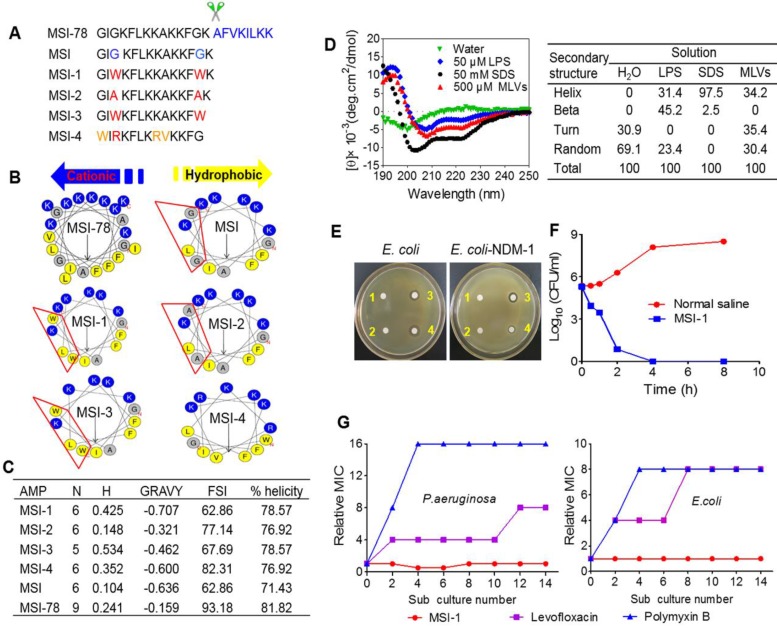
Figure 1.
Structure-activity relationships of peptides. (A) The amino acid sequences of different peptide mutants. (B) Helical wheel projections of peptides. Amino acids in blue are positively charged, while in yellow are hydrophobic. (C) The key physicochemical properties of different peptide mutants. N: Net Charges; H: Hydrophobic; MW: Molecular Weight; GRAVY: the grand average of hydropathy; FSI: Fat-soluble Index. (D) CD spectra of MSI-1 in water, 50 μM LPS, 50 mM SDS and 500 μM MLVs (0.2 DPPG/DPPE +DPPG molar ratio system). (E) Disk diffusion antibacterial assay. 1: normal saline; 2: 10 µg penicillin G sodium salt; 3: 10 µg MSI-1; 4: 10 µg polymyxin. (F) Time-kill kinetics of MSI-1 (4× MIC) against ndm-1-carrying recombinant E. coli ('superbug'). (G) Drug resistance test for MSI-1.