports of TG2 induction (5, 6, 28), we observed that exposure to SuHypoxia for 3-weeks significantly induced glycolytic enzymes HK2 and PKM2 in mouse lung tissues compared to normoxia controls as determined by Western blot analysis (Figure 2). In contrast to the mRNA data (Figure 1), protein expression of the rate-limiting glycolytic enzyme PFKFB3 was not significantly affected after 3-weeks of SuHypoxia exposure (Figure 2). In determining the relationship between SuHypoxia-induced TG2 activity and glycolytic enzyme expression, we observed that ERW1041E treatment that inhibited TG2 activity (5, 28) did not block the expression of glycolytic enzymes HK2, PFKFB3 and PKM2 (Figure 2), indicating that they are not a downstream target of TG2 activity. Rather, ERW1041E treatment significantly induced the expression of PFKFB3 and PKM2 (Figure 2), suggesting that TG2 inhibition is associated with increased glycolysis.
Figure 2. Transglutaminase 2 inhibition further increased the elevated glycolytic enzyme expression in SuHypoxia mouse lung tissues.
Wild-type mice were exposed to 3-week normoxia (room air; Nor) or Sugen+Hypoxia (10.5% O2; SuHyp). SuHyp mice were either treated with vehicle control (DMSO) or small molecule transglutaminase 2 (TG2) activity inhibitor, ERW1041E (ERW), as noted in Methods. Western blots of lung tissue protein extracts showing hexokinase 2 (HK2; 102 kDa), 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase isoform 3 (PFKFB3; 60 kDa), pyruvate kinase muscle isoform 2 (PKM2; 58 kDa) and β-actin (42 kDa) expression. Each lane corresponds to protein extracts from an individual mouse. Bar graph demonstrating fold change in HK2, PFKFB3 and PKM2 expression normalized to β-actin by densitometry analysis. n=3–5 mice/ group; mean±SEM. Statistical analysis was performed by One Way ANOVA Tukey post-hoc test. p<0.05 *compared to control normoxia; #compared to SuHyp+DMSO.
Up-regulation of PKM2 and TG2 in LVs of a chronic pressure overloaded mouse model produced by transverse aortic constriction.
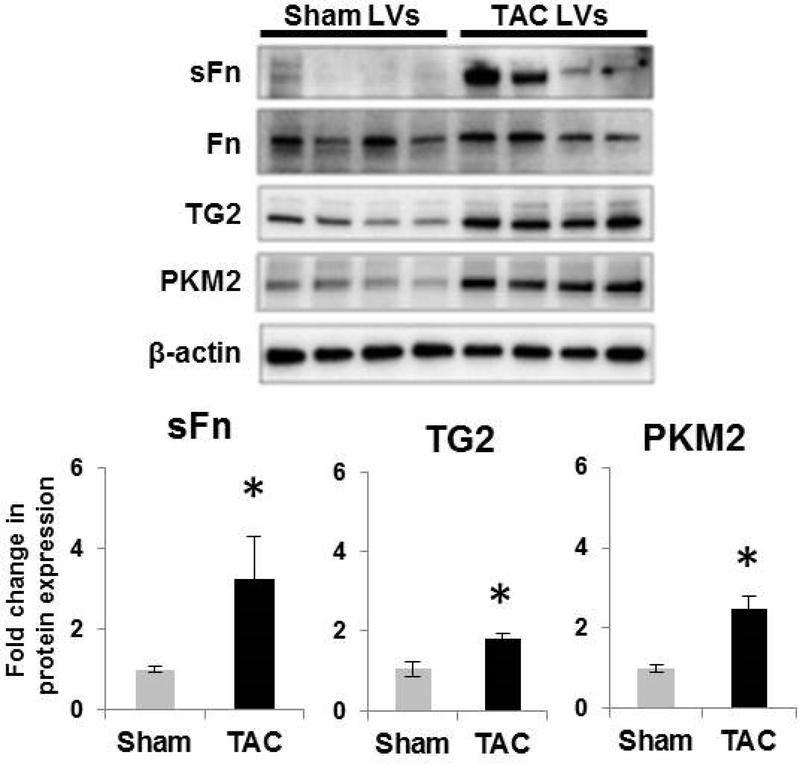
Since glycolysis (shown in Figure 1) and TG2 (5) are elevated in response to tissue-specific anaerobic glycolysis rather than the hypoxic environment itself, we examined the protein expression levels of glycolytic enzyme and TG2 in an aerobic glycolysis experimental mouse model of TAC where it is well known that elevated ventricular wall pressure is an inciting agent for glycolysis. LV fibrosis and cardiac dysfunction have been shown to occur in response to TAC-induced chronic pressure overload in these mice (14). In this report, we now additionally show that the glycolytic enzyme that catalyzes the final step in glycolysis, i.e. PKM2, and TG2 activity (serotonylation of fibronectin; sFn) and expression are significantly elevated in LVs of mice after 10-weeks of TAC surgery compared to sham-operated controls (Figure 3). These findings are consistent with a role for PKM2 (27) and TG2 (31, 32) induction in a pressure overload model of myocardial fibrosis.
Figure 3. Transglutaminase 2 activity and expression and PKM2 expression are increased in left ventricular tissue of a chronic pressure overload mouse model.
Wild-type mice were subjected to transverse aortic surgery (TAC) or sham surgery without aortic constriction (Sham). Western blot images of left ventricular (LV) protein extracts showing the effect of 10-weeks of TAC-induced chronic pressure overload compared to Sham control on serotonylated fibronectin (sFn; 220 kDa), fibronectin (Fn; 220 kDa) transglutaminase 2 (TG2; 78 kDa), pyruvate kinase muscle isoform 2 (PKM2; 58 kDa) and β-actin (45 kDa). Each lane corresponds to protein extracts from an individual mouse. Bar graph demonstrating fold change increase in TG2 activity as measured by sFn expression normalized to total Fn; TG2 and PKM2 expression normalized to β-actin by densitometry analysis. n=4 mice /group; mean±SEM. Statistical analysis was performed by t-test. p<0.05 *compared to sham surgery.
Up-regulation of PKM2 and TG2 expression in a hyperglycemic mouse model.
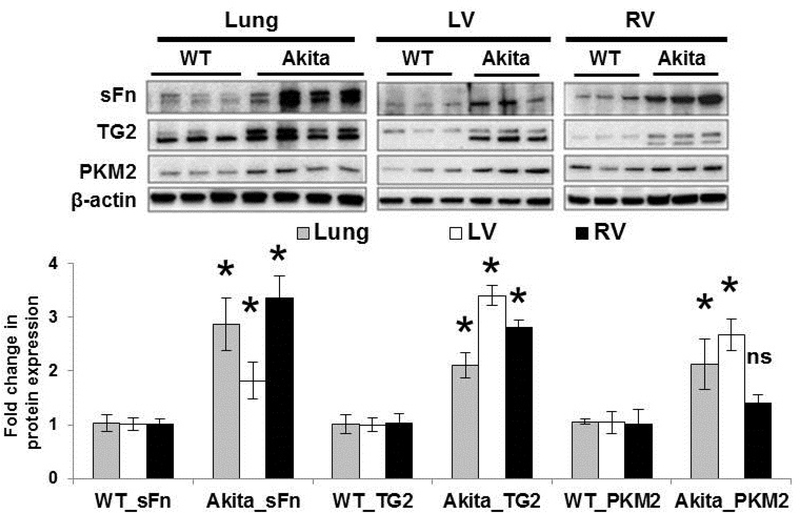
Previous studies reported that Akita mice spontaneously develop diabetes associated with significantly increased blood glucose levels (15). To further examine the role of aberrant glucose metabolism and TG2 induction, we analyzed the terminal glycolytic enzyme PKM2 expression and TG2 activity and expression levels in this chronic type 1 diabetic mouse model with hyperglycemia. We found significantly elevated PKM2 expression levels in lung and LV tissues while the increase in PKM2 expression in RVs was only minimal in the hyperglycemic Akita mice compared to age-matched WT mice. In addition, we found a significant increase in TG2 activity and expression in lung and cardiac left and right ventricular tissues of Akita mice compared to WT mice (Figure 4), indicating an association of hyperglycemia with TG2 induction.
Figure 4. Transglutaminase 2 activity and expression are increased in lung and cardiac tissues of a hyperglycemic mouse model.
Wild-type (WT) and Akita mouse lung and cardiac tissues were compared. Representative Western blots of lung and cardiac left (LV) and right (RV) ventricle protein extracts showing serotonylated fibronectin (sFn; 220 kDa), transglutaminase 2 (TG2; 78 kDa), pyruvate kinase muscle isoform 2 (PKM2; 58 kDa) and β-actin (45 kDa) levels. Each lane corresponds to protein extracts from an individual mouse. Bar graph demonstrating fold change increase in TG2 activity as measured by sFn expression normalized to total Fn; TG2 and PKM2 expression normalized to β-actin by densitometry analysis. n=3–4 mice/ group; mean±SEM. Statistical analysis was performed by t-test. p<0.05 *compared to WT mice; ns = not significant.
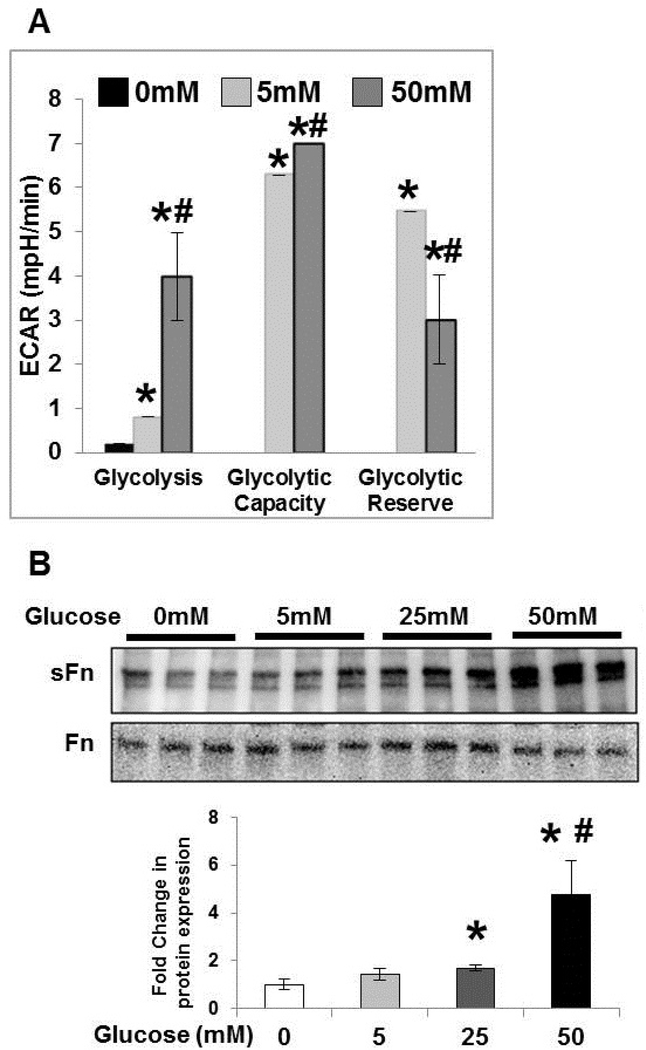
High glucose induces aerobic glycolysis and TG2 activity in fibroblasts.
To determine the effects of glucose exposure on glycolysis and TG2 activity in vitro, PA adventitial fibroblasts were cultured overnight in glucose-free media (reduced serum; 0.2% FBS), followed by stimulation with normal (5 mM) and high (25 and 50mM) glucose concentrations for 1 hour. We analyzed cellular bioenergetics using extracellular acidification rate (ECAR) for measuring real-time aerobic glycolysis with a Seahorse XFe96 analyzer. Similar to a previous report (18), increasing concentrations of glucose in media significantly induced the glycolysis rate and glycolytic capacity of these cells (Figure 5A). Inhibiting glycolysis did not affect normal glucose and high glucose induced ECAR, indicating no change in glycolytic reserve (Figure 5A). In addition, similar to our previous studies with hypoxic exposure (5), Western blot analysis showed that serotonylated fibronectin, a measure of TG2 activity, was significantly increased in response to glucose in a concentration-dependent manner (Figure 5B).
Figure 5. High glucose induces glycolysis and transglutaminase 2 crosslinking activity.
Pulmonary artery adventitial fibroblasts were starved overnight in glucose-free media followed by (A) live-cell Seahorse analysis showing the concentration-dependent effect of extracellular glucose. Glycolysis rate is calculated after 0, 5 and 50 mM glucose injections. Glycolytic capacity is compared after the addition of oligomycin (ATP synthase inhibitor). Finally, 2-deoxyglucose (glycolysis inhibitor) is used to assess the glycolytic reserve. n=3 replicates/ group; mean±SEM. Statistical analysis was performed by One Way ANOVA Tukey post-hoc test. p<0.05 *compared to 0 mM glucose concentration; p<0.05 #compared to 5 mM glucose concentration. (B) Stimulation with normal (5 mM) or high (25 and 50 mM) glucose media for 1 hour followed by protein extraction. Representative Western blot images of protein extracts from fibroblasts showing the effect of glucose concentrations (0 – 50 mM) on serotonylated fibronectin (sFn) and total fibronectin (Fn) expression. Bar graph demonstrating fold change in transglutaminase 2 activity assessed by measuring sFn levels normalized to total Fn by densitometry analysis. n=3 replicates/ group; mean±SEM. Statistical analysis was performed by One Way ANOVA Tukey post-hoc test. p<0.05 *compared to glucose-free media; #compared to 5 mM glucose media.
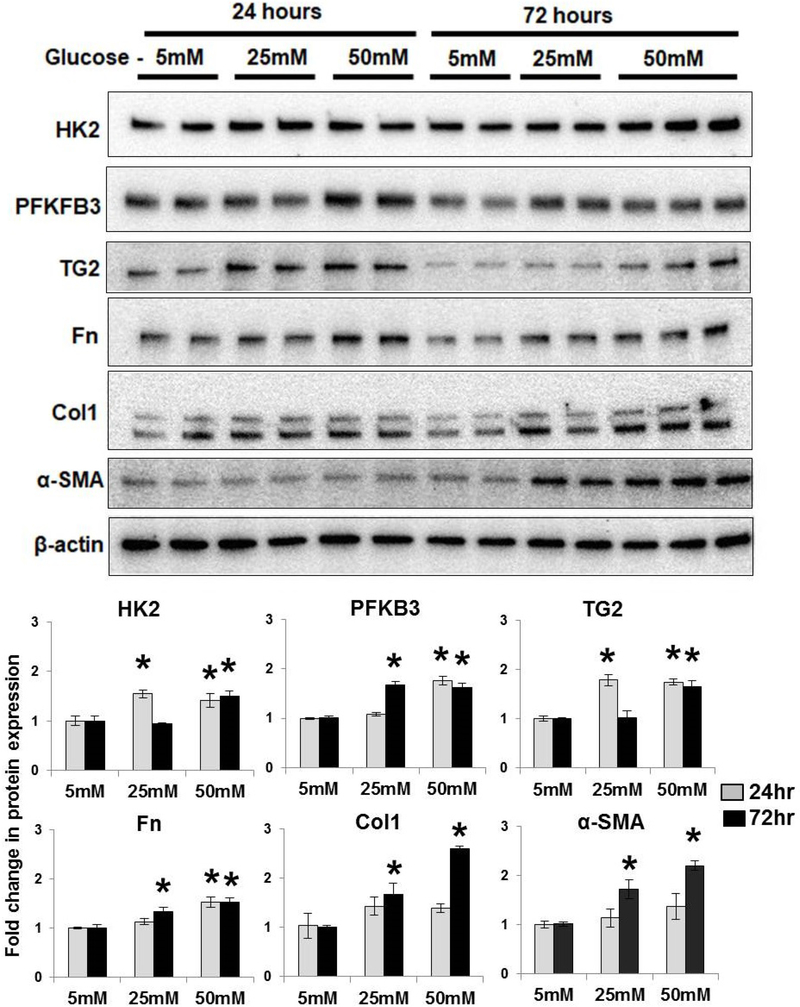
Time course of high glucose induced rate-limiting glycolytic enzymes, TG2 and fibrogenic markers in fibroblasts.
To examine the role of high glucose in vitro, PA adventitial fibroblasts were cultured overnight in glucose-free and reduced-serum media followed by stimulation with normal (5mM) and high (25 and 50mM) glucose concentrations for 24 hours and 72 hours. Western blot analysis showed that high glucose concentrations significantly increased the expression of key aerobic glycolytic enzyme (HK2), rate-limiting glycolytic enzyme (PFKFB3) and TG2 at 24 hours (Figure 6). As shown in Figure 6, fibronectin (Fn) expression was significantly increased in response to high glucose concentrations at 24 hours and 72 hours. High glucose exposure also significantly increased fibrogenic markers (Col1 and α-SMA) at 72 hours (Figure 6), suggesting a role for hyperglycemia on glycolysis, TG2 and fibrogenesis.
Figure 6. High glucose induced glycolytic enzymes and fibrogenesis.
Pulmonary artery adventitial fibroblasts were starved overnight in glucose-free media followed by stimulation with normal (5 mM) or high (25 and 50 mM) glucose media for 24 hours and 72 hours. Representative Western blot images of fibroblast protein extracts showing hexokinase 2 (HK2; 100 kDa), 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase isoform 3 (PFKFB3; 60 kDa), transglutaminase 2 (TG2; 78 kDa), fibronectin (Fn; 220 kDa), type1 collagen (Col1; 147 kDa), α-smooth muscle actin (α-SMA; 42 kDa) and β-actin (42 kDa) levels. Bar graph demonstrating changes in HK2, PFKFB3, TG2, Fn, Col1 and α-SMA expression normalized to β-actin levels by densitometry analysis. n=4–6 replicates/ treatment group; mean±SEM. Statistical analysis was performed by t-test. p<0.05 *compared to normal glucose at respective time-points.
PKM2 regulates high glucose induced TG2 activity and expression and fibrogenesis in fibroblasts.
To determine the role of the pro-glycolytic enzyme PKM2 on TG2 induction in vitro, PA adventitial fibroblasts were cultured for 24 hours after overnight glucose and serum deprivation as noted above. PA adventitial fibroblasts were cultured in media supplemented with normal and high glucose concentrations for 72 hours. As shown in Figure 7A, Western blot analysis showed that Shikonin, a pharmacological inhibitor of glycolysis and PKM2, blocked high glucose-induced TG2 activity (sFn) and expression. Shikonin treatment also significantly attenuated high glucose-induced α-smooth muscle actin expression, a fibroblast activation marker (Figure 7A).
Figure 7. Pharmacological inhibition and molecular knockdown of PKM2 attenuate high glucose-induced transglutaminase 2 activity and fibrogenesis.
Pulmonary artery adventitial fibroblasts were starved overnight in glucose-free media followed by stimulation with normal (NG; 5 mM) or high (HG; 50mM) glucose media for 72 hours. (A) Fibroblasts were exposed to NG or HG media after pretreatment with vehicle control or Shikonin (Shi; 0.5 – 5 μM). Representative Western blot images of protein extracts showing serotonylated fibronectin (sFn; 220 kDa), transglutaminase 2 (TG2; 78 kDa), α-smooth muscle actin (α-SMA; 42 kDa) and β-actin (42 kDa) expression. Bar graph demonstrating fold change in expression normalized to β-actin by densitometry analysis. n=4–6 replicates/ group; mean±SEM. Statistical analysis was performed by One Way ANOVA Tukey post-hoc test. p<0.05 *compared to NG+vehicle; #compared to HG+vehicle. (B) Fibroblasts were exposed to NG or HG media for 72-hours after transfection with control siRNA or pyruvate kinase muscle isoform 2 (PKM2) siRNA as described in methods section. Representative Western blot images of protein extracts showing PKM2 (58 kDa), sFn (220 kDa), type 1 collagen (Col1; 145 kDa), TG2 (78 kDa) and β-actin (42 kDa) expression. Bar graph demonstrating fold change in expression normalized to β-actin by densitometry analysis. n=4 replicates/ group; mean±SEM. Statistical analysis was performed by One Way ANOVA Tukey post-hoc test. p<0.05 *compared to NG+control siRNA; #compared to HG+control siRNA.
To further confirm the importance of PKM2 on TG2 induction, PA adventitial fibroblasts were transfected with PKM2 or control siRNA for 6 hours, followed by overnight serum starvation and treatment with high glucose media for 72 hours. As shown in Figure 7B, Western blot analysis showed that PKM2 siRNA transfection resulted in significant down-regulation of PKM2 protein levels compared to cells transfected with control siRNA. PKM2 siRNA transfection significantly decreased the high glucose-induced TG2 activity (sFn) compared with control siRNA (Figure 7B). Furthermore, PKM2 siRNA transfection significantly down-regulated TG2 expression levels in cells cultured in both normal and high glucose media compared to control siRNA (Figure 7B). We also found that PKM2 siRNA transfection resulted in significant reduction of type 1 collagen (Figure 7B), indicating an important role for PKM2 in stimulation of TG2 and fibrogenesis.
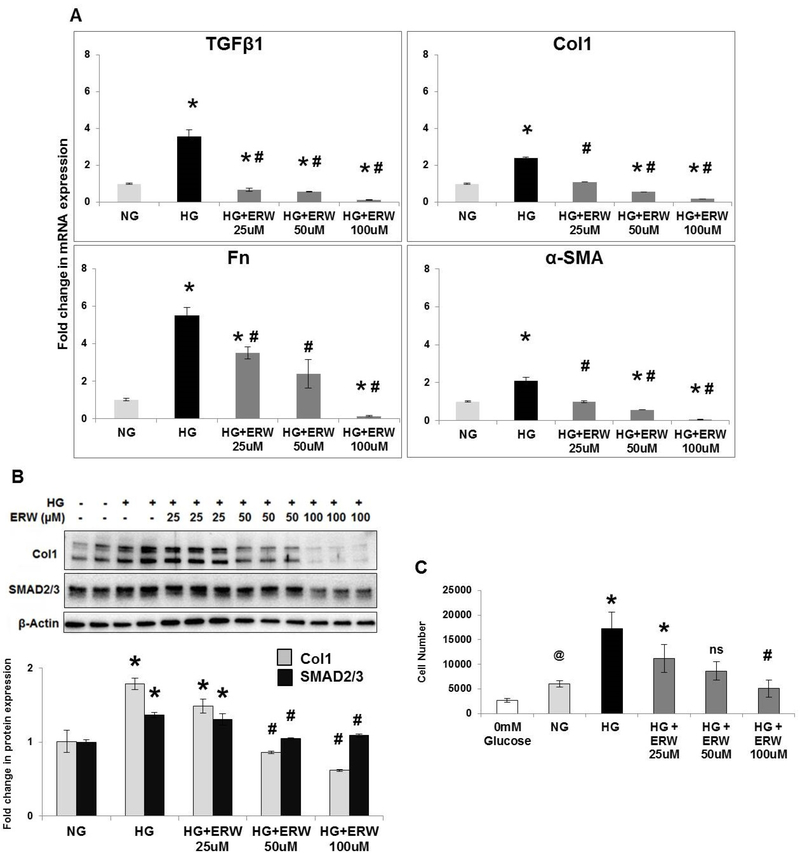
TG2 regulates high glucose-induced fibrogenic signaling and growth responses in fibroblasts.
To assess the functional role for TG2 in high glucose mediated fibrogenic signaling in vitro, we exposed PA adventitial fibroblasts to high glucose concentrations with or without ERW1041E, a small molecule selective TG2 inhibitor, for 72 hours. qPCR analysis showed that pharmacological inhibition of TG2 activity with ERW10141E significantly attenuated the high glucose-induced mRNA expression of transforming growth factor β1 (TGFβ1), type 1 collagen (Col1), fibronectin (Fn) and myofibroblast marker α-smooth muscle actin (α-SMA) (Figure 8A). Next, we further confirmed the role for TG2 on TGFβ1-dependent SMAD signaling and fibrogenesis. Western blot analysis indeed showed that pretreatment with ERW1041E significantly attenuated the high glucose-induced expression of type 1 collagen and SMAD2/3 protein levels (Figure 8B). In accordance with our previously published reports on TG2 and hypoxia (5, 7, 28), these observations further suggest the importance of glycolysis-mediated TG2 activation in fibrogenesis.
Figure 8. Transglutaminase 2 inhibition reduces high glucose-induced fibrogenesis and fibroblast proliferation.
Pulmonary artery adventitial fibroblasts were starved overnight in glucose-free media followed by stimulation with normal (NG; 5 mM) or high (HG; 50 mM) glucose media for 72 hours in the presence of vehicle control or the transglutaminase 2 (TG2) inhibitor, ERW1041E (ERW). (A) Bar graph demonstrating fold-change in mRNA expression of transforming growth factor β1 (TGFβ1), type 1 collagen (Col1), fibronectin (Fn), α-smooth muscle actin (α-SMA) assessed by real-time RT-PCR analysis. n=3–4 treatments/ group; mean±SEM. Statistical analysis was performed by One Way ANOVA Tukey post-hoc test. p<0.05 *compared to NG+vehicle; #compared to HG+vehicle. (B) Representative Western blots of protein extracts from fibroblasts showing Col1 (145 kDa), SMAD2/3 (52/60 kDa) and β-actin (42 kDa) expression. Bar graph demonstrating fold change in Col1 and SMAD2/3 expression normalized to β-actin levels by densitometry analysis. n=4–6/group; mean±SEM. Statistical analysis was performed by One Way ANOVA Tukey post-hoc test. p<0.05 *compared to NG+vehicle; #compared to HG+vehicle. (C) Cell growth was assessed after adding CellTiter-Glo reagent followed by measurement of luminescent signal. Cell proliferation analysis showing the effect of glucose-free (0mM), normal glucose (NG; 5mM) and high glucose (HG; 50mM) media for 72 hours in the presence of vehicle control or the TG2 inhibitor, ERW1041E (ERW). n=6 replicates/ group; mean±SEM. Statistical analysis was performed by One Way ANOVA Tukey post-hoc test. p<0.05 @compared to glucose-free media; *compared to NG+vehicle; #compared to HG+vehicle.
As shown in Figure 8C, increasing glucose concentrations in the media significantly increased fibroblast cell growth. We found that both normal (5 mM) and high (50 mM) glucose concentrations in the media stimulate cell growth compared to cells exposed to glucose-free media (Figure 8C), indicating a role for glucose (and glycolysis) in fibroblast proliferation. Importantly, ERW1041E pre-treatment impaired the high glucose-induced cell growth at 72 hours in a concentration-dependent manner (Figure 8C).
Discussion
Our present study provides evidence indicating that glycolysis-initiated TG2 induction participates in cardiopulmonary tissue fibrosis. Specifically, we identified that: 1) chronic exposure to experimental hypoxia in combination with Sugen, cardiac pressure overload and hyperglycemia all induce TG2 expression and activation in pulmonary and cardiac tissues in vivo; 2) high glucose exposure induces glycolysis-mediated fibrogenesis in PA adventitial fibroblasts; 3) the glycolytic enzyme PKM2 regulates TG2 expression and activation and 4) pharmacological inhibition of TG2 attenuates high glucose -induced fibroblast activation, TGFβ1 signaling, matrix protein type 1 collagen and fibronectin accumulation and cell proliferation in these fibroblasts. Taken together, these results suggest that TG2 plays a central pathogenic role in driving a pro-fibrotic phenotype in PA adventitial fibroblasts and may influence glycolysis-mediated tissue dysfunction. These findings have potentially important implications for patients with PH, diabetes mellitus and HF by providing new mechanistic insights into cardiopulmonary remodeling/ fibrogenesis and identifying TG2 as a possible new target of therapy.
Baandrup et al reported that TG2 expression in the RVs is increased in experimental rats exposed to both PA constriction (RV pressure overload) and chronic hypoxia (33). Independent studies from our lab and others have suggested that TG2-mediated fibrosis is implicated in several physiological conditions that are associated with enhanced tissue glycolysis and metabolic dysfunction (5, 6, 8, 31, 32). As demonstrated in the present investigations, these include chronic exposures to experimental hypoxia, LV pressure overload and hyperglycemia. Since TG2 is known to cross-link protein and stimulate synthesis of collagen (5, 8), we carried out a series of experiments presented in this report to better define the relationships between hyperglycemia, glycolysis, TG2 and fibrogenesis.
We previously reported that pharmacologic inhibition of TG2 activity using the small molecule inhibitor, ERW10141E, blunted the rise in RV systolic pressure in an in vivo mouse model of SuHypoxia that produces experimental PH (28). In addition to our recently published report on TG2-mediated tissue fibrosis (5), our present studies show that TG2 inhibition unexpectedly elevates the expression of glycolytic enzymes in these mouse lung tissues (Figure 2). In keeping with these observations, further studies are needed to confirm the existence of a direct interaction between TG2 inhibition and feed-back regulation of glycolysis. On this note, Piacentini et al previously reported that TG2 deficiency resulted in induction of aerobic glycolysis via impaired mitochondrial autophagy and dysfunction in fibroblasts (22, 34). Contrary to these findings with fibroblasts, previous studies by Kumar et al with mammary epithelial cells reported that over-expression of TG2 induces glycolysis and further showed that molecular knockdown of TG2 reduced glucose uptake, lactate production and glycolysis (35). Taken together, these studies show that regulation of glycolysis and TG2 are important factors in driving energy metabolism, and that these effects may be cell-specific. Future studies will be needed to unravel novel interplaying mechanisms.
Metabolic dysfunction associated with increased glycolysis has been implicated elsewhere in fibrotic tissue remodeling (18, 20, 36). Xiong et al also showed that isoform switch from PKM1 to PKM2 is required for a pro-glycolytic phenotype in the cardiac biventricular pressure overload model of Group 2 PH (27). Although our previous work (5, 7, 28) has focused on PH, where the presence of glycolytic reprogramming has been reported (37, 38), the same considerations may have more widespread usage in applications for metabolic syndrome and diastolic dysfunction leading to congestive heart failure. Recent studies have associated PH and left heart disease with metabolic disorders including diabetes and obesity (3). On this note, we report elevated levels of the PKM2 and TG2 in LVs of a TAC –induced pressure overload model (Figure 3) and in Akita mouse lung and cardiac tissues (Figure 4) compared to their respective controls. Thus, our observations suggest that PKM2 and TG2 may play important roles in the development of changes in tissue fibrosis produced by a variety of physiological effects and that interruption of this process may provide a form of therapy for this malfunction.
Fibrogenic remodeling in the systemic (39, 40) and pulmonary (41, 42) vasculature is associated with increased stiffness and reduced compliance that may directly contribute to pressure overload. Extracellular matrix remodeling of the PA is a significant contributor to pulmonary vascular dysfunction in PH pathogenesis (43). Defined by its location, the vascular adventitial layer is made up of multiple mesenchymal cell-types, predominantly fibroblasts. Fibroblasts are known to regulate tissue matrix composition and activity that are central features of tissue wound repair and fibrotic remodeling (44). Like interstitial fibroblasts, adventitial fibroblasts are known for their action on extracellular matrix protein deposition and secretion of inflammatory cytokines and chemokines (19). Also, adventitial fibroblasts are well-documented to transdifferentiate into myofibroblasts identified by expression of the contractile protein α-smooth muscle actin.
Real-time glycolysis rate measurements on a Seahorse analyzer indicated that PA adventitial fibroblasts exposed to high glucose media produced elevated ECAR levels (Figure 5A), suggesting an increased conversion of pyruvate to lactic acid. Notably, high glucose media increased TG2 activity (Figure 5B) and the expression of TG2 and glycolytic and fibrogenic markers in PA adventitial fibroblasts (Figure 6). Recently, Jeon et al demonstrated that insulin treatment attenuated high glucose –induced TG2 activation and signaling in pulmonary microvascular endothelial cells (45). These authors also report that TG2 deficiency prevents hyperglycemia-induced pulmonary vascular permeability in diabetic mice. Taken together, these studies describe a role for high glucose mediated TG2 activation in pulmonary vascular dysfunction.
The mechanism underlying the contribution of altered glucose metabolism and glycolytic enzyme activity to adverse fibrogenic remodeling is not well defined. Our results indicate that TG2 is under regulatory control by glycolysis and, in the case of these studies, specifically by the glycolytic enzyme PKM2 (Figure 7). These findings are consistent with studies by Altuntas et al where they showed that PKM2 interacts with TG2 and PKM2 inhibition results in reduced TG2 crosslinking activity (22). In the current study, we report that regulation of PA adventitial fibroblast functions, i.e., transformation to myofibroblasts and synthesis of collagen also are under the direction of PKM2 (Figure 7) and TG2 (Figure 8). This concept is illustrated in Figure 9 and the specific mechanism by which enhanced glycolysis leads to TG2 activation and subsequent tissue fibrosis needs further evaluation.
Figure 9. Central role of transglutaminase 2 in glycolysis mediated tissue fibrogenesis.
Metabolic adaptation to hypoxia, pressure overload and hyperglycemia increases glycolysis-mediated transglutaminase 2 (TG2) induction via glycolytic enzyme, pyruvate kinase M2. TG2, in turn, mediates fibroblast cell activation and fibrogenic signaling that plays a major role in cardiopulmonary tissue fibrosis.
Cancer cells with increased glycolysis universally express an M2 isoform of PK and these regulate cell proliferation; thus, small molecule inhibitors for PKM2 have been sought (46). On this note, glucose transporter (18) and glycolytic enzymes (47) other than PKM2 have also been implicated in fibrogenic remodeling. Our in vitro studies with high glucose exposure increased the expression of glycolytic enzymes, HK2 and PFKFB3. Although we found hyperglycemia induced PKM2 expression in vivo (Figure 4), we observed no such elevation of PKM2 expression at 24-, 48- and 72-hour time-points (example shown in Figure 7C) in PA adventitial fibroblasts. Importantly, our in vitro studies showed a significant effect with PKM2 pharmacological inhibition and siRNA knockdown on TG2 and fibrogenic phenotype in PA adventitial fibroblasts (Figure 7). We speculate that these effects may be cell-specific and phenotypic switch or nuclear translocation from cytoplasmic to nuclear protein kinase might be involved in the observed fibrogenic response. Prior studies by Shirai et al have demonstrated that high utilization of glucose induces mitochondrial oxidative stress and mediates nuclear translocation of PKM2, which then promotes inflammation (21). In addition to the well-documented role for hypoxia-inducible factor-1α (HIF1α) in hypoxic, inflammatory and glycolytic environments (48, 49), previous studies reported that high glucose concentrations stabilize HIF1α (50) and stimulate TG2 activity (8) and fibrogenesis in kidney epithelial cells. There is also considerable evidence that glycolysis mediated PKM2 is associated with HIF1 regulation (22). Interestingly, previous studies from our lab showed that hypoxia –induced TG2 expression was mediated by HIF1α in PA smooth muscle cells. In addition, it has been previously shown that both reactive oxygen species (51) and IL6 stimulate TG2 activation (52). Further investigations focusing on the relationships between mitochondrial dysfunction, glycolysis and inflammation and the specific effects of these pathways on TG2, tissue fibrosis and reduced tissue compliance are needed.
Recent studies have molecularly associated TG2 with both vascular (53) and cardiac (54) stiffness. Although we have focused on TG2 as a cause for fibrosis in this communication, other cellular pathways that may be influential on the development of tissue fibrosis and decreased compliance have been proposed. These include lysyl oxidase activity (55) and the YAP/TAZ pathway (56). It has been previously reported that metabolic reprogramming is required for YAP/TAZ transcriptional activity (57) and myofibroblast contractility and differentiation (58). A relationship between TG2 and YAP/TAZ has been proposed in cancer cells (59, 60). Thus, future studies may reveal novel signaling pathways related to glycolysis-mediated tissue fibrogenesis. Glycolytic activity also stimulates uptake of serotonin (61), a known substrate for activating TG2 (30), further supporting that TG2 is an important intermediary in glycolysis-regulated fibroblast function. Accordingly, our previous (5, 7) and present studies show that serotonylation of fibronectin is significantly elevated in cells and tissues undergoing increased glycolysis. Furthermore, TG2 has been previously shown to be implicated in fibroblast cell survival (62). In this study we have demonstrated that TG2 is involved in glycolysis-mediated fibroblast proliferative phenotype (Figure 8C).
Thus, in summary, we demonstrate for the first-time an up-regulation of TG2 activity and expression in response to glycolytic shift, which then affects fibrogenesis (Figure 9). Future studies are needed to assess the signaling pathways associated with the glycolysis-TG2-fibrosis axis that causes tissue rigidity in general and could lead to a better understanding of the pathogenesis and possibly therapy of pulmonary vascular and cardiac dysfunction.
Acknowledgements:
We gratefully acknowledge the support from Dr. Chaitan Khosla, Stanford University, Stanford, CA, for supplying small molecule TG2 inhibitor, ERW10141E, for in vivo studies. The authors are grateful to Bo Wang and Yali Zhang of Tufts Medical Center, Boston, MA, for assistance with Akita mice.
Sources of Funding: This study was supported by research funding from Gilead Sciences Research Scholars Program in Pulmonary Arterial Hypertension (KCP), an American Heart Association Career Development Award 18CDA34140005 (KCP) and the National Institutes of Health HL107713 (BLF) and HL074876 (JBG).
Abbreviations
- α-SMA
alpha-smooth muscle actin
- Col1
Type 1 collagen
- ECAR
Extracellular acidification rate
- ERW1041E
(S)-Quinolin-3-ylmethyl 2-((((S)-3-Bromo-4,5-dihydroisoxazol-5-yl)methyl)carbamoyl)pyrrolidine-1-carboxylate
- Fn
Fibronectin
- HF
Heart failure
- HG
High glucose
- HK2
Hexokinase 2
- LV
Left ventricle
- NG
Normal glucose
- PA
Pulmonary artery
- PFKFB3
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3
- PH
Pulmonary hypertension
- PKM2
Pyruvate kinase muslce isoform 2
- qPCR
Quantitative real-time polymerase chain reaction
- RV
Right ventricle
- sFn
Serotonylated fibronectin
- siRNA
small interfering RNA
- SuHypoxia
Sugen 5416 + chronic hypoxia
- TAC
Transverse aortic constriction
- TG2
Transglutaminase 2
- TGFβ1
Transforming growth factor beta-1
- WT
Wild-type
Footnotes
Disclosures: None.
References
- 1.Al-Naamani N, Preston IR, Paulus JK, Hill NS, and Roberts KE (2015) Pulmonary Arterial Capacitance Is an Important Predictor of Mortality in Heart Failure With a Preserved Ejection Fraction. JACC Heart Fail 3, 467–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rain S, Handoko ML, Trip P, Gan CT, Westerhof N, Stienen GJ, Paulus WJ, Ottenheijm CA, Marcus JT, Dorfmuller P, Guignabert C, Humbert M, Macdonald P, Dos Remedios C, Postmus PE, Saripalli C, Hidalgo CG, Granzier HL, Vonk-Noordegraaf A, van der Velden J, and de Man FS (2013) Right ventricular diastolic impairment in patients with pulmonary arterial hypertension. Circulation 128, 2016–2025, 2011–2010 [DOI] [PubMed] [Google Scholar]
- 3.Lai YC, Wang L, and Gladwin MT (2019) Insights into the pulmonary vascular complications of heart failure with preserved ejection fraction. J Physiol 597, 1143–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jia G, Hill MA, and Sowers JR (2018) Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ Res 122, 624–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penumatsa KC, Toksoz D, Warburton RR, Kharnaf M, Preston IR, Kapur NK, Khosla C, Hill NS, and Fanburg BL (2017) Transglutaminase 2 in pulmonary and cardiac tissue remodeling in experimental pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 313, L752–L762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei L, Warburton RR, Preston IR, Roberts KE, Comhair SA, Erzurum SC, Hill NS, and Fanburg BL (2012) Serotonylated fibronectin is elevated in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 302, L1273–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penumatsa KC, Toksoz D, Warburton RR, Hilmer AJ, Liu T, Khosla C, Comhair SA, and Fanburg BL (2014) Role of hypoxia-induced transglutaminase 2 in pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol 307, L576–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skill NJ, Johnson TS, Coutts IG, Saint RE, Fisher M, Huang L, El Nahas AM, Collighan RJ, and Griffin M (2004) Inhibition of transglutaminase activity reduces extracellular matrix accumulation induced by high glucose levels in proximal tubular epithelial cells. J Biol Chem 279, 47754–47762 [DOI] [PubMed] [Google Scholar]
- 9.Michiels C (2004) Physiological and pathological responses to hypoxia. Am J Pathol 164, 1875–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nascimben L, Ingwall JS, Lorell BH, Pinz I, Schultz V, Tornheim K, and Tian R (2004) Mechanisms for increased glycolysis in the hypertrophied rat heart. Hypertension 44, 662–667 [DOI] [PubMed] [Google Scholar]
- 11.Kolwicz SC Jr., and Tian R (2011) Glucose metabolism and cardiac hypertrophy. Cardiovasc Res 90, 194–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sankaralingam S, and Lopaschuk GD (2015) Cardiac energy metabolic alterations in pressure overload-induced left and right heart failure (2013 Grover Conference Series). Pulm Circ 5, 15–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Guo H, Xu D, Xu X, Wang H, Hu X, Lu Z, Kwak D, Xu Y, Gunther R, Huo Y, and Weir EK (2012) Left ventricular failure produces profound lung remodeling and pulmonary hypertension in mice: heart failure causes severe lung disease. Hypertension 59, 1170–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapur NK, Paruchuri V, Aronovitz MJ, Qiao X, Mackey EE, Daly GH, Ughreja K, Levine J, Blanton R, Hill NS, and Karas RH (2013) Biventricular remodeling in murine models of right ventricular pressure overload. PLoS One 8, e70802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Welzig CM, Picard KL, Du C, Wang B, Pan JQ, Kyriakis JM, Aronovitz MJ, Claycomb WC, Blanton RM, Park HJ, and Galper JB (2014) Glycogen synthase kinase-3beta inhibition ameliorates cardiac parasympathetic dysfunction in type 1 diabetic Akita mice. Diabetes 63, 2097–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu H, Forbes RA, and Verma A (2002) Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem 277, 23111–23115 [DOI] [PubMed] [Google Scholar]
- 17.Greiner EF, Guppy M, and Brand K (1994) Glucose is essential for proliferation and the glycolytic enzyme induction that provokes a transition to glycolytic energy production. J Biol Chem 269, 31484–31490 [PubMed] [Google Scholar]
- 18.Cho SJ, Moon JS, Lee CM, Choi AM, and Stout-Delgado HW (2017) Glucose Transporter 1-Dependent Glycolysis Is Increased during Aging-Related Lung Fibrosis, and Phloretin Inhibits Lung Fibrosis. Am J Respir Cell Mol Biol 56, 521–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, Wang D, Li M, Plecita-Hlavata L, D’Alessandro A, Tauber J, Riddle S, Kumar S, Flockton A, McKeon BA, Frid MG, Reisz JA, Caruso P, El Kasmi KC, Jezek P, Morrell NW, Hu CJ, and Stenmark KR (2017) Metabolic and Proliferative State of Vascular Adventitial Fibroblasts in Pulmonary Hypertension Is Regulated Through a MicroRNA-124/PTBP1 (Polypyrimidine Tract Binding Protein 1)/Pyruvate Kinase Muscle Axis. Circulation 136, 2468–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding H, Jiang L, Xu J, Bai F, Zhou Y, Yuan Q, Luo J, Zen K, and Yang J (2017) Inhibiting aerobic glycolysis suppresses renal interstitial fibroblast activation and renal fibrosis. Am J Physiol Renal Physiol 313, F561–F575 [DOI] [PubMed] [Google Scholar]
- 21.Shirai T, Nazarewicz RR, Wallis BB, Yanes RE, Watanabe R, Hilhorst M, Tian L, Harrison DG, Giacomini JC, Assimes TL, Goronzy JJ, and Weyand CM (2016) The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med 213, 337–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altuntas S, Rossin F, Marsella C, D’Eletto M, Diaz-Hidalgo L, Farrace MG, Campanella M, Antonioli M, Fimia GM, and Piacentini M (2015) The transglutaminase type 2 and pyruvate kinase isoenzyme M2 interplay in autophagy regulation. Oncotarget 6, 44941–44954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao X, Wang H, Yang JJ, Liu X, and Liu ZR (2012) Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol Cell 45, 598–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta V, and Bamezai RN (2010) Human pyruvate kinase M2: a multifunctional protein. Protein Sci 19, 2031–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong G, Mao Q, Xia W, Xu Y, Wang J, Xu L, and Jiang F (2016) PKM2 and cancer: The function of PKM2 beyond glycolysis. Oncol Lett 11, 1980–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang W, and Lu Z (2013) Regulation and function of pyruvate kinase M2 in cancer. Cancer Lett 339, 153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong PY, Tian L, Dunham-Snary KJ, Chen KH, Mewburn JD, Neuber-Hess M, Martin A, Dasgupta A, Potus F, and Archer SL (2018) Biventricular Increases in Mitochondrial Fission Mediator (MiD51) and Proglycolytic Pyruvate Kinase (PKM2) Isoform in Experimental Group 2 Pulmonary Hypertension-Novel Mitochondrial Abnormalities. Front Cardiovasc Med 5, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiRaimondo TR, Klock C, Warburton R, Herrera Z, Penumatsa K, Toksoz D, Hill N, Khosla C, and Fanburg B (2014) Elevated transglutaminase 2 activity is associated with hypoxia-induced experimental pulmonary hypertension in mice. ACS Chem Biol 9, 266–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ciuclan L, Bonneau O, Hussey M, Duggan N, Holmes AM, Good R, Stringer R, Jones P, Morrell NW, Jarai G, Walker C, Westwick J, and Thomas M (2011) A novel murine model of severe pulmonary arterial hypertension. Am J Respir Crit Care Med 184, 1171–1182 [DOI] [PubMed] [Google Scholar]
- 30.Penumatsa KC, and Fanburg BL (2014) Transglutaminase 2-mediated serotonylation in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 306, L309–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shinde AV, Su Y, Palanski BA, Fujikura K, Garcia MJ, and Frangogiannis NG (2018) Pharmacologic inhibition of the enzymatic effects of tissue transglutaminase reduces cardiac fibrosis and attenuates cardiomyocyte hypertrophy following pressure overload. J Mol Cell Cardiol 117, 36–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Stuckey DJ, Murdoch CE, Camelliti P, Lip GYH, and Griffin M (2018) Cardiac fibrosis can be attenuated by blocking the activity of transglutaminase 2 using a selective small-molecule inhibitor. Cell Death Dis 9, 613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baandrup JD, Markvardsen LH, Peters CD, Schou UK, Jensen JL, Magnusson NE, Orntoft TF, Kruhoffer M, and Simonsen U (2011) Pressure load: the main factor for altered gene expression in right ventricular hypertrophy in chronic hypoxic rats. PLoS One 6, e15859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossin F, D’Eletto M, Falasca L, Sepe S, Cocco S, Fimia GM, Campanella M, Mastroberardino PG, Farrace MG, and Piacentini M (2015) Transglutaminase 2 ablation leads to mitophagy impairment associated with a metabolic shift towards aerobic glycolysis. Cell Death Differ 22, 408–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar S, Donti TR, Agnihotri N, and Mehta K (2014) Transglutaminase 2 reprogramming of glucose metabolism in mammary epithelial cells via activation of inflammatory signaling pathways. Int J Cancer 134, 2798–2807 [DOI] [PubMed] [Google Scholar]
- 36.Wei Q, Su J, Dong G, Zhang M, Huo Y, and Dong Z (2019) Glycolysis inhibitors suppress renal interstitial fibrosis via divergent effects on fibroblasts and tubular cells. Am J Physiol Renal Physiol 316, F1162-F1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Archer SL, Fang YH, Ryan JJ, and Piao L (2013) Metabolism and bioenergetics in the right ventricle and pulmonary vasculature in pulmonary hypertension. Pulm Circ 3, 144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulin R, and Michelakis ED (2014) The metabolic theory of pulmonary arterial hypertension. Circ Res 115, 148–164 [DOI] [PubMed] [Google Scholar]
- 39.Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell G, Cohen RA, and Seta F (2013) Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension 62, 1105–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh YS (2018) Arterial stiffness and hypertension. Clin Hypertens 24, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z, Schreier DA, Abid H, Hacker TA, and Chesler NC (2017) Pulmonary vascular collagen content, not cross-linking, contributes to right ventricular pulsatile afterload and overload in early pulmonary hypertension. J Appl Physiol (1985) 122, 253–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schafer M, Myers C, Brown RD, Frid MG, Tan W, Hunter K, and Stenmark KR (2016) Pulmonary Arterial Stiffness: Toward a New Paradigm in Pulmonary Arterial Hypertension Pathophysiology and Assessment. Curr Hypertens Rep 18, 4. [DOI] [PubMed] [Google Scholar]
- 43.Thenappan T, Chan SY, and Weir EK (2018) Role of extracellular matrix in the pathogenesis of pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 315, H1322-H1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kendall RT, and Feghali-Bostwick CA (2014) Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol 5, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeon HY, Seo JA, Jung SH, Lee YJ, Han ET, Park WS, Hong SH, Kim YM, and Ha KS (2019) Insulin prevents pulmonary vascular leakage by inhibiting transglutaminase 2 in diabetic mice. Life Sci, 116711 [DOI] [PubMed] [Google Scholar]
- 46.Vander Heiden MG, Christofk HR, Schuman E, Subtelny AO, Sharfi H, Harlow EE, Xian J, and Cantley LC (2010) Identification of small molecule inhibitors of pyruvate kinase M2. Biochem Pharmacol 79, 1118–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie N, Tan Z, Banerjee S, Cui H, Ge J, Liu RM, Bernard K, Thannickal VJ, and Liu G (2015) Glycolytic Reprogramming Mediates Myofibroblast Differentiation and Promotes Lung Fibrosis. Am J Respir Crit Care Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stenmark KR, Tuder RM, and El Kasmi KC (2015) Metabolic reprogramming and inflammation act in concert to control vascular remodeling in hypoxic pulmonary hypertension. J Appl Physiol (1985) 119, 1164–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimoda LA, and Laurie SS (2014) HIF and pulmonary vascular responses to hypoxia. J Appl Physiol (1985) 116, 867–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Isoe T, Makino Y, Mizumoto K, Sakagami H, Fujita Y, Honjo J, Takiyama Y, Itoh H, and Haneda M (2010) High glucose activates HIF-1-mediated signal transduction in glomerular mesangial cells through a carbohydrate response element binding protein. Kidney Int 78, 48–59 [DOI] [PubMed] [Google Scholar]
- 51.Lee ZW, Kwon SM, Kim SW, Yi SJ, Kim YM, and Ha KS (2003) Activation of in situ tissue transglutaminase by intracellular reactive oxygen species. Biochem Biophys Res Commun 305, 633–640 [DOI] [PubMed] [Google Scholar]
- 52.Suto N, Ikura K, and Sasaki R (1993) Expression induced by interleukin-6 of tissue-type transglutaminase in human hepatoblastoma HepG2 cells. J Biol Chem 268, 7469–7473 [PubMed] [Google Scholar]
- 53.Steppan J, Sikka G, Jandu S, Barodka V, Halushka MK, Flavahan NA, Belkin AM, Nyhan D, Butlin M, Avolio A, Berkowitz DE, and Santhanam L (2014) Exercise, vascular stiffness, and tissue transglutaminase. J Am Heart Assoc 3, e000599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oh YJ, Pau VC, Steppan J, Sikka G, Bead VR, Nyhan D, Levine BD, Berkowitz DE, and Santhanam L (2017) Role of tissue transglutaminase in age-associated ventricular stiffness. Amino Acids 49, 695–704 [DOI] [PubMed] [Google Scholar]
- 55.Nave AH, Mizikova I, Niess G, Steenbock H, Reichenberger F, Talavera ML, Veit F, Herold S, Mayer K, Vadasz I, Weissmann N, Seeger W, Brinckmann J, and Morty RE (2014) Lysyl oxidases play a causal role in vascular remodeling in clinical and experimental pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 34, 1446–1458 [DOI] [PubMed] [Google Scholar]
- 56.Liu F, Lagares D, Choi KM, Stopfer L, Marinkovic A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, Rosas IO, Fredenburgh LE, Feghali-Bostwick C, Varelas X, Tager AM, and Tschumperlin DJ (2015) Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol 308, L344–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Enzo E, Santinon G, Pocaterra A, Aragona M, Bresolin S, Forcato M, Grifoni D, Pession A, Zanconato F, Guzzo G, Bicciato S, and Dupont S (2015) Aerobic glycolysis tunes YAP/TAZ transcriptional activity. EMBO J 34, 1349–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bernard K, Logsdon NJ, Ravi S, Xie N, Persons BP, Rangarajan S, Zmijewski JW, Mitra K, Liu G, Darley-Usmar VM, and Thannickal VJ (2015) Metabolic Reprogramming Is Required for Myofibroblast Contractility and Differentiation. J Biol Chem 290, 25427–25438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu CY, Pobbati AV, Huang Z, Cui L, and Hong W (2017) Transglutaminase 2 Is a Direct Target Gene of YAP/TAZ-Letter. Cancer Res 77, 4734–4735 [DOI] [PubMed] [Google Scholar]
- 60.Lee J, Condello S, Yakubov B, Emerson R, Caperell-Grant A, Hitomi K, Xie J, and Matei D (2015) Tissue Transglutaminase Mediated Tumor-Stroma Interaction Promotes Pancreatic Cancer Progression. Clin Cancer Res 21, 4482–4493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee SL, and Fanburg BL (1987) Glycolytic activity and enhancement of serotonin uptake by endothelial cells exposed to hypoxia/anoxia. Circ Res 60, 653–658 [DOI] [PubMed] [Google Scholar]
- 62.Boroughs LK, Antonyak MA, and Cerione RA (2014) A novel mechanism by which tissue transglutaminase activates signaling events that promote cell survival. J Biol Chem 289, 10115–10125 [DOI] [PMC free article] [PubMed] [Google Scholar]