Highlights
-
•
Magnetocardiography non-invasively detects coronary artery stenosis.
-
•
Emergency department chest pain patients are further evaluated in observation unit.
-
•
Patients underwent 90-second magnetocardiography scan using novel analysis system.
-
•
Results compared to usual care with stress testing and coronary angiography.
-
•
Magnetocardiography shows promise as feasible and comparable testing option.
Keywords: Magnetocardiography, Chest pain, Coronary stenosis, Emergency department, Observation unit
Abstract
Background
Magnetocardiography (MCG) has been shown to non-invasively detect coronary artery stenosis (CAS). Emergency department (ED) patients with possible acute coronary syndrome (ACS) are commonly placed in an observation unit (OU) for further evaluation. Our objective was to compare a novel MCG analysis system with stress testing (ST) and/or coronary angiography (CA) in non-high risk EDOU chest pain patients.
Methods
This is a prospective pilot study of non-high risk EDOU chest pain patients evaluated with ST and/or CA that underwent a resting 90-second MCG scan between August 2017 and February 2018. A positive MCG scan was defined as having current dipole deviations with dispersion or splitting during the repolarization phase. ST, CA and major adverse cardiac events (MACE) 30 days and 6 months post-discharge assessed.
Results
Of 101 study patients, mean age was 56 years and 53.6% were male. MCG scan sensitivity with 95% CI was 27.3% [7.3%, 60.7%], specificity 77.8% [67.5%, 85.6%], PPV 13.0% [3.4%, 34.7%] and NPV 89.7% [80.3%, 95.2%] compared to ST, and 33.3% [7.5%, 70.7%], 78.3% [68.4%, 86.2%], 13% [5.2%, 29.0%] and 92.3% [88.2%, 95.1%] respectively compared to ST and CA. No patients had positive ST, CA or MACE 30 days and 6 months post-discharge.
Conclusion
This pilot study suggests a resting 90-second MCG scan shows promise in evaluating EDOU chest pain patients for CAS and warrants further study as an alternative testing modality to identify patients safe for discharge. Larger studies are needed to assess accuracy of MCG using this novel analysis system.
1. Introduction
1.1. Background and importance
Cardiovascular disease remains the leading cause of death among men and women in the United States, representing over 25% of all-cause mortality [1]. Approximately 8 million Americans present to the Emergency Department (ED) with chest pain, making it the second most common chief complaint [2]. Emergency physicians are tasked with rapidly identifying patients with acute coronary syndrome (ACS) to optimize care and outcomes, while also identifying patients who can be safely discharged. Only a minority of patients are high-risk for ACS and will rule in for ACS with either ECG changes consistent with ischemia or infarction, positive serial cardiac biomarkers, or other high-risk features [3]. The majority will not have a diagnosis of ACS after initial ED evaluation and have a normal or non-diagnostic ECG and normal cardiac biomarkers [3], [4]. These patients where there is still a concern for possible ACS are commonly placed in an observation unit (OU) for further monitoring, cardiac diagnostic testing and/or cardiology consultation [4], [5]. This results in further hospital resource utilization, including a longer hospital stay and often diagnostic testing that exposes patients to radiation or more invasive tests.
Magnetocardiography (MCG) is a method of noninvasive measurement and mapping of the magnetic field arising from the physiologic electrical activity of the heart. The first reports of using MCG technology in the United States was over 50 years ago [6], [7]. Similar to an ECG, MCG has morphological features such as a QRS complexes and P-and T-waves. Information about the electrical activity of the heart is obtained passively. The benefit of MCG is that data can be collected rapidly, at rest, and without radiation. MCG does not require contact with the patient’s skin as it is much less affected by conductivity variations of different tissues in the body [8]. In the 1980′s, the development of superconducting quantum interference (SQUID) technology which can detect weak magnetic fields generated by cardiac electrical currents significantly improved MCG sensitivity [9]. This allowed for human studies to compare ECG with MCG in patients with various cardiac disorders, including studying cardiac depolarization abnormalities [10], [11]. Until the 1990s, only single-channel MCG was available and cardiac field maps had to be performed by recording the patient over sequential positions. Subsequently, multichannel MCG mapping, which involves simultaneous mapping from multiple locations, was introduced [12], [13]. This enabled localization of cardiac arrhythmias and arrhythmogenic risk assessment and became one of the first clinical applications of MCG.
Clinical application of MCG in the detection of coronary artery disease (CAD) also followed. An early study by Chaikovsky et al. found that MCG data analyzed in current density vector maps and current line maps of healthy patients without CAD showed a homogeneous distribution of currents whereas maps from patients with CAD showed additional current areas with a deviated direction that was posited to reflect the anatomy of ischemic area and possibly the corresponding involved coronary artery [14].
Clinical studies have investigated MCG in the detection of CAD in patients with chest pain [15], [16], [17]. MCG can detect magnetic field strengths created by cardiac ion currents within myocytes and can more accurately detect depolarization and repolarization abnormalities seen in cardiac ischemia compared with ECG [18], [19]. Studies have also found MCG detects abnormalities in patients with normal ECGs and negative cardiac biomarkers [17], [20]. An early study by Sato et al. found that MCG was more sensitive in detection of myocardial ischemia than a 12-lead ECG or echocardiography [21]. More recent studies have also demonstrated MCG to be superior to echocardiography in detection of acute ischemia [22], [23].
Most studies evaluating MCG in patients with chest pain include non-ED patients with known CAD, or patients with ACS or at high risk for ACS evaluated with coronary angiography (CA) [17], [20], [22], [23], [24], [25]. Of great importance to the emergency physician is which patients presenting with acute chest pain without a clear diagnosis of ACS or non-ACS but with possible ACS after initial ED evaluation can be safely discharged. No study has prospectively evaluated the use of MCG in these non-high-risk ED chest pain patients to assess for coronary artery stenosis (CAS) or directly compared MCG to traditional cardiac diagnostic testing performed in this patient population. Cardioflux (CF) is a novel MCG imaging and analysis system (developed by Genetesis, Inc. 5412 Courseview Drive Suite 150, Mason, OH 45,040 Mason, OH) that uses a series of diagnostic algorithms to convert and interpret magnetic field data into dynamic images with a total imaging time of 90 s. The aims of this pilot study were to evaluate the utilization of a 90-second resting MCG scan using this novel imaging and analysis system to assess for CAS in non-high risk EDOU patients and compare these results to current diagnostic testing, stress testing (ST) and/or CA.
2. Methods
2.1. Study design and setting
This was a prospective, single center pilot study of ED patients placed in an Emergency Department Observation Unit (EDOU) for evaluation of non-high risk chest pain that underwent a 90-second MCG scan between August 2017 and February 2018 in an urban teaching hospital with >100 000 ED patient visits per year. The EDOU is a 30-bed unit located directly above the ED, staffed by attending emergency medicine physicians. This study was approved by the hospital Institutional Review Board and registered in ClinicalTrials.gov (NCT03255772).
2.2. Selection of participants
This was a convenience sample of potentially eligible patients that were approached during regular business hours when a research assistant was available. Consents were obtained for study participation and release of medical information. Each patient was assigned a chronologic study number.
Patients presenting to our ED with acute chest pain suspicious for ACS undergo a standard clinical evaluation that includes ECG and two cTnT blood draws three hours apart. Our hospital uses cTnT measured in a central laboratory with a lower limit of detection of 0.01 mcg/L and a 10% coefficient of variation of 0.03–0.06 mcg/L. A decision limit for normal of <0.03 mcg/L and a positive value as ≥0.10 mcg/L is used. Patients with a diagnosis of ACS after initial ED evaluation undergo standard of care therapy with emergent CA or medical management and admission to an inpatient cardiac telemetry unit. Patients with non-ACS are discharged from the ED. Per our ED protocol, non-high risk ED chest pain patients with possible ACS are those that have a normal or non-diagnostic ECG and negative cTnT results and are then placed in the EDOU for further monitoring, stress testing and /or cardiology consultation. Patients with ECG findings of ischemia or patients with new ST-T ischemic changes compared to the basal ECG (if available) are admitted and treated for ACS and not placed in the EDOU.
2.3. Study protocol
Study inclusion criteria were non-high risk EDOU patients ≥18 years of age presenting to the ED with acute chest pain and possible ACS with a normal or non-diagnostic ECG and two negative cardiac troponin T (cTnT) results 3 h apart that consented to having an MCG scan. Exclusion criteria included patients with metallic items in the chest area, claustrophobia, non-ambulatory, unable to fit into the MCG device or lie supine for 2 min, atrial fibrillation with rapid ventricular response, prisoners, and repeat participants.
Diagnostic evaluation with ST followed our EDOU protocol: persantine stress test (PST) if patients were unable to ambulate and had either a LBBB, previous coronary intervention, automated external defibrillator, pacemaker or cardiomyopathy with low ejection fraction; patients that did not qualify for PST underwent stress echocardiography (SE) if able to do treadmill exercise or dobutamine echocardiography (DE) if unable. PST was performed with single photon emission computed tomography myocardial perfusion imaging (SPECT-MPI) after tetrofosmin injection and at 2 to 4 h of rest after first injection. A positive ST was defined as positive if a new or worsened segmental wall motion abnormality was detected on echocardiography, or if a new or worsened perfusion defect was present on nuclear imaging. When the endocardium was not adequately visualized during echocardiography, intravenous contrast was infused. The decision to undergo CA was left to the discretion of the consulting cardiologist. A positive CA was defined as ≥50% stenosis of at least one coronary artery branch of first or secondary order.
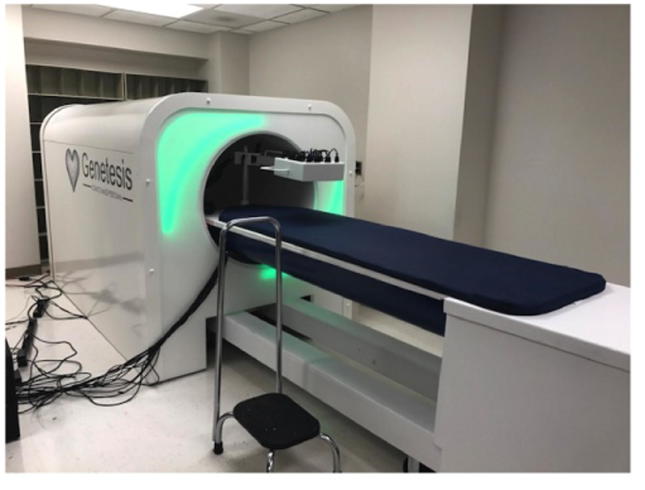
The MCG device was located in a non-magnetically shielded room down the hall from the EDOU comprised of a bed on rails and shielding chamber to prevent outside magnetic interference. (Fig. 1) The device was plugged into a standard (U.S.) 120 V electrical outlet. The device is FDA 510 K pending and there was no requirement for Investigational Device Exemption (IDE) due to minimal risk determination by the IRB. With the patient supine, a sensor plate containing 14 optically pumped magnetic field sensors was positioned about an inch above the chest area, the patient was moved into the chamber and a 90-second scan was obtained and stored in an encrypted database and sent to a HIPAA secure cloud. Patients underwent MCG scanning either prior to ST or CA, or immediately after ST.
Fig. 1.
Cardioflux device.
The MCG device signal quality was evaluated by an automated function of the software, and secondarily by Genetesis personnel. The MCG scan data was aggregated and processed into 3 components: averaged MCG waveforms, Equivalent Current Dipole (ECD), and magnetic field maps. ECD provides a mathematical model to measure and localize the movement of current wavefront within the myocardium at different points in the cardiac cycle. These components were analyzed by the CF software algorithms to look for significant deviations from a referenced database of normal MCG imaging. Because cardiac ischemia causes biologic injury currents and repolarization abnormalities reflected as abnormalities in the magnetic field pictures, it was theorized that shifts in dipole angulation or disorganization in the magnetic field map during repolarization would indicate coronary artery stenosis, and the greater these changes the greater the degree of stenosis. Patients without significant coronary artery stenosis would have organized magnetic dipole orientation without dispersion or splitting during the repolarization phase. Therefore, an automated report of negative MCG scan was defined as having no current dipole deviation pattern and a positive MCG scan was defined as having current dipole deviation with dispersion or splitting during the repolarization phase compared to a referenced database of normal MCG imaging.
Hospital data were collected using the hospital electronic medical record (EMR) and included patient demographics, cardiac risk factors, cardiac co-morbidities, laboratory reports, consultant reports, diagnostic and operative reports, and discharge diagnoses. Assessment of further diagnostic testing with ST or CA and MACE 30 days and 6 months post-discharge as well as 30-day ED re-visits was performed via review of the hospital EMR.
The treating emergency physicians and cardiologists were blinded to the results of the MCG scans and the study team was blinded to the results of the MCG scans until after patient discharge and all index visit testing results were in the hospital EMR. All patient and hospital data and diagnostic testing results were entered into a secure Research Electronic Data Capture (REDCap) form that was blinded to the Genetesis personnel.
2.4. Data analysis
Characteristics of the study group were described using the mean and standard deviation for continuous variables and frequency distributions for categorical variables. MCG scan results were compared first with only ST results. In a separate analysis, if a patient underwent both an ST and CA, the result of the CA was used in the data analysis instead of the ST result to compare with the MCG scan results as CA is the more accurate diagnostic test for CAD. Data analysis were performed with SPSS v. 24.0.
3. Results
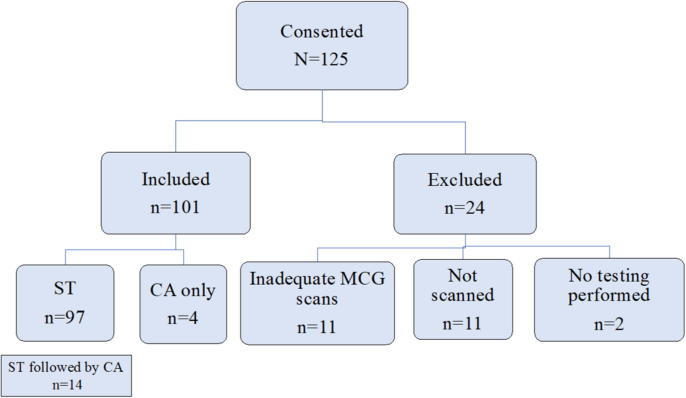
Of 125 consented patients, 101 underwent MCG scanning and were included in the data analysis. See Fig. 2. A total of 24 patients were excluded. Eleven patients were not scanned due to: body habitus (5 patients), claustrophobia (3 patients), metal in thorax (1 patient), vasovagal episode (1 patient) or leaving the OU (1 patient) prior to scanning. Eleven MCG scans were inadequate and had incomplete sensor capture due to body habitus and patient movement; and two patients did not undergo any further testing after cardiology evaluation so were excluded since there was no diagnostic testing performed for comparison. A total of 97 patients underwent ST and 18 patients underwent CA. There were 14 patients who underwent both ST followed by CA during the index visit and 4 patients who underwent only CA. Examples of negative and positive MCG scans are seen in Fig. 3.
Fig. 2.
Patient flow through study. *ST (stress tests) CA (coronary angiography) MCG (magnetocardiography).
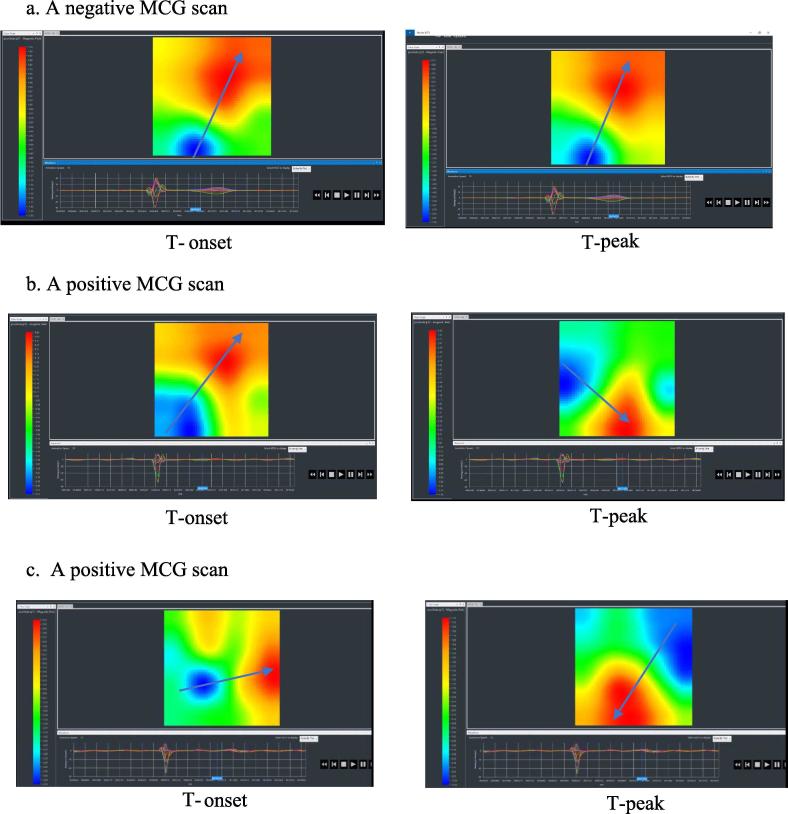
Fig. 3.
Magnetocardiogram examples 3a) A normal magnetocardiography (MCG) scan associated with no coronary artery obstructive disease with no significant current deviations within the myocardium as demonstrated by lack of angle shift (see blue arrow) between the positive red pole and negative blue pole between T wave onset (T-onset) and T wave peak (T-peak). 3b) An abnormal MCG scan demonstrating significant dipole angle deviation (see blue arrow) at T-peak compared to T-onset. 3c) An abnormal MCG scan with significant disruption of myocardial current demonstrated by significant, near reversal of magnetic pole orientation (see blue arrow) at T-peak compared to T-onset. *MCG (magnetocardiography), T-onset (onset of the T wave), T-peak (peak of the T wave).
Of the 101 study patients included in the data analysis, mean age was 56 years, 53.6% were male and 56.5% African American. See Table 1. A history of myocardial infarction or percutaneous coronary intervention was found in 9.9% of patients (no patient had a history of coronary artery bypass grafting), and 5.0% had a history of heart failure or valvular heart disease. Mean number of cardiac risk factors was two and 28.7% had ≥ 3 risk factors for CAD. Of the 97 ST performed, 55 were stress echocardiograms (SE), 16 dobutamine echocardiograms (DE), and 27 persantine stress tests (PST).
Table 1.
Patient demographics, cardiac history, cardiac risk factors, and stress test information.
| Frequency (n=101) | Percent | |
|---|---|---|
| Patient Demographics | ||
| Age | ||
| Mean age | 56 | |
| Min age | 19 | |
| Max age | 81 | |
| Gender | ||
| Male | 53 | 52.47% |
| Female | 47 | |
| Ethnicity | ||
| White | ||
| Black | ||
| Other | ||
| History of Coronary Artery Disease | ||
| MI | 7 | 6.9% |
| PCI | 3 | 3.0% |
| CABG | 0 | 0% |
| History of Other Cardiovascular Disease | ||
| Valvular Heart Disease | 0 | 0% |
| Heart Failure | 5 | 5.0% |
| Cardiac Risk Factors | ||
| Hypertension | 42 | 59.4% |
| Hyperlipidemia | 16 | 41.6% |
| Diabetes | 40 | 15.8% |
| Smoking | 37 | 39.6% |
| Family History | 29 | 36.6% |
| ≥3 Cardiac Risk Factors | 60 | 28.7% |
| Frequency (n=97) | Percent | |
| Stress Test | ||
| Stress Echo | 55 | 56.7% |
| Dobutamine Echo | 16 | 16.5% |
| Persantine | 26 | 26.8% |
Table 2 shows results of MCG scan compared to ST (2a) and compared to ST and CA (2b) where CA result was used instead of ST result in patients who underwent both. Of the patients with a negative ST, a total of 67 patients had a corresponding negative MCG scan. Of 11 patients with a positive ST, 3 had a corresponding positive MCG scan. Of the 10 patients with a negative CA, 6 patients had a corresponding negative MCG and 2 had a corresponding negative ST. Three patients did not undergo ST, only CA. Of the patients with a positive CA, 3 had a corresponding MCG scan and 5 had a corresponding ST. One patient did not undergo ST, only CA. Of these 8 patients with positive CA, 4 underwent percutaneous intervention, 1 underwent coronary artery bypass grafting and 3 were treated medically without intervention.
Table 2.
Comparison results between 3a) stress test and magnetocardiography scan, and 2b) coronary angiography and magnetocardiography scan or stress test.
| 2a. Stress Test Compared to Magnetocardiography Scan Results | ||
|---|---|---|
| ST Negative (n = 86) |
MCG Negative (n = 67) |
MCG Positive (n = 19) |
| Stress Echo (n = 48) |
40 | 8 |
| Dob Echo (n = 15) |
11 | 4 |
| Persantine (n = 23) |
16 | 7 |
| ST Positive (n = 11) |
MCG Positive (n = 3) |
MCG Negative (n = 8) |
|---|---|---|
| Stress Echo (n = 7) |
2 | 5 |
| Dob Echo (n = 1) |
0 | 1 |
| Persantine (n = 3) |
1 | 2 |
| 2b. coronary Angiography Compared to Magnetocardiography Scan And Stress Test Results | ||
|---|---|---|
| Negative CA (n = 10) | ||
| Study No. | MCG Result | Stress Test Type/Result |
| 37 | Negative | Stress Echo/Positive |
| 38 | Negative | Stress Echo/Positive |
| 76 | Negative | Stress Echo/Positive |
| 35 | Negative | Persantine/Positive |
| 45 | Negative | No stress test performed |
| 46 | Negative | No stress test performed |
| 89 | Positive | Stress Echo/Negative |
| 107 | Positive | Stress Echo/Positive |
| 71 | Positive | Persantine/Negative |
| 119 | Positive | No stress test performed |
| Positive CA (n = 8) | |||
|---|---|---|---|
| Study No. | CA Result | MCG Result | Stress Test Type/Result |
| 49 | 75% LAD | Positive | Stress Echo/Positive |
| 137 | 90% RCA, 80–90% 1st/3rd OM | Positive | Stress Echo/Negative |
| 141 | 100% LAD 1st diag | Positive | Persantine/Positive |
| 101 | 99% LAD | Negative | Stress Echo/Positive |
| 64 | 60% LAD 1st diag | Negative | Stress Echo/Negative |
| 105 | 70% LAD, 100% RCA, 80% LCx | Negative | Dob Echo/Positive |
| 120 | 80% LAD 1st diag | Negative | Persantine/Positive |
| 121 | 80% LCx, 50% LAD | Negative | No stress test performed |
*ST (stress tests).
MCG (magnetocardiography).
CA (coronary angiography).
The sensitivity with 95% CI of MCG scans compared to ST was 27.3% [7.3%, 60.7%], specificity 77.8% [67.5%, 85.6%], PPV 13.0% [3.4%, 34.7%] and NPV 89.7% [80.3%, 95.2%]. See Table 3. The sensitivity with 95% CI of MCG scans compared to ST and CA (where CA result was used instead of ST result in patients who underwent both) was 33.3% [7.5%, 70.7%], specificity 78.3% [68.4%, 86.2%], PPV 13% [5.2%, 29.0%] and NPV 92.3% [88.2%, 95.1%].
Table 3.
Comparison of magnetocardiography scan vs. stress test results, and magnetocardiography scan vs. stress test and coronary angiography results.
| MCG Scans (n = 101) vs. ST (n = 97) |
MCG Scans (n = 101) vs. ST (n = 83) + CA (n = 18) |
|
|---|---|---|
| Sensitivity | 27.3% [7.3%, 60.7%] | 33.3% [7.5%, 70.7%] |
| Specificity | 77.8% [67.5%, 85.6%] | 78.3% [68.4%, 86.2%] |
| PPV | 13.0% [3.4%, 34.7%] | 13% [5.2%, 29.0%] |
| NPV | 89.7% [80.3%, 95.2%] | 92.3% [88.2%, 95.1%] |
*ST (stress tests).
CA (coronary angiography).
MCG (magnetocardiography).
No patients underwent ST or CA or had MACE on 30-day follow-up. On 6-month follow-up, one patient underwent ST (negative) after an initial positive CA with stenting and two patients underwent CA (negative); both had corresponding negative MCG and ST. There were no reported MACE 6-months post discharge. The 30-day ED re-visit rate was 12.9% (13/101). None were admitted, one patient went to the EDOU for a complaint of dizziness then discharged, the rest were discharged directly from the ED.
4. Discussion
In the ED, identifying which patients with chest pain or other anginal equivalent symptoms and an initial negative work-up can be safely discharged is challenging. Placing them in an observation unit for further diagnostic testing with ST or CA is currently a common option but does result in the patient staying frequently overnight in the hospital. Reported pooled ST results including those of SE, DE and vasodilator nuclear MPI from multiple studies and meta-analyses show a specificity of 77–82% for detection of ≥50% stenosis as defined by quantitative CA. [26] The results of this study suggest that a resting non-invasive, 90-second MCG using a novel imaging and analysis system that does not require a magnetically shielded room shows promise as a feasible and comparable testing option and continues to evolve. When compared to data from other MCG studies evaluating specificity or NPV, results from this pilot study are also comparable. Agarwal et al. reported in a meta-analysis of prospective studies evaluating MCG in magnetically shielded and non-shielded rooms for the detection of CAD a pooled specificity of 77% [27]. The retrospective study by Kwon et al. [20] used a multichannel MCG system in a magnetically shielded room with a total imaging time of <10 min. They found that MCG using this system in acute chest pain patients with ACS but without ST-elevation ECG changes had an NPV of 70.4 in a subgroup of 238 patients with negative biomarkers. Further exclusion of patients with ischemic ECG findings in this subgroup yielded an NPV of 70.7%. The pilot study by Tolstrup et al. [17] utilized a 36 channel MCG system in a magnetically non-shielded room with an imaging time of six minutes. They found that in a subgroup of 108 inpatients and outpatients with chest pain patients with a non-ischemic ECG and biomarkers the NPV was 86.7%.
Incorporation into the ED workflow of evaluation of chest pain patients should also be explored. Instead of an extended, often overnight stay in the ED or OU due to limited availability of cardiac testing [28], MCG would be completed within minutes and could potentially be performed 24 h per day. Risks to the patient associated with ST, including radiation, adverse reactions to pharmacologic and contrast agents, as well as risks associated with hospitalization would be avoided with a rapid means of evaluating cardiac function. Furthermore, cost savings to patients and the hospital could be substantial.
5. Limitations
The MCG device used in this pilot study is not portable nor applicable at bedside. Currently, functional testing performed in observation units are also not portable nor used at bedside such as stress testing and coronary CT angiography. Other limitations were that patients with metal in the chest area were excluded. It is not known if all metals or objects with a small mass would interfere with the MCG device signal, however in this pilot we chose to exclude patients with any metal regardless of size to eliminate this variable. The diameter of the shielding chamber was also a limiting factor. Patients with larger torsos had incomplete sensor capture likely due to sensor plate contact with the torso during scanning.
As with any new technology reliant upon computer algorithms, machine learning may improve accuracy [29]. In this pilot study, MCG scans were compared to ST or CA using a deep learning computer algorithm which was naïve to recognition of myocardial ischemia. It could be hypothesized that the software needs further machine learning to better recognize various forms of negatives and positives. An example of this is two of the false positive MCG scans where patients had negative corresponding CA (one had a corresponding negative PST and no ST was performed in the other patient). In both these cases, the patients had a low ejection fraction <35%, which is still clinically significant (one patient required a life vest prior to discharge), however differentiation of non-ischemic vs. ischemic cardiomyopathy is important. Moreoever, the small number of positive ST or CA (n = 9) which would be expected in this population of non-high risk chest pain patients is a limitation to assessing accuracy as seen in the large sensitivity or PPV confidence intervals.
This pilot study was not designed to examine the performance characteristics of a new diagnostic modality, but rather to illustrate the potential of a new technology in a real-world clinical setting. Sensitivity and specificity with confidence intervals were calculated to provide guidance for future studies. Evaluation of MCG in patients with various risk profiles and prevalence versus just the population in this study with low prevalence of disease as well as further development of the computer algorithm is the focus of ongoing studies. Additionally, further improvements in the device such as increasing the diameter of the shielding chamber aim to accommodate patients who were excluded for body habitus.
6. Conclusions
This pilot study suggests that a resting non-invasive, 90-second MCG scan using a novel imaging and analysis system shows promise in evaluating EDOU patients for CAS. This warrants further study as an alternative testing modality compared to current testing to identify patients safe for discharge. Larger studies are needed to assess accuracy.
7. Financial Support
Grant from Genetesis, Inc. for administrative support.
Declaration of Competing Interest
The authors declare that they have no known competing financial in-terests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by funding from Genetesis, Inc.
Footnotes
"This author takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation".
Contributor Information
Margarita E. Pena, Email: Margarita.pena@ascension.org.
Claire L. Pearson, Email: Cpearson@med.wayne.edu.
Marc P. Goulet, Email: Marc.goulet@ascension.org.
Viviane M. Kazan, Email: Viviane.kazan@wayne.edu.
Alexandra L. DeRita, Email: Alexandra.derita@ascension.org.
Susan M. Szpunar, Email: Susan.szpunar@ascension.org.
Robert B. Dunne, Email: Robert.dunne@ascension.org.
References
- 1.Heron M. Deaths: Leading Causes for 2014. Natl. Vital Stat. Rep. 2016;65(5):1–96. [PubMed] [Google Scholar]
- 2.Pitts S.R., Niska R.W., Xu J., Burt C.W. National Hospital Ambulatory Medical Care Sur-vey: 2006 emergency department summary. Natl. Health Stat. Report. 2008;7:1–38. [PubMed] [Google Scholar]
- 3.O'Connor R.E., Brady W., Brooks S.C. Part 10: acute coronary syndromes: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S787–S817. doi: 10.1161/CIRCULATIONAHA.110.971028. [DOI] [PubMed] [Google Scholar]
- 4.Amsterdam E.A., Kirk J.D., Bluemke D.A. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation. 2010;122(17):1756–1776. doi: 10.1161/CIR.0b013e3181ec61df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moseley M.G., Hawley M.P., Caterino J.M. Emergency department observation units and the older patient. Clin. Geriatr. Med. 2013;29(1):71–89. doi: 10.1016/j.cger.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baule G., McFee R. Detection of the magnetic field of the heart. Am. Heart J. 1963;66:95–96. doi: 10.1016/0002-8703(63)90075-9. [DOI] [PubMed] [Google Scholar]
- 7.Stratbucker R.A., Hyde C.M., Wixson S.E. The magnetocardiogram–a new approach to the fields surrounding the heart. IEEE Trans. Biomed. Eng. 1963;10:145–149. doi: 10.1109/tbmel.1963.4322823. [DOI] [PubMed] [Google Scholar]
- 8.Malmivuo J., Plonsey R. Bioelectromagnetism : principles and applications of bioelec-tric and biomagnetic fields. 1995;xxii:482 pp.. [Google Scholar]
- 9.Cohen D.E.E., Zimmerman J. Magnetocardiograms taken inside a shielded room with a superconducting point-contact magnetometer. Appl. Phys. 1970:278–280. [Google Scholar]
- 10.Saarinen M., Karp P.J., Katila T.E., Siltanen P. The magnetocardiogram in cardiac di-sorders. Cardiovasc. Res. 1974;8(6):820–834. doi: 10.1093/cvr/8.6.820. [DOI] [PubMed] [Google Scholar]
- 11.Fenici R., Romani G.L., Barbanera S., Zeppilli P., Carelli P., Modena I. High resolution magnetocardiography. Non-invasive investigation of the His-Purkinje system activity in man. G Ital. Cardiol. 1980;10(10):1366–1370. [PubMed] [Google Scholar]
- 12.Makijarvi M., Nenonen J., Toivonen L., Montonen J., Katila T., Siltanen P. Magnetocar-diography: supraventricular arrhythmias and preexcitation syndromes. Eur. Heart J. 1993;14(Suppl E):46–52. doi: 10.1093/eurheartj/14.suppl_e.46. [DOI] [PubMed] [Google Scholar]
- 13.Nenonen J., Makijarvi M., Toivonen L. Non-invasive magnetocardiographic lo-calization of ventricular pre-excitation in the Wolff-Parkinson-White syndrome using a re-alistic torso model. Eur. Heart J. 1993;14(2):168–174. doi: 10.1093/eurheartj/14.2.168. [DOI] [PubMed] [Google Scholar]
- 14.L. Chaikovsky, J. Kohler, T. Hecker, et al., Detection of coro-nary artery disease in patients with normal or unspecifical-ly changed ECG on the basis of magnetocardiography. In: Biomag 2000 Proceedings of the 12th International Confer- ence on Biomagnetism. Helsinki: University of Technology, Espoo, 2001, pp. 565–568.
- 15.Park J.W., Reichert U., Maleck A., Klabes M., Schäfer J., Jung F. Sensitivity and predictivity of magnetocardiography for the diagnosis of ischemic heart disease in patients with acute chest pain: preliminary results of the hoyerswerda registry study. Crit. Pathw. Cardiol. 2002;1(4):253–254. [Google Scholar]
- 16.Hailer B., Chaikovsky I., Auth-Eisernitz S., Schafer H., Van Leeuwen P. The value of magnetocardiography in patients with and without relevant stenoses of the coronary ar-teries using an unshielded system. Pacing. Clin. Electrophysiol. 2005;28(1):8–16. doi: 10.1111/j.1540-8159.2005.09318.x. [DOI] [PubMed] [Google Scholar]
- 17.Tolstrup K., Madsen B.E., Ruiz J.A. Non-invasive resting magnetocardiographic imaging for the rapid detection of ischemia in subjects presenting with chest pain. Cardiology. 2006;106(4):270–276. doi: 10.1159/000093490. [DOI] [PubMed] [Google Scholar]
- 18.Hopenfeld B., Stinstra J.G., Macleod R.S. Mechanism for ST depression associated with contiguous subendocardial ischemia. J. Cardiovasc. Electrophysiol. 2004;15(10):1200–1206. doi: 10.1046/j.1540-8167.2004.04072.x. [DOI] [PubMed] [Google Scholar]
- 19.Hanninen H., Takala P., Makijarvi M. Detection of exercise-induced myocardial ischemia by multichannel magnetocardiography in single vessel coronary artery disease. Ann. Noninvasive Electrocardiol. 2000;5(2):147–157. [Google Scholar]
- 20.Kwon H., Kim K., Lee Y.H. Non-invasive magnetocardiography for the early diagnosis of coronary artery disease in patients presenting with acute chest pain. Circ. J. 2010;74(7):1424–1430. doi: 10.1253/circj.cj-09-0975. [DOI] [PubMed] [Google Scholar]
- 21.M. Sato, Y. Terada, T. Mitsui, T. Miyashita, A. Kandori, K. Tsukada (Eds.), Detection of myocardial ischemia by magnetocardiogram using 64-channel SQUID system, in: Biomag 2000 Proceedings of the 12th International Conference on Bio-magnetism; Espoo, Helsinki: University of Technology; 2001. pp. 523–526.
- 22.Li Y., Che Z., Quan W. Diagnostic outcomes of magnetocardiography in patients with coronary artery disease. Int. J. Clin. Exp. Med. 2015;8(2):2441–2446. [PMC free article] [PubMed] [Google Scholar]
- 23.Park J.W., Hill P.M., Chung N., Hugenholtz P.G., Jung F. Magnetocardiography predicts coronary artery disease in patients with acute chest pain. Ann. Noninvasive Electrocardiol. 2005;10(3):312–323. doi: 10.1111/j.1542-474X.2005.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park J.W., Leithauser B., Vrsansky M., Jung F. Dobutamine stress magnetocardiog-raphy for the detection of significant coronary artery stenoses - a prospective study in comparison with simultaneous 12-lead electrocardiography. Clin. Hemorheol. Microcirc. 2008;39(1–4):21–32. [PubMed] [Google Scholar]
- 25.Shin E.S., Lam Y.Y., Her A.Y., Brachmann J., Jung F., Park J.W. Incremental diagnostic value of combined quantitative and qualitative parameters of magnetocardiography to detect coronary artery disease. Int. J. Cardiol. 2017;228:948–952. doi: 10.1016/j.ijcard.2016.11.165. [DOI] [PubMed] [Google Scholar]
- 26.Arbab-Zadeh A. Stress testing and non-invasive coronary angiography in patients with suspected coronary artery disease: time for a new paradigm. Heart Int. 2012;7(1):e2. doi: 10.4081/hi.2012.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agarwal R., Saini A., Alyousef T., Umscheid C.A. Magnetocardiography for the diagno-sis of coronary artery disease: a systematic review and meta-analysis. Ann. Noninvasive Electrocardiol. 2012;17(4):291–298. doi: 10.1111/j.1542-474X.2012.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinhardt S.W., Lin C.J., Novak E., Brown D.L. Noninvasive cardiac testing vs clinical evaluation alone in acute chest pain: a secondary analysis of the ROMICAT-II randomized clinical trial. JAMA Intern. Med. 2018;178(2):212–219. doi: 10.1001/jamainternmed.2017.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tantimongcolwat T., Naenna T., Isarankura-Na-Ayudhya C., Embrechts M.J., Pracha-yasittikul V. Identification of ischemic heart disease via machine learning analysis on mag-netocardiograms. Comput. Biol. Med. 2008;38(7):817–825. doi: 10.1016/j.compbiomed.2008.04.009. [DOI] [PubMed] [Google Scholar]