Abstract
Background:
Complicated grief (CG) is characterized by persistent, impairing grief after losing a loved one. Little is known about sleep disturbance in CG. Baseline prevalence of subjective sleep disturbance, impact of treatment on sleep, and impact of mid-treatment sleep on CG and quality of life outcomes were examined in adults with CG in secondary analyses of a clinical trial.
Methods:
Patients with CG (n=395, Mage=53.0; 78% female) were randomized to: CGT+placebo, CGT+citalopram (CIT), CIT, or placebo. Subjective sleep disturbance was assessed by a grief-anchored sleep item (Pittsburgh Sleep Quality Index: PSQI-1) and a 4-item sleep subscale of the Quick Inventory of Depressive Symptomatology (QIDS-4). Sleep disturbance was quantified as at least one QIDS-4 item with severity ≥2 or grief-related sleep disturbance ≥3 days a week for PSQI-1. Outcomes included the Inventory of Complicated Grief (ICG), Work and Social Adjustment Scale (WSAS), and Clinical Global Impressions Scale.
Results:
Baseline sleep disturbance prevalence was 91% on the QIDS-4 and 46% for the grief-anchored PSQI-1. Baseline CG severity was significantly associated with sleep disturbance (QIDS-4: p=0.015; PSQI-1: p=0.001) after controlling for comorbid depression and PTSD. Sleep improved with treatment; those receiving CGT+CIT vs. CIT evidenced better endpoint sleep (p=0.027). Mid-treatment QIDS-4 significantly predicted improvement on outcome measures (all p<0.01), though only WSAS remained significant after adjustment for mid-treatment ICG (p=0.02).
Conclusions:
Greater CG severity is associated with poorer sleep beyond PTSD and depression comorbidity. Additional research including objective sleep measurement is needed to optimally elucidate and address sleep impairment associated with CG.
Keywords: grief/bereavement/Complicated Grief, quality of life, sleep disorders, treatment, antidepressants
Introduction
Complicated grief (CG), also known as prolonged grief disorder (PGD), is a disorder occurring in approximately 10% of people bereaved by natural causes (Lundorff, Holmgren, Zachariae, Farver-Vestergaard, & O’Connor, 2017) and is characterized by persistent, impairing grief with difficulty adapting after the loss of a loved one. Criteria sets for a persistent grief related diagnosis have been included in International Classification of Diseases, 11th Edition ICD-11 (World Health Organization, 2018) and provisionally included in the DSM-5 (American Psychiatric Association, 2013). The ICD-11 symptoms include persistent and pervasive longing for the deceased and/or preoccupation with the deceased, accompanied by intense emotional pain, such as difficulty accepting the death, feeling one has lost a part of one’s self, inability to experience positive mood, emotional numbness, and difficulty in engaging with social or other activities, with these symptoms lasting more than six months (Organization, 2018). Other common symptoms of CG include intense yearning and reactivity to or excessive avoidance of reminders of the deceased.
Sleep disturbance occurs in many psychiatric disorders, including major depressive disorder (MDD), generalized anxiety disorder (GAD) and post-traumatic stress disorder (PTSD). Although official criteria for persistent impairing grief are not yet finalized, criteria sets under consideration have not included sleep impairment (American Psychiatric Association, 2013; Organization, 2018; Prigerson et al., 2009; Shear, 2015). Some available evidence suggests that subjective sleep quality, onset, and maintenance may be impaired in patients with CG due to nighttime rumination about the loss and dreaming of the deceased (Hardison, Neimeyer, & Lichstein, 2005). Further, in a large population-based study of 5,421 older adults (ages 45 and above), normal and complicated grief were associated with shorter sleep duration and poorer sleep quality as measured by the Pittsburgh Sleep Quality Index (PSQI; Milic et al., 2019). Germain et al (2005) documented clinically significant sleep disturbance as measured by the PSQI in the absence as well as the presence of comorbid depression in patients with CG. This group also found that dreams in patients with CG were characterized by more family characters (including the deceased) than dreams of non-CG patients (Germain et al., 2013).
Treatment strategies for improving sleep-related difficulties include medications as well as psychotherapeutic approaches.Complicated Grief Treatment (CGT), an evidence-based CG-focused psychotherapy, has demonstrated efficacy in several large randomized controlled trials (Shear, Frank, Houck, & Reynolds, 2005; Shear et al., 2016; Shear et al., 2014). CGT includes specific procedures to assist in grief-related emotion regulation. Disrupted sleep has been shown to interfere with emotion regulation and consolidation of extinction learning in PTSD and some anxiety disorders (Pace-Schott, Germain, & Milad, 2015). While CG is not a fear-based disorder, sleep disturbances nonetheless might similarly impair learning and/or consolidation of new learning from core interventions, such as telling the story of the death in CGT. Hypothetically, reductions in CG symptoms with CGT would likely be associated with improved sleep in patients with CG; however, preliminary evidence suggests that subjective sleep impairment may persist, with some improvement, even after treatment (Germain et al., 2005; Germain et al., 2006).
The interaction between sleep quality and CG symptoms has not been well explored, including how treatment of CG, such as with CGT, may impact sleep outcomes. In the current study, we examined the prevalence of sleep impairment at baseline, its association with CG symptom severity, and the impact of treatment type on sleep outcomes as a secondary analysis of a four-arm clinical trial of pharmacotherapy with citalopram or placebo with or without CGT psychotherapy (Shear et al., 2016). Based on prior data suggesting a potential association between CG and poor sleep quality, as well as data linking sleep, emotion regulation, and extinction learning, we hypothesized that 1) baseline subjective sleep disturbance would be associated with greater CG symptom severity, 2) treatment with CGT would have better effects on sleep outcomes than treatment that does not include CGT, and 3) better sleep during the middle phase of CGT (especially during exposure-based procedures) would be associated with more improvement in grief and quality of life outcomes.
Materials and Methods
Study design
The current study is a secondary analysis of sleep data from 395 participants (Mean age = 53.0 ± 14.5, 78% female) in a four site, double-blind, randomized 2×2 clinical trial of adults with a primary diagnosis of CG (Trial Registration: clinicaltrials.gov Identifier: ; Shear et al., 2016). Study arms were: 1) citalopram alone (CIT; n = 101), 2) placebo alone (PLA; n = 99), 3) CGT + CIT (n = 99), and 4) CGT + PLA (n = 96). Randomization was stratified by site and presence of baseline comorbid MDD. Inclusion criteria included a score of 30 or greater on the Inventory of Complicated Grief (ICG; Prigerson et al., 1995) and a judgment by an experienced clinician that CG was the most important problem, based on a supplemental clinical interview for CG (Bui et al., 2015). Key exclusion criteria included: current substance use disorder, history of psychotic disorder or bipolar I disorder, active suicidal plans requiring hospitalization, significant cognitive impairment (Montreal Cognitive Assessment score less than 21), or concurrent psychotherapy or antidepressant pharmacotherapy. More detailed descriptions of study design, recruitment, randomization procedures, and primary outcomes are presented elsewhere (Shear et al., 2016).
Assessments
Trained clinical independent evaluators blinded to treatment assignment conducted assessments at baseline, and weeks 4, 8, 16, and 20 (treatment endpoint). Evaluators administered the Structured Clinical Interview for Complicated Grief (Bui et al., 2015). Comorbid diagnoses, including MDD and PTSD, were assessed using the Structured Clinical Interview for DSM-IV (First & Gibbon, 2004).
Subjective sleep disturbance was assessed by both a single, grief-anchored item adapted from the Pittsburgh Sleep Quality Index (PSQI-1; Buysse, Reynolds, Monk, Berman, & Kupfer, 1989) and by the Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR; Rush et al., 2003). The PSQI-1 inquires about the frequency of trouble sleeping due to grief over the past month (0 = not at all to 3= three or more times a week). Single item sleep measures have been used in previous studies (e.g., Cappelleri et al., 2009; Pien, Sammel, Freeman, Lin, & DeBlasis, 2008), with evidence that the single-item component of subjective sleep quality correlated most highly with global scores on the full PSQI (Carpenter & Andrykowski, 1998). The four items representing sleep disturbance on the QIDS were added to create a sleep disturbance total severity score (QIDS-4). QIDS-4 items assess difficulty falling asleep, sleeping during the night, waking up too early, and sleeping too much on a 0 to 3 point Likert scale, with higher scores indicating greater disturbance. We operationally defined sleep disturbance in two ways: 1) proportions of individuals who reported at least 1 item at a severity of ≥2 on the QIDS-4 and 2) proportion reporting trouble sleeping three or more days a week on the PSQI-1 (score = 3).
Treatment response was assessed with the Clinical Global Impressions Scale (CGI; Guy, 1976) modified for grief (Shear et al., 2016), with response defined as an improvement score of 1 or 2 (i.e., much or very much improved). CG symptom severity was measured with the Inventory of Complicated Grief (ICG; Prigerson et al., 1995), and quality of life impairment with the Work and Social Adjustment Scale (WSAS; Mundt, Marks, Shear, & Greist, 2002).
Interventions
Complicated Grief Treatment.
CGT was delivered in a manualized, 16-session format, as described in three randomized controlled trials (Shear et al., 2005; Shear et al., 2014), including the parent trial (Shear et al., 2016). The treatment consists of seven core modules with accompanying procedures delivered in 16 sessions and aimed at facilitating adaptation to loss and resolving complicating factors, such as counterfactual beliefs and excessive avoidance. The full CGT manual is available online (https://complicatedgrief.columbia.edu/). All therapists participated in a didactic seminar and completed at least two training cases prior to working, under ongoing supervision, with study patients.
Pharmacotherapy.
Patients receiving pharmacotherapy with either citalopram or placebo and grief-focused clinical management, completed 12 weeks of the intervention to limit maximal exposure to a pill placebo without psychotherapy condition. Medication was flexibly dosed to the maximum allowable (i.e., 40mg) based on tolerability and response.
Data analysis
First, we report the rate of endorsement of sleep disturbance on the QIDS-4 and PSQI-1. We used a multiple linear regression to examine the baseline association of CG symptom severity (ICG) with QIDS-4 total scores, including covariates for baseline current MDD, PTSD, and demographics (i.e., age, gender), which were chosen a priori. Similarly, a logistic regression with the same variables examined the association of CG severity with binary PSQI-1 (with 1 = endorsement of 3 or more nights of poor sleep) at baseline.
Next, we performed a longitudinal analysis of the QIDS-4 total scores (outcome) using a linear mixed effects model with subject-specific random intercepts to account for repeated measures over time. The model also included fixed effects for treatment (4 arms), time (baseline, Weeks 4, 8, 12, 16, and 20), and time by treatment interactions as predictors, covariates for comorbid MDD at baseline, site, and ethnicity. Ethnicity was included as there was evidence of imbalance across treatment arms in the parent study (Shear et al., 2016). Model-based least-squares means were obtained for endpoint QIDS-4 (Week 20) for each treatment group and the adjusted mean differences among the treatment groups were tested.
To examine the magnitude of sleep improvement over time in the subsample of individuals who completed the full study with non-missing endpoint data, we completed a paired samples t-test examining QIDS-4 score changes from baseline to Week 20 and a McNemar’s test for the binary PSQI-1.
Finally, we assessed the impact of mid-treatment sleep disturbance, as measured by the QIDS-4, on CG treatment response (CGI), CG symptoms (ICG) and grief-related functional impairment (WSAS) at Week 20. Mid-treatment QIDS-4 scores were quantified as the average of Week 4 and 8 QIDS-4 (with substitution for missing data if available a different week within this week 4 to 8 timeframe). Consistent with the parent paper, a weighted logistic or linear regression model for each outcome was fit that controlled for randomization stratification variables (site, baseline MDD status), baseline covariates found to be imbalanced across treatment arms (ethnicity), treatment arm, and baseline levels of sleep disturbance as measured by the QIDS-4. Inverse Probability Weighting (IPW), a standard strategy to account for missing assessment data (Little et al., 2012; Seaman & White, 2013), provided weights in the model. In the first set of models, the impact of mid-treatment QIDS-4 scores on outcome was assessed. In the second set of models, mid-treatment QIDS-4 scores on outcome were assessed while also controlling for mid-treatment ICG scores (Week 8). Baseline analyses of proportions reporting sleep disturbance and linear regressions were conducted in SPSS, Version 23. The remaining analyses were conducted using R version 3.5.1.
Results
Frequency and impact of sleep disturbance in CG
Baseline participant characteristics are presented in Table 1. Fully 91% of the CG sample (n = 355) reported at least 1 QIDS-4 item score of ≥2. Thirty-three percent of patients reported 1 area of disrupted sleep, 30% reported 2 sleep problems, 26% reported 3 sleep problems, and 2% reported all four types of disrupted sleep. On the PSQI-1, 46% of patients reported trouble sleeping due to grief three or more times per week over the past month. Of note, although the relationship to the deceased varied (see Table 1), and it is plausible that the effect of loss of a partner (e.g., who shared a bed) might have a greater impact on sleep, there was no difference by relationship type (quantified as romantic partner vs. not romantic partner) on subjective sleep disturbance for either QIDS-4 (t(385) = 0.12, p = 0.90) or PSQI-1 (χ2(2, N = 249) = 0.51, p = 0.53). Similarly, while co-occurring depression and PTSD might be expected to and were associated with poorer sleep, regression analyses adjusting for baseline MDD and PTSD demonstrated that CG symptom severity significantly predicted sleep disturbance on both the QIDS-4 (B = 0.34 p = 0.015) and the PSQI-1 item (B = 0.04 p = 0.001) above and beyond the effect of MDD or PTSD comorbidity (Table 2). We did not detect an association between baseline sleep disturbance and time since loss on either the QIDS-4 (r(387) = −0.07, p = 0.17) or PSQI-1 (r(392) = −0.04, p = 0.39).
Table 1.
Baseline clinical and demographic characteristics
| Total (n = 395) |
CIT (n = 101) |
PLA (n = 99) |
CGT + CIT (n = 99) |
CGT + PLA (n = 96) |
|
|---|---|---|---|---|---|
| Age (Mean, SD) | 53.0 (14.5) | 52.4 (13.1) | 53.9 (13.8) | 52.1 (15.3) | 53.5 (16.0) |
| Gender (n, % female) | 308 (78) | 82 (81.2) | 69 (69.7) | 78 (78.8) | 79 (82.3) |
| Other | 31 (7.8) | 6 (5.9) | 11 (11.1) | 7 (7.1) | 7 (7.3) |
| Hispanic (n, %)* | 45 (11.4) | 10 (9.9) | 7 (7.1) | 8 (8.1) | 20 (20.8) |
| ≥4 year college | 211 (53.4) | 56 (55.4) | 52 (52.5) | 53 (53.5) | 50 (52.1) |
| Widowed | 138 (34.9) | 34 (33.7) | 36 (36.4) | 35 (35.4) | 33 (34.4) |
| Other | 58 (14.7) | 13 (12.9) | 12 (12.1) | 16 (16.2) | 17 (17.7) |
| Years since loss (M, SD) | 4.7 (7.2) | 4.6 (5.8) | 5.3 (8.7) | 4.7 (7.4) | 4.3 (6.7) |
| Current MDD (n, %) | 262 (66.3) | 68 (67.3) | 66 (66.7) | 64 (64.6) | 64 (66.7) |
| Current PTSD (n, %) | 154 (39.0) | 41 (40.6) | 36 (36.4) | 37 (37.4) | 40 (41.7) |
| QIDS-4 sleep total score (M, SD) | 5.3 (2.5) | 5.1 (2.5) | 5.4 (2.6) | 5.2 (2.5) | 5.7 (2.3) |
| 3 or more days/week | 182 (46.1) | 45 (44.6) | 43 (43.4) | 47 (47.5) | 47 (49.0) |
| Inventory of Complicated Grief (ICG) (M, SD) | 42.8 (8.9) | 43.2 (8.5) | 42.2 (9.4) | 42.6 (9.4) | 43.0 (8.3) |
| Work and Social Adjustment Scale (WSAS) (M, SD) | 22.3 (9.8) | 22.0 (9.6) | 23.2 (10.1) | 21.8 (9.6) | 22.2 (9.9) |
| Severely/extremely ill | 66 (16.7) | 17 (16.8) | 20 (20.2) | 17 (17.2) | 12 (12.5) |
Note. MDD = major depressive disorder; PTSD = posttraumatic stress disorder; QIDS-4 = Quick Inventory of Depression Symptoms 4 sleep disturbance items; PSQI-1 = Pittsburgh Sleep Quality Index, grief-anchored sleep disturbance item,
No significant differences between groups were found on any characteristics except for Hispanic demographic status.
Table 2.
Regression results predicting QIDS-4 sleep subscale and PSQI-1 scores from grief severity
| QIDS-4 | PSQI-1 | |||
|---|---|---|---|---|
| Statistic | P-value | Statistic | P-value | |
| Model | F(5, 383) = 7.58 | <.001 | ||
| ICG score | B = 0.034 | .015 | B = 0.04 | .001 |
| MDD comorbidity | B = 0.92 | .001 | B = −0.73 | .002 |
| PTSD comorbidity | B = 0.75 | .003 | B = −0.65 | .003 |
| Gender | B = −0.26 | .38 | B = 0.05 | .86 |
| Age | B = 0.03 | .55 | B = 0.003 | .64 |
Note. QIDS-4 = Quick Inventory of Depression Symptoms 4 sleep disturbance items; PSQI-1 = Pittsburgh Sleep Quality Index, grief-anchored sleep disturbance item; ICG = Inventory of Complicated Grief; MDD = major depressive disorder; PTSD = posttraumatic stress disorder. Model included gender, age, and comorbid baseline diagnoses of MDD and/or PTSD.
Impact of CGT on sleep disturbance
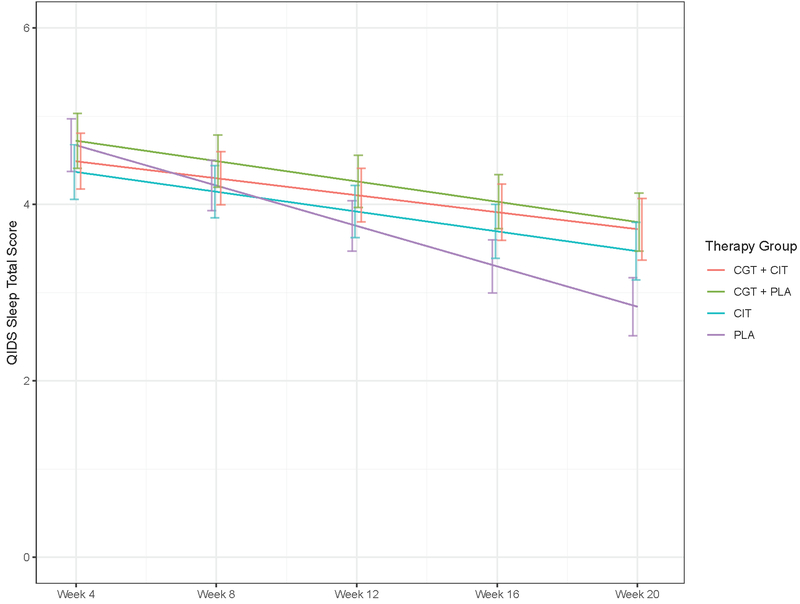
The effects of treatment, time, and their interaction on total score for the QIDS-4 were examined with a linear mixed effects model covarying for baseline MDD, site, and ethnicity. From these models, we estimated adjusted mean differences among treatment groups at endpoint to identify the main comparisons in which there were differences in sleep disturbance by treatment. There was a significant difference between CGT + CIT vs. CIT (adjusted mean difference = −0.96, t(1025) = −2.65, p=0.008, 95% CI: −1.669 to −0.25) and a non-significant trend level effect for the CGT + CIT vs. CGT + PLA comparison (adjusted mean difference = −0.63, t(1025) = −1.79, p=0.07, 95% CI: −1.319 to 0.061) on endpoint sleep. Other comparisons including CIT vs. placebo, CGT + PLA vs. PLA were not significant. See Figure 1.
Figure 1.
Trajectories of QIDS-4 sleep scores across treatment groups over time. CGT = Complicated Grief Treatment; CIT = citalopram; PLA = placebo.
In an exploratory analysis with a paired samples t-test of study completers who had Week 20 endpoint sleep measures available, sleep disturbance as measured by the QIDS-4 was significantly reduced at Week 20 compared to baseline (t(135) = 9.58, p < 0.001). In a McNemar’s test for the PSQI-1, sleep disturbance was also significantly reduced at Week 20 in the completers subsample (χ2 = 77.7, p < 0.001). Overall, the proportion of the sample reporting at least 1 area of disturbed sleep on the QIDS-4 dropped from 91% at baseline to 74% at endpoint. Similarly, on the PSQI-1 the proportion of the sample reporting at least 3 days of disturbed sleep per week dropped from 49% to 15%.
Impact of disrupted sleep at baseline and during mid-treatment on treatment outcomes
In a weighted linear regression model, baseline sleep measured by the QIDS-4 was not significantly predictive of treatment response based on the CGI (p = 0.81), ICG (p = 0.93), or WSAS (p = 0.22) at Week 20. However, mid-treatment sleep significantly predicted treatment response (OR: 0.78, p = 0.01, 95% CI: 0.63 to 0.90), grief severity (slope=1.43, p =.001, 95% CI: 0.62 to 2.25) and quality of life (slope=1.22, p <.001, 95% CI: 0.61 to 1.83) outcomes at Week 20, when adjusting for baseline QIDS-4, comorbid MDD, and other covariates. When accounting for mid-treatment (Week 8) ICG scores to determine if the sleep effect was independent of change in grief symptoms occurring by midpoint, the effect of mid-treatment QIDS-4 on grief outcomes at endpoint was no longer significant, while the effect of mid-treatment sleep at Week 8 on quality of life at endpoint remained significant (p =.02).
Discussion
This study supports recent findings that sleep is significantly impaired in individuals with CG and advances this research by elucidating the relationship between sleep quality and CG symptom severity. This study adds further evidence to indicate that impaired sleep quality is associated with greater CG severity above and beyond the effects of PTSD or depression, as previously reported (Germain et al., 2005). Further, the combination of a CG-targeted psychological intervention with a pharmacological treatment (CGT+CIT) appears to improve sleep quality more than antidepressants alone.
It is important to note that, in this study, CGT or CIT alone did not outperform placebo in terms of endpoint sleep outcomes. This may highlight the importance of both biological and psychological processes in the pathophysiology and treatment of sleep disturbance in CG. SSRIs do have documented negative effects on sleep, including increased nocturnal awakenings, decreased total sleep time, and REM suppression (as reviewed by Jindal et al., 2003). Thus, while we are unable to directly address this from study data, it is possible that CIT alone had a variable effect on sleep in this population - improving sleep quality alongside improvements in CG and depressive symptoms for some, while potentially perturbing sleep in others as a result of this well-documented side effect. Further, study completers showed a significant decline in sleep disturbance, though this sample also includes those in the placebo group. However, a percentage of individuals continue to report ongoing sleep difficulties in all treatment groups. This suggests that some individuals may require more specific sleep-focused interventions in addition to psychological and/or pharmacological interventions.
Notably, this research also provides more evidence for the clinical importance of sleep quality, particularly at midpoint, as this was significantly predictive of endpoint treatment response, quality of life, and CG severity outcomes. When controlling for mid-ICG, effects were no longer observed on CG severity outcome, suggesting sleep effects may be explained by greater CG severity; however, this may be due to an association between CG severity and sleep disturbance, which appear to be significantly correlated at baseline in this study and others (e.g., Germain et al., 2005; Germain et al., 2006). Further, previous research has demonstrated that patients with CG report quality of life impairments that are not better explained by depression, anxiety, or PTSD (Boelen & Prigerson, 2007; Silverman et al., 2000). However, little research has examined the association between sleep and quality of life in CG. A previous study of older bereaved individuals did find that sleeping more predicted better social functioning, fewer emotional role limitations, and better emotional health, though sleep quality was not evaluated (Chen, Gill, & Prigerson, 2005). Additionally, a body of evidence does support that both CG and sleep disturbance are independently associated with poorer physical health and risk of other medical conditions, such as heart disease, stroke, and diabetes (Cappuccio, D’Elia, Strazzullo, & Miller, 2010; Lannen, Wolfe, Prigerson, Onelov, & Kreicbergs, 2008). Greater focus on sleep as both a potential criterion for CG diagnosis and treatment target may be warranted.
This study includes some limitations. First, dropout was higher than expected in the medication conditions, and the sample had limited racial diversity and was predominantly women. Second, we did not have systematic data on sleep-related breathing disorders, which could also be sources of sleep disturbances, particularly in mid and later life. Third, while our analyses controlled for baseline MDD diagnosis, changes in depressed mood were not accounted for in our models. The parent trial (Shear et al., 2016) found a significant difference in change in depression severity in the CGT + CIT group vs. other groups. This change in depression severity may also be correlated with changes in sleep or quality of life. Finally, subjective sleep assessment was limited to two self-report measures, a single item of the PSQI adapted for grief, and sleep subscale using four items from the QIDS; future studies should use more refined sleep measures. With little known about sleep architecture in CG, future studies should also examine objective sleep with polysomnography. A growing literature suggests that sleep, and REM in particular, is critical to the consolidation and emotional processing of affective memories (Walker & van der Helm, 2009). In fear-based disorders, hyperarousal, which often leads to sleep disruption, can interfere with emotion regulation and learning consolidation, and can negatively impact treatment outcomes (Pace-Schott et al., 2015). This is an especially important consideration given that better sleep at mid-treatment, which is when the CGT component focused on processing the story of the death occurred, was found to be predictive of treatment outcomes. Future research should examine both subjective and objective sleep measures during CGT to identify how they change with treatment, and whether they interfere with processing of these emotional memories and/or grief outcomes. Future research with both objective and subjective sleep measures and their relationship to CG will inform whether future iterations of the DSM should consider including sleep disturbance as a diagnostic criterion and/or risk factor for CG.
Conclusion
Overall, sleep disturbance is common in CG and warrants attention in clinical practice. Additional research is needed to better understand the nature of sleep impairment, its connection to CG, and how to best address its treatment with therapy and/or medication.
Acknowledgements
This work was supported by grants R01MH60783, R01MH085297, R01MH085288, R01MH085308, and P30 MH90333 from the National Institutes of Health and by grant LSRG-S-172–12 from the American Foundation for Suicide Prevention. The funding sources did not have any role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Footnotes
Conflict of Interest
Naomi M. Simon reports the following conflicts of interest: 1) research grants from the Department of Defense, NIH, and PCORI, 2) speaking/CME/consulting from Axovant Sciences, Springworks, PraxisTherapeutics, and Aptinyx, and 3) equity (spouse) from G1 Therapeutics. Kristin Szuhany, Allison Young, Christine Mauro, Angel Garcia de la Garza, Julia Spandorfer, Rebecca Lubin, Natalia Skritskaya, Susanne S. Hoeppner, Meng Li, Ed Pace-Schott, Sidney Zisook, Charles F. Reynolds, and M. Katherine Shear have no conflicts of interest to report.
Clinical Trial Registration: clinicaltrials.gov identifier:
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®): American Psychiatric Pub. [Google Scholar]
- Boelen PA, & Prigerson HG (2007). The influence of symptoms of prolonged grief disorder, depression, and anxiety on quality of life among bereaved adults: a prospective study. Eur Arch Psychiatry Clin Neurosci, 257(8), 444–452. doi: 10.1007/s00406-007-0744-0 [DOI] [PubMed] [Google Scholar]
- Bui E, Mauro C, Robinaugh DJ, Skritskaya NA, Wang Y, Gribbin C, … Reynolds C (2015). The structured clinical interview for complicated grief: reliability, validity, and exploratory factor analysis. Depression and anxiety, 32(7), 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Cappelleri JC, Bushmakin AG, McDermott AM, Sadosky AB, Petrie CD, & Martin S (2009). Psychometric properties of a single-item scale to assess sleep quality among individuals with fibromyalgia. Health Qual Life Outcomes, 7, 54. doi: 10.1186/1477-7525-7-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, D’Elia L, Strazzullo P, & Miller MA (2010). Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep, 33(5), 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter JS, & Andrykowski MA (1998). Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res, 45(1), 5–13. [DOI] [PubMed] [Google Scholar]
- Chen JH, Gill TM, & Prigerson HG (2005). Health behaviors associated with better quality of life for older bereaved persons. J Palliat Med, 8(1), 96–106. doi: 10.1089/jpm.2005.8.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, & Gibbon M (2004). The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II).
- Germain A, Caroff K, Buysse DJ, & Shear MK (2005). Sleep quality in complicated grief. J Trauma Stress, 18(4), 343–346. doi: 10.1002/jts.20035 [DOI] [PubMed] [Google Scholar]
- Germain A, Shear K, Monk TH, Houck PR, Reynolds CF, Frank E, & Buysse DJ (2006). Treating complicated grief: effects on sleep quality. Behav Sleep Med, 4(3), 152–163. doi: 10.1207/s15402010bsm0403_2 [DOI] [PubMed] [Google Scholar]
- Germain A, Shear KM, Walsh C, Buysse DJ, Monk TH, Reynolds CF 3rd, … Silowash R (2013). Dream content in complicated grief: a window into loss-related cognitive schemas. Death Stud, 37(3), 269–284. doi: 10.1080/07481187.2011.641138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W (1976). Clinician Global Impression (CGI). In: ECDEU Assessment Manual for Psychopharmacology. [Google Scholar]
- Hardison HG, Neimeyer RA, & Lichstein KL (2005). Insomnia and complicated grief symptoms in bereaved college students. Behav Sleep Med, 3(2), 99–111. doi: 10.1207/s15402010bsm0302_4 [DOI] [PubMed] [Google Scholar]
- Jindal RD, Friedman ES, Berman SR, Fasiczka AL, Howland RH, & Thase ME (2003). Effects of sertraline on sleep architecture in patients with depression. J Clin Psychopharmacol, 23(6), 540–548. doi: 10.1097/01.jcp.0000095345.32154.9a [DOI] [PubMed] [Google Scholar]
- Lannen PK, Wolfe J, Prigerson HG, Onelov E, & Kreicbergs UC (2008). Unresolved grief in a national sample of bereaved parents: impaired mental and physical health 4 to 9 years later. J Clin Oncol, 26(36), 5870–5876. doi: 10.1200/jco.2007.14.6738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJ, D’Agostino R, Cohen ML, Dickersin K, Emerson SS, Farrar JT, … Stern H (2012). The prevention and treatment of missing data in clinical trials. N Engl J Med, 367(14), 1355–1360. doi: 10.1056/NEJMsr1203730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundorff M, Holmgren H, Zachariae R, Farver-Vestergaard I, & O’Connor M (2017). Prevalence of prolonged grief disorder in adult bereavement: A systematic review and meta-analysis. J Affect Disord, 212, 138–149. doi: 10.1016/j.jad.2017.01.030 [DOI] [PubMed] [Google Scholar]
- Milic J, Saavedra Perez H, Zuurbier LA, Boelen PA, Rietjens JA, Hofman A, & Tiemeier H (2019). The Longitudinal and Cross-Sectional Associations of Grief and Complicated Grief With Sleep Quality in Older Adults. Behav Sleep Med, 17(1), 31–40. doi: 10.1080/15402002.2016.1276016 [DOI] [PubMed] [Google Scholar]
- Mundt JC, Marks IM, Shear MK, & Greist JH (2002). The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry, 180, 461–464. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Germain A, & Milad MR (2015). Sleep and REM sleep disturbance in the pathophysiology of PTSD: the role of extinction memory. Biol Mood Anxiety Disord, 5, 3. doi: 10.1186/s13587-015-0018-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pien GW, Sammel MD, Freeman EW, Lin H, & DeBlasis TL (2008). Predictors of sleep quality in women in the menopausal transition. Sleep, 31(7), 991–999. [PMC free article] [PubMed] [Google Scholar]
- Prigerson HG, Horowitz MJ, Jacobs SC, Parkes CM, Aslan M, Goodkin K, … Neimeyer RA (2009). Prolonged grief disorder: Psychometric validation of criteria proposed for DSM-V and ICD-11. PLoS medicine, 6(8), e1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigerson HG, Maciejewski PK, Reynolds CF 3rd, Bierhals AJ, Newsom JT, Fasiczka A, … Miller M (1995). Inventory of Complicated Grief: a scale to measure maladaptive symptoms of loss. Psychiatry Res, 59(1–2), 65–79. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, … Keller MB (2003). The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry, 54(5), 573–583. [DOI] [PubMed] [Google Scholar]
- Seaman SR, & White IR (2013). Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res, 22(3), 278–295. doi: 10.1177/0962280210395740 [DOI] [PubMed] [Google Scholar]
- Shear MK (2015). Clinical practice. Complicated grief. N Engl J Med, 372(2), 153–160. doi: 10.1056/NEJMcp1315618 [DOI] [PubMed] [Google Scholar]
- Shear MK, Frank E, Houck PR, & Reynolds CF 3rd. (2005). Treatment of complicated grief: a randomized controlled trial. Jama, 293(21), 2601–2608. doi: 10.1001/jama.293.21.2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear MK, Reynolds CF 3rd, Simon NM, Zisook S, Wang Y, Mauro C, … Skritskaya N (2016). Optimizing Treatment of Complicated Grief: A Randomized Clinical Trial. JAMA Psychiatry, 73(7), 685–694. doi: 10.1001/jamapsychiatry.2016.0892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear MK, Wang Y, Skritskaya N, Duan N, Mauro C, & Ghesquiere A (2014). Treatment of complicated grief in elderly persons: a randomized clinical trial. JAMA Psychiatry, 71(11), 1287–1295. doi: 10.1001/jamapsychiatry.2014.1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman GK, Jacobs SC, Kasl SV, Shear MK, Maciejewski PK, Noaghiul FS, & Prigerson HG (2000). Quality of life impairments associated with diagnostic criteria for traumatic grief. Psychol Med, 30(4), 857–862. [DOI] [PubMed] [Google Scholar]
- Walker MP, & van der Helm E (2009). Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull, 135(5), 731–748. doi: 10.1037/a0016570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2018). International statistical classification of diseases and related health problems. 11th Revision. [Google Scholar]