Abstract
G protein-coupled receptors (GPCRs) comprise the largest group of membrane receptors in eukaryotic genomes and collectively they regulate nearly all cellular processes. Despite the widely recognized importance of this class of proteins, many GPCRs remain understudied. Gpr27 is an orphan GPCR that displays high conservation during vertebrate evolution. Although, GPR27 is known to be expressed in tissues that regulate metabolism including the pancreas, skeletal muscle, and adipose tissue, its functions are poorly characterized. Therefore, to investigate the potential roles of Gpr27 in energy metabolism, we generated a whole body gpr27 knockout zebrafish line. Loss of gpr27 potentiated the elevation in glucose levels induced by pharmacological or nutritional perturbations. We next leveraged a mass spectrometry metabolite profiling platform to identify other potential metabolic functions of Gpr27. Notably, genetic deletion of gpr27 elevated medium-chain acylcarnitines, in particular C6-hexanoylcarnitine, C8-octanoylcarnitine, C9-nonanoylcarnitine, and C10-decanoylcarnitine, lipid species known to be associated with insulin resistance in humans. Concordantly, gpr27 deletion in zebrafish abrogated insulin-dependent Akt phosphorylation and glucose utilization. Finally, loss of gpr27 increased the expression of key enzymes in carnitine-shuttle complex, in particular the homolog to the brain-specific isoform of CPT1C which functions as a hypothalamic energy senor. In summary, our findings shed light on the biochemical functions of Gpr27 by illuminating its role in lipid metabolism, insulin signaling, and glucose homeostasis.
Keywords: insulin resistance, lipid metabolism, g protein-coupled receptors, metabolomic profiling, carnitine palmitoyltransferase I
Introduction
A diverse range of extracellular ligands activate GPCRs including hormones, neurotransmitters, light, peptides, and lipids. Ligand binding triggers interaction with heterotrimeric G proteins and the subsequent activation of intracellular signaling pathways that modulate an incredible array of physiological and disease processes (1–4). Notably, pharmacological modulation of GPCRs has proven to be one of the most successful stories in modern medicine; ~34% of all the FDA-approved drugs target human GPCRs (5). Thus, GPCRs are widely recognized as an important class of proteins in human biology. However, of the ~800 GPCRs in the genome, there are ~140 GPCRs for which the endogenous ligands and physiological functions are unknown (2). Developing tools in model organisms to characterize these GPCRs may uncover many previously unappreciated functions for orphan GPCRs which may enable the future development of therapeutic agents (6).
Zebrafish have emerged as a powerful model system to elucidate the function of poorly characterized genes (7, 8). The advantages of this model system are that each female produces hundreds of offspring weekly and that the larvae develop all major organ systems in ~5 days. Moreover, due to their genetic and physiological similarities to humans, zebrafish are a well-established vertebrate organism to study human diseases (9–13). Compared to humans, there is a high degree of conservation in metabolically relevant tissues and biochemical pathways that regulate the endocrine system, skeletal muscle biology, insulin regulation, lipid metabolism, and glucose homeostasis (14–19). Here, we use this model system to investigate GPR27, an orphan GPCR that belongs to the Superconserved Receptors Expressed in the Brain (SREB) family of GPCRs and displays very high conservation among vertebrates (20–23).
Human gene expression data from the Genotype-Tissue Expression (GTEx) project demonstrated high expression of GPR27 in the brain. There is also low level expression in several other tissues including the cardiovascular system, pancreas, adrenal glands, skeletal muscle, and adipose tissue, suggesting peripheral functions as well (24). Using an siRNA screen, Ku et al previously showed that, knockdown of Gpr27 in mouse pancreatic beta cells reduced insulin promoter activity and glucose-stimulated insulin secretion (GSIS) (25), implicating its role in cellular energy metabolism. However, to date, studies using whole organism models of loss-of-function Gpr27 have not been reported. Given that GPR27 in expressed in several tissues relevant to metabolism, we hypothesized that genetic deletion of GPR27 in a whole organism would lead to defects in glucose homeostasis. Furthermore, the application of metabolite profiling approaches to model systems has the potential to reveal novel insights into the effects of genetic perturbations on organismal metabolism and may identify additional metabolic pathways regulated by GPR27.
The growing appreciation for the relationship between circulating metabolites and human health has shed light on the pathogenesis of type 2 diabetes. For example, the development of insulin resistance and diabetes in humans has been associated with the accumulation of medium- and long-chain acylcarnitines in the plasma which is indicative of incomplete fatty acid oxidation (26–29). Several lines of evidence in cell and animal models have shown that medium- and long-chain acylcarnitines act as lipid signaling molecules that diminish skeletal muscle response to insulin, thereby providing a potential link between acylcarnitine accumulation and the development of insulin resistance (30–33). However, this phenomenon is not well understood at the molecular level. In particular, the upstream molecular mechanisms that drive acylcarnitine accumulation have yet to be fully elucidated.
In the present work, we generated a zebrafish gpr27 knockout line and subjected it to energy homeostasis assays to determine the impact of gpr27 deletion on glucose homeostasis and insulin sensitivity. Further, to identify the potential metabolic actions of gpr27, we leveraged a mass spectrometry platform and evaluated a total of 150 polar metabolites. Cumulatively, this study identifies a novel role for Gpr27 in acylcarnitine metabolism and proposes a potential mechanism by which Gpr27 regulates glucose homeostasis. In addition, our zebrafish line provides a new in vivo tool to study the molecular underpinnings leading to altered acylcarnitine metabolism and defects in insulin-mediated energy homeostasis.
Materials and Methods
Zebrafish.
Animals were maintained and embryos were obtained according to standard fish husbandry protocols. Zebrafish embryos were grown at 28°C in HEPES-buffered Tübingen E3 medium inside light/dark cycle incubators. gpr27 knockout zebrafish were generated by CRISPR-Cas9 mediated genome editing. The zebrafish gpr27 gene (ENSDARG00000006607) contains a single exon. The sequence of the target site used to generate the gRNA is GGCATAATTCTGGAGCGAGGGG (the PAM nucleotides are underlined). At the 1-cell stage ~100 ng/μl of sgRNA and 150 ng/μl of Cas9 mRNA were co-injected into the embryo using a glass microcapillary pipette attached to a micromanipulator under a stereomicroscope. The gpr27 mutant line generated has a 19-bp deletion at position +39. Genome edits were confirmed by Sanger Sequencing. Founders were outcrossed to establish the F1 generation. F2 generation fish were genotyped using the following PCR primers: GGGACCAGGACTGCTTAATG (forward) and AGCCTTGTGGAGTGAGCTGT (reverse). The PCR products were run on a 2.5% agarose gel. The wildtypes exhibit a 226 base pair product whereas the knockouts exhibit a 207 base pair band. All methods were carried out in accordance with the regulations and guidelines of the Animal Welfare Act and the American Association for Accreditation of Laboratory Animal Care. All experimental protocols were approved by the IACUC committee at MGH and BIDMC.
RT-qPCR.
Zebrafish embryos were maintained at 28°C until the desired developmental stages. Subsequently, mRNAs were extracted by TRIzol™ (Invitrogen) following manufacturer’s instruction and further purified using RNA Clean & Concentrator™−5 (Zymo Research). cDNAs were synthesized using the QuantiTect Reverse Transcription kit (Qiagen). The quantitative PCR was performed using iTaq™ Universal SYBR® Green Supermix (Bio-Rad). The following PCR primers were used zEF1a-F, CTGGAGGCCAGCTCAAACAT; zEF1a-R, ATCAAGAAGAGTAGTACCGCTAGCATTAC; zINS-F, TCCACCACCATATCCACCATTC; zINS-R, CACTGGACACGACCAACAGG; zGPR27-F, CGCCAAAACAAGAACGCAGA; zGPR27-R, GGCCGAAAACAACCCAAGAC; zPCK1-F, TGGAGGAGGAGTCAGTCAGC; zPCK1-R, CATGCTGAAGGGGATCACGTA; zGLU2-F, TGCTACTGCTGGTGTGTCCA; zGLUT2-R, CTGCCTTCATTTCGGCAATGTC; zCPT1A-F, GCTCTTCGGCAAGTCTATCTC; zCPT1A-R, AACACCAGCACGAACCC; zCPT1B-F, TCATGGGCTGACTCTCCTATC; zCPT1B-R, CAATGTCCCTCTGCTGTGTATC; zCPT1C-F, TGTCTTACCAAGCCCTCAATC; zCPT1C-R, CTAGCTGGATAAGCACCCTTAAT; zCPT2-F, TCTAAATACCACGGGCAACTC; zCPT2-R, GTGCCATTCCTTTCGAATTAGC; zCRAT-F, GCTATTCAGCTTGCCTACTACA; zCRAT-R, CGAATGTAGTCTGTTCGTCCTC; zCRATA-F, GAACTCCTCCTTACAGACCAAC; zCRATA-R, GCTCTCACGGACTCCTTATTG; zACSL1-F, GCTGCCATCACCACATACT; zACSL1-R, GGATAGAGCGACGTGCATATT; zSLC25A20-F, GACGGCTCCAGAAGGTAAAT; zSLC25A20-R, GGCGTTGAAGCCCTTATAGA. The expression of elongation factor 1 alpha (zEF1a) was used for normalization.
Glucose measurement.
Metformin and glyburide were purchased from Sigma-Aldrich. Larval zebrafish were treated for 24 hours and subsequently glucose was measured. The assay was carried out on larval zebrafish loaded in 96-well plates containing HEPES-buffered Tübingen E3 medium (n=1 per well). Following exposure to treatments, larvae were frozen at −80C. Larvae were homogenized in a volume of 50 μl using a motorized pestle and centrifuged. Glucose levels in the lysates were measured using a glucose oxidase assay (34) from Invitrogen (Amplex Red Glucose Assay Kit). Data is represented as the glucose levels per larva.
Insulin injections.
At 4 d.p.f. zebrafish larvae were briefly exposed to tricaine and then placed onto an agarose gel. Pulled glass capillary needles were loaded with vehicle or 10 μg/mL insulin and 1 nL was injected into the circulation (35). The animals were then placed into HEPES-buffered Tübingen E3 medium and returned to the incubator. These studies were conducted in 4 d.p.f. larvae because it has been previously shown that larvae are sensitive to intravascularly administrated insulin at this timepoint (35). Further, at later stages as the embryonic tissues thicken, microneedle tip clogging becomes an issue which causes variation in the amount of insulin delivered between animals.
Standard and high-fat diets.
By 7 d.p.f. the nutrients in the yolk sac have been consumed, therefore this timepoint was chosen for feeding experiments (36). A well-established and widely used high-fat diet for zebrafish is chicken egg yolks (37). Zebrafish larvae were feed a solution of egg whites (standard diet) or a solution of egg yolks (high-fat diet) for 24 hours, as previously described (38).
Western blots.
Zebrafish were lysed in RIPA buffer supplemented with protease and phosphatase inhibitors using a motorized homogenizer. Lysates were run on Bis-Tris gels (4–20%) in MOPS buffer and transferred to PVDF membranes. Antibodies for AKT and pAKT ser473 were purchased from Cell Signaling (2920S and 4060S).
Mass Spectrometry.
Our platform uses targeted, multiple reaction monitoring MS data acquisition to measure approximately 150 water soluble metabolites that fall into 8 classes: a) amines; b) amino acids and amino acid conjugates; c) bile acids; d) sugars and sugar phosphates; e) indoles and indole derivatives; f) organic acids; g) purines and pyrimidines; h) lipids (39). Briefly, metabolites that ionize in the positive ion mode were extracted using acetonitrile and methanol (10 larvae per sample, 10–15 biological replicates per experimental condition). We used 6 d.pf. larvae as deyolking the embryos to mitigate the effect of highly abundant metabolites suppressing the ionization of other less abundant metabolites is not required. The extracts were separated using hydrophilic interaction liquid chromatography (HILIC) on a 1260 HPLC binary system (Agilent) and the MS analyses were performed in positive ion mode on the QTrap 4000 (Applied Biosystems/Sciex). The coefficients of variation (CV) for analyte measurements generally ≤15%, and closer to 6% for abundant analytes such as amino acids. Metabolites with CVs ≥ 30% are excluded from analysis. The metabolite data is displayed as the relative delta [((KO-WT)/WT)*100]. We corrected for multiple comparisons by using a Bonferroni adjusted p-value (p ≤ 0.05/150 = 0.00033).
Statistics.
The data is represented as the mean ± the standard deviation. Significance was determined using a Student’s t-test.
Results
Generation of the gpr27 knockout line in zebrafish.
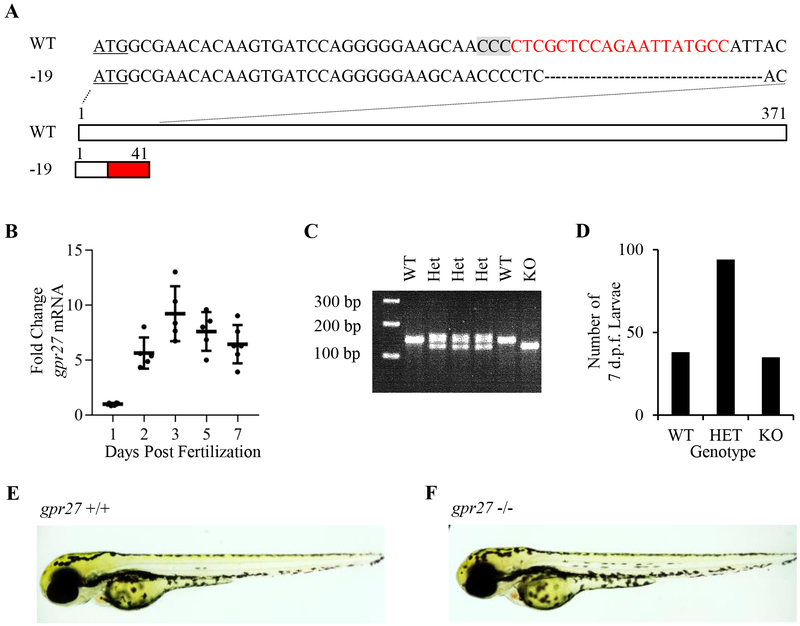
The zebrafish gpr27 gene contains a single exon that produces a 371 amino acid protein (Figure 1A). To determine if developing zebrafish embryos express gpr27, quantitative real-time PCR was used. Steadily increasing expression of gpr27 mRNA was detected in whole zebrafish embryos from 1 to 3 days post fertilization (d.p.f.); an 8.5 ± 2.5 fold increase in gpr27 mRNA was observed in 3 d.p.f. embryos compared to 1 d.p.f. embryos (Figure 1B). To target gpr27, we used CRISPR-Cas9 mediated genome editing and confirmed genome edits by Sanger sequencing (Supplemental Figure 1). One mutant exhibited a 19-nucleotide deletion starting at position +39, which is expected to cause a frame shift, the generation of a premature stop codon, and the formation of a truncated 41-amino acid protein (Figure 1A and Supplemental Figure 1). The founder was raised to adulthood and outcrossed to wildtype fish. PCR-based genotyping yielded a 226 base pair product for wildtype gpr27 and a 207 base pair product for knockout gpr27 (Figure 1C). Embryos obtained from heterozygous F1 in-crosses yielded a Mendelian ratio of offspring (Figure 1D, n=168). Further, the knockout embryos displayed normal gross morphology (Figure 1E–F) and the knockout adults lived a normal lifespan.
Figure 1. Generation of the gpr27 knockout zebrafish.
A) The zebrafish gpr27 gene contains one exon. The start codon is underlined. The reverse complement of the CRISPR target sequence is marked in red. The protospacer adjacent motif (PAM) of the target site is highlighted by a gray box. A mutant line with a 19-bp deletion (denoted by dashes) was identified. The predicted protein truncation begins at residue 15 (red box). B) gpr27 mRNA levels in 1–7 d.p.f. embryos (n = 5–6 samples of 10 larvae, vertical bars: ± standard deviation, horizontal bars: mean). C) Image of an agarose gel containing PCR products that represent gpr27 wildtype and knockout genotypes. D) The number of wildtypes, heterozygotes, and knockouts generated from a heterozygous cross. E-F) Light micrographs of a wildtype and a knockout larva at 5 d.p.f.
Basal glucose levels and insulin gene expression are unaffected in gpr27 knockout zebrafish.
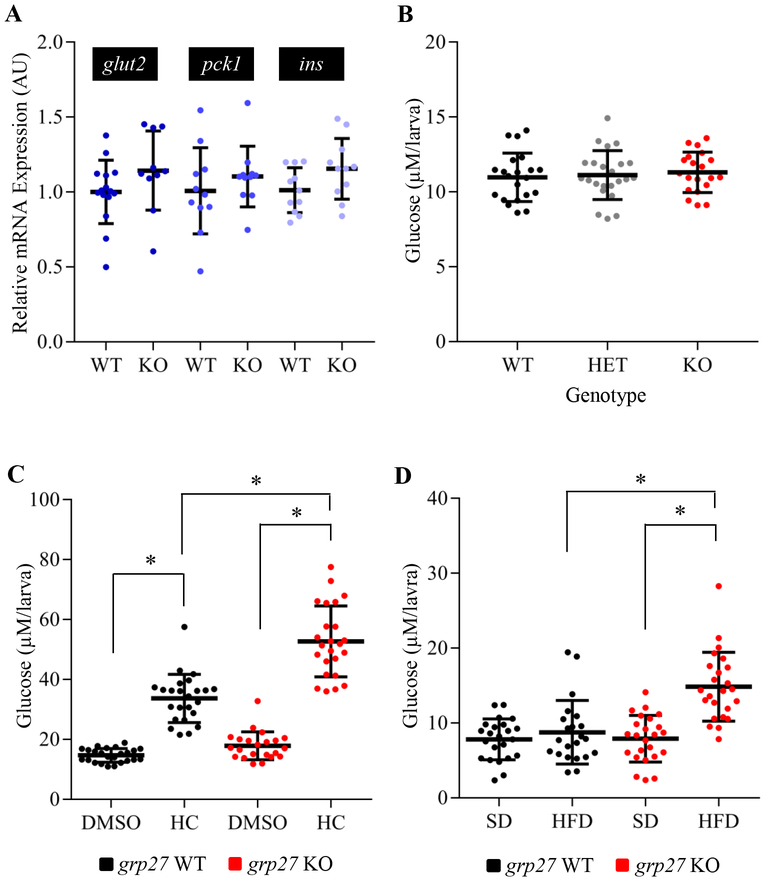
Since a previous study has demonstrated that Gpr27 regulates insulin promoter activity in MIN6 cells (25), we next assessed insulin expression in gpr27 knockout animals. We measured insulin (ins) expression in 6 d.p.f. larvae using RT-qPCR and found that there was not a significant difference in ins mRNA levels between wildtype and knockout larvae (Figure 2A). Additionally, pdx1, a transcriptional activator of the ins gene, and glut2, a glucose transporter involved in glucose-stimulated insulin secretion, were also unaffected in the gpr27 knockouts (Figure 2A). We then asked if loss of gpr27 affects basal glucose levels. Using an established assay (34), glucose was measured at 6 d.p.f. in individual larvae derived from heterozygous crosses. We found that basal glucose levels in the wildtype (n=21), heterozygote (n=23), and knockout (n=20) larvae were not significantly different (Figure 2B).
Figure 2. Pharmacological and nutritional perturbations that increase glucose levels are exacerbated by loss of gpr27.
A) Relative expression of ins, pck1 and glut2 mRNA in gpr27 wildtype and knockout larvae (n = 11 samples of 10 larvae). B) Glucose levels in 6 d.p.f. larvae generated from heterozygous crosses (n = 20–22 larvae, experiments were replicated at least 3 times). Glucose levels in gpr27 wildtype and knockout larvae treated with C) DMSO or 100 μM hydrocortisone (n = 22–24 larvae, experiments were replicated at least 3 times). Glucose levels in gpr27 wildtype and knockout larvae fed a D) standard diet or a high fat diet (n = 22–24 larvae, experiments were replicated at least 3 times). Statistically significant differences between groups are denoted by lines and asterisks (p < 1E-07). Vertical bars denote the stand deviation and horizontal bars denote the mean.
Loss of gpr27 potentiates the effects of pharmacological and nutritional perturbations that increase glucose levels.
Although basal glucose levels were normal in gpr27 knockouts, we hypothesized that pharmacological and nutritional perturbations which elevate glucose levels would reveal an underlying defect in glucose homeostasis. To increase glucose levels we administered hydrocortisone, a known gluconeogenic hormone in mammals and zebrafish (34, 40). As expected, the wildtype larvae treated with 100 μM hydrocortisone for 24 hours exhibited significantly increased glucose levels compared to the vehicle treated larvae (33.6 ± 1.6 vs 14.6 ± 2.1 μM, p=1E-11; Figure 2C). However, gpr27 knockouts exhibited an even greater response to the gluconeogenic stimulus compared to wildtypes. The gpr27 KO larvae treated with hydrocortisone displayed an ~57% higher glucose concentration compared to the gpr27 WT larvae treated with hydrocortisone (52.6 ± 2.3 versus 33.6 ± 1.6 μM, p=8E-8; Figure 2C).
We next assessed the impact of a short exposure to a high fat diet on glucose homeostasis in gpr27 knockouts. Zebrafish have been widely used to study lipid metabolism and the effects of elevated dietary fat intake (17, 37). Further, the regulation of energy homeostasis and the effects of a high fat diet on energy metabolism are conserved between zebrafish and mammals (37). Thus, we fed gpr27 WT and gpr27 KO larvae (7 d.p.f.) a standard diet which consisted of mostly proteins or a high fat diet for 24 hours and subsequently measured glucose. The wildtype and knockout larvae that were fed a standard diet exhibited similar glucose levels (7.5 ±1.2 versus 8.7 ± 1.7 μM; Figure 2D). Moreover, the high fat diet-fed wildtype larvae were able to maintain normal glucose levels as compared to the standard diet-fed wildtype larvae (7.9 ± 1.2 versus 7.5 ±1.2 μM; Figure 2D). However, when the gpr27 KO larvae were fed a high fat diet, their glucose levels increased ~87% compared to the wildtype larvae fed a high fat diet (14.8 ± 2.6 versus 7.9 ± 1.2 μM, p=3E-07; Figure 2D). These data suggest an underlying defect in glucose regulation in gpr27 knockouts. Collectively, the exacerbated elevation in glucose levels in response to the gluconeogenic stimulus or a high fat diet demonstrates that Gpr27 plays an important role in glucose homeostasis in vivo.
gpr27 deletion in zebrafish results in increased medium-chain acylcarnitines.
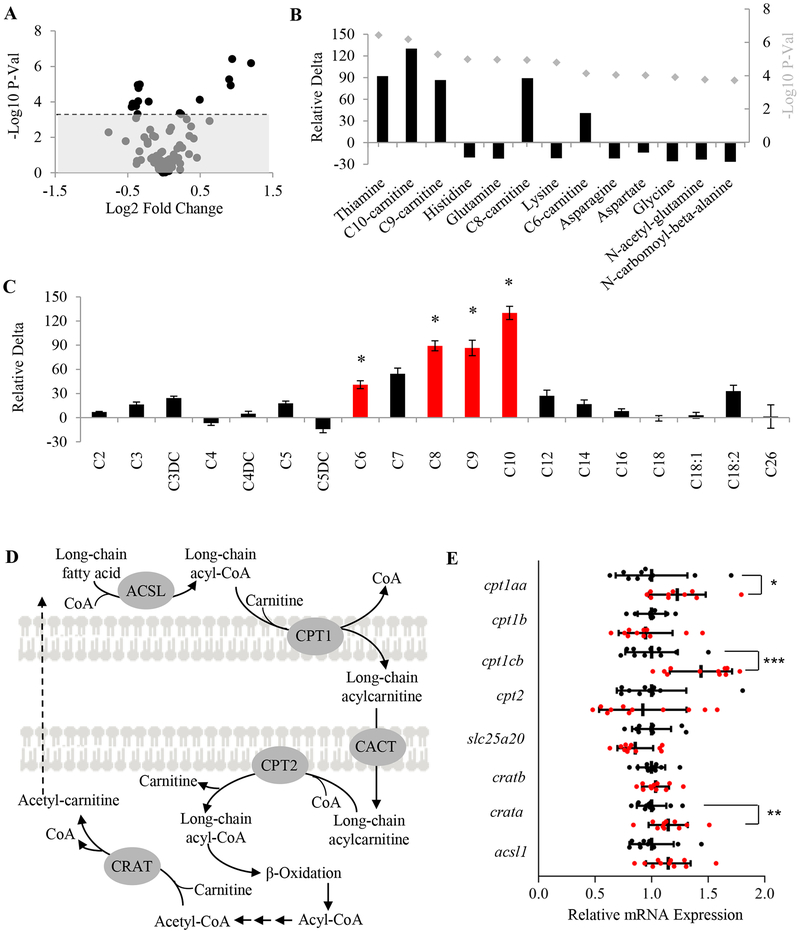
Glucose dysregulation is often interconnected with alterations in other metabolic pathways. Thus, to further characterize the metabolic phenotypes of gpr27 knockouts and to identify the potential metabolic functions of Gpr27 in an unbiased manner, we used a mass spectrometry platform that captures 150 polar metabolites including amino acids, biogenic amines, tryptophan derivatives, urea cycle intermediates, nucleotides, methyltransferase substrates, acylcarnitines, n-acetyl-l-amino acids, and other polar metabolites (Supplemental Table 1). Metabolites were extracted from gpr27 WT and gpr27 KO larvae at 6 d.p.f. and subjected to metabolite profiling. We used a Bonferroni adjusted p-value to correct for multiple comparisons (p ≤ 0.0003) and found 13 metabolites that were significantly different in gpr27 knockouts (Figure 3A). Loss of gpr27 led to a significant decrease in several amino acids including histidine, glutamine, lysine, asparagine, aspartate, and glycine, as well as in n-acetyl-glutamine and n-carbonyl-beta-alanine (Figure 3B). Additionally, we observed a significant increase in 4 metabolites that are acylcarnitine species (Figure 3B).
Figure 3. Loss of gpr27 in zebrafish results in increased medium-chain acylcarnitines.
A) Volcano plot depicting the P-value and fold change of the 150 metabolites measured in gpr27 knockouts compared to wildtype (n = 10–15 samples of 10 pooled larvae). Metabolites above the gray box reach Bonferroni adjusted significance. B) Relative delta [((KO-WT)/WT)*100] of the 13 Bonferroni significant metabolites identified by mass spectrometry (p ≤ 0.0003). The overlaid dot plot depicts the P-value. C) Relative delta of the 19 acylcarnitines measured on the platform. Red bars and asterisks denote Bonferroni significant metabolites (p ≤ 0.0003). D) Pictorial representation of the carnitine-shuttle complex enzymes in the mitochondria. E) Relative expression of cpt1aa, cpt1b, cpt1cb, cpt2, slc25a20, cratb, crata, and acsl1 mRNA in gpr27 wildtype and knockout larvae (n = 11 samples of 10 larvae, vertical bars: ± SD, horizontal bars: mean, *p=0.05, **p=0.03 and ***p=0.008).
Acylcarnitines are formed during the transport of long-chain fatty acids into the mitochondria (41, 42). Subsequently, beta-oxidation results in the complete catabolism of long-chain fatty acids or, in the case of incomplete catabolism, the export of acylcarnitines of varying carbon chain lengths out of the mitochondria. To generate the acylcarnitine profile, our platform captures a total of 19 acylcarnitines spanning distinct fatty acid species containing short-, medium-, and long-carbon chains (Figure 3C). Interestingly, genetic deletion of gpr27 resulted in a highly significant and specific elevation in medium-chain acylcarnitines, in particular C6-hexanoylcarnitine (+40.9 ± 4.9; p=7E-05), C8-octanoylcarnitine (+89.2 ± 6.1; p=1E-05), C9-nonanoylcarnitine (+86.5 ± 9.6; p=5E-06), and C10-decanoylcarnitine (+130.1 ± 8.2; p=6E-07). However, short (C2, C3, C3DC, C4, C2DC, C5, C5DC) and long chain (C12, C14, C16, C18, C18:1, C18:2, C26) acylcarnitines remained unchanged or modestly changed (Figure 3C, red denotes Bonferroni significant metabolites). Additionally, free L-carnitine levels were unchanged (Supplemental Table 1). Hence, these data demonstrate that genetic deletion of gpr27 in zebrafish leads to a specific and significant increase in medium-chain acylcarnitines which brings to light a potential role for gpr27 in the beta-oxidation of fatty acids.
cpt1 gene expression is increased in gpr27 knockouts.
Beta-oxidation of fatty acids occurs in the mitochondria. To enter the mitochondria, long-chain fatty acids must use the carnitine-shuttle complex (Figure 3D) (41, 42). Thus, the expression levels of enzymes in this complex control the rate of beta-oxidation. To determine if Gpr27 effects the expression of enzymes in the carnitine-shuttle, we measured the mRNA levels of zebrafish homologs of carnitine palmitoyltransferase I (isoforms CPT1A, CPT1B and CPT1C), carnitine palmitoyltransferase II (CPTII), carnitine-acylcarnitine translocase (SLC25A20), carnitine acetyl-CoA transferase (CRAT) and long-chain acyl-CoA ligase (ACSL1).
In gpr27 KO larvae, cpt1cb was increased by 1.44 fold (p=0.008) compared to WT larvae (Figure 3E). In addition, crata and cpt1aa was increased by 1.15 and 1.33 fold, respectively (p=0.03 and p=0.05). These findings demonstrate that loss of gpr27 affects the expression of key enzymes in carnitine-shuttle complex, in particular the homologs to the brain and liver isoforms of CPT1 (cpt1cb and cpt1aa). These data also suggest that Gpr27 may regulate the levels of Cpt1 thereby altering the levels of acylcarnitine species. Given that acylcarnitines are not merely units of energy but act as lipid signaling molecules, changes in the levels of acylcarnitines might also alter organismal physiology in gpr27 KOs. Further, since the accumulation of medium- and long-chain acylcarnitines has been linked to the development of insulin resistance (28, 30–32), we hypothesized that loss of gpr27 affects insulin sensitivity.
gpr27 knockouts are resistant to the glucose lowering effect of exogenous insulin.
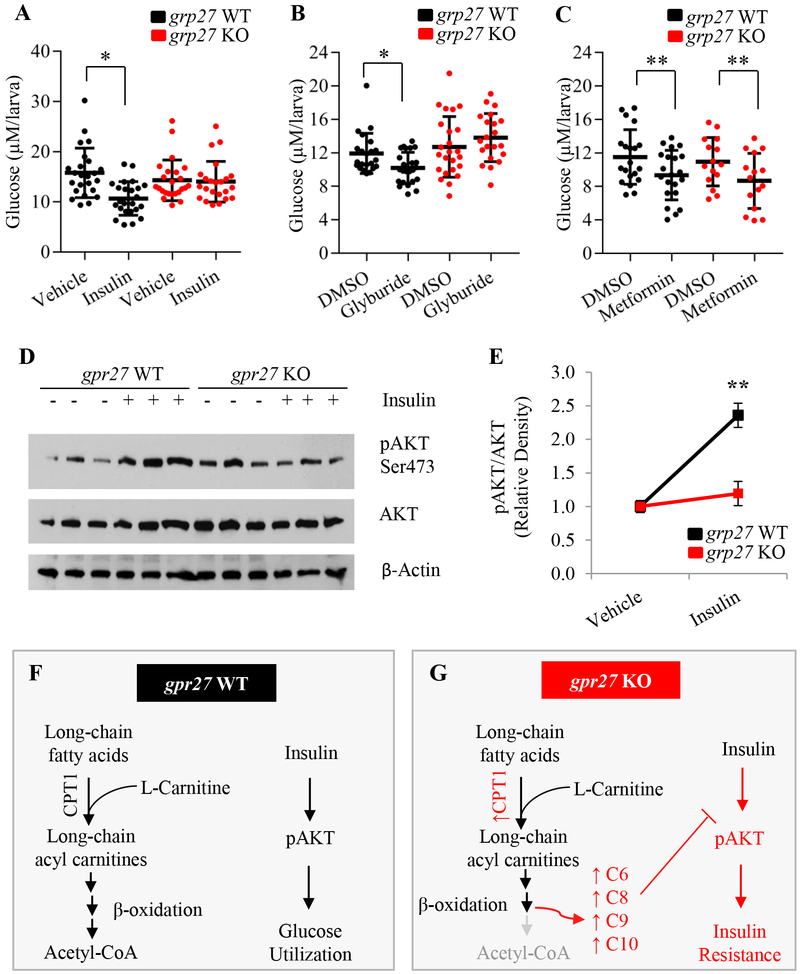
We next investigated the possibility that gpr27 deletion contributes to the development of insulin resistance in zebrafish. As in mammalian models, injection of human insulin in the circulation of zebrafish larvae (4 d.p.f.) leads to a decrease in glucose level and an increase in phosphorylated Akt (35). To test if insulin-stimulated glucose utilization is affected by the loss of gpr27, we measured glucose levels in larvae treated with insulin. At 4 d.p.f. vehicle or insulin was injected into the circulation of gpr27 WT or gpr27 KO larvae. In wildtype larvae, insulin induced an ~30% reduction in glucose levels (10.6 ± 0.4 versus 15.7 ± 0.5 μM, p=0.0001; Figure 4A). In contrast, in the gpr27 knockout larvae, insulin did not significantly affect glucose levels (14.0 ± 0.7 versus 14.3 ± 0.4 μM, Figure 4A). These data demonstrate that gpr27 knockouts are resistant to the glucose lowering effect of exogenous insulin.
Figure 4. Loss of gpr27 abrogates insulin-mediated Akt phosphorylation and glucose utilization.
A) Glucose levels in gpr27 WT or gpr27 KO larvae injected with vehicle or insulin (n = 22–24 larvae, experiments were replicated at least 3 times). Glucose levels in gpr27 wildtype and knockout larvae treated with B) DMSO or 100 nM glyburide, or C) DMSO or 100 μM metformin (n = 22–24 larvae, experiments were replicated at least 3 times). D) A representative Western blot for pAKT-Ser473 and total Akt in gpr27 WT or gpr27 KO larvae injected with vehicle or insulin (3 biological replicates of 10 pooled embryos). E) Quantification of Western blot results (9 biological replicates of 10 pooled embryos). F-G) In wildtype larvae, long-chain fatty acids enter the mitochondria using the carnitine-shuttle complex and undergo beta-oxidation to form acetyl-CoA. However, loss of grp27 increases the expression of CPT1 enzymes and leads to incomplete beta-oxidation which results in increased medium-chain acylcarnitines, abrogated pAKT levels, and diminished insulin-stimulated glucose utilization. Data is represented as the mean ± standard deviation. Statistically significant differences between groups are denoted by lines and asterisks (*p < 0.009, **p ≤ 0.02).
We next performed an orthogonal test using a compound that stimulates insulin secretion. We treated gpr27 WT and gpr27 KO larvae with the insulin secretagogue glyburide and measured glucose concentration. As expected, in the wildtype larvae, treatment with 100 nM glyburide induced an ~15% decrease in glucose levels compared to the vehicle-treated larvae (p=0.009; Figure 4B). In contrast, glyburide did not elicit a significant change in glucose levels in gpr27 knockouts (Figure 4B), corroborating our findings that gpr27 mutants are resistant to the glucose lowering effects of insulin administration (Figure 4A). In comparison, we found that both wildtype and knockout larvae responded to metformin, an anti-diabetic drug that primarily reduces glucose levels by inhibiting gluconeogenesis in the liver. Metformin (100 μM) significantly decreased the level of glucose in wildtype larvae by ~19% (p=0.01) and in knockout larvae by ~20% (Figure 4C; p=0.02). Currently, insulin protein cannot yet be reliably measured in zebrafish (37), therefore we are unable to confirm that glyburide induces similar levels of insulin secretion in wildtypes and knockouts. However, collectively our findings that both insulin injection and glyburide treatment do not lower glucose levels in gpr27 knockouts imply that loss of gpr27 diminishes insulin action.
Loss of gpr27 abrogates Akt-dependent insulin signaling.
We next sought to determine if there was a defect in insulin signaling in gpr27 KO zebrafish. Akt signaling plays a central role in insulin-stimulated glucose uptake in peripheral tissues in both mammals and zebrafish (43, 44); therefore, we examined if insulin-mediated Akt phosphorylation was affected by genetic deletion of gpr27. Following a 2-hour incubation period, lysates were generated and subjected to Western blotting for p-Akt (Ser473) and total Akt (Figure 4D–E). As expected, administration of insulin to wildtype larvae resulted in a significant 2.35 fold increase in p-Akt levels compared to vehicle-treated larvae (p=0.04). In contrast, gpr27 KO larvae showed only a slight increase in Akt phosphorylation in response to insulin treatment. Insulin-treated gpr27 KO larvae displayed significantly less insulin-induced p-Akt (Ser 473) compared to insulin-treated gpr27 WT larvae (1.19 fold increase vs 2.35 fold increase, p=0.02; Figure 4E). Finally, there was not a statistically significant difference in total AKT levels. These data demonstrate that insulin-dependent Akt phosphorylation is abrogated in gpr27 KO zebrafish and suggest that loss of gpr27 contributes to insulin insensitivity.
In summary, this study shows that loss of gpr27 results in diet-induced glucose dysregulation, increased CPT1 expression and increased medium-chain acylcarnitines, abrogated insulin-mediated Akt phosphorylation, and reduced insulin-stimulated glucose utilization. Taken together, the phenotypes in our whole body gpr27 knockout line revealed connections between gpr27, acylcarnitine metabolism, and lipid-induced insulin resistance (Figure 4F–G).
Discussion
Insulin resistance is a major cause of type 2 diabetes and a risk factor for other debilitating health conditions such as cardiovascular disease, cancer, and obesity (45, 46). Although it is known that the development of insulin resistance contributes to the etiology of several human diseases, the phenomenon remains poorly understood at the molecular level (47, 48). This study identifies Gpr27 as a previously unknown player in the development of insulin resistance and presents new opportunities for dissecting the molecular pathways leading to defects in insulin action.
By generating and phenotyping the first whole-body gpr27 knockout line, we found that loss of gpr27 leads to defects in glucose homeostasis and insulin signaling. Administration of glucose-elevating perturbations, such as a high fat diet or a glucocorticoid, resulted in significantly higher glucose level in gpr27 KO zebrafish compared to wildtypes. However, in contrast to a previous study that found decreased insulin gene expression by siRNA-mediated Gpr27 knockdown in mouse pancreatic beta cells (25), we did not find a significant difference in the insulin mRNA levels between the gpr27 wildtype and knockout zebrafish. However, GPR27 is also known to be expressed by several other tissues in addition to the pancreas. Moreover, glucose homeostasis is modulated by intricate intra-tissue communications that cannot be fully replicated in cell-based assays. Our data indicate that insulin sensitivity is diminished in gpr27 KO animals as evidenced by abrogated Akt phosphorylation and glucose utilization upon insulin treatment. These findings suggest that dysregulation of the GPR27 signaling pathway may have an unappreciated role in the development of insulin resistance in humans.
Insulin resistance in diabetic and nondiabetic individuals has been associated with elevations in free fatty acids such as acylcarnitines (26, 29, 32). Using a metabolite profiling approach, we found that gpr27 knockouts manifested a specific acylcarnitine accumulation phenotype. Genetic deletion of gpr27 resulted in elevated medium-chain acylcarnitines, whereas short- and long-chain acylcarnitines were unaffected. Together, these data provide mechanistic insight into the role of gpr27 in energy homeostasis by suggesting that loss of gpr27 leads to incomplete fatty acid oxidation. Fatty acid oxidation occurs in the mitochondria and the rate limiting step in beta-oxidation is CPT1 (41, 42). We found that loss of gpr27 leads to increase expression of the liver isoform of CPT1 and a highly significant increase in expression of the zebrafish homologue CPT1C. CPT1C is predominantly expressed in the brain (49). Interestingly, CPT1C does not play a role in fatty acid oxidation, rather it acts as a hypothalamic energy sensor (50). These findings suggest that Gpr27 may regulate lipid metabolism by transcriptional regulation of CPT1 enzymes.
Following transport into the mitochondria via CPT1 enzymes, if fatty acids are not completely oxidized to acetyl-CoA, mitochondria-derived incompletely oxidized acylcarnitines are able to cross cell membranes, enter the circulation, and act as lipid signaling molecules. Therefore, the acylcarnitine profile of gpr27 knockouts raises the possibility that the primary defect in gpr27 knockout animals is altered lipid metabolism which subsequently leads to lipid-induced insulin resistance. Our findings are consistent with previous studies that suggest a causative role for acylcarnitines in the genesis of insulin resistance. Mice that were treated for 2 weeks with medium-chain acylcarnitines (C6 and C8) exhibited normal fasting glucose levels (32). However, a glucose challenge revealed an underlying glucose intolerance phenotype (32). Moreover, skeletal muscle cells treated with C4, C14 and C16 acylcarnitines displayed decreased insulin-stimulated glucose uptake and decreased insulin-mediated Akt phosphorylation (30). Our data extend prior studies by implicating a role for GPR27 in fatty acid metabolism, thereby providing new insights into the regulation of acylcarnitines and their impact on insulin sensitivity.
Although a growing body of literature from both animal and human studies has shown that acylcarnitines contribute causally to the development of insulin resistance, prior to the development of overt disease, the upstream molecular mechanisms that precede these defects are largely unknown (26, 29, 32). We speculate that Gpr27 regulates acylcarnitine metabolism and that the subsequent accumulation of acylcarnitines influences insulin-mediated glucose homeostasis (Figure 4F–G). Thus, further investigations in the gpr27 zebrafish model may enhance our understanding of how an altered lipid profile leads to impaired insulin signaling. For instance, lipid overload has been linked to oxidative stress, reduced mitochondrial respiratory capacity, and inhibition of glucose-coupled insulin secretion (51). Therefore, future studies to determine if gpr27 knockouts exhibit mitochondrial dysfunction and to measure mitochondrial capacity in gpr27 knockouts are warranted. Notably, gpr27 knockouts exhibit normal basal glucose levels and normal gross morphology suggesting that there is not generalized failure of mitochondrial function in these animals. On the other hand, lipid overload may lead to metabolic inflexibility. The metabolic inflexibility theory postulates that an imbalance between glucose and fatty acid oxidation results in defects in fuel switching, such as in the fasting-to-fed transition, which may subsequently cause glucose utilization defects (52). Hence, it will be important to determine if loss of gpr27 affects the rates of fatty acid and glucose oxidation, in addition to fuel selection. Finally, a detailed analysis of the insulin receptor signaling axis may reveal crucial links between the metabolic phenotypes and the insulin signal transduction defects in the gpr27 knockouts.
Our study does have some limitations. First, we cannot discern the tissue source of accumulated acylcarnitines and insulin resistance. In the future, tissue-specific analyses may shed new light on the crosstalk between tissue and systemic phenotypes in these fish. Moreover, it will be useful to generate tissue-specific gpr27 knockouts in order to determine whether it functions in the brain, muscle, or other peripheral tissues. Second, we proposed that alterations in fatty acid metabolism rather than glucose metabolism are the primary defect in gpr27 KO zebrafish based on the following findings: 1) basal glucose levels are normal in the knockouts in spite of elevated acylcarnitines and 2) exposure to a high fat diet leads to glucose dysregulation. However, we acknowledge that gpr27 may also affect lipid transport. Therefore, to fully delineate the role of Gpr27 in lipid biology, a comprehensive lipid metabolite analysis following standard and high fat diets will be required in addition to lipid transport studies. Finally, an exciting alternative interpretation of our data is that Gpr27 is a lipid sensor that regulates lipid metabolism. Indeed, many lipid species such as free fatty acids, glycerophospholipids, and sphingolipids are known ligands of GPCRs (53, 54). Thus, future investigations will be conducted to determine if acylcarnitines or other lipid species are bona fide ligands of Gpr27.
In conclusion, elucidating the functions of orphan GPCRs in a tractable whole organism model such as the zebrafish is a powerful approach that can reveal unappreciated insights into their in vivo biology. Further, applying metabolic analysis to model systems can help guide mechanistic understanding of phenotypes observed in model organisms. In the present study, we coupled glucose assays with metabolomics analysis and discovered a novel role for gpr27 in acylcarnitine metabolism, insulin sensitivity, and glucose homeostasis in zebrafish. These findings suggest that dysregulated GPR27 signaling may play a role in diabetes or other metabolic syndromes in humans. Finally, this zebrafish line will enable future investigations on the molecular connections between gpr27, lipid metabolism, and insulin-mediated energy homeostasis.
Supplementary Material
Acknowledgements
This work is dedicated to Maria del Carmen who passed away on January 16, 2019. Her unyielding passion for science and her hard work helped this project taking shape in its early stages. A.K.N., J.M., Z.Z.C., Z.L., M.delC.V., and M.L.K. performed the experiments. A.K.N and J.Y. conceived the project and supervised the work. A.K.N. wrote the original draft of the manuscript. A.K.N. and J.Y. revised the manuscript. R.E.G and R.T.P. contributed to the ideas of the project and the final manuscript. A.K.N., R.E.G., R.T.P., and J.Y. provided the resources for the project. This work was supported by NIH U01 MH105027 (J.Y.), NIH R21 HG010392 (A.K.N.) and NIH R01 HL132320-01 (R.E.G.). The authors have no competing interests.
Nonstandard Abbreviations
- Gpr27
g protein-coupled receptor 27
- d.p.f.
days post fertilization
- SREB
superconserved receptors expressed in the brain
- C2
acetylcarnitine
- C3
propionylcarnitine
- C3DC
malonylcarnitine
- C4
butyrylcarnitine
- C4DC
methylmalonylcarnitine
- C5
valerylcarnitine
- C5DC
glutarylcarnitine
- C6
hexanoylcarnitine
- C7
heptanoylcarnitine
- C8
octanoylcarnitine
- C9
nonanoylcarnitine
- C10
decanoylcarnitine
- C12
dodecanoylcarnitine
- C14
tetradecanoylcarnitine
- C16
hexadecanoylcarnitine
- C18
octadecanoylcarnitine
- C18:1
octadecenoylcarnitine
- C18:2
octadecedienylcarnitine
- C26
hexacosanoylcarnitine
References
- 1.Reimann F, and Gribble FM (2016) G protein-coupled receptors as new therapeutic targets for type 2 diabetes. Diabetologia 59, 229–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lagerstrom MC, and Schioth HB (2008) Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov 7, 339–357 [DOI] [PubMed] [Google Scholar]
- 3.Hamm HE (1998) The many faces of G protein signaling. J Biol Chem 273, 669–672 [DOI] [PubMed] [Google Scholar]
- 4.Foster SR, Roura E, Molenaar P, and Thomas WG (2015) G protein-coupled receptors in cardiac biology: old and new receptors. Biophys Rev 7, 77–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauser AS, Attwood MM, Rask-Andersen M, Schioth HB, and Gloriam DE (2017) Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov 16, 829–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oprea TI, Bologa CG, Brunak S, Campbell A, Gan GN, Gaulton A, Gomez SM, Guha R, Hersey A, Holmes J, Jadhav A, Jensen LJ, Johnson GL, Karlson A, Leach AR, Ma’ayan A, Malovannaya A, Mani S, Mathias SL, McManus MT, Meehan TF, von Mering C, Muthas D, Nguyen DT, Overington JP, Papadatos G, Qin J, Reich C, Roth BL, Schurer SC, Simeonov A, Sklar LA, Southall N, Tomita S, Tudose I, Ursu O, Vidovic D, Waller A, Westergaard D, Yang JJ, and Zahoranszky-Kohalmi G (2018) Unexplored therapeutic opportunities in the human genome. Nat Rev Drug Discov 17, 317–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kettleborough RN, Busch-Nentwich EM, Harvey SA, Dooley CM, de Bruijn E, van Eeden F, Sealy I, White RJ, Herd C, Nijman IJ, Fenyes F, Mehroke S, Scahill C, Gibbons R, Wali N, Carruthers S, Hall A, Yen J, Cuppen E, and Stemple DL (2013) A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature 496, 494–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shim H, Kim JH, Kim CY, Hwang S, Kim H, Yang S, Lee JE, and Lee I (2016) Function-driven discovery of disease genes in zebrafish using an integrated genomics big data resource. Nucleic Acids Res 44, 9611–9623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assuncao JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Elliot D, Threadgold G, Harden G, Ware D, Begum S, Mortimore B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Lloyd C, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J, Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P, Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper J, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A, Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Urun Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberlander M, Rudolph-Geiger S, Teucke M, Lanz C, Raddatz G, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Schuster SC, Carter NP, Harrow J, Ning Z, Herrero J, Searle SM, Enright A, Geisler R, Plasterk RH, Lee C, Westerfield M, de Jong PJ, Zon LI, Postlethwait JH, Nusslein-Volhard C, Hubbard TJ, Roest Crollius H, Rogers J, and Stemple DL (2013) The zebrafish reference genome sequence and its relationship to the human genome. Nature 496, 498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santoriello C, and Zon LI (2012) Hooked! Modeling human disease in zebrafish. J Clin Invest 122, 2337–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinner MF, Tucker NR, Lunetta KL, Ozaki K, Smith JG, Trompet S, Bis JC, Lin H, Chung MK, Nielsen JB, Lubitz SA, Krijthe BP, Magnani JW, Ye J, Gollob MH, Tsunoda T, Muller-Nurasyid M, Lichtner P, Peters A, Dolmatova E, Kubo M, Smith JD, Psaty BM, Smith NL, Jukema JW, Chasman DI, Albert CM, Ebana Y, Furukawa T, Macfarlane PW, Harris TB, Darbar D, Dorr M, Holst AG, Svendsen JH, Hofman A, Uitterlinden AG, Gudnason V, Isobe M, Malik R, Dichgans M, Rosand J, Van Wagoner DR, Consortium M, Consortium AF, Benjamin EJ, Milan DJ, Melander O, Heckbert SR, Ford I, Liu Y, Barnard J, Olesen MS, Stricker BH, Tanaka T, Kaab S, and Ellinor PT (2014) Integrating genetic, transcriptional, and functional analyses to identify 5 novel genes for atrial fibrillation. Circulation 130, 1225–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu LY, Fox CS, North TE, and Goessling W (2013) Functional validation of GWAS gene candidates for abnormal liver function during zebrafish liver development. Dis Model Mech 6, 1271–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner BK, Gilbert TJ, Hanai J, Imamura S, Bodycombe NE, Bon RS, Waldmann H, Clemons PA, Sukhatme VP, and Mootha VK (2011) A small-molecule screening strategy to identify suppressors of statin myopathy. ACS Chem Biol 6, 900–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gut P, Reischauer S, Stainier DYR, and Arnaout R (2017) Little Fish, Big Data: Zebrafish as a Model for Cardiovascular and Metabolic Disease. Physiol Rev 97, 889–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlegel A, and Gut P (2015) Metabolic insights from zebrafish genetics, physiology, and chemical biology. Cell Mol Life Sci 72, 2249–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang W, Wang G, Delaspre F, Vitery Mdel C, Beer RL, and Parsons MJ (2014) Retinoic acid plays an evolutionarily conserved and biphasic role in pancreas development. Dev Biol 394, 83–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinlivan VH, and Farber SA (2017) Lipid Uptake, Metabolism, and Transport in the Larval Zebrafish. Front Endocrinol (Lausanne) 8, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nevis K, Obregon P, Walsh C, Guner-Ataman B, Burns CG, and Burns CE (2013) Tbx1 is required for second heart field proliferation in zebrafish. Dev Dyn 242, 550–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prince VE, Anderson RM, and Dalgin G (2017) Zebrafish Pancreas Development and Regeneration: Fishing for Diabetes Therapies. Curr Top Dev Biol 124, 235–276 [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto M, Saito T, Takasaki J, Kamohara M, Sugimoto T, Kobayashi M, Tadokoro M, Matsumoto S, Ohishi T, and Furuichi K (2000) An evolutionarily conserved G-protein coupled receptor family, SREB, expressed in the central nervous system. Biochem Biophys Res Commun 272, 576–582 [DOI] [PubMed] [Google Scholar]
- 21.Dupuis N, Laschet C, Franssen D, Szpakowska M, Gilissen J, Geubelle P, Soni A, Parent AS, Pirotte B, Chevigne A, Twizere JC, and Hanson J (2017) Activation of the Orphan G Protein-Coupled Receptor GPR27 by Surrogate Ligands Promotes beta-Arrestin 2 Recruitment. Mol Pharmacol 91, 595–608 [DOI] [PubMed] [Google Scholar]
- 22.Yanai T, Kurosawa A, Nikaido Y, Nakajima N, Saito T, Osada H, Konno A, Hirai H, and Takeda S (2016) Identification and molecular docking studies for novel inverse agonists of SREB, super conserved receptor expressed in brain. Genes Cells 21, 717–727 [DOI] [PubMed] [Google Scholar]
- 23.Martin AL, Steurer MA, and Aronstam RS (2015) Constitutive Activity among Orphan Class-A G Protein Coupled Receptors. PLoS One 10, e0138463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Consortium GT (2013) The Genotype-Tissue Expression (GTEx) project. Nat Genet 45, 580–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ku GM, Pappalardo Z, Luo CC, German MS, and McManus MT (2012) An siRNA screen in pancreatic beta cells reveals a role for Gpr27 in insulin production. PLoS Genet 8, e1002449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mai M, Tonjes A, Kovacs P, Stumvoll M, Fiedler GM, and Leichtle AB (2013) Serum levels of acylcarnitines are altered in prediabetic conditions. PLoS One 8, e82459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mihalik SJ, Goodpaster BH, Kelley DE, Chace DH, Vockley J, Toledo FG, and DeLany JP (2010) Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring) 18, 1695–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nowak C, Hetty S, Salihovic S, Castillejo-Lopez C, Ganna A, Cook NL, Broeckling CD, Prenni JE, Shen X, Giedraitis V, Arnlov J, Lind L, Berne C, Sundstrom J, Fall T, and Ingelsson E (2018) Glucose challenge metabolomics implicates medium-chain acylcarnitines in insulin resistance. Sci Rep 8, 8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, Hwang DH, Newman JW, and Garvey WT (2009) Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr 139, 1073–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aguer C, McCoin CS, Knotts TA, Thrush AB, Ono-Moore K, McPherson R, Dent R, Hwang DH, Adams SH, and Harper ME (2015) Acylcarnitines: potential implications for skeletal muscle insulin resistance. FASEB J 29, 336–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolf M, Chen S, Zhao X, Scheler M, Irmler M, Staiger H, Beckers J, de Angelis MH, Fritsche A, Haring HU, Schleicher ED, Xu G, Lehmann R, and Weigert C (2013) Production and release of acylcarnitines by primary myotubes reflect the differences in fasting fat oxidation of the donors. J Clin Endocrinol Metab 98, E1137–1142 [DOI] [PubMed] [Google Scholar]
- 32.Batchuluun B, Al Rijjal D, Prentice KJ, Eversley JA, Burdett E, Mohan H, Bhattacharjee A, Gunderson EP, Liu Y, and Wheeler MB (2018) Elevated Medium-Chain Acylcarnitines Are Associated With Gestational Diabetes Mellitus and Early Progression to Type 2 Diabetes and Induce Pancreatic beta-Cell Dysfunction. Diabetes 67, 885–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samuel VT, Petersen KF, and Shulman GI (2010) Lipid-induced insulin resistance: unravelling the mechanism. Lancet 375, 2267–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nath AK, Ryu JH, Jin YN, Roberts LD, Dejam A, Gerszten RE, and Peterson RT (2015) PTPMT1 Inhibition Lowers Glucose through Succinate Dehydrogenase Phosphorylation. Cell Rep 10, 694–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marin-Juez R, Jong-Raadsen S, Yang S, and Spaink HP (2014) Hyperinsulinemia induces insulin resistance and immune suppression via Ptpn6/Shp1 in zebrafish. J Endocrinol 222, 229–241 [DOI] [PubMed] [Google Scholar]
- 36.Schlegel A, and Stainier DY (2006) Microsomal triglyceride transfer protein is required for yolk lipid utilization and absorption of dietary lipids in zebrafish larvae. Biochemistry 45, 15179–15187 [DOI] [PubMed] [Google Scholar]
- 37.Zang L, Maddison LA, and Chen W (2018) Zebrafish as a Model for Obesity and Diabetes. Front Cell Dev Biol 6, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.den Broeder MJ, Moester MJB, Kamstra JH, Cenijn PH, Davidoiu V, Kamminga LM, Ariese F, de Boer JF, and Legler J (2017) Altered Adipogenesis in Zebrafish Larvae Following High Fat Diet and Chemical Exposure Is Visualised by Stimulated Raman Scattering Microscopy. Int J Mol Sci 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O’Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, and Gerszten RE (2011) Metabolite profiles and the risk of developing diabetes. Nat Med 17, 448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lecocq FR, Mebane D, and Madison LL (1964) The Acute Effect of Hydrocortisone on Hepatic Glucose Output and Peripheral Glucose Utilization. J Clin Invest 43, 237–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schulz H (1991) Beta oxidation of fatty acids. Biochim Biophys Acta 1081, 109–120 [DOI] [PubMed] [Google Scholar]
- 42.Indiveri C, Iacobazzi V, Tonazzi A, Giangregorio N, Infantino V, Convertini P, Console L, and Palmieri F (2011) The mitochondrial carnitine/acylcarnitine carrier: function, structure and physiopathology. Mol Aspects Med 32, 223–233 [DOI] [PubMed] [Google Scholar]
- 43.Sharma N, Arias EB, Bhat AD, Sequea DA, Ho S, Croff KK, Sajan MP, Farese RV, and Cartee GD (2011) Mechanisms for increased insulin-stimulated Akt phosphorylation and glucose uptake in fast- and slow-twitch skeletal muscles of calorie-restricted rats. Am J Physiol Endocrinol Metab 300, E966–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maddison LA, Joest KE, Kammeyer RM, and Chen W (2015) Skeletal muscle insulin resistance in zebrafish induces alterations in beta-cell number and glucose tolerance in an age- and diet-dependent manner. Am J Physiol Endocrinol Metab 308, E662–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Avgerinos KI, Spyrou N, Mantzoros CS, and Dalamaga M (2019) Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 92, 121–135 [DOI] [PubMed] [Google Scholar]
- 46.Laakso M, and Kuusisto J (2014) Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol 10, 293–302 [DOI] [PubMed] [Google Scholar]
- 47.Friesen M, and Cowan CA (2019) Adipocyte Metabolism and Insulin Signaling Perturbations: Insights from Genetics. Trends Endocrinol Metab 30, 396–406 [DOI] [PubMed] [Google Scholar]
- 48.Yang Q, Vijayakumar A, and Kahn BB (2018) Metabolites as regulators of insulin sensitivity and metabolism. Nat Rev Mol Cell Biol 19, 654–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Price N, van der Leij F, Jackson V, Corstorphine C, Thomson R, Sorensen A, and Zammit V (2002) A novel brain-expressed protein related to carnitine palmitoyltransferase I. Genomics 80, 433–442 [DOI] [PubMed] [Google Scholar]
- 50.Wolfgang MJ, Kurama T, Dai Y, Suwa A, Asaumi M, Matsumoto S, Cha SH, Shimokawa T, and Lane MD (2006) The brain-specific carnitine palmitoyltransferase-1c regulates energy homeostasis. Proc Natl Acad Sci U S A 103, 7282–7287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chow L, From A, and Seaquist E (2010) Skeletal muscle insulin resistance: the interplay of local lipid excess and mitochondrial dysfunction. Metabolism 59, 70–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goodpaster BH, and Sparks LM (2017) Metabolic Flexibility in Health and Disease. Cell Metab 25, 1027–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Costanzi S, Neumann S, and Gershengorn MC (2008) Seven transmembrane-spanning receptors for free fatty acids as therapeutic targets for diabetes mellitus: pharmacological, phylogenetic, and drug discovery aspects. J Biol Chem 283, 16269–16273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanson MA, Roth CB, Jo E, Griffith MT, Scott FL, Reinhart G, Desale H, Clemons B, Cahalan SM, Schuerer SC, Sanna MG, Han GW, Kuhn P, Rosen H, and Stevens RC (2012) Crystal structure of a lipid G protein-coupled receptor. Science 335, 851–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.