Abstract
Background: Extended-spectrum beta-lactamase producing Enterobacteriaceae (ESBL-E) threaten human health; and, in areas of sub-Saharan Africa (sSA) where carbapenems are not available, may render ESBL-E infections untreatable. Gut mucosal colonisation probably occurs before infection, making prevention of colonisation an attractive target for intervention, but the epidemiology of ESBL-E in sSA is poorly described.
Objectives: Describe ESBL-E colonisation prevalence in sSA and risk factors associated with colonisation.
Methods: Studies included were prospective cross-sectional or cohort studies reporting gut mucosal ESBL-E colonisation in any population in sSA. We searched PubMed and Scopus on 18 December 2018. We summarise the range of prevalence across sites and tabulated risk factors for colonisation. The protocol was registered (Prospero ID CRD42019123559).
Results: From 2975 abstracts we identified 32 studies including a total of 8619 participants from a range of countries and settings. Six studies were longitudinal; no longitudinal studies followed patients beyond hospital discharge. Prevalence varied between 5 and 84% with a median of 31%, with a relationship to setting: pooled ESBL-E colonisation in community studies was 18% (95% CI 12 to 28, 12 studies); in studies recruiting people at admission to hospital colonisation was 32% (95% CI 24 to 41% 8 studies); and for inpatients, colonisation was 55% (95% CI 49 to 60%, 7 studies). Antimicrobial use was associated with increased risk of ESBL-E colonisation, and protected water sources or water treatment by boiling may reduce risk.
Conclusions: ESBL-E colonisation is common in sSA, but how people become carriers and why is not well understood. To inform the design of interventions to interrupt transmission in this setting requires longitudinal, community studies.
Keywords: ESBL, Extended-spectrum beta-lactamase, Africa south of the Sahara, Antimicrobial resistance
Introduction
Extended-spectrum beta-lactamase producing Enterobacteriaceae (ESBL-E) are a significant threat to human health, and have been identified by the World Health Organisation as pathogens of critical importance 1. In sub-Saharan Africa (sSA), it is increasingly clear that a significant proportion of invasive Enterobacteriaceae infections are ESBL-E and the absence of second line antimicrobials can render infections with these pathogens locally untreatable 2. Strategies to interrupt ESBL-E transmission that can be practically deployed at scale in low resource settings are urgently needed.
Gut mucosal colonisation with Enterobacteriaceae is thought to precede invasive infection 3, 4, and so preventing ESBL-E colonisation is an attractive strategy for prevention of invasive disease. Data describing the basic epidemiology of ESBL-E colonisation in sSA, will help inform the design of interventions targeted at reducing colonisation. A 2016 meta-analysis of community ESBL-E colonisation prevalence among healthy individuals found only four studies from sSA with a pooled prevalence of 15% (95% CI 4–31%), and significant between-study heterogeneity 5. No studies described risk factors from Africa. We were aware of a number of studies that had been published since 2016 including a number that described ESBL-E colonisation in any population, so undertook a systematic review and meta-analysis with two aims: firstly, to describe the prevalence of ESBL-E gut mucosal colonisation in sSA; and secondly, to describe any risk factors associated with colonisation. In terms of the PRISMA (preferred reporting items for systematic reviews and meta analyses) PICOS (participants, interventions, comparisons, outcomes and study design) approach, our questions can be framed as: what is the prevalence of ESBL-E gut mucosal colonisation (the outcome) and risk factors for colonisation (comparisons) in any population in sSA (the population) as measured in prospective cross-sectional or cohort studies (study design).
Methods
Inclusion criteria were any prospective cross-sectional or cohort study that had screened for gut mucosal colonisation of ESBL-E in any population in sSA for which it was possible to extract a numerator and denominator to calculate an ESBL-E colonisation prevalence. Exclusion criteria were studies in which the sampled population was not clearly defined in a reproducible way (i.e. laboratory-based studies), or if the laboratory techniques aimed to isolate only a particular organism or type of organism (e.g. Enteropathogenic E. coli). PubMed and Scopus were searched in all fields using the search terms given in Table 1, on 18 December 2018. Abstracts were extracted into Endnote X7.8 (Thomson Reuters, United States) and independently reviewed against the inclusion criteria by two authors (JL and RL), with disagreements settled by consensus.
Table 1. Systematic review search terms.
| ((ESBL) OR Extended-spectrum beta-lactamase)) AND (((Angola OR Benin OR Botswana OR Burkina Faso OR Burundi OR
Cameroon OR Cape Verde OR Central African Republic OR Chad OR Comoros OR Republic of the Congo OR Congo Brazzaville OR Democratic republic of the Congo OR Cote d’Ivoire OR Djibouti OR Equatorial Guinea OR Eritrea OR Ethiopia OR Gabon OR The Gambia OR Ghana OR Guinea OR Guinea-Bissau OR Kenya OR Lesotho OR Liberia OR Madagascar OR Malawi OR Mali OR Mauritania OR Mauritius OR Mozambique OR Namibia OR Niger OR Nigeria OR Reunion OR Rwanda OR Sao Tome and Principe OR Senegal OR Seychelles OR Sierra Leone OR Somalia OR South Africa OR Sudan OR Swaziland OR Eswatini OR Tanzania OR Togo OR Uganda OR Western Sahara OR Zambia OR Zimbabwe) OR Africa)) |
Full-text review of included studies was then undertaken, with studies assessed against the same inclusion criteria, again with disagreements settled by consensus. Data were then extracted into a Microsoft Excel for Mac v16.27 spreadsheet (Microsoft, United States): study title and authors, year of publication, dates of sample collection, inclusion criteria, median age or participants, details of microbiologic testing procedures, number of participants and number of participants from whom ESBL-E were isolated, and any risk factors for ESBL-E that were assessed and/or found to be associated with ESBL-E colonisation. Two authors extracted data independently (RL and JL) and any inconsistencies corrected by re-review of the original paper. For cohort studies only the baseline prevalence was included. Prevalence was presented as forest plots with exact binomial confidence intervals. Age group (neonate, child, adult, as per study definition) and location of sampling (community, outpatient [including health centre attendees], on hospital admission, [defined as a hospital inpatient for < 24hr] hospitalised, [defined as a hospital inpatient for > 24hr]) were selected as a priori subgroups that we hypothesised may explain heterogeneity in ESBL-E prevalence, and analyses were stratified by these subgroups. Studies were additionally classified as being carried out in a special population if they were carried out in a subpopulation of a subgroup (for example, pregnant women in the community). Effect size of risk factors for ESBL-E colonisation were presented as odds ratios; if odds ratios were not provided by the original studies then they were calculated, with 0.5 added to zero cells. Pooled random effect summary estimates of prevalence, where calculated, were generated using the metaprop package in R using the inverse variance method with a logit transformation. All analysis was undertaken using R v3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
Risk of bias of included studies was assessed with a modified Critical Appraisal Skills Programme (CASP) checklist, designed to fit our research question (full tool available as extended data). The risk of bias assessment was performed by JL and RL, and any disagreements were resolved by consensus.
The protocol of this review was published on PROSPERO (PROSPERO ID CRD42019123559) and the review was undertaken as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (PRISMA checklist available Reporting guidelines).
Results
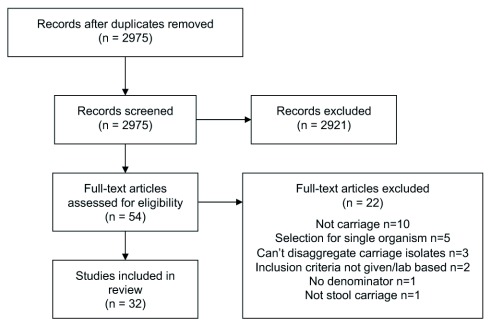
Of 2975 identified unique studies, 32 were included in this review 6– 37 ( Figure 1), from 19 countries in sSA ( Table 2). Studies from three countries – Tanzania (n=7), Madagascar (n=4) and Cameroon (n=4) - together made up 15/32 (47%) of the available studies. In total, 8619 participants were included and for 7232/8619 (84%) it was possible to disaggregate the participants into age groups: 4313/7232 (60%) were adults, 2470/7232 (34%) children and 449/7232 (6%) neonates. 2302/8619 (27%) of included participants were community members, 1729/8619 (20%) were outpatients, 2836/8619 (33%) were sampled on admission to hospital, and 1534/8619 (18%) were inpatients. 6/32 studies were cohort studies; all of these studies followed patients up whilst hospitalised only. Many studies were carried out in special populations, including the majority of community studies: 9/12 community studies were in special populations, as well as 3/7 outpatient studies, 3/8 studies of participants on hospital admission and 2/7 inpatient studies. It was not possible to classify patients from two studies into our predefined categories: one sampled staff and children of an orphanage, and the other hospital workers and their families. These studies were excluded from the pooled analyses. Details of the microbiological testing procedures are shown in Table 3.
Figure 1. Flow chart of included studies.
Table 2. Details of included studies.
CAR = Central African Republic; ART = antiretroviral therapy; UTI = urinary tract infection; NR = not reported. yr = year; m = months, d = days, hr = hours. * = mean rather than media.
| Study | Year
Pub. |
Study
Period |
Country | Study
Type |
Inclusion
Population: details |
Age
group |
Median
age |
n |
|---|---|---|---|---|---|---|---|---|
| COMMUNITY STUDIES | ||||||||
| Albrechtova 2012 | 2012 | 2009 | Kenya | Cross sec. | General population | Adults | NR | 23 |
| Mshana 2016 | 2016 | 2014 | Tanzania | Cross sec. | General population | both | 10yr | 334 |
| Katakweba 2018 | 2018 | 2011–13 | Tanzania | Cross sec. | General population | Adults | NR | 70 |
| Ruppe 2009 | 2009 | NR | Senegal | Cross sec. | Special population (remote villages) | Children | 6.9yr * | 20 |
| Lonchel 2012 | 2012 | 2009 | Cameroon | Cross sec. | Special population (students) | Adults | 24.7yr * | 150 |
| Chereau 2015 | 2015 | 2013–14 | Madagascar | Cross sec. | Special population (pregnant women) | Adults | 26yr * | 356 |
| Farra 2016 | 2016 | 2013 | CAR | Cross sec. | Special population (healthy controls in a
diarrhoea study) |
Children | 10.5m | 134 |
| Ribeiro 2016 | 2016 | 2013 | Angola | Cross sec. | Special population (no antibiotics/hospital
exposure last 3 mo) |
Adults | NR | |
| Tellevik 2016 | 2016 | 2010–11 | Tanzania | Cross sec. | Special population: <2yr attending health
centre for vaccine |
Children | NR | 250 |
| Moremi 2017 | 2017 | 2015 | Tanzania | Cross sec. | Special population (street children) | Children | 14.2yr * | 107 |
| Chirindze 2018 | 2018 | 2016 | Mozambique | Cross sec. | Special population (Students in the
community) |
Adults | NR | 275 |
| Sanneh 2018 | 2018 | 2015 | The Gambia | Cross sec. | Special population (Food handlers in schools) | Adults | 37yr * | 565 |
| HOSPITAL OUTPATIENTS | ||||||||
| Herindrainy 2011 | 2011 | 2009 | Madagascar | Cross sec. | Outpatients | Adults | NR | 306 |
| Lonchel 2012 | 2012 | 2009 | Cameroon | Cross sec. | Outpatients | Adults | 36.9yr * | 208 |
| Magoue 2013 | 2013 | 2010 | Cameroon | Cross sec. | Outpatients | Adults | NR | 232 |
| Outpatients | Children | NR | 147 | |||||
| Djuikoue 2016 | 2016 | 2011–12 | Cameroon | Cross sec. | Special population (outpatient women with
susp. UTI) |
Adults | NR | 86 |
| Wilmore 2017 | 2017 | 2014–15 | Zimbabwe | Cross sec. | Special population (outpatient, HIV infected,
stable on ART) |
Children | 11yr | 175 |
| Herindrainy 2018 | 2018 | 2015–16 | Madagascar | Cross sec. | Special population (Pregnant women at
delivery) |
Adults | 26yr * | 275 |
| Stanley 2018 | 2018 | 2017 | Uganda | Cross sec. | Special population (participants who reared
animals, attending health facility with a fever and/or diarrhoea but without malaria) |
both | 21.7yr * | 300 |
| ON HOSPITAL ADMISSION | ||||||||
| Andriatahina 2010 | 2010 | 2008 | Madagascar | Cohort | On hospital admission | Children | 38.3m | 244 |
| Kurz 2016 | 2016 | 2014 | Rwanda | Cohort | On hospital admission | both | 29yr | 753 |
| Magwenzi 2017 | 2017 | 2015 | Zimbabwe | Cohort | On hospital admission | Children | 1.0yr | 164 |
| Founou 2018 | 2018 | 2017 | South Africa | Cohort | On hospital admission | Adults | NR | 43 |
| Moremi 2018 | 2018 | 2014–15 | Tanzania | Cohort | On hospital admission | Adults | NR | 930 |
| Woerther 2011 | 2011 | 2007–08 | Niger | Cohort | Special population (Children with SAM) | Children | 16.3m * | 55 |
| Isendahl 2012 | 2012 | 2010 | Guinea-Bissau | Cross sec. | Special population (Children att. hospital w/
fever or tachycardia) |
Children | NR | 408 |
| Nelson 2014 | 2014 | 2013 | Tanzania | Cohort | Special population (Pregnant women and
neonates, inpatient) |
Neonate | 0d | 126 |
| Adults | 26.5yr * | 113 | ||||||
| INPATIENTS | ||||||||
| Lonchel 2013 | 2013 | 2009 | Cameroon | Cross sec. | Inpatients | Adults | 46.8yr * | 121 |
| Magoue 2013 | 2013 | 2010 | Cameroon | Cross sec. | Inpatients | Adults | NR | 208 |
| Schaumburg
2013 |
2013 | 2010–11 | Gabon | Cross sec. | Inpatients | Children | NR | 200 |
| Desta 2016 | 2016 | 2012 | Ethiopia | Cross sec. | Inpatients | Adults | 35yr | 154 |
| Inpatients | Children | 7yr | 94 | |||||
| Inpatients | Neonate | 9d | 19 | |||||
| Tellevik 2016 | 2016 | 2010–11 | Tanzania | Cross sec. | Inpatients | Children | NR | 353 |
| Nikema
Pessinaba 2018 |
2018 | 2015–16 | Togo | Cross sec. | Special population (<5yr with febrile
gastroenteritis) |
Children | NR | 81 |
| Marando 2018 | 2018 | 2016 | Tanzania | Cross sec. | Special population (Neonates with sepsis) | Neonate | 6d | 304 |
| OTHER | ||||||||
| Tande 2009 | 2009 | 2003 | Mali | Cross sec. | Orphanage children | Children | NR | 38 |
| Orphanage staff | Adults | NR | 30 | |||||
| Magoue 2013 | 2013 | 2010 | Cameroon | Cross sec. | Hospital workers and their families | Adults | NR | 87 |
| Relatives and carers of inpatients | Adults | NR | 63 | |||||
Table 3. Details of microbiologic testing procedures.
NR = not reported; API = analytical profile index; MALDI-TOF = Matrix-Assisted Laser Desorption/Ionization-Time of Flight.
| Study | Sample type | Screening method | Speciation
method |
ESBL confirmation
method |
|---|---|---|---|---|
| Ruppe 2009 | Stool | Drigalski and chromagar | NR | Double disc |
| Tande 2009 | Stool | Drigalski with cephalosporin | API | Double disc |
| Andriatahina 2010 | Rectal Swab | Drigalski with cephalosporin | API | Double disc |
| Herindrainy 2011 | Stool | Drigalski with cephalosporin | API | Double disc |
| Woerther 2011 | Stool | Chromagar | API | PCR |
| Albrechtova 2012 | Rectal Swab | Mackonkey with cephalosporin | API | Double disc |
| Isendahl 2012 | Rectal Swab | Chromagar | Vitek | Vitek |
| Lonchel 2012 | Stool | Mackonkey or Drigalski and cephalosporin | MALDI-TOF | Double disc |
| Lonchel 2013 | Stool | Mackonkey or Drigalski and cephalosporin | MALDI-TOF | Double disc |
| Magoue 2013 | Stool | Mackonkey or Drigalski and cephalosporin | NR | Double disc |
| Schaumburg 2013 | Rectal Swab | Chromagar | Vitek | Double disc |
| Nelson 2014 | Rectal Swab | Mackonkey with cephalosporin | Biochemical | Double disc |
| Chereau 2015 | Stool | Drigalski with cephalosporin | API | Double disc |
| Desta 2016 | Stool | Chromagar | Vitek | Vitek |
| Djuikoue 2016 | Stool | Drigalski with cephalosporin | MALDI-TOF | Double disc |
| Farra 2016 | Stool | Chromagar | NR | Double disc |
| Kurz 2016 | Rectal Swab | Chromagar | API | Combination disc |
| Mshana 2016 | Stool | Mackonkey with cephalosporin | API | Chromagar and vitek |
| Ribeiro 2016 | Stool | Chromagar | MALDI-TOF | PCR |
| Tellevik, 2016 | Stool | Chromagar | MALDI-TOF | Combination disc |
| Magwenzi 2017 | Stool or
Rectal Swab |
Chromagar and Mackonkey with cephalosporin
and nutrient broth with cephalosporin |
API | Double disc |
| Moremi 2017 | Stool | Mackonkey with cephalosporin | Biochemical | Double disc |
| Wilmore 2017 | Stool | CLEDwith cephalosproin | API and
MALDI |
Combination disc |
| Chirindze 2018 | Stool | Mackonkey with cephalosporin | API | Double disc |
| Founou 2018 | Rectal Swab | Mackonkey with cephalosporin | API | Combination disc |
| Herindrainy 2018 | Stool or
Rectal Swab |
Chromagar | MALDI-TOF | Double disc |
| Katakweba 2018 | Stool | Mackonkey with cephalosporin | MALDI-TOF | Double disc |
| Marando 2018 | Rectal swab | Mackonkey with cephalosporin | Biochemical | Double disc |
| Moremi 2018 | Rectal swab | Mackonkey with cephalosporin | vitek | vitek |
| Nikema Pessinaba
2018 |
Stool | Drigalski with cephalosporin | NR | NR |
| Sanneh 2018 | Stool | Drigalski And Cephalosporin | NR | Double disc |
| Stanley 2018 | Stool | AST | BD phoenix | BD phoenix |
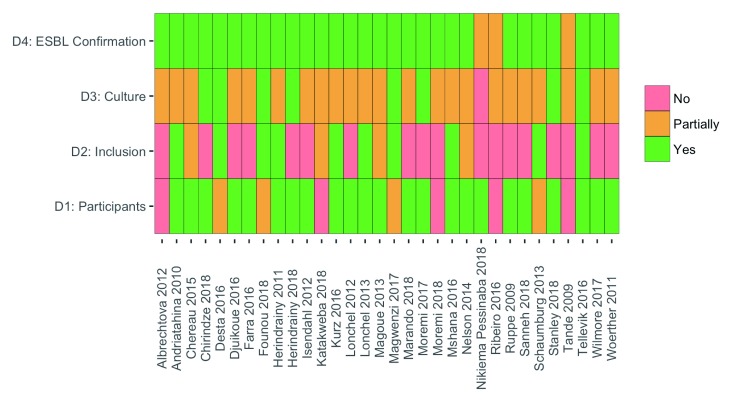
The results of the risk of bias assessment are shown in Figure 2. The most notable potential for biased ESBL-E prevalence estimates resulted from selection of study populations. Several studies recruited a selected group, which we defined as a special population: pregnant women, street children, children and staff of an orphanage, or food handlers in schools. These are likely to produce a biased estimate of community prevalence. Though microbiological culture methods were frequently described in a reproducible manner, few studies reported quality control procedures, resulting in an assessment of moderate risk of bias for the majority of studies across this domain.
Figure 2. Results of risk of bias assessment.
Domain 1: Are the characteristics of the participants included in the study adequately described? Domain 2: Are the eligibility criteria to enter the study explicit and appropriate? Domain 3: Were stool culture results precise and reported? Domain 4: Were the methods of extended-spectrum beta-lactamase (ESBL) confirmatory testing precise?
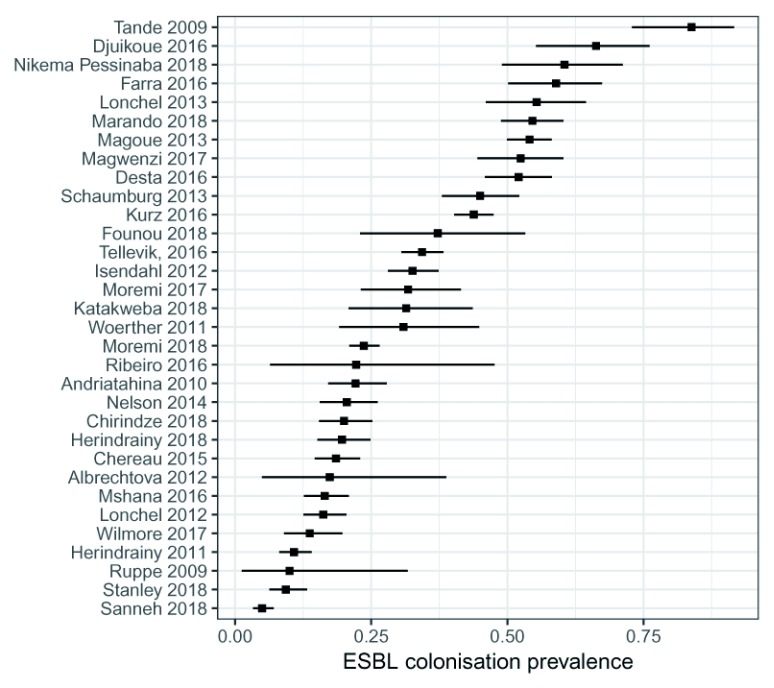
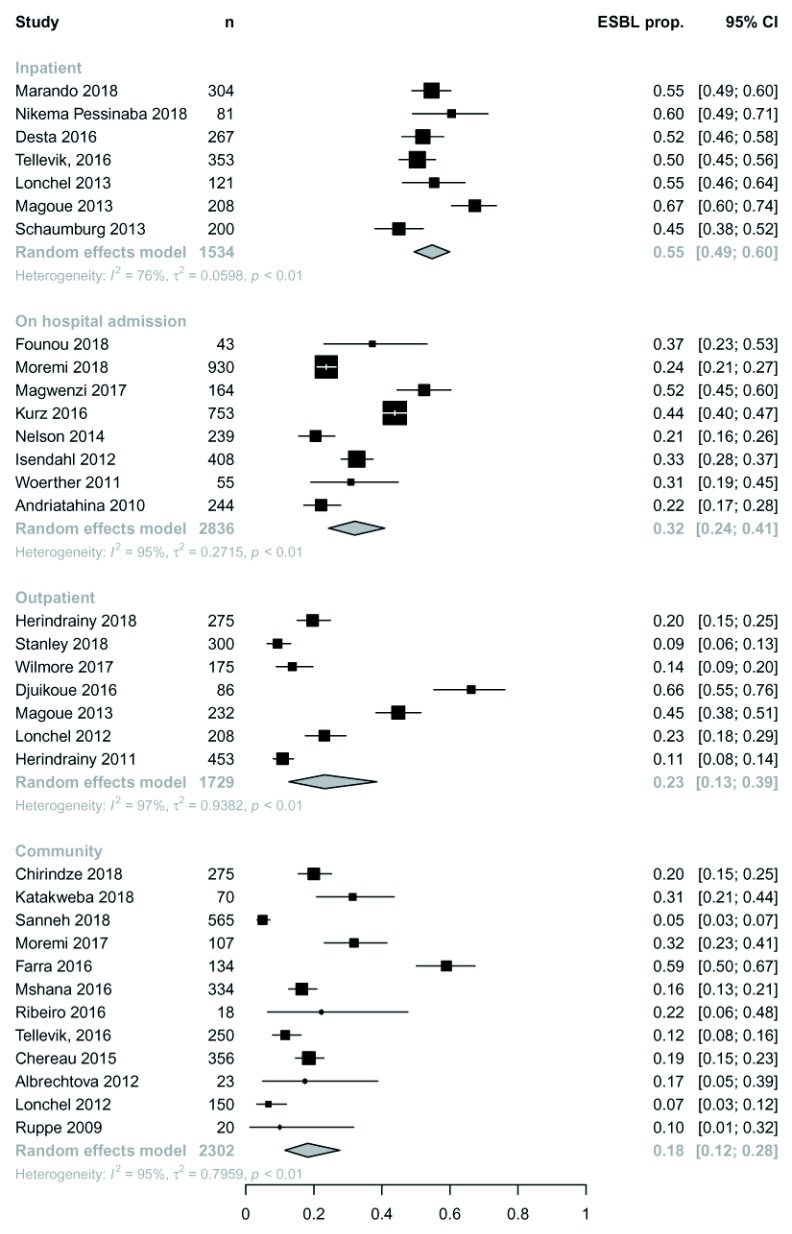
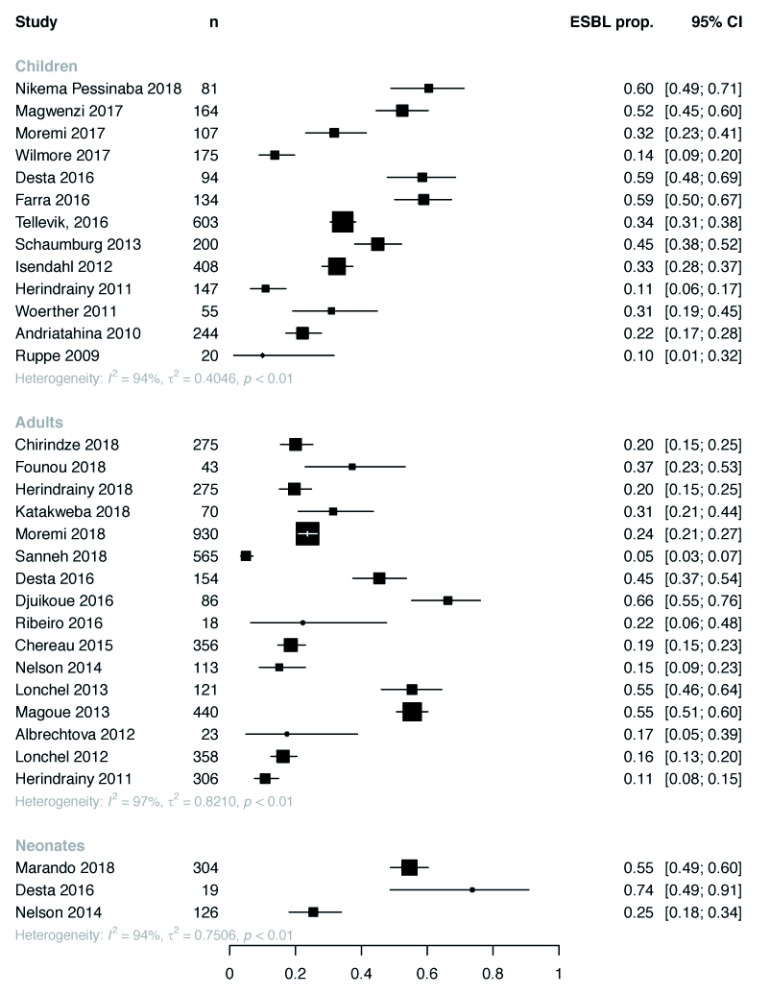
Overall ESBL-E colonisation prevalence was extremely heterogeneous across studies ranging from 5–84% (median 31%) with no trend by year of publication ( Figure 3). Some heterogeneity was explained by location of sampling ( Figure 4): inpatients tended to have the highest colonisation prevalence with community members the least. There was no clear difference in prevalence between neonates, children or adults ( Figure 5). Pooled random-effect summary estimates were therefore calculated for differing location of sampling: community members (18% [95% CI 11–28%]), outpatients (23% [95% CI 13-39%]), inpatients on hospital admission (32% [95% CI 24–41%]) and inpatients (55% [95% CI 49-60%]), though in each stratum significant heterogeneity remained (I 2 76–97%) so these summary estimates should be treated with caution ( Figure 4).
Figure 3. Overall extended-spectrum beta-lactamase producing Enterobacteriaceae (ESBL-E) colonization prevalence by study.
Figure 4. Extended-spectrum beta-lactamase (ESBL) colonisation by study with pooled random effect summary estimates stratified by location of sampling.
ESBL prop. = proportion of ESBL producing Enterobacteriaceae.
Figure 5. Extended-spectrum beta-lactamase producing Enterobacteriaceae (ESBL-E) carriage prevalence stratified by age group.
Two-thirds (21/32) of studies performed an analysis to identify factors associated with ESBL-E colonisation ( Table 4). Prior hospitalisation was assessed as a risk factor in 13 studies, and a statistically significant association found in 4/13, with odds ratios of 2.1-8.5. Antimicrobial exposure was assessed in 13 studies, and a statistically significant association found in 5/13 with odds ratios of 1.6-27.0. Using water from a borehole 28, boiling water before drinking 14 and having private inside access to drinking water 10 were found to be associated with a lower prevalence of ESBL-E colonisation in three different studies. One study found that a higher socio-economic status was associated with a lower ESBL-E prevalence 29, and one the opposite 13. Only two studies addressed the association between HIV status and ESBL-E colonisation status; one, in adults found no association 9, whereas the other, in children, found a strong association 17. Only one study assessed the association between animals in the home as ESBL-E colonisation 10, finding no association.
Table 4. Assessed and significant risk factors in the included studies.
mv = multivariate, uv = univariate, HH = household, abx = antibiotics, SES = socio-economic status, HC = health centre, ART = antiretroviral therapy, VL = viral load, PROM = premature rupture of membranes, WASH = water, sanitation and hygiene. UTI = urinary tract infection, NR = not reported. * confidence interval crosses 1; original publication used fisher’s exact test and found p < 0.05.
| Study | Risk factors assessed | Analysis | Significant risk factors | Odds ratio (95%
CI) |
|---|---|---|---|---|
| Tande 2009 | Adults with direct contact with the children in
orphanage |
uv | Contact with orphanage children | 19.7 (3.2 - 201.3) |
| Andriatahina
2010 |
Age, gender, patient origin (home vs health
facility), abx or hospitalisation last 30days, admitting dx, infection on admission |
mv | Hospitalisation last 30d | 7.4 (2.9-18.3) |
| Herindrainy 2011 | SES, no. of rooms occupied, ratio occupants:
room |
mv | Occupation HH head unemployed
vs manager |
9.1 (1.6-53.9) |
| Isendahl 2012 | Age, gender, weight, MUAC, breastfeeding,
bedsharing, children in HH, abx, hospitalisation |
uv | Bedsharing | 1.9 (1.0 - 3.4) |
| Lonchel 2013 | Age, gender, hospital, diagnosis, abx within
3m, hospitalisation within 1yr |
mv | Hospitalisation during the previous
year |
4.13 (1.37–12.78) |
| Admission with infection | 0.30 (0.10–0.82) | |||
| Intermediate vs tertiary hospital | 4.10 (1.77–9.59) | |||
| Schaumburg
2013 |
Age, hospitalisation, residence, sex, diagnosis,
abx use |
mv | Age <=5 | 2.2 (1.1–4.8) |
| Hospitalization 5–7 days vs < 5 | 5.1 (1.6–18.4) | |||
| Hospitalization for =7 days vs < 5 | 30.6 (5.8–566.0) | |||
| Hospital stay during the past
12 months |
2.1 (1.1–4.0) | |||
| Nelson 2014 | For neonates: Gestation, birthweight, gender,
delivery method, ward, abx use |
uv | Antibiotic use | 10.8 (0.6 - 186) * |
| For mothers: Delivery mode, admission within
30d, abx within 3m, abx within 30d, current abx, catheter, HIV status |
Nothing | |||
| Chereau 2015 | Study area, age, education, marital status,
type house, electricity, type of birth attendant, toilets, water, animals at home, hospitalisation, abx use |
mv | Private inside access to drinking
water |
0.3 (0.1–0.8) |
| Desta 2016 | Higher maximum bed capacity per room,
increasing number of patients admitted in single room |
uv | Sharing room vs not | 4.0 (2.3 to 5.3) |
| Djuikoue 2016 | Age, pregnancy, abx last 3m, hospital last 3m | uv | None | |
| Farra 2016 | Age, gender, comorbidity, SES, nutritional
status, animals at home, toilets, urban/rural, hh members, meals |
mv | Highest SES class vs lowest | 31.06 (2.49–387.13) |
| Kurz 2016 | Age, gender , residence, ward, referral, other
healthcare 3m, abx 3m, education, SES, water source, food, time to HC, caregiver ESBL status |
mv | ESBL colonised caregiver, | 2.88 (1.80-4.61) |
| Antibiotics within 3 months, | 2.70 (1.59-4.58) | |||
| Frequently consume eggs | 6.52 (1.75-24.31) | |||
| Boil water prior to drinking | 0.59 (0.37-0.92) | |||
| Mshana 2016 | Age, region, no of children in house, abx use
within 1m, admission within 1yr |
mv | Older age (per yr), | 1.07 (1.04–1.10) |
| Hospital admission last yr | 7.4 (1.43–38.5) | |||
| Abx last 3m | 27 (6.63–116), | |||
| Tellevik, 2016 | Age, gender, residence, parental education,
child group, nutritional status, use of abx within 14 days |
mv | HIV vs no HIV, | 9.99 (2.52–39.57), |
| Kinondoni district, | 2.62 (1.49–4.60) | |||
| Abx last 14d | 1.61 (1.07–2.41) | |||
| Moremi 2017 | Age, education, herb use, source of income,
source of food, street child type |
mv | Local herb use, | 3.3 (1.31–8.31), |
| Sleep on streets vs not | 2.8 (1.04–7.65) | |||
| Wilmore 2017 | Age, gender, CD4, VL, ART duration, admitted
to hospital with pneumonia in last 12m, adm to hospital in at 12 m |
mv | ART <1yr | 8.47 (2.22–2.27) |
| Admission with pneumonia in last
12m |
8.47 (1.12–64.07) | |||
| Marando 2018 | Age, gender, weight, admission where, clinical
factors, abx use, PROM |
mv | Current abx use | 1.73 (1.00-2.97), |
| ESBL colonised mother | 2.19 (1.26-3.79) | |||
| Moremi 2018 | Age, gender, history of antibiotic use, history of
admission, history of surgery |
mv | Older age (per year) | 1.01 (1.00–1.02) |
| Nikema
Pessinaba 2018 |
Age, gender, site, drinking water source, time
to sample analysis |
mv | Drink non borehole water vs
borehole |
3.47 (1.22-9.82) |
| Sanneh 2018 | WASH behaviours, hospitalised within 3m,
invasive procedures, abx within 3m, abx from street, completing abx, diarrhoea/UTI 3m, food handling training |
uv | Lack of food handling training and
knowledge of the principle of food safety |
NR |
| Abx within 3m | NR | |||
| Stanley
2018 |
Age, gender, health facility, presentation | uv | none |
Of the 6 cohort studies, all sampled participants on admission to hospital and on discharge, a median 5.6-8 days later, and all found an increase in ESBL-E colonisation prevalence between the two sampling points ( Table 5). No study longitudinally sampled ESBL colonisation in the community, either in community dwellers or in those discharged from hospital.
Table 5. Longitudinal ESBL prevalence in included cohort studies.
NR = not reported. * = median not given but admission length was 2–10 days.
| Study | Study population | ESBL prevalence | Median follow up | |
|---|---|---|---|---|
| Admission | Discharge | |||
| Andriatahina 2010 | Children | 51/244 (21%) | 88/154 (57%) | 5.7d |
| Woerther 2011 | Children | 17/55 (31%) | 15/16 (94%) | 8d |
| Nelson 2014 | Neonates | 32/126 (25%) | 77/126 (61%) | 7d |
| Kurz 2016 | Adults and children | 195/392 (50%) | 173/208 (83%) | 6d |
| Magwenzi 2017 | Children | 86/164 (52%) | 115/164 (70%) | 5.6d |
| Moremi 2018 | Adults | 220/930 (24%) | 143/272 (53%) | NR * |
Discussion
ESBL-E colonisation is common across sSA, though with significant unexplained heterogeneity between study locations and populations. Community ESBL-E colonisation ranges from 5% in adults in Gambia in 2015 to 59% in children in the Central African Republic in 2013, the latter comparable to the highest described colonisation prevalence in the world 5. Our pooled estimate suggests 18% (95% CI 11–29%) of people in sSA are colonised with ESBL-E, a higher prevalence than in high income settings. In Europe, community prevalence of ESBL-E colonisation is reported to range from 3.7% in Spain in 2004 to 7.3% in the UK in 2014 38– 41, similar to the United States where a community prevalence of 3.4% was reported in healthy children 42. In many of the estimates of studies included in this review, the reported prevalence of ESBL-E is more comparable to that reported in Asia (46% [95% CI 29–63%] 5).
The profound differences in community ESBL-E colonisation prevalence between sSA and high-resource settings warrants further investigation, beyond the assessment of risk factors we have identified in this review. Hospitalisation and antimicrobial use are likely drivers of colonisation in the studies, with higher prevalence seen in hospitalised individuals and prior hospitalisation and antimicrobial exposure frequently identified as risk factors for colonisation. Obversely and consistent with a putative faecal-oral transmission route, use of borehole water, a private indoor water source and boiling water before drinking were associated with reduced ESBL-E colonisation risk, and it may be that poor water, sanitation and hygiene (WASH) infrastructure and practices in sSA are driving high ESBL-E colonisation prevalence. This speaks to a role for poverty in driving ESBL-E colonisation; however, this is likely complex, and context-dependant, as evidenced by conflicting findings of the effect of socio-economic status on colonisation from two studies in different settings.
More broadly, this review highlights areas where data that could inform interventions to interrupt ESBL-E transmission are lacking. In the community, long-term longitudinal ESBL-E colonisation studies are necessary to understand the dynamics of community ESBL-E transmission, particularly the role of within household transmission, and the role of household animals. In health facilities, the determinants of apparent ESBL-E acquisition need to be clearly identified to design pragmatic intervention studies in the context of limited resources. Surprisingly, the role of HIV in driving the high ESBL-E colonisation prevalence in sSA is unknown. HIV is known to profoundly affect gut function, but we identified only two studies which have assessed HIV status as a risk factor for ESBL-E colonisation.
There are limitations of our review. Our search strategy may have missed studies that would otherwise be included. However, using broader inclusion criteria than a recent review of worldwide ESBL-E community colonisation prevalence 5, we have identified many more studies from sSA. Risk of bias assessment in observational studies is difficult, with no gold standard, and the tool we have used may misclassify studies with regard to bias. Significant heterogeneity remaining despite stratification warrants caution in interpreting summary estimates.
In conclusion, ESBL-E colonisation in sSA is common, and in places comparable to the highest prevalence in the world, though with significant unexplained heterogeneity between countries and populations. Hospitalisation, antimicrobial use, and poor WASH infrastructure and practices may be contributing to high prevalence; the roles of HIV and animal-human transmission remain unknown. Given the threat to human health of ESBL-E, data to fully characterise routes and drivers of transmission in sSA are necessary to design interventions to interrupt transmission in this setting.
Data availability
Underlying data
All data underlying the results are available as part of the article and no additional source data are required.
Extended data
Zenodo: Risk of bias tool and PRISMA checklist used for the publication: Gut mucosal colonisation with extended-spectrum beta-lactamase producing Enterobacteriaceae in sub-Saharan Africa: a systematic review and meta-analysis, http://doi.org/10.5281/zenodo.3478278 43
This project contains the following extended data:
-
-
Risk of bias tool used in the study
Reporting guidelines
Zenodo: PRISMA checklist for: Gut mucosal colonisation with extended-spectrum beta-lactamase producing Enterobacteriaceae in sub-Saharan Africa: a systematic review and meta-analysis, http://doi.org/10.5281/zenodo.3478278 43.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Funding Statement
JL and RL are supported by Wellcome Trust Clinical PhD Fellowships (109105z/15/a and University of Liverpool block award 347 [203919/Z/16/Z] respectively). NF is funded by the Antimicrobial Resistance Cross-Council Initiative through a grant from the Medical Research Council, a Council of UK Research and Innovation, and the National Institute for Health Research. This award is part of the EDCTP2 programme supported by the European Union (Grant number MR/S004793/1).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- 1. World Health Organisation: Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug-resistant bacterial infections, including tuberculosis. Geneva.2017. Reference Source [Google Scholar]
- 2. Musicha P, Cornick JE, Bar-Zeev N, et al. : Trends in antimicrobial resistance in bloodstream infection isolates at a large urban hospital in Malawi (1998–2016): a surveillance study. Lancet Infect Dis. 2017;17(10):1042–52. 10.1016/S1473-3099(17)30394-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Denis B, Lafaurie M, Donay JL, et al. : Prevalence, risk factors, and impact on clinical outcome of extended-spectrum beta-lactamase-producing Escherichia coli bacteraemia: a five-year study. Int J Infect Dis. 2015;39:1–6. 10.1016/j.ijid.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 4. Gorrie CL, Mirceta M, Wick RR, et al. : Antimicrobial-Resistant Klebsiella pneumoniae Carriage and Infection in Specialized Geriatric Care Wards Linked to Acquisition in the Referring Hospital. Clin Infect Dis. 2018;67(2):161–70. 10.1093/cid/ciy027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karanika S, Karantanos T, Arvanitis M, et al. : Fecal Colonization With Extended-spectrum Beta-lactamase-Producing Enterobacteriaceae and Risk Factors Among Healthy Individuals: A Systematic Review and Metaanalysis. Clin Infect Dis. 2016;63(3):310–8. 10.1093/cid/ciw283 [DOI] [PubMed] [Google Scholar]
- 6. Ruppé E, Woerther PL, Diop A, et al. : Carriage of CTX-M-15-producing Escherichia coli isolates among children living in a remote village in Senegal. Antimicrob Agents Chemother. 2009;53(7):3135–7. 10.1128/AAC.00139-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tandé D, Jallot N, Bougoudogo F, et al. : Extended-spectrum beta-lactamase-producing Enterobacteriaceae in a Malian orphanage. Emerg Infect Dis. 2009;15(3):472–4. 10.3201/eid1503.071637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schaumburg F, Alabi A, Kokou C, et al. : High burden of extended-spectrum β-lactamase-producing Enterobacteriaceae in Gabon. J Antimicrob Chemother. 2013;68(9):2140–3. 10.1093/jac/dkt164 [DOI] [PubMed] [Google Scholar]
- 9. Nelson E, Kayega J, Seni J, et al. : Evaluation of existence and transmission of extended spectrum beta lactamase producing bacteria from post-delivery women to neonates at Bugando Medical Center, Mwanza-Tanzania. BMC Res Notes. 2014;7:279. 10.1186/1756-0500-7-279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chereau F, Herindrainy P, Garin B, et al. : Colonization of extended-spectrum-β-lactamase- and NDM-1-producing Enterobacteriaceae among pregnant women in the community in a low-income country: a potential reservoir for transmission of multiresistant Enterobacteriaceae to neonates. Antimicrob Agents Chemother. 2015;59(6):3652–5. 10.1128/AAC.00029-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Desta K, Woldeamanuel Y, Azazh A, et al. : High Gastrointestinal Colonization Rate with Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Hospitalized Patients: Emergence of Carbapenemase-Producing K. pneumoniae in Ethiopia. PLoS One. 2016;11(8):e0161685. 10.1371/journal.pone.0161685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Djuikoue IC, Woerther PL, Toukam M, et al. : Intestinal carriage of Extended Spectrum Beta-Lactamase producing E. coli in women with urinary tract infections, Cameroon. J Infect Dev Ctries. 2016;10(10):1135–9. 10.3855/jidc.7616 [DOI] [PubMed] [Google Scholar]
- 13. Farra A, Frank T, Tondeur L, et al. : High rate of faecal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae in healthy children in Bangui, Central African Republic. Clin Microbiol Infect. 2016;22(10):891.e1–891.e4. 10.1016/j.cmi.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 14. Kurz MS, Bayingana C, Ndoli JM, et al. : Intense pre-admission carriage and further acquisition of ESBL-producing Enterobacteriaceae among patients and their caregivers in a tertiary hospital in Rwanda. Trop Med Int Heal. 2017;22(2):210–20. 10.1111/tmi.12824 [DOI] [PubMed] [Google Scholar]
- 15. Mshana SE, Falgenhauer L, Mirambo MM, et al. : Predictors of bl aCTX-M-15 in varieties of Escherichia coli genotypes from humans in community settings in Mwanza, Tanzania. BMC Infect Dis. 2016;16:187. 10.1186/s12879-016-1527-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ribeiro TG, Novais Â, Peixe L, et al. : Atypical epidemiology of CTX-M-15 among Enterobacteriaceae from a high diversity of non-clinical niches in Angola. J Antimicrob Chemother. 2016;71(5):1169–73. 10.1093/jac/dkv489 [DOI] [PubMed] [Google Scholar]
- 17. Tellevik MG, Blomberg B, Kommedal Ø, et al. : High Prevalence of Faecal Carriage of ESBL-Producing Enterobacteriaceae among Children in Dar es Salaam, Tanzania. PLoS One. 2016;11(12):e0168024. 10.1371/journal.pone.0168024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andriatahina T, Randrianirina F, Hariniana ER, et al. : High prevalence of fecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a pediatric unit in Madagascar. BMC Infect Dis. 2010;10:204. 10.1186/1471-2334-10-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Magwenzi MT, Gudza-Mugabe M, Mujuru HA, et al. : Carriage of antibiotic-resistant Enterobacteriaceae in hospitalised children in tertiary hospitals in Harare, Zimbabwe. Antimicrob Resist Infect Control. 2017;6:10. 10.1186/s13756-016-0155-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moremi N, Claus H, Vogel U, et al. : Faecal carriage of CTX-M extended-spectrum beta-lactamase-producing Enterobacteriaceae among street children dwelling in Mwanza city, Tanzania. PLoS One. 2017;12(9):e0184592. 10.1371/journal.pone.0184592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilmore SMS, Kranzer K, Williams A, et al. : Carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae in HIV-infected children in Zimbabwe. J Med Microbiol. 2017;66(5):609–15. 10.1099/jmm.0.000474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chirindze LM, Zimba TF, Sekyere JO, et al. : Faecal colonization of E. coli and Klebsiella spp. producing extended-spectrum beta-lactamases and plasmid-mediated AmpC in Mozambican university students. BMC Infect Dis. 2018;18(1):244. 10.1186/s12879-018-3154-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Founou RC, Founou LL, Essack SY: Extended spectrum beta-lactamase mediated resistance in carriage and clinical gram-negative ESKAPE bacteria: a comparative study between a district and tertiary hospital in South Africa. Antimicrob Resist Infect Control. 2018;7:134. 10.1186/s13756-018-0423-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herindrainy P, Rabenandrasana MAN, Andrianirina ZZ, et al. : Acquisition of extended spectrum beta-lactamase-producing enterobacteriaceae in neonates: A community based cohort in Madagascar. PLoS One. 2018;13(3):e0193325. 10.1371/journal.pone.0193325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Katakweba AAS, Muhairwa AP, Lupindu AM, et al. : First Report on a Randomized Investigation of Antimicrobial Resistance in Fecal Indicator Bacteria from Livestock, Poultry, and Humans in Tanzania. Microb Drug Resist. 2018;24(3):260–8. 10.1089/mdr.2016.0297 [DOI] [PubMed] [Google Scholar]
- 26. Marando R, Seni J, Mirambo MM, et al. : Predictors of the extended-spectrum-beta lactamases producing Enterobacteriaceae neonatal sepsis at a tertiary hospital, Tanzania. Int J Med Microbiol. 2018;308(7):803–11. 10.1016/j.ijmm.2018.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moremi N, Claus H, Rutta L, et al. : High carriage rate of extended-spectrum beta-lactamase-producing Enterobacteriaceae among patients admitted for surgery in Tanzanian hospitals with a low rate of endogenous surgical site infections. J Hosp Infect. 2018;100(1):47–53. 10.1016/j.jhin.2018.05.017 [DOI] [PubMed] [Google Scholar]
- 28. Nikiema Pessinaba C, Landoh DE, Dossim S, et al. : Screening for extended-spectrum beta-lactamase-producing Enterobacteriaceae intestinal carriage among children aged under five in Lomé, Togo. Med Mal Infect. 2018;48(8):551–4. 10.1016/j.medmal.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 29. Herindrainy P, Randrianirina F, Ratovoson R, et al. : Rectal carriage of extended-spectrum beta-lactamase-producing gram-negative bacilli in community settings in Madagascar. PLoS One. 2011;6(7):e22738. 10.1371/journal.pone.0022738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sanneh B, Kebbeh A, Jallow HS, et al. : Prevalence and risk factors for faecal carriage of Extended Spectrum β-lactamase producing Enterobacteriaceae among food handlers in lower basic schools in West Coast Region of The Gambia. PLoS One. 2018;13(8):e0200894. 10.1371/journal.pone.0200894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stanley IJ, Kajumbula H, Bazira J, et al. : Multidrug resistance among Escherichia coli and Klebsiella pneumoniae carried in the gut of out-patients from pastoralist communities of Kasese district, Uganda. PLoS One. 2018;13(7): 10.1371/journal.pone.0200093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Woerther PL, Angebault C, Jacquier H, et al. : Massive increase, spread, and exchange of extended spectrum β-lactamase-encoding genes among intestinal Enterobacteriaceae in hospitalized children with severe acute malnutrition in Niger. Clin Infect Dis. 2011;53(7):677–85. 10.1093/cid/cir522 [DOI] [PubMed] [Google Scholar]
- 33. Albrechtova K, Dolejska M, Cizek A, et al. : Dogs of nomadic pastoralists in northern Kenya are reservoirs of plasmid-mediated cephalosporin- and quinolone-resistant Escherichia coli, including pandemic clone B2-O25-ST131. Antimicrob Agents Chemother. 2012;56(7):4013–7. 10.1128/AAC.05859-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Isendahl J, Turlej-Rogacka A, Manjuba C, et al. : Fecal carriage of ESBL-producing E. coli and K. pneumoniae in children in Guinea-Bissau: a hospital-based cross-sectional study. PLoS One. 2012;7(12):e51981. 10.1371/journal.pone.0051981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lonchel CM, Meex C, Gangoué-Piéboji J, et al. : Proportion of extended-spectrum ß-lactamase-producing Enterobacteriaceae in community setting in Ngaoundere, Cameroon. BMC Infect Dis. 2012;12:53. 10.1186/1471-2334-12-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lonchel CM, Melin P, Gangoué-Piéboji J, et al. : Extended-spectrum β-lactamase-producing Enterobacteriaceae in Cameroonian hospitals. Eur J Clin Microbiol Infect Dis. 2013;32(1):79–87. 10.1007/s10096-012-1717-4 [DOI] [PubMed] [Google Scholar]
- 37. Magoué CL, Melin P, Gangoué-Piéboji J, et al. : Prevalence and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae in Ngaoundere, Cameroon. Clin Microbiol Infect. 2013;19(9):E416–20. 10.1111/1469-0691.12239 [DOI] [PubMed] [Google Scholar]
- 38. McNulty CAM, Lecky DM, Xu-McCrae L, et al. : CTX-M ESBL-producing Enterobacteriaceae: estimated prevalence in adults in England in 2014. J Antimicrob Chemother. 2018;73(5):1368–88. 10.1093/jac/dky007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wielders CCH, van Hoek AHAM, Hengeveld PD, et al. : Extended-spectrum β-lactamase- and pAmpC-producing Enterobacteriaceae among the general population in a livestock-dense area. Clin Microbiol Infect. 2017;23(2):120.e1–120.e8. 10.1016/j.cmi.2016.10.013 [DOI] [PubMed] [Google Scholar]
- 40. Ny S, Löfmark S, Börjesson S, et al. : Community carriage of ESBL-producing Escherichia coli is associated with strains of low pathogenicity: a Swedish nationwide study. J Antimicrob Chemother. 2017;72(2):582–8. 10.1093/jac/dkw419 [DOI] [PubMed] [Google Scholar]
- 41. Valverde A, Coque TM, Sanchez-Moreno MP, et al. : Dramatic increase in prevalence of fecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae during nonoutbreak situations in Spai. J Clin Microbiol. 2004;42(10):4769–75. 10.1128/JCM.42.10.4769-4775.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Islam S, Selvarangan R, Kanwar N, et al. : Intestinal Carriage of Third-Generation Cephalosporin-Resistant and Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Healthy US Children. J Pediatric Infect Dis Soc. 2018;7(3):234–240. 10.1093/jpids/pix045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lewis JM, Lester R, Garner P, et al. : Risk of bias tool used for the publication: Gut mucosal colonisation with extended-spectrum beta-lactamase producing Enterobacteriaceae in sub-Saharan Africa: a systematic review and meta-analysis (Version v1.0). Zenodo. 2019. 10.5281/zenodo.3478278 [DOI] [PMC free article] [PubMed]