Abstract
Purpose
To identify the associations between MetS and its components and chronic kidney disease (CKD) in a population with arterial hypertension (AH), or diabetes mellitus (DM) accompanied by the Primary Health Care (PHC).
Patients and methods
A cross-sectional study with 788 individuals diagnosed with AH and/or DM followed by PHC of Viçosa, Brazil. Anthropometric, biochemical and clinical measures were performed for the diagnosis of MetS and CKD. MetS was identified using the NCEP-ATPIII criteria. CKD was identified by estimating the glomerular filtration rate using the CKD-EPI equation. Logistic regression models were used to estimate the chances of CKD associated with MetS and its components and specific combinations of components.
Results
The prevalence of MetS reported in the population was 65.4%, that of hidden CKD was 15.4%. The prevalence of CKD among participants with MetS was 75.2%. The most prevalent component of MetS in the population was AH (96.7%). Elevated fasting blood glucose, central obesity, and reduced HDL-c were significantly associated with an increased chance of CKD (OR = 2.80, 95% CI 1.76–4.45, OR = 1.68, 95% CI, 05–2.71, OR = 1.61, CI 95% 1.03–2.50, respectively). For the multivariate adjustment, the participants with MetS were 2 times more likely to have CKD than those without MetS (OR = 2.07; 95% CI, 1.25–3.44). The combination of three components of MetS high blood pressure, abdominal obesity and elevated fasting blood glucose and the combination of four components of MetS high blood pressure, reduced HDL-c, high fasting blood glucose and abdominal obesity were associated with increased odds of CKD (OR = 2.67, CI 95% 1.70–4.20, OR = 2.50, CI 95% 1.55–4.02, respectively).
Conclusion
MetS, as well as its individual or combined components were independently associated with CKD in the population with AH and/or DM accompanied by PHC.
Keywords: high blood pressure, abdominal obesity, hypertriglyceridemia, fasting blood glucose, HDL-c, estimated glomerular filtration rate
Introduction
Metabolic syndrome (MetS) is an asymptomatic pathophysiological condition characterized by obesity, insulin resistance, arterial hypertension (AH), hyperglycemia and dyslipidemia.1 It was first described by Reaven in 1988,2 whose study demonstrated that resistance to insulin-stimulated glucose uptake was involved in the etiology of non-insulin-dependent diabetes mellitus (DM), AH and coronary artery disease.
Even with all divergences regarding the diagnostic criteria and terminology used, the term SM is accepted in cases where the clinical condition includes multiple metabolic risk factors for cardiovascular diseases (CVD) and DM. Among the main comorbidities known and associated with MetS, most of the individuals present abdominal obesity and insulin resistance. These two conditions seem to be strongly related to the appearance of metabolic risk factors.1,3
Regarding the epidemiology of MetS, its prevalence in the United States of America adjusted for age was approximately 34.7% among the years 2011–2012.4 The prevalence of MetS in Latin America was 21% between 2003 and 2005, ranging from 14% to 27%, according to the territories studied.5 In Brazil, the prevalence was even higher, varying around 30% between individuals aged between 19 and 64 years in different regions of the country.3
Previous studies have shown that MetS is a constellation of metabolic risk factors for cardiovascular disease (CVD), type 2 DM, and all-cause mortality.6–8 All components that fall within the definition of MetS (hyperglycemia, elevated blood pressure, elevated total cholesterol, reduced HDL cholesterol, and central fat deposition) are also involved in the development of CKD.9
However, to our knowledge, there are sparse data on the association between MetS and CKD in a developing country such as Brazil, especially in a population that is accompanied by PHC and has an AH and/or DM. Thus, the objective of this study was to report the associations between the presence of MetS, its individual and grouped components, and CKD in a population diagnosed with AH and/or DM accompanied by PHC.
Materials and Methods
Study Design and Population
A cross-sectional study based on a sample of the urban population of individuals with AH, or DM over 18 years old, accompanied by 16 PHC teams from the municipality of Viçosa, Brazil, from August 2017 to April 2018.
As inclusion criteria, participants should be 18 years of age or older, have an AH diagnosis and, or DM, be accompanied by PHC services and agree to participate in the study after due clarification. Pregnant women were excluded, individuals who could not move to the collection site (the PHC unit), individuals with a history of alcohol abuse and/or other drugs, who had severe clinical conditions that required specialized care or had a diagnosis of established CKD.
The sample was defined considering the reference population with AH and/or DM in the year 2017 of the municipality (6624 individuals), a prevalence of 50% of the phenomenon studied, 5% sample margin of error, 50% of the conglomerate effect, 10% of refusals and, or losses, 20% to control confounders and 95% confidence level. The sample calculation resulted in a minimum sample of 719 individuals. The final study sample consisted of 788 individuals.
Participants were selected using the two-step cluster sampling method. First, health units (territories) were selected, and then individuals with hypertension and/or diabetes were drawn from a list provided by the health service. The sample calculation was performed using the Statcalc program of Epi-Info® version 7.2.
The research was conducted in accordance with the Norms and Ethical Guidelines of the Resolution of the National Health Council 510/2016 of the Ministry of Health of Brazil and with the Declaration of Helsinki. The Research Ethics Committee of the Federal University of Viçosa under the number 1203173/2015 approved it. After the reading and signing of the Informed Consent Term, all participants were submitted to anamnesis, clinical, laboratory and anthropometric assessments.
Data Collection and Measures
The data were collected in the PHC units from August 2017 to April 2018. We used a semi-structured questionnaire with sociodemographic, clinical, lifestyle, and anthropometric data, blood pressure measurement, and conducting biochemical tests of blood and urine.
The definition of MetS used in this study follows the criteria of the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III).10 Participants who had three or more of the five criteria were considered with MetS: a) waist circumference ≥102 cm for men and ≥88 cm for women; b) serum triglyceride ≥150 mg/dL; c) HDL-cholesterol <40 mg/dL in men and <50 mg/dL in women; d) Blood pressure ≥130/85 mmHg; and e) serum fasting blood glucose level ≥100 mg/dL. Treatment with antidiabetic, antihypertensive, or triglyceride reducing agents was considered as variables for the definition of MetS.
Serum creatinine and albumin/creatinine ratio (ACR) were evaluated for renal function. ACR was used as a diagnostic tool to assess albumin excretion. An ACR ≥ 30 mg/g is recognized as a marker of renal damage and increased glomerular permeability, known as albuminuria. The CKD-EPI formula was used to identify the occurrence of CKD. To classify the CKD stages, the following criteria were applied: (1) GFR ≥ 90 mL/min/1.73 m2; (2) GFR entre 60 e 89 mL/min/1.73 m2; (3A) GFR entre 45 e 59 mL/min/1.73 m2; (3B) GFR entre 30 e 44 mL/min/1.73 m2; (4) GFR entre 15 e 29 mL/min/1.73 m2; (5) GFR < 15 mL/min/1.73 m2. Individuals with GFR < 60 mL/min/1.73 m2 (stages 3A, 3B, 4 and 5) or with GFR > 60 mL/min/1.73 m2 with coexisting albuminuria (ACR ≥ 30 mg/g) were considered affected by the CKD. The ACR was classified as follows: A1 <30mg/g (normal to slightly increased); A2 between 30 and 300 mg/g (moderately increased); A3 >300mg/g (severely increased). Confirming the presence of CKD, creatinine and albuminuria tests were repeated after 3 months to confirm the diagnosis, as recommended by KDIGO.11
Blood and urine samples were collected after 12 hr overnight fasting of participants. The biochemical parameters analyzed were fasting blood glucose, glycosylated hemoglobin, triglycerides, total cholesterol and fractions, phosphorus, calcium, serum albumin, estimated glomerular filtration rate and albuminuria. The collection and analysis of the biological material was performed in a single-accredited laboratory in the municipality of Viçosa, MG, using commercial kits. The techniques and the classification criteria of the values found were those of reference of the laboratory.
Blood pressure was measured by a trained professional with a mercury sphygmomanometer applied to the left arm, with the subject sitting in position. The BMI was calculated by dividing the individual’s weight in kilograms by the square of the height in meters (kg/m2). Abdominal obesity was defined according to the waist circumference equal to or greater than 94 cm in men and 80 cm in women and was measured with an inelastic tape measure at the midpoint between the iliac crest and the lower rib with the patient standing. The perimeter of the hip was measured in the largest proportion of the gluteal region with the participant standing, using an inelastic tape measure. The waist-to-hip ratio (WHR) and waist-to-height ratio (WHtR) were calculated by dividing the waist circumference in centimeters by the perimeter of the hip and the height by centimeters, respectively. The reference values of the WHR are equal to or greater than 0.85 for women and 0.90 for men; for the WHtR the cut-off point is 0.5 for both sexes.
Statistical Analyses
Population characteristics were described using estimates of frequencies, means, medians, standard deviations, interquartile ranges and percentages according to the type of variable. To verify the associations between categorical variables by CKD status, we used Pearson’s chi-square test. Continuous variables were tested for normal distribution by the Kolmogorov Smirnov test followed by the parametric (Student’s t-test) or nonparametric (Mann–Whitney) according to the normality test result. For all tests, the significance level of 95% was set.
The MetS status, the presence of its individual components, and the number (1 to 5) of MetS components were determined. Multiple logistic regression models and 95% confidence intervals were used to estimate the chances of having CKD associated with the status of MetS, individual components of MetS, and number of MetS components when age, sex, and serum albumin were kept constant.
Participants who had one component of MetS were compared to those who had 2, 3, 4 and 5 components. Participants with MetS were compared to those with fewer than three components of MetS. We also evaluated the odds ratios for having CKD with 10 combinations of three components and five combinations of four components of MetS and compared with the odds ratio of participants with one component to determine the combinations with the highest probabilities, controlling for covariates. All analyses were performed in the SPSS program (Statistical Package for the Social Science, version 22; SPSS Inc. Chicago, USA).
Results
Characteristics of Participants and Comparison of Study Participants by DRC Status
Most participants were female (62.7%) with a median age of 62 years and most married or friendly (62.7%). The median level of education was 4 years of complete schooling. Other sociodemographic characteristics of the population studied are present in Table 1.
Table 1.
Sociodemographic, Clinical, Biochemical, Anthropometric Characteristics of the Population Studied in Viçosa, Minas Gerais in 2018 and Univariate Analysis
| Total Population | CKD | p-value | ||
|---|---|---|---|---|
| Yes | No | |||
| Sex£ | 0.321 | |||
| Male | 294 (37.3%) | 50 (17.0%) | 244 (83.0%) | |
| Female | 494 (62.7%) | 71 (14.4%) | 423 (85.6%) | |
| Age# | 62 (54–69) | 66 (58–74) | 62 (53–68) | <0.001c |
| Civil Status£ | 0.570 | |||
| Single | 78 (10.4%) | 11 (14.1%) | 67 (85.9%) | |
| Married/Friends | 470 (62.7%) | 74 (15.7%) | 396 (84.3%) | |
| Separated/Shacked | 71 (9.5%) | 7 (9.9%) | 64 (90.1%) | |
| Widower | 131 (17.5%) | 22 (16.8%) | 109 (83.2%) | |
| Color£ | 0.354 | |||
| Black | 173 (23.3%) | 21 (12.1%) | 152 (87.9%) | |
| Brown/Yellow/Indigenous | 325 (43.9%) | 50 (15.4%) | 275 (84.6%) | |
| White | 243 (32.8%) | 42 (17.3%) | 201 (82.7%) | |
| Education (years of study)$ | 4 (3–6) | 4 (2–6) | 4 (3–7) | 0.253 |
| Tobacco£ | 0.006a | |||
| Smoker | 86 (11.7%) | 4 (4.7%) | 82 (95.3%) | |
| Ex-smoker | 218 (29.7%) | 42 (19.3%) | 176 (80.7%) | |
| Never smoked | 431 (58.6%) | 66 (15.3%) | 365 (84.7%) | |
| Alcohol£ | 0.407 | |||
| Yes | 205 (27.8%) | 27 (13.2%) | 178 (86.8%) | |
| No | 532 (72.2%) | 83 (15.6%) | 449 (84.4%) | |
| Base Disease | <0.001ª | |||
| Hypertension | 440 (55.8%) | 45 (10.2%) | 395 (89.8%) | |
| Diabetes | 62 (7.9%) | 8 (12.9%) | 54 (87.1%) | |
| Hypertension and Diabetes | 286 (36.3%) | 68 (23.8%) | 218 (76.2%) | |
| Heart Attack£ | 0.119 | |||
| Yes | 42 (5.7%) | 10 (23.8%) | 32 (76.2%) | |
| No | 693 (94.8%) | 103 (14.9%) | 590 (85.1%) | |
| Stroke£ | 0.268 | |||
| Yes | 48 (6.5%) | 10 (20.8%) | 38 (79.2%) | |
| No | 692 (93.5%) | 103 (14.9%) | 589 (85.1%) | |
| Number of drugs used $ | 3 (1–4) | 3 (1–5) | 2 (1–4) | 0.117 |
| Glycosylated hemoglobin (mg/dL)$ | 6.0 (5.6–7.1) | 6.6 (5.9–7.8) | 6.0 (5.6–6.8) | <0.001b |
| Total cholesterol (mg/dL)# | 191.0 (40.5) | 191.4 (39.6) | 191.4 (39.6) | 0.527 |
| LDL-c (mg/dL)$ | 107.0 (87.2–132.6) | 101.4 (81.0–134.2) | 107.6 (88.0–132.4) | 0.179 |
| VLDL-c (mg/dL)$ | 25.2 (19.0–34.6) | 26.4 (17.6–36.4) | 25.0 (19.0–34.0) | 0.664 |
| Serum phosphorus (mg/dL)$ | 3.4 (3.0–3.8) | 3.5 (3.1–3.8) | 3.4 (3.0–3.8) | 0.184 |
| Calcium (mg/dL)$ | 9.5 (9.3–9.8) | 9.5 (9.3–9.8) | 9.5 (9.2–9.7) | 0.188 |
| Serum albumin (mg/dL)# | 4.4 (0.28) | 4.4 (0.39) | 4.5 (0.26) | 0.001 b |
| MetS£ | 0.013ª | |||
| Yes | 515 (65.4%) | 91 (17.7%) | 424 (82.3%) | |
| No | 273 (34.6%) | 30 (11.0%) | 243 (89.0%) | |
| BMI (kg/m2)$ | 28.3 (25.2–32.1) | 29.3 (25.0–32.7) | 28.3 (25.3–32.0) | 0.469 |
| Central obesity (measured by WC in cm)# | 93.77 (11.4) | 97.05 (12.4) | 93.18 (11.0) | 0.001c |
| Systolic blood pressure (mmHg)$ | 130.0 (120.0–140.0) | 136.0 (124.0–150.0) | 130.0 (120.0–140.0) | 0.003 b |
| Diastolic pressure (mmHg)$ | 80.0 (80.0–90.0) | 80.0 (80.0–90.0) | 80.0 (80.0–90.0) | 0.245 |
| Fasting blood glucose (mg/dL)$ | 98.0 (88.0–129.0) | 107.0 (90.0–141.0) | 97.0 (87.0–125.0) | 0.021 b |
| Triglycerides (mg/dL)$ | 126.0 (95.0–175.0) | 134.0 (90.0–189.0) | 125.0 (95.0–171.0) | 0.372 |
| HDL-c (mg/dL)$ | 49.0 (41.0–59.0) | 46.0 (41.0–53.0) | 49.0 (41.0–60.0) | 0.019b |
Notes: £number and percentage; $median and interquartile range; #mean and standard deviation; astatistically significant p-value by Pearson’s Chi-square test; bp-value statistically significant by the Mann–Whitney U-test; cp-value statistically significant by the Student’s t-test.
Abbreviations: CKD, chronic kidney disease; MetS, metabolic syndrome; LDL-c, low-density lipoprotein cholesterol; VLDL-c, very low-density lipoprotein cholesterol; HDL-c, high-density lipoprotein cholesterol; BMI, body mass index; WC, waist circumference.
Participants who presented CKD were older (median age 66 vs 62 years, p = <0.001) and reported to be ex-smokers or never smokers (p = 0.006). Regarding the biochemical characteristics, participants with CKD had a higher median glycosylated hemoglobin (6.5 vs 5.9 mg/dL, p = <0.001) and lower median serum albumin (4.4 vs 4.5 mg/dL, p = 0.001). They also had a higher mean WC (97.05 vs 93.18 cm, p = 0.001), higher median systolic blood pressure (136.0 vs 130.0 mmHg, p = 0.003), and fasting blood glucose (107 vs 97 mg/dL, p = 0.021); and lower median HDL-c (46 vs 49 mg/dL, p = 0.019). More results can be seen in Table 1.
Individual Components of MetS, Prevalence of MetS and CKD
The overall prevalence of MetS found was 65.4%. The most prevalent component of MetS in the study population was hypertension observed in 498 participants (96.7%), followed by low HDL-c in 417 participants (81.0%), hypertriglyceridemia in 397 participants (77.1%), high fasting blood glucose in 348 participants (67.6%) and central obesity in 340 participants (66.0%). The prevalence of CKD was 15.4%. Among individuals with CKD, the prevalence of MetS reached 75.2%.
Association Between MetS, Its Individual and Grouped Components and CKD
Table 2 presents the unadjusted and adjusted odds ratios for each individual component of MetS and by the number of components of MetS among the study population. The multivariate adjustment model showed that the presence of high fasting blood glucose, central obesity and reduced HDL-c were significantly associated with CKD (OR = 2.80, 95% CI 1.76 to 4.45, OR = 1.68, 95% CI 1.05–2.71, OR = 1.61, 95% CI 1.03–2.50, respectively).
Table 2.
Unadjusted and Adjusted DRC Probabilities for Each Individual Component, Number of MetS Components and MetS (Yes/No) Among the Study Population in Viçosa, Minas Gerais, in 2018
| Variables | CKD | |||||
|---|---|---|---|---|---|---|
| Not Adjusted | Adjusted by Sex and Age | Multivariate Adjustmentª | ||||
| OR (95% IC) | p-valueb | OR (95% IC) | p-valueb | OR (95% IC) | p-valueb | |
| Fasting blood glucose ≥100 mg/dL | 2.73 (1.81–4.12) | 0.000 | 2.68 (1.77–4.07) | 0.000 | 2.80 (1.76–4.45) | 0.000 |
| Triglycerides ≥150 mg/dL | 1.15 (0.79–1.67) | 0.476 | 1.05 (0.72–1.55) | 0.787 | 1.19 (0.78–1.82) | 0.420 |
| High blood pressure (systolic BP: ≥130 mmHg; Diastolic BP: ≥85 mmHg) | 1.54 (0.53–4.41) | 0.426 | 1.10 (0.38–3.24) | 0.859 | 1.55 (0.43–5.62) | 0.508 |
| Central obesity (WC: >102 cm in men, >88 cm in women) | 1.41 (0.95–2.08) | 0.088 | 1.65 (1.06–2.58) | 0.026 | 1.68 (1.05–2.71) | 0.032 |
| HDL-c, mg/dL (<40 mg/dL in men, <50 mg/dL in women) | 1.526 (1.03–2.25) | 0.034 | 1.49 (1.00–2.22) | 0.052 | 1.61 (1.03–2.50) | 0.037 |
| 1 | Reference | 0.004 | Reference | 0.005 | Reference | 0.005 |
| 2 | 1.82 (0.78–4.26) | 0.167 | 1.94 (0.82–4.62) | 0.133 | 1.68 (0.64–4.39) | 0.290 |
| 3 | 2.07 (0.91–4.73) | 0.083 | 2.37 (1.01–5.52) | 0.046 | 2.71 (1.07–6.85) | 0.036 |
| 4 | 2.21 (0.97–5.01) | 0.058 | 2.27 (0.98–5.26) | 0.056 | 2.29 (0.91–5.76) | 0.078 |
| 5 | 4.17 (1.83–9.49) | 0.001 | 4.55 (1.92–10.78) | 0.001 | 4.73 (1.84–12.13) | 0.001 |
| MetSc | 1.74 (1.12–2.71) | 0.014 | 1.76 (1.11–2.78) | 0.015 | 2.07 (1.25–3.44) | 0.005 |
Notes: ªAdjusted for age, sex, smoking, serum albumin and serum calcium; bp-values calculated by logistic regression; ccompared to participants with less than 3 components of the metabolic syndrome.
Abbreviations: OR, odds ratio; CI, confidence interval; SM, metabolic syndrome; BP, blood pressure; HDL-c, high-density lipoprotein cholesterol; WC, waist circumference.
The odds ratios adjusted for age and gender indicated that participants with MetS had 76% more chances of CKD than those without MetS (OR = 1.76 CI 85% 1.11–2.78). For the multivariate adjustment considering the variables gender, age, smoking, serum albumin and serum calcium, participants with MetS were 2 times more likely to have CKD than those without MetS (OR = 2.07, 95% CI, 1.25–3, 44).
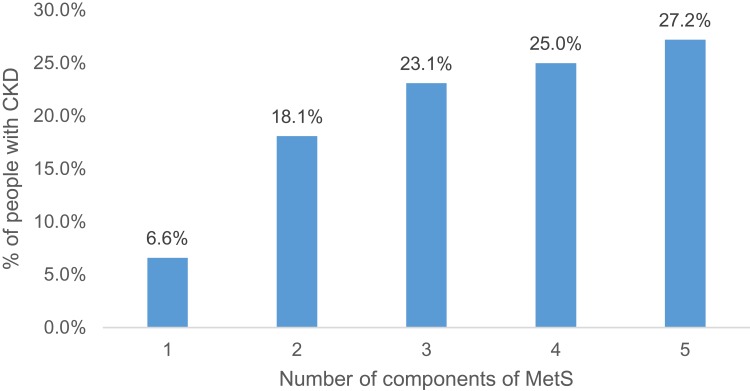
An increase in the prevalence of CKD was observed with the increase in the number of components of MetS (Figure 1), however, the increase was not statistically significant.
Figure 1.
Prevalence of CKD according to the number of components of MetS among the study population, Viçosa, 2018.
Association Between Combinations of SM and DRC Components
Table 3 shows the odds ratios for each of the 15 combinations of MetS components tested (10 combinations of three components and five combinations of four components). The result of the multivariate adjustment of the 10 MetS three-component combinations revealed that the highest associations with CKD were: 1) HBP+AO+HFBG – high blood pressure, abdominal obesity and high fasting blood glucose (OR= 2.67; CI 95% 1.70–4.20); 2) AO+LHDL+HFBG – abdominal obesity, low HDL cholesterol, and elevated fasting blood glucose (OR = 2.49, 95% CI 1.55–4.00); 3) LHDL+HTGL+HFBG – low HDL cholesterol, high triglycerides, high fasting blood glucose (OR = 2.34, 95% CI 1.43–3.82). By the multivariate adjustment of the five MetS four-component combinations, the combinations that presented the most significant associations with CKD were: 1) HBP+LHDL+HFBG+AO – high blood pressure, low HDL cholesterol, high fasting blood glucose, abdominal obesity (OR = 2.50, 95% CI 1.55–4.02); 2) HBP+HTGL+HFBG+AO – high blood pressure, elevated triglycerides, high fasting blood glucose, abdominal obesity (OR = 2.31, 95% CI 1.41–3.78); 3) HTGL+LHDL+HFBG+AO – high blood pressure, elevated triglycerides, high fasting blood glucose, low HDL cholesterol (OR = 2.31, 95% CI 1.40–3.81).
Table 3.
Unadjusted and Adjusted Odds of Having CKD by Combinations of Components of MetS Among the Study Population in Viçosa, Minas Gerais, in 2018
| CKD | ||||||
|---|---|---|---|---|---|---|
| Not adjusted | Adjusted by Sex and Age | Multivariate Adjustmentª | ||||
| OR (95% CI) | p-value b | OR (95% CI) | p-value b | OR (95% CI) | p-value b | |
| HBP+AO+LHDL | 1.74 (1.17–2.59) | 0.006 | 1.90 (1.23–2.93) | 0.004 | 1.80 (1.14–2.85) | 0.012* |
| HBP+AO+HTGL | 1.44 (0.96–2.16) | 0.081 | 1.45 (0.94–2.25) | 0.092 | 1.56 (0.98–2.48) | 0.062 |
| HBP+AO+HFBG | 2.36 (1.58–3.52) | 0.000 | 2.48 (1.62–3.77) | 0.000 | 2.67 (1.70–4.20) | 0.000* |
| HBP+LHDL+HTGL | 1.33 (0.91–1.94) | 0.141 | 1.20 (0.82–1.77) | 0.352 | 1.30 (0.85–1.97) | 0.235 |
| HBP+LHDL+HFBG | 2.20 (1.50–3.22) | 0.000 | 2.03 (1.37–2.99) | 0.000 | 2.31 (1.51–3.53) | 0.000* |
| AO+LHDL+HTGL | 1.72 (1.17–2.54) | 0.006 | 1.58 (1.07–2.35) | 0.022 | 1.82 (1.18–2.80) | 0.006* |
| AO+HFBG+HTGL | 1.52 (1.00–2.31) | 0.049 | 1.55 (0.99–2.43) | 0.054 | 1.55 (0.96–2.49) | 0.072 |
| AO+LHDL+HFBG | 2.48 (1.63–3.76) | 0.000 | 2.58 (1.65–4.01) | 0.000 | 2.49 (1.55–4.00) | 0.000* |
| LHDL+HTGL+HFBG | 2.17 (1.41–3.35) | 0.000 | 2.24 (1.42–3.52) | 0.000 | 2.34 (1.43–3.82) | 0.001* |
| HBP+HTGL+HFBG | 1.80 (1.21–2.67) | 0.004 | 1.65 (1.11–2.47) | 0.014 | 1.78 (1.15–2.75) | 0.010* |
| HBP+HTGL+AO+LHDL | 1.48 (0.97–2.26) | 0.069 | 1.48 (0.94–2.32) | 0.089 | 1.52 (0.94–2.44) | 0.087 |
| HBP+HTGL+HFBG+LHDL | 1.87 (1.25–2.80) | 0.002 | 1.69 (1.13–2.54) | 0.011 | 1.86 (1.20–2.89) | 0.006* |
| HBP+HTGL+HFBG+AO | 2.14 (1.38–3.33) | 0.001 | 2.16 (1.37–3.42) | 0.001 | 2.31 (1.41–3.78) | 0.001* |
| HBP+LHDL+HFBG+AO | 2.50 (1.63–3.82) | 0.000 | 2.53 (1.62–3.96) | 0.000 | 2.50 (1.55–4.02) | 0.000* |
| HTGL+LHDL+HFBG+AO | 2.26 (1.44–3.53) | 0.000 | 2.31 (1.45–3.68) | 0.000 | 2.31 (1.40–3.81) | 0.001* |
Notes: ªAdjusted for age, sex, smoking, serum albumin and serum calcium; bp-values calculated by logistic regression; *statistically significant values. Abdominal obesity, defined by a waist circumference ≥102 cm in men and ≥88 cm in women; high blood pressure, defined as systolic blood pressure ≥130 mmHg, or diastolic blood pressure ≥85 mmHg, medical diagnosis and/or use of antihypertensive drugs; elevated plasma triglycerides (≥150 mg/dL) or treated dyslipidemia; low fasting HDL cholesterol (men <40 mg/dL and women <50 mg/dL) and high fasting blood glucose (≥100 mg/dL) or use of antidiabetic medication. Metabolic syndrome defined as having three or more of the following components: abdominal obesity, high blood pressure, elevated fasting blood glucose, high triglycerides and low HDL cholesterol.
Abbreviations: OR, odds ratio; CI, confidence interval; HBP, high blood pressure; BHDL, low-density lipoprotein cholesterol; OA, abdominal obesity; HTGL, high triglycerides; HFBG, high fasting blood glucose.
Discussion
Significant relationships were found between MetS, the presence of its components, the number of components, as well as the combinations of MetS components and CKD, regardless of gender, age, smoking, serum albumin, and calcium levels. The outcomes found in this study have important clinical and public health implications for two reasons: 1) the prevalence of components of MetS (high blood pressure, high blood glucose and abdominal obesity) is high in the Brazilian adult population12,13 and; 2) data on the prevalence of MetS and CKD and the relationship between both in the Brazilian population are scarce and vary according to the criteria used. Thus, the present study contributes to the literature that documents the association between MetS and CKD among individuals who are at high risk of developing both.
The prevalence of MetS found in the population that presented CKD was 75.2% and is considered high in relation to other studies that evaluated the prevalence of MetS in adults and elderly with CKD.14,15 The results suggest that among the population studied, MetS significantly increased the chances of the individual presenting with CKD.
Multivariate models using the NCEP-ATPIII definition demonstrated that both the presence of MetS and its individual components, high fasting blood glucose, central obesity, and reduced HDL-c are independently associated with CKD in participants (OR = 2, 80, 95% CI 1.76–4.45, OR = 1.68, 95% CI 1.05–2.71, OR = 1.61, 95% CI 1.03–2.50, respectively). These findings are consistent with other studies conducted with the adult population14,16 that used the same diagnostic criteria for MetS.
In MetS, visceral obesity is an essential component that induces the increase of lipolysis, increasing free fatty acids, decreasing the insulin sensitivity in the target organs, favoring hepatic gluconeogenesis and the synthesis of triglycerides, leading to the release of glucose in the chain and perpetuating insulin resistance.17–19 Insulin resistance, a prominent feature of the MetS, can lead to progressive loss of renal function by worsening renal hemodynamics through multiple pathways, including sympathetic nervous system activation, sodium retention, and downregulation of the natriuretic peptide system20,21. Abdominal obesity is also associated with CKD, regardless of general adiposity and increased BMI.22,23 It is also associated with glomerular hyperfiltration23 because it causes persistent efferent arteriolar vasoconstriction, which leads to glomerular hypertrophy to maintain normal GFR. Lee et al24 demonstrated in adult and postmenopausal men and women that subcutaneous adipose tissue showed increased association with glomerular hyperfiltration. This association was explained by the increase in leptin levels that are released by subcutaneous adipose tissue. Pinto-Sietsma et al25 demonstrated that a central fat distribution pattern seems to be important for renal impairment since renal abnormalities were associated with central body fat, decreased filtration and proteinuria regardless of BMI, hypertension, and glycemia.
Differently from other cross-sectional studies14–16 there was no significant positive association and a linear trend between the number of MetS components and the prevalence of CKD. However, although the odds ratio is significant only with 3 and 5 components of MetS, it is possible to observe the increase of its absolute value and the frequency of CKD with the increase in the number of components of MetS.
Among combinations of three components of MetS, the combination of abdominal obesity, high fasting blood glucose, and reduced HDL-c showed the highest strength of association with CKD (OR = 2.67, 95% CI 1.70–4.20). Among the four-component combinations of MetS, the one with the greatest strength of association with CKD was the combination of high blood pressure, low HDL cholesterol, high fasting blood glucose, abdominal obesity (OR = 2.50 95% CI 1.55–4.02). Remarkably, high fasting blood glucose was in all combinations that had the highest chances of CKD. This pattern reaffirms the impact of diabetes on the risk of CKD among individuals in the study population.1,18,26,27 More studies are needed to explore these relationships, particularly in the Brazilian population with AH and/or DM.
Considering the findings of this study and taking into account the increase in the prevalence of Non-communicable Diseases (NCDs), the “Strategic Action Plan to Confront Non-communicable Chronic Diseases (NCDs) in Brazil, 2011–2022”28 defines and prioritizes actions and investment needed to prepare the country to confront and detain NCDs over the 10 years. The objective of the Plan is to promote the development and implementation of effective, integrated, sustainable and evidence-based public policies for the prevention and control of NCDs and their risk factors and to strengthen health services for these diseases.28 Thus, the National Kidney Disease Outcomes Quality Initiative (NKF KDOQI) guidelines recommend that subjects with AH and/or DM be screened for hidden CKD during regular clinical visits because studies have shown that when these patients maintain blood glucose and blood pressure under control the onset of renal disease is prevented or delayed.29,30
Thus, PHC is of strategic importance in the follow-up and monitoring of these patients. Identify individuals with AH and DM, who are risk factors for many diseases such as MetS and CKD in their early stages and informing them of their condition, will help prevent the progression to cardiovascular diseases or end-stage renal disease. Unfortunately, data from the Brazilian Dialysis Census show that even with the estimated number of patients who began treatment in Brazil in 2016 being 39,714, there are large regional (and state) variations, so renal treatment rates may be underestimated.31
Data from a study in the U.S.A.32 that developed projections of incidence and prevalence of end-stage renal disease from population rates of obesity, hypertension, diabetes, age, and race revealed that population changes in age and racial distribution, prevalence of obesity and diabetes, and survival of ESRD will result in an increment of 11–18% incidence rate in the gross 2015–2030. Once again, the main link between the patient and his/her appropriate treatment is in PHC.
The consensus paper33 published in 2018 by the Working Group of the European Society for Hypertension, Diabetes, and High-Risk Patients and the European Association for the Study of Obesity revealed that obesity-related cardiovascular risk should be detected early on by health education in schools and communities. Also, the document emphasized the importance that proper management of overweight and obesity has about the reduction of financial and social costs for health systems and people.
This study has many strengths. The first is the methodological rigor that reinforced the robustness of the results presented, strengthening the internal validity of the research and the ability to generalize its findings in the planning of health services not only in the investigated region but in other small and medium-sized Brazilian cities who have PHC coverage. A multi-stage stratified cluster-sampling procedure was also used to obtain a representative sample of the population followed by PHC in the Brazilian municipality studied, aged over 18 years. However, the study also has some limitations. Among the limitations of this study, we highlight the possible reverse causality bias inherent in cross-sectional studies, which restricts some of the associations found in that it is not possible to establish causal relationships between exposures and the outcome due to the lack of temporality between the occurrence of the events of interest. And, second, the presence or absence of CKD was calculated using the CKD-EPI formula which, while widely used and recommended, may not provide an exact estimate of GFR.
Conclusion
The study showed that MetS is prevalent in adults and the elderly with hypertension and/or diabetes mellitus. In individuals with CKD, MetS was highly prevalent. The individual components of high fasting blood glucose MetS, abdominal obesity, and reduced HDL-c, as well as the number and combinations of components, were independently associated with CKD. Critical combinations of MetS containing mainly high fasting blood glucose accounted for the greatest strength of association with CKD. The findings of this study highlight the importance of PHC in the early diagnosis of diseases such as CKD. It also highlights the need for prevention, targeted screening, and appropriate treatments regarding MetS components in adults and the elderly who already have risk factors in developing it.
Acknowledgments
The present study was supported by the Minas Gerais Research Support Foundation (FAPEMIG) for the research project entitled “Prevention of diseases and diseases in patients with arterial hypertension in the context of primary health care: chronic renal disease on the agenda”. Process Number: CSA-APQ-03510-13.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International. Circulation. 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 2.Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. [DOI] [PubMed] [Google Scholar]
- 3.Vidigal FC, Bressan J, Babio N, Salas-Salvadó J. Prevalence of metabolic syndrome in Brazilian adults: a systematic review. BMC Public Health. 2013;13:1198. doi: 10.1186/1471-2458-13-1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aguilar M, Bhuket T, Torres S, Liu B, Wong R. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA. 2015;313:1973–1974. [DOI] [PubMed] [Google Scholar]
- 5.Escobedo J, Schargrodsky H, Champagne B, et al. Prevalence of the metabolic syndrome in Latin America and its association with sub-clinical carotid atherosclerosis: the CARMELA cross sectional study. Cardiovasc diabetol. 2009;9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Muntner P, Hamm L, et al. The metabolic syndrome and chronic kidney disease in U.S.adults. Ann Intern Med. 2004;140:167–174. doi: 10.7326/0003-4819-140-3-200402030-00007 [DOI] [PubMed] [Google Scholar]
- 7.Wilson PWF, Agostino RBD, Parise H, Sullivan L, Meigs JB. Metabolic Syndrome as a Precursor of Cardiovascular Disease and Type 2 Diabetes Mellitus. Cirulation 2005; [DOI] [PubMed] [Google Scholar]
- 8.Sheng W, Zhong H, Suzanne L. Metabolic Syndrome and All-Cause Mortality: A Meta-Analysis of Prospective Cohort Studies. Eur J Epidemiol. 2010;25(6):375–384. doi: 10.1007/s10654-010-9459-z. [DOI] [PubMed] [Google Scholar]
- 9.Lastra G, Manrique C, Sowers JR. Obesity, cardiometabolic syndrome, and chronic kidney disease: the weight of the evidence. Adv Chronic Kidney Dis. 2006;13:365–373. doi: 10.1053/j.ackd.2006.07.011 [DOI] [PubMed] [Google Scholar]
- 10.National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III) final report. Circulation. 2002;106:3144–3421. [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 12.Instituto Brasileiro de Geografia e Estatística - IBGE. Pesquisa De Orçamentos Familiares (POF) 2008-2009: Antropometria e Estado Nutricional De Crianças, Adolescentes e Adultos No Brasil. Rio de Janeiro: IBGE; 2009. [Google Scholar]
- 13.Brasil. Ministério Da Saúde. Secretaria de Vigilancia em Saúde. Departamento de Vigilância de Doenças e Agravos não Transmissíveis e Promoção da Saúde. VIGITEL BRASIL 2017 - Vigilância De Fatores De Risco e Proteção Para Doenças Crônicas Por Inquérito Telefônico. Brasília: Ministério da Saúde; 2018. Available from: http://portal.saude.gov.br/portal/arquivos/pdf/vigitel_2010_preliminar_web.pdf. [Google Scholar]
- 14.Chen J, Kong X, Jia X, et al. Association between metabolic syndrome and chronic kidney disease in a Chinese urban population. Clin Chim Acta. 2017;470:103–108. doi: 10.1016/j.cca.2017.05.012 [DOI] [PubMed] [Google Scholar]
- 15.Huh JH, Yadav D, Kim JS, et al. An association of metabolic syndrome and chronic kidney disease from a 10-year prospective cohort study. Metabolism. 2017;67:54–61. doi: 10.1016/j.metabol.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 16.Mendy VL, Azevedo MJ, Sarpong DF, et al. The association between individual and combined components of metabolic syndrome and chronic kidney disease among African Americans: the Jackson Heart Study. PLoS One. 2014;9:1–7. doi: 10.1371/journal.pone.0101610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopes HF, Corrêa-Giannella ML, Consolim-Colombo FM, Egan BM. Visceral adiposity syndrome. Diabetol Metab Syndr. 2016;8:1–8. doi: 10.1186/s13098-016-0156-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trujillo J, Chirino Yolanda I, Martínez-Tagüeña N, Pedraza-Chaverri J. Renal damage in the metabolic syndrome (MetSx): disorders implicated. Eur J Pharmacol. 2018;818:554–568. doi: 10.1016/j.ejphar.2017.11.032 [DOI] [PubMed] [Google Scholar]
- 19.Stefansson VTN, Schei J, Solbu MD, Jenssen TG, Melsom T, Eriksen BO. Metabolic syndrome but not obesity measures are risk factors for accelerated age-related glomerular filtration rate decline in the general population. Kidney Int. 2018;93:1183–1190. doi: 10.1016/j.kint.2017.11.012 [DOI] [PubMed] [Google Scholar]
- 20.Spoto B, Pisano A, Zoccali C. Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Physiol. 2016;311:F1087–F1108. doi: 10.1152/ajprenal.00340.2016 [DOI] [PubMed] [Google Scholar]
- 21.Ding C, Yang Z, Wang S, Sun F, Zhan S. The associations of metabolic syndrome with incident hypertension, type 2 diabetes mellitus and chronic kidney disease: a cohort study. Endocrine. 2018;60:282–291. doi: 10.1007/s12020-018-1552-1 [DOI] [PubMed] [Google Scholar]
- 22.Kwakernaak AJ, Zelle DM, Bakker SJL, Navis G. Central body fat distribution associates with unfavorable renal hemodynamics independent of body mass index. J Am Soc Nephrol. 2013;24:987–994. doi: 10.1681/ASN.2012050460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burton JO, Gray LJ, Webb DR, et al. Association of anthropometric obesity measures with chronic kidney disease risk in a non-diabetic patient population. Nephrol Dial Transplant. 2012;27:1860–1866. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, Kim HJ, Cho B, et al. Abdominal adipose tissue was associated with glomerular hyperfiltration among non-diabetic and normotensive adults with a normal body mass index. PLoS One. 2015;10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinto-Sietsma S-J, Navis G, Janssen WMT, de ZD, Gans ROB, De Jong PE. A central body fat distribution is related to renal function impairment, even in lean subjects. Am J Kidney Dis. 2003;41:733–741. doi: 10.1016/S0272-6386(03)00020-9 [DOI] [PubMed] [Google Scholar]
- 26.McCracken E, Monaghan M, Sreenivasan S. Pathophysiology of the metabolic syndrome. Clin Dermatol. 2018;36:14–20. doi: 10.1016/j.clindermatol.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 27.Gluba A, Mikhailidis DP, Lip GYH, Hannam S, Rysz J, Banach M. Metabolic syndrome and renal disease. Int J Cardiol. 2013;164:141–150. doi: 10.1016/j.ijcard.2012.01.013 [DOI] [PubMed] [Google Scholar]
- 28.BRASIL. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Análise de Situação de Saúde. Plano de ações estratégicas para o enfrentamenteo das Doenças Crônias Não Transmissíveis (DCNT) no Brasil 2011-2022; 2011. doi: 10.1017/CBO9781107415324.004. [DOI]
- 29.NICE Clinical Guidelines 182. Chronic Kidney Disease: Early Iden- Tification and Management of Chronic Kidney Disease in Adults in Primary and Secondary Care. National Institute of Health and Care Excellence: London; 2014. [Google Scholar]
- 30.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 31.Sesso RC, Lopes AA, Thomé FS, Lugon JR, Martins CT. Brazilian chronic dialysis survey 2016. J Bras Nefrol. 2017;39:261–266. doi: 10.5935/0101-2800.20170049 [DOI] [PubMed] [Google Scholar]
- 32.McCullough KP, Morgenstern H, Saran R, Herman WH, Robinson BM. Projecting ESRD incidence and prevalence in the United States through 2030. J Am Soc Nephrol. 2019;30:127–135. doi: 10.1681/ASN.2018050531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kotsis V, Tsioufis K, Antza C, et al. Obesity and cardiovascular risk. J Hypertens. 2018;36:1441–1455. doi: 10.1097/HJH.0000000000001731 [DOI] [PubMed] [Google Scholar]