Abstract
Background
Glycopyrrolate/formoterol fumarate metered dose inhaler (GFF MDI) is a long-acting muscarinic antagonist/long-acting β2-agonist fixed-dose combination therapy delivered by MDI, formulated using innovative co-suspension delivery technology. The PINNACLE-4 study evaluated the efficacy and safety of GFF MDI in patients with moderate-to-very severe chronic obstructive pulmonary disease (COPD) from Asia, Europe, and the USA. This article presents the results from the China subpopulation of PINNACLE-4.
Methods
In this randomized, double-blind, placebo-controlled, parallel-group Phase III study (NCT02343458), patients received GFF MDI 18/9.6 µg, glycopyrrolate (GP) MDI 18 µg, formoterol fumarate (FF) MDI 9.6 µg, or placebo MDI (all twice daily) for 24 weeks. The primary endpoint was change from baseline in morning pre-dose trough forced expiratory volume in 1 second at Week 24. Secondary lung function endpoints and patient-reported outcome measures were also assessed. Safety was monitored throughout the study.
Results
Overall, 466 patients from China were included in the intent-to-treat population (mean age 63.6 years, 95.7% male). Treatment with GFF MDI improved the primary endpoint compared to GP MDI, FF MDI, and placebo MDI (least squares mean differences: 98, 104, and 173 mL, respectively; all P≤0.0001). GFF MDI also improved daily total symptom scores and time to first clinically important deterioration versus monocomponents and placebo MDI, and Transition Dyspnea Index focal score versus placebo MDI. Rates of treatment-emergent adverse events were similar across the active treatment groups and slightly higher in the placebo MDI group.
Conclusion
GFF MDI improved lung function and daily symptoms versus monocomponents and placebo MDI and improved dyspnea versus placebo MDI. All treatments were well tolerated with no unexpected safety findings. Efficacy and safety results were generally consistent with the global PINNACLE-4 population, supporting the use of GFF MDI in patients with COPD from China.
Keywords: bronchodilator, COPD, co-suspension delivery technology, LAMA/LABA, exacerbations
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of mortality and morbidity worldwide.1,2 In China, COPD is a serious public health concern, accounting for 910,809 deaths in 2013 (31.1% of the world total for COPD that year).3 Two recently published large cross-sectional studies estimated the prevalence of COPD to be approximately 14% of Chinese adults over the age of 40 in 2015.4,5
Glycopyrrolate/formoterol fumarate metered dose inhaler (GFF MDI) is a long-acting muscarinic antagonist (LAMA)/long-acting β2-agonist (LABA) fixed-dose combination (FDC) formulated using innovative co-suspension delivery technology. GFF MDI was first approved for the long-term maintenance treatment of airflow obstruction in patients with COPD in the USA in 20166 and is the first LAMA/LABA FDC available as an MDI. It has been shown to provide effective dose delivery to the whole lung in vivo,7 as well as consistent aerosol performance in vitro, even in the presence of simulated patient-handling errors.8 The pivotal Phase III PINNACLE-1 and PINNACLE-2 studies (NCT01854645 and NCT01854658) demonstrated the efficacy and safety of GFF MDI in patients with COPD from the USA, Australia, and New Zealand over 24 weeks.9 A 28-week safety extension of these studies (PINNACLE-3; NCT01970878) confirmed the long-term safety and efficacy of GFF MDI.10
As patients of different ethnicities can respond differently to pharmacological therapies,11,12 it is important to assess the efficacy and safety of new treatments in a range of patients from different geographical locations and ethnic backgrounds. The PINNACLE-4 study (NCT02343458) evaluated the efficacy and safety of GFF MDI in a geographically expanded population, which included patients from Asia, Europe, and the USA.13 This article presents the results from the China subpopulation and discusses these in the context of the results from the global patient population.13
Materials And Methods
Study Design And Treatment
A detailed description of the study design has been published.13 Briefly, patients in this double-blind, placebo-controlled, parallel-group Phase III study were randomized in a 7:6:6:3 scheme to receive treatment with either GFF MDI 18/9.6 µg, glycopyrrolate (GP) MDI 18 µg, formoterol fumarate (FF) MDI 9.6 µg, or matched placebo MDI (all twice daily) for 24 weeks. All treatments were administered as two actuations. Each actuation of GFF MDI contains 9 µg glycopyrrolate and 4.8 µg formoterol fumarate, and the GFF MDI 18/9.6 µg dose is equivalent to glycopyrronium/formoterol fumarate dihydrate 14.4/10 µg.
During screening, patients were provided with open-label ipratropium bromide MDI (34 µg) administered four times daily for COPD maintenance therapy. Open-label salbutamol sulfate MDI (90 µg) was provided to use as rescue medication, as needed, throughout the study.
The study was conducted at multiple sites in Asia, Europe, and the USA (including 33 sites across China) in accordance with Good Clinical Practice, including the International Council for Harmonisation and the Declaration of Helsinki, and patients provided written informed consent prior to screening. Study endpoints differed according to the regional regulatory registration requirements, and this article reports the approach that satisfies the filing requirements of Chinese regulatory authorities.
Study Population
Inclusion and exclusion criteria have previously been reported.9,13 Briefly, patients were 40–80 years of age with an established clinical history of COPD (as defined by the American Thoracic Society/European Respiratory Society),14 and a smoking history of ≥10 pack-years. Patients were required to demonstrate a forced expiratory volume in 1 second (FEV1)/forced vital capacity ratio <0.70 and FEV1 <80% predicted normal value at screening.
Assessments
The primary endpoint of this study (China approach) was the change from baseline in morning pre-dose trough FEV1 at Week 24. Secondary lung function endpoints were change from baseline in morning pre-dose trough FEV1 over 24 weeks, peak change from baseline in FEV1 within 2 hrs post-dosing at Week 24, and time to onset of action on Day 1 (defined as the first timepoint at which the difference from placebo MDI was statistically significant).
Secondary symptom and health-related quality of life (HRQoL) endpoints were Transition Dyspnea Index (TDI) focal score over 24 weeks, change from baseline in St George's Respiratory Questionnaire (SGRQ) total score at Week 24, and change from baseline in average daily rescue salbutamol use over 24 weeks.
Other endpoints included change from baseline in daily total symptom score (including clinical symptoms of cough, shortness of breath, sputum volume, night-time awakenings, and salbutamol use; complete scoring system described in Supplementary Table 1) and rate of/time to first moderate or severe exacerbation. Time to first clinically important deterioration (CID) was also assessed (defined as the first occurrence of either a decline of ≥100 mL in trough FEV1, a moderate or severe COPD exacerbation, or an increase of ≥4 units in SGRQ total score).
Safety endpoints included 12-lead electrocardiograms, clinical laboratory testing, vital sign measurements, and adverse event (AE) monitoring.
Statistical Analysis
This Chinese subgroup analysis of PINNACLE-4 was pre-specified. However, as PINNACLE-4 was a multinational trial, the sample size for China was chosen to fulfil local regulatory requirements for consistency with the overall population, rather than statistical significance. Hence, statistical analyses in the Chinese subgroup were considered exploratory and no adjustments for multiplicity were made. Treatment differences are presented with 95% confidence intervals (CIs) throughout the manuscript; p-values are provided in the tables. P-values <0.05 should only be interpreted in terms of nominal significance. The China ITT population included all patients enrolled at sites in mainland China who were randomized and received at least one dose of study treatment. The China per-protocol (PP) population was a subset of the China ITT population defined as all patients with post-randomization data obtained prior to a major protocol deviation. All patients in the China ITT population were included in the safety population, except that patients were analyzed according to treatment received instead of treatment randomized. The China symptomatic population included all patients in the China ITT population with a COPD Assessment Test (CAT) score ≥15 at screening. Patients with an average baseline rescue medication use of ≥1 puff/day were defined as the China rescue medication user population. The symptomatic and rescue medication user populations were both pre-defined in the study protocol and statistical analysis plan.
The primary endpoint, change from baseline in morning pre-dose trough FEV1 at Week 24, was analyzed in the China ITT population, using a repeated-measures (RM) linear model with unstructured covariance matrix. The linear RM model included baseline FEV1 and reversibility to salbutamol as continuous covariates and visit, treatment, and the treatment by visit interaction as categorical covariates. An additional supporting analysis of the primary endpoint in the China PP population was also performed, with treatment differences at individual timepoints estimated by the RM model.
All secondary endpoints were also analyzed using the China ITT population, except for daily rescue medication use, which was analyzed in the China rescue medication user population. TDI focal score over 24 weeks and change from baseline in SGRQ score at Week 24 were analyzed in both the China ITT and China symptomatic populations. The majority of secondary endpoints were analyzed using similar models to the primary endpoint. Time to first moderate/severe exacerbation was analyzed using the Cox regression model, adjusting for baseline % predicted FEV1, baseline COPD exacerbation history (yes/no), baseline CAT score, smoking status at baseline (former smoker/current smoker), baseline continuous eosinophil count, and inhaled corticosteroid use at baseline (yes/no). Exacerbation rates were analyzed using negative binomial regression, adjusting for the same covariates, with treatment exposure used as an offset variable. Further details regarding the statistical analysis for the full PINNACLE-4 study have been reported previously.13
Results
Patient Disposition
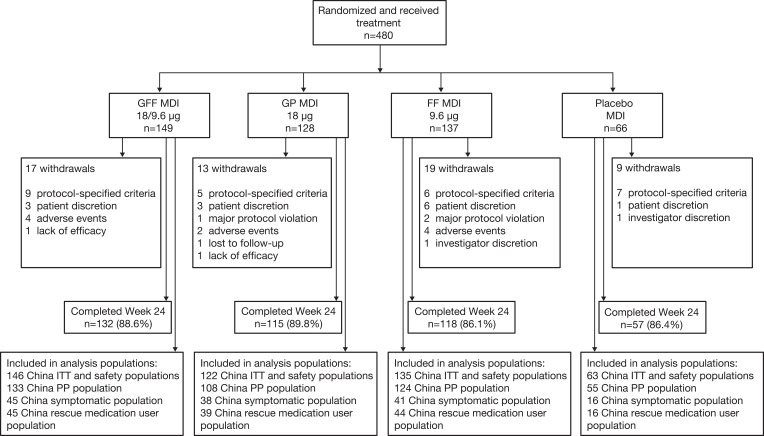
In this study, 480 patients were treated at sites located in China (globally, 1,756 patients were randomized and received treatment). Of the 466 patients who were included in the China ITT and safety populations, 422 completed Week 24. The PP, symptomatic, and rescue medication user populations included 420, 140, and 144 patients, respectively. Fifty-seven patients (12.2%) from the China ITT population discontinued from the study, with the most common reasons for withdrawal being patient discretion or protocol-specified criteria (Figure 1).
Figure 1.
Patient disposition (all patients randomized in China).
Abbreviations: FF, formoterol fumarate; GFF, glycopyrrolate/formoterol fumarate; GP, glycopyrrolate; MDI, metered dose inhaler.
The mean patient age of the China ITT population was 63.6 years and 95.7% were males. Patient demographics and characteristics were similar between treatment groups (Table 1). Although this study enrolled patients with moderate-to-very severe COPD, 17 patients were classed as having mild COPD due to the application of the Asian correction factor (0.88)15 at the time of analysis and were listed as protocol deviations.
Table 1.
Patient Demographics And Characteristics (China ITT Population)
| Parameter | GFF MDI 18/9.6 µg n=146 | GP MDI 18 µg n=122 | FF MDI 9.6 µg n=135 | Placebo MDI n=63 |
|---|---|---|---|---|
| Mean age, years (SD) | 64.1 (6.4) | 63.5 (8.0) | 63.0 (7.6) | 63.5 (6.2) |
| Male, n (%) | 140 (95.9) | 116 (95.1) | 130 (96.3) | 60 (95.2) |
| Race, n (%) | ||||
| Asian | 146 (100) | 122 (100) | 135 (100) | 63 (100) |
| Mean BMI, kg/m2 (SD) | 22.9 (3.3) | 23.3 (3.3) | 22.8 (3.3) | 23.2 (3.4) |
| Smoking status, n (%) | ||||
| Current | 50 (34.2) | 42 (34.4) | 43 (31.9) | 20 (31.7) |
| Former | 96 (65.8) | 80 (65.6) | 92 (68.1) | 43 (68.3) |
| Mean number of pack-years smokeda (SD) | 37.0 (23.8) | 34.7 (21.0) | 39.7 (22.6) | 34.9 (19.8) |
| COPD severityb, n (%) | ||||
| Mildc | 3 (2.1) | 7 (5.7) | 3 (2.2) | 4 (6.3) |
| Moderate | 78 (53.4) | 66 (54.1) | 72 (53.3) | 29 (46.0) |
| Severe | 59 (40.4) | 45 (36.9) | 56 (41.5) | 29 (46.0) |
| Very severe | 6 (4.1) | 4 (3.3) | 4 (3.0) | 1 (1.6) |
| Mean COPD duration, years (SD) | n=141 | n=122 | n=132 | n=62 |
| 4.2 (5.5) | 3.7 (5.0) | 3.5 (4.2) | 4.3 (5.5) | |
| Mean post-bronchodilator FEV1, % predicted (SD) | 53.33 (14.76) | 54.45 (14.81) | 53.55 (14.01) | 53.62 (15.84) |
| Reversibility to salbutamol | ||||
| Reversibled, n (%) | 76 (52.1) | 60 (49.2) | 63 (46.7) | 32 (50.8) |
| Mean reversibility post-bronchodilator, % (SD) | 20.9 (16.1) | 20.4 (14.7) | 19.8 (13.9) | 20.6 (15.3) |
| Prior use of ICSe, n (%) | 49 (33.6) | 39 (32.0) | 56 (41.5) | 27 (42.9) |
| Mean BDI score (SD) | n=142 | n=119 | n=129 | n=59 |
| 7.0 (2.1) | 7.1 (2.0) | 7.3 (2.1) | 6.4 (2.3) | |
| Mean baseline SGRQ total score (SD) | n=129 | n=110 | n=116 | n=53 |
| 36.0 (15.5) | 33.2 (15.3) | 32.6 (14.0) | 34.9 (12.0) | |
| Mean CAT total scoref (SD) | n=145 | n=121 | n=135 | n=63 |
| 12.0 (6.0) | 12.3 (6.8) | 11.7 (6.0) | 11.0 (5.9) | |
| Mean rescue medication useg at baseline, puffs/day (SD) | n=45 | n=39 | n=44 | n=16 |
| 3.6 (3.1) | 3.5 (2.4) | 3.2 (2.3) | 4.4 (4.4) |
Notes: aNumber of pack-years smoked = (number of cigarettes each day/20) × number of years smoked. bSeverity of COPD was based on the non-missing post-salbutamol assessment at screening. cThese patients were characterized as having mild COPD due to the application of an Asian correction factor to baseline lung function assessments at the time of analysis. dReversible is defined as improvement in FEV1 post-salbutamol administration compared to pre-salbutamol of ≥12% and ≥200 mL. eDefined as using ICS on the day of the first dose of study medication. fCAT total score is the sum of eight CAT item scores (range: 0–40). gRescue medication use was analyzed in the China rescue medication user population, defined as all patients in the China ITT population with mean baseline rescue salbutamol use of ≥1 puff/day.
Abbreviations: BDI, Baseline Dyspnea Index; BMI, body mass index; CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FF, formoterol fumarate; GFF, glycopyrrolate/formoterol fumarate; GP, glycopyrrolate; ICS, inhaled corticosteroid; ITT, intent-to-treat; MDI, metered dose inhaler; SD, standard deviation; SGRQ, St George’s Respiratory Questionnaire.
Lung Function
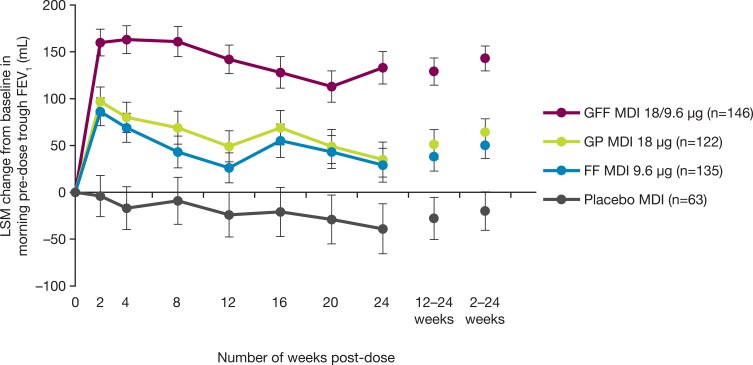
Treatment with GFF MDI improved the change from baseline in morning pre-dose trough FEV1 at Week 24 (primary endpoint) compared to placebo MDI (least squares mean [LSM] difference 173 mL [95% CI 110, 235]; Table 2 and Figure 2) and compared to GP MDI and FF MDI (LSM differences of 98 mL (48, 148) and 104 mL (55, 153), respectively (Table 2 and Figure 2). Results in the PP population were similar, with LSM differences in trough FEV1 at Week 24 for GFF MDI versus placebo MDI, GP MDI, and FF MDI of 169 mL (95% CI 102, 236), 121 mL (69, 174), and 113 mL (61, 164), respectively.
Table 2.
Primary And Secondary Lung Function Endpoints (China ITT Population)
| GFF MDI 18/9.6 µg | GP MDI 18 µg | FF MDI 9.6 µg | Placebo MDI | |
|---|---|---|---|---|
| Primary endpoint | ||||
| Change from baseline in morning pre-dose trough FEV1 at Week 24, mL | ||||
| n | 126 | 110 | 116 | 54 |
| LSM (SE) | 133 (17.3) | 35 (18.7) | 29 (18.1) | −39 (−26.6) |
| Treatment difference for GFF MDI vs monocomponents and placebo MDI | ||||
| LSM (95% CI) | NA | 98 (48, 148) | 104 (55, 153) | 173 (110, 235) |
| P-value | NA | 0.0001 | <0.0001 | <0.0001 |
| Secondary endpoints | ||||
| Change from baseline in morning pre-dose trough FEV1 over 24 weeksa, mL | ||||
| n | 143 | 121 | 131 | 60 |
| LSM (SE) | 143 (13.3) | 64 (14.5) | 50 (13.9) | −20 (20.6) |
| Treatment difference for GFF MDI vs monocomponents and placebo MDI | ||||
| LSM (95% CI) | NA | 79 (40, 118) | 93 (55, 131) | 163 (115, 212) |
| P-value | NA | <0.0001 | <0.0001 | <0.0001 |
| Peak change from baseline in FEV1 within 2 hrs post-dosing at Week 24, mL | ||||
| n | 128 | 110 | 116 | 54 |
| LSM (SE) | 377 (19.7) | 195 (21.3) | 226 (20.7) | 72 (30.3) |
| Treatment difference for GFF MDI vs monocomponents and placebo MDI | ||||
| LSM (95% CI) | NA | 182 (125, 239) | 151 (95, 207) | 305 (234, 376) |
| P-value | NA | <0.0001 | <0.0001 | <0.0001 |
| Onset of action on Day 1 (change from baseline in FEV1), mL | ||||
| Difference vs placebo MDI at 5 mins post-dose | ||||
| n | 119 | 104 | 118 | 52 |
| LSM (95% CI) | 184 (150, 219) | 34 (−1, 70) | 169 (135, 204) | NA |
| P-value | <0.0001 | 0.0557 | <0.0001 | NA |
| Difference vs placebo MDI at 15 mins post-dose | ||||
| n | 143 | 117 | 132 | 62 |
| LSM (95% CI) | 225 (189, 262) | 76 (38, 113) | 193 (156, 230) | NA |
| P-value | <0.0001 | <0.0001 | <0.0001 | NA |
Note: aMorning pre-dose trough FEV1 over 24 weeks was based on assessments at Weeks 2, 4, 8, 12, 16, 20, and 24. All p-values <0.05 should only be interpreted in terms of nominal significance as the analyses in the Chinese subgroup were considered exploratory.
Abbreviations: CI, confidence interval; FEV1, forced expiratory volume in 1 second; FF, formoterol fumarate; GFF, glycopyrrolate/formoterol fumarate; GP, glycopyrrolate; ITT, intent-to-treat; LSM, least squares mean; MDI, metered dose inhaler; NA, not applicable; SE, standard error.
Figure 2.
Least squares mean change (±SE) from baseline in morning pre-dose trough FEV1 over 24 weeks (China ITT population).
Note: Morning pre-dose trough FEV1 over 24 weeks was based on assessments at Weeks 2, 4, 8, 12, 16, 20, and 24.
Abbreviations: FEV1, forced expiratory volume in 1 second; FF, formoterol fumarate; GFF, glycopyrrolate/formoterol fumarate; GP, glycopyrrolate; ITT, intent-to-treat; LSM, least squares mean; MDI, metered dose inhaler; SE, standard error.
Improvements in the secondary lung function endpoints (change from baseline in morning pre-dose trough FEV1 over 24 weeks and peak change from baseline in FEV1 within 2 hrs post-dosing at Week 24) were also larger in the GFF MDI treatment group compared to placebo MDI and monocomponents (Table 2). Compared to placebo MDI, GFF MDI and FF MDI demonstrated a benefit in FEV1 within 5 mins post-dose, and GP MDI demonstrated a benefit within 15 mins post-dose (Table 2).
Symptoms And HRQoL
Estimates for TDI focal score over 24 weeks showed a clinically meaningful improvement above 1 unit16 with GFF MDI compared to placebo MDI (LSM difference 1.11, 95% CI 0.52, 1.69) in the China ITT population and in the China symptomatic population (LSM difference 1.22, 95% CI –0.02, 2.45; Table 3). The estimated difference for change from baseline in SGRQ score at Week 24 with GFF MDI versus placebo MDI was greater in the Chinese symptomatic population (LSM difference −5.04 [95% CI –13.66, 3.58]) than in the Chinese ITT population (LSM difference −3.24 [95% CI −6.76, 0.28]; Table 3). No clear trend was observed for GFF MDI versus monocomponents (Table 3).
Table 3.
Secondary Patient-Reported Outcome Endpoints (China ITT Population/China Symptomatic Population)
| China ITT Population | China Symptomatic Populationa | |||||||
|---|---|---|---|---|---|---|---|---|
| GFF MDI 18/9.6 µg |
GP MDI 18 µg |
FF MDI 9.6 µg |
Placebo MDI | GFF MDI 18/9.6 µg |
GP MDI 18 µg | FF MDI 9.6 µg | Placebo MDI | |
| TDI focal score over 24 weeksb | ||||||||
| n | 142 | 119 | 129 | 59 | 43 | 37 | 39 | 15 |
| LSM (SE) | 2.5 (0.16) | 2.0 (0.18) | 2.3 (0.17) | 1.4 (0.25) | 2.2 (0.32) | 1.7 (0.34) | 2.1 (0.33) | 1.0 (0.54) |
| Treatment difference for GFF MDI vs monocomponents and placebo MDI | ||||||||
| LSM | NA | 0.42 | 0.20 | 1.11 | NA | 0.46 | 0.10 | 1.22 |
| 95% CI | NA | −0.05, 0.89 | −0.26, 0.66 | 0.52, 1.69 | NA | −0.46, 1.38 | −0.81, 1.01 | −0.02, 2.45 |
| P-value | NA | 0.0780 | 0.3924 | 0.0002 | NA | 0.3234 | 0.8267 | 0.0543 |
| Change from baseline in SGRQ total score at Week 24 | ||||||||
| n | 129 | 110 | 116 | 53 | 38 | 34 | 35 | 13 |
| LSM (SE) | −8.5 (0.97) | −7.3 (1.05) | −9.4 (1.02) | −5.3 (1.51) | −12.0 (2.17) | −12.3 (2.33) | −13.7 (2.28) | −7.0 (3.75) |
| Treatment difference for GFF MDI vs monocomponents and placebo MDI | ||||||||
| LSM | NA | −1.21 | 0.87 | −3.24 | NA | 0.26 | 1.70 | −5.04 |
| 95% CI | NA | −4.02, 1.61 | −1.90, 3.64 | −6.76, 0.28 | NA | −6.05, 6.57 | −4.54, 7.94 | −13.66, 3.58 |
| P-value | NA | 0.3996 | 0.5373 | 0.0714 | NA | 0.9352 | 0.5894 | 0.2489 |
Notes: aThe China symptomatic population was defined as patients in the China ITT population with a CAT score ≥15 at screening. bTDI focal score over 24 weeks was based on assessments at Weeks 4, 8, 12, 16, 20, and 24. All p-values <0.05 should only be interpreted in terms of nominal significance as the analyses in the Chinese subgroup were considered exploratory.
Abbreviations: CAT, COPD Assessment Test; CI, confidence interval; COPD, chronic obstructive pulmonary disease; FF, formoterol fumarate; GFF, glycopyrrolate/formoterol fumarate; GP, glycopyrrolate; ITT, intent-to-treat; LSM, least squares mean; MDI, metered dose inhaler; NA, not applicable; SE, standard error; SGRQ, St George’s Respiratory Questionnaire; TDI, Transition Dyspnea Index.
Of the 466 patients in the China ITT population, 144 had a mean baseline rescue salbutamol use of ≥1 puff/day and were included in the rescue medication user population. In these patients, GFF MDI treatment reduced daily rescue medication use over 24 weeks compared to GP MDI (LSM difference −1.42 puffs/day, 95% CI –2.26, –0.59). Treatment differences with GFF MDI versus FF MDI and placebo MDI were −0.63 (–1.45, 0.19) and −1.11 (–2.24, 0.02) puffs/day, respectively; Supplementary Table 2).
GFF MDI improved daily, daytime, and night-time symptom scores over 24 weeks compared to placebo MDI, with treatment differences that were more pronounced for daytime symptoms than for night-time symptoms (LSM treatment differences [95% CI] between GFF MDI and placebo MDI of −2.12 [–2.94, –1.30], −1.25 [–1.72, –0.78], and −0.91 [–1.33, –0.49] for daily, daytime, and night-time symptom scores, respectively; Supplementary Table 3). Treatment differences between GFF MDI and monocomponent MDIs are summarized in Supplementary Table 3.
COPD Exacerbations And CID
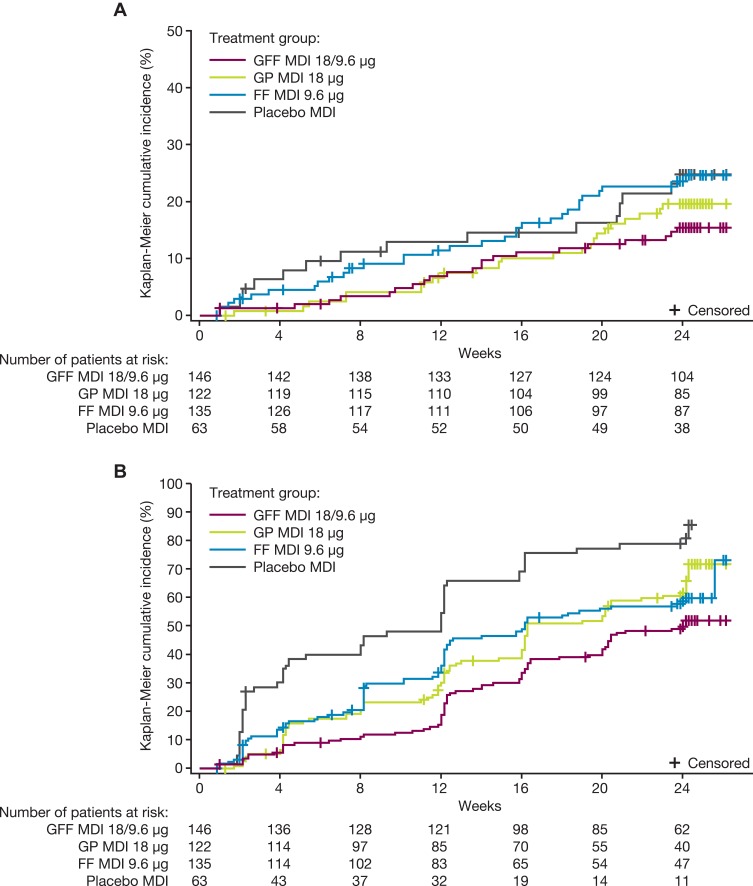
While the PINNACLE-4 study was not powered for formal comparisons in exacerbation outcomes, the reduction in risk of a moderate or severe COPD exacerbation for GFF MDI compared to GP MDI, FF MDI, and placebo MDI was estimated to be 29%, 43%, and 43%, respectively, with hazard ratios (95% CIs) for time to first moderate or severe exacerbation for GFF MDI versus comparators of 0.71 (0.39, 1.28), 0.57 (0.33, 1.00), and 0.57 (0.29, 1.10), respectively (Figure 3A, Table 4). The model-estimated rate of moderate or severe COPD exacerbations (per year) was 0.38 in the GFF MDI group versus 0.46, 0.58, and 0.60 for GP MDI, FF MDI, and placebo MDI (Table 4). Treatment with GFF MDI reduced the risk of a CID versus GP MDI, FF MDI, and placebo MDI, with hazard ratios for time to first CID of 0.65 (95% CI 0.47, 0.90), 0.71 (0.52, 0.99), and 0.36 (0.25, 0.52), respectively (Figure 3B, Table 4).
Figure 3.
Kaplan–Meier curves for (A) time to first moderate or severe COPD exacerbationa and (B) time to first CIDb (both China ITT population).
Notes: aTime to first moderate or severe exacerbation (weeks) = (date of first COPD exacerbation – first treatment administration date + 1)/7. bTime to CID (weeks) = (date of CID – first treatment administration date + 1)/7.
Abbreviations: CID, clinically important deterioration; COPD, chronic obstructive pulmonary disease; FF, formoterol fumarate; GFF, glycopyrrolate/formoterol fumarate; GP, glycopyrrolate; ITT, intent-to-treat; MDI, metered dose inhaler.
Table 4.
Exacerbations And CIDa (China ITT Population)
| GFF MDI 18/9.6 µg n=146 |
GP MDI 18 µg n=122 |
FF MDI 9.6 µg n=135 |
Placebo MDI n=63 |
|
|---|---|---|---|---|
| Exacerbations | ||||
| Patients with a moderate or severe exacerbation | ||||
| n (%) | 22 (15.1) | 23 (18.9) | 31 (23.0) | 15 (23.8) |
| Time to first moderate or severe COPD exacerbation | ||||
| GFF MDI vs monocomponents and placebo MDI | ||||
| Hazard ratio | NA | 0.71 | 0.57 | 0.57 |
| 95% CI | NA | 0.39, 1.28 | 0.33, 1.00 | 0.29, 1.10 |
| P-value | NA | 0.2543 | 0.0501 | 0.0950 |
| Rate of moderate or severe COPD exacerbations (per year)b | ||||
| Rate | 0.44 | 0.47 | 0.62 | 0.65 |
| Model-estimated rate | 0.38 | 0.46 | 0.58 | 0.60 |
| GFF MDI vs monocomponents and placebo MDI | ||||
| Model-estimated rate ratio | NA | 0.82 | 0.65 | 0.63 |
| 95% CI | NA | 0.46, 1.49 | 0.38, 1.12 | 0.32, 1.22 |
| P-value | NA | 0.5200 | 0.1224 | 0.1689 |
| CID | ||||
| Patients with a CID | ||||
| n (%) | 73 (50.0) | 80 (65.6) | 77 (57.0) | 51 (81.0) |
| Median time to CIDc (weeks) | 24.1 | 16.3 | 16.1 | 12.0 |
| Time to first CID | ||||
| GFF MDI vs monocomponents and placebo MDI | ||||
| Hazard ratio | NA | 0.65 | 0.71 | 0.36 |
| 95% CI | NA | 0.47, 0.90 | 0.52, 0.99 | 0.25, 0.52 |
| P-value | NA | 0.0095 | 0.0428 | <0.0001 |
Notes: aCID is defined as the first occurrence of one of the following events: ≥100 mL decline in trough FEV1; a treatment-emergent moderate or severe COPD exacerbation; or ≥4-unit increase in SGRQ total score. bRate of exacerbations per year = total number of exacerbations/total years of exposure across all patients for the treatment. cTime to CID (weeks) = (date of CID – first treatment administration date + 1)/7. All p-values <0.05 should only be interpreted in terms of nominal significance as the analyses in the Chinese subgroup were considered exploratory.
Abbreviations: CI, confidence interval; CID, clinically important deterioration; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FF, formoterol fumarate; GFF, glycopyrrolate/formoterol fumarate; GP, glycopyrrolate; ITT, intent-to-treat; MDI, metered dose inhaler; NA, not applicable; SGRQ, St George’s Respiratory Questionnaire.
Safety
The incidence of treatment-emergent AEs (TEAEs) was lowest in the FF MDI group (51.1%) and highest in the placebo MDI group (66.7%; Table 5). The rate of serious TEAEs related to study treatment ranged from 0.7% (GFF MDI) to 3.2% (placebo MDI) across treatment groups. The most commonly occurring AEs are listed in Table 5.
Table 5.
Summary Of AEs (China Safety Population)
| Parameter | GFF MDI 18/9.6 µg n=146 | GP MDI 18 µg n=122 | FF MDI 9.6 µg n=135 | Placebo MDI n=63 |
|---|---|---|---|---|
| Patients with ≥1 TEAE | 83 (56.8) | 71 (58.2) | 69 (51.1) | 42 (66.7) |
| Patients with TEAEs relateda to study treatment | 18 (12.3) | 18 (14.8) | 17 (12.6) | 7 (11.1) |
| Patients with serious TEAEs | 15 (10.3) | 10 (8.2) | 13 (9.6) | 7 (11.1) |
| Patients with serious TEAEs relateda to study treatment | 1 (0.7) | 2 (1.6) | 3 (2.2) | 2 (3.2) |
| Patients with TEAEs leading to early discontinuation | 10 (6.8) | 6 (4.9) | 9 (6.7) | 6 (9.5) |
| Deaths (all cause) during treatment period | 1 (0.7) | 0 | 1 (0.7) | 0 |
| Deaths (all cause) during treatment period + 14 days | 1 (0.7) | 0 | 1 (0.7) | 0 |
| AEs occurring in ≥4% of patients in any treatment arm (preferred term), n (%) | ||||
| Upper respiratory tract infection | 18 (12.3) | 20 (16.4) | 18 (13.3) | 11 (17.5) |
| Viral upper respiratory tract infection | 12 (8.2) | 15 (12.3) | 15 (11.1) | 4 (6.3) |
| Chronic obstructive pulmonary diseaseb | 7 (4.8) | 5 (4.1) | 5 (3.7) | 6 (9.5) |
| Blood glucose increased | 2 (1.4) | 4 (3.3) | 2 (1.5) | 4 (6.3) |
| Hyperlipidemia | 3 (2.1) | 6 (4.9) | 1 (0.7) | 2 (3.2) |
| Protein urine present | 5 (3.4) | 1 (0.8) | 0 | 3 (4.8) |
| Blood triglycerides increased | 3 (2.1) | 1 (0.8) | 2 (1.5) | 3 (4.8) |
Notes: Data are n (%). aRelated = possibly, probably, or definitely related in the opinion of the Investigator. bWorsening of COPD defined as a COPD exacerbation since the patient’s last visit. COPD exacerbations were only recorded as an AE if they were considered to be a serious TEAE.
Abbreviations: AE, adverse event; COPD, chronic obstructive pulmonary disease; FF, formoterol fumarate; GFF, glycopyrrolate/formoterol fumarate; GP, glycopyrrolate; MDI, metered dose inhaler; TEAE, treatment-emergent adverse event.
Overall, 2 deaths occurred during the study in the China safety population (due to metastatic lung cancer [n=1; GFF MDI] and hypoglycemic coma [n=1; FF MDI]). Neither of these deaths were judged by the investigator to be related to study drug treatment.
Discussion
In the PINNACLE-4 study, treatment with GFF MDI led to improvements in lung function compared to monocomponents and placebo MDI in the global patient population.13 In this Chinese subgroup analysis of the PINNACLE-4 study, patient demographics and baseline characteristics were generally similar to the global population, although there was a higher proportion of male patients, a lower mean body mass index, and a lower proportion of current smokers in the China population than in the global population.13
In Chinese patients, GFF MDI led to improvements in the primary and secondary lung function endpoints compared to the monocomponent and placebo MDIs, as in the global population. The LSM differences in trough FEV1 at 24 weeks (primary endpoint) for GFF MDI versus GP MDI, FF MDI, and placebo MDI were 59, 72, and 165 mL, respectively, in the global population,13 versus 98, 104, and 173 mL, respectively, in the China population. Treatment differences between GFF MDI and monocomponents in lung function endpoints tended to be larger in the China population than in the global population, while differences versus placebo were similar in both populations.
The treatment differences between GFF MDI and the monocomponent and placebo MDIs in TDI and SGRQ were generally consistent with those in the overall study population.13 Due to the smaller sample size in the China population, this subgroup analysis was not designed with sufficient power to reach a significance level of P<0.05 for treatment comparisons, especially in the China symptomatic population. However, the improvement with GFF MDI compared to placebo MDI reached the minimum clinically important difference for the TDI focal score (≥1 unit)16 in both the ITT and symptomatic populations, and for the SGRQ total score (≥4 units)17 in the symptomatic population. Relative to baseline, TDI scores and improvements in SGRQ scores were larger than those reported for the global population with all active treatments (GFF MDI and monocomponent MDIs) as well as placebo MDI.13 SGRQ scores in clinical trials have previously been reported to improve to a greater degree in low-/medium-income countries compared to high-income countries, for bronchodilator treatments as well as placebo.18 GFF MDI treatment also improved daily, daytime, and night-time symptom scores compared to placebo MDI in Chinese patients, with improvements versus monocomponents observed in both daily and daytime symptoms. Similar results were seen in previous studies of GFF MDI in patients from the USA, Australia, and New Zealand.9,19
It should be acknowledged that our study was not powered for assessing exacerbations in the global or China ITT populations, and thus CIs for this endpoint were wide. However, our data showed that the effect of GFF MDI on the time to first moderate or severe COPD exacerbation compared to monocomponents and placebo MDI in Chinese patients was similar to, or larger than, in the global PINNACLE-4 population, in terms of lower hazard ratio estimates (provided in Supplementary Table 4). This is in agreement with results from the PINNACLE-3 study, where time to first exacerbation was generally longer in the GFF MDI treatment group compared to GP MDI and FF MDI.10 In addition, GFF MDI reduced the risk of a first CID in Chinese patients compared to monocomponent and placebo MDIs, which was comparable with the findings in the global population (provided in Supplementary Table 4). A similar trend was observed in the PINNACLE-1 and PINNACLE-2 studies,20 although the effects seen in Chinese patients in the current study were larger. The reduction in risk of CID relative to monocomponents and placebo is consistent with those reported in studies of the LAMA/LABA FDCs umeclidinium/vilanterol and aclidinium/formoterol, although there were slight differences in the definition of a CID event.21,22 As CID is a composite endpoint encompassing lung function, symptoms, and exacerbations, it provides a more comprehensive assessment of disease status than individual outcomes. Limiting disease progression is a major objective of COPD management,23 and therefore identifying treatments that prevent deterioration is an important clinical goal.
Across all treatment groups, no unexpected safety signals were identified in Chinese patients. The safety profile of GFF MDI in patients from China was generally consistent with the global patient population13 and also with the safety profiles of other LAMA/LABA FDCs in predominantly Caucasian patient populations.24–27 In line with our results, previous studies of glycopyrrolate or formoterol fumarate,28–30 as well as other LAMA/LABA FDCs,31–33 have also found that treatment effects and safety outcomes were generally similar between Asian and predominantly Caucasian patient populations.
Increasing availability and choice in effective treatment therapies is essential to address the burden of COPD in China, given the high prevalence of the disease and the low treatment rate.4 In 2014–2015, only 11.7% of Chinese patients with COPD were receiving treatment.4 Currently, two LAMA/LABA FDCs (glycopyrrolate/indacaterol and umeclidinium/vilanterol) are available in China for the treatment of COPD, both of which are delivered by dry powder inhaler. GFF MDI is the first LAMA/LABA FDC delivered using an MDI. As device familiarity may improve treatment outcomes for inhaled therapies,34,35 and short-acting bronchodilators are most commonly prescribed in an MDI,36 GFF MDI may provide a convenient alternative option to LAMA/LABA dry powder inhalers for patients with COPD. This may be especially important for patients who lack the necessary inspiratory flow to use a dry powder inhaler.37 In addition, GFF MDI can be used with a spacer,38 which can improve lung deposition for patients who have difficulty coordinating actuation and inhalation.39 In a Phase III study in patients with COPD, use of a spacer device resulted in only minor differences in the pharmacokinetic profiles of glycopyrronium and formoterol and did not affect the lung function benefits of GFF MDI.38
The limited number of patients in the Chinese subgroup relative to the overall population, particularly in the symptomatic and rescue medication user populations, should be considered when interpreting the study findings. A lower proportion of the China ITT population was included in the symptomatic and rescue medication populations (30.0% and 30.9% of patients, respectively) compared to the global ITT population (48.3% and 47.2%, respectively). However, based on local regulatory requirements, the sample size of the Chinese subgroup was chosen to evaluate the consistency of the results with the overall population, rather than statistical significance, and the overall direction and magnitude of the treatment effects observed in the China population were consistent with the findings in the global population. An additional limitation is the fact that a large majority of Chinese patients enrolled in PINNACLE-4 were male (~96%). This reflects the fact that a smoking history of ≥10 pack-years, which was a requirement for inclusion, is rare in Chinese women (in 2015, more than 90% of female patients with COPD were never-smokers, versus approximately one-quarter of male patients).5 A key strength of this study was the international multicenter design, which provided confirmation of the efficacy and safety of GFF MDI in Chinese patients, as well as allowing for a direct comparison between Chinese patients and the overall patient population.
Conclusions
GFF MDI improved lung function and daily symptoms compared to monocomponent and placebo MDIs in Chinese patients in the PINNACLE-4 study. Both efficacy and safety results were generally consistent with the global patient population, supporting the use of GFF MDI for the long-term maintenance treatment of airflow obstruction in patients with COPD from China.
Acknowledgments
The authors would like to thank all the patients and their families and the team of investigators, research nurses, and operations staff involved in these studies. Medical writing support, under the direction of the authors, was provided by Julia King, PhD, of CMC Connect, a division of McCann Health Medical Communications Ltd, Glasgow, UK, funded by AstraZeneca, Gaithersburg, USA, in accordance with Good Publication Practice (GPP3) guidelines.40
Abbreviations
AE, adverse event; BDI, Baseline Dyspnea Index; BMI, body mass index; CAT, COPD Assessment Test; CI, confidence interval; CID, clinically important deterioration; COPD, chronic obstructive pulmonary disease; FDC, fixed-dose combination; FEV1, forced expiratory volume in 1 second; FF, formoterol fumarate; GFF, glycopyrrolate/formoterol fumarate; GP, glycopyrrolate; HRQoL, health-related quality of life; ICS, inhaled corticosteroid; ITT, intent-to-treat; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; LSM, least squares mean; MDI, metered dose inhaler; PP, per protocol; RM, repeated measures; SD, standard deviation; SE, standard error; SGRQ, St George’s Respiratory Questionnaire; TDI, Transition Dyspnea Index; TEAE, treatment-emergent adverse event.
Ethics Approval And Informed Consent
Patients provided written informed consent prior to screening, and the study was conducted in accordance with Good Clinical Practice, including the Declaration of Helsinki and the International Council for Harmonisation. The protocol was approved by local institutional review boards (names and protocol numbers are provided in Supplementary Table 5).
Data Availability
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Author Contributions
CR is the guarantor and takes responsibility for the content of this manuscript, including the data and analysis. All authors participated in the analysis and interpretation of data reported. AM and PNA made significant contributions to the statistical analysis of the data. RC, NZ, HYW, LZ, XM, ZQ and JH participated in the acquisition of reported data. AM, PNA, SS, CR, and UJM made substantial contributions to the conception or design of the study. All authors reviewed or critically revised the manuscript, provided final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Funding
The PINNACLE studies were supported by AstraZeneca.
Disclosure
RC is an advisory committee member and speaker for AstraZeneca. NZ has received consultancy and lecture fees from Boehringer Ingelheim and Novartis and was on the advisory board of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) committee. XM and ZQ are speakers for AstraZeneca. JH and PNA are employees of AstraZeneca Shanghai, People's Republic of China. AM, SS, UJM, and CR are employees of AstraZeneca, with stock options. The authors report no other conflicts of interest in this work.
References
- 1.GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–1210. doi: 10.1016/S0140-6736(17)32152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. doi: 10.1016/S2213-2600(17)30293-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin P, Wang H, Vos T, et al. A subnational analysis of mortality and prevalence of COPD in China from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Chest. 2016;150(6):1269–1280. doi: 10.1016/j.chest.2016.08.1474 [DOI] [PubMed] [Google Scholar]
- 4.Fang L, Gao P, Bao H, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med. 2018;6(6):421–430. doi: 10.1016/S2213-2600(18)30103-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi: 10.1016/S0140-6736(18)30841-9 [DOI] [PubMed] [Google Scholar]
- 6.AstraZeneca Pharmaceuticals LP. Bevespi aerosphere™ prescribing information. 2019. Available from: http://www.azpicentral.com/bevespi/bevespi_pi.pdf. Accessed July2, 2019.
- 7.Taylor G, Warren S, Dwivedi S, et al. Gamma scintigraphic pulmonary deposition study of glycopyrronium/formoterol metered dose inhaler formulated using co-suspension delivery technology. Eur J Pharm Sci. 2018;111:450–457. doi: 10.1016/j.ejps.2017.10.026 [DOI] [PubMed] [Google Scholar]
- 8.Doty A, Schroeder J, Vang K, et al. Drug delivery from an innovative LAMA/LABA co-suspension delivery technology fixed-dose combination MDI: evidence of consistency, robustness, and reliability. AAPS PharmSciTech. 2018;19(2):837–844. doi: 10.1208/s12249-017-0891-1 [DOI] [PubMed] [Google Scholar]
- 9.Martinez FJ, Rabe KF, Ferguson GT, et al. Efficacy and safety of glycopyrrolate/formoterol metered dose inhaler formulated using co-suspension delivery technology in patients with COPD. Chest. 2017;151(2):340–357. doi: 10.1016/j.chest.2016.11.028 [DOI] [PubMed] [Google Scholar]
- 10.Hanania NA, Tashkin DP, Kerwin EM, et al. Long-term safety and efficacy of glycopyrrolate/formoterol metered dose inhaler using novel Co-Suspension™ delivery technology in patients with chronic obstructive pulmonary disease. Respir Med. 2017;126:105–115. doi: 10.1016/j.rmed.2017.03.015 [DOI] [PubMed] [Google Scholar]
- 11.Yasuda SU, Zhang L, Huang S-M. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther. 2008;84(3):417–423. doi: 10.1038/clpt.2008.141 [DOI] [PubMed] [Google Scholar]
- 12.Cazzola M, Rogliani P, Sanduzzi A, Matera MG. Influence of ethnicity on response to asthma drugs. Expert Opin Drug Metab Toxicol. 2015;11(7):1089–1097. doi: 10.1517/17425255.2015.1047341 [DOI] [PubMed] [Google Scholar]
- 13.Lipworth BJ, Collier DJ, Gon Y, et al. Improved lung function and patient-reported outcomes with co-suspension delivery technology glycopyrrolate/formoterol fumarate metered dose inhaler in COPD: a randomized Phase III study conducted in Asia, Europe, and the USA. Int J Chron Obstruct Pulmon Dis. 2018;13:2969–2984. doi: 10.2147/COPD.S171853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Celli BR, MacNee W. ATS/ERS task force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304 [DOI] [PubMed] [Google Scholar]
- 15.Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky KH, Barr RG. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the multi-ethnic study of atherosclerosis (MESA) lung study. Chest. 2010;137(1):138–145. doi: 10.1378/chest.09-0919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahler DA, Witek TJ Jr. The MCID of the transition dyspnea index is a total score of one unit. COPD. 2005;2(1):99–103. doi: 10.1081/COPD-200050666 [DOI] [PubMed] [Google Scholar]
- 17.Jones PW. St. George’s respiratory questionnaire: MCID. COPD. 2005;2(1):75–79. doi: 10.1081/COPD-200050513 [DOI] [PubMed] [Google Scholar]
- 18.Jones PW, Gelhorn H, Wilson H, et al. Socioeconomic status as a determinant of health status treatment response in COPD trials. Chronic Obstr Pulm Dis. 2017;4(2):150–158. doi: 10.15326/jcopdf.4.2.2017.0132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez FJ, Fabbri LM, Ferguson GT, et al. Effect of glycopyrrolate/formoterol fumarate fixed-dose combination metered dose inhaler (GFF MDI) delivered by novel Co-Suspension™ delivery technology on daily symptoms in patients with COPD. Am J Respir Crit Care Med. 2017;195:A5469. doi: 10.1164/rccm.201701-0150WS [DOI] [Google Scholar]
- 20.Rabe KF, Martinez FJ, Rodriguez-Roisin R, et al. LAMA/LABA glycopyrrolate/formoterol fixed-dose combination, delivered using a novel MDI Co-Suspension™ delivery technology reduces risk of clinically important deteriorations in COPD versus placebo and monocomponent MDIs. Am J Respir Crit Care Med. 2017;195:A3594. doi: 10.1164/rccm.201701-0150WS [DOI] [Google Scholar]
- 21.Singh D, Maleki-Yazdi MR, Tombs L, Iqbal A, Fahy WA, Naya I. Prevention of clinically important deteriorations in COPD with umeclidinium/vilanterol. Int J Chron Obstruct Pulmon Dis. 2016;11:1413–1424. doi: 10.2147/COPD.S101612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh D, D’Urzo AD, Chuecos F, Muñoz A, Garcia Gil E. Reduction in clinically important deterioration in chronic obstructive pulmonary disease with aclidinium/formoterol. Respir Res. 2017;18(1):106. doi: 10.1186/s12931-017-0583-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of COPD. 2018. (May 2, 2018). Available from: http://www.goldcopd.org. Accessed October30, 2018.
- 24.Singh D, Jones PW, Bateman ED, et al. Efficacy and safety of aclidinium bromide/formoterol fumarate fixed-dose combinations compared with individual components and placebo in patients with COPD (ACLIFORM-COPD): a multicentre, randomised study. BMC Pulm Med. 2014;14:178. doi: 10.1186/1471-2466-14-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahler DA, Kerwin E, Ayers T, et al. FLIGHT1 and FLIGHT2: efficacy and safety of QVA149 (indacaterol/glycopyrrolate) versus its monocomponents and placebo in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(9):1068–1079. doi: 10.1164/rccm.201505-1048OC [DOI] [PubMed] [Google Scholar]
- 26.Buhl R, Maltais F, Abrahams R, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2-4). Eur Respir J. 2015;45(4):969–979. doi: 10.1183/09031936.00136014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donohue JF, Maleki-Yazdi MR, Kilbride S, Mehta R, Kalberg C, Church A. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107(10):1538–1546. doi: 10.1016/j.rmed.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 28.Wang C, Sun T, Huang Y, et al. Efficacy and safety of once-daily glycopyrronium in predominantly Chinese patients with moderate-to-severe chronic obstructive pulmonary disease: the GLOW7 study. Int J Chron Obstruct Pulmon Dis. 2015;10:57–68. doi: 10.2147/COPD.S72650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minakata Y, Iijima H, Takahashi T, et al. Efficacy and safety of formoterol in Japanese patients with COPD. Intern Med. 2008;47(4):217–223. doi: 10.2169/internalmedicine.47.0494 [DOI] [PubMed] [Google Scholar]
- 30.Fukushima Y, Nakatani Y, Ide Y, et al. Randomized, double-blind, placebo-controlled trial to assess the efficacy and safety of three doses of co-suspension delivery technology glycopyrronium MDI in Japanese patients with moderate-to-severe COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:1187–1194. doi: 10.2147/COPD.S159246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng J, Zhong N, Newlands A, Church A, Goh AH. Efficacy and safety of once-daily inhaled umeclidinium/vilanterol in Asian patients with COPD: results from a randomized, placebo-controlled study. Int J Chron Obstruct Pulmon Dis. 2015;10:1753–1767. doi: 10.2147/COPD.S81053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wedzicha JA, Zhong N, Ichinose M, et al. Indacaterol/glycopyrronium versus salmeterol/fluticasone in Asian patients with COPD at a high risk of exacerbations: results from the FLAME study. Int J Chron Obstruct Pulmon Dis. 2017;12:339–349. doi: 10.2147/COPD.S125058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bai C, Ichinose M, Lee SH, et al. Lung function and long-term safety of tiotropium/olodaterol in East Asian patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:3329–3339. doi: 10.2147/COPD.S137719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bosnic-Anticevich S, Chrystyn H, Costello RW, et al. The use of multiple respiratory inhalers requiring different inhalation techniques has an adverse effect on COPD outcomes. Int J Chron Obstruct Pulmon Dis. 2016;12:59–71. doi: 10.2147/COPD.S117196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price D, Chrystyn H, Kaplan A, et al. Effectiveness of same versus mixed asthma inhaler devices: a retrospective observational study in primary care. Allergy Asthma Immunol Res. 2012;4(4):184–191. doi: 10.4168/aair.2012.4.4.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lavorini F, Corrigan CJ, Barnes PJ, et al. Retail sales of inhalation devices in European countries: so much for a global policy. Respir Med. 2011;105(7):1099–1103. doi: 10.1016/j.rmed.2011.03.012 [DOI] [PubMed] [Google Scholar]
- 37.Mahler DA. Peak inspiratory flow rate as a criterion for dry powder inhaler use in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2017;14(7):1103–1107. doi: 10.1513/AnnalsATS.201702-156PS [DOI] [PubMed] [Google Scholar]
- 38.Fakih F, Spangenthal S, Sigal B, et al. Randomized study of the effects of Aerochamber Plus® Flow-Vu® on the efficacy, pharmacokinetics and safety of glycopyrronium/formoterol fumarate dihydrate metered dose inhaler in patients with chronic obstructive pulmonary disease. Respir Med. 2018;138:74–80. doi: 10.1016/j.rmed.2018.03.033 [DOI] [PubMed] [Google Scholar]
- 39.Lavorini F, Fontana GA. Targeting drugs to the airways: the role of spacer devices. Expert Opin Drug Deliv. 2009;6(1):91–102. doi: 10.1517/17425240802637862 [DOI] [PubMed] [Google Scholar]
- 40.Battisti WP, Wager E, Baltzer L, et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med. 2015;163(6):461–464. doi: 10.7326/M15-0288 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- AstraZeneca Pharmaceuticals LP. Bevespi aerosphere™ prescribing information. 2019. Available from: http://www.azpicentral.com/bevespi/bevespi_pi.pdf. Accessed July2, 2019.
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.