Abstract
New strategies for HIV testing services (HTS) are needed to achieve UN 90-90-90 targets, including diagnosis of 90% of people living with HIV. Task-sharing HTS to trained lay providers may alleviate health worker shortages and better reach target groups. We conducted a systematic review of studies evaluating HTS by lay providers using rapid diagnostic tests (RDTs). Peer-reviewed articles were included if they compared HTS using RDTs performed by trained lay providers to HTS by health professionals, or to no intervention. We also reviewed data on end-users’ values and preferences around lay providers preforming HTS. Searching was conducted through 10 online databases, reviewing reference lists, and contacting experts. Screening and data abstraction were conducted in duplicate using systematic methods. Of 6113 unique citations identified, 5 studies were included in the effectiveness review and 6 in the values and preferences review. One US-based randomized trial found patients’ uptake of HTS doubled with lay providers (57% vs. 27%, percent difference: 30, 95% confidence interval: 27–32, p<.001). In Malawi, a pre/post study showed increases in HTS sites and tests after delegation to lay providers. Studies from Cambodia, Malawi, and South Africa comparing testing quality between lay providers and laboratory staff found little discordance and high sensitivity and specificity (≥98%). Values and preferences studies generally found support for lay providers conducting HTS, particularly in non-hypothetical scenarios. Based on evidence supporting using trained lay providers, a WHO expert panel recommended lay providers be allowed to conduct HTS using HIV RDTs. Uptake of this recommendation could expand HIV testing to more people globally.
Keywords: HIV testing, HTS, lay providers, task shifting, task sharing
Introduction
The first of the United Nation’s 90–90–90 global HIV targets is to diagnose 90% of people with HIV globally (UNAIDS, 2014a). Achieving this goal will require a range of approaches to delivering HIV testing services (HTS) tailored toward different epidemic contexts, groups most at risk for HIV, and people who remain undiagnosed and underserved (WHO, 2015). Rapid diagnostic tests (RDTs) can facilitate this by providing HIV test results in minutes rather than days. However, in many settings, a critical shortage of healthcare providers hampers expansion, and traditional testing approaches may poorly reach key populations and other high-risk groups (UNAIDS, 2014b).
Task-sharing – the rational redistribution of tasks from higher-level health provider cadres to lower-level cadres – could expand HTS availability by shifting the role of test-provider from doctors or nurses to lay providers. The World Health Organization (WHO) has defined a lay health worker as “any health worker who performs functions related to health-care delivery; was trained in some way in the context of the intervention; but has received no formal professional or paraprofessional certificate or tertiary education degree” (WHO, 2014). WHO recommends that lay health workers can provide a range of clinical services (WHO, 2007, 2013, 2014).
A recent analysis of national policies for HIV testing across 50 countries showed that 42% allowed lay providers to perform testing using RDTs (64% in African countries) and even more allowed lay providers to perform pre- and post-test counseling (56% overall and 80% in Africa) (Flynn, 2017). However, several countries limit these roles to trained healthcare providers due to concerns about lay providers’ ability to perform RDTs (whether fingerstick blood or oral fluid) and administer HIV-testing-related services, including pre- and post-test counseling, linkage to appropriate prevention and clinical care services, and coordination with laboratory services to ensure the delivery of correct test results (WHO, 2015).
In 2015, WHO sought to review the evidence for lay providers conducting HTS to inform WHO guidelines. We conducted a systematic review of the literature to answer the question: Should trained lay providers perform HIV testing using RDTs?
Methods
Search strategy and screening
To be included in the review, a study had to meet the following inclusion criteria: (1) be published in a peer-reviewed journal, (2) employ a comparative study design (either pre/post or multi-arm) where participants receiving RDT-based HTS by trained lay providers (following the WHO definition above) are compared to participants receiving HTS conducted by trained health professionals, or to no intervention, and (3) measure one or more of the following outcomes: (a) Measures of testing quality assurance/control (lost, damaged, uninterpretable specimens); (b) Accurate results (sensitivity, specificity); (c) Adverse events (e.g. coercion, inter-partner violence, self-harm, psychosocial, stigma/discrimination); (d) HTS uptake; (e) CD4 measurement among HIV-infected individuals; (f) Linkage to medical care following HIV diagnosis; (g) Antiretroviral treatment (ART) initiation. No restrictions were based on intervention location or language.
Ten electronic databases were searched through 3 September 2014: PubMed, Scopus, CINAHL, LILACS, WHO Global Health Libraries, Ovid Global Health, Sociological Abstracts, PsycINFO, EMBASE, and POPLINE. Search strategies included terms for HIV, health provider cadres, HIV testing, comparative study designs, and elimination of irrelevant terms. Appendix 1 presents the full search strategy for one database (PubMed).
Secondary reference searching was conducted on all included studies and on those included in seven related reviews, mostly focused on lay providers in HIV care and treatment services (Emdin, Chong, & Millson, 2013; Iwu & Holzemer, 2014; Mdege, Chindove, & Ali, 2013; Mwai et al., 2013; Penazzato, Davies, Apollo, Negussie, & Ford, 2014; Rackal et al., 2011; Wong, Luk, & Kidd, 2012). Expert members of the WHO guideline development group identified additional articles.
Titles, abstracts, and citation information were screened independently in duplicate. Full-text articles were assessed independently by two reviewers for final study eligibility. Articles not meeting inclusion criteria but presenting complementary information, such as review articles, were used as background material.
Data analysis
Data were extracted by two reviewers using standardized forms. Differences in data extraction were resolved through discussion and referral to a senior study team member when necessary. The following information was gathered from each included study:
Study description: Citation information; objectives; location; population characteristics; intervention description; study design; sample size; follow-up periods; loss to follow-up
Outcomes: Analytic approach; outcome measures; comparison groups; effect sizes; confidence intervals; significance levels; conclusions; limitations
For randomized controlled trials (RCTs), risk of bias was assessed using the Cochrane Collaboration’s tool for assessing risk of bias (Higgins & Green, 2011). For other study designs, rigor was assessed through the Evidence Project’s quality assessment tool used in other HTS systematic reviews (Denison, O’Reilly, Schmid, Kennedy, & Sweat, 2008; Fonner, Denison, Kennedy, O’Reilly, & Sweat, 2012; Kennedy et al., 2013).
Data were analyzed according to coding categories and outcomes. Due to the lack of combinable studies, meta-analysis was not possible.
Values and preferences review
The same search was used to identify studies presenting information on end-users’ values and preferences. Studies were included if they presented primary data examining people’s preferences regarding different cadres of health providers and HIV testing. These studies could be qualitative or quantitative in nature, but had to present primary data collection; opinion pieces and review articles were excluded.
Results
Search results
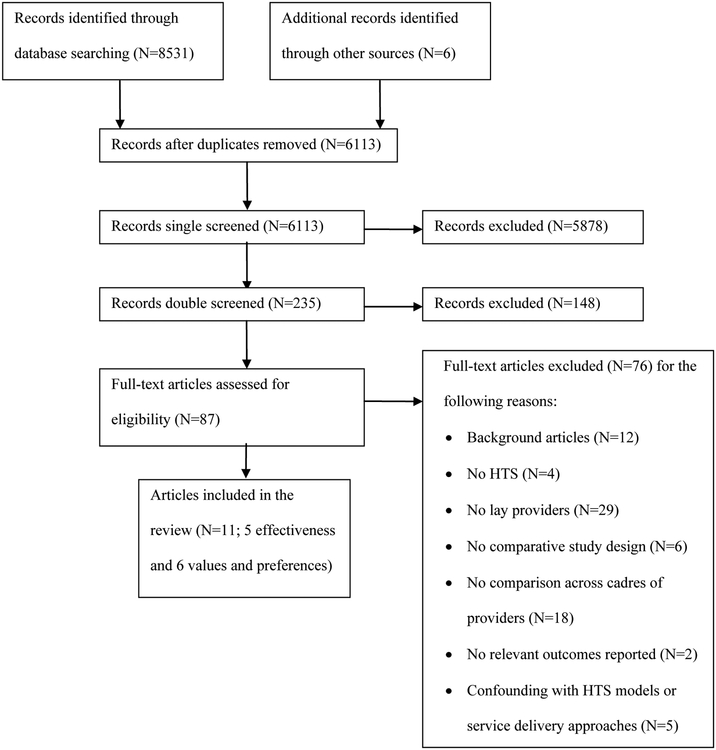
Initial database searching yielded 8531 citations, with six identified through other means; 6113 remained after removing duplicates (Figure 1). Initial screening excluded 5878 records and secondary screening 148 for not meeting the inclusion criteria; the kappa statistic for inter-rater reliability of screening in duplicate was moderately good at 0.571. After thoroughly reviewing and discussing the remaining 87 articles, 76 were excluded, of which 12 were used for background.
Figure 1:
Disposition of citations during the search and screening process
Four initially included studies (reported in five articles) were later dropped because comparisons of lay providers with healthcare providers were confounded by comparisons of different HTS models or service delivery approaches. These studies examined either home-based HTS using lay providers compared with clinic-based HTS using health workers (Fylkesnes et al., 2013; Jurgensen, Sandoy, Michelo, & Fylkesnes, 2013; Lugada et al., 2010), or provider-initiated testing using health workers compared with client-initiated testing using lay providers (Leon, Naidoo, Mathews, Lewin, & Lombard, 2010; Seewald et al., 2013).
Ultimately, five studies were included in the effectiveness review (Table 1), and six studies were included in the values and preferences review.
Table 1.
Study descriptions and results for studies included in effectiveness review.
| Citation | Study location and setting | Study design and sample size | Lay provider definition | Outcomes |
|---|---|---|---|---|
| Bemelmans et al., 2010 | Thyolo District, Malawi Rural health facilities |
Pre-post study 39 health facility sites |
Trained health surveillance assistant (HSA) counsellors (community cadre originally responsible for preventive activities and organizing disease outbreak via 10 week basic training, now given additional 3 week formal training and Ministry of Health-certified to give HIV testing and counseling at hospital; HSA/Lay counsellor at health center; Lay counsellor and HSA at improved health post | Uptake of HTS: 2003: 1300 tests per month 2009: 6500 tests per month 2003: 14 HTS sites (average of 93 tests per month per site) 2009: 39 HTS sites (average of 167 tests per month per site). |
| Jackson et al., 2013 | Umzimkhulu subdistrict of Sosonke district, KwaZulu-Natal, South Africa Home-based HTS |
Quality comparison 3986 samples tested |
Counsellors who completed a 10-day nationally accredited HIV testing and counseling course, 3 months shadowing facility lay counselors and nurse-supervised testing experience, 1-day training on obtaining/packaging dried blood spot samples | Test result concordance: 3963/3986; Of the 23 discordant results, only 2 cases were considered lay provider “critical errors” Sensitivity: 98.0% (95% CI: 96.3– 98.9%) Specificity: 99.6% (95% CI: 99.4–99.7%). |
| Kanal et al., 2005 | Cambodia PMTCT sites |
Quality comparison 563 samples tested |
Midwives working for antenatal care/delivery ward with no previous lab experience, given half-day training (how to use a pipette, how to process whole blood samples with chase buffer, how to read test result) | Test result concordance: 559/563; the 4 discordant results were lab errors, not lay provider errors |
| Molesworth et al., 2010 | Chilumba, Karonga district, Malawi Home-based HTS |
Quality comparison 2911 samples tested |
Counsellors trained and certified by Ministry of Health staff to perform HIV counselling, whole-blood rapid testing and specimen collection by finger-prick, using standard training procedures | Test result concordance: 2907/2911; 3 of the 4 discrepant results likely resulted from “sample peculiarities” because results from several parallel tests were discordant Sensitivity: 99.6% Specificity: 100.0%. |
| Walensky, Reichmann, et al., 2011 | Boston, MA, USA Hospital emergency department |
RCT Lay provider arm: N=2446 Trained healthcare provider arm: N=2409 |
Dedicated HIV counsellor (without other clinical responsibilities) | Uptake of HTS: Lay provider arm: 57% (1,382/2,446) Healthcare provider arm: 27% (643/2,409) p<.001 |
The five studies included in the effectiveness review were diverse in location: two were conducted in Malawi (Bemelmans et al., 2010; Molesworth et al., 2010), one in South Africa (Jackson et al., 2013), one in Cambodia (Kanal et al., 2005), and one in the United States (Walensky, Reichmann, et al., 2011). Of the six studies presenting values and preferences data, four were conducted in sub-Saharan Africa (one each in Botswana (Ledikwe et al., 2013), Malawi (DeGraft-Johnson, Paz-Soldan, Kasote, & Tsui, 2005), Zambia (Jurgensen, Sandoy, Michelo, Fylkesnes, et al., 2013), and Zimbabwe (Chirawu et al., 2010)), while two were conducted in the United States (Donnell-Fink et al., 2011; Hecht, Smith, Radonich, Kozlovskaya, & Totten, 2011). Table 2 presents an assessment of study design and rigor for each included study.
Table 2.
Risk of bias and quality assessment table for studies included in the effectiveness review.
| Cochrane Risk of Bias for Randomized Controlled Trials | |||||||
|---|---|---|---|---|---|---|---|
| Study | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) | Other bias |
| Walensky, Reichmann, et al., 2011 | Low risk | Low risk | High risk | Low risk | Low risk | Uncertain risk | Low risk |
| Evidence Project Study Design and Rigor Assessment for Observational Studies | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Cohort | Control or comparison group | Pre/post intervention data | Random assignment of participants to the intervention | Random selection of participants for assessment | Follow-up rate of 80% or more | Comparison groups equivalent on socio-demographics | Comparison groups equivalent at baseline on outcome measure |
| Bemelmans et al., 2010 | No | No | Yes | No | No | Not applicable | Not reported | Not applicable |
| Jackson et al., 2013 | No | Yes | No | Yes1 | No2 | Not applicable | Yes3 | Not applicable |
| Kanal et al., 2005 | No | Yes | No | No | No4 | Not applicable | Yes3 | Not applicable |
| Molesworth et al., 2010 | No | Yes | No | No | No5 | Not applicable | Yes3 | Not applicable |
Samples came from a larger cluster-randomized trial where every eligible individual living in the study clusters was sampled.
Systematic sample: All HIV positive and indeterminate samples were sent for laboratory testing. During the first few months of the study, all HIV-negative samples were sent for laboratory testing, and thereafter a systematic sample of HIV-negative specimens was sent for laboratory testing.
The same samples were tested by both lay providers and laboratory technicians, so socio-demographic characteristics of the patients tested were identical.
Census sample: all blood samples from pregnant women who wanted to be tested during the study period.
Systematic sample: all positive and every tenth negative specimen.
Study findings: effectiveness review
In Boston, USA, an RCT called the USHER study (Universal Screening for HIV Infection in the Emergency Room) compared two HTS provision models in an emergency department setting: HTS by lay providers (trained HIV counselors) compared with HTS by regular emergency department healthcare providers (emergency service assistants, ESAs) (Walensky, Reichmann, et al., 2011). In the lay provider arm, trained HIV counselors performed all services from test consent to test result delivery and referral for confirmatory testing. In the healthcare provider arm, ESAs (generally a two-year college degree) performed the tests but physicians gave results and follow-up. Both lay providers and ESAs received the same one-day training and successfully completed the accompanying competency test. This study was classified as having low or uncertain risk of bias across all measures except blinding of participants and personnel; while participants, counselors, and providers could not be masked to study arm assignment, neither were they incentivized to complete the testing process. Kappa for inter-rater reliability of risk of bias was good at 0.739. Uptake of HTS among emergency department patients was 57% (1382/2446) in the lay provider arm compared with 27% in the healthcare provider arm (643/2409), a 30% difference (95% CI: 27–32, p<.001). The authors suggested that lower testing rates by providers may have been a function of insufficient time in patient encounters and competing demands “in the face of patient acuity and other duties” in a busy emergency department.
One study examined HIV testing uptake before and after the use of lay providers for HTS (Bemelmans et al., 2010). Conducted in rural Thyolo District, Malawi, this study employed various programmatic efforts to enable rapid scale-up of HIV care and treatment services, including task-sharing to increase the number of health workers in HIV care, decentralization of care to health centers and community sites, simplification of testing and treatment protocols, community engagement to increase capacity and support program sustainability, and health system strengthening. HTS was delegated to health surveillance assistant (HSA) counsellors, who received a 10-week basic HSA training and an additional 3-week HTS counselor training. After delegating HTS to lay providers, uptake of testing increased from 1,300 tests per month in 2003 to 6,500 tests per month in 2009. HTS increased from 14 sites in 2003 to 39 sites in 2009, growing from an average of 93 to 167 tests per month per site. Likely due in part to this expanded coverage, the proportion of clients testing positive decreased from 36% in 2003 (5,612/15,618) to 16% in 2009 (12,364/77,736). While the study also reported numbers of patients initiated on ART, the additional changes in the health system described above seriously limited the ability to link these outcome changes with the HTS-providing cadre changes.
Three studies conducted quality comparisons between lay providers and laboratory staff. In Sisonke District, South Africa, the Good Start cluster-randomized trial evaluated an integrated, scalable infant health package delivered by community health workers (Jackson et al., 2013). As part of the intervention arm, lay providers conducted home-based HTS. These lay providers completed a 10-day nationally accredited HTS course, learning how to use two finger-prick HIV RDTs. They shadowed facility counselors for three months, gained nurse-supervised testing experience at local health facilities, and received one-day training on dried blood spot sample collection from laboratory technicians. Lay provider and laboratory results from HIV tests were concordant in all but 23 of 3986 matched cases. Further examination revealed only two “critical error” cases where the lay provider found a HIV-positive result and the laboratory had a negative result; the rest had at least one indeterminate result, mostly cases of cautious lay providers waiting for laboratory confirmation. Overall, sensitivity was calculated as 98.0% (95% CI: 96.3–98.9%) and specificity as 99.6% (95% CI: 99.4–99.7%).
In rural Karonga District, Malawi, the Karonga Prevention Study examined the quality of home-based rapid testing (Molesworth et al., 2010). Lay providers were trained and certified by Ministry of Health staff to perform HIV counseling, serial venous whole-blood rapid testing, and finger-prick specimen collection. Of 10,819 samples, 2911 were sent for laboratory quality control or confirmation, retesting every tenth negative and all positive specimens. Lay provider and laboratory results were concordant in all but four cases, considered the result of “sample peculiarities” after several parallel discordant tests. Results showed a sensitivity of 99.6% and specificity of 100.0%, as well as a 99.9% positive predictive value and 99.9% negative predictive value.
In Cambodia, a study compared results of rapid HIV testing by lay providers working in a prevention of mother-to-child transmission site with results from laboratory technicians (Kanal et al., 2005). Lay providers were trained HTS counsellors: midwives without any laboratory or phlebotomy experience. They received a half-day training on HTS and how to use Determine™ HIV1/2 (Abbott Japan Co Ltd, Tokyo, Japan) test kits using finger-stick whole-blood samples. Laboratory technicians routinely did the same test and returned the report of test results to lay providers. A total of 563 samples were tested by both lay providers and laboratory technicians; study authors confirmed that these were all blood samples from pregnant women desiring HTS during the study period. Lay provider and laboratory results of HTS were concordant in all but four cases, which the authors found were caused by “human error” in laboratory write-ups.
Study findings: values and preferences review
Six studies reported on values and preferences related to lay providers conducting HTS services.
The one RCT identified above (Walensky, Reichmann, et al., 2011) published related results from a patient satisfaction survey (Donnell-Fink et al., 2011). Of 2025 HTS clients, 1616 (79.8%) completed the survey and most (91.5%) reported being very satisfied with their HTS experience. While overall satisfaction was high, results suggested slightly higher satisfaction with lay providers compared with healthcare providers. In multivariate analyses, patients in the healthcare provider arm were more likely to be less than “very satisfied” compared with those in the lay provider arm (aOR: 1.50; 95% CI: 1.00–2.24). Less than optimal satisfaction with “time spent on HIV testing” was significantly more likely among participants tested by a healthcare provider (13%) than among those tested by a lay provider (8%) (aOR: 1.73; 95% CI: 1.20–2.51). Almost all participants expressed optimal satisfaction with the tester’s ability to answer questions (lay providers: 99.6%, healthcare providers: 99.5%).
A second study examined preferences towards HTS in emergency departments in the United States (Hecht et al., 2011). Surveys, completed by 457 patients and 85 emergency department staff, asked about hypothetical preferences, not actual experiences. Both patients and staff preferred HIV test results delivery by a physician compared with lay providers (HIV counselors) or other staff members (nurses, physician assistants, or social workers); exact statistics were not presented.
Studies from sub-Saharan Africa were more diverse and used both quantitative and qualitative methods. The strongest of these was a study from Botswana, which conducted exit interviews with clients who had received HTS from lay providers (Ledikwe et al., 2013). Most clients (n=46; 97.9%) reported being satisfied with the HTS services received; the same felt comfortable returning for such services in the future.
The remaining three studies did not examine clients’ actual experiences with HTS by lay providers but provided insight into desired HTS provision characteristics. In rural Malawi, a survey of 648 men and 868 women examined preferences for different ways of HIV test result notification (DeGraft-Johnson et al., 2005). Almost all participants who desired testing were willing to learn their results from a counselor at the test site and on the same day of the test (>90%). A majority of men (61%) and women (59%) also were open to obtaining their results from an anonymous posting using a patient number; about half of women (55%) and men (44%) were willing to learn their results from a community counselor at their homes. In Zimbabwe, a qualitative study suggested that clients preferred testing personnel to come from outside the community due to confidentiality concerns (Chirawu et al., 2010). Another qualitative study from Zambia, embedded within a larger trial of community-based HTS, found that clients wanted trustworthy providers; trust was based on professional conduct, knowledge, politeness, adeptness in dealing with sensitive issues, and listening ability (Jurgensen, Sandoy, Michelo, Fylkesnes, et al., 2013).
Discussion
The existing literature generally supports using trained lay providers to perform HTS using HIV RDTs. While the evidence base is very limited, findings from one RCT and an observational study suggest that using trained lay providers can increase HIV testing uptake, and findings from three quality comparison studies suggest that lay providers can achieve similar testing quality as trained healthcare providers. Unfortunately, no studies measured adverse events following testing, nor linkage to care. Values and preferences studies, though also limited in number, generally found support for lay providers conducting HTS, particularly in the strongest study designs that examined preferences among people who had actually undergone HTS with a lay provider, rather than hypothetical scenarios.
Based on this evidence, program experience supporting feasibility in many settings (Flynn, 2017; WHO, 2015), and cost considerations, the WHO expert panel made the following recommendation (WHO, 2015): Lay providers who are trained and supervised to use rapid diagnostic tests (RDTs) can independently conduct safe and effective HIV testing services.
As many countries still require HTS to be performed only by nurses, doctors, or other trained heath care professionals, adopting this recommendation could increase availability of HTS services worldwide. Estimates suggest almost 40% of people living with HIV in sub-Saharan Africa are unaware of their status (UNAIDS, 2016); an increase in HTS services globally is thus crucial to help individuals learn their serostatus and engage in appropriate treatment and prevention services. Men, adolescents, and people from key populations are particularly underserved by current HTS approaches, partially because they less frequently attend health facilities (UNAIDS, 2014b). Providing more acceptable community-based HTS options should be prioritized to overcome this disparity.
Using trained lay providers may also enable more cost-effective HIV testing services. Lay providers generally receive lower salaries than trained health professionals, although full program costs (including training and supervision), cost-effectiveness, and affordability vary across settings. The USHER trial embedded a cost-effectiveness study (Walensky, Morris, et al., 2011): estimated HIV screening costs in the healthcare provider and lay provider arms averaged US $8.10 and $31.00 per result received. The healthcare provider strategy (compared to no screening) had an incremental cost-effectiveness ratio of $58,700/quality-adjusted life year (QALY) and the lay provider strategy (compared to the healthcare provider strategy) a ratio of $64,500/QALY. The authors concluded that provider-based screening was cheaper on a per-result basis, but fewer overall results were given because providers were already overstretched in terms of time and other clinical activities. As different program factors can dramatically impact cost-effectiveness in different situations, further research on the costs and cost-effectiveness of lay providers in HTS is warranted to provide important information as HIV programs seek increased efficiency.
The limited evidence base identified through this review suggests a need for additional research on the effectiveness and values and preferences around using lay providers for HTS. Future studies could examine the effect of task-shifting HTS services to lay providers on uptake of HTS, linkage to care and ART initiation, adverse events, sexual behavior, HIV serostatus disclosure, and other health and well-being outcomes. Findings from multiple settings could also help elucidate how these issues differ across diverse health systems and HIV epidemics.
Our review had limitations. We only included studies published in peer-reviewed journals, which provided some assurance of study quality given our otherwise broad inclusion criteria, but may have excluded other relevant studies. The limited evidence base did not permit us to judge aspects of the setting, training, or characteristics of lay providers that might yield better outcomes.
Task-shifting to lay providers has been recommended for a range of clinical care services, and the existing evidence supports allowing lay providers to conduct HTS using RDTs. HIV testing is the entry point to HIV care and treatment services and is the critical first step to achieving the UN 90–90–90 targets. Allowing lay providers to conduct HTS will expand the range of options available to countries as they work to meet these goals.
Acknowledgments
We wish to thank Rachel Rieder, Kathleen Ridgeway, Shristi Pandey and Kate Perepezko for their help with abstract screening. We also thank the members of the WHO consolidated HIV testing guideline development group for their feedback and suggestions. This research was supported by the World Health Organization, Department of HIV/AIDS.
Funding
This work was supported by the World Health Organization, Department of HIV/AIDS.
Appendix 1: List of search terms used in PubMed
Concept 1: HIV/AIDS
“HIV”[mh] OR “HIV”[mesh] OR AIDS[all] OR “HIV-1”[mh] OR “HIV-2”[mh] OR “HIV”[all] OR “HIV-1”[all] OR “HIV-2”[all] OR “Human immunodeficiency viruses”[all] OR “AIDS virus”[all] OR “AIDS viruses”[all] OR “HTLV-III”[all] OR “Human Immunodeficiency Virus”[all] OR “Human Immunodeficiency Viruses”[all] OR “Acquired Immune Deficiency”[all] OR “Acquired Immuno-Deficiency Syndrome”[all] OR “Acquired Immunodeficiency Syndrome”[Mesh] or “Acquired Immunodeficiency Syndrome”[all]
Concept 2: Cadres of healthcare providers
“Health personnel”[mesh] OR “health personnel”[tw] OR “health educators”[mesh] or “health educator”[tw] or “health educators”[tw] OR “nurses”[mesh] or “nurse”[tw] OR “nurses”[tw] OR “physicians”[mesh] OR “physicians, primary care”[mesh] OR “physician”[tw] OR “physicians”[tw] OR “nurses, community health”[mesh] OR “community health nurse”[tw] OR “community health nurses”[tw] OR “community health workers”[mesh] OR “community health worker”[tw] OR “community health workers”[tw] OR “community health aides”[mesh] OR “community health aide”[tw] OR “community health aides”[tw] OR “village health worker”[mesh] OR “village health worker”[tw] OR “village health workers”[tw] OR “barefoot doctor”[mesh] OR “barefoot doctor”[tw] OR “barefoot doctors”[tw] OR “lay provider”[mesh] OR “lay provider”[tw] OR “lay providers”[tw] OR “lay worker”[mesh] OR “lay worker”[tw] OR “primary care physician”[mesh] OR “primary care physician”[tw] OR “primary care physicians”[tw] OR “lay workers”[tw] OR “lay health provider”[mesh] OR “lay health provider”[tw] OR “lay health providers”[tw] OR “lay health worker”[mesh] OR “lay health worker”[tw] OR “community health nurse”[mesh] OR “community health nurse”[tw] OR “community health nurses”[tw] OR “visiting nurse”[mesh] OR “visiting nurses”[tw] OR “visiting nurse”[tw] OR “home nurse”[mesh] OR “home nurse”[tw] OR “home nurses”[tw] OR “health visitor”[mesh] OR “health visitor”[tw] OR “health visitors”[tw] OR “lay health workers”[mesh] OR “lay health workers”[tw] OR “lay health advisor”[mesh] OR “lay health advisor”[tw] OR “lay health advisors”[tw] OR “lay healthcare provider”[mesh] OR “lay healthcare provider”[tw] OR “lay healthcare providers”[tw] OR “lay healthcare worker”[mesh] OR “lay healthcare worker”[tw] OR “lay healthcare workers”[tw] OR “lay health care provider”[mesh] OR “lay health care provider”[tw] OR “lay health care providers”[tw] OR “lay health care worker”[mesh] OR “lay health care worker”[tw] OR “lay health care workers”[tw] OR “lay health advisor”[mesh] OR “lay health advisor”[tw] OR “lay health advisors”[tw] OR “doctor”[mesh] OR “doctor”[tw] OR “doctors”[tw] OR “doctors”[mesh] OR “peer navigator”[mesh] OR “peer navigator”[tw] OR “peer navigators”[tw] OR “CHW”[mesh] OR”CHW”[tw] OR “CHWs”[tw] OR “peer health worker”[mesh] OR “peer health worker”[tw] OR “peer health workers”[tw] OR “peer healthcare worker”[mesh] OR “peer healthcare worker”[tw] OR “peer healthcare workers”[tw] OR “peer health care worker”[mesh] OR “peer health care worker”[tw] OR “peer health care workers”[tw] OR “community health nurse”[mesh] OR “community health nurse”[tw] OR “community health nurses”[tw] OR “healthcare professionals”[mesh] OR “healthcare professionals”[tw] OR “healthcare professional”[tw] OR “healthcare provider”[mesh] OR “healthcare provider”[tw] OR “healthcare providers”[tw] OR “health care professionals”[mesh] OR “health care professionals”[tw] OR “health care professional”[tw] OR “health care provider”[mesh] OR “health care provider”[tw] OR “health care providers”[tw] OR “fieldworker”[mesh] OR “fieldworker”[tw] OR “fieldworkers”[tw] OR “field worker”[mesh] OR “field worker”[tw] OR “field workers”[tw] OR “mobile health units”[Mesh] OR “mobile health unit”[tw] OR “mobile health units”[tw] OR “cadre”[mesh] OR “cadre”[tw] OR “cadres”[tw] OR “task shifting”[mesh] OR “task shifting”[tw] OR “task-shifting”[mesh] OR “task-shifting”[tw]
Concept 3:HIV testing
“diagnosis”[mesh] OR “diagnosis”[tw] OR “AIDS serodiagnosis”[mesh] OR “AIDS serodiagnosis”[tw] OR “hiv infections/diagnosis*”[mesh] OR “HIV antibodies/diagnostic use”[mesh] OR “AIDS serodiagnosis/methods*”[mesh] OR “AIDS serodiagnosis”[tw] OR “rapid test”[tw] OR “HIV serodiagnosis”[tw] OR “testing”[tw] OR “screening”[tw] OR “hiv test”[tw] OR “hiv tests”[tw] OR “hiv testing”[tw]
Concept 4: Comparative studies
“Health Care Quality, Access, and Evaluation”[mesh] OR “feasibility studies”[Mesh] OR “feasibility study”[tw] OR “feasibility studies”[tw] OR “Randomized Controlled Trial”[Publication Type] OR “randomized controlled trial”[tw] OR “randomized controlled trials”[tw] OR “randomized control trial”[tw] OR “randomized control trials”[tw] OR “prospective studies”[mesh] OR “prospective study”[tw] OR “prospective studies”[tw] OR “retrospective studies”[mesh] OR “retrospective study”[tw] OR “retrospective studies”[tw] OR “comparative study”[publication type] OR “comparative study”[tw] OR “comparative studies”[tw] OR “longitudinal studies”[mesh] OR “longitudinal study”[tw] OR “longitudinal studies”[tw] “outcome assessment (health care)”[mesh] OR “outcome assessment”[tw] OR “noninferiority”[tw] OR “non-inferiority”[tw] OR “cluster randomized trial”[tw] OR “cluster randomized trials”[tw] OR “task shifting”[tw] OR “interprofessional relations”[mesh] OR “Cluster randomized controlled trial”[tw] OR “cluster randomised controlled trial”[tw] OR “cluster randomized control trial”[tw] OR “cluster randomized controlled trial”[tw]
Concept 5: Elimination of irrelevant terms
NOT (“animals”[mh] NOT (“animals”[mh] AND “humans”[mh]))
NOT “hearing aids”[tw]
References
- Bemelmans M, Van Den Akker T, Ford N, Philips M, Zachariah R, Harries A, … Massaquoi M (2010). Providing universal access to antiretroviral therapy in Thyolo, Malawi through task shifting and decentralization of HIV/AIDS care. Tropical Medicine and International Health, 15(12), 1413–1420. doi: 10.1111/j.1365-3156.2010.02649.x [DOI] [PubMed] [Google Scholar]
- Chirawu P, Langhaug L, Mavhu W, Pascoe S, Dirawo J, & Cowan F (2010). Acceptability and challenges of implementing voluntary counselling and testing (VCT) in rural Zimbabwe: evidence from the Regai Dzive Shiri Project. AIDS Care, 22(1), 81–88. doi: 10.1080/09540120903012577 [DOI] [PubMed] [Google Scholar]
- DeGraft-Johnson J, Paz-Soldan V, Kasote A, & Tsui A (2005). HIV voluntary counseling and testing service preferences in a rural Malawi population. AIDS and Behavior, 9(4), 475–484. doi: 10.1007/s10461-005-9018-x [DOI] [PubMed] [Google Scholar]
- Denison JA, O’Reilly KR, Schmid GP, Kennedy CE, & Sweat MD (2008). HIV voluntary counseling and testing and behavioral risk reduction in developing countries: a meta-analysis, 1990--2005. AIDS and Behavior, 12(3), 363–373. doi: 10.1007/s10461-007-9349-x [DOI] [PubMed] [Google Scholar]
- Donnell-Fink L, Reichmann WM, Arbelaez C, Case AL, Katz JN, Losina E, & Walensky RP (2011). Patient satisfaction with rapid HIV testing in the emergency department. Annals of Emergency Medicine, 58(1 SUPPL), S49–S52. doi: 10.1016/j.annemergmed.2011.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdin CA, Chong NJ, & Millson PE (2013). Non-physician clinician provided HIV treatment results in equivalent outcomes as physician-provided care: A meta-analysis. Journal of the International AIDS Society, 16. doi: 10.7448/IAS.16.1.18445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn DE, Johnson C, Sands A, Wong V, Figueroa C, & Baggaley R (2017). Can trained lay providers perform HIV testing services? A review of national HIV testing policies. BMC Research Notes, 10:20. doi: 10.1186/s13104-016-2339-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonner VA, Denison J, Kennedy CE, O’Reilly K, & Sweat M (2012). Voluntary counseling and testing (VCT) for changing HIV-related risk behavior in developing countries. Cochrane Database of Systematic Reviews, 9, CD001224. doi: 10.1002/14651858.CD001224.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fylkesnes K, Sandoy IF, Jurgensen M, Chipimo PJ, Mwangala S, & Michelo C (2013). Strong effects of home-based voluntary HIV counselling and testing on acceptance and equity: A cluster randomised trial in Zambia. Social Science and Medicine, 86, 9–16. doi: 10.1016/j.socscimed.2013.02.036 [DOI] [PubMed] [Google Scholar]
- Hecht CR, Smith MD, Radonich K, Kozlovskaya O, & Totten VY (2011). A comparison of patient and staff attitudes about emergency department-based HIV testing in 2 urban hospitals. Annals of Emergency Medicine, 58(1 SUPPL), S28–S32. doi: 10.1016/j.annemergmed.2011.03.020 [DOI] [PubMed] [Google Scholar]
- Higgins JPT, & Green S (2011). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Retrieved from http://www.cochrane-handbook.org/
- Iwu EN, & Holzemer WL (2014). Task shifting of HIV management from doctors to nurses in Africa: clinical outcomes and evidence on nurse self-efficacy and job satisfaction. AIDS Care, 26(1), 42–52. doi: 10.1080/09540121.2013.793278 [DOI] [PubMed] [Google Scholar]
- Jackson D, Naik R, Tabana H, Pillay M, Madurai S, Zembe W, & Doherty T (2013). Quality of home-based rapid HIV testing by community lay counsellors in a rural district of South Africa. Journal of the International AIDS Society, 16. doi: 10.7448/IAS.16.1.18744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgensen M, Sandoy IF, Michelo C, & Fylkesnes K (2013). Effects of home-based Voluntary Counselling and Testing on HIV-related stigma: Findings from a cluster-randomized trial in Zambia. Social Science and Medicine, 81, 18–25. doi: 10.1016/j.socscimed.2013.01.011 [DOI] [PubMed] [Google Scholar]
- Jurgensen M, Sandoy IF, Michelo C, Fylkesnes K, Mwangala S, & Blystad A (2013). The seven Cs of the high acceptability of home-based VCT: Results from a mixed methods approach in Zambia. Social Science and Medicine, 97, 210–219. doi: 10.1016/j.socscimed.2013.07.033 [DOI] [PubMed] [Google Scholar]
- Kanal K, Chou TL, Sovann L, Morikawa Y, Mukoyama Y, & Kakimoto K (2005). Evaluation of the proficiency of trained non-laboratory health staffs and laboratory technicians using a rapid and simple HIV antibody test. AIDS Research and Therapy, 2(1). doi: 10.1186/1742-6405-2-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy CE, Fonner VA, Sweat MD, Okero FA, Baggaley R, & O’Reilly KR (2013). Provider-initiated HIV testing and counseling in low- and middle-income countries: a systematic review. AIDS and Behavior, 17(5), 1571–1590. doi: 10.1007/s10461-012-0241-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledikwe JH, Kejelepula M, Maupo K, Sebetso S, Thekiso M, Smith M, … Semo BW (2013). Evaluation of a well-established task-shifting initiative: the lay counselor cadre in Botswana. PLoS One, 8(4), e61601. doi: 10.1371/journal.pone.0061601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon N, Naidoo P, Mathews C, Lewin S, & Lombard C (2010). The impact of provider-initiated (opt-out) HIV testing and counseling of patients with sexually transmitted infection in Cape Town, South Africa: a controlled trial. Implementation Science, 5(8), [11] p. doi: 10.1186/1748-5908-5-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugada E, Levin J, Abang B, Mermin J, Mugalanzi E, Namara G, … Bunnell R (2010). Comparison of home and clinic-based HIV testing among household members of persons taking antiretroviral therapy in Uganda: results from a randomized trial. Journal of Acquired Immune Deficiency Syndromes, 55(2), 245–252. doi: 10.1097/QAI.0b013e3181e9e069 [DOI] [PubMed] [Google Scholar]
- Mdege ND, Chindove S, & Ali S (2013). The effectiveness and cost implications of task-shifting in the delivery of antiretroviral therapy to HIV-infected patients: a systematic review. Health Policy and Planning, 28(3), 223–236. doi: 10.1093/heapol/czs058 [DOI] [PubMed] [Google Scholar]
- Molesworth AM, Ndhlovu R, Banda E, Saul J, Ngwira B, Glynn JR, … French N (2010). High accuracy of home-based community rapid HIV testing in rural Malawi. Journal of Acquired Immune Deficiency Syndromes, 55(5), 625–630. doi: 10.1097/QAI.0b013e3181f98628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwai GW, Mburu G, Torpey K, Frost P, Ford N, & Seeley J (2013). Role and outcomes of community health workers in HIV care in sub-Saharan Africa: A systematic review. Journal of the International AIDS Society, 16. doi: 10.7448/IAS.16.1.18586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penazzato M, Davies MA, Apollo T, Negussie E, & Ford N (2014). Task shifting for the delivery of pediatric antiretroviral treatment: a systematic review. Journal of Acquired Immune Deficiency Syndromes, 65(4), 414–422. doi: 10.1097/qai.0000000000000024 [DOI] [PubMed] [Google Scholar]
- Rackal JM, Tynan AM, Handford CD, Rzeznikiewiz D, Agha A, & Glazier R (2011). Provider training and experience for people living with HIV/AIDS. Cochrane Database of Systematic Reviews, (6), CD003938. doi: 10.1002/14651858.CD003938.pub2 [DOI] [PubMed] [Google Scholar]
- Seewald R, Bruce RD, Elam R, Tio R, Lorenz S, Friedmann P, … Perlman DC (2013). Effectiveness and feasibility study of routine HIV rapid testing in an urban methadone maintenance treatment program. The American Journal of Drug and Alcohol Abuse, 39(4), 247–251. doi: 10.3109/00952990.2013.798662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. (2014a). 90–90–90. Ambitious treatment targets: writing the final chapter of the AIDS epidemic – a discussion paper. Retrieved from: http://www.unaids.org/en/resources/documents/2014/90-90-90
- UNAIDS. (2014b). The Gap Report. Retrieved from: http://files.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2014/UNAIDS_Gap_report_en.pdf
- UNAIDS. (2016). Prevention Gap Report. Retrieved from: http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf
- Walensky RP, Morris BL, Reichmann WM, Paltiel AD, Arbelaez C, Donnell-Fink L, … Losina E (2011). Resource utilization and cost-effectiveness of counselor- vs. provider-based rapid point-of-care HIV screening in the emergency department. PLoS One, 6(10), e25575. doi: 10.1371/journal.pone.0025575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walensky RP, Reichmann WM, Arbelaez C, Wright E, Katz JN, Seage IGR, … Losina E (2011). Counselor-versus provider-based HIV screening in the emergency department: Results from the universal screening for HIV Infection in the Emergency Room (USHER) randomized controlled trial. Annals of Emergency Medicine, 58(1 SUPPL), S126–S132. doi: 10.1016/j.annemergmed.2011.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. (2007). Treat train retain. Task shifting: global recommendations and guidelines. Retrieved from: http://www.who.int/healthsystems/task_shifting/en/
- WHO. (2013). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. Retrieved from: http://www.who.int/hiv/pub/guidelines/arv2013/download/en/ [PubMed]
- WHO. (2014). Optimizing health worker roles to improve access to key maternal and newborn health interventions through task-shifting. Retrieved from: http://www.who.int/reproductivehealth/publications/maternal_perinatal_health/978924504843/en/ [PubMed]
- WHO. (2015). Consolidated guidelines on HIV testing services. Retrieved from: http://www.who.int/hiv/pub/guidelines/hiv-testing-services/en/
- Wong WCW, Luk CW, & Kidd MR (2012). Is there a role for primary care clinicians in providing shared care in HIV treatment? A systematic literature review. Sexually Transmitted Infections, 88(2), 125–131. doi: 10.1136/sextrans-2011-050170 [DOI] [PubMed] [Google Scholar]