Abstract
Articular cartilage defects are a common source of joint pain and dysfunction. We hypothesized that sustained low-dose dexamethasone (DEX) delivery via an acellular osteochondral implant would have a dual pro-anabolic and anti-catabolic effect, both supporting the functional integrity of adjacent graft and host tissue while also attenuating inflammation caused by iatrogenic injury. An acellular agarose hydrogel carrier with embedded DEX-loaded poly(lactic-co-glycolic) acid (PLGA) microspheres (DLMS) was developed to provide sustained release for at least 99 days. The DLMS implant was first evaluated in an in vitro pro-inflammatory model of cartilage degradation. The implant was chondroprotective, as indicated by maintenance of Young’s modulus (EY) (p=0.92) and GAG content (p=1.0) in the presence of interleukin-1β insult. In a subsequent preliminary in vivo experiment, an osteochondral autograft transfer was performed using a pre-clinical canine model. DLMS implants were press-fit into the autograft donor site and compared to intra-articular DEX injection (INJ) or no DEX (CTL). Functional scores for DLMS animals returned to baseline (p=0.39), whereas CTL and INJ remained significantly worse at 6 months (p<0.05). DLMS knees were significantly more likely to have improved OARSI scores for proteoglycan, chondrocyte, and collagen pathology (p<0.05). However, no significant improvements in synovial fluid cytokine content were observed. In conclusion, utilizing a targeted DLMS implant, we observed in vitro chondroprotection in the presence of IL-1-induced degradation and improved in vivo functional outcomes. These improved outcomes were correlated with superior histological scores but not necessarily a dampened inflammatory response, suggesting a primarily pro-anabolic effect.
Keywords: Osteochondral Repair, Microspheres, Dexamethasone, Targeted Drug Delivery, Preclinical Models
Graphical Abstract
1. Introduction
The avascular nature and dense extracellular matrix of articular cartilage confers a poor healing capacity whereby localized regions of tissue damage can lead to joint degeneration and osteoarthritis (OA) [36, 44]. The effectiveness and longevity of cartilage restoration treatments, including osteochondral autografts and allografts and autologous chondrocyte implantation (ACI), can be limited by the impact of proinflammatory cytokines on graft incorporation and integrity [24, 41, 61, 75]. Synovial fluid from injured knees is known to negatively affect chondrogenesis [85], with interleukins specifically linked to adverse integrative repair [83]. Iatrogenic cartilage injury can further hamper outcomes; chondrocyte death can occur due to osmolarity differences in saline, contact with blood, or drying during open procedures [2, 38, 71]. Furthermore, cartilage grafts often suffer from reduced viability and metabolic activity caused by extended preservation [60] or method of graft harvest and delivery [71]. Development of more effective treatment of cartilage defects may shorten rehabilitation times, prevent the progression of OA, and reduce the need for total joint replacement.
Intra-articular glucocorticoid injections have been used for decades to treat joint inflammation and pain [18, 33, 35, 52, 72]. However, injection doses are necessarily high due to the high clearance rate of the steroid from the joint space, where serum levels can peak within hours to a couple of days after administration [7, 34]. Repeated high-dose injections [8, 42] have been associated with adverse effects on growth of articular and growth plate chondrocytes [20, 26], synoviocytes [72], and osteoblasts [1]. These side effects, as well as risks of systemic absorption, typically limit patients to 2–4 injections per year [7, 11, 34, 55, 58, 86].
Dexamethasone (DEX), a synthetic glucocorticoid, is a common media supplement for chondrogenic cultures. Used at relatively low doses, it stimulates anabolism in immature tissues and contributes to maintenance of mature tissues in vitro [12, 47, 49]. Lu and co-workers have reported that DEX concentrations as low as 1 nM were sufficient to protect cartilage explants against tumor necrosis factor-α (TNF-α), and concentrations of 100 nM to 10 μM enhanced cartilage explant sulfate incorporation and suppressed GAG loss to the media [52]. This is significantly lower than the theoretical peak concentration from a clinical DEX injection (7.7 mM; based on a 4 mg/ml injection) [17].
To the authors’ knowledge, an optimal delivery system for sustained low (therapeutic) dose of DEX to the synovial joint is not currently available for clinical use in cartilage restoration. In an early attempt to use local sustained DEX delivery as a disease modifying OA drug (DMOAD), DEX was conjugated to avidin and introduced by intra-articular injection. While some benefits were observed over the 3-week rabbit anterior cruciate ligament (ACL) transection study, the nanocarrier induced cartilage GAG content loss [9]. Another strategy for targeted low dose intra-articular DEX delivery has been proposed using magnetic PLGA nanoparticles [15]. However, drug penetration can be hindered by dense cartilage matrix [9], potentially necessitating delivery directly to the site of chondral defect treatment. Furthermore, it is anticipated that free-floating particulates would become embedded in surrounding tissue, contributing to both inflammation and cartilage wear [74] as intra-articular microspheres (MS) may persist for more than 70 days [67].
We hypothesized that sustained low-dose DEX delivery to the site of cartilage damage would have a dual anti-catabolic and pro-anabolic effect, preventing further cartilage degeneration while simultaneously supporting the functional integrity of osteochondral autografts. Toward this goal, it has been demonstrated that in vitro local sustained DEX release from poly(lactic-co-glycolic) acid (PLGA) MS promotes development of functional engineered cartilage and confers protection from pro-inflammatory cytokine-induced tissue degradation [64]. PLGA is the focus of intensive research due to its biocompatibility, biodegradability, mechanical strength, ease of formulation into different drug delivery devices, and FDA-approved status [22, 43]. Using a single emulsion/solvent extraction technique to manufacture MS, a controlled release of DEX can be maintained over at least a 52-day period with a sustained linear release [66]. Based on these safety and mechanistic data, DEX-PLGA-MS has the potential to provide a clinically applicable method for enhancing cartilage restoration. Therefore, the present study was designed to provide preclinical data towards addressing a critical unmet need in orthopaedic surgery.
To facilitate targeted drug delivery to the joint, an acellular agarose (copolymer in phase III clinical trials [69, 70]) hydrogel carrier with embedded DEX-eluting PLGA microspheres (DLMS) was developed. We hypothesized that this DLMS implant will prevent cartilage degradation and stimulate growth in an in vitro inflammatory environment. To test this, we first assessed drug penetration and activity; DLMS carriers were co-cultured with engineered cartilage constructs and evaluated for p57Kip2 expression, an indicator of glucocorticoid binding [68] (Study 1a). Separately, to evaluate chondroprotection, carriers and engineered cartilage constructs were co-cultured for two weeks ± interleukin-1 (IL-1) (Study 1b). Engineered tissues were used for in vitro validation (Study 1) due to their consistent geometry, composition, and growth, which allow for reliable mechanical and biochemical assessments with higher sample size. Behavior of engineered adult cartilage has been validated against explanted tissues [49, 59].
We then moved to clinically relevant osteochondral autograft transfer (OAT) for in vivo study, as it is an established procedure that may benefit from expedited host integration, support of chondrocyte growth, and reduced donor site morbidity [71]. Specifically, we hypothesized that the DLMS implant will modulate the post-surgical inflammatory environment and support expedited return to function and improved repair integrity. To answer this, DLMS implants were combined with a clinically-relevant bone substrate to aid in integration [40] and press-fit into the autograft donor site and compared to intra-articular DEX injection and standard-of-care controls. The 6-month study end point was selected as the critical window for healing, integration, and cartilage restoration needed for successful functional outcomes in this preclinical model. Overall, we anticipate that DEX can protect against iatrogenic injury by promoting healing via protection from pro-inflammatory cytokines and promotion of anabolic chondrocyte activities.
2. Methods
2.1. Microsphere Fabrication, Characterization, and Dosing
Dexamethasone sodium phosphate (DSP; MW 516.4; Sigma D1159), a 1:1 molar equivalent of dexamethasone base (MW 392.5) [5, 6], was encapsulated in PLGA (75:25 lactide:glycolide, MW: 66,000–107,000; Sigma P1941) as previously described to create DLMS [64, 66]. Unloaded PLGA microspheres (ULMS) were fabricated similarly without the addition of DSP. This microsphere formulation was previously characterized [63]. Briefly, DLMS and ULMS had mean diameter of 46 ± 17 μm (n=543) and 25 ± 15 μm (n=411), respectively. The theoretical drug loading of DLMS was 9.1% (w/w). The actual loading capacity, determined via complete polymer hydrolysis and spectrophotometric analysis at 242 nm, was 6.3% (w/w).
To estimate proper dosing, the release profile of the microsphere formulation in agarose was characterized [63]. Briefly, DLMS were encapsulated in 2% (w/v) agarose (Sigma; A9414) and punched into cylindrical (Ø 6 mm × 2.34 mm thickness) constructs (5.33 mg MS/ml; 350 μg MS per carrier). Constructs (n=4) were placed in individual microcentrifuge tubes with 1 ml PBS (pH 7.4), which was replaced at regular time points over 100 days. Supernatant was assayed spectrophotometrically at 242 nm and absorbance compared to a standard curve. There was an initial burst release of approximately 9.2% of the total encapsulated drug in the first 24 hours. Fitting the subsequent release data to a line over the next 99 days (R2=0.99), an average release rate of approximately 0.9% per day was determined.
Synovial fluid volume in the canine knee (stifle) can range from <1 ml in normal animals to 3 ml or more with induced synovitis [56]. Therefore, we estimated that ~1 ng DSP would be sufficient to meet the 1 nM DEX critical threshold for chondroprotection and ~10 μg DSP to reach the upper threshold for a pro-anabolic effect (10 μM DEX). Dosing for subsequent experiments was chosen to target this specific therapeutic window identified by Lu, et al [52].
2.2. Preparation and Culture of Engineered Cartilage Constructs (Study 1)
Articular cartilage was harvested from the knees of adult dogs (~1-year-old; n=4 joints) euthanatized for unrelated purposes. Briefly, chondrocytes were isolated via collagenase digestion, and pooled passage 2 (P2) cells were encapsulated in agarose (2% (w/v) Type VII Agarose, Sigma A4018) to form cylindrical constructs (Ø 4 mm × 2.23 mm thickness) with an initial composition of 30 × 106 cells/ml [59]. Constructs were initially cultured to maturity (Day 42, Young’s modulus (EY) ~ 200kPa) in chondrogenic medium (CM) supplemented with 10 ng/ml TGFβ3 (R&D Systems #243B3200), 100 nM dexamethasone base (Sigma D4902), and 50 μg/ml ascorbic acid-2-phosphate (Sigma A8960).
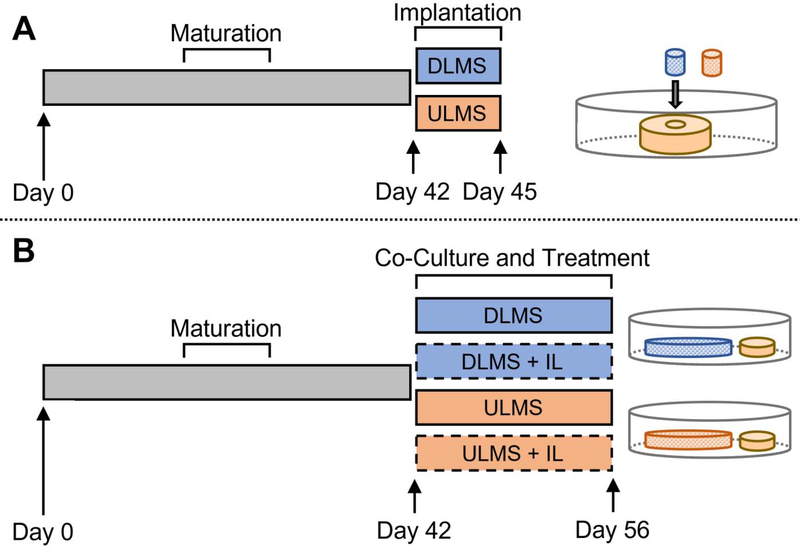
At culture maturity, constructs were concentrically cored and replaced with a 2% (w/v) agarose hydrogel carrier (Ø 1.5 mm × 2.23 mm thickness) containing DLMS or ULMS (3.3 mg MS/ml) (Fig. 1A). Engineered cartilage ring-implant core (ring-core) pairs were cultured for 3 days in CM supplemented with 10 ng/ml TGFβ3 and 50 μg/ml ascorbic acid-2-phosphate (Study 1a).
Figure 1.
Study 1 Schematic. (A) Following a 42-day maturation period, engineered cartilage constructs (~200 kPa) were cored and implanted with DLMS or ULMS carriers for 3 days (Study 1a). (B) Mature engineered cartilage constructs were divided into six experimental groups for a 14-day interleukin-1β (IL) stimulation period where they were co-cultured with DLMS or ULMS carriers.
For Study 1b, 2% (w/v) agarose hydrogel carriers (Ø 10 mm × 2.23 mm thickness) were encapsulated with DLMS (5.0 mg MS/ml; approximately 880 μg MS and 55 μg DSP per carrier). Mature cartilage constructs were co-cultured with the DLMS carriers for two weeks with or without application of 10 ng/ml IL-1 (recombinant human interleukin 1β; ThermoFisher PHC0816) (n=6) (Fig. 1B). To achieve the desired dosage range, four hydrogel carriers were placed in a total of 25 ml media, resulting in an estimated burst delivery of 20. μg DSP (1.6 μM DEX) in the first 24 hours followed by 1.9 μg DSP (150 nM DEX) per day.
Negative control carriers contained ULMS (5.0 mg MS/ml; 880 μg MS). Separate controls were continuously supplemented with 100 nM dexamethasone base, a standard concentration and dosage form for cultures, which facilitated comparisons with previous chondroprotection studies [49, 59, 64]. In each group, half of the media was replaced with fresh media 3x per week. All constructs were supplemented with TGF-β3 and ascorbic acid-2-phosphate for the entire culture period.
2.2.1. In Vitro Evaluation of Delivery to Engineered Cartilage Constructs (Study 1)
Study 1a specimens were fixed in formalin, paraffin embedded, and sectioned (20 μm). Heat mediated epitope retrieval was performed in citrate buffer at 60°C overnight, followed by incubating in rabbit monoclonal [EP2515Y] to p57Kip2 (Abcam ab75974) and then goat anti-rabbit IgG secondary antibody (Alexa Fluor 488; ThermoFisher A11034). Pixel intensity was analyzed to quantify cell DEX uptake radially away from the core (ImageJ).
For Study 1b, Young’s (EY) and dynamic (G) moduli were measured under unconfined compression conditions [54]. Following mechanical testing, individual specimens were solubilized using proteinase K (MP Biomedicals #02193981-CF) prior to being assayed for DNA, glycosaminoglycans (GAG), and collagen (COL) using the Picogreen Assay (ThermoFisher P11496), dimethylmethylene blue dye assay, and the orthohydroxyproline assay, respectively. Values were normalized to specimen wet weight (ww). Half of each specimen was fixed in formalin and subsequently embedded in paraffin, sectioned (8 μm), and stained with safranin-o. Resulting images were processed in ImageJ (convert to 8-bit, invert black and white) and relative pixel intensity quantified using the “plot profile” function.
2.3. Preclinical Canine Osteochondral Autograft Model (Study 2)
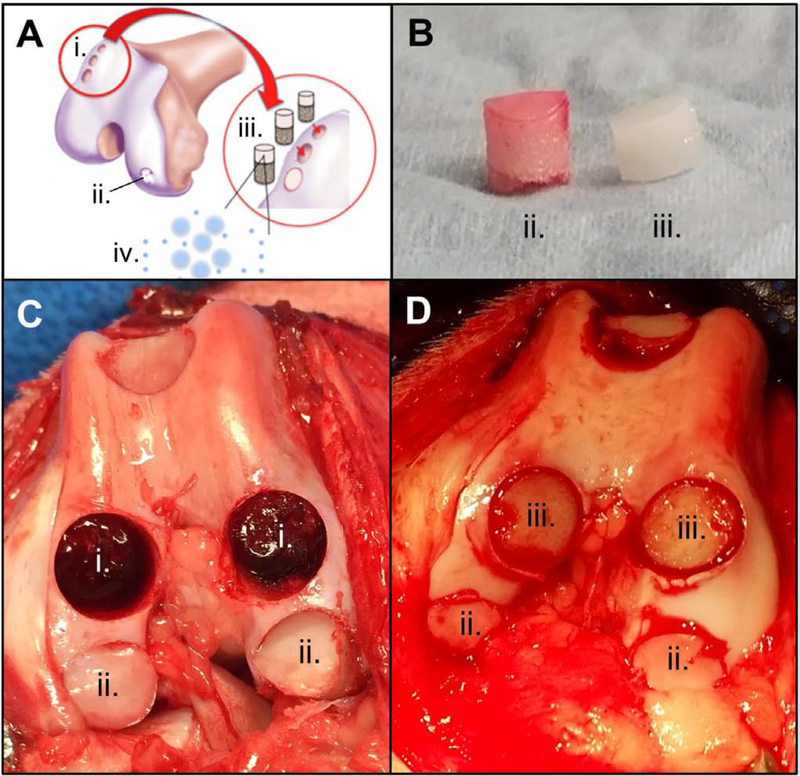
In preparation for the procedure, devitalized bovine trabecular bone cores (Ø 8 mm × 5 mm thickness) were infused with 2% (w/v) agarose hydrogel mixed with DLMS (33 mg MS/ml; 3.3 mg MS and 210 μg DSP per carrier) to produce multilayered acellular osteochondral implants (1 mm gel-only region, 1 mm gel bone interface, and 4 mm bone-only) (Fig. 2, iii). From release studies, this was expected to deliver a 24-hour burst of 19 μg DSP (15–75 μM DEX; assuming 0.5–2.5 ml volume [57]) per implant followed by 1.8 μg DSP (1.4–7.0 μM DEX; assuming 0.5–2.5 ml volume) per implant per day. To confirm expected release, extra implants (n=4) were placed in PBS and supernatant was collected and assayed as in Section 2.1, above.
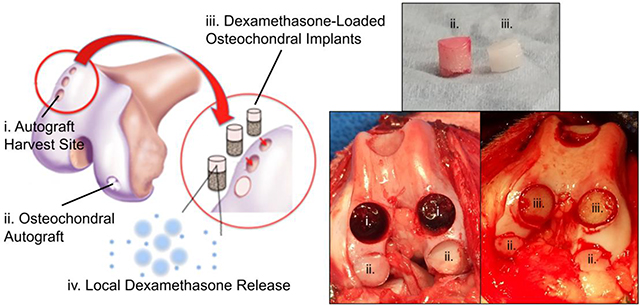
Figure 2.
(A) Schematic of Study 2 showing autograft donor site (i.), repair site (ii.), DLMS implant (iii.), and dexamethasone-PLGA microsphere release (iv.); (B) cartilage autograft (ii.) and DLMS implant (iii.); (C) autograft donor (i.) and repair (ii.) sites; (D) DLMS implant (iii.) and repair (ii.) sites.
On the day of surgery, adult mongrel dogs (10 ± 1 months, 25.3 ± 3.5 kg) were premedicated, anesthetized and prepared for aseptic surgery of the right knee (University of Missouri-Columbia IACUC #9167; complied with ARRIVE guidelines). Briefly, 2 doses of cefazolin (antibiotic) were given perioperatively and 2 morphine intramuscular doses plus 2 doses of oral tramadol were given for pain management. Post-operatively, a soft padded bandage was kept on the operated right hindlimb for 1 week with oral cefpodoxime (antibiotic) for 10 days.
Osteochondral autografts (Ø 8 mm diameter) (Fig. 2, ii) were obtained from the trochlear ridge and sulcus terminalis and transferred to size-matched recipient defects on the neighboring trochlea or medial/lateral femoral condyle of the same knee (n=3 grafts per knee) using the Osteochondral Autograft Transfer System® (OATS®; Arthrex, Naples, FL). The empty graft donor sites (Fig. 2, i) were filled with press-fit DLMS-loaded implants (OATS-DLMS; n=6 animals, n=3 implants per animal). Dogs receiving this implant were compared to those for which there was one post-surgical DSP injection of 4 mg/knee (OATS-INJ; n=5 animals, n=3 grafts per animal; no implant) or no DSP injection (OATS-CTL; n=5 animals, n=3 grafts per animal; no implant).
Possible systemic effects of dexamethasone were assessed by daily monitoring by veterinarians, animal weights, and post mortem examination (necropsy).
2.3.1. Clinically Based Assessments (Study 2)
Animals were examined by a board-certified veterinary orthopaedic surgeon (JLC). Clinical lameness, functional gait, comfortable range of motion (CROM), pain, and effusion [14] were assessed both pre-surgery (T=0) and at the conclusion of the study period (T=6 months). Synovial fluid composition was assessed at monthly intervals (T=0, 1, 2, 3 months) with a Luminex Multiplex Assay for IL-6 [77], IL-8 [51], MMP-2 [27], and MMP-3 [62] (ThermoFisher).
2.3.2. Histological Scoring (Study 2)
At the terminal time point (T=6 months), animals were sacrificed and tissue harvested for histopathology assessment. Osteochondral treatment sites including adjacent cartilage and bone were collected, fixed in formalin, and stained with H&E, picrosirius red, and toluidine blue. Synovium was harvested from four separate locations (medial/lateral trochlea and medial/lateral femoral condyle), fixed in formalin, and stained with H&E.
A modified osteochondral (OC) scoring system was used to evaluate the autograft recipient sites [19]. The scoring consisted of evaluating cartilage fill, cartilage edge integration at host-graft junction, cartilage surface congruity of construct and host-construct junction, fibrosis, and inflammation.
A modified OARSI method was used to evaluate surrounding cartilage structure, chondrocyte pathology, proteoglycan staining, collagen integrity, tidemark, and subchondral bone plate [21]. The OARSI method was also used to evaluate synovial pathology through microscopic examination of the lining cell, lining, and cell infiltration characteristics [21]. All scoring was performed by two blinded board-certified veterinary pathologists.
DLMS implants were scanned using Micro-CT at T=0 and T=6 months to assess changes to bone fill and structure at the donor site as previously described [50].
2.4. Statistics
Data sets were tested for normality (Kolmogorov-Smirnov Test) and homogeneity (Bartlett’s Test). When necessary, data was log-transformed to achieve normality or evaluated using equivalent nonparametric tests. For Study 1, one-way ANOVA with Tukey post hoc test (α=0.05) was used. Young’s modulus data was analyzed using the Kruskall-Wallis test with Dunn’s post hoc comparisons (α=0.05). For Study 2, functional and synovial fluid measures were compared using two-way ANOVA or corresponding mixed-effects analysis (group, time) with time as a repeated measures factor and Tukey post-hoc test (α=0.05). This data was presented as mean and standard deviation, unless otherwise noted. Analyses were performed using GraphPad Prism 8.
Ordinal scores (OARSI and OC) were analyzed using a Generalized Linear Model. Specifically, data was fit to an ordinal multinomial probability distribution and cumulative logit link function with generalized estimating equations correction for repeated measures (location, scorer). The dependent (response) variable was the score and the independent variables (predictors) were the categorical factors: group and/or repair location. Total OARSI scores were grouped into ordered categories for regression analysis to increase the number of observations per level of the dependent variable. Odds ratios (OR) and corresponding 95% CI were computed from the model’s parameter estimates. Averages of non-normal datasets were presented as median (95% CI). Ordinal regression analyses were performed using IBM SPSS Statistics 25. The results of a pilot study were used to determine the sample size needed in Study 2 to achieve at least 80% power with G*Power 3 (α=0.05) [30].
3. Results
3.1. In Situ Evaluation of DEX Receptor Binding (Study 1a)
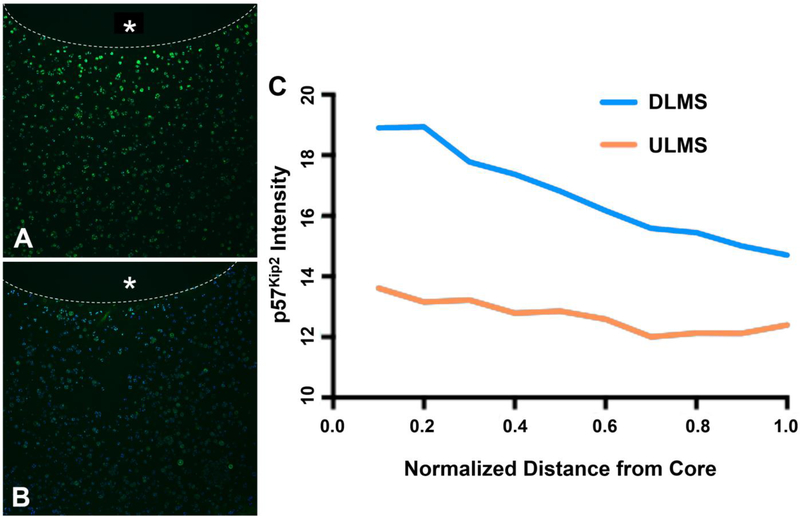
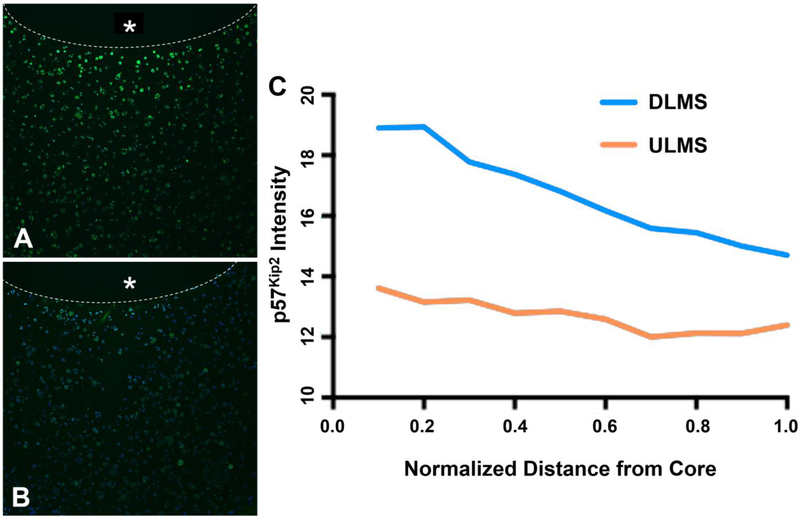
Cells in the surrounding cartilage ring were exposed to a radially-dependent concentration of DEX, with cartilage immediately adjacent to the DLMS-laden core expressing the most intense staining for p57Kip2 that declined toward the edge of the construct (Fig. 3). In contrast, for ULMS constructs, mean intensity of the stain remained at baseline levels.
Figure 3.
Representative immunohistochemical stain for p57Kip2 expression (green) counterstained with DAPI. Inner core containing either (A) DLMS or (B) ULMS marked with * and boundary outlined with dotted line; (C) Relative pixel intensity of immunohistochemical stain expression as a function of distance away from the microsphere embedded core (n=1).
3.2. In Vitro Chondroprotection Via Dexamethasone-loaded Microsphere Carriers (Study 1b)
At day 42, Young’s modulus (EY) of the engineered cartilage constructs was 213 ± 29 kPa (Fig. 4A). Following the 14-day treatment period (day 56), EY of ULMS+IL specimens was significantly lower than ULMS (247 vs. 141 kPa; between-group difference, 106 kPa; p=0.048). Meanwhile, EY was not significantly affected by IL insult in DLMS (256 vs. 197 kPa; between-group difference, 59.0 kPa; p=0.92). No significant between-group differences were observed in dynamic modulus (G) (Fig. 4B).
Figure 4.
(A) Young’s modulus (EY); (B) Dynamic modulus (G); (C) GAG/ww (%); (D) COL/ww (%); *p<0.05. (bars show mean and 95% CI).
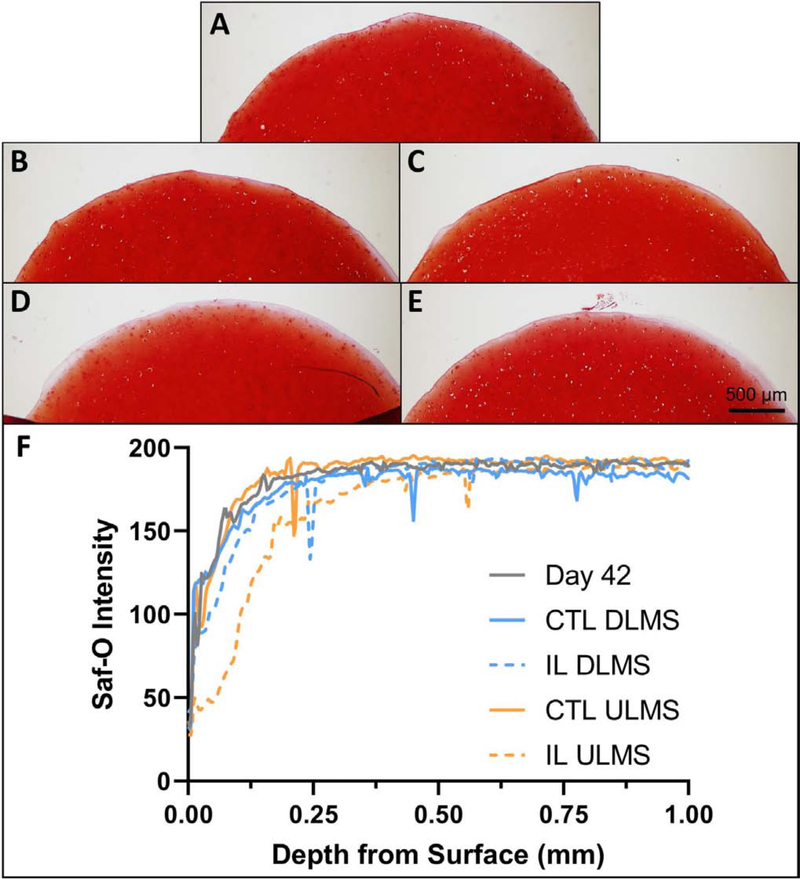
GAG/ww reached 5.5 ± 0.6% by day 42 (Fig. 4C). Following the 14-day treatment period, GAG/ww of ULMS+IL specimens was significantly lower than ULMS (5.8 vs. 4.3%; between-group difference, 1.5%; p<0.001). GAG distribution was visually more diffuse in the periphery of the IL ULMS group (Fig. 5). Safranin-O intensity was similar between groups at depths greater than ~250 μm. Meanwhile, GAG/ww was not significantly affected by IL insult in DLMS (6.1 vs. 5.9%; between-group difference, 0.2%; p=1.0) (Fig. 4C). GAG/ww in the DLMS+IL group was significantly greater than ULMS+IL (p<0.0001).
Figure 5.
Representative safranin-o (saf-o) histology of the cartilage construct cross-section for (A) Day 42, (B) CTL ULMS, (C) CTL DLMS, (D) IL ULMS, (E) IL DLMS, and (F) corresponding relative staining intensity.
COL/ww was not significantly affected by IL insult, however DLMS had significantly lower content than ULMS (p=0.022) and day 42 (p<0.001) (Fig. 4D).
Continuous bolus addition of DEX base confirmed similar maintenance of construct properties (data not shown).
3.3. Confirmation of DLMS Implant Release Profile
A burst release of 64 μg DSP was observed in the first 48 hours. Fitting the subsequent release data to a line (R2=0.98) over the next 8 days, a daily release rate of 1.4 μg DSP per day (95% CI, 1.1 to 1.6 μg DSP per day).
3.4. Clinically Based Assessments of In Vivo OATS Repair (Study 2)
Gait was significantly worse at 6 months compared to baseline in OATS-CTL (p=0.031) and INJ (p=0.008) groups (Table 1). Meanwhile, gait score was similar to baseline in DLMS specimens (p=0.39). Lameness was elevated in CTL compared to both DLMS (1.8 vs. 1.3; between-group difference, 0.5; p=0.099) and INJ (1.8 vs. 1.4; between-group difference, 0.4; p=0.20).
Table 1.
Clinical outcome scores at 6 months
| Mean (95% CI) |
Mean Difference from Baseline (95% CI) |
|||
|---|---|---|---|---|
| Measurement | Baseline | OATS-CTL (N=5) | OATS-DLMS (N=6) | OATS-INJ (N=5) |
| Gait | 10.0 (10.0 to 10.0) | −2.9 (−5.5 to −0.2)* | −1.3 (−3.7 to 1.1) | −3.6 (−6.2 to −0.9)** |
| CROM (°) | 108 (107 to 109) | −12 (−20 to −4)** | −8 (−15 to −0.0)* | −9 (−17 to −1)* |
| Lameness | 0.0 (0.0 to 0.0) | 1.8 (1.2 to 2.4)**** | 1.3 (0.8 to 1.9)**** | 1.4 (0.8 to 2.0)**** |
| Pain | 0.0 (0.0 to 0.0) | 1.3 (0.2 to 2.4)* | 1.1 (0.2 to 2.1)* | 1.0 (0.0 to 2.1) |
| Effusion | 0.0 (0.0 to 0.0) | 2.0 (0.9 to 3.0)*** | 1.8 (0.9 to 2.8)*** | 1.3 (0.3 to 2.4)* |
p<0.05
p<0.01
p<0.001
p<0.0001
OATS, osteochondral autograft transfer system; CTL, no dexamethasone supplementation group; DLMS, dexamethasone-loaded microsphere implant group; INJ, dexamethasone injection group
We did not observe significant between-group differences in animal’s change in weight at the 6-month time point.
3.5. Histological Assessment of OATS Repair (Study 2)
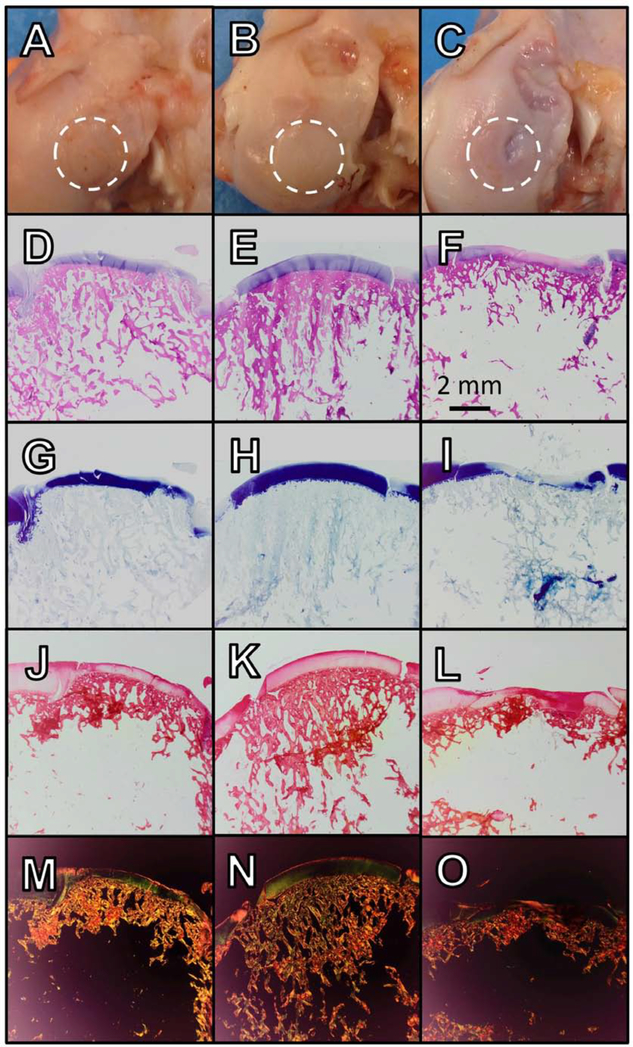
Cartilage was visually intact in each group, with some evidence of lesions in OATS-INJ specimens (Fig. 6A–C). The combined OARSI cartilage score of DLMS specimens were more than twice as likely to be improved compared to CTL (p=0.003), but there was not a significantly increased likelihood compared to the INJ group (p=0.47) (Table 2). OC scores, which specifically evaluated the graft-host junction, showed little pathology across groups (Table 3).
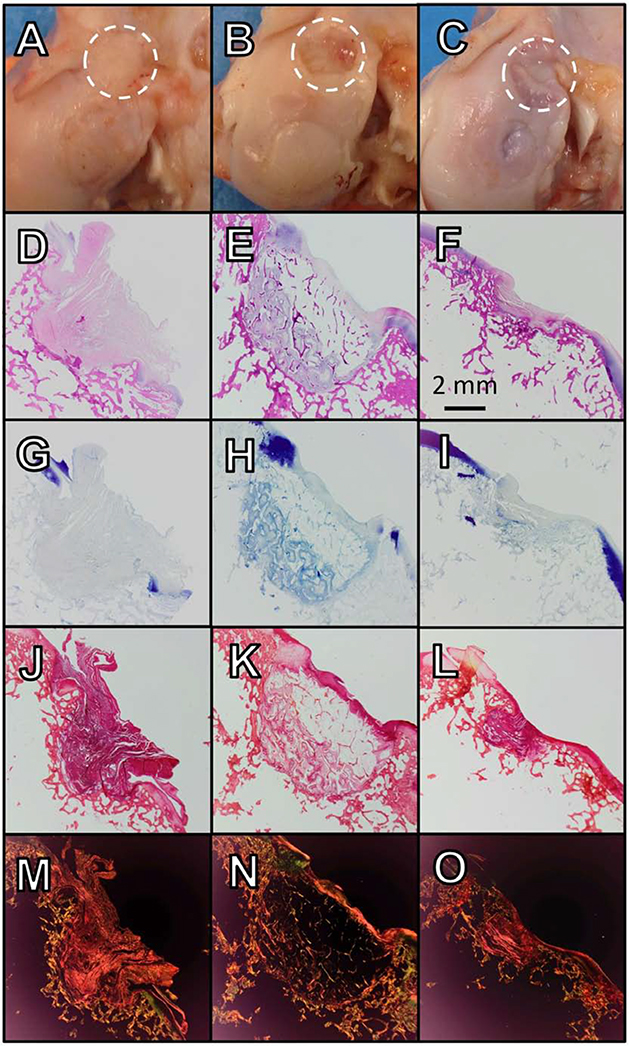
Figure 6.
(A-C) Gross imaging, (D-F) H&E, (G-I) toluidine blue, and (J-L) picrosirius red staining and (M-O) picrosirius red with polarized light for selected graft recipient sites; OATS-CTL (A, D, G, J, M; 92-FCL), OATS DLMS (B, E, H, K, N; 88-FCL), OATS-INJ (C, F, I, L, O; 112-FCL)
Table 2.
OARSI cartilage scores and sub-scores
| Median (95% CI) |
Odds Ratio (95% CI) |
|||||
|---|---|---|---|---|---|---|
| Measurement | OATS-CTL (N=5) | OATS-INJ (N=5) | OATS-DLMS (N=6) | INJ vs. CTL | DLMS vs. CTL | DLMS vs. INJ |
| OARSI (Cartilage, combined) | 12 (9 to 17) | 12 (7 to 15) | 8 (6 to 12) | 1.3 (0.6 to 2.7) | 2.2 (1.3 to 3.6)** | 1.6 (0.7 to 3.7) |
| Structure | 2 (2 to 3) | 2 (1 to 3) | 2 (1 to 3) | 1.8 (0.9 to 3.5) | 1.5 (0.9 to 2.8) | 0.9 (0.4 to 2.0) |
| Chondrocytes | 4 (2 to 4) | 4 (2 to 4) | 2 (1 to 3) | 1.1 (0.4 to 3.2) | 2.7 (1.5 to 5.1)** | 2.6 (0.9 to 7.4) |
| Proteoglycans | 3 (2 to 4) | 3 (1 to 4) | 2 (1 to 2) | 1.0 (0.3 to 3.5) | 3.3 (1.4 to 7.5)** | 3.4 (1.0 to 11.4)* |
| Collagen | 2 (1 to 3) | 2 (1 to 3) | 1 (1 to 2) | 1.1 (0.4 to 2.9) | 2.8 (1.5 to 5.1)** | 2.5 (0.9 to 6.8) |
| Tidemark | 1 (0 to 2) | 0 (0 to 2) | 0 (0 to 2) | 1.8 (1.2 to 2.8)** | 1.7 (1.1 to 2.8)* | 1.0 (0.6 to 1.4) |
| Bone | 1 (0 to 3) | 0 (0 to 3) | 3 (0 to 3) | 1.1 (0.6 to 2.4) | 0.7 (0.2 to 2.1) | 0.6 (0.2 to 1.8) |
p<0.05
p<0.01
p<0.001
OATS, osteochondral autograft transfer system; CTL, no dexamethasone supplementation group; DLMS, dexamethasone-loaded microsphere implant group; INJ, dexamethasone injection group
Table 3.
OC scores.
| Median (95% CI) |
|||
|---|---|---|---|
| Measurement | OATS-CTL (N=5) | OATS-DLMS (N=6) | OATS-INJ (N=5) |
| OC (Total) | 2 (1 to 4) | 2 (1 to 3) | 2 (1 to 3) |
| Fill | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) |
| Edge Integration | 1 (1 to 1) | 1 (1 to 1) | 1 (1 to 1) |
| Surface Congruity | 0 (0 to 1) | 1 (0 to 1) | 0 (0 to 1) |
| Calcified Cartilage | 0 (0 to 1) | 0 (0 to 0) | 0 (0 to 1) |
| Fibrosis | 0 (0 to 1) | 0 (0 to 1) | 0 (0 to 1) |
| Inflammation | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) |
OATS, osteochondral autograft transfer system; CTL, no dexamethasone supplementation group; DLMS, dexamethasone-loaded microsphere implant group; INJ, dexamethasone injection group
Examining sub-scores (Table 2), DLMS were nearly three times more likely to have better chondrocyte scores and more than three times more likely to have better proteoglycan sub-scores than both CTL (p=0.001 and 0.005, respectively) and INJ (p=0.078 and 0.048, respectively). Meanwhile, INJ was not significantly likely to differ from CTL in chondrocyte or proteoglycan score (p=0.92 and 0.95, respectively). Superior proteoglycan deposition in DLMS samples was evident from toluidine blue staining as well (Fig. 6G–I).
DLMS were more than twice as likely to have a better collagen sub-score than both CTL (p=0.001) and INJ (p=0.068), which was further evidenced in picrosirius red staining (Fig. 6J–L). Collagen pathology in INJ was not significantly likely to differ from CTL (p=0.82).
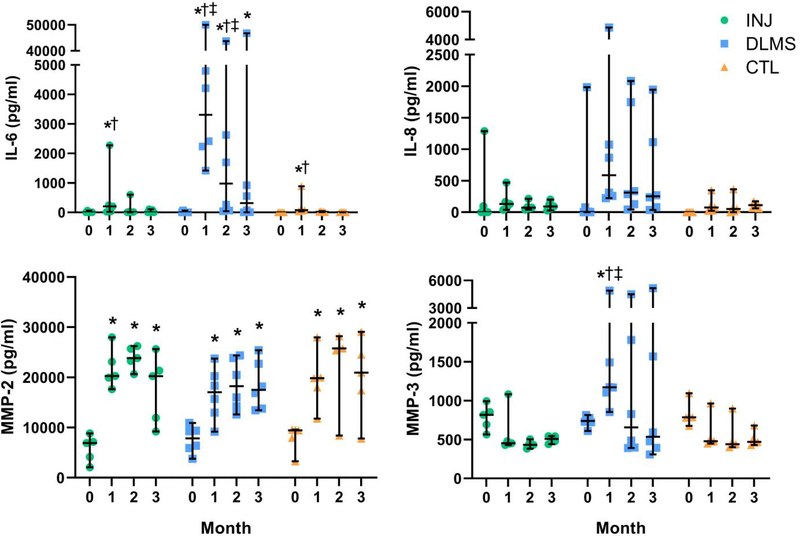
3.6. Modulation of In Vivo Inflammatory Environment (Study 2)
Synovial fluid IL-6 levels peaked at 1 month in each group (p<0.01) (Fig. 7). The concentration of IL-6 at 1-month was significantly elevated in the OATS-DLMS group relative to CTL (p=0.004) and INJ (p=0.011). Significant differences were not detected in IL-8 levels.
Figure 7.
Synovial Fluid Composition; *p<0.05 compared to Day 0 (same group), †p<0.05 compared to OATS CTL (same time point), ‡p<0.05 compared to OATS-INJ (same time point). (bars show median and 95% CI).
MMP-2 was elevated at 1 month and remained significantly elevated at 3 months in all groups (p<0.001). MMP-3 levels peaked at 1 month in the DLMS group only (p=0.012). By 2 months, MMP-3 levels in DLMS had returned to baseline (p=0.78). The concentration of MMP-3 was significantly elevated in the DLMS group relative to CTL (p=0.009) and INJ (p=0.008) at the 1-month time point.
No significant differences were observed in OARSI synovium scores, which were largely in the range of mild to moderate pathology (Table 4).
Table 4.
Histological scoring of the synovium (OARSI)
| Median (95% CI) |
|||
|---|---|---|---|
| Measurement | OATS-CTL (N=5) | OATS-DLMS (N=6) | OATS-INJ (N=5) |
| OARSI (Synovium Total) | 7 (5 to 9) | 7 (6 to 11) | 8 (6 to 10) |
| Lining Cells | 2 (1 to 3) | 2 (1 to 3) | 3 (2 to 3) |
| Lining Characteristics | 3 (3 to 5) | 4 (2 to 5) | 4 (3 to 4) |
| Cell Infiltration | 1 (0 to 2) | 2 (1 to 3) | 1 (1 to 2) |
OATS, osteochondral autograft transfer system; CTL, no dexamethasone supplementation group; DLMS, dexamethasone-loaded microsphere implant group; INJ, dexamethasone injection group
3.7. Evaluation of DLMS Implant in Graft Donor Sites
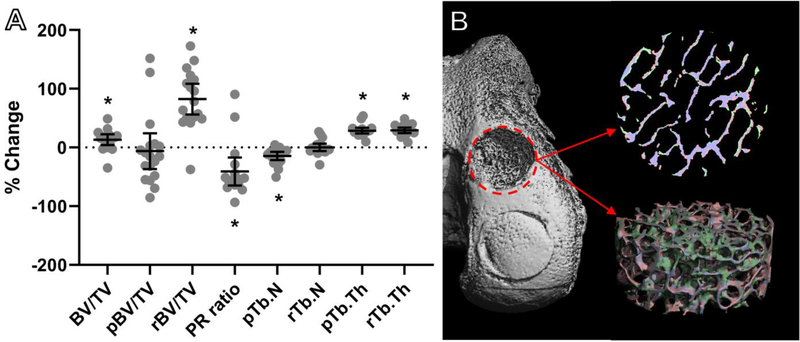
Micro-CT reconstructions of the DLMS implant bone bases revealed significant bone fill-in and ingrowth (Fig. 8). Specifically, bone volume density (BV/TV) of the bone scaffold increased 17 ± 23% over the implantation period (p=0.009). There was also a significant decrease in plate-rod (PR) ratio (p<0.001).
Figure 8.
(A) μCT revealed significant bone remodeling in the DLMS osteochondral implant; 30 (B) Reconstruction of DLMS bone base showing resorbed bone (pink), new bone (green), and unchanged bone (blue); Bone volume density (BV/TV), plate density (pBV/TV), rod density (rBV/TV), plate-rod ratio (PR ratio), number of plate trabeculi (pTb.N), number of rod trabeculi (rTb.N), plate trabecular thickness (pTb.Th), and rod trabecular thickness (rTb.Th); *p<0.05 vs. baseline.
Representative histology of the graft donor site, which also served as the DLMS implant site, showed a degree of fibrous tissue formation in CTL and INJ groups (Fig. 9).
Figure 9.
(A-C) Gross imaging, (D-F) H&E, (G-I) toluidine blue, and (J-L) picrosirius red staining and (M-O) picrosirius red with polarized light for selected graft donor sites; OATS-CTL (A, D, G, J, M; 92-FCL), OATS DLMS (B, E, H, K, N; 88-FCL), OATS-INJ (C, F, I, L, O; 112-FCL).
4. Discussion
The current study examined the influence of targeted, sustained low-dose DEX delivery, via PLGA microspheres embedded in an acellular agarose carrier, on growth and repair of neighboring focal articular cartilage injuries. While periodic intra-articular glucocorticoid injections have long been used to manage joint inflammation and pain [18], only recently have they been proposed as an early intervention aimed at dampening the inflammatory cascade following joint injury and surgical repair [33, 35, 39]. To overcome issues with low residence time and negative local and systemic effects, MS glucocorticoid delivery platforms have entered development. One product, an already FDA-approved intra-articular microsphere injection of triamcinolone acetonide (TA), has demonstrated extended local drug release and improvements in pain, however no impact on tissue integrity [13, 45, 76]. We anticipated that DEX, due to its elevated anti-inflammatory potency relative to TA [16] and demonstrated strong pro-anabolic effects in cartilage cultures at low doses [12, 33, 59], would be a prime candidate to expedite and augment cartilage repair and restoration procedures that can be limited by graft durability and viability [32, 60, 71] as well as iatrogenic injury [2, 38, 71]. Overall, we accept our hypothesis that the DLMS implant provides chondroprotection and improved functional osteochondral integrity in both an in vitro cytokine-challenged environment (Study 1) and a preclinical model of cartilage restoration surgery (Study 2).
Study 1a confirmed DEX bioavailability not only to the bathing media (i.e. joint space), but also directly to the adjacent dense cartilage matrix. Glucocorticoid receptor binding, indicated by p57Kip2 expression, demonstrated a radially-dependent concentration of DEX, with cartilage immediately adjacent to the DLMS-laden core expressing the most intense staining that declined toward the edge of the construct (Fig. 3). This local delivery rendered special targeting mechanisms, such as conjugating with avidin [9], unnecessary.
In Study 1b, engineered cartilage derived from adult canine chondrocytes reached native levels for Young’s modulus, dynamic modulus, and GAG content by day 42 in culture [59]. And as anticipated, EY and GAG/ww were not significantly affected by IL insult in the DLMS group, indicating that the carrier was chondroprotective to these mature constructs in vitro. Meanwhile, specimens without DEX supplementation (ULMS), a positive control for IL-induced cartilage degradation [64], decreased significantly in EY and GAG content when subjected to IL insult. ULMS+IL specimens had significantly lower EY than CTL ULMS, which was likely due to the significant decrease in GAG/ww (Fig. 4A, C) in the periphery of the constructs (Fig. 5). Cytokine treatment did not significantly decrease collagen content in IL-treated groups, regardless of microsphere treatment, which is consistent with literature studies of two-week cytokine stimulation of engineered and explanted cartilage tissues [49, 79].
Counterintuitively, in the absence of IL, DLMS constructs had significantly lower COL/ww compared to ULMS. This was partially a byproduct of higher GAG content (i.e. wet weight) in the DLMS group, however absolute levels of collagen were still approximately 20% below pre-treatment values. In juvenile engineered tissues, there is evidence that collagen deposition later in culture can be higher without DEX [49], which may have played a role here. It is also possible that DEX levels accumulated too quickly in vitro and approached a deleterious level. DLMS groups ostensibly had variable DEX concentrations, which were dependent on release kinetics and media volume (estimated to be 1.6 μM DSP in the first 24 hours followed by 150 nM DSP per day) as well as drug clearance (half of media replaced 3x per week). However, even with accumulated DEX over the course of the two week culture, concentrations likely remained at least an order of magnitude below the peak seen in a clinical setting (7.7 mM; based on 4 mg, 1 ml injection [17]).
A potential limitation of Study 1b was the smaller average diameter of ULMS compared to DLMS (25 and 46 μm, respectively). Smaller ULMS have a higher surface area-to-volume ratio, potentially leading to faster water permeation and matrix degradation [23]. Although PLGA is considered biocompatible, increased acidic degradation products in ULMS may have caused local pH changes that artificially increased inflammation in those groups. In one in vitro study, 80 mg/ml predegraded PLGA microspheres changed pH of saline from 6.3 to 3.8 over 4 days [37]. However, Study 1b utilized a much lower mass of PLGA, approximately 140 μg/ml, likely lessening this effect. Furthermore, PLGA microspheres under 300 μm in diameter have been shown to have equivalent degradation in the core and outer surface for both in vitro and in vivo systems [3], so controlling for microsphere mass was expected to be more relevant to our studies. This expectation was ultimately borne out, as evidence of elevated baseline tissue degradation was not observed in ULMS samples.
In Study 2, DLMS carriers were formulated to deliver an estimated burst release of 15–75 μM DSP in the first 24 hours followed by 1.4–7.0 μM DSP per carrier per day (based on estimated 0.5–2.5 ml synovial fluid volume). An in vitro release study of actual DLMS implants confirmed this estimate. While it is anticipated that in vivo release kinetics would be expedited compared to in vitro [25], studies have shown that extended in vivo release (at least 70 days) still occurs [25, 67]. For PLGA-DEX microspheres, in vitro release is predictive of in vivo release kinetics [87]. Although expected to at times be higher than minimum values established to be chondroprotective (1 nM-10 μM) [52], we anticipated that concentrations would remain well below deleterious levels. The total dosage used in this study was estimated to be 0.63 mg DSP (3 implants per knee), whereas clinical injections contain 4 mg DSP.
At the terminal time point, the OATS-DLMS group showed superior knee function based on return to pre-surgery gait scores and improved lameness grades. Between-group differences in pain were not observed, likely due to the fact that OAT procedures are generally successful in restoring joint function [53]. Further benefits of low dose DEX were observed in OARSI cartilage score, with DLMS specimens more than twice as likely to be improved compared to controls. Examining sub-scores, DLMS were nearly three times more likely to have better chondrocyte scores and more than three times more likely to have better proteoglycan sub-scores than both CTL and INJ. This supported the results of Study 1b, which showed enhanced GAG/ww in the DLMS group.
In our studies, DLMS had a mean diameter of approximately 46 μm, which was similar to TA-loaded PLGA MS products on the market administered via intra-articular injection [46]. In advanced OA patients, these TA-MS injections provided better pain relief and longer joint residence time than bolus TA injection [45, 76]. However, TA-loaded MS did not affect cartilage pathology in this clinical trial [76] or an in vivo rat model of OA [67]. This may have been a result of TA toxicity; TA has been reported to be toxic to chondrocytes [29] and to significantly decrease the proliferative rate and inhibit chondrogenic differentiation at an equivalent DEX level established to be chondroprotective (1 μM TA ≈ 200 nM DEX) [52, 81]. Alternatively, free floating MS in the joint space may have led to increased joint friction and cartilage wear [28]. End point histological analyses of rats did not show visible TA-MS [67], indicating that free-floating MS may not remain in place. To prevent this, our DLMS implant was composed of agarose-encapsulated MS. Furthermore, DLMS carriers were constructed on a bone scaffold base to aid integration (Fig. 2B), ensuring that the carriers remained in place for the duration of the study (Fig. 9).
DLMS were more than twice as likely to have a better collagen sub-score than both CTL and INJ, which was further evidenced by pronounced picrosirius red staining. While contrary to the results of Study 1b, DEX at 100 nM is known to promote the osteoblast phenotype and in vivo bone formation by transplanted human osteoblasts on collagen sponges [84]. It should also be emphasized that in vitro studies were based only on activities of chondrocytes seeded into the construct, whereas in vivo experiments incorporated the contributions of a complex milieu of other joint tissues, including synovial- and bone marrow-derived stem and immune cells.
Meanwhile, chondrocyte and proteoglycan scores were not significantly likely to differ from CTL in the high dose DEX group (OATS-INJ). In a rabbit PTOA model, frequent injections of high dose DEX were chondroprotective over three weeks but had severe systemic side effects, including weight loss and organ necrosis [39]. We did not observe significant weight gain or loss for any dog in the study and necropsy results showed no evidence for systemic pathology. Furthermore, neither impairment of healing nor infections were observed in our studies, each of which can be undesired side effects of glucocorticoids [72].
Donor site morbidity is still a significant concern associated with the OAT procedure [4, 73]. Despite limited reports that describe filling donor sites with porous or solid plugs [15] and the availability of commercial products to backfill donor sockets after OATS, the standard of care is to leave the sockets empty [14,16]. Taken together with the expense of performing large preclinical animal studies, this preliminary study did not include donor site backfill with agarose-bone (DEX-free) implant controls but rather a clinically-relevant intra-articular dexamethasone injection group (OATS-INJ) and empty socket group (OATS-CTL). As we did not observe donor site morbidity in any of the experimental groups, we ascribe the enhanced tissue repair with DLMS implants to the dexamethasone released from microspheres. However, we acknowledge that the contribution of plugging the donor socket site cannot be completely ruled out and contend that the encouraging findings of the current study merit further investigation to more fully establish the benefits of local, sustained release of dexamethasone from within the joint on cartilage repair. Other DEX delivery methods, such as an intra-articular DLMS patch, are also feasible if concerns regarding donor site morbidity persist or if other cartilage restoration techniques, such as OCA, ACI, or MACI, are selected.
Cytokine levels in the synovial fluid were inconclusive regarding a potential anti-inflammatory effect. Counterintuitively, both IL-6 and MMP-3 concentrations were increased at the 1-month time point in the OATS-DLMS group relative to CTL and INJ (Fig. 7). This transient increase may have been due to local pH effects caused by PLGA degradation, however previous work has suggested that DEX-delivery largely attenuates this effect [87]. While elevated levels of these markers are typically associated with inflammation and arthritis [31, 65], in our study they were paired with the relative absence of synovitis and improved cartilage tissue quality in DLMS groups. IL-6 has been implicated as an anti-inflammatory mediator in OA, showing chondroprotective and anabolic effects [31, 82]. Meanwhile, MMP-2 and MMP-3 can be an indicator of wound healing early in repair [65]. Further, MMP-3 is mainly expressed by fibroblasts and endothelial cells [62], indicating that changes to the synovium are in fact contributing to observations in Study 2. Previous in vitro work has suggested that DEX modulates synovium behavior to IL-1 insult [78], however the role of MMPs on synovium function is unclear. Ultimately, additional studies will be required to elucidate the mechanism of DEX-induced matrix remodeling in vivo.
It is unlikely that increased cytokine concentration was a pro-inflammatory reaction to agarose, which is widely reported to be biocompatible [22, 70]. We speculate that the bone substrate used with the DLMS carrier may have contributed to elevated levels, however devitalized bone has been successfully used by others [80]. We previously reported negative in vitro effects of bone with juvenile chondrocytes, but did not see any with adult chondrocytes [48]. Overall, conclusions based on measures of inflammation (synovitis and cytokines) were potentially limited by having multiple repairs per knee. If only one DLMS implant failed, one might expect to observe a global spike in synovial fluid cytokines while simultaneously seeing improved local tissue quality at other repair sites. To capitalize on the positive effects of DEX, future studies may utilize other porous base scaffolds such as titanium [10].
This preliminary in vivo experiment was potentially limited by sample size, which was improved by creating multiple defects per knee. Based on previous canine studies in our laboratory, we determined a priori that a sample size of approximately 15 repairs per group would be required to achieve a power of at least 0.8. In addition, the 6-month end point provides only an initial assessment of healing, integration, and cartilage restoration for functional outcomes with longer term studies needed prior to clinical application. Nevertheless, trends and significant between-group differences were observed for the outcome measures, providing evidence for expedited early repair and providing rationale for long-term studies of sustained, targeted low-dose DEX delivery to the joint.
5. Conclusions
Using in vitro models (Study 1) and confirmatory in vivo models (Study 2) of cartilage restoration provide guidance for optimizing localized DEX delivery strategies to maximize cartilage graft survival and function. Utilizing a targeted DLMS carrier implant, we observed in vitro chondroprotection in the presence of IL-1-induced degradation and improved in vivo functional outcomes. These improved outcomes were correlated with superior histological cartilage scores and minimal-to-no comorbidity, but not necessarily a dampened inflammatory response. DEX, a potent glucocorticoid with concomitant anti-catabolic and pro-anabolic effects on cartilage, may serve as an adjuvant for other cartilage repair strategies, including allografts, microfracture, ACI, MACI.
Statement of Significance.
Articular cartilage defects are a common source of joint pain and dysfunction. Effective treatment of these injuries may prevent the progression of osteoarthritis and reduce the need for total joint replacement. Dexamethasone, a potent glucocorticoid with concomitant anti-catabolic and pro-anabolic effects on cartilage, may serve as an adjuvant for a variety of repair strategies. Utilizing a dexamethasone-loaded osteochondral implant with controlled release characteristics, we demonstrated in vitro chondroprotection in the presence of IL-1-induced degradation and improved in vivo functional outcomes. These improved outcomes were correlated with superior histological cartilage scores and minimal-to-no comorbidity, which is a risk with high dose dexamethasone injections. Using this model of cartilage restoration, we have for the first time shown the application of targeted, low-dose dexamethasone for improved healing in a preclinical model of focal defect repair.
Acknowledgments
We thank Chantelle Bozynski and Keiichi Kuroki for conducting histology and clinical scoring. Thank you to Brendan Roach for help with microsphere fabrication and characterization. Thank you to Krista Durney for help with study coordination, preparation and assessment of osteochondral implants.
Disclosures
This work was supported by grants from the Musculoskeletal Transplant Foundation (AMS, GAA, JLC, CTH), NIH (XEG, AMS, GAA, KGM, JLC, CTH), and NYSTEM predoctoral fellowship (RMS). The funding sources had no role in the study design, data collection, data analysis, or in the preparation and submission of the manuscript. One or more authors have received honorarium for being deputy editor of Journal of Orthopaedic Research (CTH), royalties from Allosource (GAA, CTH), and pending license royalties from MTF (CTH). JLC discloses the following financial interests: paid consultant for Artelon, IP royalties; paid consultant; paid presenter or speaker; research support from Arthrex, Inc, research support from ConforMIS, the Coulter Foundation, and DePuy, A Johnson & Johnson Company, paid consultant; research support from Eli Lilly, editorial or governing board for Journal of Knee Surgery, research support from Merial, board or committee member at Midwest Transplant Network, board or committee member; research support from Musculoskeletal Transplant Foundation, paid consultant for Schwartz Biomedical, Publishing royalties, financial or material support from Thieme, research support from the U.S. Department of Defense, and research support from Zimmer-Biomet. The remaining authors (RMS, AJL, ART, KMD, SSH, YH, XEG, AMS, KGM) certify that they have no commercial associations that might pose a conflict of interest in connection with the submitted article.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allen DB. GROWTH SUPPRESSION BY GLUCOCORTICOID THERAPY. Endocrinol. Metab. Clin. North Am 1996;25:699–717. [DOI] [PubMed] [Google Scholar]
- 2.Amin AK, Simpson AHRW, Hall AC. Iatrogenic articular cartilage injury: the elephant in the operating theatre. Bone Jt. J 2017;99-B:1555–1556. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev 2012;64:72–82. [DOI] [PubMed] [Google Scholar]
- 4.Andrade R, Vasta S, Pereira R, Pereira H, Papalia R, Karahan M, Oliveira JM, Reis RL, Espregueira-Mendes J. Knee donor-site morbidity after mosaicplasty - a systematic review. J. Exp. Orthop 2016;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anon. Dexamethasone Sodium Phosphate Injection, USP: 2014. [Google Scholar]
- 6.Anon. DailyMed - CORTAREN CORTICOSTEROID/ANTI-INFLAMMATORY SYSTEM- dexamethasone sodium phosphate. Available at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=84fffebe-8468-42be-adab-6220e737499a [Accessed May 31, 2019].
- 7.Armstrong RD, English J, Gibson T, Chakraborty J, Marks V. Serum methylprednisolone levels following intra-articular injection of methylprednisolone acetate. Ann. Rheum. Dis 1981;40:571–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Backes JR, Bentley JC, Politi JR, Chambers BT. Dexamethasone Reduces Length of Hospitalization and Improves Postoperative Pain and Nausea After Total Joint Arthroplasty: A Prospective, Randomized Controlled Trial. J. Arthroplasty 2013;28:11–17. [DOI] [PubMed] [Google Scholar]
- 9.Bajpayee A, Scheu M, Varady N, Yannatos I, Brown L, Krishnan Y, Fitzsimons T, Bhattacharya P, Frank E, Grodzinsky A, Porter R. Sustained intra-cartilage delivery of low dose dexamethasone using a cationic carrier for treatment of post traumatic osteoarthritis. Eur. Cell. Mater 2017;34:341–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bal BS, Rahaman MN, Jayabalan P, Kuroki K, Cockrell MK, Yao JQ, Cook JL. In vivo outcomes of tissue-engineered osteochondral grafts. J. Biomed. Mater. Res. B Appl. Biomater 2010;93B:164–174. [DOI] [PubMed] [Google Scholar]
- 11.Berthelot J-M, Le Goff B, Maugars Y. Side effects of corticosteroid injections: What’s new? Joint Bone Spine. 2013;80:363–367. [DOI] [PubMed] [Google Scholar]
- 12.Bian L, Stoker AM, Marberry KM, Ateshian GA, Cook JL, Hung CT. Effects of Dexamethasone on the Functional Properties of Cartilage Explants During Long-Term Culture. Am. J. Sports Med 2010;38:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bodick N, Lufkin J, Willwerth C, Kumar A, Bolognese J, Schoonmaker C, Ballal R, Hunter D, Clayman M. An Intra-Articular, Extended-Release Formulation of Triamcinolone Acetonide Prolongs and Amplifies Analgesic Effect in Patients with Osteoarthritis of the Knee: A Randomized Clinical Trial. J. Bone Jt. Surg.-Am. Vol 2015;97:877–888. [DOI] [PubMed] [Google Scholar]
- 14.Bozynski CC, Kuroki K, Stannard JP, Smith PA, Stoker AM, Cook CR, Cook JL. Evaluation of Partial Transection versus Synovial Debridement of the ACL as Novel Canine Models for Management of ACL Injuries. J. Knee Surg 2015;28:404–410. [DOI] [PubMed] [Google Scholar]
- 15.Butoescu N, Seemayer CA, Foti M, Jordan O, Doelker E. Dexamethasone-containing PLGA superparamagnetic microparticles as carriers for the local treatment of arthritis. Biomaterials. 2009;30:1772–1780. [DOI] [PubMed] [Google Scholar]
- 16.Buttgereit F, Silva JAPD, Boers M, Burmester G-R, Cutolo M, Jacobs J, Kirwan J, Köhler L, van Riel P, Vischer T, Bijlsma JWJ. Standardised nomenclature for glucocorticoid dosages and glucocorticoid treatment regimens: current questions and tentative answers in rheumatology. Ann. Rheum. Dis 2002;61:718–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardone DA. Joint and Soft Tissue Injection. 2002;66:6. [PubMed] [Google Scholar]
- 18.Cederlöf S, Jonson G. Intraarticular prednisolone injection for osteoarthritis of the knee. A double blind test with placebo. Acta Chir. Scand 1966;132:532–537. [PubMed] [Google Scholar]
- 19.Chang EY, Pallante-Kichura AL, Bae WC, Du J, Statum S, Wolfson T, Gamst AC, Cory E, Amiel D, Bugbee WD, Sah RL, Chung CB. Development of a Comprehensive Osteochondral Allograft MRI Scoring System (OCAMRISS) With Histopathologic, Micro–Computed Tomography, and Biomechanical Validation. CARTILAGE. 2014;5:16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chrysis D, Ritzen EM, Sävendahl L. Growth retardation induced by dexamethasone is associated with increased apoptosis of the growth plate chondrocytes. J. Endocrinol 2003;176:331–337. [DOI] [PubMed] [Google Scholar]
- 21.Cook JL, Kuroki K, Visco D, Pelletier J-P, Schulz L, Lafeber. The OARSI histopathology initiative – recommendations for histological assessments of osteoarthritis in the dog. Osteoarthritis Cartilage. 2010;18:S66–S79. [DOI] [PubMed] [Google Scholar]
- 22.Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: An overview of biomedical applications. J. Controlled Release. 2012;161:505–522. [DOI] [PubMed] [Google Scholar]
- 23.Dawes GJS, Fratila-Apachitei LE, Mulia K, Apachitei I, Witkamp G-J, Duszczyk J. Size effect of PLGA spheres on drug loading efficiency and release profiles. J. Mater. Sci. Mater. Med 2009;20:1089–1094. [DOI] [PubMed] [Google Scholar]
- 24.Djouad F, Rackwitz L, Song Y, Janjanin S, Tuan RS. ERK1/2 Activation Induced by Inflammatory Cytokines Compromises Effective Host Tissue Integration of Engineered Cartilage. Tissue Eng. Part A 2009;15:2825–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doty AC, Weinstein DG, Hirota K, Olsen KF, Ackermann R, Wang Y, Choi S, Schwendeman SP. Mechanisms of in vivo release of triamcinolone acetonide from PLGA microspheres. J. Controlled Release. 2017;256:19–25. [DOI] [PubMed] [Google Scholar]
- 26.Dragoo JL, Danial CM, Braun HJ, Pouliot MA, Kim HJ. The chondrotoxicity of single-dose corticosteroids. Knee Surg. Sports Traumatol. Arthrosc 2012;20:1809–1814. [DOI] [PubMed] [Google Scholar]
- 27.Duerr S, Stremme S, Soeder S, Bau B, Aigner T. MMP-2/gelatinase A is a gene product of human adult articular chondrocytes and is increased in osteoarthritic cartilage. :6. [PubMed]
- 28.Estell EG. Modulation of Synovium Mechanobiology and Tribology in the Osteoarthritic Environment. 2018.
- 29.Euppayo T, Siengdee P, Buddhachat K, Pradit W, Chomdej S, Ongchai S, Nganvongpanit K. In vitro effects of triamcinolone acetonide and in combination with hyaluronan on canine normal and spontaneous osteoarthritis articular cartilage. Vitro Cell. Dev. Biol. - Anim 2016;52:723–735. [DOI] [PubMed] [Google Scholar]
- 30.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007;39:175–191. [DOI] [PubMed] [Google Scholar]
- 31.Gaissmaier C, Koh JL, Weise K. Growth and differentiation factors for cartilage healing and repair. Injury. 2008;39:88–96. [DOI] [PubMed] [Google Scholar]
- 32.Gomoll AH, Filardo G, Almqvist FK, Bugbee WD, Jelic M, Monllau JC, Puddu G, Rodkey WG, Verdonk P, Verdonk R, Zaffagnini S, Marcacci M. Surgical treatment for early osteoarthritis. Part II: allografts and concurrent procedures. Knee Surg. Sports Traumatol. Arthrosc 2012;20:468–486. [DOI] [PubMed] [Google Scholar]
- 33.Grodzinsky AJ, Wang Y, Kakar S, Vrahas MS, Evans CH. Intra-articular dexamethasone to inhibit the development of post-traumatic osteoarthritis. J. Orthop. Res 2017;35:406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Habib GS. Systemic effects of intra-articular corticosteroids. Clin. Rheumatol 2009;28:749–756. [DOI] [PubMed] [Google Scholar]
- 35.Heard BJ, Barton KI, Chung M, Achari Y, Shrive NG, Frank CB, Hart DA. Single intra-articular dexamethasone injection immediately post-surgery in a rabbit model mitigates early inflammatory responses and post-traumatic osteoarthritis-like alterations. J. Orthop. Res 2015;33:1826–1834. [DOI] [PubMed] [Google Scholar]
- 36.Hehenberger K, Kratz G, Hansson A, Brismar K. Fibroblasts derived from human chronic diabetic wounds have a decreased proliferation rate, which is recovered by the addition of heparin. J. Dermatol. Sci 1998;16:144–151. [DOI] [PubMed] [Google Scholar]
- 37.Hickey T, Kreutzer D, Burgess DJ, Moussy F. In vivo evaluation of a dexamethasone/PLGA microsphere system designed to suppress the inflammatory tissue response to implantable medical devices. J. Biomed. Mater. Res 2002;61:180–187. [DOI] [PubMed] [Google Scholar]
- 38.Hooiveld M, Roosendaal G, Wenting M, van den Berg M, Bijlsma J, Lafeber F. Short-Term Exposure of Cartilage to Blood Results in Chondrocyte Apoptosis. Am. J. Pathol 2003;162:943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huebner KD, Shrive NG, Frank CB. Dexamethasone inhibits inflammation and cartilage damage in a new model of post-traumatic osteoarthritis. J. Orthop. Res 2014;32:566–572. [DOI] [PubMed] [Google Scholar]
- 40.Hung CT, Lima EG, Mauck RL, Taki E, LeRoux MA, Lu HH, Stark RG, Guo XE, Ateshian GA. Anatomically shaped osteochondral constructs for articular cartilage repair. J. Biomech 2003;36:1853–1864. [DOI] [PubMed] [Google Scholar]
- 41.Hunter CJ, Levenston ME. Maturation and integration of tissue-engineered cartilages within an in vitro defect repair model. Tissue Eng. 2004;10:736–746. [DOI] [PubMed] [Google Scholar]
- 42.Ikeuchi M, Kamimoto Y, Izumi M, Fukunaga K, Aso K, Sugimura N, Yokoyama M, Tani T. Effects of dexamethasone on local infiltration analgesia in total knee arthroplasty: a randomized controlled trial. Knee Surg. Sports Traumatol. Arthrosc 2014;22:1638–1643. [DOI] [PubMed] [Google Scholar]
- 43.Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21:2475–2490. [DOI] [PubMed] [Google Scholar]
- 44.Kloth LC. Electrical Stimulation for Wound Healing: A Review of Evidence From In Vitro Studies, Animal Experiments, and Clinical Trials. Int. J. Low. Extrem. Wounds 2005;4:23–44. [DOI] [PubMed] [Google Scholar]
- 45.Kraus VB, Conaghan PG, Aazami HA, Mehra P, Kivitz AJ, Lufkin J, Hauben J, Johnson JR, Bodick N. Synovial and systemic pharmacokinetics (PK) of triamcinolone acetonide (TA) following intra-articular (IA) injection of an extended-release microsphere-based formulation (FX006) or standard crystalline suspension in patients with knee osteoarthritis (OA). Osteoarthritis Cartilage. 2018;26:34–42. [DOI] [PubMed] [Google Scholar]
- 46.Kumar A, Bendele AM, Blanks RC, Bodick N. Sustained efficacy of intra-articular FX006 in a rat model of osteoarthritis. 2012. Available at: https://linkinghub.elsevier.com/retrieve/pii/S1063458412005705 [Accessed May 31, 2019]. [DOI] [PubMed]
- 47.Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, Tuan RS, Ateshian GA, Hung CT. The Beneficial Effect of Delayed Compressive Loading on Tissue-Engineered Cartilage Constructs Cultured with TGF-β3. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc 2007;15:1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lima EG, Grace Chao P, Ateshian GA, Bal BS, Cook JL, Vunjak-Novakovic G, Hung CT. The effect of devitalized trabecular bone on the formation of osteochondral tissue-engineered constructs. Biomaterials. 2008;29:4292–4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lima EG, Tan AR, Tai T, Bian L, Stoker AM, Ateshian GA, Cook JL, Hung CT. Differences in Interleukin-1 Response Between Engineered and Native Cartilage. Tissue Eng. Part A 2008;14:1721–1730. [DOI] [PubMed] [Google Scholar]
- 50.Liu XS, Sajda P, Saha PK, Wehrli FW, Bevill G, Keaveny TM, Guo XE. Complete Volumetric Decomposition of Individual Trabecular Plates and Rods and Its Morphological Correlations With Anisotropic Elastic Moduli in Human Trabecular Bone. J. Bone Miner. Res 2007;23:223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lotz M, Terkeltaub R, Villiger PM. Cartilage and joint inflammation. Regulation of IL-8 expression by human articular chondrocytes. J. Immunol 1992;148:466–473. [PubMed] [Google Scholar]
- 52.Lu YC, Evans CH, Grodzinsky AJ. Effects of short-term glucocorticoid treatment on changes in cartilage matrix degradation and chondrocyte gene expression induced by mechanical injury and inflammatory cytokines. Arthritis Res. Ther 2011;13:R142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma H-L, Hung S-C, Wang S-T, Chang M-C, Chen T-H. Osteochondral autografts transfer for post-traumatic osteochondral defect of the knee—2 to 5 years follow-up. Injury. 2004;35:1286–1292. [DOI] [PubMed] [Google Scholar]
- 54.Mauck RL, Soltz MA, Wang CCB, Wong DD, Chao P-HG, Valhmu WB, Hung CT, Ateshian GA. Functional Tissue Engineering of Articular Cartilage Through Dynamic Loading of Chondrocyte-Seeded Agarose Gels. J. Biomech. Eng 2000;122:252–260. [DOI] [PubMed] [Google Scholar]
- 55.McAlindon TE, LaValley MP, Harvey WF, Price LL, Driban JB, Zhang M, Ward RJ. Effect of Intra-articular Triamcinolone vs Saline on Knee Cartilage Volume and Pain in Patients With Knee Osteoarthritis: A Randomized Clinical Trial. JAMA. 2017;317:1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Myers SL, Brandt KD, Eilam O. Even low-grade synovitis significantly accelerates the clearance of protein from the canine knee: implications for measurement of synovial fluid “markers” of osteoarthritis. Arthritis Rheum. 1995;38:1085–1091. [DOI] [PubMed] [Google Scholar]
- 57.Nade S, Newbold PJ. Factors determining the level and changes in intra-articular pressure in the knee joint of the dog. J. Physiol. 1983;338:21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neustadt DH. Intra-articular injections for osteoarthritis of the knee. Cleve. Clin. J. Med 2006;73:897–8, 901,–4, 906–11. [DOI] [PubMed] [Google Scholar]
- 59.Ng KW, Lima EG, Bian L, O’Conor CJ, Jayabalan PS, Stoker AM, Kuroki K, Cook CR, Ateshian GA, Cook JL, Hung CT. Passaged Adult Chondrocytes Can Form Engineered Cartilage with Functional Mechanical Properties: A Canine Model. Tissue Eng. Part A 2010;16:1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nover AB, Stefani RM, Lee SL, Ateshian GA, Stoker AM, Cook JL, Hung CT. Long-term storage and preservation of tissue engineered articular cartilage. J. Orthop. Res 2015;34:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Obradovic B, Martin I, Padera RF, Treppo S, Freed LE, Vunjak-Navakovic G. Integration of engineered cartilage. J. Orthop. Res 2001;19:1089–1097. [DOI] [PubMed] [Google Scholar]
- 62.Okada Y, Shinmei M, Tanaka O, Naka K, Kimura A, Nakanishi I, Bayliss MT, Iwata K, Nagase H. Localization of matrix metalloproteinase 3 (stromelysin) in osteoarthritic cartilage and synovium. Lab. Investig. J. Tech. Methods Pathol 1992;66:680–690. [PubMed] [Google Scholar]
- 63.Roach BL. Modulation of the in vitro mechanical and chemical environment for the optimization of tissue-engineered articular cartilage. 2017.
- 64.Roach BL, Kelmendi-Doko A, Balutis EC, Marra KG, Ateshian GA, Hung CT. Dexamethasone Release from Within Engineered Cartilage as a Chondroprotective Strategy Against Interleukin-1α. Tissue Eng. Part A 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rose BJ, Kooyman DL. A Tale of Two Joints: The Role of Matrix Metalloproteases in Cartilage Biology. Dis. Markers. 2016;2016. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4961809/ [Accessed October 21, 2019]. [DOI] [PMC free article] [PubMed]
- 66.Rubin JP, DeFail A, Rajendran N, Marra KG. Encapsulation of adipogenic factors to promote differentiation of adipose-derived stem cells. J. Drug Target 2009;17:207–215. [DOI] [PubMed] [Google Scholar]
- 67.Rudnik-Jansen I, Colen S, Berard J, Plomp S, Que I, van Rijen M, Woike N, Egas A, van Osch G, van Maarseveen E, Messier K, Chan A, Thies J, Creemers L. Prolonged inhibition of inflammation in osteoarthritis by triamcinolone acetonide released from a polyester amide microsphere platform. J. Controlled Release 2017;253:64–72. [DOI] [PubMed] [Google Scholar]
- 68.Samuelsson MKR, Pazirandeh A, Davani B, Okret S. p57Kip2, a Glucocorticoid-Induced Inhibitor of Cell Cycle Progression in HeLa Cells. Mol. Endocrinol 1999;13:1811–1822. [DOI] [PubMed] [Google Scholar]
- 69.Selmi TAS, Neyret P, Verdonk PCM, Barnouin L. Autologous Chondrocyte Transplantation in Combination With an Alginate-Agarose Based Hydrogel (Cartipatch). Tech. Knee Surg 2007;6:253–258. [Google Scholar]
- 70.Selmi TAS, Verdonk P, Chambat P, Dubrana F, Potel J-F, Barnouin L, Neyret P. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: OUTCOME AT TWO YEARS. J. Bone Joint Surg. Br 2008;90-B:597–604. [DOI] [PubMed] [Google Scholar]
- 71.Sgaglione N, Kerker J. Autologous Osteochondral Transplantation. OKOJ. 2008;6:22. [Google Scholar]
- 72.Sherman SL, James C, Stoker AM, Cook CR, Khazai RS, Flood DL, Cook JL. In Vivo Toxicity of Local Anesthetics and Corticosteroids on Chondrocyte and Synoviocyte Viability and Metabolism. CARTILAGE. 2015;6:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shimozono Y, Seow D, Yasui Y, Fields K, Kennedy JG. Knee-to-Talus Donor-Site Morbidity Following Autologous Osteochondral Transplantation: A Meta-Analysis with Best-case and Worst-case Analysis. Clin. Orthop 2019;477:1915–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silverstein AM, Stefani RM, Sobczak E, Tong EL, Attur MG, Shah RP, Bulinski JC, Ateshian GA, Hung CT. Toward understanding the role of cartilage particulates in synovial inflammation. Osteoarthritis Cartilage. 2017;25:1353–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spalazzi JP, Dagher E, Doty SB, Guo XE, Rodeo SA, Lu HH. In vivo evaluation of a multiphased scaffold designed for orthopaedic interface tissue engineering and soft tissue-to-bone integration. J. Biomed. Mater. Res. A 2008;86A:1–12. [DOI] [PubMed] [Google Scholar]
- 76.Spitzer AI, Richmond JC, Kraus VB, Gomoll A, Jones DG, Huffman KM, Peterfy C, Cinar A, Lufkin J, Kelley SD. Safety and Efficacy of Repeat Administration of Triamcinolone Acetonide Extended-release in Osteoarthritis of the Knee: A Phase 3b, Open-label Study. Rheumatol. Ther 2019;6:109–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stannus O, Jones G, Cicuttini F, Parameswaran V, Quinn S, Burgess J, Ding C. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage. 2010;18:1441–1447. [DOI] [PubMed] [Google Scholar]
- 78.Stefani RM, Halder SS, Estell EG, Lee AJ, Silverstein AM, Sobczak E, Chahine NO, Ateshian GA, Shah RP, Hung CT. A Functional Tissue-Engineered Synovium Model to Study Osteoarthritis Progression and Treatment. Tissue Eng. Part A 2018. Available at: https://www.liebertpub.com/doi/full/10.1089/ten.TEA.2018.0142 [Accessed November 16, 2018]. [DOI] [PMC free article] [PubMed]
- 79.Tan AR, VandenBerg CD, Attur M, Abramson SB, Knight MM, Bulinski JC, Ateshian GA, Cook JL, Hung CT. Cytokine preconditioning of engineered cartilage provides protection against interleukin-1 insult. Arthritis Res. Ther 2015;17:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tognana E, Padera RF, Chen F, Vunjak-Novakovic G, Freed LE. Development and remodeling of engineered cartilage-explant composites in vitro and in vivo. Osteoarthritis Cartilage. 2005;13:896–905. [DOI] [PubMed] [Google Scholar]
- 81.Weiss A, Livne E, Silbermann M. Glucocorticoid hormone adversely affects the growth and regeneration of cartilage in vitro. Growth Dev. Aging GDA. 1988;52:67–75. [PubMed] [Google Scholar]
- 82.Westacott CI, Sharif M. Cytokines in osteoarthritis: Mediators or markers of joint destruction? Semin. Arthritis Rheum. 1996;25:254–272. [DOI] [PubMed] [Google Scholar]
- 83.Wilusz RE, Weinberg JB, Guilak F, McNulty AL. Inhibition of integrative repair of the meniscus following acute exposure to interleukin-1 in vitro. J. Orthop. Res 2008;26:504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yamanouchi K, Satomura K, Gotoh Y, Kitaoka E, Tobiume S, Kume K, Nagayama M. Bone Formation by Transplanted Human Osteoblasts Cultured Within Collagen Sponge with Dexamethasone In Vitro. J. Bone Miner. Res 2001;16:857–867. [DOI] [PubMed] [Google Scholar]
- 85.Yang KGA, Saris D b. f., Verbout A j., Creemers L b., Dhert W j. a. The Effect of Synovial Fluid from Injured Knee Joints on in Vitro Chondrogenesis. Tissue Eng. 2006;12:2957–2964. [DOI] [PubMed] [Google Scholar]
- 86.Younes M, Neffati F, Touzi M, Hassen-Zrour S, Fendri Y, Béjia I, Ben Amor A, Bergaoui N, Najjar MF. Systemic effects of epidural and intra-articular glucocorticoid injections in diabetic and non-diabetic patients. Joint Bone Spine. 2007;74:472–476. [DOI] [PubMed] [Google Scholar]
- 87.Zolnik BS, Burgess DJ. Evaluation of in vivo–in vitro release of dexamethasone from PLGA microspheres. J. Controlled Release 2008;127:137–145. [DOI] [PubMed] [Google Scholar]