Abstract
Previous studies have revealed faster detection of snake images in humans and non-human primates (NHPs), suggesting automatic detection of evolutionary fear-relevant stimuli. Furthermore, human studies have indicated that general fear-relevance rather than evolutionary relevance is more effective at capturing attention. However, the issue remains unclarified in NHPs. Thus, in the present study, we explored the attentional features of laboratory-reared monkeys to evolutionary and general fear-relevant stimuli (e.g., images of snakes, capturing gloves). Eye-tracking technology was utilized to assess attentional features as it can provide more accurate latency and variables of viewing duration and frequency compared with visual search task (VST) and response latency adopted in previous studies. In addition, those with autism spectrum disorder (ASD) show abnormal attention to threatening stimuli, including snake images. Rett syndrome (RTT) is considered a subcategory of ASD due to the display of autistic features. However, the attentional features of RTT patients or animal models to such stimuli remain unclear. Therefore, we also investigated the issue in MECP2 gene-edited RTT monkeys. The influence of different cognitive loads on attention was further explored by presenting one, two, or four images to increase stimulus complexity. The eye-tracking results revealed no significant differences between RTT and control monkeys, who all presented increased viewing (duration and frequency) of snake images but not of aversive stimuli compared with control images, thus suggesting attentional preference for evolutionary rather than general fear-relevant visual stimuli. Moreover, the preference was only revealed in visual tasks composed of two or four images, suggesting its cognitive-load dependency.
Keywords: Non-human primates, Attention, Snake, Evolutionary relevance
INTRODUCTION
Animals can acquire fear response to neutral stimuli through associative learning. For instance, laboratory-reared rodents can learn fear response to neutral sounds/environments through fear conditioning (Maren, 2001). However, animals demonstrate instant fear to evolutionary-relevant predators. For example, rodents display fear response to cat odor despite never having encountered a live cat, suggesting the development of innate fear to natural predators over eons of evolution (Dielenberg & McGregor, 2001). In contrast, early non-human primate (NHP) studies by Mineka et al. (1980) have suggested the requirement of associative learning for the development of fear response to snakes, a primary predator of NHPs from an evolutionary perspective (Isbell, 2006; Öhman, 2009). For instance, their studies have indicated that wild monkeys demonstrate intense snake fear, probably due to encountering snakes in the wild, whereas laboratory-reared monkeys do not (Cook & Mineka, 1990; Mineka et al., 1980). However, laboratory-reared monkeys have been shown to acquire a preferential fear response to toy snakes rather than to flowers by observational learning, suggesting differential perception of evolutionary fear-relevant and -irrelevant stimuli (Cook & Mineka, 1989, 1990).
Earlier research proposed an evolved module for fear learning, suggesting automatic detection and unconscious perception of evolutionary fear-relevant stimuli by a primitive evolved subcortical brain network (Öhman & Mineka, 2001, 2003). Subsequent human and NHP studies indeed revealed faster detection of snake images compared with images of flowers or mushrooms using the visual search task (VST), thus supporting the fear learning module (Blanchette, 2006; Kawai & Koda, 2016; Shibasaki & Kawai, 2009; Soares et al., 2014). However, faster detection of general fear-relevant stimuli, such as knifes, guns, and syringes, has also been revealed in humans, suggesting more importance of general fear-relevance than evolutionary relevance in capturing attention (Blanchette, 2006; Brown et al., 2010; Forbes et al, 2011). With the issue remaining unclarified in NHPs, we explored the attentional features of laboratory-reared NHPs to general fear-relevant stimuli (e.g., images of capturing gloves, nets) in addition to evolutionary fear-relevant stimuli (e.g., images of snakes). In regard to the accuracy of response latency, the stimulus detection speed in previous VST studies is inevitably affected by the additional body and hand responses after the initial target detection. Thus, in the current study, we utilized eye-tracking, a widely adopted technology in previous NHP attentional studies, as it provides more accurate latency and additional variables of attention, such as viewing duration and frequency (Dal Monte et al., 2015; Gothard et al., 2004; Machado et al., 2011; Zhang et al., 2012).
Rett syndrome (RTT), which is considered a category of autism spectrum disorder (ASD) due to its display of autistic features, exhibits unique attentional patterns (Rose et al., 2013, 2016). Although ASD patients demonstrate abnormal attention to threatening stimuli, including snake images (Isomura et al., 2015; Milosavljevic et al., 2017), their attentional features to such stimuli remain unclear. Changes in methyl-CpG-binding protein 2 (MECP2) gene expression are associated with neurodevelopmental disorders, such as ASD and RTT (Qiu, 2018). Therefore, we established the first MECP2 gene mutant RTT monkey model to explore the cognitive phenotypes and potential behavioral interventions of the syndrome (Chen et al., 2017). We recently reported increased attention of RTT monkeys to salient social stimuli (conspecific stare and profile faces) compared with control monkeys, indicating social valence-related attentional preference (Zhang et al., 2019b). Thus, we further investigated the attentional features of RTT monkeys to evolutionary-related or general fear-relevant visual stimuli.
MATERIALS AND METHODS
Subjects
Seven laboratory-reared control and five gene-edited RTT cynomolgus monkeys (Macaca fascicularis, females, 26–49 months old, weighing 2.2–3.9 kg) were used in the present study. All were born and raised at the Yunnan Key Laboratory of Primate Biomedical Research Center, Kunming, China. They were housed in a controlled environment during the experiment (temperature: 22±1 °C; humidity: 50%±5% RH) under a 12 h light/12 h dark cycle (lights on at 0800h). All animals were feed a commercial monkey diet twice a day, with fruit and vegetable enrichment provided once a day and with tap water provided ad libitum. The animal facility is accredited by AAALAC International and all experimental and animal care procedures were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the Yunnan Key Laboratory of Primate Biomedical Research.
Eye-tracking experiment
The noninvasive head restraint method for NHP eye-tracking has been described in our previous reports (Zhang et al., 2012, 2019b). Briefly, the restraint helmet was made with thermoplastic mesh sheets (30 cm wide×30 cm long×0.32 cm thick) bordered on four sides by a hard, plastic frame, through which the helmet was attached to the primate chair with wing bolts. The helmet was made pliable by soaking in warm water, then stretched by the experimenter and gently placed and molded closely over the monkey's head (subjects were sedated with ketamine hydrochloride, 5–10 mg/kg, im). The front part of the helmet was then cut to expose the eye and snout regions once the thermoplastic sheet became rigid after cooling.
The detailed eye-tracking procedures, including the apparatus setup, behavioural adaptation, and pre-test calibration, have been described previously (Zhang et al., 2019b). Briefly, a Tobii Pro TX300 Eye Tracker (Tobii Technology AB, Danderyd, Sweden) was set in front of each monkey at a distance of 65 cm, with infrared illumination and cameras used to induce and detect pupil and corneal reflections, respectively, which were then integrated with an internal model to enable calculation of gaze data. Gaze data were sampled at 300 Hz and integrated with a 23 inch monitor at a resolution of 1 920×1 080 pixels, with a single fixation then registered for each gaze point falling within a 0.5° visual angle radius over 75 ms. Visual stimulus presentation and gaze data were generated and collected on a PC with Tobii Studio software (v3.4.7). Monkeys were gradually habituated to the testing environment and manipulations in the testing room with lights turned off to diminish distraction. Pre-test calibration was performed by presenting small circles or audio cartoon symbols at pre-set locations to attract attention. Results were then presented graphically on the screen, with a small green dot in the center of a circle representing qualified high-quality calibration (Figure 1C). Low-quality calibration resulted in green colored lines extending from the circle, with the number, length, and dispersion of the lines representing the extent of mismatch between acquired data and actual calibration image location. Acceptance of calibration results was determined by visual inspection by an experienced experimenter. The calibration routine was repeated to attain satisfactory results within 5 min, or else the task was terminated for the subject.
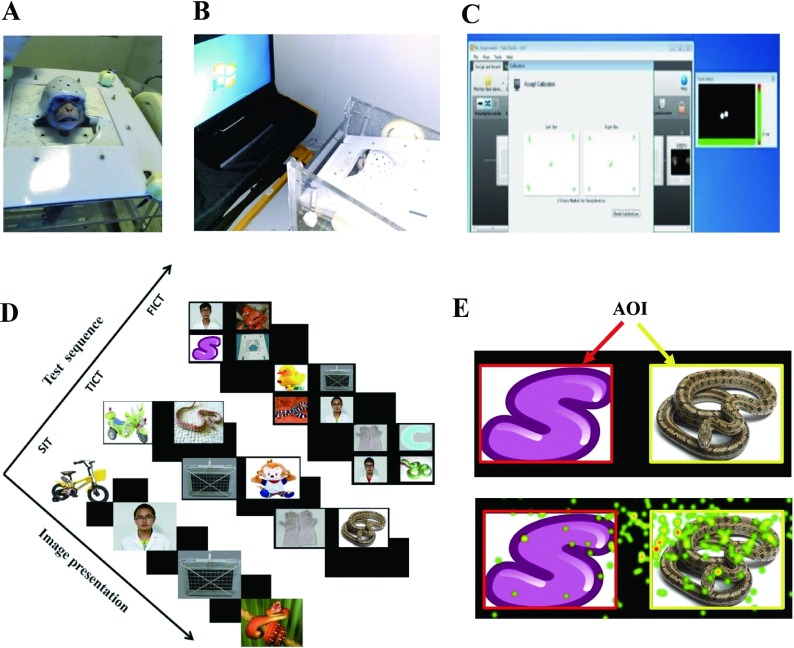
1. Demonstrations of the eye-tracking apparatus, procedure and visual stimuli.
A: Non-invasive head restraint method. B: Behavioral eye-tracking setup, in which a conscious monkey wearing a restraint helmet sits in a primate chair and faces the Tobii Pro TX300 Eye Tracker. C: Five-point calibration results for both eyes indicated by green dots. D: Description of three tasks performed sequentially, including single image test (SIT), two image comparison test (TICT), and four image comparison test (FICT). E: Top panel, example of manually drawn areas of interest (AOIs, red and yellow squares); bottom panel, heatmap example of eye-tracking results within different AOIs, with red representing most attended area. B and C are quoted and modified from our previous report (Zhang et al., 2019b).
Four categories of visual stimuli were used, including evolutionary fear-relevant stimuli (snakes), general fear-relevant aversive stimuli (capturing gloves, nets), control stimuli (toys, such as ducks and hello kitty; abstract graphics, such as letter S in Figure 1E) and familiar human neutral faces. Images of conspecific faces were used in previous investigation and, therefore, were not included in the present study so as to avoid the potential influence of stimulus overexposure (Zhang et al., 2019b). To explore the influence of cognitive load on attention, increased complexity of visual stimuli was introduced by presenting one image, two paired images, and then four paired images on the screen across paradigms of single image test (SIT), two image comparison test (TICT), and four image comparison test (FICT), respectively. For SIT, eight images were used in each category (dimension 570×380 pixels). For TICT, three categories of paired images were used, including aversive stimuli-control, aversive stimuli-snake, and snake-control, with eight pairs for each category (dimension 1 700×550 pixels). For FICT, 10 paired images were used (dimension 1 700×1 050 pixels), with each image composed of one picture from each category. Each image was presented once consequentially, with 32 trials in SIT, 24 trials in TICT, and 10 trials in FICT. The sequence of tasks was SIT, TICT, and then FICT, with each task performed once (Figure 1D). The order of presented images was randomized by Tobii software and the presentation duration of each image was 5 s in SIT, 8 s in TICT, and 15 s in FICT, with 2 s of blank image as an inter-trial interval.
Data analyses
Rectangular areas of interest (AOIs) were hand drawn by the analyzer on each image (Figure 1E), with Tobii Studio software (v3.4.7) automatically calculating total viewing duration, frequency, and latency within different AOIs. All variables were subject to square root transformation to generate normally distributed data for further statistical analyses using SPSS 19.0 (SPSS Inc, Chicago, Illinois, USA). The data were assessed by analysis of variance (ANOVA) with repeated measures, with groups being the between-subjects factor and stimuli being the within-subjects factor. All data were presented as means±SEM and differences were considered significant at P<0.05.
RESULTS
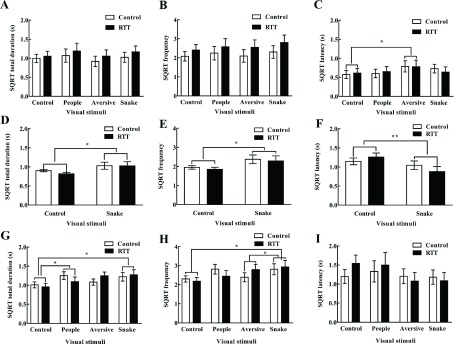
Two-way repeated ANOVA revealed no significant interaction between group and stimuli in SIT (duration F(3,30)=0.157, frequency F(3,30)=0.189, latency F(3,30)=0.513), snake-control comparison (duration F(1,10)=0.288, frequency F(1,10)=0.01, latency F(1,10)=3.452), and FICT (duration F(3,30)=1.186, frequency F(3,30)=1.35, latency F(3,30)=1.104) (all P>0.05). Therefore, the main effects of group and stimuli were further explored. ANOVA also revealed no significant group effect in SIT (durationF(1,10)=0.33, frequency F(1,10)=0.78, latency F(1,10)=0), snake-control comparison (duration F(1,10)=0.328, frequency F(1,10)=0.21, latency F(1,10)=0.042), and FICT (duration F(1,10)=0.001, frequency F(1,10)=0.01, latency F(1,10)=0.069) (all P>0.05). Results indicated similar attentional features between RTT and control monkeys, independent of differential cognitive load. However, ANOVA revealed a significant stimulus effect in the snake-control comparison (durationF(1,10)=5.182, frequency F(1,10)=6.388) and FICT (duration F(3,30)=3.099, frequency F(3,30)=3.356) (all P<0.05). The subjects presented significantly increased viewing (duration and frequency) of snakes compared with control images in the snake-control comparison (Figure 2D, E) and FICT (post hoc comparison, duration P=0.022, frequency P=0.015) (Figure 2G, H). In contrast, ANOVA revealed no significant stimulus effect in SIT (duration F(3,30)=1.805, frequency F(3,30)=1.813, all P>0.05) (Figure 2A, B). Additionally, results showed significantly reduced latency viewing of snakes compared with control images in the snake-control image comparison (F(1,10)=10.165, P=0.01) (Figure 2F), but not in SIT or FICT (Figure 2C, I), suggesting task-dependent faster detection of snake images. Thus, these results suggest task-dependent attentional preference for snake images compared with control images in both groups of monkeys.
2. Single image test with variables of viewing duration (A), frequency (B), and latency (C). Snake-control comparison with variables of viewing duration (D), frequency (E), and latency (F). Four image comparison with variables of viewing duration (G), frequency (H), and latency (I).
All variables were subjected to square root transformation and represented as means±SEM. SQRT: Square root transformation, *: P<0.05, **: P<0.01.
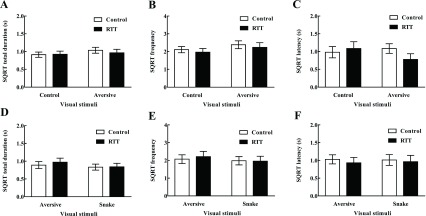
In addition to evolutionary fear-relevant snakes, the present study also included general fear relevant aversive stimuli, such as capturing gloves and nets, which the subjects have encountered and presented fear responses to in daily life. Two-way ANOVA revealed no significant differences between aversive and control image viewing in SIT and FICT (all P> 0.05) (Figure 2A, B, G, H) and TICT (duration F(1,10)=2.5, frequency F(1,10)=4.735, all P>0.05) (Figure 3A, B), indicating no attentional preference for aversive stimuli compared with control images. Together with the snake attentional preference findings, these results suggest attentional preference for evolutionary fear-relevant rather than general fear-relevant stimuli in both groups of monkeys. However, direct comparison of snake-aversive stimuli revealed no significant viewing difference (duration F(1,10)=2.025, frequency F(1,10)=1.435, latency F(1,10)=0.003, all P>0.05) (Figure 3D–F), indicating task/comparison dependency of the preference.
3. Aversive-control comparison with variables of viewing duration (A), frequency (B), and latency (C). Aversive-snake comparison with variables of viewing duration (D), frequency (E), and latency (F).
All variables were subjected to square root transformation and represented as means±SEM. SQRT: Square root transformation.
DISCUSSION
Compared with non-primate animals, NHPs share considerably high similarities with humans in various aspects, such as their genomes and highly developed brains, which make them ideal models for translational medical research (Izpisua Belmonte et al., 2015; Kaas, 2013; Wu et al., 2017; Zhang, 2017, 2019a). For instance, macaque monkeys (genus Macaca) are suitable for visual cognition research as they possess trichromatic color vision and high visual acuity (Jacobs, 2008; Orban et al., 2004). Snakes are primary predators of NHPs from an evolutionary perspective (Isbell, 2006; Öhman, 2009), and previous studies have indicated NHPs can acquire fear of snakes via associative learning (Cook & Mineka, 1990; Mineka et al., 1980). Subsequent studies have also found behavioral inhibition (Nelson et al., 2003) and faster detection of snake images (Kawai & Koda, 2016; Shibasaki & Kawai, 2009) in laboratory-reared monkeys, consistent with the evolved fear learning module, which suggests automatic detection of evolutionary fear-relevant snakes (Öhman & Mineka, 2001, 2003).
Related attentional studies have mainly utilized the VST paradigm and consequential behavioral response latency to assess visual attention, with accuracy inevitably affected due to the combination of both hand and body responses (Blanchette, 2006; Kawai & Koda, 2016; Shibasaki & Kawai, 2009; Soares et al., 2014). In contrast, we explored snake attention in laboratory-reared monkeys based on eye-tracking, which provides more accurate attentional latency and additional variables of viewing duration and frequency. In addition to evolutionary fear-relevant snake attention, faster detection of general fear-relevant stimuli has been revealed in humans (Blanchette, 2006; Brown et al., 2010; Forbes et al., 2011; Smith et al., 2003). However, the attentional features to general fear-relevant stimuli remain to be elucidated in NHPs. As such, we included general fear-relevant images, such as capturing gloves and nets, to explore the issue in laboratory-reared monkeys. We recently established the first MECP2 gene mutant RTT monkey model to explore the visual cognitive phenotypes associated with the syndrome and found that social valence increased attention in the monkeys (Zhang et al., 2019b). In the present study, we further investigated the attentional features of RTT monkeys to evolutionary and general fear-relevant stimuli.
We found similar attentional features between RTT and control monkeys, both demonstrating increased viewing duration and frequency of snake images but not of aversive stimuli compared with control images (Figure 2D, E, G, H; Figure 3A, B). The monkeys also demonstrated faster detection of snake images and delayed detection of aversive stimuli, as indicated by decreased (Figure 2F) and increased (Figure 2C, P=0.03) latency, respectively. These results suggest attentional preference (increased viewing and faster detection) for evolutionary related rather than general fear-relevant stimuli, providing the first NHP eye-tracking evidence for the previously proposed evolved fear learning module (Öhman & Mineka, 2001). This null preference for general fear-relevant aversive stimuli is inconsistent with the faster detection findings in humans (Blanchette, 2006; Brown et al., 2010; Forbes et al., 2011; Smith et al., 2003). This inconsistency may be caused by differences in testing paradigms, as VST requires additional hand touching response after the initial visual detection of the target image compared with the eye-tracking procedure. Alternatively, this inconsistency may be due to the unique ability of humans to use oral/written language for information processing and transmission. For instance, humans can learn that general fear-relevant stimuli (e.g., knife, gun) are threatening from various indirect sources such as parents, books, and news reports. In contrast, animals can only acquire fear response to stimuli through direct experience or observational learning.
We utilized images with different complexity to explore the potential influence of cognitive load on visual attention. Results revealed increased viewing and faster detection of snake images in TICT and FICT (Figure 2), and delayed detection of aversive stimuli in SIT (Figure 2C, latency P=0.03), suggesting cognitive load-affected attention. The SIT only requires passive viewing, whereas TICT and FICT require advanced selective attention and comparison. Thus, this may contribute to the subjects demonstrating increased viewing duration and frequency across SIT (duration 1 s, frequency two counts), TICT (duration 2 s, frequency four counts), and FICT (duration 4 s, frequency eight counts). Alternatively, the increased viewing may be caused by increased duration of image presentation across tests (5 s in SIT, 8 s in TICT, and 15 s in FICT). We also found increased viewing (duration 1 s, frequency two counts) of single images presented in SIT (Figure 2A, B) compared with our previous findings (duration 0.6 s, frequency one count) (Zhang et al., 2012). This difference could potentially be caused by different variables, e.g., subjects (female cynomolgus monkeys vs. male rhesus monkeys), visual stimuli (four categories vs. conspecific profile faces), testing apparatus (Tobii Pro TX300 vs. T60 Eye Tracker), and definition of one fixation (gaze maintained within 0.5° visual angle for 75 vs. 100 ms).
Consistent with the present eye-tracking findings of snake attentional preference, previous studies have provided electrophysiological evidence of faster detection of snake images in the pulvinar neurons of macaque monkeys (Le et al., 2016; Van Le et al., 2013). However, automatic attention of snakes does not necessarily mean automatic negative emotion (such as fear) (Purkis & Lipp, 2007). Therefore, further studies are required to validate the direct association between snake attentional preference and fear emotion/response. It would be informative to know whether monkeys present fear-associated biological changes during eye-tracking investigations, such as increased blood pressure, heart rhythm, pupil size, and amygdala activities.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS' CONTRIBUTIONS
B. Z. designed the experiment, analyzed the data, and prepared the manuscript. Z.G.Z. and Y.Z. performed the experiments. Y.C.C. participated in experimental design and manuscript preparation. All authors read and approved the final version of the manuscript.
Funding Statement
This work was supported by the National Natural Science Foundation of China (U1602224), Guangxi Key Laboratory of Brain and Cognitive Neuroscience, China (GKLBCN-20190101), Yunnan Basic Research Program, China (2018FB114), the National Key Research and Development Program of China (2016YFA0101401, 2017YFC1001902, 2018YFA0107902)
References
- 1.Blanchette I Snakes, spiders, guns, and syringes: how specific are evolutionary constraints on the detection of threatening stimuli? Quarterly Journal of Experimental Psychology. 2006;59(8):1484–1504. doi: 10.1080/02724980543000204. [DOI] [PubMed] [Google Scholar]
- 2.Brown C, El-Deredy W, Blanchette I Attentional modulation of visual-evoked potentials by threat: investigating the effect of evolutionary relevance. Brainand Cognition. 2010;74(3):281–287. doi: 10.1016/j.bandc.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Yu J, Niu Y, Qin D, Liu H, Li G, Hu Y, Wang J, Lu Y, Kang Y, Jiang Y, Wu K, Li S, Wei J, He J, Wang J, Liu X, Luo Y, Si C, Bai R, Zhang K, Liu J, Huang S, Chen Z, Wang S, Chen X, Bao X, Zhang Q, Li F, Geng R, Liang A, Shen D, Jiang T, Hu X, Ma Y, Ji W, Sun YE Modeling rett syndrome using talen-edited MECP2 mutant cynomolgus monkeys. Cell. 2017;169(5):945–955.e10. doi: 10.1016/j.cell.2017.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook M, Mineka S Observational conditioning of fear to fear-relevant versus fear-irrelevant stimuli in rhesus monkeys. Journal of Abnormal Psychology. 1989;98(4):448–459. doi: 10.1037/0021-843X.98.4.448. [DOI] [PubMed] [Google Scholar]
- 5.Cook M, Mineka S Selective associations in the observational conditioning of fear in rhesus monkeys. Journal of Experimental Psychology Animal Behavior Processes. 1990;16(4):372–389. doi: 10.1037/0097-7403.16.4.372. [DOI] [PubMed] [Google Scholar]
- 6.Dal Monte O, Costa VD, Noble PL, Murray EA, Averbeck BB Amygdala lesions in rhesus macaques decrease attention to threat. Nature Communications. 2015;6:10161. doi: 10.1038/ncomms10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dielenberg RA, Mcgregor IS Defensive behavior in rats towards predatory odors: a review. Neuroscience and Biobehavioral Reviews. 2001;25(7–8):597–609. doi: 10.1016/s0149-7634(01)00044-6. [DOI] [PubMed] [Google Scholar]
- 8.Forbes SJ, Purkis HM, Lipp OV Better safe than sorry: simplistic fearrelevant stimuli capture attention. Cognition and Emotion. 2011;25(5):794–804. doi: 10.1080/02699931.2010.514710. [DOI] [PubMed] [Google Scholar]
- 9.Gothard KM, Erickson CA, Amaral DG How do rhesus monkeys (Macaca mulatta) scan faces in a visual paired comparison task? Animal Cognition. 2004;7(1):25–36. doi: 10.1007/s10071-003-0179-6. [DOI] [PubMed] [Google Scholar]
- 10.Isbell LA Snakes as agents of evolutionary change in primate brains. Journal of Human Evolution. 2006;51(1):1–35. doi: 10.1016/j.jhevol.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Isomura T, Ogawa S, Shibasaki M, Masataka N Delayed disengagement of attention from snakes in children with autism. Frontiers in Psychology. 2015;6:241. doi: 10.3389/fpsyg.2015.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izpisua Belmonte JC, Callaway EM, Caddick SJ, Churchland P, Feng G, Homanics GE, Lee KF, Leopold DA, Miller CT, Mitchell JF, Mitalipov S, Moutri AR, Movshon JA, Okano H, Reynolds JH, Ringach D, Sejnowski TJ, Silva AC, Strick PL, Wu J, Zhang F Brains, genes, and primates. Neuron. 2015;86(3):617–631. doi: 10.1016/j.neuron.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs GH Primate color vision: a comparative perspective. Visual Neuroscience. 2008;25(5–6):619–633. doi: 10.1017/S0952523808080760. [DOI] [PubMed] [Google Scholar]
- 14.Kaas JH The evolution of brains from early mammals to humans. Wiley Interdisciplinary Reviews Cognitive Science. 2013;4(1):33–45. doi: 10.1002/wcs.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawai N, Koda H Japanese monkeys (Macaca fuscata) quickly detect snakes but not spiders: evolutionary origins of fear-relevant animals . Journal of Comparative Psychology. 2016;130(3):299–303. doi: 10.1037/com0000032. [DOI] [PubMed] [Google Scholar]
- 16.Le QV, Isbell LA, Matsumoto J, Le VQ, Nishimaru H, Hori E, Maior RS, Tomaz C, Ono T, Nishijo H Snakes elicit earlier, and monkey faces, later, gamma oscillations in macaque pulvinar neurons. Scientific Reports. 2016;6:20595. doi: 10.1038/srep20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machado CJ, Bliss-Moreau E, Platt ML, Amaral DG Social and nonsocial content differentially modulates visual attention and autonomic arousal in Rhesus macaques. PLoS One. 2011;6(10):e26598. doi: 10.1371/journal.pone.0026598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maren S Neurobiology of Pavlovian fear conditioning. Annual Review of Neuroscience. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- 19.Milosavljevic B, Shephard E, Happe FG, Johnson MH, Charman T Anxiety and attentional bias to threat in children at increased familial risk for Autism Spectrum Disorder. Journal of Autism and Developmental Disorders. 2017;47(12):3714–3727. doi: 10.1007/s10803-016-3012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mineka S, Keir R, Price V Fear of snakes in wild- and laboratory-reared rhesus monkeys (Macaca mulatta) . Animal Learning & Behavior. 1980;8(4):653–663. [Google Scholar]
- 21.Nelson EE, Shelton SE, Kalin NH Individual differences in the responses of naive rhesus monkeys to snakes. Emotion. 2003;3(1):3–11. doi: 10.1037/1528-3542.3.1.3. [DOI] [PubMed] [Google Scholar]
- 22.Öhman A Of snakes and faces: an evolutionary perspective on the psychology of fear. Scandinavian Journal of Psychology. 2009;50(6):543–552. doi: 10.1111/j.1467-9450.2009.00784.x. [DOI] [PubMed] [Google Scholar]
- 23.Öhman A, Mineka S Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychological Review. 2001;108(3):483–522. doi: 10.1037/0033-295X.108.3.483. [DOI] [PubMed] [Google Scholar]
- 24.Öhman A, Mineka S The malicious serpent: snakes as a prototypical stimulus for an evolved module of fear. Current Directions in Psychological Science. 2003;12(1):5–9. doi: 10.1111/1467-8721.01211. [DOI] [Google Scholar]
- 25.Orban GA, Van Essen D, Vanduffel W Comparative mapping of higher visual areas in monkeys and humans. Trends in Cognitive Sciences. 2004;8(7):315–324. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Purkis HM, Lipp OV Automatic attention does not equal automatic fear: preferential attention without implicit valence. Emotion. 2007;7(2):314–323. doi: 10.1037/1528-3542.7.2.314. [DOI] [PubMed] [Google Scholar]
- 27.Qiu Z Deciphering MECP2-associated disorders: disrupted circuits and the hope for repair. Current Opinion in Neurobiology. 2018;48:30–36. doi: 10.1016/j.conb.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Rose SA, Djukic A, Jankowski JJ, Feldman JF, Fishman I, Valicenti-Mcdermott M Rett syndrome: an eye-tracking study of attention and recognition memory. Developmental Medicine & Child Neurology. 2013;55(4):364–371. doi: 10.1111/dmcn.12085. [DOI] [PubMed] [Google Scholar]
- 29.Rose SA, Djukic A, Jankowski JJ, Feldman JF, Rimler M Aspects of attention in Rett Syndrome. Pediatric Neurology. 2016;57:22–28. doi: 10.1016/j.pediatrneurol.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Shibasaki M, Kawai N Rapid detection of snakes by Japanese monkeys (Macaca fuscata): an evolutionarily predisposed visual system. Journal of Comparative Psychology. 2009;123(2):131–135. doi: 10.1037/a0015095. [DOI] [PubMed] [Google Scholar]
- 31.Smith NK, Cacioppo JT, Larsen JT, Chartrand TL May I have your attention, please: electrocortical responses to positive and negative stimuli. Neuropsychologia. 2003;41(2):171–183. doi: 10.1016/S0028-3932(02)00147-1. [DOI] [PubMed] [Google Scholar]
- 32.Soares SC, Lindstrom B, Esteves F, Öhman A The hidden snake in the grass: superior detection of snakes in challenging attentional conditions. PLoS One. 2014;9(12):e114724. doi: 10.1371/journal.pone.0114724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Le Q, Isbell LA, Matsumoto J, Nguyen M, Hori E, Maior RS, Tomaz C, Tran AH, Ono T, Nishijo H Pulvinar neurons reveal neurobiological evidence of past selection for rapid detection of snakes. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(47):19000–19005. doi: 10.1073/pnas.1312648110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu SH, Liao ZX, Rizak JD, Zheng N, Zhang LH, Tang H, He XB, Wu Y, He XP, Yang MF, Li ZH, Qin DD, Hu XT Comparative study of the transfection efficiency of commonly used viral vectors in rhesus monkey (Macaca mulatta) brains. Zoological Research. 2017;38(2):88–95. doi: 10.24272/j.issn.2095-8137.2017.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang B Consequences of early adverse rearing experience(EARE) on development: insights from non-human primate studies. Zoological Research. 2017;38(1):7–35. doi: 10.13918/j.issn.2095-8137.2017.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang B, Noble PL, Winslow JT, Pine DS, Nelson EE Amygdala volume predicts patterns of eye fixation in rhesus monkeys. Behavioural Brain Research. 2012;229(2):433–437. doi: 10.1016/j.bbr.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang B, Xiong F, Ma Y, Li B, Mao Y, Zhou Z, Yu H, Li J, Li C, Fu J, Wang J Chronic phencyclidine treatment impairs spatial working memory in rhesus monkeys. Psychopharmacology. 2019a;236(7):2223–2232. doi: 10.1007/s00213-019-05214-2. [DOI] [PubMed] [Google Scholar]
- 38.Zhang B, Zhou Z, Zhou Y, Zhang T, Ma Y, Niu Y, Ji W, Chen Y Social valence related increased attention in Rett syndrome (RTT) cynomolgus monkeys: An eye-tracking study. Autism Research. 2019b;12(11):1585–1597. doi: 10.1002/aur.2189. [DOI] [PubMed] [Google Scholar]