Abstract
Objectives:
This study was undertaken as an attempt to assess radiographic temporomandibular joint (TMJ) changes in relation to rheumatoid factor (RF), anticitrullinated protein (ACCP) antibodies and disease activity score 28 (DAS28) in rheumatoid arthritis (RA) patients to find the best predictor of rheumatoid affection of the TMJ with the ultimate goal of maintaining TMJ function and preventing joint damage.
Methods:
20 Rheumatoid Arthritis patients as well as 20 volunteers were included in this study. RA group were assessed for RF, ACCP, DAS28. Both groups were assessed by CBCT for TMJ dimensions and radiographic osteoarthritic changes. All data were statistically analyzed.
Results:
Rheumatoid Arthritis group showed significantly less condylar height and more radiographic osteoarthritic changes than the control group. RF showed no significant correlation with either TMJ measurements or TMJ radiographic osteoarthritic changes. ACCP showed significant inverse correlation with condylar height and anteroposterior (AP) dimensions, but non-significant relation with mediolateral dimension and radiographic osteoarthritic changes. DAS28 showed significant inverse correlation with condylar AP and mediolateral dimensions. It also showed significant correlation with flattening of the TMJ condylar head and flattening of the articular fossa. Patients with high and moderate disease activity showed significantly smaller AP TMJ dimension than patients with low disease activity. Disease activity showed statistically significant direct correlation with all osteoarthritic changes except for erosions of the glenoid fossa and condyle.
Conclusion:
Disease Activity Score28 score and disease activity are strong indicators of TMJ affection in RA patients when compared to RF and ACCP. ACCP is a better indicator of changes in condylar measurements than TMJ osteoarthritic changes. While RF is the least efficient indicator of TMJ involvement in RA patients.
Keywords: Arthritis, Rheumatoid/diagnosis, Rheumatology/methods, Rheumatoid Arthritis, Rheumatoid Factor, Anti CitrullinatedProtein Antibodies, Disease Activity Score, Temporomandibular Joint, Cone-Beam Computed Tomography, Disability Evaluation, Disease Progression, Predictive Value of Tests
Introduction
Rheumatoid arthritis (RA) is one of the most common autoimmune diseases, affecting 1–1.5% of the population worldwide.1–5 It is characterized by persistent synovitis, systemic inflammation, and autoantibodies particularly to rheumatoid factor and citrullinated peptide.6–8 The hallmark feature of the disease is persistent polyarthritis (synovitis) that may affect many synovial joints.2–5,9–11
The reported prevalence of TMJ involvement by RA patients varies widely from 4.7 to 88%.12 Rheumatoid affection of the TMJ may lead to severe sequelae and disabilities including pain at pre-auricular region, morning stiffness of TMJ which may last for 30 min, as well as difficulty in eating and ankylosis.12–17
RF is an important diagnostic tool for assessment of RA. It is considered one of the diagnostic criteria of RA in the European League Against Rheumatism (EULAR) system.1,18,19 ACCP is a prognostic indicator for RA with a reported 80% sensitivity and 98% specificity.6 The “Disease Activity Score using 28 joint counts” (DAS28) is a combined index that has been developed in the eighties to measure the disease activity in patients with RA. It has been extensively validated for its use in clinical trials in combination with the EULAR response criteria.3
RF, ACCP and DAS28 are common, reliable and regularly used methods of assessment of RA.1,18,19 If these indices correlate with TMJ changes in RA patients, then they can be used as potential predictors of TMJ affection in RA patients with the ultimate goal of providing dedicated TMJ treatment options for these patients and avoiding mutilating TMJ sequalae.
CBCT allows accurate radiographic assessment of incipient bony TMJ changes that less advanced radiographic techniques could not reveal because of overlapping and distortion.20–24 Therefore, the aim of the present study is to assess the correlation of radiographic TMJ changes with RF, ACCP and DAS28 in rheumatoid arthritis patients using CBCT.
Patients and methods
Inclusion and exclusion criteria
Rheumatoid group: This group comprised 20 female patients with previously established and confirmed diagnosis of RA with an age range of 30–65 years old. The diseased group patients were selected from the inpatient and outpatient clinics of the Rheumatology Department of Ain-Shams University. All patients were confirmed as having RA according to the criteria of the new classification of the American College of Rheumatology with the EULAR in 20101 where the cut point for RA patient was six points or more of the following :
-
Joint involvement (0–5)
One medium-to-large joint (0)
Two to ten medium-to-large joints (1)
One to three small joints (large joints not counted) (2)
Four to ten small joints (large joints not counted) (3)
More than ten joints (at least one small joint) (5)
-
Serology (0–3)
Negative RF and negative ACPA (0)
Low positive RF or low positive ACPA (2)
High positive RF or high positive ACPA (3)
-
Acute-phase reactants (0–1)
Normal CRP and normal ESR (0)
Abnormal CRP or abnormal ESR (1)
-
Duration of symptoms (0–1)
Less than 6 weeks (0)
6 weeks or more (1)
Control group: This group comprised 20 asymptomatic female volunteers randomly chosen from the outpatient clinics of Faculty of Dentistry at the same institute with their age matched to the study group. They had no clinical (present or past) complaints or signs and symptoms of TMD or RA but needed other dental treatment which required CBCT radiographic imaging including pre-surgical planning for removal of impacted teeth, maxillofacial pathology not affecting TMJ and dental implant planning.
The study was approved by the Research Ethics Committee of the Faculty of Dentistry Ain-Shams University, approval number FDASU-REC ID 011414. It approved the written informed consent which was signed by all subjects after explaining to them the nature of the study. A copy was given to each patient and a note was written that she can leave the study at any time.
Methods of evaluation
I-Serology
A serum sample was aspirated from each patient of the rheumatoid group and the following were assessed: a-RF measured in IU/ml positivity ≥15 IU ml−1 b-ACCP measured in U/ml positivity ≥5 U ml−1
II-DAS28
DAS28 for each patient of the Rheumatoid group was assessed as follows
Number of swollen joints (in 28 joints which are: 2 shoulders, 2 elbows, 2 wrists, 5 metacarpophalyngeal joints on each side, 5 proximal interphalyngeal joints on each side and 2 knees). Joints were assessed by observing and palpating for evidence of swelling.
Number of tender joints (in the same 28 joints). Tender joints were assessed by palpating and were based upon the patient’s reaction to palpation.
ESR is measured in mm/h.
Patient global assessment of disease activity during the last week using visual analogue scale (0–100 mm).
All these data were fed to the DAS calculator (DAS28 calculator excel sheet v. 1.1-beta for DAS28 with four variables), the result appeared immediately. The patient’s DAS28 (range 0–9.4) was then classified as follows: remission <2.6, low activity ≥2.6 to <3.2, moderate activity ≥3.2 to ≤5.1, high activity >5.1.
III-Imaging
CBCT scans were performed using i-CAT imaging system (Imaging sciences International, Hatfield, PA). Exposure was done at 120 KV, 37.07 mA and 26.9 s acquisition time. The voxel dimension selected was 0.2 mm. The Image detector was a flat panel measuring 20 × 25 cm, images were acquired in a single 360° rotation. The field of view was 8 × 14 cm to cover the TMJ with the inferior orbital margin as the upper limit.
During scanning, the patient position was set according to manufacturer instructions marked by a laser beam of the machine. Another vertical laser beam was aligned 3.8 cm (1.5 inch) in front of the condyle. Patients were instructed to swallow and bite on a bite block. Patient's head was supported as recommended by the manufacturer using forehead support and chin rest.
The reconstructed data sets were exported as Digital Imaging and Communications in Medicine (DICOM) image stacks and then transferred to another work station to view the images using On Demand software (On demand 3D™, Cybermed, South Korea). Images were viewed using Dell monitor (22'' Full HD 1920 x 1080 display) in dimmed light room.
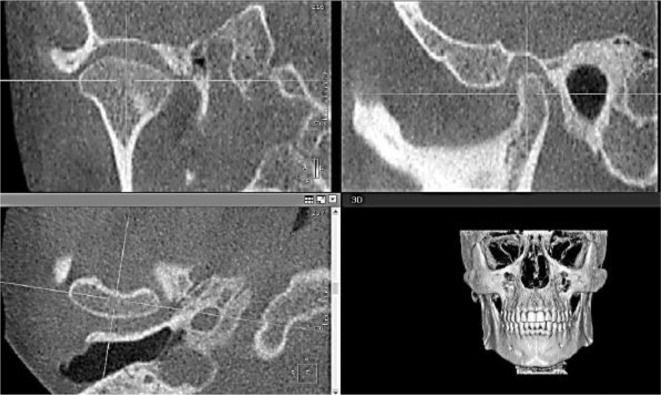
Right and left condyles were assessed separately. On the MPR screen; the coronal section was oriented on the axial window in order to pass through the widest condyle ML dimension. Then, the sagittal section was oriented on the axial window in order to bisect the coronal section. (Figure 1)
Figure 1. .
Standardized orientation of the MPR views. The coronal plane was oriented on the axial window so that it passes through the widest condyle dimension mediolaterally. The sagittal plane was oriented on the axial window so that it bisects the coronal plane. MPR, multiplanar reconstruction.
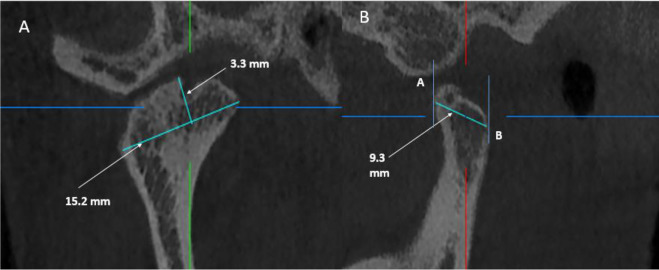
Condylar assessment was performed as follows: (Figure 2)
Figure 2. .
Condylar head measurements. (A) On the corrected coronal view, mediolateral dimension and height were measured. (B) On the corrected sagittal view, antero posterior dimension was measured.
1-Condyle ML dimension: was measured on the corrected coronal view as the distance between the most prominent medial (M) and lateral (L) points of the condylar head.
2-Condyle height: was measured on the corrected coronal view as the linear distance between the most superior point of the condyle (S) and the line measuring the condyle’s medio-lateral dimension.
3-Condyle AP dimension: was measured on the corrected sagittal view as the distance between the most prominent anterior (A) and posterior (P) points of the condylar head.
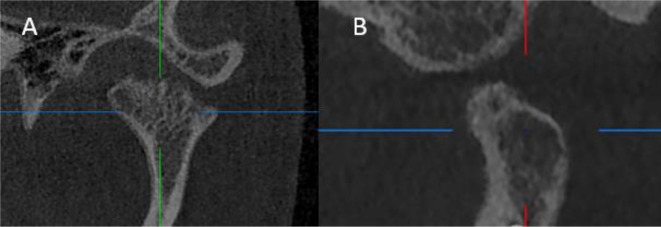
4-Osteoarthritic features: Osteoarthritic features were assessed on the corrected sagittal and corrected coronal views including; erosion in condylar head, erosion in fossa, flattening in condylar head, flattening in fossa, osteophytes and subchondral cyst. (Figure 3)
Figure 3. .
TMJ radiographic osteoartheritic changes. (A) corrected coronal view showing condylar head erosions. (B) corrected sagittal view, showing subchondral cyst. TMJ, temporomandibular joint.
IV-Statisticalanalysis
Qualitative data were presented as frequencies and percentages. χ2 test or Fisher’s Exact test when applicable were used for the comparisons. Numerical data were explored for normality by checking the distribution of data and using tests of normality (Kolmogorov–Smirnov and Shapiro–Wilk tests). Age and TMJ measurements data showed normal (parametric) distribution while RF, ACCP and DAS28 scores showed non-parametric distribution. Parametric data were presented as mean and standard deviation (SD) values while non-parametric data were presented as median and range values.
For parametric data; Student’s t-test was used to compare between the two groups. One-way ANOVA followed by Bonferroni’s post-hoc test were used to compare between more than two groups. For non-parametric data; Mann–Whitney U test was used to compare between the two groups. Spearman’s correlation coefficient was used to determine significant correlations between the different variables. The significance level was set at p ≤ 0.05. Statistical analysis was performed with IBM® SPSS® Statistics v. 20 for Windows.
N.B. the Serology sample, DAS28 and imaging were done for each patient at the same day.
Results
There was no statistically significant difference between mean age of the two groups (p-value = 0.900). the mean age of the RA group was 48 years while the mean age of the control group was 47.4 years.
The mean (SD) of RF in the rheumatoid group were 73.4 (98.7) IU/mL with a minimum of 5 and a maximum of 371 IU ml−1. The mean (SD) of ACCP in the rheumatoid group were 136.8 (186.1) U/ml with a minimum of 0.5 and a maximum of 831.5 U ml−1. The mean (SD) of Total DAS28 score in the rheumatoid group were 5.4 (1.4) with a minimum of 2.8 and a maximum of 7.6. In the rheumatoid group, 2 subjects (10%) had low disease activity, 6 subjects (30%) had moderate disease activity while 12 subjects (60%) had high disease activity.
Rheumatoid group showed significantly lower mean condylar height than the control group (p-value = 0.002). However, there was no statistically significant difference between mean ML and AP condylar dimensions in the two groups (p-value = 0.813 and 0.131 successively) (Table 1). The rheumatoid group showed significantly higher prevalence of all the radiographic osteoarthritic changes than the control group (p-value < 0.001 for each change). (Table 2)
Table 1. .
Mean, SD values and results of Student’s t-test for comparisons between TMJ measurements in the two groups
| TMJ measurements | Rheumatoid group (n = 40 Joints) |
Control group (n = 40 Joints) |
p-value |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Mediolateral dimension | 17.89 (1.94) | 17.99 (1.84) | 0.813 |
| Height | 4.3 (0.75) | 4.87 (0.87) | 0.002a |
| Anteroposterior dimension | 8.66 (1.13) | 8.27 (1.12) | 0.131 |
SD, standard deviation; TMJ, temporomandibular joint.
Significant at p ≤ 0.05
Table 2. .
Frequencies, percentages and results of χ2 test for comparisons between osteoarthritic changes in the two groups
| Osteoarthritic changes | Rheumatoid group (n = 40 Joints) |
Control group (n = 40 Joints) |
p-value |
|---|---|---|---|
| n (%) | n (%) | ||
| Erosion in condylar head | 16 (40) | 5 (12.5) | 0.0006a |
| Erosion in fossa | 12 (30) | 0 (0) | <0.001a |
| Flattening in condylar head | 32 (80) | 2 (5) | <0.001a |
| Flattening in fossa | 32 (80) | 1 (2.5) | <0.001a |
| Osteophytes | 26 (65) | 0 (0) | <0.001a |
| Subchondral cyst | 26 (65) | 0 (0) | <0.001a |
Significant at p ≤ 0.05
Rheumatoid factor level did not correlate significantly with TMJ measurements or radiographic osteoarthritic changes. (Correlation coefficient = 0.070, 0.70 and −0.259 for condylar height, ML and AP dimensions respectively, p-value > 0.05).
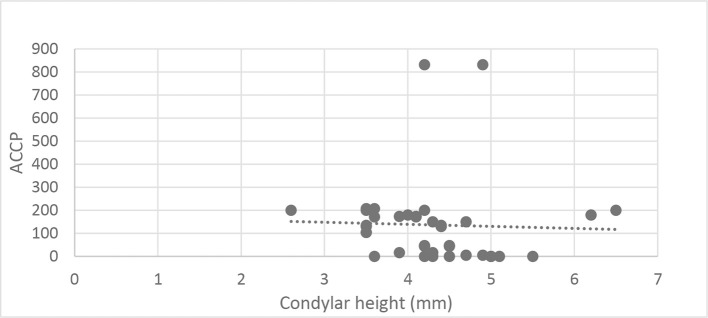
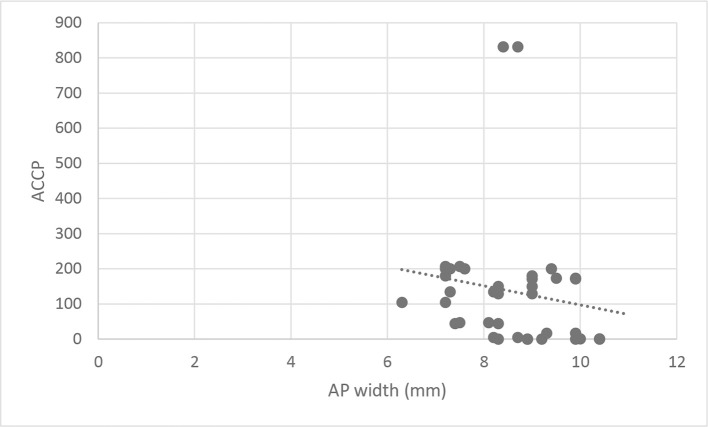
There was a statistically significant inverse correlation between ACCP levels and condylar height and AP joint dimension (Correlation coefficient = −0.325 and −0.348, p-value = 0.047 and 0.032 respectively). But there was no significant correlation between ACCP levels and ML condylar dimension (Correlation coefficient = −0.188, p-value = 0.258). Moreover, there was no significant correlation between ACCP levels in rheumatoid patients and radiographic osteoarthritic changes (Figures 4 and 5).
Figure 4. .
Scatter diagram representing inverse correlation between condylar height and ACCP level. ACCP,anticitrullinated protein.
Figure 5. .
Scatter diagram representing inverse correlation between anteroposterior joint width and ACCP level. ACCP, anticitrullinated protein.
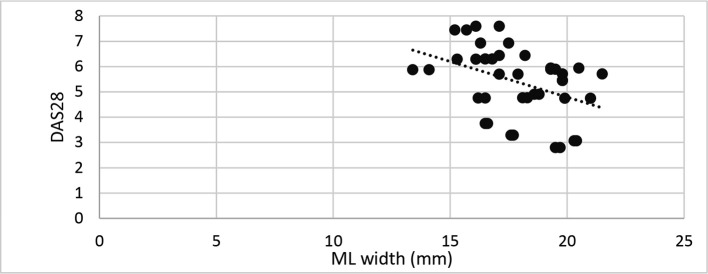
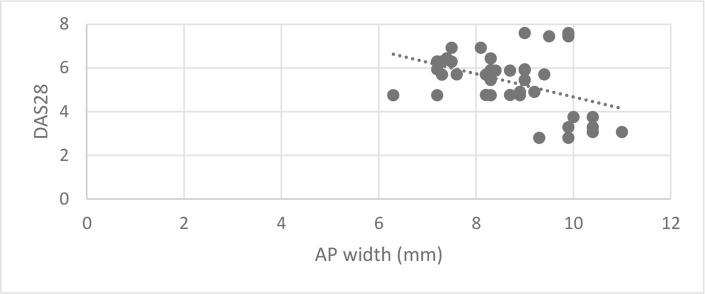
A statistically significant inverse correlation was found between DAS28 score and ML and AP joint dimensions (Correlation coefficient = −0.454 and −0.363, p-value = 0.003 and 0.021 successively) (Figures 6 and 7). DAS28 score did not correlate with condylar height (Correlation coefficient = −0.275, p-value = 0.086). Patients with signs of flattening of condylar head and/or fossa showed significantly higher median DAS28 score than patients without signs (p-value = 0.038 and 0.038, respectively) (Table 3). Other radiographic osteoarthritic changes did not correlate significantly with DAS28 scores.
Figure 6. .
Scatter diagram representing inverse correlation between ML joint dimension and DAS28 score. DAS28, disease activity score 28; ML,mediolateral.
Figure 7. .
Scatter diagram representing inverse correlation between anteroposterior joint dimension and DAS28 score. DAS28, disease activity score 28.
Table 3. .
Median, range values and results of Mann–Whitney U test for comparisons between DAS28 score in rheumatoid patients with and without signs of osteoarthritic changes
| Osteoarthritic changes | Positive OA | Negative OA | r-value | P-value |
|---|---|---|---|---|
| Median (Range) DAS28 | Median (Range) DAS28 | |||
| Erosion in condylar head | 5.8 (3.3–7.6) | 5.7 (2.8–6.9) | 0.09999 | 0.643 |
| Erosion in fossa | 5.6 (3.3–5.9) | 5.9 (2.8–7.6) | 0.09999 | 0.322 |
| Flattening in condylar head | 5.8 (3.3–7.6) | 4.8 (2.8–5.9) | 0.4769a | 0.038a |
| Flattening in fossa | 5.8 (3.3–7.6) | 4.8 (2.8–5.9) | 0.4769a | 0.038a |
| Osteophytes | 5.7 (3.3–7.6) | 5.8 (2.8–6.9) | −0.2271 | 0.663 |
| Subchondral cyst | 5.7 (3.3–7.6) | 5.8 (2.8–6.9) | 0.1062 | 0.663 |
DAS28, disease activity score 28; OA, osteoarthritis.
Significant at p ≤ 0.05
There was a significant difference between AP joint widths in patients with different disease activities (p-value = 0.005). Pairwise comparisons revealed that patients with low disease activity showed significantly higher mean width. There was no significant difference between patients with moderate and high disease activity. ML joint dimension and condylar height did not differ significantly between patients with different disease activities (p-value = 0.055 and 0.601 successively) (Table 4).
Table 4. .
Mean, SD values and results of one-way ANOVA test for comparisons between TMJ measurements in rheumatoid patients with different disease activities
| TMJ measurements | Low activity (n = 4 Joints) |
Moderate activity (n = 12 Joints) |
High activity (n = 24 Joints) |
P-value |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Mediolateral dimension | 19.98 (0.44) | 17.98 (1.47) | 17.5 (2.1) | 0.055 |
| Height | 4.28 (0.67) | 4.48 (0.67) | 4.21 (0.83) | 0.601 |
| Anteroposterior dimension | 10.15 (0.72) A | 8.87 (1.26) B | 8.3 (0.89) B | 0.005a |
SD, standard deviation; TMJ, temporomandibular joint.
Significant at p ≤ 0.05, Different superscripts in the same row are significantly different
Rheumatoid patients with flattening in condylar head and flattening of fossa showed significantly higher prevalence of high and moderate disease activity than patients without signs (p-value = 0.001 and 0.001, respectively). Rheumatoid patients with osteophytes and subchondral cyst showed significantly higher prevalence of high and moderate disease activity than patients without these OA signs (p-value = 0.012 and 0.012, respectively) (Table 5). There was no statistically significant difference between disease activity in rheumatoid patients with and without signs of erosion in condylar head and erosion in fossa (p-value = 0.170 and 0.532, respectively).
Table 5. .
Frequencies, percentages and results of Fisher’s exact test for the association between disease activity in rheumatoid patients with and without signs of osteoarthritic changes
| Osteoarthritic changes | Positive OA | Negative OA | P-value |
|---|---|---|---|
| n (%) Disease activity | n (%) Disease activity | ||
| Erosion in condylar head | 0.170 | ||
| Low activity | 0 (0) | 4 (16.7) | |
| Moderate activity | 4 (25) | 8 (33.3) | |
| High activity | 12 (75) | 12 (50) | |
| Erosion in fossa | 0.532 | ||
| Low activity | 0 (0) | 4 (14.3) | |
| Moderate activity | 4 (33.3) | 8 (28.6) | |
| High activity | 8 (66.7) | 16 (57.1) | |
| Flattening in condylar head | 0.001a | ||
| Low activity | 0 (0) | 4 (50) | |
| Moderate activity | 10 (31.2) | 2 (25) | |
| High activity | 22 (68.8) | 2 (25) | |
| Flattening in fossa | 0.001a | ||
| Low activity | 0 (0) | 4 (50) | |
| Moderate activity | 10 (31.2) | 2 (25) | |
| High activity | 22 (68.8) | 2 (25) | |
| Osteophytes | 0.012a | ||
| Low activity | 0 (0) | 4 (28.6) | |
| Moderate activity | 10 (38.5) | 2 (14.3) | |
| High activity | 16 (61.5) | 8 (57.1) | |
| Subchondral cyst | 0.012a | ||
| Low activity | 0 (0) | 4 (28.6) | |
| Moderate activity | 10 (38.5) | 2 (14.3) | |
| High activity | 16 (61.5) | 8 (57.1) |
OA, osteoarthritis.
Significant at p ≤ 0.05.
Discussion
This study was undertaken as an attempt to assess TMJ changes in rheumatoid patients and their relation to RF, ACCP and DAS scores using CBCT for better understanding, early diagnosis and effective timely treatment and appropriate follow up of RA patients with TMJ involvement.
TMJ affection in RA is frequently overlooked by many rheumatologists or by the patients themselves, because rheumatoid treatment is usually focused on other joints for upper extremity function or weight-bearing.12–14
Only females were included in this study because RA occurs 2–3 times more in females than in males.2 Decreasing the variables ensures that differences found between groups are actually due to RA. The control group was chosen to be age and sex matched.
All patients of RA group were confirmed as having RA according to the criteria of the latest classification of the American College of Rheumatology with the EULAR in 2010.1 The EULAR system reassesses RA by focusing on features of disease, rather than defining the disease by its late-stage criteria.1 Paying attention to earlier diagnosis can prevent or decrease the occurrence of the sequelae of RA.1
There are 2 types of DAS28, DAS28 CRP (C-reactive protein) and DAS28 ESR. The DAS28 ESR is considered a better assessment tool than DAS28 CRP, as DAS28 CRP underestimates the disease in comparison to DAS28 ESR.25,26 That is why it was preferred in our patients assessment.
CBCT is an indispensable method for TMJ assessment as it allows multiplanar reformation in axial, coronal, sagittal as well as oblique image planes which provides easy detection of morphological changes in osseous parts of the TMJ. Moreover, CBCT provides accurate and reliable linear measurements of dental and maxillofacial structures.22,27–30
CBCT field of view was adjusted to cover both arches reaching up to the inferior orbital margins to facilitate applying the reference lines and points needed to perform the radiographic analysis of the TMJ.27,28,31,32 The multiplanar view was used for TMJ analysis instead of the limited TMJ view as it gives a wide view for the TMJ and allows the application of the reference lines and points yielding more accurate and reproducible measurements.
There is no available radiographic scoring method to measure and evaluate TMJ changes in RA like Larsen’s classification that is used to assess other individual joints, therefore TMJ changes were assessed as condylar dimensional changes and radiographic osteoarthritic features.
In the current study, 60% (12 subjects) of RA patients group had high disease activity. This may indicate the reason why they came to the clinic as they seriously needed treatment for their disease condition.
Rheumatoid arthritis patients had significantly decreased height of the mandibular condyle than the control group. While the ML and AP dimensions did not differ significantly between the two groups. This indicates bone destruction in the upper condylar surface with less or no destruction on the other directions. Most of the previous studies did not include measurements of condylar dimensions in assessment of RA and they focused only on clinical TMJ condition,33,34 or radiographic osteoarthritic changes.35–38 One paper described decreased condylar height in RA patients with no mention of the other dimensions.39
Rheumatoid arthritis patients had significantly more osteoarthritic changes than the control group. All the studied aspects were significantly more apparent in the RA group including erosion in condylar head, erosion in glenoid fossa, flattening in condylar head, flattening in glenoid fossa, osteophytes and subchondral cysts. Previous studies agreed that there is an increase in TMJ radiographic osteoarthritic changes in RA patients in comparison to normal individuals.35,37,40 This implies the presence of abnormal inflammatory process and indicates the importance of radiographic examination to assess such changes in RA patients.
Although RF is considered one of the important diagnostic examinations for RA, it showed no statistically significant relation with any of the TMJ measurements or any of the radiographic osteoarthritic changes in the RA patients’ group. So, RF level is a poor predictor of condylar size or radiographic osteoarthritic changes in RA patients. Although previous studies agreed that RF level is high in most of RA patients with TMJ involvement, none of these studies assessed the TMJ changes in RA patients in relation to RF level.33,35,37,40
Anticyclic citrullinated peptides showed statistically significant inverse relation with both height and AP dimensions of the condyle. Thus, ACCP is a better representative of changes in dimensions of the condyle in RA patients. Its higher levels may be an indicator of more destructive disease of the TMJ. On the contrary, ACCP had no statistically significant relation with any of the radiographic osteoarthritic changes in TMJ. None of previous studies of TMJ in RA patients correlated changes in condylar head dimensions or radiographic osteoarthritic changes to the level of ACCP.
DAS28 showed statistically significant inverse relation with both AP and ML dimensions of the condyle in RA patients. High DAS28 score may be an indicator of RA associated changes in condylar head dimensions. Condylar dimensions in relation to DAS28 score in RA patients were not assessed in any of the previous studies.
Out of the eight studied radiographic osteoarthritic changes, DAS28 had a significant relation only with flattening of the condyle and flattening of the eminence. So, DAS28 may reflect TMJ radiographic osteoarthritic changes. Previous studies showed high correlation between DAS28 score and clinical TMJ assessment with high DAS28 as a risk factor for more severe TMJ pain and dysfunction.33,41 Gheita et al37 showed significant correlation between DAS28 and TMJ osteoarthritic change as a whole.37
According to their DAS28, RA patients were classified into remission, low, moderate and high disease activity. None of the RA patients were in remission disease activity stage. Patients with high and moderate disease activity showed significantly smaller AP condylar dimension than patients with low disease activity. Moreover, disease activity showed a significant direct correlation with all radiographic osteoarthritic changes except for erosions of the glenoid fossa and condyle.
From the current study, it could be concluded that DAS28 score and disease activity are strong indicators of TMJ affection in RA patients when compared to RF and ACCP. ACCP is a better indicator of decreased condylar measurements than it represents osteoarthritic changes in TMJs involved with RA. While RF is the least efficient indicator of TMJ involvement in RA patients.
Rheumatoid arthritis patients with high DAS28 or high ACCP should be referred for CBCT of the TMJ for early diagnosis of its involvement offering the patient early access to dedicated TMJ treatment to maintain joint function and prevent joint damage in addition to the general RA treatment fast acting first line and slow acting second line RA drugs. Treatment of affected TMJs can be done by physiotherapy, functional orthodontic devices (e.g. soft splints and activator splints) as well as intra-articular corticosteroids injections and open joint surgery.17,42 On the other hand, patients having radiographic osteoarthritic changes of the TMJ should be referred for RF, ACCP and DAS28 investigations for early identification of RA.
REFERENCES
- 1.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against rheumatism collaborative initiative. Arthritis Rheum 2010; 62: 2569–81. doi: 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 2.Littlejohn EA, Monrad SU. Early diagnosis and treatment of rheumatoid arthritis. Prim Care 2018; 45: 237–55. doi: 10.1016/j.pop.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 3.Aletaha D. Management of rheumatoid arthritis: what happens and what does not happen in real life. Rheumatol Int 2016; 36: 167–8. doi: 10.1007/s00296-015-3402-2 [DOI] [PubMed] [Google Scholar]
- 4.Fiehn C, Krüger K. Management of rheumatoid arthritis. Internist 2016; 57: 1042–51. doi: 10.1007/s00108-016-0132-9 [DOI] [PubMed] [Google Scholar]
- 5.Hamburger J. Orofacial manifestations in patients with inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol 2016; 30: 826–50. doi: 10.1016/j.berh.2016.09.013 [DOI] [PubMed] [Google Scholar]
- 6.Marcelletti JF, Nakamura RM. Assessment of serological markers associated with rheumatoid arthritis: diagnostic autoantibodies and conventional disease activity markers. Clinical and Applied Immunology Reviews 2003; 4: 109–23. [Google Scholar]
- 7.Kung TN, Bykerk VP. Detecting the earliest signs of rheumatoid arthritis: symptoms and examination. Rheum Dis Clin North Am 2014; 40: 669–83. doi: 10.1016/j.rdc.2014.07.009 [DOI] [PubMed] [Google Scholar]
- 8.D'Agostino MA, Haavardsholm EA, van der Laken CJ. Diagnosis and management of rheumatoid arthritis; what is the current role of established and new imaging techniques in clinical practice? Best Pract Res Clin Rheumatol 2016; 30: 586–607. doi: 10.1016/j.berh.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 9.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. The Lancet 2016; 388: 2023–38. doi: 10.1016/S0140-6736(16)30173-8 [DOI] [PubMed] [Google Scholar]
- 10.Rais R, Saeed M, Haider R, Jassani Z, Riaz A, Perveen T. Rheumatoid arthritis clinical features and management strategies at an urban tertiary facility in Pakistan. J Pak Med Assoc 2014; 64: 1435–7. [PubMed] [Google Scholar]
- 11.Nam JL. Rheumatoid arthritis management of early disease. Curr Opin Rheumatol 2016; 28: 267–74. doi: 10.1097/BOR.0000000000000276 [DOI] [PubMed] [Google Scholar]
- 12.Lin Y-C, Hsu M-L, Yang J-S, Liang T-H, Chou S-L, Lin H-Y. Temporomandibular joint disorders in patients with rheumatoid arthritis. J Chin Med Assoc 2007; 70: 527–34. doi: 10.1016/S1726-4901(08)70055-8 [DOI] [PubMed] [Google Scholar]
- 13.Yamakawa M, Ansai T, Kasai S, Ohmaru T, Takeuchi H, Kawaguchi T, et al. Dentition status and temporomandibular joint disorders in patients with rheumatoid arthritis. CRANIO® 2002; 20: 165–71. doi: 10.1080/08869634.2002.11746207 [DOI] [PubMed] [Google Scholar]
- 14.Jayachandran S, Khobre P. Temporomandibular joint in rheumatoid arthritis: clinicoradiological aspects. Indian Journal of Rheumatology 2016; 11: 174–6. [Google Scholar]
- 15.Hirahara N, Kaneda T, Muraoka H, Fukuda T, Ito K, Kawashima Y. Characteristic magnetic resonance imaging findings in rheumatoid arthritis of the temporomandibular joint: focus on abnormal bone marrow signal of the mandibular condyle, Pannus, and lymph node swelling in the parotid glands. J Oral Maxillofac Surg 2017; 75: 735–41. doi: 10.1016/j.joms.2016.09.051 [DOI] [PubMed] [Google Scholar]
- 16.Zou L, He D, Ellis E. A comparison of clinical follow-up of different total temporomandibular joint replacement prostheses: a systematic review and meta-analysis. J Oral Maxillofac Surg 2017;. [DOI] [PubMed] [Google Scholar]
- 17.O'Connor RC, Fawthrop F, Salha R, Sidebottom AJ. Management of the temporomandibular joint in inflammatory arthritis: involvement of surgical procedures. Eur J Rheumatol 2017; 4: 151–6. doi: 10.5152/eurjrheum.2016.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conigliaro P, Triggianese P, Chimenti MS, Lucchetti R, Kroegler B, Perricone R. Serological markers associated with disease activity in patients with rheumatoid arthritis treated with rituximab. J Int Med Res 2016; 44(1 suppl): 53–7. doi: 10.1177/0300060515593240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodkinson B, Meyer PWA, Musenge E, Ally MMT, Wadee AA, Anderson R, et al. The diagnostic utility of the anti-CCP antibody test is no better than rheumatoid factor in South Africans with early rheumatoid arthritis. Clin Rheumatol 2010; 29: 615–8. doi: 10.1007/s10067-010-1374-x [DOI] [PubMed] [Google Scholar]
- 20.Tamimi D, Jalali E. CBCT Evaluation of the TMJ. 143 Contemporary Management of Temporomandibular Disorders: Fundamentals and Pathway to Diagnosis; 2019. [Google Scholar]
- 21.Caruso S, Storti E, Nota A, Ehsani S, Gatto R. Temporomandibular joint anatomy assessed by CBCT images. Biomed Res Int 2017; 2017: 10: 2916953. doi: 10.1155/2017/2916953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machado GL. CBCT imaging - A boon to orthodontics. Saudi Dent J 2015; 27: 12–21. doi: 10.1016/j.sdentj.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishnamoorthy B, Mamatha N, Kumar VA. TMJ imaging by CBCT: current scenario. Ann Maxillofac Surg 2013; 3: 80–3. doi: 10.4103/2231-0746.110069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Vos W, Casselman J, Swennen GRJ. Cone-Beam computerized tomography (CBCT) imaging of the oral and maxillofacial region: a systematic review of the literature. Int J Oral Maxillofac Surg 2009; 38: 609–25. doi: 10.1016/j.ijom.2009.02.028 [DOI] [PubMed] [Google Scholar]
- 25.Cetin P, Solmaz D, Gulluoglu H, Sari I, Birlik M, Akar S, et al. difference Between The Two Versions Of Disease Activity Score Based On C-reactive Protein and Erythrocyte Sedimentation Rate.: 2248. Arthritis & Rheumatism 2013; 65: S956. [Google Scholar]
- 26.Sengul I, Akcay-Yalbuzdag S, Ince B, Goksel-Karatepe A, Kaya T. Comparison of the DAS28-CRP and DAS28-ESR in patients with rheumatoid arthritis. Int J Rheum Dis 2015; 18: 640–5. doi: 10.1111/1756-185X.12695 [DOI] [PubMed] [Google Scholar]
- 27.Tamimi D, Jalali E, Hatcher D, Imaging TJ. Temporomandibular joint imaging. Radiol Clin North Am 2018; 56: 157–75. doi: 10.1016/j.rcl.2017.08.011 [DOI] [PubMed] [Google Scholar]
- 28.Hammer MR, Kanaan Y. Imaging of the pediatric temporomandibular joint. Oral Maxillofac Surg Clin North Am 2018; 30: 25–34. doi: 10.1016/j.coms.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 29.Boeddinghaus R, Whyte A. Trends in maxillofacial imaging. Clin Radiol 2018; 73: 4–18. doi: 10.1016/j.crad.2017.02.015 [DOI] [PubMed] [Google Scholar]
- 30.Kiljunen T, Kaasalainen T, Suomalainen A, Kortesniemi M. Dental cone beam CT: a review. Phys Med 2015; 31: 844–60. doi: 10.1016/j.ejmp.2015.09.004 [DOI] [PubMed] [Google Scholar]
- 31.Shaffer SM, Brismee JM, Sizer PS, Courtney CA, disorders T. Part 1: anatomy and examination/diagnosis. J Man Manip Ther 2014; 22: 2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sağlam AA, Şanli G. Condylar asymmetry measurements in patients with temporomandibular disorders. J Contemp Dent Pract 2004; 5: 59–65. doi: 10.5005/jcdp-5-3-59 [DOI] [PubMed] [Google Scholar]
- 33.Ahmed N, Mustafa HM, Catrina AI, Alstergren P. Impact of temporomandibular joint pain in rheumatoid arthritis. Mediators Inflamm 2013; 2013: 1–6. doi: 10.1155/2013/597419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida A, Higuchi Y, Kondo M, Tabata O, Ohishi M. Range of motion of the temporomandibular joint in rheumatoid arthritis: relationship to the severity of disease. CRANIO® 1998; 16: 162–7. doi: 10.1080/08869634.1998.11746054 [DOI] [PubMed] [Google Scholar]
- 35.Rehan OM, Saleh HAK, Raffat HA, Abu-Taleb NS. Osseous changes in the temporomandibular joint in rheumatoid arthritis: a cone-beam computed tomography study. Imaging Sci Dent 2018; 48: 1–9. doi: 10.5624/isd.2018.48.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Çeliker R, Gökçe-Kutsal Y, Eryilmaz M. Temporomandibular joint involvement in rheumatoid arthritis. Relationship with disease activity. Scand J Rheumatol 1995; 24: 22–5. doi: 10.3109/03009749509095149 [DOI] [PubMed] [Google Scholar]
- 37.Gheita T, Dahaba M, Ahmed E, Khalifa S, Basmy A. Using clinical and multislice computer tomographic features to assess temporomandibular joint osseous involvement in rheumatoid arthritis: a preliminary Study/Romatid Artritte Osseöz Temporomandibüler Eklem Tutulumunun Klinik ve Çok Kesitli Bilgisayarli Tomografi Özellikleri: Ön Çalisma. Turkish Journal of Rheumatology 2012; 27: 47. [Google Scholar]
- 38.dos Anjos Pontual ML, Freire JSL, Barbosa JMN, Frazão MAG, dos Anjos Pontual A, Fonseca da Silveira MM. Evaluation of bone changes in the temporomandibular joint using cone beam CT. Dentomaxillofac Radiol 2012; 41: 24–9. doi: 10.1259/dmfr/17815139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamimi D, Kocasarac HD, Mardini S. Imaging of the temporomandibular joint. Semin Roentgenol 2019; 54: 282–301. doi: 10.1053/j.ro.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 40.Sodhi A, Naik S, Pai A, Anuradha A. Rheumatoid arthritis affecting temporomandibular joint. Contemp Clin Dent 2015; 6: 124–7. doi: 10.4103/0976-237X.149308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moen K, Bertelsen LT, Hellem S, Jonsson R, Brun JG. Salivary gland and temporomandibular joint involvement in rheumatoid arthritis: relation to disease activity. Oral Dis 2005; 11: 27–34. doi: 10.1111/j.1601-0825.2004.01054.x [DOI] [PubMed] [Google Scholar]
- 42.Cascos-Romero J, Vázquez Delgado E, Vázquez Rodríguez E, Gay Escoda C. The use of tricyclic antidepressants in the treatment of temporomandibular joint disorders: systematic review of the literature of the last 20 years. Medicina Oral, Patología Oral y Cirugia Bucal 2009; 14(num 1): 3–7p. [PubMed] [Google Scholar]