Abstract
Tripartite motif 34 (TRIM34) is a member of TRIM family that can be highly induced by type I Interferon. Currently little is known about the subcellular localization and biological function of TRIM34. In the present study, confocal microscope assay showed that TRIM34 proteins were mainly distributed in the cytoplasm and part of TRIM34 proteins were localized to the mitochondria in human embryonic kidney 293T (HEK293T) cells. Western blot results demonstrated FLAG-TRIM34 could also be identified in the mitochondrial fractions of HEK293T cells transfected with the 5′FLAG-pcDNA3.1-TRIM34 vector. The CCK-8 assay further demonstrated that TRIM34 significantly decreased the viability of HEK293T cells. Nevertheless, TRIM34 had no apparent effect on the cell cycle distribution. Interestingly, flow cytometry showed that TRIM34 could obviously induce apoptosis in HEK293T cells. Moreover, we discovered that TRIM34 promoted apoptosis by inducing the loss of mitochondrial membrane potential (MMP) in HEK293T cells, leading to the release of cytochrome c from mitochondia. In short, these results demonstrate that TRIM34 proteins can localize to the mitochondria and induce apoptosis via the depolarization of MMP in HEK293T cells.
Keywords: Cell biology, Immunology, Microbiology, Proteins, Molecular biology, Colocalization, Tripartite motif 34 (TRIM34), Mitochondria, Apoptosis
Cell biology; Immunology; Microbiology; Proteins; Molecular Biology; Colocalization; Tripartite motif 34 (TRIM34); Mitochondria; Apoptosis.
1. Introduction
TRIM34 belongs to the tripartite motif family and contains a RING domain, two B-Box domains and a coiled-coil domain (RBCC) at the N-terminal region [1, 2]. It has been shown that TRIM34 possesses at least three kinds of isoforms [3]. The medium isoform consists of RBCC-B30.2 domain and is the main form of TRIM34 in various human organs [3]. The long isoform of TRIM34 is composed of RBCC-RBCC-B30.2 domain and the short isoform possesses only RBCC domain. Human TRIM34 gene is located on the chromosomal 11p15, clustering with a group of TRIM homologous genes containing TRIM6, TRIM5 and TRIM22 [4,5]. Similarly to many TRIM family members, TRIM34 can be stimulated dramatically by Type I interferons in HeLa cells which predicates that TRIM34 protein potentially mediates the biological function of interferons [3]. The basal expression level of TRIM34 is low in unstimulated human primary macrophages and lymphocytes. However, TRIM34 is significantly induced by type I or type II interferon in human primary macrophages or lymphocytes [6]. Besides, TRIM34 is up-regulated by influenza A virus (H1N1) or phosphorothioate CpG DNA stimulus in mouse macrophages and DC, which is dependent on type I interferon signaling passway [7].
Previous studies have revealed that TRIM34 plays certain roles in antiviral action. TRIM5α from rhesus monkey is characterized by blocking HIV-1 replication through targeting viral capsid, leading to premature disassembly before reverse transcription [8, 9]. The RBCC domain from rhesus monkey TRIM5α can be substituted by corresponding domain of TRIM34 and the novel recombined proteins effectively suppress the HIV-1 replication [10]. TRIM34 can bind the capsid protein of HIV-1, however it cannot obviously suppress the HIV-1 infection [11]. In addition, TRIM34 possesses a weak but specific restriction on HIV-2/SIV (MAC) and EIAV [12]. Recently, evidences show that TRIM34 is associated with Parkinson's disease_ENREF_9. Epigenetic modifications of specific genes, such as methylated CpGs of TRIM34 and PDE4D, were related to the susceptibility of Parkinson's disease to some degree [13].
Although many aspects of TRIM34 have been shown, the subcellular location of TRIM34 and its function on cell cycle and apoptosis remain unknown. In this study, we found that TRIM34 proteins were distributed mainly in the cytoplasm and could localize to the mitochondria in HEK293T cells. The CCK-8 assay showed that TRIM34 overexpression significantly decreased the viability of HEK293T cells, nevertheless TRIM34 had no apparent effect on the cell cycle distribution. Interestingly, flow cytometry showed that TRIM34 could induce apoptosis in HEK293T cells. We speculate that TRIM34 potentially contributes to apoptosis through the mitochondria passway. Thus, we also examined the effect of TRIM34 on the MMP using Rodamine123, JC-1 or MitoTracker Deep Red staining.
2. Methods
2.1. Plasmids construct
Human TRIM34 cDNAs were obtained by RT-PCR from HeLa cells stimulated by INFα and cloned into pEGFP-N3 vector (Clontech) using XhoⅠ and HindⅢ restriction enzymes. For construction of the 5′FLAG-pcDNA3.1-TRIM34 vector, TRIM34 cDNA fragment was subcloned at the ClaⅠ and XhoⅠ sites into 5′FLAG-pcDNA3.1 vector (Invitrogen). Human TRIM34 or TRIM22 cDNAs were also subcloned into the XhoⅠ/HindⅢ sites of pDsRed1-N1 (Clontech) respectively. The constructs described here were verified by sequencing. The pEGFP-LC3B-h vector was purchased from Wuhan Miaoling Bioscience & Technology.
2.2. Cell culture and transfection
HEK293T cells were cultured in DMEM (Hyclone) containing 10% fetal bovine serum, 4.5 g/L glucose, 4.0 mM L-glutamine and sodium pyruvate. Cells were maintained at 37 °C in humidified atmosphere of 5% CO2. For transfection, HEK293T cells were seeded in culture plates (CORNING) or confocal dishes (NEST) for 20 h and transfected with plasmids using Lipofectamine 2000 (Invitrogen) following the protocol of manufacturer. Mitochondria were visualized by incubating cells with MitoTracker Deep Red (100 nM) (Molecular Probes: M22426) for 30 min at 37 °C with 5% CO2.
2.3. Cell counting kit 8 (CCK8) assay
HEK293T cells were seeded in 96-well plates at density of 1×104 cells per well and incubated at 37 °C for 24 h. Five duplicate wells were set for each group. The cells were transfected with 5′FLAG-pcDNA3.1-TRIM34 or 5′FLAG-pcDNA3.1 (0.2 μg/well) for 24 h. Then 10 μl of CCK-8 (Dojindo, Japan) was added and the cells were incubated for 2 h at 37 °C. At the end, the optical density was examined by Multiskan FC microplate photometer (Thermo) at the wave length of 450 nm.
2.4. Cell cycle analysis by flow cytometry
HEK293T cells were cultured in 6 well plates at density of 3×105/plate. The cells were transfected with the 5′FLAG-pcDNA3.1-TRIM34 vector or 5′FLAG-pcDNA3.1 vector (3 μg/well) for 24 h. The cells were released by trypsinization and washed with cold PBS. Then the cell pellets were suspended in 500 μl of propidium iodide solution (3.8 mM sodium citrate, 20 μg/mL propidiumiodide, 0.1% Triton X-100, 20 μg/mL RNase B) and incubated at 4 °C in the dark for 30 min. Finally, the stained cells were analyzed by FACS Calibur flow cytometer (Becton-Dickinson). Twenty thousand events were acquired and the data were analyzed by Modfit software.
2.5. Apoptosis detection
HEK293T cells were seeded in 12-well plates and transfected with vectors when cells reached 70%~80% of growth density, according to Lipofectamine 2000 instructions. At 24 h post-transfection, the cells were trypsinized, washed twice and suspended in 100 μl 1×binding buffer. Then cells were incubated with 5 μl AnnexinV-PE and 5 μl 7-AAD (Becton-Dickinson) for 15 min at room temperature in the dark. Finally, 400 μl of 1×binding buffer was added to each tube and the samples were detected by flow cytometry within 1 h.
2.6. Confocal microscope assays
HEK293T cells were washed with PBS and fixed with 4% paraformaldehyde at room temperature for 15 min. Then the cells were permeabilized (0.1% Triton X-100) for 5 min and stained with DAPI for 10 min. Images were analyzed by Leica TCS SP5 confocal microscope equipped with 63×oil immersion objectives. The results were acquired in sequential scan mode and the specimens were excited at 405 nm, 488 nm, 543 nm or 633 nm wave length.
To quantify the colocalization degree in some confocal microscope results, JACoP analysis plugin of ImageJ 1.50i was used to calculate the Pearson's correlation coefficients (PCC). The extent of colocalization from PCC value was sorted as very strong (0.85–1.0), strong (0.49–0.84), moderate (0.1–0.48), weak (-0.26 to 0.09) etc [14].
2.7. Subcellular fractionation
Two different methods were applied to prepare the cell extracts. The cells were washed with ice-cold PBS and lysed with ice-cold buffer A containing 1.0% (vol/vol) Nonidet P40, 20 mM Tris-HCl (pH 8.0), 150 mM NaCl, 10% (vol/vol) glycerol, 1 mM NaF, 1 mM sodium pyrophosphate, 2 mM sodium orthovanadate and protease inhibitor cocktail (Roche). After incubation on ice for 15 min, the supernatants were obtained by centrifuging at 12,000×g for 15 min and the protein concentration was detected by the BCA method.
The cells (2×107) were harvested by trypsinization, washed once in ice-cold PBS and resuspended in 800 μl hypotonic buffer B (10 mM Tris-Cl, pH 7.5, 1.5 mM MgCl2, 10 mM KCl, 1 mM Na-EDTA, 1 mM DTT, 0.5 mM PMSF and protease inhibitor cocktail) for 5 min on ice. Then, the cells were homogenized with 30 strokes using the pre-cooled dounce tissue grinder on ice. The sucrose concentration was adjusted to 250mM with 2M sucrose and the homogenates were centrifuged at 700×g for 10 min at 4 °C to remove the nuclei and unbroken cells. The supernatant was collected and pelleted by centrifugation at 10,000×g for 15 min at 4 °C. Then, the supernatant was carefully collected and the pellet contained the mitochondria fraction. Afterwards, the supernatant was centrifuged at 15000×g for 15 min at 4 °C as the mitochondria-free cytoplasmic fraction. The pelleted mitochondrial fraction was washed with 500 μl buffer C (10 mM Tris-Cl, pH 7.5, 20 mM EDTA, 250mM sucrose), and then were dissolved in 100 μl RIPA buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% Nonidet P-40) containing DTT and protease inhibitors. The protein concentrations were determined by the BCA method.
2.8. Western blot analysis
Proteins from different groups were separated in 10% or 12% SDS-polyacrylamide gel and transferred to the nitrocellulose membrane (Pall). Then the membranes were washed with TBS-T and blocked in 5% powdered-milk solution in TBS-T for 1 h. After washing with TBS-T, the membranes were separately probed with antibodies of anti-FLAG (sigma, F3165), β-actin (Cell Sigmaling, #4970), cytochrome c (Santa Cruz Biotechnology, sc-13156) for 1 h. After washing with TBS-T, the membranes were incubated with the secondary HRP-labeled anti-mouse antibody (SouthernBiotech, 6170-05) or anti-rabbit antibody (SouthernBiotech, 4030-05) for 1 h at room temperature. Proteins were visualized by the ECL Plus Western Blotting Substrate (Thermo) according to the manufacturer's directions.
2.9. Measurement of the MMP by Rhodamine 123
The MMP was monitored by Rhodamine 123 staining, which can be selectively absorbed into the mitochondria. The depolarization of MMP led to the loss of Rhodamine 123 from the mitochondria. Briefly, HEK293T cells were seeded in 12-well plates to reach 50%–60% confluence and were transfected with the 5′-Flag-pcDNA3.1 or 5′-Flag-pcDNA3.1-TRIM34 vectors (1.5 μg/well). At 24 h post-transfection, Rhodamine 123 (final concentration 1 μg/ml) was added into the cell culture medium and incubated for 15 min at 37 °C in the dark. After detachment by trypsinization, the cells were washed twice with cold PBS and immediately analyzed by flow cytometer at 488 nm excitation wave length.
2.10. Detection of the MMP by JC-1
The MMP was also measured using JC-1 Assay Kit (Shanghai Yeasen Biological Technology Company). HEK293T cells were seeded in 12-well plates and transfected with the 5′-Flag-pcDNA3.1 or 5′-Flag-pcDNA3.1-TRIM34 vectors (1.5 μg/well). After 24 h, the cells were collected by mild trypsinisation and centrifugation. The pellets were resuspended in the JC-1 staining solution and incubated for 20 min at 37 °C in a CO2 incubator. Then the cells were washed twice with the JC-1 staining buffer, resuspended in 500 μl staining buffer and analyzed by flow cytometry. Excited by the laser at 488 nm wave length, the JC-1 aggregates produced red fluorescence detected at the FL-2 channel, while the JC-1 monomers produced green fluorescence detected at the FL-1 channel. The data were analyzed by FlowJo VX software.
2.11. Detection of the MMP by MitoTracker Deep Red
HEK293T cells were seeded in 12-well plates and transfected with the pEGFP-N3 (control) or pEGFP-N3-TRIM34 vectors (1.5 μg/well). At 24 h post-transfection, the cells were stained with MitoTracker Deep Red (5 nM) for 30 min at 37 °C with 5% CO2. After detachment by trypsinization, the cells were washed twice with cold PBS and analyzed by flow cytometry at 488 nm and 633 excitation wave length.
2.12. Statistical analysis
Each experiment was repeated at least three times, and the data were expressed as mean ± standard deviation (SD). All the statistical analyses were conducted by unpaired student's t-test using GraphPad Prism7.0 software (San Diego, USA). The differences were considered statistically significant when the p-value was less than 0.05.
3. Results
3.1. TRIM34 localized to both the mitochondria and cytosol
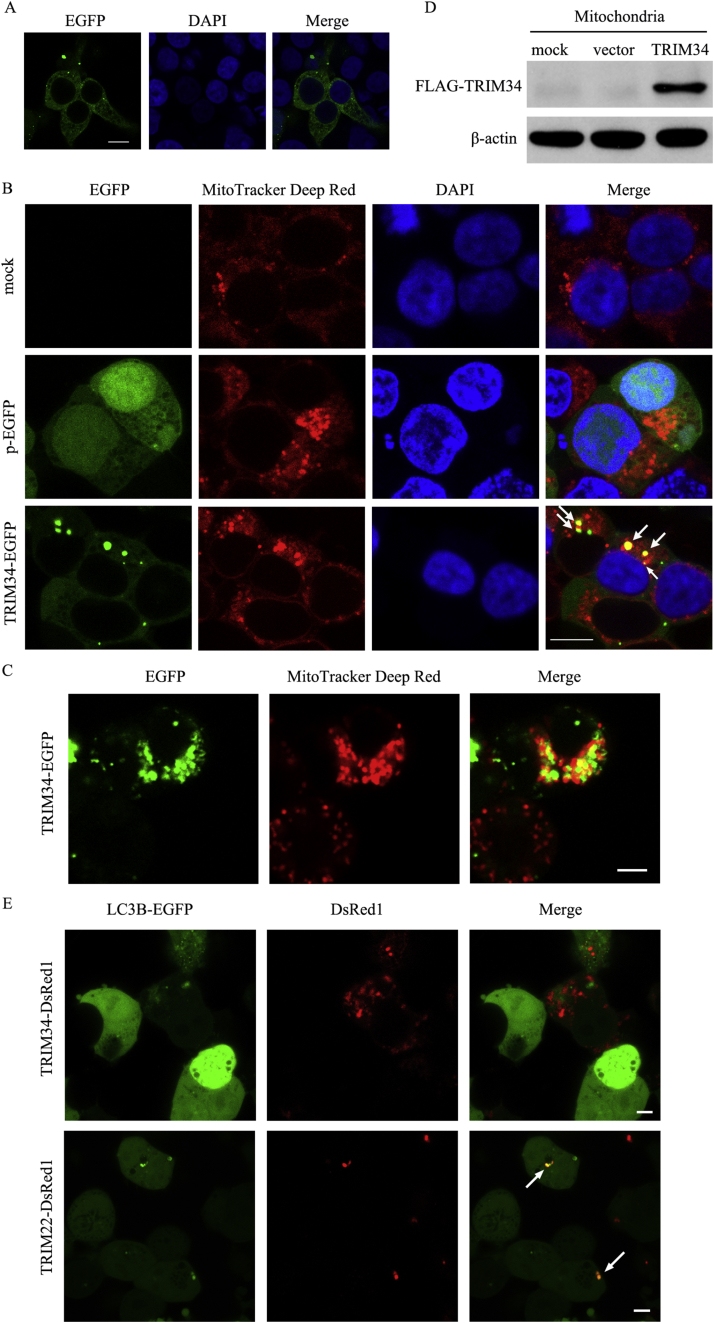
Confocal microscope showed that TRIM34-EGFP was distributed mainly in the cytoplasm and formed aggregates in HEK293T cells (Figure 1A). Interestingly, part of the TRIM34-EGFP fusion proteins localized to the mitochondria (Figure 1B). However, the EGFP proteins alone did not localize to the mitochondria (Figure 1B). The Pearson's correlation coefficient for the TRIM34-EGFP versus mitochondria was 0.607 analyzed by JACoP analysis plugin of ImageJ 1.50i software (Figure 1C). Moreover, western blot analysis revealed that the FLAG-TRIM34 proteins could be detected in the mitochondrial proteins (Figure 1D). At the same time, we found that TRIM34-DsRed1 did not colocalize with LC3B-EGFP, an autophagy marker, while TRIM22-DsRed1 colocalized with LC3B-EGFP markedly (Figure 1E). This result suggested that TRIM34 might have no effect on the autophagy-mediated changes of mitochondrial mass.
Figure 1.
TRIM34 localized to both the mitochondria and cytosol. (A) HEK293T cells were transfected with the pEGFP-N3-TRIM34 vectors for 24 h. Then the cells were fixed, stained for nuclei using DAPI (blue) and analyzed by confocal microscope. Scale bar, 10 μm. (B) HEK293T cells were transfected with pEGFP-N3-TRIM34 or pEGFP-N3 vectors (control) for 24 h, then the mitochondria were stained with MitoTracker Deep Red and the nuclei were stained with DAPI (blue). Images were visualized at × 63 oil immersion objectives by confocal microscope. The scale bar represented 10 μm. The localization of TRIM34-EGFP to the mitochondria was marked with arrows. Representative results were presented, two additional experiments yielded similar results. (C) The Pearson's correlation coefficients were calculated by JACoP analysis plugin of ImageJ 1.50i. The scale bar represented 5 μm. (D) HEK293T cells were transfected with 5′FLAG-pcDNA3.1 (vector), 5′FLAG-pcDNA3.1-TRIM34 (TRIM34) or subjected to mock transfection for 24 h. The mitochondria fraction was prepared and the expression of TRIM34 was examined by western blot analysis. Representative results were presented and two additional experiments yielded similar results. (E) HEK293T cells were cotransfected with LC3B-EGFP/TRIM34-DsRed1 or LC3B-EGFP/TRIM22-DsRed1 for 48 h. Images were visualized at × 63 oil immersion objectives using confocal microscope. The scale bar represented 5 μm. Representative results were presented and two additional experiments yielded similar results.
3.2. Effects of TRIM34 on the cell viability and cell cycle
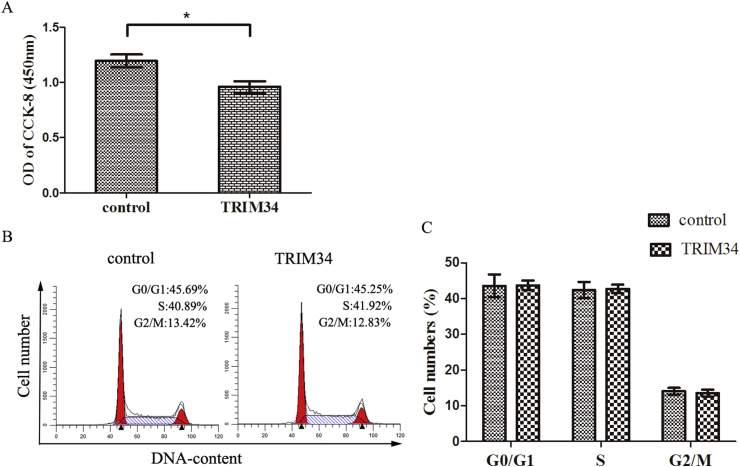
The CCK8 assay was performed to examine the role of TRIM34 on the viability of HEK293T cells. HEK293T cells were transfected with the 5′FLAG-pcDNA3.1-TRIM34 or 5′FLAG-pcDNA3.1 vectors for 24 h, and then the changes of cell viability was evaluated. As shown in Figure 2A, TRIM34 significantly reduced the cell viability compared with the control group.
Figure 2.
Effects of TRIM34 on the cell viability and cell cycle. (A) HEK293T cells were transfected with 5′FLAG-pcDNA3.1-TRIM34 or control vectors for 24 h, and then subjected to the CCK8 assay. The cell viability was detected by absorbance which was measured at 450 nm. Results were obtained from at least three independent experiments, and data were showed as mean ± SD. (B) HEK293T cells were transfected with 5′FLAG-pcDNA3.1- TRIM34 or 5′FLAG-pcDNA3.1 vectors for 24 h. The cells were then harvested and stained with propidium iodide. The cell cycle distribution was analyzed by flow cytometry. Images were representative of three independent tests. (C) The cell cycle distribution was quantitative analyzed. Each bar represented means ± SD from three independent experiments.
The effect of TRIM34 on the cell cycle distribution was investigated by flow cytometry in HEK293T cells. As shown in Figures 2B and 2C, there were no significant differences between the TRIM34 group and the control group in the cell cycle distribution.
3.3. Apoptosis induced by TRIM34
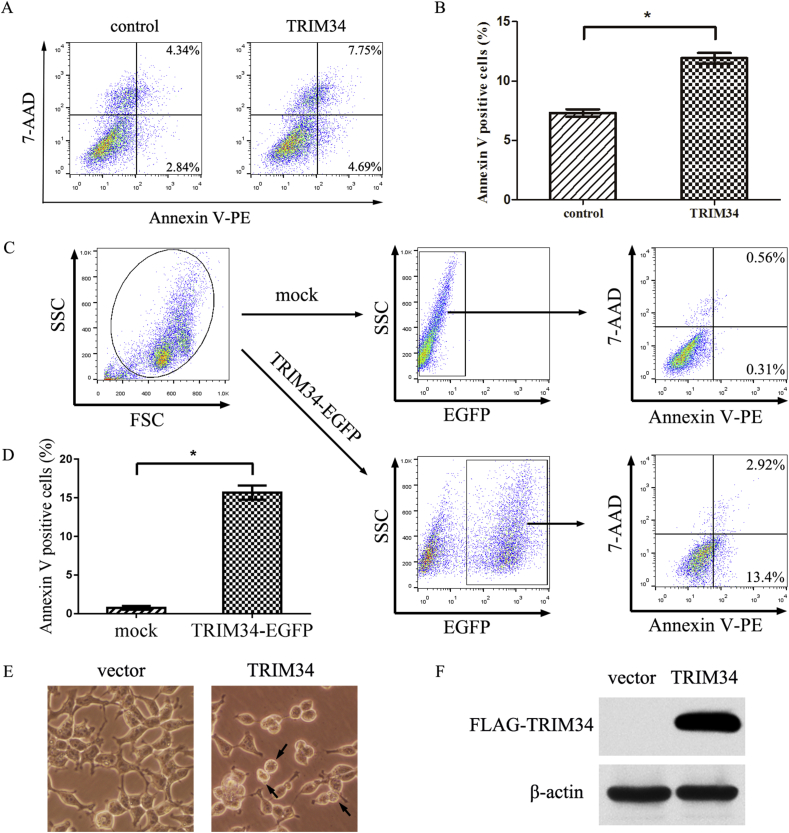
To ascertain whether TRIM34 could induce apoptosis, HEK293T cells were transfected with 5′FLAG-pcDNA3.1-TRIM34 or 5′FLAG-pcDNA3.1 vector for 24 h, then the cells were analyzed by flow cytometry using Annexin V-PE and 7-AAD double staining (Figure 3A). The proportion of AnnexinV-PE+ cells that underwent apoptosis in early (AnnexinV-PE+/7-AAD-) and late phases (AnnexinV-PE+/7-AAD+) increased to 12.44 % in TRIM34 overexpressing group compared with the control group (7.07%) (Figure 3B). This result indicated that TRIM34 decreased the cell viability by inducing apoptosis. HEK293T cells were also transfected with the pEGFP-N3-TRIM34 vector or subjected to mock transfection for 24 h, followed by AnnexinV-PE and 7-AAD double staining. Flow cytometry analysis showed that the TRIM34-EGFP positive cells displayed increased levels of apoptosis compared with that of mock transfection group (Figure 3C and D). At 24 h post-transfection, the microscope images indicated that the cells undergoing apoptosis displayed typical morphological features, such as cell shrinkage, membrane blebbing (Figure 3E). Western blot analysis showed the expression level of FLAG-TRIM34 in the transfection group compared with the control group (Figure 3F).
Figure 3.
TRIM34 induced the apoptosis in HEK293T cells. (A) HEK293T cells were transfected with 5′FLAG-pcDNA3.1-TRIM34 or 5′FLAG-pcDNA3.1 vectors (1.5μg each) for 24 h. Transfected cells were digested by trypsin and were double-stained with AnnexinV-PE and 7-AAD. The apoptosis rate was analyzed by flow cytometer. (B) The percentage of apoptotic cells was quantitatively presented. Error bars indicated mean ± SD for three independent experiments. *P < 0.05, compared to the control. (C) HEK293T cells were transfected with pEGFP-N3-TRIM34 vectors (1.5μg) or subjected to mock transfection for 24 h. FSC/SSC plot was used to gate the viable HEK293T cells. The percentages of AnnexinV-PE+ cells were determined by flow cytometry in HEK293T cells. (D) Histograms showed the percentage of apoptosis cells (AnnexinV-PE+ cells). Results were presented as mean ± SD (n = 3). *P < 0.05, compared to the mock transfection group. (E) TRIM34 induced morphological features of apoptosis. (F) The expression of FLAG-TRIM34 was measured by western blot analysis using anti-FLAG antibody. House-keeping gene β-actin was used as a loading control.
3.4. TRIM34 enhanced the mitochondrial depolarization leading to cytochrome c release
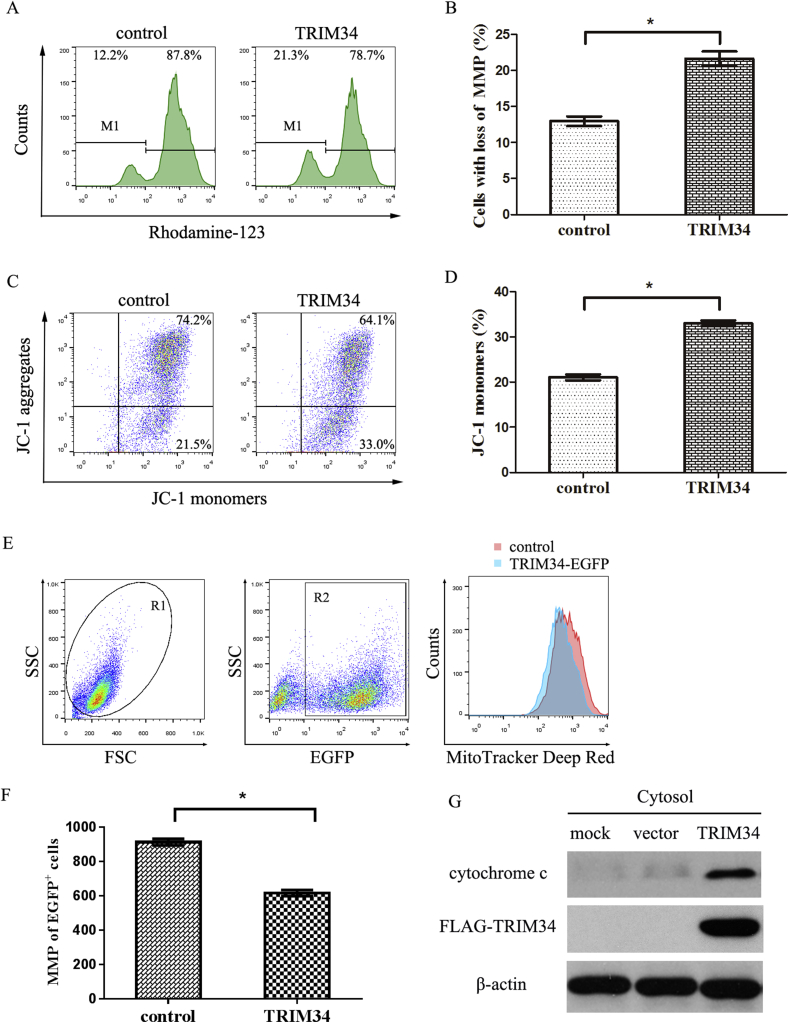
The MMP is an important parameter for the function of mitochondria. The effects of TRIM34 on the MMP were first examined by Rhodamine 123 staining. TRIM34 increased the percentage of HEK293T cells with low MMP to 21.3%, compared with the control group (12.2%) (Figure 4A). Quantitative data confirmed a significant decrease of the MMP in TRIM34-overexpressing group compared with the control group (Figure 4B).
Figure 4.
TRIM34 enhanced the mitochondrial depolarization and release of cytochrome c. (A) TRIM34-induced loss of MMP was measured by Rhodamine 123 method. HEK293T cells were transfected with 5′FLAG-pcDNA3.1- TRIM34 or 5′FLAG-pcDNA3.1 vectors for 24 h. Thereafter, the cells were stained with Rhodamine-123 and analyzed by flow cytometry. Representative histograms were shown and the percentage of cells in depolarized zone (M1) was illustrated. (B) Quantification of the cells with low MMP in Figure 4A. Results were presented as mean ± SD in triplicates. *P < 0.05, compared to the control group. (C) TRIM34-induced mitochondrial depolarization was measured by JC-1 method. HEK293T cells were transfected with 5′FLAG-pcDNA3.1-TRIM34 or 5′FLAG-pcDNA3.1 for 24 h. Then the cells were stained with JC-1 and subjected to flow cytometry analysis. Flow cytometry analysis showed the distribution of JC-1 aggregates (healthy cells, red fluorescence) and JC-1 monomers (apoptotic cells, green fluorescence). (D) Histograms showed the percentage of JC-1 monomer-positive cells. Results were presented as mean ± SD (n = 3). *P < 0.05, difference versus control vectors. (E) TRIM34-induced mitochondrial depolarization was measured by MitoTracker Deep Red method. HEK293T cells were transfected with pEGFP-N3-TRIM34 or pEGFP-N3 vectors (control) for 24 h. Thereafter, the cells were stained with MitoTracker Deep Red and analyzed by flow cytometry. FSC/SSC plot was used to gate (R1) the viable HEK293T cells. Next, the R1 gated events were analysed for the EGFP positive cells (R2). The MMP was measured by the MFI of MitoTracker Deep Red, gating of events using R2. Overlays of the TRIM34-EGFP group (blue) and the pEGFP-N3 control group (red) were shown. (F) Histogram showed the MFI of MitoTracker Deep Red between the TRIM34-EGFP group (blue) and the pEGFP-N3 control group, gating on the EGFP-positive cells. Results were presented as mean ± SD (n = 3). *P < 0.05, difference versus control vectors. (G) HEK293T cells were transfected with 5′FLAG-pcDNA3.1-TRIM34 (TRIM34), 5′FLAG-pcDNA3.1 (vector) or subjected to mock transfection for 24 h. The mitochondria-free cytoplasmic fraction was prepared and the cytochrome c level was examined by western blot analysis. Representative results were presented and two additional experiments yielded similar results.
The influence of TRIM34 on the MMP was also measured by JC-1 staining. As shown in Figure 4C, TRIM34 resulted in a significant increase of JC-1 monomers in the TRIM34-overexpressing group (33.0%) compared with the control group (21.5%). The quantitative analysis confirmed the effect of TRIM34 on the increase of JC-1 monomer, which showed in green fluorescence and implied low membrane potential (Figure 4D).
The role of TRIM34 on the MMP was also measured by MitoTracker Deep Red staining. Flow cytometry showed that the mean fluorescence intensity (MFI) of MitoTracker Deep Red from TRIM34-EGFP+ cells was evidently decreased compared with the EGFP+ control cells (Figure 4E and F). These results indicated that TRIM34 caused a substantial loss of the electric potential across the membrane of mitochondria. Western blot also revealed that the release of cytochrome c was increased in the TRIM34-overexpressing group compared with the control group (Figure 4G).
4. Discussion
TRIM family play important roles in innate immunity and contain about 80 members [15]. TRIM proteins are characterized as E3 ubiquitin ligases for RING-finger domain and categorized into 11 subgroups (C–I to C-XI) on the basis of domain composition [4, 16, 17]. Rhesus monkey TRIM5α is the first member of TRIM family associated with HIV-1 restriction [9]. In composition, TRIM34 is similar to TRIM5 and belongs to the C-IV subgroup of TRIM family, containing the N-terminal RBCC domain and C-terminal SPRY domain [4]. Full length of TRIM34 was first cloned in 2000, however the subcellular localization and biological function of TRIM34 remained unclear [3]. By MitoTracker Deep Red staining, confocal analysis showed that TRIM34-EGFP was localized to the mitochondria in HEK293T cells (Figure 1B). Western blot analysis also showed that FLAG-TRIM34 could be detected in the mitochondrial fraction (Figure 1D). Moreover, the CCK-8 assay demonstrated that TRIM34 suppressed the viability of HEK293T cells (Figure 2A). However, TRIM34 had no significant effect on the cell cycle distribution (Figure 2B and C), suggesting TRIM34 might impair the cell viability through other means.
Apoptosis is very important on the physiological cell death and disease and can be activated by the extrinsic or intrinsic pathways [18]. The extrinsic pathway is initiated by the death receptors upon ligand binding, while the intrinsic pathway can be triggered by the loss of MMP [18]. Flow cytometry analysis demonstrated that TRIM34 induced the apoptosis evidently in HEK293T cells (Figure 3B and D). Moreover, TRIM34 could also lead to the typical apoptosis morphological changes, such as cell shrinkage and membrane blebbing (Figure 3E). As TRIM34 localized to the mitochondria, it would be impossible that TRIM34 might regulate the apoptosis via influencing the function of mitochondria.
Mitochondria are critical in the cell energy production and apoptosis regulation [19, 20]. Previous studies demonstrate that certain proteins translocate to the mitochondria and induce the MMP depolarization. For instance, the Bax protein can insert into the mitochondrial outer membrane, resulting in high permeability [21]. Dynamin related protein (Drp1) facilitates the Bax protein to translocate to the mitochondria in response to irradiation [22]. Besides, ING1 can translocate from the nucleus to the mitochondria and accelerate the apoptosis via interacting with the Bax protein [23]. On the contrary, some proteins can enhance the MMP. Foxg1 localizes to the mitochondria in primary neuronal or glial cell cultures, boosting the MMP and accelerating the mitochondrial fission and mitosis [24]. Mfn2 proteins can also localize to the mitochondria and increase the MMP, accompanied by activating the mitochondrial metabolism such as Kreb's cycle and glucose oxidation [25].
The depolarization of MMP is supposed as the early event of apoptosis, resulting in the release of cytochrome c and the activation of caspase cascade. In this study, three types of fluorescent dye were used to show the changes of MMP. First, we found that TRIM34 promoted the loss of MMP in HEK293T cells (Figure 4A), using fluorescent voltage-sensitive dye Rhodamine-123 [26,27]. Second, the fluorescent cationic dye JC-1 is also selectively accumulated to the mitochondria [28]. At low MMP, JC-1 exists in the form of monomers and emits green fluorescence after laser excitation. At high MMP, JC-1 forms aggregates and emits red fluorescence [29, 30]. Figure 4C showed that TRIM34 increased the percentage of the cells with low MMP compared with the control cells. Third, MitoTracker Deep Red is a new type of mitochondrion-selective fluorescent dye and it can be retained in the mitochondria after fixation. We found that TRIM34 significantly decreased the MFI of MitoTracker Deep Red compared with the control group (Figure 4E and F). Moreover, western blot analysis demonstrated that TRIM34 remarkably enhanced the release of cytochrome c from mitochondria compared with the control group (Figure 4G).
Accumulated evidences indicate that TRIM34 can be induced by type I interferon or viruses. Rajsbaum et al. found that influenza virus stimulated the expression of TRIM34 in mouse macrophages and myeloid DC in a type I interferon-dependent manner [7]. Moreover, the expression of TRIM34 and TRIM22 were also enhanced upon type I interferon stimulation in human macrophages [6]. Jiang et al. observed that TRIM34 was up-regulated remarkably by the toll-like receptor 3/4 stimulation in THP1-derived macrophages [31]. Interestingly, TRIM22 enhanced the expression and oligomerization of the pro-apoptotic protein Bak in THP-1 monocytes [32]. As a result, TRIM22 improved the apoptotic rate of THP-1 monocytes treated with lipopolysaccharides (LPS) [32]. We hypothesized that TRIM34 might cooperate with TRIM22 to negatively regulate immune response to avoid harm.
In short, our findings demonstrate that TRIM34 brings about the mitochondria-dependent apoptosis by inducing the disruption of MMP in HEK293T cells. This study provides new insights into the role of TRIM34 in negatively regulating the function of mitochondria.
Declarations
Author contribution statement
X. An: Performed the experiments.
B. Ji: Contributed reagents, materials, analysis tools or data.
D. Sun: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by Projects of Medical and Health Technology Development Program in Shandong province (grant numbers 2018WSB30002); the Natural Science Foundation of Shandong Province, China, (grant numbers ZR2012CM009); Science and Technology Development Plan Project of Shandong Province (grant number 2011YD18015).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Hatakeyama S. TRIM family proteins: roles in autophagy, immunity, and carcinogenesis. Trends Biochem. Sci. 2017;42:297–311. doi: 10.1016/j.tibs.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Versteeg G.A., Rajsbaum R., Sanchez-Aparicio M.T., Maestre A.M., Valdiviezo J., Shi M., Inn K.S., Fernandez-Sesma A., Jung J., Garcia-Sastre A. The E3-ligase TRIM family of proteins regulates signaling pathways triggered by innate immune pattern-recognition receptors. Immunity. 2013;38:384–398. doi: 10.1016/j.immuni.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orimo A., Tominaga N., Yoshimura K., Yamauchi Y., Nomura M., Sato M., Nogi Y., Suzuki M., Suzuki H., Ikeda K., Inoue S., Muramatsu M. Molecular cloning of ring finger protein 21 (RNF21)/interferon-responsive finger protein (ifp1), which possesses two RING-B box-coiled coil domains in tandem. Genomics. 2000;69:143–149. doi: 10.1006/geno.2000.6318. [DOI] [PubMed] [Google Scholar]
- 4.Rajsbaum R., Garcia-Sastre A., Versteeg G.A. TRIMmunity: the roles of the TRIM E3-ubiquitin ligase family in innate antiviral immunity. J. Mol. Biol. 2014;426:1265–1284. doi: 10.1016/j.jmb.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawyer S.L., Emerman M., Malik H.S. Discordant evolution of the adjacent antiretroviral genes TRIM22 and TRIM5 in mammals. PLoS Pathog. 2007;3 doi: 10.1371/journal.ppat.0030197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carthagena L., Bergamaschi A., Luna J.M., David A., Uchil P.D., Margottin-Goguet F., Mothes W., Hazan U., Transy C., Pancino G., Nisole S. Human TRIM gene expression in response to interferons. PLoS One. 2009;4 doi: 10.1371/journal.pone.0004894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajsbaum R., Stoye J.P., O'Garra A. Type I interferon-dependent and -independent expression of tripartite motif proteins in immune cells. Eur. J. Immunol. 2008;38:619–630. doi: 10.1002/eji.200737916. [DOI] [PubMed] [Google Scholar]
- 8.Turrini F., Di Pietro A., Vicenzi E. Lentiviral effector pathways of TRIM proteins. DNA Cell Biol. 2014;33:191–197. doi: 10.1089/dna.2014.2374. [DOI] [PubMed] [Google Scholar]
- 9.Stremlau M., Owens C.M., Perron M.J., Kiessling M., Autissier P., Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- 10.Li X., Li Y., Stremlau M., Yuan W., Song B., Perron M., Sodroski J. Functional replacement of the RING, B-box 2, and coiled-coil domains of tripartite motif 5alpha (TRIM5alpha) by heterologous TRIM domains. J. Virol. 2006;80:6198–6206. doi: 10.1128/JVI.00283-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y., Brandariz-Nunez A., Fricke T., Ivanov D.N., Sarnak Z., Diaz-Griffero F. Binding of the rhesus TRIM5alpha PRYSPRY domain to capsid is necessary but not sufficient for HIV-1 restriction. Virology. 2014;448:217–228. doi: 10.1016/j.virol.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang F., Hatziioannou T., Perez-Caballero D., Derse D., Bieniasz P.D. Antiretroviral potential of human tripartite motif-5 and related proteins. Virology. 2006;353:396–409. doi: 10.1016/j.virol.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 13.Kaut O., Schmitt I., Tost J., Busato F., Liu Y., Hofmann P., Witt S.H., Rietschel M., Frohlich H., Wullner U. Epigenome-wide DNA methylation analysis in siblings and monozygotic twins discordant for sporadic Parkinson's disease revealed different epigenetic patterns in peripheral blood mononuclear cells. Neurogenetics. 2017;18:7–22. doi: 10.1007/s10048-016-0497-x. [DOI] [PubMed] [Google Scholar]
- 14.Zinchuk V., Wu Y., Grossenbacher-Zinchuk O. Bridging the gap between qualitative and quantitative colocalization results in fluorescence microscopy studies. Sci. Rep. 2013;3:1365. doi: 10.1038/srep01365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Versteeg G.A., Benke S., Garcia-Sastre A., Rajsbaum R. InTRIMsic immunity: positive and negative regulation of immune signaling by tripartite motif proteins. Cytokine Growth Factor Rev. 2014;25:563–576. doi: 10.1016/j.cytogfr.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomar D., Singh R. TRIM family proteins: emerging class of RING E3 ligases as regulator of NF-kappaB pathway. Biol. Cell. 2015;107:22–40. doi: 10.1111/boc.201400046. [DOI] [PubMed] [Google Scholar]
- 17.Koliopoulos M.G., Esposito D., Christodoulou E., Taylor I.A., Rittinger K. Functional role of TRIM E3 ligase oligomerization and regulation of catalytic activity. EMBO J. 2016;35:1204–1218. doi: 10.15252/embj.201593741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfeffer C.M., Singh A.T.K. Apoptosis: a target for anticancer therapy. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Picard M., McEwen B.S., Epel E.S., Sandi C. An energetic view of stress: focus on mitochondria. Front. Neuroendocrinol. 2018;49:72–85. doi: 10.1016/j.yfrne.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke P.J. Mitochondria, bioenergetics and apoptosis in cancer. Trends Cancer. 2017;3:857–870. doi: 10.1016/j.trecan.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosentino K., Garcia-Saez A.J. Bax and Bak pores: are we closing the circle? Trends Cell Biol. 2017;27:266–275. doi: 10.1016/j.tcb.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang P., Liu B., Zhao J., Pang Q., Agrawal S.G., Jia L., Liu F.T. Dynamin-related protein Drp1 is required for Bax translocation to mitochondria in response to irradiation-induced apoptosis. Oncotarget. 2015;6:22598–22612. doi: 10.18632/oncotarget.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bose P., Thakur S., Thalappilly S., Ahn B.Y., Satpathy S., Feng X., Suzuki K., Kim S.W., Riabowol K. ING1 induces apoptosis through direct effects at the mitochondria. Cell Death Dis. 2013;4:e788. doi: 10.1038/cddis.2013.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pancrazi L., Di Benedetto G., Colombaioni L., Della Sala G., Testa G., Olimpico F., Reyes A., Zeviani M., Pozzan T., Costa M. Foxg1 localizes to mitochondria and coordinates cell differentiation and bioenergetics. Proc. Natl. Acad. Sci. U. S. A. 2015;112:13910–13915. doi: 10.1073/pnas.1515190112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schrepfer E., Scorrano L. Mitofusins, from mitochondria to metabolism. Mol. Cell. 2016;61:683–694. doi: 10.1016/j.molcel.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 26.Chen X., Shi X., Zhao C., Li X., Lan X., Liu S., Huang H., Liu N., Liao S., Zang D., Song W., Liu Q., Carter B.Z., Dou Q.P., Wang X., Liu J. Anti-rheumatic agent auranofin induced apoptosis in chronic myeloid leukemia cells resistant to imatinib through both Bcr/Abl-dependent and -independent mechanisms. Oncotarget. 2014;5:9118–9132. doi: 10.18632/oncotarget.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chao M.W., Chen C.H., Chang Y.L., Teng C.M., Pan S.L. alpha-Tomatine-mediated anti-cancer activity in vitro and in vivo through cell cycle- and caspase-independent pathways. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perelman A., Wachtel C., Cohen M., Haupt S., Shapiro H., Tzur A. JC-1: Alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis. 2012;3:e430. doi: 10.1038/cddis.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah B.P., Pasquale N., De G., Tan T., Ma J., Lee K.B. Core-shell nanoparticle-based peptide therapeutics and combined hyperthermia for enhanced cancer cell apoptosis. ACS Nano. 2014;8:9379–9387. doi: 10.1021/nn503431x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salido M., Gonzalez J.L., Vilches J. Loss of mitochondrial membrane potential is inhibited by bombesin in etoposide-induced apoptosis in PC-3 prostate carcinoma cells. Mol. Cancer Ther. 2007;6:1292–1299. doi: 10.1158/1535-7163.MCT-06-0681. [DOI] [PubMed] [Google Scholar]
- 31.Jiang M.X., Hong X., Liao B.B., Shi S.Z., Lai X.F., Zheng H.Y., Xie L., Wang Y., Wang X.L., Xin H.B., Fu M., Deng K.Y. Expression profiling of TRIM protein family in THP1-derived macrophages following TLR stimulation. Sci. Rep. 2017;7:42781. doi: 10.1038/srep42781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen C., Zhao D., Fang S., Chen Q., Cheng B., Fang X., Shu Q. TRIM22-Mediated apoptosis is associated with Bak oligomerization in monocytes. Sci. Rep. 2017;7:39961. doi: 10.1038/srep39961. [DOI] [PMC free article] [PubMed] [Google Scholar]