Summary
We conducted a feasibility study of a telehealth intervention (an electronic pill box) and an m-health intervention (an app on a smartphone) for improving medication adherence in older adults with heart failure. A secondary aim was to compare patient acceptance of the devices. The participants were 60 adults with HF (65% male). Their average age was 69 years and 83% were Caucasian. Patients were randomized using a 2 × 2 design to one of four groups: pillbox silent, pillbox reminding, smartphone silent, smartphone reminding. We examined adherence to 4 medications over 28 days. The overall adherence rate was 78% (SD 35). People with the telehealth device adhered 80% of the time and people with the smartphone adhered 76% of the time. Those who received reminders adhered 79% of the time, and those with passive medication reminder devices adhered 78% of the time, i.e. reminding did not improve adherence. Patients preferred the m-health approach. Future interventions may need to address other contributors to poor adherence such as motivation.
Introduction
Heart failure (HF) is a common chronic medical condition which affects over five million Americans.1 Treatment typically includes multiple medications, and medication adherence is often poor. Long-term adherence is 56–83%.2 For a single monitored medication, 11% of HF patients took fewer than 80% of the prescribed pills.3 If full adherence is defined as the patient having enough medication for an entire year, then the percentage of HF patients who attain full adherence may be as low as 10%.4 Factors contributing to low adherence by patients with HF include complex medication regimens5 and mild cognitive dysfunction6, amongst other social, emotional and economic factors.7
Meta-analysis reveals that interventions designed to improve medication adherence provide little effect, with increases in adherence of 4–11%.8 Medication reminding using forms of telemedicine (such as use of a home telephone line to transmit health information) or m-health (using mobile devices to transmit health information) may improve medication adherence. A recent meta-analysis of randomized controlled trials9 and a systematic review10 support the effectiveness of medication reminders for medication adherence, although patients with HF have not been studied. Reminder devices (e.g. pagers, mobile phones) are typically well accepted by patients10, which suggests that reminding may be an effective way of improving medication adherence in HF. However, there have been no previous trials of reminding for medication adherence in HF.
The present study aimed to determine whether reminding using a telehealth intervention (electronic pillbox) or an m-health intervention (smartphone application) would improve medication adherence in older adults with HF. A secondary aim was to evaluate feasibility and participant acceptance of the interventions.
The hypotheses were:
Reminding would be associated with higher adherence for both the telehealth intervention and the m-health intervention.
Patients would prefer the m-health intervention to the telehealth intervention.
Methods
The trial evaluated adherence in outpatients with systolic and diastolic HF (trial no ). HF was confirmed by medical chart review. Patients were randomized to one of four groups: smartphone silent, smartphone reminding, pillbox silent, pillbox reminding.
Interventions
Telehealth.
An electronic pillbox (Medsignals, Austin TX), the telehealth medication container, was used as one medication reminder device. Each telehealth medication container had four compartments for the patients’ medications. The pillboxes were either programmed with reminder alarms for the times the participants were prescribed to take each of the four monitored pills, or the alarms were not programmed, rendering the device a passive adherence monitor. Each telehealth medication container was connected to a telephone line and data were transferred using a built-in modem to a central server supported by the manufacturer.
One week after completing the first assessment session, study personnel arrived at the participant’s home where a 35-day supply of medication was placed in each of the medication bins of the telehealth medication container. The participant was then taught how to use the telehealth medication container. Participants were tested on their ability to use the device. Participants then used the telehealth medication container for 28 days of monitoring. Participants were given the telephone numbers of study personnel, whom they were encouraged to call for assistance when needed.
M-health.
A smartphone (iPhone, Apple, Inc., Cupertino CA) was used as the second medication reminder device. A medication adherence app (iRx Reminder LLC, Akron OH) provided medication reminders and acted as a passive medication-taking log. After selecting the app icon or tapping on an alert message (in the reminding condition only), participants were able to record taking their medication with five options: take each medication individually, take all medications, skip all, as well as two snooze options. Participants could also view a list of their medications with information on each medication, including special instructions, such as taking it with a meal or before bedtime. The data were transferred over the cellular network to a secure central server supported by the service.
One week after completing the first assessment session, study personnel arrived at the participant’s home. The participant was trained on how to use the smartphone’s features, including the clock, calendar, text messaging, email, weather, camera, calculator and notes. Learning was confirmed by a skills-based test. Afterwards, participants were trained on how to use the medication adherence application. All participants were taught how to access the application, navigate the features within the application, and record a medication-taking event (taking a medication or skipping it).
Participants in the silent (no reminder) condition were equipped with similar smartphones that did not provide reminders. Individuals in the active (reminder) condition were equipped with smartphones programmed to provide reminders and were taught about medication reminders that appeared via text messages that linked to the medication adherence application. All participants were then tested on their ability to use the smartphone in general. Participants used the smartphone application for 28 days of monitoring. Participants were given the telephone numbers of study personnel, whom they were encouraged to call for assistance when needed.
Selection criteria
Inclusion criteria were: age 45–90 years; English speaking; New York Heart Association (NYHA) class II or III for three or more months; history of HF managed by the patient for at least 3 months; systolic HF with LVEF <40% documented using left ventricular angiography, nuclear wall motion study, or echocardiography within 12 months of study enrolment, or diastolic HF confirmed by chart diagnosis; willing to allow a device to be attached to their telephone for the duration of the study; living within 50 km of the performance site (Summa Health System in Akron, Ohio).
Exclusion criteria were: history of a neurological disorder or injury; moderate or severe head injury defined as >10 min of loss of consciousness; past or current history of psychotic disorders or bipolar disorder; history of alcohol or drug abuse as defined by DSM-IV criteria; history of a learning disorder or developmental disability that could interfere with functions of daily living as defined by DSM-IV criteria; renal failure requiring dialysis; sleep apnoea not managed by continuous positive airway pressure (CPAP) therapy; history of coronary artery bypass grafting within three months prior to enrolment; terminal illness (e.g. life expectancy <6 months). Although cognitive impairment is common in HF, people with severe cognitive impairment were not included in the study as they would not be reasonably expected to manage their medication.
Measures
Medication adherence.
The primary outcome measure was medication adherence. Four medications were monitored. A daily adherence value was calculated by dividing the number of recorded medication-taking events by the number of scheduled medication-taking events for all four medications. Therefore, medication adherence was measured by pillbox bin openings for people with the telehealth intervention and electronic self-report for people with the m-health intervention. This was the sole method for measuring medication adherence.
The recorded medication-taking events were uploaded from each of the intervention devices to a server. The number of scheduled medication-taking events was derived from the patients’ prescribed medication regimens. All 28 daily adherence values for each medication were included in the analyses to preserve intra-individual differences. All medications monitored were prescribed 1–3 times each day. All the chosen medications were used to treat cardiovascular disease.
Mastery of the intervention.
Following training, participants were tested on their ability to use the medication-related functions of the devices. Participants demonstrated their knowledge of how the device was powered, how to use the device, how to open and close the medication container bins (for the telehealth intervention), and how to open and use the smartphone application (for the m-health intervention).
Device ratings.
A survey was created to assess participant acceptance of the device to which they were randomized (see Appendix). The survey assessed factors such as helpfulness, quality of life, willingness to recommend the device to a friend and satisfaction. At the final study session, participants completed the questionnaire about their opinion of the device. Participants rated how much they agreed with each statement on a 5-point scale, from strongly disagree to strongly agree. The 14 items were summed into a total score, where higher scores represented greater participant acceptance. The range of possible scores was 14–70.
Design and procedure
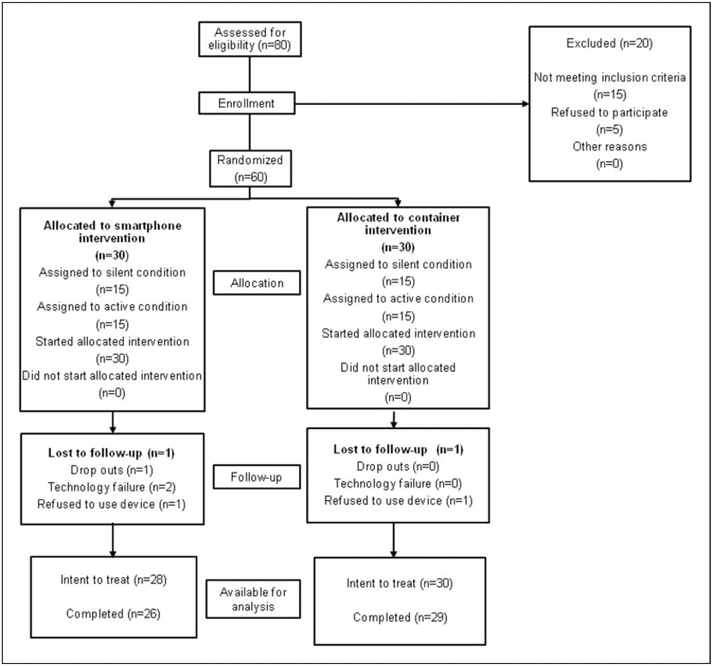
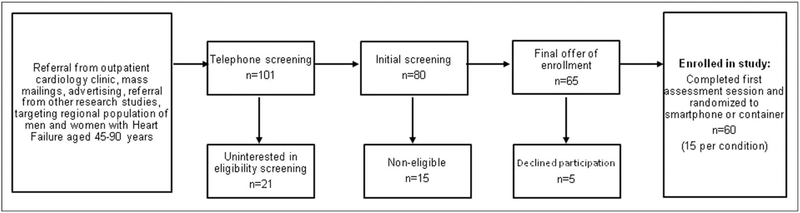
Patients were enrolled from June 2011 to May 2012. Participants were enrolled from an outpatient hospital-affiliated cardiology clinic. Patient characteristics are summarized in Table 1. The flow of patients through the trial is shown in Figure 1. Patient accrual from recruiting efforts and eligibility screening is shown in Figure 2.
Table 1.
Characteristics of the participants.
| Total sample (n = S8) | Smartphone (n = 28) | Pillbox (n = 30) | |
|---|---|---|---|
| Age, y (SD) | 69.3 (10.9) | 69.0 (10.6) | 69.6 (11.3) |
| Sex (% female) | 20 (35) | 9 (32) | 11 (37) |
| Race (% non-white) | 11 (19) | 5 (18) | 6 (20) |
| Education level (% of sample) | |||
| 9–11th Grade | 4 (7) | 2 (7) | 2 (7) |
| High School | 18 (31) | 9 (32) | 9 (30) |
| Technical or Trade School | 7 (12) | 2 (7) | 5 (17) |
| Some College | 18 (31) | 11 (39) | 7 (23) |
| Bachelor’s Degree | 8 (14) | 3 (11) | 5 (17) |
| Master’s Degree | 3 (5) | 1 (4) | 2 (7) |
| Comorbidities (% of sample) | |||
| Hypertension | 32 (55) | 17 (61) | 15 (50) |
| Myocardial infarction | 2 (3) | 0 (0) | 2 (3) |
| Diabetes | 19 (33) | 10 (36) | 9 (30) |
| Sleep apnoea | 5 (9) | 2 (7) | 3 (10) |
Figure 1.
CONSORT chart.
Figure 2.
Patient accrual from recruiting efforts and eligibility screening.
The study was approved by the appropriate ethics committees. Prospective participants were interviewed by telephone and were screened for eligibility. Prospective participants who were eligible according to their interview answers were asked for consent, after which they were enrolled. The study used a 2 × 2, open-label, randomized study design with a 1:1 allocation ratio. The random allocation sequence that was used to assign participants to one of the four groups (smartphone silent, smartphone reminding, pillbox silent, pillbox reminding) was determined using a random number generator. The sample was limited to 60 participants. Sample size was chosen prior to the initiation of the trial, but was not pre-calculated because it was a feasibility trial.
Participants completed the first assessment session in either the research office or in their home. After participants completed the first assessment session, which consisted of health interviews, cognitive testing and psychosocial questionnaires, patients were randomized. Participants were randomized to 28 days of the telehealth intervention or the m-health intervention, either with or without reminders, based on a generated random number.
After 28 days of monitoring, the device was collected and participants completed additional questionnaires assessing health status, psychosocial factors and acceptance of the intervention. The third study session also took place in the participants’ homes.
Concealment of treatment allocation was not possible. Participants and study personnel completed the first assessment session without knowledge of the group to which the participant would be assigned. Study personnel determined the participant’s condition prior to the second assessment session by procedures described above.
Analysis
The primary outcome measure was medication adherence. Hierarchical Linear Modeling (HLM) was used to examine adherence between conditions and devices.11 Analyses were conducted based on both intention-to-treat and those completing the intervention. For intention-to-treat analyses, baseline values were carried forward when postintervention values were missing. “Completers” were defined as people who completed the last assessment, who were not hospitalized for one week or more during the monitoring period, who reported attempting to use the device through the study, and whose data were not lost due to technical failure. Data were analysed using standard packages (HLM 7, Scientific Software International, Skokie IL and IBM SPSS Statistics version 19).
Differences in HLM regression coefficients at level-1 for each participant were predicted by level-2 equations. The lack of a medication-taking event was considered non-adherence rather than missing data. Data were lost for two participants due to equipment failure in the smartphone group, and these people were excluded from all analyses. In addition, two participants chose to stop using the m-health intervention and one chose to stop using the telehealth intervention during the monitoring period.
Results
Participants were 60 adults with NYHA Class II or III HF (65% male) of average age 69 years (SD 11). Most were Caucasian 83%). Demographic, educational and comorbidities were similar between groups (Table 1). The average Mini Mental Examination score was 28.7 (SD 1.6), suggesting that the participants were oriented and did not have dementia.
The hypotheses were first tested using intention-to-treat analyses. The data were normally distributed. The unconditional model indicated that differences in adherence existed between individuals and that the data were appropriate for hierarchical linear modelling. The intra-class correlation (ICC) was also calculated to determine the percentage of variance in adherence that was attributable to the person level and to repeated measures level. The result suggested that 57% of the variance in adherence was at the person level.
In the final model, device type was not significantly associated with medication adherence (P = 0.87). In addition, condition was not significantly associated with medication adherence (P = 0.48). There was no significant interaction between device and condition (P = 0.33).
Mastery of the device
In all groups, all participants were able to use the device according to observation by study personnel. All participants demonstrated their knowledge of how the device was powered and how to use the device. Participants with the telehealth device were all able to open and close the telehealth medication container bins. All participants with the m-health device demonstrated their ability to use the smartphone application.
Medication adherence
According to intention-to-treat analyses, the overall adherence rate for both devices and conditions was 78% (SD 35), see Table 2. Overall, people with the telehealth device adhered 80% of the time and people with the smartphone adhered 76% of the time. Those who received reminders adhered 79% of the time, and those with passive medication reminder devices adhered 78% of the time.
Table 2.
Adherence based on intention-to-treat.
| Pillbox | Smartphone | Total | |
|---|---|---|---|
| Active, % (SD) | 84 (32) | 73 (39) | 79 (36) |
| Passive, % (SD) | 76 (33) | 79 (33) | 78 (33) |
| Total, % (SD) | 80 (33) | 76 (36) |
According to completers analyses, the overall adherence rate for both devices and conditions was 82%. People with the telehealth device adhered 83% of the time and people with the smartphone adhered 82% of the time. Those who received reminders adhered 85% of the time, and those with passive medication reminder devices adhered 80% of the time.
Device ratings
In both conditions (reminding or passive), participants preferred the m-health intervention to the telehealth intervention (P < 0.001). Participants who received smartphones with the m-health app (mean score 48.7) rated their device significantly higher on patient acceptance and device helpfulness (P < 0.001) than people who received telehealth medication containers (mean score 33.4). The same was true for completers. At the conclusion of data collection, most participants who received a passive device spontaneously reported that a reminding function would probably improve the device.
Discussion
The present study showed that medication reminding via an alarmed electronic pillbox (telehealth) or a smartphone app (m-health) produced similar medication adherence in patients with HF. Contrary to the original hypotheses, there were no differences in the main outcome measure (medication adherence) in the two groups. Overall adherence was high, reaching nearly 80%. It is remarkable that some patients using the smartphone app consistently logged medication-taking for 4 medications with no reminders. However, for completers, the lowest quartile was below 71%, which would not be considered satisfactory adherence.12 These findings are somewhat surprising since previous studies have estimated adherence to long-term therapies for patients with chronic illness in industrialised nations to be about 50%.13–15 Studies with HF populations have found a wide range of adherence values, although most HF patients achieved medication adherence which was suboptimum.2
The high adherence rates observed in our study may have been due to sample characteristics and features of the trial design. That is, they may reflect a well-managed sample of largely adherent patients willing to volunteer to try new technologies. For example, since study participants reported that they were willing to learn to use the devices, their adherence may have been influenced by their interest in learning to use the device.16 In fact, the non-alarmed smartphone app intended as a control condition may have improved adherence by providing a convenient means of self-monitoring medication-taking behaviour. Furthermore, the high adherence may have contributed to a ceiling effect, perhaps obscuring differences in adherence between the study groups and conditions. Although the relatively short monitoring period ensured that factors such as obtaining refills and cost of medications did not reduce adherence, it may not have allowed for measurement reactivity to subside. Studies published after our trial ended have suggested that interventions using telehealth/m-health technologies occurring over many months of monitoring may minimize measurement reactivity.17 For the roughly 25% of our sample who remained poorly adherent, medication reminding was ineffective.
Our intervention did not address motivation. Although healthcare providers and researchers assume that HF patients aim for full adherence, perhaps this is not true of everyone. Future interventions may need to address motivation and other patient attitudes (e.g. health beliefs) that can affect adherence. Furthermore, future studies should analyse adherence by timing (e.g. morning or evening) and complexity (e.g. once daily or three times daily) to clarify how telemedicine devices affect adherence. Finally, future studies should also measure baseline adherence, as high baseline adherence can contribute to a ceiling effect, which would affect the conclusions of future pilot trials and interventions.
It is promising that nearly all participants were able to use the technologies trialled, and we did not see any evidence that older adults with a chronic disease could not use telehealth or smartphones after appropriate training. Some participants refused to use the device: one participant who received the smartphone refused due to rehospitalization, while another refused to use it due to lack of interest. One person in the telehealth medication container group reported disliking the device too much to continue using it.
People who were given the smartphone app rated their device much higher than people given the telehealth medication container. Similar to previous literature (e.g. Haberer et al.18), although participants were willing to use the devices, some of them found the reminding features of the telehealth medication containers annoying. It is possible that participants opened bins in the active condition merely to silence the alarms, thus recording a medication-taking event, whether or not a pill was taken. In contrast, the high ratings for the smartphone app may have been partly due to the ease of integrating the intervention into their everyday lives and existing medication-taking systems. Future research should consider the use of devices capable of capturing medication-taking errors, such as incorrect doses.
Clearly, more work is needed to design effective medication adherence interventions for patients with HF. Dynamic interventions, which are administered to all medication takers, but which provide real-time adherence feedback to non-adherent patients over the course of the intervention are especially promising.19
We only monitored four medications, although HF patients often have many more. The telehealth intervention was only capable of monitoring four medications, while the m-health intervention had no limit. The m-health and telehealth interventions were the only sources of adherence data. Pill counts and pharmacy refills were not compared to intervention-derived medication adherence rates in the present study.
Limitations
The present study had some limitations. It was a small trial, to evaluate feasibility and patient acceptance of the interventions. Some participants were recruited by responding to mailings and advertising, which may represent an inherently motivated sample. We did not collect baseline data on adherence rates before administering the intervention.
The smartphone application was unable to capture overdoses due to the design of the smartphone application. In fact, the method of ascertaining adherence varied between devices: bin openings vs. self-report. This could have resulted in differential measurement of adherence. Future studies should avoid this confounding factor, perhaps by using one intervention or by using electronic monitoring of adherence with each intervention tested. Future studies could use an additional measure of medication adherence such as a medication possession ratio or a proportion of days covered by pharmacy refills. Also, providing a fully functioning smartphone to participants randomized to the m-health condition may have influenced differences in participant satisfaction.
In conclusion, reminding via smartphone app or alarmed pillbox did not improve adherence. Overall adherence was high, which may reflect a well-managed sample of patients who were well-rehearsed at their medication regimens. However, a subset exhibited relatively poor adherence, and reminding alone did not appear sufficient to improve adherence in this group. Future interventions may need to address other contributors to poor adherence such as motivation.
Acknowledgements
We thank the research personnel, the cardiology practice and the participants for their time and commitment. Dr Anthony Sterns is the CEO of a company (iRxReminder, LLC) that created an intervention used in the present study, but he was not involved in the data collection or the analysis of the results. The research was supported by grants from the National Heart Lung and Blood Institute, National Institutes of Health (1 R01 HL096710-01A1), the Kent State University Initiative for Clinical and Translational Research, and the Frances Payne Bolton School of Nursing, Case Western Reserve University.
Appendix 1. Final evaluation of device
On a scale of 1 to 5 where 1 is strongly disagree and 5 is strongly agree, rate how much you agree with the following statements. Note: “MRS” means medication reminder system.
| I found the MRS easy to use. | 1 2 3 4 5 |
| The MRS helped me to remember my medications. | 1 2 3 4 5 |
| The MRS made my life easier. | 1 2 3 4 5 |
| I liked using the MRS. | 1 2 3 4 5 |
| I would recommend the MRS to a friend. | 1 2 3 4 5 |
| I have told people I know good things about this MRS. | 1 2 3 4 5 |
| I would buy this MRS for a loved one with heart failure if it was affordable. | 1 2 3 4 5 |
| Other people like me would find this MRS helpful. | 1 2 3 4 5 |
| Overall, I was satisfied with this MRS. | 1 2 3 4 5 |
| This MRS fulfilled my needs. | 1 2 3 4 5 |
| I would buy this product for myself if it was affordable. | 1 2 3 4 5 |
| This MRS improved my quality of life. | 1 2 3 4 5 |
| This product was well-designed. | 1 2 3 4 5 |
| The MRS was user-friendly. | 1 2 3 4 5 |
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics – 2012 update: a report from the American Heart Association. Circulation 2012;125:e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Persistent use of evidence-based pharmacotherapy in heart failure is associated with improved outcomes. Circulation 2007;116:737–44. [DOI] [PubMed] [Google Scholar]
- 3.Granger BB, Swedberg K, Ekman I, et al. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial. Lancet 2005;366:2005–11. [DOI] [PubMed] [Google Scholar]
- 4.Monane M, Bohn RL, Gurwitz JH, Glynn RJ, Avorn J. Noncompliance with congestive heart failure therapy in the elderly. Arch Intern Med 1994;154:433–7. [PubMed] [Google Scholar]
- 5.Wu JR, Moser DK, Chung ML, Lennie TA. Predictors of medication adherence using a multidimensional adherence model in patients with heart failure. J Card Fail 2008;14:603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pressler SJ. Cognitive functioning and chronic heart failure: a review of the literature (2002-July 2007). J Cardiovasc Nurs 2008;23:239–49. [DOI] [PubMed] [Google Scholar]
- 7.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–97. [DOI] [PubMed] [Google Scholar]
- 8.Peterson AM, Takiya L, Finley R. Meta-analysis of trials of interventions to improve medication adherence. Am J Health Syst Pharm 2003;60:657–65. [DOI] [PubMed] [Google Scholar]
- 9.Fenerty SD, West C, Davis SA, Kaplan SG, Feldman SR. The effect of reminder systems on patients’ adherence to treatment. Patient Prefer Adherence 2012;6:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vervloet M, Linn AJ, van Weert JC, de Bakker DH, Bouvy ML, van Dijk L. The effectiveness of interventions using electronic reminders to improve adherence to chronic medication: a systematic review of the literature. J Am Med Inform Assoc 2012;19:696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raudenbush SW, Byrk AS. Hierarchical Linear Models: applications and data analysis methods. Newbury Park, CA: Sage Publications, 1992. [Google Scholar]
- 12.Wu JR, Moser DK, De Jong MJ, et al. Defining an evidence-based cutpoint for medication adherence in heart failure. Am Heart J 2009;157:285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogner HR, de Vries HF. Integrating type 2 diabetes mellitus and depression treatment among African Americans: a randomized controlled pilot trial. Diabetes Educ 2010;36:284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosworth HB, Olsen MK, Gentry P, et al. Nurse administered telephone intervention for blood pressure control: a patient-tailored multifactorial intervention. Patient Educ Couns 2005;57:5–14. [DOI] [PubMed] [Google Scholar]
- 15.Burkhart PV, Sabaté E. Adherence to long-term therapies: evidence for action. J Nurs Scholarsh 2003;35:207. [PubMed] [Google Scholar]
- 16.Heinrich CK, Kuiper RA. Using handheld devices to promote medication adherence in chronic illness. J Nurse Pract 2012;8:288–93. [Google Scholar]
- 17.Cook P, Schmiege S, McClean M, Aagaard L, Kahook M. Practical and analytic issues in the electronic assessment of adherence. West J Nurs Res 2012;34:598–620. [DOI] [PubMed] [Google Scholar]
- 18.Haberer JE, Robbins GK, Ybarra M, et al. Real-time electronic adherence monitoring is feasible, comparable to unannounced pill counts, and acceptable. AIDS Behav 2012;16:375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cutrona SL, Choudhry NK, Fischer MA, et al. Targeting cardiovascular medication adherence interventions. J Am Pharm Assoc (2003) 2012;52:381–97. [DOI] [PubMed] [Google Scholar]