Abstract
Objective:
Corneal nerve damage may result in neuropathic corneal pain (NCP). Autologous serum tears (AST) have been shown to results in nerve regeneration and may help alleviate corneal pain. This study aimed to evaluate the efficacy of AST in the treatment of NCP.
Methods:
This was a retrospective case-control study. Sixteen patients suffering from severe NCP and no current ocular surface disease were compared to 12 controls. In vivo confocal microscopy (IVCM) (HRT3/RCM; Heidelberg, Germany) of the central corneas was performed bilaterally. Change in pain severity (scale of 0-10), corneal nerve density, tortuosity, reflectivity and presence of beading and microneuromas before and after treatment were recorded.
Results:
All patients had severe pain of 9.1 ± 0.2 (range 8-10). Before treatment, subbasal nerves were significantly decreased compared to controls, including total nerve length (10,935.5 ±1,264.3 vs. 24,714.4 ±1,056.2 μm/mm2; p<0.0001) and total number of nerves (10.5±1.4 vs. 28.6±2.0; p<0.0001), respectively. Morphologically, significantly increased reflectivity (2.9±0.2 vs. 1.2±0.1; p=0.00008) and tortuosity (2.4±0.2 vs. 1.7±0.1; p=0.001), both graded on a scale of 0-4, were noted. After 3.8 ±0.5 months (range 1-8 months) of AST treatment, pain severity decreased to 3.1 ± 0.3 (range 0-4), (p<0.0001). Further, IVCM demonstrated a significant improvement (p<0.005) in total nerve length (17,351.3±1,395.6 μm/mm2) and number (15.1±1.6) as well as significant decrease in reflectivity (2.4±0.2; p=0.001) and tortuosity (2.2±0.2; p = 0.001).
Conclusion:
IVCM demonstrates underlying alterations of the subbasal corneal nerve plexus in patients suffering from debilitating NCP. AST-induced nerve regeneration is seen following treatment with AST, which correlates with improvement in patient symptoms of NCP.
Keywords: Corneal neuropathic pain, corneal allodynia, in vivo confocal microscopy, autologous serum tears, corneal nerve regeneration
INTRODUCTION
Despite poor understanding of the disease mechanism, most cornea specialists now agree that neuropathic corneal pain (NCP) is an increasingly prevalent and a very challenging condition to diagnose and treat. Corneal neuralgia, keratoneuralgia and corneal allodynia are all terms that fall under the same disease entity of NCP.1 NCP develops as a result of direct damage to corneal nerve axons that carry nociceptors responsible for detection and processing of painful stimuli. The axonal damage and local inflammation leads to up regulation of the nociceptors, which may develop abnormal ectopic activity and results in hyper-responsiveness to stimuli.2-5 Further, peripheral axonal injury may also alter the signaling pathways in the higher order neuronal pathways leading to central sensitization.5 This leads to increased perception of pain in response to even normally unpainful stimuli (allodynia). The increased neuronal excitability (hyperalgesia) persists even when the initial damaging stimulus is no longer present and the damaged tissue has apparently healed. This is the basis of the discordance between the symptoms of pain and apparently normal clinical examination.6-9
The debilitating discomfort from chronic NCP has an immense negative impact on quality of life of patients, resulting in impaired functioning and inability to carry out activities of daily living. Despite the significant impact on the quality of life, NCP remains unrecognized and severely under-treated by ophthalmologists. This is due to limited understanding of the pathophysiology, absence of clinical signs on standard slit-lamp examination, overlap of symptoms with dry eye disease in mild to moderate cases, and a lack of standardized therapy.1
According to the International Association for the Study of Pain – “The diagnosis of neuropathic pain requires confirmation of injury or disease affecting somatosensory pathways of peripheral and/or central nervous systems”.10 In the absence of clinical signs, it is critical to demonstrate damage to corneal nerves to establish the diagnosis of corneal neuropathy. With the advent of sophisticated imaging modalities such as high-resolution laser in vivo confocal microscopy (IVCM), it is now possible to obtain real time, high resolution corneal imaging noninvasively.11-14 Thus, IVCM now confers the ability to analyze the corneal ultrastructure layer-by layer and directly visualize of the corneal nerves both in heath and disease.15-20 This aids in making a diagnosis of NCP and allows for monitoring treatment efficacy.6,21,22,23
Given that the basis of NCP is damage to the somatosensory nervous system, in this case to corneal nerves, we hypothesized that therapeutic strategies targeted at reversing this damage through neuronal regeneration may be effective to treat NCP. 24, 25 Autologous plasma26 and serum tears (AST) contain several neurotrophic and epithelial growth factors, and have been shown efficacy in regeneration of corneal nerves.6, 27 Through the direct visualization of corneal nerves, IVCM can be used as a diagnostic tool to detect nerve degeneration and subsequent nerve regeneration in response to therapies.6, 28, 29 We have previously demonstrated successful treatment of a subset of patients with corneal neuropathy that presented with photoallydynia, but not pain or neuralgia. Although not clear what the underlying pathophysiological mechanisms are that differentiate these patients to have pain versus light sensitivity (or sometimes both), we hypothesize that by supporting neuronal regeneration, AST may be useful to treat symptoms of NCP
Thus, the purpose of our study is to correlate corneal nerve changes in patients with NCP and to evaluate the clinical efficacy of autologous serum tears treatment using IVCM. Herein, we demonstrate that the patients’ pain severity levels decreased and corneal nerve density, as assessed by IVCM, improved significantly following treatment with AST.
MATERIALS AND METHODS
Design and Patients
This study is a retrospective cohort study with a comparison control group. It was conducted at the Massachusetts Eye & Ear Infirmary, Department of Ophthalmology, Harvard Medical School, Boston, Massachusetts.
Sample size calculation was performed, assuming pain severity as the primary endpoint. Assuming an alpha of 0.05 and beta of 0.9, and assuming a baseline pain score of 8 with standard deviation of 1, to detect a 2 point reduction in the pain score (e.g., from 8 to 6), 6 patients would be needed at baseline and post-intervention. We then performed an additional sample size calculation assuming total nerve length as the primary end point. Assuming an alpha of 0.05 and beta of 0.9, and assuming a baseline total nerve length of 11,000 μm with standard deviation of 1,500 μm, to detect a 3,000 μm improvement in the total nerve length (e.g., from 11,000 to 14,000 μm), 6 patients would be needed at baseline and post-intervention. Thus, we determined that we needed data for a minimum of 6 patients pre and post-intervention in order for the study to be appropriately powered.
We included 16 patients with chief complaints of severe corneal pain (greater or equal than 7/10), refractory to all previous treatment, including frequent lubrication with artificial tears, topical steroid therapy and other anti-inflammatory therapies, without any relief in symptoms. Autologous serum tears 20%, were added for treatment of all patients for the purpose of nerve regeneration to help alleviate pain. All consecutive patients with NCP (pain≥7) who were treated with AST within our study time frame (October 2012 to March 2014; 15 months) were included. The visual analogue scale was used for pain grading; the pain was documented from 0-10, 10 being the worst pain. These patients had an absence of ocular surface disease on slit-lamp examination, including lack of staining with vital dye stains and normal tear break-up time at the time of baseline examination. Exclusion criteria included any other pathology that could cause symptoms of ocular pain, including corneal infections, abrasions, recurrent erosion syndrome, iridocyclitis or uveitis. The patients were identified by one observer and verified by the senior author. All the clinical examination was carried out by the senior observer. (P.H.). These patients were compared to 12 age- and sex-matched controls. These controls were chosen from an existing normative database. In this data registry, subjects are enrolled prospectively. Only subjects without any ocular history or ocular signs on examination are included. These controls were confirmed to have no active ocular surface signs i.e. Schirmer’s test, tear breakup time were within normal limits and there was no vital dye staining of the surface.
The Institutional Review Board/ Ethics Committee approved the protocol. We ensured compliance with the Health Insurance Portability and Accountability Act (HIPAA) and adherence to the tenets of the Declaration of Helsinki.
Clinical Chart Review
We conducted a thorough chart review and recorded clinical parameters at two time points – before treatment with autologous serum tears and post treatment. After starting AST therapy, the patients were followed and severity of patient-reported pain was recorded and IVCM was conducted in all subsequent visits. The quantitative IVCM analysis time point post-treatment was chosen at the time of maximum improvement in patient-reported symptoms.
The parameters recorded included patient reported symptoms, specifically corneal pain, pain severity, description of effect of pain on the activities of daily living, and previous ophthalmic history. Slit-lamp bio-microscopic findings and corneal fluorescein staining, the treatment regimen and duration of treatment were also recorded.
In Vivo Confocal Microscopy and Image Analysis
Laser IVCM (Heidelberg Retina Tomograph 3 with the Rostock Cornea Module [HRT3/RCM], Heidelberg Engineering GmbH, Heidelberg, Germany) was conducted on central corneas of all patients and controls, bilaterally (C.C.), as previously described.16 Equipped with a 63× objective immersion lens with a numerical aperture of 0.9 (Olympus, Tokyo, Japan), this microscope uses a 670-nm red wavelength diode laser source to produce an image representing a coronal section of the cornea of 400 × 400 μm (horizontal x vertical). Digital images are recorded at of 30 frames/s. Adjacent images are separated by 1 μm, with a lateral resolution of 1 μm/pixel. To perform this procedure, both eyes were topically anesthetized using 0.5% proparacaine hydrochloride (Alcaine; Alcon, Fort Worth, TX). This was followed by administration of a drop of hydroxypropyl methylcellulose 2.5% (GenTeal gel, Novartis Ophthalmics, East Hanover, NJ) to improve the optical coupling with the cornea module of the microscope. The cornea module was mounted with a disposable, sterile polymethylmethacrylate cap (Tomo-Cap; Heidelberg Engineering GmbH), filled with a layer of hydroxypropyl methylcellulose 2.5% (GenTeal gel; Novartis Ophthalmics), gel was also applied to the surface of the cap. The equipment is manually advanced until the gel on the cap comes in contact with the surface of the central cornea.
Out of a total of six to eight sequence scans performed on the full thickness of the central cornea, resulting in a total of 50-100 images of the corneal subbasal, a masked observer chose three images most representative of the subbasal nerve plexus. The subbasal plexus is seen in subepithelial area, immediately at or posterior to the basal epithelial layer and anterior to the Bowman's layer, typically at a depth of 50 to 80 μm. The criteria to select the images were the best focused images, in a single layer, without folds and a good contrast.
Two masked observers (S.A.; A.K.) then evaluated the confocal images for morphology and density of the subbasal plexus. In case of any discrepancy, the images were analyzed by a third observer. The quantitative parameters considered were number and length of main nerves, nerve branches and total nerves. Presence of beading, microneuromas, tortuosity and reflectivity were used as qualitative parameters. Microneuromas: thickening of nerves at point of injury and were identified as stumps of a nerve fiber on confocal images; and beading: discrete small beads along the length of the nerves.
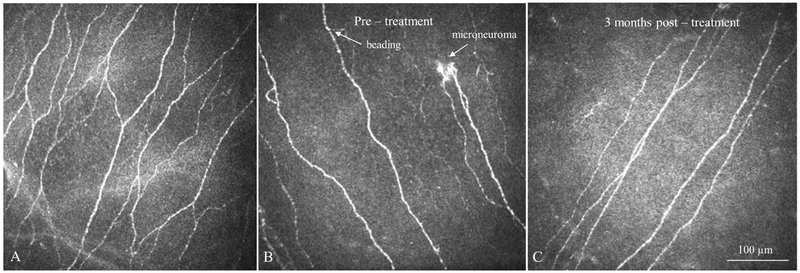
We performed quantitative nerve analysis using the semi-automated tracing program NeuronJ (http://www.imagescience.org/meijering/software/neuronj/),30 a plug-in for ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/http://rsb.info.nih.gov/ij/) as previously described.16-18 We quantified all quantitative data as density (μm/mm2) ± SEM. Qualitatively, we classified tortuosity and reflectivity in four grades, according to a grading scale reported by Oliveira-Soto and Efron.31 We reported microneuromas and beading based on presence or absence of findings (Figure 1) as previously described.6
Figure 1: Laser In Vivo Confocal Microscopy (IVCM) Images.
IVCM images obtained at the level of corneal subbasal nerve plexus demonstrate changes in corneal nerves in patients with neuropathic corneal pain. A. Normal corneal subbasal nerve plexus. B. Nerve plexus at baseline, before starting autologous serum therapy (AST) in a patient with neuropathic corneal pain. Note decreased density of nerves, presence of microneuromas and beading, as well as increased reflectivity. C. Nerve plexus of the same patient at 3 months after AST therapy. Note improved nerve plexus and absence of microneuromas. However with decreased nerves compared to normal controls. Size bar = 100 μm.
Preparation and Administration of Autologous Serum Tears
The compounding pharmacy at our institution prepared the AST in the following manner: After centrifuging blood for 10 minutes at 3000 rpm, the serum was separated in a sterile manner and filtered using MILLLEX – HP PES 5 micron low protein binding filter. After diluting to 20% using sterile saline solution, the final preparation was aliquoted into 5 ml bottles and kept frozen at −20°C until ready for use, for a maximum of 3 months. Expiration at 4°C was 2 weeks (unopened) and 1 week (after opening). We instructed the patients to keep the bottles under refrigeration and to use the provided amber colored sleeve to protect from light. Patients used AST topically 8 times daily.
Statistical Analysis:
We performed statistical analysis with statistical analysis software in Microsoft Excel 2010 (Analysis Toolpak). We used Student's t-test and analysis of variance (ANOVA) to compare the different groups and paired t-test was used to compare pre- and post-treatment groups. P values less than 0.05 were considered statistically significant. Normality of data was tested using the Shapiro-Wilk test.
Literature Search:
We performed a systematic search of all relevant publications between 1966 and 2018, from PubMed, PubMed Central, MEDLINE (National Library of Medicine), Cochrane Database and OVID. Search terms included the following: pain, neuropathic pain, peripheral pain, central pain, keratoneuralgia, corneal neuropathic pain, corneal neuropathy, corneal neuralgia, pain post refractive surgery, ocular surface disease, dry eye, ocular discomfort, allodynia, photoallodynia, confocal microscopy. We considered all systematic reviews, meta-analyses, randomized controlled trials (RCTs), retrospective studies, case series, and case reports. Studies were evaluated according to the Oxford Centre for Evidence-Based Medicine levels of evidence.
RESULTS
Demographics
Sixteen eyes of 16 patients suffering from extreme corneal pain (severity level 7 and higher) were studied and the data collected was compared with 12 age- and sex-matched control eyes. Demographic data of patients and controls are presented in Table 1.
Table 1.
Demographics and distribution of the study population
| Controls | Patients | P value | |
|---|---|---|---|
| Number | 12 | 16 | |
| Age (years) (Mean ± SEM) |
56.4 ± 3.1 (Range 25–74) |
61.8 ± 4.4 (Range 27–88) |
P = 0.43 |
| Gender (Male / Female) |
6 / 6 | 6 / 10 | P = 0.82 |
SEM- Standard error of mean
Clinical Features
All patients complained of severe corneal pain with average pain being 9.1 ±0.2 (range 8-10). Review of clinical history revealed that the majority of the patients (9/16; 56.25%) had undergone refractive surgery in the past (8 with laser in situ keratomileusis [LASIK] and 1 with photorefractive keratectomy [PRK]), 3 patients had previously suffered from dry eye disease (DED), 2 patients had been exposed to ultraviolet (UV) radiation, 1 patient had a history of herpes zoster ophthalmicus (HZO) and 1 patient suffered from autonomic neuropathy (Table 2). Clinical examination at the time of treatment initiation was within normal limits with none of the patients having active ocular surface disease. Slit-lamp bio-microscopy of the eyelid margins and conjunctiva did not reveal any currently active blepharitis, meibomian gland dysfunction, or conjunctivitis.
Table 2.
Clinical history of the study patients
| Patient | Likely Cause of Neuropathic Pain |
Time since surgery/initial affliction (years) |
Duration of Pain (years) |
Pain Level (0-10) |
Exacerbating Factors |
Other symptoms |
Active ocular surface disease* |
Other treatments | Other neurological/psychiatric diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| Refractive causes | |||||||||
| 1 | LASIK | 1 | 1 | 10 | Light | Light sensitivity | None | AT, topical loteprednol etabronate 0.5% | |
| 2 | LASIK | 2 | 2 | 10 | Light | Light sensitivity, foreign body sensation | None | AT, topical loteprednol etabronate 0.5%, topical cyclosporine 0.05% | |
| 3 | LASIK | 1 | 1 | 8 | Wind | None | None | AT, topical prednisolone acetate 1% | |
| 4 | LASIK | 0.75 | 0.5 | 10 | Use of digital devices | Redness, ocular discomfort | None | AT, topical loteprednol etabronate 0.5%, oral TCA | Depression |
| 5 | LASIK | 6 | 6 | 9 | Light | Light sensitivity | None | AT, topical loteprednol etabronate 0.5%, oral TCA | |
| 6 | LASIK | 1 | 0.5 | 9 | Wind/Cold air | Foreign body sensation | None | AT, topical prednisolone acetate 1%, oral gabapentin | Depression, Chronic pain syndrome |
| 7 | LASIK | 3 | 2 | 8 | Use of digital devices/Reading | Redness, tearing | None | AT, topical loteprednol etabronate 0.5%, topical cyclosporine | |
| 8 | LASIK | 1 | 0.8 | 10 | Light | Light sensitivity | None | AT, topical prednisolone acetate 1%, oral TCA | Depression |
| 9 | PRK | 5 | 5 | 10 | Reading | None | None | AT, topical loteprednol etabronate 0.5% | |
| Non – refractive causes | |||||||||
| 10 | Dry eye disease | 3 | 9 | Wind | Foreign body sensation | None | AT, topical cyclosporine 0.05% | Chronic pan syndrome | |
| 11 | Dry eye disease | 2 | 9 | Wind/cold air | Redness, tearing, foreign body sensation | None | AT, prednisolone acetate 1% | Fibromyalgia | |
| 12 | Dry eye disease | 2 | 8 | Use of digital devices/Reading | Redness | None | AT, prednisolone acetate 1% | ||
| 13 | Autonomic neuropathy | 2 | 9 | Reading | None | None | AT, topical loteprednol etabronate 0.5% | ||
| 14 | Herpes zoster ophthalmicus | 1 | 3 | 10 | Periocular discomfort | None | AT, topical loteprednol etabronate 0.5%, oral gabapentin | Post herpetic neuralgia | |
| 15 | UV light exposure | 0.67 | 3 | 8 | Light | Tearing, foreign body sensation | None | AT, topical loteprednol etabronate 0.5%, oral TCA | Depression |
| 16 | UV light exposure | 0.5 | 0.5 | 9 | Use of digital devices | Foreign body sensation | None | AT, topical loteprednol etabronate 0.5% |
Lasik – laser assisted in situ keratomileusis; PRK – photorefractive keratectomy; UV – ultraviolet; AT – artificial tears; TCA – tricyclic antidepressants
Based on Schirmer’s, Tear breakup time test and vital staining of surface
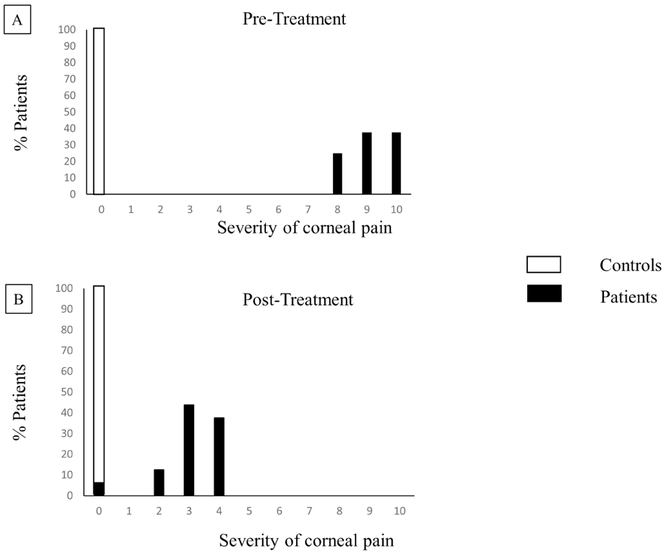
The mean pain severity was 9.1 ± 0.2 (range 8-10). After a mean of 3.8 ±0.5 months (range 1-8 months) of AST therapy, symptom severity decreased to a mean of 3.1 ±0.3 (range 0-4) (p<0.0001; Figure 2). The time point of post-treatment was the time point when patients reported improvement in symptoms.
Figure 2: Severity of Neuropathic Corneal Pain Pre- and Post-Treatment.
Bar graphs showing severity of corneal pain A. pre-treatment and B. post-treatment.
In Vivo Confocal Microscopy Findings
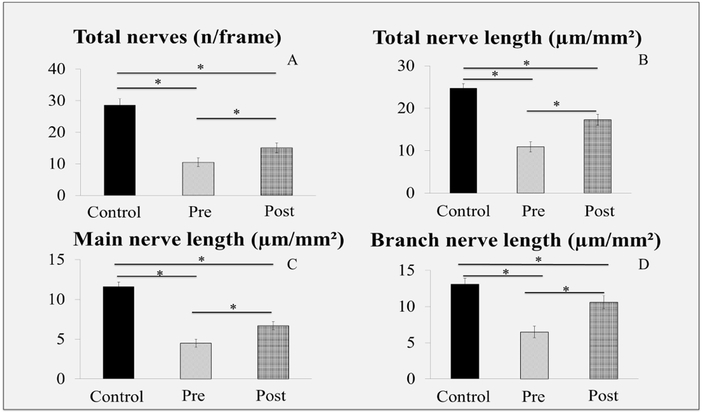
A summary of the corneal subbasal nerve parameters for the patients, pre- and post-treatment with autologous serum tears, and the normal control group is presented in Table 3. The subbasal nerve plexus in patients pre-treatment was significantly decreased for all parameters as compared to controls, including total nerve length (10,935.5±1,264.3 μm/mm2 vs. 24,714.4±1,056.2 μm/mm2) (p<0.0001) total number of nerves (10.5±1.4 vs. 28.6±2.0) (p<0.0001), main nerve length (4,390.3±753.8 μm/mm2 vs. 11,194 ±683.4 μm/mm2), number of main nerves (0.47±0.6 vs. 4.1 ±2.8), branch nerve length (6545.5±838.3 μm/mm2 vs. 13519.8 ± 807.4 μm/mm2), and branch nerve number (10.1±0.9 vs. 24.3 ±2.0), respectively. Morphologically, the nerves showed significant increase in reflectivity (2.9±0.2 vs. 1.2±0.1) (p<0.01) and increased nerve tortuosity (2.4±0.2 vs. 1.7±0.1) (p<0.01), presence of microneuromas and beading in 100% and 75% of patients respectively (Figures 3-4, Table 3). None of the controls had neuromas or beading.
Table 3.
In Vivo Confocal Microscopy (IVCM) Parameters Pre- and Post-Treatment
| IVCM Parameter | Controls | Patients | |
|---|---|---|---|
| Pre-Treatment | Post-Treatment | ||
| No. of total nerves (n/frame) | 28.6 ± 2.0 | 10.5 ± 1.4* | 15.1 ± 1.6*† |
| Total nerve length (μm/mm2) | 24,714.4 ± 1,056.2 | 10,935.5 ± 1,264.3* | 17,351.3 ± 1,395.6*† |
| No. of nerve branches (n/frame) | 24.3 ± 2 | 10.1 ± 0.9* | 12.9 ± 1.5*† |
| Length of nerve branches (μm/mm2) | 13,519.8 ± 807.4 | 6,545.5 ± 838.3* | 8,925.6 ± 1,228.7*† |
| Tortuosity (0-4) | 1.7 ±0.1 | 2.4 ± 0.2* | 2.2 ± 0.2* |
| Reflectivity (0-4) | 1.2 ± 0.1 | 2.9 ± 0.2* | 2.4 ± 0.2* |
Statistically significant compared to controls, p < 0.0001,
Statistically significant as compared to pre-treatment group (p<0.005).
Mean ± Standard error of mean
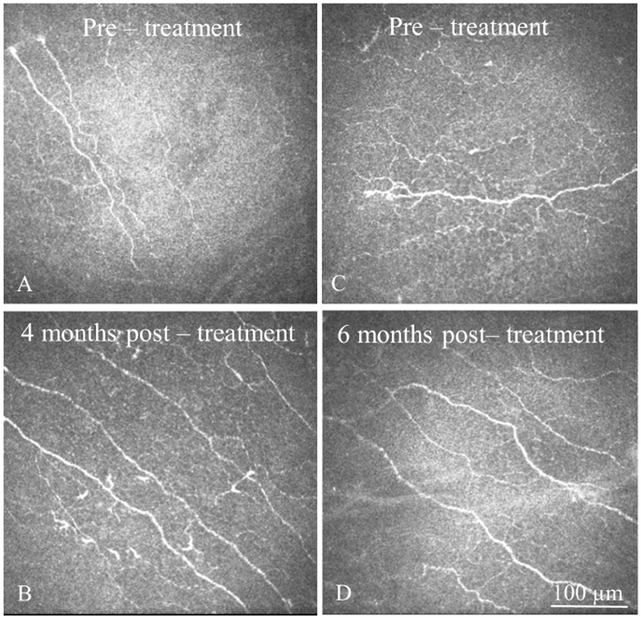
Figure 3: In Vivo Confocal Microscopy (IVCM) Images Pre- and Post-Treatment.
IVCM images obtained at the level of corneal subbasal nerve plexus demonstrate improvement in corneal nerves in patients with neuropathic corneal pain after treatment with autologous serum tears (AST). A, C. Subbasal nerve plexus at baseline. Note decreased density of nerves, presence of microneuromas and beading, as well as increased reflectivity. B, D. Subbasal nerve plexus of the same patients after 4 and 6 months after AST therapy, respectively. Note improved subbasal nerve plexus morphology and increase in density of nerves. Size bar = 100 μm
Figure 4:
Comparison of Subbasal Nerve Density of Normal Controls with Patients with Neuropathic Corneal Pain Pre- and Post-treatment
Bar graphs showing corneal nerve density pre-treatment, post-treatment, and control eyes. A. Total nerve number. B. Length of total nerve nerves. C. Length of main nerves. D. Length of nerve branches. * P < 0.001.
Post-treatment, IVCM showed a significant improvement (p<0.005) in density (total nerve length 17,351.3±1,395.6 μm/mm2 and number 15.1±1.6). Nerve morphology also showed improvement. Microneuromas and beading were significantly decreased and were now seen in 6.25% and 37.5% patients respectively (p<0.05). Nerve reflectivity (2.4±0.2) and tortuosity (2.2±0.2) were also decreased, although not statistically significant (p>0.05) (Figure 3-4, Table 3).
DISCUSSION
Neuropathic corneal pain is a relatively new disease entity, and recent advances in the field of corneal neurobiology have helped in understanding the pathophysiology of corneal pain mechanisms1, 2, 5 Understanding the function and adaption of nociceptors in response to stimuli is critical to the understanding the mechanism of corneal neuropathic pain. The cornea is one the most richly innervated tissues of the body with an estimated central corneal nerve density in humans of 7000 nerve terminals per square millimeter. The peripheral sensory fibers are derived from the ophthalmic division of the trigeminal nerve. The nerve endings penetrate the cornea in a radial fashion in the stroma, ascend anteriorly and penetrate the Bowman’s layer to form the subbasal nerve plexus and terminate as nerve endings in the epithelial layer.32, 33 These peripheral nerve endings are densely populated with a variety of morphologically and functionally heterogeneous nociceptors, which respond to noxious stimuli and help in protecting, restoring and sustaining health of the ocular surface.2,3
Cellular injury or exposure to noxious stimuli leads to nociceptor activation and forms the basis of acute physiological pain, also known as nociceptive pain. The inflammatory mediators released in response to tissue injury including prostaglandins and cytokines, like leukotrienes, tumor necrosis factor, bradykinin and neuropeptides from stimulated nociceptor terminals induce activation of ion channels and transduction into electrical impulses.4, 5,34, 35 These impulses are processed at various levels along the neuronal pain axis, including the trigeminal brainstem complex, trigeminal subnucleus interpolaris/caudalis transition region, and the upper cervical cord junction. These are then projected to the posterior thalamus, and finally processed at the cerebral cortex.13, 36, 37
While the physiological pain response is a protective response, damage to peripheral nerve endings and persistent inflammation result in complex adaptive mechanisms, namely peripheral and central sensitization, as well as neuronal plasticity, and subsequently result in transition from acute pain responses to chronic neuropathic pain, which has no protective value.5 The neuropathic pain special interest group of the international association of study of pain has defined neuropathic pain as “pain arising as a direct mechanism of a lesion or disease affecting the somatosensory system”.38 These mechanisms of neuropathic pain have been studied extensively in the non-ocular pain systems.39-43 With axonal injury, the damaged axons seal the injured stump and forms terminal bulbs with small fine branches in an attempt to regenerate. These stumps are called microneuromas.44, 45 Microneuroma formation is accompanied by sustained release of inflammatory mediators, which causes vasodilation and further augmentation of the inflammatory cascade. These inflammatory mediators then cause modification of transducing ion channels and lead to upregulation of ion channels in the nociceptors, modification of intrinsic membrane potentials, and generate oscillating aberrant nerve activity known as ectopic impulses. Generation of these ectopic nerve stimuli at the level of microneuromas renders the nociceptors abnormally hypersensitive to mechanical and chemical stimuli, and subsequently lowers the firing threshold – known as peripheral sensitization.46-50 This aberrant altered input from peripheral nerve endings projects to the above described central pain pathway and produces central sensitization.51, 52 In addition to the polymodal nociceptors, the low threshold mechanoreceptors and cold receptors also undergo neuronal modulation and contribute to peripheral and central sensitization, thereby perpetuating the pain and dysesthesia caused by low threshold stimuli.5 This neuronal maladaptation may become permanent and the distorted neuronal hypersensitivity persists even after apparent healing of the injured tissue and absence of initial inciting event resulting in neuropathic pain.35,53-55
As reviewed by Dieckmann et al.,56 various triggers cause corneal nerve damage and may result in neuropathic corneal pain. These include ocular surface diseases, such as DED and recurrent corneal erosions, 57, 58 keratorefractive surgeries,59 infections such as herpes zoster keratitis,60 systemic conditions such as diabetic neuropathy,61 and exposure to drugs, such as isotretinoin treatments, or radiation. Rosenthal and Borsook described the corneal maladaptive changes after the above mentioned triggers, which may explain the chronic self-sustained symptoms in absence of signs, called corneal “pain without stain” or “phantom cornea’.7-9
In this study, our patient population also suffered from severe corneal pain with unremarkable slit-lamp examination this lack of correlation between the severity of symptoms and clinical examination suggested that the source of the pain was neuropathic of origin. Review of clinical history of our patients further included diseases resulting in corneal nerve damage that may have led to the development of neuropathic corneal pain. The majority of our patients (56.25%) had a history of having undergone refractive surgery in the past (time since surgery ranging from 6 months to 2 years back), while others had past ocular history significant for DED, exposure to ultraviolet light and herpes zoster ophthalmicus. Neuropathic corneal pain is an under-evaluated component of dry eye disease. As such, many of our patients also reported dry eye-like symptoms, including tearing, light sensitivity, foreign body sensation and ocular discomfort, in addition to severe pain. Further, a few of these patients had a diagnosis of depression, chronic pain syndrome and other co-morbid pain syndromes, which have also been shown to be associated frequently with ocular neuropathic pain.53, 58
While symptoms of corneal pain without clinical findings on slit lamp biomicroscopy is suggestive of corneal neuropathic pain, it is critical to demonstrate damage to the corneal nerves to make a definitive diagnosis. The ASIP recommends tissue biopsy to show nerve damage and thus establish diagnosis of neuropathic pain.62 However, as corneal biopsy is not feasible, IVCM is a very useful modality in aiding diagnosis of neuropathic cornea pain, as it can be used to study both qualitative and quantitative changes of the corneal nerve plexus and also provides an objective measure of objective nerve damage. Previous IVCM studies on patients with corneal neuropathy have shown decreased density of subbasal nerves. The subbasal nerves also become very tortuous and hyper-reflective. Increased microneuroma formation and beading of nerves has been shown in corneal neuropathy as well.1, 6, 56 Similar to previous studies, in our current study, IVCM of the corneal subbasal plexus showed significantly decreased density of the corneal nerve plexus compared to controls and morphological alteration of the nerves, including increased reflectivity, tortuosity, nerve beading and presence of microneuromas, thus supporting the diagnosis of neuropathic corneal pain.
After topical administration of AST for a mean duration of 3.8 months, neuropathic corneal pain patients showed significant decrease in symptoms. IVCM at the post-treatment time period showed increased corneal nerve density and decrease in the morphological changes including nerve beading and presence of microneuromas. Our working hypothesis for the treatment of these neuropathic corneal pain patients with autologous serum tears was based on the assumed underlying pathophysiology of aberrant nerve regeneration subsequent to nerve injury. Autologous serum consists of a large number of neurotrophic and epithelial growth factors, such as the nerve growth factor (NGF), insulin-like growth factor-1, transforming growth factor β, fibronectin, and epidermal growth factors.63, 64 NGF is present in high concentrations in serum tears compared to natural tears, and has been shown to play an important role in nerve regeneration, through induction of neuronal sprouting and restoration of function of injured neurons.24, 25 NGF also aids in the differentiation and growth of sensory and sympathetic neurons, as demonstrated by various in vitro and in vivo studies.65-67 Further, several studies have reported successful use of AST in dry eye disease and for neurotrophic epithelial defects.68-71
We have previously reported successful reduction in photoallodynia, i.e. severe light sensitivity (without pain) in the setting of corneal neuropathy with regeneration of corneal nerves, after approximately 4 months of treatment with topical AST as demonstrated by IVCM.5 While most patients responded within 3-4 months, some took longer to experience improvement. Further, while some patients may experienced some improvement early on, it took a longer period of time to achieve maximal degree of improvement. Thus, if some response was elicited, patients were asked to continue their treatment eight times daily until maximum effect was achieved and the improvement plateaued. For the purpose of the IVCM analysis, the post-treatment time point was when maximum benefit was noted and not initial improvement. Further, in our experience, the more recent the initial insult has been, the faster patients respond. Majority of the patients showed improvement in pain with administration of proparacaine suggesting peripheral sensitization. As such earlier diagnosis, prior to centralization of pain is crucial. However prospective studies are needed to further elucidate the timing and efficacy of AST therapy in NCP.
Our study has several limitations. First, it is retrospective in nature and includes a relatively small number of patients. However, despite the small sample size, we found statistically significant results. In addition, given that this disease is not common, larger prospective studies would require inclusion of multiple clinical sites. Nevertheless, the results are highly promising for this refractory disease and warrant larger prospective clinical studies. Second, we have reported clinical severity of symptoms on a subjective patient-reported scale. For future prospective studies, objective pain assessment tools, which also assesses effect on the quality of life of patients should be used.72 Third, there may be concern that as IVCM obtains images from a 400 micrometer square are of the cornea at one time, it may be difficult to establish that the post-treatment scan is of the same area as the pre-treatment. However we have previously shown that there is no significant difference in IVCM parameters when three representative images are chosen for analysis versus analyzing composite corneal IVCM images.73 We are currently developing a fully automated artificial intelligence system to analyze the corneal nerve images, which will help reduce the potential bias in manual selection and analysis.74, 75 Fourth, we don’t have IVCM data from refractive surgery patients who do not have symptoms to NCP as these patients are not routinely scanned in the clinic. We have a study currently underway to study the nerve changes in the sub-group of NCP patients with history of refractive surgery to refractive surgery patients without NCP and believe that it will add to our understanding of this condition. Finally, the follow-up time is relatively short. However, despite the relatively short follow-up time, our results are statistically and clinically significant with profound reduction in pain in this population. Long-term studies of these patients will help to understand if the outcomes of AST continue to improve with long-term use or if the effects are reversible after stopping treatment. Therefore prospective randomized clinical trials are needed to assess long-term effects of AST for neuropathic corneal pain.
In conclusion, AST with concurrent use of IVCM for diagnosis and to monitor therapeutic response, is a highly effective management strategy for neuropathic corneal pain, which has hitherto remained underdiagnosed and a challenging condition to treat.
Acknowledgments
Financial Support: NIH K08-EY020575, Research to Prevent Blindness Career Development Award, Falk Medical Research Trust, New England Corneal Transplant Research Fund
The funding organizations had no role in the design or conduct of this research
Footnotes
Meeting Presentation: This work has been presented in part at the Annual American Academy of Ophthalmology Meeting in Chicago, October 2014 and at the annual ARVO meeting in Orlando, May 2014.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES: None of the authors have any conflicts of interest
Conflict of interest: No conflicting relationship exists for any author
REFERENCES
- 1.Goyal S, Hamrah P. Understanding Neuropathic Corneal Pain-Gaps and Current Therapeutic Approaches. Semin Ophthalmol 2016;31:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res 2004;78:513–25. [DOI] [PubMed] [Google Scholar]
- 3.Belmonte C, Aracil A, Acosta MC, Luna C, Gallar J. Nerves and sensations from the eye surface. Ocul Surf 2004;2:248–53. [DOI] [PubMed] [Google Scholar]
- 4.Stapleton F, Marfurt C, Golebiowski B, Rosenblatt M, Bereiter D, Begley C, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the subcommittee on neurobiology. Invest Ophthalmol Vis Sci 2013;54:TFOS 71–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belmonte C, Nichols JJ, Cox SM, Brock JA, Begley C, Bereiter D, et al. TFOS DEWS II pain and sensation report. Ocul Surf 2017;15:TFOS404–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal S, Kheirkhah A, Cavalcanti BM, Cruzat A, Colon C, Brown E, et al. Autologous Serum Tears for Treatment of Photoallodynia in Patients with Corneal Neuropathy: Efficacy and Evaluation with In Vivo Confocal Microscopy. Ocul Surf 2015;13:250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenthal P, Baran I, Jacobs DS. Corneal pain without stain: is it real? Ocul Surf 2009;7:28–40. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal P, Borsook D. Ocular neuropathic pain. Br J Ophthalmol 2016;100:128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenthal P, Borsook D. The corneal pain system. Part I: the missing piece of the dry eye puzzle. Ocul Surf 2012;10:2–14. [DOI] [PubMed] [Google Scholar]
- 10.Dworkin RH, O'Connor AB, Kent J, Mackey SC, Raja SN, Stacey BR, et al. Interventional management of neuropathic pain: NeuPSIG recommendations. Pain. 2013;154:2249–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruzat A, Pavan-Langston D, Hamrah P. In vivo confocal microscopy of corneal nerves: analysis and clinical correlation. Semin Ophthalmol 2010;25:171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guthoff RF, Zhivov A, Stachs O. In vivo confocal microscopy, an inner vision of the cornea - a major review. Clin Experiment Ophthalmol 2009;37:100–17. [DOI] [PubMed] [Google Scholar]
- 13.Patel DV, McGhee CN. Quantitative analysis of in vivo confocal microscopy images: a review. Surv Ophthalmol 2013;58:466–75. [DOI] [PubMed] [Google Scholar]
- 14.Zhivov A, Winter K, Peschel S, Guthoff RF, Stachs O, Harder V, et al. [Quantitative analysis of corneal subbasal nerve plexus with in vivo confocal laser scanning microscopy]. Klin Monbl Augenheilkd 2011;228:1067–72. [DOI] [PubMed] [Google Scholar]
- 15.Alzubaidi R, Sharif MS, Qahwaji R, Ipson S, Brahma A. In vivo confocal microscopic corneal images in health and disease with an emphasis on extracting features and visual signatures for corneal diseases: a review study. Br J Ophthalmol 2016;100:41–55. [DOI] [PubMed] [Google Scholar]
- 16.Cruzat A, Witkin D, Baniasadi N, Zheng L, Ciolino JB, Jurkunas UV, et al. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci 2011;52:5136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamrah P, Cruzat A, Dastjerdi MH, Prüss H, Zheng L, Shahatit BM, et al. Unilateral herpes zoster ophthalmicus results in bilateral corneal nerve alteration: an in vivo confocal microscopy study. Ophthalmology 2013;120:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamrah P, Cruzat A, Dastjerdi MH, Shahatit BM, Bayhan HA, et al. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology 2010;117:1930–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tavakoli M, Marshall A, Pitceathly R, Fadavi H, Gow D, Roberts ME, et al. Corneal confocal microscopy: a novel means to detect nerve fibre damage in idiopathic small fibre neuropathy. Exp Neurol 2010;223:245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tavakoli M, Quattrini C, Abbott C, Kallinikos P, Marshall A, Finnigan J, et al. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care 2010;33:1792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qazi Y, Aggarwal S, Cavalcanti BM, Hamrah P. In vivo confocal microscopy demonstrates a profound increase in immune dendritic cells and decrease in corneal nerves in patients with post-refractive surgery keratoneuralgia. Invest Ophthalmol Vis Sci 2013;54: ARVO E-Abstract 3711 [Google Scholar]
- 22.Li Y, Xu J, Hong J, Le Q. Dry eye like symptoms without dessicated signs implies corneal neuropathy: an in vivo confocal microscopy study. Invest Ophthalmol Vis Sci 2014;55: ARVO E-Abstract 3646 [Google Scholar]
- 23.Hamrah P, Qazi Y, Shahatit B, Dastjerdi MH, Pavan-Langston D, Jacobs DS, Rosenthal P. Corneal Nerve and Epithelial Cell Alterations in Corneal Allodynia: An In Vivo Confocal Microscopy Case Series. Ocul Surf 2017; 15:139–51. [DOI] [PubMed] [Google Scholar]
- 24.Cirillo G, Cavaliere C, Bianco MR, De Simone A, Colangelo AM, Sellitti S,et al. Intrathecal NGF administration reduces reactive astrocytosis and changes neurotrophin receptors expression pattern in a rat model of neuropathic pain. Cell Mol Neurobiol 2010;30:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colangelo AM, Bianco MR, Vitagliano L, Cavaliere C, Cirillo G, De Gioia L, et al. A new nerve growth factor-mimetic peptide active on neuropathic pain in rats. J Neurosci 2008;28:2698–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao K, Leveque C, Pflugfelder SC. Corneal nerve regeneration in neurotrophic keratopathy following autologous plasma therapy. Br J Ophthalmol 2010;94:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abedi F, Hamrah P. Corneal Subbasal Nerve Recovery in an Acute Case of Ultraviolet Keratitis Treated with Autologous Serum Eye Drops. J Ophthalmol 2018: 4905487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cruzat A, Hamrah P, Cavalcanti BM, Zheng L, Colby K, Pavan-Langston D. Corneal Reinnervation and Sensation Recovery in Patients With Herpes Zoster Ophthalmicus: An In Vivo and Ex Vivo Study of Corneal Nerves. Cornea 2016;35:619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller RT, Abedi F, Cruzat A, , Witkin D, Baniasadi N, Cavalcanti BM, et al. Degeneration and Regeneration of Subbasal Corneal Nerves after Infectious Keratitis: A Longitudinal In Vivo Confocal Microscopy Study. Ophthalmology 2015;122:2200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meijering E, Jacob M, Sarria JC, Steiner P, Hirling H, Unser M. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A 2004;58:167–76. [DOI] [PubMed] [Google Scholar]
- 31.Oliveira-Soto L, Efron N. Morphology of corneal nerves using confocal microscopy. Cornea 2001;20:374–84. [DOI] [PubMed] [Google Scholar]
- 32.Labbe A, Alalwani H, Van Went C, Brasnu E, Georgescu D, Baudouin C. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci 2012;53:4926–31. [DOI] [PubMed] [Google Scholar]
- 33.Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res 2003;76:521–42. [DOI] [PubMed] [Google Scholar]
- 34.Acosta MC, Tan ME, Belmonte C, Gallar J. Sensations evoked by selective mechanical, chemical, and thermal stimulation of the conjunctiva and cornea. Invest Ophthalmol Vis Sci 2001;42:2063–7. [PubMed] [Google Scholar]
- 35.Belmonte C, Acosta MC, Merayo-Lloves J, Gallar J. What Causes Eye Pain? Curr Ophthalmol Rep 2015;3:111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aicher SA, Hermes SM, Hegarty DM. Corneal afferents differentially target thalamic- and parabrachial-projecting neurons in spinal trigeminal nucleus caudalis. Neuroscience 2013;232:182–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirata H, Okamoto K, Tashiro A, Bereiter DA. A novel class of neurons at the trigeminal subnucleus interpolaris/caudalis transition region monitors ocular surface fluid status and modulates tear production. J Neurosci 2004;24:4224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci 2009;32:611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 2007;10:1361–8. [DOI] [PubMed] [Google Scholar]
- 40.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett 2004;361:184–7. [DOI] [PubMed] [Google Scholar]
- 41.von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 2012;73:638–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 2009;32:1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ro LS, Chang KH. Neuropathic pain: mechanisms and treatments. Chang Gung Med J 2005;28:597–605. [PubMed] [Google Scholar]
- 44.Beuerman RW, Schimmelpfennig B. Sensory denervation of the rabbit cornea affects epithelial properties. Exp Neurol 1980;69:196–201. [DOI] [PubMed] [Google Scholar]
- 45.Lee BH, McLaren JW, Erie JC, Hodge DO, Bourne WM. Reinnervation in the cornea after LASIK. Invest Ophthalmol Vis Sci 2002;43:3660–4. [PubMed] [Google Scholar]
- 46.Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. Sodium channels in normal and pathological pain. Annu Rev Neurosci 2010;33:325–47. [DOI] [PubMed] [Google Scholar]
- 47.Everill B, Kocsis JD. Reduction in potassium currents in identified cutaneous afferent dorsal root ganglion neurons after axotomy. J Neurophysiol 1999;82:700–8. [DOI] [PubMed] [Google Scholar]
- 48.Liu CY, Lu ZY, Li N, Yu LH, Zhao YF, Ma B. The role of large-conductance, calcium-activated potassium channels in a rat model of trigeminal neuropathic pain. Cephalalgia 2015;35:16–35. [DOI] [PubMed] [Google Scholar]
- 49.Bhave G, Gereau RWt. Posttranslational mechanisms of peripheral sensitization. J Neurobiol 2004;61:88–106. [DOI] [PubMed] [Google Scholar]
- 50.Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev 2006;51:240–64. [DOI] [PubMed] [Google Scholar]
- 51.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature 1983;306:686–8. [DOI] [PubMed] [Google Scholar]
- 52.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152:S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galor A, Levitt RC, Felix ER, Martin ER, Sarantopoulos CD. Neuropathic ocular pain: an important yet underevaluated feature of dry eye. Eye (Lond) 2015;29:301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618–25. [DOI] [PubMed] [Google Scholar]
- 55.Seifert F, Maihofner C. Functional and structural imaging of pain-induced neuroplasticity. Curr Opin Anaesthesiol 2011;24:515–23. [DOI] [PubMed] [Google Scholar]
- 56.Dieckmann G, Goyal S, Hamrah P. Neuropathic Corneal Pain: Approaches for Management. Ophthalmology 2017;124:S34–S47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belmonte C Eye dryness sensations after refractive surgery: impaired tear secretion or "phantom" cornea? J Refract Surg 2007;23:598–602. [DOI] [PubMed] [Google Scholar]
- 58.Kalangara JP, Galor A, Levitt RC, Felix ER, Alegret R, Sarantopoulos CD. Burning Eye Syndrome: Do Neuropathic Pain Mechanisms Underlie Chronic Dry Eye? Pain Med 2016;17:746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chao C, Golebiowski B, Stapleton F. The role of corneal innervation in LASIK-induced neuropathic dry eye. Ocul Surf 2014;12:32–45. [DOI] [PubMed] [Google Scholar]
- 60.Rowbotham MC, Petersen KL. Zoster-associated pain and neural dysfunction. Pain 2001;93:1–5. [DOI] [PubMed] [Google Scholar]
- 61.Nitoda E, Kallinikos P, Pallikaris A, Moschandrea J, Amoiridis G, Ganotakis ES, et al. Correlation of diabetic retinopathy and corneal neuropathy using confocal microscopy. Curr Eye Res 2012;37:898–906. [DOI] [PubMed] [Google Scholar]
- 62.Dworkin RH, O'Connor AB, Kent J, Mackey SC, Raja SN, Stacey BR, et al. Interventional management of neuropathic pain: NeuPSIG recommendations; International Association for the Study of Pain Neuropathic Pain Special Interest Group. Pain 2013;154:2249–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bradley JC, Bradley RH, McCartney DL, Mannis MJ. Serum growth factor analysis in dry eye syndrome. Clin Experiment Ophthalmol 2008;36:717–20. [DOI] [PubMed] [Google Scholar]
- 64.Pan Q, Angelina A, Marrone M, Stark WJ, Akpek EK. Autologous serum eye drops for dry eye. Cochrane Database Syst Rev. 2017. February 28;2:CD009327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Di Fausto V, Fiore M, Tirassa P, Lambiase A, Aloe L. Eye drop NGF administration promotes the recovery of chemically injured cholinergic neurons of adult mouse forebrain. Eur J Neurosci 2007;26:2473–80. [DOI] [PubMed] [Google Scholar]
- 66.Takemura Y, Imai S, Kojima H, Katagi M, Yamakawa I, Kasahara T, et al. Brain-derived neurotrophic factor from bone marrow-derived cells promotes post-injury repair of peripheral nerve. PLoS One 2012;7:e44592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wiesmann C, de Vos AM. Nerve growth factor: structure and function. Cell Mol Life Sci 2001;58:748–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geerling G, Maclennan S, Hartwig D. Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol 2004;88:1467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kojima T, Ishida R, Dogru M, Goto E, Matsumoto Y, Kaido M, et al. The effect of autologous serum eyedrops in the treatment of severe dry eye disease: a prospective randomized case-control study. Am J Ophthalmol 2005;139:242–6. [DOI] [PubMed] [Google Scholar]
- 70.Matsumoto Y, Dogru M, Goto E, Ohashi Y, Kojima T, Ishida R, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology 2004; 111:1115–20. [DOI] [PubMed] [Google Scholar]
- 71.Noda-Tsuruya T, Asano-Kato N, Toda I, Tsubota K. Autologous serum eye drops for dry eye after LASIK. J Refract Surg 2006;22:61–6. [DOI] [PubMed] [Google Scholar]
- 72.Qazi Y, Hurwitz S, Khan S, Jurkunas UV, Dana R, Hamrah P. Validity and Reliability of a Novel Ocular Pain Assessment Survey (OPAS) in Quantifying and Monitoring Corneal and Ocular Surface Pain. Ophthalmology 2016;123:1458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kheirkhah A, Müller R, Mikolajczak J, Ren A, Kadas EM, Zimmermann H, et al. Comparison of Standard versus Wide-field Composite Images of the Corneal Subbasal Layer by In Vivo Confocal Microscopy. Invest Ophthalmol Vis Sci 2015; 56:5801–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koseoglu ND, Beam A, Hamrah P. The utilization of artificial intelligence for corneal nerve analyses of in vivo confocal microscopy images for the diagnosis of neuropathic corneal pain ARVO Abstract, 2018, HI, USA [Google Scholar]
- 75.Hamrah P, Koseoglu D, Kovler I et al. Deep Learning Convolutional Neural Network for the Classification and Segmentation of In Vivo Confocal Microscopy Images ARVO Abstract, 2018, HI. [Google Scholar]