Abstract
Childhood abuse confers risk for psychopathology and pathophysiology in midlife through intermediate pathways that remain unclear. Systemic inflammation was tested in the present study as one pathway that may link physical abuse in childhood to the adult functioning of corticolimbic brain circuits broadly implicated in risk for poor mental and physical health. Midlife adults (N = 303; 30–51 years of age; 149 women without psychiatric, immune, or cardiovascular diagnoses provided retrospective reports of childhood physical abuse. Functional connectivity between corticolimbic brain areas (amygdala, hippocampus, ventromedial prefrontal cortex [vmPFC], anterior cingulate cortex [ACC]) was measured at rest using functional magnetic resonance imaging. Circulating levels of interleukin(IL)-6, a pro-inflammatory cytokine previously linked to childhood abuse and corticolimbic functionality were measured via blood draw. Consistent with prior studies, retrospectively reported childhood physical abuse was associated positively with circulating IL-6, and negatively with connectivity between the amygdala and vmPFC. IL-6 was also associated negatively with several corticolimbic functional connections, including amygdala-vmPFC connectivity. Moreover, path analyses revealed an indirect effect of IL-6 that partially explained the association between childhood physical abuse and adult amygdala-vmPFC connectivity. Consistent with recent neurobiological models of early life influences on disease risk across the lifespan, associations between childhood physical abuse and adulthood corticolimbic circuit functionality may be partially explained by inflammatory processes
Keywords: amygdala, childhood abuse, functional connectivity, inflammation, interleukin-6, ventromedial prefrontal cortex
1. INTRODUCTION
Physical abuse in childhood is a form of early-life adversity that confers risk for poor mental and physical health across the life course. Specifically, individuals who experience childhood physical abuse are more likely to develop neuropsychiatric disorders, including major depressive disorder and post-traumatic stress disorder in adulthood (Green et al., 2010; McLaughlin, Conron, Koenen, & Gilman, 2010). In addition, childhood physical abuse not only associates with premature death (E. Chen, Turiano, Mroczek, & Miller, 2016), but also with chronic physical illnesses in adulthood that are comorbid with poor mental health. The latter include coronary heart disease, hypertension, and Type II diabetes (Basu, McLaughlin, Misra, & Koenen, 2017; Wegman & Stetler, 2009). Presently unclear however, are the neurobiological pathways that may link childhood physical abuse to poor health outcomes later in life.
One speculation is that childhood physical abuse confers risk for poor health later in life by influencing the development of corticolimbic brain systems; namely, the amygdala, hippocampus, anterior cingulate cortex (ACC), and ventromedial prefrontal cortex (vmPFC) (Teicher, Samson, Anderson, & Ohashi, 2016). These corticolimbic brain systems are implicated in detecting and maintaining vigilance towards threatening information, generating and regulating emotions, and encoding contextual and self-relevant memories (Davis & Whalen, 2001; Hiser & Koenigs, 2018; Price & Drevets, 2012; Simons & Spiers, 2003). From an evolutionary perspective, corticolimbic alterations following childhood physical abuse may be adaptive in the short term (e.g., serving to detect danger and avoid harm), yet maladaptive if maintained in the long-term or later life [e.g., resulting in the contextually inappropriate maintenance of hypervigilance and dysphoric mood, (Takesian & Hensch, 2013)]. In support of this speculation, animal models of early-life caregiver maltreatment [e.g., limited nesting environments, (Raineki, Moriceau, & Sullivan, 2010)] increase offspring dendritic spine numbers, in-vitro synaptic responses, and c-Fos expression in the amygdala (Guadagno, Wong, & Walker, 2018; Raineki, Cortés, Belnoue, & Sullivan, 2012). These maltreatment paradigms also reduce offspring neural activity in the mPFC (Rincón-Cortés & Sullivan, 2016). Findings from human brain imaging studies of adults also show associations of reported childhood physical abuse with alterations in the structure (Bremner et al., 1997; Gorka, Hanson, Radtke, & Hariri 2014; Tomoda et al., 2009) and function (Banihashemi, Sheu, Midei, & Gianaros, 2015; Grant, Cannistraci, Hollon, Gore, & Shelton, 2011; Hein & Monk, 2017; Redlich et al., 2015) of corticolimbic areas (Cisler et al., 2013; Teicher, Anderson Ohashi & Polcari, 2014). Moreover, childhood physical abuse associates with decreased functional connectivity (i.e., cross-correlated activity) between limbic (amygdala, hippocampus) and cortical (PFC, ACC) structures in both animal (Guadagno Kang, et al., 2018; Yan et al., 2017) and human (Birn, Patriat, Phillips, Germain, & Herringa, 2014; Grant et al., 2014; Herringa et al., 2013) studies. Reduced functional connectivity in the above corticolimbic brain systems in turn has been linked to negative affective states and neuropsychiatric disorders [e.g., major depression, social anxiety, (Brown et al., 2014; Hahn et al., 2011; Tang et al., 2013)]. Finally, similar corticolimbic brain alterations associated with childhood abuse have been reported in samples with and without neuropsychiatric disorders, suggesting these alterations may reflect a latent neurobiological or pre-clinical vulnerability for psychopathology (McCrory & Viding, 2015; Teicher & Samson, 2013). In light of these observations, however, there is limited understanding of the intermediate pathways that link childhood physical abuse to corticolimbic circuit function in adulthood. Here, we test the putative role of peripheral inflammatory physiology.
To elaborate, it has been proposed that psychosocial and environmental conditions in childhood affect the developing immune system, and exposure to adverse conditions such as childhood physical abuse sensitizes peripheral immune cells to promote systemic inflammation, manifesting as a so-called “pro-inflammatory phenotype” (G. E. Miller, Chen, & Parker, 2011). In animal models, early life stress upregulates pro-inflammatory gene transcription in peripheral monocytes (S. W. Cole et al., 2012) and increases circulating markers of systemic inflammation (Raineki et al., 2017; Wieck, Andersen, & Brenhouse, 2013). Similarly in epidemiological studies, childhood abuse associates with elevated circulating markers of inflammation (M. Chen & Lacey, 2018; Danese, Pariante, Caspi, Taylor, & Poulton, 2007; Matthews, Chang, Thurston, & Bromberger, 2014), inflammatory responses to stress (Carpenter et al., 2010; Pace et al., 2006), and the co-occurrence of systemic inflammation and depression (Danese et al., 2008) in adulthood. Notably, a recent meta-analysis reported reliable associations in particular between childhood physical abuse and elevations of the pro-inflammatory cytokine, interleukin(IL)-6, in adulthood (Baumeister, Akhtar, Ciufolini, Pariante, & Mondelli, 2016). In parallel, animal models show that IL-6 in the periphery can directly and indirectly promote central neuroinflammatory processes that impact functional neural activity, neurogenesis, and long-term potentiation (Ekdahl, Claasen, Bonde, Kokaia, & Lindvall, 2003; Frenois et al., 2007; Katsuki et al., 1990). In contrast, other circulating markers of systemic inflammation, such as the acute phase reactant C-reactive protein (CRP), are generally thought to be incapable of directly accessing the central nervous system unless there are disruptions in blood-brain barrier permeability or clinical manifestations of pathology and clinically elevated circulating levels_(Elwood et al., 2017; Kuhlmann et al., 2009). Human laboratory and brain imaging studies that experimentally stimulate increases in circulating levels of IL-6 [e.g., using endotoxin, (Fong et al., 1989)], report decreased functional connectivity within amygdala-PFC and hippocampal-PFC circuits (Harrison et al., 2009; Kraynak, Marsland, Wager, & Gianaros, 2018). In sum, peripheral inflammatory processes, in particular those involving IL-6, reliably associate with childhood physical abuse, and are implicated in corticolimbic functionality, yet their contribution to the latter in the context of childhood physical abuse is presently unclear.
To review, parallel lines of evidence suggest physical abuse in childhood relates to both elevated systemic inflammation and reduced corticolimbic functionality in adulthood. In view of this evidence, Nusslock and Miller formulated the neuroimmune network hypothesis, which posited that “early-life adversity amplifies crosstalk between peripheral inflammation and neural circuitries subserving threat-, reward- and executive control-related processes” [cf. (Nusslock & Miller, 2016)]. At present, however, what is unclear is the extent to which systemic inflammation explains associations between childhood physical abuse and brain connectivity phenotypes previously linked to poor health in adulthood in line with a neuroimmune network framework.
Accordingly, we studied a community sample of midlife adults to test the hypotheses that reports of childhood physical abuse would be associated with (a) decreased functional connectivity at rest between corticolimbic brain areas—focusing on amygdala-PFC and hippocampal-PFC connections, as well as (b) elevated circulating IL-6. Hence, we aimed to replicate prior findings in these areas using a community sample of midlife adults. We further hypothesized that elevated IL-6 would be associated with decreased corticolimbic connectivity. Finally, path analyses tested the hypothesis that IL-6 would partly explain the statistical association between childhood physical abuse and corticolimbic connectivity. Ancillary analyses examined the specificity of our findings to childhood physical abuse and IL-6, and tested for additional associations with individual differences in negative affect and subclinical depressive symptoms.
2. METHODS
2.1. Participants
Participants were from the Pittsburgh Imaging Project (PIP), comprising a community sample of healthy midlife adults (N = 331; age 30 – 51) recruited using mass mailings to residents of Allegheny County, Pennsylvania. Those responding to the mass mailings were phone screened to determine initial eligibility, and those meeting initial eligibility appeared for a medical history interview conducted by a trained research assistant to screen for chronic medical conditions and medication usages. Full exclusion criteria for this study included self-reports of: (1) any history of clinical cardiovascular disease (CVD) or a CVD event (including stage II hypertension, stroke, myocardial infarction, congestive heart failure, or arrhythmia); (2) cardiovascular surgery; (3) cancer, a chronic kidney or liver condition, type 1 or 2 diabetes mellitus, or any pulmonary or respiratory disease; (4) current or past psychiatric diagnoses of substance abuse or mood disorders [verified by the Patient Health Questionnaire (Spitzer, Kroenke, & Williams, 1999)]; (5) prior cerebrovascular trauma; (6) neurosurgery or any neurological condition; (7) pregnancy (verified by urine test in females); (8) color-blindness; (9) claustrophobia; (10) ferromagnetic implants; or (11) any use of psychotropic, lipid-lowering, weight loss, insulin, glucocorticoid, hypoglycemic, or cardiovascular medications. All participants provided informed consent, and the University of Pittsburgh Institutional Review Board granted study approval.
2.2. Protocol and Measures
2.2.1. Self-report measures
Self-report measures examined childhood physical abuse and other forms of childhood adversity, adult stress, subclinical symptoms of depression and anxiety, personality factors related to negative affect, and adult health behaviors. Childhood physical abuse was measured using the Childhood Trauma Questionnaire [CTQ; (Bernstein et al., 1994, 2003)]. The CTQ comprises five subscales measuring different domains of childhood maltreatment: physical abuse, emotional abuse, sexual abuse, physical neglect, and emotional neglect. Each subscale comprises five questions regarding experiences in childhood (e.g., “People in my family hit me so hard that it left bruises or marks”) and participants respond using five-point Likert scales (i.e., “never true” through “very often true”). The CTQ has a cutoff score of 8 for the physical abuse subscale, which approximately 19% of the present sample met or exceeded, consistent with other community samples (Matthews et al., 2014). The CTQ has demonstrated adequate test-retest reliability, as well as internal consistency and convergent validity with clinician-rated interviews of childhood abuse (Fink, Bernstein, Handelsman, Foote, & Lovejoy, 1995). Given our hypotheses, primary analyses used the physical abuse subscale. For completeness of reporting, other forms of abuse and neglect as measured by the CTQ were examined in ancillary tests (see Supplemental Material). Two indices of childhood socioeconomic disadvantage were collected: parental education and perceived parental social standing (Adler & Stewart, 2007). Parental education was defined as the highest education attained by either parent during the participant’s childhood, as measured by years of schooling (i.e., 1 = grade school; 9 = high school; 13 = college; 20 = postgraduate). Perceived parental social standing was measured by standardizing and averaging maternal and paternal ratings from the MacArthur Scale of Subjective Social Status (Adler & Stewart, 2007).
Recent negative life events were assessed using the Life Events Checklist (Cohen, Tyrrell, & Smith, 1991). Perceived stress was measured using the 10-item Perceived Stress Scale (Cohen, Kamarck, & Mermelstein, 1983). These scales were used to test whether findings could be accounted for by adult levels of stress or adversity as opposed to childhood physical abuse. Depressive symptoms were measured using the Beck Depression Inventory-II [BDI-II, (Beck, Steer, & Brown, 1996)]. Because of the role of inflammatory cytokines in sickness behaviors (Swardfager, Rosenblat, Benlamri, & McIntyre, 2016), ancillary analyses also considered the somatic-affective dimension of the BDI, computed using published factor loadings (Steer, Ball, Ranieri, & Beck, 1999). Trait anxiety was measured using the Trait scale of the Spielberger State-Trait Anxiety Inventory (STAI-T) (Spielberger, Gorsuch, & Lushene, 1970). Finally, because personality factors related to negative affect could bias retrospective recall of childhood abuse (Watson & Pennebaker, 1989), trait neuroticism was measured using the NEO personality inventory (Costa & McCrae, 1992). Adult health behaviors included measures of smoking status (never, former, or current) as well as alcohol use (reported number of alcoholic drinks consumed in the past month).
2.2.2. Systemic inflammation
All participants refrained from eating and exercising, as well as consuming caffeine, tobacco, and alcohol for at least 8 hours prior to the morning of MRI assessment. A morning blood sample was drawn immediately prior to MRI assessment (7:00 to 11:00 AM), and the visit was rescheduled if the participant reported symptoms of acute infection, use of antibiotics or antivirals, or vaccination in the previous two weeks. Blood was collected in citrated tubes, with harvested plasma frozen at −80°C until batch analysis. IL-6 was determined by a high-sensitivity quantitative sandwich enzyme immunoassay kit (R&D Systems, Minneapolis, MN, standard range = 0.156–10 pg/mL). IL-6 levels were extrapolated from a standard curve with linear regression from a log-linear curve. IL-6 samples were run twice (mean coefficient of variation 4.24). Log transformation was used to correct the distributional skew of IL-6 values. Blood samples were also used to determine high-sensitivity CRP (see Supplemental Material).
2.2.3. Magnetic resonance imaging (MRI) acquisition and preprocessing
Functional MRI (fMRI) data were acquired within a 3T Trio TIM whole-body MRI scanner (Siemens, Erlangen, Germany), equipped with a 12-channel phased-array head coil. Blood-oxygen level-dependent (BOLD) images were acquired over a 5-minute eyes-open resting period with a gradient-echo EPI sequence using the following parameters: field-of-view (FOV) = 205 × 205 mm2, matrix size = 64 × 64 mm2, repetition time (TR) = 2,000 ms, echo time (TE) = 28 ms, and flip angle (FA) = 90. Thirty-nine slices (3 mm thickness, no gap) were obtained in an interleaved sequence in an inferior-to-superior direction, yielding 150 BOLD images (three initial discarded images, allowing for magnetic equilibration). For spatial coregistration of BOLD images, T1- weighted 3D magnetization-prepared rapid gradient echo (MPRAGE) neuroanatomical images were acquired over 7 min 17 s by these parameters: FOV = 256 × 208 mm2, matrix size = 256 × 208 mm2, TR = 2,100 ms, time-to-inversion (TI) = 1,100 ms, TE = 3.29 ms, and FA = 8.
Spatial preprocessing steps were conducted using Statistical Parametric Mapping 8 (SPM8; http://www.fil.ion.ucl.ac.uk/spm). Functional resting-state BOLD images were realigned to the first image using a six-parameter rigid-body transformation; the output movement parameters were used for subsequent motion regression preprocessing steps. Functional images were then coregistered to the T1-weighted structural image and normalized by 12-parameter affine transformation to the International Consortium for Brain Mapping 152 template. Functional images were spatially smoothed using a 6-mm full-width-at-half-maximum (FWHM) Gaussian kernel. Additional preprocessing steps were conducted using the CONN toolbox, version 17e (Whitfield-Gabrieli & Nieto-Castanon, 2012). Functional images were detrended and corrected for motion and physiological factors by removing via multiple regression the following timeseries: the six detrended motion correction parameters produced by the realignment step, their temporal derivatives, the first two principal components of white matter timeseries extracted using principal component analysis within a standard group atlas mask, and the first three principal components of cerebrospinal fluid timeseries extracted using principal component analysis within a standard group atlas mask (Behzadi, Restom, Liau, & Liu, 2007). Residual functional data were temporally bandpass filtered (0.009 Hz < f < 0.08) to preserve low frequency signal fluctuations of interest.
2.3. Region-of-interest (ROI) definition
We selected the following six a priori ROIs comprising limbic, vmPFC, and ACC areas, according to their hypothesized role in childhood physical abuse and inflammatory physiology. Amygdala and hippocampus ROI masks were created from the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) using the Wake Forest University PickAtlas Toolbox (Maldjian, Laurienti, Kraft, & Burdette, 2003). Because there were not a priori hypotheses about laterality, left and right regions were combined into a bilateral mask for each structure. The vmPFC mask was created by combining rectus and orbital gyrus regions labeled in the AAL atlas (Tzourio-Mazoyer et al., 2002). Masks pertaining to three subdivisions of the ACC (i.e., dorsal [dACC], perigenual [pgACC], and subgenual [sgACC]) were created from the IBASPM 71 atlas (Aleman-Gomez, Melie-García, & Valdés-Hernandez, 2006) in accordance with prior work (Gianaros et al., 2014).
2.4. Functional connectivity estimation
Connectivity metrics were computed in the CONN toolbox using ROI-to-ROI procedures. For each pair of four cortical (i.e., vmPFC, dACC, pgACC, sgACC) and two limbic (i.e., amygdala, hippocampus) ROIs, functional connectivity between two extracted and mean-centered timeseries was estimated using Pearson’s correlation and transformed using the Fisher r-to-z transform, resulting in estimates for 8 corticolimbic connections. Ancillary analyses tested the latent factor structure of these corticolimbic connections using Factor Analysis (see Supplemental Material). Individual corticolimbic connections, as well as these latent connectivity factors, were examined in association with physical abuse and IL-6.
2.5. Covariates
All analyses controlled for age and sex. In addition, due to the role of adiposity in systemic inflammation (Mohamed-Ali et al., 1997), body mass index (BMI; derived using participant height and weight) was included in all analyses. Finally, head motion during fMRI acquisition might also bias functional connectivity estimates, due to motion-induced increases in the BOLD signal across the brain (Van Dijk, Sabuncu, & Buckner, 2012). Therefore, in addition to minimizing within-participant motion confounds during fMRI preprocessing, participant head motion during fMRI acquisition was treated as a between-participant covariate. Specifically, for each participant, we calculated mean framewise displacement (FD), an index of frame-to-frame head motion (Power, Schlaggar, & Petersen, 2015) that we previously linked to IL-6 (Marsland et al., 2017), and entered FD as a between-participant covariate in regression models.
2.6. Statistical Analysis
Statistical analysis was conducted using R (R Development Core Team, 2016). Associations between childhood physical abuse, circulating IL-6, and extracted corticolimbic connectivity estimates, adjusting for covariates, were conducted using separate linear regression models.
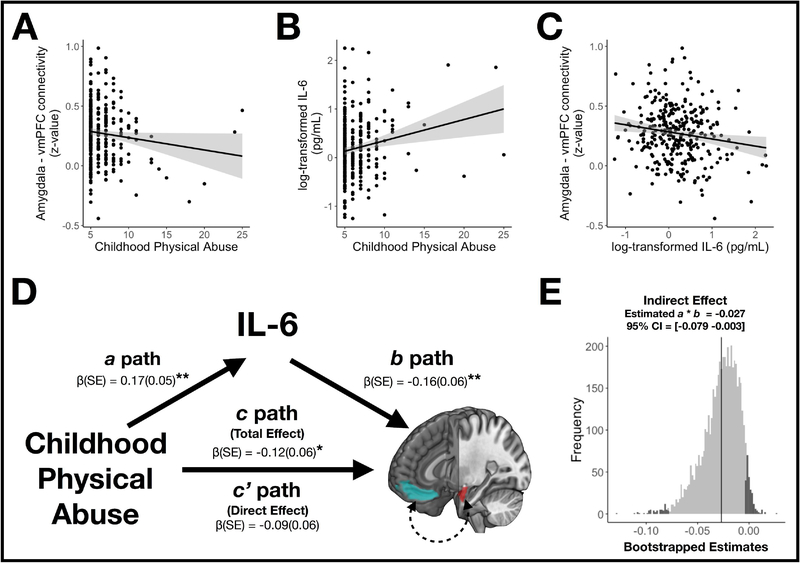
To test predictions from the neuroimmune network hypothesis (Nusslock & Miller, 2016), we conducted path analyses (Baron & Kenny, 1986) (Figure 1D). According to this model, associations between childhood physical abuse and corticolimbic connectivity without controlling for IL-6 were tested as the total effect of the independent variable (X) on the dependent variable (Y), or Path c. Associations of abuse with IL-6 were tested as the effect of X on the mediator variable (M), corresponding to Path a. Associations of IL-6 with connectivity, controlling for abuse, were tested as the effect of M on Y, corresponding to Path b. Associations between abuse and connectivity while controlling for IL-6 were tested as the direct effects of X on Y, or Path c’. Finally, the indirect path effect of the association of abuse and connectivity – as mediated by IL-6, was tested as the indirect effects of X on Y through M, and calculated as the product of Paths a and b. Statistical significance of the indirect path was assessed by bootstrapping (5000 iterations) the indirect path coefficients (Preacher & Hayes, 2008), with 95% confidence intervals (CIs) generated using the bias-corrected and accelerated method in the Causal Mediation Analysis package (Tingley, Yamamoto, Hirose, Keele, & Imai, 2014).
Figure 1.
(A) Adulthood resting connectivity between the amygdala and ventromedial prefrontal cortex (vmPFC) shown as a function of retrospectively reported childhood physical abuse. (B) Circulating levels of adulthood interleukin-6 (IL-6) shown as a function of childhood physical abuse. (C) Amygdala – vmPFC connectivity shown as a function of IL-6. The scatter plots shown in A-C are unadjusted for covariates for illustration purposes. (D) Path model summarizing the association of childhood physical abuse and amygdala – vmPFC connectivity, as mediated by circulating IL-6. Regions-of-interest corresponding to the amygdala and vmPFC are depicted in red and blue, respectively. The values correspond to each estimate [β(SE)] for the indirect (a, b), direct (c’), and total (c) paths. (E) Distribution of 5000 bootstrap samples of the indirect (a × b) effect for the mediation results shown in D. The gray-shaded area of the distribution encompasses a × b indirect effects falling within a 95% bias corrected and accelerated confidence interval (CI).
β, standardized beta; CI, Confidence intervals; CTQ, Childhood Trauma Questionnaire; IL-6, interleukin-6; SE, standard error; vmPFC, ventromedial prefrontal cortex * p < .05. ** p < .01
We also explored the specificity of our findings and the influence of other factors by examining associations of IL-6 and corticolimbic connectivity with other childhood (e.g., socioeconomic disadvantage) and adulthood stressors (e.g., negative life events), demographics (age, sex, race, socioeconomic status), adult health behaviors, subclinical depressive symptoms, trait anxiety, and neuroticism. Owing to the cross-sectional nature of the present data, the above path analyses were modified in post-hoc analyses in which the proposed mediator (i.e., adult circulating IL-6) and outcome (i.e., adult amygdala-vmPFC connectivity) were exchanged in a new path analysis model. Finally, a separate set of ancillary analyses tested interactive effects between childhood physical abuse and inflammation on connectivity (Hostinar, Davidson, et al., 2017) (detailed in Supplemental Material).
3. RESULTS
3.1. Descriptive statistics
After quality control review of the complete PIP sample (N = 331), 28 participants were excluded on account of missing or high levels (i.e., greater than 10 pg/mL) of circulating IL-6 data (N = 17); self-reporting elevated symptoms of depression (Beck Depression Inventory score > 20 and Patient Health Questionnaire score > 12) (N = 1); missing, poor quality, or artifactual MRI data (N = 10). Demographic characteristics of the complete analytic sample (N = 303) are in Table 1. Participants included in analyses did not differ statistically from excluded participants on any study variable (all p > .15).
Table 1.
Summary of participant characteristics (N = 303).
| Characteristic | Mean or N | SD or % |
|---|---|---|
| Age (years) | 40.3 | 6.24 |
| Race | ||
| White | 213 | 70.30 |
| Nonwhite | 90 | 29.70 |
| Body mass index (kg/m2) | 26.8 | 5.03 |
| Smoking status | ||
| Never | 191 | 63.04 |
| Former | 60 | 19.8 |
| Current | 52 | 17.16 |
| Alcohol use (number of drinks in past month) | 10.84 | 15.82 |
| Childhood Trauma Questionnaire | ||
| Total score | 36.29 | 11.42 |
| Physical abuse subscale | 6.44 | 2.54 |
| Meets clinical criteria for physical abuse * | 56 | 18.48 |
| Occupant-adjusted income (thousand dollars / year) | 30.57 | 7.45 |
| Depressive symptoms (BDI-II) | ||
| Total | 3.46 | 3.38 |
| Somatic symptoms | 1.05 | 1.19 |
| Trait Anxiety (STAI-T) | 32.91 | 7.2 |
| Neuroticism (NEO-N) | 76.47 | 20.71 |
| IL-6 (pg/mL) | ||
| Raw | 1.48 | 1.21 |
| Log-transformed | 0.19 | 0.6 |
| Framewise Displacement (mm) | 0.24 | 0.18 |
Determined using cutoffs as defined in ref. (Bernstein et al., 2003).
BDI, Beck Depression Inventory; STAI-T, State Trait Anxiety Inventory - Trait subscale; NEO-N, Neuroticism, Extraversion, Openness Personality Inventory - Neuroticism subscale.
3.2. Associations of childhood physical abuse, adult IL-6, and adult corticolimbic connectivity
Retrospective reports of childhood physical abuse covaried negatively with functional connectivity between the amygdala and vmPFC in linear regression models adjusting for a priori covariates (β = −0.12, p = .039). Physical abuse did not associate with other corticolimbic connections (all p > .2), nor did it associate with latent factors of connectivity (both p > .2). Retrospective reports of childhood physical abuse also covaried positively with circulating IL-6 in linear regression models adjusted for covariates (β = 0.17, p = .002).
Circulating levels of IL-6 covaried negatively with connectivity between the amygdala and three cortical ROIs: the vmPFC (β = −0.18, p = .004), sgACC (β = −0.14, p = .020), and pgACC (β = −0.13, p = .030). IL-6 also covaried negatively with connectivity between the hippocampus and the vmPFC (β = −0.15, p = .012). Finally, these results agreed with those indicating that a latent factor of ventral PFC – limbic connectivity, comprising connections between the VMPFC, pgACC, sgACC, and both limbic regions (amygdala and hippocampus), similarly associated with circulating IL-6 (β = −0.17, p = .006).
3.3. Exploratory path analyses of childhood physical abuse, adult IL-6, and adult amygdala-vmPFC connectivity
Path analyses were used to test whether individual differences in circulating IL-6 accounted for associations between childhood physical abuse and corticolimbic connectivity (Baron & Kenny, 1986). Results from all path analysis models are depicted in Table 2, and results from the model including amygdala-vmPFC connectivity are depicted in Figure 1D. Notably, there was a total effect of childhood physical abuse on amygdala-vmPFC connectivity (β = −0.12, p = .039), but not a direct effect when including IL-6 (β = −0.09, p = .11). Consistent with the absence of a direct effect, the indirect (a × b) effect of childhood physical abuse on amygdala-vmPFC connectivity – as mediated by circulating IL-6 – exhibited an effect size of β = −0.027. Bootstrapped estimations demonstrated that a 95% bias-corrected and accelerated confidence interval of the latter indirect effect estimate did not include zero [−0.079 −0.003], indicating significant mediation (Figure 1E). The indirect (a × b) effect reported above 17 accounted for 22.2% of the association between childhood physical abuse and amygdala-vmPFC connectivity.
Table 2.
Standardized point estimates (β) and corresponding standard errors (SE) in parentheses for mediation models depicting relationships between childhood physical abuse, interleukin-6, and individual corticolimbic connectivity outcomes. See Figure 1 for the organization of the hypothesized relationships. Estimates for Path a are equivalent across models (i.e., all β(SE) = 0.17(0.05), p = .002) and therefore are not depicted in the Table. See Supplemental Material for descriptions of the ventral and dorsal connectivity factors.
| Corticolimbic Connectivity | Total Effect c β (SE) | Path b β (SE) | Direct Effect c’ β (SE) | Indirect Effect a × b β [95% Cl] |
|---|---|---|---|---|
| Amyg - vmPFC | −0.12* (0.06) | −0.16** (0.06) | −0.09 (0.06) | −0.027 [−0.079 −0.003] |
| Amyg - sgACC | −0.07 (0.06) | −0.13 * (0.06) | −0.04 (0.06) | −0.022 [−0.068 −0.001] |
| Amyg - pgACC | −0.05 (0.06) | −0.13 * (0.06) | −0.03 (0.06) | −0.021 [−0.066 −0.001] |
| Amyg - dACC | −0.05 (0.06) | 0.06 (0.06) | −0.06 (0.06) | 0.010 [−0.006 0.043] |
| Hipp - vmPFC | −0.05 (0.06) | −0.15 * (0.06) | −0.03 (0.06) | −0.025 [−0.078 −0.002] |
| Hipp - sgACC | 0.00 (0.06) | −0.06 (0.06) | 0.01 (0.06) | −0.01 [−0.047 0.009] |
| Hipp - pgACC | −0.04 (0.06) | −0.11 (0.06) | −0.02 (0.06) | −0.018 [−0.055 0.001] |
| Hipp - dACC | −0.02 (0.06) | −0.03 (0.06) | −0.01 (0.06) | −0.005 [−0.033 0.011] |
| Ventral Factor | −0.06 (0.06) | −0.16 ** (0.06) | −0.04 (0.06) | −0.027 [−0.076 −0.003] |
| Dorsal Factor | −0.02 (0.06) | 0.01 (0.06) | −0.02 (0.06) | 0.001 [−0.018 0.023] |
Amyg, amygdala; β, standardized beta; CI, confidence interval; dACC, dorsal anterior cingulate cortex; Hipp, hippocampus; pgACC, perigenual anterior cingulate cortex; SE standard error; sgACC, subgenual anterior cingulate cortex; vmPFC, ventromedial prefrontal cortex.
p < .05.
p < .01
3.4. Ancillary tests of relationships with other forms of childhood adversity and inflammatory physiology
We examined associations of other forms of childhood adversity (e.g., socioeconomic disadvantage) with IL-6 and corticolimbic connectivity (see Table S5 in Supplemental Material). In addition to physical abuse, circulating IL-6 was associated with the CTQ sum score (r = .12, p = .040), as well as the physical neglect subscale (r = .12, p = .041); however, these indices of childhood adversity did not statistically relate to IL-6 in regression models (p’s > .05). Moreover, IL-6 was unrelated to other childhood experiential factors, such as emotional abuse and socioeconomic disadvantage. Separately, we examined associations of CRP with physical abuse and corticolimbic connectivity; whereas CRP associated negatively with amygdala-vmPFC connectivity (β = −0.19, p = .004), it did not statistically associate with childhood physical abuse (β = 0.02, p = .751; see Supplemental Material for full details).
3.5. Ancillary tests of relationships with adult demographics, health behaviors, stress, depression, trait anxiety, and neuroticism
There were statistical race differences in retrospectively reported childhood physical abuse, with white participants reporting lower levels of childhood physical abuse than nonwhite participants (β = −0.17, p = .003). Smoking status (dichotomized into current/former vs never) was also statistically associated with childhood physical abuse (t = 2.18, p = .03). In contrast, there were not statistical sex differences in retrospectively reported abuse, and abuse was not statistically associated with age, adult occupant-adjusted income, alcohol use, perceived stress, subclinical depressive symptoms, trait anxiety, or neuroticism (all p’s > .12). IL-6 associated with adult occupant-adjusted income (β = −0.22, p < .001), smoking status (t = 3.41 p < .001), perceived stress (β = 0.13, p = .017), subclinical depressive symptoms (β = 0.12, p = .025), as well as the somatic-affective dimension of depressive symptoms (β = 0.12, p = .025). There were no differences by sex or race in IL-6, and IL-6 did not significantly correlate with age, alcohol use, neuroticism, or trait anxiety (all p > .05). Although smoking status associated with both childhood physical abuse and adult IL-6, the association of physical abuse and IL-6 remained in previously described regression models that also included smoking status (β = 0.15, p = .006). Finally, there were no statistical differences by sex, race, nor smoking status in corticolimbic connectivity, and connectivity estimates did not statistically associate with age, alcohol use, depressive symptoms, trait anxiety, or neuroticism (all p > .05).
3.6. Ancillary path analyses focusing on adult amygdala-vmPFC connectivity as mediator and circulating IL-6 as outcome
In a post-hoc analysis we modified the a priori path model above, using the same covariates, except treating amygdala-vmPFC connectivity as the proposed mediator (M) and circulating IL-6 as the proposed outcome (Y). There was a total effect of childhood physical abuse on circulating IL-6 (β = 0.17, p = .002), as well as a statistical direct effect when including adult-vmPFC connectivity in the model (β = 0.15, p = .006). The indirect effect of childhood physical abuse on amygdala-IL-6 via circulating amygdala-vmPFC connectivity exhibited an effect size of β = 0.017, 95% confidence intervals 0.001 to 0.057. The indirect effect accounted for 10% of the association between childhood physical abuse and circulating IL-6.
4. DISCUSSION
Four sets of novel findings from the present study suggest that reported experiences of physical abuse in childhood may relate to corticolimbic functionality in adulthood, and that this association may be partly explained by systemic inflammation. First, retrospective reports of physical abuse in childhood associated with resting connectivity between the amygdala and vmPFC in a midlife community sample. Second, abuse associated with elevated levels of circulating IL-6. Third, IL-6 levels associated with amygdala-vmPFC connectivity, as well as several other corticolimbic connections. Importantly, and consistent with our hypotheses as well as core predictions of the neuroimmune network hypothesis (Nusslock & Miller, 2016), the effect of childhood physical abuse on amygdala-vmPFC connectivity was partly explained (i.e., statistically mediated) by circulating IL-6 (Figure 1D–E). To our knowledge, this is the first study to examine childhood physical abuse, peripheral inflammation, and measures of corticolimbic circuit functionality as measured by resting state fMRI in a healthy midlife sample from the community that was unconfounded by clinical disease or medication status. Likewise, these novel results also appear to support the main predictions of the neuroimmune network hypothesis; specifically, that elevated levels of systemic inflammation attributable to childhood physical abuse may plausibly affect corticolimbic circuits important for mental and physical health in adulthood (Nusslock & Miller, 2016). Broadly, our findings are largely in line with hypothesized corticolimbic brain alterations that follow childhood abuse and persist into later life. Prior studies reported similar associations of childhood maltreatment and amygdala-vmPFC connectivity at rest in adolescent samples (Herringa et al., 2013), as well as adult samples with comorbid psychiatric diagnoses (Birn et al., 2014). Together, these findings point to connectivity within the corticolimbic circuit as a potential adult neurobiological correlate of childhood physical abuse. Notably, the specificity of our findings with regard to childhood abuse and corticolimbic functionality appear consistent with dimensional perspectives on childhood adversity and the brain (McLaughlin, Sheridan, & Lambert, 2014). Specifically, according to these latter perspectives, the dimension of threat (including childhood physical abuse) is thought to exert specific effects on threat-related corticolimbic circuits, whereas the conceptually distinct dimension of deprivation (e.g., caregiver neglect, institutionalization) is thought to exert broader and less specific effects on brain development, particularly manifesting as reduced global metrics of gray matter thickness (McLaughlin, Sheridan, Winter, et al., 2014; Sheridan, Fox, Zeanah, McLaughlin, & Nelson, 2012), as well as functional changes in sensory and association cortices (Chugani et al., 2001). Indeed, in our sample, amygdala-vmPFC connectivity was statistically associated with childhood physical abuse, and not physical or emotional neglect (Table S5). Interestingly, subjective reports of parental social status, an approximation of childhood socioeconomic disadvantage, was associated with amygdala-sgACC connectivity. It should be noted, however, that socioeconomic disadvantage is not thought to reflect the dimension of deprivation per se, but rather a mix of deprivation and threat (McLaughlin, Sheridan, & Lambert, 2014).
Our observation of an association between childhood physical abuse and adulthood IL-6 agrees with cumulative and meta-analytic evidence (Baumeister et al., 2016). The precise mechanisms underlying this association are unclear, but may involve a combination of neuroendocrine influences (Heim, Newport, Mletzko, Miller, & Nemeroff, 2008), increased vulnerability to infections (Gilbert et al., 2009), and engagement in health-risk behaviors (Raposa, Bower, Hammen, Najman, & Brennan, 2014). Regarding the latter pathway, the association of childhood physical abuse and inflammation in our study did not appear to be accounted for or confounded by smoking status, alcohol use, or body mass index. Prior studies have reported associations between systemic inflammation and diverse childhood adversities (Carroll et al., 2013; G. E. Miller et al., 2009), yet associations with IL-6 observed in this study were relatively specific to physical abuse. With the exception of overall maltreatment (i.e., total CTQ score) and physical neglect, we did not observe statistical associations between IL-6 and other forms of childhood adversity. Moreover, we did not find a statistical association between childhood physical abuse and adult CRP, which is consistent with cumulative meta-analytic evidence (Baumeister et al., 2016). Indeed, the latter meta-analysis on early life adversity and adult inflammation indicated that the presence of clinical disorders might augment the effects of early life adversity on CRP; the nonsignificant association in this healthy adult sample is consistent with this notion. Differences in these aspects of childhood adversity, such as their timing, exposure, chronicity, or other interpersonal or environmental characteristics, as well as the presence of psychiatric disorders later in life could explain differences in associations with peripheral physiology (Tottenham & Sheridan, 2010). Taken together, our findings linking childhood physical abuse to IL-6 adds to growing evidence that childhood adversity is a biobehavioral risk factor for systemic inflammation in midlife, which itself is a risk factor for diverse physical health outcomes (Geovanini & Libby, 2018; Libby, Ridker, & Maseri, 2002; Vaziri & Rodríguez-Iturbe, 2006).
Circulating IL-6 was also associated with several features of resting corticolimbic connectivity in the present study, primarily comprising connections between the amygdala, hippocampus, vmPFC, and ventral portions of the ACC. These findings are largely consistent with a recent neuroimaging meta-analysis, which showed peripheral inflammatory physiology consistently associates with changes in activity within the amygdala, hippocampus, and vmPFC (Kraynak, Marsland, Wager, et al., 2018). In extension of this literature on peripheral inflammation and corticolimbic activity, to our knowledge this study is the first to report associations between inflammation and corticolimbic connectivity at rest in a large, representative sample of midlife adults. For example, a recent study reported negative associations between CRP and amygdalavmPFC connectivity in a sample of depressed adults (Mehta et al., 2018). Ancillary analyses focusing on CRP in the present sample showed a similar negative association between CRP and amygdala-vmPFC connectivity (see Supplemental Material). The latter study also reported associations of amygdala-vmPFC connectivity with symptoms of anxiety, which we did not observe in our asymptomatic midlife sample. A more recent paper from this group used large-scale network-based analyses and reported negative associations between CRP and connectivity within a widely distributed circuit encompassing the amygdala, parahippocampal gyrus, and orbitofrontal cortices, which anatomically aligns considerably with our ventral connectivity factor (Yin et al., in press). Separately, we recently reported associations between circulating IL-6 and connectivity of the default mode network, which comprises corticolimbic regions including the vmPFC (Marsland et al., 2017). However, in the latter study we did not find inflammation-associated connectivity with canonical limbic areas, such as the amygdala or hippocampus. Taken together, our findings accord with a growing literature which suggests the corticolimbic circuit may be uniquely linked to peripheral inflammatory physiology (Harrison et al., 2009).
The physiological basis underlying the association of IL-6 and corticolimbic connectivity may be accounted for by bidirectional pathways linking peripheral inflammation and the brain (Irwin & Cole, 2011). First, peripheral IL-6 can influence corticolimbic connectivity in a ‘bottom-up’ or body-to-brain manner by accessing the brain and influencing local physiological processes, such as neurotransmitter metabolism, long term potentiation, synaptic plasticity, and structural remodeling in specific areas (Marsland, Gianaros, Abramowitch, Manuck, & Hariri, 2008; Marsland et al., 2015; Yirmiya & Goshen, 2011). In contrast, corticolimbic brain circuits can regulate peripheral inflammatory processes via ‘top-down’ or brain-to-body autonomic and neuroendocrine pathways, particularly during stress (Beissner, Meissner, Bär, & Napadow, 2013; Ginty, Kraynak, Fisher, & Gianaros, 2017; Kraynak, Marsland, & Gianaros, 2018).
Regarding the latter top-down pathway, it is plausible that experiences of childhood physical abuse could directly sensitize corticolimbic connectivity, resulting in dysregulated autonomic and neuroendocrine outflow in the periphery and thereafter promoting systemic inflammation. Indeed, this alternative formulation to the a priori model tested here accords with the neuroimmune network hypothesis (Nusslock & Miller, 2016), as well as evidence that connections between the vmPFC and limbic and brainstem visceromotor areas are implicated in stress reactivity and autonomic control (Critchley, Nagai, Gray, & Mathias, 2011; Ginty, Kraynak, Kuan, & Gianaros, in press; Roy, Shohamy, & Wager, 2012). We examined this alternative explanation in post-hoc path analyses, and results suggested that while there was a statistical indirect effect of childhood physical abuse on circulating IL-6 via adult amygdala-vmPFC connectivity (i.e., confidence intervals of indirect path estimates did not include zero), the direct effect of abuse on IL-6 remained statistically significant, and the indirect path explained 10% of the association between abuse and IL-6 (versus 22.2% in our original model). Hence, we cautiously speculate these data appear more in line with afferent (body-to-brain) effects linking IL-6 and corticolimbic connectivity (Figure 1D) (Critchley & Harrison, 2013; Goehler et al., 2000). Future studies that longitudinally examine relationships between peripheral IL-6 and corticolimbic connectivity have the potential to address these uncertainties regarding bidirectional pathways that link childhood abuse to later health (Hostinar, Nusslock, & Miller, 2017).
Moreover, despite the uncertainties described above, our results reinforce the notion that childhood abuse exerts broad effects on multiple biological systems throughout the life course (Hostinar, Nusslock, et al., 2017). Hence, these results suggest peripheral inflammatory processes and their interaction with corticolimbic circuit functionality in particular may comprise a key pathway linking childhood abuse to multimorbidity in adulthood which spans mental and physical health conditions (Anisman & Hayley, 2012). Supporting this, exploratory path analyses indicated circulating IL-6 accounted for 22.2% of the association between childhood physical abuse and adult amygdala-vmPFC connectivity. The above suggests that while a sizeable portion of the association between childhood abuse and adult connectivity may be attributable to inflammatory processes, there is nonetheless a larger proportion not explained by IL-6 which our study did not address. We speculate this larger proportion of variance may be potentially explained by other important mediating factors, including neuroendocrine processes (Heim et al., 2008), physical exercise (Erickson et al., 2011), and engagement in health-risk behaviors (Raposa et al., 2014). Future studies should incorporate intensive sampling of these and other factors to examine parallel mediating pathways linking childhood abuse to later health. Finally, we note that ancillary moderation analyses were inconsistent with the notion of childhood abuse moderating (or upregulating) links between IL-6 and corticolimbic connectivity [(Hostinar, Davidson, et al., 2017), see Supplemental Material]. Rather, our primary analyses are consistent with models by which childhood abuse affects later corticolimbic circuit functionality and peripheral inflammation, the latter potentially serving as a mediating pathway.
A separate question regarding the mechanisms underlying our results involves the developmental timing of neurobiological and immune changes following childhood abuse. For example, prior studies of adolescents and young adults have reported associations of childhood maltreatment with elevated systemic inflammation (Danese et al., 2011; Slopen, Kubzansky, McLaughlin, & Koenen, 2013) and reduced corticolimbic connectivity (Herringa et al., 2013). Hence, it is possible that associations as reported in our study of midlife adults could have manifested earlier in life. Accordingly, future studies in adolescent and young adult samples should explicitly examine peripheral inflammation as a pathway emerging in adolescence that could impact developing corticolimbic functionality as well as its cognitive and affective sequelae.
Given the above associations, we further tested for associations with subclinical symptoms of depression, trait anxiety, and the personality feature of neuroticism. Our findings raise several open questions and potential inferences. First, null associations between reported childhood abuse with adulthood features of negative affect (e.g., trait anxiety and neuroticism) in our sample suggest these dispositions did not bias reports of childhood abuse, which is a major concern of retrospective measures (Watson & Pennebaker, 1989). Second, the positive association between IL-6 and depressive symptoms replicates findings from several prior cross-sectional studies (Jokela, Virtanen, Batty, & Kivimäki, 2016; A. H. Miller, Maletic, & Raison, 2009; Stewart, Rand, Muldoon, & Kamarck, 2009). This association also accords with mechanistic accounts by which experimental manipulations of systemic inflammation reliably induce a constellation of mood and affective changes, termed ‘sickness behaviors’, which overlap considerably with several features of depression (Dantzer, O’Connor, Freund, Johnson, & Kelley, 2008; Maier & Watkins, 1998; A. H. Miller et al., 2009). Third, while we did not observe statistical associations between corticolimbic connectivity and depressive symptoms, we speculate such associations could become more apparent in clinical samples or among samples with a wider range of symptomatology. In extension, it may be that the majority of this sample exhibited elevated levels of resilience to the effects of childhood abuse on adult symptomatology and risk for clinical psychiatric disorders. In other words, there were many individuals in the present sample who reported physical abuse, but did not go on to develop mood, anxiety, or other disorders.
Limitations, Future Directions, and Conclusion
Limitations to the present study warrant attention. First, the study design was cross-sectional, which precludes causal inference and may have biased reported effect sizes (Maxwell & Cole, 2007). However, to our knowledge, no prospective studies have examined the effects of childhood physical abuse on both neurobiological and inflammatory changes in adulthood. Hence, the present study provides a preliminary reference point for future prospective studies in this area [e.g., using longitudinal mediational modeling, (D. A. Cole & Maxwell, 2003)], indicating a need to focus specifically on physical abuse-related forms of childhood adversity and corticolimbic connectivity. Second, the present sample of midlife adults was screened to be free of major physical health conditions and diagnosed psychiatric disorders. Possibly as a result, the base-rate of childhood physical abuse was lower than in other samples with known trauma or diagnosed psychiatric disorders, as were facets of negative affect and depressive symptomatology. As a result, we may have been underpowered to detect significant adversity-related inflammatory or brain outcomes that have been reported elsewhere in the literature. Third, the retrospective nature of reporting childhood abuse has the potential to introduce recall and other forms of memory or reporting biases (Pope & Hudson, 1995; Usher & Neisser, 1993). However, it should be noted that prospective studies of childhood physical abuse are potentially subject to other complications and sources of bias, including low reporting rates and selective attrition (Teicher et al., 2016). Fourth, we only examined one marker of peripheral inflammation in our primary analyses, although we tested for effects of CRP in post-hoc analyses, which did not support our overall model (see Supplemental Material). Future studies in this area should utilize multiplex assays that test for a broad array of peripheral inflammatory indicators (e.g., IL-1β, Tumor Necrosis Factor alpha) to identify patterns of peripheral inflammatory physiology that may be affect by childhood physical abuse. Finally, we only examined one of many hypothesized pathways implicated in the neuroimmune network hypothesis (Nusslock & Miller, 2016). Accordingly, future research should also consider predictions from the neuroimmune network hypothesis that pertain to other brain systems, namely reward and executive control networks. Indeed, the potential effects of childhood adversity on these latter brain systems may differ from those reported here, in terms of timing, effect size, and specific adversity type. Similarly, our study only tested systemic inflammation as a peripheral physiological pathway linking childhood physical abuse and the brain. Hence, future studies could integrate inflammatory measures as reported here with measures of autonomic (e.g., cardiac vagal) and neuroendocrine (e.g., hypothalamic-pituitary-adrenal axis) function, which are similarly thought to be implicated in childhood abuse and adult health (Gee et al., 2013; Heim et al., 2008; Shenk, Noll, Putnam, & Trickett, 2010).
Limitations notwithstanding, the present findings offer support for several predictions from the neuroimmune network hypothesis; namely, associations between childhood physical abuse and resting brain corticolimbic connectivity in adulthood appear to be partially explained by systemic inflammation. As such, we speculate that peripheral inflammatory processes may correspond to an intermediate pathway and a possible target for interventions or preventative strategies to reduce the adverse physical and mental consequences of childhood adversity.
Supplementary Material
Pathways linking childhood abuse with adult health are poorly understood.
Tested roles of systemic inflammation and corticolimbic functionality.
Childhood abuse associated with increased IL-6 and reduced amygdala-vmPFC connectivity.
IL-6 associated with reduced amygdala-vmPFC connectivity.
Path analyses showed indirect effect of abuse on amygdala-vmPFC connectivity via IL-6.
Acknowledgments
Funding Sources: This work was supported by the National Institutes of Health (NHLBI P01 040962, NHLBI R01 HL089850, and NHLBI T32 HL007560).
Footnotes
Disclosures
The authors attest that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler N, & Stewart J (2007). The MacArthur scale of subjective social status. MacArthur Research Network on SES & Health. Retrieved from Http://Www.Macses.Ucsf.Edu/Research/Psychosocial/Subjective.Php. [Google Scholar]
- Aleman-Gomez Y, Melie-García L, & Valdés-Hernandez P (2006). IBASPM: toolbox for automatic parcellation of brain structures. 12th Annual Meeting of the Organization for Human Brain Mapping, 27, 11–15. [Google Scholar]
- Anisman H, & Hayley S (2012). Inflammatory factors contribute to depression and its comorbid conditions. Science Signaling, 5(244), pe45 10.1126/scisignal.2003579 [DOI] [PubMed] [Google Scholar]
- Banihashemi L, Sheu LK, Midei AJ, & Gianaros PJ (2015). Childhood physical abuse predicts stressor-evoked activity within central visceral control regions. Social Cognitive and Affective Neuroscience, 10(4), 474–485. 10.1093/scan/nsu073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, & Kenny DA (1986). The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology, 51(6), 1173. [DOI] [PubMed] [Google Scholar]
- Basu A, McLaughlin KA, Misra S, & Koenen KC (2017). Childhood Maltreatment and Health Impact: The Examples of Cardiovascular Disease and Type 2 Diabetes Mellitus in Adults. Clinical Psychology: Science and Practice, 24(2), 125–139. 10.1111/cpsp.12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister D, Akhtar R, Ciufolini S, Pariante CM, & Mondelli V (2016). Childhood trauma and adulthood inflammation: A meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Molecular Psychiatry, 21(5), 642–649. 10.1038/mp.2015.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). BDI-II, Beck depression inventory: Manual. San Antonio, Tex.; Boston: Psychological Corp.; Harcourt Brace. [Google Scholar]
- Behzadi Y, Restom K, Liau J, & Liu TT (2007). A Component Based Noise Correction Method (CompCor) for BOLD and Perfusion Based fMRI. NeuroImage, 37(1), 90–101. 10.1016/j.neuroimage.2007.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissner F, Meissner K, Bär K-J, & Napadow V (2013). The autonomic brain: An activation likelihood estimation meta-analysis for central processing of autonomic function. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 33(25), 10503–10511. 10.1523/JNEUROSCI.1103-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, ... Ruggiero J (1994). Initial reliability and validity of a new retrospective measure of child abuse and neglect. The American Journal of Psychiatry, 151(8), 1132–1136. 10.1176/ajp.151.8.1132 [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, ... Zule W (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect, 27(2), 169–190. [DOI] [PubMed] [Google Scholar]
- Birn RM, Patriat R, Phillips ML, Germain A, & Herringa RJ (2014). Childhood Maltreatment and Combat Posttraumatic Stress Differentially Predict Fear-Related Fronto-Subcortical Connectivity. Depression and Anxiety, 31(10), 880–892. 10.1002/da.22291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, ... Charney DS (1997). Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—A preliminary report. Biological Psychiatry, 41(1), 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VM, LaBar KS, Haswell CC, Gold AL, Mid-Atlantic MIRECC Workgroup, Beall undefined S. K., ... Morey RA (2014). Altered Resting-State Functional Connectivity of Basolateral and Centromedial Amygdala Complexes in Posttraumatic Stress Disorder. Neuropsychopharmacology, 39(2), 361–369. 10.1038/npp.2013.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, & Price LH (2010). Association between Plasma IL-6 Response to Acute Stress and Early-Life Adversity in Healthy Adults. Neuropsychopharmacology, 35(13), 2617–2623. 10.1038/npp.2010.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Gruenewald TL, Taylor SE, Janicki-Deverts D, Matthews KA, & Seeman TE (2013). Childhood abuse, parental warmth, and adult multisystem biological risk in the Coronary Artery Risk Development in Young Adults study. Proceedings of the National Academy of Sciences, 110(42), 17149–17153. 10.1073/pnas.1315458110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Turiano NA, Mroczek DK, & Miller GE (2016). Association of Reports of Childhood Abuse and All-Cause Mortality Rates in Women. JAMA Psychiatry, 73(9), 920–927. 10.1001/jamapsychiatry.2016.1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, & Lacey RE (2018). Adverse childhood experiences and adult inflammation: Findings from the 1958 British birth cohort. Brain, Behavior, and Immunity, 69, 582–590. 10.1016/j.bbi.2018.02.007 [DOI] [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhász C, Nagy F, & Chugani DC (2001). Local Brain Functional Activity Following Early Deprivation: A Study of Postinstitutionalized Romanian Orphans. NeuroImage, 14(6), 1290–1301. 10.1006/nimg.2001.0917 [DOI] [PubMed] [Google Scholar]
- Cisler JM, James GA, Tripathi S, Mletzko T, Heim C, Hu XP, ... Kilts CD (2013). Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychological Medicine, 43(3), 507–518. 10.1017/S0033291712001390 [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A Global Measure of Perceived Stress. Journal of Health and Social Behavior, 24(4), 385–396. 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- Cohen S, Tyrrell DA, & Smith AP (1991). Psychological stress and susceptibility to the common cold. The New England Journal of Medicine, 325(9), 606–612. 10.1056/NEJM199108293250903 [DOI] [PubMed] [Google Scholar]
- Cole DA, & Maxwell SE (2003). Testing mediational models with longitudinal data: Questions and tips in the use of structural equation modeling. Journal of Abnormal Psychology, 112(4), 558–577. 10.1037/0021-843X.112.4.558 [DOI] [PubMed] [Google Scholar]
- Cole SW, Conti G, Arevalo JMG, Ruggiero AM, Heckman JJ, & Suomi SJ (2012). Transcriptional modulation of the developing immune system by early life social adversity. Proceedings of the National Academy of Sciences of the United States of America, 109(50), 20578–20583. 10.1073/pnas.1218253109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, & McCrae RR (1992). NEO PI-R Professional Manual. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Critchley HD, & Harrison NA (2013). Visceral influences on brain and behavior. Neuron, 77(4), 624–638. 10.1016/j.neuron.2013.02.008 [DOI] [PubMed] [Google Scholar]
- Critchley HD, Nagai Y, Gray MA, & Mathias CJ (2011). Dissecting axes of autonomic control in humans: Insights from neuroimaging. Autonomic Neuroscience, 161(1–2), 34–42. 10.1016/j.autneu.2010.09.005 [DOI] [PubMed] [Google Scholar]
- Danese A, Caspi A, Williams B, Ambler A, Sugden K, Mika J, ... Arseneault L (2011). Biological embedding of stress through inflammation processes in childhood. Molecular Psychiatry, 16(3), 244–246. 10.1038/mp.2010.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, & Caspi A (2008). Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Archives of General Psychiatry, 65(4), 409–415. 10.1001/archpsyc.65.4.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, & Poulton R (2007). Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences, 104(4), 1319–1324. 10.1073/pnas.0610362104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, & Kelley KW (2008). From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience, 9(1), 46–56. 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, & Whalen PJ (2001). The amygdala: Vigilance and emotion. Molecular Psychiatry, 6(1), 13–34. 10.1038/sj.mp.4000812 [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen J-H, Bonde S, Kokaia Z, & Lindvall O (2003). Inflammation is detrimental for neurogenesis in adult brain. Proceedings of the National Academy of Sciences of the United States of America, 100(23), 13632–13637. 10.1073/pnas.2234031100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwood E, Lim Z, Naveed H, & Galea I (2017). The effect of systemic inflammation on human brain barrier function. Brain, Behavior, and Immunity, 62, 35–40. 10.1016/j.bbi.2016.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, ... Kramer AF (2011). Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences, 108(7), 3017–3022. 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink LA, Bernstein D, Handelsman L, Foote J, & Lovejoy M (1995). Initial reliability and validity of the childhood trauma interview: A new multidimensional measure of childhood interpersonal trauma. The American Journal of Psychiatry, 152(9), 1329–1335. 10.1176/ajp.152.9.1329 [DOI] [PubMed] [Google Scholar]
- Fong Y, Moldawer LL, Marano M, Wei H, Tatter SB, Clarick RH, ... Sehgal PB (1989). Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. The Journal of Immunology, 142(7), 2321–2324. [PubMed] [Google Scholar]
- Frenois F, Moreau M, O’Connor J, Lawson M, Micon C, Lestage J, ... Castanon N (2007). Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology, 32(5), 516–531. 10.1016/j.psyneuen.2007.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, ... Tottenham N (2013). Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences, 110(39), 15638–15643. 10.1073/pnas.1307893110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geovanini GR, & Libby P (2018). Atherosclerosis and inflammation: Overview and updates. Clinical Science, 132(12), 1243–1252. 10.1042/CS20180306 [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Marsland AL, Kuan DC-H, Schirda BL, Jennings JR, Sheu LK, ... Manuck SB (2014). An inflammatory pathway links atherosclerotic cardiovascular disease risk to neural activity evoked by the cognitive regulation of emotion. Biological Psychiatry, 75(9), 738–745. 10.1016/j.biopsych.2013.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert R, Kemp A, Thoburn J, Sidebotham P, Radford L, Glaser D, & Macmillan HL (2009). Recognising and responding to child maltreatment. Lancet (London, England), 373(9658), 167–180. 10.1016/S0140-6736(08)61707-9 [DOI] [PubMed] [Google Scholar]
- Ginty AT, Kraynak TE, Fisher JP, & Gianaros PJ (2017). Cardiovascular and autonomic reactivity to psychological stress: Neurophysiological substrates and links to cardiovascular disease. Autonomic Neuroscience, 207(Supplement C), 2–9. 10.1016/j.autneu.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginty AT, Kraynak TE, Kuan DCH, & Gianaros PJ (in press). Ventromedial prefrontal cortex connectivity during and after psychological stress in women. Psychophysiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RPA, Hansen MK, Anderson K, Maier SF, & Watkins LR (2000). Vagal immune-to-brain communication: A visceral chemosensory pathway. Autonomic Neuroscience, 85(1–3), 49–59. 10.1016/S1566-0702(00)00219-8 [DOI] [PubMed] [Google Scholar]
- Gorka AX, Hanson JL, Radtke SR, & Hariri AR (2014). Reduced hippocampal and medial prefrontal gray matter mediate the association between reported childhood maltreatment and trait anxiety in adulthood and predict sensitivity to future life stress. Biology of Mood & Anxiety Disorders, 4(1), 12 10.1186/2045-5380-4-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MM, Cannistraci C, Hollon SD, Gore J, & Shelton R (2011). Childhood trauma history differentiates amygdala response to sad faces within MDD. Journal of Psychiatric Research, 45(7), 886–895. 10.1016/j.jpsychires.2010.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MM, White D, Hadley J, Hutcheson N, Shelton R, Sreenivasan K, & Deshpande G (2014). Early life trauma and directional brain connectivity within major depression. Human Brain Mapping, 35(9), 4815–4826. 10.1002/hbm.22514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2010). Childhood Adversities and Adult Psychiatric Disorders in the National Comorbidity Survey Replication I: Associations With First Onset of DSM-IV Disorders. Archives of General Psychiatry, 67(2), 113–123. 10.1001/archgenpsychiatry.2009.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagno A, Kang MS, Devenyi GA, Mathieu AP, Rosa-Neto P, Chakravarty M, & Walker C-D (2018). Reduced resting-state functional connectivity of the basolateral amygdala to the medial prefrontal cortex in preweaning rats exposed to chronic early-life stress. Brain Structure and Function, 223(8), 3711–3729. 10.1007/s00429-018-1720-3 [DOI] [PubMed] [Google Scholar]
- Guadagno A, Wong TP, & Walker C-D (2018). Morphological and functional changes in the preweaning basolateral amygdala induced by early chronic stress associate with anxiety and fear behavior in adult male, but not female rats. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 81, 25–37. 10.1016/j.pnpbp.2017.09.025 [DOI] [PubMed] [Google Scholar]
- Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, ... Lanzenberger R (2011). Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. NeuroImage, 56(3), 881–889. 10.1016/j.neuroimage.2011.02.064 [DOI] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, & Critchley HD (2009). Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biological Psychiatry, 66(5), 407–414. 10.1016/j.biopsych.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, & Nemeroff CB (2008). The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology, 33(6), 693–710. 10.1016/j.psyneuen.2008.03.008 [DOI] [PubMed] [Google Scholar]
- Hein TC, & Monk CS (2017). Research Review: Neural response to threat in children, adolescents, and adults after child maltreatment – a quantitative meta-analysis. Journal of Child Psychology and Psychiatry, 58(3), 222–230. 10.1111/jcpp.12651 [DOI] [PubMed] [Google Scholar]
- Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, & Essex MJ (2013). Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proceedings of the National Academy of Sciences, 110(47), 19119–19124. 10.1073/pnas.1310766110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiser J, & Koenigs M (2018). The Multifaceted Role of the Ventromedial Prefrontal Cortex in Emotion, Decision Making, Social Cognition, and Psychopathology. Biological Psychiatry, 83(8), 638–647. 10.1016/j.biopsych.2017.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Davidson RJ, Graham EK, Mroczek DK, Lachman ME, Seeman TE, ... Miller GE (2017). Frontal brain asymmetry, childhood maltreatment, and low-grade inflammation at midlife. Psychoneuroendocrinology, 75, 152–163. 10.1016/j.psyneuen.2016.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Nusslock R, & Miller GE (2017). Future directions in the study of early-life stress and physical and emotional health: Implications of the neuroimmune network hypothesis. Journal of Clinical Child & Adolescent Psychology, 1–15. 10.1080/15374416.2016.1266647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, & Cole SW (2011). Reciprocal regulation of the neural and innate immune systems. Nature Reviews Immunology, 11(9), 625–632. 10.1038/nri3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokela M, Virtanen M, Batty GD, & Kivimäki M (2016). Inflammation and Specific Symptoms of Depression. JAMA Psychiatry, 73(1), 87–88. 10.1001/jamapsychiatry.2015.1977 [DOI] [PubMed] [Google Scholar]
- Katsuki H, Nakai S, Hirai Y, Akaji K, Kiso Y, & Satoh M (1990). Interleukin-1 beta inhibits long-term potentiation in the CA3 region of mouse hippocampal slices. European Journal of Pharmacology, 181(3), 323–326. [DOI] [PubMed] [Google Scholar]
- Kraynak TE, Marsland AL, & Gianaros PJ (2018). Neural Mechanisms Linking Emotion with Cardiovascular Disease. Current Cardiology Reports, 20(12), 128 10.1007/s11886-018-1071-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraynak TE, Marsland AL, Wager TD, & Gianaros PJ (2018). Functional neuroanatomy of peripheral inflammatory physiology: A meta-analysis of human neuroimaging studies. Neuroscience & Biobehavioral Reviews, 94, 76–92. 10.1016/j.neubiorev.2018.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann CRW, Librizzi L, Closhen D, Pflanzner T, Lessmann V, Pietrzik CU, ... Luhmann HJ (2009). Mechanisms of C-reactive protein-induced blood-brain barrier disruption. Stroke, 40(4), 1458–1466. 10.1161/STROKEAHA.108.535930 [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, & Maseri A (2002). Inflammation and Atherosclerosis. Circulation, 105(9), 1135–1143. 10.1161/hc0902.104353 [DOI] [PubMed] [Google Scholar]
- Maier SF, & Watkins LR (1998). Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychological Review, 105(1), 83–107. 10.1037/0033-295X.105.1.83 [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, & Burdette JH (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19(3), 1233–1239. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, & Hariri AR (2008). Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biological Psychiatry, 64(6), 484–490. 10.1016/j.biopsych.2008.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Kuan DC-H, Sheu LK, Krajina K, & Manuck SB (2015). Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain, Behavior, and Immunity, 48, 195–204. 10.1016/j.bbi.2015.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Kuan DC-H, Sheu LK, Krajina K, Kraynak TE, Manuck SB, & Gianaros PJ (2017). Systemic inflammation and resting state connectivity of the default mode network. Brain, Behavior, and Immunity, 62, 162–170. 10.1016/j.bbi.2017.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Chang Y-F, Thurston RC, & Bromberger JT (2014). Child abuse is related to inflammation in mid-life women: Role of obesity. Brain, Behavior, and Immunity, 36, 29–34. 10.1016/j.bbi.2013.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell SE, & Cole DA (2007). Bias in cross-sectional analyses of longitudinal mediation. Psychological Methods, 12(1), 23–44. 10.1037/1082-989X.12.1.23 [DOI] [PubMed] [Google Scholar]
- McCrory EJ, & Viding E (2015). The theory of latent vulnerability: Reconceptualizing the link between childhood maltreatment and psychiatric disorder. Development and Psychopathology, 27(2), 493–505. 10.1017/S0954579415000115 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Conron KJ, Koenen KC, & Gilman SE (2010). Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: A test of the stress sensitization hypothesis in a population-based sample of adults. Psychological Medicine, 40(10), 1647–1658. 10.1017/S0033291709992121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, & Lambert HK (2014). Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neuroscience & Biobehavioral Reviews, 47, 578–591. 10.1016/j.neubiorev.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Winter W, Fox NA, Zeanah CH, & Nelson CA (2014). Widespread Reductions in Cortical Thickness Following Severe Early-42 Life Deprivation: A Neurodevelopmental Pathway to Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry, 76(8), 629–638. 10.1016/j.biopsych.2013.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta ND, Haroon E, Xu X, Woolwine BJ, Li Z, & Felger JC (2018). Inflammation negatively correlates with amygdala-ventromedial prefrontal functional connectivity in association with anxiety in patients with depression: Preliminary results. Brain, Behavior, and Immunity. 10.1016/j.bbi.2018.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, & Raison CL (2009). Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biological Psychiatry, 65(9), 732–741. 10.1016/j.biopsych.2008.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, ... Kobor MS (2009). Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Sciences, 106(34), 14716–14721. 10.1073/pnas.0902971106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Parker KJ (2011). Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin, 137(6), 959–997. 10.1037/a0024768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, ... Coppack SW (1997). Subcutaneous Adipose Tissue Releases Interleukin-6, But Not Tumor Necrosis Factor-α, in Vivo. The Journal of Clinical Endocrinology & Metabolism, 82(12), 4196–4200. 10.1210/jcem.82.12.4450 [DOI] [PubMed] [Google Scholar]
- Nusslock R, & Miller GE (2016). Early-life adversity and physical and emotional health across the lifespan: A neuroimmune network hypothesis. Biological Psychiatry, 80(1), 23–32. 10.1016/j.biopsych.2015.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TWW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, & Heim CM (2006). Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. The American Journal of Psychiatry, 163(9), 1630–1633. 10.1176/ajp.2006.163.9.1630 [DOI] [PubMed] [Google Scholar]
- Pope HG, & Hudson JI (1995). Can memories of childhood sexual abuse be repressed? Psychological Medicine, 25(1), 121–126. 10.1017/S0033291700028142 [DOI] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, & Petersen SE (2015). Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage, 0, 536–551. 10.1016/j.neuroimage.2014.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40(3), 879–891. [DOI] [PubMed] [Google Scholar]
- Price JL, & Drevets WC (2012). Neural circuits underlying the pathophysiology of mood disorders. Trends in Cognitive Sciences, 16(1), 61–71. 10.1016/j.tics.2011.12.011 [DOI] [PubMed] [Google Scholar]
- R Development Core Team. (2016). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. [Google Scholar]
- Raineki C, Bodnar TS, Holman PJ, Baglot SL, Lan N, & Weinberg J (2017). Effects of early-life adversity on immune function are mediated by prenatal environment: Role of prenatal alcohol exposure. Brain, Behavior, and Immunity, 66, 210–220. 10.1016/j.bbi.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Cortés MR, Belnoue L, & Sullivan RM (2012). Effects of Early-Life Abuse Differ across Development: Infant Social Behavior Deficits Are Followed by Adolescent Depressive-Like Behaviors Mediated by the Amygdala. Journal of Neuroscience, 32(22), 7758–7765. 10.1523/JNEUROSCI.5843-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Moriceau S, & Sullivan RM (2010). Developing a Neurobehavioral Animal Model of Infant Attachment to an Abusive Caregiver. Biological Psychiatry, 67(12), 1137–1145. 10.1016/j.biopsych.2009.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposa EB, Bower JE, Hammen CL, Najman JM, & Brennan PA (2014). A developmental pathway from early life stress to inflammation: The role of negative health behaviors. Psychological Science, 25(6), 1268–1274. 10.1177/0956797614530570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlich R, Stacey D, Opel N, Grotegerd D, Dohm K, Kugel H, ... Dannlowski U (2015). Evidence of an IFN-γ by early life stress interaction in the regulation of amygdala reactivity to emotional stimuli. Psychoneuroendocrinology, 62, 166–173. 10.1016/j.psyneuen.2015.08.008 [DOI] [PubMed] [Google Scholar]
- Rincón-Cortés M, & Sullivan RM (2016). Emergence of social behavior deficit, blunted corticolimbic activity and adult depression-like behavior in a rodent model of maternal maltreatment. Translational Psychiatry, 6(10), tp2016205 10.1038/tp.2016.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Shohamy D, & Wager TD (2012). Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences, 16(3), 147–156. 10.1016/j.tics.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk CE, Noll JG, Putnam FW, & Trickett PK (2010). A prospective examination of the role of childhood sexual abuse and physiological asymmetry in the development of psychopathology. Child Abuse & Neglect, 34(10), 752–761. 10.1016/j.chiabu.2010.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, & Nelson CA (2012). Variation in neural development as a result of exposure to institutionalization early in childhood. Proceedings of the National Academy of Sciences, 109(32), 12927–12932. 10.1073/pnas.1200041109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, & Spiers HJ (2003). Prefrontal and medial temporal lobe interactions in long-term memory. Nature Reviews Neuroscience, 4(8), 637–648. 10.1038/nrn1178 [DOI] [PubMed] [Google Scholar]
- Slopen N, Kubzansky LD, McLaughlin KA, & Koenen KC (2013). Childhood adversity and inflammatory processes in youth: A prospective study. Psychoneuroendocrinology, 38(2), 188–200. 10.1016/j.psyneuen.2012.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, & Lushene RE (1970). STAI Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press Inc. [Google Scholar]
- Spitzer RL, Kroenke K, & Williams JB (1999). Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA, 282(18), 1737–1744. [DOI] [PubMed] [Google Scholar]
- Steer RA, Ball R, Ranieri WF, & Beck AT (1999). Dimensions of the Beck Depression Inventory-II in clinically depressed outpatients. Journal of Clinical Psychology, 55(1), 117–128. [DOI] [PubMed] [Google Scholar]
- Stewart JC, Rand KL, Muldoon MF, & Kamarck TW (2009). A prospective evaluation of the directionality of the depression–inflammation relationship. Brain, Behavior, and Immunity, 23(7), 936–944. 10.1016/j.bbi.2009.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swardfager W, Rosenblat JD, Benlamri M, & McIntyre RS (2016). Mapping inflammation onto mood: Inflammatory mediators of anhedonia. Neuroscience & Biobehavioral Reviews, 64, 148–166. 10.1016/j.neubiorev.2016.02.017 [DOI] [PubMed] [Google Scholar]
- Takesian AE, & Hensch TK (2013). Chapter 1—Balancing Plasticity/Stability Across Brain Development. In Merzenich MM, Nahum M, & Van Vleet TM (Eds.), Progress in Brain Research (pp. 3–34). 10.1016/B978-0-444-63327-9.00001-1 [DOI] [PubMed] [Google Scholar]
- Tang Y, Kong L, Wu F, Womer F, Jiang W, Cao Y, ... Wang F (2013). Decreased functional connectivity between the amygdala and the left ventral prefrontal cortex in treatment-naive patients with major depressive disorder: A resting-state functional magnetic resonance imaging study. Psychological Medicine, 43(9), 1921–1927. 10.1017/S0033291712002759 [DOI] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Ohashi K, & Polcari A (2014). Childhood Maltreatment: Altered Network Centrality of Cingulate, Precuneus, Temporal Pole and Insula. Biological Psychiatry, 76(4), 297–305. 10.1016/j.biopsych.2013.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, & Samson JA (2013). Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. The American Journal of Psychiatry, 170(10), 1114–1133. 10.1176/appi.ajp.2013.12070957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, & Ohashi K (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews Neuroscience, 17(10), 652–666. 10.1038/nrn.2016.111 [DOI] [PubMed] [Google Scholar]
- Tingley D, Yamamoto T, Hirose K, Keele L, & Imai K (2014). Mediation: R package for causal mediation analysis. Journal of Statistical Software, 59(5), 1–38.26917999 [Google Scholar]
- Tomoda A, Suzuki H, Rabi K, Sheu Y-S, Polcari A, & Teicher MH (2009). Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. NeuroImage, 47 Suppl 2, T66–71. 10.1016/j.neuroimage.2009.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, & Sheridan MA (2010). A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Frontiers in Human Neuroscience, 3 10.3389/neuro.09.068.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, ... Joliot M (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15(1), 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Usher JA, & Neisser U (1993). Childhood amnesia and the beginnings of memory for four early life events. Journal of Experimental Psychology: General, 122(2), 155–165. 10.1037/0096-3445.122.2.155 [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, & Buckner RL (2012). The influence of head motion on intrinsic functional connectivity MRI. NeuroImage, 59(1), 431–438. 10.1016/j.neuroimage.2011.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri ND, & Rodríguez-Iturbe B (2006). Mechanisms of Disease: Oxidative stress and inflammation in the pathogenesis of hypertension. Nature Reviews Nephrology, 2(10), 582–593. 10.1038/ncpneph0283 [DOI] [PubMed] [Google Scholar]
- Watson D, & Pennebaker JW (1989). Health complaints, stress, and distress: Exploring the central role of negative affectivity. Psychological Review, 96(2), 234–254. 10.1037/0033-295X.96.2.234 [DOI] [PubMed] [Google Scholar]
- Wegman HL, & Stetler C (2009). A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosomatic Medicine, 71(8), 805–812. 10.1097/PSY.0b013e3181bb2b46 [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connectivity, 2(3), 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Wieck A, Andersen SL, & Brenhouse HC (2013). Evidence for a neuroinflammatory mechanism in delayed effects of early life adversity in rats: Relationship to cortical NMDA receptor expression. Brain, Behavior, and Immunity, 28, 218–226. 10.1016/j.bbi.2012.11.012 [DOI] [PubMed] [Google Scholar]
- Yan C-G, Rincón-Cortés M, Raineki C, Sarro E, Colcombe S, Guilfoyle DN, ... Castellanos FX (2017). Aberrant development of intrinsic brain activity in a rat model of caregiver maltreatment of offspring. Translational Psychiatry, 7(1), e1005 10.1038/tp.2016.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Xu X, Chen G, Mehta ND, Haroon E, Miller AH, ... Felger JC (in press). Inflammation and decreased functional connectivity in a widely-distributed network in depression: Centralized effects in the ventral medial prefrontal cortex. Brain, Behavior, and Immunity. 10.1016/j.bbi.2019.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, & Goshen I (2011). Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain, Behavior, and Immunity, 25(2), 181–213. 10.1016/j.bbi.2010.10.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.