Abstract
Antibodies targeting CTLA4 induce durable responses in some patients with melanoma and are being tested in a variety of human cancers. However, a majority of patients across tumor types fail to respond. Further understanding the immune alterations induced by these therapies may enable the development of novel strategies to enhance tumor control and biomarkers to identify patients most likely to respond. In murine models anti-CTLA4 efficacy depends on interactions between the Fc region of anti-CTLA4 antibodies and Fc receptors (FcRs). Anti-CTLA4 binding to FcRs has been linked to depletion of intratumoral Tregs. In agreement with previous studies we find that intratumoral Tregs have the highest expression of surface CTLA4 (sCTLA4) and anti-CTLA4 leads to Fc/FcR-dependent depletion of intratumoral Tregs. This Treg depletion coincided with activation and degranulation of intratumoral NK cells. Similarly, in patient-derived tumor tissue Tregs have the highest sCTLA4 expression, and intratumoral Tregs express more sCTLA4 than circulating Tregs. Interestingly, patients who benefit from Ipilimumab have higher intratumoral CD56 expression. Furthermore, in a murine model combination therapy with anti-CTLA4 plus IL15/IL15Rα complexes enhanced tumor control compared to either monotherapy.
Keywords: Anti-CTLA4, NK cells, immunotherapy, IL15, Regulatory T cells
Introduction
Combination therapy with immune checkpoint inhibitors targeting PD1 (i.e. Nivolumab) and CTLA4 (i.e. Ipilimumab) for advanced melanoma is dramatically successful with up to a 60% response rate(1), and recent data have shown that combination therapy with Nivolumab and Ipilimumab leads to higher response rates than Nivolumab alone in patients with brain metastases(2). Likewise, recent clinical trials have shown efficacy of combination therapy with Nivolumab and Ipilimumab in a subset of patients with renal cell carcinoma (RCC) and non-small cell lung cancer (NSCLC)(3, 4). However, across tumor types a majority of patients still fail to respond to immunotherapies. Thus, a major goal in tumor immunology is to further understand the immunomodulation induced by these therapies. This will enable the development of novel therapeutic strategies to enhance responses and foster development of biomarkers to identify patients most likely to respond to specific therapies.
Immune checkpoint inhibitors are designed to prevent the interaction of these checkpoint molecules with their ligands, and thus prevent immune inhibition. However, in addition to blocking activity, some antibodies can mediate effector functions when their Fc domain interacts with Fc receptors (FcRs). In murine models, anti-CTLA4 can lead to the death of cells expressing CTLA4 on their cell surface in an Fc/FcR-dependent manner(5–10). While CTLA4 is largely intracellular, it is expressed on the surface of T regulatory cells (Tregs) infiltrating murine tumor models(5–7). Importantly, Tregs can limit anti-tumor immune response. Thus, anti-CTLA4 depletes intratumoral Tregs in these models, and this Treg depletion correlates with the anti-tumor activity of anti-CTLA4(5–7). In B16 tumor-bearing mice treated with a GM-CSF secreting vaccine, Treg depletion has been linked to FcRs on macrophages and non-classical monocytes(5). However, GM-CSF may induce intratumoral accumulation and activation of myeloid-derived suppressor cells in addition to macrophages and non-classical monocytes. NK cells are also able to mediate the depletion of antibody opsonized cells in an FcR-dependent manner. Thus, additional studies are necessary to determine the effect of anti-CTLA4 on intratumoral NK cells. Such studies may suggest combination strategies likely to enhance the efficacy of anti-CTLA4 therapies.
In part because Tregs are not reduced in the blood of patients receiving anti-CTLA4(11–13), it has been thought that antibody-dependent cell-mediated cytotoxicity (ADCC)-mediated depletion of Tregs is not a mechanism of action of anti-CTLA4 in patients. However, in murine models intratumoral Tregs have elevated surface CTLA4 (sCTLA4) expression, and anti-CTLA4 specifically depletes intratumoral Tregs but not peripheral Tregs(5, 7, 13). More recently it has been found that Ipilimumab, a human IgG1 antibody, can induce ADCC of Tregs, and reduced intratumoral Treg levels following Ipilimumab treatment may correlate with efficacy in patients(12, 14). Additionally, in patients with tumors with a high mutation burden the response to Ipilimumab is associated with the presence of the high-affinity CD16-V158F germline SNP(8). Despite these data the ability of Ipilimumab to deplete intratumoral Tregs in patients remains controversial. Therefore, further investigations are required to determine if Tregs infiltrating tumors in patients also express higher levels of surface CTLA4 (sCTLA4), which would provide a rationale for how anti-CTLA4 therapies specifically deplete intratumoral Tregs in patients.
Here we show that Tregs infiltrating patient-derived NSCLC tissue express more sCTLA4 than Tregs from matched patient blood or intratumoral effector T cells. Similarly, in a majority of patient-derived melanoma tissue intratumoral Tregs express more sCTLA4 than effector T cells. Interestingly, using a murine model we found that anti-CTLA4 therapies induce NK cell activation and degranulation specifically within the tumor microenvironment. This NK cell activation coincided with depletion of intratumoral Tregs. Furthermore, combination of anti-CTLA4 plus IL15/IL15Rα complexes enhanced tumor control in comparison to either monotherapy. Consistently, patients who benefited from Ipilimumab had higher intratumoral expression of the NK cell marker CD56.
Materials and Methods
Study approval.
Patient studies were conducted in accordance with ethical guidelines including the Declaration of Helsinki, The Belmont Report and the U.S. Common Rule. Patient-derived tumor tissue specimens were collected in accordance to the Institutional Review Boards at HFGCC of Christiana Health Care System (NSCLC) or The University of Pennsylvania (melanoma). Informed consent was received from participants prior to inclusion in the study.
Mice.
Murine experiments were approved by the Institutional Animal Care and Use Committees of The Wistar Institute or Dartmouth University. C57BL/6J mice were obtained from The Jackson Laboratories (USA) and BALB/C mice were obtained from Taconic (USA) and kept under specific pathogen free condition in The Wistar Institute’s Animal Facility (Philadelphia, PA, USA).
Unless otherwise indicated, 5×105 CT26 tumor cells were subcutaneously transplanted in the right flank of the mouse with 27g needles in 200 μl of PBS. 2.5 × 105 to 5 × 105 YUMM1.7 cells or 1× 106 B16-F1 cells were transplanted s.c. on day 0 where indicated. In experiments in which mice were treated with anti-CTLA4, unless otherwise noted, mice were treated with 300 μg of the indicated anti-CTLA4 clone or appropriate isotype (both from BioXcell) diluted in PBS, and mice were euthanized 5 days post-anti-CTLA4 administration for analysis of intratumoral T cells. Where indicated, mice were injected i.v. with 200 μg of anti-FcγRIV (clone 9E9) 1 hour prior to injection of anti-CTLA4. Where indicated, mice were treated i.p. on days 6 and 8 with 200 μl IL15/IL15Rα complexes, containing 0.5 μg human IL-15 (NCI biorepository) and 2.33 μg of soluble murine IL15Rα-Fc (R&D Systems/Fisher Scientific) per dose, and on days 9, 12, 15 and 17 with 200 μg anti-CTLA4 (clone 9D9). After treatment initiation tumor size was measured every two to four days and mice were euthanized when tumors reached 12 × 12 mm2. In experiments where tumor-bearing mice were euthanized at a set time point, tumors were weighed upon euthanasia.
Induction of autochthonous tumors.
Tyr::CreER+BrafCA/+Ptenfl/fl mice were kindly provided by Marcus Bosenberg (Yale), and bred in-house onto a C57BL/6 background, with >98% purity confirmed by congenic testing (DartMouse™). For induction of autochthonous tumors, 80 μg of 4-hydroxy-tamoxifen (4-HT; Sigma) in DMSO was intradermally injected in the right flanks of 4-week-old mice. Tumors and skin samples from age-matched mice were harvested 5 weeks later.
Flow cytometry.
Tumors were extracted, minced and digested in 5 ml HBSS with 0.5% FBS and 1% HEPES containing 1 mg/ml collagenase type-I from Clostridium histolyticum (Sigma-Aldrich) for 30 minutes at 37°C. Cells were then washed and filtered through 70 μm cell strainers.
Autochthonous tumors and murine skin samples were harvested, minced, and digested for 45 minutes at 37°C in 2 ml HBSS containing 7 mg/ml collagenase D (Roche) and 200 μg/ml DNase I (Roche), using magnetic bar stirring at 300 RPM. Tissue fragments were mechanically dissociated through a 40 μm nylon mesh filter. Samples were washed in RPMI-1640 media containing 10% FBS and 2mM EDTA.
For surface CTLA4 (sCTLA4) staining, cells were processed as before then two rounds of FASER-PE (Miiltenyi Biotec) staining were performed according to manufacturer instructions. For mouse Fc receptor staining, cell suspensions were incubated with anti-mouse FcγRIV (CD16.2, clone 9E9, Biolegend) for 20 minutes at 4°C, washed twice, incubated with anti-mouse CD16/32 (clone 2.4G2, BD Bioscience) for 10 minutes at 4°C, then stained with all the other surface antibodies. Fixation and intracellular staining was accomplished with eBioscience FOXP3/Transcription factor staining buffer set.
Patient-derived tumor tissues were cut in small pieces and processed with Tumor Dissociation Kit, human (Miltenyi Biotech) according to manufacturer instructions. Red blood cell lysis buffer was used to remove RBCs from blood specimens. Cells were Fc blocked with Fc blocking reagent (Miltenyi Biotec), and then cells were stained similarly to murine cells. Patient-derived tumor tissues were processed for flow cytometry the day of excision.
Samples were analyzed using LSR IIs (BD Bioscience) equipped with 4 lasers or a CELESTA (BD Bioscience) flow cytometer equipped with 3 lasers or a Gallios Flow Cytometer (Beckman Coulter). MFI indicates geometric mean of the indicated fluorescence. Data were analyzed using FlowJo versions 9 or 10 (TreeStar).
Generation and testing of IL15/IL15Rα complexes.
IL15/IL15Rα complexes were generated by combining 0.5 μg human IL-15 (NCI biorepository) with 2.33 μg of soluble murine IL15Rα-Fc (R&D Systems/Fisher Scientific) per dose for 30 mins at 37 degrees. PBS was used to q.s. IL15 complexes to 200 μl per dose, aliquoted and stored at −80. Batches of IL15 complexes were validated once by i.p. injecting a mouse with IL15 complexes containing 0.5 μg human IL-15 (NCI biorepository) and 2.33 μg of soluble murine IL15Rα-Fc (R&D Systems/Fisher Scientific) per dose on days 1, 3, and 5. Spleens from mice treated with IL15 complexes were then analyzed for size, NK cell frequency and frequency of activated CD8 T cells compared to control spleen on day 7.
Analysis of RNA-seq data.
Relative expression of the NK cell genes NCAM1 (CD56), FCGR3A, NCR1, NCR2, and KLRK1, CD3D, CD8A, CD4, CD14, CD74, CD163 and CX3CR1 in melanoma tissue from patients who received benefit or little to no benefit from anti-CTLA4 CX3CR1 published by Snyder et al., 2014 or Van Allen et al., 2015 were compared. NCAM1 expression in melanoma tissue from patients who received benefit or little to no benefit from anti-CTLA4 as published by Snyder et al., 2014 was chosen for further analysis. Statistical difference in expression of NCAM1 was only found when analysis was restricted to samples from cutaneous melanoma.
Statistics.
Prism 7 (GraphPad) was used for statistical data analysis. To determine statistical significance, paired or two-group t-test was used as indicated. For multiple groups of data analysis, ANOVA with Tukey’s post-hoc multiple comparison was used to determine statistical significance between two groups. For comparisons of multiple groups to a single group, Dunnett’s post-hoc multiple comparisons test was used to determine statistical significance. For data that doesn’t follow normal distribution, Wilcoxon rank-sum test was used to determine statistical significance. To determine if indicated treatments induced significant difference in tumor progression, the trends of mean tumor volume over time were compared between groups using a linear mixed effects model with mouse as the random effect. A likelihood ratio testing nested model was used to examine if slopes are significantly different between groups and overall. p < 0.05 was considered statistically significant.
Results
Anti-CTLA4 induces NK cell activation in the tumor microenvironment.
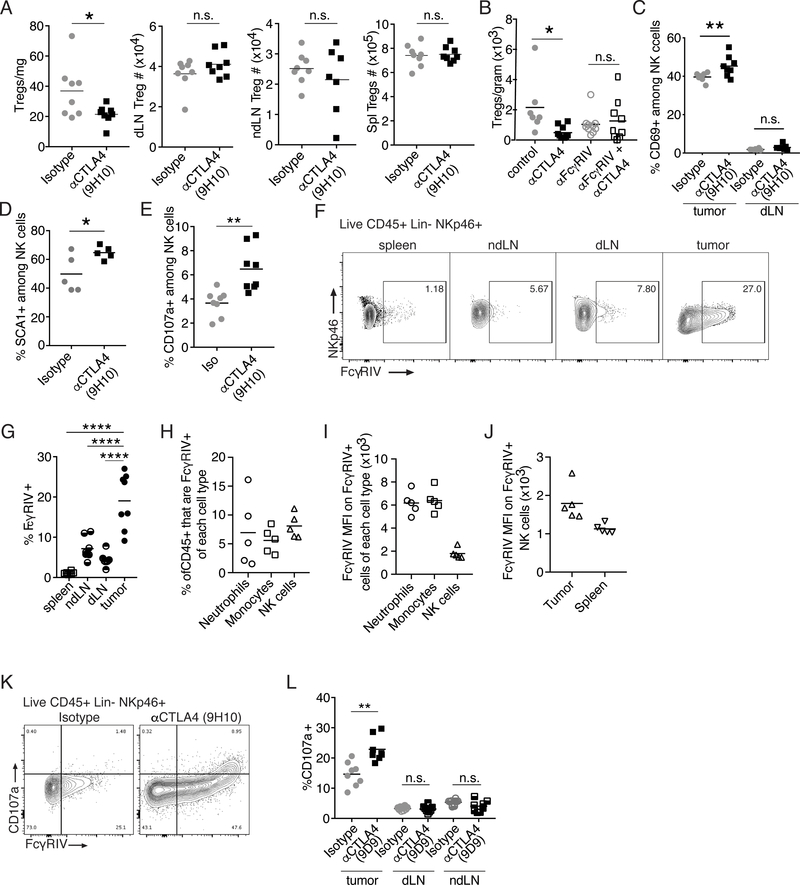
As expected, administration of anti-CTLA4 clone 9H10, a Syrian hamster IgG, to mice bearing CT26 tumors led to depletion of Tregs specifically within the tumor microenvironment (Figure 1A). Anti-CTLA4 clone 9H10 has been shown to bind to FcγRIV, and mediate depletion of Tregs infiltrating B16 tumors in an FcγRIV-dependent manner(5). This and other data suggest that anti-CTLA4 induced depletion of intratumoral Tregs may be mediated by non-classical monocytes(5, 14). Furthermore, we found that pre-treatment with an antibody to FcγRIV (clone 9E9), which has been reported to be a blocking antibody, prevented anti-CTLA4-mediatied depletion of Tregs infiltrating CT26 tumors (Figure 1B). We note, however, that to our knowledge the ability of anti-FcγRIV clone 9E9 to deplete FcγRIV expressing cells has not been investigated, and thus a possibility is that administration of FcγRIV depleted FcγRIV+ cells, which may also express multiple other FcRs. Nonetheless, our data further support the now prevailing evidence that anti-CTLA4 clone 9H10 depletes intratumoral Tregs in an FcR-dependent manner.
Figure 1.
Anti-CTLA4 induces NK cell activation and degranulation specifically in the tumor microenvironment.
(A) Twelve days post-transplantation of CT26 cells, mice were treated with anti-CTLA4 (clone 9H10) or isotype (Isotype) control and 3 days later (day 15) the number of Tregs (Live CD45+CD19-CD11c-Ly6G-CD3+CD4+FOXP3+) per mg of tumor tissue or within the draining lymph node (dLN), a non-draining lymph node (ndLN) or the spleen (Spl) was determined by flow cytometry. (B) Seven days post-transplantation of CT26 tumors mice were i.v. injected with anti-FcγRIV (clone 9E9) or Armenian Hamster isotype control. 1 hour later anti-CTLA4 clone 9H10 or Syrian Hamster isotype control was administered (i.p.). On day 14, five days post-administration of antibodies, the number of Tregs (Live CD45+ B220-CD11b-CD11c-Ly6G-CD3+) per gram of tumor tissue was determined by flow cytometry. (C-E) CT26 cells were transplanted s.c. and on the indicated day post tumor-transplantation, mice were treated with anti-CTLA4 (clone 9H10) or isotype (Isotype) control. (C) Graphs show the frequency of NK cells (Live CD45+ NKp46+) within the tumor or spleen that express CD69 on day 15 when mice were treated with anti-CTLA4 clone 9H10 on day 10. (D) Graphs show the frequency of NK cells (Live CD45+CD19-Ly6G-CD3-NKp46+) that express SCA1 on day 14 when mice were treated with anti-CTLA4 clone 9H10 on day 9. (E) Graphs show the frequency of NK cells (Live CD45+CD19-CD11c-Ly6G-CD3-NKp46+) that display CD107a on their surface when mice were treated with anti-CTLA4 clone 9H10 on day 12. (F-G) Representative contour plots (F) and graph (G) showing expression of FcγRIV (CD16.2) on NK cells (Live CD45+CD19-CD11c-Ly6G-CD3-NKp46+) from indicated organs 15 days after transplantation of CT26 tumors. (H) Graph shows the frequency of Live intratumoral CD45+ cells that are FcγRIV+ neutrophils (NKp46-CD11B+LY6G+), monocytes (NKp46-LY6G-LY6CHi) and NK cells (CD3-NKp46+) 13 days post-transplantation of CT26 tumors. (I-J) FcγRIV MFI on FcγRIV+ population of the indicated cell type within the tumor (I) or on intratumoral and splenic NK cells (J) 13 days post transplantation of CT26 tumor cells. (K) Representative contour plots showing expression of FcγRIV versus CD107 on NK cells (Live CD45+CD19-CD11c-Ly6G-NKp46+) 3 days post administration of anti-CTLA4 clone 9H10. (L) Graphs show the frequency of NK cells (Live CD45+CD19-CD11c-Ly6G-CD3-NKp46+) in indicated organs (ndLN, non-tumor-draining lymph node) expressing CD107a 5 days post administration of anti-CTLA4 clone 9D9. To determine statistical significance, t-test for two groups was used. For comparison between multiple groups in 1C, ANOVA with Tukey’s post-hoc for multiple comparisons, and for 1G, ANOVA with Dunnett’s post-hoc for multiple comparison were used to determine statistical significance between two groups. n.s. indicates non-significant, * indicates p < 0.05, ** indicates p < 0.01, **** indicates p< 0.0001.
NK cells also express FcRs and are able to mediate FcR-dependent functions of antibodies. Thus, we investigated the effect of anti-CTLA4 treatment on NK cells. We found that 5 days post-administration of a single dose of anti-CTLA4 clone 9H10, an increased frequency of intratumoral NK cells were activated as judged by NK cell expression of CD69 and SCA1 (Figure 1C–D). However, NK cell activation appeared to be limited to the tumor microenvironment as the frequency of activated NK cells was not increased outside of the tumor microenvironment (Figure 1C). Thus, anti-CTLA4 leads to NK cell activation specifically within the tumor microenvironment.
Anti-CTLA4 induces NK cell degranulation in the tumor microenvironment.
As the binding of the Fc region of antibodies to activating FcRs on NK cells can lead to NK cell activation, one possibility is that anti-CTLA4 activates NK cells to kill cells opsonized by anti-CTLA4. In agreement with this we find that three days post-administration of a single dose of anti-CTLA4 clone 9H10, a time point that correlates with the timing of Treg depletion (Figure 1A)(5, 6), an increased frequency of intratumoral NK cells display CD107a on their cell surface directly ex vivo (Figure 1E, Supplemental Figure 1A). Surface CD107a expression indicates that these NK cells have recently degranulated.
While expression of FcγRIV on NK cells in periphery is rare, a fraction of tumor-infiltrating NK cells in B6 mice do indeed express FcγRIV(5). Similarly, we find that while peripheral NK cells in BALB/c mice express little to no FcγRIV, a substantial proportion of NK cells infiltrating CT26 tumors in BALB/c mice express FcγRIV (Figure 1F–G). Furthermore, we find that FcγRIV+ NK cells are present within tumors at a similar frequency to FcγRIV+ neutrophils and monocytes (Figure 1H). However, we note that the per cell expression of FcγRIV is higher on intratumoral FcγRIV+ neutrophils and monocytes than FcγRIV+ NK cells (Figure 1I). Nonetheless, the expression of FcγRIV+ is increased on intratumoral FcγRIV+ NK cells compared to splenic NK cells (Figure 1J). Furthermore, consistent with the dependence of anti-CTLA4 clone 9H10 on FcγRIV for intratumoral Treg depletion, surface CD107a+ NK cells are largely FcγRIV+ (Figure 1K).
Anti-CTLA4 clone 9H10 is a hamster anti-mouse antibody and thus may bind to FcRs differently than mouse anti-mouse antibodies. Thus, to determine if NK cell activation following anti-CTLA4 administration is specific to anti-CTLA4 clone 9H10 or may be broadly applicable across anti-CTLA4 therapies able to mediate ADCC, we investigated NK cell phenotype following administration of anti-CTLA4 clone 9D9, a murine IgG2b antibody. Murine antibodies of the IgG2b isotypes are more traditionally considered able to induce NK cell-mediated ADCC. As with anti-CTLA4 clone 9H10, anti-CTLA4 clone 9D9 led to NK cells degranulation (Figure 1L). Consistent with anti-CTLA4 inducing Treg depletion specifically within the tumor microenvironment, the increase in NK cell degranulation is specific to the tumor microenvironment (Figure 1L).
NK cells infiltrating CT26 tumors express little to no CTLA4.
NK cells are able to express CTLA4 when cultured in the presence of IL2(15). Thus, a possibility was that anti-CTLA4 activates NK cells by blocking CTLA4 expressed by NK cells. To investigate this possibility, we used flow cytometry to assess surface and intracellular CTLA4 expression by NK cells infiltrating CT26 tumors on day 18. As expected, we find that intratumoral Tregs express sCTLA4, however, little to no CTLA4 expression by NK cells was detected (Supplemental Figure 1B). From these results we conclude it is unlikely that anti-CTLA4 activates NK cells by relieving a ‘brake’ imposed on NK cells by their own expression of CTLA4.
Intratumoral Tregs express the highest levels of sCTLA4 in many murine tumor models.
The ability of anti-CTLA4 to specifically mediate intratumoral Treg depletion (Figure 1A) is consistent with intratumoral Tregs expressing the highest levels of sCTLA4 (Supplemental Figure 2A), and this has previously been shown of Tregs infiltrating B16-BL6 and CT26 tumor models(5, 6). In agreement with this, anti-CTLA4 therapy leads to depletion of Tregs with the highest CTLA4 expression(5, 6). However, activated effector T cells can also express high levels of CTLA4, and it has previously been found that CD4 effector T cells responding to chronic stimulation can further upregulate CTLA4(16). Furthermore, CTLA4 surface retention can be enhanced by stimulation(17). In agreement with this, we find that sCTLA4+ Tregs and CD4 effector cells infiltrating well-established YUMM1.7 tumors, a transplanted BrafV600E/Pten−/− /Cdkn2a−/− melanoma model(18), express similar levels of sCTLA4 (Supplemental Figure 2B). However, Tregs express the most sCTLA4 of the T cell subsets infiltrating autochthonous melanomas in a conditionally-induced BrafV600E/Pten−/− melanoma mouse model (Supplemental Figure 2C). Importantly, using this model we also find that melanoma-infiltrating Tregs express more sCTLA4 than peripheral or skin Tregs (Supplemental Figure 2D). Nonetheless, these data demonstrate that in some tumor microenvironments, a portion of effector T cells may express sCTLA4 at levels similar to Tregs. Thus, as anti-CTLA4 therapies that mediate ADCC induce depletion of cells with the highest sCTLA4 expression(5, 6), it was important to determine the relative sCTLA4 expression by T cells infiltrating patient-derived tumor tissue.
Higher surface CTLA4 expression by Tregs versus effector T cells infiltrating patient-derived NSCLC tissue.
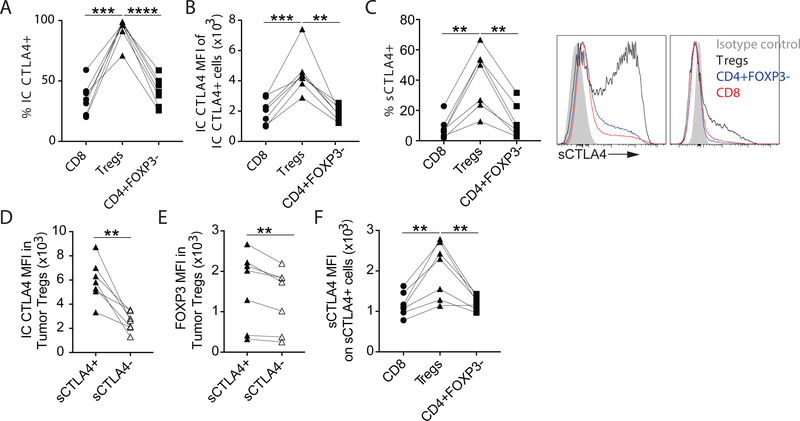
Tregs infiltrating previously untreated patient-derived NSCLC tissue express high levels of intracellular CTLA4 (IC CTLA4, Figure 2A–B)(8). However, a majority of CTLA4 is intracellular(17, 19), and little is known about the expression of sCTLA4 on T cell subsets infiltrating patient-derived tumor tissue directly ex vivo. Using flow cytometry we found that while nearly all Tregs infiltrating patient-derived NSCLC tissue express IC CTLA4 (Figure 2A), only a fraction of intratumoral Tregs express sCTLA4 (Figure 2C). Interestingly, sCTLA4+ Tregs express more IC CTLA4 and FOXP3 compared to sCTLA4- Tregs (Figure 2D–E). Nonetheless, a higher frequency of Tregs than CD8 or CD4 effector T cells infiltrating patient-derived NSCLC tissue express sCTLA4 (Figure 2C). Importantly, in nearly all NSCLC tissues analyzed, sCTLA4+ Tregs express more sCTLA4 on a per cell basis than the sCTLA4+ intratumoral CD8 or CD4 effector T cells from the same tumor tissue (Figure 2F).
Figure 2.
Expression of sCTLA4 of T cell subsets infiltrating patient-derived NSCLC tissue.
Single cell suspensions from fresh patient-derived NSCLC tissue were analyzed by flow cytometry. (A) Graph shows the frequency of intratumoral CD8, Tregs (CD4+FOXP3+) and CD4+FOXP3- T cells (Live CD45+CD20-CD14-CD11c-CD66b-CD3+) expressing intracellular (IC) CTLA4. (B) Graph shows the level of IC CTLA4 expression by the IC CTLA4+ fraction of these intratumoral T cell subsets. (C) Left, graph shows the frequency of intratumoral CD8, Tregs and CD4+FOXP3- T cells expressing surface CLTA4 (sCTLA4). Right, Representative histograms of the two types of sCTLA4 expression patterns seen (Tregs, black histogram; CD4+FOXP3-, blue histogram; CD8+, red histogram). (D-E) Graphs show the level of IC CTLA4 (D) and FOXP3 (E) expression by sCTLA4+ and sCTLA4- intratumoral Tregs. (F) Graph shows the level of sCTLA4 expression by the sCTLA4+ fraction of these intratumoral T cell subsets. To determine statistical significance paired t-tests were used. ** indicates p < 0.01, p indicates < 0.001, **** indicates p < 0.0001.
Higher expression of sCTLA4 on Tregs infiltrating patient-derived NSCLC tissue compared to circulating Tregs.
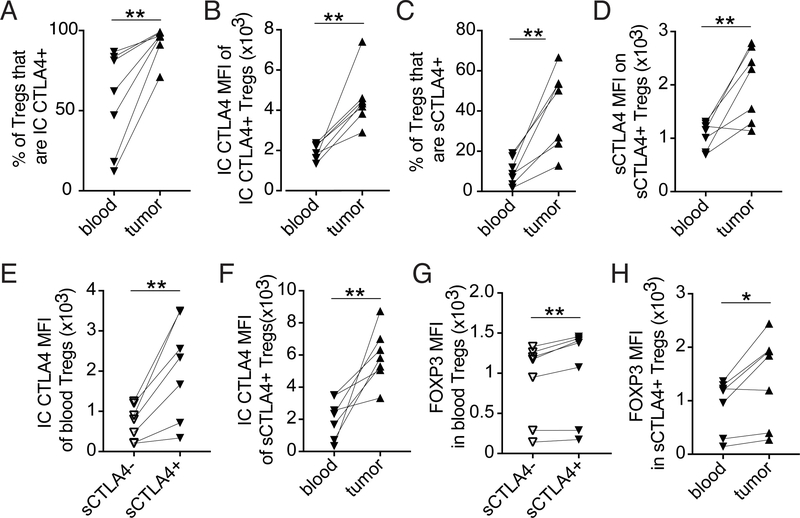
Tregs infiltrating patient-derived NSCLC tissue express higher levels of intracellular CTLA4 than Tregs from matched patient blood (Figure 3A–B)(8). However, even in intratumoral Tregs a majority of CTLA4 is intracellular. Thus, to better understand if, similar to what has been found in murine models, Tregs infiltrating patient-derived tumor tissue express more sCTLA4 than peripheral Tregs, and thus if anti-CTLA4 is likely to specifically target intratumoral Tregs in patients, we compared the level of sCTLA4 expression on intratumoral and peripheral Tregs. Comparing the data from Figure 2 to co-stained circulating Tregs from matched patient blood, we found that both the frequency of sCTLA4+ Tregs and the level of expression of sCTLA4 is higher on Tregs infiltrating patient-derived NSCLC tissue compared to circulating Tregs from matched patient blood (Figure 3C–D).
Figure 3.
Tregs infiltrating patient-derived NSCLC tissue have higher expression of sCTLA4 compared to peripheral Tregs.
Comparison of T cell subsets from patient-derived NSCLC tissue (from Figure 2) with T cell subsets in matched patient blood collected the morning of biopsy. (A) Graph shows the frequency of circulating and intratumoral Tregs (CD4+FOXP3+) expressing IC CTLA4. (B) Graph shows the level of IC CTLA4 expression by the IC CTLA4+ fraction of circulating and intratumoral Tregs. (C) Graph shows the frequency of circulating and intratumoral Tregs expressing sCTLA4. (D) Graph shows the level of sCTLA4 expression by the sCTLA4+ fraction of circulating and intratumoral Tregs. (E) Graph shows the level of IC CTLA4 expression by circulating sCTLA4+ or sCTLA4- Tregs. (F) Graph shows the level of IC CTLA4 expression by circulating or intratumoral sCTLA4+ Tregs. (G) Graph shows the level of FOXP3 expression by circulating sCTLA4+ or sCTLA4- Tregs. (H) Graph shows the level of FOXP3 expression by circulating or intratumoral sCTLA4+ Tregs. To determine statistical significance paired t-tests were used. * indicates p < 0.05, ** indicates p < 0.01.
To begin to understand factors that contribute to higher sCTLA4 levels on intratumoral compared to circulating Tregs, we evaluated IC CTLA4 and FOXP3 levels in sCTLA4+ Tregs infiltrating the tumor and in circulation. While sCTLA4+ circulating Tregs expressed higher levels of IC CTLA4 than sCTLA4- circulating Tregs (Figure 3E), they expressed lower IC CTLA4 levels compared to intratumoral sCTLA4+ Tregs (Figure 3F). Likewise, while sCTLA4+ circulating Tregs expressed higher levels of FOXP3 than sCTLA4- circulating Tregs (Figure 3G), these Tregs had lower FOXP3 levels than intratumoral sCTLA4+ Tregs (Figure 3H). This data suggests that the level of sCTLA4 on sCTLA4+ Tregs in part reflects the level of total expression of CTLA4 and also associates with the level of FOXP3 expression.
Expression of sCTLA4 by intratumoral T cells infiltrating patient-derived melanoma tissue.
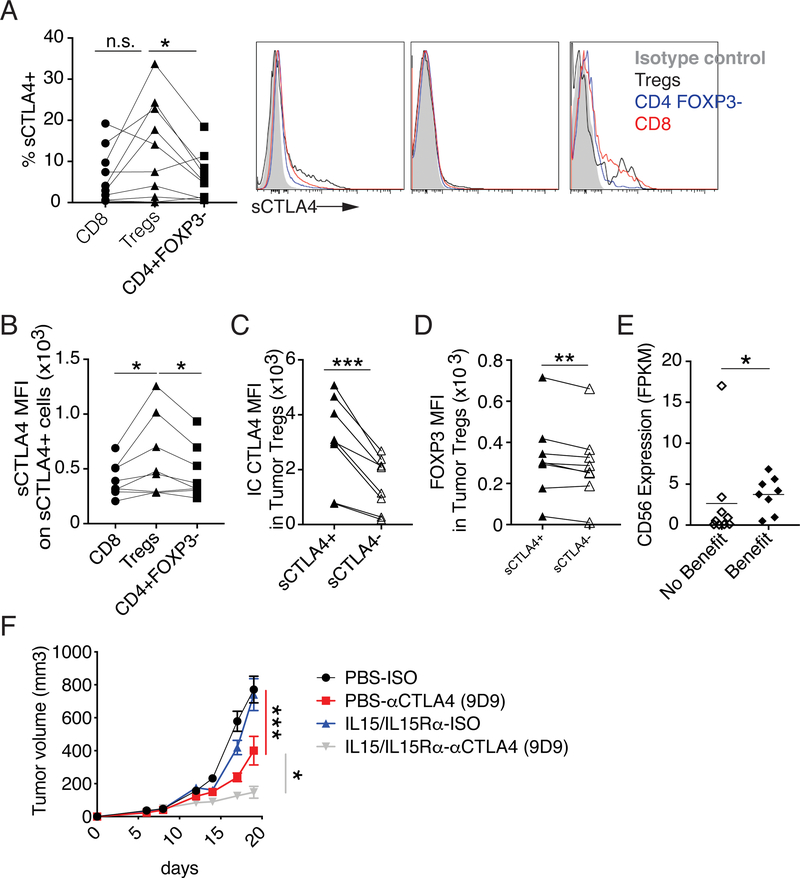
Using flow cytometry, we find that in a majority of patient-derived melanoma tissue a higher frequency of Tregs express sCTLA4 than CD4 effector T cells from the same tissue (Figure 4A). Furthermore, as with Tregs infiltrating patient-derived NSCLC tissue, sCTLA4+ Tregs infiltrating patient-derived melanoma tissue express more sCTLA4 on a per cell basis compared to sCTLA4+ CD8 or CD4 effector T cells from the same tumor tissue (Figure 4B). As was seen in Tregs infiltrating NSCLC, sCTLA4+ Tregs infiltrating patient-derived melanoma tissue expressed more FOXP3 and IC CTLA4 than sCTLA4- Tregs from the same samples (Figure 4C–D).
Figure 4.
Expression of sCTLA4 on T cell subsets infiltrating patient-derived melanoma tissue.
Single cell suspensions from fresh patient-derived melanoma tissue were analyzed by flow cytometry. (A) Left, graph shows the frequency of intratumoral CD8, Tregs (CD4+FOXP3+CD127lo) and CD4+FOXP3-T cells expressing sCTLA4. Right, Histograms of sCTLA4 expression on T cell (Clive CD45+CD20-CD14-CD11c-CD66b-CD3+) subsets from three of the patient-derived melanoma tissues (Isotype control, filled gray histogram; Tregs, black histogram; CD4+FOXP3-, blue histogram; CD8 T cells, red histogram). (B) Graph shows the expression (MFI) on indicated sCTLA4+ cells. (C-D) Graphs show IC CTLA4 (C), or FOXP3 (D) expression by intratumoral sCTLA4+ or sCTLA4- Tregs (CD4+FOXP3+CD127lo) as determine by flow cytometry. (E) Relative expression of CD56 in cutaneous melanoma samples from patients with benefit or minimal or no benefit from Ipilimumab as published by Snyder et al., 2014. (F) Mice were treated with +/− anti-CTLA4 (clone 9D9, days 9, 12, 15 and 17) +/− IL15/IL15Rα complexes (days 6 and 8, containing 0.5 μg human IL-15 and 2.33 μg of soluble murine IL15Rα-Fc per dose) and tumor progression was followed (data shown is from one experiment with 10 mice per a group). To determine statistical significance paired t-tests were used. For data that doesn’t follow normal distribution, Wilcoxon rank-sum test was used to determine statistical significance. The trends of mean tumor volume over time were compared between groups using a linear mixed effects model with mouse as the random effect. A likelihood ratio testing nested models was used to examine if slopes are significantly different between groups and overall. n.s. indicates non-significant, * indicates p < 0.05, ** indicates p < 0.01, *** indicates p< 0.001.
Higher intratumoral CD56 expression associates with benefit from Ipilimumab.
Productive depletion of intratumoral Tregs may enhance the efficacy of anti-CTLA4 in patients. In a murine model we found that depletion of intratumoral Tregs following administration of anti-CTLA4 coincided with activation and degranulation of NK cells. Thus, a possibility is that patients with higher levels of intratumoral NK cells will be more likely to benefit from anti-CTLA4 therapies. In support of this model, analysis of available RNA-seq data(20) revealed that a higher intratumoral CD56 expression is associated with benefit from Ipilimumab in cutaneous melanoma patients (Figure 4E).
Anti-CTLA4 plus IL15/IL15Rα complexes enhance tumor control compared to either monotherapy.
Anti-CTLA4 activates intratumoral NK cells coincident to intratumoral Treg depletion in a murine model and intratumoral levels of the NK cell marker CD56 are increased in cutaneous melanoma patients who benefit from Ipilimumab. Thus, a possibility was that combination therapy with anti-CTLA4 plus IL15/IL15Rα complexes, which have been shown to increase intratumoral NK cells (21), would enhance tumor control. To test this possibility, mice bearing CT26 tumors were treated with anti-CTLA4 (clone 9D9), IL15/IL15Rα complexes (containing 0.5 μg human IL-15 and 2.33 μg of soluble murine IL15Rα-Fc per dose (22)) or combination therapy. As expected, anti-CTLA4 monotherapy moderately limited tumor progression, but, in this model, monotherapy with IL15/IL15Rα complexes was ineffective. However, combination therapy with anti-CTLA4 plus IL15/IL15Rα complexes enhanced tumor control compared to either monotherapy (Figure 4F).
Discussion
We show that Tregs infiltrating not only murine tumor models but also patient-derived tumors preferentially express sCTLA4. Consistently, anti-CTLA4 specifically depletes intratumoral Tregs in an FcR-dependent manner. We further find that anti-CTLA4 therapies induce NK cell activation and degranulation specifically in the tumor microenvironment, and this corresponds with anti-CTLA4 mediated depletion of intratumoral Tregs. Moreover, increased intratumoral CD56 expression associates with benefit from Ipilimumab, and in a murine model anti-CTLA4 plus IL15/IL15Rα complexes enhanced tumor control compared to either monotherapy. Thus enhanced intratumoral NK cell activity may contribute to the activity of anti-CTLA4 therapy.
A possibility from our data is anti-CTLA4 opsonizing intratumoral Tregs leads to the activation of FcR+ cells, including NK cells, which can then mediate ADCC of these anti-CTLA4 opsonized Tregs. Thus, NK cells may contribute to anti-CTLA4-mediated depletion of intratumoral Tregs (Supplemental Figure 3). In agreement with this, NK cells have been shown to be able to mediate ADCC of CTLA4+ Tregs in the presence of a human anti-human CTLA4 antibody(23). Furthermore, Ipilimumab is able to induce the activation and degranulation of human NK cells. Concomitantly, Ipilimumab also induces downregulation of CD16 expression on NK cell surface (Supplemental Figure 4). Alternatively, intratumoral Tregs may inhibit NK cells, and anti-CTLA4 mediated depletion of intratumoral Tregs may allow for NK cell activation and degranulation when these NK cells target tumor cells. This activation of NK cells following Treg depletion may contribute to the durable responses induced by anti-CTLA4.
The cytokine IL15 supports NK cell proliferation, survival and cytolytic activity, and administration of IL15/IL15Rα complexes has been shown to enhance intratumoral NK cell levels (21). Thus, while do not rule out the likelihood that the effect of IL15/IL15Ra complexes on CD8 T cells is important for the increased tumor control seen in mice co-treated with IL15 super agonists and anti-CTLA4, a possibility from our data is that increased intratumoral NK cell levels and activity is important for the synergy IL15 complexes have with anti-CTLA4 in controlling tumor progression. Consistently, increased intratumoral levels of the NK cell marker CD56 is associated with benefit from Ipilimumab in patients with cutaneous melanoma, further suggesting that increased intratumoral NK cell levels enhance anti-CTLA4 efficacy.
The ability of Ipilimumab to deplete intratumoral Tregs in patients remains controversial. While a decrease in intratumoral Treg levels following Ipilimumab treatment may correlate with efficacy in patients, circulating Tregs are not decreased following administration of anti-CTLA4. We found that surface CTLA4 expression is enhanced on Tregs infiltrating patient-derived tumor tissue compared to circulating Tregs from matched patient blood. This data provides a potential rationale for why anti-CTLA4 therapy has been found to specifically reduce intratumoral but not circulating Treg levels in patients. Thus, anti-CTLA4 therapies may preferentially deplete intratumoral Tregs in patients, and specifically intratumoral Tregs with enhanced expression of not only CTLA4 but also FOXP3.
In conclusion we show that anti-CTLA4 therapy induces activation and degranulation of intratumoral NK cells and that combination therapy with IL15/IL15Rα complexes enhances tumor control. Additionally, increased intratumoral levels of the NK cell marker CD56 is associated with benefit from Ipilimumab in patients with advanced cutaneous melanoma. Nonetheless, it remains to be determined if NK cells contribute to anti-CTLA4-mediated Treg depletion and the enhanced tumor control induced by combination therapy with anti-CTLA4+ IL15/IL15Rα complexes. Thus, future research should investigate the role of NK cells in anti-CTLA4 efficacy and if increased intratumoral NK cells enhance anti-CTLA4 efficacy.
Supplementary Material
Acknowledgements
The authors are grateful to Sophia Simon, Autumn Powell, Keira McAfee and Claire Cohen (Wistar) for genotyping and mouse line maintenance assistance. The authors thank Patricia Swanson and Lori Huelsenbeck-Dill (HFGCC-Christiana Health Care System) for assistance with patient recruitment. The authors are especially grateful for scientific discussions with the Stone Lab and members of The Wistar Institute.
• Financial support. This research was sponsored by The Wistar Institute Cancer Center and an American Cancer Society-IRG grant. The research was funded by a Bristol-Myers Squibb-MRA Young Investigator Award (ELS), a Wistar/UPenn Specialized Program of Research Excellence (SPORE) grant in Skin Cancers Developmental Research Award (ELS), and a grant from BNY Mellon Wealth Management through The Martha W. Rogers Charitable Trust (ELS). ELS was funded in part by 1K01DK095008 (NIDDK) and PA CURE grants. JK was funded in part by a PENNPort IRACDA postdoctoral fellowship. ES was funded in part by a Fellowship from Istituto Pasteur Italia – Fondazione Cenci Bolognetti. Core Facilities were supported by Cancer Center Support Grant (CCSG) CA010815 to The Wistar Institute. BAA and JEH are supported in part by MUSC. MJT and TBS are supported by NIH grants R21CA209375-01, R01CA225028 and R01CA205965. The authors are grateful for support from The Tara Miller Melanoma Foundation.
Footnotes
• Conflict of interest statement. The authors have no conflicts of interest with regard to the manuscript in question.
References
- 1.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino MS, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbe C, Ascierto PA, Long GV, Cebon J, Sosman J, Postow MA, Callahan MK, Walker D, Rollin L, Bhore R, Hodi FS, Larkin J. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. The New England journal of medicine. 2017;377(14):1345–56. Epub 2017/09/12. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, Wilmott JS, Edwards J, Gonzalez M, Scolyer RA, Menzies AM, McArthur GA. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. The Lancet Oncology. 2018;19(5):672–81. Epub 2018/04/01. doi: 10.1016/s1470-2045(18)30139-6. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthelemy P, Porta C, George S, Powles T, Donskov F, Neiman V, Kollmannsberger CK, Salman P, Gurney H, Hawkins R, Ravaud A, Grimm MO, Bracarda S, Barrios CH, Tomita Y, Castellano D, Rini BI, Chen AC, Mekan S, McHenry MB, Wind-Rotolo M, Doan J, Sharma P, Hammers HJ, Escudier B. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. The New England journal of medicine. 2018;378(14):1277–90. Epub 2018/03/22. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers S, Salman P, Borghaei H, Ramalingam SS, Brahmer J, Reck M, O’Byrne KJ, Geese WJ, Green G, Chang H, Szustakowski J, Bhagavatheeswaran P, Healey D, Fu Y, Nathan F, Paz-Ares L. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. The New England journal of medicine. 2018. Epub 2018/04/17. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, Peggs KS, Ravetch JV, Allison JP, Quezada SA. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. The Journal of experimental medicine. 2013;210(9):1695–710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bulliard Y, Jolicoeur R, Windman M, Rue SM, Ettenberg S, Knee DA, Wilson NS, Dranoff G, Brogdon JL. Activating Fc γ receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. The Journal of experimental medicine. 2013;210(9):1685–93. doi: 10.1084/jem.20130573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, Korman AJ. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer immunology research. 2013;1(1):32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 8.Arce Vargas F, Furness AJS, Litchfield K, Joshi K, Rosenthal R, Ghorani E, Solomon I, Lesko MH, Ruef N, Roddie C, Henry JY, Spain L, Ben Aissa A, Georgiou A, Wong YNS, Smith M, Strauss D, Hayes A, Nicol D, O’Brien T, Martensson L, Ljungars A, Teige I, Frendeus B, Pule M, Marafioti T, Gore M, Larkin J, Turajlic S, Swanton C, Peggs KS, Quezada SA. Fc Effector Function Contributes to the Activity of Human Anti-CTLA-4 Antibodies. Cancer Cell. 2018;33(4):649–63.e4. Epub 2018/03/27. doi: 10.1016/j.ccell.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingram JR, Blomberg OS, Rashidian M, Ali L, Garforth S, Fedorov E, Fedorov AA, Bonanno JB, Le Gall C, Crowley S, Espinosa C, Biary T, Keliher EJ, Weissleder R, Almo SC, Dougan SK, Ploegh HL, Dougan M. Anti-CTLA-4 therapy requires an Fc domain for efficacy. Proc Natl Acad Sci U S A. 2018;115(15):3912–7. Epub 2018/03/28. doi: 10.1073/pnas.1801524115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du X, Tang F, Liu M, Su J, Zhang Y, Wu W, Devenport M, Lazarski CA, Zhang P, Wang X, Ye P, Wang C, Hwang E, Zhu T, Xu T, Zheng P, Liu Y. A reappraisal of CTLA-4 checkpoint blockade in cancer immunotherapy. Cell research. 2018;28(4):416–32. Epub 2018/02/24. doi: 10.1038/s41422-018-0011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kavanagh B, O'Brien S, Lee D, Hou Y, Weinberg V, Rini B, Allison JP, Small EJ, Fong L. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood. 2008;112(4):1175–83. doi: 10.1182/blood-2007-11-125435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarhini AA, Edington H, Butterfield LH, Lin Y, Shuai Y, Tawbi H, Sander C, Yin Y, Holtzman M, Johnson J, Rao UNM, Kirkwood JM. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PloS one. 2014;9(2):e87705. doi: 10.1371/journal.pone.0087705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furness AJS, Vargas FA, Peggs KS, Quezada SA. Impact of tumour microenvironment and Fc receptors on the activity of immunomodulatory antibodies. Trends in immunology. 2014;35(7):290–8. doi: 10.1016/j.it.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Romano E, Kusio-Kobialka M, Foukas PG, Baumgaertner P, Meyer C, Ballabeni P, Michielin O, Weide B, Romero P, Speiser DE. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(19):6140–5. doi: 10.1073/pnas.1417320112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stojanovic A, Fiegler N, Brunner-Weinzierl M, Cerwenka A. CTLA-4 is expressed by activated mouse NK cells and inhibits NK Cell IFN-gamma production in response to mature dendritic cells. J Immunol. 2014;192(9):4184–91. Epub 2014/04/02. doi: 10.4049/jimmunol.1302091. [DOI] [PubMed] [Google Scholar]
- 16.Crawford A, Angelosanto JM, Kao C, Doering TA, Odorizzi PM, Barnett BE, Wherry EJ. Molecular and transcriptional basis of CD4+ T cell dysfunction during chronic infection. Immunity. 2014;40(2):289–302. doi: 10.1016/j.immuni.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linsley PS, Bradshaw J, Greene J, Peach R, Bennett KL, Mittler RS. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4(6):535–43. [DOI] [PubMed] [Google Scholar]
- 18.Meeth K, Wang JX, Micevic G, Damsky W, Bosenberg MW. The YUMM lines: a series of congenic mouse melanoma cell lines with defined genetic alterations. Pigment cell & melanoma research. 2016;29(5):590–7. doi: 10.1111/pcmr.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung HT, Bradshaw J, Cleaveland JS, Linsley PS. Cytotoxic T lymphocyte-associated molecule-4, a high-avidity receptor for CD80 and CD86, contains an intracellular localization motif in its cytoplasmic tail. The Journal of biological chemistry. 1995;270(42):25107–14. [DOI] [PubMed] [Google Scholar]
- 20.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA. Genetic basis for clinical response to CTLA-4 blockade in melanoma. The New England journal of medicine. 2014;371(23):2189–99. Epub 2014/11/20. doi: 10.1056/NEJMoa1406498. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epardaud M, Elpek KG, Rubinstrain MP, Yonekura A, Bellemare-Pelletier A, Bronson R, Hamerman JA, Goldrath AW, Turley SJ. Interleukin-15/Interleukin-15Ra complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer Res. 2008;68(8):2972–83. [DOI] [PubMed] [Google Scholar]
- 22.Doedens AL, Rubinstein MP, Gross ET, Best JA, Craig DH, Baker MK, Cole DJ, Bui JD Goldrath AW. Molecular Programming of Tumor-Infiltrating CD8+ T cells and IL15 resistance. Cancer immunology research. 2016;4(9):799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gombos RB, Gonzalez A, Manrique M, Chand D, Savitsky D, Morin B, Breous-Nystrom E, Dupont C, Ward RA, Mundt C, Duckless B, Tang H, Findeis MA, Schuster A, Waight JD, Underwood D, Clarke C, Ritter G, Merghoub T, Schaer D, Wolchok JD, van Dijk M, Buell JS, Cuillerot JM, Stein R, Drouin EE, Wilson NS. Toxicological and pharmacological assessment of AGEN1884, a novel human IgG1 anti-CTLA-4 antibody. PLoS One. 2018;13(4):e0191926. Epub 2018/04/05. doi: 10.1371/journal.pone.0191926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.