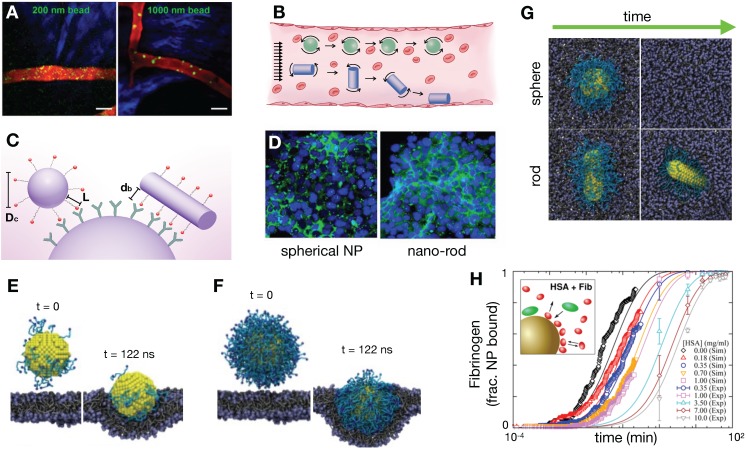
Figure 5.
NP size, shape and coating impact kinetics at different spatial scales. (A) IVM quantifies spherical polystyrene beads in the postcapillary venule of mouse ears, showing that larger NPs marginate in the blood vessels more than smaller NPs (Adapted with permission from 107 copyright 2013 Springer Nature). (B) NP shape impacts vessel margination, as variable forces and torques exerted on rods under flow promote drift towards the vessel wall, where they may bind to receptors or extravasate through endothelial gaps (Modified from 106). (C) Shape, ligand length, and polymer flexibility all contribute to the active fractional area of a nano-carrier (AFAC). For a sphere, the AFAC is defined as (L-db)/Dc, where L is the length of the ligand, db is the binding distance between the nanoparticle and the receptor, and Dc is the diameter of the nano-carrier (Modified from 106). (D) Shape dependent NP uptake is exemplified here with increased anti-HER2 trastuzumab-coated nano-rod accumulation in HER2+ breast cancer cells compared to spherical NPs (Adapted with permission from 120, copyright 2013 National Academy of Sciences, U.S.A.). (E-F) Dissipative particle dynamics modeling simulates the effect of PEGylation density (blue-green) on the NP surface and the effect of shape (G) on internalization dynamics. Increased PEGylation and spherical shape promote internalization (Adapted with permission from 121, 123, copyright 2014, 2015 Elsevier, Royal Society of Chemistry). (H) Coarse-grained modeling of competitive protein adsorption onto silica NPs was used to simulate the competitive adsorption between human serum albumin and fibrinogen at differing solution concentrations. Simulations based on the non-Langmuir differential rate equation (which enabled extrapolations of findings over long time scales, > 1 h, open symbols) showed that protein adsorption is a competitive process. These findings correlated well with experimental data (symbols with error bars; Adapted with permission from 125, copyright 2016 ACS Publications).