Abstract
Object
This study aimed at investigating the clinical significance and biological function of ubiquitination factor E4B (UBE4B) in human renal cell carcinoma (RCC).
Methods
19 paired clear cell renal cell carcinoma (ccRCC) tumor samples and the matched neighboring non-tumor samples were used to detect the expression of UBE4B in RCC tumor by Western blotting and RT-qPCR. UBE4B expression was also detected in 151 ccRCC paraffin-embedded tumor samples by using immunohistochemistry. Overall survival (OS) in different UBE4B expression groups were compared with Log rank test. The prognostic value of UBE4B expression in OS was evaluated with the univariate and multivariate Cox regression models. UBE4B was knocked down by small interfering RNA (siRNA) technology, and the effect of UBE4B on cell proliferation, colony formation, metastasis, apoptosis and cell cycle of RCC cells were examined in vitro.
Results
Both protein and mRNA levels of UBE4B were up-regulated in ccRCC tumor tissues in contrast to the corresponding adjacent nontumor ones. UBE4B expression was positively associated with tumor-node-metastasis (TNM) stage and distant metastasis in ccRCC patients. Survival analyses indicated that low expression of UBE4B was associated with increased OS in ccRCC patients. Functional analyses demonstrated that siRNA silencing of UBE4B expression in SKRC39 and ACHN cells further reduced the growth, motility and invasiveness of RCC cells. Moreover, siRNA silencing of UBE4B in the RCC cell lines did not induce apoptosis, and an increase in the cell population was observed during the G0/G1 phase of the cell cycle.
Conclusion
UBE4B might act as an oncogene in regulating RCC development. Therefore it could be served as an effective indicator to predict OS and a potential biomarker for targeted therapy of RCC patients.
Keywords: renal cell carcinoma, UBE4B, prognosis, oncogene
Renal cell carcinoma (RCC) is a fatal genitourinary malignancy, and the most common adult kidney cancer.1,2 Regarded as effective curative therapies for RCC, nephrectomy and radiotherapy offer the opportunity of favorable long-term prognosis for early RCC patients. In addition, RCC is sensitive to immunotherapy and targeted therapy while highly resistant to both radiotherapy and chemotherapy.3 Nevertheless, more than 20% of patients with localized RCC suffer from tumor recurrence or progress to metastasis after nephrectomy,4,5 and it still causes nearly 120,000 deaths each year worldwide.6,7 Apart from that, the molecular mechanism involved in the pathogenesis of RCC has not been clarified yet. Hence, a novel molecular marker suitable for early diagnosis and more effective targeted therapy to ameliorate the outcome of RCC patients are urgently needed.
The ubiquitination factor E4B gene (UBE4B), also referred to as UFD2a, is one of the two human homolog of the UFD2 yeast gene.8,9 UBE4B is located in chromosome 1p36 and encodes ubiquitin conjugation factor E4 that is involved in multiubiquitin chain assembly.10,11 Ubiquitination is critical in cell functions such as proliferation, apoptosis, cell cycle regulation and DNA repair, and deregulation of certain types of protein that are involved in the tumorigenesis of many types of tumor.12,13 E4 is a key newly discovered class of ubiquitination enzyme during protein ubiquitination. Generally, dysfunction of anti-oncogene, e.g.p53, is crucial during tumor progression.14–16 Recent studies showed that UBE4B facilitated the polyubiquitination and degeneration of p53.17–20 Moreover, UBE4B aberrant expression has been reported in several kinds of cancers, such as breast cancer,21 promyelocytic leukemia22 and nasopharyngeal carcinoma.23 Our previous study also demonstrated that UBE4B overexpression promoted the tumorigenesis of hepatocellular carcinoma (HCC) and was related to a poor outcome in HCC patients.24 As far as we know, the expression and prognostic value of UBE4B in tumorigenesis of RCC has not been clarified yet.
Aiming to reveal the clinical value of UBE4B in RCC, the expression and prognostic values of UBE4B in RCC was investigated. Additionally, the effect of UBE4B in RCC progression was also evaluated by using different RCC cell lines.
Materials and Methods
Human Clinical Samples
Since clear cell renal cell carcinoma (ccRCC) comprises approximately 75% of RCC in surgical series,25,26 ccRCC subtype was selected to be the research subject. 151 ccRCC patients who had undergone nephrectomy without preoperative radiotherapy or chemotherapy were selected from the Sun Yat-sen University Cancer Center (SYSUCC) between 2012 and 2014, and their corresponding paraffin-embedded tumor samples were obtained. All the RCC patients’ postoperative histopathology diagnosis were confirmed as ccRCC by qualified Pathologists based on World Health Organization (WHO) classification standard,27 and their pathological grading were graded following the nuclear grading system (Fuhrman et al 1982).28 The TNM stage was staged by radiographic findings and postoperative pathological data according to 2010 American Joint Committee on Cancer (AJCC) TNM classification.29 The patient demographics and follow-up data were obtained from the follow-up center of SYSUCC. Overall survival (OS) was employed as an index to evaluate the prognosis, and it was defined as the time from the date of operation to death or the latest follow-up. In addition, 19 paired ccRCC tumor samples and the matched neighboring non-tumor samples were acquired from ccRCC patients undergoing surgical resection at SYSUCC in 2015.
Cell Lines
Five human RCC cell lines (786-O, CaKi-1, SKRC39, ACHN and A498) were acquired from the American Type Culture Collection (ATCC, Rockville, MD, USA). All the cells were cultured in RPMI1640 medium (Gibco, Grand Island, NY, USA) added with 10% FBS (Gibco, Grand Island, NY, USA) and 1% penicillin-streptomycin in 5% CO2 humidified atmosphere at 37°C.
Cell Transfection with Small Interfering RNA (siRNA)
As shown in Table 1, four siRNAs (siUBE4B #1-4) and a negative control (siNC) were purchased from GenePharma (GenePharma, Suzhou, China). The siUBE4B and siNC were transfected transiently into SKRC39 and ACHN cells via Lipofectamine 2000 (Invitrogen, Carlsbad, CA,USA) based on the product’s specification. The transfected cells were named as SKRC39/siUBE4B, SKRC39/siNC, ACHN/siUBE4B, and ACHN/siNC, respectively.
Table 1.
Small Interfering RNA (siRNA) Sequences That Target Ubiquitination Factor E4B (siUBE4B) and Negative Control siRNA (siNC)
| siRNA | Sense Sequence | Anti-Sense Sequence |
|---|---|---|
| siUBE4B #1 | 5ʹ-GCCCUCUAAUAGCCUUGAATT-3’ | 5ʹ-UUCAAGGCUAUUAGAGGGCTT-3’ |
| siUBE4B #2 | 5ʹ-GACAGUGACUACUUUAAAUTT-3’ | 5ʹ-AUUUAAAGUAGUCACUGUCTT-3’ |
| siUBE4B #3 | 5ʹ-GCAGUUUGUUCGCUAUAUATT-3’ | 5ʹ-UAUAUAGCGAACAAACUGCTT-3’ |
| siUBE4B #4 | 5ʹ-CUGCAAUGCUGAACUUUAATT-3’ | 5ʹ-UUAAAGUUCAGCAUUGCAGTT-3’ |
| siNC | 5ʹ-UUCUCCGAACGUGUCACGUTT-3’ | 5ʹ-ACGUGACACGUUCGGAGAATT-3’ |
Real-Time Quantitative PCR (RT-qPCR)
Firstly, total RNA was extracted from RCC tissues utilizing TRIzol (Invitrogen, Carlsbad, CA, USA). Then, each sample’s total RNA was reversely transcribed to cDNA utilizing the ReverTraAce qPCR RT Kit (TOYOBO, Shanghai, China) following the product’s specification. The target cDNA was amplified by realtime PCR employing the SYBR Green Realtime PCR master mix (Invitrogen, Carlsbad, CA,USA). The comparative threshold cycle (2-ΔΔCT) method was applied to quantify the relative transcript levels of the UBE4B gene which was normalized by the corresponding values of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The sequences of all primers used were as follows: the reference gene, GAPDH, forward 5ʹ-CTCCTCCTGTTCGACAGTCAGC-3ʹ, reverse 5ʹ-CCCAATACGACCAAATCCGTT-3ʹ; the target gene, UBE4B, forward 5ʹ-CCAAAGAAGCTGTTGGACCAACTG-3ʹ, reverse 5ʹ-GGGTGTCCATCAGAGGGTCTCTG-3ʹ.
Western Blotting
Radio-Immunoprecipitation Assay (RIPA) Lysis Buffer (Beyotime, Shanghai, China) was used to extract total proteins. The concentration of total proteins was determined using the BCA Protein Assay Kit (Bio-Rad; Hercules, CA, USA), and the optical density (OD) was recorded at 562 nm by a microplate spectrophotometer (EPOCH2, Bio-Tek, Winooski, USA). Western blotting and the quantification of band intensity were performed as described previously.30 The following antibodies were applied: rabbit polyclonal antibody against UBE4B (1:1000 dilution; Proteintech, Chicago, IL,USA, Catalogue number:18148-1-AP), rabbit anti-GAPDH polyclonal antibody (1:10,000 dilution, Proteintech, Chicago, IL, USA, Catalogue number: 10494-1-AP) and anti-rabbit IgG, horseradish peroxidase (HRP)-conjugated secondary antibody (1:2000 dilution, Cell Signaling Technology, Danvers, MA, USA, Catalogue number: 7074S). The UBE4B protein expression levels were normalized to that of GAPDH.
Immunohistochemistry Analysis
Serial 2 μm sections that cut from 151 ccRCC paraffin-embedded samples were used for UBE4B immunohistochemistry. Rabbit polyclonal antibody against UBE4B (1:200 dilution; Proteintech, Chicago, IL, USA, Catalogue number:18148-1-AP) and anti-Mo&Rb IgG, HRP-conjugated secondary antibody (Envision™ Detection Kit, Gene Tech, Shanghai, China, Catalogue number:GK500705) were applied. Immunohistochemistry staining and a semi-quantitative scoring criterion for the assessment of UBE4B immunostaining degree were performed as indicated in our previous study.30 For negative controls, tissue sections were immunoreacted with PBS under the same experimental conditions. The quantification of UBE4B expression levels in ccRCC paraffin-embedded samples were evaluated according to the staining intensity and the positively stained areas of tumor cells by 2 independent pathological professionals (HXQ and ZZQ) who were blinded to the clinicopathological parameters under a light microscope (Nikon, ECLIPSE E100, Japan). If the two evaluations of one sample were different, a third pathologist would participate to determine the final evaluation. The semi-quantitative scoring criterion was as follows: the percent of positive staining was defined as 0 (<5%, negative), 1 (5%–25%, sporadic), 2 (25%– 50%, focal), or 3 (>50%, diffuse); staining intensity was classified as 0 (no staining), 1 (weak staining), 2 (moderate staining) and 3 (strong staining). The two values were multiplied to give sum scores of the immunostaining between 0 and 9. These were used to define the expressions of UBE4B as follows: “−” (score 0–1), “+” (score 2–3), “++” (score 4–5) or “+++” (>6 points). The sampled patients were divided into low UBE4B expression (UBE4B- or UBE4B+) or high UBE4B expression groups (UBE4B++ or UBE4B+++).
Proliferation Assay
A density of 800 transfected cells per well were plated and cultured in 96-well plates. Cell proliferation was evaluated for 7 consecutive days using the methanethiosulfonate (MTS) assay. In brief, 20 μL MTS reagent (5 mg/mL, Sigma-Aldrich, Shanghai, China) was added to the plated cells and the optical density (OD) was recorded at 490 nm by a spectrophotometric reader (EPOCH2, Bio-Tek, Winooski, USA) after 3 hrs’ incubation. All experiments were repeated in triplicate.
Colony Formation Assay
A density of 1000 transfected cells per well were plated and cultured in 6-well plates in triplicate. After 12 days, the living cells were fixed and stained. The colony-forming efficiency was evaluated as our previous study.30
Cell Cycle Analysis
To determine cell cycle distribution, the targeted cells were preprocessed and treated with cell cycle detection kit (Bestbio, Shanghai, China) according to the product’s specification. DNA content of cells was tested using a flow cytometry (Beckman Coulter, Fullerton, CA, USA). Cell Quest and ModFit software (Beckman Coulter) were used to calculate the proportion of cells at different cell cycle phase. All experiments were conducted in triplicate.
Apoptosis Assay
The Annexin V-FITC Apoptosis Detection Kit (BD Biosciences, Bedford, MA, USA) was used to examine the apoptosis rates of cells following the product’s instruction by flow cytometry (Beckman Coulter, Fullerton, CA, USA). The experiments were repeated three times.
Cell Migration and Invasion Assays in vitro
2×105 cells (migration) or 3×105 cells (invasion) were resuspended in 200 μL RPMI 1640 without serum and planted in pore polycarbonate membrane inserts (8 μm, Corning Incorporated, Corning, NY, USA) with or without a filmy coating of Matrige (0.5 mg/mL, BD, Franklin Lakes, NJ, USA) respectively. Then, the inserts were placed on 24-well plates which contained 500 μL RPMI 1640 with 10% FBS. The above cells were cultured for 24 h (migration) or 48 h (invasion), and then, the cells on the reverse side of membrane were fixed and stained. After that, cotton swabs were applied to remove cells on the surface of the membrane, and five random microscopic fields of each membrane were selected to count the stained cells by microscopy. All experiments were repeated three times.
Statistical Analysis
Data analysis in this study was realized with SPSS software 21.0 (SPSS Inc., Chicago, IL, USA). The Pearson’s X2 test was used for qualitative variables.and the Student’s t-test was applied for quantitative variables to assess the correlations between UBE4B expression and the baseline variables of RCC patients. OS in different UBE4B expression groups were compared with Log rank test. The prognostic value of UBE4B expression in OS was evaluated with the univariate and multivariate Cox regression models. Two-sided P<0.05 was considered as statistical significance.
Results
The Expression of UBE4B in RCC Samples and RCC Cell Lines
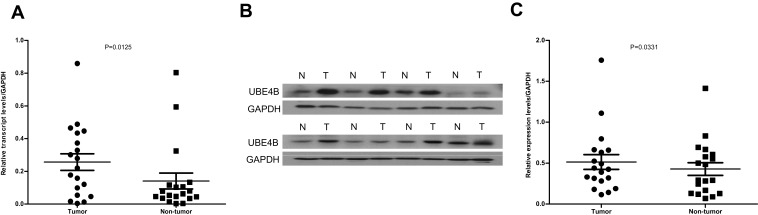
RT-qPCR was applied to determine the mRNA levels of UBE4B in 19 pairs of RCC tissues and the matched nontumor tissues. Compared with the corresponding non-tumor tissues, the mRNA levels of UBE4B were remarkably up-regulated in 14/19 (73.7%) RCC tissues (P= 0.0125; Figure 1A). Similar to the RT-qPCR results, the protein expression levels of UBE4B were remarkably higher in 15/19 (78.9%) of the RCC tissues than the corresponding nontumor tissues (P= 0.0331; Figure 1B and C).
Figure 1.
Expression of UBE4B mRNA and protein in human ccRCC surgical specimens was evaluated by RT-qPCR and Western blotting. (A) RT-qPCR revealed that the relative expression of UBE4B was significantly higher in tumor tissues compared to the matched adjacent non-cancerous tissues (n=19;P=0.0125). (B) Representative Western blotting analysis of UBE4B protein expression in eight paired ccRCC tissues and the matched adjacent non-cancerous tissues (N, matched non-cancerous tissues; T, ccRCC tissues). (C) Relative UBE4B protein expression was higher in tumor tissues than the matched adjacent non-tumor tissues (n =19; P = 0.0331).
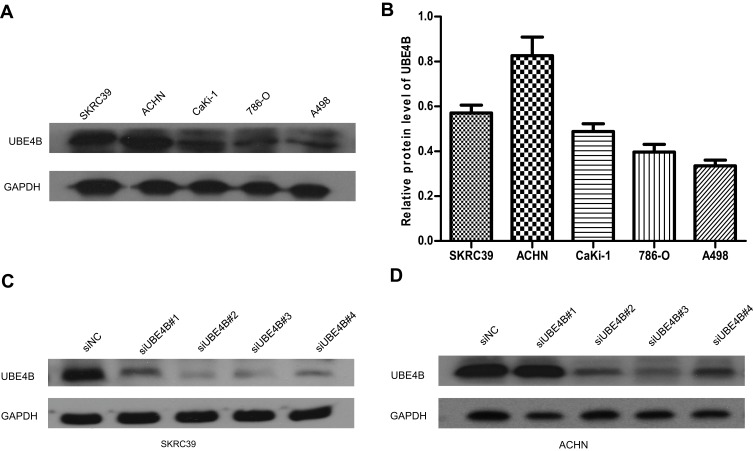
UBE4B expression was tested in five human RCC cell lines so as to choose the most suitable cells to be transfected. Relatively higher expression of UBE4B was found in SKRC39 and ACHN cells than the other cell lines examined by Western blotting (Figure 2A and B). Accordingly, SKRC39 and ACHN were selected as the optimal cells to be transfected with four targeting-siRNAs (siUBE4B#1-4). After 48 hrs’ transfection, the knockdown efficiency of UBE4B was assessed by Western blotting. The outcomes suggested that the level of UBE4B expression was inhibited efficiently in both cell lines transfected with either siUBE4B #2 or siUBE4B #3 (Figure 2C and D).
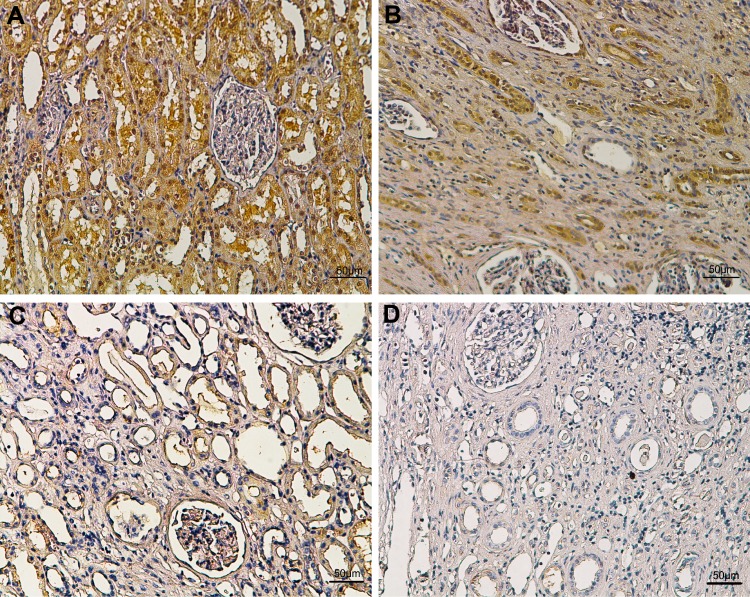
Figure 3.
Representative immunohistochemical images of different staining intensity in ccRCC and surrounding non-tumor tissues. (A) Strong UBE4B staining in ccRCC tissues. (B) Intermediate UBE4B staining in ccRCC tissues. (C) Weak UBE4B staining in ccRCC tissues. (D) Negative staining in surrounding non-tumor tissues. Scale bars: 50μm. Original magnification: ×100.
Figure 2.
Expression of UBE4B protein in RCC cell lines by Western blotting. (A) Representative Western blotting of UBE4B protein expression in five human RCC cell lines. (B) Expression of UBE4B protein in human RCC cell lines was upregulated in ACHN, A498, 786-O, CaKi-1 and SKRC39 cells. Relative protein levels of UBE4B in different cell lines were shown as mean ± SD. (C, D) Among the four UBE4B-targeted small interfering RNAs (siUBE4B), siUBE4B#2 and siUBE4B#3 showed higher knockdown efficiencies after 48 hrs’ transfection. siNC represents negative control small interfering RNAs.
Immunohistochemical UBE4B Intensity and Its Association with the Baseline Variables of RCC Patients
To explore the clinical significance of UBE4B in RCC, the relationship between the expression of UBE4B and baseline features were examined. As indicated in Figure 3, the positive staining of UBE4B was mostly distributed in the cell membrane and/or cytoplasm. The 151 patients were divided into the high UBE4B expression group (n=72) or low UBE4B expression group (n=79). The relationship between UBE4B expression and the baseline variables of RCC were shown in Table 2. X2 test analyses revealed that UBE4B expression in RCC tissues was obviously associated with TNM stage (P<0.001). Moreover, the higher incidence of distant metastases was observed in the high UBE4B expression group (P=0.005), and none of other baseline features were related to UBE4B expression.
Table 2.
Relationship Between UBE4B Expression and Clinic Pathological Features of Patients with ccRCC
| Variables | UBE4B Expression | P value | |
|---|---|---|---|
| Low(79) | High(72) | ||
| Mean±SD age (years) | 49.1±12.2 | 52.4±13.2 | 0.114 |
| Gender | 0.274 | ||
| Male | 56 | 45 | |
| Female | 23 | 27 | |
| Tomor size (cm) | 0.816 | ||
| <5 | 41 | 36 | |
| ≥5 | 38 | 36 | |
| Mean±SD serum AFP(ug/l) | 2.52±1.66 | 2.54±1.67 | 0.919 |
| Fuhrman grade | 0.177 | ||
| Clear cell Ⅰ-Ⅱ | 69 | 57 | |
| Clear cell Ⅲ-Ⅳ | 10 | 15 | |
| TNM Staging | 0.001* | ||
| Ⅰ-Ⅱ | 74 | 53 | |
| Ⅲ-Ⅳ | 5 | 19 | |
| Hematuria | 0.939 | ||
| Negative | 50 | 46 | |
| Positive | 29 | 26 | |
| Albuminuria | 0.644 | ||
| Negative | 70 | 62 | |
| Positive | 9 | 10 | |
| Distant metastasis | 0.005* | ||
| Yes | 5 | 16 | |
| No | 74 | 56 | |
| Renal capsular invasion | 0.871 | ||
| Yes | 38 | 36 | |
| No | 41 | 36 | |
Note: *P < 0.05 was considered statistically significant.
Abbreviations: AFP, alpha-fetoprotein; SD, standard deviation.
Association of UBE4B Expression with RCC Patient Survival
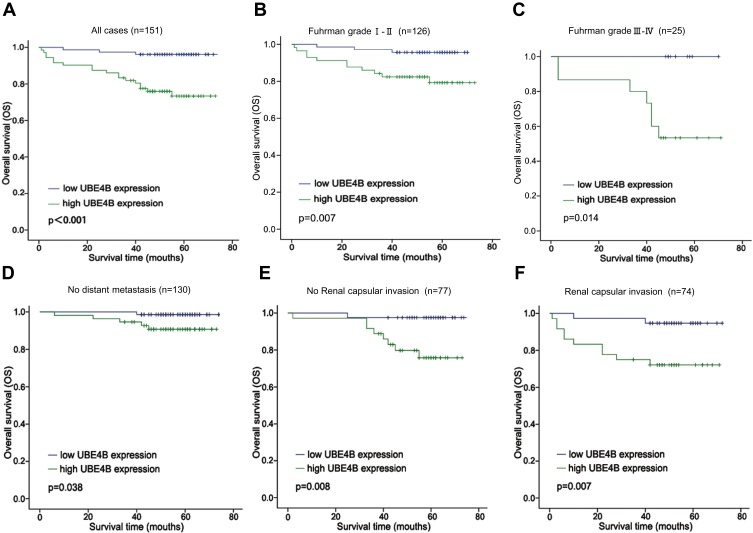
The relationship between UBE4B expression and patient survival was analyzed to assess the prognostic value of UBE4B expression in RCC patients. Kaplan-Meier analyses indicated that worse OS was found in patients of the UBE4B high expression group (P<0.001, Figure 4A). Univariate Cox regression analyses revealed that gender (P=0.036), tumor sizer (P=0.012), histological grade (P=0.036), UBE4B expression (P=0.001) and distant metastasis (P<0.001) were all remarkable risk factors (Table 3). Multivariate Cox regression analyses showed that high UBE4B expression was an independent factor of poor outcome in RCC patients (P= 0.034, Table 3).
Figure 4.
Analysis of overall survival (OS) for patients with ccRCC stratified by UBE4B expression. (A) Kaplan–Meier analysis of OS of all cases (n=151). (B) OS for the subgroup with Fuhrman grade I-II (n = 126). (C) OS for the subgroup with Fuhrman grade III-IV (n = 25). (D) OS for the subgroup with no Distant metastasis (n = 130). (E) OS for the subgroup with no Renal capsular invasion (n=77). (F) OS for the subgroup with Renal capsular invasion (n=74). P-values were calculated using the Log rank test.
Table 3.
Univariate and Multivariate Analysis of Overall Survival in ccRCC
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.009 (0.974–1.046) |
0.603 | — | — |
| Gender (female vs Malea) |
2.505 (1.063–5.902) |
0.036* | 2.374 (0.884–6.089) |
0.087 |
| Tumor size (≥ 5 vs < 5 cma) |
3.616 (1.325–9.873) |
0.012* | 2.374 (0.690–8.165) |
0.170 |
| Fuhrman grade (Ⅲ-Ⅳ vs.Ⅰ-Ⅱa) |
2.642 (1.065–6.559) |
0.036* | 0.668 (0.227–1.961) |
0.668 |
| TNM stage (III + IV vs I + IIa) |
11.971 (4.936–29.013) |
<0.001* | 0.434 (0.078–2.401) |
0.339 |
| AFP | 0.918 (0.686–1.229) |
0.566 | — | — |
| Hematuria (positive vs negativea) |
0.396 (0.133–1.178) |
0.096 | — | — |
| Albuminuria (positive vs negativea) |
0.691 (0.161–2.970) |
0.620 | — | — |
| Renal capsular invasion (positive vs negativea) |
0.343 (0.640–3.615) |
0.343 | — | — |
| UBE4B (high vs lowa) |
7.477 (2.201–25.396) |
0.001* | 4.470 (1.121–17.829) | 0.034* |
| Distant metastasis (positive vs negativea) |
27.272 (10.392–71.570) | <0.001* | 34.945 (7.220–169.138) | <0.001* |
Notes: *P < 0.05 was considered statistically significant. aReference group.
Abbreviations: HR, hazard ratio; CI, confidence interval; AFP, alfa fetoprotein; TNM, tumor, node, metastasis.
To further prove the prognostic value of UBE4B expression in RCC, subgroup analysis based on several clinical variables was conducted. The prognostic value of UBE4B expression was analyzed when patients were grouped based on Fruhman grade, Renal capsular invasion (RCI) and distant metastasis (DM). We found that high and low UBE4B expression groups displayed remarkably different OS according to Fruhman grade (Figure 4B and C), DM (Figure 4D) and RCI (Figure 4E and F). On the whole, these findings showed that UBE4B expression could effectively predict the outcome of RCC patients.
Effect of UBE4B on the Growth of RCC Cells
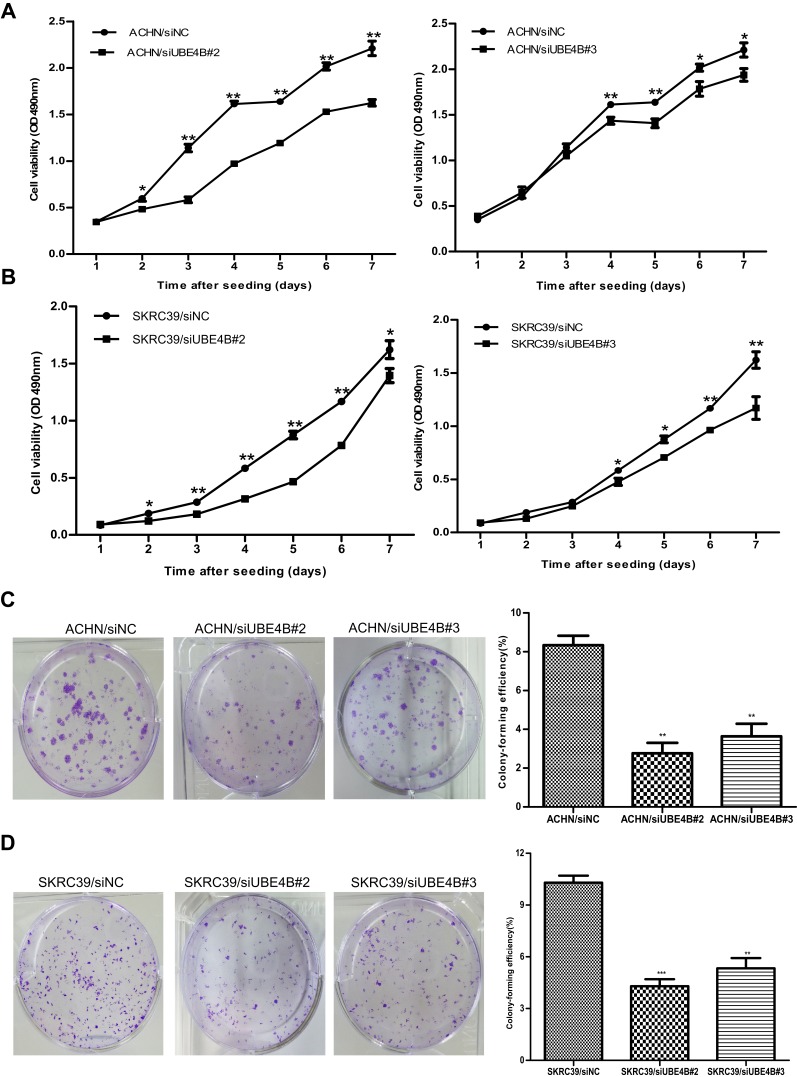
Cell proliferation and colony formation assays were carried out to find out whether UBE4B could influence tumor growth in vitro. SKRC39/siUBE4B and ACHN/siUBE4B cells showed inhibited proliferation (all P<0.05, Figure 5A and B) and colony-forming abilities compared with SKRC39/siNC and ACHN/siNC cells (all P<0.001, Figure 5C and D). These findings revealed that UBE4B may enhance the growth of RCC cells in vitro.
Figure 5.
Inhibition of RCC cell growth ability by UBE4B silencing. (A, B) Proliferation assay results showed that silencing of UBE4B expression inhibited proliferation of ACHN (A) and SKRC39 (B) cells. (C, D) Left: representative images of inhibited colony formation in a monolayer culture by silencing UBE4B. Right: colony-forming efficiencies were calculated after 12 days’ conventional culture. Measurements were carried out in triplicate, and experiments were repeated three times. Data was presented as mean ± SD. P values were calculated using Student’s t-test. *P<0.05, **P <0.01, and ***P<0.001, versus cells transfected with siNC. siNC represents negative control small interfering RNAs.
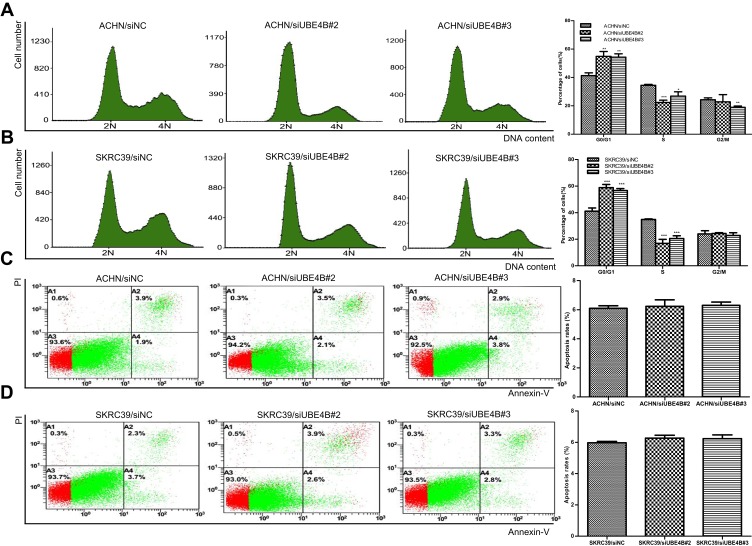
Effect of UBE4B in Cell Cycle and Apoptosis in RCC Cells
Apoptosis and cell cycle analyses were performed to examine whether the UBE4B knockdown-mediated inhibition of RCC cells growth is related to cell cycle arrest or an induction of apoptosis. Results revealed that knockdown of UBE4B in SKRC39 and ACHN cells resulted in more percentages of cells in the G0/G1 phase and fewer percentages of cells in the S phase (P<0.05, Figure 6A and B), and no remarkable differences were found in the percentages of apoptotic cells between RCC cells transfected with siUBE4B and those transfected with siNC (both P>0.05, Figure 6C and D).
Figure 6.
Effects of UBE4B silencing on the cell cycle and apoptosis of of ACHN and SKRC39 cells. (A, B) Effect of UBE4B knockdown on the cell cycle was determined using flow cytometry. UBE4B overexpression caused G0/G1 phase arrest in ACHN (A) and SKRC39 (B) cells. (C, D) Effect of UBE4B knockdown on apoptosis of RCC cells was determined with Annexin V-propidium iodide (PI) staining method. Apoptosis rates were not significantly different between ACHN(C) and SKRC39(D) cells transfected with siUBE4B and those transfected with siNC. P values were calculated using Student’s t-test. *P<0.05, **P<0.01, and ***P<0.001, versus cells transfected with siNC. siNC represents negative control small interfering RNAs.
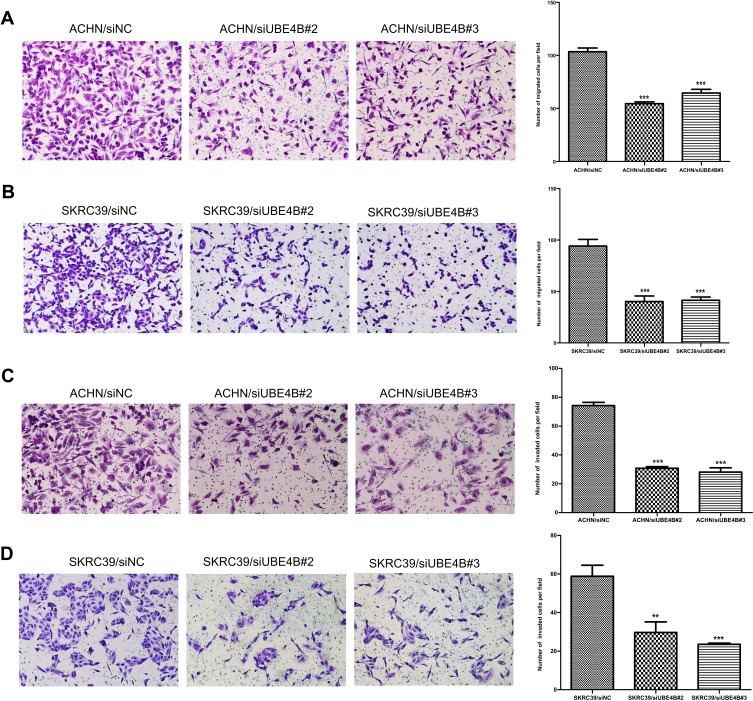
Effect of UBE4B on the Metastasis of RCC Cells
Previous results indicated that UBE4B expression was obviously associated with tumor distant metastasis (Table 2). Subsequent analyses were conducted to ascertain the effect on metastasis in RCC through Boyden chamber migration and Matrigel invasion assays. Transwell assay displayed that the metastasis ability of ACHN and SKRC39 cells transfected with siUBE4B were significantly suppressed compared to controlled cells (all P<0.01, Figure 7A–D).
Figure 7.
Suppression of RCC cell migration and invasion ability by UBE4B silencing. (A, B) UBE4B knockdown using specific siRNAs inhibited the migration ability of ACHN (A) and SKRC39 (B) cells in a Transwell migration assay. (C, D) UBE4B silencing using specific siRNAs remarkably attenuated the invasion ability of ACHN (C) and SKRC39 (D) cells in a Matrigel invasion assay. Representative images are shown on the left (×200 magnification) with quantification of the numbers of cells in ten randomly-selected fields shown on the right. Data was shown as mean ± SD of three independent experiments. P-values were calculated using the Student’s t-test; **P <0.01, ***P <0.001, versus cells transfected with siNC. siNC represents negative control small interfering RNAs.
Discussion
Recent findings on the role of UBE4B in the tumorigenesis of cancer are controversial. In this study, a series of ccRCC tissue samples were used to investigate UBE4B expression and its prognostic value in RCC patients. Meanwhile, a series of in vitro experiments were performed to clarify the potential mechanism of UBE4B in the progression of RCC. Our research showed that UBE4B expression was significantly up-regulated at both transcriptional and translational levels of RCC tissues compared with the corresponding non-tumor tissues, which was similar to findings on breast cancer,21 nasopharyngeal carcinoma23 and hepatocellular carcinoma.24 Immunohistochemical analysis also indicated that the up-regulated UBE4B expression was remarkably associated with advanced TNM stage and distant metastasis. These findings indicated that UBE4B could be served as an oncogene in several kinds of cancers.
This study revealed that high UBE4B expression may be related to a poor clinical outcome in RCC patients. Moreover, multivariate analyses displayed that UBE4B expression was an independent prognostic indicator for OS. Similarly, our previous research had reported the relation between the magnifying expression of UBE4B and unfavorable prognosis in HCC.24 Conversely, studies by Zage et al31 and Sarah et al32 reported that low UBE4B expression was related to an unfavorable prognosis in patients with neuroblastoma. These findings implied that UBE4B might act as a new prognostic indicator for patients with various malignancies.
Previous studies have identified that Histological differentiation and Renal capsular invasion could be independent prognostic variables for ccRCC.33,34 Multivariate Cox regression analyses in this study indicated that distant metastasis was an independent prognostic factor in RCC. To further prove the prognostic value of UBE4B in RCC, we conducted subgroup analysis based on Fruhman grade, Renal capsular invasion and Distant metastasis. The results suggested that no matter with low or high Furhman Grade or other independent prognostic variables, RCC patients which demonstrated high UBE4B expression had poorer OS. All the results of survival analysis highlighted the expression of UBE4B could be an independent prognostic indicator for OS in RCC patients either with low or high tumor burden.
Considering that UBE4B has oncogenic potential, a string of functional studies in RCC cell lines in vitro were conducted to explore whether the reduction of UBE4B expression by siRNA could suppress tumor growth and metastasis. Proliferation and colony formation assays confirmed that silencing of UBE4B suppressed RCC cells growth and motility. Several studies had reported similar cellular functions of UBE4B in various types of solid cancers.21,23,24,35 Weng et al23 discovered that reduction of UBE4B expression could suppress Nasopharyngeal carcinoma cell growth. Zhang et al24 reported that lowering UBE4B expression by siRNA suppressed the growth and mobility ability of HCC cells. Wu et al35 knocked down UBE4B in wild-type human HCT116 colorectal cancer cells with siRNA and assessed growth ability in a mouse model, and they found that the suppression of UBE4B expression could prohibit tumor formation.
Recently, there is immense interest in restoring p53 activity in tumor cells by inhibiting the murine double minute 2 (MDM2, HDM2 in humans). Many relative studies concur that the interaction between MDM2 and p53 is a primary mechanism for inhibition of the p53 function in cancers retaining wild-type p53, and they found that inhibiting the MDM2 expression could reactivate p53 function, triggering p53-dependent cell cycle arrest or apoptosis in tumor cells with functional p53.36–39 Blocking the MDM2-p53 interaction to reactivate the p53 function has been recognized as a promising cancer therapeutic strategy,40,41 and a specific inhibitor of MDM2-p53 interaction is still under research. Currently, it has been reported that UBE4B functions as an E4 ligase is essential and required for MDM2 to promote the polyubiquitination and degradation of p53, thus negatively regulating the stability and function of p53.22,35 Wu et al35 founded that the reduction of UBE4B expression facilitated the ability of p53 to retard BJT fibroblasts in the G1 phase of the cell cycle, which was similar to our finding that lowering UBE4B expression in RCC cells blocked cell cycle progression. Additionally, we learnt that MDM2-p53 interaction played a specific role in regulating the cell cycle in RCC cells.39 Therefore, it is inferred that the function of UBE4B in cell cycle regulation may be involved in the MDM2-p53 pathway in human RCC cells. Our future work will focus on investigating whether UBE4B plays a part in regulating cell cycle-related molecular mechanisms in RCC.
To sum up, this study manifested that UBE4B was overexpressed in RCC, and high UBE4B expression was remarkably related to poor outcome in RCC patients after surgery. Apart from that, cellular function analysis indicated that inhibiting UBE4B expression could restrain RCC cell growth and motility ability, while inducing cell cycle arrest. These results suggested that UBE4B might act as an oncogene in the carcinogenesis of RCC and might serve as an effective indicator to predict OS and a potential biomarker for targeted therapy of RCC patients.
Acknowledgments
This work was supported by grants from the National Key R&D Program of China (No. 2018YFC1313400) and the Frontier and Key Technology Innovation Special Grant from the Department of Science and Technology of Guangdong province (No. 2017B020227003).
Ethical Conduct of Research
Approval for this research was obtained from the SYSUCC Ethics Committee and the patients’ informed consent was acquired before the study. Each experimental procedure that involves human specimens was performed based on the ethical standards.
Author Contributions
All authors were contributors in conception and design, analysis and interpretation of data. All authors took part in drafting the article or revising it critically for important intellectual content. Every author gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ljungberg B, Campbell SC, Choi HY, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60(4):615–621. doi: 10.1016/j.eururo.2011.06.049 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 3.Itsumi M, Tatsugami K. Immunotherapy for renal cell carcinoma. Clin Dev Immunol. 2011;2010(1740–2522):284581. doi: 10.1155/2010/284581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.A I C, J S L, R A F, et al. Surveillance strategies for renal cell carcinoma patients following nephrectomy. Rev Urol. 2006;8(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Girgis AH, Iakovlev VV, Beheshti B, et al. Multilevel whole-genome analysis reveals candidate biomarkers in clear cell renal cell carcinoma. Cancer Res. 2012;72(20):5273–5284. doi: 10.1158/0008-5472.can-12-0656 [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):169–190. doi: 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 7.Srinivasan R, C J R, Sourbier C, et al. New strategies in renal cell carcinoma: targeting the genetic and metabolic basis of disease. Clin Cancer Res. 2015;21(1):10–17. doi: 10.1158/1078-0432.CCR-13-2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lefeuvre M, Gunduz M, Nagatsuka H, et al. Fine deletion analysis of 1p36 chromosomal region in oral squamous cell carcinomas. J Oral Pathol Med. 2009;38(1):94–98. doi: 10.1111/j.1600-0714.2008.00666.x [DOI] [PubMed] [Google Scholar]
- 9.Mammen AL, Mahoney JA, St Germain A, et al. A novel conserved isoform of the ubiquitin ligase UFD2a/UBE4B is expressed exclusively in mature striated muscle cells. PLoS One. 2011;6(12):e28861. doi: 10.1371/journal.pone.0028861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koegl M, Hoppe T, Schlenker S, Ulrich HD, Mayer TU, Jentsch S. A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly. Cell. 1999;96(5):635–644. doi: 10.1016/S0092-8674(00)80574-7 [DOI] [PubMed] [Google Scholar]
- 11.Jordan VK, Zaveri HP, Scott DA. 1p36 deletion syndrome: an update. Appl Clin Genet. 2015;8:189–200. doi: 10.2147/TACG.S65698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nussinov R, Tsai CJ, Liu J. Principles of allosteric interactions in cell signaling. J Am Chem Soc. 2014;136(51):17692–17701. doi: 10.1021/ja510028c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loveless TB, Topacio BR, Vashisht AA, et al. DNA damage regulates translation through β-TRCP targeting of CReP. PLoS Genet. 2015;11(6):e1005292. doi: 10.1371/journal.pgen.1005292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pomerantz JH, Blau HM. Tumor suppressors: enhancers or suppressors of regeneration? Development. 2013;140(12):2502–2512. doi: 10.1242/dev.084210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown CJ, Lain S, Verma CS, Fersht AR, Lane DP. Awakening guardian angels: drugging the p53 pathway. Nat Rev Cancer. 2009;9(12):862–873. doi: 10.1038/nrc2763 [DOI] [PubMed] [Google Scholar]
- 16.Soussi T. The p53 pathway and human cancer. Brit J Surg. 2005;92(11):1331–1332. doi: 10.1002/bjs.5177 [DOI] [PubMed] [Google Scholar]
- 17.Wu H, Leng RP. UBE4B, a ubiquitin chain assembly factor, is required for MDM2-mediated p53 polyubiquitination and degradation. Cell Cycle. 2011;10(12):1912–1915. doi: 10.4161/cc.10.12.15882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi D, Pop MS, Kulikov R, Love IM, Kung AL, Grossman SR. CBP and p300 are cytoplasmic E4 polyubiquitin ligases for p53. Proc Natl Acad Sci USA. 2009;106(38):16275–16280. doi: 10.1073/pnas.0904305106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pant V, Lozano G. Limiting the power of p53 through the ubiquitin proteasome pathway. Genes Dev. 2014;28(16):1739–1751. doi: 10.1101/gad.247452.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du C, Wu H, Leng RP. UBE4B targets phosphorylated p53 at serines 15 and 392 for degradation. Oncotarget. 2016;7(3):2823–2836. doi: 10.18632/oncotarget.6555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Lv Y, Zhang Y, Gao H. Regulation of p53 level by UBE4B in breast cancer. PLoS One. 2014;9(2):e90154. doi: 10.1371/journal.pone.0090154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heuze ML, Lamsoul I, Moog-Lutz C, Lutz PG. Ubiquitin-mediated proteasomal degradation in normal and malignant hematopoiesis. Blood Cells Mol Dis. 2008;40(2):200–210. doi: 10.1016/j.bcmd.2007.07.011 [DOI] [PubMed] [Google Scholar]
- 23.Weng C, Chen Y, Wu Y, et al. Silencing UBE4B induces nasopharyngeal carcinoma apoptosis through the activation of caspase3 and p53. Onco Targets Ther. 2019;12:2553–2561. doi: 10.2147/OTT.S196132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang XF, Pan QZ, Pan K, et al. Expression and prognostic role of ubiquitination factor E4B in primary hepatocellular carcinoma. Mol Carcinog. 2016;55(1):64–76. doi: 10.1002/mc.22259 [DOI] [PubMed] [Google Scholar]
- 25.De Vivar Chevez AR, Finke J, Bukowski R. The role of inflammation in kidney cancer. Adv Exp Med Biol. 2014;816:197–234. doi: 10.1007/978-3-0348-0837-8_9 [DOI] [PubMed] [Google Scholar]
- 26.Escudier B, Eisen T, Porta C, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(7):65–71. doi: 10.1093/annonc/mds227 [DOI] [PubMed] [Google Scholar]
- 27.Eble JN, Sauter G, Epstein JI, Sesterhen IA. Worle Health Organization Classification of Tumour. Pathology and Genetics of Tumors of the Urinary Systerm and Male Genital Organs. Lyon: IARC Press; 2004. [Google Scholar]
- 28.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Patho. 1982;6(7):655–663. doi: 10.1097/00000478-198210000-00007 [DOI] [PubMed] [Google Scholar]
- 29.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual (7th Ed). New York: Springer; 2010. [Google Scholar]
- 30.Huang XQ, Zhou ZQ, Zhang XF, et al. Overexpression of SMOC2 attenuates the tumorigenicity of hepatocellular carcinoma cells and is associated with a positive postoperative prognosis in human hepatocellular carcinoma. J Cancer. 2017;8(18):3812–3827. doi: 10.7150/jca.20775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zage PE, Sirisaengtaksin N, Liu Y, et al. UBE4B levels are correlated with clinical outcomes in neuroblastoma patients and with altered neuroblastoma cell proliferation and sensitivity to epidermal growth factor receptor inhibitors. Cancer. 2013;119(4):915–923. doi: 10.1002/cncr.27785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodfield SE, Guo RJ, Liu Y, et al. Neuroblastoma patient outcomes, tumor differentiation, and ERK activation are correlated with expression levels of the ubiquitin ligase UBE4B. Genes Cancer. 2016;7(1–2):13–26. doi: 10.18632/genesandcancer.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ha US, Lee KW, Jung JH, et al. Renal capsular invasion is a prognostic biomarker in localized clear cell renal cell carcinoma. Sci Rep. 2018;8(1):202–210. doi: 10.1038/s41598-017-18466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh RR, Murugan P, Patel LR, et al. Intratumoral morphologic and molecular heterogeneity of rhabdoid renal cell carcinoma: challenges for personalized therapy. Mod Pathol. 2015;28(9):1225–1235. doi: 10.1038/modpathol.2015.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu H, Pomeroy SL, Ferreira M, et al. UBE4B promotes Hdm2-mediated degradation of the tumor suppressor p53. Nat Med. 2011;17(3):347–355. doi: 10.1038/nm.2283 [DOI] [PubMed] [Google Scholar]
- 36.De Rozieres S, Maya R, Oren M, Lozano G. The loss of mdm2 induces p53 mediated apoptosis. Oncogene. 2000;19(13):1691–1697. doi: 10.1038/sj.onc.1203468 [DOI] [PubMed] [Google Scholar]
- 37.Safiran YJ, Oberoi P, Kenten JH, Philliphs AC, Weissman AM, Vousden KH. Small molecule inhibitors of HDM2 ubiquitin ligase activity stabilize and activate p53 in cells. Cancer Cell. 2005;7:547–559. doi: 10.1016/j.ccr.2005.04.029 [DOI] [PubMed] [Google Scholar]
- 38.Ringshausen I, O’Shea CC, Finch AJ, Swigart LB, Evan GI. Mdm2 is critically and continuously required to suppress lethal p53 activity in vivo. Cancer Cell. 2006;10:501–514. doi: 10.1016/j.ccr.2006.10.010 [DOI] [PubMed] [Google Scholar]
- 39.Warburton HE, Brady M, Vlatkovic´ N, Linehan WH, Parsons K, Boyd MT. p53 regulation and function in renal cell carcinoma. Cancer Res. 2005;65(15):6498–6503. doi: 10.1158/0008-5472.CAN-05-0017 [DOI] [PubMed] [Google Scholar]
- 40.Chène P. Inhibiting the p53–MDM2 interaction: an important target for cancer therapy. Nat Rev Cancer. 2003;3(2):102–109. doi: 10.1038/nrc991 [DOI] [PubMed] [Google Scholar]
- 41.Shangary S, Wang S. Targeting the MDM2-p53 interaction for cancer therapy. Clin Cancer Res. 2008;14(17):5318–5324. doi: 10.1158/1078-0432.CCR-07-5136 [DOI] [PMC free article] [PubMed] [Google Scholar]